One hand clapping: lateralization of motor control
- 1Neuroscience Paris Seine, CNRS UMR8246, Inserm U1130, Sorbonne Universités, UPMC UM119, Paris, France
- 2Inserm U1127, CNRS UMR 7225, Sorbonne Universités, UPMC UMR S1127, Institut du Cerveau et de la Moelle épinière, ICM, Paris, France
- 3Département des Maladies du Système Nerveux, AP-HP, Hôpital Pitié Salpêtrière, Paris, France
Lateralization of motor control refers to the ability to produce pure unilateral or asymmetric movements. It is required for a variety of coordinated activities, including skilled bimanual tasks and locomotion. Here we discuss the neuroanatomical substrates and pathophysiological underpinnings of lateralized motor outputs. Significant breakthroughs have been made in the past few years by studying the two known conditions characterized by the inability to properly produce unilateral or asymmetric movements, namely human patients with congenital “mirror movements” and model rodents with a “hopping gait”. Whereas mirror movements are associated with altered interhemispheric connectivity and abnormal corticospinal projections, abnormal spinal cord interneurons trajectory is responsible for the “hopping gait”. Proper commissural axon guidance is a critical requirement for these mechanisms. Interestingly, the analysis of these two conditions reveals that the production of asymmetric movements involves similar anatomical and functional requirements but in two different structures: (i) lateralized activation of the brain or spinal cord through contralateral silencing by cross-midline inhibition; and (ii) unilateral transmission of this activation, resulting in lateralized motor output.
Introduction
Lateralization of motor control is required for a variety of coordinated movements, including skilled bimanual tasks and locomotion. To our knowledge, only two conditions are associated with the inability to produce asymmetric movements in mammals: human “mirror movements” and rodent “hopping gait”.
Mirror movements are involuntary symmetrical movements of one side of the body that mirror voluntary movements of the other side. The affected individuals are unable to perform purely unimanual movements and have difficulties to perform tasks requiring independent actions with the two hands such as holding a cup while filling it with water, opening a jar or playing a musical instrument. During these tasks, the effectors produce different motor outputs that are usually bound together by a shared, object-directed goal.
Quadrupedal locomotion is characterized by coordinated, alternating bilateral activation of limb muscles, in which effectors repeatedly produce similar motor outputs in a specific temporal order. A “hopping gait” is a switch from alternate to synchronous activity of the limbs during locomotion that is observed in rodent mutants with impaired axonal guidance.
Here we discuss the neuroanatomical substrates and pathophysiological underpinnings of lateralized motor output through the study “mirror movements” and “hopping gait”. Whereas mirror movements are associated with altered interhemispheric connectivity and abnormal corticospinal projections, abnormal spinal cord interneurons trajectory is responsible for the “hopping gait”. Interestingly, the analysis of these two conditions indicates that the production of asymmetric movements involves similar anatomical and functional requirements but in two different structures, the cerebral cortex and the spinal cord, and it emphasizes the importance of proper commissural axon guidance in this process.
The “Mirror Movement” Paradigm: Inability to Produce Asymmetric Skilled Hand Movements
Humans have a greater ability than other species to produce purposeful handling movements, most of them being asymmetric. With training, we can master highly complex skills ranging from the fluid movements of the virtuoso pianist to the precise life-saving gestures of the heart surgeon. In humans, execution of unimanual movements requires lateralized activation of the primary motor cortex (M1), which then transmits the motor command to the contralateral hand through the crossed corticospinal tract (CST; Figure 1A; Chouinard and Paus, 2010; Galléa et al., 2011).
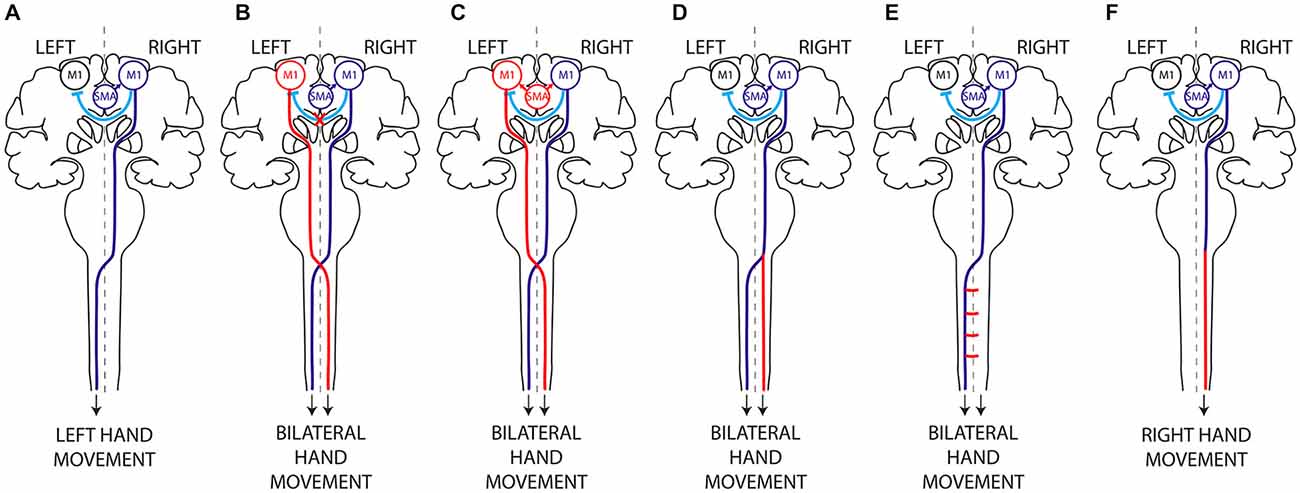
Figure 1. Hypothetical mechanisms of mirror movements. (A) In humans, execution of a unilateral left hand movement requires both lateralized activation of the right primary motor cortex (M1) by interhemispheric inhibition (IHI) and proper motor planning and then transmission of the motor command to the contralateral (left) hand alone, through a crossed corticospinal tract. There are two main mechanisms underlying MM: (i) abnormal IHI (B) or abnormal delivery of the motor plan from the supplementary motor area (SMA) to M1 (C), resulting in bilateral activation of the primary motor cortices; and (ii) abnormal decussation of the CST (D) or abnormal branching of the CST in the spinal cord (E), resulting in bilateral transmission of the motor command to the spinal cord. Mirror movements have not been described in horizontal gaze palsy with progressive scoliosis (HGPPS), despite the absence of CST decussation in these patients (F). This suggests that MM are related to the presence of bilateral spinal cord projections arising from a single primary motor cortex rather than to abnormal decussation of the CST per se. Dark Blue, normal mechanism; Red, abnormal mechanism; Light blue, IHI.
Loss of this lateralization results in mirror movements (MM), which consist of involuntary symmetrical movements of one side of the body that mirror voluntary movements of the other side. Congenital mirror movement disorder (CMM) is a rare genetic disorder transmitted in autosomal dominant manner in which mirror movements are the only clinical abnormality. These mirror movements predominate in the distal upper limbs, leaving affected individuals unable to perform independent actions with the two hands or to perform purely unimanual movements. They usually have hand clumsiness and pain in the upper limbs during sustained manual activities. The two main culprit genes are Dcc (deleted in colorectal cancer) and Rad51 (Srour et al., 2010; Depienne et al., 2011, 2012; Méneret et al., 2014a). A third gene, Dnal4, might also be involved (Ahmed et al., 2014; Méneret et al., 2014b). Dcc plays a key role in CST midline crossing (Finger et al., 2002), while Rad51 is well known for its role in DNA repair and may also have a major role in motor system development (Depienne et al., 2012; Gallea et al., 2013). In addition to isolated congenital mirror movements caused by Dcc or Rad51 mutations, syndromic forms of MM may be accompanied by numerous other symptoms, in disorders such as Dandy walker syndrome, Joubert’s syndrome, X-linked Kallmann syndrome, Klippel Feil syndrome and congenital hemiparesis (Vulliemoz et al., 2005; Galléa et al., 2011; Peng and Charron, 2013).
CMM provides a unique paradigm for studying the lateralization of motor control (Carson, 2005; Galléa et al., 2011; Peng and Charron, 2013). Two main non exclusive mechanisms may account for MM: (i) abnormal interhemispheric communication resulting in bilateral activation of primary motor areas (Figures 1B,C); and (ii) a corticospinal tract abnormality leading to bilateral downstream transmission of the motor command (Figures 1D,E; Gallea et al., 2013).
Interhemispheric Connectivity and Motor Lateralization
In humans, the default set-up of motor behavior is probably a mirror program (Chan and Ross, 1988; Meyer et al., 1995; Cincotta and Ziemann, 2008). Unilateral and bilateral voluntary movements are preceded by slow negativity on EEG recordings, known as the Bereitschaftpotential (Shibasaki and Hallett, 2006), which starts 2 s before movement onset and is distributed over the two hemispheres. This Bereitschaftpotential may reflect bilateral activation of the supplementary motor areas (SMA) and dorsal premotor cortices (dPMC) during motor planning. Just before movement onset, cortical activity is restricted to the primary motor cortex and dPMC contralateral to the intended movement (Shibasaki and Hallett, 2006). An active mechanism is required to restrict motor activation to one hemisphere during execution of a pure unimanual movement.
Our current understanding of this “non mirror transformation” derives mainly from the study of “physiological” mirror movements. Healthy subjects have a default tendency to produce minimal mirror movements when performing highly complex and effortful unimanual tasks (Koerte et al., 2010; Sehm et al., 2010; Beaulé et al., 2012). Activation of the mirror M1 (ipsilateral to the voluntary movement) is the main explanation for this tendency (Mayston et al., 1999; Cincotta et al., 2004; Zijdewind et al., 2006; Hübers et al., 2008). In order to achieve this “non mirror transformation”, the active M1 (contralateral to the intended movement) inhibits the mirror M1 via fibers that pass through the corpus callosum (transcallosal tract, TCT), thereby restricting the motor output to the active M1. This inhibition of one motor cortex by the other is called interhemispheric inhibition (IHI). IHI is thought to rely on transcallosal glutamatergic connections to inhibitory interneurons that in turn innervate pyramidal cells in the receiving hemisphere (Meyer et al., 1995; Reis et al., 2008). Several lines of evidence support the importance of TCT-mediated IHI in the lateralization of motor control. For example, the gradual disappearance of minimal MM frequently observed in young children correlates with the degree of TCT myelination and with the level of IHI (Koerte et al., 2010; Beaulé et al., 2012). Also, experimental modulation of IHI directed from the active M1 to the mirror M1 affects mirror activity: a transient increase in IHI is associated with a decrease in mirror activity, and vice versa (Hübers et al., 2008).
IHI between the two primary motor cortices is modulated differently during the different phases of unimanual movements. IHI is balanced between the two motor cortices at the onset of movement preparation, then shifts towards the ipsilateral M1 (ipsilateral to the voluntary movement) at the end of movement preparation and at movement onset (Murase et al., 2004; Duque et al., 2007). In parallel, IHI of the contralateral M1 decreases during movement preparation and shifts towards facilitation at movement onset (Murase et al., 2004; Perez and Cohen, 2008). These subtle time-dependent bilateral variations of IHI are necessary to avoid premature execution (Duque and Ivry, 2009), and to prevent mirror activity in the ipsilateral M1 (Giovannelli et al., 2009). Impairment of IHI may thus result in bilateral M1 activation and transmission of the motor command to both hands through the two crossed CSTs.
In patients with CMM and X-linked Kallmann syndrome, several studies have revealed abnormal, bilateral M1 activation during voluntary unimanual movements and have confirmed that activation of the mirror M1 is not a sensory consequence of the mirror movement but rather participates actively in the mirroring motor activity (Shibasaki and Nagae, 1984; Cohen et al., 1991; Mayer et al., 1995; Cincotta et al., 1996; Krams et al., 1997; Verstynen et al., 2007). However, studies based on indirect methods have failed to demonstrate consistent impairment of IHI mechanisms in CMM patients (Cincotta et al., 1996, 2002; Papadopoulou et al., 2010). Using dual-site transcranial magnetic stimulation (TMS), a more direct method (Perez and Cohen, 2008), we found that CMM patients with Rad51 mutations had abnormal IHI between the primary motor cortices at rest, together with morphological abnormalities of the TCT (Figure 1B; Gallea et al., 2013). It has been proposed that this impaired IHI is due to an abnormal input of the transcallosal glutamatergic connections onto the inhibitory interneurons in the receiving hemisphere. It is noteworthy that most individuals lacking a corpus callosum do not exhibit mirror movements, suggesting that the absence of the corpus callosum and interhemispheric connections alone might not be sufficient to generate MM. Finally, a study of a CMM patient with complete agenesis of the corpus callosum concluded that the absence of TCT played little part in the pathophysiology of MM (Lepage et al., 2012).
Interhemispheric pathways are not limited to direct M1-M1 interactions and IHI but also include circuits linking secondary motor areas (SMA and PMd) to contralateral motor areas. These circuits might be involved in restricting the generation of motor output to the active hemisphere during movement preparation. For these reasons it has been proposed that abnormal motor planning and/or abnormal transmission of the motor plan from the secondary motor areas to the primary motor areas might also be involved in MM generation (Chan and Ross, 1988; Cincotta et al., 2004; Duque et al., 2010; Galléa et al., 2011; Gallea et al., 2013). Evidence of abnormal motor planning associated with MM was first obtained through studies of two CMM patients and a patient with Kallmann’s syndrome, who showed an abnormal, bilateral (instead of unilateral) distribution of the Bereitschaftpotential during movement preparation (Shibasaki and Nagae, 1984; Cohen et al., 1991). However, two other studies argued against a role of abnormal movement planning in MM: the first showed that movement-related cortical EEG potentials were identical (that is to say, lateralized and not bilateral) in healthy volunteers and in six CMM patients (Mayer et al., 1995), while the second study, a case report, showed normal, unilateral cortical activation during fMRI imaging of imagined movements closely related to motor planning (Verstynen et al., 2007). More recently, we found that the SMA activation pattern and connectivity are abnormal during both unimanual and bimanual movements in Rad51-mutated CMM patients (Figure 1C; Gallea et al., 2013) This suggested that cortical activation and connectivity might be modified in CMM patients during movement preparation, resulting in inappropriate delivery of the motor program from the SMA to both primary motor cortices.
Together, these results suggest that interhemispheric connectivity is critical for lateralized activation of the motor cortex when a unilateral movement is intended.
The Corticospinal Tract and Motor Lateralization
The CST is a crossed tract that transmits the motor command from one motor cortex to the contralateral spinal cord. The CST first appeared in mammals and was likely critical for the development of voluntary skilled movements through evolution (Vulliemoz et al., 2005). Selective lesions of the CST in humans, non human primates and rodents impair skilled digit movements such as reaching (Schieber, 2007). The CST is massively crossed in humans. About 70–95% of all CST axons cross the midline at the junction between the medulla and the spinal cord, forming the so-called “pyramidal decussation”, and establish direct contacts with the motor neurons located in the anterior horn of the spinal cord (Vulliemoz et al., 2005). The approximately 10% of CST axons that do not decussate at the medulla remain ipsilateral, and this ipsilateral tract is mainly located in the ventral part of the spinal cord in both humans and rodents (Brösamle and Schwab, 1997; Vulliemoz et al., 2005). The ipsilateral CST component does not target motor neurons innervating distal limb muscles but rather motor neurons innervating the proximal or axial musculatures (Bawa et al., 2004; Vulliemoz et al., 2005). In humans, cats and rodents, the CST initially establishes strong bilateral projections to the spinal cord. The ipsilateral projections consist of uncrossed CST axons (Joosten et al., 1992; Brösamle and Schwab, 1997; Eyre et al., 2001), and/or of normally crossed CST axons that recross the midline within the spinal cord (Li and Martin, 2000; Rosenzweig et al., 2009). This CST projection pattern is refined during early post-natal development, resulting in the elimination of the majority of the ipsilateral projections (Joosten et al., 1992; Eyre et al., 2000, 2001; Li and Martin, 2000). This refinement of the ipsilateral projections is an activity-dependent process of competition with the crossed CST fibers originating from the contralateral motor cortex (Martin and Lee, 1999; Eyre et al., 2001; Eyre, 2003; Martin et al., 2004; Friel and Martin, 2007; Friel et al., 2014).
Human MM could result from the presence of CST projections to both the ipsilateral and contralateral spinal cord. In patients with CMM, Kallmann syndrome, Klippel-Feil syndrome or congenital hemiparesis, unilateral stimulation of the primary motor cortex hand area at rest by TMS elicits bilateral hand muscle responses with identical latencies, whereas in healthy volunteers the muscle response is strictly contralateral to the stimulated hemisphere (Nass, 1985; Farmer et al., 1990; Benecke et al., 1991; Mayston et al., 1997; Alagona et al., 2001; Staudt et al., 2002; Cincotta et al., 2003; Bawa et al., 2004; Srour et al., 2010; Depienne et al., 2011; Gallea et al., 2013). This reveals the presence of fast-conducting corticospinal projections from the hand area of one primary motor cortex to both sides of the spinal cord in CMM patients and suggests an anatomic-functional link between anomalies in the CST trajectory and the inability to produce lateralized movements.
Bilateral corticospinal projections to the spinal cord could be due to: (i) abnormal pyramidal decussation resulting in an aberrant uncrossed ipsilateral CST (Figure 1D); or (ii) aberrant branching of crossed CST axons in the spinal cord (Figure 1E). In both cases, the aberrant CST projection pattern could result from abnormal guidance of the CST axons or from an abnormal persistence of the ipsilateral CST projections that are normally eliminated during development. An elegant TMS study of two CMM patients supports the existence of a separate uncrossed ipsilateral CST (Cincotta et al., 2003). Diffusion tensor imaging (DTI) was used to study the precise anatomy of the pyramidal decussation in Rad51-mutated patients, confirming abnormal CST decussation (Gallea et al., 2013), although Dcc-mutated CMM patients have yet to be studied. Rad51 expression pattern in the mouse central nervous system (Depienne et al., 2012), and the known role of DCC in commissural axons guidance (Kennedy et al., 1994; Serafini et al., 1994; Keino-Masu et al., 1996; Finger et al., 2002), suggest that abnormal axonal guidance rather than impaired CST maturation is responsible for the bilateral CST projections observed in Rad51- and Dcc-mutated patients. Electrophysiological studies also support the existence of an aberrant uncrossed CST in X-linked Kallmann patients (Mayston et al., 1997; Farmer et al., 2004). In patients with congenital hemiparesis, MM may be explained by an abnormal maturation of the CST due to the unequal activity between the affected and unaffected motor cortices (Eyre et al., 2001; Eyre, 2003; Friel et al., 2014). This would lead to the maintenance and reinforcement of the ipsilateral CST from the unaffected motor cortex, combined with aberrant branching of corticospinal fibers in the spinal cord (Benecke et al., 1991; Alagona et al., 2001; Staudt et al., 2002; Galléa et al., 2011; Friel et al., 2014). Mirror movements have not been described in patients with horizontal gaze palsy with progressive scoliosis (HGPPS), despite their lack of CST decussation. HGPPS is linked to mutations in the axon guidance receptor ROBO3 (Jen et al., 2004). The CST is completely uncrossed in HGPPS patients, and each hemisphere thus projects in a strictly ipsilateral manner to the spinal cord (Figure 1F). Together, these findings suggest that MM are related to the presence of bilateral spinal cord projections arising from a single primary motor cortex rather than to abnormal decussation of the CST per se.
Study of MM patients enlightened the critical importance of two mechanisms for the generation of asymmetric movements: (i) lateralized activation of the brain through contralateral silencing by IHI and proper motor planning; and (ii) unilateral transmission of the motor command to the contralateral spinal cord via the CST. Both abnormal interhemispheric connectivity and an altered CST trajectory could be responsible for MM, but the respective importance of each factor is unclear.
Control of Left-Right Alternation During Locomotion: New Insights from Genetically Modified Mice with Developmental Motor System Anomalies
Quadrupedal locomotion requires repeated coordinated activity of each limb in a specific temporal sequence. Alternating left-right activity of the forelimbs and hindlimbs is observed at low locomotor frequencies (walking and trotting), while synchronized activity of the homologous limbs is observed at high locomotor frequencies (galloping) in mice, cats, horses and dogs (Forssberg et al., 1980; Dickinson et al., 2000; Serradj and Jamon, 2009). Lateralized motor output is thus a crucial aspect of locomotion, especially at low motor frequencies. In the past decade, careful analysis of genetically modified mice with a “hopping gait” has shed light on the respective contributions of the corticospinal tract and spinal central pattern generators (CPG) to left-right alternation during mouse locomotion.
The Corticospinal Tract and Left-Right Alternation During Locomotion
The CST forms a crossed (lateralized) motor circuit controlling voluntary movements of the four limbs. In rodents, the CST is composed of neurons originating from cortical layer V, projecting mainly to the contralateral side of the spinal cord and eventually connecting to motor neurons via a multisynaptic pathway (Figure 2; Canty and Murphy, 2008). A role of the CST in the control of alternating left-right activity during mouse locomotion was initially suggested by the “hopping gait” described in mice with genetically induced alterations of CST projections (mice with mutations of the EphA4 signaling pathway and kanga mice). EphA4 (a member of the Eph family of tyrosine-kinase receptors) and its ligand ephrinB3 are involved in axonal guidance of the CST during development. Deletion of EphA4, Ephrin-B3 or proteins involved in the EphA4 downstream signaling pathway (α2-chimaerin, Nck, RhoA) results in a hopping-gait phenotype (Dottori et al., 1998; Kullander et al., 2001a,b; Yokoyama et al., 2001; Beg et al., 2007; Fawcett et al., 2007; Iwasato et al., 2007; Mulherkar et al., 2013). In EphA4 and EphrinB3 knockout mice, the CST trajectory is normal from the cortex to the pyramidal decussation. In the spinal cord, CST axons re-cross the midline, resulting in bilateral innervation of the spinal cord by each of the two hemispheres. In wild-type animals, EphA4-expressing CST axons are repelled by ephrin-B3 secreted at the midline, deterring them from re-crossing the midline at the spinal level (Dottori et al., 1998; Kullander et al., 2001a,b; Yokoyama et al., 2001). These findings suggested that the hopping gait might be explained by transmission of motor commands to both sides of the spinal cord through abnormally re-crossed CST axons. Similarly to mice with genetic alterations of the EphA4 signaling pathway, a mutant mouse line carrying a viable mutation of the DCC receptor have a “kangaroo-like” hopping gait phenotype and are thus named “kanga” (Finger et al., 2002). The DCC ligand Netrin-1 belongs to the netrin family of extracellular guidance molecules. Netrin-1 has an attractive effect on growth cones when it interacts with the DCC receptor (Keino-Masu et al., 1996). This attraction allows commissural axons to approach and cross the midline (Kennedy et al., 1994; Serafini et al., 1994). DCC is expressed within the main forebrain descending tracts during their development (Shu et al., 2000). In kanga mice, the CST fails to cross the midline at the pyramidal decussation and projects exclusively to the ipsilateral side of the spinal cord (Finger et al., 2002).
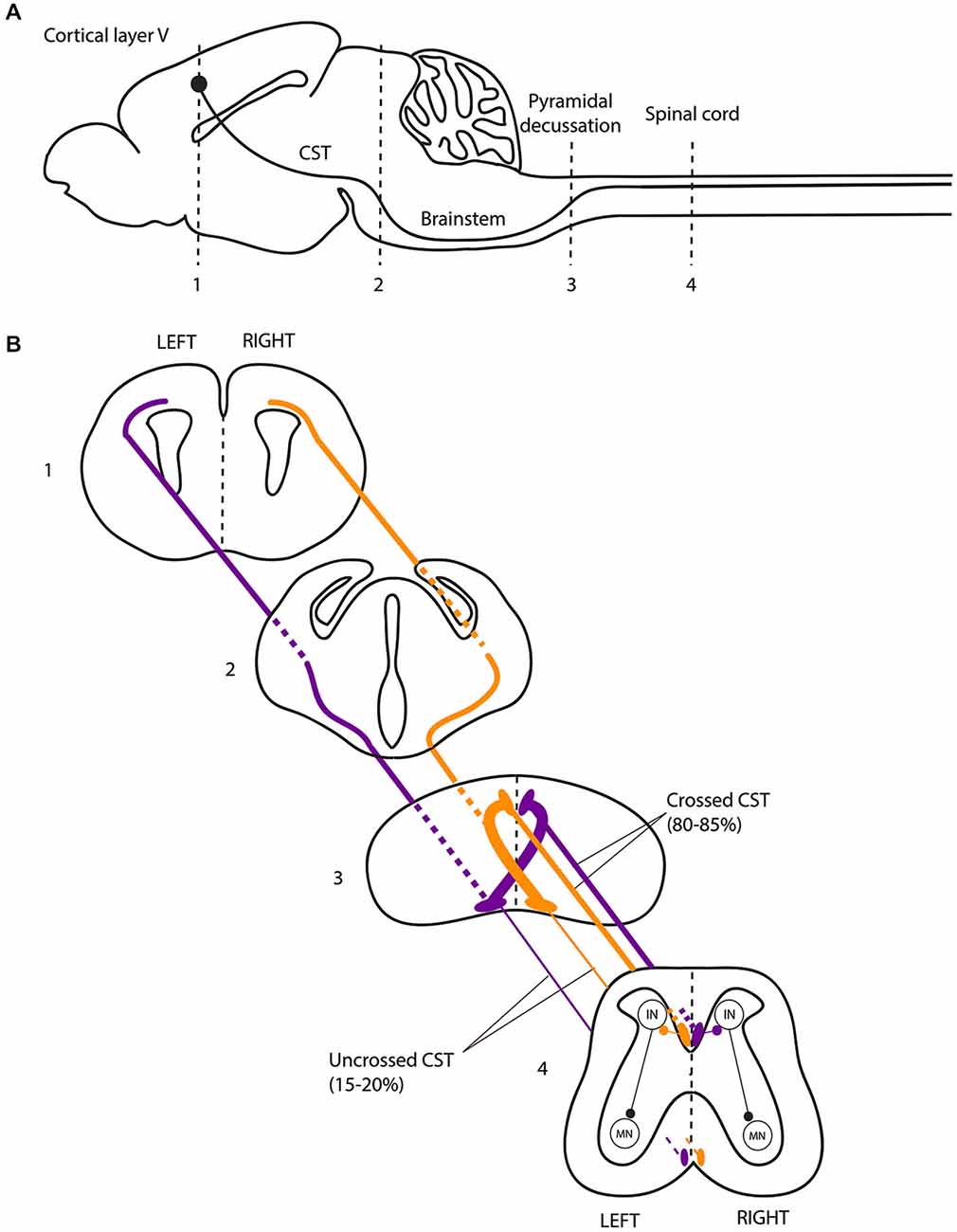
Figure 2. The corticospinal tract forms a crossed motor system in mice. (A) Sagittal view of the mouse central nervous system and corticospinal tract (CST). (B) Coronal views of the CST trajectory. The level of each coronal schematic section is indicated in figure A. At the junction between the hindbrain and the spinal cord (pyramidal decussation, level 3), the vast majority (80–85%) of corticospinal tract (CST) axons cross the midline and continue their trajectory through the most ventral part of the dorsal funiculus within the half of the spinal cord contralateral to their hemisphere of origin. In the spinal cord, the CST undergoes collateral branching principally at the level of the cervical and lumbar enlargement, eventually transmitting motor commands to the forelimb and hindlimb muscles, respectively, via a multisynaptic pathway involving interneurons mainly located in the dorsal horn of the spinal cord. CST, corticospinal tract; IN, interneurons; MN, motor neurons.
However, other experimental findings do not support a major contribution of the CST to alternating left-right activity during locomotion. Indeed, abnormal CST midline crossing is not systematically associated with synchronized activity of the limbs during locomotion: mutants for L1 (Cohen et al., 1998; Jakeman et al., 2006), NCAM (Rolf et al., 2002), Sema6A and PlexinA3/PlexinA4 (Faulkner et al., 2008; Runker et al., 2008), exhibit normal locomotion despite having an abnormal CST. In rodents, a lateralized lesion of the cortex or CST, occurring during the first week of life, leads to sprouting of the remaining CST across the midline and thus to bilateral spinal cord projections (Leong and Lund, 1973; Kartje-Tillotson et al., 1987). This results in altered skilled forelimb movements without affecting left-right alternation during locomotion (Kunkel-Bagden et al., 1992; Whishaw et al., 1993; Whishaw, 2000; Metz and Whishaw, 2002; Tennant and Jones, 2009). Thus, abnormal CST projections do not necessarily induce a hopping gait.
It is important to recall that the genetic alterations induced in EphA4, ephrin-B3 and DCC kanga mutant mice not only impact CST development but also affect commissural cell populations expressing these proteins, such as pre-cerebellar commissural neurons (Hashimoto et al., 2012), and commissural spinal cord interneurons (Kullander et al., 2003; Beg et al., 2007; Iwasato et al., 2007; Rabe Bernhardt et al., 2012). This implies that the hopping gait observed in these mice is not necessarily due to their CST abnormalities. Two recent studies took advantage of the conditional knockout mouse Emx1::cre;EphA4flox/flox in which genetic deletion of EphA4 is restricted to the forebrain. These mice exhibit normal stereotypical locomotion despite bilateral CST projections to the spinal cord (Borgius et al., 2014; Serradj et al., 2014). Together, these results show that proper CST wiring is not necessary for stereotypic left-right alternation.
Supra-spinal control plays a critical role in voluntary movements and adaptive locomotion when sensory-motor integration is required (for example when stepping over an obstacle). Emx1::cre;EphA4flox/flox mice with bilateral CST projections to the spinal cord exhibit symmetric voluntary movements under conditions when asymmetric limbs movements are normally produced (Borgius et al., 2014; Friel et al., 2014; Serradj et al., 2014). These results emphasize the role of the CST in voluntary asymmetric movements.
In addition to the CST, supra-spinal structures playing an important role in the control of gait are located in the cerebral cortex, the cerebellum and in the brainstem, and constitute an interconnected network. There is no clear evidence implicating a supra-spinal control for left-right alternation and lateralization of motor control during gait. Among the locomotor centers with direct spinal projections, the mesencephalic locomotor region (MLR) is of particular interest for our purpose. Electrical stimulation of the brainstem in decerebrate cats placed on a treadmill recapitulates normal alternate locomotion without the need of descending commands from the cortex (Shik et al., 1966, 1967). The MLR, which comprises the pedunculopontine (PPN) and cuneiform (CN) nuclei, sends outputs to the basal ganglia, the cerebellar and the cerebral locomotor areas. The MLR plays a major role in gait initiation and in internal generation of adaptive lower limb movement during the automated gait cycle (Alam et al., 2011; Grabli et al., 2012). The MLR could be involved in the control of gait cadence (Piallat et al., 2009; Karachi et al., 2010), but this involvement is more likely related to higher-order functions during faster gait rather than basic motor control as suggested by rodent models (Winn, 2008). Dysfunction of the MLR and cerebral locomotor centers is observed in patients with Parkinson disease and freezing of gait (Fling et al., 2013, 2014), which is the inability to move the feet despite the effort to overcome the motor block and move forward. These patients exhibit alteration of gait rhythm, gait symmetry and bilateral coordination in stepping (Plotnik and Hausdorff, 2008; Plotnik et al., 2008). However, freezing of gait and bilateral coordination problems are triggered by particular circumstances, when adaptive locomotion is needed (Grabli et al., 2012). In addition, freezing also occurs during writing and speech, although the MLR is not involved in such tasks. MPTP monkeys with selective loss of cholinergic neurons in the PPN have gait impairments but no specific problem in the alternation of lower limb movements (Karachi et al., 2010). In cats, electrical stimulation of the PPN suppresses postural muscle tone, whereas CN stimulation elicits locomotor movements (Takakusaki et al., 2003). In humans, activity of the PPN seems to be modulated during rhythmical stepping, but the increased demands of postural control and attention during stepping could not be cancelled out (Fraix et al., 2013). Therefore, the structures constituting the MLR might play different roles in gait control, but none of them is known to be specifically involved in left-right alternation of the lower limbs.
In non mammalian vertebrates the descending motor pathways are mainly composed of reticulospinal tracts originating from the hindbrain (Vulliemoz et al., 2005). In zebrafish, descending motor pathways include Mauthner cells and other reticulospinal neurons (MiD2cm, MiD3cm and MiD3cl). This crossed network plays a critical role in adaptive locomotor activity such as escape behavior: a stimulus delivered to one side of the head results first in tail bending towards the opposite side, followed by a counter-bend that enables efficient propulsion (Kohashi and Oda, 2008; Jain et al., 2014). DCC mutations, leading to midline guidance defects of MiD2cm and MiD3cm neurons that project bilaterally instead of contralaterally, cause an abnormal counter-bend in the same direction as the first. Escape behavior of these mutant zebrafish is thereby compromised. This phenotype is rescued by ablation of the aberrantly projecting MiD2cm and MiD3cm neurons, demonstrating that supra-spinal pathways predominate over spinal circuitry during adaptive locomotion (Jain et al., 2014).
Altogether, these results suggest that supra-spinal control plays a critical role in motor lateralization during voluntary movements and adaptive locomotion but is not involved in left-right alternation during stereotypic locomotion.
Spinal Control of Left-Right Alternation During Locomotion
The importance of local spinal circuitry in locomotion is supported by “fictive locomotion” experiments performed in vitro. Exposure of isolated rodent spinal cords to neurotransmitter agonists such as serotonin and dopamine produces rhythmic activity at the lumbar level lasting several hours. This activity is characterized by alternating ipsilateral flexor-extensor activity and alternating left-right activity (Smith and Feldman, 1987; Kiehn and Kjaerulff, 1996). Successful replication of left-right alternation in spinal cords isolated from the forebrain strongly suggests that the spinal neuronal network plays a critical role in locomotion. This network is called the central pattern generator (CPG), and its role in swimming and walking has been extensively studied (Grillner, 2003; Goulding, 2009; Kiehn et al., 2010; Kiehn, 2011). The CPG generates rhythm, ipsilateral flexor-extensor alternation, and left-right alternation. Spinal commissural interneurons (CIN), mostly located in the ventromedial spinal cord (lamina VIII), play a key role in left-right alternation (Stokke et al., 2002). Fictive locomotion experiments in vitro have shown that removal of the dorsal part of the spinal cord does not affect left-right alternation, whereas sectioning of the ventral spinal cord commissure completely abolishes it (Kjaerulff and Kiehn, 1996). When inhibitory GABAa or glycinergic CIN are neutralized by the use of antagonists, spinal left-right alternating activity switches to synchronous activity, demonstrating that this cross-midline inhibition is critical for lateralized motor activity (Cowley and Schmidt, 1995). Conversely, suppression of glutamatergic excitatory transmission in the spinal cord of Vglut2 mutants does not affect the generation of left-right alternation or locomotor rhythms (Gezelius et al., 2006; Wallén-Mackenzie et al., 2006).
The specific characteristics and fate of spinal cord interneurons are determined by the progenitor subtype from which they originate. During the early phases of CNS development, transcription-factor gradients result in dorsoventral patterning of spinal neurons. There are 11 progenitor domains in the spinal cord, six dorsal (dI1-dI6) and five ventral (V0, V1, V2, motor neurons and V3 interneurons, in dorsal-to-ventral order; Jessell, 2000; Arber, 2012). Delineation of the CPG circuitry through the use of mutant mice improved our understanding of spinal alternating left-right activity.
By connecting the two sides of the spinal cord, CIN determine the excitatory/inhibitory balance over the midline. Most CIN involved in left-right coordination originate from the ventral spinal cord, from V0 and V3 progenitors (Kiehn, 2011; Chédotal, 2014), but the role of dorsally derived interneurons was recently highlighted (Andersson et al., 2012; Vallstedt and Kullander, 2013). Cross-midline inhibition relies on a dual inhibitory pathway (Figure 3A) composed mainly of V0-derived CIN and comprising: (i) a group of dorsal inhibitory CIN (V0D) that project monosynaptically to contralateral motor neurons; and (ii) a group of ventral glutamatergic CIN (V0V) which provide indirect inhibition via multisynaptic connections with ipsilateral inhibitory interneurons such as Renshaw cells (RC) and Ia inhibitory interneurons (Moran-Rivard et al., 2001; Pierani et al., 2001; Lanuza et al., 2004; Goulding, 2009; Kiehn et al., 2010). This system allows contralateral silencing during left-right alternation, in a frequency-dependent manner (Talpalar et al., 2013). In contrast, an excitatory pathway (Figure 3B) composed of glutamatergic CIN derived from V3 progenitors and projecting directly to contralateral motor neurons provides support for left-right synchrony (Zhang et al., 2008; Rabe et al., 2009; Borowska et al., 2013). This organization has been described in rodents (Quinlan and Kiehn, 2007; Restrepo et al., 2009) and cats (Jankowska et al., 2009). Additionally, ipsilaterally projecting interneurons are key components of multisynaptic pathways that provide indirect cross-midline inhibition and, as such, also participate in left-right alternation (Crone et al., 2008, 2009).
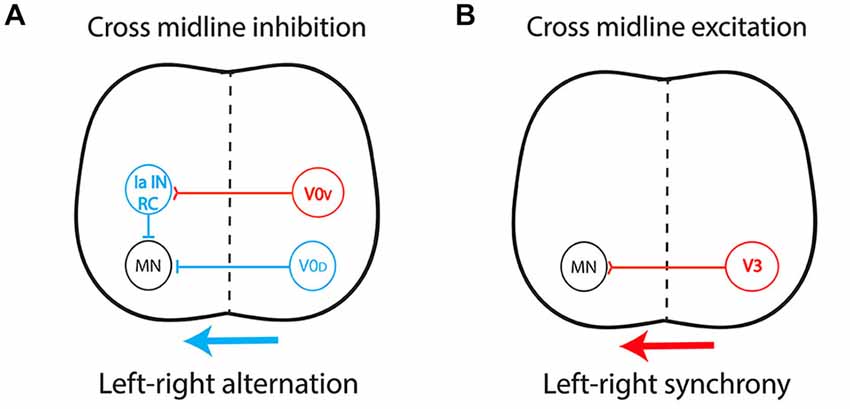
Figure 3. Central pattern generator circuitry underlying left-right coordination during locomotion. (A) The dual inhibitory pathway (glycinergic/GABAergic) is composed of V0-derived interneurons including: (i) a group of dorsal inhibitory commissural interneurons (CIN) that project monosynaptically to contralateral motor neurons (V0D); and (ii) a group of ventral glutamatergic CIN (V0V) which provide indirect inhibition via a multisynaptic pathway with ipsilateral inhibitory interneurons such as Renshaw cells (RC) and Ia inhibitory interneurons (Ia IN). This system produces cross-midline inhibition that allows contralateral silencing during left-right alternation, in a frequency-dependent manner. (B) The excitatory pathway is composed of glutamatergic CIN (V3) projecting directly to contralateral motor neurons, providing the support for left-right synchronicity. Red, excitatory interneurons; Blue, inhibitory interneurons.
Mutant mice with commissural axon guidance defects have been critical for studying the spinal locomotor circuitry (Figure 4). Spinal CIN cross the midline at the floor plate, a structure located in the ventral spinal cord that secretes several molecules such as Ephrin-B3 and Netrin-1 involved in commissural axon guidance (Nawabi and Castellani, 2011). EphA4 and Ephrin-B3 knockout mice both have a hopping-gait phenotype (Dottori et al., 1998; Kullander et al., 2001b; Yokoyama et al., 2001). Fictive locomotion was studied with isolated spinal cords from EphA4 and ephrin-B3 null mutants aged between post-natal day 0 (P0) and P5, a period when the CST has not yet reached the lumbar spinal cord (Gianino et al., 1999). A switch from left-right alternating activity to synchronous activity was observed, together with an increased number of CIN in the ventral spinal cord. Reinforcement of cross-midline inhibition by GABA/glycine uptake blockers completely reversed this effect (Kullander et al., 2003). It was postulated that EphA4 is expressed in a population of excitatory interneurons projecting ipsilaterally, and that loss of EphA4 or ephrin-B3 leads to aberrant midline crossing of this population, resulting in “pseudo-commissural” excitatory connections. This would push the excitatory/inhibitory balance over the midline towards excitation (Figure 4B). In keeping with this hypothesis, specific deletion of EphA4 in the spinal cord or in glutamatergic interneurons is sufficient to induce a hopping gait both in vivo and in vitro (Borgius et al., 2014).
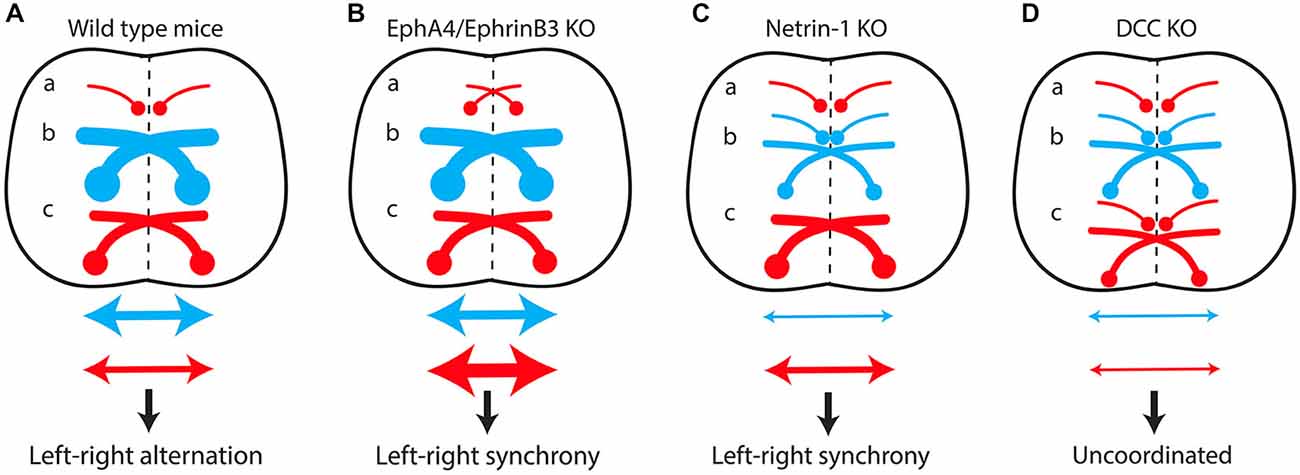
Figure 4. The excitatory/inhibitory balance over the midline in the spinal cord determines the lateralization of motor output. (A) In wild-type mice, predominant recruitment of the dual-inhibitory pathway at low locomotor frequencies produces cross-midline inhibition resulting in left-right alternating activity. A switch from alternating to synchronous left-right activity results from increased cross-midline excitation due to the formation of “pseudo-commissural” excitatory neurons (a) in EphA4/EphrinB3 knockout mice (B) or to misguidance of several populations of inhibitory commissural interneurons (b) in Netrin-1 knockout mice (C). Conversely, the loss of both inhibitory (b) and excitatory commissural interneurons (c) in DCC mutants (D) produces uncoordinated left-right activity. (a) Excitatory ipsilateral interneurons. (b) Inhibitory commissural interneurons. (c) Excitatory commissural interneurons. Red arrow, cross-midline excitation; Blue arrow, cross-midline inhibition.
Netrin-1 knockout mice lack several inhibitory CIN populations, whereas their excitatory CIN are unaffected (Rabe et al., 2009). The inhibitory/excitatory balance over the midline is therefore shifted toward excitation, resulting in synchronous left-right locomotor activity in vitro (Figure 4C; Rabe et al., 2009). Surprisingly, suppression of the expression of DCC, the Netrin-1 receptor, leads to a different phenotype. DCC knockout mice exhibit uncoordinated left-right activity in vitro, reflecting the preservation of the excitatory/inhibitory balance over the midline, due to the loss of both inhibitory and excitatory CIN populations (Figure 4D; Rabe Bernhardt et al., 2012).
A hopping gait has also been described in Nkx mutant mice (Holz et al., 2010). Nkx transcription factors are involved in the development of the floor plate. The misguidance of V0 and dI6 CIN might be responsible for the phenotype of Nkx mutant mice (Holz et al., 2010).
Lateralization of motor output between the two sides of the spinal cord during stereotypic locomotion mainly relies on the excitatory/inhibitory balance over the midline. Recruitment of inhibitory pathways results in cross-midline inhibition and left-right alternation, whereas recruitment of the excitatory pathway results in a shift toward excitation and left-right synchrony. Supra-spinal control and descending pathways (CST in mammals, reticulospinal tracts in non mammalian vertebrates) do not participate in stereotypic left-right alternation but rather contribute to motor lateralization during voluntary movements and adaptive locomotion.
Conclusion
The study of human “mirror movements” and rodent “hopping gait” reveals analogous mechanisms underlying the generation of asymmetric movements. Lateralized activation of the brain or spinal cord is first achieved through contralateral silencing by cross-midline inhibition. In the brain, this inhibition relies on excitatory neurons of the transcallosal tract that connect to inhibitory interneurons in the receiving hemisphere, while in the spinal cord both direct and indirect inhibition is involved during locomotion. Unilateral transmission of these activations results in lateralized motor output. When commissural axon guidance is compromised during development, the formation of projections to both sides of the spinal cord results in bilateral motor output. In mice, the formation of aberrant crossed excitatory connections in the spinal cord induces a hopping gait, while abnormal guidance of the CST in humans results in mirror movements.
Author’s Role
QW, ID, CG and ER drafted the manuscript. QW, CG produced the figures. QW, ID, CG and ER critically reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahmed, I., Mittal, K., Sheikh, T. I., Vasli, N., Rafiq, M. A., Mikhailov, A., et al. (2014). Identification of a homozygous splice site mutation in the dynein axonemal light chain 4 gene on 22q13.1 in a large consanguineous family from pakistan with congenital mirror movement disorder. Hum. Genet. 133, 1419–1429. doi: 10.1007/s00439-014-1475-8
Alagona, G., Delvaux, V., Gérard, P., De Pasqua, V., Pennisi, G., Delwaide, P. J., et al. (2001). Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke 32, 1304–1309. doi: 10.1161/01.str.32.6.1304
Alam, M., Schwabe, K., and Krauss, J. K. (2011). The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 134, 11–23. doi: 10.1093/brain/awq322
Andersson, L. S., Larhammar, M., Memic, F., Wootz, H., Schwochow, D., Rubin, C. J., et al. (2012). Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488, 642–646. doi: 10.1038/nature11399
Arber, S. (2012). Motor circuits in action: specification, connectivity and function. Neuron 74, 975–989. doi: 10.1016/j.neuron.2012.05.011
Bawa, P., Hamm, J. D., Dhillon, P., and Gross, P. A. (2004). Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp. Brain Res. 158, 385–390. doi: 10.1007/s00221-004-2031-x
Beaulé, V., Tremblay, S., and Théoret, H. (2012). Interhemispheric control of unilateral movement. Neural Plast. 2012:627816. doi: 10.1155/2012/627816
Beg, A. A., Sommer, J. E., Martin, J. H., and Scheiffele, P. (2007). α2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron 55, 768–778. doi: 10.1016/j.neuron.2007.07.036
Benecke, R., Meyer, B. U., and Freund, H. J. (1991). Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp. Brain Res. 83, 419–426. doi: 10.1007/bf00231167
Borgius, L., Nishimaru, H., Caldeira, V., Kunugise, Y., Löw, P., Reig, R., et al. (2014). Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits. J. Neurosci. 34, 3841–3853. doi: 10.1523/JNEUROSCI.4992-13.2014
Borowska, J., Jones, C. T., Zhang, H., Blacklaws, J., Goulding, M., and Zhang, Y. (2013). Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J. Neurosci. 33, 18553–18565. doi: 10.1523/JNEUROSCI.2005-13.2013
Brösamle, C., and Schwab, M. E. (1997). Cells of origin, course and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J. Comp. Neurol. 386, 293–303. doi: 10.1002/(SICI)1096-9861(19970922)386:2%3C293::AID-CNE9%3E3.0.CO;2-X
Canty, A. J., and Murphy, M. (2008). Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog. Neurobiol. 85, 214–235. doi: 10.1016/j.pneurobio.2008.02.001
Carson, R. G. (2005). Neural pathways mediating bilateral interactions between the upper limbs. Brain Res. Brain Res. Rev. 49, 641–662. doi: 10.1016/j.brainresrev.2005.03.005
Chan, J. L., and Ross, E. D. (1988). Left-handed mirror writing following right anterior cerebral artery infarction: evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology 38, 59–63. doi: 10.1212/wnl.38.1.59
Chédotal, A. (2014). Development and plasticity of commissural circuits: from locomotion to brain repair. Trends Neurosci. 37, 551–562. doi: 10.1016/j.tins.2014.08.009
Chouinard, P. A., and Paus, T. (2010). What have we learned from “Perturbing” the human cortical motor system with transcranial magnetic stimulation? Front. Hum. Neurosci. 4:173. doi: 10.3389/fnhum.2010.00173
Cincotta, M., Borgheresi, A., Balestrieri, F., Giovannelli, F., Rossi, S., Ragazzoni, A., et al. (2004). Involvement of the human dorsal premotor cortex in unimanual motor control: an interference approach using transcranial magnetic stimulation. Neurosci. Lett. 367, 189–193. doi: 10.1016/j.neulet.2004.06.003
Cincotta, M., Borgheresi, A., Balzini, L., Vannucchi, L., Zeloni, G., Ragazzoni, A., et al. (2003). Separate ipsilateral and contralateral corticospinal projections in congenital mirror movements: neurophysiological evidence and significance for motor rehabilitation. Mov. Disord. 18, 1294–1300. doi: 10.1002/mds.10545
Cincotta, M., Borgheresi, A., Boffi, P., Vigliano, P., Ragazzoni, A., Zaccara, G., et al. (2002). Bilateral motor cortex output with intended unimanual contraction in congenital mirror movements. Neurology 58, 1290–1293. doi: 10.1212/wnl.58.8.1290
Cincotta, M., Lori, S., Gangemi, P. F., Barontini, F., and Ragazzoni, A. (1996). Hand motor cortex activation in a patient with congenital mirror movements: a study of the silent period following focal transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 101, 240–246. doi: 10.1016/0924-980x(96)95621-0
Cincotta, M., and Ziemann, U. (2008). Neurophysiology of unimanual motor control and mirror movements. Clin. Neurophysiol. 119, 744–762. doi: 10.1016/j.clinph.2007.11.047
Cohen, L. G., Meer, J., Tarkka, I., Bierner, S., Leiderman, D. B., Dubinsky, R. M., et al. (1991). Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain 114(Pt. 1B), 381–403. doi: 10.1093/brain/114.1.381
Cohen, N. R., Taylor, J. S., Scott, L. B., Guillery, R. W., Soriano, P., and Furley, A. J. (1998). Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 8, 26–33. doi: 10.1016/s0960-9822(98)70017-x
Cowley, K. C., and Schmidt, B. J. (1995). Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J. Neurophysiol. 74, 1109–1117.
Crone, S. A., Quinlan, K. A., Zagoraiou, L., Droho, S., Restrepo, C. E., Lundfald, L., et al. (2008). Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 60, 70–83. doi: 10.1016/j.neuron.2008.08.009
Crone, S. A., Zhong, G., Harris-Warrick, R., and Sharma, K. (2009). In mice lacking V2a interneurons, gait depends on speed of locomotion. J. Neurosci. 29, 7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009
Depienne, C., Bouteiller, D., Méneret, A., Billot, S., Groppa, S., Klebe, S., et al. (2012). RAD51 haploinsufficiency causes congenital mirror movements in humans. Am. J. Hum. Genet. 90, 301–307. doi: 10.1016/j.ajhg.2011.12.002
Depienne, C., Cincotta, M., Billot, S., Bouteiller, D., Groppa, S., Brochard, V., et al. (2011). A novel DCC mutation and genetic heterogeneity in congenital mirror movements. Neurology 76, 260–264. doi: 10.1212/WNL.0b013e318207b1e0
Dickinson, M. H., Farley, C. T., Full, R. J., Koehl, M. A., Kram, R., and Lehman, S. (2000). How animals move: an integrative view. Science 288, 100–106. doi: 10.1126/science.288.5463.100
Dottori, M., Hartley, L., Galea, M., Paxinos, G., Polizzotto, M., Kilpatrick, T., et al. (1998). EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc. Natl. Acad. Sci. U S A 95, 13248–13253. doi: 10.1073/pnas.95.22.13248
Duque, J., Davare, M., Delaunay, L., Jacob, B., Saur, R., Hummel, F., et al. (2010). Monitoring coordination during bimanual movements: where is the mastermind? J. Cogn. Neurosci. 22, 526–542. doi: 10.1162/jocn.2009.21213
Duque, J., and Ivry, R. B. (2009). Role of corticospinal suppression during motor preparation. Cereb. Cortex 19, 2013–2024. doi: 10.1093/cercor/bhn230
Duque, J., Murase, N., Celnik, P., Hummel, F., Harris-Love, M., Mazzocchio, R., et al. (2007). Intermanual differences in movement-related interhemispheric inhibition. J. Cogn. Neurosci. 19, 204–213. doi: 10.1162/jocn.2007.19.2.204
Eyre, J. A. (2003). Development and plasticity of the corticospinal system in man. Neural Plast. 10, 93–106. doi: 10.1155/np.2003.93
Eyre, J. A., Miller, S., Clowry, G. J., Conway, E. A., and Watts, C. (2000). Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 123(Pt. 1), 51–64. doi: 10.1093/brain/123.1.51
Eyre, J. A., Taylor, J. P., Villagra, F., Smith, M., and Miller, S. (2001). Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology 57, 1543–1554. doi: 10.1212/wnl.57.9.1543
Farmer, S. F., Harrison, L. M., Mayston, M. J., Parekh, A., James, L. M., and Stephens, J. A. (2004). Abnormal cortex-muscle interactions in subjects with X-linked Kallmann’s syndrome and mirror movements. Brain 127, 385–397. doi: 10.1093/brain/awh047
Farmer, S. F., Ingram, D. A., and Stephens, J. A. (1990). Mirror movements studied in a patient with Klippel-Feil syndrome. J. Physiol. 428, 467–484. doi: 10.1113/jphysiol.1990.sp018222
Faulkner, R. L., Low, L. K., Liu, X. B., Coble, J., Jones, E. G., and Cheng, H. J. (2008). Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Dev. 3:21. doi: 10.1186/1749-8104-3-21
Fawcett, J. P., Georgiou, J., Ruston, J., Bladt, F., Sherman, A., Warner, N., et al. (2007). Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc. Natl. Acad. Sci. U S A 104, 20973–20978. doi: 10.1073/pnas.0710316105
Finger, J. H., Bronson, R. T., Harris, B., Johnson, K., Przyborski, S. A., and Ackerman, S. L. (2002). The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J. Neurosci. 22, 10346–10356.
Fling, B. W., Cohen, R. G., Mancini, M., Carpenter, S. D., Fair, D. A., Nutt, J. G., et al. (2014). Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One 9:e100291. doi: 10.1371/journal.pone.0100291
Fling, B. W., Cohen, R. G., Mancini, M., Nutt, J. G., Fair, D. A., and Horak, F. B. (2013). Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136, 2405–2418. doi: 10.1093/brain/awt172
Forssberg, H., Grillner, S., Halbertsma, J., and Rossignol, S. (1980). The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol. Scand. 108, 283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x
Fraix, V., Bastin, J., David, O., Goetz, L., Ferraye, M., Benabid, A. L., et al. (2013). Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS One 8:e83919. doi: 10.1371/journal.pone.0083919
Friel, K. M., and Martin, J. H. (2007). Bilateral activity-dependent interactions in the developing corticospinal system. J. Neurosci. 27, 11083–11090. doi: 10.1523/jneurosci.2814-07.2007
Friel, K. M., Williams, P. T., Serradj, N., Chakrabarty, S., and Martin, J. H. (2014). Activity-based therapies for repair of the corticospinal system injured during development. Front. Neurol. 5:229. doi: 10.3389/fneur.2014.00229
Galléa, C., Popa, T., Billot, S., Méneret, A., Depienne, C., and Roze, E. (2011). Congenital mirror movements: a clue to understanding bimanual motor control. J. Neurol. 258, 1911–1919. doi: 10.1007/s00415-011-6107-9
Gallea, C., Popa, T., Hubsch, C., Valabregue, R., Brochard, V., Kundu, P., et al. (2013). RAD51 deficiency disrupts the corticospinal lateralization of motor control. Brain 136, 3333–3346. doi: 10.1093/brain/awt258
Gezelius, H., Wallén-Mackenzie, A., Enjin, A., Lagerström, M., and Kullander, K. (2006). Role of glutamate in locomotor rhythm generating neuronal circuitry. J. Physiol. Paris 100, 297–303. doi: 10.1016/j.jphysparis.2007.05.001
Gianino, S., Stein, S. A., Li, H., Lu, X., Biesiada, E., Ulas, J., et al. (1999). Postnatal growth of corticospinal axons in the spinal cord of developing mice. Brain Res. Dev. Brain Res. 112, 189–204. doi: 10.1016/s0165-3806(98)00168-0
Giovannelli, F., Borgheresi, A., Balestrieri, F., Zaccara, G., Viggiano, M. P., Cincotta, M., et al. (2009). Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J. Physiol. 587, 5393–5410. doi: 10.1113/jphysiol.2009.175885
Goulding, M. (2009). Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518. doi: 10.1038/nrn2608
Grabli, D., Karachi, C., Welter, M. L., Lau, B., Hirsch, E. C., Vidailhet, M., et al. (2012). Normal and pathological gait: what we learn from Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 83, 979–985. doi: 10.1136/jnnp-2012-302263
Grillner, S. (2003). The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586. doi: 10.1038/nrn1137
Hashimoto, M., Ito, R., Kitamura, N., Namba, K., and Hisano, Y. (2012). Epha4 controls the midline crossing and contralateral axonal projections of inferior olive neurons. J. Comp. Neurol. 520, 1702–1720. doi: 10.1002/cne.23008
Holz, A., Kollmus, H., Ryge, J., Niederkofler, V., Dias, J., Ericson, J., et al. (2010). The transcription factors Nkx2.2 and Nkx2.9 play a novel role in floor plate development and commissural axon guidance. Development 137, 4249–4260. doi: 10.1242/dev.053819
Hübers, A., Orekhov, Y., and Ziemann, U. (2008). Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur. J. Neurosci. 28, 364–371. doi: 10.1111/j.1460-9568.2008.06335.x
Iwasato, T., Katoh, H., Nishimaru, H., Ishikawa, Y., Inoue, H., Saito, Y. M., et al. (2007). Rac-GAP alpha-chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell 130, 742–753. doi: 10.1016/j.cell.2007.07.022
Jain, R. A., Bell, H., Lim, A., Chien, C. B., and Granato, M. (2014). Mirror movement-like defects in startle behavior of zebrafish dcc mutants are caused by aberrant midline guidance of identified descending hindbrain neurons. J. Neurosci. 34, 2898–2909. doi: 10.1523/JNEUROSCI.2420-13.2014
Jakeman, L. B., Chen, Y., Lucin, K. M., and McTigue, D. M. (2006). Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur. J. Neurosci. 23, 1997–2011. doi: 10.1111/j.1460-9568.2006.04721.x
Jankowska, E., Bannatyne, B. A., Stecina, K., Hammar, I., Cabaj, A., and Maxwell, D. J. (2009). Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J. Physiol. 587, 401–418. doi: 10.1113/jphysiol.2008.159236
Jen, J. C., Chan, W. M., Bosley, T. M., Wan, J., Carr, J. R., Rüb, U., et al. (2004). Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304, 1509–1513. doi: 10.1126/science.1096437
Jessell, T. M. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20–29. doi: 10.1038/35049541
Joosten, E. A., Schuitman, R. L., Vermelis, M. E., and Dederen, P. J. (1992). Postnatal development of the ipsilateral corticospinal component in rat spinal cord: a light and electron microscopic anterograde HRP study. J. Comp. Neurol. 326, 133–146. doi: 10.1002/cne.903260112
Karachi, C., Grabli, D., Bernard, F. A., Tandé, D., Wattiez, N., Belaid, H., et al. (2010). Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 120, 2745–2754. doi: 10.1172/JCI42642
Kartje-Tillotson, G., O’Donoghue, D. L., Dauzvardis, M. F., and Castro, A. J. (1987). Pyramidotomy abolishes the abnormal movements evoked by intracortical microstimulation in adult rats that sustained neonatal cortical lesions. Brain Res. 415, 172–177. doi: 10.1016/0006-8993(87)90283-6
Keino-Masu, K., Masu, M., Hinck, L., Leonardo, E. D., Chan, S. S., Culotti, J. G., et al. (1996). Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185. doi: 10.1016/s0092-8674(00)81336-7
Kennedy, T. E., Serafini, T., de la Torre, J. R., and Tessier-Lavigne, M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435. doi: 10.1016/0092-8674(94)90421-9
Kiehn, O. (2011). Development and functional organization of spinal locomotor circuits. Curr. Opin. Neurobiol. 21, 100–109. doi: 10.1016/j.conb.2010.09.004
Kiehn, O., Dougherty, K. J., Hägglund, M., Borgius, L., Talpalar, A., and Restrepo, C. E. (2010). Probing spinal circuits controlling walking in mammals. Biochem. Biophys. Res. Commun. 396, 11–18. doi: 10.1016/j.bbrc.2010.02.107
Kiehn, O., and Kjaerulff, O. (1996). Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J. Neurophysiol. 75, 1472–1482.
Kjaerulff, O., and Kiehn, O. (1996). Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J. Neurosci. 16, 5777–5794.
Koerte, I., Eftimov, L., Laubender, R. P., Esslinger, O., Schroeder, A. S., Ertl-Wagner, B., et al. (2010). Mirror movements in healthy humans across the lifespan: effects of development and ageing. Dev. Med. Child Neurol. 52, 1106–1112. doi: 10.1111/j.1469-8749.2010.03766.x
Kohashi, T., and Oda, Y. (2008). Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J. Neurosci. 28, 10641–10653. doi: 10.1523/jneurosci.1435-08.2008
Krams, M., Quinton, R., Mayston, M. J., Harrison, L. M., Dolan, R. J., Bouloux, P. M., et al. (1997). Mirror movements in X-linked Kallmann’s syndrome II. A PET study. Brain 120(Pt. 7), 1217–1228. doi: 10.1093/brain/120.7.1217
Kullander, K., Butt, S. J., Lebret, J. M., Lundfald, L., Restrepo, C. E., Rydström, A., et al. (2003). Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299, 1889–1892. doi: 10.1126/science.1079641
Kullander, K., Croll, S. D., Zimmer, M., Pan, L., McClain, J., Hughes, V., et al. (2001a). Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 15, 877–888. doi: 10.1101/gad.868901
Kullander, K., Mather, N. K., Diella, F., Dottori, M., Boyd, A. W., and Klein, R. (2001b). Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron 29, 73–84. doi: 10.1016/s0896-6273(01)00181-7
Kunkel-Bagden, E., Dai, H. N., and Bregman, B. S. (1992). Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp. Neurol. 116, 40–51. doi: 10.1016/0014-4886(92)90174-o
Lanuza, G. M., Gosgnach, S., Pierani, A., Jessell, T. M., and Goulding, M. (2004). Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42, 375–386. doi: 10.3410/f.1019388.218551
Leong, S. K., and Lund, R. D. (1973). Anomalous bilateral corticofugal pathways in albino rats after neonatal lesions. Brain Res. 62, 218–221. doi: 10.1016/0006-8993(73)90630-6
Lepage, J. F., Beaulé, V., Srour, M., Rouleau, G., Pascual-Leone, A., Lassonde, M., et al. (2012). Neurophysiological investigation of congenital mirror movements in a patient with agenesis of the corpus callosum. Brain Stimul. 5, 137–140. doi: 10.1016/j.brs.2011.02.004
Li, Q., and Martin, J. H. (2000). Postnatal development of differential projections from the caudal and rostral motor cortex subregions. Exp. Brain Res. 134, 187–198. doi: 10.1007/s002210000454
Martin, J. H., Choy, M., Pullman, S., and Meng, Z. (2004). Corticospinal system development depends on motor experience. J. Neurosci. 24, 2122–2132. doi: 10.1523/jneurosci.4616-03.2004
Martin, J. H., and Lee, S. J. (1999). Activity-dependent competition between developing corticospinal terminations. Neuroreport 10, 2277–2282. doi: 10.1097/00001756-199908020-00010
Mayer, M., Bötzel, K., Paulus, W., Plendl, H., Pröckl, D., and Danek, A. (1995). Movement-related cortical potentials in persistent mirror movements. Electroencephalogr. Clin. Neurophysiol. 95, 350–358. doi: 10.1016/0013-4694(95)00100-d
Mayston, M. J., Harrison, L. M., Quinton, R., Stephens, J. A., Krams, M., and Bouloux, P. M. (1997). Mirror movements in X-linked Kallmann’s syndrome. I. A neurophysiological study. Brain 120(Pt. 7), 1199–1216. doi: 10.1093/brain/120.7.1199
Mayston, M. J., Harrison, L. M., and Stephens, J. A. (1999). A neurophysiological study of mirror movements in adults and children. Ann. Neurol. 45, 583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w
Méneret, A., Depienne, C., Riant, F., Trouillard, O., Bouteiller, D., Cincotta, M., et al. (2014a). Congenital mirror movements: mutational analysis of RAD51 and DCC in 26 cases. Neurology 82, 1999–2002. doi: 10.1212/wnl.0000000000000477
Méneret, A., Trouillard, O., Vidailhet, M., Depienne, C., and Roze, E. (2014b). Congenital mirror movements: no mutation in DNAL4 in 17 index cases. J. Neurol. 261, 2030–2031. doi: 10.1007/s00415-014-7505-6
Metz, G. A., and Whishaw, I. Q. (2002). Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing and co-ordination. J. Neurosci. Methods 115, 169–179. doi: 10.1016/s0165-0270(02)00012-2
Meyer, B. U., Roricht, S., Grafin Von Einsiedel, H., Kruggel, F., and Weindl, A. (1995). Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118(Pt. 2), 429–440. doi: 10.1093/brain/118.2.429
Moran-Rivard, L., Kagawa, T., Saueressig, H., Gross, M. K., Burrill, J., and Goulding, M. (2001). Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron 29, 385–399. doi: 10.1016/s0896-6273(01)00213-6
Mulherkar, S., Liu, F., Chen, Q., Narayanan, A., Couvillon, A. D., Shine, H. D., et al. (2013). The small GTPase RhoA is required for proper locomotor circuit assembly. PLoS One 8:e67015. doi: 10.1371/journal.pone.0067015
Murase, N., Duque, J., Mazzocchio, R., and Cohen, L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 55, 400–409. doi: 10.1002/ana.10848
Nass, R. (1985). Mirror movement asymmetries in congenital hemiparesis: the inhibition hypothesis revisited. Neurology 35, 1059–1062. doi: 10.1212/wnl.35.7.1059
Nawabi, H., and Castellani, V. (2011). Axonal commissures in the central nervous system: how to cross the midline? Cell. Mol. Life Sci. 68, 2539–2553. doi: 10.1007/s00018-011-0691-9
Papadopoulou, M., Chairopoulos, K., Anagnostou, E., Kokotis, P., Zambelis, T., and Karandreas, N. (2010). Concurrent bilateral projection and activation of motor cortices in a patient with congenital mirror movements: a TMS study. Clin. Neurol. Neurosurg. 112, 824–828. doi: 10.1016/j.clineuro.2010.06.016
Peng, J., and Charron, F. (2013). Lateralization of motor control in the human nervous system: genetics of mirror movements. Curr. Opin. Neurobiol. 23, 109–118. doi: 10.1016/j.conb.2012.08.007
Perez, M. A., and Cohen, L. G. (2008). Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J. Neurosci. 28, 5631–5640. doi: 10.1523/jneurosci.0093-08.2008
Piallat, B., Chabardes, S., Torres, N., Fraix, V., Goetz, L., Seigneuret, E., et al. (2009). Gait is associated with an increase in tonic firing of the sub-cuneiform nucleus neurons. Neuroscience 158, 1201–1205. doi: 10.1016/j.neuroscience.2008.10.046
Pierani, A., Moran-Rivard, L., Sunshine, M. J., Littman, D. R., Goulding, M., and Jessell, T. M. (2001). Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29, 367–384. doi: 10.1016/s0896-6273(01)00212-4
Plotnik, M., Giladi, N., and Hausdorff, J. M. (2008). Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur. J. Neurosci. 27, 1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x
Plotnik, M., and Hausdorff, J. M. (2008). The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov. Disord. 23(Suppl. 2), S444–S450. doi: 10.1002/mds.21984
Quinlan, K. A., and Kiehn, O. (2007). Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J. Neurosci. 27, 6521–6530. doi: 10.1523/jneurosci.1618-07.2007
Rabe, N., Gezelius, H., Vallstedt, A., Memic, F., and Kullander, K. (2009). Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J. Neurosci. 29, 15642–15649. doi: 10.1523/jneurosci.5096-09.2009
Rabe Bernhardt, N., Memic, F., Gezelius, H., Thiebes, A. L., Vallstedt, A., and Kullander, K. (2012). DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev. Biol. 366, 279–289. doi: 10.1016/j.ydbio.2012.03.017
Reis, J., Swayne, O. B., Vandermeeren, Y., Camus, M., Dimyan, M. A., Harris-Love, M., et al. (2008). Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 586, 325–351. doi: 10.1113/jphysiol.2007.144824
Restrepo, C. E., Lundfald, L., Szabó, G., Erdelyi, F., Zeilhofer, H. U., Glover, J. C., et al. (2009). Transmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J. Comp. Neurol. 517, 177–192. doi: 10.1002/cne.22144
Rolf, B., Bastmeyer, M., Schachner, M., and Bartsch, U. (2002). Pathfinding errors of corticospinal axons in neural cell adhesion molecule-deficient mice. J. Neurosci. 22, 8357–8362.
Rosenzweig, E. S., Brock, J. H., Culbertson, M. D., Lu, P., Moseanko, R., Edgerton, V. R., et al. (2009). Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J. Comp. Neurol. 513, 151–163. doi: 10.1002/cne.21940
Runker, A. E., Little, G. E., Suto, F., Fujisawa, H., and Mitchell, K. J. (2008). Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 3:34. doi: 10.1186/1749-8104-3-34
Schieber, M. H. (2007). Chapter 2 comparative anatomy and physiology of the corticospinal system. Handb. Clin. Neurol. 82, 15–37. doi: 10.1016/s0072-9752(07)80005-4
Sehm, B., Perez, M. A., Xu, B., Hidler, J., and Cohen, L. G. (2010). Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb. Cortex 20, 34–45. doi: 10.1093/cercor/bhp075
Serafini, T., Kennedy, T. E., Galko, M. J., Mirzayan, C., Jessell, T. M., and Tessier-Lavigne, M. (1994). The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424. doi: 10.1016/0092-8674(94)90420-0
Serradj, N., and Jamon, M. (2009). The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behav. Brain Res. 201, 59–65. doi: 10.1016/j.bbr.2009.01.030
Serradj, N., Paixao, S., Sobocki, T., Feinberg, M., Klein, R., Kullander, K., et al. (2014). EphA4-mediated ipsilateral corticospinal tract misprojections are necessary for bilateral voluntary movements but not bilateral stereotypic locomotion. J. Neurosci. 34, 5211–5221. doi: 10.1523/JNEUROSCI.4848-13.2014
Shibasaki, H., and Hallett, M. (2006). What is the Bereitschaftspotential? Clin. Neurophysiol. 117, 2341–2356. doi: 10.1016/j.clinph.2006.04.025
Shibasaki, H., and Nagae, K. (1984). Mirror movement: application of movement-related cortical potentials. Ann. Neurol. 15, 299–302. doi: 10.1002/ana.410150317
Shik, M. L., Severin, F. V., and Orlovskii, G. N. (1966). [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika 11, 659–666.
Shik, M. L., Severin, F. V., and Orlovskii, G. N. (1967). [Structures of the brain stem responsible for evoked locomotion]. Fiziol. Zh. SSSR Im. I M Sechenova 53, 1125–1132.
Shu, T., Valentino, K. M., Seaman, C., Cooper, H. M., and Richards, L. J. (2000). Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J. Comp. Neurol. 416, 201–212. doi: 10.1002/(sici)1096-9861(20000110)416:2<201::aid-cne6>3.0.co;2-z
Smith, J. C., and Feldman, J. L. (1987). In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J. Neurosci. Methods 21, 321–333. doi: 10.1016/0165-0270(87)90126-9
Srour, M., Rivière, J. B., Pham, J. M., Dube, M. P., Girard, S., Morin, S., et al. (2010). Mutations in DCC cause congenital mirror movements. Science 328:592. doi: 10.1126/science.1186463
Staudt, M., Grodd, W., Gerloff, C., Erb, M., Stitz, J., and Krageloh-Mann, I. (2002). Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain 125, 2222–2237. doi: 10.1093/brain/awf227
Stokke, M. F., Nissen, U. V., Glover, J. C., and Kiehn, O. (2002). Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J. Comp. Neurol. 446, 349–359. doi: 10.1002/cne.10211
Takakusaki, K., Habaguchi, T., Ohtinata-Sugimoto, J., Saitoh, K., and Sakamoto, T. (2003). Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119, 293–308. doi: 10.1016/s0306-4522(03)00095-2
Talpalar, A. E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., and Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500, 85–88. doi: 10.1038/nature12286
Tennant, K. A., and Jones, T. A. (2009). Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J. Neurosci. Methods 181, 18–26. doi: 10.1016/j.jneumeth.2009.04.009
Vallstedt, A., and Kullander, K. (2013). Dorsally derived spinal interneurons in locomotor circuits. Ann. N Y Acad. Sci. 1279, 32–42. doi: 10.1111/j.1749-6632.2012.06801.x
Verstynen, T., Spencer, R., Stinear, C. M., Konkle, T., Diedrichsen, J., Byblow, W. D., et al. (2007). Ipsilateral corticospinal projections do not predict congenital mirror movements: a case report. Neuropsychologia 45, 844–852. doi: 10.1016/j.neuropsychologia.2006.08.019
Vulliemoz, S., Raineteau, O., and Jabaudon, D. (2005). Reaching beyond the midline: why are human brains cross wired? Lancet Neurol. 4, 87–99. doi: 10.1016/s1474-4422(05)00990-7
Wallén-Mackenzie, A., Gezelius, H., Thoby-Brisson, M., Nygård, A., Enjin, A., Fujiyama, F., et al. (2006). Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J. Neurosci. 26, 12294–12307. doi: 10.3410/f.1052770.504685
Whishaw, I. Q. (2000). Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology 39, 788–805. doi: 10.1016/s0028-3908(99)00259-2
Whishaw, I. Q., Pellis, S. M., Gorny, B., Kolb, B., and Tetzlaff, W. (1993). Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 56, 59–76. doi: 10.1016/0166-4328(93)90022-i
Winn, P. (2008). Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat. Disord. 14(Suppl. 2), S194–S198. doi: 10.1016/j.parkreldis.2008.04.030
Yokoyama, N., Romero, M. I., Cowan, C. A., Galvan, P., Helmbacher, F., Charnay, P., et al. (2001). Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron 29, 85–97. doi: 10.1016/s0896-6273(01)00182-9
Zhang, Y., Narayan, S., Geiman, E., Lanuza, G. M., Velasquez, T., Shanks, B., et al. (2008). V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60, 84–96. doi: 10.3410/f.1125790.582877
Keywords: mirror movement, hopping gait, corticospinal tract, corpus callosum, spinal cord, axon guidance
Citation: Welniarz Q, Dusart I, Gallea C and Roze E (2015) One hand clapping: lateralization of motor control. Front. Neuroanat. 9:75. doi: 10.3389/fnana.2015.00075
Received: 31 March 2015; Accepted: 17 May 2015;
Published online: 02 June 2015.
Edited by:
Yun-Qing Li, The Fourth Military Medical University, ChinaReviewed by:
José A. Armengol, University Pablo de Olavide, SpainYu-Qiang Ding, Tongji Unversity, China
Copyright © 2015 Welniarz, Dusart, Gallea and Roze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuel Roze, Département des Maladies du Système Nerveux, AP-HP, Hôpital Pitié Salpêtrière, 47–83 boulevard de l’Hôpital, 75013 Paris, France, emmanuel.flamand-roze@psl.aphp.fr
 Quentin Welniarz
Quentin Welniarz Isabelle Dusart
Isabelle Dusart Cécile Gallea
Cécile Gallea Emmanuel Roze
Emmanuel Roze