Estimating frontal and parietal involvement in cognitive estimation: a study of focal neurodegenerative diseases
- 1Penn Frontotemporal Degeneration Center, Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA
- 2Department of Linguistics, University of Pennsylvania, Philadelphia, PA, USA
We often estimate an unknown value based on available relevant information, a process known as cognitive estimation. In this study, we assess the cognitive and neuroanatomic basis for quantitative estimation by examining deficits in patients with focal neurodegenerative disease in frontal and parietal cortex. Executive function and number knowledge are key components in cognitive estimation. Prefrontal cortex has been implicated in multilevel reasoning and planning processes, and parietal cortex has been associated with number knowledge required for such estimations. We administered the Biber cognitive estimation test (BCET) to assess cognitive estimation in 22 patients with prefrontal disease due to behavioral variant frontotemporal dementia (bvFTD), to 17 patients with parietal disease due to corticobasal syndrome (CBS) or posterior cortical atrophy (PCA) and 11 patients with mild cognitive impairment (MCI). Both bvFTD and CBS/PCA patients had significantly more difficulty with cognitive estimation than controls. MCI were not impaired on BCET relative to controls. Regression analyses related BCET performance to gray matter atrophy in right lateral prefrontal and orbital frontal cortices in bvFTD, and to atrophy in right inferior parietal cortex, right insula, and fusiform cortices in CBS/PCA. These results are consistent with the hypothesis that a frontal-parietal network plays a crucial role in cognitive estimation.
Introduction
Throughout the course of a day, individuals make decisions on the basis of estimations — Do I have enough gas to get to work this morning? How long will my afternoon meeting take? How much bread do I need for the week? Our decisions regarding what we buy, how we plan our day, and any of a number of other activities are strongly influenced by quantities that we calculate using estimates based on relevant knowledge. In this study, we assess the cognitive and neuroanatomic basis for deficits in quantitative estimation in groups of patients with focal neurodegenerative disease in frontal and parietal cortex.
Cognitive estimation is the strategic process of generating a mental approximation based on available but incomplete information (Shallice and Evans, 1978). Beyond the necessary semantic knowledge of the relevant concepts and working memory needed to maintain relevant information in an active state, cognitive estimation requires quantitative reasoning about familiar concepts and probabilistic processes in the face of imprecise information in order to develop a reasonable final estimated quantity (Shallice and Evans, 1978; Bullard et al., 2004). There appear to be two major components to cognitive estimation: executive resources and number knowledge. These components facilitate the integration of information from semantic memory using strategic reasoning skills to derive a probabilistic evaluation, a process that is central to cognitive estimation. For example, when estimating how long it will take to read this article, one must identify and retrieve the appropriate knowledge about reading from semantic memory, and integrate this with quantitative information about reading speed, to derive an appropriate estimate (e.g., it’s an academic article with small font so it might take longer than most reading).
In the current study, we sought to identify the neuroanatomic areas contributing to the neural network supporting cognitive estimation. Based on functional imaging studies in healthy adults and patients with focal brain damage, we hypothesize that these two components depend in part on two interacting brain regions.
Dorsolateral prefrontal cortex (dlPFC) is implicated in executive functioning (Duncan and Owen, 2000; Aron et al., 2004). Seem to be of particular importance for cognitive estimation. fMRI studies of healthy adults engaged in tasks requiring probability show activation in dlPFC (Casey et al., 2001). An fMRI study examining the neural correlates of tactile estimation showed greater levels of activation in dlPFC during a texture estimation task as compared to a nearly identical task not requiring any estimation (Kitada et al., 2005). Complementary to the neuroimaging evidence, individuals with frontal lobe damage demonstrate severe limitations in cognitive estimation abilities (Shallice and Evans, 1978). Smith and Milner (1984) showed impairment following right-lateralized dlPFC damage in patients with surgical treatment for epilepsy.
To examine the role of dlPFC in cognitive estimation, we assessed estimation abilities in non-aphasic individuals with the behavioral variant frontotemporal dementia (bvFTD), a neurodegenerative condition that is associated with GM atrophy encompassing dlPFC (Rosen et al., 2002; Grossman et al., 2004) and frontal WM disease (Whitwell et al., 2010a). Symptomatically, bvFTD is characterized by executive impairment as well as behavioral disinhibition and personality changes (Kramer et al., 2003; Libon et al., 2006; Rascovsky et al., 2011; Possin et al., 2013). We assessed cognitive estimation in these patients to minimize confounds associated with semantic knowledge, visuospatial and language abilities where their performance is generally preserved, but show limitations in reasoning, organization, and social judgment. Given the collective evidence of estimation deficits in people with executive dysfunction, and the association of these deficits with dlPFC damage, we expected bvFTD patients to be impaired compared to healthy controls in a test of cognitive estimation, and that their impaired cognitive estimation performance would relate to cortical atrophy including at least right dlPFC.
Number knowledge is also necessary to produce appropriate quantitative estimations. This is the domain of knowledge over which cognitive estimations often operate. Numerical knowledge is said to involve an analog number system that depends in part on the representation of ratios between quantities on a logarithm-like number line (Dehaene, 1997). Some have argued that the representation of precise numbers larger than 4 may depend in part on an external algorithm involving language (Dehaene et al., 1999), although we have shown that number knowledge is compromised in non-aphasic patients with CBS and PCA (Koss et al., 2010; Spotorno et al., 2014). Multiple fMRI studies using a variety of techniques have demonstrated that the parietal lobe, and particularly the intraparietal sulcus and adjacent inferior parietal lobule, play a crucial role in the representation of number knowledge (Piazza and Dehaene, 2004; Pinel et al., 2004; Danker and Anderson, 2007; Nieder and Dehaene, 2009). This includes knowledge of quantity that is mediated both symbolically by Arabic numerals and non-symbolic representations of number such as quantities of filled circles. Furthermore, the inferior parietal lobule seems to be associate with magnitude and quantitative processing, possible because of the spatial magnitude component involved in these processes. An fMRI study tested healthy volunteers during number comparisons and showed bilateral activation of the inferior parietal lobes, with higher activation on the right side (Chochon et al., 1999). Dehaene et al. (1999) also found increased fMRI and ERP activation in the inferior parietal lobe during approximation calculations as compared to exact calculations, which they attributed to non-linguistic numerical processing during approximations.
In the present study, we assessed the quantitative or numeric component of cognitive estimation in patients with parietal disease, including CBS and PCA. CBS is an extrapyramidal disorder with involuntary movements associated with basal ganglia disease and a variety of clinical features attributable to parietal disease including apraxia and cortical sensory loss (Murray et al., 2007; Armstrong et al., 2013). PCA is a variant of Alzheimer’s disease with visuospatial deficits due to parietal–occipital disease (Crutch et al., 2012). We have shown that patients with CBS and PCA have significant deficits with number knowledge, including impairments on measures involving single-digit calculations and Arabic numeral-dot matching (Koss et al., 2010; Morgan et al., 2011). Numerical and quantitative processing, crucial components of cognitive estimation, are associated with parietal lobe functioning, therefore, we expected that CBS and PCA patients with parietal damage would also show deficits in cognitive estimation.
To evaluate the specificity of the hypothesized frontal and parietal contributions to cognitive estimation we additionally evaluated patients with mild cognitive impairment (MCI). MCI is characterized by mild memory impairments (Feldman and Jacova, 2005; Albert et al., 2011) and is typically considered a prodromal form of Alzheimer’s disease (Boyle et al., 2006; Gauthier et al., 2006; Meyer et al., 2007). Critically, MCI patients have relatively preserved executive function and intact number knowledge (Zamarian et al., 2007; Aretouli and Brandt, 2010); therefore, despite cognitive impairments and likely neurodegenerative disease, we hypothesize that these patients will have relatively preserved cognitive estimation.
Complex behaviors such as cognitive estimation depend on multiple GM nodes that are integrated by WM projections. dlPFC and intraparietal sulcus hypothesized to play a role in cognitive estimation may be linked by dorsal and ventral WM streams. The dorsal stream is mediated by the superior longitudinal fasciculus (SLF), and the ventral stream by the inferior frontal-occipital fasciculus (IFO) or some combination of the uncinate fasciculus (UNC) and inferior longitudinal fasciculus (ILF). To help define the WM projections integrating the neuroanatomic network underlying cognitive estimation, we also obtained diffusion-weighted imaging studies. We expected that some combination of dorsal and ventral stream projections would also be implicated in cognitive estimation by imaging studies.
Materials and Methods
Participants
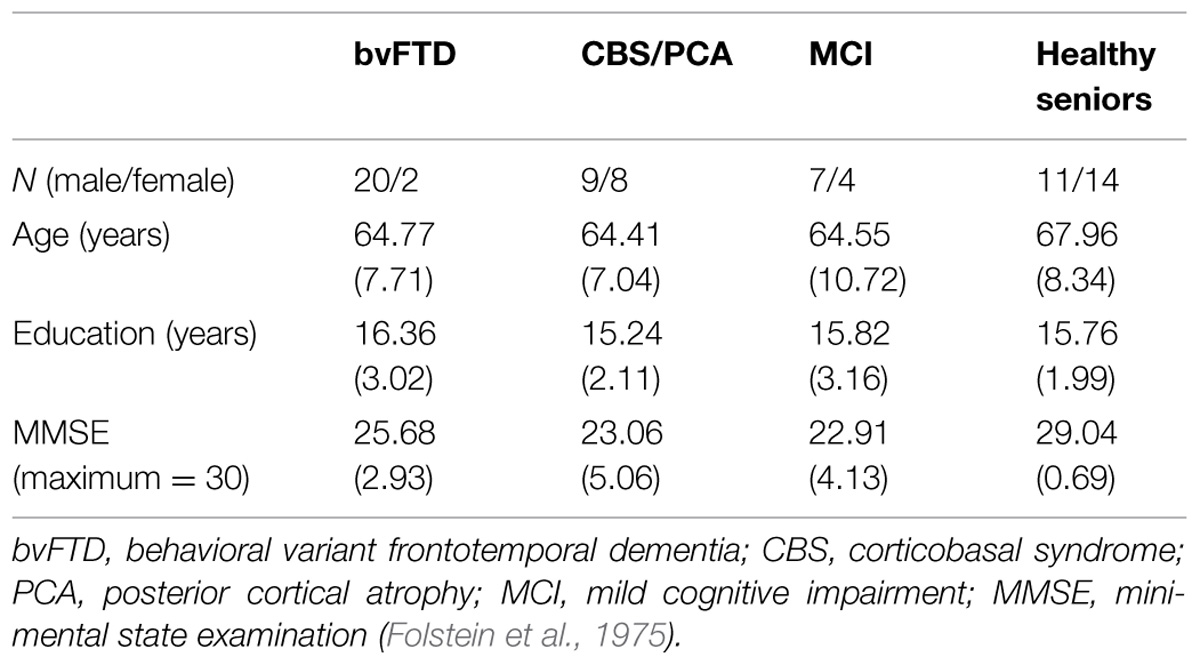
Thirty-nine patients with a targeted neurodegenerative disease, including patients with bvFTD (N = 22) or CBS/PCA (N = 17), 11 brain-damaged controls with MCI not involving the frontal lobe or the parietal lobe, and 25 age- and education-matched healthy controls, participated in the experiment. All participants were native English speakers. Patients were diagnosed by board-certified neurologists (M.G. and D.J.I.). bvFTD diagnosis was verified through a consensus procedure using published criteria (Rascovsky et al., 2011). The CBS/PCA group consisted of 11 patients diagnosed with CBS and six PCA patients. Consensus verification of CBS used published criteria (Armstrong et al., 2013), while verification of PCA was based on reports from the literature (e.g., Crutch et al., 2012, 2013a,b). As bvFTD patients can develop language impairments associated with semantic variant primary progressive aphasia (svPPA), any patients with symptomatic evidence of svPPA were excluded from the sample population. A brain-damaged control group consisted of patients with MCI and were diagnosed using published criteria (Albert et al., 2011). Other types of dementia such as vascular disease, head trauma or hydrocephalus were excluded through clinical evaluation. Patients with primary psychiatric or medical diagnoses that can impact cognition were excluded. Some patients may have been taking small doses of medically necessary medications such as non-sedating anti-depressants. Control subjects confirmed their status through negative self-report of a neurological and psychiatric history. Study participation was voluntary and in accordance with the informed consent procedures approved by the University of Pennsylvania Institutional Review Board.
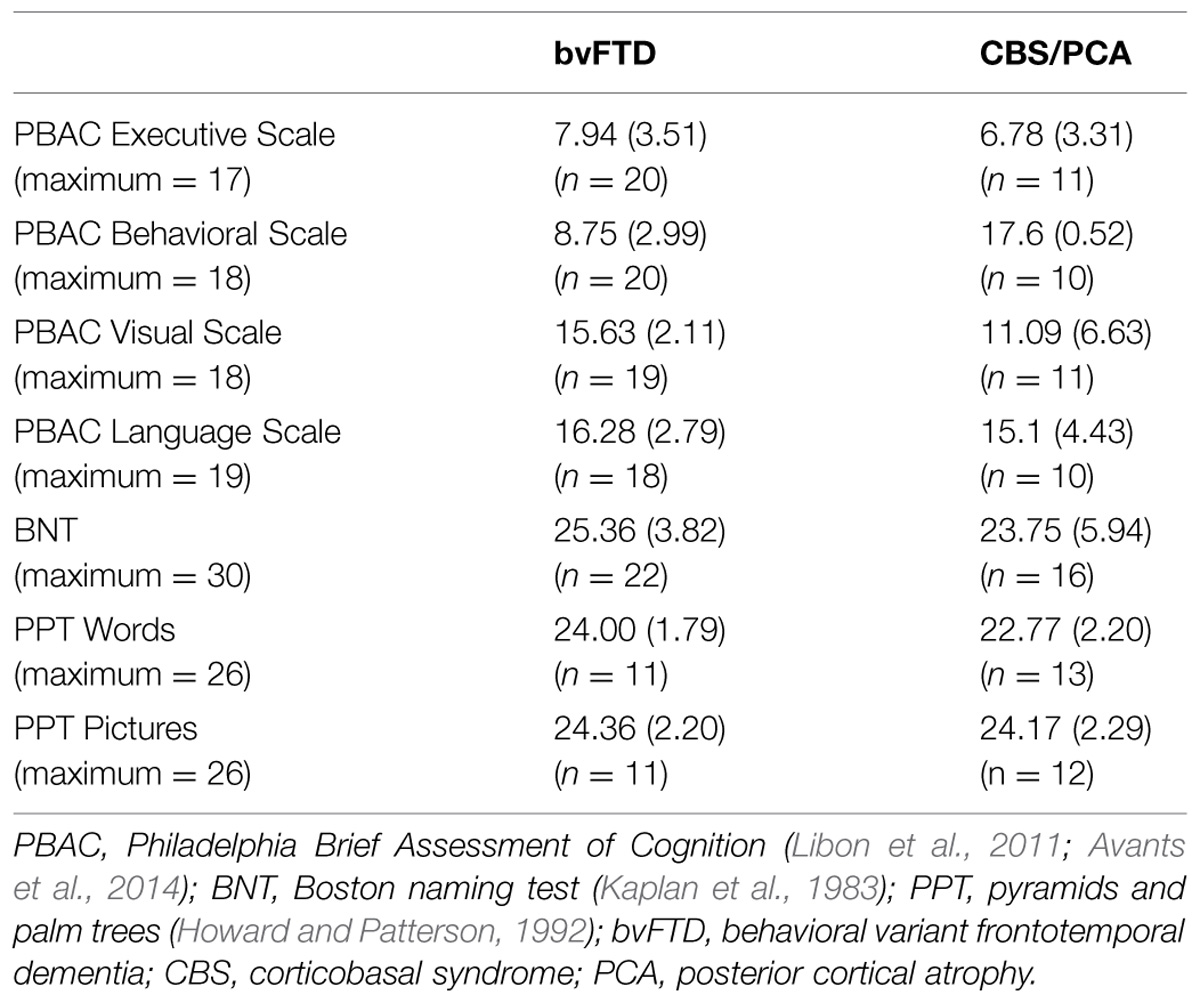
Participants’ overall cognitive status was assessed using the MMSE (Folstein et al., 1975). Only patients with an MMSE score of 15 (out of 30 possible points) or above were included in the research sample, in order to restrict the patient participants to mild or moderate levels of dementia. Controls were required to score at least 28 on the MMSE to participate. Demographics for each group are summarized in Table 1. All three dementia groups had lower mean MMSE scores than the control group [χ2(3) = 34.28, p < 0.001]. Comparison of the three dementia groups revealed that these groups did not differ in their MMSE scores [χ2(2) = 4.20, p = 0.12]. To develop a neuropsychological profile for our focal patient groups (bvFTD and CBS/PCA), we assessed cognitive abilities in the domains of executive, visuospatial, language and memory functioning using the PBAC (Libon et al., 2011; Avants et al., 2014). Moreover, to assess comprehension of the verbal stimulus materials, we also administered the BNT (Kaplan et al., 1983) and the PPT (Howard and Patterson, 1992) to these patient groups. The neuropsychological characteristics of these groups are summarized in Table 2. The patient groups also did not differ in their scores on the PBAC scales [Executive Scale: U(29) = 96.00, p = 0.61; Visual Scale: U(28) = 63.50, p = 0.08; Language Scale: U(26) = 77.50, p = 0.56], with the exception of the behavioral scale for which bvFTD patients showed more severe behavioral ratings than CBS/PCA patients [U(28) = 0.00, p < 0.001].
Behavioral Materials and Procedures
The BCET, originally developed by Bullard et al. (2004) was verbally administered to each participant as one measure in a larger battery that tested decision-making abilities and executive functioning.
The BCET asks participants to mentally calculate numerical approximations of imprecise quantitative values based on relevant information (e.g., How many slices of bread are there in a one pound loaf?). The 20-item task includes estimations across four modalities: quantity, weight, distance, and time. The task instructions emphasize that participants often will not be able to give an exact answer, but should instead provide their “best guess.” They are asked to answer with a specific number, not a range; and, they are asked to specify the units of their answer (e.g., 30 slices of bread). Whenever participants answered with a range or without specifying a unit of measurement, they were prompted to be more specific.
Participants’ responses were transformed to Z-scores based on published normative data (Bullard et al., 2004). As there were no a priori predictions with regard to the direction of estimation errors (i.e., over versus under estimation), the absolute values of the calculated Z-scores were used for analysis. The means of each participant’s Z-scores for all BCET responses were calculated and analyzed. Three patients were identified as outliers based on a mean Z-score greater than 5.0. These patients were excluded from all analyses reported herein. All mean Z-scores healthy control participants were within the normative range according to the published data from Bullard et al. (2004).
T1 Structural Gray Matter Imaging Acquisition and Analysis
A structural T1-weighted 3-dimensional spoiled gradient-echo sequence was obtained on a Siemens 3.0T Trio scanner with an 8-channel head coil for 18 bvFTD and 13 CBS/PCA participants (sequence parameters: TR = 1620 ms, TE = 3 ms, flip angle = 15°, matrix = 192 × 256, slice thickness = 1 mm, and in-plane resolution = 1 × 1 mm). Reasons for exclusion included health and safety (e.g., metallic implants, shrapnel, claustrophobia), intercurrent medical illness, and lack of interest in an imaging study. Imaging was acquired on average within 125 (±75) days of performing the task. The subsets of patients for whom imaging data were available did not differ (p > 0.3) on any demographic, neuropsychological, or language performance measures from the total set of participants in each group. Imaging was also collected on 38 healthy controls. The three imaged groups were comparable in age and education, as determined by one-way ANOVAs [F(2,66) = 0.254, p = 0.777 and F(2,66) = 2.313, p = 0.107, respectively].
Gray matter images were normalized to a standard space and segmented using the PipeDream interface1 to the ANTS toolkit2 (Avants et al., 2014). The ANTS toolkit implements a diffeomorphic and symmetric registration and normalization method that is the most reliable tool available (Avants et al., 2008; Klein et al., 2010). A local T1 template with 1 mm isotropic resolution was built using ANTS from 25 healthy seniors and 25 frontotemporal lobar degeneration patients. Subject images were registered to the local template. The Atropos tool in ANTS (Avants et al., 2011) used template-based priors to guide three-tissue segmentation (GM, WM, and cerebrospinal fluid), and GM probability (GMP) images were calculated as a quantitative measure of GM atrophy. GMP images were then transformed into MNI space for statistical analysis and smoothed in SPM83 using a 4-mm full-width half-maximum Gaussian kernel to minimize individual gyral variations. Finally, images were down-sampled to 2 mm isotropic resolution in order to attain a more anatomically relevant voxel size.
We used the randomise tool in FSL4 to perform two separate non-parametric, permutation-based statistical analysis (permutations = 10,000) to compare GM density between bvFTD patients and controls and CBS/PCA patients and controls. Analyses were restricted to voxels containing GM using the same explicit mask generated from the average GMP map of all imaged subjects. For each density comparison, we considered only clusters that exceeded an extent threshold of 50 voxels and a height threshold of p < 0.0001 (Bonferroni-corrected for multiple comparisons with threshold free cluster enhancement). We then used the randomise tool to perform regression analyses that related GM density to the BCET mean Z-score for each patient group (permutations = 10,000). Regression analyses were restricted to areas of GM disease for each patient group, as determined by the respective GM density analyses. Clusters with a height threshold of p < 0.05 (uncorrected for multiple comparisons) and an extent threshold of 10 voxels were considered significant.
Diffusion Tensor Imaging White Matter Acquisition and Analysis
Diffusion tensor imaging was available for the same patients with bvFTD (N = 18) and controls (N = 38) with T1 imaging, and 12 of the 13 CBS/PCA patients with T1 imaging. A 30-directional DWI sequence was collected using single-shot, spin-echo, diffusion-weighted echo planar imaging (FOV = 240 mm; matrix size = 128 × 128; number of slices = 70; voxel size = 2 mm isotropic; TR = 8100 ms; TE = 83 ms; fat saturation). Thirty volumes with diffusion weighting (b = 1000 s/mm2) were collected along 30 non-collinear directions, and either one or five volumes without diffusion weighting (b = 0 s/mm2) were collected per subject. The PipeDream processing pipeline utilized ANTS (Avants et al., 2014) and Camino (Cook et al., 2006) to preprocess DWI. Motion and distortion artifacts were removed using affine co-registration of each diffusion-weighted image to the average of the unweighted (b = 0) images. Using a weighted linear least-squares algorithm (Salvador et al., 2005) implemented in Camino, DTs were computed. FA was computed from the DT image, and distortion between the subject’s T1 and DT image was corrected by registering the FA to the T1 image. DT images were then relocated to the local template (mentioned above) for statistical analysis by applying the FA-to-T1 and T1-to-local template warps, and tensors were reoriented using the preservation of principal direction algorithm (Alexander et al., 2001). Each participant’s FA image was recomputed from the DT image in local template space and smoothed using a 4 mm full-width half-maximum isotropic Gaussian kernel.
Healthy WM is vital for communication between different brain regions. Therefore, disruption in any WM tracts associated with the GM regions related to BCET performance may contribute to impaired cognition by disrupting the large-scale GM and WM network underlying cognitive estimation. Using group-level FA comparisons, we assessed the integrity of the WM tracts that integrate the GM regions associated with each patient group’s BCET mean Z-score regression analysis. To do this, we first inflated the GM regression results 3 voxels in all directions to assure that the regression clusters extended into the WM. We then performed two deterministic tractography procedures (maximum angle over 5 mm = 75°, minimum FA considered WM = 0.25) to identify all WM tracts associated with the GM regression results for each of the two patient groups (see Supplementary Figure S1 for results of tracking procedure). The DT image used to generate the streamlines for both deterministic tractography images was a healthy senior population template image, created by averaging each DT image after its preprocessing and spatial normalization to the local template (as above). Next, we converted each tractography image into a volumetric WM image, one capturing the WM regions associated with the GM regression results of the patients with bvFTD, and one capturing the WM regions associated with the GM regression results for the patients with CBS/PCA. These volumetric WM images were used as masks for two FA comparisons that were performed using the randomise tool in FSL5 (permutations = 10,000), one between patients with bvFTD and controls, and one between patients with CBS/PCA and controls. For each FA comparison, we considered only clusters that exceeded an extent threshold of 50 voxels and a height threshold of p < 0.001 (uncorrected).
Results
Behavioral Results
To assess participant performance on the BCET, we calculated the mean Z-score across all questions. Two participants did not respond to all questions, therefore the mean Z-score for their responses did not include the questions that were not answered. The analyses were performed comparing each group separately because disease in specific neuroanatomical regions for each group was thought to lead to distinct forms of estimation impairment. Inspection of Q–Q Plots revealed that the distribution of scores was not normal. This was confirmed using a Shapiro–Wilks test of Normality. Therefore, non-parametric tests were used for all analyses.
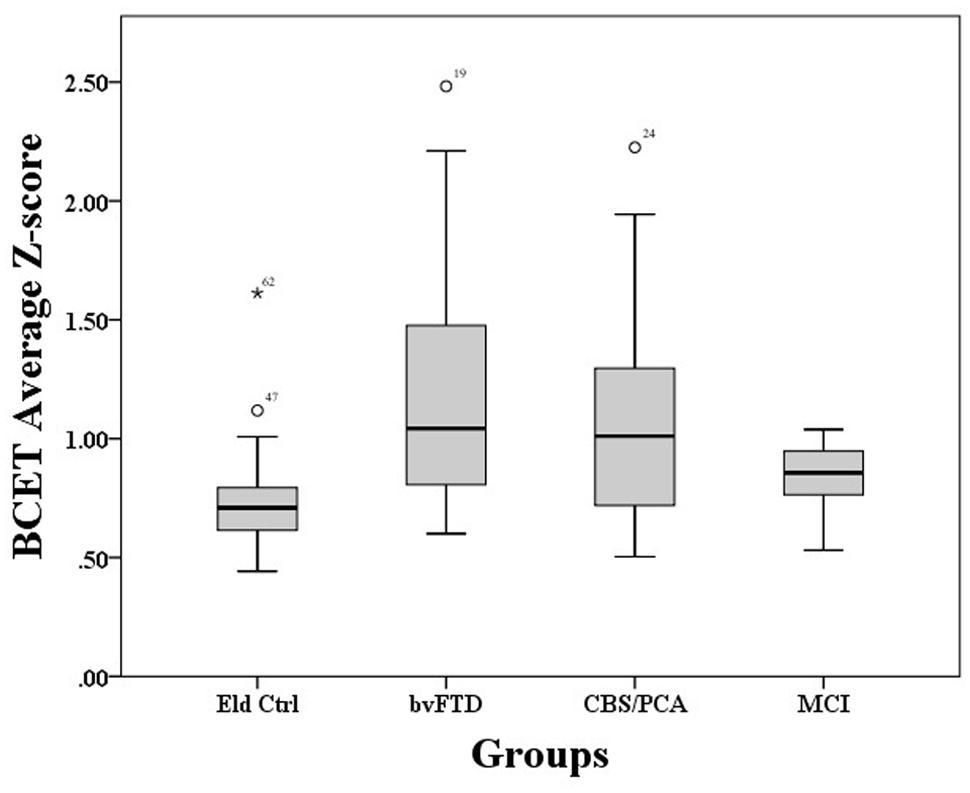
A Kruskal–Wallis test showed that performance on the BCET differed between the four groups, χ2(3) = 15.22, p = 0.002. As illustrated in Figure 1, pairwise post hoc comparisons using Mann–Whitney with Bonferroni correction revealed that both the bvFTD [U(45) = 108.00, p < 0.001] and CBS/PCA [U(40) = 115.00, p = 0.012] groups differed from controls; however, the MCI group did not differ from controls, (U(34) = 88.00, p = 0.093). Further comparisons revealed that the bvFTD and CBS/PCA groups did not differ from each other in their performance on the BCET (U(37) = 164.00, p = 0.52). To confirm that the results of the CBS/PCA group were not driven by only one of those groups, we conducted a Mann–Whitney test comparing BCET performance in the PCA patients and the CBS patients and found no significant difference in performance between the two groups [U(15) = 29.00, p = 0.69].
To explore whether BCET impairment could be related to disease severity, correlations were conducted between BCET mean Z-scores and patient MMSE scores. These correlations were not significant for any of the patient groups (bvFTD: r = 0.11, p = 0.63; CBS/PCA: r = −0.40, p = 0.11; MCI: r = −0.42, p = 0.20).
Structural Gray Matter Imaging Results
Significant regions of reduced GMP and regression relating GMP to task performance for bvFTD and CBS/PCA patients are shown in Figure 2. Peak coordinates of GM atrophy and regression analyses are summarized in Tables 2 and 3 respectively. MRI data for the MCI group was not analyzed as this group was not impaired on the BCET.
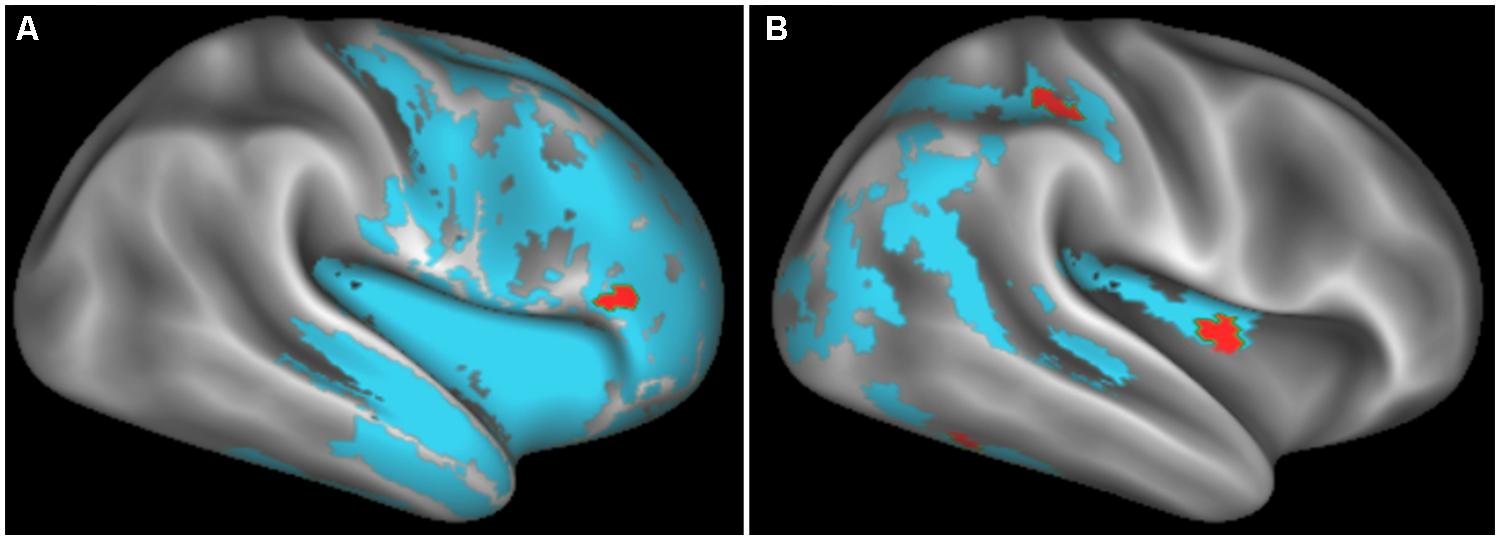
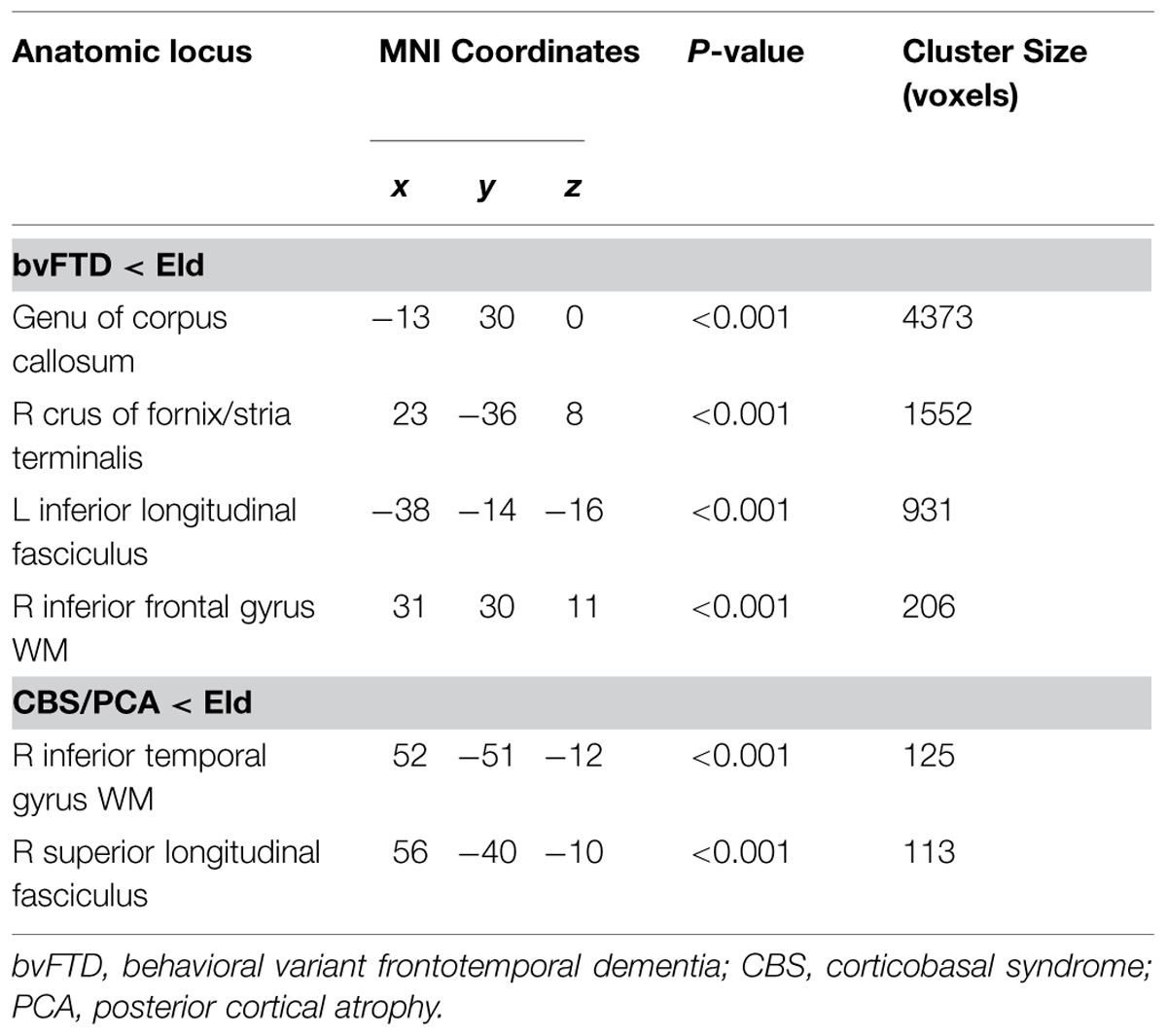
FIGURE 2. Regions of significantly reduced GM density relative to controls (blue) and regression relating GM density to task performance (red) for behavioral variant frontotemporal degeneration (A) and corticobasal syndrome and posterior cortical atrophy (B).
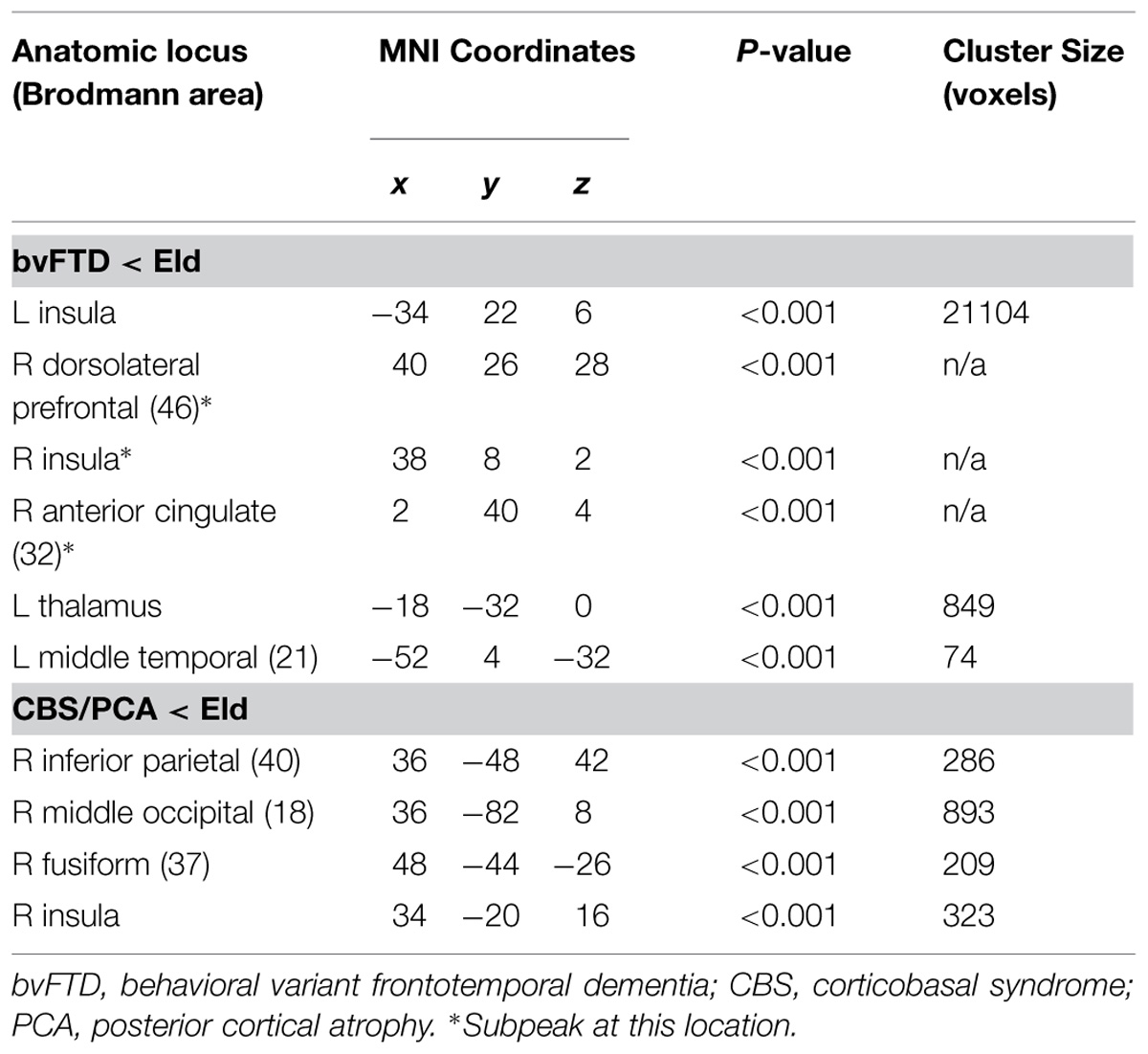
Figure 2 illustrates significant GM atrophy in bvFTD patients that is apparent bilaterally in lateral frontal, medial frontal, anterior temporal, and medial temporal cortices. Coordinates for peak voxels are provided in Table 3, suggesting more extensive atrophy on the right. Peak coordinates are provided in Table 4 for the regression analysis relating BCET mean Z-score cognitive estimation performance to reduced GMP. As illustrated in Figure 2, this implicated right lateral prefrontal cortex in cognitive estimation in bvFTD, as well as right orbital frontal, right hippocampus and left thalamus.
In the CBS/PCA patient group, Figure 2 shows that atrophy is restricted to the right hemisphere, focused in parietal, occipital, insular, and superior and posterior temporal cortices. Peak voxels for the atrophy analysis are summarized in Table 3. The regression analysis in CBS/PCA, summarized in Figure 2 and Table 4, related reduced cognitive estimation performance to GMP in right inferior parietal cortex, as well as right insula and fusiform cortices.
White Matter Imaging Results
After determining the GM regions related to cognitive estimation deficits in patients with bvFTD and patients with CBS/PCA, we examined the WM tracts related to GM atrophy to determine if they also displayed disease. As illustrated in Figure 3, we observed distinct patterns of reduced FA (shown in orange) relative to controls for each of the patient groups. This showed that WM associated with the GM regions related to cognitive estimation mean Z-score is also diseased. We display the tracts from the tractography analysis that are associated with (i.e., pass through) the reduced FA clusters (Figure 3, RGB streamlines). Peak coordinates of reduced FA are summarized in Table 5.
FIGURE 3. Significantly reduced FA in WM tracts (RGB) associated with GM atrophy regions related to cognitive estimation task performance (cyan) for patients with behavioral variant frontotemporal degeneration (A) and for patients with corticobasal syndrome and posterior cortical atrophy (B). We also illustrate WM regions with reduced FA (orange). Please refer to Table 5. *Red: left–right, green: anterior–posterior, blue: inferior–superior.
In patients with bvFTD, there is significantly reduced FA in WM related to the regions implicated in the regression analysis relating GM atrophy to cognitive estimation performance. These regions include bilateral genu of the corpus callosum, the ILF, the rostral portion of the inferior fronto-occipital fasciculus, as well as WM in right inferior frontal gyrus that is proximal to the right inferior frontal cortical region implicated in the GM regression analysis with cognitive estimation performance.
Patients with CBS/PCA also show reduced FA in WM regions related to results of regression analysis of GM atrophy and cognitive estimation performance. However, in contrast to the patients with bvFTD, the patients with CBS/PCA displayed regions of significantly reduced FA in WM of the right temporal-parietal region, including vertical portions of the SLF.
We did not observe a significant correlation between FA and BCET performance.
Discussion
Cognitive estimation is a complex process that we use commonly in the face of imprecise knowledge about quantities associated with familiar objects. Central components of cognitive estimation include executive resources needed to formulate a reasonable probability, and knowledge of numbers. We found that patients with bvFTD and CBS/PCA, but not MCI, are significantly impaired with cognitive estimation. Thus, they have difficulty estimating the number of slices in a loaf of bread, and other common, day-to-day estimates involving quantity, time and distance. This appears to be due in part to disease that compromises a frontal-parietal network that plays a crucial role in probabilistic and quantitative components of cognitive estimation. We consider each of these issues below.
Patients with bvFTD have significant deficits with executive resources and social functioning. This results in one of the cardinal clinical features of bvFTD – an impairment of judgment that manifests clinically as inappropriate behaviors largely unmodulated by social norms (Rascovsky et al., 2011). Difficulty estimating the range of appropriate responses in a social situation is analogous to the difficulty that we observe when bvFTD patients are attempting to estimate a quantity associated with a familiar object like a loaf of bread. A range of responses is provided by healthy controls when asked to estimate a quantity such as the number of slices in a loaf of bread. This is because we generally do not have a precise representation of quantities associated with these kinds of objects. Nevertheless, we can provide a reasonable estimate that is close to the actual quantity. This can be inferred semi-quantitatively through a multistep process such as estimating the thickness of a bread slice, estimating the length of a loaf of bread, and then estimating the number slices in the loaf. Alternately, a reasonable approximation can be offered that is within the rough Poisson distribution of one’s experiences with a loaf of bread. Regardless of the specific basis for estimation, patients with bvFTD tend to provide an estimate that is more distant from the average quantity provided by a reference population of young healthy controls. For example, evidence has been shown suggesting that bvFTD patients have difficulty developing a multistep strategy that can support the estimation process (Carey et al., 2008; Johns et al., 2009; Poletti et al., 2011), and it is possible that this strategic deficit limits the ability to formulate a reasonable estimation. Alternately, fMRI work has shown that probability estimation activates dlPFC (Casey et al., 2001), an area that is compromised in bvFTD, and these patients may have difficulty with developing a Poisson distribution that captures the likelihood of a particular quantity associated with the target concept. While some work has shown a deficit in number knowledge in bvFTD patients (Halpern et al., 2003), it is less likely that this can explain the estimation impairment. The number deficit in these patients thus appeared to depend specifically on the performance of complex calculations rather than a deficit in number knowledge. It is unlikely that these patients did not understand the target concept since there was no correlation between their estimation performance and measures on tasks requiring semantic memory. While bvFTD patients can be apathetic or distractible, these characteristics are unlikely to account for their poor estimates of quantity since their responses were not totally unrelated to the range of quantities for the estimation; for example, they never responded with a very small number like “1” or a three-digit quantity when asked to estimate the number of slices in a bread loaf. It does not appear that working memory limitations can explain their deficit because they were asked to judge only one object concept at a time and there was no interference that could have compromised their ability to hold the concept in mind while they were making a judgment.
Patients with bvFTD have disease predominantly in prefrontal regions of the brain. Many studies have associated deficits on measures of executive functioning with atrophy of the frontal lobe (Manes et al., 2002; Kramer et al., 2003; Alvarez and Emory, 2006; Libon et al., 2009). In the present study, we found that frontal lobe atrophy is associated with impaired cognitive estimation as well. In particular, regression analyses associated their deficit in cognitive estimation with atrophy in dlPFC and orbital frontal cortex. dlPFC is an area that has been associated with probability estimation in fMRI studies of healthy controls (Casey et al., 2001). This area also has been associated with the strategic development of multistep planning such as that required to perform a task like the Tower of London (Baker et al., 1996; Newman et al., 2003). Orbital frontal cortex has been associated with mental flexibility (Milner, 1963; Kim and Ragozzino, 2005; Eslinger et al., 2007; Stalnaker et al., 2007; Evans et al., 2015), and disease in this area may be contributing to performance by limiting the ability to assess more than a limited range of possible quantitative estimates.
There is also disease in WM regions in bvFTD, and this appears to contribute to the cognitive estimation deficit in these patients. WM disease in the anterior corpus callosum may interrupt information transfer between hemispheres. Disease in ILF may interfere with relating visual or imaged representations of objects in visual association cortex to frontal regions important for estimation. Diseased U-fibers in orbital frontal regions may be important for mental flexibility.
Cognitive estimation also depends in part on number knowledge. The current study shows that patients with CBS/PCA have difficulty with cognitive estimation; we attribute their deficit to limited knowledge of numbers. The estimation process thus is being applied over the domain of a quantitative property of a familiar object, and knowledge of quantity is necessary to support this process. Much work has demonstrated a significant deficit in number knowledge in these patients (Halpern et al., 2004; Koss et al., 2010; Morgan et al., 2011; Spotorno et al., 2014). Some have observed executive limitations in patients with CBS (Huey et al., 2009), and we cannot rule out that this also may be contributing to their deficit.
Many imaging studies (Whitwell et al., 2007, 2010b; Lehmann et al., 2011) and autopsy-verified assessments (Alladi et al., 2007; Murray et al., 2007; Pantelyat et al., 2011) underline the distribution of disease in the parietal lobe in CBS/PCA. Extensive fMRI work in healthy adults has associated the parietal lobe with number knowledge (Rueckert et al., 1996; Pinel et al., 1999), and thus it is not surprising that these patients have difficulty with processing a quantity – in this case, developing a quantity estimation. Consistent with these findings, the regression analysis found that cognitive estimation in CBS/PCA is related to cortical atrophy in the right inferior parietal lobule. The insula may be playing a role in evaluating the value of a correct response on this measure (Singer et al., 2009). We also observed WM disease in CBS/PCA that is associated with these GM regions. Disease in the vertical portion of the SLF and posterior temporal regions may contribute to relating visual or imaged object representations processing in visual association cortex to parietal regions important for quantity processing.
Specificity for our proposed fronto-parietal network comes from our observation that MCI patients, our dementia control group, were not impaired in their estimation abilities. This observation suggests that cognitive estimation difficulties are related to a specific neuroanatomic network and that our results are not simply related to non-specific or global neurodegeneration of the brain. These participants were matched with the focal patient groups for MMSE, a brief neuropsychological evaluation of cognitive functioning. Furthermore, we did not find a significant correlation relating disease severity to estimation abilities. These results further support our hypothesis that a network specifically involving frontal and parietal regions is involved in cognitive estimation.
Several caveats should be kept in mind when evaluating our findings. We examined a small number of patients, and a larger cohort is needed to evaluate estimation performance. Because of power limitations, we used a relatively liberal threshold to relate performance to regional cortical atrophy. Nevertheless, we believe that these findings are likely to be valid because regressions are in areas of known disease for each patient group, the regressions encompass areas of disease consistent with predictions based on previous work, and the findings are unique to each group. We also did not observe a direct correlation between FA and BCET performance. It is possible that heterogeneity between groups such as the amount of WM disease in individuals associated with distinct underlying pathological sources (McMillan et al., 2013) may have obscured our findings. Additional work in larger and pathologically confirmed samples are necessary to further explore the potential contributions of WM to cognitive estimation. While regression analyses relating performance to structural imaging provide converging evidence implicating the estimation process and knowledge of quantity in the estimation deficit in these patients, it would be useful to have additional evidence about estimation with this task from other sources such as fMRI activation or rTMS in healthy adults. With these caveats in mind, our findings are consistent with the hypothesis that a frontal-parietal network plays a central role in the process of cognitive estimation. Based on studies of patients with focal neurodegenerative disease, we observed that patients with predominantly prefrontal disease and patients with predominantly parietal disease have difficulty with estimating a quantitative property of a familiar object. This is consistent with the claim that a large-scale frontal-parietal network plays a crucial role in cognitive estimation.
Author Contributions
All authors participated in drafting and revising the manuscript for intellectual content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported in part by grants from the National Institute of Health (AG017586, NS044266, AG032953, AG043503, and NS053488) and the Wyncote Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnhum.2015.00317/abstract
Figure S1 | Tracts used in RGB mask for comparisons of fractional anisotropy for behavioral variant frontotemporal degeneration (A) and for the combined corticobasal syndrome and posterior cortical atrophy (B). Red: left–right, green: anterior–posterior, blue: inferior–superior
Abbreviation
BCET, Biber cognitive estimation test; BNT, Boston naming test; bvFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; dlPFC, dorsolateral prefrontal cortex; DT, diffusion tensor; DTI, diffusion tensor imaging; DWI, diffusion weighted imaging; FA, fractional anisotropy; GM, gray matter; GMP, gray matter probability; MMSE, mini-mental state examination; PBAC, Philadelphia Brief Assessment of Cognition; PCA, posterior cortical atrophy; PFC, prefrontal cortex; PPT, pyramid and palm tree test; WM, white matter.
Footnotes
- ^http://www.sourceforge.net/projects/neuropipedream/
- ^http://www.picsl.upenn.edu/ANTS/
- ^http://www.fil.ion.ucl.ac.uk/spm/software/spm8
- ^http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise
- ^http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Alexander, D. C., Pierpaoli, C., Basser, P. J., and Gee, J. C. (2001). Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans. Med. Imaging 20, 1131–1139. doi: 10.1109/42.963816
Alladi, S., Xuereb, J., Bak, T., Nestor, P., Knibb, J., Patterson, K., et al. (2007). Focal cortical presentations of Alzheimer’s disease. Brain 130, 2636–2645. doi: 10.1093/brain/awm213
Alvarez, J. A., and Emory, E. (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42. doi: 10.1007/s11065-006-9002-x
Aretouli, E., and Brandt, J. (2010). Everyday functioning in mild cognitive impairment and its relationship with executive cognition. Int. J. Geriatr. Psychiatry 25, 224–233. doi: 10.1002/gps.2325
Armstrong, M. J., Litvan, I., Lang, A. E., Bak, T. H., Bharia, K. P., Borroni, B., et al. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503. doi: 10.1212/WNL.0b013e31827f0fd1
Aron, A. R., Robbins, T. W., and Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177. doi: 10.1016/j.tics.2004.02.010
Avants, B. B., Epstein, C. L., Grossman, M., and Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41. doi: 10.1016/j.media.2007.06.004
Avants, B. B., Libon, D. J., Rascovsky, K., Boller, A., McMillan, C. T., Massimo, L., et al. (2014). Sparse canonical correlation analysis relates network-level atrophy to multivariate cognitive measures in a neurodegenerative population. Neuroimage 84, 698–711. doi: 10.1016/j.neuroimage.2013.09.048
Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., and Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. doi: 10.1016/j.neuroimage.2010.09.025
Baker, S. C., Rogers, R. D., Owen, A. M., Frith, C. D., Dolan, R. J., Frackowiak, R. S. J., et al. (1996). Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia 34, 515–526. doi: 10.1016/0028-3932(95)00133-6
Boyle, P. A., Wilson, R. S., Aggarwal, N. T., Tang, Y., and Bennett, D. A. (2006). Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 67, 441–445. doi: 10.1212/01.wnl.0000228244.10416.20
Bullard, S. E., Fein, D., Gleeson, M. K., Tischer, N., Mapou, R. L., and Kaplan, E. (2004). The Biber cognitive estimation test. Arch. Clin. Neuropsychol. 19, 835–846. doi: 10.1016/j.acn.2003.12.002
Carey, C. L., Woods, S. W., Damon, J., Halabi, C., Dean, D., Delis, D. C., et al. (2008). Discriminant validity and neuroanatomical correlates of rule monitoring in frontotemporal dementia and Alzheimer’s disease. Neuropsychologia 46, 1081–1087. doi: 10.1016/j.neuropsychologia.2007.11.001
Casey, B. J., Forman, S. D., Franzen, P., Berkowitz, A., Braver, T. S., Nystrom, L. E., et al. (2001). Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum. Brain Mapp. 13, 26–33. doi: 10.1002/hbm.1022
Chochon, F., Cohen, L., van de Moortele, P. F., and Dehaene, S. (1999). Differential contributions of the left and right inferior patietal lobules to number processing. J. Cogn. Neurosci. 11, 617–630. doi: 10.1162/089892999563689
Cook, P. A., Bai, Y., Seunarine, K. K., Hall, M. G., Parker, G. J., and Alexander, D. C. (2006). “Camino: open-source diffusion-MRI reconstruction and processing,” in Proceedings of the International Society for Magnetic Resonance in Medicine, Seattle, WA, 2759.
Crutch, S. J., Lehmann, M., Schott, J. M., Rabinovici, G. D., Rossor, M. N., and Fox, N. C. (2012). Posterior cortical atrophy. Lancet Neurol. 11, 170–178. doi: 10.1016/S1474-4422(11)70289-7
Crutch, S. J., Lehmann, M., Warren, J. D., and Rohrer, J. D. (2013a). The language profile of posterior cortical atrophy. J. Neurol. Neurosurg. Psychiatry 84, 460–466. doi: 10.1136/jnnp-2012-303309
Crutch, S. J., Schott, J. M., Rabinovici, G. D., Boeve, B. F., Cappa, S. F., Dickerson, B. C., et al. (2013b). Shining a light on posterior cortical atrophy. Alzheimer’s Dementia 9, 463–4653. doi: 10.1016/j.jalz.2012.11.004
Danker, J. F., and Anderson, J. R. (2007). The roles of prefrontal and posterior parietal cortex in algebra problem solving: a case of using cognitive modeling to inform neuroimaging data. Neuroimage 35, 1365–1377. doi: 10.1016/j.neuroimage.2007.01.032
Dehaene, S. (1997). The Number Sense: How the Mind Creates Mathematics. New York, NY: Oxford University Press.
Dehaene, S., Spelke, E., Pinel, P., Stanescu, R., and Tsivkin, S. (1999). Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284, 970–974. doi: 10.1126/science.284.5416.970
Duncan, J., and Owen, A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23, 475–483. doi: 10.1016/S0166-2236(00)01633-7
Eslinger, P. J., Moore, P., Troiani, V., Antani, S., Cross, K., Kwok, S., et al. (2007). Oops! resolving social dilemmas in frontotemporal dementia. J. Neurol. Neurosurg. Psychiary 78, 457–460. doi: 10.1136/jnnp.2006.098228
Evans, J., Olm, C., McCluskey, L. F., Elman, L. B., Boller, A., Moran, E., et al. (2015). Impaired cognitive flexibility in amyotrophic lateral sclerosis. Cogn. Behav. Neurol 28, 17–26. doi: 10.1097/wnn.0000000000000049
Feldman, H. H., and Jacova, C. (2005). Mild cognitive impairment. Am. J. Geriatr. Psychiatry 13, 645–655. doi: 10.1097/00019442-200508000-00003
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Gauthier, S., Reisberg, B., Zaudig, M., Petersen, R. C., Ritchie, K., Broich, K., et al. (2006). Mild cognitive impairment. Lancet 367, 1262–1270. doi: 10.1016/S0140-6736(06)68542-5
Grossman, M., McMillan, C. T., Moore, P., Ding, L., Glosser, G., Work, M., et al. (2004). What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia, and corticobasal degeneration. Brain 127, 628–649. doi: 10.1093/brain/awh075
Halpern, C., Glosser, G., Clark, R., Gee, J. C., Moore, P., Dennis, K., et al. (2004). Dissociation of numbers and objects in corticobasal degeneration and semantic dementia. Neurology 62, 1163–1170. doi: 10.1212/01.WNL.0000118209.95423.96
Halpern, C., McMillan, C. T., Moore, P., Dennis, K., and Grossman, M. (2003). Calculation impairment in neurodegenerative diseases. J. Neurol. Sci. 208, 31–38. doi: 10.1016/S0022-510X(02)00416-1
Howard, D., and Patterson, K. (1992). The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures. Bury St. Edmunds: Thames Valley Test Company.
Huey, E. D., Goveia, E. N., Pavol, S., Pardini, M., Krueger, F., Zamboni, G., et al. (2009). Executive dysfunction in frontotemporal dementia and corticobasal syndrome. Neurology 72, 453–459. doi: 10.1212/01.wnl.0000341781.39164.26
Johns, E. K., Phillips, N. A., Belleville, S., Goupil, D., Babin, L., Kelner, N., et al. (2009). Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology 23, 765–777. doi: 10.1037/a0016792
Kaplan, E., Goodglass, H., and Weintraub, S. (1983). Boston Naming Test. Philadelphia: Lea & Febiger.
Kim, J., and Ragozzino, M. E. (2005). The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol. Leaarn. Mem. 83, 125–133. doi: 10.1016/j.nlm.2004.10.003
Kitada, R., Hashimoto, T., Kochiyama, T., Kito, T., Okada, T., Matsumura, M., et al. (2005). Tactile estimation of the roughness of gratings yields a graded response in the human brain: an fMRI study. Neuroimage 25, 90–100. doi: 10.1016/j.neuroimage.2004.11.026
Klein, A., Ghosh, S. S., Avants, B., Yeo, B. T. T., Fischl, B., Ardekani, B., et al. (2010). Evaluation of volume-based and surface-based brain image registration methods. Neuroimage 51, 214–220. doi: 10.1016/j.neuroimage.2010.01.091
Koss, S., Clark, R., Vesely, L., Weinstein, J., Anderson, C., Richmond, L., et al. (2010). Numerosity impairment in corticobasal syndrome. Neuropsychology 24, 476–492. doi: 10.1037/a0018755
Kramer, J. H., Jurik, J., Sha, S. J., Rankin, K. P., Rosen, H. J., and Johnson, J. K. (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn. Behav. Neurol. 16, 211–218. doi: 10.1097/00146965-200312000-00002
Lehmann, M., Crutch, S. J., Ridgway, G. R., Ridha, B. H., Barnes, J., Warrington, E. K., et al. (2011). Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. Neurobiol. Aging 32, 1466–1476. doi: 10.1016/j.neurobiolaging.2009.08.017
Libon, D. J., McMillan, C. T., Gunawardena, D., Powers, C, Massimo, L., Khan, A., et al. (2009). Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology 73, 535–542. doi: 10.1212/WNL.0b013e3181b2a4f5
Libon, D. J., Price, C. C., Heilman, K. M., and Grossman, M. (2006). Alzheimer’s “other dementia.” Cogn. Behav. Neurol. 19, 112–116. doi: 10.1097/01.wnn.0000209870.69522.a3
Libon, D. J., Rascovsky, K., Gross, R. G., White, M. T., Xie, S. X., Dreyfuss, M., et al. (2011). The Philadelphia brief assessment of cognition (PBAC): a validated screening measure for dementia. Clin. Neuropsychol. 25, 1314–1330. doi: 10.1080/13854046.2011.631585
Manes, F., Sahakian, B., Clark, L., Rogers, R., Antoun, N., Aitken, M., et al. (2002). Decision-making processes following damage to the prefrontal cortex. Brain 125, 624–639. doi: 10.1093/brain/awf049
McMillan, C. T., Irwin, D. J., Avants, B. B., Powers, J., Cook, P. A., Toledo, J. B., et al. (2013). White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 84, 949–955. doi: 10.1136/jnnp-2012-304418
Meyer, J. S., Huang, J., and Chowdhury, M. H. (2007). MRI confirms mild cognitive impairment prodromal for Alzheimer’s, vascular and Parkinson-Lewy body dementias. J. Neurol. Sci. 257, 97–104. doi: 10.1016/j.jns.2007.01.016
Milner, B. (1963). Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 9, 90–100. doi: 10.1001/archneur.1963.00460070100010
Morgan, B., Gross, R. G., Clark, R., Dreyfuss, M., Boller, A., Camp, E., et al. (2011). Some is not enough: quantifier comprehension in corticobasal syndrome and behavioral variant frontotemporal dementia. Neuropsychologia 49, 3532–3541. doi: 10.1016/j.neuropsychologia.2011.09.005
Murray, R., Neumann, M., Forman, M. S., Farmer, J., Massimo, L., Rice, A., et al. (2007). Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology 68, 1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3
Newman, S. D., Carpenter, P. A., Varma, S., and Just, M. A. (2003). Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia 41, 1668–1682. doi: 10.1016/S0028-3932(03)00091-5
Nieder, A., and Dehaene, S. (2009). Representation of number in the brain. Annu. Rev. Neurosci. 32, 185–208. doi: 10.1146/annurev.neuro.051508.135550
Pantelyat, A., Dreyfuss, M., Moore, P., Gross, R., Schuck, T., Irwin, D., et al. (2011). Acalculia in autopsy-proven corticobasal degeneration. Neurology 76, S61–S63. doi: 10.1212/WNL.0b013e31820c34ca
Piazza, M., and Dehaene, S. (2004). “From number neurons to mental arithmetic: the cognitive neuroscience of number sense,” in The Cognitive Neurosciences, 3rd Edn, ed. M. Gazzaniga (Cambridge: MIT Press), 865–875.
Pinel, P., Le Clec’H, G., van de Moortele, P.-F., Naccache, L., Le Bihan, D., and Dehaene, S. (1999). Event-related fMRI analysis of the cerebral circuit for number comparison. Neuroreport 10, 1473–1479. doi: 10.1097/00001756-199905140-00015
Pinel, P., Piazza, M., Le Bihan, D., and Dehaene, S. (2004). Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron 41, 983–993. doi: 10.1016/S0896-6273(04)00107-2
Poletti, M., Borelli, P., and Bonuccelli, U. (2011). The neuropsychological correlates of pathological lying: evidence from behavioral variant frontotemporal dementia. J. Neurol. 258, 2009–2013. doi: 10.1007/s00415-011-6058-1
Possin, K. L., Feigenbaum, D., Rankin, K. P., Smith, G. E., Boxer, A. L., Wood, K., et al. (2013). Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology 80, 2180–2185. doi: 10.1212/WNL.0b013e318296e940
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. doi: 10.1093/brain/awr179
Rosen, H. J., Gorno Tempini, M. L., Goldman, W. P., Perry, R. J., Schuff, N., Weiner, M., et al. (2002). Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208. doi: 10.1212/WNL.58.2.198
Rueckert, L., Lange, N., Partiot, A., Appollonio, I., Litvan, I., Le Bihan, D., et al. (1996). Visualizing cortical activation during mental calculation with functional MRI. Neuroimage 3, 97–103. doi: 10.1006/nimg.1996.0011
Salvador, R., Peña, A., Menon, D. K., Carpenter, T. A., Pickard, J. D., and Bullmore, E. T. (2005). Formal characterization and extension of the linearized diffusion tensor model. Hum. Brain Mapp. 24, 144–155. doi: 10.1002/hbm.20076
Shallice, T., and Evans, M. E. (1978). The involvement of the frontal lobes in cognitive estimation. Cortex 14, 294–303. doi: 10.1016/S0010-9452(78)80055-0
Singer, T., Critchley, H. D., and Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. doi: 10.1016/j.tics.2009.05.001
Smith, M. L., and Milner, B. (1984). Differential effects of frontal-lobe lesions on cognitive estimation and spatial memory. Neuropsychologia 22, 697–705. doi: 10.1016/0028-3932(84)90096-4
Spotorno, N., McMillan, C. T., Power, J. P., Clark, R., and Grossman, M. (2014). Counting or chunking? Mathematical and heuristic abilities in patients with corticobasal syndrome and posterior cortical atrophy. Neuropsychologia 64, 176–183. doi: 10.1016/j.neuropsychologia.2014.09.030
Stalnaker, T. A., Franz, T. M., Singh, T., and Schoenbaum, G. (2007). Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron 54, 51–58. doi: 10.1016/j.neuron.2007.02.014
Whitwell, J. L., Avula, R., Senjem, M. L., Kantarci, K., Weigand, S. D., Samikoglu, A., et al. (2010a). Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74, 1279–1287. doi: 10.1212/WNL.0b013e3181d9edde
Whitwell, J. L., Jack, C. R. J., Boeve, B. F., Parisi, J. E., Ahlskog, J. E., Drubach, D. A., et al. (2010b). Imaging correlates of pathology in corticobasal syndrome. Neurology 75, 1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8
Whitwell, J. L., Jack, C. R. J., Kantarci, K., Weigand, S. D., Boeve, B. F., Knopman, D. S., et al. (2007). Imaging correlates of posterior cortical atrophy. Neurobiol. Aging 28, 1051–1061. doi: 10.1016/j.neurobiolaging.2006.05.026
Keywords: cognitive estimation, behavioral variant frontotemporal degeneration, corticobasal syndrome, posterior cortical atrophy, prefrontal cortices, parietal cortices
Citation: Bisbing TA, Olm CA, McMillan CT, Rascovsky K, Baehr L, Ternes K, Irwin DJ, Clark R and Grossman M (2015) Estimating frontal and parietal involvement in cognitive estimation: a study of focal neurodegenerative diseases. Front. Hum. Neurosci. 9:317. doi: 10.3389/fnhum.2015.00317
Received: 04 February 2015; Accepted: 18 May 2015;
Published online: 04 June 2015.
Edited by:
Srikantan S. Nagarajan, University of California, San Francisco, USAReviewed by:
Arun Bokde, Trinity College Dublin, IrelandKamalini Gayathree Ranasinghe, University of California, San Francisco, USA
Zachary Adam Miller, University of California, San Francisco Memory and Aging Center, USA
Copyright © 2015 Bisbing, Olm, McMillan, Rascovsky, Baehr, Ternes, Irwin, Clark and Grossman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Murray Grossman, Penn Frontotemporal Degeneration Center, Department of Neurology, University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 1904-4283, USA, mgrossma@mail.med.upenn.edu
 Teagan A. Bisbing
Teagan A. Bisbing Christopher A. Olm
Christopher A. Olm Corey T. McMillan
Corey T. McMillan Katya Rascovsky1
Katya Rascovsky1  Kylie Ternes
Kylie Ternes David J. Irwin
David J. Irwin Robin Clark
Robin Clark Murray Grossman
Murray Grossman