Stress during adolescence increases novelty seeking and risk taking behavior in male and female rats
- 1 School of Biomedical Sciences, University of Nottingham, Nottingham, UK
- 2 Laboratory of Behavioral Genetics, Brain Mind Institute, École Polytechnique FÉdÉrale de Lausanne, Lausanne, Switzerland
Adolescence is a period of major physical, hormonal, and psychological change. It is also characterized by a significant increase in the incidence of psychopathologies and this increase is gender-specific. Likewise, stress during adolescence is associated with the development of psychiatric disorders later in life. Previously, using a rat model of psychogenic stress (exposure to predator odor followed by placement on an elevated platform) during the pre-pubertal period (postnatal days 28–30), we reported sex-specific effects on auditory and contextual fear conditioning. Here, we study the short-term impact of psychogenic stress before and during puberty (postnatal days 28–42) on behavior (novelty seeking, risk taking, anxiety, and depression) and hypothalamus–pituitary–adrenocortical (HPA) axis activation during late adolescence (postnatal days 45–51). Peri-pubertal stress decreased anxiety-like behavior and increased risk taking and novelty seeking behaviors during late adolescence (measured with the elevated plus maze, open field and exposure to novel object tests and intake of chocopop pellets before or immediate after stress). Finally neither depressive-like behavior (measured at the forced-swim test) nor HPA response to stress (blood corticosterone and glucose) were affected by peri-pubertal stress. Nevertheless, when controlling for the basal anxiety of the mothers, animals exposed to peri-pubertal stress showed a significant decrease in corticosterone levels immediate after an acute stressor. The results from this study suggest that exposure to mild stressors during the peri-pubertal period induces a broad spectrum of behavioral changes in late adolescence, which may exacerbate the independence-building behaviors naturally happening during this transitional period (increase in curiosity, sensation-seeking, and risk-taking behaviors).
Introduction
Adolescence is a critical developmental period characterized by change. Despite its gradual onset and offset there are major physical, hormonal, and behavioral differences between adolescence and childhood or adulthood. Adolescence is characterized by major developmental changes in the brain and the hypothalamic–pituitary–adrenal axis (HPA axis). The HPA axis matures before the brain pathways that regulate emotion, cognition, and learning (such as the prefrontal cortex, hippocampus, amygdala, and ventral striatum; Giedd et al., 1999; Sowell et al., 1999). Dynamic changes in stress-responsive regions are theorized to contribute to the unique behavior of adolescents (Cunningham et al., 2002; Casey et al., 2010).
This major biological transition is hypothesized to render the adolescent more vulnerable to stress and the development of psychopathologies. Stressful experiences during childhood and adolescence have been associated with the development of psychiatric disorders later in life (Kessler and Magee, 1993; Penza et al., 2003; Heim et al., 2004). Yet, only a percentage of the adolescents exposed to stress develop psychopathologies (Fergus and Zimmerman, 2005).
In the rat, adolescence is considered to last from either postnatal days 21–59 (Tirelli et al., 2003) or from postnatal days 28 till 42 (Spear and Brake, 1983; Spear, 2000). Recently, there has been an increase in the number of rat models developed to study the short and long-term effects of stress during adolescence. These animal models use various stressors ranging from purely social stress to exclusively physical stress (Table 1). While the majority of these models focus on the rat, a few have recently used mice (Ito et al., 2010; Schmidt et al., 2010a,b; Sterlemann et al., 2010; Peleg-Raibstein and Feldon, 2011). Stressors in these models are applied either before puberty, around puberty, after puberty, or during the entire adolescence (Table 1). Importantly, despite the original focus on males, inclusion of male and females in studies is progressively increasing investigation into the effects of peri-pubertal stress are sex specific (Table 1).
Until now most studies focused on the long-term effects of peri-adolescent stress. They investigated the impact on adult behavior, endocrine responses, gene expression, neuronal, and brain morphology, cell proliferation in the dentate gyrus and drug sensitization (Table 1). While some recent studies have investigated the short-term effects of stress during adolescence (Table 1), there is no sufficient information about the short-term effects of stress on risk taking and novelty seeking behaviors, and whether there are gender differences on those behaviors. Previously, using a rat model of psychogenic stress during the pre-pubertal period, we reported sex-specific effects on auditory and contextual fear conditioning (Toledo-Rodriguez and Sandi, 2007). In the current study, we aimed to investigate the impact of psychogenic stress experienced during the peri-pubertal period, on the behavior (novelty seeking, risk taking, anxiety, and depression) and HPA axis activation displayed by animals during late adolescence. Additionally, we explored whether the anxiety trait of the parents had any influence on the offspring’s reactivity to stress. For this purpose, we modified our rat model of psychogenic stress prolonging the stress period to last the entire male and female peri-pubertal phase (from 28 to 42 days of age). The stress days were chosen to cover pre-puberty (28–30 days old), and puberty (34 and 36 days old for the female, Lewis et al., 2002; and 40 and 42 days old for the male, Lewis et al., 2002; Fernandez-Fernandez et al., 2005). After 2 days recovery from the peri-pubertal stress protocol, control, and stressed animals were submitted to a battery of behavioral tests and hormonal analyses. We focused on risk taking, basal anxiety, novelty seeking, and depressive-like behaviors. Hormonal assessments included plasma corticosterone and glucose levels immediately after exposure to a mild acute stressor.
Materials and Methods
Subjects
Subjects were the offspring of rats purchased from Charles River Laboratories, France. Fifteen male and 18 female Wistar Han rats from different liters were weaned at postnatal day 21. To avoid litter effects rats from different litters were mixed, placing equivalent numbers of animals from each litter in the stress and control groups. Rats were housed in same sex cages (three or four per cage) in standard plastic cages on a 12-h light-dark cycle (light on at 7:00 AM). Food and water were available ad libitum. All the procedures described were conducted in conformity with the Swiss National Institutional Guidelines on Animal Experimentation, and approved by the Swiss Cantonal Veterinary Office Committee for Animal Experimentation.
Peri-Pubertal Stress Procedure
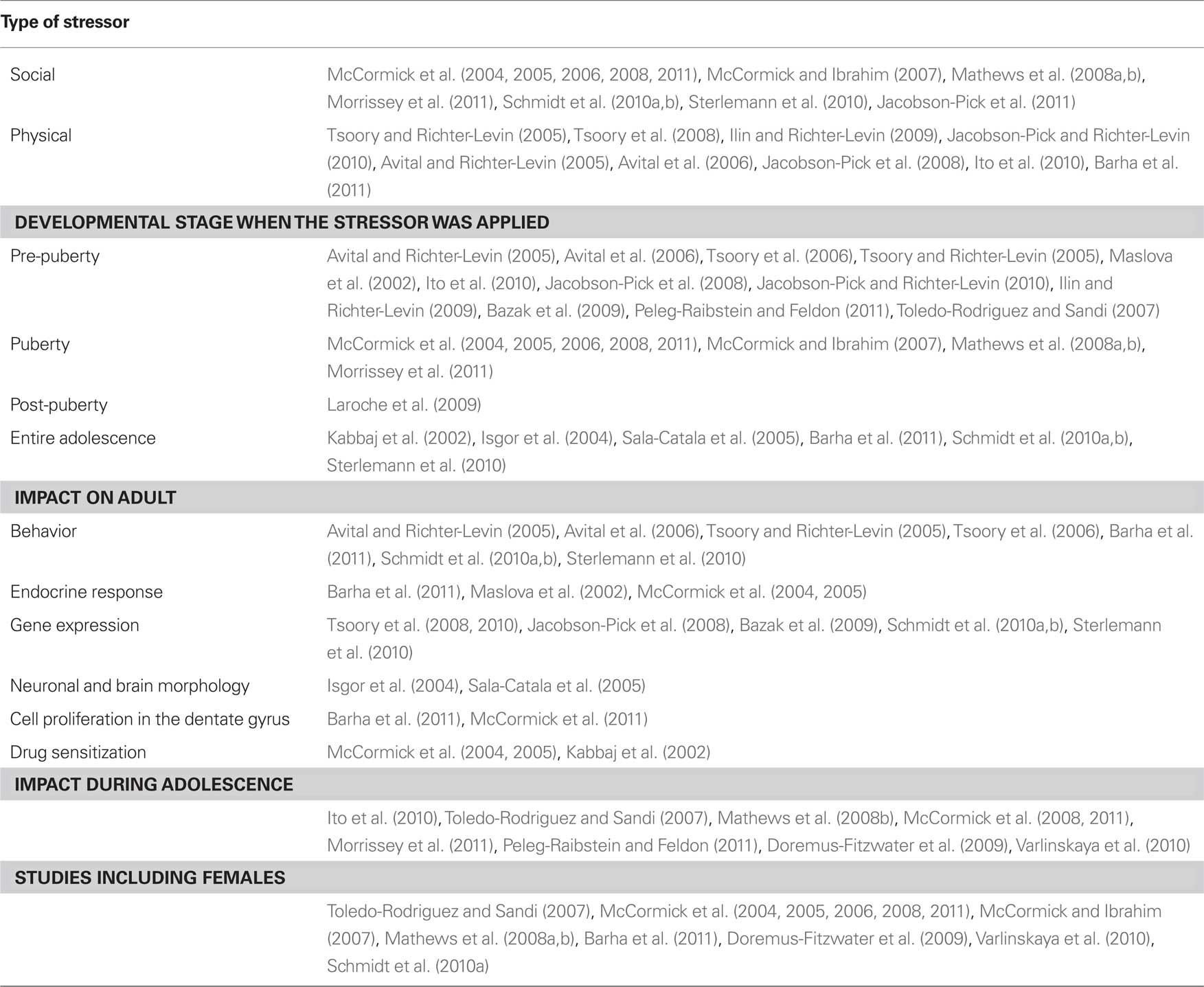
The stress protocol used was a longer version of the one previously developed in our laboratory (Toledo-Rodriguez and Sandi, 2007). Animals underwent stress during postnatal days 28–30 (three consecutive days pre-puberty), 34, 36 (female puberty), 40, and 42 (male puberty). During the days of stress animals were exposed to one or two consecutive stressors (see Figure 1). The stressors were: (a) placement for 5 min on a novel location (rectangular box) for 5 min (only the first day of stress); (b) exposure to trimethylthiazoline (TMT) odor (Phero Tech Inc., Delta, BC, Canada) during 25 min in a closed box (38 cm × 27.5 cm × 31 cm dimensions) under bright light (200–250 lx) containing a cloth impregnated with 10 mg TMT or (c) placement on an elevated platform (of 12 cm × 12 cm located 94.5 cm from the ground) during 25 min under direct bright light (500–550 lx). After stress animals were placed for 15 min in a clean cage without sawdust fitted with perforated perpex partitions. In this way animals could see hear and smell, but not touch each other. The timing of the stress was random (unpredictable for the animal) but always during the light period. See Figure 1 for a schematic representation of the stress protocol and behavioral tests’ schedule. During the days of stress, control rats were manipulated for 2 min by the experimenter.
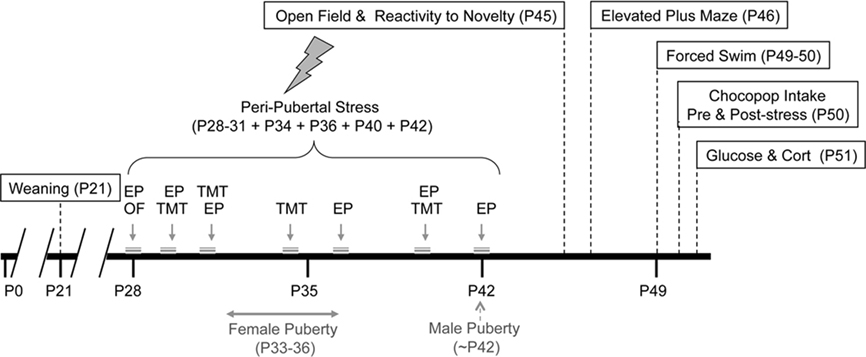
Figure 1. Stress and behavioral test procedures employed in this study. P, indicates the postnatal day; EP, Elevated Platform; OF, Open Field, TMT, exposure to trimethylthiazoline odor.
Activity Box (Open Field) and Reactivity to Novelty (Novel Object)
After the stress period all rats (stressed and control) were handled during 2 min daily for 2 days before testing their spontaneous locomotor activity and reactivity to novelty in an activity box (at 45 days of age). The activity box consisted in a rectangular arena (of 37 cm × 57 cm) divided in nine zones of identical size. The test was started by placing the animals in the center of the arena and consisted in two blocks of 10 and 5 min. During the first 10 min the animal’s reactivity to a novel location was measured. Afterward a novel object (dark glass bottle) was placed at the center of the arena and the reactivity of the animal toward the object was measured. The locomotor activity was monitored by a video camera, mounted on the ceiling and a computerized tracking system (Ethovision 1.90, Noldus IT, Wageningen, The Netherlands) recorded the total distance moved, speed, percentage of time spent in each zone, and latency to enter the center. An observer (blind to the animal’s experimental condition) scored the animal’s sniffing and rearing onto the object. The floor of the arena and the object were washed after each test with 0.1% acetic acid solution to remove odors left by previous subjects. The test was performed simultaneously to all the animals in the cage by using three or four adjacent arenas.
Elevated Plus Maze
Twenty-four hours after the activity box and reactivity to novelty tests, anxiety-like behavior of stressed and control rats was evaluated using the elevated plus maze (EPM) test. The EPM consisted of two opposing open arms (45 cm × 10 cm) and two closed arms (45 cm × 10 cm × 50 cm) that extended from a central platform (10 cm × 10 cm) elevated 65 cm above the floor. Rats were placed individually on the central platform facing a closed arm and were allowed to freely explore the maze for 5 min. The behavior of each rat was monitored using a video camera and the movement of the rats automatically registered and analyzed with a computerized tracking system (Ethovision 1.90, Noldus IT, The Netherlands). Entry into an arm was defined as entry of all four paws into the arm. Total distance moved, speed, time spent in the open and closed arms, number of times the animal entered each type of arm, latency before entering an open arm and number of defecations were measured. An observer (blind to the animal’s experimental condition) scored the animal’s grooming, stretching, and rearing behaviors as well as the exploration outside the maze (head-dipping). The floor of the EPM was washed after each testing with 0.1% acetic acid solution to remove odors left by previous subjects.
The parents of the experimental animals used in this study were also assessed for their anxiety-like behavior in the EPM test before breeding took place.
Chocopop Pellet Intake
On postnatal days 45–49, animals were habituated to eat Chocopop flakes (Kellogg’s, Switzerland) by feeding four flakes per animal in their homecages daily. Afterward two test of food intake were performed:
Chocopop intake in basal conditions. On the afternoon of postnatal day 50 (and at least 3 h before the forced-swim test) animals were placed in a clean cage without sawdust fitted with perforated perpex partitions. On the floor of each partition there were 10 Chocopop flakes. Animals were allowed to explore freely the partition and eat the flakes for 2 min.
Chocopop intake immediate after Forced-Swim stress. Immediate after forced-swim test (postnatal day 50, see below) animals were placed in a clean cage without sawdust fitted with perforated perpex partitions with 10 Chocopop flakes on the floor. Animals were allowed to eat the flakes for 2 min.
In all cases the number of flakes eaten by the animal were measured. The floor and walls of the arena were washed after each testing with 0.1% EtOH solution to remove odors left by previous subjects. The test was performed simultaneously to all the animals in the cage by using three or four adjacent arenas.
Forced Swim
To evaluate depression-like behavior animals underwent forced-swim test as previously described (Toledo-Rodriguez and Sandi, 2007). In brief, animals were individually placed in plastic cylinders (25 cm diameter, 46 cm deep) filled with 30 cm of water (25 ± 1°C). The session’s duration was 15 min for the training and 5 min for the test. The test session was performed 24 h after training. Behavior was recorded with a video camera and the time spent immobile (making only those movements necessary to keep the snout above the water), swimming, climbing, or diving was quantified manually by an observer blind to the animal’s experimental condition. Tests were performed simultaneously to all the animals in the cage by using three or four adjacent cylinders.
Plasma Corticosterone and Glucose Measurement
On postnatal day 51 rats were placed individually in cylinders (25 cm diameter) for 30 min. Immediately after, rats were decapitated and their trunk blood collected in heparinized tubes. Plasma corticosterone and glucose levels were measured using an enzyme immunoassay kit (Correlate-EIA from Assay Designs Inc., USA) and (RTU BioMerieux ref 61269, BioMerieux (Suisse SA) respectively.
Statistics
All data from the adolescent animals was analyzed using two-way ANOVA. When the sex × treatment interaction was significant, Student’s t-tests were performed as post hoc analyses. ANCOVA analysis was employed to study the impact of the covariate “parental basal anxiety” (measured as time spent in the open arms of the EPM by either the mothers or the fathers before breeding took place) on the levels of corticosterone of their offspring during testing. PASW Statistics 18.0 statistical package was used for the analyses. Data represent the mean ± SEM. Significance of results was accepted at p < 0.05.
Results
Spontaneous Locomotor Activity (Open Field Test)
First, we studied whether peri-pubertal stress affected the spontaneous locomotor activity of adolescent animals. Two days after the end of the stress period (i.e., 45 days of age) the basal locomotor activity and exploratory behavior of stressed and control animals were measured. Stressed animals showed significantly longer latency to reach the center of the arena [F (1,29) = 6.553, p = 0.016; Figure 2A] but did not differ from control animals in the time spent at the center of the arena [F (1,29) = 1.049, p = 0.314]. Additionally, two-way ANOVA revealed that, while there was not a treatment or gender effect, there was a significant treatment × sex interaction in the distance moved at the corners of the arena [F (1,29) = 6.123, p = 0.019]. Post hoc comparisons indicate that in the control group females walked longer distances [t (13) = −2.89, p = 0.013 (Figure 2B). No sex differences were observed in this test.
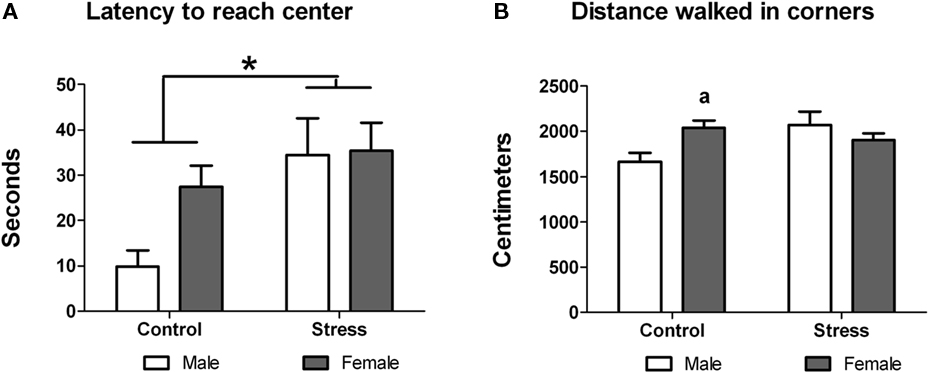
Figure 2. Effect of peri-pubertal stress on behavior at the open field test. Mean (SEM) behavioral measures in the open field test for male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control). (A) Latency to reach center and (B) distance walked in corners. *p < 0.05 treatment effect; a p < 0.05 post hoc comparison between control male and females.
Reactivity to Novelty (Novel Object)
Next we examined whether peri-pubertal stress influenced the reactivity to explore a novel object. Stress during adolescence had a significant effect on the exploratory behavior of the animals. Stressed animals showed significant lower latency to approach the object [F (1,29) = 5.75, p = 0.023; Figure 3A), spent more time sniffing the object [F (1,29) = 8.701, p = 0.006; Figure 3B) and sniffed the object more times [F (1,29) = 4.961, p = 0.034; Figure 3C) than controls. No sex or treatment differences were found in any for these parameters. However, a significant treatment × sex interaction was observed in the number of times the animals reared on the object [F (1,29) = 10.46, p = 0.003]. Post hoc comparisons indicated that within the control group males reared more times on the object [t (13) = 3.80, p = 0.002] while with females peri-pubertal stress lead to a significant increase in the number of rearings on the object [t (16) = −3.15 (two-tailed), p = 0.006; Figure 3D). No sex differences were observed in this test.
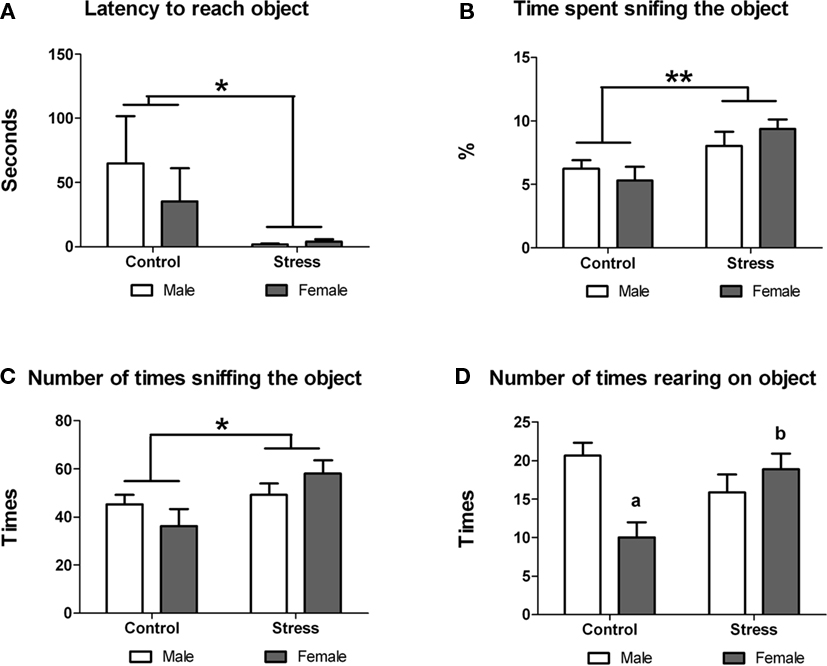
Figure 3. Effect of peri-pubertal stress on behavior at the novel object test. Mean (SEM) behavioral measures of reactivity to novelty (novel object test) for male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control). (A) Latency to reach object, (B) time spent sniffing the object, (C) number of times sniffing the object, and (D) number of times rearing on the object. *p < 0.05 treatment effect; **p < 0.01 treatment effect; a p < 0.01 post hoc comparison between control male and females; b p < 0.01 post hoc comparison between stressed and control females.
Anxiety-Like Behavior (EMP)
On postnatal day 46, animals underwent the EMP test to measure their anxiety-like levels. Two-way ANOVA revealed that stress during adolescence had a significant effect on anxiety-like behavior in the adolescent rat. Stressed animals: (a) spent more time in the open arms [F (1,29) = 5.73, p = 0.023; Figure 4A]; (b) entered more times the open arms [F (1,29) = 6.81, p = 0.014; Figure 4B] and center [F (1,29) = 6.00, p = 0.021]; (c) walked longer distances at the EPM [F (1,29) = 7.69, p = 0.010; Figure 4C]; and (d) had a faster mean velocity [F (1,29) = 7.51, p = 0.010; Figure 4D]. Moreover, stress during adolescence had also a significant effect on the behaviors displayed by the adolescent rats at the EPM. Stressed animals: (a) reared more times at the EPM [F (1,29) = 13.53, p < 0.001; Figure 4F] and (b) spent more time head-dipping at the EPM [F (1,29) = 5.03, p = 0.033; Figure 4G]. A two-way ANOVA also revealed a significant sex effect. Female rats: (a) entered more times into the open arms [F (1,29) = 5.56, p = 0.025; Figure 4B] and center [F (1,29) = 4.89, p = 0.035]; (b) spent more time rearing [F (1,29) = 9.39, p = 0.005; Figure 4E]; (c) reared more times at the EPM [F (1,29) = 19.33, p < 0.000; Figure 4F]; (d) spent more time head-dipping at the EPM [F (1,29) = 6.80, p = 0.014; Figure 4G]; and (e) performed head-dipping more frequently [F (1,29) = 4.81, p = 0.036; Figure 4H]. No treatment × sex interactions were found.
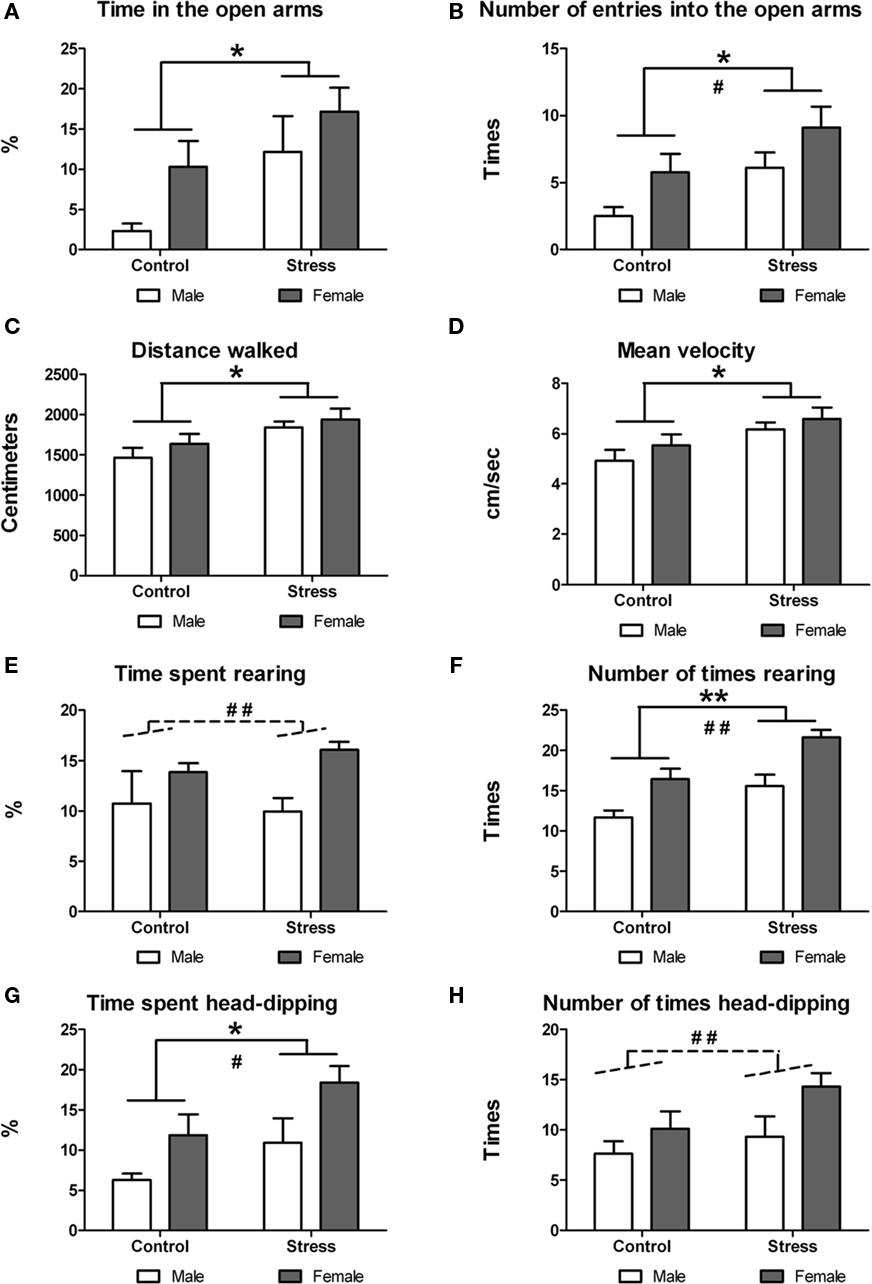
Figure 4. Effect of peri-pubertal stress on behavior at the elevated plus maze (EPM) test. Mean (SEM) behavioral measures in the EPM test for male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control). (A) Time in the open arms, (B) number of entries into the open arms, (C) distance walked, (D) mean velocity, (E) time spent rearing, (F) number of times rearing, (G) time spent head-dipping, and (H) number of times head-dipping. *p < 0.05 treatment effect; **p < 0.01 treatment effect, #p < 0.05 sex effect, ##p < 0.01 sex effect.
Depressive-Like Behavior (Forced-Swim Test)
On postnatal day 50 peri-pubertal stressed and control animals underwent forced-swim test (training took place 24 h before). Exposure to peri-pubertal stress did not affect swimming behavior as revealed by two-way ANOVA performed on the percentage of time spent floating [treatment effect, F (1,29) = 2.14, p = 0.154; treatment × sex interaction F (1,29) = 0.445, p = 0.510; Figure 5A]. There was a significant sex effect on the number of times the animals floated during the test [F (1,29) = 9.774, p = 0.004; Figure 5B], with females showing more floating episodes than males, though there was neither a treatment effect, nor a treatment × sex interaction.
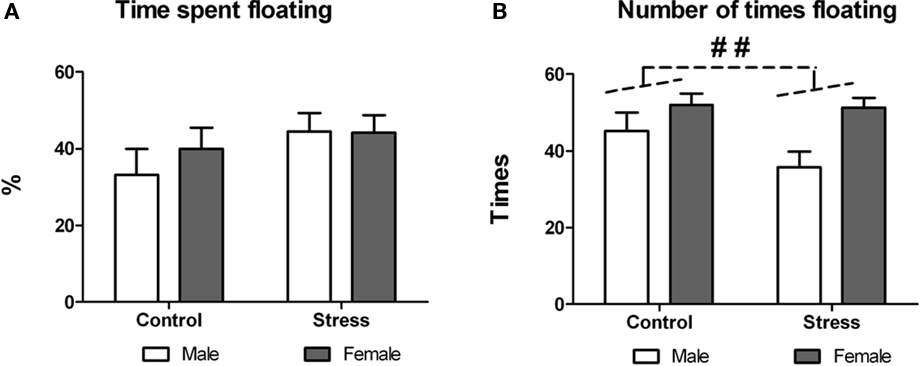
Figure 5. Effect of peri-pubertal stress on behavior at the forced-swim test. Mean (SEM) behavioral measures in the forced-swim test for male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control). (A) Time spent floating and (B) number of times floating. ##p < 0.01 sex effect.
Low Novelty Reactivity Under Basal and Stressful Conditions (Chocopop Intake)
In this test, performed on postnatal day 50, each rat was allowed 2 min to eat 10 chocopop pellets. Statistical analysis showed significant treatment effect with stressed animals eating significantly more chocopop pellets [F (1,29) = 7.57, p = 0.010; Figure 6A]. Next, we studied whether an additional acute stress (forced swim) affected chocopop intake during 2 min. For this purpose we measured the number of chocopops eaten by each animal immediately after the forced-swim test. Two-way ANOVA revealed that despite the acute stress, animals than underwent peri-pubertal stress ate significantly more chocopop pellets [F (1,29) = 7.95, p = 0.009; Figure 6B]. No sex differences or treatment × sex interaction were observed in both tests.
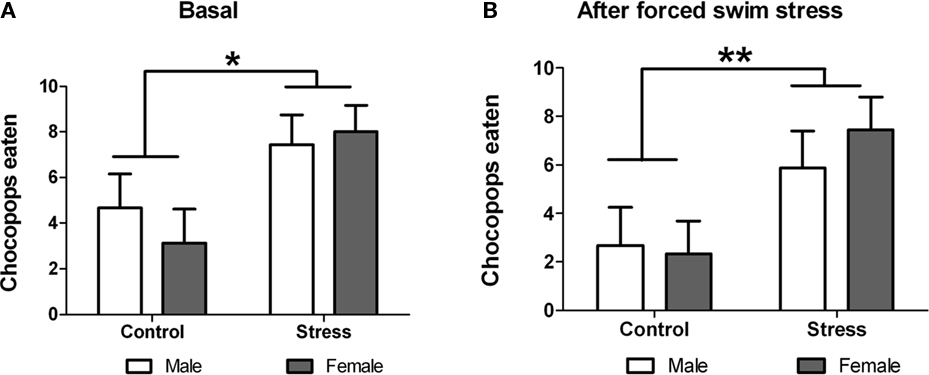
Figure 6. Effect of peri-pubertal stress on behavior at the Chocopop pellet test. Mean (SEM) number of Chocopop pellets eaten by male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control) in their home cage (A) or immediately after stress (B). *p < 0.05 treatment effect; **p < 0.01 treatment effect.
Plasma Glucose and Corticosterone Levels
On postnatal day 51, animals were exposed to a novel environment for 30 min and immediate afterward blood samples were taken to assess plasma corticosterone and glucose levels. Two-way ANOVA analysis revealed that while peri-pubertal stress did not have any effect on glucose levels [F (1,29) = 0.29, p = 0.5935; Figure 7A], it induced a mild but not significant decrease in corticosterone levels [F (1,28) = 3.31, p = 0.080; Figure 7B]. No sex differences or treatment × sex interaction were observed in both tests.
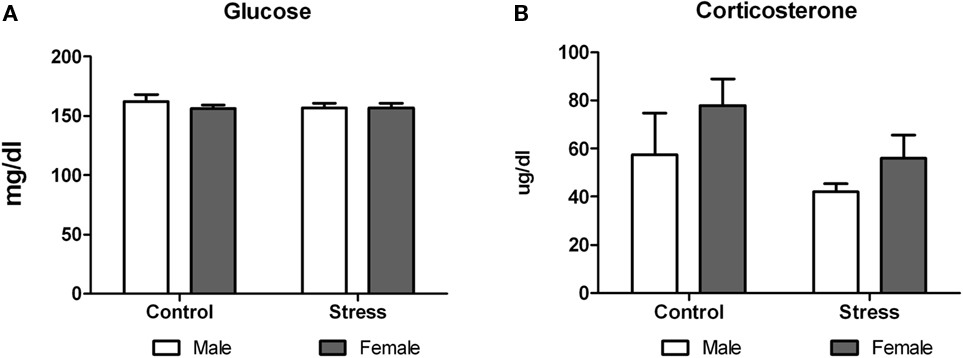
Figure 7. Effect of peri-pubertal stress on blood glucose and corticosterone. Mean (SEM) plasma corticosterone (A) and glucose (B) concentrations immediately after being at a novel location (acute stress) for male and female rats that underwent either peri-pubertal stress (Stress) or no stress (Control).
Influence of Parental Anxiety on Adolescent Pups Behavior and Reactivity to Stress
Finally, we evaluated whether the behavior and reactivity to stress of the adolescent rats was influence by the parental anxiety-like traits. For this purpose, we repeated the previous analyses including the covariate “basal anxiety of either the mother of the father before breeding” (measured as the time spent in the open arms of the EPM; see Materials and Methods).
ANCOVA analysis revealed that the covariate “basal anxiety of the mothers before breeding” was significantly related only to the offspring’s latency to approach the object [F (1,28) = 4.85, p = 0.005, r = 0.38] and blood corticosterone levels [F (1,27) = 9.20, p = 0.005, r = 0.50]. Interestingly, the effect of peri-pubertal stress on corticosterone levels became significant after controlling for the mothers’ basal trait anxiety [F (1,27) = 5.06, p = 0.033]. Controlling for the mothers’ basal trait anxiety did not change the significance of the effect of stress on latency to approach the object [F (1,28) = 6.60, p = 0.016].
The covariate “basal anxiety of the fathers before breeding” was marginally but not significantly related to the offspring’s blood corticosterone levels [F (1,27) = 3.45, p = 0.074, r = 0.37] and significantly related to the offspring’s’ distance moved at the center and corners of the open field [F (1,28) = 4.34, p = 0.046, r = 0.37] and [F (1,28) = 6.40, p = 0.017, r = 0.43] respectively. Controlling for the fathers’ basal trait anxiety did not change the significance of stress on offspring behavior (i.e., non-significant effect for distance walked in the center [F (1,28) = 0.64, p = 0.429] and significant sex × treatment interaction of the distance walked in the corners [F (1,28) = 6.50, p = 0.017]).
Discussion
The present study investigated the behavioral and hormonal effects of psychogenic stress (exposure to predator odor and placement on an elevated platform) during the peri-pubertal period in adolescent male and female rats. Our findings indicate that peri-pubertal stress reduces anxiety-like behaviors without affecting depressive-like behavior in late adolescence. Stressed animals exhibited increases in: (a) risk-taking behaviors (measured as time spent in the open arms of the EPM, time spent head-dipping, and number of entries to the EPM open arms) and (b) novelty seeking behaviors (measured as latency to reach a novel object, distance walked at the EPM, frequency, and time spent sniffing the object and number of chocopop pellets eaten under basal or stress conditions). Yet, stressed animals did not differ from controls in: (a) time spent floating during the forced-swim test or (b) blood corticosterone and glucose levels immediate after an acute stress.
At first glance our results may seem paradoxical when compared with previous studies reporting an increase of anxiety-like behaviors in rats exposed to stress during adolescence (Avital et al., 2006; Tsoory et al., 2006; Ilin and Richter-Levin, 2009). However, this contradiction can be explained by the fact that most these studies investigated behavior in adulthood. Indeed recent reports studying the effects of stress on behavior during adolescence show a decrease in anxiety-like and in risk-taking behaviors (i.e., increased time spent in the open arms of the EPM and increased locomotor activity in the open field and EPM; McCormick et al., 2008; Burke et al., 2010; Ito et al., 2010; Jacobson-Pick and Richter-Levin, 2010; Jacobson-Pick et al., 2011; Peleg-Raibstein and Feldon, 2011).
The increase in risk-taking behaviors in animals that underwent stress during adolescence seems to be restricted to the adolescent period. In fact, many of the above cited studies reported that the decrease in anxiety-like behaviors following adolescent stress disappeared when animals were tested in adulthood, both for males (Peleg-Raibstein and Feldon, 2011; Jacobson-Pick et al., 2011; and females, McCormick et al., 2008; Jacobson-Pick et al., 2011). Risk taking and novelty seeking behaviors emerge around puberty and are considered by many the hallmarks of adolescent behavior (Spear, 2000; Macri et al., 2002; Dahl, 2004; Kelley et al., 2004). Our results suggest that exposure to mild psychogenic stress before and during puberty exaggerates the independence-building behaviors naturally happening during this transition period (Macri et al., 2002).
What could be the implications of the increase in novelty seeking and risk-taking behaviors resulting from exposure to stress during adolescence? Early life stress has been hypothesized to lead to an increase in vulnerability to develop psychopathologies later in life (Kessler and Magee, 1993; Penza et al., 2003; Heim et al., 2004; Avital and Richter-Levin, 2005; Tsoory et al., 2006; Mathews et al., 2008b). Excessive novelty seeking and risk taking are characteristics of sensation-seeking behavior. Sensation-seeking behavior has been correlated with drug experimentation and recklessness (Laviola et al., 2003). Interestingly, chronic stress during adolescence was shown to enhance locomotor sensitization to amphetamine (Mathews et al., 2008a) or cocaine (Lepsch et al., 2005) in both male and female rats tested days after the stress period.
At this stage, it is not possible to indicate what is the predictive value of the stress-induced changes in behavioral reactivity for maladaptive behavior later in life. Further studies are required in order to determine how these alterations relate to behavioral responses in adulthood.
The use of males and female rats in our study enabled us to investigate whether the effects of peri-pubertal stress were sex specific. We found a significant interaction between treatment and sex factors in some parameters of the open field and novel object tests, but not in the standard variables from these tests (such as those related to visits to the center of the arena or to the exploration of the novel object). Interestingly, there was a significant sex difference in the behavior at the EPM with females showing increased risk-taking behaviors. So far there are only a handful of studies investigating the effects of stress during adolescence on behavior at the EPM in males and females. The results from these studies are not consistent. Exposure to chronic social stress during adolescence was shown to result in only females displaying higher risk-taking behaviors at the EPM, when tested in late adolescence (McCormick et al., 2008). Nevertheless, a recent study reports no sex differences in the behavior at the EPM of adolescent rats that did not undergo stress (Lynn and Brown, 2010), while other study reports only treatment effect on rats stressed and tested before puberty (Jacobson-Pick and Richter-Levin, 2010). Currently we cannot identify the source of such discrepancy on the effects of adolescent stress on males and females. Yet we can only speculate that it may be due to either the stage of sexual maturation of the animals during stress and test (Spear, 2000) or to differences in the nature of the stressors and/or stress protocols.
In addition, we found that the basal anxiety of the mothers was significantly related to the offspring’s blood corticosterone levels after acute stress. Future experiments should address the molecular and/or behavioral mechanisms underlying this link between maternal anxiety and the offspring’s HPA reactivity after adolescent stress.
One should be cautious when comparing the results of the present study with human adolescence, since brain maturation in rodents and humans follows different developmental time patterns. For example, while the rodent hippocampus continues to develop well into adolescence (Meyer et al., 1978), the human hippocampus is fully developed by 2 years old (Lupien et al., 2009).
It should be noted that a shortcoming in the present study is the fact that only one measure of the HPA axis and glucose responses to acute stress was performed, with basal levels not being included. Given that corticosterone (McCormick et al., 2005) and glucose (Bialik et al., 1988, 1989) show dynamic changes that can last up to 2 h post stress, a single datapoint only provides partial information on the HPA axis response.
Adolescence, and in particular the peri-pubertal period, is a critical developmental period when major changes take place in the brain (i.e., synapse pruning, fiber sprouting, and myelination; Giedd et al., 1999; Cunningham et al., 2002). It has been hypothesized that the different speeds in maturation of brain areas involved in emotional regulation and cognitive function may be partly responsible for the sharp increase in risk taking and novelty seeking observed during adolescence (Rosenberg and Lewis, 1995; Cunningham et al., 2002; Erickson and Lewis, 2002; Cruz et al., 2003). Due to incomplete maturation of the HPA negative-feedback system, adolescent rats show prolonged activation of the HPA axis when exposed to stress (Romeo et al., 2004), which might be implicated in the observed changes in curiosity and risk-taking behaviors observed in the stressed rats.
Juvenile stress has been reported to delay the maturational changes in expression of GABAa receptor subunits in the amygdala (Jacobson-Pick et al., 2008) and of the cell adhesion molecules PSA-NCAM (Tsoory et al., 2008) and L1 (Tsoory et al., 2010) in the basolateral amygdala and hippocampus. Future studies should address the cellular and molecular changes induced by the stress protocol used in our study and explore their potential impact on later life behaviors.
In conclusion, this set of experiments shows that exposure to stress during the peri-pubertal period results in an increase of novelty seeking and risk-taking behavior in adolescent male and female rats. These data suggest that peri-pubertal stress exacerbates the independence-building behavior characteristic of this transitional period. This behavioral change may reflect alterations in the plastic maturational changes undergone by brain regions regulating emotionality and stress responsiveness during this developmental stage.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Coralie Siegmund and Clara Rosetti for their invaluable experimental assistance, Dr. M. Isabel Cordero and Dr. Cristina Marquez for her help with the behavioral analyses and Prof. Charles Marsden for his useful comments on the manuscript. This work was supported by the Roche Research Foundation, and grants from the EU 7th Framework Program (MemStick), the Swiss National Science Foundation (310000-120791; Sinergia CRSIK0-122697 and CRSIK0-122691).
References
Avital, A., Ram, E., Maayan, R., Weizman, A., and Richter-Levin, G. (2006). Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res. Bull. 68, 419–424.
Avital, A., and Richter-Levin, G. (2005). Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 8, 163–173.
Barha, C. K., Brummelte, S., Lieblich, S. E., and Galea, L. A. (2011). Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. doi: 10.1002/hipo.20829. [Epub ahead of print].
Bazak, N., Kozlovsky, N., Kaplan, Z., Matar, M., Golan, H., Zohar, J., Richter-Levin, G., and Cohen, H. (2009). Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology 34, 844–858.
Bialik, R. J., Smythe, J. W., and Roberts, D. C. (1988). Alpha 2-adrenergic receptors mediate the increase in blood glucose levels induced by epinephrine and brief footshock stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 12, 307–314.
Bialik, R. J., Smythe, J. W., Sardelis, M., and Roberts, D. C. (1989). Adrenal demedullation blocks and brain norepinephrine depletion potentiates the hyperglycemic response to a variety of stressors. Brain Res. 502, 88–98.
Burke, A. R., Renner, K. J., Forster, G. L., and Watt, M. J. (2010). Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res. 1352, 147–156.
Casey, B. J., Jones, R. M., Levita, L., Libby, V., Pattwell, S. S., Ruberry, E. J., Soliman, F., and Somerville, L. H. (2010). The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev. Psychobiol. 52, 225–235.
Cruz, D. A., Eggan, S. M., and Lewis, D. A. (2003). Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J. Comp. Neurol. 465, 385–400.
Cunningham, M. G., Bhattacharyya, S., and Benes, F. M. (2002). Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 453, 116–130.
Dahl, R. E. (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 1021, 1–22.
Doremus-Fitzwater, T. L., Varlinskaya, E. I., and Spear, L. P. (2009). Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol. Behav. 97, 484–494.
Erickson, S. L., and Lewis, D. A. (2002). Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J. Comp. Neurol. 448, 186–202.
Fergus, S., and Zimmerman, M. A. (2005). Adolescent resilience: a framework for understanding healthy development in the face of risk. Annu. Rev. Public Health 26, 399–419.
Fernandez-Fernandez, R., Navarro, V. M., Barreiro, M. L., Vigo, E. M., Tovar, S., Sirotkin, A. V., Casanueva, F. F., Aguilar, E., Dieguez, C., Pinilla, L., and Tena-Sempere, M. (2005). Effects of chronic hyperghrelinemia on puberty onset and pregnancy outcome in the rat. Endocrinology 146, 3018–3025.
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., Paus, T., Evans, A. C., and Rapoport, J. L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863.
Heim, C., Plotsky, P. M., and Nemeroff, C. B. (2004). Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29, 641–648.
Ilin, Y., and Richter-Levin, G. (2009). Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS ONE 4, e4329. doi: 10.1371/journal.pone.0004329
Isgor, C., Kabbaj, M., Akil, H., and Watson, S. J. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14, 636–648.
Ito, H., Nagano, M., Suzuki, H., and Murakoshi, T. (2010). Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology 58, 746–757.
Jacobson-Pick, S., Audet, M. C., Nathoo, N., and Anisman, H. (2011). Stressor experiences during the juvenile period increase stressor responsivity in adulthood: transmission of stressor experiences. Behav. Brain Res. 216, 365–374.
Jacobson-Pick, S., Elkobi, A., Vander, S., Rosenblum, K., and Richter-Levin, G. (2008). Juvenile stress-induced alteration of maturation of the GABAA receptor alpha subunit in the rat. Int. J. Neuropsychopharmacol. 11, 891–903.
Jacobson-Pick, S., and Richter-Levin, G. (2010). Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav. Brain Res. 214, 268–276.
Kabbaj, M., Isgor, C., Watson, S. J., and Akil, H. (2002). Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience 113, 395–400.
Kelley, A. E., Schochet, T., and Landry, C. F. (2004). Risk taking and novelty seeking in adolescence: introduction to part I. Ann. N. Y. Acad. Sci. 1021, 27–32.
Kessler, R. C., and Magee, W. J. (1993). Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychol. Med. 23, 679–690.
Laroche, J., Gasbarro, L., Herman, J. P., and Blaustein, J. D. (2009). Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology 150, 2351–2358.
Laviola, G., Macri, S., Morley-Fletcher, S., and Adriani, W. (2003). Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 27, 19–31.
Lepsch, L. B., Gonzalo, L. A., Magro, F. J., Delucia, R., Scavone, C., and Planeta, C. S. (2005). Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addict. Biol. 10, 251–256.
Lewis, E. M., Barnett, J. F., Jr., Freshwater, L., Hoberman, A. M., and Christian, M. S. (2002). Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug Chem. Toxicol. 25, 437–458.
Lupien, S. J., Mcewen, B. S., Gunnar, M. R., and Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445.
Lynn, D. A., and Brown, G. R. (2010). The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev. Psychobiol. 52, 731–739.
Macri, S., Adriani, W., Chiarotti, F., and Laiola, G. (2002). Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim. Behav. 64, 541–543.
Maslova, L. N., Bulygina, V. V., and Popova, N. K. (2002). Immediate and long-lasting effects of chronic stress in the prepubertal age on the startle reflex. Physiol. Behav. 75, 217–225.
Mathews, I. Z., Mills, R. G., and Mccormick, C. M. (2008a). Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev. Psychobiol. 50, 451–459.
Mathews, I. Z., Wilton, A., Styles, A., and Mccormick, C. M. (2008b). Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav. Brain Res. 190, 33–40.
McCormick, C. M., and Ibrahim, F. N. (2007). Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacol. Biochem. Behav. 86, 92–102.
McCormick, C. M., Merrick, A., Secen, J., and Helmreich, D. L. (2006). Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. J. Neuroendocrinol. 19, 116–126.
McCormick, C. M., Nixon, F., Thomas, C., Lowie, B., and Dyck, J. (2011). Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav. Brain Res. 208, 23–29.
McCormick, C. M., Robarts, D., Gleason, E., and Kelsey, J. E. (2004). Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm. Behav. 46, 458–466.
McCormick, C. M., Robarts, D., Kopeikina, K., and Kelsey, J. E. (2005). Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm. Behav. 48, 64–74.
McCormick, C. M., Smith, C., and Mathews, I. Z. (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res. 187, 228–238.
Meyer, G., Ferres-Torres, R., and Mas, M. (1978). The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 155, 108–112.
Morrissey, M. D., Mathews, I. Z., and Mccormick, C. M. (2011). Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol. Learn. Mem. 95, 46–56.
Peleg-Raibstein, D., and Feldon, J. (2011). Differential effects of post-weaning juvenile stress on the behaviour of C57BL/6 mice in adolescence and adulthood. Psychopharmacology (Berl.) 214, 339–351.
Penza, K. M., Heim, C., and Nemeroff, C. B. (2003). Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch. Womens Ment. Health 6, 15–22.
Romeo, R. D., Lee, S. J., Chhua, N., Mcpherson, C. R., and Mcewen, B. S. (2004). Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 79, 125–132.
Rosenberg, D. R., and Lewis, D. A. (1995). Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 358, 383–400.
Sala-Catala, J., Torrero, C., Regalado, M., Salas, M., and Ruiz-Marcos, A. (2005). Movements restriction and alterations of the number of spines distributed along the apical shafts of layer V pyramids in motor and primary sensory cortices of the peripubertal and adult rat. Neuroscience 133, 137–145.
Schmidt, M. V., Scharf, S. H., Liebl, C., Harbich, D., Mayer, B., Holsboer, F., and Muller, M. B. (2010a). A novel chronic social stress paradigm in female mice. Horm. Behav. 57, 415–420.
Schmidt, M. V., Scharf, S. H., Sterlemann, V., Ganea, K., Liebl, C., Holsboer, F., and Muller, M. B. (2010b). High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology 35, 635–643.
Sowell, E. R., Thompson, P. M., Holmes, C. J., Jernigan, T. L., and Toga, A. W. (1999). In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 2, 859–861.
Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463.
Spear, L. P., and Brake, S. C. (1983). Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 16, 83–109.
Sterlemann, V., Rammes, G., Wolf, M., Liebl, C., Ganea, K., Muller, M. B., and Schmidt, M. V. (2010). Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus 20, 540–549.
Tirelli, E., Laviola, G., and Adriani, W. (2003). Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci. Biobehav. Rev. 27, 163–178.
Toledo-Rodriguez, M., and Sandi, C. (2007). Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007, 71203.
Tsoory, M., Cohen, H., and Richter-Levin, G. (2006). Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur. Neuropsychopharmacol. 17, 245–256.
Tsoory, M., Guterman, A., and Richter-Levin, G. (2008). Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology 33, 378–393.
Tsoory, M., and Richter-Levin, G. (2005). Learning under stress in the adult rat is differentially affected by “juvenile” or “adolescent” stress. Int. J. Neuropsychopharmacol. 1–16.
Tsoory, M. M., Guterman, A., and Richter-Levin, G. (2010). “Juvenile stress” alters maturation-related changes in expression of the neural cell adhesion molecule L1 in the limbic system: relevance for stress-related psychopathologies. J. Neurosci. Res. 88, 369–380.
Keywords: adolescence, gender differences, stress, anxiety, risk taking, novelty seeking, resilience, vulnerability
Citation: Toledo-Rodriguez M and Sandi C (2011) Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front. Behav. Neurosci. 5:17. doi: 10.3389/fnbeh.2011.00017
Received: 02 February 2011;
Accepted: 25 March 2011;
Published online: 07 April 2011.
Edited by:
Nuno Sousa, University of Minho, PortugalReviewed by:
Cesar Venero, National University of Distance Education, SpainOsborne F. Almeida, University of Minho, Portugal
Copyright: © 2011 Toledo-Rodriguez and Sandi. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Maria Toledo-Rodriguez, School of Biomedical Sciences, Medical School, Queen’s Medical Centre, Nottingham NG7 2UH, UK.e-mail: maria.toledo@nottingham.ac.uk