- 1Department of MRI, Shaanxi Provincial People’s Hospital, Xi’an, China
- 2Department of Graduate, Xi’an Medical University, Xi’an, China
- 3Department of Diagnostic Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
The role of the cerebellum in type 2 diabetes mellitus (T2DM) has been receiving increased attention. However, the functional connectivity (FC) between the cerebellar subregions and the cerebral cortex has not been investigated in T2DM. Therefore, the purpose of this study was to investigate cerebellar-cerebral FC and the relationship between FC and clinical/cognitive variables in patients with T2DM. A total of 34 patients with T2DM and 30 healthy controls were recruited for this study to receive a neuropsychological assessment and undergo resting-state FC. We selected four subregions of the cerebellum (bilateral lobules IX, right and left Crus I/II, and left lobule VI) as regions of interest (ROIs) to examine the differences in cerebellar-cerebral circuits in patients with T2DM compared to healthy controls. Correlation analysis was performed to examine the relationship between FC and clinical/cognitive variables in the patients. Compared to healthy controls, patients with T2DM showed significantly decreased cerebellar-cerebral FC in the default-mode network (DMN), executive control network (ECN), and visuospatial network (VSN). In the T2DM group, the FC between the left cerebellar lobule VI and the right precuneus was negatively correlated with the Trail Making Test A (TMT-A) score (r = −0.430, P = 0.013), after a Bonferroni correction. In conclusion, patients with T2DM have altered FC between the cerebellar subregions and the cerebral networks involved in cognitive and emotional processing. This suggests that a range of cerebellar-cerebral circuits may be involved in the neuropathology of T2DM cognitive dysfunction.
Introduction
Type 2 diabetes mellitus (T2DM) is a risk factor for Alzheimer’s disease (AD) and vascular dementia (Curb et al., 1999; Luchsinger et al., 2001; Cukierman et al., 2005). It causes emotional abnormalities and multiple cognitive dysfunctions, such as executive function and visual space (Macpherson et al., 2017). Brain network disorders and abnormal neuronal activity are the neural bases of cognitive impairment. Studies have found, for example, that disruption of the default-mode network (DMN) may be related to episodic memory impairment (Qi et al., 2017) and depression (Cui et al., 2015), whereas disruption of the executive control network (ECN) may lead to reduced working memory (Alvarenga et al., 2010) in patients with T2DM. In addition, T2DM studies have demonstrated abnormal neuronal activity in the core regions (posterior parietal and occipital cortex) of the visuospatial network (VSN) (Cui et al., 2014; Peng et al., 2016; Wang et al., 2017).
Many neuroimaging studies on the cognitive networks have mainly focused on the cerebrum. Several clinical and neuroimaging studies have demonstrated that the cerebellum is also involved in multiple cognitive functions, such as working memory, executive function, emotional processing, and attention (Middleton and Strick, 2000; Timmann and Daum, 2007; Baillieux et al., 2010; Grimaldi and Manto, 2012). Moreover, different cerebellar subregions have distinct functional heterogeneity, and some cerebellar subregions have functional connectivity (FC) with networks related to cognition. Habas et al. (2009) found that the right and left cerebellar Crus I/II interact with cerebral executive control circuitry contralaterally, and bilateral lobule IX with the DMN, which was confirmed by later studies (Sang et al., 2012; Li et al., 2013; Marien and Beaton, 2014). In addition, a meta-analysis of neuroimaging studies showed the left lobule VI is engaged in visuospatial processing (Stoodley and Schmahmann, 2009). Furthermore, patients with cerebellar lesions have been found to experience abnormal executive functions (Karatekin et al., 2000), visuospatial dysfunction (Levisohn et al., 2000), and depressive symptoms (Kim et al., 2017).
Although accumulating evidence has highlighted the involvement of the cerebellum in cognitive function, studies exploring the potential role of the cerebellum in T2DM are relatively rare. A plausible reason is that the cerebellum is thought to be protected by hypoglycemia (Ertas et al., 1997) and may prevent damage from hyperglycemia (Heikkila et al., 2010), although such theories are being challenged by some recent studies. For example, it has been found that the density of cerebellar gray matter (Garcia-Casares et al., 2014) and blood flow (Dai et al., 2017) are decreased in patients with T2DM. In addition, several studies that used resting-state functional magnetic resonance imaging (fMRI) have demonstrated abnormalities in the spontaneous activity of neurons in the cerebellum, and impairment of FC among other regions in patients with T2DM (Xia et al., 2013; Cui et al., 2014; Garcia-Casares et al., 2014; Peng et al., 2016; Tan et al., 2019). These studies provide evidence that the cerebellum may be a vulnerable region in T2DM.
It is currently accepted that the core neuropathological features in the AD brain are extensive extracellular neuritic amyloid plaques leading to dystrophic neurites and intracellular neurofibrillary tangles consisting of tau proteins; these hallmarks ultimately lead to synapse atrophy and neuron loss (Lemche, 2018). The pathological mechanism of cognitive impairment in T2DM is believed to be similar to that of AD (Bedse et al., 2015). That is, insulin resistance leads to the phosphorylation of tau and the production of amyloid-β plaques, which tend to accumulate in cognitively related cortical hubs, disrupting the connectivity among these regions, and thus, causing cognitive impairment (Buckner et al., 2009). Therefore, T2DM-induced cognitive decline may be due to a disconnection syndrome. Resting-state FC can be used to evaluate interregional cooperation between different brain regions. Previous studies that examined different regions of interest (ROIs) other than the cerebellum have revealed the abnormal patterns of FC in T2DM (Musen et al., 2012; Chen et al., 2015; Sun et al., 2018).
The cerebellum appears to suffer from neuropathological damage, as the cerebrum does, because amyloid-β plaque deposits have been found in the cerebellum of patients with AD (Ciavardelli et al., 2010). Furthermore, primate research has found that phosphorylated tau is increased in the cerebellum of diabetic monkeys (Morales-Corraliza et al., 2016). However, it is still unclear whether the cerebellum, together with the cerebrum, participates in cognitive impairment in T2DM. Fang et al. (2017) found that diabetes-related cognitive deficits are associated with abnormal cerebellar-cerebral circuits, structurally. To the best of our knowledge, however, no study has investigated functional changes in cerebellar-cerebral circuits in patients with T2DM. Therefore, it is crucial to explore the intrinsic FC patterns of the cerebellum in T2DM.
The purpose of the present study was to investigate the intrinsic FC of cerebellar subregions related to cognitive function in patients with T2DM using resting-state fMRI, and reveal the neuropathological mechanisms of cerebellar-related cognitive impairment in T2DM. We hypothesized that patients with T2DM have decreased cerebellar-cerebral FC that is associated with cognitive impairment.
Materials and Methods
Participants
Thirty-eight patients with T2DM from the Department of Endocrinology of Shaanxi Provincial People’s Hospital and 33 euglycemic healthy controls from the local community were enrolled in our study from March 2018 to May 2019. All the participants were 40–70 years old, right-handed, and received at least 6 years of education. The patients met the diagnostic criteria of T2DM according to the American Diabetes Association in 2014, and were on stable therapy (diet, oral medications, and/or insulin). Patients were excluded from the study if they had a history of hypoglycemic episodes and macrovascular diseases (e.g., cerebrovascular or cardiovascular diseases), or had hypoglycemia (blood glucose <3.9 mmol/L) or hyperglycemia (blood glucose >33.3 mmol/L) during their hospital stay. The exclusion criteria of both groups were a self-reported history of known brain injury, epilepsy, stroke, alcohol or other substance dependence, Parkinson’s disease, major depression or other disorders that could affect cognitive function, major medical illnesses (e.g., cancer), or MRI contraindications. Details of the complications and therapeutic agents for T2DM are provided in Supplementary Tables S1, S2.
All patients were instructed to control their blood glucose according to their doctor’s orders on the day of the scan. They arrived at the Department of MRI between 6:30 and 7: 00 pm after dinner. A structured clinical interview and a series of psychological tests were conducted for approximately 30 min. Then, the patients were prepared for the MRI scan. Only one patient was scheduled each day to ensure that each patient’s MRI scan time was completed between 7:30 and 8:30 pm. Healthy controls had to have normal fasting glucose levels, and their test procedure and scan time were consistent with those of the patients. All the participants were awake during the scan and did not feel discomfort. The study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital. The study protocol was explained to all the participants and signed informed consent was obtained before they participated in the study.
Clinical Data and Neuropsychological Tests
The medical history and clinical data of the patients were obtained from the medical records and questionnaires. The clinical data of the healthy controls were collected from the outpatient medical examination center, and included weight, height, blood pressure, and body mass index. Blood pressure was measured while sitting at three different times during the day and then averaged. Blood samples were obtained, after overnight fasting for at least 8 h, to test the levels of fasting blood glucose, triglycerides, total cholesterol, low-density lipoprotein, and glycated hemoglobin. A battery of neuropsychological tests was used to evaluate the participants’ mental status and cognitive domains. The Mini Mental State Examination and the Montreal Cognitive Assessment were used to assess general cognitive function. Information processing speed and attention were tested by the Trail Making Test A (TMT-A). Executive function and visuospatial skills were evaluated by the Clock Drawing Test, and the Beck Depression Inventory was used to evaluate depressive symptoms. The neuropsychological tests were performed by a psychiatrist with more than 5 years of work experience.
Image Acquisition
The MRI scan was performed on a 3.0-Tesla MR scanner (Philips Ingenia, Best, Netherlands) using a 16-channel phased-array head coil. All participants were instructed to keep their eyes closed but stay awake during the scan. Foam pads and headphones were used to control head motion and decrease scanner noise as much as possible. Conventional T2WI and FLAIR scans were also acquired to exclude visible brain lesions. Sagittal 3-dimensional T1-weighted images were acquired with the following parameters: TR = 7.5 ms, TE = 3.5 ms, FA = 8°, FOV = 250 mm × 250 mm, matrix = 256 × 256, slice thickness = 1 mm, no gap, and 328 sagittal slices. Resting-state functional BOLD images were obtained using a gradient-echo planar sequence with the following parameters: TR = 2000 ms, TE = 30 ms, slices = 34, thickness = 4 mm, gap = 0 mm, FOV = 230 mm × 230 mm, matrix = 128 × 128, FA = 90°, and 200 volumes.
Imaging Data Analysis
Functional data analyses were conducted using the programs in Data Processing & Analysis for Brain Imaging v3.01 that are based on Statistical Parametric Mapping v12 (SPM12).2 The slice timing and realignment for head motion correction were performed after discarding the first 10 time points. Any head motion >1.5 mm or translation >1.5° rotation in any direction was excluded. Then, normalization was performed based on the resulting images using a unified segmentation of anatomical images (resampling voxel size = 3 mm × 3 mm × 3 mm). Multiple regression models were used to remove the effects of covariates of no interest, which involved 24 motion parameters, cerebrospinal fluid signals, and white matter signals. The obtained images were smoothened using an isotropic Gaussian smooth kernel with a FWHM of 6, followed by detrending and filtering (0.01–0.08 Hz).
Four ROIs were defined in standard MNI space using the map of the Automated Anatomical Labeling atlas. The ROIs were the bilateral cerebellar lobules IX, the left and right cerebellar Crus I/II, and the left cerebellar lobule VI (Figure 1). Correlation analysis was performed between the mean signal change and the time series of every voxel of the whole brain for each ROI. The resulting correlation coefficients (r) were converted by the Fisher’s r-to-z transformation to improve the Gaussianity of their distribution. The ROIs and intergroup analysis results were visualized using the BrainNet Viewer package.3
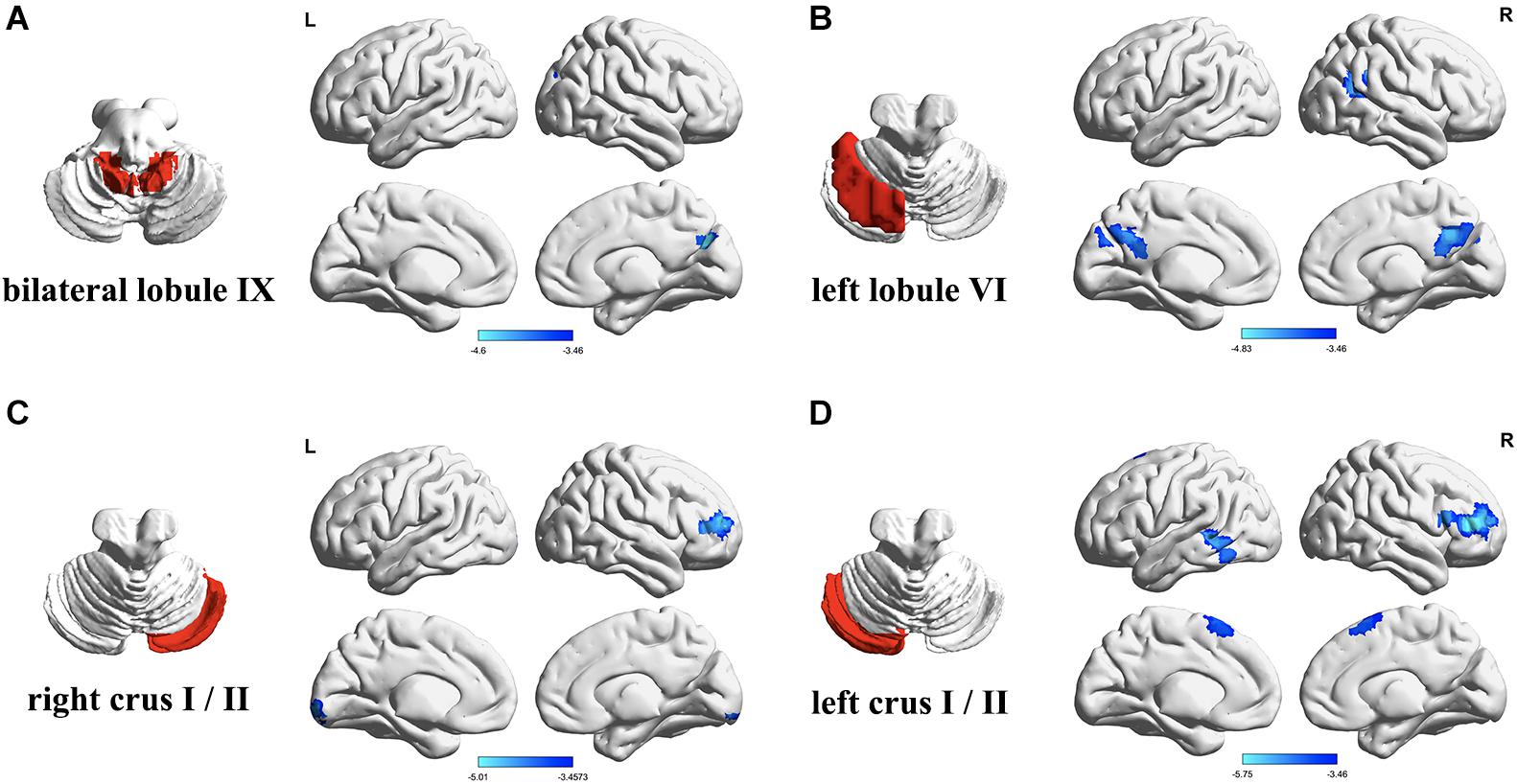
Figure 1. Differences in resting-state functional connectivity between the patients with type 2 diabetes mellitus (T2DM) and the healthy controls (two-sample t-test, P < 0.05, Gaussian random field-corrected). (A) Lower connectivity of bilateral lobule IX in T2DM. (B) Lower connectivity of left lobule VI in T2DM. (C) Lower connectivity of right Crus I/II in T2DM. (D) Lower connectivity of left Crus I/II in T2DM. L, left; R, right.
An age-related white matter change scale was used to quantitatively evaluate lacunar infarcts and white matter hyperintensity based on fluid attenuated inversion recovery images, excluding subjects with a rating score >2. Seven participants were excluded from the final statistical analysis: four (three with T2DM and two controls) were excluded for excessive motion, and two (one with T2DM and one control) were excluded for a rating score of white matter hyperintensity >2.
Statistical Analysis
Statistical analyses were performed using SPSS v17.0 (SPSS Inc., Chicago, IL, United States). Independent sample t-tests (two-tailed) were used to determine group differences for normally distributed variables. Variables that were not normally distributed were analyzed using the Mann–Whitney U-test. The chi-square (χ2) test was used to assess intergroup differences in gender and smoking. The significance level was set at P < 0.05.
Voxel-wise two-sample t-tests embedded in Data Processing & Analysis for Brain Imaging were performed to evaluate group differences in resting-state FC for each ROI after controlling for years of education. The significance was determined using Gaussian random field correction with P < 0.05 (voxel P < 0.001, cluster size >46).
The mean FC values of the functionally altered brain areas between the groups were extracted from the patients with T2DM. Partial correlation analyses were conducted to identify the relationships between the mean FC and clinical/cognitive variables, by controlling for years of education.
Results
Clinical and Neuropsychological Data
A total of 34 patients with T2DM and 30 healthy controls were enrolled in the study. The demographic, clinical, and cognitive data of the two groups are presented in Table 1. There were no significant group differences in age, gender, smoking, body mass index, total cholesterol, triglycerides, low-density lipoprotein, blood pressure, or cognitive scores (P > 0.05). However, level of education was higher in the control group than the T2DM group (P < 0.01). In addition, the T2DM group had increased levels of fasting blood glucose, glycated hemoglobin, and Beck Depression Inventory score compared to the control group (all P-values < 0.001). In the T2DM group, there were 15 patients with no complications and 19 patients with complications including nephropathy, peripheral neuropathy, retinopathy (Supplementary Table S1). Eleven patients received dietary restriction, two received insulin, 16 patients received oral medication (including metformin, sulfonylureas, and acarbose), and five patients received a combination of insulin and oral medication (Supplementary Table S2). Insulin was administered via subcutaneous injection.
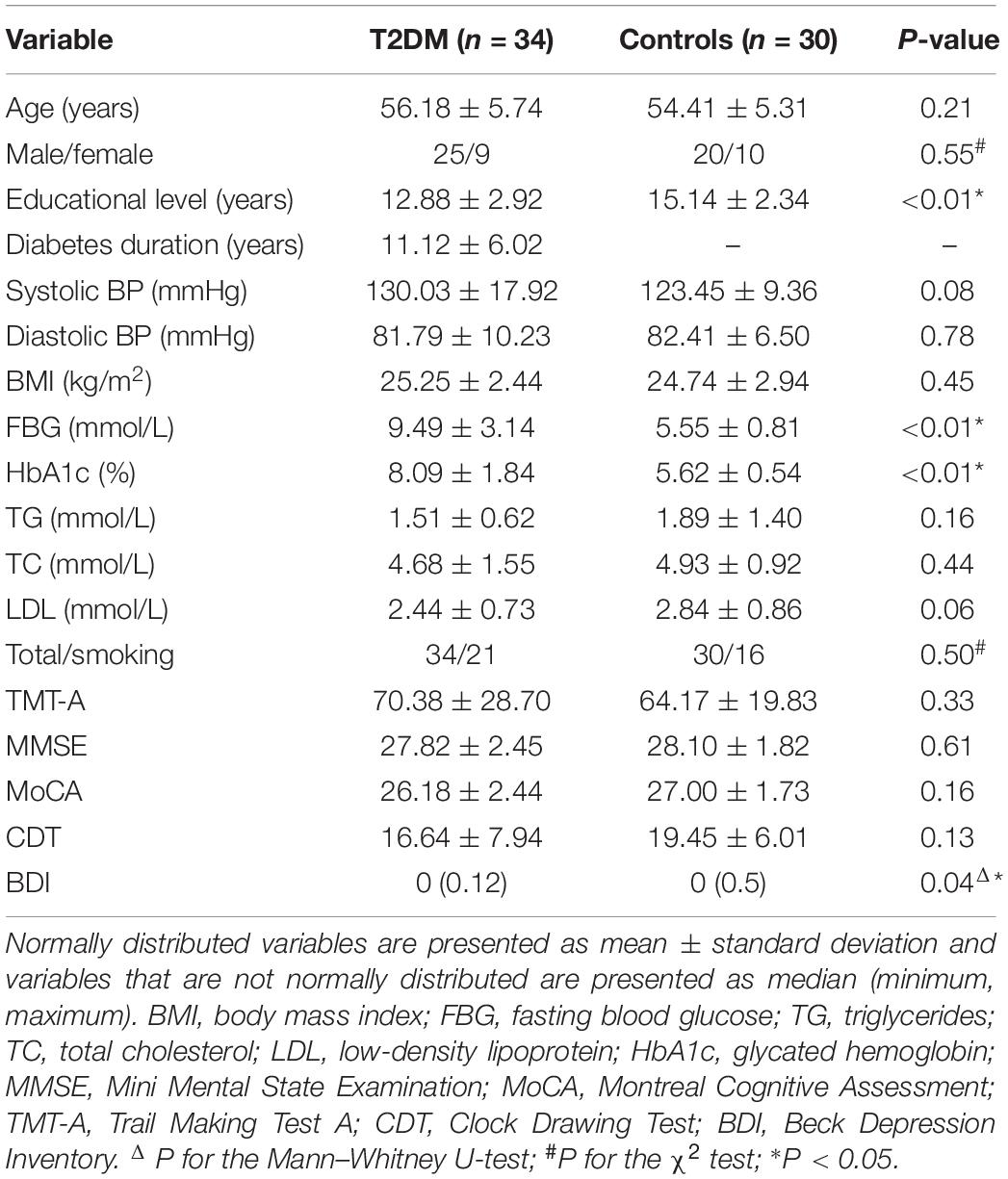
Table 1. Demographic, clinical, and cognitive data of the patients with type 2 diabetes mellitus (T2DM) and the healthy controls.
The FC Between Cerebellar Subregions and the Cerebral Cortex
The FC between the bilateral cerebellar lobules IX and the right cuneus/precuneus was significantly lower in the T2DM group than the control group, after controlling for years of education (P < 0.05). The T2DM group also had significantly lower FC (all P-values < 0.05): (a) between the right cerebellar Crus I/II and the right dorsolateral prefrontal cortex and right lingual; (b) between the left cerebellar Crus I/II and the right dorsolateral prefrontal cortex, left middle/inferior temporal gyrus, and bilateral supplementary motor area. In addition, the T2DM group had significantly lower FC between the left cerebellar lobule VI and the bilateral precuneus/posterior cingulate, right angular gyrus/supramarginal gyrus, and right superior/middle temporal gyrus. We did not find any regions in which FC was significantly higher in the T2DM group than the control group (Table 2 and Figure 1).
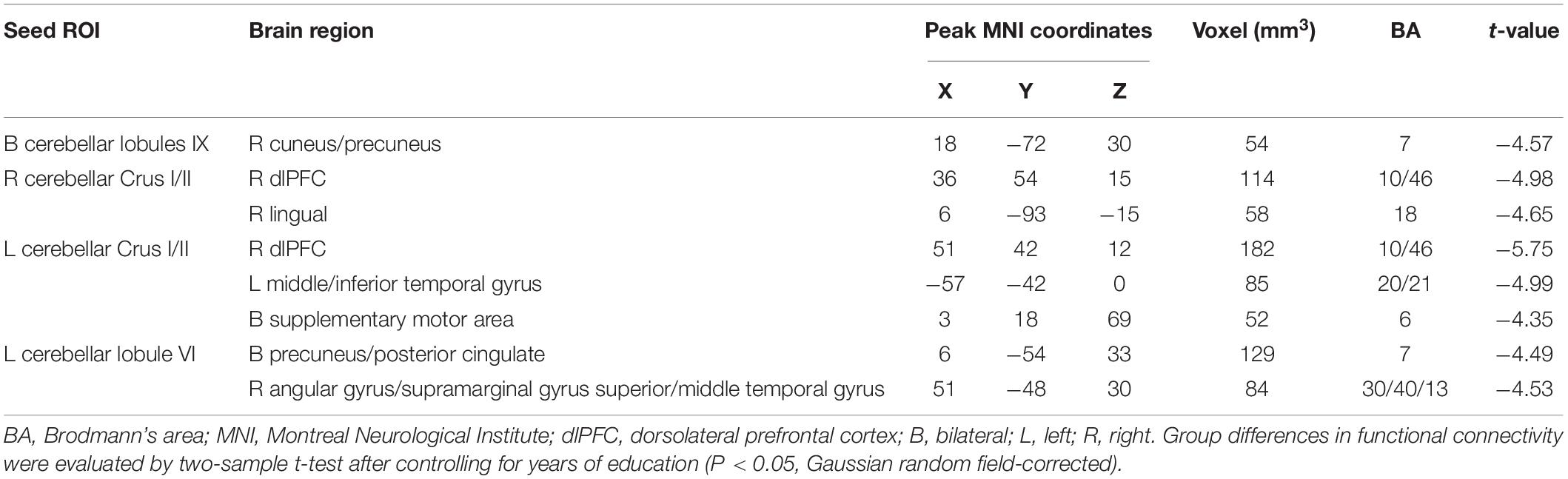
Table 2. Abnormal functional connectivity in the patients with type 2 diabetes mellitus group compared to the healthy controls.
Correlation Between the FC and Clinical/Cognitive Variables
After controlling for years of education (P < 0.05). The FC between the left cerebellar lobule VI and the right precuneus was negatively correlated with TMT-A score (r = −0.430, P = 0.013), after the Bonferroni correction for P (Figure 2). There were no significant correlations between the cerebellar-cerebral FC and other clinical/cognitive variables.
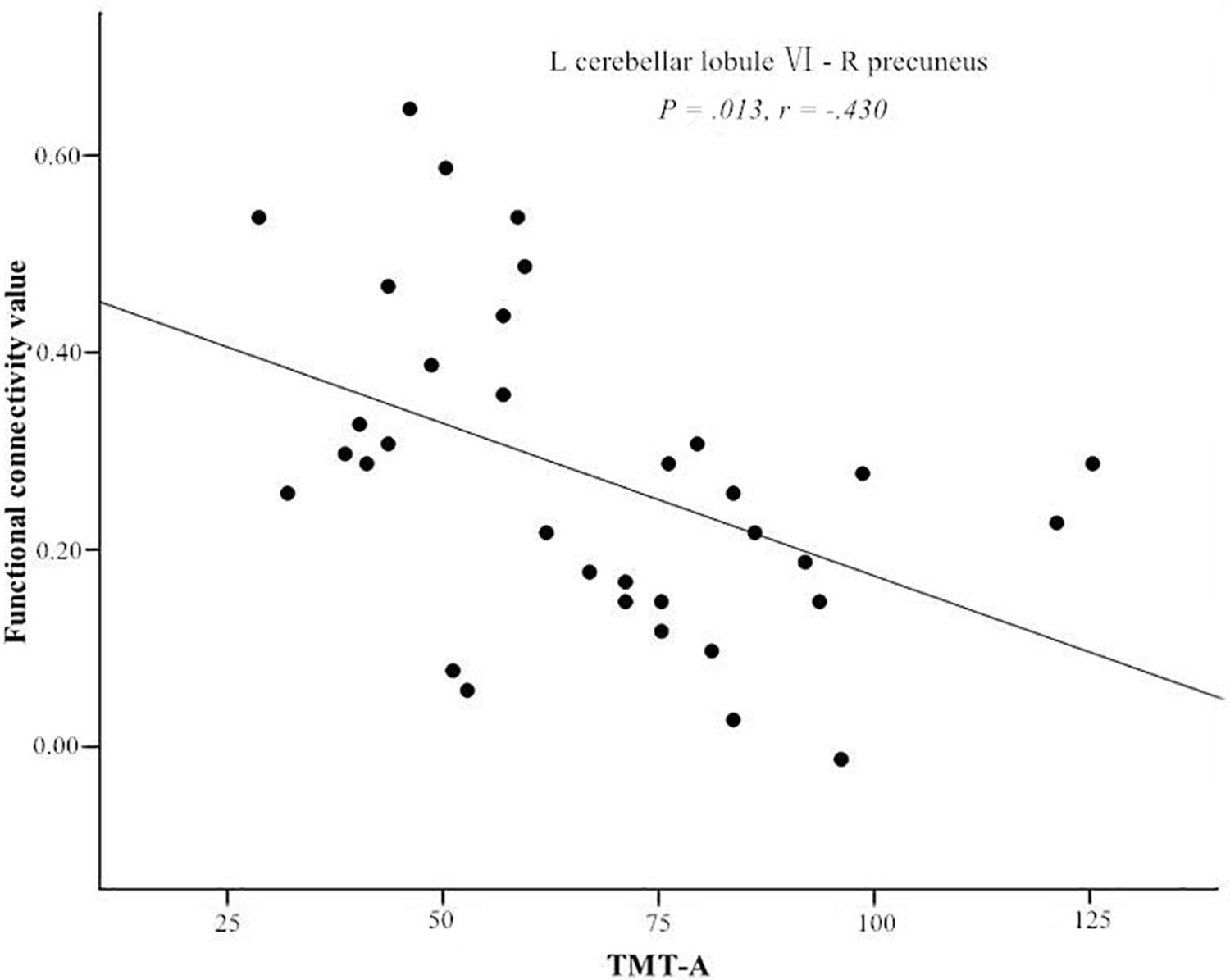
Figure 2. Significant negative correlations of the Trail Making Test A (TMT-A) score and the functional connectivity between left cerebellar lobule VI and right precuneus. L, left; R, right.
Discussion
This study explored the patterns of resting-state cerebellar-cerebral FC in T2DM. We found that cerebellar-cerebral FC decreased in the patients with T2DM compared to healthy controls, including the DMN, ECN, and VSN. This result suggests that cognitive-related cerebellar subregions are involved in the neuropathology of cognitive impairment in T2DM.
Decreased Connectivity of the Cerebellum-Cerebral DMN in T2DM
The DMN is involved in cognitive functions such as episodic memory retrieval, self-referencing, and emotion management (Buckner et al., 2008). Previous studies have found decreased gray-matter volume in DMN-related regions (Liu et al., 2017), and abnormal FC within the DMN as well as between the DMN and other brain regions (including the cerebellum) in patients with T2DM (Zhou et al., 2010; Musen et al., 2012; Zhang et al., 2015; Yang et al., 2016). The present study provides further evidence for the impairment of the cerebellum-cerebral DMN connection in patients with T2DM, especially a disconnection between the bilateral cerebellar region IX and the right cuneus/precuneus.
The precuneus is a pivotal hub of the DMN and plays a vital role in emotion management (Sheline et al., 2009). The cerebellum is also involved in emotion regulation, and abnormal connectivity between the left cerebellum and the posterior cingulate cortex/precuneus might be a predictor of suicidal behavior in depressed adolescent patients (Zhang et al., 2016). Several studies suggest that the relationship between depression and T2DM is bi-directional or co-morbid (Mezuk et al., 2008; O’Connor et al., 2009; Stuart and Baune, 2012). The depression scores of our patients with T2DM were significantly higher than that of healthy participants, which suggests that the patients may be prone to depression. Therefore, the decreased FC between the cerebellar region IX and the precuneus might underlie the dysregulation of emotion.
Decreased Connectivity of the Cerebellum-Cerebral ECN in T2DM
Multiple studies have found that biphasic projections and functional coupling exist in the prefrontal cortex and cerebellar Crus I/II (Middleton and Strick, 1994; Kelly and Strick, 2003; Strick et al., 2009). Salmi et al. (2010) showed that the cerebellar Crus I/II is linked to lateral prefrontal cortex activity with increased working memory task load. In addition, the supplementary motor area is considered to be involved in the executive system of attention and responsible for the planning of motor actions (Marek et al., 2010). Here, we demonstrated that the cerebellar Crus I/II had decreased FC with the dorsolateral prefrontal cortex and supplementary motor area. This result suggests that the bilateral cerebellum-cerebral ECN circuit is disconnected in T2DM.
Interestingly, we found that the right cerebellar Crus I/II had decreased FC with the ipsilateral rather than the contralateral dorsolateral prefrontal cortex. This result is contradictory to the theory that the bilateral Crus I/II is coupled with the contralateral ECN. Krienen and Buckner (2009) found that the cerebellar Crus I/II was slightly connected (20–30%) to the ipsilateral prefrontal cortex in healthy individuals. Therefore, we speculate that the abnormal FC in T2DM may be related to damage to the inherent pattern of the ECN. Structural research has also found decreased connectivity between the right Crus II and the right superior frontal gyrus instead of the left (Fang et al., 2017). Based on the consistent results of structural and functional studies, we believe that altered cerebellum-cerebral ECN is mainly manifested in cross-projecting functional abnormalities in T2DM.
Several studies have found that the lingual gyrus and lateral temporal lobes participate in and maintain working memory processing (Zamora et al., 2016; Moon and Jeong, 2017; Fan et al., 2019). A study on a similar diabetic population also reported an association between poor working memory in patients and disrupted connectivity of the occipital network anchored in the lingual gyrus (Cui et al., 2016). Therefore, the lower FC between the cerebellar Crus I/II and the lingual gyrus and lateral temporal gyrus may indicate impaired working memory in T2DM.
Decreased Connectivity of the Cerebellum-Cerebral VSN in T2DM
Cerebellar lobule VI receives peripheral and central inputs from the visual systems (Schmahmann, 2019). Based on independent component analysis, previous studies have shown that the posterior parietal cortex (angular gyrus and supramarginal gyrus), precuneus, and posterior cingulate are the main regions of the VSN (Beckmann et al., 2005; Smitha et al., 2017). Many studies reported that the VSN has abnormal neural intensity, reduced gray matter volume, and/or decreased perfusion of the precuneus and posterior cingulate (Cui et al., 2014; Peng et al., 2016; Liu et al., 2017; Wang et al., 2017; Wu et al., 2017), which is associated with impaired visuospatial function in T2DM (Cui et al., 2017). Here, our results suggest that the left cerebellar lobule VI is related to the impairment of visuospatial function in patients with T2DM.
Trail Making Test A is not only useful for assessing neural flexibility and attention, but it may be a particularly useful tool for detecting changes in visuospatial abilities (Vaportzis et al., 2019). In the T2DM group, we found that the decreased FC between the left cerebellar lobule VI and the right precuneus was negatively correlated with TMT-A score. This result is consistent with a tractography study of T2DM (Fang et al., 2017), which also found decreased anatomical connections between cerebellar lobule VI and the precuneus. The bilateral lobule VI and the precuneus participate in memory-guided visuospatial attention processes and have considerably greater activity during such processing (Rosen et al., 2018). The left cerebellar lobule VI may, in fact, play a vital role in controlling visuospatial attention (Striemer et al., 2015). Therefore, the abnormal connection between the left cerebellar lobule VI and the precuneus is indicative of impaired visuospatial attention in patients with T2DM.
In normal elderly individuals, amyloid-β deposition is most likely to occur in the core regions of DMN such as the precuneus (Mormino et al., 2011). In T2DM patients, insulin resistance can further accelerate amyloid-β and tau deposition (Verdile et al., 2015), increasing the possibility of T2DM developing into AD. Studies have found that the typical AD tau pattern mainly involves the dorsal attention and higher visual network (Hansson et al., 2017). Considering the similar pathological mechanism of T2DM and AD, we speculated that this may be the neural basis of abnormal visuospatial attention in patients with T2DM. Whether the FC disruption of these brain regions is related to abnormal amyloid-β and tau deposition in T2DM will be investigated in our future research.
Limitations
Several limitations of this study should be mentioned. First, there were no significant group differences in cognitive scores, which may be related to the small sample size of the study. Second, the educational level of the healthy controls was significantly higher than that of the T2DM group. This difference may produce a certain bias in the results, although the educational level is not the only factor that affects cognitive function (Crowe et al., 2013), and we have controlled it in the data analysis. Third, many of the patients received various medications with or without insulin, and therefore, we could not control the effects of medication and insulin on the results. For instance, metformin may affect the cognitive functions of patients with diabetes (Moore et al., 2013). In addition, seven patients included in this study were treated with subcutaneous insulin injection to better control blood glucose, although peripheral insulin may be involved in the modulation of plasma and cerebrospinal fluid amyloid-β levels and cognitive function, theoretically (Morris and Burns, 2012). However, more studies suggest that peripheral insulin therapy is often related to muscle and fat metabolism (Russell-Jones and Khan, 2007; McFarlane, 2009). Fourth, the serum glucose level of patients was not measured immediately before the MRI scan, and future studies should examine the relationship between blood glucose control and neuronal dysfunction. Finally, most of the patients with T2DM in this study were re-visiting the hospital and had a relatively long duration of disease; hence, the results may not be applicable to patients with early T2DM.
Conclusion
This study identified patterns of abnormal FC between cognitive-related cerebellar subregions and the cerebrum in patients with T2DM. The results suggest that a range of cerebellar-cerebral circuits may be involved in the neuropathology of T2DM, which indicates new directions for exploring cognitive dysfunction in T2DM. Further clinical studies are needed to confirm whether treatments that target the cerebellar-cerebral circuits can improve the cognitive functioning of patients with T2DM.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shaanxi Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DZ and FQ drafted the manuscript, designed the experiment, and performed the statistical analysis. JG contributed to performing the experiments and revised the manuscript. MT, XZ, and XY collected the data. MC, MW, QX, and YS provided technical support. YW contributed to the manuscript review and critique. XZ made contributions to the design of the experiment and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (81270416 and 81801327), the Key Research and Development Program of Shaanxi Province of China (2018ZDXM-SF-038), and the Social Development Science and Technology Research Project of Shaanxi Province of China (2019SF-131).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2020.571210/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; DMN, default-mode network; ECN, executive control network; FC, functional connectivity; ROI, region of interest; T2DM, type 2 diabetes mellitus; TMT-A, Trail Making Test A; VSN, visuospatial network.
Footnotes
References
Alvarenga, P. P., Pereira, D. S., and Anjos, D. M. (2010). Functional mobility and executive function in elderly diabetics and non-diabetics. Rev. Bras. Fisioter. 14, 491–496.
Baillieux, H., De Smet, H. J., Dobbeleir, A., Paquier, P. F., De Deyn, P. P., and Marien, P. (2010). Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex 46, 869–879. doi: 10.1016/j.cortex.2009.09.002
Beckmann, C. F., DeLuca, M., Devlin, J. T., and Smith, S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1001–1013. doi: 10.1098/rstb.2005.1634
Bedse, G., Di Domenico, F., Serviddio, G., and Cassano, T. (2015). Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front. Neurosci. 9:204. doi: 10.3389/fnins.2015.00204
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29, 1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009
Chen, Y. C., Xia, W., Qian, C., Ding, J., Ju, S., and Teng, G. J. (2015). Thalamic resting-state functional connectivity: disruption in patients with type 2 diabetes. Metab. Brain Dis. 30, 1227–1236. doi: 10.1007/s11011-015-9700-2
Ciavardelli, D., Silvestri, E., Del Viscovo, A., Bomba, M., De Gregorio, D., Moreno, M., et al. (2010). Alterations of brain and cerebellar proteomes linked to Abeta and tau pathology in a female triple-transgenic murine model of Alzheimer’s disease. Cell Death Dis. 1:e90. doi: 10.1038/cddis.2010.68
Crowe, M., Clay, O. J., Martin, R. C., Howard, V. J., Wadley, V. G., Sawyer, P., et al. (2013). Indicators of childhood quality of education in relation to cognitive function in older adulthood. J. Gerontol. A Biol. Sci. Med. Sci. 68, 198–204. doi: 10.1093/gerona/gls122
Cui, Y., Jiao, Y., Chen, H. J., Ding, J., Luo, B., Peng, C. Y., et al. (2015). Aberrant functional connectivity of default-mode network in type 2 diabetes patients. Eur. Radiol. 25, 3238–3246. doi: 10.1007/s00330-015-3746-8
Cui, Y., Jiao, Y., Chen, Y. C., Wang, K., Gao, B., Wen, S., et al. (2014). Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63, 749–760. doi: 10.2337/db13-0519
Cui, Y., Li, S. F., Gu, H., Hu, Y. Z., Liang, X., Lu, C. Q., et al. (2016). Disrupted brain connectivity patterns in patients with type 2 diabetes. AJNR Am. J. Neuroradiol. 37, 2115–2122. doi: 10.3174/ajnr.A4858
Cui, Y., Liang, X., Gu, H., Hu, Y., Zhao, Z., Yang, X. Y., et al. (2017). Cerebral perfusion alterations in type 2 diabetes and its relation to insulin resistance and cognitive dysfunction. Brain Imaging Behav. 11, 1248–1257. doi: 10.1007/s11682-016-9583-9
Cukierman, T., Gerstein, H. C., and Williamson, J. D. (2005). Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 48, 2460–2469. doi: 10.1007/s00125-005-0023-4
Curb, J. D., Rodriguez, B. L., Abbott, R. D., Petrovitch, H., Ross, G. W., Masaki, K. H., et al. (1999). Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology 52, 971–975. doi: 10.1212/wnl.52.5.971
Dai, W., Duan, W., Alfaro, F. J., Gavrieli, A., Kourtelidis, F., and Novak, V. (2017). The resting perfusion pattern associates with functional decline in type 2 diabetes. Neurobiol. Aging 60, 192–202. doi: 10.1016/j.neurobiolaging.2017.09.004
Ertas, N. K., Hanoglu, L., Kirbas, D., and Hatemi, H. (1997). Cerebellar syndrome due to hypoparathyroidism. J. Neurol. 244, 338–339. doi: 10.1007/s004150050099
Fan, F. M., Xiang, H., Wen, Y., Zhao, Y. L., Zhu, X. L., Wang, Y. H., et al. (2019). Brain abnormalities in different phases of working memory in schizophrenia: an integrative multi-modal MRI study. J. Nerv. Ment. Dis. 207, 760–767. doi: 10.1097/NMD.0000000000001001
Fang, P., An, J., Tan, X., Zeng, L. L., Shen, H., Qiu, S., et al. (2017). Changes in the cerebellar and cerebro-cerebellar circuit in type 2 diabetes. Brain Res. Bull. 130, 95–100. doi: 10.1016/j.brainresbull.2017.01.009
Garcia-Casares, N., Jorge, R. E., Garcia-Arnes, J. A., Acion, L., Berthier, M. L., Gonzalez-Alegre, P., et al. (2014). Cognitive dysfunctions in middle-aged type 2 diabetic patients and neuroimaging correlations: a cross-sectional study. J. Alzheimers Dis. 42, 1337–1346. doi: 10.3233/JAD-140702
Grimaldi, G., and Manto, M. (2012). Topography of cerebellar deficits in humans. Cerebellum 11, 336–351. doi: 10.1007/s12311-011-0247-4
Habas, C., Kamdar, N., Nguyen, D., Prater, K., Beckmann, C. F., Menon, V., et al. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 29, 8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009
Hansson, O., Grothe, M. J., Strandberg, T. O., Ohlsson, T., Hagerstrom, D., Jogi, J., et al. (2017). Tau pathology distribution in Alzheimer’s disease corresponds differentially to cognition-relevant functional brain networks. Front. Neurosci. 11:167. doi: 10.3389/fnins.2017.00167
Heikkila, O., Makimattila, S., Timonen, M., Groop, P. H., Heikkinen, S., and Lundbom, N. (2010). Cerebellar glucose during fasting and acute hyperglycemia in nondiabetic men and in men with type 1 diabetes. Cerebellum 9, 336–344. doi: 10.1007/s12311-010-0166-9
Karatekin, C., Lazareff, J. A., and Asarnow, R. F. (2000). Relevance of the cerebellar hemispheres for executive functions. Pediatr. Neurol. 22, 106–112. doi: 10.1016/s0887-8994(99)00128-9
Kelly, R. M., and Strick, P. L. (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003
Kim, N. Y., Lee, S. C., Shin, J. C., Park, J. E., and Kim, Y. W. (2017). Voxel-based lesion symptom mapping analysis of depressive mood in patients with isolated cerebellar stroke: a pilot study. Neuroimage Clin. 13, 39–45. doi: 10.1016/j.nicl.2016.11.011
Krienen, F. M., and Buckner, R. L. (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex 19, 2485–2497. doi: 10.1093/cercor/bhp135
Lemche, E. (2018). Early life stress and epigenetics in late-onset Alzheimer’s dementia: a systematic review. Curr. Genomics 19, 522–602. doi: 10.2174/1389202919666171229145156
Levisohn, L., Cronin-Golomb, A., and Schmahmann, J. D. (2000). Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 123(Pt 5), 1041–1050. doi: 10.1093/brain/123.5.1041
Li, W., Han, T., Qin, W., Zhang, J., Liu, H., Li, Y., et al. (2013). Altered functional connectivity of cognitive-related cerebellar subregions in well-recovered stroke patients. Neural Plast. 2013:452439. doi: 10.1155/2013/452439
Liu, J., Liu, T., Wang, W., Ma, L., Ma, X., Shi, S., et al. (2017). Reduced gray matter volume in patients with type 2 diabetes mellitus. Front. Aging Neurosci. 9:161. doi: 10.3389/fnagi.2017.00161
Luchsinger, J. A., Tang, M. X., Stern, Y., Shea, S., and Mayeux, R. (2001). Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 154, 635–641. doi: 10.1093/aje/154.7.635
Macpherson, H., Formica, M., Harris, E., and Daly, R. M. (2017). Brain functional alterations in Type 2 Diabetes – A systematic review of fMRI studies. Front. Neuroendocrinol. 47, 34–46. doi: 10.1016/j.yfrne.2017.07.001
Marek, T., Fafrowicz, M., Golonka, K., Mojsa-Kaja, J., Oginska, H., Tucholska, K., et al. (2010). Diurnal patterns of activity of the orienting and executive attention neuronal networks in subjects performing a Stroop-like task: a functional magnetic resonance imaging study. Chronobiol. Int. 27, 945–958. doi: 10.3109/07420528.2010.489400
Marien, P., and Beaton, A. (2014). The enigmatic linguistic cerebellum: clinical relevance and unanswered questions on nonmotor speech and language deficits in cerebellar disorders. Cerebellum Ataxias 1:12. doi: 10.1186/2053-8871-1-12
McFarlane, S. I. (2009). Insulin therapy and type 2 diabetes: management of weight gain. J. Clin. Hypertens. (Greenwich) 11, 601–607. doi: 10.1111/j.1751-7176.2009.00063.x
Mezuk, B., Eaton, W. W., Albrecht, S., and Golden, S. H. (2008). Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 31, 2383–2390. doi: 10.2337/dc08-0985
Middleton, F. A., and Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266, 458–461. doi: 10.1126/science.7939688
Middleton, F. A., and Strick, P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 31, 236–250. doi: 10.1016/s0165-0173(99)00040-5
Moon, C. M., and Jeong, G. W. (2017). Functional and morphological alterations associated with working memory dysfunction in patients with generalized anxiety disorder. Acta Radiol. 58, 344–352. doi: 10.1177/0284185116649794
Moore, E. M., Mander, A. G., Ames, D., Kotowicz, M. A., Carne, R. P., Brodaty, H., et al. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987. doi: 10.2337/dc13-0229
Morales-Corraliza, J., Wong, H., Mazzella, M. J., Che, S., Lee, S. H., Petkova, E., et al. (2016). Brain-wide insulin resistance, tau phosphorylation changes, and hippocampal neprilysin and amyloid-beta alterations in a monkey model of type 1 diabetes. J. Neurosci. 36, 4248–4258. doi: 10.1523/JNEUROSCI.4640-14.2016
Mormino, E. C., Smiljic, A., Hayenga, A. O., Onami, S. H., Greicius, M. D., Rabinovici, G. D., et al. (2011). Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex 21, 2399–2407. doi: 10.1093/cercor/bhr025
Morris, J. K., and Burns, J. M. (2012). Insulin: an emerging treatment for Alzheimer’s disease dementia? Curr. Neurol. Neurosci. Rep. 12, 520–527. doi: 10.1007/s11910-012-0297-0
Musen, G., Jacobson, A. M., Bolo, N. R., Simonson, D. C., Shenton, M. E., McCartney, R. L., et al. (2012). Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61, 2375–2379. doi: 10.2337/db11-1669
O’Connor, P. J., Crain, A. L., Rush, W. A., Hanson, A. M., Fischer, L. R., and Kluznik, J. C. (2009). Does diabetes double the risk of depression? Ann. Fam. Med. 7, 328–335. doi: 10.1370/afm.964
Peng, J., Qu, H., Peng, J., Luo, T. Y., Lv, F. J., Chen, L., et al. (2016). Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. Eur. J. Radiol. 85, 607–615. doi: 10.1016/j.ejrad.2015.12.024
Qi, D., Wang, A., Chen, Y., Chen, K., Zhang, S., Zhang, J., et al. (2017). Default mode network connectivity and related white matter disruption in type 2 diabetes mellitus patients concurrent with amnestic mild cognitive impairment. Curr. Alzheimer Res. 14, 1238–1246. doi: 10.2174/1567205014666170417113441
Rosen, M. L., Stern, C. E., Devaney, K. J., and Somers, D. C. (2018). Cortical and subcortical contributions to long-term memory-guided visuospatial attention. Cereb. Cortex 28, 2935–2947. doi: 10.1093/cercor/bhx172
Russell-Jones, D., and Khan, R. (2007). Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes. Metab. 9, 799–812. doi: 10.1111/j.1463-1326.2006.00686.x
Salmi, J., Pallesen, K. J., Neuvonen, T., Brattico, E., Korvenoja, A., Salonen, O., et al. (2010). Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 22, 2663–2676. doi: 10.1162/jocn.2009.21382
Sang, L., Qin, W., Liu, Y., Han, W., Zhang, Y., Jiang, T., et al. (2012). Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61, 1213–1225. doi: 10.1016/j.neuroimage.2012.04.011
Schmahmann, J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688, 62–75. doi: 10.1016/j.neulet.2018.07.005
Sheline, Y. I., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z., et al. (2009). The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U.S.A. 106, 1942–1947. doi: 10.1073/pnas.0812686106
Smitha, K. A., Akhil Raja, K., Arun, K. M., Rajesh, P. G., Thomas, B., Kapilamoorthy, T. R., et al. (2017). Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 30, 305–317. doi: 10.1177/1971400917697342
Stoodley, C. J., and Schmahmann, J. D. (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501. doi: 10.1016/j.neuroimage.2008.08.039
Strick, P. L., Dum, R. P., and Fiez, J. A. (2009). Cerebellum and nonmotor function. Annu. Rev. Neurosci. 32, 413–434. doi: 10.1146/annurev.neuro.31.060407.125606
Striemer, C. L., Chouinard, P. A., Goodale, M. A., and de Ribaupierre, S. (2015). Overlapping neural circuits for visual attention and eye movements in the human cerebellum. Neuropsychologia 69, 9–21. doi: 10.1016/j.neuropsychologia.2015.01.024
Stuart, M. J., and Baune, B. T. (2012). Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci. Biobehav. Rev. 36, 658–676. doi: 10.1016/j.neubiorev.2011.10.001
Sun, Q., Chen, G. Q., Wang, X. B., Yu, Y., Hu, Y. C., Yan, L. F., et al. (2018). Alterations of white matter integrity and hippocampal functional connectivity in type 2 diabetes without mild cognitive impairment. Front. Neuroanat. 12:21. doi: 10.3389/fnana.2018.00021
Tan, X., Liang, Y., Zeng, H., Qin, C., Li, Y., Yang, J., et al. (2019). Altered functional connectivity of the posterior cingulate cortex in type 2 diabetes with cognitive impairment. Brain Imaging Behav. 13, 1699–1707. doi: 10.1007/s11682-018-0017-8
Timmann, D., and Daum, I. (2007). Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum 6, 159–162. doi: 10.1080/14734220701496448
Vaportzis, E., Niechcial, M. A., and Gow, A. J. (2019). A systematic literature review and meta-analysis of real-world interventions for cognitive ageing in healthy older adults. Ageing Res. Rev. 50, 110–130. doi: 10.1016/j.arr.2019.01.006
Verdile, G., Fuller, S. J., and Martins, R. N. (2015). The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 84, 22–38. doi: 10.1016/j.nbd.2015.04.008
Wang, Z. L., Zou, L., Lu, Z. W., Xie, X. Q., Jia, Z. Z., Pan, C. J., et al. (2017). Abnormal spontaneous brain activity in type 2 diabetic retinopathy revealed by amplitude of low-frequency fluctuations: a resting-state fMRI study. Clin. Radiol. 72, 340.e1–340.e7. doi: 10.1016/j.crad.2016.11.012
Wu, G., Lin, L., Zhang, Q., and Wu, J. (2017). Brain gray matter changes in type 2 diabetes mellitus: a meta-analysis of whole-brain voxel-based morphometry study. J. Diabetes Complications 31, 1698–1703. doi: 10.1016/j.jdiacomp.2017.09.001
Xia, W., Wang, S., Sun, Z., Bai, F., Zhou, Y., Yang, Y., et al. (2013). Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology 38, 2493–2501. doi: 10.1016/j.psyneuen.2013.05.012
Yang, S. Q., Xu, Z. P., Xiong, Y., Zhan, Y. F., Guo, L. Y., Zhang, S., et al. (2016). Altered Intranetwork and internetwork functional connectivity in type 2 diabetes mellitus with and without cognitive impairment. Sci. Rep. 6:32980. doi: 10.1038/srep32980
Zamora, L., Corina, D., and Ojemann, G. (2016). Human temporal cortical single neuron activity during working memory maintenance. Neuropsychologia 86, 1–12. doi: 10.1016/j.neuropsychologia.2016.04.004
Zhang, H., Hao, Y., Manor, B., Novak, P., Milberg, W., Zhang, J., et al. (2015). Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64, 1025–1034. doi: 10.2337/db14-1000
Zhang, S., Chen, J. M., Kuang, L., Cao, J., Zhang, H., Ai, M., et al. (2016). Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry 16:337. doi: 10.1186/s12888-016-1047-7
Keywords: type 2 diabetes mellitus, resting-state fMRI, functional connectivity, cerebellum, subregion, neuroimaging
Citation: Zhang D, Qi F, Gao J, Yan X, Wang Y, Tang M, Zhe X, Cheng M, Wang M, Xie Q, Su Y and Zhang X (2020) Altered Cerebellar-Cerebral Circuits in Patients With Type 2 Diabetes Mellitus. Front. Neurosci. 14:571210. doi: 10.3389/fnins.2020.571210
Received: 10 June 2020; Accepted: 19 August 2020;
Published: 24 September 2020.
Edited by:
Etienne Challet, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Erwin Lemche, King’s College London, United KingdomArturo Ortega, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV), Mexico
Copyright © 2020 Zhang, Qi, Gao, Yan, Wang, Tang, Zhe, Cheng, Wang, Xie, Su and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Zhang, zxl.822@163.com
†These authors have contributed equally to this work
 Dongsheng Zhang
Dongsheng Zhang Fei Qi2†
Fei Qi2† Jie Gao
Jie Gao Xia Zhe
Xia Zhe