- Department of Biology, University of Toronto Mississauga, Mississauga, ON, Canada
A vital feature in the success of Ecdysozoa is their ability to shed their exoskeleton (a process called ecdysis) such that they can grow or change their morphology. In holometabolous insects, these behaviors are orchestrated by the sequential actions of neuropeptides, one of which is crustacean cardioactive peptide (CCAP). Little is known about the control of ecdysis in hemimetabolous insects. Here, we report that CCAP is essential for successful ecdysis in the hemimetabolous insect, Rhodnius prolixus; the vector of Chagas disease. The first indication of CCAP's involvement in ecdysis was the observation of decreased staining intensity of CCAP-containing neurons immediately following ecdysis, indicative of the release of CCAP. The critical importance of the CCAP signaling pathway was further demonstrated by knockdown (as determined by qPCR and immunohistochemistry) of the CCAP and CCAPR transcripts utilizing dsRNA. This technique reduced the staining intensity of CCAP-containing neurons, and knocked down the transcript levels by up to 92%, with lethal consequences to the insect. Insects with these transcripts knocked down had very high mortality (up to 84%), typically at the expected time of the ecdysis sequence, or had ecdysis extremely delayed. This is the first report of the susceptibility of R. prolixus to dsRNA knockdown of neuropeptide and receptor transcripts, and the data clearly demonstrates the conserved nature of the CCAP signaling pathway in ecdysis between holometabolous and hemimetabolous insects.
Introduction
Groups of Ecdysozoa including Arthropoda, Nematoda, and Cephalorhyncha possess a hard cuticle. This exoskeleton serves as protection against injury and desiccation, as a barrier against microorganisms, and as a framework for the attachment of muscles; however, this exoskeleton has the potential to interrupt the growth of Ecdysozoa (Aguinaldo et al., 1997). Thus, Ecdysozoa shed their exoskeleton in order to grow or change their morphology. The process of shedding the cuticle, or ecdysis, involves innate behaviors that are exhibited in distinct phases. Ecdysis provides an ideal model to analyze or understand a behavior at the molecular and cellular level.
Ecdysis consists of three distinct stages: pre-ecdysis, ecdysis, and post-ecdysis. Contractions of specific skeletal muscles occur in each of the stages and are orchestrated by the combined actions of hormonal and neural components involving peptidergic signaling pathways (Truman, 2005; Žitňan et al., 2007). Direct and indirect evidence in arthropods suggest that the major components of these peptidergic signaling pathways are pre-ecdysis triggering hormone (PETH), ecdysis triggering hormone (ETH), eclosion hormone (EH), crustacean cardioactive peptide (CCAP), and bursicon (Ewer and Truman, 1996; Gammie and Truman, 1997; Žitňan et al., 1999; Park et al., 2003a,b; Clark et al., 2004; Kim et al., 2006a,b; Arakane et al., 2008; Roller et al., 2010). There is considerable evidence from Crustacea and holometabolous insects that CCAP is a neuropeptide that regulates some of the ecdysis behavior. For example, CCAP is released around the time of ecdysis and activates the ecdysis motor program by turning off pre-ecdysis in Manduca sexta (Gammie and Truman, 1997; Žitňan and Adams, 2000; Fuse and Truman, 2002). Ablation of neurons expressing CCAP leads to failure of pupal ecdysis in Drosophila melanogaster (Park et al., 2003a; Clark et al., 2004; Kim et al., 2006a) and recently using a genetic analysis approach it was shown that Drosophila lacking both CCAP and pburs (partner of bursicon gene) function expressed a more severe defect at pupation than flies lacking either hormone (Lahr et al., 2012). Other studies have shown that reducing CCAP and its receptor transcript levels by RNA interference (RNAi) in Tribolium castaneum leads to a failure in ecdysis (Arakane et al., 2008; Li et al., 2011). CCAP levels are also increased up to 30-fold or more than 100-fold in the haemolymph during ecdysis in a crab, Carcinus maenas and a crayfish, Orconectes limosus, respectively, and drop to basal levels after ecdysis (Phlippen et al., 2000). These studies suggest that CCAP is critical during ecdysis in Crustacea and holometabolous insects; however, little is known about hemimetabolous insects, and nothing is known for the important vector of Chagas disease, Rhodnius prolixus. This medically-important insect provides an ideal model for studies in growth and development, including ecdysis, since in the unfed condition each instar remains in a state of arrested development and gorging on a blood meal initiates all of the physiological and endocrinological events leading to the next instar. Thus, these events can be easily timed and therefore studied accurately.
Recently, we cloned and characterized the CCAP gene in R. prolixus and mapped the expression of CCAP to cells in the CNS, and not in other tissues (Lee and Lange, 2011; Lee et al., 2011). We have also cloned and characterized the CCAP receptor gene (RhoprCCAPR) and have shown that RhoprCCAPR is upregulated prior to ecdysis in the heart and plays a role in regulating heartbeat rate (Lee et al., 2013). In the current study, we have investigated the developmental expression pattern of both RhoprCCAP and RhoprCCAPR during ecdysis in the central nervous system (CNS) and peripheral tissues of R. prolixus. We observed a reduction in the staining intensity in cell bodies and processes of CCAP-like immunoreactivity (IR) immediately following ecdysis. Utilizing RNAi, we have successfully knocked down RhoprCCAP and RhoprCCAPR transcripts, confirmed by qPCR and peptide-staining levels. Finally, we demonstrate that RhoprCCAP is critically important in R. prolixus ecdysis since interrupting the RhoprCCAP signaling pathway using RNAi leads to high mortality at ecdysis or delayed ecdysis. This is the first study to confirm the involvement of CCAP in ecdysis in a hemimetabolous insect, and illustrates the potential of using R. prolixus as a model system for examining peptidergic signaling during ecdysis.
Materials and Methods
Animals
Fourth instars R. prolixus were allowed to gorge on defibrinated rabbit blood (Hemostat Laboratories, Dixon, CA, USA; supplied by Cedarlane, Burlington, ON, Canada) and then held in an incubator at 28°C in a 16:8 h light/dark cycle. The humidity of the chamber was 30%.
Ecdysis Behavioral Sequence
Ecdysis of fully gorged 4th instar R. prolixus was investigated during the photophase. From the day that the insects were fed, their behavior during the molting cycle was observed. Some insects from Day 8 post-fed were transferred during the photophase to a Petri dish that contained a filter paper on which ecdysis took place. Specific behaviors exhibited during pre-ecdysis, ecdysis, and post-ecdysis were observed and photographed (Cybershot, Sony).
Quantitative Real Time PCR (qPCR) Analyses
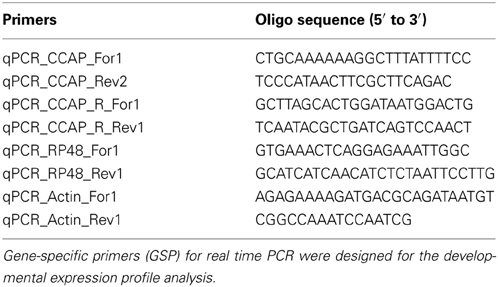
CNS, salivary gland, and hindgut of 4th or 5th instar R. prolixus were dissected in RNase free physiological saline and stored in RNA later solution (Ambion, Austin, TX). Tissues were dissected from R. prolixus 4th instar (Day 4–9 post-fed) and 5th instar (Day 1–6 post-ecdysis). Total RNA was isolated from tissues using the Trizol® reagent (Ambion, Austin, TX) and first-strand cDNA was synthesized using 100 ng of total RNA as previously described (Lee et al., 2013). The reaction was diluted 20-fold with nuclease free water and qPCR analyses were carried out on a CFX96Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA) using the Ssofast™ EvaGreen supermix (Bio-Rad Laboratories Inc., Hercules, CA, USA). Gene specific primers for RhoprCCAP, RhoprCCAPR, and the reference genes (ribosomal protein 49, rp49, and beta-actin) (Table 1) were designed to amplify target fragments of similar size across all samples. The reference genes have been validated as stable targets and used previously (Lee et al., 2013). Each primer set was designed with one primer over an exon/exon boundary and the primer efficiency was determined for each target. The relative expression was determined following the ΔΔCt method (Livak and Schmittgen, 2001; Pfaffl, 2001) and fold-differences were normalized to both of the reference genes, RP49 and beta-actin. qPCRs were repeated for a total of three biological replicates with three technical replicates each and included a no template control and a no reverse-transcriptase control. The means of the biological replicates were calculated along with their standard errors. In the dsRNA knockdowns, CNS or peripheral tissues (salivary glands, hindgut, and pool of tissues including dorsal vessel, fat body, and trachea) were collected from 4th instars 5 days post-injection with dsRNA or no injection, and quantified as above. qPCRs were repeated for a total of four biological replicates with three technical replicates each that included a no template control and a no reverse-transcriptase control.
Immunohistochemisty
Whole mount immunohistochemistry was performed to determine the level of CCAP in the CNS (brain, subesophageal ganglion, prothoracic ganglion, mesothoracic ganglionic mass) during developmental stages as described by Lee and Lange (2011) with slight modifications as described below. The CNS of 4th (6 days post-fed) and 5th instar (1 h and 6 days post-ecdysis) R. prolixus was dissected under physiological saline (NaCl, 150 mmol L−1, KCl, 8.6 mmol L−1, CaCl2, 2 mmol L−1, Glucose, 34 mmol L−1, NaHCO3, 4 mmol L−1, MgCl2, 8.5 mmol L−1, HEPES, 5 mmol L−1, pH 7.0). The tissues were fixed with 2% paraformaldehyde at 4°C overnight and processed for immunohistochemistry (Lee and Lange, 2011). CCAP like-IR was observed under a confocal microscope with LSM image browser software (Zeiss, Jena, Germany). The CNS of 4th instar R. prolixus 5 days post-injection (either with dsCCAP, dsARG, or saline) was also dissected and processed for immunohistochemistry. Pre-absorption of the CCAP antiserum with 10−5 M CCAP at 4°C for 24 h eliminated all staining indicating that the antiserum was specific for CCAP.
Double-Stranded RNA (dsRNA) Synthesis
Two overlapping fragments of the DNA template for dsRNA synthesis (see Table 2) were prepared with PCR by conjugating the T7 RNA polymerase promoter to the 5′ end (5′-taatacgactcactatagggaga-3′) on RhoprCCAP, RhoprCCAPR, or ampicillin resistant gene (ARG) as previously described (Lee et al., 2013). The PCR products were used as template for double stranded RNA (dsRNA) synthesis using the T7 Ribomax Express RNAi System (Promega, Madison, WI, USA). After synthesis, the dsRNA was precipitated with isopropanol and eluted in DEPC-treated water. It was then quantified at 260 nm wavelength using nanodrop. The quality of the dsRNA products was verified by 1% agarose gel electrophoresis and kept at −80°C until use. Before injection, the dsRNA was resuspended with DEPC-treated water at a concentration of 2 μg/μl.

Table 2. Primers for generating templates of dsCCAP, dsCCAPR or the ampicillin resistance gene (dsARG).
dsRNA Delivery
Fourth instar R. prolixus were anesthetized with CO2 for 10 s and 2 μg of dsRNA was injected into the thoracic/abdominal haemocoel at the base of the metathoracic legs using a 5 μl Hamilton syringe. Five differently treated groups, each consisting of 20–30 bugs were used in this experiment as follows: (1) dsCCAP injected, (2) dsCCAPR injected, (3) dsCCAP and dsCCAPR injected, (4) negative control dsARG injected, and (5) no injection. All bugs were left for 1 h at room temperature to recover and then placed into an incubator at 28°C on a 16:8 h light/dark cycle. Mortality, abnormal behavior, and ecdysis (timing and behavior) were monitored daily. This experiment was repeated 3 times.
Results
R. Prolixus Ecdysis Behavioral Sequence
In 4th instar R. prolixus the ecdysis behavioral sequence commences on Day 9 post-feeding on defibrinated rabbit blood under our experimental conditions. Characteristic stages of the ecdysis behavioral sequence are observed, namely pre-ecdysis, ecdysis, and post-ecdysis as previously described (Ampleford and Steel, 1982a,b). Pre-ecdysis behavior occurs approximately 2 h prior to the ecdysis sequence (Figure 1). During that period R. prolixus are observed to repeatedly touch their antennae with their prothoracic legs and rub their legs against each other several times. They then invert themselves and remain vertically upside-down on filter paper for at least 2 h. As the timing of the ecdysis sequence approaches they bob their heads up and down, swallow air, and push up repeatedly using their forelegs. The ecdysis behavioral sequence in our regime (16L: 8D) occurs at 11 h after lights off, i.e., 3 h into the light. During the ecdysis sequence, R. prolixus bend their heads downward and in the middle of ecdysis, their proboscis extends with the tip pointing toward the abdomen (Figure 1). At this time, the new cuticle on the dorsal surface of the thorax and the first two abdominal segments is exposed due to longitudinal contraction of intersegmental skeletal muscles. During the last third of the ecdysis sequence, they are quiescent—a time devoted to shedding the cuticle from the delicate foregut and tracheae. Toward the end of the ecdysis sequence, all of the legs are free from the old cuticle and the hind legs are used to push the body forward and out of the old cuticle. The R. prolixus ecdysis sequence takes 22 ± 3 min (n = 20, Mean ± SE). After shedding the old cuticle, the insects remain close to their exuvia for up to 5 min. The new cuticle of the head, legs, and wing pads is very soft and light orange in color. Post-ecdysis behavior is observed after shedding the old cuticle. During this time the soft exoskeleton becomes darker and harder (Figure 1). Post-ecdysis behavior lasts for up to 4 h (n = 20).
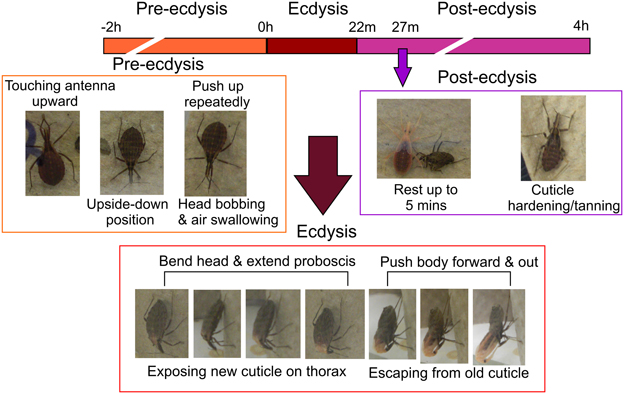
Figure 1. Temporal sequence of pre-ecdysis, ecdysis, and post-ecdysis behaviors in 4th instar Rhodnius prolixus. All characteristic behaviors during the different stages are boxed in different colors. Purple arrow denotes the end of the 5 min quiescent stage at the beginning of post-ecdysis. Observations based on 20 insects.
Developmental Expression Profile of RhoprCCAP and RhoprCCAPR Transcript
RhoprCCAP and RhoprCCAPR transcript expression is present throughout the days studied (Figure 2). In the hindgut, RhoprCCAPR transcript expression which was present on all days tested, was significantly (ANOVA p < 0.0004; Tukey's test p < 0.01) increased Day 4 post-ecdysis (Day 4 PE). (Figure 2).
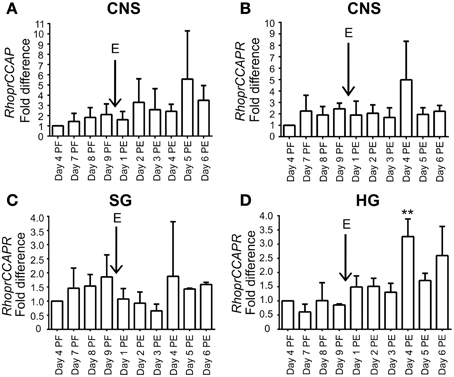
Figure 2. Expression profile of RhoprCCAP and RhoprCCAPR transcript levels during development in different tissues of 4th and 5th instar R. prolixus. (A) RhoprCCAP expression level in central nervous system (CNS). E represents day of ecdysis. (B) RhoprCCAPR transcript expression level in CNS. (C) RhoprCCAPR transcript expression in salivary gland (SG). (D) RhoprCCAPR transcript expression in hindgut (HG) was significantly increased Day 4 post-ecdysis (Day 4 PE) compared to Day 4 post-fed (Day 4 PF) as determined by ANOVA (P < 0.0004; Tukey's Test **P < 0.01). Values are expressed as the mean ± s.e.m. (n = 3).
Developmental Profile of RhoprCCAP
There is a substantial decrease in staining intensity of cell bodies and processes containing CCAP like-IR in the CNS 1 h post-ecdysis (PE) compared to Day 6 post-fed (PF) and Day 6 PE (Figure 3). In particular, CCAP like-IR in processes in the corpus cardiacum (CC) which forms an extensive neurohaemal plexus of terminal endings and varicosities were absent 1 h PE as were the extensive neuropilar regions in the brain and nerve cord.
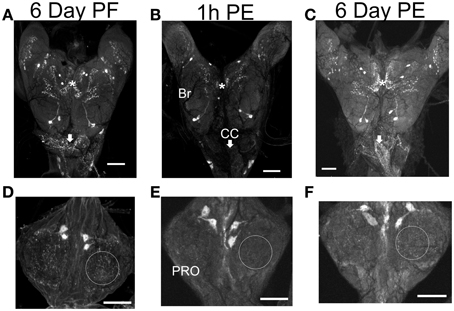
Figure 3. CCAP-like immunoreactive staining in CNS. Central nervous systems were dissected on Day 6 post-fed (PF) (A,D), 1 h post-ecdysis (PE) (B,E) and Day 6 PE (C,F). Reduction in staining intensity was observed in the neuropile and cell bodies in the brain (BR) (compare regions indicated by asterisks), processes in the corpus cardiacum (CC; compare regions indicated by arrows) and neuropile region in the prothoracic ganglion (PRO; compare regions indicated by circle) 1 h PE (B,E). Staining intensity increased by Day 6 PE. n = 30/time point. Scale bars: 100 μm.
RNAi-Mediated Knockdown of RhoprCCAP and RhoprCCAPR
Overall, RhoprCCAP and RhoprCCAPR transcript levels were dramatically reduced by 5 days after dsRNA injection (Day 9 PF) (Figure 4A). Control injections with dsARG did not reduce transcript levels relative to no injection (96 ± 3% of no injection). In the CNS, RhoprCCAP and RhoprCCAPR transcript levels were reduced by 82.6 ± 8.5% and 80.1 ± 1.3%, respectively. The percentage knockdown of the RhoprCCAPR transcript level was quantified in three peripheral tissues including the salivary glands, hindgut and a pool of tissues containing the dorsal vessel, trachea, and fat body and was reduced by 73.3 ± 12.4%, 92.7 ± 3.9%, and 70.4 ± 9.7%, respectively compared to the dsARG control group. In the double knockdown group (dsCCAP and dsCCAPR), the RhoprCCAP transcript level was decreased by 78 ± 18% in CNS, and the RhoprCCAPR transcript level was decreased in the CNS, pool of tissues containing the dorsal vessel, trachea, and fat body and the salivary glands with percentage knockdown of 63.1 ± 5.1%, 58.2 ± 0.2%, and 74.8 ± 6.0%, respectively (Figure 4B). dsRNA covering different regions of the same transcript was injected into R. prolixus and produced the same results, confirming the specificity of RNAi. Also, immunohistochemical analysis verified the reduction in peptide level caused by dsCCAP (Figure 5). Compared to the dsARG control group, the staining of cell bodies and processes containing CCAP-like IR was either absent or greatly reduced in the group injected with dsCCAP.
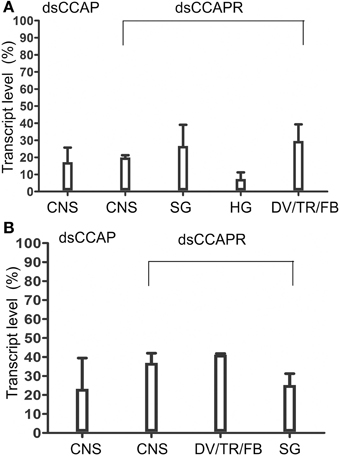
Figure 4. Verification of dsRNA efficiency by real time qPCR. (A) RhoprCCAP or RhoprCCAPR expression levels were reduced in 4th instar central nervous system (CNS), salivary gland (SG), hindgut (HG) and pools of tissues including dorsal vessel/trachea/fat body (DV/TR/FB) following injection of dsCCAP or dsCCAPR, relative to control dsARG injection. (B) RhoprCCAP and RhoprCCAP receptor expression levels were similarly reduced by injection of dsCCAP and dsCCAPR concurrently, compared with the control, dsARG. Values are expressed as the mean ± s.e.m. (n = 4).
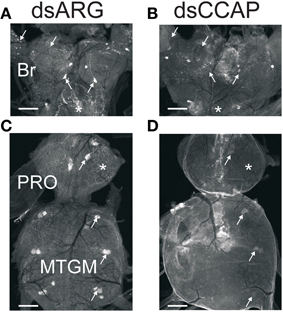
Figure 5. Verification of dsRNA knockdown by immunohisto chemistry. Central nervous systems from insects injected with dsARG or dsCCAP were examined Day 5 post-injection. (A,C) Controls (dsARG) display bright CCAP-like immunoreactive staining in cell bodies and neuropile processes (arrows). Asterisks denote staining in the corpus cardiacum (A) and neuropile (C). (B,D) dsCCAP-treated insects were devoid of staining or had reduced staining intensity. Arrows indicate location of cell bodies and neuropile processes. Asterisks denote location of the corpus cardiacum (B) and neuropile (D). Br, Brain; PRO, prothoracic ganglion; MTGM, mesothoracic ganglionic mass. n = 30/treatment. Scale bars: 100 μm.
The Effects of Disrupting the CCAP Signaling Pathway
Mortality
R. prolixus injected with dsCCAP, dsCCAPR or both dsCCAP and dsCCAPR have high mortality when monitored over a time span of 24 days (Figure 6A). The mortality of insects injected with dsCCAP was 51.1 ± 6.1% and was significantly different from insects injected with dsARG as a control (unpaired t-test, p < 0.001). When dsCCAPR was injected, 66.5 ± 9.2% of the population died (unpaired t-test, p < 0.0001) and this was not significantly different from insects injected with dsCCAP (unpaired t-test, p = 0.084). Interestingly, when both RhoprCCAP and RhoprCCAPR transcripts were concurrently knocked down, the mortality rate increased to 84.4 ± 7.3% (unpaired t-test, p < 0.0001) and this knockdown was significantly higher than in those insects injected only with dsCCAP (unpaired t-test, p = 0.0069), but not with dsCCAPR (unpaired t-test, p = 0.1145).
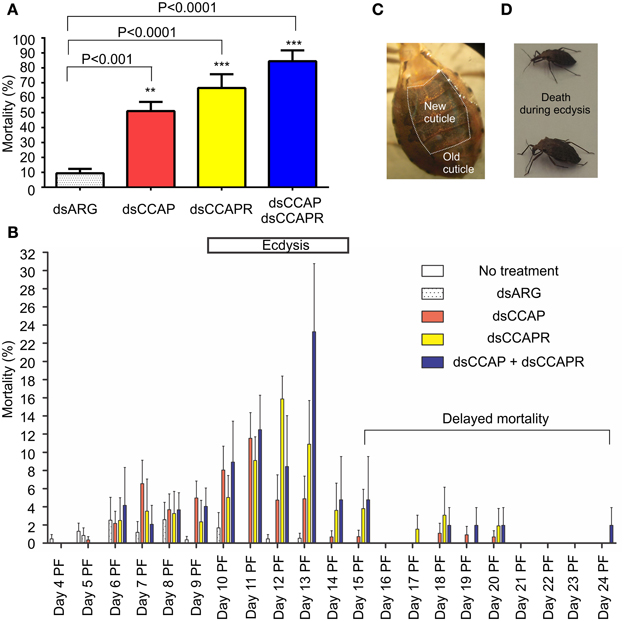
Figure 6. Mortality increases in insects injected with dsRNA of RhoprCCAP, RhoprCCAPR or both in 4th instar R. prolixus. Two micrograms of dsRNA for the ampicillin resistance gene (dsARG), the RhoprCCAP (dsCCAP), RhoprCCAPR (dsCCAPR), or both (dsCCAP and dsCCAPR) were injected into 4th instar R. prolixus on Day 4 post-fed (PF). (A) Insects injected with dsCCAP, dsCCAPR or dsCCAP and dsCCAPR have high mortality rate. Asterisks indicate significant differences compared to the control group that was injected with dsARG (unpaired t-test, **P < 0.001; ***P < 0.0001). (B) Day on which mortality occurred. Bar denotes expected time of ecdysis. (C) Photo of a bug that died during the ecdysis sequence. The new cuticle has been laid down under the old cuticle. (D) Photo of two bugs that died after initiating the ecdysis sequence. Proboscis was extended posteriorly, body partially bent, but unable to complete ecdysis. Each group consisted of 20–30 bugs and this experiment was repeated 3 times. Values are expressed as the mean ± s.e.m. (n = 3).
Interestingly, in these RNAi experiments the majority of insects formed a new cuticle, in preparation for the ecdysis behavioral sequence, which was already separated from the old cuticle (Figure 6C). Overall, death of insects injected with dsCCAP and/or dsCCAPR occurred during the expected time of the ecdysis sequence (Day 9–14) (Figure 6B). In insects injected with dsCCAP, 47.6% of the population died between Day 5 and 14 PF while 3.4% of the population died from Day 15 to 20 (delayed mortality). In insects injected with dsCCAPR, 56.2% of the population died during Day 6–14 and 10.3% of the population died from Day 15 to 20. In insects injected with both dsCCAP and dsCCAPR, 71.8% of population died during Day 6–14, and 12.6% of them died from Day 15 to 24. None of the insects that died (even those with “delayed” mortality) had successfully completed the ecdysis sequence. Examination of the insects dying indicated that they died after initiating ecdysis behavior. Their proboscis was extended posteriorly and their body partially bent but they were unable to complete ecdysis and died in this position (Figure 6D).
Ecdysis
In insects that received no injection, 97.4% of the population successfully completed the ecdysis behavioral sequence at the expected time between Days 9 and 14 PF (normal ecdysis) (Figure 7). A second set of control insects were injected with dsARG and in these 82.6% of the population successfully completed ecdysis with 7.5% showing a slightly delayed ecdysis (from Day 15 to 17 PF).
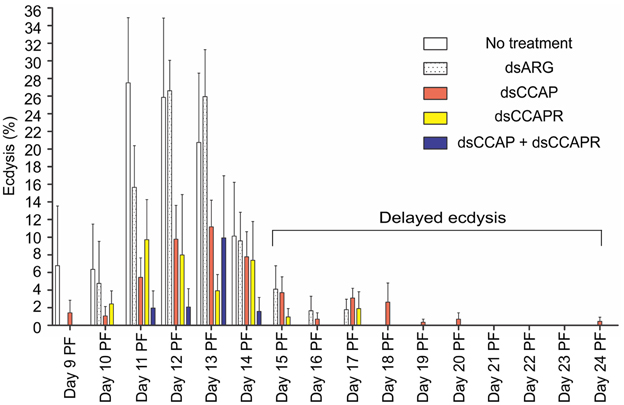
Figure 7. Some 4th instar R. prolixus that had knockdown of RhoprCCAP, RhoprCCAPR or both did not die and were still able to ecdyse; however, many of them had delayed ecdysis. Insects injected with dsCCAP, dsCCAPR, or dsCCAP and dsCCAPR displayed an ecdysis timing that was delayed (i.e., shifted to the right) compared to the control insects with some insects being delayed 24 days PF. Each group consisted of 20–30 bugs and this experiment was repeated 3 times. Values are expressed as the mean ± s.e.m. (n = 3).
Although mortality was high for the experimental groups, some of the remaining insects injected with dsCCAP and/or dsCCAPR still were capable of normal ecdysis (36.7% of the dsCCAP injected insects; 31.4% injected with dsCCAPR; 15.6% injected with both dsCCAP and dsCCAPR) (Figure 7). Some of the insects injected with dsCCAP delayed their ecdysis behavioral sequence for up to Day 24 PF (11.7%) and 2.9% of insects injected with dsCCAPR also delayed their ecdysis for up to Day 17 PF.
The data on mortality, delayed mortality, ecdysis, and delayed ecdysis are all summarized in Figure 8. Of the insects that ecdysed successfully, no defects or delays in cuticle hardening or tanning were observed.
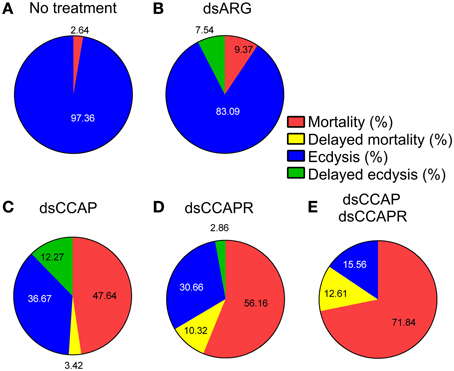
Figure 8. Summary of the effects of knockdown of CCAP and CCAPR on R. prolixus ecdysis. Four different categories were investigated; % mortality, % delayed mortality, % ecdysis and % delayed ecdysis. The percentage of mortality and delayed mortality was calculated up to Day 14 post-fed (PF), and after Day 15 PF, respectively. The percentage of ecdysis or delayed ecdysis was calculated on bugs up to Day 14 PF, and after Day15 PF, respectively. (A) No treatment. (B) Control injected with dsARG. (C) Injected with dsCCAP. (D) Injected with dsCCAPR. (E) Injected with both dsCCAP and dsCCAPR. Overall, high mortality rate and delayed ecdysis was observed in experimental groups that were treated with dsCCAP, dsCCAPR or both.
Discussion
Ecdysis is a complex process requiring a series of patterned behaviors and has been studied in detail in several holometabolous insects (see Žitňan and Adams, 2012). The ecdysis behavioral sequence is controlled by conserved neuronal networks and peptidergic signaling pathways and in holometabolous insects CCAP is one of the important neuropeptides involved in the ecdysis behavioral sequence (see Gammie and Truman, 1997; Fuse and Truman, 2002; Park et al., 2003a,b; Clark et al., 2004; Kim et al., 2006a,b; Arakane et al., 2008; Li et al., 2011; Žitňan and Adams, 2012). CCAP is co-expressed with other neuropeptides including bursicon, myoinhibiting peptides (MIPs), as well as ETH receptors (Kim et al., 2006a,b; Luan et al., 2006; Loveall and Deitcher, 2010).
In the hemimetabolous insect, R. prolixus, ecdysis timing was first studied by (Ampleford and Steel, 1982a,b). The ecdysis behavioral sequence occurs with a circadian rhythm, synchronized by lights off. R. prolixus demonstrates characteristic pre-ecdysis, ecdysis, and post-ecdysis behaviors in a similar fashion to holometabolous insects (see Gammie and Truman, 1997; Park et al., 2003a; Clark et al., 2004; Arakane et al., 2008). As might be expected for a peptide that is pleiotropic, the CCAP and CCAPR transcripts are present at all days tested, and CCAP is obviously involved in a variety of physiological processes. However, here we demonstrate that the CCAP signaling pathway is essential for successful ecdysis in R. prolixus; the first time that CCAP has been implicated in ecdysis in a hemimetabolous insect.
The RNAi technique has been used to investigate the functional roles of CCAP in ecdysis in this hemimetabolous insect, R. prolixus. Interfering with the CCAP signaling pathway disrupts ecdysis. Thus, injection of dsRNA into 4th instar R. prolixus resulted in a suppression of RhoprCCAP and RhoprCCAPR transcript levels as well as peptide content. R. prolixus with reduced transcript levels of RhoprCCAP, RhoprCCAPR or both have high mortality; the insects dying immediately prior to or during the ecdysis behavioral sequence. In a small number of cases the ecdysis behavioral sequence occurred but some were greatly delayed (up to 24 d). It is possible that in the insects that were successful at undergoing ecdysis or had delayed ecdysis, the CCAP signaling pathway had been disrupted but not to the same extent as in the insects that died during ecdysis (e.g., efficiency of knockdown). The involvement of CCAP in ecdysis is consistent with other studies. For example, the highest expression level of CCAP was observed during pre-moult and the lowest level was observed during post-molt in Crustacea (Chung et al., 2006). Also, peak CCAP receptor expression was observed during late pupal stages in D. melanogaster and Anopheles gambiae (Cazzamali et al., 2003; Belmont et al., 2006; Estevez-Lao et al., 2013). In T. castaneum, CCAP and its receptor transcript levels were highest during the mid-pupal stage but declined thereafter (Arakane et al., 2008). We observed a substantial reduction in the staining intensity of cell bodies and processes containing CCAP like-IR 1 h PE. Since we did not observe any decrease in CCAP transcript levels at these times, this decrease in staining is suggestive of CCAP being released from its stores (although we cannot discount that the stored CCAP is being enzymatically degraded). In D. melanogaster larval and pupal ecdysis, a reduction in the staining of CCAP-like IR in axons in the CNS was also observed (Park et al., 2003a; Clark et al., 2004).
The possible importance of CCAP in hemimetabolous insects has been suggested before. In Locusta migratoria nymphs, ecdysis behavior is associated with cGMP elevation in a set of CCAP-containing neurons (Truman et al., 1996), as it is in some other insects (Ewer and Truman, 1996; Žitňan et al., 2002; see Žitňan and Adams, 2012). Also, in Schistocerca gregaria, air swallowing—an important feature of ecdysis, is driven by the frontal ganglion, and rhythmic motor patterns are initiated from the frontal ganglion by application of ETH and CCAP (Zilberstein et al., 2006). Thus, CCAP in R. prolixus may well be acting on central neurons to initiate or modulate programmed motor output for specific sequences of muscle contraction associated with ecdysis, as has been shown in a variety of holometabolous insects (see Žitňan and Adams, 2012). CCAP and its receptor are also associated with the salivary glands and hindgut in R. prolixus. CCAP has direct myotropic actions on several insect visceral/skeletal muscles (Donini et al., 2001, 2002; Lange and Patel, 2005; Lee and Lange, 2011). It would be interesting to see if CCAP is important for modulating contractions of some of the muscles involved in ecdysis behavior. The associations of CCAP with salivary glands suggests that CCAP may be involved in the control of this tissue, but this is yet to be tested.
In addition to the need for fine control of central neurons and skeletal and visceral muscles, ecdysis also demands a regulation of cardiovascular functions which affects heartbeat performance and the redirection of haemolymph flow (Phlippen et al., 2000). It is well known that CCAP is a cardioaccelerator in arthropods (Tublitz and Evans, 1986; Lehman et al., 1993; Nichols et al., 1999; Lee and Lange, 2011). D. melanogaster heartbeat frequency is increased during the last 10 h of adult development and peaks at 1 h before the ecdysis behavioral sequence in the white stage (Baker et al., 1999). Previously, we reported that RhoprCCAPR transcript level was up-regulated 10-fold in a pool of tissues containing the heart (dorsal vessel), trachea and fat body prior to ecdysis and dropped to basal levels post-ecdysis (Lee et al., 2013). This indicates that the heart/dorsal vessel may have increased sensitivity to CCAP during the ecdysis behavioral sequence. Also, reducing the RhoprCCAPR transcript level leads to significantly decreased heartbeat frequency (31.1%) in vivo (Lee et al., 2013). In other insects, CCAP is co-released with bursicon and MIPs—neuropeptides which are also important for ecdysis (Kim et al., 2006a,b; Woodruff et al., 2008). CCAP may help to disperse these neuropeptides throughout the haemocoel during the ecdysis behavioral sequence because of its ability to increase heartbeat rate. In addition, interfering with these other physiological functions of CCAP (e.g., heart and muscle stimulatory actions) might be causes of early mortality or delayed ecdysis in some of the insects tested.
Overall, our RNAi experiments followed by behavioral analysis reveal the essential roles of CCAP in R. prolixus ecdysis and this represents the first hemimetabolous insect to be studied in this way. In addition, this work emphasizes the conserved nature of the CCAP signaling pathway throughout insects and furthermore confirms the advantages of using R. prolixus as a model in light of the ability to precisely time events associated with growth and development.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada. We are grateful to Nikki Sarkar for maintaining the R. prolixus colony.
References
Aguinaldo, A. M., Turbeville, J. M., Linford, L. S., Rivera, M. C., Garey, J. R., Raff, R. A., et al. (1997). Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387, 489–493. doi: 10.1038/387489a0
Ampleford, E. J., and Steel, C. G. (1982a). The behaviour of Rhodnius prolixus (Stål) during the imaginal ecdysis. Can. J. Zool. 60, 168–174. doi: 10.1139/z82-022
Ampleford, E. J., and Steel, C. G. (1982b). Circadian control of ecdysis in Rhodnius prolixus (Hemiptera). J. Comp. Physiol. 147, 281–286. doi: 10.1007/BF00609852
Arakane, Y., Li, B., Muthukrishnan, S., Beeman, R. W., Kramer, K. J., and Park, Y. (2008). Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech. Dev. 125, 984–995. doi: 10.1016/j.mod.2008.09.002
Baker, J. D., McNabb, S. L., and Truman, J. W. (1999). The hormonal coordination of behavior and physiology at adult ecdysis in Drosophila melanogaster. J. Exp. Biol. 202, 3037–3048.
Belmont, M., Cazzamali, G., Williamson, M., Hauser, F., and Grimmelikhuijzen, C. J. (2006). Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem. Biophys. Res. Commun. 344, 160–165. doi: 10.1016/j.bbrc.2006.03.117
Cazzamali, G., Hauser, F., Kobberup, S., Williamson, M., and Grimmelikhuijzen, C. J. (2003). Molecular identification of a Drosophila G protein-coupled receptor specific for crustacean cardioactive peptide. Biochem. Biophys. Res. Commun. 303, 146–152. doi: 10.1016/S0006-291X(03)00302-4
Chung, J. S., Wilcockson, D. C., Zmora, N., Zohar, Y., Dircksen, H., and Webster, S. G. (2006). Identification and developmental expression of mRNAs encoding crustacean cardioactive peptide (CCAP) in decapod crustaceans. J. Exp. Biol. 209, 3862–3872. doi: 10.1242/jeb.02425
Clark, A. C., del Campo, M. L., and Ewer, J. (2004). Neuroendocrine control of larval ecdysis behavior in Drosophila: complex regulation by partially redundant neuropeptides. J. Neurosci. 24, 4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004
Donini, A., Agricola, H., and Lange, A. B. (2001). Crustacean cardioactive peptide is a modulator of oviduct contractions in Locusta migratoria. J. Insect. Physiol. 47, 277–285. doi: 10.1016/S0022-1910(00)00112-8
Donini, A., Ngo, C., and Lange, A. B. (2002). Evidence for crustacean cardioactive peptide-like innervation of the gut in Locusta migratoria. Peptides 23, 1915–1923. doi: 10.1016/S0196-9781(02)00174-2
Estevez-Lao, T. Y., Boyce, D. S., Honegger, H. W., and Hillyer, J. F. (2013). Cardioacceleratory function of the neurohormone CCAP in the mosquito Anopheles gambiae. J. Exp. Biol. 216, 601–613. doi: 10.1242/jeb.077164
Ewer, J., and Truman, J. W. (1996). Increases in cyclic 3', 5'-guanosine monophosphate (cGMP) occur at ecdysis in an evolutionarily conserved crustacean cardioactive peptide-immunoreactive insect neuronal network. J. Comp. Neurol. 370, 330–341. doi: 10.1002/(SICI)1096-9861(19960701)370:3<330::AID-CNE4>3.3.CO;2-5
Fuse, M., and Truman, J. W. (2002). Modulation of ecdysis in the moth Manduca sexta: the roles of the suboesophageal and thoracic ganglia. J. Exp. Biol. 205, 1047–1058.
Gammie, S. C., and Truman, J. W. (1997). Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta. J. Neurosci. 17, 4389–4397.
Kim, Y. J., Žitňan, D., Galizia, C. G., Cho, K. H., and Adams, M. E. (2006a). A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr. Biol. 16, 1395–1407. doi: 10.1016/j.cub.2006.06.027
Kim, Y. J., Žitňan, D., Cho, K. H., Schooley, D. A., Mizoguchi, A., and Adams, M. E. (2006b). Central peptidergic ensembles associated with organization of an innate behavior. Proc. Natl. Acad. Sci. U.S.A. 103, 14211–14216. doi: 10.1073/pnas.0603459103
Lahr, E. C., Dean, D., and Ewer, J. (2012). Genetic analysis of ecdysis behavior in Drosophila reveals partially overlapping functions of two unrelated neuropeptides. J. Neurosci. 32, 6819–6829. doi: 10.1523/JNEUROSCI.5301-11.2012
Lange, A. B., and Patel, K. (2005). The presence and distribution of crustacean cardioactive peptide in the central and peripheral nervous system of the stick insect, Baculum extradentatum. Regul. Pept. 129, 191–201. doi: 10.1016/j.regpep.2005.02.011
Luan, H., Lemon, W. C., Peabody, N. C., Pohl, J. B., Zelensky, P. K., Wang, D., et al. (2006). Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J. Neurosci. 26, 573–584. doi: 10.1523/JNEUROSCI.3916-05.2006
Lee, D. H., and Lange, A. B. (2011). Crustacean cardioactive peptide in the Chagas' disease vector, Rhodnius prolixus: presence, distribution and physiological effects. Gen. Comp. Endocrinol. 174, 36–43. doi: 10.1016/j.ygcen.2011.08.007
Lee, D. H., Paluzzi, J. P., Orchard, I., and Lange, A. B. (2011). Isolation, cloning and expression of the crustacean cardioactive peptide gene in the Chagas' disease vector, Rhodnius prolixus. Peptides 32, 475–482. doi: 10.1016/j.peptides.2010.06.035
Lee, D. H., Vanden Broeck, J., and Lange, A. B. (2013). Identification and expression of the CCAP receptor in the Chagas' disease vector, Rhodnius prolixus, and its involvement in cardiac control. PLoS. ONE. 8:e68897. doi: 10.1371/journal.pone.0068897
Lehman, H. K., Murgiuc, C. M., Miller, T. A., Lee, T. D., and Hildebrand, J. G. (1993). Crustacean cardioactive peptide in the sphinx moth, Manduca sexta. Peptides 14, 735–741. doi: 10.1016/0196-9781(93)90106-Q
Li, B., Beeman, R. W., and Park, Y. (2011). Functions of duplicated genes encoding CCAP receptors in the red flour beetle, Tribolium castaneum. J. Insect. Physiol. 57, 1190–1197. doi: 10.1016/j.jinsphys.2011.05.011
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Loveall, B. J., and Deitcher, D. L. (2010). The essential role of bursicon during Drosophila development. BMC Dev. Biol. 10:92. doi: 10.1186/1471-213X-10-92
Nichols, R., Kaminski, S., Walling, E., and Zornik, E. (1999). Regulating the activity of a cardioacceleratory peptide. Peptides 20, 1153–1158. doi: 10.1016/S0196-9781(99)00118-7
Park, J. H., Schroeder, A. J., Helfrich-Forster, C., Jackson, F. R., and Ewer, J. (2003a). Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development 130, 2645–2656. doi: 10.1242/dev.00503
Park, Y., Kim, Y. J., Dupriez, V., and Adams, M. E. (2003b). Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J. Biol. Chem. 278, 17710–17715. doi: 10.1074/jbc.M301119200
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. doi: 10.1093/nar/29.9.e45
Phlippen, M. K., Webster, S. G., Chung, J. S., and Dircksen, H. (2000). Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J. Exp. Biol. 203, 521–536.
Roller, L., Žitňanova, I., Dai, L., Šimo, L., Park, Y., Satake, H., et al. (2010). Ecdysis triggering hormone signaling in arthropods. Peptides 31, 429–441. doi: 10.1016/j.peptides.2009.11.022
Truman, J., Ewer, J., and Ball, E. (1996). Dynamics of cyclic GMP levels in identified neurones during ecdysis behaviour in the locust Locusta migratoria. J. Exp. Biol. 199, 749–758.
Truman, J. W. (2005). Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam. Horm. 73, 1–30. doi: 10.1016/S0083-6729(05)73001-6
Tublitz, N. J., and Evans, P. D. (1986). Insect cardioactive peptides: cardioacceleratory peptide (CAP) activity is blocked in vivo and in vitro with a monoclonal antibody. J. Neurosci. 6, 2451–2456.
Woodruff, E. A. III., Broadie, K., and Honegger, H. W. (2008). Two peptide transmitters co-packaged in a single neurosecretory vesicle. Peptides 29, 2276–2280. doi: 10.1016/j.peptides.2008.08.023
Zilberstein, Y., Ewer, J., and Ayali, A. (2006). Neuromodulation of the locust frontal ganglion during the moult: a novel role for insect ecdysis peptides. J. Exp. Biol. 209, 2911–2919. doi: 10.1242/jeb.02339
Žitňan, D., and Adams, M. E. (2000). Excitatory and inhibitory roles of central ganglia in initiation of the insect ecdysis behavioural sequence. J. Exp. Biol. 203, 1329–1340.
Žitňan, D., and Adams, M. E. (2012). “Neuroendocrine regulation of ecdysis,” in Insect Endocrinology, ed L. I. Gilbert (London: Academic Press), 253–309.
Žitňan, D., Hollar, L., Spalovská, I., Takáě, P., Žitňanova, I., Gill, S. S., et al. (2002). Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J. Exp. Biol. 205, 3459–3473.
Žitňan, D., Kim, Y. J., Žitňanova, I., Roller, L., and Adams, M. E. (2007). Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol. 153, 88–96. doi: 10.1016/j.ygcen.2007.04.002
Keywords: Chagas disease, crustacean cardioactive peptide, G-protein coupled receptor, hemipteran, RNAi, CNS
Citation: Lee D, Orchard I and Lange AB (2013) Evidence for a conserved CCAP-signaling pathway controlling ecdysis in a hemimetabolous insect, Rhodnius prolixus. Front. Neurosci. 7:207. doi: 10.3389/fnins.2013.00207
Received: 22 August 2013; Accepted: 16 October 2013;
Published online: 05 November 2013.
Edited by:
Liliane Schoofs, Catholic University of Leuven, BelgiumReviewed by:
Jozef Vanden Broeck, Katholieke Universiteit Leuven, BelgiumDusan Zitnan, Institute of Zoology, Slovakia
Copyright © 2013 Lee, Orchard and Lange. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DoHee Lee, Department of Biology, University of Toronto Mississauga, 3359 Mississauga Road, Mississauga, ON L5L 1C6, Canada e-mail: dohee.lee@utoronto.ca
 DoHee Lee
DoHee Lee Ian Orchard
Ian Orchard Angela B. Lange
Angela B. Lange