Recent trends in wearable device used to detect freezing of gait and falls in people with Parkinson’s disease: A systematic review
- 1Laboratory of Laser Sports Medicine, South China Normal University, Guangzhou, Guangdong, China
- 2Department of Gastroenterology, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, Guangdong, China
Background: The occurrence of freezing of gait (FOG) is often observed in moderate to last-stage Parkinson’s disease (PD), leading to a high risk of falls. The emergence of the wearable device has offered the possibility of FOG detection and falls of patients with PD allowing high validation in a low-cost way.
Objective: This systematic review seeks to provide a comprehensive overview of existing literature to establish the forefront of sensors type, placement and algorithm to detect FOG and falls among patients with PD.
Methods: Two electronic databases were screened by title and abstract to summarize the state of art on FOG and fall detection with any wearable technology among patients with PD. To be eligible for inclusion, papers were required to be full-text articles published in English, and the last search was completed on September 26, 2022. Studies were excluded if they; (i) only examined cueing function for FOG, (ii) only used non-wearable devices to detect or predict FOG or falls, and (iii) did not provide sufficient details about the study design and results. A total of 1,748 articles were retrieved from two databases. However, only 75 articles were deemed to meet the inclusion criteria according to the title, abstract and full-text reviewed. Variable was extracted from chosen research, including authorship, details of the experimental object, type of sensor, device location, activities, year of publication, evaluation in real-time, the algorithm and detection performance.
Results: A total of 72 on FOG detection and 3 on fall detection were selected for data extraction. There were wide varieties of the studied population (from 1 to 131), type of sensor, placement and algorithm. The thigh and ankle were the most popular device location, and the combination of accelerometer and gyroscope was the most frequently used inertial measurement unit (IMU). Furthermore, 41.3% of the studies used the dataset as a resource to examine the validity of their algorithm. The results also showed that increasingly complex machine-learning algorithms had become the trend in FOG and fall detection.
Conclusion: These data support the application of the wearable device to access FOG and falls among patients with PD and controls. Machine learning algorithms and multiple types of sensors have become the recent trend in this field. Future work should consider an adequate sample size, and the experiment should be performed in a free-living environment. Moreover, a consensus on provoking FOG/fall, methods of assessing validity and algorithm are necessary.
Systematic Review Registration: PROSPERO, identifier CRD42022370911.
Introduction
Parkinson’s disease (PD) is an age-related progressive neurodegenerative condition clinically characterized by bradykinesia and either resting tremor or rigidity, affecting about 1% of adults older than 60 worldwide (Samii et al., 2004). The freezing of gait (FOG) occurrence is often observed in moderate to last-stage PD, increasing fall risk, reducing the quality of life, and the likelihood of independent living (Kerr et al., 2010).
As a complex and highly-variable phenomenon, FOG can be defined as a brief episode absence or marked reduction in the forward progression of the feet despite the intention to walk, which remains a persistent and incapacitating motor problem for many patients in daily life (Rahman et al., 2008). Episodes can be brief or exceed 30 s (Schaafsma et al., 2003). It is hard to anticipate the occurrence of FOG for patients with PD who live at home since FOG can occur several times a day and most commonly between doses when the medication wears off (Nantel and Bronte-Stewart, 2014; Okuma et al., 2018).
FOG management can be divided into pharmacological treatment (Nonnekes et al., 2015) and non-pharmacological treatment, such as exercise (Corcos et al., 2013), deep brain stimulation (Hacker et al., 2020), or cueing devices (Griffin et al., 2011). Meanwhile, due to the limitations and side effects of the pharmacological intervention (Obeso et al., 2000; Aquino and Fox, 2015), more attention has been focused on non-pharmacological interventions, such as resistance exercises can evaluate the severity of FOG and should run through the diagnosis and treatment. The most common evaluation methods include the Timed up and Go test (TUG; Mak and Pang, 2009; Kerr et al., 2010), Unified Parkinson’s Disease Rating Scale (UPDRS; Lun et al., 2005; Kerr et al., 2010), Freezing of Gait Questionnaire (FOG-Q; Giladi et al., 2009; Tambasco et al., 2015) and so on. Nevertheless, most of them have limited specificity and sensitivity for identifying prospective fallers in patients with PD (Boonstra et al., 2008) and may not be sufficiently sensitive to detect changes in balance and walking in the PD population with mild to moderate disease severity (Lo et al., 2010; Fox et al., 2011; Ustinova et al., 2011; Tomlinson et al., 2014).
With the development of wireless communication and microelectronics technology, wearable micro-electro-mechanical systems (MEMS), such as accelerometers and magnetometers, have become small, lightweight and low-cost (Patel et al., 2012). There is a growing interest in using wearable health technology to access FOG and falls. These sensors, generally consisting of accelerometers, gyroscopes, magnetometers and others, can capture body movements in real-time. With a significant advantage compared to clinical scales and conventional assessment tools, the wearable device can act as a personal healthcare worker to help patients evaluate the severity of PD, improve treatment, and avoid the incidence of privacy breaches (Patel et al., 2012; Del Din et al., 2016).
However, owing to the high degree of diversity and complexity of FOG, a huge body of research investigated the feasibility of numerous sensors on various body parts with different algorithms, ranging from machine learning and threshold approaches. There is little agreement on the most effective system design. Meanwhile, most current review articles about FOG detection with wearable sensors ignored the relationship between technology and time. Therefore, we provide a systematic review of the use of wearable systems detect FOG and falls in PD, and the development of this technology, to help guide future research.
Review methodology
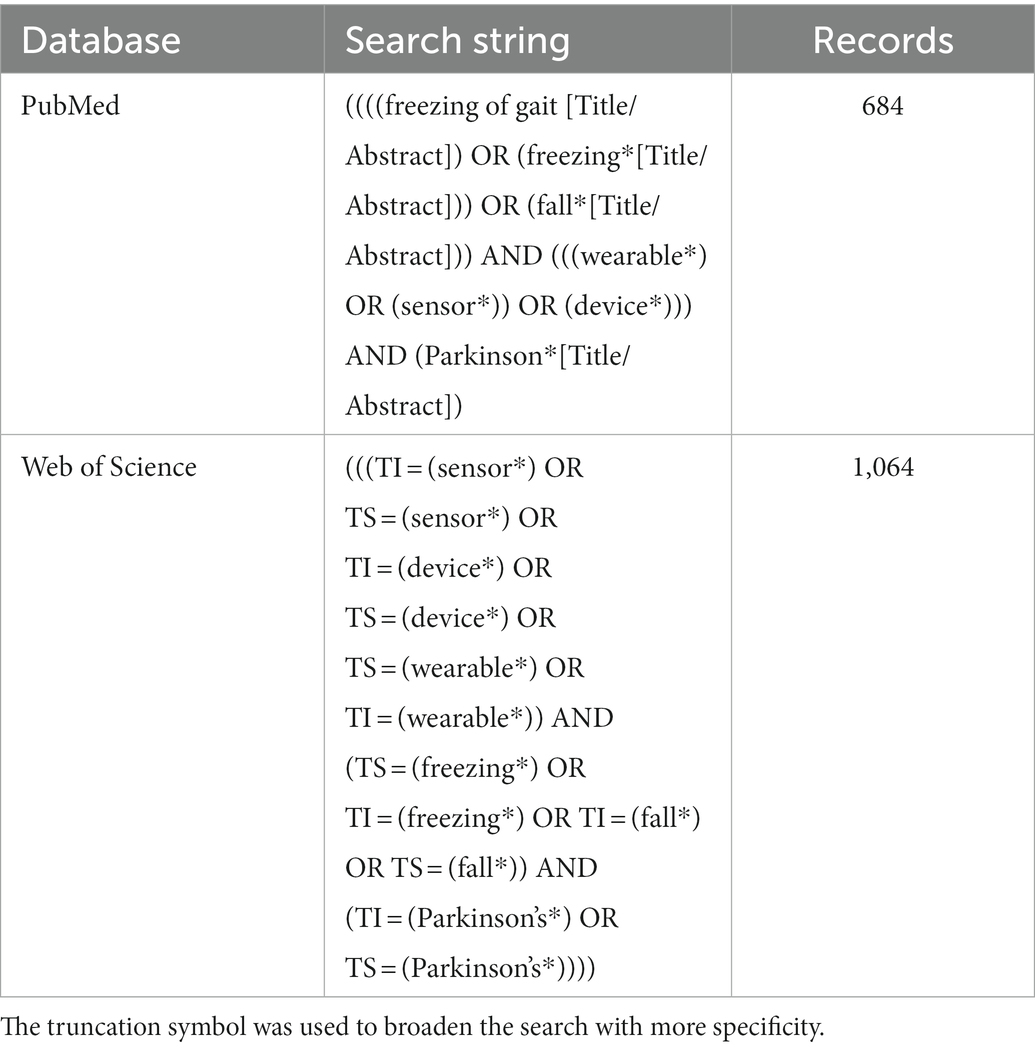
A literature review was performed according to the guidelines of the PRISMA statement. An electronic database search of titles and abstracts was performed by searching Pubmed and Web of Science, and the final search was completed on September 26, 2022. These databases were chosen to allow both medical and engineering journals to be included in the search process. The final search query is summarized in Table 1.
Only original, full-text, peer-reviewed journal articles published in English to access FOG and falls in people with PD were considered in this systematic review. Duplicate findings were removed, and the remaining pieces were relevant according to their title and abstract. Leaving documents were reviewed in full.
Articles were screened based on a series of eligibility standards:
1. Use wearable devices (a single or combination of wearable devices) to collect data as input.
2. Involve people with PD or a dataset of PD.
3. Present original research on the validation of wearable sensors to detect, predict or measure FOG, falls or fall risk.
Studies were excluded:
1. Only examined cueing function for FOG.
2. Only use non-wearable devices to detect or predict FOG or falls.
3. Did not provide sufficient details about the study design and results.
Two reviewers independently screened titles and abstracts included in electronic databases according to eligibility standards. Two reviewers screened the full text of those selected for eligibility. Disagreements between reviewers were resolved by consensus, if needed, after the consultation of a third reviewer. Variable was extracted from chosen research and classified in a predefined table. Authorship, details of the experimental object (i.e., study population, age and medication status), type of sensor, device site, activities, year of publication, evaluation in real-time, the algorithm to process data and classifier performance were all recorded.
Results
Studies selection
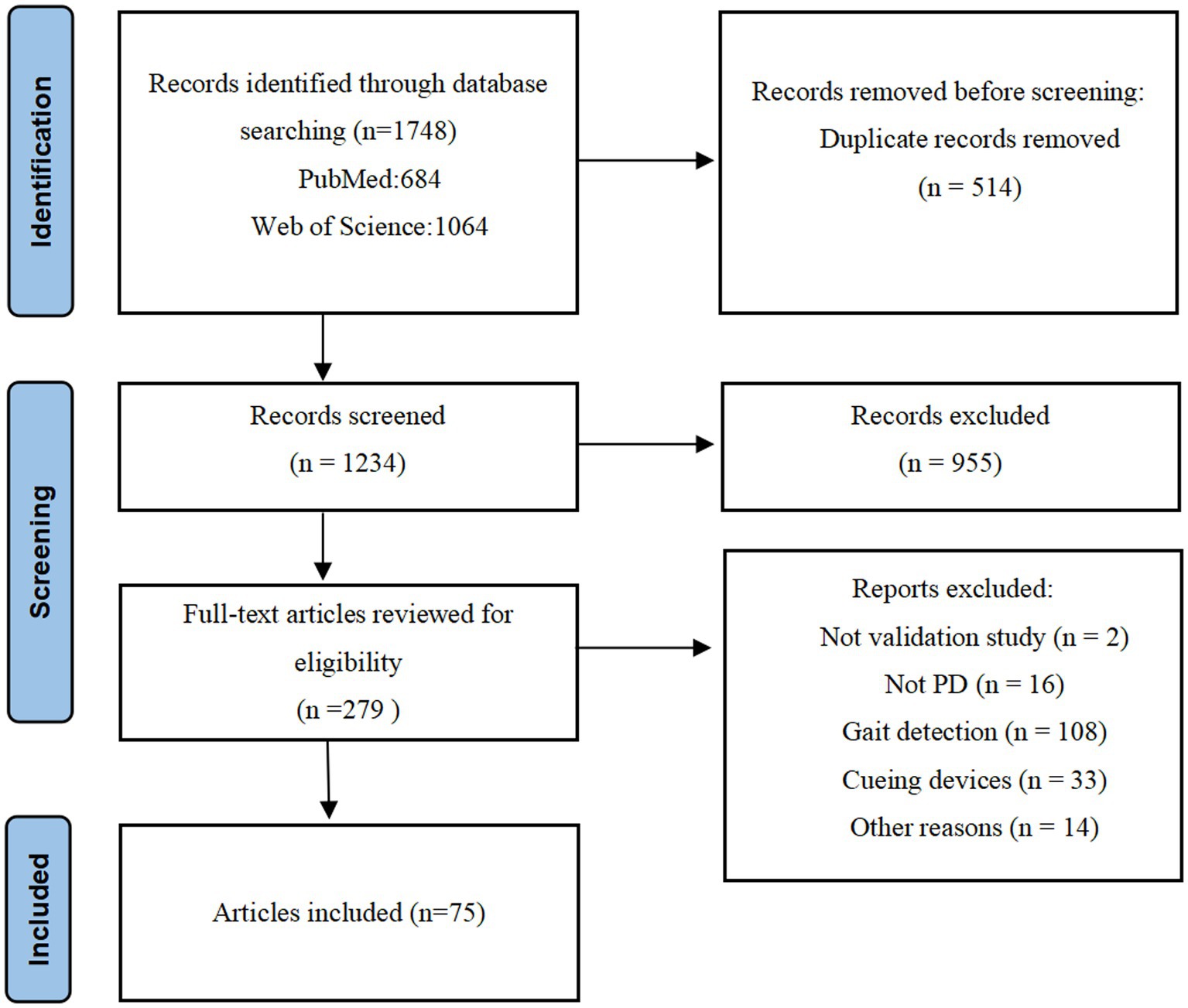
An initial database search identified 1,748 articles that were potentially eligible for inclusion. 514 articles were excluded as duplicates, resulting in 1,234 papers being screened (955 records excluded). The remaining 279 articles were screened by full text. Following screening and eligibility assessment, 75 pieces were shortlisted in this systemic review (72 on FOG detection and 3 on fall detection of PD patients). A complete overview of the selection process is summarized in Figure 1.
FOG detection
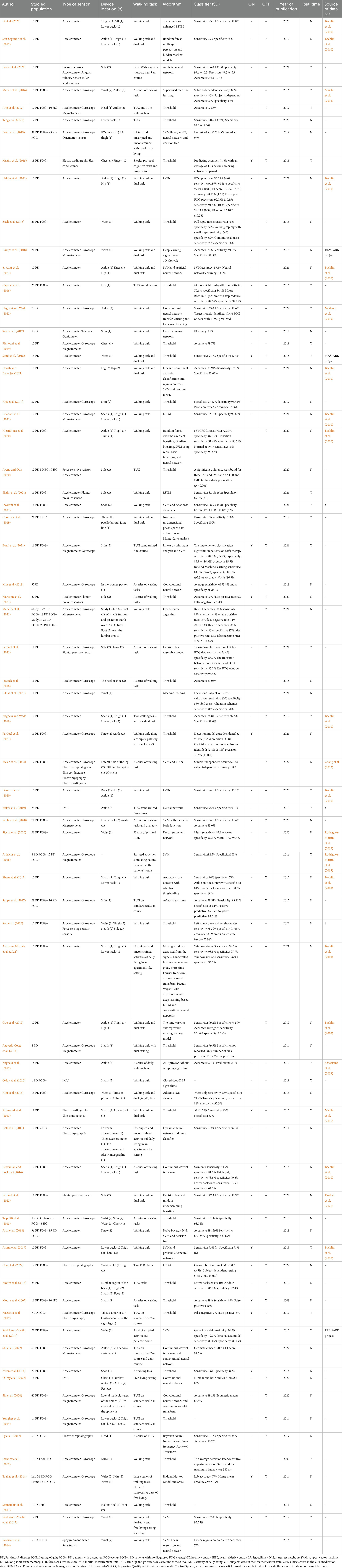
For FOG detection, 72 papers investigated the usage of wearable devices to access FOG in PD (Table 2; Mazilu et al., 2015, 2016; Zach et al., 2015; Capecci et al., 2016; Ahn et al., 2017; Kita et al., 2017; Saad et al., 2017; Camps et al., 2018; Samà et al., 2018; Borzì et al., 2019; Chomiak et al., 2019; Pierleoni et al., 2019; San-Segundo et al., 2019; Ayena and Otis, 2020; Kleanthous et al., 2020; Li et al., 2020; Tang et al., 2020; Dvorani et al., 2021; El-Attar et al., 2021; Esfahani et al., 2021; Ghosh and Banerjee, 2021; Halder et al., 2021; Prado et al., 2021; Shalin et al., 2021; Naghavi and Wade, 2022). The number of subjects used to test the validity of the FOG detection system varied significantly between studies, from 1 (O’day et al., 2020) to 131 (Borzì et al., 2019) (MED = 12). The studied population consisted of patients with Parkinson’s disease, PD patients with diagnosed FOG events (n = 35), PD patients with no diagnosed FOG events (n = 6), healthy control (n = 6) and healthy elderly control (n = 1). Furthermore, 43.1% of papers included in this review (n = 31) used the data set as a resource to examine the validity of their algorithm. The most commonly used data set was from Bachlin et al. (2010).
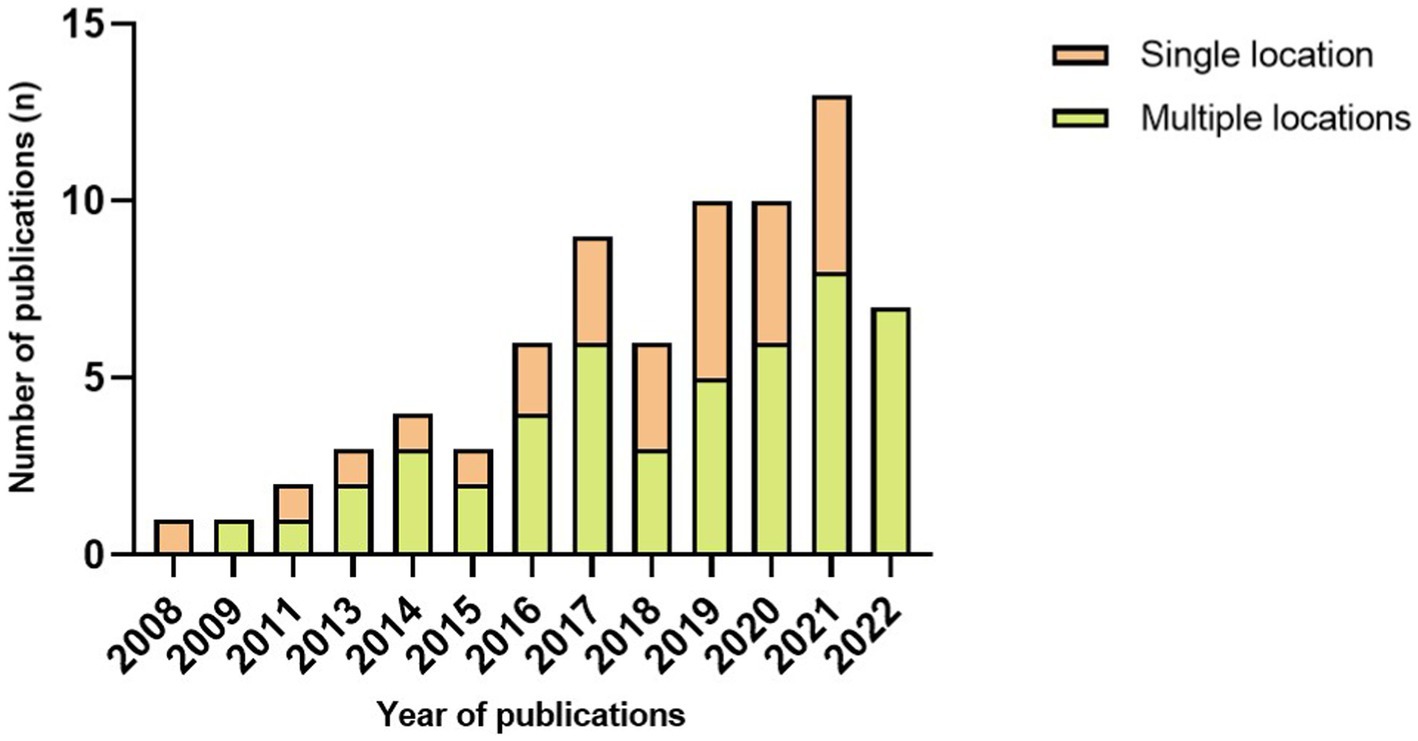
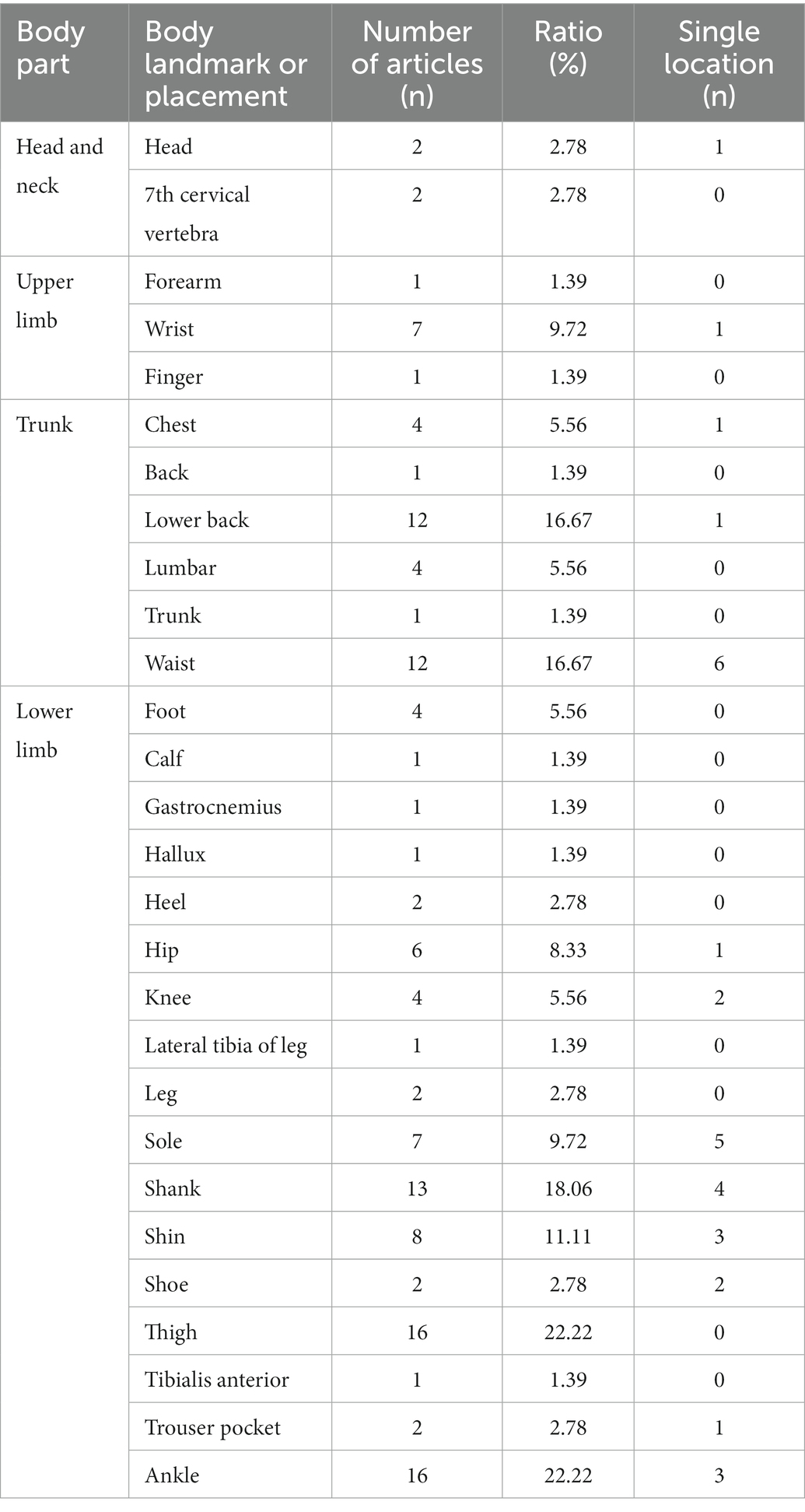
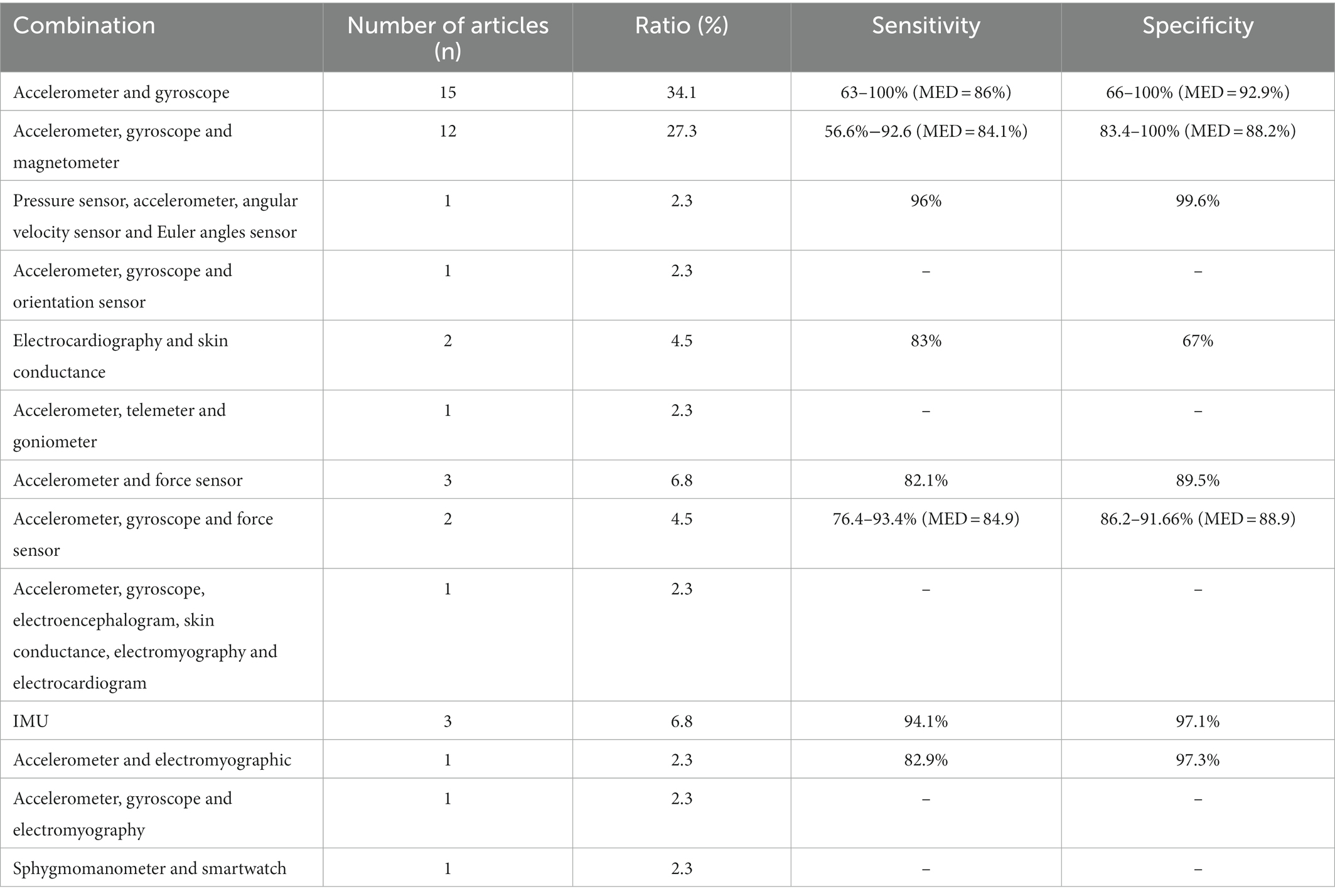
Device type and placement are remarkably diverse between studies. Concerning the type of sensor, 27 papers used a single type of wearable device to implement FOG detection, including 25 articles that used an accelerometer, two with electroencephalography and one with plantar pressure sensors. It is important to note that 45 articles used multiple wearable device types to access FOG detection (Figure 2). The combination of accelerometers and gyroscopes was the choice of 15 papers, and 12 pieces combined accelerometers, gyroscopes and magnetometers to access FOG detection. Likewise, wearable devices are located on various parts of the human body. Of the 72 included studies, the same number of papers reported placing a wearable device on the thigh and ankle (22.22% of studies, n = 16, 3 times as the single site on the ankle), the shank (19.44% of studies, n = 14, 4 times as the single location), the waist (16.67% of studies, n = 12) and the lower back (16.67% of studies, n = 12, 6 times as the single location). Details on the studies included in this systematic review that reported placement are summarized in Figure 3 and Table 3.
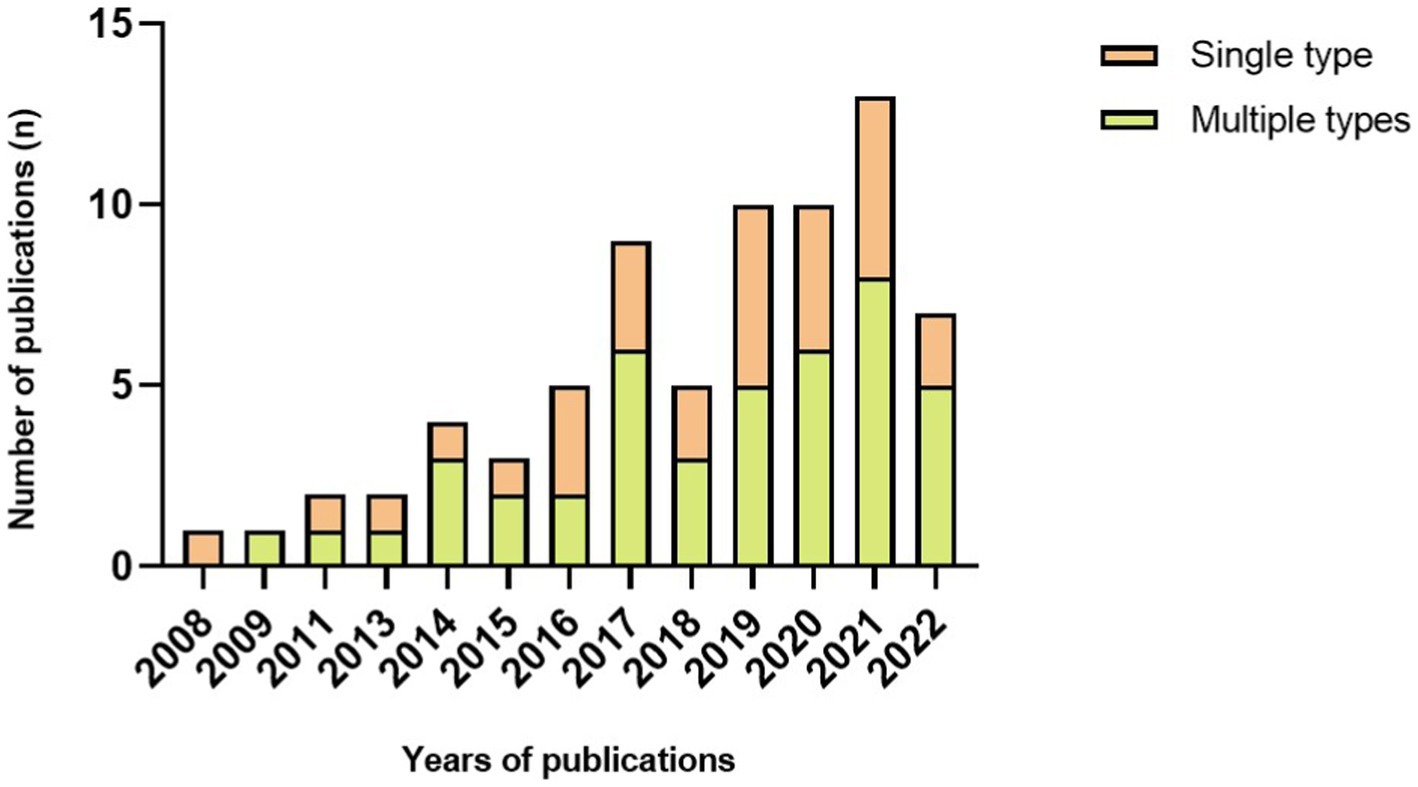
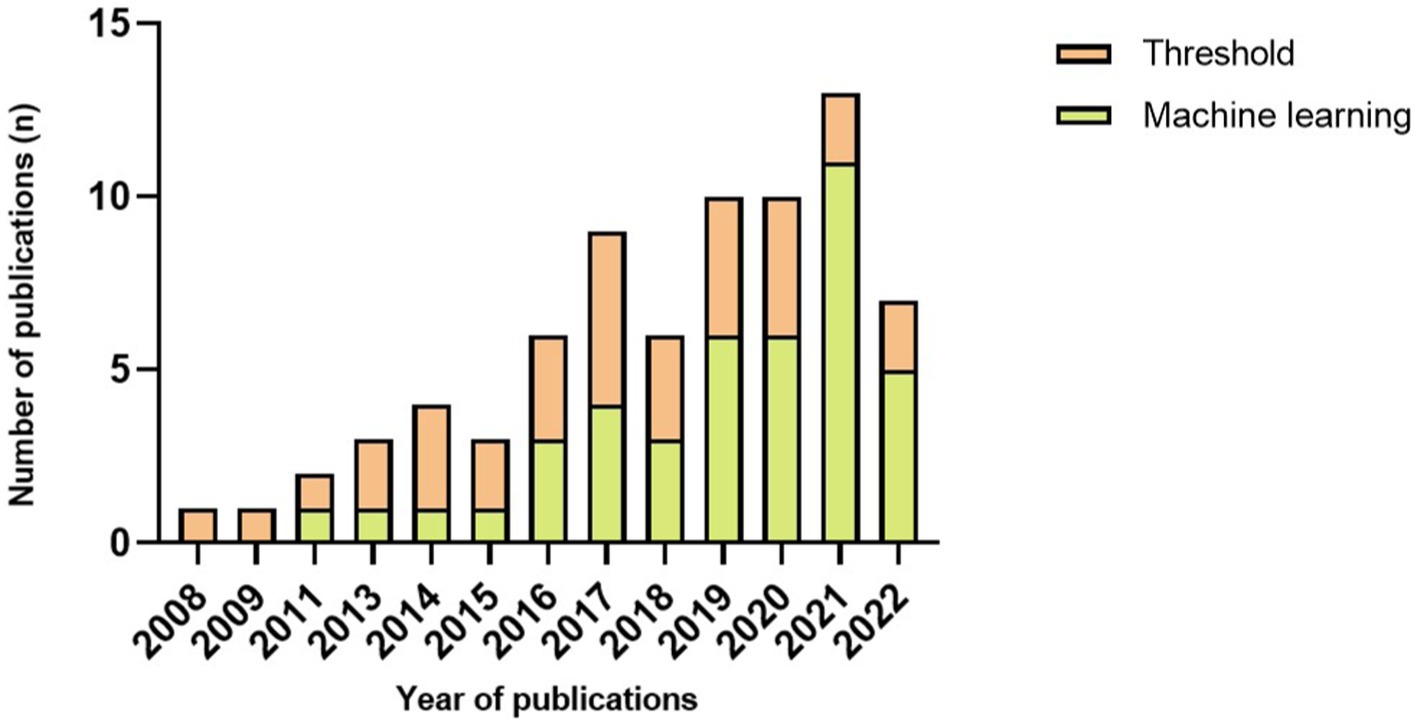
The algorithm plays a vital role in FOG detection and varies in complexity. Generally, it can be categorized into threshold and machine learning. Of 72 papers, 30 used threshold-based algorithms to detect FOG, leaving 42 pieces used machine learning. In Figure 4, we observed that the number of articles that used thresholds was more than or equal to articles that used machine learning before 2019. Since then, more papers have used machine learning than the threshold, even five times higher in 2021. Evaluation in real-time was the choice of 24 articles. Machine learning algorithms were used in 15 of the 24 articles, leaving 9 papers that used threshold algorithms to detect a FOG episode as it occurs.
Among the 73 articles investigating FOG detection, a vast majority of studies (n = 71) reported measures of validation performance [e.g., sensitivity, specificity, accuracy, area under the curve (AUC) or f-score], and 2 studies did not report validity measures (Stamatakis et al., 2011; Yungher et al., 2014). Overall, the sensitivities reported in the reviewed studies ranged from 63 to 100%, from 59 to 100% for specificity, from 71.3 to 99.7% for accuracy, AUC ranged from 76 to 97% and f-score ranged from 77.98 to 92.10% (Table 4).
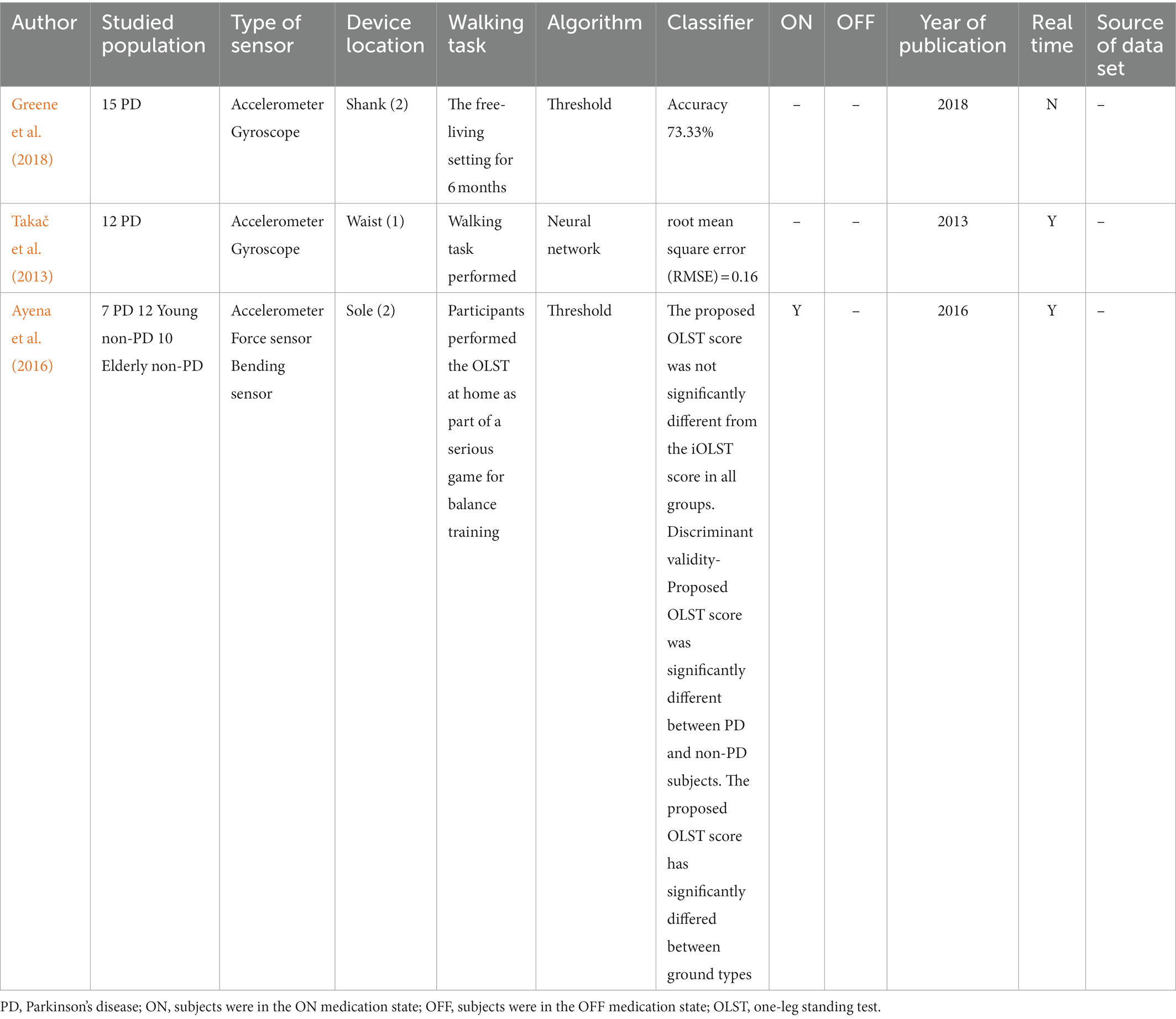
Fall detection
A total of 3 papers on fall detection were included and varied in the study population, approach and performance (Table 5). The number of subjects ranged from 12 to 29 (MED = 15), and the studied population can be categorized into patients with PD (n = 3), healthy control (n = 1) and healthy elderly control (n = 1). None of them used a data set.
All articles used multiple wearable devices. However, the type of sensor and placement are remarkably diverse between studies. Two pieces used accelerometers and gyroscopes to detect falls, while the remaining one used an accelerometer, force sensor and bending sensor. As for device location, 1 article placed sensors on the shank, 1 on the waist and 1 on the insole. Regarding the algorithm, 2 papers used threshold to process data, leaving 1 article used machine learning. Three articles reported fall detection performance, but only two performed fall detection in real-time. Meanwhile, the measure of validation performance was varied. One piece used accuracy (73.33%), and one used root mean square error (0.16), leaving one article mentioning the data difference.
Discussion
This systematic review aimed to examine the articles of FOG and fall detection area to determine the best type of wearable devices, the most appropriate device locations, and the most effective approaches to processing data, which can balance accuracy and immediacy. This paper also discussed the recent trend of related technologies. A total of 75 articles were included in this review, 72 on FOG and 3 on falls.
FOG/falls detection apparatus
The apparatus used in FOG or fall detection can be generally divided into wearable devices and context-aware systems. Due to the development of wireless communication and microelectronics technology, many researchers focus on wearable devices to detect FOG or falls. In this review, the type of sensors and the combination are remarkably diverse between studies. Twenty-eight studies used a single type of wearable device to detect FOG, and 92.9% of them relied on accelerometers only (n = 26), and the sensitivity of using an accelerometer only ranged from 70.1 to 99.2% (MED = 88.52%), and the specificity ranged from 59 to 99.83% (MED = 88%). Meanwhile, 2 studies used electroencephalography only, while Pardoel et al. (2022), the pressure sensor was the only device for FOG detection, its sensitivity ranged from 77.3 to 84.2%, and specificity ranged from 82.9 to 88%. These results indicated that the type of sensor would not affect the accuracy of using a single type of sensor. The use of single kind of sensor can reduce the calculation and complexity of the FOG detection system.
In this review, we found a large proportion of studies using IMU, which often consists of more than one type of sensor, have become popular in FOG and fall detection applications. As shown in Table 2, a total of 44 papers utilized IMU for FOG detection, 3 of them only mentioned IMU, remaining 41 articles illustrated the type of sensors. The combination consisting of an accelerometer and a gyroscope was the most popular in this review, 15 papers used this combination, and the combination of an accelerometer, a gyroscope and a magnetometer was the choice of 12 articles. The difference in validation performance (e.g., sensitivity and specificity) between combinations were slight, except for the specificity of the combination of electrocardiography and skin conductance (67%). Multiple types of sensors were the choice of 3 articles to detect falls in patients with PD. There might be several reasons behind this trend. First, the IMUs can provide multidimensional data to measure body movement of FOG and fall detection, improving the validation performance. Second, the rapid development of MEMS facilitated lower energy consumption and small-sized chips with low cost, which makes the placement of wearable IMUs much easier. Third, as machine-learning technology advances rapidly, researchers can process vast quantities of data and conclude with high accuracy.
Device location
As mentioned above, various protocols were described concerning the device’s location on the human body to detect FOG and falls. Generally, the human body is divided into the head and neck, trunk, upper limb and lower limb. Of the 72 studies included in this review, 84.7% studies used the lower limb as a wearable device location (n = 61). The most popular placements were the thigh (n = 16) and the ankle (n = 16). Besides, the sole was the most common single placement on the lower limb. The results also showed that the waist (n = 12) and lower back (n = 12) were the most used on the trunk, and the waist (n = 6) was the most frequent single placement on the human body. Considering fall detection, 2 articles used the lower limb as a wearable sensor location, leaving 1 article placed sensors on the upper limb. The critical task of the lower limb is to support the entire body. Changes in the lower limb (e.g., velocity, direction and speed) can intuitively reflect the status of patients.
FOG/fall detection algorithms
FOG and fall detection approaches vary in complexity. Threshold-based algorithm appeared to be the most straightforward method in FOG and fall detection. A total of 30 articles included in this review use threshold-based algorithms in FOG detection. As for fall detection, 2 papers used threshold-based algorithms. With threshold-based algorithms, the occurrence of FOG and falls are considered to be detected if indicators are beyond a specific threshold. Otherwise, the event of FOG/fall does not exist. With the advantage of being computationally efficient, threshold methods can process data in a short period, making them easily used in real-time systems. However, the drawback of the threshold-based algorithm is obvious. Generally, a high threshold may lead to a low false positive rate but also ignore some occurrences of FOG/fall, and vice versa. This is the conundrum that almost current researchers have to face.
To improve the accuracy of FOG and fall detection, machine learning algorithms, including SVM (Tzallas et al., 2014; Ahlrichs et al., 2016; Iakovakis et al., 2016; Rodríguez-Martín et al., 2017; Aich et al., 2018; Arami et al., 2019; Borzì et al., 2019, 2021; Kleanthous et al., 2020; Reches et al., 2020; Dvorani et al., 2021; El-Attar et al., 2021; Ghosh and Banerjee, 2021; Mesin et al., 2022), k-NN (Aich et al., 2018; Borzì et al., 2019; Demrozi et al., 2020; Halder et al., 2021; Mesin et al., 2022), decision trees (Aich et al., 2018; Borzì et al., 2019; Pardoel et al., 2021, 2022), hidden Markov model (Tzallas et al., 2014; San-Segundo et al., 2019), neural network (Cole et al., 2011; Iakovakis et al., 2016; Ly et al., 2017; Saad et al., 2017; Kim et al., 2018; Arami et al., 2019; Borzì et al., 2019; Mikos et al., 2019; Kleanthous et al., 2020; O’day et al., 2020, 2022; Shi et al., 2020, 2022; Sigcha et al., 2020; Ashfaque Mostafa et al., 2021; El-Attar et al., 2021; Prado et al., 2021; Naghavi and Wade, 2022), random forest (San-Segundo et al., 2019; Kleanthous et al., 2020; Ghosh and Banerjee, 2021) and LSTM (Li et al., 2020; Ashfaque Mostafa et al., 2021; Esfahani et al., 2021; Shalin et al., 2021; Guo et al., 2022), were used extensively in recent studies. Data were collected from sensors, and a training period is necessary for machine learning. Machine learning can improve the validation performance of FOG/fall detection but might require a longer time for data processing. With the development of computer technology, studies have increasingly examined machine learning algorithms in real-time FOG detection. Furthermore, the utilization of machine learning algorithms to identify FOG is becoming the primary current for the sake of improving validation performance (Figure 3).
FOG/fall detection performance
The validation performance of FOG/fall detection varies, including sensitivity, specificity, accuracy, AUC and f-score. Among the 73 articles investigating FOG detection, the sensitivities ranged from 63 to 100%. The highest sensitivity (100%) was achieved by Chomiak et al. (2019) and the lowest sensitivity (63%) was reported by Naghavi et al. (2019). The specificities were from 59 to 100%. The lowest specificity (59%) was written by Zach et al. (2015) and only one article reported 100% specificity (Chomiak et al., 2019). Some papers used accuracy as a validation performance standard, ranging from 71.3 to 99.7%. The accuracy (71.3%) in Mazilu et al. (2015) was the lowest, and the highest accuracy (99.7%) was achieved by Pierleoni et al. (2019). A few studies reported AUC ranged from 76 to 97%. The highest AUC (97%) was achieved by Borzì et al. (2019) and the lowest AUC (76%) was in Palmerini et al. (2017) A few studies utilized f-score to evaluate validation performance ranging from 77.98 to 92.10%. The lowest f-score (77.98%) was reported by Ren et al. (2022) and the highest f-score (92.10%) was written by Halder et al. (2021). Meanwhile, the measure of validation performance various considerably, including accuracy (73.33%, n = 1), root mean square error (0.16, n = 1) and data difference (n = 1).
It should be noted that the conclusion of the best FOG/fall detection based on the reported validation performance is unwarranted since the collection approaches of FOG/fall data varies considerably, including methods of provoking FOG/fall, and the number of subjects varied, which might affect the validation performance.
Conclusion
Based on 75 articles on wearable device utilization for FOG and fall detection in patients with PD, this review represented the recent trend and several critical aspects in current research, including the type of sensors, device location, FOG/fall algorithms, the number of subjects (or data set) and validation performance. Research on FOG and fall detection has been developed rapidly in recent years, and emerging technology like machine learning can balance accuracy and immediacy. Furthermore, using multiple types of sensors has become the recent trend in FOG and fall detection in patients with PD. Nevertheless, the limitations in the current studies were obvious. The research was carried out with a low number of samples. A universally recognized adequate standard provoking FOG and fall is yet lacking, it might lead researchers to encounter difficulties in finding the best system based on the reported validation performance. Besides, there is little consensus on algorithm analysis. Future work should give careful consideration to address these limitations. First, an adequately studied population should be provided to support their study. Second, a consensus on provoking FOG/fall, methods of assessing validity and algorithm are necessary. Lastly, studies should carry out in a free-living environment with low-cost and low-energy consumption apparatus.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JH and TH conceived and designed the methodology of the systematic review. TH and ML extracted and collected the relevant information. TH drafted the manuscript. JH supervised the study at different steps and reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahlrichs, C., Samà, A., Lawo, M., Cabestany, J., Rodríguez-Martín, D., Pérez-López, C., et al. (2016). Detecting freezing of gait with a tri-axial accelerometer in Parkinson’s disease patients. Med. Biol. Eng. Comput. 54, 223–233. doi: 10.1007/s11517-015-1395-3
Ahn, D., Chung, H., Lee, H. W., Kang, K., Ko, P. W., Kim, N. S., et al. (2017). Smart gait-aid glasses for Parkinson’s disease patients. IEEE Trans. Biomed. Eng. 64, 2394–2402. doi: 10.1109/TBME.2017.2655344
Aich, S., Pradhan, P. M., Park, J., Sethi, N., Vathsa, V., and Kim, H. C. (2018). A validation study of freezing of gait (fog) detection and machine-learning-based fog prediction using estimated gait characteristics with a wearable accelerometer. Sensors 18:3287. doi: 10.3390/s18103287
Aquino, C. C., and Fox, S. H. (2015). Clinical Spectrum of levodopa-induced complications. Mov. Disord. 30, 80–89. doi: 10.1002/mds.26125
Arami, A., Poulakakis-Daktylidis, A., Tai, Y. F., and Burdet, E. (2019). Prediction of gait freezing in parkinsonian patients: a binary classification augmented with time series prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 1909–1919. doi: 10.1109/TNSRE.2019.2933626
Ashfaque Mostafa, T., Soltaninejad, S., McIsaac, T. L., and Cheng, I. (2021). A comparative study of time frequency representation techniques for freeze of gait detection and prediction. Sensors 21:6446. doi: 10.3390/s21196446
Ayena, J. C., and Otis, M. J. D. (2020). Validation of minimal number of force sensitive resistors to predict risk of falling during a timed up and go test. J. Med. Biol. Eng. 40, 348–355. doi: 10.1007/s40846-020-00512-z
Ayena, J. C., Zaibi, H., Otis, M. J. D., and Menelas, B. A. J. (2016). Home-based risk of falling assessment test using a closed-loop balance model. IEEE Trans. Neural Syst. Rehabil. Eng. 24, 1351–1362. doi: 10.1109/TNSRE.2015.2508960
Azevedo Coste, C., Sijobert, B., Pissard-Gibollet, R., Pasquier, M., Espiau, B., and Geny, C. (2014). Detection of freezing of gait in Parkinson disease: preliminary results. Sensors 14, 6819–6827. doi: 10.3390/s140406819
Bachlin, M., Plotnik, M., Roggen, D., Maidan, I., Hausdorff, J. M., Giladi, N., et al. (2010). Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans. Inf. Technol. Biomed. 14, 436–446. doi: 10.1109/TITB.2009.2036165
Bikias, T., Iakovakis, D., Hadjidimitriou, S., Charisis, V., and Hadjileontiadis, L. J. (2021). Deepfog: an Imu-based detection of freezing of gait episodes in Parkinson’s disease patients via deep learning. Front. Robot. AI 8:537384. doi: 10.3389/frobt.2021.537384
Boonstra, T. A., Van Der Kooij, H., Munneke, M., and Bloem, B. R. (2008). Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr. Opin. Neurol. 21, 461–471. doi: 10.1097/WCO.0b013e328305bdaf
Borzì, L., Mazzetta, I., Zampogna, A., Suppa, A., Olmo, G., and Irrera, F. (2021). Prediction of freezing of gait in Parkinson’s disease using wearables and machine learning. Sensors 21:614. doi: 10.3390/s21020614
Borzì, L., Varrecchia, M., Olmo, G., Artusi, C. A., Fabbri, M., Rizzone, M. G., et al. (2019). Home monitoring of motor fluctuations in Parkinson’s disease patients. J. Reliab. Intell. Environ. 5, 145–162. doi: 10.1007/s40860-019-00086-x
Camps, J., Samà, A., Martín, M., Rodríguez-Martín, D., Pérez-López, C., Moreno Arostegui, J. M., et al. (2018). Deep learning for freezing of gait detection in Parkinson’s disease patients in their homes using a waist-worn inertial measurement unit. Knowl. Based Syst. 139, 119–131. doi: 10.1016/j.knosys.2017.10.017
Capecci, M., Pepa, L., Verdini, F., and Ceravolo, M. G. (2016). A smartphone-based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait Posture 50, 28–33. doi: 10.1016/j.gaitpost.2016.08.018
Chomiak, T., Xian, W. B., Pei, Z., and Hu, B. (2019). A novel single-sensor-based method for the detection Ofgait-cycle breakdown and freezing of gait in Parkinson’s disease. J. Neural Transm. 126, 1029–1036. doi: 10.1007/s00702-019-02020-0
Cole, B T, Roy, S H, and Nawab, S H. (2011). Detecting freezing-of-gait during unscripted and unconstrained activity; Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Boston, MA, Aug 30-Sep 03.
Corcos, D. M., Robichaud, J. A., David, F. J., Leurgans, S. E., Vaillancourt, D. E., Poon, C., et al. (2013). A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov. Disord. 28, 1230–1240. doi: 10.1002/mds.25380
Del Din, S., Godfrey, A., Mazzà, C., Lord, S., and Rochester, L. (2016). Free-living monitoring of Parkinson’s disease: lessons from the field. Mov. Disord. 31, 1293–1313. doi: 10.1002/mds.26718
Demrozi, F., Bacchin, R., Tamburin, S., Cristani, M., and Pravadelli, G. (2020). Toward a wearable system for predicting freezing of gait in people affected by Parkinson’s disease. IEEE J. Biomed. Health Inform. 24, 2444–2451. doi: 10.1109/JBHI.2019.2952618
Dvorani, A., Waldheim, V., Jochner, M. C. E., Salchow-Hömmen, C., Meyer-Ohle, J., Kühn, A. A., et al. (2021). Real-time detection of freezing motions in Parkinson’s patients for adaptive gait phase synchronous cueing. Front. Neurol. 12:516. doi: 10.3389/fneur.2021.720516
El-Attar, A., Ashour, A. S., Dey, N., Abdelkader, H., Abd el-Naby, M. M., and Sherratt, R. S. (2021). Discrete wavelet transform-based freezing of gait detection in Parkinson’s disease. J. Exp. Theor. Arti. Intell. 33, 543–559. doi: 10.1080/0952813X.2018.1519000
Esfahani, A. H., Dyka, Z., Ortmann, S., and Langendorfer, P. (2021). Impact of data preparation in freezing of gait detection using feature-less recurrent neural network. IEEE Access 9, 138120–138131. doi: 10.1109/ACCESS.2021.3117543
Fox, S. H., Katzenschlager, R., Lim, S.-Y., Ravina, B., Seppi, K., Coelho, M., et al. (2011). The movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 26, S2–S41. doi: 10.1002/mds.23829
Ghosh, N., and Banerjee, I. (2021). Iot-based freezing of gait detection using Grey relational analysis. Internet Things 13:100068. doi: 10.1016/j.iot.2019.100068
Giladi, N., Tal, J., Azulay, T., Rascol, O., Brooks, D. J., Melamed, E., et al. (2009). Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov. Disord. 24, 655–661. doi: 10.1002/mds.21745
Greene, B. R., Caulfield, B., Lamichhane, D., Bond, W., Svendsen, J., Zurski, C., et al. (2018). Longitudinal assessment of falls in patients with Parkinson’s disease using inertial sensors and the timed up and go test. J. Rehabilit. Assist. Technol. Eng. 5:2055668317750811.
Griffin, H. J., Greenlaw, R., Limousin, P., Bhatia, K., Quinn, N. P., and Jahanshahi, M. (2011). The effect of real and virtual visual cues on walking in Parkinson’s disease. J. Neurol. 258, 991–1000. doi: 10.1007/s00415-010-5866-z
Guo, Y. Z., Huang, D. B., Zhang, W., Wang, L., Li, Y., Olmo, G., et al. (2022). High-accuracy wearable detection of freezing of gait in Parkinson’s disease based on pseudo-multimodal features. Comput. Biol. Med. 146:105629. doi: 10.1016/j.compbiomed.2022.105629
Guo, Y. Z., Wang, L. P., Li, Y., Guo, L., and Meng, F. (2019). The detection of freezing of gait in Parkinson’s disease using asymmetric basis function Tv-Arma time-frequency spectral estimation method. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 2077–2086. doi: 10.1109/TNSRE.2019.2938301
Hacker, M. L., Turchan, M., Heusinkveld, L. E., Currie, A. D., Millan, S. H., Molinari, A. L., et al. (2020). Deep brain stimulation in early-stage Parkinson disease: five-year outcomes. Neurology 95, E393–E401. doi: 10.1212/WNL.0000000000009946
Halder, A., Singh, R., Suri, A., and Joshi, D. (2021). Predicting state transition in freezing of gait via acceleration measurements for controlled cueing in Parkinson’s disease. IEEE Trans. Instrum. Meas. 70, 1–16. doi: 10.1109/TIM.2021.3090153
Iakovakis, D. E., Papadopoulou, F. A., and Hadjileontiadis, L. J. (2016). Fuzzy logic-based risk of fall estimation using smartwatch data as a means to form an assistive feedback mechanism in everyday living activities. Healthc. Technol. Lett. 3, 263–268. doi: 10.1049/htl.2016.0064
Jovanov, E, Wang, E, Verhagen, L, Fredrickson, M, and Fratangelo, R. (2009). Defog - a real time system for detection and unfreezing of gait of Parkinson’s patients. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, Sep 03–06, 2009.
Kerr, G. K., Worringham, C. J., Cole, M. H., Lacherez, P. F., Wood, J. M., and Silburn, P. A. (2010). Predictors of future falls in Parkinson disease. Neurology 75, 116–124. doi: 10.1212/WNL.0b013e3181e7b688
Kim, H. B., Lee, H. J., Lee, W. W., Kim, S. K., Jeon, H. S., Park, H. Y., et al. (2018). Validation of freezing-of-gait monitoring using smartphone. Telemedicine E-Health 24, 899–907. doi: 10.1089/tmj.2017.0215
Kim, H, Lee, H J, Lee, W, Kwon, S., Kim, S. K., Jeon, H. S., et al. (2015). Unconstrained detection of freezing of gait in Parkinson’s disease patients using smartphone; Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, Aug 25–29, 2015.
Kita, A., Lorenzi, P., Rao, R., and Irrera, F. (2017). Reliable and robust detection of freezing of gait episodes with wearable electronic devices. IEEE Sensors J. 17, 1899–1908. doi: 10.1109/JSEN.2017.2659780
Kleanthous, N., Hussain, A. J., Khan, W., and Liatsis, P. (2020). A new machine learning based approach to predict freezing of gait. Pattern Recogn. Lett. 140, 119–126. doi: 10.1016/j.patrec.2020.09.011
Kwon, Y., Park, S. H., Kim, J. W., Ho, Y., Jeon, H. M., Bang, M. J., et al. (2014). A practical method for the detection of freezing of gait in patients with Parkinson’s disease. Clin. Interv. Aging 9, 1709–1719. doi: 10.2147/CIA.S69773
Li, B. C., Yao, Z. M., Wang, J. G., Wang, S., Yang, X., and Sun, Y. (2020). Improved deep learning technique to detect freezing of gait in Parkinson’s disease based on wearable sensors. Electronics 9:1919. doi: 10.3390/electronics9111919
Lo, A. C., Chang, V. C., Gianfrancesco, M. A., Friedman, J. H., Patterson, T. S., and Benedicto, D. F. (2010). Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: a pilot study. J. Neuroeng. Rehabil. 7:51. doi: 10.1186/1743-0003-7-51
Lun, V., Pullan, N., Labelle, N., Adams, C., and Suchowersky, O. (2005). Comparison of the effects of a self-supervised home exercise program with a physiotherapist-supervised exercise program on the motor symptoms of Parkinson’s disease. Mov. Disord. 20, 971–975. doi: 10.1002/mds.20475
Ly, Q T, Handojoseno, A M A, Gilat, M, Rifai, C, Ehgoetz Martens, KA, Georgiades, M, et al. (2017). Detection of turning freeze in Parkinson’s disease based on S-transform decomposition of Eeg signals. Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), South Korea, Jul 11–15, 2017.
Mak, M. K. Y., and Pang, M. Y. C. (2009). Balance confidence and functional mobility are independently associated with falls in people with Parkinson’s disease. J. Neurol. 256, 742–749. doi: 10.1007/s00415-009-5007-8
Mancini, M., Shah, V. V., Stuart, S., Curtze, C., Horak, F. B., Safarpour, D., et al. (2021). Measuring freezing of gait during daily-life: an open-source, wearable sensors approach. J. Neuroeng. Rehabil. 18:1. doi: 10.1186/s12984-020-00774-3
Marcante, A., di Marco, R., Gentile, G., Pellicano, C., Assogna, F., Pontieri, F. E., et al. (2021). Foot pressure wearable sensors for freezing of gait detection in Parkinson’s disease. Sensors 21:128. doi: 10.3390/s21010128
Mazilu, S., Blanke, U., Calatroni, A., Gazit, E., Hausdorff, J. M., and Tröster, G. (2016). The role of wrist-mounted inertial sensors in detecting gait freeze episodes in Parkinson’s disease. Pervasive Mob. Comput. 33, 1–16. doi: 10.1016/j.pmcj.2015.12.007
Mazilu, S, Blanke, U, Roggen, D, Blanke, U., Roggen, D., Tröster, G., et al. (2013). Engineers Meet Clinicians: Augmenting Parkinson’s Disease Patients to Gather Information for Gait Rehabilitation. Proceedings of the 4th Augmented Human International Conference, Stuttgart, Germany, 124–127.
Mazilu, S., Calatroni, A., Gazit, E., Mirelman, A., Hausdorff, J. M., and Troster, G. (2015). Prediction of freezing of gait in Parkinson’s from physiological wearables: an exploratory study. IEEE J. Biomed. Health Inform. 19, 1843–1854. doi: 10.1109/JBHI.2015.2465134
Mazzetta, I., Zampogna, A., Suppa, A., Gumiero, A., Pessione, M., and Irrera, F. (2019). Wearable sensors system for an improved analysis of freezing of gait in Parkinson’s disease using. Electromyography And Inertial Signals. Sensors 19:948. doi: 10.3390/s19040948
Mesin, L., Porcu, P., Russu, D., Farina, G., Borzì, L., Zhang, W., et al. (2022). A multi-modal analysis of the freezing of gait phenomenon in Parkinson’s disease, 2613. Sensors 22. doi: 10.3390/s22072613
Mikos, V., Heng, C. H., Tay, A., Yen, S. C., Chia, N. S. Y., Koh, K. M. L., et al. (2019). A wearable, patient-adaptive freezing of gait detection system for biofeedback cueing in Parkinson’s disease. IEEE Trans. Biomed. Circuits Syst. 13, 503–515. doi: 10.1109/TBCAS.2019.2914253
Moore, S. T., Macdougall, H. G., and Ondo, W. G. (2007). Ambulatory monitoring of freezing of gait in Parkinson’s disease. Mov. Disord. 22:S79-S. doi: 10.1016/j.jneumeth.2007.08.023
Moore, S. T., Yungher, D. A., Morris, T. R., Dilda, V., MacDougall, H. G., Shine, J. M., et al. (2013). Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental Accelerometry. J. Neuroeng. Rehabil. 10:19. doi: 10.1186/1743-0003-10-19
Naghavi, N., Miller, A., and Wade, E. (2019). Towards real-time prediction of freezing of gait in patients with Parkinson’s disease: addressing the class imbalance problem. Sensors 19:3898. doi: 10.3390/s19183898
Naghavi, N., and Wade, E. (2019). Prediction of freezing of gait in Parkinson’s disease using statistical inference and lower-limb acceleration data. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 947–955. doi: 10.1109/TNSRE.2019.2910165
Naghavi, N., and Wade, E. (2022). Towards real-time prediction of freezing of gait in patients with Parkinson’s disease: a novel deep one-class classifier. IEEE J. Biomed. Health Inform. 26, 1726–1736. doi: 10.1109/JBHI.2021.3103071
Nantel, J., and Bronte-Stewart, H. (2014). The effect of medication and the role of postural instability in different components of freezing of gait (fog). Parkinsonism Relat. Disord. 20, 447–451. doi: 10.1016/j.parkreldis.2014.01.017
Nonnekes, J., Snijders, A. H., Nutt, J. G., Deuschl, G., Giladi, N., and Bloem, B. R. (2015). Freezing of gait: a practical approach to management. Lancet Neurol. 14, 768–778. doi: 10.1016/S1474-4422(15)00041-1
O’Day, J., Lee, M., Seagers, K., Hoffman, S., Jih-Schiff, A., Kidziński, Ł., et al. (2022). Assessing inertial measurement unit locations for freezing of gait detection and patient preference. J. Neuroeng. Rehabil. 19:20. doi: 10.1186/s12984-022-00992-x
Obeso, J. A., Olanow, C. W., and Nutt, J. G. (2000). Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 23, S2–S7. doi: 10.1016/S1471-1931(00)00031-8
O’day, J J, Kehnemouyi, Y M, Petrucci, M N, Anderson, R., Herron, J., and Bronte-Stewart, H. (2020). Demonstration of kinematic-based closed-loop deep brain stimulation for mitigating freezing of gait in people with Parkinson’s disease. Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, Canada, Jul 20–24, 2020.
Okuma, Y., Silva De Lima, A. L., Fukae, J., Bloem, B. R., and Snijders, A. H. (2018). A prospective study of falls in relation to freezing of gait and response fluctuations in Parkinson’s disease. Parkinsonism Relat. Disord. 46, 30–35. doi: 10.1016/j.parkreldis.2017.10.013
Palmerini, L., Rocchi, L., Mazilu, S., Gazit, E., Hausdorff, J. M., and Chiari, L. (2017). Identification of characteristic motor patterns preceding freezing of gait in Parkinson’s disease using wearable sensors. Front. Neurol. 8:394. doi: 10.3389/fneur.2017.00394
Pardoel, S., Nantel, J., Kofman, J., and Lemaire, E. D. (2022). Prediction of freezing of gait in Parkinson’s disease using unilateral and bilateral plantar-pressure data. Front. Neurol. 13:831063. doi: 10.3389/fneur.2022.831063
Pardoel, S., Shalin, G., Lemaire, E. D., Kofman, J., and Nantel, J. (2021). Grouping successive freezing of gait episodes has neutral to detrimental effect on freeze detection and prediction in Parkinson’s disease. PLoS One 16:e0258544. doi: 10.1371/journal.pone.0258544
Pardoel, S., Shalin, G., Nantel, J., Lemaire, E. D., and Kofman, J. (2021). Early detection of freezing of gait during walking using inertial measurement unit and plantar pressure distribution data. Sensors 21:2246. doi: 10.3390/s21062246
Patel, S., Park, H., Bonato, P., Chan, L., and Rodgers, M. (2012). A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 9:21. doi: 10.1186/1743-0003-9-21
Pham, T. T., Moore, S. T., Lewis, S. J. G., Nguyen, D. N., Dutkiewicz, E., Fuglevand, A. J., et al. (2017). Freezing of gait detection in Parkinson’s disease: a subject-independent detector using anomaly scores. IEEE Trans. Biomed. Eng. 64, 2719–2728. doi: 10.1109/TBME.2017.2665438
Pierleoni, P., Belli, A., Bazgir, O., Maurizi, L., Paniccia, M., and Palma, L. (2019). A smart inertial system for 24h monitoring and classification of tremor and freezing of gait in Parkinson’s disease. IEEE Sensors J. 19, 11612–11623. doi: 10.1109/JSEN.2019.2932584
Prado, A., Kwei, S. K., Vanegas-Arroyave, N., and Agrawal, S. K. (2021). Continuous identification of freezing of gait in Parkinson’s patients using artificial neural networks and instrumented shoes. IEEE Trans. Med. Robot. Bionics 3, 554–562. doi: 10.1109/TMRB.2021.3091526
Prateek, G. V., Skog, I., McNeely, M. E., Duncan, R. P., Earhart, G. M., and Nehorai, A. (2018). Modeling, detecting, and tracking freezing of gait in Parkinson disease using inertial sensors. IEEE Trans. Biomed. Eng. 65, 2152–2161. doi: 10.1109/TBME.2017.2785625
Rahman, S., Griffin, H. J., Quinn, N. P., and Jahanshahi, M. (2008). The factors that induce or overcome freezing of gait in Parkinson’s disease. Behav. Neurol. 19, 127–136. doi: 10.1155/2008/456298
Reches, T., Dagan, M., Herman, T., Gazit, E., Gouskova, N., Giladi, N., et al. (2020). Using wearable sensors and machine learning to automatically detect freezing of gait during a fog-provoking test. Sensors 20:4474. doi: 10.3390/s20164474
Ren, K., Chen, Z. L., Ling, Y., and Zhao, J. (2022). Recognition of freezing of gait in Parkinson’s disease based on combined wearable sensors. BMC Neurol. 22:229. doi: 10.1186/s12883-022-02732-z
Rezvanian, S., and Lockhart, T. E. (2016). Towards real-time detection of freezing of gait using wavelet transform on wireless accelerometer data. Sensors 16:475. doi: 10.3390/s16040475
Rodríguez-Martín, D., Pérez-López, C., Samà, A., Català, A., Moreno Arostegui, J., Cabestany, J., et al. (2017). A waist-worn inertial measurement unit for long-term monitoring of Parkinson’s disease patients. Sensors 17:827. doi: 10.3390/s17040827
Rodriguez-Martin, D., Samà, A., Pérez-López, C., Cabestany, J., Català, A., and Rodríguez-Molinero, A. (2015). Posture transition identification on Pd patients through a Svm-based technique and a single waist-worn accelerometer. Neurocomputing 164, 144–153. doi: 10.1016/j.neucom.2014.09.084
Rodríguez-Martín, D., Samà, A., Pérez-López, C., Català, A., Moreno Arostegui, J. M., Cabestany, J., et al. (2017). Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS One 12:e0171764. doi: 10.1371/journal.pone.0171764
Saad, A., Zaarour, I., Guerin, F., Bejjani, P., Ayache, M., and Lefebvre, D. (2017). Detection of freezing of gait for Parkinson’s disease patients with multi-sensor device and Gaussian neural networks. Int. J. Mach. Learn. Cybern. 8, 941–954. doi: 10.1007/s13042-015-0480-0
Samà, A., Rodríguez-Martín, D., Pérez-López, C., Català, A., Alcaine, S., Mestre, B., et al. (2018). Determining the optimal features in freezing of gait detection through a single waist accelerometer in home environments. Pattern Recogn. Lett. 105, 135–143. doi: 10.1016/j.patrec.2017.05.009
Samii, A., Nutt, J. G., and Ransom, B. R. (2004). Parkinson’s disease. Lancet 363, 1783–1793. doi: 10.1016/S0140-6736(04)16305-8
San-Segundo, R., Torres-Sánchez, R., Hodgins, J., and de la Torre, F. (2019). Increasing robustness in the detection of freezing of gait in Parkinson’s disease. Electronics 8:119. doi: 10.3390/electronics8020119
Schaafsma, J. D., Balash, Y., Gurevich, T., Bartels, A. L., Hausdorff, J. M., and Giladi, N. (2003). Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 10, 391–398. doi: 10.1046/j.1468-1331.2003.00611.x
Shalin, G., Pardoel, S., Lemaire, E. D., Nantel, J., and Kofman, J. (2021). Prediction and detection of freezing of gait in Parkinson’s disease from plantar pressure data using long short-term memory neural-networks. J. Neuroeng. Rehabil. 18:167. doi: 10.1186/s12984-021-00958-5
Shi, B. H., Tay, A., Au, W. L., Tan, D. M. L., Chia, N. S. Y., and Yen, S. C. (2022). Detection of freezing of gait using convolutional neural networks and data from lower limb motion sensors. IEEE Trans. Biomed. Eng. 69, 2256–2267. doi: 10.1109/TBME.2022.3140258
Shi, B H, Yen, S C, Tay, A, Tan, D. M. L., Chia, N. S. Y, Au, W. L., et al. (2020). Convolutional neural network for freezing of gait detection leveraging the continuous wavelet transform on lower extremities wearable sensors data. Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, Canada, Jul 20–24.
Sigcha, L., Costa, N., Pavón, I., Costa, S., Arezes, P., López, J. M., et al. (2020). Deep learning approaches for detecting freezing of gait in Parkinson’s disease patients through on-body acceleration sensors. Sensors 20:1895. doi: 10.3390/s20071895
Stamatakis, J, Cremers, J, Maquet, D, Macq, B, and Garraux, G. (2011). Gait feature extraction in Parkinson’s disease using low-cost accelerometers. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Boston, MA, Aug 30-Sep 03, 2011.
Suppa, A., Kita, A., Leodori, G., Zampogna, A., Nicolini, E., Lorenzi, P., et al. (2017). L-Dopa and freezing of gait in Parkinson’s disease: objective assessment through a wearable wireless system. Front. Neurol. 8:406. doi: 10.3389/fneur.2017.00406
Takač, B., Català, A., Rodríguez Martín, D., van der Aa, N., Chen, W., and Rauterberg, M. (2013). Position and orientation tracking in a ubiquitous monitoring system for Parkinson disease patients with freezing of gait symptom. JMIR Mhealth Uhealth 1:e14. doi: 10.2196/mhealth.2539
Tambasco, N., Simoni, S., Eusebi, P., Ripandelli, F., Brahimi, E., Sacchini, E., et al. (2015). The validation of an Italian version of the freezing of gait questionnaire. Neurol. Sci. 36, 759–764. doi: 10.1007/s10072-014-2037-5
Tang, S. T., Tai, C. H., Yang, C. Y., and Lin, J.‑. H. (2020). Correction to: feasibility of smartphone-based gait assessment for Parkinson’s disease. J. Med. Biol. Eng. 40, 770–771. doi: 10.1007/s40846-020-00564-1
Tomlinson, C. L., Herd, C. P., Clarke, C. E., Meek, C., Patel, S., Stowe, R., et al. (2014). Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Syst. Rev. 6:Cd002815. doi: 10.1002/14651858.CD002815.pub2
Tripoliti, E. E., Tzallas, A. T., Tsipouras, M. G., Rigas, G., Bougia, P., Leontiou, M., et al. (2013). Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput. Methods Prog. Biomed. 110, 12–26. doi: 10.1016/j.cmpb.2012.10.016
Tzallas, A. T., Tsipouras, M. G., Rigas, G., Tsalikakis, D., Karvounis, E., Chondrogiorgi, M., et al. (2014). Perform: a system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 14, 21329–21357. doi: 10.3390/s141121329
Ustinova, K., Chernikova, L., Bilimenko, A., Telenkov, A., and Epstein, N. (2011). Effect of robotic locomotor training in an individual with Parkinson’s disease: a case report. Disabil. Rehabil. Assist. Technol. 6, 77–85. doi: 10.3109/17483107.2010.507856
Yungher, D. A., Morris, T. R., Dilda, V., Shine, J. M., Naismith, S. L., Lewis, S. J. G., et al. (2014). Temporal characteristics of high-frequency lower-limb oscillation during freezing of gait in Parkinson’s disease. Parkinsons Disease 2014, 1–7. doi: 10.1155/2014/606427
Zach, H., Janssen, A. M., Snijders, A. H., Delval, A., Ferraye, M. U., Auff, E., et al. (2015). Identifying freezing of gait in Parkinson’s disease during freezing provoking tasks using waist-mounted Accelerometry. Parkinsonism Relat. Disord. 21, 1362–1366. doi: 10.1016/j.parkreldis.2015.09.051
Keywords: wearable device, Parkinson’s disease, freezing of gait (FOG), fall – Wound, FOG detection algorithm
Citation: Huang T, Li M and Huang J (2023) Recent trends in wearable device used to detect freezing of gait and falls in people with Parkinson’s disease: A systematic review. Front. Aging Neurosci. 15:1119956. doi: 10.3389/fnagi.2023.1119956
Edited by:
Corinne A. Jones, The University of Texas at Austin, United StatesReviewed by:
Joan Cabestany, Universitat Politecnica de Catalunya, SpainRobert Fekete, New York Medical College, United States
Copyright © 2023 Huang, Li and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwei Huang, ✉ gmu_jianwei_huang@163.com
†These authors have contributed equally to this work and share first authorship
 Tinghuai Huang
Tinghuai Huang Meng Li1†
Meng Li1†