Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation
- 1Department of Microbiology, Dr. Harisingh Gour Vishwavidyalaya (A Central University), Sagar, India
- 2Department of Biochemistry and Microbiology, Rhodes University, Makhanda, South Africa
The gut microbiota in the human body is an important component that plays a pivotal role in the ability of the host to prevent diseases and recover from these diseases. If the human microbiome changes for any reason, it affects the overall functioning of the host. Healthy and vigorous gut microbiota require dietary fiber supplementation. Recently, oligosaccharides have been found to play a significant role in the modulation of microbiota. Several such oligosaccharides, i.e., xylooligosaccharides (XOS), mannooligosaccharides (MOS), and arabino-xylooligosaccharides (AXOS), are derived from hemicellulosic macromolecules such as xylan, mannan, and arabino-xylan, respectively. These oligosaccharides serve as substrates for the probiotic production of health-promoting substances (short-chain fatty acids, branched chain amino acids etc.), which confer a variety of health benefits, including the prevention of some dreaded diseases. Among hemicellulose-derived oligosaccharides (HDOs), XOS have been largely explored, whereas, studies on MOS and AXOS are currently underway. HDOs, upon ingestion, help reduce morbidities by lowering populations of harmful or pathogenic bacteria. The ATP-binding cassette (ABC) transporters are mainly utilized for the uptake of oligosaccharides in probiotics. Butyrate generated by the selective fermentation of oligosaccharides, along with other short-chain fatty acids, reduces gut inflammation. Overall, oligosaccharides derived from hemicelluloses show a similar potential as conventional prebiotics and can be supplemented as functional foods. This review summarizes the role of HDOs in the alleviation of autoimmune diseases (inflammatory bowel disease, Crohn's disease), diabetes, urinary tract infection, cardiovascular diseases, and antimicrobial resistance (AMR) through the modulation of the gut microbiota. The mechanism of oligosaccharide utilization and disease mitigation is also explained.
Introduction
Humans are very interested in controlling and minimizing the risk of their personal health, and modern consumers have a strong preference for natural food over processed food (1). An adequate consumption of dietary short-chain carbohydrates, such as dietary fibers and oligosaccharides, decreases the risk of development of diseases such as colorectal cancer (CRC), cardiovascular diseases, obesity, diabetes, etc. (2). The human gut comprises ~1013-1015 microbial cells that amount to a complex and diverse microbial community. Host gut microbiota vary among individuals and have a close relation with different factors such as the sex, age, health, diet, genetic makeup, and immune system of the host. However, Firmicutes, Bacteroidetes, and Actinobacteria comprise the most common microbiota residing in the human gut (3). Together, these communities contain much more genomic information (100-fold) than the host itself, which leads to functional expansion of the abilities of the host. Functional oligosaccharides directly influence the gut microbiota and help to produce different key health-promoting metabolites, which are directly associated with the physiology of the host (4, 5). For this reason, the physicochemical and physiological properties of non-digestible carbohydrate fibers have drawn the attention of food scientists who explore them as functional food ingredients. Different types of oligosaccharides, like galactooligosaccharides (GOS), fructooligosaccharides (FOS), and inulooligosaccharides (IOS), are already recognized as nutraceuticals and are frequently used in synbiotic pharmaceutical preparations. Hemicellulose-derived oligosaccharides (HDOs) such as XOS and mannooligosaccharides (MOS) are rapidly emerging prebiotics, which fall in this category and have similar bioactive properties as conventionally used oligos (FOS) (6). XOS and MOS are easily produced from different low-value substrates (locust bean gum, guar gum, and konjac gum), as well as different agro-wastes (corn cob, copra meal, palm kernel cake, and corn cob), by enzymatic hydrolysis using hemicellulases such as endo-β-(1 → 4)-xylanase and endo-β-(1 → 4)-mannanase (7, 8). These oligosaccharides contribute to the important physiological functions of dietary fibers: (1) Their consumption does not increase the blood glucose level or spike the secretion of insulin because of the formation of a gel in the gut through which it dissolves, (2) their nature is non-cariogenic and low calorific (0–3 kcal/g of sugar), (3) they stimulate the growth of specific microorganisms that enrich the gut environment by decreasing pH, and (4) they ameliorate the absorption of the minerals (mainly calcium) through the intestinal cells. Thus, HDOs work as a silent health promoter, thus lowering the risk of different complex health issues (9, 10).
From a commercial point of view, the oligosaccharide market is increasing rapidly and is expected to reach a total turnover of 7.37 billion USD by 2023. In the case of XOS, the market is growing at a rate of ~4.1% per annum and is expected to reach a projected value of 130 million USD by 2025 from 94 million USD in 2018 (11). This high growth is mainly because of the advanced scientific research and continuous development in the field of oligosaccharides and their product development. These oligosaccharides are also being utilized in pharmaceuticals, feeds, cosmetics, and as immunostimulating agents and bulking agents (12). Some of the commercially produced oligosaccharides and their uses are indicated in Table 1. The nutritional significance of oligosaccharides (including HDOs) has been indicated in various diseases, like heart infections, autoimmune diseases, osteoporosis, and many chronic diseases. Conventional oligosaccharides such as FOS are well-documented, while HDOs are emerging and scientists are trying to establish their role in the amelioration of diseases. This review summarizes the biochemical properties of HDOs and their impact in reducing the development of diseases through the modulation of gut microbiota.
Gut Microbiota and HDOs
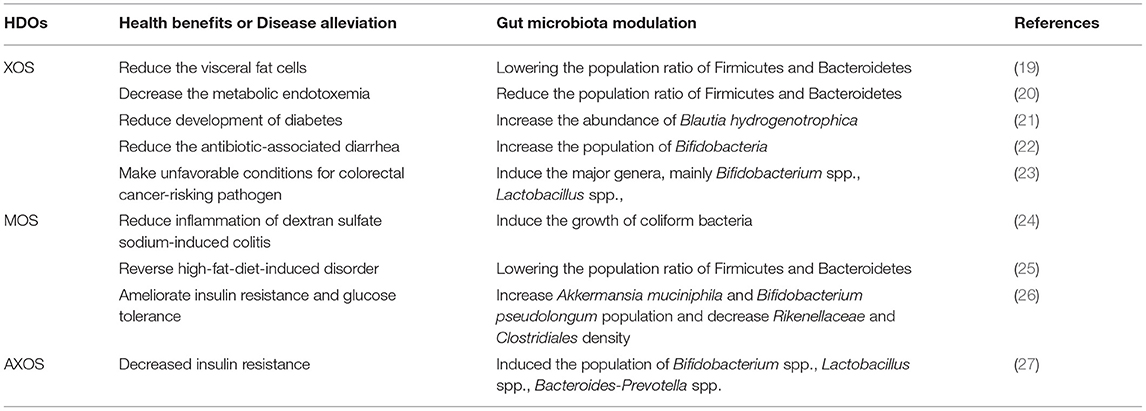
Gut microbiota are a collective environment of microorganisms, like bacteria, fungi, viruses, and protozoans present in the gastrointestinal tract (GIT) (13). These microorganisms act as regulators of the metabolism of the host. The role of gut microbiota in disease control has drawn a significant attention over the past few decades, which were kept hidden for a long time in the absence of metagenomic techniques and suitable cultivation media. Currently, an analysis of the collective genome of the gut microbiota through the next-generation sequencing has helped us in unraveling complex gut ecosystem (14). Gut microbiota of humans start developing in the fetus itself and are strongly influenced by the microbiome of the mother. Alterations in the microbiome completely depend upon several factors, such as the process of parturition, surrounding environment, infant feeding method, lifestyle, stress, and diet. The key taxa involved in the gut microbial diversity of individuals include Lactobacillus, Ruminococcus, Bifidobacterium, Clostridium, Eubacterium, Akkermansia, Butyrivibrio, Roseburia, Prevotella, Faecalibacterium, Bacillus, Oxalobacter, Lachnospiraceae, and Blautia (15). Breast milk contains higher amounts of Bifidobacterium and Lactobacillus; therefore, infants on high-breast-milk diet tend to have a greater preponderance of the two probiotic species (16). In addition, the Firmicutes/Bacteroidetes ratio may be an important biomarker in the case of humans that exhibit morbidity (17, 18). Thus, it may be concluded that gut microbiota play a pivotal role in human physiology and have an impact on the alleviation of diseases for better health. The gut microbiota can be modulated favorably using prebiotic oligosaccharides. Apart from the frequently used FOS, and more recently, HDOs have been found to confer a selective advantage to gut microbiota (Table 2). The intake of HDOs was found to diversify the human gastrointestinal microbiota and increase the defense against different chronic non-communicable diseases (28). XOS were found to reduce gut disturbance, as well as gut inflammation, by lowering the Firmicutes/Bacteroidetes ratio and Enterobacteriaceae in obese rats (20). XOS have also been indicated in resisting weight gain by increasing the population of Bifidobacteria and Lachnospiraceae in cecum microbiota (29), while MOS have been found to reduce the gut inflammation by decreasing the Clostridium content in gut microbiota of piglets (30).
Types of HDOs
The two major plant hemicelluloses are xylan and mannan, and accordingly, XOS and MOS make up most of the potential HDOs. Due to the diverse and heteropolymeric nature of xylans and mannans, the nature of derived oligosaccharides varies. In general, a repertoire of hydrolyzing enzymes is employed to achieve the controlled degradation of the hemicelluloses.
Xylooligosaccharides
Xylooligosaccharides are short oligomers composed of xylose moieties and are commonly derived from corn cob, bamboo shoots, wheat straw, sugarcane bagasse, and hardwood xylan. Among these, corn cob contains the highest amount of xylan (35–38%), and hence, it can be exploited as a cheap and renewable source of XOS (31). Based on the sugar units and linkages present, the exact composition and the quantity of the produced XOS vary from one plant source to another. XOS generated after the hydrolysis of xylan are short-chain XOS and consist of xylobiose, xylotriose, and xylotetraose. XOS can be produced by the action of xylan-degrading enzymes such as endo-β-1,4-xylanase (EC 3.2.1.8) and xylan 1, 4-β-xylosidase (EC 3.2.1.37), with the help of other side chain-degrading enzymes, like α-glucuronidase (EC 3.2.1.139), acetyl xylan esterase (EC 3.1.1.72), α-L-feruloyl esterase (EC 3.1.1.73), and arabinofuranosidase (EC 3.2.1.55) (32). The sources and structural details of xylan-derived MOS are presented in Figure 1.
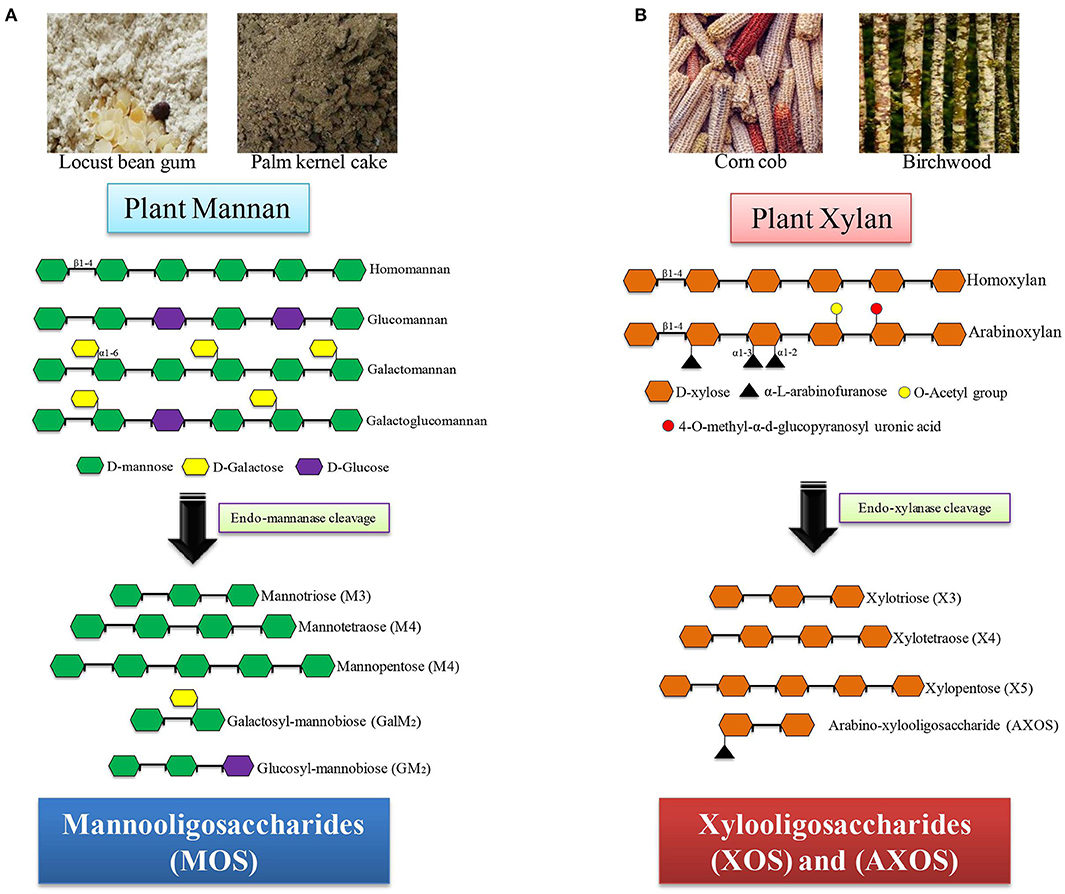
Figure 1. Sources and structure of hemicelluloses-derived oligosaccharides (HDOs). (A) Mannan containing substrates (locust bean gum, palm kernel cake) produce different mannooligomers after mannanase treatment. (B) Xylan containing substrates (corn cob, birchwood) produce xylooligomers after xylanase treatment.
Mannooligosaccharides
Mannooligosaccharides are short chains of repeating units of mannose linked by glycosidic bonds. Based on the source, the MOS are divided into two major groups, namely, α- and β-MOS. α-MOS are commonly obtained from the physicochemical hydrolysis of the cell wall of the yeast (Saccharomyces cerevisiae). α-MOS are commonly used in the animal agriculture sector as a feed additive. β-MOS are plant-derived MOS obtained after enzymatic, alkaline, or acidic hydrolysis of plant β-mannan and are commonly found in locust bean gum, konjac gum, and guar gum. β-MOS can be generated by applying a combination of β-1, 4-mannanase (EC 3.2.1.78), β-mannosidase (EC3.2.1.25), α-galactosidase (EC 3.2.1.22), and β-glucosidase (EC 3.2.1.21) for the hydrolysis of plant mannans such as LBG, GG, and KG (33, 34). The sources and structural details of mannan-derived MOS are given in Figure 1.
Mechanism of Transport of Oligosaccharides
Probiotic bacteria supplemented with prebiotic oligosaccharides are reported to impart several health benefits, including improved immunity and relief in gastrointestinal disorders, but the precise route of specific oligosaccharide uptake is yet to be determined. According to reports available thus far, three main transport systems, such as ATP-binding cassette (ABC) transporters, major facilitator superfamily (MFS) transporters, and the phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS) (Figure 2), are used to transport these oligosaccharides (35). ABC transporters import oligosaccharides by utilizing energy from ATP hydrolysis and are regulated by the two-component systems (TCSs), alongside the involvement of a different gene cluster for polymer hydrolysis (Table 3) (43). The drivers of ABC transporters are the nucleotide-binding domains (NBDs) that conduct the transport of substrates across cell membranes by ATP binding and hydrolysis. The xyloside ABC transporter functions as a membrane permease that promotes the transport of XOS across the cell membrane (36). The ATPase present in the ABC transporter has several domains that interact with more than one ABC transporter, and it simultaneously functions with transporters that form a complex network of ABC transporters (44). Streptomyces thermoviolaceus has been shown to have two integral ABC transporters in the cell membrane containing a conserved EAA (glutamic acid-alanine-alanine) domain. These two proteins are regulated by a transcriptional regulator protein that directs the uptake of XOS along with the degradation the xylan polymer (45). A MFS transporter acts as a symporter like XOS in bacteria and performs the transport through xyloside/Na+ (H+) symporters. Xyloside/Na+ (H+) symporters are the multipass transmembrane proteins in the cell membrane that transport XOS alongside with sodium as a proton motive force (36). The PTS operates through a facilitated diffusion where imported sugar is phosphorylated and the modification does not allow these sugars to diffuse back from the cell. During evolution, the PTS has emerged in the late bacterial evolution stage and, therefore, could not be found in many early bacterial lineages. It is found mainly in lactic acid bacteria and enterobacteria. In particular, homo-fermentative lactobacilli have significant PTS transporters compared to hetero-fermentative lactobacilli (46).
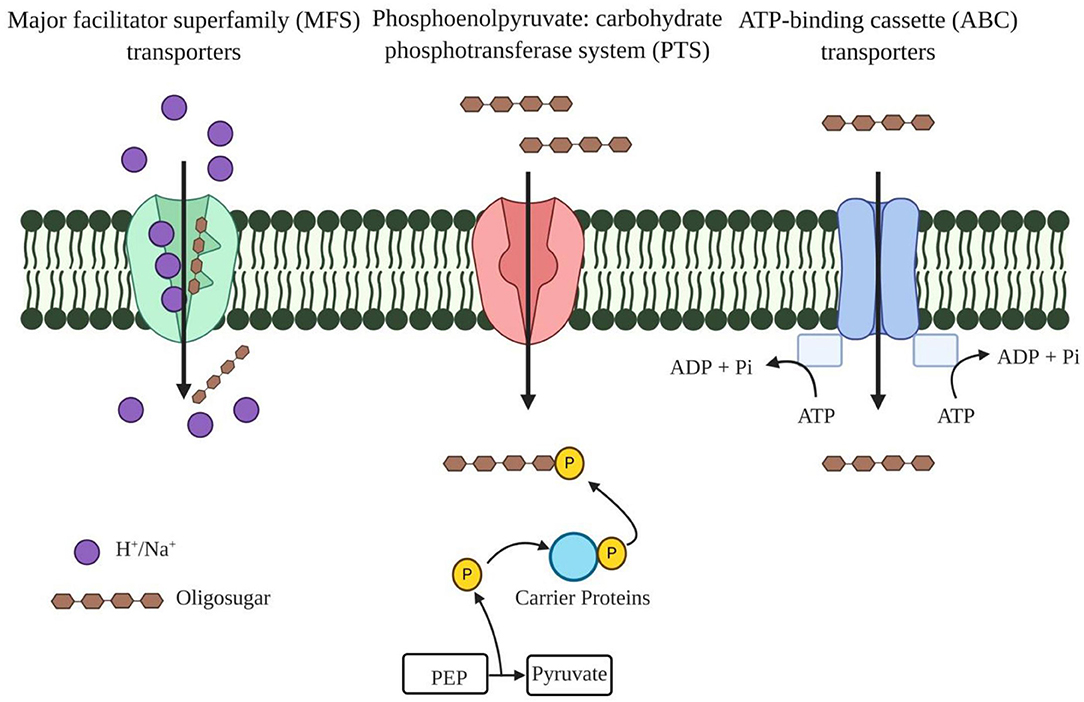
Figure 2. Different oligosaccharide transporters present in the plasma membrane of bacteria. Major facilitator superfamily (MFS) transporters function as a symporter that transport oligosaccharide alongside with Na+/H+ as a proton motive factor. Phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS) transports the oligosaccharide and transported oligosaccharides are further phosphorylated to diffuse back from the cell. ATP binding cassette transporters (ABC) have nucleotide binding domains (NBDs) as these power the transport of substrates across cell membranes by ATP-binding and hydrolysis.
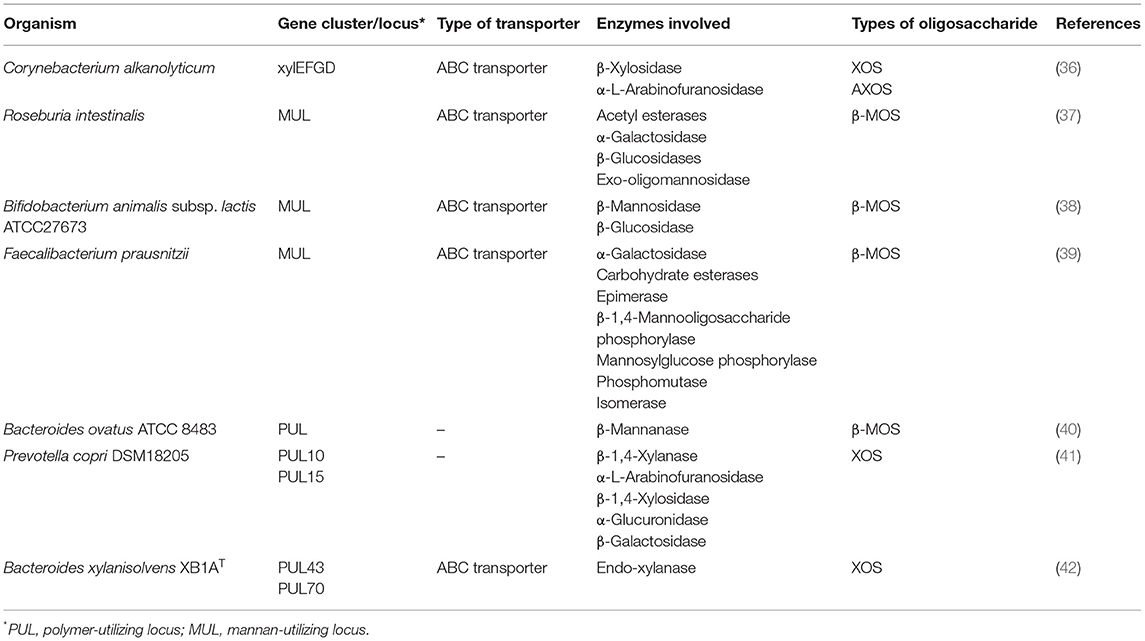
Table 3. Gene clusters and enzymes involved in polysaccharide utilization by microorganisms present in the gut.
The β-MOS ABC transporter in Bifidobacterium animalis has two oligosaccharide-specific extracellular lipid-anchored solute-binding protein (SBP) genes. This transporter recognizes the mannosyl unit of MOS at position 2 through the asparagine and glycine amino acids, respectively (38). La Rosa et al. (37) reported that the Firmicute, Roseburia intestinalis, has an ABC transporter containing three subunits that transported the smaller MOS from all types of mannans (glucomannan, galactomannan, acetyl-galactoglucomannan, and undecorated mannan), which were further completely hydrolyzed by the intracellular enzyme cocktail. Unlike the sugar/cation symporters, mannoside/Na+ (H+) symporters were also noticed in Bacteroides fragilis that transferred MOS into the bacterial cell (47).
Mode of Action of Oligosaccharides Through SCFAs
Different studies have concluded that oligosaccharides prevent gut damage caused by pathogenic bacteria or a diet containing harmful chemicals such as lectin (48). The oligosaccharides enhance the population of probiotic bacteria and increase the production of short chain fatty acids (SCFA's), including acetate, propionate, and butyrate (7, 49). These acids have an important role in increasing the transepithelial fluid transport and epithelial defense barrier and in decreasing mucosal inflammation and oxidative stress. They play a significant role in hypercholesterolemia, insulin resistance, hemoglobinopathies, and genetic metabolic diseases. SCFAs are absorbed and utilized by the enteric cells as the main source of energy (50). The transportation of the SCFAs in the colonic epithelium and mucosal immune cells is directed through different transporters like monocarboxylate transporters, G-protein-coupled receptors (GPCRs), intracellular receptors, and several other enzymes. The main transporters involved in SCFA transportation are monocarboxylate transporters 1 and 4 (MCT-1 and MCT4), which are basically proton-coupled transporters, e.g., sodium-coupled monocarboxylate transporter 1 (SMCT-1) (Figure 3). The MCT-1 is expressed in both the apical and basolateral sides of the colonic cells, while MCT-4 is found at the basolateral side (Figure 3). SCFAs are used as ligands by many GPCRs, mainly GPR41, GPR43, and GPR109A for initially different signaling cascades. Other two less explored transportation mechanisms are through the ABC superfamily G member 2 (ABCG2) and SCFA−/ exchanger. ABCG2 is expressed in the apical colonic cells where it binds with butyrate as a substrate for efflux in the intestinal cells. The SCFA−/ exchanger is present in the small intestine and colon, and the secretion of leads to an increased uptake of SCFA− into the vesicles (51, 52). Butyrate has an inhibitory effect on histone deacetylase (HDAC), which leads to the modulation of different oncogenic signaling pathways such as the JAK2/STAT3 and VEGF pathways, and it can also influence the extrinsic apoptotic and mitochondrial apoptotic pathways. Butyrate is shown to relieve gut inflammation by regulating the Treg cell differentiation and NF-κB and STAT3 pathways (53, 54).
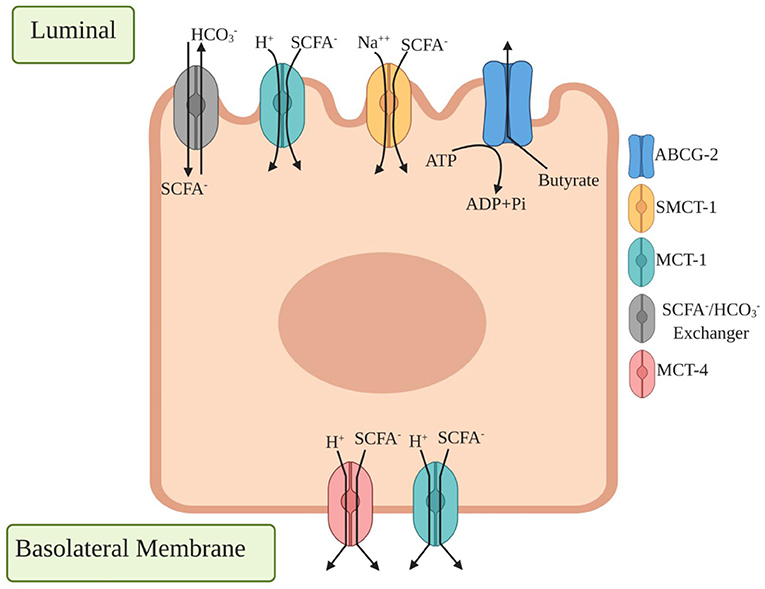
Figure 3. Different transporter proteins expressed in colonic epithelium cells for transport of short chain fatty acids (SCFA). Luminal side expressed transporters are /SCFA− exchangers which secrete that result in SCFA− transport across the apical membrane, monocarboxylate transporter 1 (MCT1) binds a proton first followed by monocarboxylates such as lactate, pyruvate, and transport to the cell where basolateral side expressed monocarboxylate transporter 4 (MCT4) functions similarly by exporting SCFA− from the basolateral membrane. ATP binding cassette transporters G family Member 2 (ABCG2) is expressed in apical colonic cells where they bind with butyrate as a substrate for efflux in intestinal cells.
Role of HDOs in Disease Alleviation
Cardiovascular Diseases
Cardiovascular diseases are a major health concern in low- and middle-income countries causing an estimated 31% of all the global deaths (https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). The abnormality of lipid content in serum is a biomarker of cardiovascular risk, which is influenced by daily diet components. Diets with plant-based food such as “Mediterranean diet” and “Prudent diet” have been shown to be protective compared to the “Western diet” (55). The high-fat diet and low fiber intake result in the Western diet higher growth of Firmicutes and lowering of Bacteroidetes in the gut, which is an important marker of obesity (56). Different studies using different model organisms showed that plant-derived, non-digestible oligosaccharides, such as XOS and MOS, have a high impact on serum lipoprotein and cholesterol levels, which compensate for the higher risk of cardiovascular disease (57). XOS supplementation reduced lipogenesis by decreasing the activity of different lipogenic enzymes such as fatty acid synthetase, malic enzyme, and others (58). On the other hand, it also upregulated the lipoprotein lipase, which was responsible for breaking down fat in the form of triglycerides (lipolysis) (59). Moreover, the XOS diet mainly reduced the visceral fat cells by downregulating the Mcp 1 gene, rather than the blood and liver fat cells, by lowering the population ratio of obesity-related microbiota Firmicutes and Bacteroidetes (19). XOS, especially xylobiose, was able to downregulate the lipogenic and adipogenic genes in mesenteric fat and liver in case of a high-fat-diet-supplemented mice (60). A high-fat diet induced macrophage infiltration in adipose tissue, insulin resistance, metabolic endotoxemia, inflammatory stress, and other biochemical issues in the body system. AXOS treatment in diet-induced obese mice altered the gut microbiota, resulting in higher levels of appetite suppressing satietogenic peptides, which reduced macrophage infiltration in the adipose tissues (27).
Obesity
Obesity is a metabolic disorder that occurs due to excessive fat accumulation in the body after being fed a high-fat diet. This lifestyle disease is a result of energy-balance dysregulation, an unhealthy sedentary routine, and also individual genetic traits (25). According to the WHO, 1.9 billion adults were found to be overweight, and among these, over 650 million adults were obese. In 2016, data showed that 13% of the adult populations (11% of men and 15% of women) of the world were obese. The increase in obesity nearly tripled between 1975 and 2016, which is not only a problem in high-income countries but also a rising problem in low- and middle-income countries (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). A healthy diet could modulate the gut microbiota, which supported a loss in body weight. It was reported that an abundance of a particular bacterial group like Firmicutes/Bacteroidetes had a positive effect in overcoming obesity in mice. Different studies have indicated that the gut microbiota can be modulated by different oligosaccharides, which have a preventive effect on obesity. MOS intake could reverse the high-fat-diet-induced disorder by restructuring the overall composition of the gut microbiota, including lowering the Firmicutes/Bacteroidetes ratio (25). Coffee-based MOS, either as a mixture or in the pure form, had a different effect on the fat deposition in the tissue. The MOS mixture was reported to decrease the gain in body weight, body fat, and visceral adipose tissue, whereas, purified MOS had no such effect on these parameters (61). Prebiotic oligosaccharides were shown to have gender-specific activity in the reduction of fat. In a study, men consuming an MOS containing beverage experienced a greater weight loss than their female counterparts, which signified the importance of MOS for strategic weight management and improvement in adipose tissue distribution (62). MOS can also control excess appetite-causing genes and modulate the expression of appetite-related hormones like leptin, proopiomelanocortin (POMC), cocaine- and amphetamine-regulated transcript (CART), and neuropeptide Y (NPY) (63). XOS consumption decreased metabolic endotoxemia and reduced the Firmicutes/Bacteroidetes ratio in the obese rat (20). Monocyte chemoattractant protein 1 (MCP-1) is one of the major proteins found in white adipose tissue that is overexpressed in the obese compared to those persons of proper weight. MCP-1 helps to differentiate the adipocyte, which decreases insulin-stimulated glucose uptake (64). XOS significantly reduced the plasma levels of MCP-1 by lowering the mRNA expression in high-fat diet-induced obesity (65).
Type 2 Diabetes
Type 2 diabetes is a chronic metabolic condition that arises when pancreatic β-cells lose their function (66, 67). According to the IDF (International Diabetes Federation) Diabetes Atlas (9th edition, 2019), ~463 million adults aged between 20 and 79 years have diabetes, and it will increase to 700 million by 2045. Among these, 374 million people will have the risk of developing Type 2 diabetes (https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html). There is a significant role of the gut microbiota in managing Type 2 diabetes. Previous reports suggested that members of the genera Roseburia, Faecalibacterium, Bacteroides, and Akkermansia reduced T2D, whereas, Fusobacterium, Ruminococcus, and Blautia had a positive association with T2D (68). XOS derived from rice husk reduced insulin resistance and signaling and enhanced glucose uptake by altering the gut microbiota and mitigating endotoxemia. Glucose transporter 4 (GLUT-4) is known as the major glucose-transporting protein that promotes glucose uptake into the skeletal muscles and also controls glucose homeostasis in the body. XOS administration decreased the expression of GLUT-4 and transferred it from the cytosolic compartment to the plasma membrane by Akt (protein kinase B) activation through phosphorylation (69). Prediabetic adults had a higher percentage of Howardella, Enterorhabdus, and Slackia populations in their stools. XOS treatment increased the abundance of positively associated microflora species such as Blautia hydrogenotrophica (21). Similarly, MOS obtained from konjac glucomannan modulated the gut microbiota by increasing the population of Akkermansia muciniphila and Bifidobacterium pseudolongum, while decreasing Rikenellaceae and Clostridiales density (26). At the molecular level, MOS improved glucose and insulin tolerance by modulating the insulin signaling pathway through the activation of GLUT-2 and its translocation into the membrane. MOS also upregulated the expression of leptin-associated protein and downregulated the negative regulators of the insulin signaling pathway proteins, protein tyrosine phosphatase 1B, and suppressor of cytokine signaling 3 (70).
Autoimmune Diseases [Inflammatory Bowel Disease]
Inflammatory bowel disease covers two major diseases [Crohn's disease (CD) and ulcerative colitis (UC)], which severely affect the GI tract, resulting in chronic and relapsing conditions. In the diseased state, patients with CD face intense inflammation in the GI tract, whereas, UC results in tissue damage of the deep areas of the colon and rectum (71). According to the Center for Disease Control and Prevention, 3 million (1.3%) US adults were diagnosed with IBD in 2015 (https://www.cdc.gov/ibd/data-statistics.htm). The main etiology in dysbiosis of gut microbiota is the reduction in community of commensal microorganisms due to an abnormal T-cell-mediated immune response (72). Several studies have demonstrated that supplements such as non-digestible carbohydrate fibers in a diet can enhance the growth of selective commensal microorganisms through anaerobic fermentation and attenuate the disease complications by alleviating gut inflammation. The fiber supplementation also improved the disrupted cell morphology, which developed due to the diseased state of the colon. Hemicellulose-derived XOS not only reduced the inflammation, but also helped to maintain the colon crypt cell integrity in attenuated chronic colitis in rats (73). These oligomers are fermented with varied efficiency depending upon the gut microbiota diversity. β-Diversity of the gut microbiota in patients with UC was significantly promoted by XOS treatment, whereas, α-diversity could not utilize and ferment XOS (74). In CD, different related genes and proteins like nucleotide-binding oligomerization domain-containing protein 2 (NOD2), immunity-related GTPase family M (IRGM), autophagy-related 16 Like 1 (ATG16L1), the toll-like receptor 4 (TLR4), and proinflammatory cytokines (IL-10, IL-1a, IL-1b) were found to be present, while the deterioration of α- and β-diversity of gut microbiota was also recorded (72). Treatment with synbiotic XOS and Bifidobacterium infantis downregulated the proinflammatory cytokines (TNF-α and IL-1β) and upregulated the anti-inflammatory cytokines (IL-10) in the colon cells of a dextran sodium sulfate-induced mouse. XOS supplementation significantly enhanced the expression of different junction proteins, including claudin-1 tight junction (TJ), zonula occludens-1 (ZO-1), and occluding junction in the colon tissue (75). Apart from XOS, MOS have also been found to alleviate IBD symptoms. MOS administration to dextran sulfate sodium-induced colitis mice enhanced the growth of coliform bacteria and lowered the expression of different proinflammatory cytokines (IL-5, IL-1a, IL-1b, G-CSF, and MCP-1). Also, MOS normalized the expression of muc2 (intestinal mucin) in the goblet cells of the colon and small intestine (24).
Colorectal Cancer
Cancer is an abnormal growth of normal cells in any site of the body. It is the cause of most deaths globally with an estimate of 9.6 million in 2018 (https://www.who.int/news-room/fact-sheets/detail/cancer). CRC is one of the major causes of death and arises due to an increased intake of animal-based food diet rather than a plant-based diet (76). A plant-based diet contains high amounts of non-digestible short-chain carbohydrates that retard the growth of CRC by either fermenting it with the help of gut microbiota or directly binding to the cell surface receptor (77). XOS reduced 1, 2-dimethylhydrazine (DMH)-induced artificial colon cancer by activating glutathione-S-transferase and catalase present in the liver and colonic mucosa (78). The fermentation of oligosaccharides resulted in the production of metabolites such as SCFAs, which lowered the pH of the gut and produced the unfavorable condition of CRC-risking pathogens by enhancing the growth of lactobacilli and bifidobacteria. SCFAs also lowered the carcinogenic products and suppressed the bacterial conversion of pro-carcinogens to carcinogens (23). Among the SCFAs, butyrate was more lethal to CRC cells and inhibited their growth by inducing histone hyperacetylation and blocking the histone deacetylase (79). The synbiotic approach was useful in this case—a XOS and Weissella cibaria combination acted differently, where XOS significantly increased the acidification rate, while W. cibaria reduced cancer cell proliferation by inhibiting the different proteins, namely, TLR4, MyD88, MD2, and NF-κb (80). It was also reported that in some instances, XOS had a more pronounced inhibitory effect on the precancerous colon lesions than conventional oligosaccharides such as FOS and decreased the amount of aberrant crypt foci in the colon (81).
Diarrhea
Diarrhea is a diseased state when colon cells are unable to absorb fluid sufficiently. Secretory diarrhea mainly results in lower absorption of essential ions due to infection and the release of toxins by pathogenic microorganisms and the depletion of beneficial microflora (82). Approximately 88% of diarrheal patients die because of insufficient hygiene, unsafe water, and inadequate medication. Among these patients, rotavirus infections in children below 5 years cause about 40% of hospitalizations for diarrhea (https://www.cdc.gov/healthywater/pdf/global/programs/globaldiarrhea508c.pdf). Nutraceuticals such as oligosaccharides may play an important role in alleviating diarrhea by selectively enhancing the population of favorable microorganisms (9). XOS lead to a significant increase in the Bifidobacterium population and also enhance the total counts of anaerobic bacteria and Bacteroides fragilis (83). Similarly, a synbiotic containing Lactobacillus paracasei with XOS and arabinogalactan significantly reduced diarrhea compared to the placebo group in children (84). A healthy human gut usually has a low bacterial translocation (BT) but extensive pathogen invasion increases the BT, which leads to a disruption of the gut barrier function (85). Consumption of AXOS not only increased the population of bifidobacteria in the gut but also decreased the disorders linked with antibiotic-associated diarrhea. In addition, AXOS intake enhanced the butyrate production in the colon, which further maintained the gut biological barrier function (22). In addition, a high intake of fermentable but poorly digestible carbohydrates has also been shown to confer negative effects to humans and animals. A high intake of XOS induced diarrhea due to subchronic oral toxicity and carbotoxicity (86). In general, high XOS consumption in human subjects was responsible for transient gastrointestinal discomfort (87).
Urinary Tract Infections
Urinary tract infections are the most common infections occurring in the urinary system, including the urethra, bladder, ureters, or kidneys. UTIs are diagnosed in over 150 million people worldwide each year. UTIs are significantly more common in infants, older men, and women of all ages (88). Among these, it has been reported that women are most affected with a 12.6% annual incidence of this disease, compared to men showing only 3% annual incidence (89). The main causative agent for the disease is uropathogenic Escherichia coli (UPEC), and 70–80% cases of UTIs also showed the presence of Staphylococcus, Klebsiella, and Enterococcus species (90, 91). These pathogens make up the first line of infection through binding of the oligosaccharides present on the epithelial layer with the help of their own carbohydrate-binding proteins, and therefore, the presence of external oligosaccharides will be the first-line defense (92). Among all the HDOs, arabino-xyloglucan oligosaccharides have been found to be effective in the prevention of UTIs. Cranberry (Vaccinium macrocarpon) juice containing arabino-xyloglucan oligosaccharides showed an anti-adhesion effect against P-fimbriated E. coli in swine (93). The oligosaccharides have an adverse effect on different surface proteins which are involved in substrate translocation, including sugar across the bacterial cell membrane (94). In another study, high mannose-containing glycoprotein oligosaccharides attached to the pathogenic fimbriated E. coli were found to be the reason for mannose-inhibited hemagglutination (95).
Antimicrobial Resistance
Antimicrobial resistance is acquired when pathogenic microorganisms develop the ability to overcome drug treatment. AMR is also frequently termed as antibiotic-resistant infection. According to the CDC, every year about 2.8 million people are diagnosed with infection of antibiotic-resistant bacteria. The first case of AMR was identified in 1942 with penicillin-resistant Staphylococcus aureus as the causal organism. There are three ways a microorganism can develop AMR: (1) selective pressure, where selective antimicrobial gene carrying survivors dominate the population after sudden treatment with antimicrobial agents; (2) mutations in different genes help a microorganism to survive against antimicrobial agents; and (3) horizontal gene transfer, where a drug resistance gene gets transferred to the non-drug-resistant microorganism. Salmonella enteritidis is one of the major causal agents of salmonellosis in birds and mammals. Multidrug-resistant phenotypes of S. enteritidis enter the human body via animal food consumption through the food chain. For this reason, poultry animals infected with S. enteritidis are a major source of infection in humans. Feed supplementation with MOS and XOS reduces S. enteritidis in poultry animals by increasing specific health-promoting microorganisms (96). MOS increased the population of Bifidobacterium spp., while decreasing the populations of Enterobacteriaceae members and Enterococcus spp. The increased population of Bifidobacteria produces SCFAs that reduce the pH. XOS promoted the growth of diverse groups of commensals like Lactiplantibacillus and Levilactobacillus, to produce high amounts of organic acids, which had an antimicrobial effect on both pathogenic bacteria and fungi (97). It has been shown that the antimicrobial activity of XOS is limited to the gastrointestinal digestion tract and that its effects are greatly reduced in later stages (98). Corncob XOS exhibited an increased digestion and lowering of lipid vacuolization in fish, Dicentrarchus labrax, challenged with the pathogen Aeromonas hydrophila. Dietary supplementation with XOS also enhanced the serum immunoglobulin level, serum protein content, and lysozyme activity (99). The high intake of these oligosaccharides induces the production of the antimicrobial peptides (AMPs). AMPs are short, positively charged, defense peptides that kill pathogenic microorganisms directly or indirectly. MOS supplementation induced higher transcriptional levels of antimicrobial peptides in the head-kidney and spleen of Ctenopharyngodon idella (100, 101).
Conclusion and Future Prospects
Hemicellulose-derived oligosaccharides offer themselves as a viable and cost-effective alternative to the conventional prebiotics, such as FOS, GOS, and trans-galactooligosaccharides (TOS). Information on the modulation of microbiota and disease recovery of the host upon the ingestion of HDOs is rapidly emerging. The dynamics of microbial diversity and gut environment upon the consumption of HDOs, under both normal and diseased conditions, is being explored at a faster pace. Noteworthy mechanisms delineating the utilization of HDOs, leading to the favorable modulation of gut microbiota, are being unraveled. Knowledge of their interaction with commensal and pathogenic bacteria will lead to the development of a more efficient HDO-based prebiotic formulation that would target the specific gut microbiota for better health.
In spite of a large number of biochemical investigations, well-designed clinical trials are necessary for establishing the specific alteration in gut microbiota at the taxa level by the HDOs. Clinical trials are usually limited to animal models, but can be utilized for deciphering the particular mechanism of action of HDOs in health-promoting activities. In vitro studies with particular groups of microorganisms will also be helpful in revealing information about the gut environment. Statistical approaches and machine learning with multi-omics will provide further details and unravel the interactions between the host, microbes, and HDOs.
Author Contributions
UJ provided the idea and was involved in conceptualization, visualization, writing the original draft, and writing the review and editing. NK and BP were involved in supervision, resources, and writing the review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author UJ is grateful to the Indian Council of Medical Research (ICMR), New Delhi, for providing financial assistance as a Senior Research Fellow (SRF). The infrastructural support of sophisticated instrumentation center and DST-PURSE (II) at Dr. Harisingh Gour Vishwavidyalaya, Sagar, is duly acknowledged. Figures 2, 3 were generated with the help of Biorender (Biorender.com). BP acknowledges the financial support received from Rhodes University.
References
1. Román S, Sánchez-Siles LM, Siegrist M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci Technol. (2017) 67:44–57. doi: 10.1016/j.tifs.2017.06.010
2. Li YO, Komarek AR. Dietary fibre basics: health, nutrition, analysis, and applications. Food Qual Saf. (2017) 1:47–59. doi: 10.1093/fqs/fyx007
3. Erejuwa OO, Sulaiman SA, Ab Wahab MS. Oligosaccharides might contribute to the antidiabetic effect of honey: a review of the literature. Molecules. (2012) 17:248–66. doi: 10.3390/molecules17010248
4. Dahiya DK, Renuka Puniya M, Shandilya UK, Dhewa T, Kumar N, et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front Microbiol. (2017) 8:563. doi: 10.3389/fmicb.2017.00563
5. Kho ZY, Lal SK. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. (2018) 9:1–23. doi: 10.3389/fmicb.2018.01835
6. Jana UK, Suryawanshi RK, Prajapati BP, Kango N. Prebiotic mannooligosaccharides: synthesis, characterization, and bioactive properties. Food Chem. (2020) 342:128328. doi: 10.1016/j.foodchem.2020.128328
7. Jana UK, Kango N. Characteristics and bioactive properties of mannooligosaccharide derived from agro-waste mannans. Int J Biol Macromol. (2020) 149:931–40. doi: 10.1016/j.ijbiomac.2020.01.304
8. Khangwal I, Nath S, Kango N, Shukla P. Endo-xylanase induced xylooligosaccharide production from corn cobs, its structural features, and concentration-dependent antioxidant activities. Biomass Convers Biorefinery. (2020). doi: 10.1007/s13399-020-00997-3
9. Qiang X, YongLie C, QianBing W. Health benefit application of functional oligosaccharides. Carbohydr Polym. (2009) 77:435–41. doi: 10.1016/j.carbpol.2009.03.016
10. Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcif Tissue Int. (2018) 102:443–79. doi: 10.1007/s00223-017-0339-3
11. Santibáñez L, Henríquez C, Corro-Tejeda R, Bernal S, Armijo B, Salazar O. Xylooligosaccharides from lignocellulosic biomass: a comprehensive review. Carbohydr Polym. (2021) 251:117118. doi: 10.1016/j.carbpol.2020.117118
12. Samanta AK, Jayapal N, Jayaram C, Roy S, Kolte AP, Senani S, et al. Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact Carbohydrates Diet Fibre. (2015) 5:62–71. doi: 10.1016/j.bcdf.2014.12.003
13. Cresci GAM, Izzo K. Gut microbiome. In: Corrigan ML, Roberts K, Steiger E, editors. Adult Short Bowel Syndrome. London: Academic Press (2019). p. 4554. doi: 10.1016/B978-0-12-814330-8.00004-4
14. Lepage P, Leclerc MC, Joossens M, Mondot S, Blottière HM, Raes J, et al. A metagenomic insight into our gut's microbiome. Gut. (2013) 62:146–58. doi: 10.1136/gutjnl-2011-301805
15. Ashman S, Krishnamurthy H. The gut microbiome. In: Yafi FA, Yafi NR, editors. Effects of Lifestyle on Men's Health. London: Academic Press (2019). p. 61–98. doi: 10.1016/B978-0-12-816665-9.00004-4
16. Manley GCA, Lee YK, Zhang Y. Gut microbiota and immunology of the gastrointestinal tract. In: Rao SSC, Lee YY, Ghoshal UC, editors. Clinical and Basic Neurogastroenterology and Motility. London: Academic Press (2019). p. 63–78. doi: 10.1016/B978-0-12-813037-7.00004-2
17. Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, et al. A new genomic blueprint of the human gut microbiota. Nature. (2019) 568:499–504. doi: 10.1038/s41586-019-0965-1
18. De R, Mukhopadhyay AK, Dutta S. Metagenomic analysis of gut microbiome and resistome of diarrheal fecal samples from Kolkata, India, reveals the core and variable microbiota including signatures of microbial dark matter. Gut Pathog. (2020) 12:1–48. doi: 10.1186/s13099-020-00371-8
19. Long J, Yang J, Henning SM, Woo SL, Hsu M, Chan B, et al. Xylooligosaccharide supplementation decreases visceral fat accumulation and modulates cecum microbiome in mice. J Funct Foods. (2019) 52:138–46. doi: 10.1016/j.jff.2018.10.035
20. Thiennimitr P, Yasom S, Tunapong W, Chunchai T, Wanchai K, Pongchaidecha A, et al. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition. (2018) 54:40–7. doi: 10.1016/j.nut.2018.03.005
21. Yang J, Summanen PH, Henning SM, Hsu M, Lam H, Huang J, et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front Physiol. (2015) 6:1–11. doi: 10.3389/fphys.2015.00216
22. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol. (2016) 7:979. doi: 10.3389/fmicb.2016.00979
23. Raman M, Ambalam P, Doble M. Probiotics and Bioactive Carbohydrates in Colon Cancer Management. New Delhi: Springer India (2016). doi: 10.1007/978-81-322-2586-7
24. Ferenczi S, Szegi K, Winkler Z, Barna T, Kovács KJ. Oligomannan prebiotic attenuates immunological, clinical and behavioral symptoms in mouse model of inflammatory bowel disease. Sci Rep. (2016) 6:2–11. doi: 10.1038/srep34132
25. Wang H, Zhang X, Wang S, Li H, Lu Z, Shi J, et al. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. (2018) 9:3916–29. doi: 10.1039/C8FO00209F
26. Zheng J, Li H, Zhang X, Jiang M, Luo C, Lu Z, et al. Prebiotic Mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J Agric Food Chem. (2018) 66:5821–31. doi: 10.1021/acs.jafc.8b00829
27. Neyrinck AM, Van Hée VF, Piront N, De Backer F, Toussaint O, Cani PD, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. (2012) 2:e28. doi: 10.1038/nutd.2011.24
28. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. (2017) 8:172–84. doi: 10.1080/19490976.2017.1290756
29. Berger K, Burleigh S, Lindahl M, Bhattacharya A, Patil P, Stålbrand H, et al. Xylooligosaccharides increase Bifidobacteria and Lachnospiraceae in mice on a high-fat diet, with a concomitant increase in short-chain fatty acids, especially butyric acid. J Agric Food Chem. (2021) 69:3617–25. doi: 10.1021/acs.jafc.0c06279
30. Agazzi A, Perricone V, Zorini FO, Sandrini S, Mariani E, Jiang X, et al. Inflammatory response and improve duodenal villi height in post-weaning piglets improving feed efficiency. Animals. (2020) 4:1–14. doi: 10.3390/ani10081283
31. Poletto P, Pereira GN, Monteiro CRM, Pereira MAF, Bordignon SE, de Oliveira D. Xylooligosaccharides: transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem. (2020) 91:352–63. doi: 10.1016/j.procbio.2020.01.005
32. Saleh SAA, Abdel Wahab WA, El-Dein AN, Abdelwahab WA, Ahmed AAM, Helmy WA, et al. Characterization of Aspergillus niger MK981235 xylanase with extraction of anti-hepatotoxic, antioxidant, hypocholesterolemic and prebiotic Corchorus olitorius stems xylooligosaccharides. Int J Biol Macromol. (2021) 166:677–86. doi: 10.1016/j.ijbiomac.2020.10.225
33. Jana UK, Suryawanshi RK, Prajapati BP, Soni H, Kango N. Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae. Bioresour Technol. (2018) 268:308–14. doi: 10.1016/j.biortech.2018.07.143
34. Suryawanshi RK, Jana UK, Prajapati BP, Kango N. Immobilization of Aspergillus quadrilineatus RSNK-1 multi-enzymatic system for fruit juice treatment and mannooligosaccharide generation. Food Chem. (2019) 289:95–102. doi: 10.1016/j.foodchem.2019.03.035
35. Andersen JM, Barrangou R, Hachem MA, Lahtinen SJ, Goh YJ, Svensson B, et al. Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genomics. (2013) 14:312. doi: 10.1186/1471-2164-14-312
36. Watanabe A, Hiraga K, Suda M, Yukawa H, Inui M. Functional characterization of Corynebacterium alkanolyticum β-xylosidase and xyloside ABC transporter in Corynebacterium glutamicum. Appl Environ Microbiol. (2015) 81:4173–83. doi: 10.1128/AEM.00792-15
37. La Rosa SL, Leth ML, Michalak L, Hansen ME, Pudlo NA, Glowacki R, et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat Commun. (2019) 10:1–14. doi: 10.1038/s41467-019-08812-y
38. Ejby M, Guskov A, Pichler MJ, Zanten GC, Schoof E, Saburi W, et al. Two binding proteins of the ABC transporter that confers growth of Bifidobacterium animalis subsp. lactis ATCC27673 on β-mannan possess distinct manno-oligosaccharide-binding profiles. Mol Microbiol. (2019) 112:114–30. doi: 10.1111/mmi.14257
39. Lindstad LJ, Lo G, Leivers S, Lu Z, Michalak L, Pereira GV, et al. Human gut Faecalibacterium prausnitzii deploy a highly efficient conserved system to cross-feed on β-mannan-derived oligosaccharides. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.12.23.424282
40. Bagenholm V, Reddy SK, Bouraoui H, Morrill J, Kulcinskaja E, Bahr CM, et al. Galactomannan catabolism conferred by a polysaccharide utilization locus of Bacteroides ovatus: Enzyme synergy and crystal structure of a β-mannanase. J Biol Chem. (2017) 292:229–43. doi: 10.1074/jbc.M116.746438
41. Linares-Pastén JA, Hero JS, Pisa JH, Teixeira C, Nyman M, Adlercreutz P, et al. Novel xylan degrading enzymes from polysaccharide utilizing loci of Prevotella copri DSM18205. bioRxiv [Preprint]. (2020) doi: 10.1101/2020.12.10.419226
42. Despres J, Forano E, Lepercq P, Comtet-Marre S, Jubelin G, Chambon C, et al. Xylan degradation by the human gut Bacteroides xylanisolvens XB1AT involves two distinct gene clusters that are linked at the transcriptional level. BMC Genomics. (2016) 17:1–14. doi: 10.1186/s12864-016-2680-8
43. Shulami S, Zaide G, Zolotnitsky G, Langut Y, Feld G, Sonenshein AL, et al. A two-component system regulates the expression of an ABC transporter for xylo-oligosaccharides in Geobacillus stearothermophilus. Appl Environ Microbiol. (2007) 73:874–84. doi: 10.1128/AEM.02367-06
44. Webb AJ, Homer KA, Hosie AHF. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J Bacteriol. (2008) 190:168–78. doi: 10.1128/JB.01509-07
45. Tsujibo H, Kosaka M, Ikenishi S, Sato T, Miyamoto K, Inamori Y. Molecular characterization of a high-affinity xylobiose transporter of Streptomyces thermoviolaceus OPC-520 and its transcriptional regulation. J Bacteriol. (2004) 186:1029–37. doi: 10.1128/JB.186.4.1029-1037.2004
46. Zúñiga M, Comas I, Linaje R, Monedero V, Yebra MJ, Esteban CD, et al. Horizontal gene transfer in the molecular evolution of mannose PTS transporters. Mol Biol Evol. (2005) 22:1673–85. doi: 10.1093/molbev/msi163
47. Senoura T, Ito S, Taguchi H, Higa M, Hamada S, Matsui H, et al. New microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem Biophys Res Commun. (2011) 408:701–6. doi: 10.1016/j.bbrc.2011.04.095
48. Freed DLJ. Do dietary lectins cause disease? The evidence is suggestive—and raises interesting possibilities for treatment. BMJ. (1999) 318:1023–4. doi: 10.1136/bmj.318.7190.1023
49. Suryawanshi RK, Kango N. Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chem. (2021) 334:127428. doi: 10.1016/j.foodchem.2020.127428
50. Załȩski A, Banaszkiewicz A, Walkowiak J. Butyric acid in irritable bowel syndrome. Prz Gastroenterol. (2013) 8:350–3. doi: 10.5114/pg.2013.39917
51. Sivaprakasam S, Bhutia YD, Yang S, Ganapathy V. Short-chain fatty acid transporters: role in colonic homeostasis. Compr Physiol. (2018) 8:299–314. doi: 10.1002/cphy.c170014
52. Venegas DP, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. doi: 10.3389/fimmu.2019.01486
53. Canani RB, Costanzo M Di, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. (2011) 17:1519–28. doi: 10.3748/wjg.v17.i12.1519
54. Chen J, Zhao KN, Vitetta L. Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients. (2019) 11:1–26. doi: 10.3390/nu11051026
55. Misra BB, Puppala SR, Comuzzie AG, Mahaney MC, VandeBerg JL, Olivier M, et al. Analysis of serum changes in response to a high fat high cholesterol diet challenge reveals metabolic biomarkers of atherosclerosis. PLoS ONE. (2019) 14:e0214487. doi: 10.1371/journal.pone.0214487
56. Barczynska R, Kapusniak J, Litwin M, Slizewska K, Szalecki M. Dextrins from maize starch as substances activating the growth of Bacteroidetes and Actinobacteria simultaneously inhibiting the growth of Firmicutes, responsible for the occurrence of obesity. Plant Foods Hum Nutr. (2016) 71:190–6. doi: 10.1007/s11130-016-0542-9
57. Anderson JW, Hanna TJ. Impact of non-digestible carbohydrates on serum lipoproteins and risk for cardiovascular disease. J Nutr. (1999) 129:1457–66S doi: 10.1093/jn/129.7.1457S
58. Guerreiro I, Oliva-Teles A, Enes P. Improved glucose and lipid metabolism in European sea bass (Dicentrarchus labrax) fed short-chain fructooligosaccharides and xylooligosaccharides. Aquaculture. (2015) 441:57–63. doi: 10.1016/j.aquaculture.2015.02.015
59. Abasubong KP, Li XF, Zhang DD, Jia ET, Xiang-Yang Y, Xu C, et al. Dietary supplementation of xylooligosaccharides benefits the growth performance and lipid metabolism of common carp (Cyprinus carpio) fed high-fat diets. Aquac Nutr. (2018) 24:1416–24. doi: 10.1111/anu.12678
60. Lim SM, Kim E, Shin JH, Seok PR, Jung S, Yoo SH, et al. Xylobiose prevents high-fat diet induced mice obesity by suppressing mesenteric fat deposition and metabolic dysregulation. Molecules. (2018) 23:705. doi: 10.3390/molecules23030705
61. Smith DL, Nagy TR, Wilson LS, Dong S, Barnes S, Allison DB. The effect of mannan oligosaccharide supplementation on body weight gain and fat accrual in C57Bl/6J mice. Obesity. (2010) 18:995–9. doi: 10.1038/oby.2009.308
62. St-Onge MP, Salinardi T, Herron-Rubin K, Black RM. A weight-loss diet including coffee-derived mannooligosaccharides enhances adipose tissue loss in overweight men but not women. Obesity. (2012) 20:343–8. doi: 10.1038/oby.2011.289
63. Yan S, Shi R, Li L, Ma S, Zhang H, Ye J, et al. Mannan oligosaccharide suppresses lipid accumulation and appetite in western-diet-induced obese mice via reshaping gut microbiome and enhancing short-chain fatty acids production. Mol Nutr Food Res. (2019) 63:1–13. doi: 10.1002/mnfr.201900521
64. Panee J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine. (2012) 60:1–12. doi: 10.1016/j.cyto.2012.06.018
65. Fei Y, Wang Y, Pang Y, Wang W, Zhu D, Xie M, et al. Xylooligosaccharide modulates gut microbiota and alleviates colonic inflammation caused by high fat diet induced obesity. Front Physiol. (2020) 10:1–12. doi: 10.3389/fphys.2019.01601
66. Khunti K. SGLT2 inhibitors in people with and without T2DM. Nat Rev Endocrinol. (2021) 17:75–6. doi: 10.1038/s41574-020-00453-2
67. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. (2017) 389:2239–51. doi: 10.1016/S0140-6736(17)30058-2
68. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. (2020) 51:102590. doi: 10.1016/j.ebiom.2019.11.051
69. Khat-udomkiri N, Toejing P, Sirilun S, Chaiyasut C, Lailerd N. Antihyperglycemic effect of rice husk derived xylooligosaccharides in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rat model. Food Sci Nutr. (2020) 8:428–44. doi: 10.1002/fsn3.1327
70. Zhu D, Yan Q, Li Y, Liu J, Liu H, Jiang Z. Effect of Konjac Mannan oligosaccharides on glucose homeostasis via the improvement of insulin and leptin resistance in vitro and in vivo. Nutrients. (2019) 11:1705. doi: 10.3390/nu11081705
71. Del Fabbro S, Calder PC, Childs CE. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc Nutr Soc. (2020) 79:468–78. doi: 10.1017/S0029665120006953
72. Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. (2012) 3:544–55. doi: 10.4161/gmic.22156
73. Grisham MB, DeMichele SJ, Garleb KA, Specian RD. Sulfasalazine or enteral diets containing fish oil or oligosaccharides attenuate chronic colitis in rats. Inflamm Bowel Dis. (1996) 2:178–88. doi: 10.1097/00054725-199609000-00004
74. Li Z, Wang X, Dai N, Yan XE, Yang Y. Tu1783 – in vitro effects of xylo-oligosaccharide on gut microbiota in patients with ulcerative colitis. Gastroenterology. (2019) 156:S1121. doi: 10.1016/S0016-5085(19)39769-0
75. Sheng K, He S, Sun M, Zhang G, Kong X, Wang J, et al. Synbiotic supplementation containing: Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. (2020) 11:3964–74. doi: 10.1039/D0FO00518E
76. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
77. Prado SBR do, Castro-Alves VC, Ferreira GF, Fabi JP. Ingestion of non-digestible carbohydrates from plant-source foods and decreased risk of colorectal cancer: a review on the biological effects and the mechanisms of action. Front Nutr. (2019) 6:1–17. doi: 10.3389/fnut.2019.00072
78. Aachary AA, Gobinath D, Srinivasan K, Prapulla SG. Protective effect of xylooligosaccharides from corncob on 1,2-dimethylhydrazine induced colon cancer in rats. Bioact Carbohydrates Diet Fibre. (2015) 5:146–52. doi: 10.1016/j.bcdf.2015.03.004
79. Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. (2002) 132:1012–7. doi: 10.1093/jn/132.5.1012
80. Le B, Ngoc APT, Yang SH. Synbiotic fermented soymilk with Weissella cibaria FB069 and xylooligosaccharides prevents proliferation in human colon cancer cells. J Appl Microbiol. (2020) 128:1486–96. doi: 10.1111/jam.14551
81. Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr. (2004) 134:1523–8. doi: 10.1093/jn/134.6.1523
82. de Vrese M, Offick B. Probiotics and prebiotics: effects on diarrhea. In: Watson RR, Preedy VR, editors. Bioactive Foods in Promoting Health. London: Academic Press (2010). p. 205–27. doi: 10.1016/B978-0-12-374938-3.00014-1
83. Finegold SM, Li Z, Summanen PH, Downes J, Thames G, Corbett K, et al. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. (2014) 5:436–45. doi: 10.1039/c3fo60348b
84. Passariello A, Terrin G, Cecere G, Micillo M, De Marco G, Di Costanzo M, et al. Randomised clinical trial: efficacy of a new synbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhoea. Aliment Pharmacol Ther. (2012) 35:782–8. doi: 10.1111/j.1365-2036.2012.05015.x
85. Assimakopoulos SF, Triantos C, Maroulis I, Gogos C. The role of the gut barrier function in health and disease. Gastroenterol Res. (2018) 11:261–3. doi: 10.14740/gr1053w
86. Kroemer G, Madeo F, Cabo R De, Platforms M, Roussy G, Campus C, et al. Carbotoxicity-noxious effects of carbohydrates. Cell. (2019) 175:605–14. doi: 10.1016/j.cell.2018.07.044
87. Gao Y, Wang Y, Li Y, Han R, Li C, Xiao L, et al. Repeated sub-chronic oral toxicity study of xylooligosaccharides (XOS) in dogs. Regul Toxicol Pharmacol. (2017) 86:379–85. doi: 10.1016/j.yrtph.2017.04.009
88. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Diagnosis, differential and treatment options. Nat Rev Microbiol. (2000) 13:269–84. doi: 10.1038/nrmicro3432
89. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med J. (2017) 29:131–7. doi: 10.4103/tcmj.tcmj_53_17
90. Foxman B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. (2014) 28:1–13. doi: 10.1016/j.idc.2013.09.003
91. Lacerda Mariano L, Ingersoll MA. The immune response to infection in the bladder. Nat Rev Urol. (2020) 17:439–58. doi: 10.1038/s41585-020-0350-8
92. Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. (1996) 347:1017–21. doi: 10.1016/S0140-6736(96)90150-6
93. Coleman CM, Auker KM, Killday KB, Azadi P, Black I, Ferreira D. Arabinoxyloglucan oligosaccharides may contribute to the antiadhesive properties of porcine urine after cranberry consumption. J Nat Prod. (2019) 82:589–605. doi: 10.1021/acs.jnatprod.8b01043
94. Ebersbach T, Andersen JB, Bergström A, Hutkins RW, Licht TR. Xylo-oligosaccharides inhibit pathogen adhesion to enterocytes invitro. Res Microbiol. (2012) 163:22–7. doi: 10.1016/j.resmic.2011.10.003
95. Rosenstein IJ, Stoll MS, Mizuochi T, Childs RA, Hounsell EF, Feizi T. New type of adhesive specificity revealed by oligosaccharide probes in Escherichia coli from patients with urinary tract infection. Lancet. (1988) 2:1327–30. doi: 10.1016/S0140-6736(88)90868-9
96. Pourabedin M, Chen Q, Yang MM, Zhao X. Mannan- and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal Salmonella enteritidis colonisation in young chickens. FEMS Microbiol Ecol. (2017) 93:1–11. doi: 10.1093/femsec/fiw226
97. Abouloifa H, Khodaei N, Rokni Y, Karboune S, Brasca M, D'Hallewin G, et al. The prebiotics (Fructo-oligosaccharides and Xylo-oligosaccharides) modulate the probiotic properties of Lactiplantibacillus and Levilactobacillus strains isolated from traditional fermented olive. World J Microbiol Biotechnol. (2020) 36:1–12. doi: 10.1007/s11274-020-02961-9
98. Costa JR, Amorim M, Vilas-Boas A, Tonon RV, Cabral LMC, Pastrana L, et al. Impact of: in vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah grape pomace extract. Food Funct. (2019) 10:1856–69. doi: 10.1039/C8FO02534G
99. Abdelmalek BE, Driss D, Kallel F, Guargouri M, Missaoui H, Chaabouni SE, et al. Effect of xylan oligosaccharides generated from corncobs on food acceptability, growth performance, haematology and immunological parameters of Dicentrarchus labrax fingerlings. Fish Physiol Biochem. (2015) 41:1587–96. doi: 10.1007/s10695-015-0110-5
100. Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. (2016) 6:1–12. doi: 10.3389/fcimb.2016.00194
Keywords: gut microbiota, hemicelluloses, probiotic, oligosaccharide, nutrition
Citation: Jana UK, Kango N and Pletschke B (2021) Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 8:670817. doi: 10.3389/fnut.2021.670817
Received: 22 February 2021; Accepted: 21 June 2021;
Published: 27 July 2021.
Edited by:
Amalia Yanni, Harokopio University, GreeceReviewed by:
Ahmad Ud Din, Southwest Medical University, ChinaSylvie Françoise Rebuffat, Muséum National d'Histoire Naturelle, France
Copyright © 2021 Jana, Kango and Pletschke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naveen Kango, nkango@gmail.com; Brett Pletschke, b.pletschke@ru.ac.za
 Uttam Kumar Jana
Uttam Kumar Jana Naveen Kango
Naveen Kango Brett Pletschke
Brett Pletschke