- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Background: Risk stratification of asymptomatic carotid artery stenosis (ACAS) is still an issue for carotid revascularization. We sought to identify factors associated with symptomatic carotid artery stenosis (SCAS) using multimodal imaging techniques.
Methods: We retrospectively collected data on patients who underwent carotid artery revascularization. Results from duplex sonography, computerized tomography angiography, brain magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), perfusion-weighted imaging, and demographic profiles were compared between ACAS and SCAS patients. Differences in baseline characteristics between the two groups were balanced by the propensity matching score method. Multivariable regression analysis was performed to identify factors associated with symptomaticity of carotid artery stenosis. We compared the strength of associations between significant imaging factors and symptomatic carotid stenosis using C statistics.
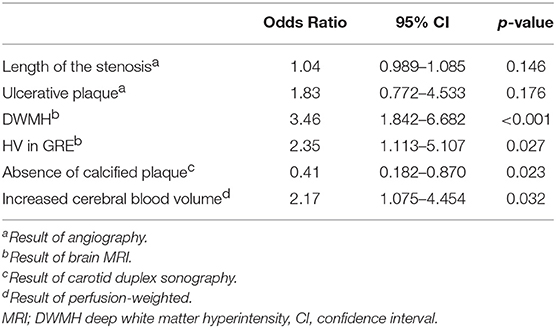
Results: A total of 259 patients (asymptomatic 57.1%, symptomatic 42.9%) with carotid stenosis were included. After 1:1 propensity score matching, the multivariable regression analysis revealed that the absence of plaque calcification [Odds ratio 0.41, 95% confidence interval (CI) 0.182–0.870, p = 0.023], deep white matter hyperintensity (DWMH; Odds ratio 3.46, 95% CI 1.842–6.682, p < 0.001), susceptibility vessel sign seen on gradient-echo MRI (Odds ratio 2.35, 95% CI 1.113–5.107, p = 0.027), and increased cerebral blood volume (CBV) seen on perfusion-weighted MRI (CBV; Odds ratio 2.17, 95% CI 1.075–4.454, p = 0.032) were associated with SCAS. The combination of these variables had a fair accuracy to classify SCAS (Area under the curve 0.733, 95% CI 0.662–0.803).
Conclusions: We identified several multimodal imaging markers independently associated with SCAS. These markers may provide information to identify ACAS patients with high risk of ischemic stroke. Future studies are needed to predict SCAS using our findings in other independent cohorts.
Background
Carotid artery stenosis is one of the major causes of stroke. In the population over 65 years old, 5–10% of people have over a 50%-degree of stenosis in the proximal carotid artery and 0.3–2.0% of patients with asymptomatic carotid artery stenosis (ACAS) have an ischemic stroke event each year (1). The mechanisms of stroke or transient ischemic attack in proximal carotid stenosis are artery-to-artery embolism from an unstable plaque or hypo-perfusion due to severe stenosis with poor collaterals. Unfortunately, only 15% of patients with ACAS have warning signs before an ischemic stroke (2, 3).
Therefore, imaging predictors of ischemic stroke should be identified to identify high-risk patients with ACAS and make a clinical decision on the necessity of preventive revascularization.
We hypothesized that atherosclerotic plaque characteristics, white matter hyperintensities, and perfusion abnormalities would be associated with symptomatic carotid stenosis. Plaque characteristics have been extensively studied. Among various plaque characteristics, stenosis degree has been frequently reported to one of the risk factors. Previous studies suggested high-degree stenosis is more associated with symptomatic carotid artery stenosis (SCAS) (4, 5). However, recent studies did not reveal any significant relationship between stenosis severity and risk of stroke (2, 3). An echolucent plaque, plaque ulceration, thinning of the fibrous cap, intraplaque hemorrhage, length of stenosis, and microembolic signals are also suggested as risk factors of SCAS (6). In contrast, perfusion status has been relatively less investigated. Ipsilateral hypoperfusion has been recently highlighted as a risk factor of SCAS (7). However, clinical decisions cannot be made based on a single variable, and it is unknown which factor is more important. In addition, we questioned how to better predict symptomatic carotid stenosis using a combination of already known factors.
In this study, we attempt to identify factors associated with SCAS using multimodal imaging techniques. Plaque characteristics from ultrasonography and angiography and various perfusion markers from brain magnetic resonance imaging (MRI) and perfusion weighted imaging (PWI) were comprehensively investigated and compared between ACAS and SCAS. We aimed to identify independent imaging markers of SCAS, which may suggest the risk of impending stroke when present in ACAS, and test if the multimodal approach (i.e., combination of different imaging markers) would be superior to each method.
Methods
Patients
We retrospectively collected data on patients who had carotid artery revascularization procedures (carotid endarterectomy or carotid artery stenting) from April 2007 to April 2016. The decision to use carotid endarterectomy or carotid artery stenting was determined by a consensus meeting based on published guidelines (8–10). Patients who had a cerebral ischemic stroke event during the 6 months before carotid endarterectomy or carotid artery stenting were categorized in the SCAS group. The other patients were categorized in the ACAS group. Only patients with atherosclerosis in the carotid artery were included, and individuals were excluded if they had a diagnosis of aneurysm or dissection of the carotid artery, fibromuscular dysplasia, Takayasu's arteritis, or post-radiotherapy. This study was approved by Institutional Review Board of Samsung Medical Center, Seoul, Korea. (IRB No. 2018-08-059) A detailed clinical chart review was performed, and the abstracted information was recorded on a standardized data collection form. The following variables were collected: sex, age, body mass index, history of smoking, presence of hyperlipidemia, diabetes mellitus, ischemic heart disease, atrial fibrillation and peripheral artery occlusive disease, and medications such as anti-platelets, anticoagulants, or statins.
Laboratory Data
Because dyslipidemia and hyperglycemia are well-known risk factors for atherosclerosis (11, 12), blood tests for lipid profile and blood sugar were given to all patients. Data on triglyceride, total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, fasting blood sugar, and lipoprotein A levels were collected. Echocardiography was performed in most patients. The ejection fraction (13) was obtained from echocardiography.
Imaging Data
Carotid duplex sonography, computed tomography angiography, MRI, magnetic resonance angiography (MRA) and PWI were performed (Figure 1). Several parameters presenting carotid plaque pathology (14) are obtained using Carotid Doppler ultrasound machine (Acuson Antares, Siemens, Munich, Germany) with a 7.5-MHz linear array probe. In carotid duplex sonography, the degree, and velocity of stenosis site and plaque nature (calcification, ulceration, and echogenicity) were recorded. The angiography values were determined from computed tomography angiography and MRA. The length of the plaque in angiography was measured from the normal to other normal side. The degree of stenosis (≥70%) and presence of contralateral carotid stenosis, ipsilateral tandem lesion, and ulceration were recorded.
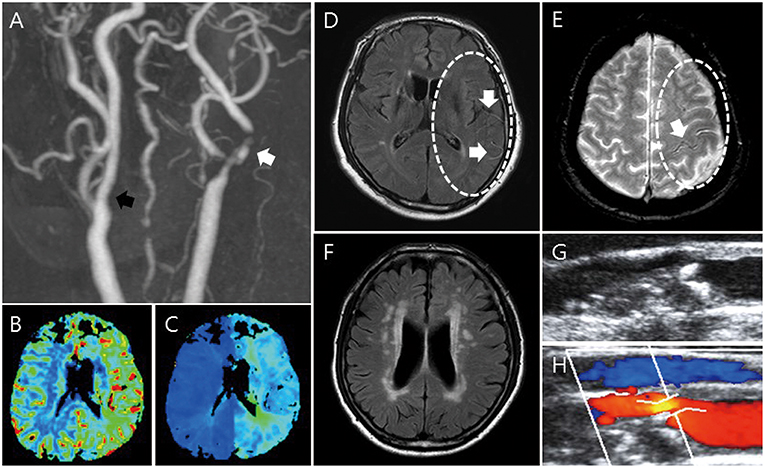
Figure 1. Imaging parameters: MRI, CTA/MRA, PWI, and carotid duplex sonography. MRA shows stenosis of the proximal internal carotid artery (A, white arrow) and contralateral carotid artery stenosis (A, black arrow). In PWI, increased CBV (B) and delayed TTP (C) are shown. FLAIR shows HVS in FLAIR (D) and PWMH/DWMH (F). GRE shows HV in GRE (E). Through duplex carotid sonography, plaque characteristics such as calcification, ulceration, echolucent, and stenosis are shown (G,H).
MRI was performed using a 3.0-T unit (Achieva; Philips Medical Systems). The typical stroke MRI protocol included at least DWI, perfusion-weighted imaging, FLAIR, and vascular images (3-dimensional time-of-flight MRA and contrast-enhanced MRA including the extracranial ICA and the vertebral artery; Figure 1). From brain MRI, the presence of deep or periventricular white matter hyperintensity (DWMH or PWMH) (15), hyperintense vessel signal (HVS) in FLAIR (16, 17), and hypointense vessels (HV) in GRE (18) were obtained by comparing to the contralateral side. On PWI, increased relative cerebral blood volume (CBV) and delayed time to peak (TTP) were evaluated by comparing with the contralateral side and posterior circulation.
Statistics
To compare the two groups (ACAS and SCAS groups), chi-square tests and independent Student t-tests were performed for dichotomous variables and continuous variables, respectively. Propensity score matching was performed to adjust for differences in baseline characteristics between ACAS and SCAS groups. Propensity scores were generated using the following variables: age, sex, body mass index, the type of procedure (carotid endarterectomy or stenting), the side of stenosis, smoking, hypertension, diabetes, hyperlipidemia, Ischemic heart disease, previous CABG or PCI, peripheral artery occlusive disease, atrial fibrillation, congestive heart failure, use of antiplatelet drug, anticoagulant, and statin, and the dose of statin. After propensity score generation, patients underwent 1:1 nearest neighbor (Greedy-type) matching of the logit of the propensity score with a caliper width of 0.25. We performed the univariable logistic regression analysis and included and adjusted variables with univariable p < 0.10 in the multivariable logistic regression model. Variables were selected after checking for multicollinearity using the correlation coefficient and variance inflation factor (VIF). Variables with a correlation coefficient above 0.3 with statistical significance or VIF > 10 were considered to have multicollinearity and excluded from the multivariable logistic regression analyses.
The receiver operating characteristic (ROC) curves were generated using various combination of imaging factors independently associated with SCAS. The area under ROC (AUC) was calculated to measure the accuracy of each combination to differentiated SCAS from ACAS (19). Statistical analyses were performed with R software (R version 3.2.5, The R Foundation for Statistical Computing).
Results
Patient Characteristics
From the total 1,096 patients during the study period, 612 patients without imaging data, 33 patients without echocardiography, 122 patients without carotid sonography, and 70 patients with incomplete laboratory data were excluded. Finally, 259 patients who had complete multimodal imaging data were included in the study, and 148 (57.1%) of these patients had ACAS while the rest (42.9%) had SCAS (Figure S1).
In the unmatched data, patients with ACAS were younger than those with SCAS (p = 0.037). Ischemic heart disease was more frequent in the ACAS group, CABG or PCI were also more often performed in the ACAS group (40.5 vs. 26.1%, p = 0.022; 34.5 vs. 18.0%, p = 0.005, respectively). The two groups did not differ in sex, body mass index, smoking history, hyperlipidemia, diabetes mellitus, hypertension, peripheral artery occlusive disease, atrial fibrillation, and congestive heart failure. Statin use was more common in the ACAS group (80.4 vs. 68.5%, p = 0.039). Usage of other medications did not show significant differences between the two groups (Table 1).
Propensity Score Matched Analysis
After propensity score matching, 95 patients in each group were balanced for baseline variables. As a result of propensity score matching, no significant differences in baseline characteristics were noted (Table 1). Table 2 shows results from univariable analyses of laboratory, echocardiography, and multimodal imaging results. Laboratory test results and echocardiography did not differ between ACAS and SCAS (Table 2). Factors associated with SCAS with univariable p < 0.1 were: longer stenosis length (p = 0.029), all variables on brain MRI and PWI (PWMH, p = 0.027; DWMH, p < 0.001; HVS in FLAIR, p = 0.007; HV in GRE, p = 0.012; increased CBV, p = 0.012; delayed TTP, p = 0.042), absent calcification of the plaque (p = 0.058), ulcerative plaques p = 0.055), and the velocity of the stenosis (p = 0.077).
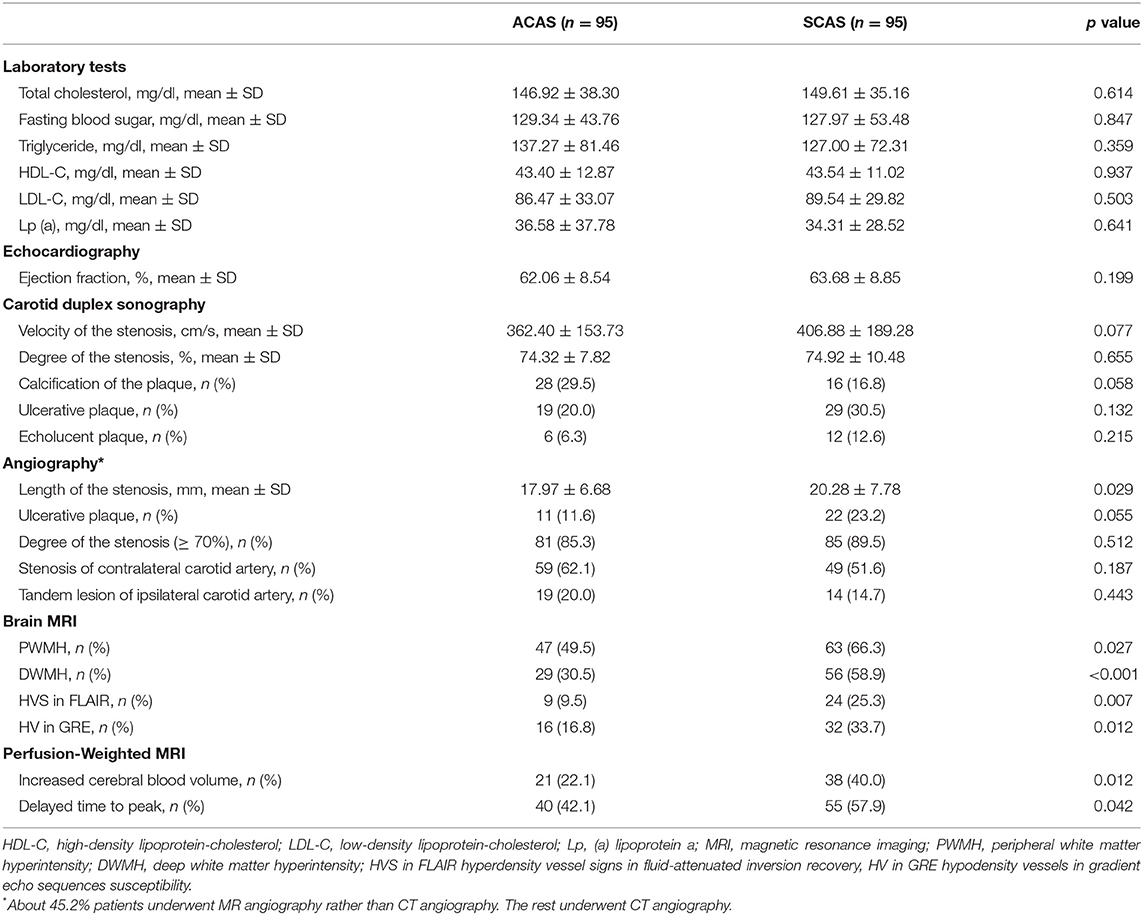
Table 2. Comparison of laboratory and imaging findings between symptomatic vs. asymptomatic carotid stenosis in propensity-matched subgroups.
Factors Independently Associated With SCAS
Among variables with univariable p < 0.1, the length of the stenosis, ulcerative plaque, calcification of the plaque, DWMH, HV in GRE, and increased CBV were included in the multivariable model after excluding variables showing significant multicollinearity (Table 3). The absence of plaque calcification (OR 0.41, 95% CI 0.182–0.870, p = 0.023), more extensive DWMH (OR 3.46, 95% CI 1.842–6.682, p < 0.001), the presence of HV in GRE (OR 2.35, 95% CI 1.113–5.107, p = 0.027), and increased CBV (OR 2.17, 95% CI 1.075–4.454, p = 0.032) were independently associated with SCAS (Table 3).
ROC Analysis
The accuracy of differentiating SCAS from ACAS was assessed using several combination of factors associated with SCAS (Figure 2): ROC curve of DWMH alone (blue line), DWMH and absent plaque calcification (orange line), DWMH, absent plaque calcification, and GRE HV sign (green line), and DWMH, absent plaque calcification, GRE HV sign, and increased cerebral blood volume (black line). The combination of all four factors (black line) yielded the best accuracy to classify SCAS (AUC = 0.733, 95% CI 0.662–0.803; Figure 2).
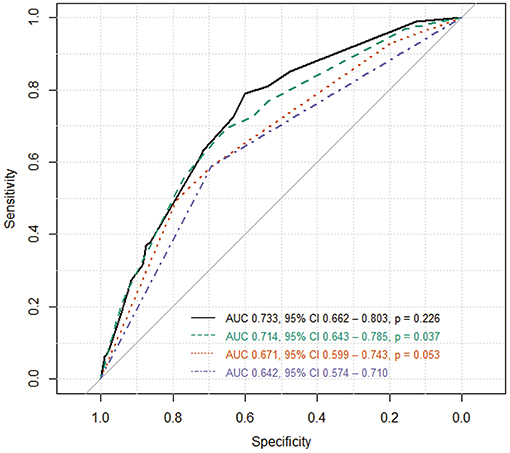
Figure 2. Receiver operating characteristic (ROC) curve. The accuracy of differentiating SCAS from ACAS was measured using several combination of factors associated SCAS: ROC curve of DWMH alone (blue line), DWMH and absent plaque calcification (orange line), DWMH, absent plaque calcification, and GRE HV sign (green line), and DWMH, absent plaque calcification, GRE HV sign, and increased cerebral blood volume (black line). The combination of all four factors (black line) yielded the best accuracy. AUC indicates area under the ROC curve; CI, confidence interval; p, p-value comparing the two AUCs.
Discussion
The major finding of our study is that in addition to the conventional risk factors for SCAS, vascular imaging and PWI parameters may provide important information about uncompensated hypoperfusion associated with SCAS. The absence of calcification of the plaque, more extensive DWMH, the presence of HV in GRE, and increased CBV were independently associated with SCAS. The combination of these factors better classified SCAS from ACAS than each factor. Multimodal imaging may therefore provide additional risk stratification in ACAS patients.
When present in ACAS, the markers of hypoperfusion that were associated with SCAS likely indicate that brain perfusion status is approaching a critical threshold for symptomatic ischemia.
In our retrospective cohorts who underwent carotid revascularization, patients had different indications for the revascularization and thus differences between baseline characteristics. Although the prospective observation of ACAS patients would yield the most unbiased results, such study requires a large number of samples and long observation time since the rate of primary outcome events (symptomatic stroke or transient ischemic attack) is low in patients with ACAS. To overcome our study design, we used the propensity score matching to approximate randomization by using baseline variables and identify matched pairs.
The two major mechanisms of transient ischemic attack or ischemic stroke in carotid stenosis are artery-to-artery embolism from an unstable plaque and low perfusion due to severe stenosis and poor collaterals. Previous studies suggested the nature of the plaque as a predictor of the risk of stroke in patients with ACAS. Plaque ulceration, echolucent plaque, and microembolic signals were suggested as risk factors of ischemic stroke in ACAS patients (20–22). Using ultrasonography, plaque instability indicated by echolucent, hypoechoic plaque (23), or heterogeneous echocontrast (24). In addition, patients with a juxtaluminal hypoechoic area of >8 mm in the plaque had a high occurrence rate of ischemic stroke (25). Plaque ulceration or intraplaque hemorrhage on magnetic resonance carotid artery imaging indicate instability of the plaque (26, 27). In our study, there were no significant associations between in plaque characteristics on in carotid duplex sonography and symptomatic status. However, after propensity matching, SCAS was associated with longer plaque length in univariable analysis and calcification of the plaque was more frequent in the ACAS group in multivariable analysis. Longer plaque length was associated with greater risk of ischemic stroke either due to small emboli from the plaque or hypoperfusion (28). Plaque calcification has been suggested as an indicator of greater plaque stability and lower embolic risk. Plaque composition analysis using CT angiography revealed calcification proportion was inversely associated with plaque ulceration (29). There is also evidence that patients with less calcification of the plaque on carotid computed tomography had more stroke events (30).
In previous MRI-based carotid stenosis studies, silent infarcts on FLAIR MRI, contralateral carotid artery stenosis on brain MRA and increased mean transit time and decreased CBF on PWI were shown to predict stroke events in patients with ACAS (29–31). In our SCAS group, DWMH, HVS in FLAIR and HV in GRE were detected more often on brain MRI. Previous studies have shown that WMH is associated with an increased risk of cerebrovascular events (32, 33), and is a predictor of stroke due to atherosclerosis (34).
DWMH represents the area of chronic hypoperfusion in the brain. The significantly higher frequency of DWMH in the SCAS group indicates a decreased threshold for ischemic events. HVS in FLAIR has usually been described in hyperacute strokes with arterial occlusion and indicate collateral vessels (17). Between the two groups, the SCAS group showed a higher rate of HVS in FLAIR than the ACAS group, but HVS was not independently associated with SCAS in the multivariable analysis. HV in GRE has been used to predict the state of cerebral hypoperfusion and is thought to represent increased oxygen extraction fraction (35). In our study, CBV was increased in the SCAS group. Increased CBV by compensatory vasodilatation can develop in patients with chronic hemodynamic insufficiency, which in turn acts as a risk factor for further ischemic events if the compensation fails to properly respond.
There are some limitations of this study. First, the study was retrospective and there was no imaging of patients with carotid lesions prior to them becoming symptomatic. Brain MRI and PWI parameters were obtained during or after the acute ischemic period in the SCAS group: MR including PWI was acquired within about 6 days, [Median 6.0 days, interquartile range (IQR) 1.0–17.0 days]. The observed CBV abnormalities may reflect chronic stenosis rather than acute stroke. Second, we included patients who underwent carotid artery revascularization procedures, so we did not include all patients who received medical treatment. In accordance with AHA/ASA guidelines, symptomatic patients with ≥50% stenosis were considered for carotid artery revascularization procedures (10). In this study, all patients had more than moderate stenosis, so our results of this study might indicate specific risk factors for patients who need revascularization procedures rather than the best medical treatment. In addition, although differences in baseline characteristics between groups were balanced using the propensity score matching, unmeasured confounding factors by variables not available in our dataset may persist. Finally, long term follow-up of moderate to severe ACAS with quantitative analysis of WMH, HVS in FLAIR, HV in GRE, and PWI parameters is necessary to validate the predictive value of the multimodal carotid imaging approach for SCAS.
Strengths of our study include the use of propensity score matching to reduce the impact of selection bias. The recent treatment guidelines for ACAS emphasize the importance of best medical treatment with strict control of risk factors (36). However, some patients with ACAS still had ischemic stroke events, despite receiving the best medical treatment. Prediction for SCAS is important to select candidates for carotid endarterectomy and carotid artery stenting. The DWMH, HVS in FLAIR, HV in GRE, and increased CBV were shown association with SCAS independently. In addition, a calcified plaque revealed a low risk for SCAS. DWMH, FLAIR HVS sign, and HV in GRE can provide information on tissue perfusion without performing PWI.
Conclusions
Multimodal imaging including computed tomography angiography, conventional MRI, and PWI may be useful in the identification of patients at high risk of ischemic stroke among patients with ACAS. Further validation with prospective patient registration and long term follow-up is required to prove the predictive value of these parameters.
Data Availability
All relevant data are within the article. Raw data can be obtained by contacting the corresponding author (kimgm@skku.edu)
Ethics Statement
The Institutional Review Board of Samsung Medical Center approved this study and each patient provided written informed consent.
Author Contributions
MP and SK: study design, data analysis, statistical analysis, and manuscript drafting. ML data analysis. KK, PJ, YP, YK, DK, OB, CC, and KL: data acquisition and data analysis. GK: study concept and design, data acquisition, data analysis, and manuscript correction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00765/full#supplementary-material
Abbreviations
ACAS, asymptomatic carotid artery stenosis; SCAS, symptomatic carotid artery stenosis; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; PWI, perfusion weighted imaging; DWMH, deep white matter hyperintensity; PWMH, periventricular white matter hyperintensity; HVS, hyperintense vessel signal; HV, hypointense vessels; CBV, cerebral blood volume; TTP, delayed time to peak; VIF, variance inflation factor; AUC, area under ROC; CI, confidence interval.
References
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
2. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:517–84. doi: 10.1161/STR.0b013e3181fcb238
3. Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. (2010) 376:1074–84. doi: 10.1016/S0140-6736(10)61197-X
4. Autret A, Pourcelot L, Saudeau D, Marchal C, Bertrand P, de Boisvilliers S. Stroke risk in patients with carotid stenosis. Lancet. (1987) 1:888–90. doi: 10.1016/S0140-6736(87)92861-3
5. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. (1995) 273:1421–8. doi: 10.1001/jama.273.18.1421
6. Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. (2000) 31:774–81. doi: 10.1161/01.STR.31.3.774
7. Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol. (2010) 9:1008–17. doi: 10.1016/S1474-4422(10)70185-X
8. Sacco RL. Clinical practice. Extracranial carotid stenosis. N Engl J Med. (2001) 345:1113–8. doi: 10.1056/NEJMcp011227
9. Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Carotid endarterectomy–an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. (2005) 65:794–801. doi: 10.1212/01.wnl.0000176036.07558.82
10. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. (2011) 57:1002–44. doi: 10.1161/CIR.0b013e31820d8d78
11. Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Dyslipidemia as a risk factor for ischemic stroke. Curr Top Med Chem. (2009) 9:1291–7. doi: 10.2174/156802609789869628
12. Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. (2004) 35:363–4. doi: 10.1161/01.STR.0000115297.92132.84
13. Pullicino P, Mifsud V, Wong E, Graham S, Ali I, Smajlovic D. Hypoperfusion-related cerebral ischemia and cardiac left ventricular systolic dysfunction. J Stroke Cerebrovasc Dis. (2001) 10:178–82. doi: 10.1053/jscd.2001.26870
14. Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. (2005) 36:253–7. doi: 10.1161/01.STR.0000152336.71224.21
15. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. (2014) 83:1228–34. doi: 10.1212/WNL.0000000000000837
16. Wolf RL. Intraarterial signal on fluid-attenuated inversion recovery images: a measure of hemodynamic stress? AJNR Am J Neuroradiol. (2001) 22:1015–6.
17. Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology. (2009) 72:1134–9. doi: 10.1212/01.wnl.0000345360.80382.69
18. Kesavadas C, Santhosh K, Thomas B. Susceptibility weighted imaging in cerebral hypoperfusion-can we predict increased oxygen extraction fraction? Neuroradiology. (2010) 52:1047–54. doi: 10.1007/s00234-010-0733-2
19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
20. Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology. (2011) 77:751–8. doi: 10.1212/WNL.0b013e31822b00a6
21. Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. (2010) 52:1486–96.e1–5. doi: 10.1016/j.jvs.2010.07.021
22. Madani A, Beletsky V, Tamayo A, Munoz C, Spence JD. High-risk asymptomatic carotid stenosis: ulceration on 3D ultrasound vs TCD microemboli. Neurology. (2011) 77:744–50. doi: 10.1212/WNL.0b013e31822b0090
23. Grogan JK, Shaalan WE, Cheng H, Gewertz B, Desai T, Schwarze G, et al. B-mode ultrasonographic characterization of carotid atherosclerotic plaques in symptomatic and asymptomatic patients. J Vasc Surg. (2005) 42:435–41. doi: 10.1016/j.jvs.2005.05.033
24. Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. (2001) 104:68–73. doi: 10.1161/hc2601.091704
25. Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg. (2013) 57:609–18.e1; discussion: 17–8. doi: 10.1016/j.jvs.2012.09.045
26. Demarco JK, Ota H, Underhill HR, Zhu DC, Reeves MJ, Potchen MJ, et al. MR carotid plaque imaging and contrast-enhanced MR angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: an in vivo study at 3T. AJNR Am J Neuroradiol. (2010) 31:1395–402. doi: 10.3174/ajnr.A2213
27. Singh N, Moody AR, Gladstone DJ, Leung G, Ravikumar R, Zhan J, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. (2009) 252:502–8. doi: 10.1148/radiol.2522080792
28. Deweese JA, May AG, Lipchik EO, Rob CG. Anatomic and hemodynamic correlations in carotid artery stenosis. Stroke. (1970) 1:149–57. doi: 10.1161/01.STR.1.3.149
29. Hougaku H, Matsumoto M, Handa N, Maeda H, Itoh T, Tsukamoto Y, et al. Asymptomatic carotid lesions and silent cerebral infarction. Stroke. (1994) 25:566–70. doi: 10.1161/01.STR.25.3.566
30. Longstreth WT Jr, Shemanski L, Lefkowitz D, O'Leary DH, Polak JF, Wolfson SK Jr. Asymptomatic internal carotid artery stenosis defined by ultrasound and the risk of subsequent stroke in the elderly. The Cardiovascular Health Study. Stroke. (1998) 29:2371–6. doi: 10.1161/01.STR.29.11.2371
31. Soinne L, Helenius J, Tatlisumak T, Saimanen E, Salonen O, Lindsberg PJ, et al. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke. (2003) 34:1655–61. doi: 10.1161/01.STR.0000075605.36068.D9
32. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. (2010) 341:c3666. doi: 10.1136/bmj.c3666
33. Kim GM, Park KY, Avery R, Helenius J, Rost N, Rosand J, et al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke. (2014) 45:479–85. doi: 10.1161/STROKEAHA.113.003004
34. Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatr. (2002) 72:576–82. doi: 10.1136/jnnp.72.5.576
35. Kaya D, Dincer A, Yildiz ME, Cizmeli MO, Erzen C. Acute ischemic infarction defined by a region of multiple hypointense vessels on gradient-echo T2* MR imaging at 3T. AJNR Am J Neuroradiol. (2009) 30:1227–32. doi: 10.3174/ajnr.A1537
36. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:3754–832. doi: 10.1161/STR.0000000000000046
Keywords: asymptomatic carotid artery stenosis, symptomatic carotid artery stenosis, risk factors, multimodal imaging, perfusion-weighted MRI
Citation: Park MS, Kwon S, Lee MJ, Kim KH, Jeon P, Park YJ, Kim DI, Kim YW, Bang OY, Chung CS, Lee KH and Kim GM (2019) Identification of High Risk Carotid Artery Stenosis: A Multimodal Vascular and Perfusion Imaging Study. Front. Neurol. 10:765. doi: 10.3389/fneur.2019.00765
Received: 17 March 2019; Accepted: 01 July 2019;
Published: 16 July 2019.
Edited by:
Bruce Campbell, The University of Melbourne, AustraliaReviewed by:
Nishant K. Mishra, Icahn School of Medicine at Mount Sinai, United StatesDavor Pavlin-Premrl, Royal Melbourne Hospital, Australia
Copyright © 2019 Park, Kwon, Lee, Kim, Jeon, Park, Kim, Kim, Bang, Chung, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyeong-Moon Kim, kimgm@skku.edu
†These authors have contributed equally to this work
 Moo-Seok Park1†
Moo-Seok Park1† Soonwook Kwon
Soonwook Kwon Mi Ji Lee
Mi Ji Lee Pyoung Jeon
Pyoung Jeon Oh Young Bang
Oh Young Bang Gyeong-Moon Kim
Gyeong-Moon Kim