- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Nuclear Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
Posterior cortical atrophy (PCA) is widely considered as an atypical variant of Alzheimer disease and is characterized by a progressive decline in visual function. PCA has been investigated from the standpoints of brain structure and metabolism, but tau deposition and its relationship to disease severity still remain unclear. Here, we used a novel tau ligand, [18F]PI2620, to visualize tau deposition in a PCA patient. The results showed that high [18F]PI2620 uptake in posterior cortical regions was associated with clinical manifestations, morphologic changes in the brain observed by magnetic resonance imaging (MRI), and hypometabolism detected by [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET). This is the first report demonstrating a clinical anatomical correspondence between [18F]PI2620 PET results, clinical manifestations, MRI, and [18F]FDG PET findings in a Chinese patient with PCA. The results also support the utility of [18F]PI2620 for visualizing tau aggregation in PCA.
Introduction
Posterior cortical atrophy (PCA) is a neurodegenerative syndrome affecting parietal, occipital, and occipitotemporal cortices that is characterized by visuospatial and visuoperceptual impairment, while episodic memory, judgment, and insight are relatively well-preserved early in the clinical course (1).
As an atypical Alzheimer disease (AD), PCA includes the amyloid plaques and neurofibrillary tangles seen in typical amnestic AD. Recent studies have reported a close relationship between tau neurofibrillary tangles and clinical deficits at autopsy, suggesting that tau pathology induces subsequent neurodegeneration. The development of selective tau tracers has made it possible to qualitatively analyze brain tau profiles in vivo (2–4). [18F]PI2620 is a next-generation tau-specific positron emission tomography (PET) tracer with high binding affinity for pathogenic tau protein but without off-target binding to β-amyloid, monoamine oxidase (MAO)-A, or MAO-B (5). Human studies have shown that [18F]PI2620 can detect tau pathology throughout the course of AD (6, 7). However, there have been few studies using [18F]PI2620 PET in PCA, and its utility in this context has yet to be demonstrated. Here, we described the case of a Chinese PCA patient presenting with typical visual disorder and described his magnetic resonance imaging (MRI), [18F] fluorodeoxyglucose (FDG) PET, [18F]AV45 PET, and [18F]PI2620 PET outcomes and neuropsychologic test results. We hypothesized that higher [18F]PI2620 uptake is related to clinical manifestation, atrophy patterns observed by MRI, and hypometabolism detected by [18F]FDG PET.
Case Report
A 56-year-old right-handed man with secondary education was admitted to our hospital complaining of visual deficit that had gradually progressed over 6 years. Initial presentation 6 years prior included misplacement of his belonging such as keys and wallet; however, as his daily life activities were not affected and he maintained independence, he continued driving and working full time. The symptoms were not sufficiently severe for the patient to seek treatment at that time. Four years ago, he started experiencing confusion at home, which led him to enter the wrong rooms; he urinated anywhere as he was unable to locate the bathroom. As the symptoms worsened, he could no longer find a cup on the table immediately in front of him and gradually developed difficulty in writing in a given area on a piece of paper. He could not track sentences in the newspaper and started listening to the radio instead of watching television. He subsequently experienced problems in performing calculations, dressing himself, and recognizing faces. He had to cease working and driving because of difficulties in identifying common objects. On the other hand, his ability to recognize different sounds and voices was preserved, and he did not experience any hallucinations, weakness, extrapyramidal symptoms, or ataxia. There were no significant events in his medical history. By probing his family history, it was revealed that his mother suffered from mild cognitive decline at 80 years old, while his father did not have any neurologic dysfunction.
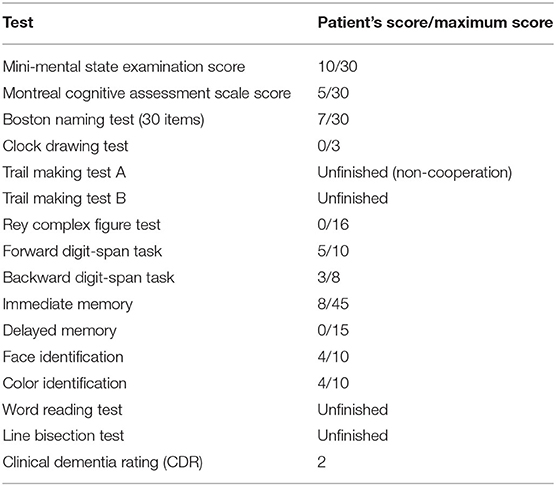
Neurologic examination revealed no cranial nerve, motor, or cortical sensory impairment. The patient's performance in neuropsychologic tests (Table 1) showed visuospatial deficits, prosopagnosia, color agnosia, and simultanagnosia, memory deficits, dyscalculia, agraphia, and alexia. The patient had difficulty completing some tests such as the trail making, line bisection, and word reading tests, as he was unable to recognize the figures. Language and executive functions were less affected. The patient was aware of his visual deficits and became silent and withdrawn as the disease progressed.
Routine laboratory test showed that blood and urine parameters, serum chemistry, and liver and renal functions were within normal limits. Lumbar puncture revealed normal cerebrospinal fluid pressure and biochemistry. Ophthalmologic examination found no abnormalities in the fundus or in intraocular pressure, while visual field and visual acuity tests were not completed because of non-cooperation. Brain MRI showed marked diffuse cortical and subcortical atrophy in bilateral parietal and occipital lobes. [18F]FDG PET revealed significant hypometabolism in diffuse regions of bilateral temporal, parietal, and occipital lobes, prominently in occipital lobes, and no metabolic changes were observed in frontal lobes, basal ganglia, and thalami; [18F]PI2620 PET showed increased tracer uptake throughout the brain, especially in temporal, parietal, and occipital lobes, while standardized uptake value ratios (SUVRs) were 3.8, 4.0, and 4.3, respectively, using cerebellum as the reference region, in close correspondence with the regions that showed the most prominent hypometabolism; [18F]AV45 PET was positive with amyloid deposited throughout the cortices (Figure 1). Whole-exome sequencing identified the p.Pro354Leu (c.1061C>T) variant of the microtubule-associated protein tau (MAPT) gene. The apolipoprotein E genotype was ε3/ε4. No other variant was detected in genes associated with early-onset AD [amyloid precursor protein (APP), presenilin 1 (PSEN1), and PSEN2]; frontotemporal dementia and parkinsonism linked to chromosome 17 [granulin (GRN), fused in sarcoma (FUS), TAR DNA-binding protein (TARDBP), charged multivesicular body protein 2b (CHMP2B), valosin-containing protein (VCP), etc.]; and other degenerative diseases. There was no expansion detected in Chromosome 9 open reading frame 72 [C9ORF72]. Genetic testing and neurologic evaluation were also proposed to the patient's parents, but they refused consent.

Figure 1. Axial, sagittal, and coronal slices from MRI and [18F] fluorodeoxyglucose ([18F]FDG), [18F]PI2620, and [18F]AV45 PET scans. (A–C) T1-weighted sequences in MRI show cortical and subcortical atrophy, especially in bilateral parietal and occipital lobes. (D–F) [18F]FDG PET revealed significant hypometabolism in diffuse regions of bilateral temporal, parietal, and occipital lobes, prominently in occipital lobes, and no metabolic change was observed in the frontal lobes, basal ganglia, and thalami. (G–I) [18F]PI2620 PET showed increased tracer uptake throughout the brain, especially in the temporal, parietal, and occipital lobes. (J–L) [18F]AV45 PET showed amyloid deposition throughout the cortex that was especially pronounced in bilateral frontal cortices.
The patient was diagnosed with PCA and received treatment with donepezil 10 mg/day and memantine 10 mg/day; additionally, psychoeducation and home-based cognitive retraining tasks were assigned to the patient and his family members, but the symptoms deteriorated.
The clinical study protocol and informed consent forms were approved by the ethics committee of Xuanwu Hospital of Capital Medical University, China. Written informed consent was obtained from the patient and his legal guardian for [18F] PI2620 PET, genetic testing, and the publication of this case report (including all data and images).
Discussion
In this report, we described a case characterized by predominant visuoperceptual and visuospatial deficits that fulfilled the diagnostic criteria for PCA (1). And the beta amyloid deposition, pathologic tau, and neurodegeneration (A+/T+/N+) fulfilled the 2018 National Institute on Aging and Alzheimer's Association (NIA-AA) guidelines, which confirmed that, in this patient, PCA was attributed to AD (8). Furthermore, the tau load revealed by [18F]PI2620 PET corresponded to prominent visual cognitive impairment, the extent of regional brain atrophy, and regions of hypometabolism in the [18F]FDG-PET scan.
In our patient, early diagnosis was confounded by the detection of p.P354L variant of MAPT, while subsequent [18F]AV45 PET confirmed the AD pathology. Mutations in MAPT—which encodes tau protein—are considered as a pathogenic factor in frontotemporal dementia (FTD) but have seldom been observed in PCA (9). Only one PCA patient carrying an MAPT p.V363I mutation has been reported (10). However, the variant detected in our patient (p.P354L) has not been previously reported and has uncertain significance according to the variant interpretation guidelines of the American College of Medical Genetics and Genomics (11). So, it was not clear whether FTD tau pathology existed as a comorbidity in this patient.
Amyloid aggregation and tau hyperphosphorylation are typical pathophysiologic events in PCA with AD pathology. In recent years, various tau imaging markers have become available to investigate tau pathology. The most extensively used ligand is [18F]AV1451. Tau PET studies in PCA using [18F]AV1451 have indicated that the tracer is taken up and selectively retained in bilateral parietal, occipital, and occipitotemporal cortices, corresponding to the regions that are clinically affected and consistent with the patterns of functional and structural deterioration and tau deposition detected in postmortem studies. However, measurable off-target binding to MAO-A or MAO-B, neuromelanin- and melanin-containing cells, choroid plexus, basal ganglia, and some other areas without tau pathology was also detected by [18F]AV1451 PET, affecting the quantitative measurement of tau burden, especially in target regions (5, 12, 13). In this study, we used [18F]PI2620, a novel tau tracer, to detect tau deposition in vivo. In vitro and animal studies have shown that [18F]PI2620 is highly selective for pathologic tau aggregates with no significant off-target binding to β-amyloid, MAO-A, or MAO-B and can be rapidly washed out from unaffected brain regions (5, 14). In-human clinical studies confirmed that tracer uptake level generally corresponds to disease severity throughout the course of AD and can clearly distinguish AD patients from healthy controls (6, 7). One study of four PCA cases showed robust increases in [18F]PI2620 uptake throughout the posterior cortical regions with relative sparing of the medial frontal and temporal lobes, which was related to disease severity (6); this is the only study to date assessing tau burden in PCA by [18F]PI2620 PET. In accordance with previous studies, in our patient, [18F]PI2620 uptake was more restricted to bilateral occipital cortices, consistent with the observed visuospatial and visuoperceptual deficits and brain atrophy and hypometabolism detected by MRI and [18F]FDG PET, respectively. Due to being in the later stage of disease, apart from prominent visual impairment, the patient also exhibited memory impairment and executive dysfunction, which was in line with brain atrophy, hypometabolism, and [18F]PI2620 uptake in bilateral temporal and parietal cortices.
This is the first study evaluating tau burden in vivo using a novel tau tracer, [18F]PI2620, in a Chinese PCA patient in relation to detailed clinical manifestations, neuropsychologic test results, and MRI, [18F]FDG PET, and [18F]AV45 PET findings. Our results demonstrate a clinical anatomical correspondence between the neuropsychological deficits, the tau uptake, and signs of neurodegeneration such as atrophy and hypometabolism seen in the MRI and [18F]FDG PET. Detection of tau aggregation has important implications for understanding the pathologic mechanisms underlying the development of PCA, as well as for disease monitoring and targeted therapy. A limitation of our study is that it involved a single case, and the patient was in the later phase of disease, which may suggest that the results must be validated by a larger sample study with varied clinical stages; nonetheless, it supports the clinical use of [18F]PI2620 for evaluating tau aggregations in living patients with PCA.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Xuanwu Hospital of Capital Medical University, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YK contributed to the patient review and medical review of the manuscript. LW and YK contributed to the study supervision and coordination. KX, XL, and YW contributed to genetic testing, mutation sequencing, and manuscript writing. HQ, YC, and DJ contributed to analysis and interpretation of brain MRI data. All authors read and approved the manuscript.
Funding
This study was supported by a grant from the National Nature Science Foundation of China (no. 81971011 to LW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the study participant.
References
1. Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. (2017) 13:870–84. doi: 10.1016/j.jalz.2017.01.014
2. Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. (2016) 43:1139–50. doi: 10.1007/s00259-015-3231-2
3. Jellinger KA, Grazer A, Petrovic K, Ropele S, Alpi G, Kapeller P, et al. Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta Neuropathol. (2011) 121:267–77. doi: 10.1007/s00401-010-0712-z
4. Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. (2016) 139:1551–67. doi: 10.1093/brain/aww027
5. Kroth H, Oden F, Molette J, Schieferstein H, Capotosti F, Mueller A, et al. Discovery and preclinical characterization of [18F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer's disease and other tauopathies. Eur J Nucl Med Mol Imaging. (2019) 46:2178–89. doi: 10.1007/s00259-019-04397-2
6. Mormino EC, Toueg TN, Azevedo C, Castillo JB, Guo W, Nadiadwala A, et al. Tau PET imaging with F-PI-2620 in aging and neurodegenerative diseases. Eur J Nucl Med Mol Imaging. (2020). doi: 10.1007/s00259-020-04923-7. [Epub ahead of print].
7. Mueller A, Bullich S, Barret O, Madonia J, Berndt M, Papin C, et al. Tau PET imaging with F-PI-2620 in patients with Alzheimer's disease and healthy controls: a first-in-human study. J Nucl Med. (2020) 61:911–9. doi: 10.2967/jnumed.119.236224
8. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. (2018) 14:535–62. doi: 10.1016/j.jalz.2018.02.018
9. Greaves CV, Rohrer JD. An update on genetic frontotemporal dementia. J Neurol. (2019) 266:2075–86. doi: 10.1007/s00415-019-09363-4
10. Rossi G, Bastone A, Piccoli E, Morbin M, Mazzoleni G, Fugnanesi V, et al. Different mutations at V363 MAPT codon are associated with atypical clinical phenotypes and show unusual structural and functional features. Neurobiol Aging. (2014) 35:408–17. doi: 10.1016/j.neurobiolaging.2013.08.004
11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
12. Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. (2016) 4:58. doi: 10.1186/s40478-016-0315-6
13. Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna L, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. (2015) 78:787–800. doi: 10.1002/ana.24517
Keywords: posterior cortical atrophy (PCA), MAPT mutation, Tau PET, [18F]PI2620, dementia
Citation: Kong Y, Xie K, Qiao H, Cui Y, Jing D, Wang Y, Li X and Wu L (2020) Case Report: [18F]PI2620 as a Tau Imaging Agent in Posterior Cortical Atrophy. Front. Neurol. 11:566667. doi: 10.3389/fneur.2020.566667
Received: 28 May 2020; Accepted: 10 November 2020;
Published: 08 December 2020.
Edited by:
Pedro Rosa-Neto, McGill University, CanadaReviewed by:
Elisa De Paula Franca Resende, Federal University of Minas Gerais, BrazilKok Pin Ng, National Neuroscience Institute (NNI), Singapore
Copyright © 2020 Kong, Xie, Qiao, Cui, Jing, Wang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyong Wu, wmywly@hotmail.com
 Yu Kong
Yu Kong Kexin Xie1
Kexin Xie1 Hongwen Qiao
Hongwen Qiao Yue Cui
Yue Cui Donglai Jing
Donglai Jing Liyong Wu
Liyong Wu