Variation and Variability in Drosophila Grooming Behavior
- 1Interdepartmental Graduate Program in Dynamical Neuroscience, University of California, Santa Barbara, Santa Barbara, CA, United States
- 2Department of Physics, University of California, Santa Barbara, Santa Barbara, CA, United States
- 3Department of Molecular, Cellular, and Developmental Biology, University of California, Santa Barbara, Santa Barbara, CA, United States
- 4Neuroscience Research Institute, University of California, Santa Barbara, Santa Barbara, CA, United States
Behavioral differences can be observed between species or populations (variation) or between individuals in a genetically similar population (variability). Here, we investigate genetic differences as a possible source of variation and variability in Drosophila grooming. Grooming confers survival and social benefits. Grooming features of five Drosophila species exposed to a dust irritant were analyzed. Aspects of grooming behavior, such as anterior to posterior progression, were conserved between and within species. However, significant differences in activity levels, proportion of time spent in different cleaning movements, and grooming syntax were identified between species. All species tested showed individual variability in the order and duration of action sequences. Genetic diversity was not found to correlate with grooming variability within a species: melanogaster flies bred to increase or decrease genetic heterogeneity exhibited similar variability in grooming syntax. Individual flies observed on consecutive days also showed grooming sequence variability. Standardization of sensory input using optogenetics reduced but did not eliminate this variability. In aggregate, these data suggest that sequence variability may be a conserved feature of grooming behavior itself. These results also demonstrate that large genetic differences result in distinguishable grooming phenotypes (variation), but that genetic heterogeneity within a population does not necessarily correspond to an increase in the range of grooming behavior (variability).
Introduction
Differences in phenotype arise from differences in genotype. Changes in DNA account for variation in traits among species, and differences between individuals of the same species. Animal behavior contains phenotypes partially under genetic control, and specific genes associated with observable differences in behavior between and within species have been uncovered (Baker et al., 2001; Johanssen, 2014). Different mouse species exhibit variation in monogamy and parental care, and different fly species show variation in courtship song, food preference, and larval digging (Bendesky et al., 2017; Kim et al., 2017; Ding et al., 2019; Markow, 2019; Auer et al., 2020). From endangered species to agricultural crops to virus variants, genetic diversity affects organismal success. Within a species, natural variations in DNA sequences produce individual mice that differ in aggression and or flies that implement different foraging strategies (Anderson, 2016; Allen et al., 2017) and advantageous variants can be selected. Mutant screens have also uncovered gene variants associated with differences in locomotion, courtship routines, and sleep patterns, among other complex behaviors (Baker et al., 2001; Sokolowski, 2001; Ayroles et al., 2015; Gaertner et al., 2015).
Some behaviors can be performed in different ways even by genetically identical organisms or by the same individual in repeated trials. Behavioral variability can be advantageous as a bet-hedging strategy against unstable environmental conditions (Kain et al., 2015). Phenotypic variability in behaviors ranging from birdsong to escape trajectories can increase individual success, but also fitness in a population, suggesting that variability itself can be a selectable trait. Experiments in Drosophila melanogaster demonstrate that the degree of behavioral variability in locomotion is partially controlled by genetic expression of proteins such as teneurin-α, a cell adhesion molecule (Honegger and de Bivort, 2018). Additionally, silencing a subset of neurons in the central complex modifies the degree of variability of locomotor behavior (Honegger and de Bivort, 2018). Differences in neurodevelopment and synaptic connectivity can also result in behavioral variability (Linneweber et al., 2020). Together, these observations suggest that factors at both at the population (genetic) and individual (neuronal) levels contribute to behavioral variability.
Drosophila grooming shows behavioral variability. Fruit flies live in dirty environments, from laboratory vials to rotting fruit, and they perform grooming actions to remove accumulated particulates. Grooming has been observed in several drosophilid species and is important for survival (Szebenyi, 1969; Spruijt et al., 1992; Zhukovskaya et al., 2013). Past work demonstrated that the leg movements used in grooming are stereotyped, but the sequences of actions are flexible as opposed to fixed. While the rules underlying grooming do exhibit observable structure in flies (Seeds et al., 2014; Mueller et al., 2019) and in mice (Fentress and Stilwell, 1973; Geuther et al., 2021), different sensory experiences and life histories may influence grooming behavior. These results lead us to ask: how much of variability in fruit fly grooming is under genetic control?
To address this question, we evaluated different features of dust-induced grooming behavior by comparing their values between groups and their range within groups. The raw behavioral data and features we examine here are schematized in Figure 1. In particular, we focus on the transition probabilities between actions that compose the grooming sequence, which we refer to as “syntax”; in linguistics, this term refers to the rules that indicate how words and phrases may be combined to form sentences, so it is borrowed here to indicate that action transitions also conform to rules.

Figure 1. Grooming variability dataset and analysis overview. (A) In total, N = 390 male flies were dusted and their activity was recorded for approximately half an hour each. Five drosophilid species, four melanogaster stock lines, one interbred melanogaster line, and six isogenic melanogaster lines were analyzed for similarity, differences, and stereotypy in grooming and non-grooming behaviors. On the left is a schematic of the different drosophilid groups included in this analysis. Higher levels of the tree indicate higher levels of genetic diversity (scale is relative, not absolute). On the right is a sample of ethograms generated by automated annotation of video. Color indicates the occurrence of the five grooming actions (F, front leg cleaning; H, head grooming; A, abdomen grooming; B, back leg cleaning; W, wing grooming) and two non-grooming actions (Wk, walking; S, standing). (B) Features scored from ethograms provide summary representations of behavior. Shown here are sample visualizations of behavioral metrics analyzed in this work. On the left, the proportion of time spent in different actions provides the coarsest description of the behavioral response to a dust stimulus. Regardless of genotype, all flies exhibit variable (not fixed) action sequences consisting of the same set of five grooming actions, walking, and standing after exposure to irritant. Next, action transition probabilities (syntax) describe the likelihood of performing consecutive actions. Arrow directions and thicknesses represent the probability of performing an action, given the identity of the previous action. Shown next is an example behavioral progression, which depicts the proportion of time spent in each action over a sliding window. Most flies follow a typical behavioral progression pattern: initial anterior grooming followed by increased posterior grooming. The amount and timing of walking and standing, however, can vary significantly between flies. Finally, action (bout) duration distributions describe the range of action lengths. All example features shown here are scored from Canton-S flies.
First, we evaluated differences in phenotype between genetically-distinct populations, such as drosophilid species. Differences in grooming behavior when members of different species are compared can reasonably be attributed to differences in their DNA sequences.
In a wild and genetically diverse population of flies, there may be mutations that change grooming behavior, but lab strains of Drosophila melanogaster are largely clonal—all individuals should have the same genotype. We next compared the grooming behavior of different lab strains, and then of individuals within a given lab strain. We hypothesized that genetic heterogeneity might contribute to the magnitude of grooming variability. By interbreeding or isogenizing melanogaster lab strains, we generated stocks with high and low genetic diversity, but we find that all groups exhibited similar variability in measured grooming features. Intra-genotypic or phenotypic variability has also been observed in fly locomotor behavior, and some populations exhibit a wide range while others exhibit a narrower one (Ayroles et al., 2015).
Genetic differences among drosophilid species and strains may underlie variation in the syntax of their cleaning movements, but all flies show variability in the exact sequence of those movements. Furthermore, even the same fly tested on sequential days revealed sequence variability. The extent of within-fly differences in syntax were similar to between-fly differences: flies were no more similar to themselves over time than they were to other flies on a given day. Finally, flies stimulated using optogenetic manipulation to induce grooming exhibited increased stereotypy, but within-individual grooming variability between stimulation sessions was not fully abolished. These data show that genetic heterogeneity plays a limited role in the variability of grooming behavior, and that differences in sensory experience contribute but do not account for all observed variability. The widespread nature of grooming variability suggests that it may be an important feature, but our experiments indicate the need to search for alternative causes, perhaps including developmental stochasticity, differences in internal state, or noisy neural circuit dynamics.
Results
In this work, N = 390 male flies were covered in dust and their grooming behavior was recorded for approximately 30 min each (Figure 1A). We analyzed flies from five drosophilid species (melanogaster, santomea, sechellia, simulans, and erecta), which are genetically distinct—separated by millions of years of evolution—and inhabit different ecological niches. We also examined four common melanogaster lab stocks (Canton-S, Oregon-R, Berlin-K, and w1118), and several isogenic lines derived from these parent stocks in our laboratory.
To analyze this large data set, we employed tools from computational ethology (Datta et al., 2019). An automated behavioral recognition system [ABRS, Ravbar et al. (2019)] was used to classify fly behavior into one of five grooming actions (front leg cleaning, head grooming, abdomen grooming, back leg cleaning, wing grooming) and two non-grooming actions (walking and standing). As a note, head grooming consists of actions that use the front legs to clean the antennae, eyes, and face, but sub-movements such as these were not easily detectable using the recording methodology employed here, so analysis was restricted to coarser spatiotemporal scales. After generating ethograms (behavioral time series records) for each fly, several grooming features were extracted (Figure 1B). We measure the average amount of time flies spend performing each of the grooming actions (plus standing and walking), the syntax of transition probabilities among these actions, the anterior-to-posterior progression, and the durations of bouts of grooming actions. We used classification analysis and various measures of stereotypy to quantify the variation (inter-species or inter-strain differences) and variability (intra-strain or intra-individual differences) of these characteristics.
Drosophilids Exhibit a Robust Grooming Response but Different Syntax After Irritant Exposure
Across the Drosophila species tested here, five grooming actions were observed consistently, indicating a conserved behavioral response (Supplementary Figure 1). Previous work showed that these actions are sufficiently stereotyped to be reliably classified by manual and automated annotation in melanogaster (ABRS, see section “Materials and Methods”) (Mueller et al., 2019; Ravbar et al., 2019). Here, the ABRS classifier was validated on training data for each species, showing comparable accuracy (see section “Materials and Methods” and Supplementary Figure 22), indicating that the movement primitives that make up grooming are stereotyped. No novel species-specific grooming actions were detected. Although some fine-scale movement differences may occur among species, they are beyond the spatial and temporal resolution of the current video and unlikely to affect the analysis of transition probabilities presented here. Analysis of mouse behavior indicates that grooming subroutines are largely stereotyped at high temporal resolution, increasing our confidence in this approach (Wiltschko et al., 2015).
To quantify the behavioral response to dusting, the proportion of time spent grooming (as opposed to walking and standing) was calculated for each fly (Figure 2A). The proportions of total grooming time between species were all statistically different (Wilcoxon rank-sum test, p < 0.05, multiple comparison correction via Holm’s method; Supplementary Figure 23), but all species spent at least 35% of the time grooming, on average. In this analysis, a single stock line (Canton-S, N = 18) was used as the representative melanogaster group. Full action proportion distributions are shown in Supplementary Figure 2.
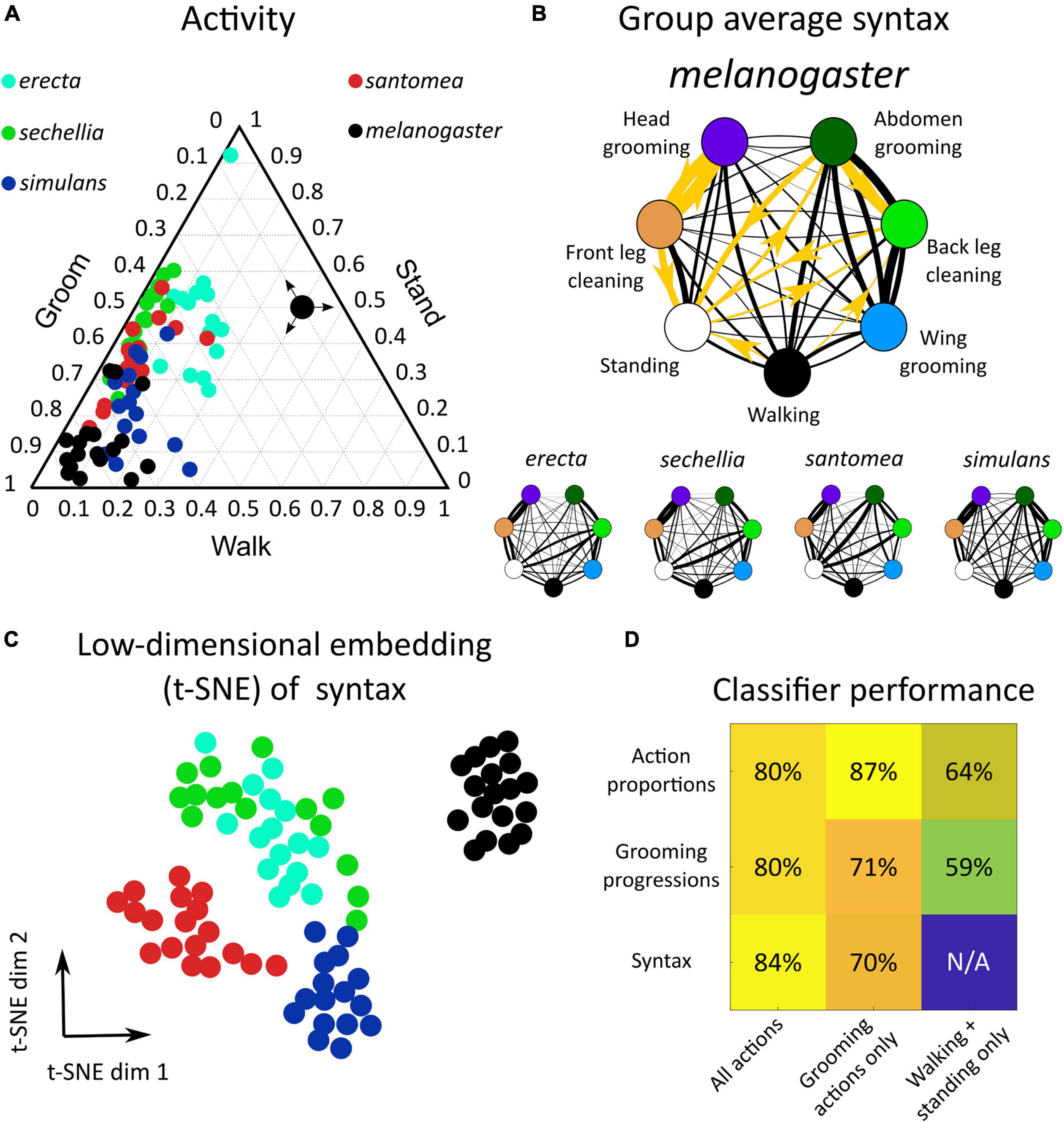
Figure 2. Drosophila species share behavioral features but exhibit between-species variation in action proportions and syntax in response to dust stimulus. (A) Dusting elicits a conserved behavioral response across drosophilids. Shown is a ternary plot of activity proportions for each species examined here (N = 65 flies total). Colored points represent a single fly, with color indicating species. The large black point with arrows indicates how to read activity proportions; the example point corresponds to 10% grooming, 40% walking, and 50% standing. (B) Drosophilid species produce a probabilistic behavioral sequence (as shown in Figure 1A), which can be characterized by the transition probabilities (syntax) between actions [as represented in Figure 1B, calculated as in Mueller et al. (2019)]. The mean syntax for each species is depicted as a graph, with nodes representing actions and edges indicating transition probability. Thicker edges indicate higher probabilities. On the melanogaster syntax graph, the 10 action transitions exhibiting the largest magnitude differences between melanogaster and non-melanogaster species are highlighted in gold. These differences are identifiable in anterior motif transitions, which use the front legs to perform grooming actions. Species also differ in their transitions between posterior grooming actions and non-grooming actions (walking and standing) (C) Each fly’s 42-dimensional syntax vector was plotted in two dimensions after dimensionality reduction using t-SNE. t-SNE preserves local distance structure, indicating that tightly grouped clusters of points are similar to one another. In this case, dimensionality reduction reveals that drosophilid species exhibit significant differences in syntax, as syntax vectors congregate by color. (D) Classification analysis confirms the qualitative clustering observed in C. Shown is a heat map of accuracy rates of 5-possibility multinomial logistic regression classifiers trained on behavioral features. For these samples, classification at chance would be 20%. Consistent classification accuracy values >20% indicate that species are highly separable by behavioral features. Simple features, such as behavioral proportions and progressions, classify individuals by species with high accuracy when grooming actions are included. Classification using only non-grooming actions (walking and standing) still yields classification above chance, indicating that species differ significantly in their overall activity levels. Syntax also allows for accurate classification, particularly when all action transitions are considered.
Behavior was then examined in more detail by considering all seven actions (five grooming movements plus walking and standing) and the progression of those actions over time, as in Figure 1B. These species exhibited a qualitatively similar behavioral progression, characterized by initial anterior grooming followed by increased posterior grooming, walking, and standing, but the relative proportions and timing of these behaviors over time differed (Supplementary Figure 3).
Syntax (the transition probabilities between discrete behaviors) was calculated from the ethogram of each dusted fly (N = 390). With seven behavioral states, 42 transitions were possible, excluding self-transitions. Thus, syntax was represented as a 42-dimensional vector for subsequent classification analysis and visualization. Syntax across all flies exhibited high transition probabilities within the anterior grooming motif (front leg cleaning, head grooming) and posterior grooming motif (abdomen grooming, back leg cleaning, wing grooming). The average syntax for each species is illustrated as a weighted, directed graph in Figure 2B.
Finally, continuous grooming action duration distributions (e.g., the distribution of how long each head grooming action was) were calculated from ethograms. Distributions of action durations were qualitatively similar across species and had probability peaks between 500 and 750 ms (Supplementary Figure 4). When considering the same action, no action duration distributions differed significantly in any pairwise comparison (e.g., comparing head grooming between erecta and santomea) between any species (two-way Kolmogorov-Smirnov test, no p values < 0.05 after correcting for multiple comparisons using Holm’s method).
Several significant differences in behavioral features between melanogaster and non-melanogaster species were identified. Supplementary Figure 5 illustrates differences in overall action proportions, around 36% of which differed between species. To compare syntax, transition probability distributions for each action transition (e.g., head cleaning to front leg rubbing) were compared between species in a pairwise manner. 38 of 42 unique syntax elements (90.5%) were significantly different between at least two species (Wilcoxon rank-sum test, p < 0.05, multiple comparison correction via Holm’s method). Overall, 125 of 420 (30%) of pairwise syntax comparisons revealed differences between species (see Supplementary Figure 24 for all p values).
Of these syntactic differences, 71 (60%) occurred between melanogaster and non-melanogaster species. In particular, posterior motif grooming transitions (transitions between abdomen grooming, back leg rubbing, and wing grooming) were consistently significantly different, on average, as were transitions between back leg rubbing, standing, and walking. Figure 2B illustrates these syntactic differences.
Figure 2C depicts a low-dimensional embedding of species syntax using t-SNE. This visualization suggests that different species possess distinguishable syntax, as points are aggregated by species. Low-dimensional visualizations of all behavioral features are illustrated in Supplementary Figures 5, 6.
Classification analysis was applied to behavioral features to verify this interpretation and quantify the degree of variation between species. Multinomial logistic regression classified flies by species according to behavioral proportions, progressions, and syntax with >80% accuracy (Figure 2D). Notably, classification was also possible with accuracy significantly above chance when only considering the proportions and progressions of non-grooming actions, walking and standing, indicating that species also vary in their overall activity levels.
Finally, entropy rates were calculated from syntax transition probabilities to quantify the degree of stereotypy in behavior. An entropy rate of zero would indicate complete stereotypy and perfectly predictable, repeated action sequences, while in this calculation, an entropy rate of one indicates an approximate 37% probability of correctly predicting the next action in a sequence (see section “Materials and Methods”). Supplementary Figure 7 shows that all species possess average entropy rates between zero and one, demonstrating that grooming sequences are neither fixed nor truly random. melanogaster flies possessed the lowest entropy (highest degree of stereotypy) due to high transition probabilities between head cleaning and front leg rubbing (Figure 2B). In summary, drosophilid species exhibit variation in grooming behavior—visible in proportion of time spent on different actions and the transition probabilities among them—but they all share common cleaning movements and variability in their exact action sequences.
Drosophila melanogaster Strains Exhibit Variation in Grooming Behavior
Next, standard Drosophila melanogaster lab strains (Canton-S, Berlin-K, Oregon-R, w1118) were analyzed for differences in grooming features (full ethograms shown in Supplementary Figure 8). Behavioral proportions, progressions, and syntax differed between stocks, allowing for classification moderately above chance levels. Comparisons of grooming features can be found in Supplementary Figures 9–12.
Overall the proportion of time grooming could account for most of the differences observed between stocks. Figure 3A shows a ternary plot of activity, showing that Canton-S flies spend more time walking than other stocks. A t-SNE embedding of the syntax of melanogaster stocks is depicted in Figure 3A. Similar to the species analysis, all action transition probability distributions were compared in a pairwise manner to look for variation in syntax.
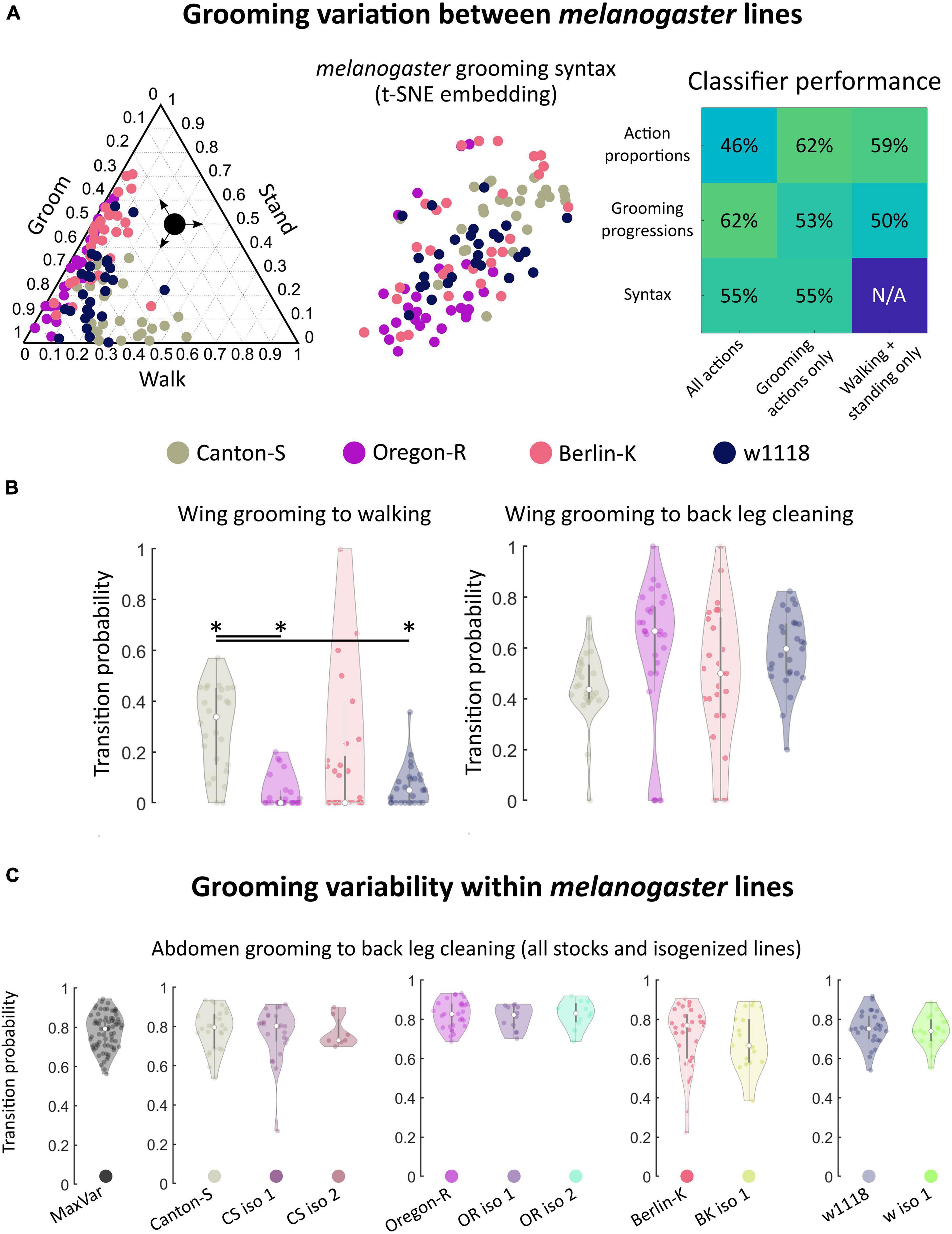
Figure 3. Within melanogaster, different stocks differences in syntax activity levels. Genetic homogeneity does not correspond to behavioral stereotypy. (A) melanogaster stocks (N = 111 flies total) exhibited variation in grooming syntax, though many features were shared. On the left is a ternary plot of grooming, walking, and standing proportions for each stock, similar to Figure 2A. Colored points represent individual flies. Shown in the middle is a t-SNE plot of syntax vectors, as in Figure 2C. The high degree of overlap in both of these plots illustrates that behavioral responses are qualitatively similar between some individuals of different stock lines. Classifier performance (similar to that shown in Figure 2D) is shown on the right. For these data, classification at chance is 25%. Performance above chance is still possible for stock lines. Classification performs similarly well for behavioral features regardless of their complexity; using just walking and standing behavioral proportions provides similar discriminability as using the full syntax. (B) Most syntax elements were similar between melanogaster stocks, but Canton-S flies walked more than other stocks. Due to differences in activity levels, some walking-related syntax elements differed between Canton-S flies and other stocks. Of the significantly different transitions, only two were within-motif transitions while the rest consisted mostly of transitions to and from walking and standing (Supplementary Figure 12). Shown on the left are the wing grooming to walking transition probability distributions for each melanogaster stock line. Significant differences in these distributions were observed between lines. On the right, distributions for a posterior grooming transition are shown; the vast majority of action transition distributions did not differ due to their large variances. (C) Variances of action transition distributions for stock lines, lines bred for maximum genetic heterogeneity (MaxVar), and lines bred to minimize genetic heterogeneity (iso) were compared (N = 252 total). Genetic homogeneity did not correspond to behavioral variability. Shown as an example are the distributions of abdomen grooming to back leg cleaning transitions. MaxVar flies did not exhibit a higher degree of variability (as measured by the variance of transition distributions) than stock lines. Isogenized lines did not exhibit a lower degree of variability than their parent stocks. *significantly different at p < 0.05.
Only 19 of 42 unique syntax element comparisons (45%) differed significantly between any two stocks and, of these, only two within-motif transition (both posterior) differed significantly. Within-motif syntax elements are of particular interest because they represent the most common, most highly stereotyped action transitions observed across flies of all genotypes (see Figure 2B for visualizations of these transitions). The syntax element exhibiting the greatest statistically significant difference was the wing grooming to walking transition, shown in Figure 3B. The other significantly different transitions also mostly involved transitions to and from walking and standing, perhaps reflecting differences in overall activity levels (Supplementary Figure 12).
Classification accuracy was moderate but above chance for all features examined; as expected, variation within melanogaster was less pronounced than variation between species (compare Figures 2D, 3A). Variation within melanogaster stocks appears to be due to differences in overall activity levels, as classification using only non-grooming features (walking and standing proportions and progressions) yielded results similar to classification using full grooming behavior syntax. This is illustrated by the fact that Canton-S flies’ higher propensity to walk after grooming their wings is reflected both in their syntax and grooming proportions in Figure 3B.
Within Canton-S, activity levels separated male and female flies, as male flies tended to walk more than females (Supplementary Figure 13). Male and female flies also possessed somewhat different syntax; classification by syntax was 71%, where chance levels would be 50% for this comparison. This level of accuracy is higher than what was achievable when classifying melanogaster stock lines using syntax, but lower than the same comparison for interspecies data.
Since all flies examined showed variability in syntax, we wondered whether the extent of this variability differed among species or strains. Figure 3B also illustrates the high degree of variability in melanogaster syntax. The wing grooming to back leg cleaning transition exhibited the largest difference between median values of any syntax element (comparison of Canton-S and Oregon-R yielded this difference), but none of these distributions possessed detectable statistical differences due to their concomitantly large variances.
Grooming Behavioral Variability Is Similar Across melanogaster Genotypes
To examine the potential relationship between genetic heterogeneity and behavioral variability, each melanogaster lab strain was compared to lines bred to maximize genetic heterogeneity (MaxVar) or minimize genetic heterogeneity (isogenic lines). These can be considered outbred and inbred strains. If variability in grooming syntax within a population is strongly related to genetic heterogeneity, we would expect populations with larger genetic heterogeneity to also contain flies with more variable syntax.
All lines, regardless of genetic heterogeneity, exhibit variable grooming (Supplementary Figure 14). To quantify variability, the variances of action transition probability distributions were calculated and compared. Only 6/252 (2.4%) transition probability distributions possessed statistically significantly different variances between MaxVar, Canton-S, and the isogenic lines out of all possible pairwise comparisons (Levene’s test, p < 05 after correction for multiple comparisons via Holm’s method). Moreover, none of these differences corresponded to within-motif transitions, indicating that variability of common transitions is similar regardless of genetic heterogeneity in a population. These findings also held for Oregon-R (8/252), Berlin-K (19/108), and w1118 (2/108) stock and isogenic comparisons. See Supplementary Figure 25 for all p values of pairwise action transition distribution variance comparisons.
Figure 3C provides the transition probability distributions for the most common posterior motif transition (abdomen grooming to back leg cleaning) for all stocks and stock-derived isogenic lines. This transition exhibits wide variability in many populations and even populations with smaller variability (CS iso 2) are not different enough to achieve statistical significance after accounting for multiple hypothesis testing.
We also examined stock lines derived from selected wild isolates (Mackay et al., 2012) to determine if these showed more or less grooming variability, as measured by syntax element variance values and Markov entropy. Their variability is comparable to that within lab stocks (Supplementary Figure 15).
Finally, we analyzed dust-induced grooming in 15 Canton-S flies that were assayed on three consecutive days. Since a given individual’s genome remains constant through the three trials, we could isolate the magnitude of grooming variability that is due to differences in sensory experience (since the dusting protocol does not allow for perfect replication of sensory experience) and life history (since flies will have been exposed to the same irritant several times by the end of the experiment). Ethograms from three example flies are provided in Figure 4A (full ethograms are shown in Supplementary Figure 16).
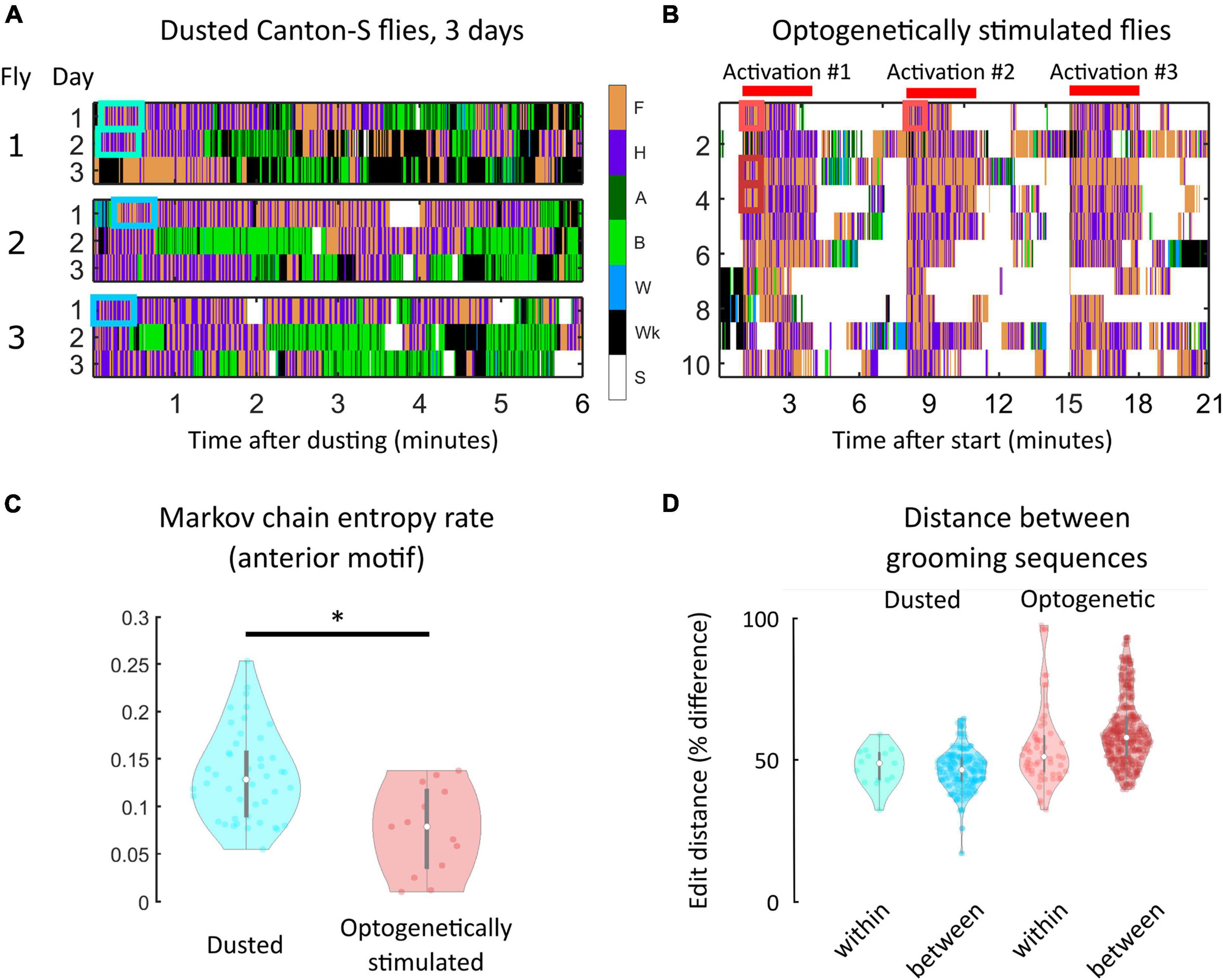
Figure 4. Within-individual grooming differences suggest that non-genetic factors account for a significant portion of variability in behavior. (A) Portions of ethograms from three Canton-S flies observed on consecutive days after dust irritant exposure. The differences in ethograms on consecutive days indicate that non-genetic factors must account for some amount of grooming variability. (B) Shown are ethograms of 10 Bristle-spGAL4-1 >CsChrimson flies (Zhang et al., 2020). Flies were optogenetically stimulated to induce anterior grooming in three separate 3-min windows, indicated by the red bars. Between these windows, flies still exhibit within-individual grooming variability even though the sensory experience is more uniform than repeated dust exposure. (C) Markov chain entropy, a measure of grooming stereotypy, was calculated from anterior grooming syntax. Optogenetically stimulated flies (right) exhibited lower entropies, corresponding to a higher degree of stereotypy, than dusted flies (left). However, optogenetically stimulated flies still exhibited differences in stereotypy between stimulation windows, implicating sources of grooming variability beyond genetic and sensory influences (Supplementary Figure 19). (D) To assess grooming stereotypy, edit distance between anterior motif repeats was computed. For dusted within-fly comparisons, we computed the edit distance between the first continuous anterior motif sequence lasting 30 s on consecutive days (light blue). For between-fly comparisons, we computed the edit distance between the first continuous anterior motif sequence lasting 30 s on the first day of experiments (dark blue). For all optogentically-stimulated flies, we computed two similar comparisons: within-session [i.e., comparing the sequences labeled “Activation #1” and “Activation #2” in panel (B); light red] and between-fly (i.e., “Activation #1” for each fly; dark red). For each comparison listed, the median edit distance computed corresponded to around 50% of the sequence length, demonstrating the low degree of stereotypy present in grooming sequences. *significantly different at p < 0.05.
Flies exhibited some longitudinal grooming trends, as the total amount of grooming decreased between the first and third days of the experiment. However, the time to completion of 50% of their total grooming did not decrease, suggesting that flies are not simply grooming quicker, but rather are grooming less consistently over time (i.e., punctuating grooming bouts with more walking and standing) (Supplementary Figure 17). Importantly, intra-individual variability in syntax across three sessions was of the same magnitude as inter-individual variation in syntax (Supplementary Figures 18, 19); that is, flies were no more similar to themselves over time than they were to other flies on a given day. This suggests that non-genetic factors account for a significant proportion of grooming variability.
Standardizing Sensory Experience Does Not Abolish Grooming Behavioral Variability
To probe the sensory contribution to within-individual variability, we used optogenetic stimulation to induce anterior grooming. 20 Bristle-spGAL4-1 > UAS-CsChrimson flies were tested (Zhang et al., 2020). Figure 4B provides ethograms from this experiment, with red bars indicating the three stimulation windows. Even when sensory experience was controlled in this way, flies exhibited variability in their grooming response.
Grooming stereotypy was again quantified using the entropy rate of the grooming syntax. The entropy rate for optogenetically-stimulated flies was lower than for dusted flies (p < 0.05, Wilcoxon rank-sum test), indicating a higher degree of stereotypy in grooming (Figure 4C). We nonetheless observed within-individual variability between stimulation windows, indicating that standardization of sensory input does not fully abolish grooming variability. Supplementary Figure 20 quantifies differences in entropy between sessions for three example flies. In addition, optogenetic stimulation resulted in strong anterior motif grooming behavior, rendering all flies’ transition probabilities very similar (Supplementary Figure 21).
Finally, grooming stereotypy was characterized using edit distance between anterior motif repeats (Figure 4D). This metric, used commonly in bioinformatics, describes the difference between two DNA sequences by calculating the minimum number of base pair substitutions, additions, or deletions that would be necessary for the sequences to be identical. Identical sequences would have an edit distance of zero between them, while maximally different sequences would have an edit distance equivalent to the total sequence length (see Supplementary Methods for details).
Since edit distance measures the similarity between two sequences (rather than the underlying rules that may generate the sequences), it provides a much stricter definition of stereotypy than Markov entropy, which we use as a measure of stereotypy earlier in our analysis. In addition, it is most useful as a stereotypy measure when it is possible to identify a synchronizing “start” signal between sequences of interest, which is not present in the previously described experiments, but is present for optogenetically-stimulated flies. Therefore, when comparing flies across recording sessions or sequences from different flies, the use of edit distance helps answer the specific question, “Do flies perform repeated, similar sequences or subsequences?”
For all dusted flies, we calculated the edit distance within flies across consecutive days to assess whether flies possess stereotyped repeats. For these comparisons, we compared the first continuous anterior motif sequence lasting at least 30 s on consecutive days, shown by the blue boxes in Figure 4A. This particular comparison was chosen to standardize the amount of dust present on the fly to the greatest extent possible and the short-term grooming history for each sequence, and to ensure that each sequence was long enough to exhibit stereotypy if it exists. Anterior motifs were chosen because they consist of only two actions with high transition probabilities between them, making these sequences the most likely candidates for exhibiting stereotypy. These comparisons yielded a minimum edit distance corresponding to a 39.6% difference between sequences. A similar calculation was made between flies, using the first continuous anterior motif sequence lasting at least 30 s on the first day of experiments. These comparisons yielded a minimum edit distance corresponding to a 41.6% difference between sequences.
Edit distance calculations were also performed for all optogenetically-stimulated flies. Within-fly comparisons (i.e., comparing the sequences labeled “Activation #1” and “Activation #2,” red boxes in Figure 4B) yielded a minimum edit distance corresponding to a 31.5% difference in sequences. Between-fly comparisons (i.e., “Activation #1” for each fly) yielded a minimum edit distance corresponding to a 42.8% difference in sequences (Figure 4D). Together, the low degree of stereotypy present in grooming sequences within and between both dusted and optogenetically-stimulated flies shows that grooming sequence variability is present even when genetics, sensory input, and behavioral history are controlled to the greatest extent possible within this experimental paradigm.
Discussion
Here, we analyzed fly grooming behavior in five different drosophilid species and four common melanogaster stocks to investigate the relationship between genetic heterogeneity and behavioral variability. Large genetic differences (species-level) correspond to identifiable differences in several grooming features, including the rules governing action transitions known as syntax. Within melanogaster, stock lines exhibited smaller variation in grooming syntax, as well as differences in overall activity levels. All flies showed variability in the details of the grooming movement sequence, but increased genetic heterogeneity did not correspond to increased behavioral variability. Analysis of 15 Canton-S flies recorded over consecutive days showed that intra-individual and inter-individual comparisons had similar—high—levels of variability. Optogenetically-stimulated flies also exhibited intra-individual variability in grooming behavior, but less. Taken together, these results demonstrate that large genetic differences result in distinguishable grooming phenotypes, but that genetic heterogeneity within a population does not necessarily correspond to an increase in the range of grooming behavior variability.
Genetic Influences on Behavioral Variation and Variability
Advantageous behavioral phenotypes that are under genetic control can be selected over evolution to produce populations with differing behaviors. Here, we identified significant inter-species variation in grooming syntax, suggesting a genetic basis for group differences in grooming behavior.
Some species differ from melanogaster in their propensity to perform anterior grooming actions; the anterior motif actions are significantly less strongly coupled in non-melanogaster drosophilids, suggesting that anterior neuronal circuitry or sensory physiology may differ. We also identified differences in grooming behavior between commonly used melanogaster stock lines and between male and female Canton-S flies; most of these differences relate to overall activity levels.
Variability itself is a trait that can also be selected for, but is often overlooked (Geiler-Samerotte et al., 2013). At the individual level, randomizing escape trajectories can be beneficial for escaping predators (Wang et al., 2020), and diversity in search paths can be useful when a group is foraging for food. The fate of the passenger pigeons, hunted to death while flocking together, illustrates the dangers of behavioral homogeneity (Murray et al., 2017). The degree of variability in behavior can be selected for as a bet-hedging strategy against unstable environmental conditions (Kain et al., 2015; Krams et al., 2021). Genetic factors contribute to variability in fly visual, olfactory, and locomotor behaviors (Ayroles et al., 2015; Honegger et al., 2019; Linneweber et al., 2020).
The prevalence of variability in Drosophila grooming action sequences suggests that non-stereotyped grooming may be advantageous, perhaps for removing diverse distributions or kinds of debris. We examined whether greater genetic heterogeneity within a population corresponded to greater behavioral variability but did not detect any significant impact.
A recent investigation of unstimulated behaviors in different Drosophila species detected differences in spontaneous grooming between species and among individuals within a species (Hernández et al., 2020). Using similar methods, they accurately assigned individuals into species categories and assessed variability among individuals. Our findings are complementary: drosophilid species show differences in stimulated grooming behaviors as well, suggesting genetic control, but individuals within a species show variability in grooming, indicating that factors other than genes can influence aspects of the behavioral sequence. Hernández et al. (2020) propose that over the long timescales measured in their assay, internal states may explain the observed fluctuation in action transition probabilities. In the shorter timescales we assayed, where flies are responding acutely to dust, we attribute the variability to inherent flexibility in the behavior itself, produced by differences in sensory input and/or intrinsic stochasticity in the neurons or circuits that coordinate the action sequences. These views are not in conflict and together establish that variability in grooming is widespread—potentially even advantageous—with both genetic and non-genetic factors influencing its expression.
Variability also encompasses individuality in animal behavior, typically defined as a trait-like feature that persists stably over several observations. Individuality has been identified in fruit fly turning (Buchanan et al., 2015), mouse roaming behavior (Freund et al., 2013), and bumblebee foraging (Klein et al., 2017), among others (Linneweber et al., 2020; Takagi and Benton, 2020). In both dust-induced and optogenetically-initiated grooming, we did not find evidence for individuality in action sequence patterns at the resolution we analyzed, but this may be because small contributions from individual tendencies are outweighed by the large amount of variability in the behavior as a whole arising from other causes.
Environmental and Stochastic Influences of Behavioral Variation and Variability
Our analysis of genetic contributions to behavioral variation and variability in the grooming suggests that at the species level, flies show significant differences in the grooming sequence, especially in the syntax of transition probabilities, that allow accurate classification. Differences in behavior between common lab wild-type stocks also support classification, but the accuracy is lower and the effect size of the differences is smaller.
Genetic factors have been implicated in spontaneous (i.e., unstimulated) grooming behavior in Drosophila melanogaster (Yanagawa et al., 2020) and in other drosophilid species (Hernández et al., 2020). Our results demonstrate that this is true for dust-induced grooming as well. Both spontaneous grooming (Hernández et al., 2020) and dust induced grooming show individual-to-individual variability within a species. The prevalence of sequence flexibility in all species and in controlled experimental conditions suggests that variability itself is a feature of grooming behavior, not a bug. Individuals with overly rigid grooming sequences might not respond as effectively to changing environmental conditions, such as different kinds of debris or the presence of a potential mate or predator.
The causes of grooming variability are still under investigation. Differences in developmental processes such as neural wiring or synaptic connectivity may contribute to behavioral differences between flies, but our experiments show that even individual flies exhibit variability in grooming over repeated trials with dust or optogenetic stimulation. This suggests that non-genetic factors such as sensory stimuli, internal state, previous experience, and circuit noise contribute to the variability we observe in grooming action sequences. The reduction of variability when sensory inputs are optogenetically controlled supports diversity of sensory stimulation as a contributor. The persistence of variability within individuals suggests that intrinsic stochasticity or noise within the neurons or circuits themselves may also play a role, which are possibilities which should be explored further.
Materials and Methods
Genetic Stocks
Canton-S, Oregon-R, Berlin-K, w1118, Bristle- spGAL4- 1 (R38B08- AD; R81E10- DBD), and 20XUAS- CsChrimson. mVenus (attp18) stocks were obtained from the Bloomington Stock Center. Isogenic (more accurately, reduced genetic variability) stocks were made by crossing single males to double-balanced stocks and then back-crossing males to the double balancer stock to isolate single second and third chromosomes. Single pairs were mated to reduce variability of X and IV. ∼ 2 independent isogenic lines from each melanogaster stock were generated; note that many attempts to isogenize result in lethality, as anecdotally reported by colleagues. Maximum Variability stocks were obtained by crossing each melanogaster strain to double balancers and then crossing the progeny together and selecting against the balancers. This allowed combination of chromosomes for all four strains. The progeny were allowed to interbreed for several generations to enable recombination in the females.
Drosophilid species stocks were obtained from Tom Turner, UCSB, and the National Drosophila Species Stock Center1.
Data Collection and Processing
Grooming was induced and analyzed as described in Seeds et al. (2014) and Zhang et al. (2020). Three chambers were used in fly dusting assay: dusting chamber (24 well Corning tissue culture plate #3524), transfer chamber and recording chamber. Recording chambers were coated with Insect-a-slip (BioQuip Products Cat #2871A) to discourage wall-climbing and cleaned daily. To control potential circadian effects during assays, trials containing flies of different genotypes were interleaved (allowing for near simultaneity of experiments), and assays were run at the same time each day. Dust-induced grooming assays were performed in 21–23°C. 4–7 day old male flies were anesthetized on ice and transferred to the middle four wells of the transfer chamber. Flies were left in the transfer chamber for 15 min to recover. Approximately 5 mg Reactive Yellow 86 dust (Organic Dyestuffs Corporation CAS 61951-86-8) was added into each of the 4 middle wells of dusting chamber. For fly dusting, the transfer chamber was aligned with the dusting chamber. Flies were tapped into the dusting chamber and shaken 10 times. After dusting, flies and dust were transferred back into the transfer chamber.
Transfer chamber was tapped against an empty pipette tip box to remove extra dust. Dusted flies were then immediately tapped into recording chamber for video recording. The entire dusting process was performed in a WS-6 downflow hood. Approximately 10 individuals were recorded for each genotype. 30 Hz videos were recorded for 50,000 frames (27.78 min) with a DALSA Falcon2 color 4 M camera. A white LED ring right was used for illumination.
Optogenetic stimulation protocol is replicated from Zhang et al. (2020). Further details can be found in the Supplementary Methods.
For each set of experimental comparisons (between species, within species, within individual), a single experimenter performed all dusting assays to eliminate experimenter-related differences that may arise. In total, 390 ethograms were recorded. This number includes species data (N = 83), melanogaster stocks and isogenic lines = 252), additional male/female Canton-S flies (N = 31), individual Canton-S flies followed for three sessions (N = 45), and optogenetically stimulated flies (N = 10).
Videos were processed through the Automated Behavior Recognition System [ABRS, Ravbar et al. (2019)], trained on a classifier using melanogaster flies to generate ethograms. Grooming actions were described previously (Seeds et al., 2014; Hampel et al., 2015). Sub-movements of the grooming actions used in this analysis have not yet been rigorously described and may occur on time scales faster than the 30 Hz recording setup can reliably capture, so they were not considered in this work.
Automated behavioral recognition system was used to generate ethograms. Briefly, the raw video frames were pre-processed to generate 3-channel spatiotemporal images (ST images). Features were extracted in three timescales and saved into different channels of ST images: 1. raw frame; 2. difference between two frames; 3. Spectral features extracted from a 0.5 s window. A convolutional network trained by ST images under different light conditions was then used to label the behavior identified in each frame. A different network was trained for classification of each species due to differences in body size and light conditions. All networks achieved >70% validation accuracy within the training protocol, which reserved 20% of frames as test data after training (see Supplementary Figure 22 for table of classifier performances).
Finally, ethograms were denoised to only include grooming actions that persisted for longer than the approximate duration of one complete leg sweep. Here, we used a cutoff of 150 ms, and eliminated any actions shorter than this duration (fewer than 1% of bouts were removed under this criterion).
Data Analysis
All ethogram features were extracted using custom-written code in MATLAB 2019a. Grooming progression vectors were generated for each fly by calculating the proportion of each action in 10 non-overlapping windows (2.78 min each), yielding a 70-dimensional vector for each fly (10 windows with 7 behavioral proportions). Grooming syntax was defined as the first-order transition probabilities between actions. Syntax for each fly was calculated as described in Mueller et al. (2019).
Bout duration distributions were generated as described in Mueller et al. (2019), using a normalized histogram with 20 bins of equal width for each behavior. Bin width was determined independently for grooming and non-grooming actions, as standing and walking exhibit longer tailed distributions than grooming actions. Thus, duration distribution vectors were 140-dimensional for each fly. Examples of progression, syntax, and duration distribution vectors can be found in the Supplementary Information.
Statistics for comparisons between grooming features were calculated using built-in MATLAB functions. t-SNE, and multinomial logistic regression classification analysis were performed using built-in MATLAB functions (Supplementary Information).
Data Availability Statement
The original data analyzed in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
Author Contributions
JS: experimental design. JS and NZ: data collection. JM: data analysis. JM and JS: manuscript writing. JM, JS, and JC: manuscript editing. JS and JC: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by HHMI Janelia transition funds (JS), NIH R01NS110866 (JS and NZ), the David and Lucile Packard Foundation, and the Institute for Collaborative Biotechnologies through Contract No.W911NF-09-D-0001 from the U.S. Army Research Office (JM and JC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We would like to thank Karen Hibbard, Barry Ganetzky, and Claire McKellar for consultations on isogenization strategies, Jordan Zawaydeh for collection of individual fly data, and Kristin Branson, Matthieu Louis, Roian Egnor, and Primoz Ravbar for advice and comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2021.769372/full#supplementary-material
Footnotes
References
Allen, A., Anreiter, I., Neville, M., and Sokolowski, M. (2017). Feeding-related traits are affected by dosage of the foraging gene in drosophila melanogaster. Genetics 205, 761–773. doi: 10.1534/genetics.116.197939
Anderson, D. (2016). Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci. 17, 692–704. doi: 10.1038/nrn.2016.125
Auer, T. O., Khallaf, M. A., Silbering, A. F., Zappia, G., Ellis, K., Álvarez-Ocaña, R., et al. (2020). Olfactory receptor and circuit evolution promote host specialization. Nature 579, 402–408. doi: 10.1038/s41586-020-2073-7
Ayroles, J., Buchanan, S., O’Leary, C., Skutt-Kakaria, K., Grenier, J., Clark, A., et al. (2015). Behavioral idiosyncracy reveals genetic control of phenotypic variability. PNAS 112, 6706–6711. doi: 10.1073/pnas.1503830112
Baker, B., Taylor, B., and Hall, J. (2001). Are complex behaviors specified by dedicated regulatory genes? reasoning from drosophila. Genetics 105, 13–24. doi: 10.1016/s0092-8674(01)00293-8
Bendesky, A., Kwon, Y., Lassance, J., Lewarch, C., Yao, S., Peterson, B., et al. (2017). The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439. doi: 10.1038/nature22074
Buchanan, S., Kain, J., and de Bivort, B. (2015). Neuronal control of locomotor handedness in drosophila. PNAS 112, 6700–6705. doi: 10.1073/pnas.1500804112
Datta, S., Anderson, D., Branson, K., Perona, P., and Leifer, A. (2019). Computational neuroethology: a call to action. Neuron 104, 11–24. doi: 10.1016/j.neuron.2019.09.038
Ding, Y., Lillvis, J., Cande, J., Berman, G., Arthur, B., Long, X., et al. (2019). Neural evolution of context-dependent fly song. Curr. Biol. 29, 1089–1099. doi: 10.1016/j.cub.2019.02.019
Fentress, J., and Stilwell, F. (1973). Grammar of a movement sequence in inbred mice. Nature 6, 52–53. doi: 10.1038/244052a0
Freund, J., Brandmaier, A., Lewejohann, L., Kirste, I., Kritzler, M., Kruger, A., et al. (2013). Emergence of individuality in genetically identical mice. Science 340, 756–759. doi: 10.1126/science.1235294
Gaertner, B., Ruedi, E., McCoy, L., Moore, J., Wolfner, M., and Mackay, T. (2015). Heritable variation in courtship patterns in drosophila melanogaster. G 3, 531–539. doi: 10.1534/g3.114.014811
Geiler-Samerotte, K., Bauer, C., Li, S., Ziv, N., Gresham, D., and Siegal, M. (2013). The details in the distributions: why and how to study phenotypic variability. Curr. Opin. Biotechnol. 24, 752–759. doi: 10.1016/j.copbio.2013.03.010
Geuther, B., Peer, A., He, H., Sabnis, G., Philip, V., and Kumar, V. (2021). Action detection using a neural network elucidates the genetics of mouse grooming behavior. Elife 10:e63207. doi: 10.7554/eLife.63207
Hampel, S., Franconville, R., Simpson, J., and Seeds, A. (2015). A neural command circuit for grooming movement control. Elife 7:e08758. doi: 10.7554/eLife.08758
Hernández, D., Rivera, C., Cande, J., Zhou, B., Stern, D., and Berman, G. (2020). A frame473 work for studying behavioral evolution by reconstructing ancestral repertoires. Biorxiv doi: 10.1101/2020.07.17.209361
Honegger, K., and de Bivort, B. (2018). Stochasticity, individuality and behavior. Curr. Biol. 28, R8–R12. doi: 10.1016/j.cub.2017.11.058
Honegger, K., Smith, M., Churgin, M., Turner, G., and de Bivort, B. (2019). Idiosyncratic neural coding and neuromodulation of olfactory individuality in drosophila. PNAS 117, 23292–23297. doi: 10.1073/pnas.1901623116
Johanssen, W. (2014). The genotype conception of heredity. Int. J. Epidemiol. 43, 989–1000. doi: 10.1093/ije/dyu063
Kain, J., Zhang, S., Akhund-Zade, J., Samuel, A., Klein, M., and de Bivort, B. (2015). Variability in thermal and phototactic preferences in drosophila may reflect an adaptive bet-hedging strategy. Evolution 69, 3171–85. doi: 10.1111/evo.12813
Kim, D., Alvarez, M., Lechuga, L., and Louis, M. (2017). Species-specific modulation of food-search behavior by respiration and chemosensation in drosophila larvae. Elife 6:e27057. doi: 10.7554/eLife.27057
Klein, S., Pasquaretta, C., Barron, A., Devaud, J., and Lihoreau, M. (2017). Inter-individual variability in the foraging behaviour of traplining bumblebees. Sci. Rep. 7:4561. doi: 10.1038/s41598-017-04919-8
Krams, I. A., Krama, T., Krams, R., Trakimas, G., Popovs, S., Jõers, P., et al. (2021). Serotoninergic modulation of phototactic variability underpins a bet-hedging strategy in Drosophila melanogaster. Front. Behav. Neurosci. 15:659331. doi: 10.3389/fnbeh.2021.659331
Linneweber, G. A., Andriatsilavo, M., Dutta, S. B., Bengochea, M., Hellbruegge, L., Liu, G., et al. (2020). A neurodevelopmental origin of behavioral individuality in the Drosophila visual system. Science 367, 1112–1119. doi: 10.1126/science.aaw7182
Mackay, T. F., Richards, S., Stone, E. A., Barbadilla, A., Ayroles, J. F., Zhu, D., et al. (2012). The drosophila melanogaster genetic reference panel. Nature 482, 173–178. doi: 10.1038/nature10811
Markow, T. (2019). Host use and host shifts in Drosophila. Curr. Opin. Insect Sci. 31, 139–145. doi: 10.1016/j.cois.2019.01.006
Mueller, J., Ravbar, P., Simpson, J., and Carlson, J. (2019). Drosophila melanogaster grooming possesses syntax with distinct rules at different temporal scales. PLoS Comp. Biol. 15:e1007105. doi: 10.1371/journal.pcbi.1007105
Murray, G. G. R., Soares, A. E. R., Novak, B. J., Schaefer, N. K., Cahill, J. A., Baker, A. J., et al. (2017). Natural selection shaped the rise and fall of passenger pigeon genomic diversity. Science 358, 951–954. doi: 10.1126/science.aao0960
Ravbar, P., Branson, K., and Simpson, J. (2019). An automatic behavior recognition system classifies animal behaviors using movements and their temporal context. J. Neuro. Methods 326:108352. doi: 10.1016/j.jneumeth.2019.108352
Seeds, A., Ravbar, P., Chung, P., Hampel, S., Midgley, F. Jr., Mensh, B., et al. (2014). A suppression hierarchy among competing motor programs drives sequential grooming in drosophila. Elife 3:e02951. doi: 10.7554/eLife.02951
Sokolowski, M. (2001). Drosophila: genetics meets behavior. Nat. Rev. Genet. 2, 879–890. doi: 10.1038/35098592
Spruijt, B., van Hooff, J., and Gispen, W. (1992). Ethology and neurobiology of grooming behavior. Physiol. Rev. 72, 825–852. doi: 10.1152/physrev.1992.72.3.825
Szebenyi, A. (1969). Cleaning behaviour in drosophila melanogaster. Anim. Behav. 17, 641–651. doi: 10.1016/s0003-3472(69)80006-0
Takagi, S., and Benton, R. (2020). Animal behavior: a neural basis of individuality. Curr. Biol. 30, R698–R720. doi: 10.1016/j.cub.2020.04.052
Wang, Y., Zhang, X., Xin, Q., Hung, W., Florman, J., Huo, J., et al. (2020). Flexible motor sequence generation during stereotyped escape responses. Elife 9:e56942. doi: 10.7554/eLife.56942
Wiltschko, A., Johnson, M., Iurilli, G., Peterson, R., Katon, J., Pashkovski, S., et al. (2015). Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135. doi: 10.1016/j.neuron.2015.11.031
Yanagawa, A., Huang, W., Yamamoto, A., Wada-Katsumata, A., Schal, C., and Mackay, T. (2020). Genetic basis of natural variation in spontaneous grooming in Drosophila melanogaster. G3 10, 3453–3460. doi: 10.1534/g3.120.401360
Zhang, N., Guo, L., and Simpson, J. (2020). Spatial comparisons of mechanosensory information govern the grooming sequence in drosophila. Curr. Biol. 30, 988–1001. doi: 10.1016/j.cub.2020.01.045
Keywords: Drosophila, variability, variation, neural circuits, motor sequence, behavior
Citation: Mueller JM, Zhang N, Carlson JM and Simpson JH (2022) Variation and Variability in Drosophila Grooming Behavior. Front. Behav. Neurosci. 15:769372. doi: 10.3389/fnbeh.2021.769372
Received: 01 September 2021; Accepted: 13 December 2021;
Published: 11 January 2022.
Edited by:
Benjamin L. De Bivort, Harvard University, United StatesReviewed by:
Sheyum Syed, University of Miami, United StatesTim Lebestky, Williams College, United States
Vivek Kumar, Jackson Laboratory, United States
Copyright © 2022 Mueller, Zhang, Carlson and Simpson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie H. Simpson, jhsimpson@ucsb.edu
 Joshua M. Mueller
Joshua M. Mueller Neil Zhang
Neil Zhang Jean M. Carlson
Jean M. Carlson Julie H. Simpson
Julie H. Simpson