- 1Department of Neurosurgery, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Anesthesiology, Zhongnan Hospital of Wuhan University, Wuhan, China
The neutrophil-to-lymphocyte ratio (NLR) plays an important role in the progression of intracerebral hemorrhage (ICH). An increasing number of studies have reported that a high NLR is correlated with poor clinical outcomes among patients with ICH. Here, we conducted a systematic review and meta-analysis to evaluate the prognostic value of NLR in the setting of ICH. We performed a comprehensive search of electronic literature databases to identify all relevant studies evaluating the prognostic role of NLR in patients with ICH. Two researchers independently screened the studies and extracted relevant data. We extracted, pooled, and weighted odds ratio (OR) and 95% confidence interval (CI) values using a generic inverse-variance method, and then evaluated the heterogeneity among studies using Q test and I2 statistic. Finally, we selected a total of 26 studies including 7,317 patients for the current study. Overall, our results indicated that a high NLR was significantly associated with a poor outcome (OR, 1.32; 95% CI, 1.19–1.46; P < 0.00001), mortality (OR, 1.05; 95% CI, 1.01–1.09; P = 0.02), and neurological deterioration (OR, 1.65; 95% CI, 1.08–2.52; P = 0.02). We did not observe a significant association between NLR and hematoma expansion (OR, 1.04; 95% CI, 0.99–1.08; P = 0.09). Our study indicated that a high NLR is significantly associated with poor clinical outcomes in patients with ICH. As NLR is a simple and easily available biomarker, future studies should focus on exploring its application in the prognostic evaluation of patients with ICH.
Introduction
Spontaneous intracerebral hemorrhage (ICH), as a common stroke subtype, is one the most devastating neurosurgical diseases, with high mortality and morbidity rates (Feigin et al., 2003; van Asch et al., 2010). ICH is characterized by an instance of sudden bleeding due to ruptured cerebral blood vessels and by the action of blood flowing into the brain parenchyma or cerebral ventricles (Keep et al., 2012). Despite the great advances and developments that have occurred in medical technology, ICH still represents the most serious type of stroke due to a persistent lack of effective therapeutic strategies (Hwang et al., 2011; Cordonnier et al., 2018). It is reported that more than one-third of patients who experience ICH die within one month after ictus, and many of the survivors are severely disabled and show persistent functional impairment (Ferro, 2006; van Asch et al., 2010; Ikram et al., 2012). ICH thus represents a substantial clinical and economic public health burden, especially in low-income countries, and it is critical to identify promising prognostic factors to evaluate the severity and prognosis of patients (Wang W. et al., 2017; Garg and Biller, 2019).
Intracerebral hemorrhage scores, consisting mainly of the Glasgow Coma Scale score, age, ICH volume, intraventricular hemorrhage, and infratentorial origin of ICH, have been extensively validated and widely used to predict poor prognosis among patients with ICH (Hemphill et al., 2001; Nisar et al., 2018). In addition to these risk factors, increasing evidence indicates that an inflammatory response is involved in the development of secondary brain injuries after ICH onset and plays a vital role in the pathophysiological mechanism of ICH (Xi et al., 2006; Wang, 2010; Chen et al., 2015; Wang T. et al., 2018). The neutrophil-to-lymphocyte ratio (NLR), defined as the ratio between the absolute neutrophil count and the absolute lymphocyte count, is an indicator of systemic inflammation that can suggest a patient’s inflammatory status (Wang Y. et al., 2018; Song et al., 2021). Recently, NLR has been widely reported as an independent predictive factor for the prognosis of ICH (Ye et al., 2017; Lattanzi et al., 2019; Liu et al., 2019). In a previous study, Liu et al. (2019) reported that a higher NLR level was significantly correlated with major disability at 90 days and a higher short-term mortality rate. Wang Z. et al. (2019) suggested that NLR can predict hematoma expansion. Finally, Lattanzi et al. (2017) indicated that NLR may be a prognostic factor for neurological deterioration. With the publications of most recent studies, there is still a need to further assess the prognostic value of NLR in terms of poor outcome, mortality, hematoma expansion, and neurological deterioration.
Methods
We completed the current study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary File 1).
Data Sources and Searches
We conducted a comprehensive database search of PubMed, Embase, the Cochrane Library, and the Web of Science, respectively, from database inception until September 2021 to identify all relevant articles. The predefined search strategy was as follows: (“neutrophil-to-lymphocyte ratio” or “neutrophil to lymphocyte ratio” or “neutrophil lymphocyte ratio” or “neutrophil/lymphocyte ratio” or “NLR”) and (“intracerebral hemorrhage” or “intracranial hemorrhage” or “cerebral hemorrhage” or “brain hemorrhage” or “ICH”). The free terms are presented in Supplementary File 2. In addition, we also attempted to manually search all reference lists of the selected studies and related review articles to identify any additional studies that may be appropriate to include.
Literature Selection and Inclusion Criteria
Two researchers independently completed the literature screening process wherein all potential articles were individually assessed for inclusion in our analysis and any disagreements were solved through discussion between them or involvement of a third researcher. Articles were enrolled if they met the following criteria: (1) included adult patients (≥18 years of age) diagnosed with ICH; (2) reported the NLR values at hospital admission; (3) reported the prognostic value of NLR and clinical outcomes (including poor outcome, mortality, hematoma expansion, and neurological deterioration); (4) provided original data with adjusted odds ratio (OR) and corresponding 95% confidence interval (CI) values in a multivariate analysis for clinical outcomes; and (5) had an observational study design (including prospective and retrospective cohort studies). We excluded articles if they satisfied any of the following criteria: (1) letters, case reports, reviews, or animal studies; (2) only provided univariate analysis data; and (3) did not contain any relevant clinical outcomes.
Outcome Measures
We included four outcome measures in the current study, including mortality, poor outcome, hematoma expansion, and neurological deterioration. We defined mortality as death from any cause during the follow-up period. We defined a poor outcome by the recording of a Modified Rankin Scale score of more than two points and/or a Glasgow Outcome Scale score of less than four points at any point during follow-up. We defined hematoma expansion by an absolute increase of more than 6 mL or a relative growth of greater than 33% in the hematoma volume using follow-up computed tomography imaging. Finally, we defined neurological deterioration by a National Institutes of Health Stroke Scale score increase of at least four points, a Glasgow Coma Scale decrease of at least two points, or the occurrence of death between the time of admission and 7 days after the hemorrhage.
Data Extraction and Quality Assessment
Two researchers independently extracted detailed information from individual studies based on a predesigned checklist, including the first author’s name, publication year, country, number of participants, sex ratio, mean or median age of the participants, NLR cut-off value, follow-up period, clinical outcomes (including poor outcome, mortality, hematoma expansion, and neurological deterioration), and corresponding ORs and 95% CI values. ORs and 95% CI values were extracted from multivariable analyses. In addition, we preferentially extracted ORs that represented the association of NLR at admission or at the early phase of admission and outcome measures when available.
We conducted a quality assessment of each included study using the Newcastle–Ottawa Quality Assessment Scale (NOS), which includes eight items, classified into the three domains of selection, comparability, and exposure, respectively. Two researchers independently assessed the quality of each study. The highest NOS score possible was nine points, and we considered studies with NOS scores of at least six points as high-quality studies (Supplementary File 3).
Statistics Analyses
We pooled the extracted OR and corresponding 95% CI values using the Review Manager version 5.4 analysis software (The Cochrane Collaboration, London, United Kingdom). Forest plots were drawn to display the pooled results. Extracted hazard ratios and 95% CIs were pooled and weighted using a generic inverse-variance method. We used I2 statistics and Cochran’s Q test to assess the heterogeneity across studies. When I2was greater than?50% or the P-value was less than 0.1, we considered the degree of heterogeneity to be substantial and adopted a random-effects model; otherwise, we adopted a fixed-effects model. To further explore the potential source of heterogeneity, we performed a series of subgroup analyses and sensitivity analyses. The subgroup analyses were conducted based on ethnicity, NLR cut-off value, and follow-up period, and the sensitivity analyses were performed by excluding studies one by one. Besides, we further assess the publication bias using a funnel plot.
Results
Study Selection and Baseline Characteristic of Included Studies
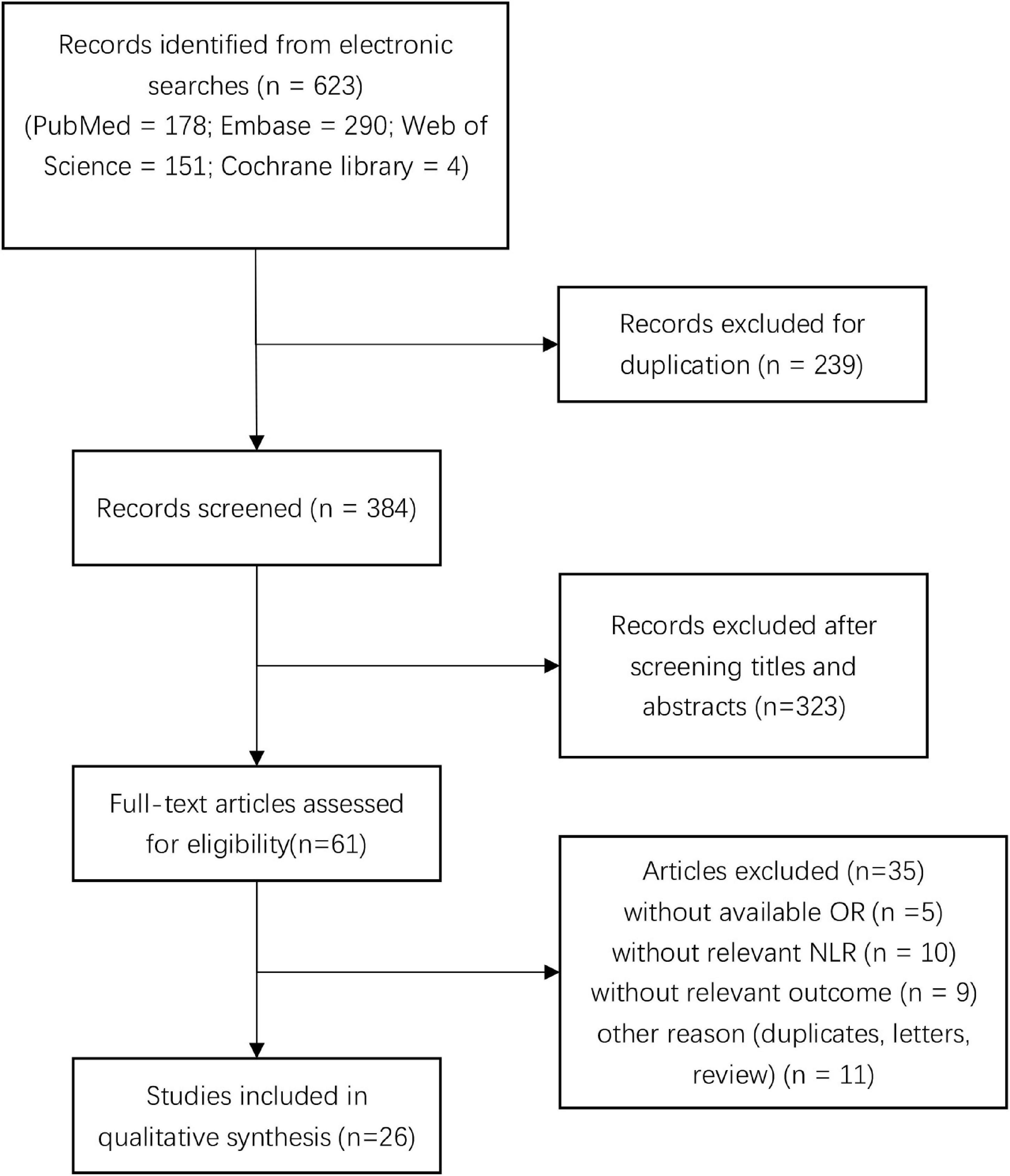
We initially screened a total of 623 studies that satisfied our preliminary screening criteria. Figure 1 shows the detailed study-selection process. First, we excluded 239 duplicates, then an additional 323 studies after screening the titles and abstracts; subsequently, 61 studies remained to undergo full-text review. Finally, we included 26 studies with 7,317 patients in the final analysis (Lattanzi et al., 2016, 2017, 2018; Wang et al., 2016; Giede-Jeppe et al., 2017; Seabra et al., 2017; Sun et al., 2017; Tao et al., 2017; Fan et al., 2018; Qi et al., 2018; Wang F. et al., 2018, 2019; Zhang et al., 2018a,b, 2019a,b, 2019c; Guo et al., 2019; Pektezel et al., 2019; Qin et al., 2019; Wang Z. et al., 2019; Chen et al., 2020; Fonseca et al., 2021; Menon et al., 2021; Mohamed et al., 2021; Radu et al., 2021), of which 14 studies reported the prognostic value of NLR for poor outcome, 12 studies reported the prognostic value of NLR for mortality, four studies reported the prognostic value of NLR for hematoma expansion, and three studies reported the prognostic value of NLR for neurological deterioration, respectively.
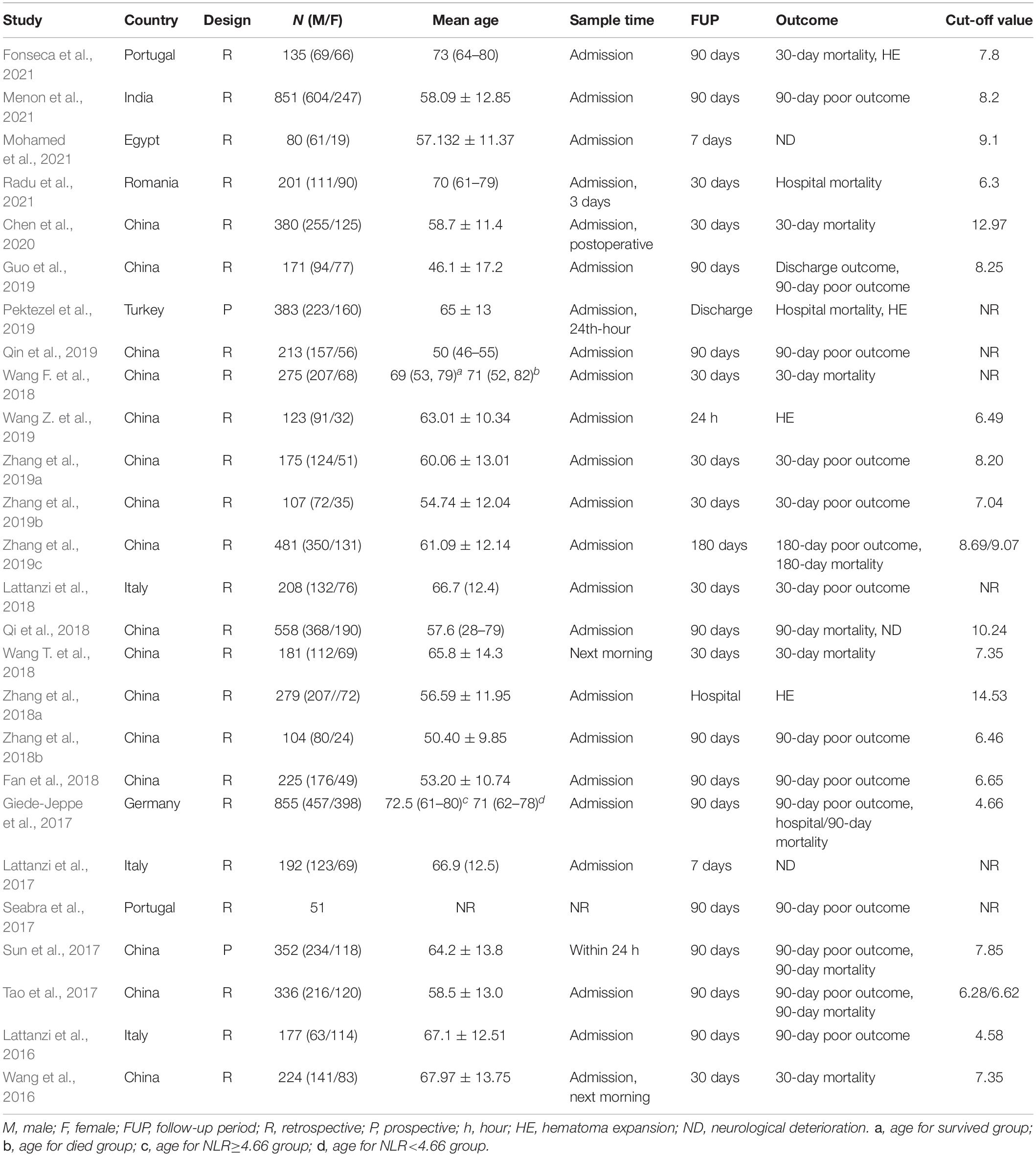
The baseline characteristics of the included studies are presented in Table 1. All studies were published after 2016, and one was a conference paper, providing limited information. Most studies were conducted in China. Except for two prospective studies, all of the included studies were retrospective investigations. The number of study participants per study ranged from 51 to 855, with a mean age range across all studies of 46.1 to 73 years. The longest follow-up period was 1 year. In all included studies, the NLR was gleaned from peripheral blood samples collected prior to treatment, and the cut-off NLR value ranged from 4.58 to 12.97.
Poor Outcome
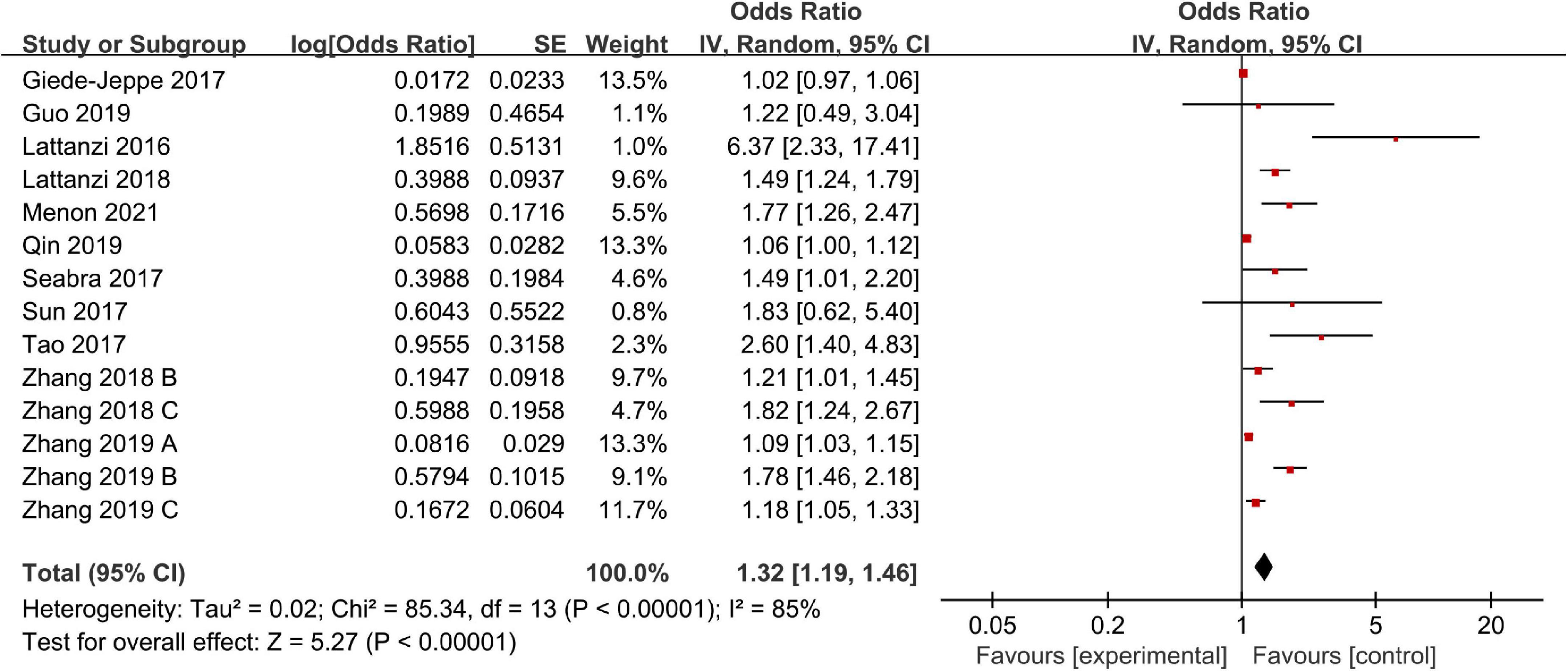
There were 14 studies including 4,306 patients that reported adjusted ORs for poor outcome. The overall analysis revealed that a high NLR was significantly associated with a poor outcome (OR, 1.32; 95% CI, 1.19–1.46; P < 0.00001), and we confirmed statistically significant heterogeneity across all studies (I2 = 85%; P < 0.00001) (Figure 2). Next, we performed a sensitivity analysis by excluding studies one by one, and we found that the meta-analysis results were robust because the ORs did not obviously change after excluding any single study.
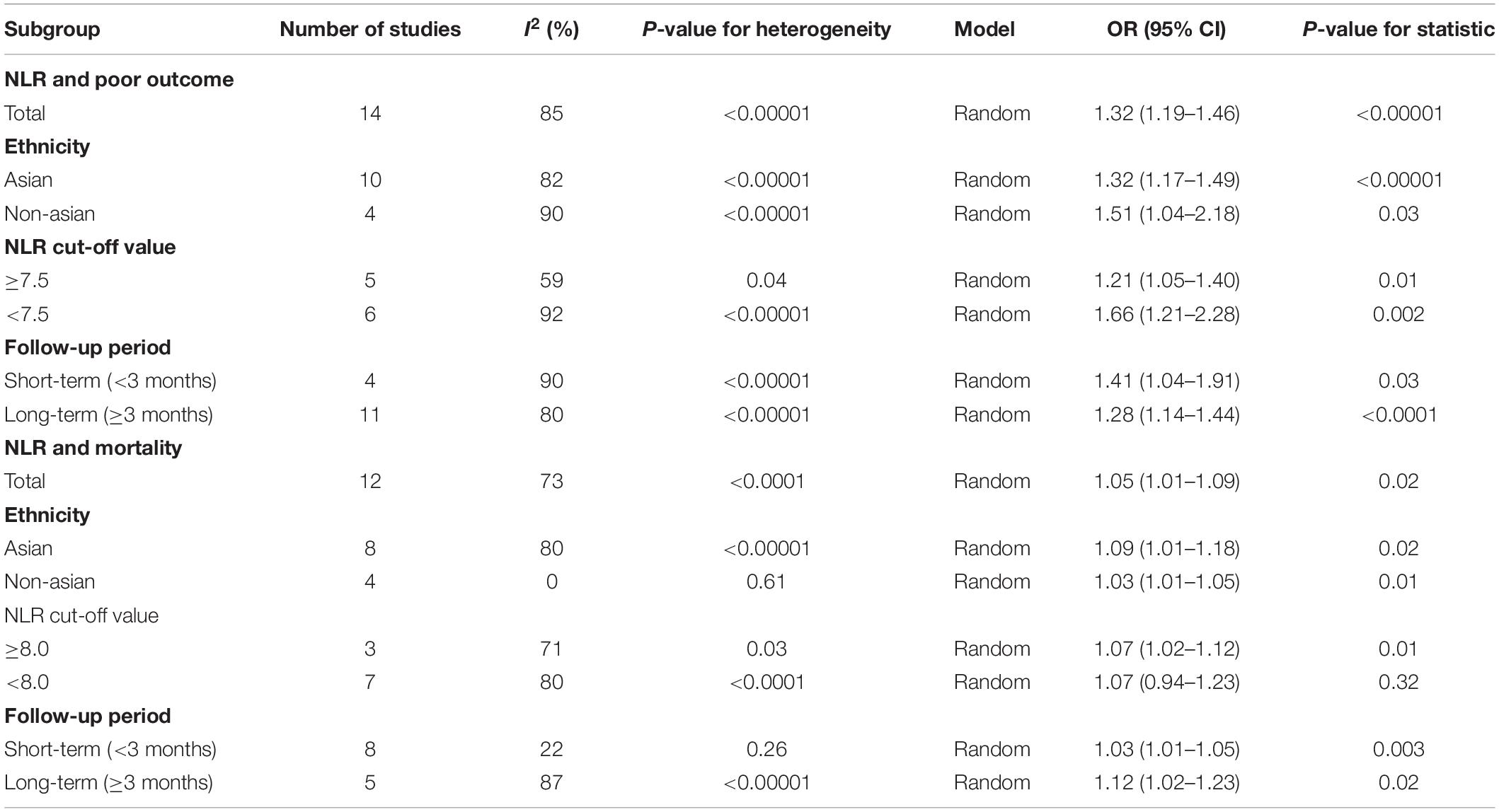
We also performed a subgroup analysis based on ethnicity, NLR cut-off value, and follow-up period. The results of the subgroup analysis stratified by ethnicity indicated that a higher NLR was significantly associated with poor outcome in both Asian (OR, 1.32; 95% CI, 1.17–1.49; P < 0.00001) and non-Asian (OR, 1.51; 95% CI, 1.04–2.18; P = 0.03) regions. According to the NLR cut-off value, we found that there was a significant association between the NLR and poor outcome in the NLR cut-off value of less than 7.5 group (OR, 1.66; 95% CI, 1.21–2.28; P = 0.002), and the NLR cut-off value of at least 7.5 group (OR, 1.21; 95% CI, 1.05–1.40; P = 0.01). The subgroup analysis indicated that heterogeneity obviously decreased in the higher NLR cut-off value subgroup. It suggests that NLR cut-off value may be the potential source of heterogeneity. Finally, according to the follow-up period, we found that a high NLR was more significantly associated with a poor short-term outcome (<3 months) (OR, 1.41; 95% CI, 1.04–1.91; P = 0.03) than a poor relatively long-term outcome (≥3 months) (OR, 1.28; 95% CI, 1.14–1.44; P < 0.0001) (Table 2).
Mortality
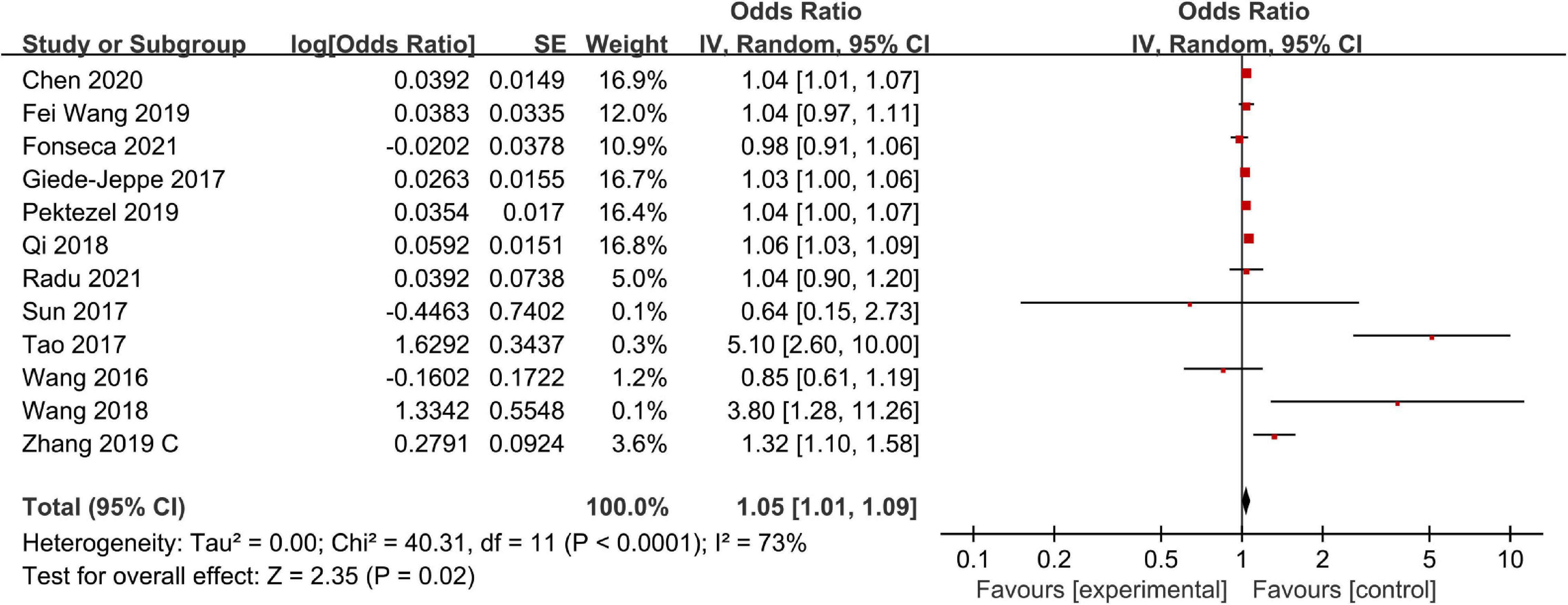
There were 12 studies including 4,361 patients that reported adjusted ORs for mortality at the final follow-up. The overall analysis revealed that a high NLR was significantly associated with mortality (OR, 1.05; 95% CI, 1.01–1.09; P = 0.02), and statistically significant heterogeneity was confirmed across all studies (I2 = 73%; P < 0.0001) (Figure 3). Next, we performed a series of sensitivity analyses and subgroup analyses to identify potential sources of heterogeneity across studies. The sensitivity analysis was conducted by excluding studies one by one. After removing Tao et al.’s (2017) study, heterogeneity was substantially decreased (I2 = 47%; P = 0.04), which indicated that it may be the potential source of the heterogeneity. However, removing this study did not change the overall pattern of results (OR = 1.04, 95% CI, 1.01–1.07; P = 0.002), which indicated that our results were robust.
Separately, we performed subgroup analysis based on ethnicity, NLR cut-off value, and follow-up period. The results of the subgroup analysis stratified by ethnicity indicated that a higher NLR was significantly associated with mortality in both Asian (OR, 1.09; 95% CI, 1.01–1.18; P = 0.02) and non-Asian (OR, 1.03; 95% CI, 1.01–1.05; P = 0.01) regions. Based on the NLR cut-off value, we found that there was a significant association between NLR and mortality in both the NLR cut-off value of less than 8.0 group (OR, 1.07; 95% CI, 0.94–1.23; P = 0.32) and the NLR cut-off value of at least 8.0 group (OR, 1.07; 95% CI, 1.02–1.12; P = 0.01). When considering the follow-up period, we found that a high NLR was significantly associated with both short-term mortality (OR, 1.03; 95% CI, 1.01–1.05; P = 0.003) and relativity long-term mortality (OR, 1.12; 95% CI, 1.02–1.23; P = 0.02) (Table 2).
Hematoma Expansion
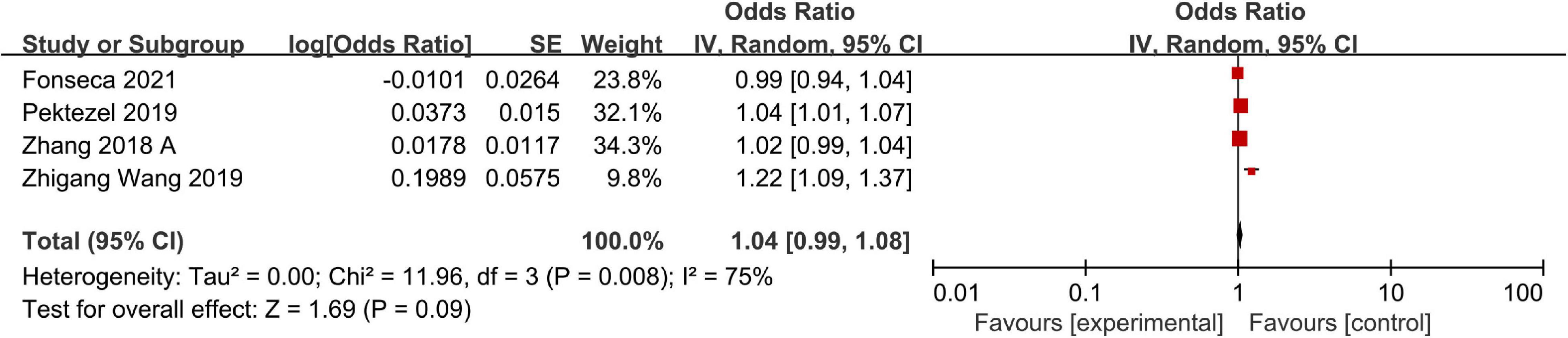
A total of four studies including a total of 920 patients reported adjusted ORs for hematoma expansion. Significant heterogeneity was observed across studies, so we adopted a random-effects model to finish the data synthesis (I2 = 75%; P = 0.008). Ultimately, the pooled results indicated that a high NLR was not significantly associated with hematoma expansion (OR, 1.04; 95% CI, 0.99–1.08; P = 0.09) (Figure 4). The sensitivity analysis indicated that the study of Wang Z. et al. (2019) maybe the main source of heterogeneity. After excluding the study, the heterogeneity was obviously decreased (I2 = 24%; P = 0.27). However, removing this study did not change the overall pattern of results (OR = 1.02, 95% CI, 1.00–1.04; P = 0.05).
Neurological Deterioration
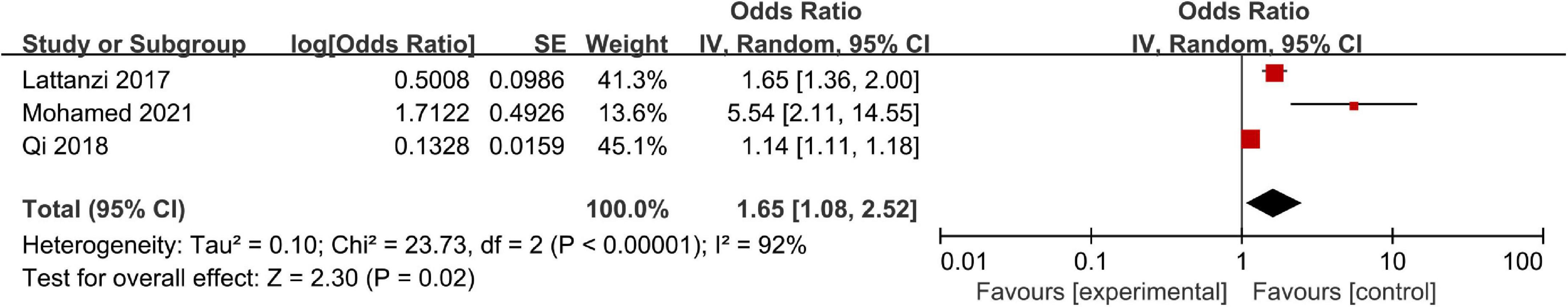
Three studies including a total of 830 patients reported adjusted ORs for neurological deterioration. Significant heterogeneity was observed across these studies, so we adopted a random-effects model to finish the data synthesis (I2 = 92%; P < 0.00001), and the pooled results indicated that a high NLR was associated with neurological deterioration (OR, 1.65; 95% CI, 1.08–2.52; P = 0.02) (Figure 5).
Publication Bias
In the current study, we only tested the publication bias for mortality and poor outcome. Overall, we did not find that obvious publication bias existed for mortality or poor outcome (Supplementary File 4).
Discussion
A high NLR has been suggested as a prognostic biomarker and is already widely used in patients with cancer and cardiovascular and cerebrovascular diseases (Templeton et al., 2014; Angkananard et al., 2018; Sharma et al., 2021). In the current study, we included 26 studies with a total of 7,317 participants that assessed the prognostic value of NLR in patients with ICH. Fourteen of the included studies evaluated the association between NLR and poor outcome, while 12 studies evaluated the association between NLR and all-cause mortality, four studies evaluated the association between NLR and hematoma expansion, and three studies evaluated the association between NLR and neurological deterioration. Our results indicated that a higher NLR level was significantly associated with all-cause mortality, poor outcome, and neurological deterioration, but we did not observe a significant association between NLR and hematoma expansion.
Neutrophil-to-lymphocyte ratio, considered to be a convenient indicator for systemic inflammation, represents the balance between the systemic inflammatory response and immune response (Maestrini et al., 2015; Malhotra et al., 2018). Increasing studies have indicated NLR may be a reliable predictor of clinical outcome in the setting of ICH (Lattanzi et al., 2019; Tschoe et al., 2020); however, the exact mechanism in this context is not fully understood. Some studies have proposed that NLR may be indicative of inflammation, and a higher NLR level indicates a higher inflammatory level (Chang et al., 2020). In patients with ICH, an inflammation response immediately occurs after the hemorrhage (Slevin et al., 2020; Nóbrega Lima Rodrigues de Morais et al., 2021); following the hemorrhage, hematoma components spread to the brain parenchyma, which can activate microglia to initiate inflammatory signaling. Subsequently, pro-inflammatory cytokines and chemokines are released to favor peripheral inflammatory infiltration, which leads to secondary damage (Lan et al., 2017; Sosa et al., 2018).
Available evidence suggests that circulating neutrophils promptly migrate to the site of hematoma and cerebral injury, and neutrophils are the earliest leukocytes to travel to the hematoma site (Wang and Doré, 2007; Hermann et al., 2018). On the one hand, elevated neutrophils can induce blood–brain barrier injury and exacerbate brain damage by the release of tumor necrosis factor, elastase, matrix metalloproteinase, myeloperoxidase, and reactive oxygen species, inducing neurotoxicity. On the other hand, elevated neutrophils can cause a state of temporary immune suppression, thereby leading to a decrease in peripheral lymphopenia (Römer et al., 2015). In addition, the abrupt release of some amount of catecholamines and steroids can weaken and even fully suppress the immune response in the early stages of hemorrhage, contributing to a decrease in peripheral lymphocytes (Liesz et al., 2013). Lymphocytes are the most important player of adaptive immune responses, and their decrease can enhance the patient’s susceptibility to infection (Wang Y. et al., 2017; Lattanzi et al., 2019). The inflammatory response and immune system are collectively involved in the disease course that results in leukocytosis and lymphocytopenia. The NLR is believed to be a composite index that reflects the balance between the innate (neutrophil) and adaptive (lymphocyte) immune responses. As such, dynamic changes in the NLR can rapidly reflect the possibility of a secondary brain injury and a patient’s vulnerability to posthemorrhage complications (Lattanzi et al., 2019; Kashiwazaki et al., 2021). Therefore, an increase in the NLR value may be a reliable predictor of poor functional outcome, mortality, growth of a hematoma, the risk of developing infection, and early neurological deterioration (Qi et al., 2018; Wang Z. et al., 2019; Fonseca et al., 2021; Menon et al., 2021).
Increasing evidence has indicated that NLR may be a potential prognostic predictor for poor prognosis in patients with ICH (Lattanzi et al., 2019; Liu et al., 2019). However, available results are contradictory. In a previous meta-analysis, Zhang et al. (2017) indicated that there was a correlation between NLR and poor functional outcome, but not the 90-day mortality rate. Ye et al. (2017) found that a high NLR was significantly associated with mortality but not poor functional outcome. Liu et al. (2019) updated a meta-analysis in 2019, and their results indicated that NLR is an independent predictor of major disability at 90 days and short-term mortality among patients with ICH but not of in-hospital mortality or 90-day mortality. Based on these conflicting results, we saw a need to perform an additional meta-analysis. Compared with previous studies, in our investigation, we included more patients and more studies published after 2019. We only included data adjusted by multivariable analysis to weak the degree of contamination by other confounding factors. In addition, we not only evaluated the prognostic value of NLR for mortality and poor outcome but also the prognostic value of NLR on hematoma expansion and neurological deterioration. Therefore, we consider our results to be more reliable and valuable than those of earlier studies.
In the current study, our results indicated that NLR was significantly related to poor outcome and mortality. We further conducted analyses based on different ethnicities, cut-off value, and follow-up period and found that a high NLR is significantly associated with mortality and poor outcome independently of variations in these characteristics. Despite a high NLR had a greater magnitude of association with mortality and poor outcome in certain subgroups, it did not significantly alter the effect of NLR. Substantial heterogeneity was also observed in the current study, and we considered that some factors may explain the origin of such heterogeneity. First, all studies included participants diagnosed with ICH but did not report the initial severity of ictus. Therefore, the initial severity of ictus may have been extremely different between studies. Second, the types and the locations of ICH were not the same. In addition, although most studies provided data at the time of admission, there were still some differences in the timing of sample collection, which can result in heterogeneity because the NLR value can change rapidly at different time points during the disease course. Therefore, we should view these results with caution.
The majority of patients with ICH had a poor prognosis. It is necessary to identify potentially severe patients early, transfer them to the intensive care unit, and initiate effective management on time. Currently, the ICH score is the most commonly used clinical predictive model for identifying ICH patients with unfavorable prognoses. However, the ICH score, just based on clinical information, ignores the predictive value of biological elements from the lab. The findings of our study fill this gap in the field and help with future guidelines addressing the topic of the prognostic value of NLR in ICH. Future studies should focus on investigating the association between NLR and clinical outcomes in specific subgroups, including stratification by subtype of hemorrhage, location of hemorrhage, or severity of ICH.
There were some limitations in this meta-analysis. First, most of the included studies were retrospective investigations, which may have led to inevitable selection and information bias. Second, there was substantial heterogeneity among the included studies. Although we explored the source of heterogeneity through sensitivity analysis and subgroup analysis, the potential source of heterogeneity could be found. Third, the cut-off value varied greatly across the included studies such that we could not determine the optimal NLR value for clinical use.
Conclusion
Our study indicated that a high NLR is significantly associated with poor clinical outcomes among patients with ICH. Given that NLR is a simple and easily available biomarker, future studies should focus on exploring its application in the prognostic evaluation of patients with ICH.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
MS took responsibility for the integrity of the data and the accuracy of the data analysis. MS and X-FL contributed significantly to data analysis, data acquisition, and manuscript preparation. MS, X-FL, T-BZ, and Q-WT made critical revision of the manuscript for important intellectual content. MP guided the research and revised the manuscript. W-YZ guided the research and contributed to supervision. All authors contribute to the conception, design, analysis, and interpretation of data of the study.
Funding
This work was supported by the Key Research and Development Plan of Hubei Science and Technology Department (No. 2020BCB033).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.825859/full#supplementary-material
Supplementary File 1 | PRISMA 2009 Checklist.
Supplementary File 2 | Free terms.
Supplementary File 3 | Quality assessment of included studies using the Newcastle Ottawa Scale for cohort studies.
Supplementary File 4 | Funnel plot of publication bias of NLR with primary outcome.
References
Angkananard, T., Anothaisintawee, T., McEvoy, M., Attia, J., and Thakkinstian, A. (2018). Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed. Res. Int. 2018:2703518. doi: 10.1155/2018/2703518
Chang, L. S., Lin, Y. J., Yan, J. H., Guo, M. M., Lo, M. H., and Kuo, H. C. (2020). Neutrophil-to-lymphocyte ratio and scoring system for predicting coronary artery lesions of Kawasaki disease. BMC Pediatr. 20:398. doi: 10.1186/s12887-020-02285-5
Chen, S., Yang, Q., Chen, G., and Zhang, J. H. (2015). An update on inflammation in the acute phase of intracerebral hemorrhage. Transl. Stroke Res. 6, 4–8. doi: 10.1007/s12975-014-0384-4
Chen, W., Wang, X., Liu, F., Tian, Y., Chen, J., Li, G., et al. (2020). The predictive role of postoperative neutrophil to lymphocyte ratio for 30-day mortality after intracerebral hematoma evacuation. World Neurosurg. 134, e631–e635. doi: 10.1016/j.wneu.2019.10.154
Cordonnier, C., Demchuk, A., Ziai, W., and Anderson, C. S. (2018). Intracerebral haemorrhage: current approaches to acute management. Lancet 392, 1257–1268. doi: 10.1016/s0140-6736(18)31878-6
Fan, Z., Hao, L., Chuanyuan, T., Jun, Z., Xin, H., Sen, L., et al. (2018). Neutrophil and platelet to lymphocyte ratios in associating with blood glucose admission predict the functional outcomes of patients with primary brainstem hemorrhage. World Neurosurg. 116, e100–e107. doi: 10.1016/j.wneu.2018.04.089
Feigin, V. L., Lawes, C. M., Bennett, D. A., and Anderson, C. S. (2003). Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2, 43–53. doi: 10.1016/s1474-4422(03)00266-7
Ferro, J. M. (2006). Update on intracerebral haemorrhage. J. Neurol. 253, 985–999. doi: 10.1007/s00415-006-0201-4
Fonseca, S., Costa, F., Seabra, M., Dias, R., Soares, A., Dias, C., et al. (2021). Systemic inflammation status at admission affects the outcome of intracerebral hemorrhage by increasing perihematomal edema but not the hematoma growth. Acta Neurol. Belg. 121, 649–659. doi: 10.1007/s13760-019-01269-2
Garg, R., and Biller, J. (2019). Recent advances in spontaneous intracerebral hemorrhage. F1000Res 8:16357. doi: 10.12688/f1000research.16357.1
Giede-Jeppe, A., Bobinger, T., Gerner, S. T., Sembill, J. A., Sprügel, M. I., Beuscher, V. D., et al. (2017). Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc. Dis. 44, 26–34. doi: 10.1159/000468996
Guo, R., Wu, Y., Chen, R., Yu, Z., You, C., Ma, L., et al. (2019). Clinical value of neutrophil-to-lymphocyte ratio in primary intraventricular hemorrhage. World Neurosurg. 127, e1051–e1056. doi: 10.1016/j.wneu.2019.04.040
Hemphill, J. C. III, Bonovich, D. C., Besmertis, L., Manley, G. T., and Johnston, S. C. (2001). The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32, 891–897. doi: 10.1161/01.str.32.4.891
Hermann, D. M., Kleinschnitz, C., and Gunzer, M. (2018). Implications of polymorphonuclear neutrophils for ischemic stroke and intracerebral hemorrhage: Predictive value, pathophysiological consequences and utility as therapeutic target. J. Neuroimmunol. 321, 138–143. doi: 10.1016/j.jneuroim.2018.04.015
Hwang, B. Y., Appelboom, G., Ayer, A., Kellner, C. P., Kotchetkov, I. S., Gigante, P. R., et al. (2011). Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc. Dis. 31, 211–222. doi: 10.1159/000321870
Ikram, M. A., Wieberdink, R. G., and Koudstaal, P. J. (2012). International epidemiology of intracerebral hemorrhage. Curr. Atheroscler. Rep. 14, 300–306. doi: 10.1007/s11883-012-0252-1
Kashiwazaki, D., Tomita, T., Shibata, T., Yamamoto, S., Hori, E., Akioka, N., et al. (2021). Impact of perihematomal edema on infectious complications after spontaneous intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 30:105827. doi: 10.1016/j.jstrokecerebrovasdis.2021.105827
Keep, R. F., Hua, Y., and Xi, G. (2012). Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11, 720–731. doi: 10.1016/s1474-4422(12)70104-7
Lan, X., Han, X., Li, Q., Yang, Q. W., and Wang, J. (2017). Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 13, 420–433. doi: 10.1038/nrneurol.2017.69
Lattanzi, S., Brigo, F., Trinka, E., Cagnetti, C., Di Napoli, M., and Silvestrini, M. (2019). Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl. Stroke Res. 10, 137–145. doi: 10.1007/s12975-018-0649-4
Lattanzi, S., Cagnetti, C., Provinciali, L., and Silvestrini, M. (2017). Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget 8, 57489–57494. doi: 10.18632/oncotarget.15423
Lattanzi, S., Cagnetti, C., Provinciali, L., and Silvestrini, M. (2016). Neutrophil-to-Lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke 47, 1654–1657. doi: 10.1161/strokeaha.116.013627
Lattanzi, S., Cagnetti, C., Rinaldi, C., Angelocola, S., Provinciali, L., and Silvestrini, M. (2018). Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J. Neurol. Sci. 387, 98–102. doi: 10.1016/j.jns.2018.01.038
Liesz, A., Rüger, H., Purrucker, J., Zorn, M., Dalpke, A., Möhlenbruch, M., et al. (2013). Stress mediators and immune dysfunction in patients with acute cerebrovascular diseases. PLoS One 8:e74839. doi: 10.1371/journal.pone.0074839
Liu, S., Liu, X., Chen, S., Xiao, Y., and Zhuang, W. (2019). Neutrophil-lymphocyte ratio predicts the outcome of intracerebral hemorrhage: A meta-analysis. Medicine 98:e16211. doi: 10.1097/md.0000000000016211
Maestrini, I., Strbian, D., Gautier, S., Haapaniemi, E., Moulin, S., Sairanen, T., et al. (2015). Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology 85, 1408–1416. doi: 10.1212/wnl.0000000000002029
Malhotra, K., Goyal, N., Chang, J. J., Broce, M., Pandhi, A., Kerro, A., et al. (2018). Differential leukocyte counts on admission predict outcomes in patients with acute ischaemic stroke treated with intravenous thrombolysis. Eur. J. Neurol. 25, 1417–1424. doi: 10.1111/ene.13741
Menon, G., Johnson, S. E., Hegde, A., Rathod, S., Nayak, R., and Nair, R. (2021). Neutrophil to lymphocyte ratio - A novel prognostic marker following spontaneous intracerebral haemorrhage. Clin. Neurol. Neurosurg. 200:106339. doi: 10.1016/j.clineuro.2020.106339
Mohamed, W. S., Kamel, A. E., Abdelwahab, A. H., and Mahdy, M. E. (2021). High neutrophil-to-lymphocyte ratio predicts early neurological deterioration in spontaneous intracerebral hemorrhage patients. Egypt. J. Neurol. Psychiatry Neurosurg. 57:267. doi: 10.1186/s41983-020-00267-z
Nisar, T., Alchaki, A., and Hillen, M. (2018). Validation of ICH score in a large urban population. Clin. Neurol. Neurosurg. 174, 36–39. doi: 10.1016/j.clineuro.2018.09.007
Nóbrega Lima Rodrigues de Morais, A., Ribeiro Baylao, V. M., Martins Silva, T., Gomes Dos Santos, A., Azevedo, M., and de Oliveira, A. (2021). Is neutrophil-lymphocyte ratio a useful tool for predicting outcome in subarachnoid hemorrhage? A systematic review. Neurosurg. Rev. 44, 3023–3028. doi: 10.1007/s10143-021-01484-7
Pektezel, M. Y., Arsava, E. M., Öge, D. D., Yildiz, O. K., and Topcuoglu, M. A. (2019). Neutrophil-to-Lymphocyte ratio and prognosis of spontaneous intracerebral hemorrhage. Turkish J. Cerebrovasc. Dis. 25, 118–124. doi: 10.5505/tbdhd.2019.87587
Qi, H., Wang, D., Deng, X., and Pang, X. (2018). Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-day mortality in spontaneous intracerebral hemorrhage. Med. Sci. Monit. 24, 9282–9291. doi: 10.12659/msm.911645
Qin, J., Li, Z., Gong, G., Li, H., Chen, L., Song, B., et al. (2019). Early increased neutrophil-to-lymphocyte ratio is associated with poor 3-month outcomes in spontaneous intracerebral hemorrhage. PLoS One 14:e0211833. doi: 10.1371/journal.pone.0211833
Radu, R. A., Terecoasă, E. O., Tiu, C., Ghita, C., Nicula, A. I., Marinescu, A. N., et al. (2021). Neutrophil-to-Lymphocyte ratio as an independent predictor of in-hospital mortality in patients with acute intracerebral hemorrhage. Medicina 57:622. doi: 10.3390/medicina57060622
Römer, C., Engel, O., Winek, K., Hochmeister, S., Zhang, T., Royl, G., et al. (2015). Blocking stroke-induced immunodeficiency increases CNS antigen-specific autoreactivity but does not worsen functional outcome after experimental stroke. J. Neurosci. 35, 7777–7794. doi: 10.1523/jneurosci.1532-14.2015
Seabra, M., Soares, A., Costa, F., Fonseca, S., Parreira, T., Azevedo, E., et al. (2017). Intracerebral haemorrhage and the role of the inflammatory response. Eur. J. Neurol. 24, 361.
Sharma, D., Spring, K. J., and Bhaskar, S. M. M. (2021). Neutrophil-lymphocyte ratio in acute ischemic stroke: Immunopathology, management, and prognosis. Acta Neurol. Scand. 2021:13493. doi: 10.1111/ane.13493
Slevin, M., Capitanescu, B., Sanfeliu, C., Zeinolabediny, Y., and AlBaradie, R. (2020). Monomeric C-Reactive protein aggravates secondary degeneration after intracerebral haemorrhagic stroke and may function as a sensor for systemic inflammation. J. Clin. Med. 9:53. doi: 10.3390/jcm9093053
Song, M., Graubard, B. I., Rabkin, C. S., and Engels, E. A. (2021). Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 11:464. doi: 10.1038/s41598-020-79431-7
Sosa, P. M., de Souza, M. A., and Mello-Carpes, P. B. (2018). Green tea and red tea from camellia sinensis partially prevented the motor deficits and striatal oxidative damage induced by hemorrhagic stroke in rats. Neural. Plast 2018:5158724. doi: 10.1155/2018/5158724
Sun, Y., You, S., Zhong, C., Huang, Z., Hu, L., Zhang, X., et al. (2017). Neutrophil to lymphocyte ratio and the hematoma volume and stroke severity in acute intracerebral hemorrhage patients. Am. J. Emerg. Med. 35, 429–433. doi: 10.1016/j.ajem.2016.11.037
Tao, C., Hu, X., Wang, J., Ma, J., Li, H., and You, C. (2017). Admission neutrophil count and neutrophil to lymphocyte ratio predict 90-day outcome in intracerebral hemorrhage. Biomark. Med. 11, 33–42. doi: 10.2217/bmm-2016-0187
Templeton, A. J., McNamara, M. G., Šeruga, B., Vera-Badillo, F. E., Aneja, P., Ocaña, A., et al. (2014). Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 106:124. doi: 10.1093/jnci/dju124
Tschoe, C., Bushnell, C. D., Duncan, P. W., Alexander-Miller, M. A., and Wolfe, S. Q. (2020). Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J. Stroke 22, 29–46. doi: 10.5853/jos.2019.02236
van Asch, C. J., Luitse, M. J., Rinkel, G. J., van der Tweel, I., Algra, A., and Klijn, C. J. (2010). Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176. doi: 10.1016/s1474-4422(09)70340-0
Wang, F., Hu, S., Ding, Y., Ju, X., Wang, L., Lu, Q., et al. (2016). Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 25, 182–187. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.013
Wang, F., Wang, L., Jiang, T. T., Xia, J. J., Xu, F., Shen, L. J., et al. (2018). Neutrophil-to-lymphocyte ratio is an independent predictor of 30-day mortality of intracerebral hemorrhage patients: a validation cohort study. Neurotox. Res. 34, 347–352. doi: 10.1007/s12640-018-9890-6
Wang, F., Xu, F., Quan, Y., Wang, L., Xia, J. J., Jiang, T. T., et al. (2019). Early increase of neutrophil-to-lymphocyte ratio predicts 30-day mortality in patients with spontaneous intracerebral hemorrhage. CNS Neurosci. Ther. 25, 30–35. doi: 10.1111/cns.12977
Wang, J. (2010). Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 92, 463–477. doi: 10.1016/j.pneurobio.2010.08.001
Wang, J., and Doré, S. (2007). Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 27, 894–908. doi: 10.1038/sj.jcbfm.9600403
Wang, T., Nowrangi, D., Yu, L., Lu, T., Tang, J., Han, B., et al. (2018). Activation of dopamine D1 receptor decreased NLRP3-mediated inflammation in intracerebral hemorrhage mice. J. Neuroinflam. 15:2. doi: 10.1186/s12974-017-1039-7
Wang, W., Jiang, B., Sun, H., Ru, X., Sun, D., Wang, L., et al. (2017). Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480?687 Adults. Circulation 135, 759–771. doi: 10.1161/circulationaha.116.025250
Wang, Y., Ju, M., Chen, C., Yang, D., Hou, D., Tang, X., et al. (2018). Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J. Thorac. Dis. 10, 273–282. doi: 10.21037/jtd.2017.12.131
Wang, Y., Liu, J., Wang, X., Liu, Z., Li, F., Chen, F., et al. (2017). Frequencies of circulating B- and T-lymphocytes as indicators for stroke outcomes. Neuropsychiatr. Dis. Treat. 13, 2509–2518. doi: 10.2147/ndt.S148073
Wang, Z., Gong, Q., Guo, C., Luo, Y., and Chen, L. (2019). Neutrophil-to-lymphocyte ratio predicts hematoma growth in intracerebral hemorrhage. J. Int. Med. Res. 47, 2970–2975. doi: 10.1177/0300060519847866
Xi, G., Keep, R. F., and Hoff, J. T. (2006). Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 5, 53–63. doi: 10.1016/s1474-4422(05)70283-0
Ye, Z., Ai, X., Fang, F., Hu, X., Faramand, A., and You, C. (2017). The use of neutrophil to lymphocyte ratio as a predictor for clinical outcomes in spontaneous intracerebral hemorrhage. Oncotarget 8, 90380–90389. doi: 10.18632/oncotarget.20120
Zhang, F., Qian, J., Tao, C., Wang, Y., Lin, S., You, C., et al. (2018a). Neutrophil to lymphocyte ratio predicts island sign in patients with intracranial hemorrhage. Medicine 97:e13057. doi: 10.1097/md.0000000000013057
Zhang, F., Tao, C., Hu, X., Qian, J., Li, X., You, C., et al. (2018b). Association of neutrophil to lymphocyte ratio on 90-day functional outcome in patients with intracerebral hemorrhage undergoing surgical treatment. World Neurosurg. 119, e956–e961. doi: 10.1016/j.wneu.2018.08.010
Zhang, F., Ren, Y., Fu, W., Wang, Y., Qian, J., Tao, C., et al. (2019a). Association between neutrophil to lymphocyte ratio and blood glucose level at admission in patients with spontaneous intracerebral hemorrhage. Sci. Rep. 9:15623. doi: 10.1038/s41598-019-52214-5
Zhang, F., Ren, Y., Fu, W., Yang, Z., Wen, D., Hu, X., et al. (2019b). Predictive accuracy of neutrophil-to-lymphocyte ratio on long-term outcome in patients with spontaneous intracerebral hemorrhage. World Neurosurg. 125, e651–e657. doi: 10.1016/j.wneu.2019.01.143
Zhang, F., Ren, Y., Shi, Y., Fu, W., Tao, C., Li, X., et al. (2019c). Predictive ability of admission neutrophil to lymphocyte ratio on short-term outcome in patients with spontaneous cerebellar hemorrhage. Medicine 98:e16120. doi: 10.1097/md.0000000000016120
Keywords: neutrophil-to-lymphocyte ratio, intracerebral hemorrhage, prognostic value, mortality, poor outcome, hematoma expansion, neurological deterioration
Citation: Shi M, Li X-f, Zhang T-b, Tang Q-w, Peng M and Zhao W-y (2022) Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Front. Neurosci. 16:825859. doi: 10.3389/fnins.2022.825859
Received: 30 November 2021; Accepted: 07 February 2022;
Published: 10 March 2022.
Edited by:
Aurel Popa-Wagner, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Jesus Miguel Pradillo, Complutense University of Madrid, SpainNaufal Arkan Abiyyu Ibrahim, University of Indonesia, Indonesia
Cristina Tiu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2022 Shi, Li, Zhang, Tang, Peng and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-yuan Zhao, zhaowenyuan2021@163.com; Mian Peng, mianpeng@whu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Min Shi
Min Shi Xiao-feng Li2†
Xiao-feng Li2† Ting-bao Zhang
Ting-bao Zhang Qing-wen Tang
Qing-wen Tang Wen-yuan Zhao
Wen-yuan Zhao