Development and Evaluation of Maze-Like Puzzle Games to Assess Cognitive and Motor Function in Aging and Neurodegenerative Diseases
- 1Gerontechnology & Rehabilitation, University of Bern, Bern, Switzerland
- 2ARTORG Center for Biomedical Engineering Research, University of Bern, Bern, Switzerland
- 3Neurocenter, Luzerner Kantonsspital, Lucerne, Switzerland
- 4Neurozentrum Siloah and Department of Neurology, Swiss Huntington’s Disease Center, University of Bern, Bern, Switzerland
- 5Department of Neurology, University Neurorehabilitation, Inselspital, Bern, Switzerland
There is currently a need for engaging, user-friendly, and repeatable tasks for assessment of cognitive and motor function in aging and neurodegenerative diseases. This study evaluated the feasibility of a maze-like Numberlink puzzle game in assessing differences in game-based measures of cognition and motor function due to age and neurodegenerative diseases. Fifty-five participants, including young (18–31 years, n = 18), older (64–79 years, n = 14), and oldest adults (86–98 years, n = 14), and patients with Parkinson’s (59–76 years, n = 4) and Huntington’s disease (HD; 35–66 years, n = 5) played different difficulty levels of the Numberlink puzzle game and completed usability questionnaires and tests for psychomotor, attentional, visuospatial, and constructional and executive function. Analyses of Numberlink game-based cognitive (solving time and errors) and motor [mean velocity and movement direction changes (MDC)] performance metrics revealed statistically significant differences between age groups and between patients with HD and older adults. However, patients with Parkinson’s disease (PD) did not differ from older adults. Correlational analyses showed significant associations between game-based performance and movement metrics and performance on neuropsychological tests for psychomotor, attentional, visuospatial, and constructional and executive function. Furthermore, varying characteristics of the Numberlink puzzle game succeeded in creating graded difficulty levels. Findings from this study support recent suggestions that data from a maze-like puzzle game provide potential “digital biomarkers” to assess changes in psychomotor, visuoconstructional, and executive function related to aging and neurodegeneration. In particular, game-based movement measures from the maze-like puzzle Numberlink games are promising as a tool to monitor the progression of motor impairment in neurodegenerative diseases. Further studies are needed to more comprehensively establish the cognitive validity and test–retest reliability of using Numberlink puzzles as a valid cognitive assessment tool.
Introduction
Neurodegenerative diseases comprise a heterogeneous group of age-related disorders with progressive loss of neuronal structure and function (Cummings and Pillai, 2016; Kovacs, 2018). Neurodegeneration can occur in normal aging and age-related neurodegenerative diseases that include Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Affecting different brain areas, patients with neurodegenerative diseases suffer from cognitive, motor, and neuropsychiatric impairments (Cummings and Pillai, 2016; Hussain et al., 2018; Kovacs, 2018). Cognitive functions commonly affected in neurodegenerative diseases include attentional, visuospatial, and constructional, executive, and motor function that detrimentally impact everyday activities and quality of life in normal cognitive aging (Deary et al., 2009; Diesing and Rizzo, 2018), AD (Rycroft and Giovannetti, 2017), PD (Ding et al., 2015; Pal et al., 2018), and HD (Dumas et al., 2013; Paulsen and Long, 2014). Strikingly, these cognitive changes vary considerably both between and within individuals in normal aging (Agrigoroaei and Lachman, 2011; Mosti et al., 2019) and patients with neurodegenerative disorders (Boller et al., 2013; White et al., 2018; Greenland et al., 2019).
Given this variability, it is important to differentiate normal cognitive aging from neurodegenerative diseases in order to detect cognitive deficits early and provide adequate treatment to attenuate further neurodegeneration and cognitive dysfunction (Massaldjieva, 2018). However, existing neuropsychological assessments have been criticized for their: (a) unsuitability for repeated use because of time demand and practice effects (i.e., test performance improvements when tests are taken repeatedly); (b) limited sensitivity to detect early and subtle cognitive changes because of ceiling and flooring effects (i.e., high rates of highest or lowest possible test scores); and (c) lack of ecological validity as many cognitive tasks do not reflect cognitive demands of everyday activities (Silverberg et al., 2011; Allard et al., 2014; Zygouris and Tsolaki, 2015; Valdes et al., 2016; Howieson, 2019).
A novel approach to address these issues is the use of video games to study age and neurodegenerative disease-related differences in cognitive function (Boot, 2015; Koo and Vizer, 2019). Video games involve solving cognitive challenges that require a range of cognitive abilities and often share properties with psychological tests (Holmgard et al., 2016). Video games provide a bulk of performance measures (e.g., reaction and solving time, number of errors and others) that can be used to assess cognitive functioning (Areàn et al., 2016; Mandryk and Birk, 2019). Furthermore, video games promote prolonged motivation and engagement, and offer performance-based adjustments of task difficulty that can help avoid practice effects with repeated use and accommodate different levels of cognitive ability (Holmgard et al., 2016; Diesing and Rizzo, 2018; Levy et al., 2018). Also, video games involve complex cognitive skills that better reflect everyday cognitive function than simple cognitive tasks (Boot et al., 2013). To date, several studies demonstrated associations between video game performance measures and performance on specific cognitive tests, suggesting that video games measure relevant cognitive abilities (Baniqued et al., 2013; Oei and Patterson, 2013; Martinovic et al., 2015) and are feasible to assess cognitive function in older adults (Thompson et al., 2012; Tong et al., 2016).
With regard to detecting age-related changes in cognitive and motor functioning, maze tasks represent a particularly promising addition to standardized cognitive assessments (de Souza et al., 2013). Mazes are complex visual-motor planning and problem-solving tasks that require finding a path from the start to the end of a maze as quickly as possible. Mazes are non-verbal, simple to understand and use, relatively independent of educational level and suitable for a wide range of older adults and persons with cognitive impairment (Marhasev et al., 2009; Silva et al., 2017). Maze tasks require an interplay between both cognitive and motor processes and are similar to complex everyday activities that require planning and problem solving (Lewis and Miller, 2007; Marhasev et al., 2009; Howieson, 2019). Solving mazes requires multiple cognitive processes that include attentional, visuospatial and visuoconstructional, and executive function (planning, foresight and problem solving) as well as visuomotor function (Snellgrove, 2005; Kirsch et al., 2006; Carlozzi, 2011; Zhao and Marquez, 2013).
To date, maze tests have been used in some studies to assess cognitive and motor functioning and demonstrated sensitivity in differentiating healthy normal aging from either mild cognitive impairment (Zhang et al., 2007; de Souza et al., 2013), Alzheimer’s dementia (Mack and Patterson, 1995), PD (Mimura et al., 2006), and HD (Deckel and Duffy, 2000; Montoya et al., 2006). Furthermore, maze task performance was shown as a strong predictor of everyday functioning ability in older adults (Mack and Patterson, 1995; Ott et al., 2003; Lewis and Miller, 2007; Staplin et al., 2013).
In the current study, a maze-like Numberlink (NL) puzzle video game adapted to assess cognitive and motor ability in aging and neurodegenerative diseases is presented. NL was first published as a newspaper puzzle column by Sam Loyd (Loyd, 1897) and later popularized in Japan as pastime puzzle books (Dudeney, 1917; Nikoli, 1989; Yoshinaka et al., 2012; Adcock et al., 2015). In 2012, NL puzzles have been released as a puzzle game for mobile applications called Flow Free® (Big Duck Games LLC) that has been downloaded over 250 million times and is among the most popular puzzle games (Newman and Newman, 2012). In a previous playtest study in older adults, we found that the NL puzzles were particularly enjoyed and rated to meet game characteristics for cognitive tasks and trainings (Chesham et al., 2017).
Similar to video games, mazes can be modified in difficulty to match the test-takers’ level of cognitive ability. A number of recent studies have further provided maze tasks with graded levels of difficulty accomplished by varying variables of the maze (McClendon, 2001; Ott et al., 2003; Blatter et al., 2005; Davis et al., 2014; Pasek, 2016). Variable maze difficulty levels can help prevent practice effects during repeated administration and reduce ceiling and flooring effects by continuously matching the task difficulty to the participant’s cognitive ability level (Davis et al., 2014; Loe and Rust, 2019). Graded difficulty levels for the NL puzzle game were generated by manipulating game characteristics. This benefits future studies to create game-based adaptive computerized cognitive assessments.
The first aim of this study was to examine the acceptance and usability of a game-based NL task in young, older, and oldest adults and persons with neurodegenerative diseases. In line with recent suggestions, we hypothesize that the use of playful elements of a puzzle video game is user-friendly and enjoyable for older people and patients with neurodegenerative diseases (Polzer and Gewald, 2019). The second aim of this study was to examine age- and NDD related differences in NL game-based cognitive (solving time and accuracy) and motor (movement velocity and direction changes) performance.
Following previous findings using maze tasks, our study tests the hypothesis that NL game-based performance measures can be used to distinguish between age groups (de Souza et al., 2013) and between healthy controls and patients with HD (Montoya et al., 2006) and PD (Mimura et al., 2006). Based on our hypothesis that NL puzzles are similar to maze tasks, we propose that NL game-based performance measures are associated with performance on the Snellgrove Maze Task (SMT) and standard measures of visuomotor, visuospatial and constructional, executive, and global cognitive function (Snellgrove, 2005; Yew et al., 2011). Finally, we assessed whether NL puzzle difficulty can be varied by manipulating game parameters. Following a previous study, we hypothesize that the difficulty of NL puzzles increases with set size and the number of paths (van Kreveld et al., 2015).
Materials and Methods
Participants
In total, 55 participants (36 females) between the ages of 18 and 98 years participated in this study (see Table 1). Five groups of participants were enrolled for this study: (1) young adult university students (YA; n = 18, 12 female, mean age 21.83, range 18–31 years); (2) older adults (OA; n = 14, 8 female, mean age 71.14, range 64–79 years); (3) oldest adults (OOA; n = 14, 12 female; mean age 89.93, range 86–98 years) living independently in senior residence apartments. The group of participants with neurodegenerative disease included; (4) patients with HD (n = 5, 2 female; mean age 49.40, range 35–66 years; Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III) 45.75 ± 10.78, range 30–54; Total Functional Capacity 8.0 ± 3.16, range 6–12; Functional Assessment: 15.75 ± 6.18, range 7–21; Independence Scale 81.25 ± 21.75%, range 60%–100%); and (5) patients with PD (n = 4, 2 female; mean age 67.50, range 59–76 years; MDS-UPDRS-III 17.75 ± 5.38, range 11.00–23.00; disease duration 9.88 ± 3.01 years, range 7.00–14.00 years). HD and PD patients were recruited from the Swiss HD Center, Neurozentrum Siloah; Department of Neurology, Bern, Switzerland; and the Neurology and Neurorehabilitation Center, Luzerner Kantonsspital, Luzern, Switzerland. All HD patients were previously given a clinical confirmed diagnosis of HD. One of five HD patients did not receive any medication, while the rest of HD patients were under medications. The medications were personalized depending on the individual symptoms and covered a wide range (Zolpidem 10 mg 1–2, Risperidone 0.5 mg, Trittico 100 mg, Citalopram 20 mg, Quetiapine 25 mg, Zoldorm 10 mg, Aripiprazole 5 mg, Eltroxin LF 0.05 mg, and Rosuvastatin 10 mg). Patients with PD were diagnosed with PD as per UK Parkinson’s Disease Brain Bank criteria and prescribed anti-Parkinsonian medication (levodopa-dose 412.5 ± 188.75 mg, range 150–600 mg). Three of the PD patients were also prescribed additional drugs depending on their symptoms (Sifrol ER 1.5 mg, Pramipexole 3 mg, Roprinirole 8 mg, and Safinamide 50 mg). All PD patients were in “ON” phase of the medication cycle when participating in this study.
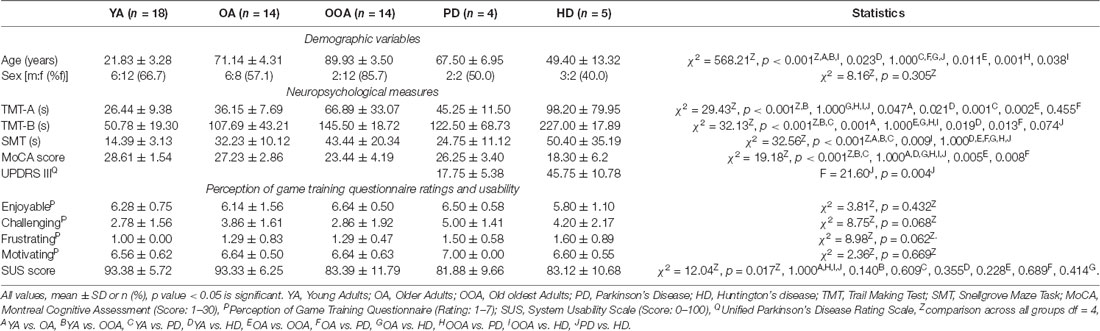
Table 1. Demographics, clinical and neuropsychological measures, acceptance, and usability by group.
Exclusion criteria for participation were insufficient coordinative, motor, and perceptual ability to handle a tablet-computer and history of any additional neurological or psychiatric deficits. All participants had normal or corrected-to-normal vision. Written informed consent was provided in accordance with the latest version of the Declaration of Helsinki prior to participation. The cantonal ethics committees of Bern, Northwest and Central Switzerland, Switzerland granted the ethical approval for this study (2016-01281).
Neuropsychological Assessment
Global cognitive, attentional, visuospatial and visuoconstructional, and executive function was evaluated in all participants. Global cognitive ability was examined with the Montreal Cognitive Assessment (MoCA) that evaluates executive, attentional and visuospatial function, memory, and language (Nasreddine et al., 2005). Patients with HD completed the Mini Mental State Examination (MMSE; Folstein et al., 1975) instead of the MoCA. MMSE scores for the HD patients were converted to MoCA scores using conversion guidelines from Roalf et al. (2013). Attention and executive functions were further assessed with the Trail Making Test part A (TMT-A) and part B (TMT-B; Schretlen et al., 1996). The TMT-A tests selective attention, visual scanning, and visuomotor processing, and the TMT-B tests divided attention and executive control (Schretlen et al., 1996; Strauss et al., 2006). Finally, the SMT was used as a screening for multiple cognitive functions that include attention, visuoconstructional ability, and executive functions of planning and foresight (Snellgrove, 2005).
Acceptance and Usability Assessment
Subjective acceptance of the NL puzzle game was assessed with the Perception of Game Training Questionnaire (Boot et al., 2013). In this questionnaire, participants rated the extent to which they found playing the mazes “enjoyable,” “challenging,” “frustrating,” as well as their motivation while playing the mazes on a seven-point Likert scale. The 10-item System Usability Scale (SUS) was used to measure user experience, usability, and learnability of the NL puzzle game. The SUS provides a composite score from 0 to 100 where a higher number indicates a higher usability (Brooke, 1996).
NL Puzzle Task
Task Description
NL puzzles are maze-like link puzzles that involve finding multiple distinct paths to connect pairs of identical objects using non-intersecting and continuous lines (Yew et al., 2012). NL puzzles are played on a grid-based (width × height) puzzle board of cells. Some cells contain colored circles with numbers or letters (“dots”) that represent start and end points of paths, while the rest of the cells are empty (Kalvelagen, 2017). Dots always come in pairs that have the same color and letter (Hartmann, 2018; see Figure 1, top row). The overall goal of NL puzzles is to connect all pairs of dots with non-intersecting and continuous lines (called “path” or “flow”) such that finally each empty cell in the grid is part of a path (Adcock et al., 2015). The following rules must be observed to solve NL puzzles: first, all pairs of identical dots must be connected with single continuous paths. Second, paths can be drawn only in horizontal or vertical direction, must go at least through one empty cell, and cannot go through an empty cell twice. Third, paths are not allowed to cross cells containing dots or intersect with other paths, as crossed paths will be overwritten. Fourth and final, all empty cells must be filled with paths once all pairs of dots are connected (see Figure 1, bottom row; Newman and Newman, 2012; Yew et al., 2012; Yoshinaka et al., 2012; Kalvelagen, 2017; Hartmann, 2018; Laurentiz, 2018). The main challenge of NL puzzles lies in completing the puzzle in as little time as possible using a minimum number of moves. Moves are counted from the moment a dot is tapped and dragged to draw a path until it is released again. Moves can result in either complete or incomplete paths between two identical dots. Paths can be deleted completely by touching the start or end dot or can be broken up at the point where the path is touched. Hence, to solve a NL puzzle with an optimal number of moves, each pair of dots should be connected exactly once; that is, the number of moves should be equal to the number of pairs of dots (Newman and Newman, 2012; van Kreveld et al., 2015).
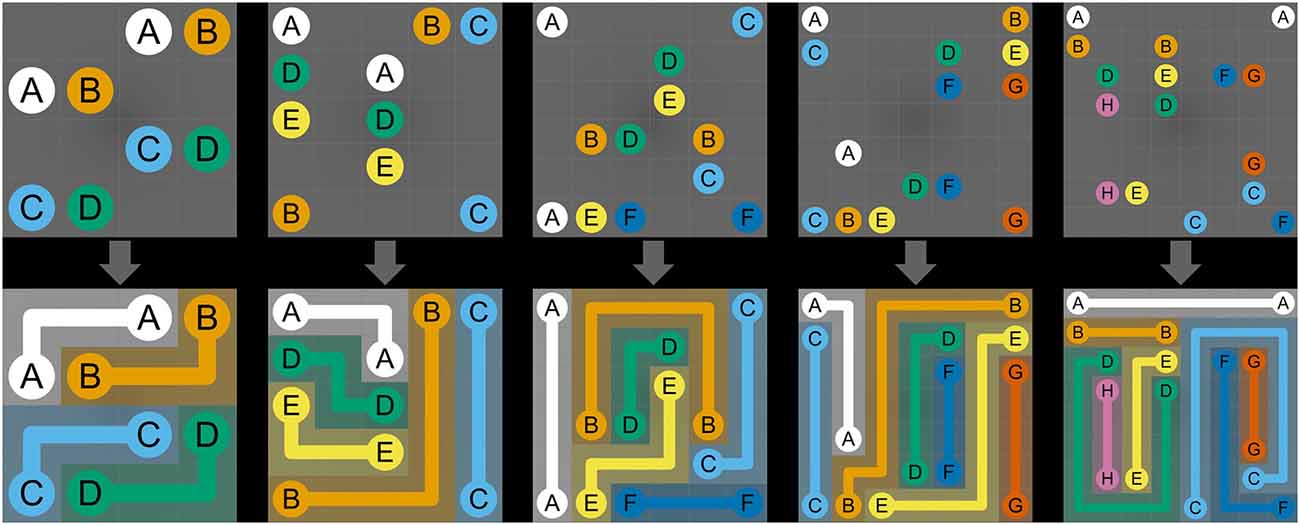
Figure 1. Numberlink puzzle difficulty levels. The manipulated variables include the width and height of the board, the number of paths, and the total number of turns. The top row depicts the initial, and the bottom row, the solved NL puzzles. The difficulty levels shown from left to right are (width, height, paths, turns) = (4, 4, 4, 4), (5, 5, 5, 5), (6, 6, 6, 6), (7, 7, 7, 7), (8, 8, 8, 8).
Comparison Between Maze Tasks and NL Puzzles
NL puzzle games share similarities with perceptual maze tasks such as paper-based and computerized versions of the Porteus Maze Tests and Wechsler Mazes (Porteus, 1945; Wechsler, 1949; Ott et al., 2003; Blatter et al., 2005), the Elithorn Perceptual Maze Test (Elithorn, 1955; Loe and Rust, 2019), and the SMT (Snellgrove, 2005). Maze tasks are composed of two-dimensional grids of cells and are made up of a complex set of branching paths (“arms”) confined by fixed walls between cells. The start (“entry”) and end (“exit”) points are located on the outer edge of the maze (Figure 2, Maze Task, left). Arms inside the maze are connected by intersections and are either open or closed (“dead-ends”). The goal of maze tasks is to draw a single continuous path from entry to the exit (Figure 2, Maze Task, right). This requires a multistep solution composed of a set of connected, directed arms within the maze. Therefore, solving a maze involves selecting multiple, consecutive correct arms and avoiding dead-ends at every intersection of the maze (Bagnall and Zatuchna, 2005; Blatter et al., 2005; Carlozzi, 2011; Pasek, 2016).
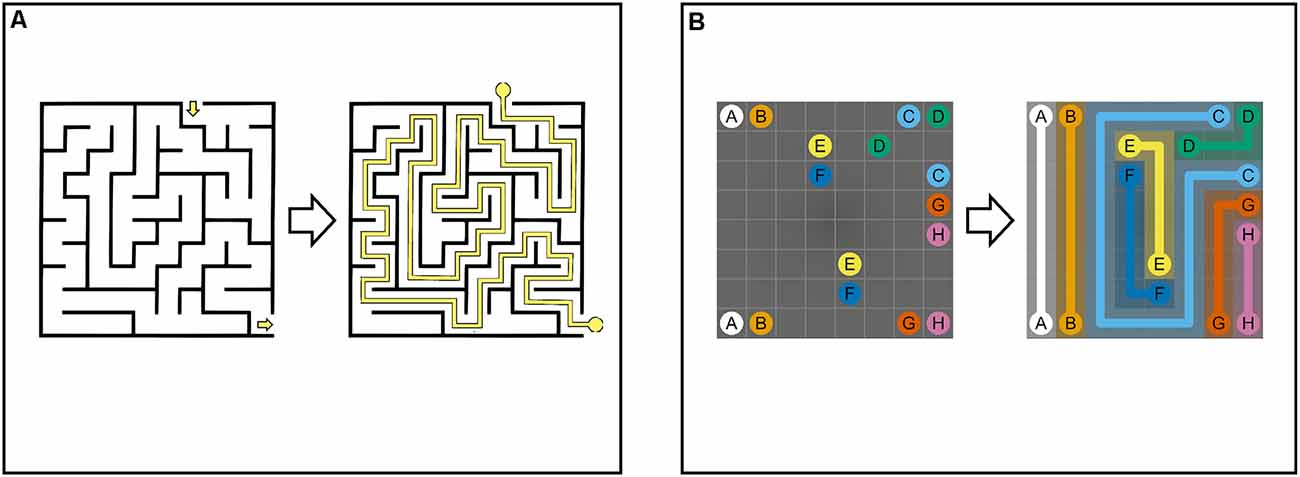
Figure 2. Maze Task (A): maze with cells separated by boundaries. The maze comprises connected paths that are open or closed (dead-ends). To solve the maze, single continuous path (i.e., sequence of connected paths) between start and end must be found. Numberlink Puzzle (B): Numberlink puzzle consisting of a two-dimensional grid with multiple start and end points (i.e., pairs of identical dots). To solve the puzzle, all pairs of dots must be connected with continuous non-intersecting lines and all empty cells must be part of a path.
Like perceptual mazes, NL puzzles are played on a two-dimensional grid of cells. Instead of finding a single path between a single entry and exit point, NL puzzles involve connecting multiple unordered start and end points (i.e., pairs of identical dots) with multiple continuous, non-intersecting paths (Figure 2, NL Puzzle). In addition, unlike mazes, paths in NL puzzles are not delimited by static boundaries, but “pre-determined” by constraints given by the rules of the game (Laurentiz, 2018). For this reason, NL puzzles are considered a set of undirected paths that connect multiple start and end points (Yew et al., 2011; Alviano et al., 2013; Hartmann, 2018).
NL Difficulty Level Generation
The NL puzzle game used in this study was adapted according to the Flow Free® App (Big Duck Games LLC; Newman and Newman, 2012). In the Flow Free® App, difficulty is governed by the size of the grid-based board with sizes ranging from 5 × 5 to 15 × 15. A recent study showed that four game characteristics can be used to model and control the difficulty level of NL puzzles: (1) the size of the board (width × height); (2) the number of paths or pairs of dots; (3) the total number of turns, that is, direction changes of paths to the left or right; and (4) the average city block distance between start and end points of paths (Mutser, 2014; van Kreveld et al., 2015).
To create fine-grained and controlled difficulty levels for the NL puzzle in this study, a set of 25 board sizes was specified by all combinations of board widths and heights varied over a range from 4 to 8: (width, height) = (4, 5, 6, 7, 8) × (4, 5, 6, 7, 8). For each of the 25 board sizes, 1 Mio. random NL puzzles were computer-generated using an open-source Numberlink Generator (Ahle, 2017). Following a recent study on determinants of NL puzzle difficulty, the generated puzzles (text files) were computer-processed and sorted by the number of paths and the total number of turns (Mutser, 2014; van Kreveld et al., 2015). In order to reduce the number of generated NL puzzles for this study, the number of paths and the number of total turns per maze were set between 4 and the maximum value of the width or height of the board. This resulted in a total number of 361 difficulty levels: (width, height, paths, turns) = (4, 5, 6, 7, 8) × (4, 5, 6, 7, 8) × [4 ≤ paths ≤ max (width, height)] × [4 ≤ turns ≤ max(width, height)]. Then, two parallel versions of difficulty levels (see Supplementary Material, Appendix) were created, by parallelizing board size and number of paths (width, height, paths). Both sets contained all 15 square (width = height) NL difficulty levels (width, height, paths) = (4, 4, 4), (5, 5, 4), (5, 5, 5), (6, 6, 4), (6, 6, 5), (6, 6, 6), (7, 7, 4), (7, 7, 5), (7, 7, 6), (7, 7, 7), (8, 8, 4), (8, 8, 5), (8, 8, 6), (8, 8, 7), (8, 8, 8). The non-square (width ≢ height) NL difficulty levels were assigned in a parallelized fashion (Set A) = (5, 4, 4), (4, 5, 5), …, (8, 7, 8), (Set B) = (4, 5, 4), (5, 4, 5), …, (7, 8, 8) but still contain all board sizes. Finally, for both sets, one instance of total number of turns (ranging from 4 to 8) was selected randomly. For both the full set A and B, three sets of difficulty levels were created (see Supplementary Material, Appendix): a short version (width, height, paths, turns) = (4, 5, 6) × (4, 5, 6) × [4 ≤ p ≤ max(width, height)] × [4 ≤ t ≤ max(w, h)] = 12 levels, a medium version (width, height, paths, turns) = (4, 5, 6, 7) × (4, 5, 6, 7) × [4 ≤ paths ≤ max(width, height)] × [4 ≤ turns ≤ max(width, height)] = 24 levels, and a long version (width, height, paths, turns) = (4, 5, 6, 7, 8) × (4, 5, 6, 7, 8) × [4 ≤ paths ≤ max(width, height)] × [4 ≤ turns ≤ max(width, height)] = 40 levels. The reasoning behind creating short, medium, and long versions of NL difficulty levels was based on preliminary experience and mainly due to time considerations and to avoid overburdening participants.
Data Preparation, Maze Performance, and Movement Metrics
NL puzzle performance metrics were calculated based on the participants’ completion time, accuracy, and touchscreen interaction (“movement”). For each played maze difficulty level, two data files were stored as text files: (1) time-stamped “screenshots” (two-dimensional arrays) representing every change of state in empty cells from between the initial and solved NL puzzle; and (2) raw touch input data from the tablet-computer consisting of time-stamped x and y coordinates of touch points.
First, three response time-based NL performance measures were calculated: total solving time (TST), motor execution time (MET), and mental planning time (MPT). TST was defined as the time elapsed from the initial touch to the solved NL puzzle (see Figure 1). Using the touch input data, single moves (“drag movements”) were defined as a sequence of touch points that fall between a touch down and release event (see Figure 3). For every move, move duration (i.e., the time between touch down and touch release) was calculated (Antal and Szabó, 2016). MET was computed by summing the durations of all moves that were required to complete the NL puzzle. Finally, MPT was calculated by subtracting MET from TST.
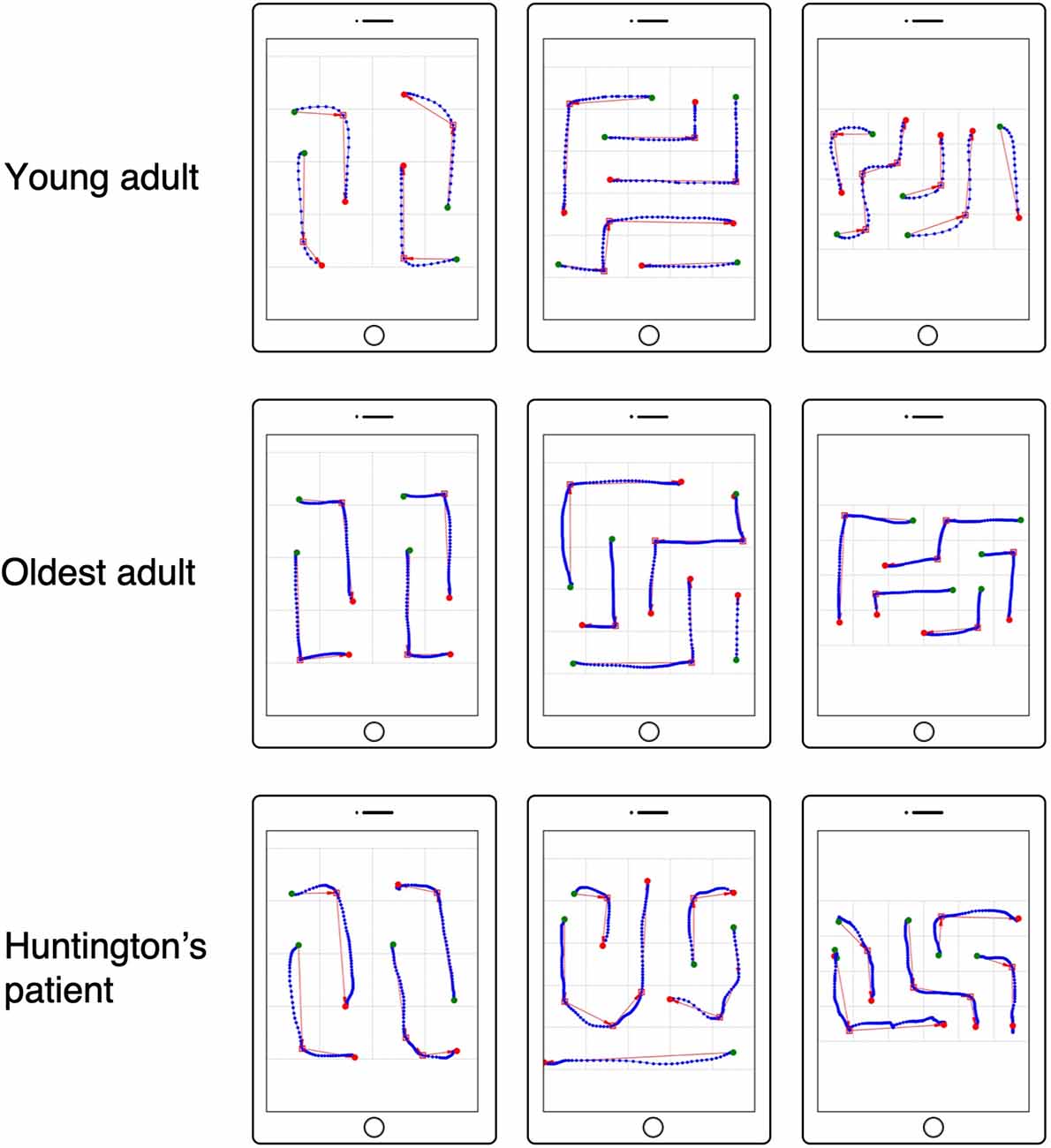
Figure 3. Numberlink motor performance based on tablet touch interaction data. Examples are shown for a young adult (top row), oldest adult (middle row), and patient with Huntington’s disease (HD; bottom row). Touch interaction data are shown for NL puzzle levels solved without errors (false paths) for difficulty levels (width, height, paths, turns) = (4, 4, 4, 4), (5, 5, 5, 5), (6, 4, 5, 6). Green circles indicate the start and red circles indicate the end of a move. Red squares represent turns in the path as detected using the Ramer–Douglas–Peucker algorithm.
Second, two accuracy-based NL performance measures were calculated: the number of false moves and excess moves. False moves were defined as complete or incomplete paths that were not part of the solved maze or, although correct, were deleted before the maze was finally solved. Excess moves were calculated by subtracting the number of paths (i.e., pairs of dots) from the total (i.e., false and correct) number of moves.
Third, movement metrics were computed from the touch data. For this, moves connecting start and end points were extracted. In NL puzzles, moves follow either straight or winding paths. Winding paths change direction from segment to segment depending on the number of turns of the path (see Figures 1, 2). Because movements in NL puzzles are constrained to vertical and horizontal direction, moves for paths with changing directions (i.e., paths involving turns) were segmented into sub-paths based on the detected path turns (see red squares for detected path turns and red arrows for sub-paths in Figure 3) using the Ramer–Douglas–Peucker algorithm (Ramer, 1972; Douglas and Peucker, 1973; Bleier, 2011). For the thus extracted sub-paths and paths without turns (straight), two quantitative movement metrics were calculated: mean velocity (MVE) and movement direction changes (MDC; MacKenzie et al., 2001).
MVE represents the average movement speed. MDC represents the number of direction changes within the axis of the detected sub-paths during drag movements. More recently, these measures have been shown successful in differentiating between groups with and without motor impairment (Keates and Trewin, 2005; Keates et al., 2005; Mertens et al., 2012; Montague et al., 2014; Papatheodorou et al., 2019).
Procedure
The study consisted of a neuropsychological assessment and a NL puzzle playing session. After written informed consent was obtained, baseline measures of general cognitive ability (MoCA), visual search and visuomotor processing (TMT-A), cognitive flexibility, divided attention, working memory and inhibition (TMT-B), and visuoconstructional ability, planning, and foresight (SMT) were collected.
Next, participants completed a practice block of three NL puzzles: (w, h, p, t) = (4, 4, 4, 0), (4, 4, 4, 4), (5, 5, 5, 5) on a tablet-computer (Apple© 12.9” iPad Pro, Apple Inc., Cupertino, CA, United States). Instructions were given that all pairs of same-colored dots must be connected using tap and drag movements to cover all empty cells in the board and that connections will be severed if they intersect. Also, participants were shown the hint button that connects two dots when pressed and encouraged to use a hint should they struggle to solve a puzzle. Thereafter, participants were assigned one of three sets of NL puzzle difficulty levels based on their individual MoCA score and TMT B performance: short version (MoCA < 23, TMT B > 120 s), medium version (MoCA ≥ 23, ≤ 28, TMT B < 120 s) and long version (MoCA > 28, TMT B < 120 s). For each difficulty level, a NL puzzle was selected randomly from the pre-generated NL puzzles. The order of presentation of difficulty levels was randomized in order to avoid learning effects (van Kreveld et al., 2015). After completing the NL puzzle difficulty levels, participants evaluated the usability and their experience with the NL puzzles by filling in the SUS and the Perception of Game Training Questionnaire.
Statistical Analysis
For group comparison, only the NL difficulty levels from the short version, completed by all participants, were analyzed. Due to non-normally distributed data (visual inspection of histogram and quantile–quantile plots and Shapiro–Wilk tests), statistical differences between the participant groups were performed using the non-parametric Kruskal–Wallis test followed by multiple comparison post hoc tests using the pgirmess package (Giraudoux, 2018) in R version 1.1.463 (R Core Team, 2018). As criterion for statistical significance, a probability level of 0.05 was used in post hoc comparisons.
To assess the concurrent validity of the NL puzzle task, associations between NL puzzle performance measures and neuropsychological test measures of attentional, visuospatial and visuoconstructional, and executive function and global cognitive ability were tested by correlational analyses (Spearman rank correlation coefficients) using the sjstats package (Lüdecke, 2019). Furthermore, partial correlation analyses (using Spearman rank correlation coefficients), controlling for participant age, were performed using the ppcor package (Kim, 2015).
Difficulty level manipulation was examined using correlational analyses (Spearman rank correlation coefficients) between total time to solve the puzzle and difficulty level. Difficulty levels were ordered based on the set size (width × height) and the number of paths of the NL puzzles. Only data from the young and older adults who completed the full range of manipulated difficulty levels were analyzed using separate correlations for young and older adults.
Results
Results for Demographic Variables and Neuropsychological Tests
Participant demographic variables and neuropsychological test data are shown in Table 1. Groups differed significantly in age ( = 568.21; p < 0.001), but not in years of education (F(4, 38) = 1.744, p = 0.161). Neuropsychological test measures revealed significant group differences in the TMT-A completion time ( = 29.43; p < 0.001) and errors ( = 11.26; p = 0.024), the TMT-B completion time ( = 32.13; p < 0.001) and errors ( = 13.04; p = 0.01) and number correct ( = 8.62; p = 0.07), the SMT solving time ( = 32.56; p < 0.001) and errors ( = 13.52; p < 0.01) and the MoCA score ( = 19.18; p < 0.001). Post hoc analyses (multiple comparison tests, p < 0.05) that young adults were significantly faster on the TMT-A and TMT-B than oldest adults (TMT-A p < 0.001, TMT-B p < 0.001) and patients with HD (TMT-A p = 0.021, TMT-B p = 0.019). Furthermore, young adults were significantly faster on the TMT-B than old adults (p = 0.001). SMT solving time was significantly different between young adults and older adults (p < 0.001), oldest adults and patients with HD (p = 0.009).
Results for Perception and Usability of the NL Puzzle Game
Ratings of enjoyment ( = 3.81; p = 0.43), challenge ( = 8.75; p = 0.07), frustration ( = 8.98; p = 0.06), and motivation ( = 2.36; p = 0.67) for the NL puzzle game did not differ significantly between groups. There was a significant difference in usability ratings between groups ( = 12.04; p = 0.02), but post hoc comparisons failed to show any significant differences between groups (YA vs. OA, OOA vs. PD, OOA vs. HD, PD vs. HD: p = 1.000, YA vs. OOA p = 0.140, YA vs. PD p = 0.609, YA vs. HD p = 0.355, OA vs. OOA p = 0.228, OA vs. PD p = 0.689, OA vs. HD p = 0.414). Overall, individual SUS ratings ranged from 67.50 (“good”) to 100.00 (“best”) with a mean SUS rating of 86.43 (“excellent”; Bangor et al., 2008).
Results for NL Performance Measures
NL puzzle game performance measures by group can be found in Table 2 and are displayed in Figure 4. For the time-based NL performance measures, there were significant group differences in TST ( = 419.51, p < 0.001), MET ( = 324.72, p < 0.001), and MPT ( = 410.97, p < 0.001). Post hoc analyses showed that both total solving (TST: YA vs. OA, YA vs. OOA, YA vs. PD, YA vs. HD, OA vs. OOA p < 0.001, OOA vs. PD p = 0.034, PD vs. HD: p = 0.002) and MPTs (MPT: YA vs. OA, YA vs. OOA, YA vs. HD, p < 1.000, YA vs. PD p = 0.009, OA vs. OOA p = 0.001, OOA vs. PD p = 0.015, OA vs. HD = 0.034, PD vs. HD: p = 0.008) were significantly different between all groups (multiple comparison test, p < 0.05), except between older adults and patients with PD (TST p = 1.000, MPT p = 1.000) and between oldest adults and patients with HD (TST p = 1.000, MPT p = 1.000). MET (MET: YA vs. OA, YA vs. OOA, YA vs. HD, p < 0.001, YA vs. PD p = 0.002, OA vs. OOA p = 0.003, OA vs. HD = 0.008, PD vs. HD: p = 0.043) differed significantly between all groups (p < 0.05) with the exception of older adults and Parkinson’s patients, oldest adults and Parkinson’s patients, and oldest adults and Huntington’s patients that were not significantly different.
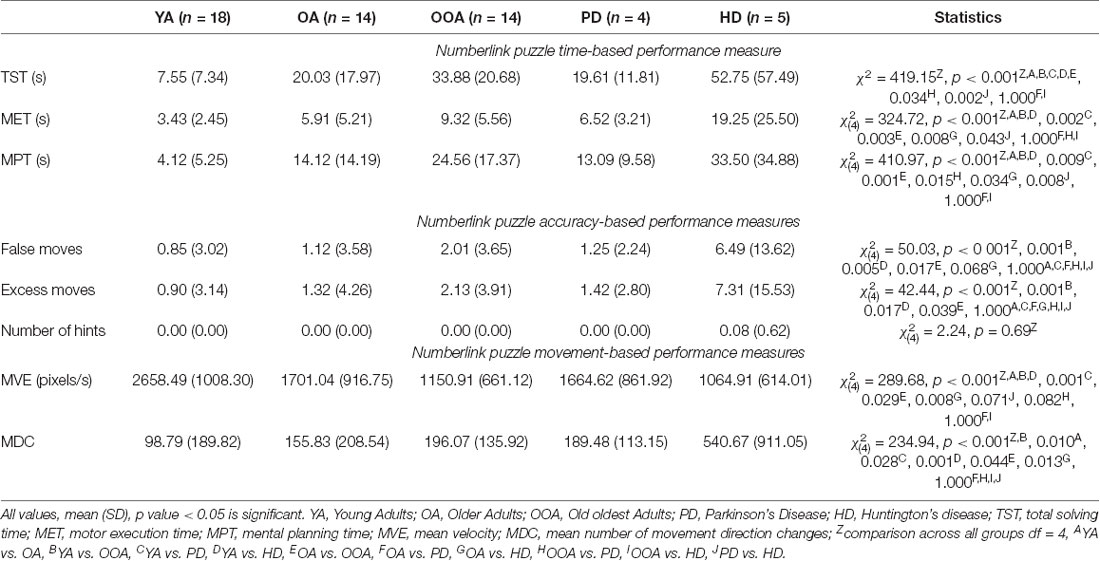
Table 2. Time, accuracy, and movement-based performance measures for the Numberlink puzzle game by group.
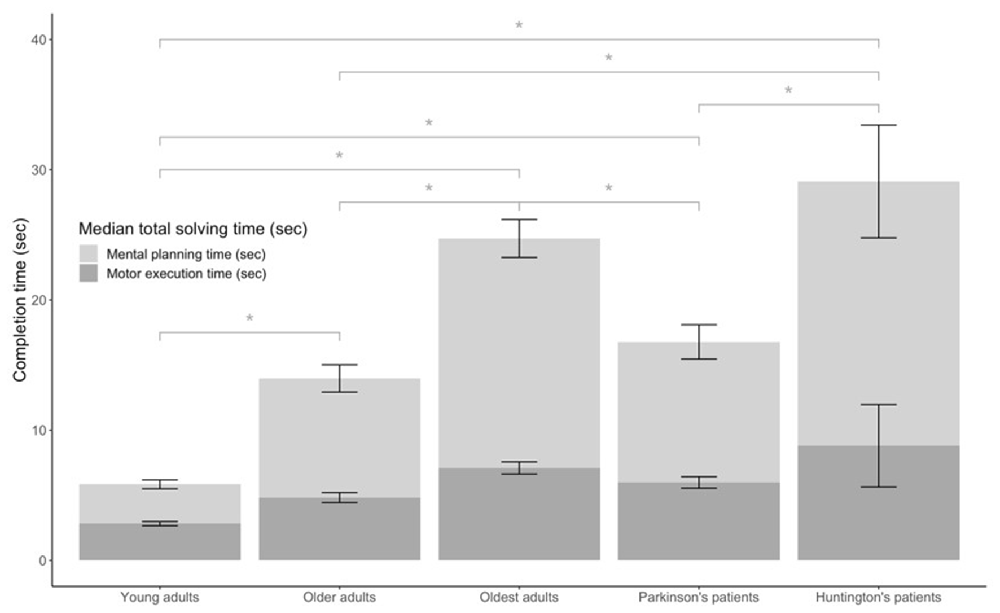
Figure 4. Median solving times of Numberlink puzzles for young, older, and oldest adults, and patients with Parkinson’s and Huntington’s disease. Total solving time (TST) is shown separately as the time needed to connect paths motor execution time (MET) and time where no movements were made mental planning time (MPT), *significant difference at the 0.05 level.
Results for accuracy-based NL performance indicators further suggest significant group differences in the number of false ( = 50.03, p < 0.001) and excess moves ( = 42.44, p < 0.001) moves. There were no significant group differences in the number of used hints ( = 2.24, p = 0.69). The number of false moves were significantly different between the young and oldest adults (p = 0.001), young adults and patients with HD (p = 0.005), as well as between the older adults and the oldest adults (p = 0.017) and the older adults and patients with HD. Post hoc comparisons further revealed that patients with HD made significantly more excess moves than both young (p = 0.017) and older adults. Oldest adults further made significantly more excess moves than young adults (p = 0.001).
Results for NL Puzzle Movement Metrics
Analysis of movement-based measures when playing the NL puzzles, revealed significant differences between groups in average movement velocity ( = 289.68, p < 0.001) and average number of MDC ( = 234.94, p < 0.001). Post hoc group comparisons revealed significant differences in average movement velocity (MVE: YA vs. OA, YA vs. OOA, YA vs. HD, p < 0.001, YA vs. PD p = 0.001, OA vs. OOA p = 0.029, OA vs. HD p = 0.008) between all groups (p < 0.05) except with older adults and patients with PD, and oldest adults compared to patients with HD and PD. In terms of the number of MDC, all group comparisons (MDC: YA vs. OA p = 0.010, YA vs. OOA p < 0.001, YA vs. PD p = 0.028, YA vs. HD p = 0.001, OA vs. OOA p = 0.044, OA vs. HD: p = 0.013) were significant, except for patients with HD compared to oldest adults and patients with PD and patients with PD compared to older and oldest adults.
Correlation Between NL Puzzle Performance and Neuropsychological Test Measures
Correlational analyses (see Table 3) revealed significant associations between time-based NL puzzle performance (median TST) and performance in visuomotor and visuospatial (TMT-A time), executive (shifting, inhibition; TMT-B time), visuoconstructional and executive (planning and foresight) function (SMT time), and global cognitive ability (MoCA). Partial correlations controlling for age remained significant. Similarly, accuracy-based NL puzzle measures (number of false and excess moves) were significantly correlated with TMT-A and -B time, SMT time, and the MoCA score. However, when controlling for age with partial correlation, the correlations with SMT time and MoCA did not remain significant. Finally, movement-based measures from the NL puzzles (MVE, MDC) all showed significant correlations with SMT time, TMT-A and -B time, and MoCA score. After controlling for age, these correlations remained significant with the exception of correlations between MDC and global cognitive ability (MoCA).
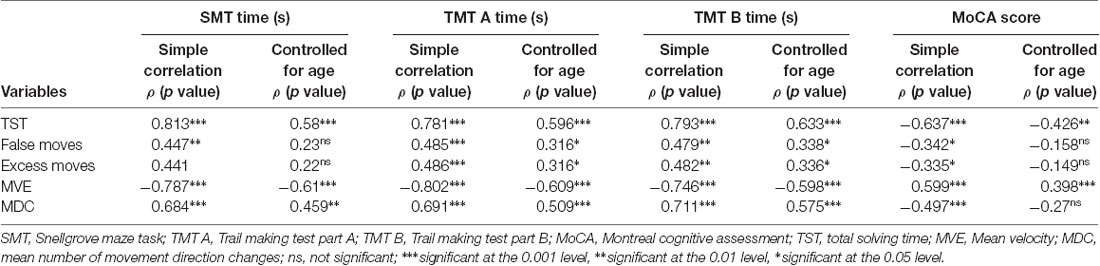
Table 3. Correlations between Numberlink puzzle performance and movement metrics and neuropsychological test measures.
Results for Task Difficulty Manipulation
Total NL puzzle solving time as a function of difficulty level are shown separately for young and older adults that completed the full range of NL difficulty levels in Figure 5. Separate Spearman correlation analyses between total puzzle solving time and ranked difficulty levels revealed a significant positive association for both the young adults (r(17) = 0.583, p < 0.001) and the older adults (r(13) = 0.496, p < 0.001).
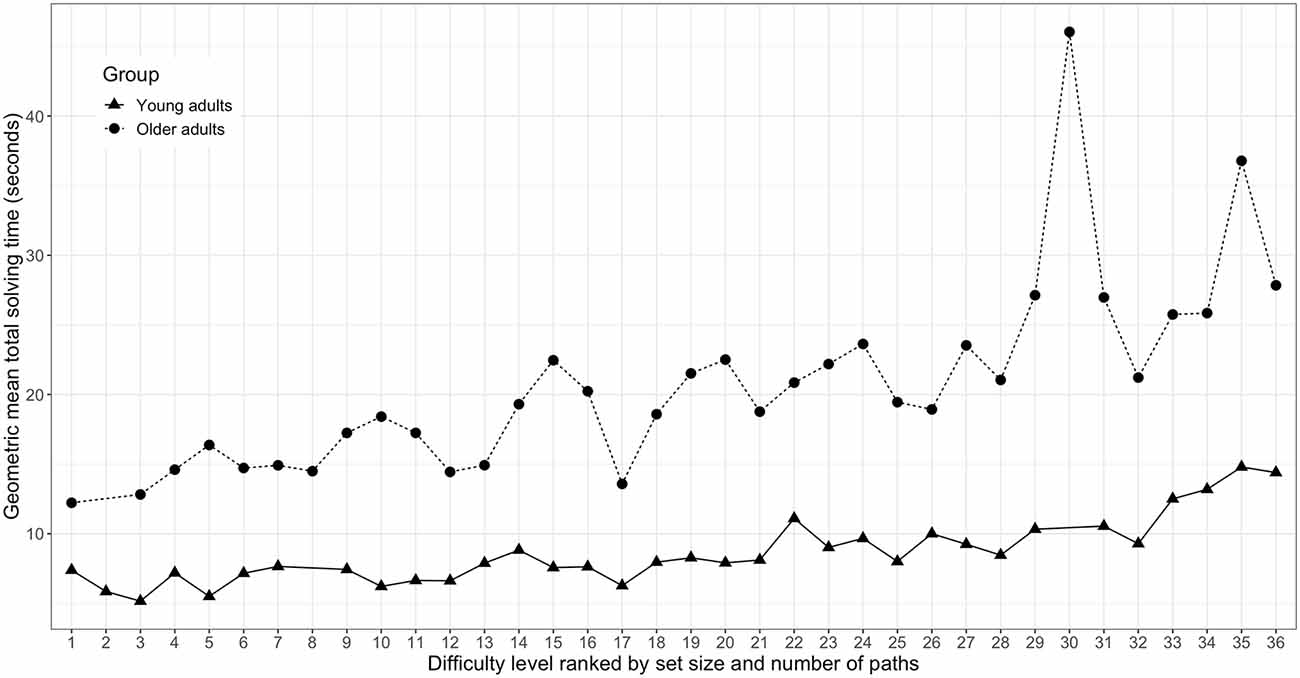
Figure 5. Total Numberlink puzzle solving time as a function of ranked difficulty level for young (n = 18) and older adults (n = 14). Difficulty levels are defined by set size and the number of paths of the respective Numberlink puzzles.
Discussion
The aim of this study was to evaluate the feasibility and preliminary validity of a maze-like NL puzzle video game as a tool to assess cognitive and motor differences in older adults and patients with NDDs. Results from this study show that NL puzzles are enjoyable, motivating, and user-friendly for older adults and patients with motor difficulties in neurodegenerative diseases. Analyses of game-based measures of cognitive and motor performance showed significant differences in executive and motor function within the different age groups and between the disease groups. However, there were no differences in total solving, mental planning, and MET between HD and the oldest age (OOA) group and between PD and the older age (OA) group. Initial concurrent validation showed that the NL puzzle game performance correlates with a standardized maze task (SMT) and tests for cognitive abilities relevant to cognitive decline due to aging and NDD. This study further shows that characteristics of the game can be adjusted to create graded levels of difficulty.
Analyses of performance on the played NL puzzle game levels showed significant age-group differences. Overall, older adults took longer to solve NL puzzles than young adults, and oldest adults were slower compared to older adults. The differences in NL puzzle performance between the age groups are consistent with age-related changes in motor and executive functioning (Zhang et al., 2007). One explanation for the effect of age on NL puzzle game performance might simply be a decline in motor processing speed with age (Salthouse, 2000; Ebaid et al., 2017). Findings from this study do show that MET (i.e., total time needed to manually connect paths in the maze) and average velocity of these movements in the NL puzzle game were significantly different between young, older, and oldest adults. However, the movement velocity and MDC did not differ between the oldest groups and the neurodegenerative disease groups. Reasons may lie in the visuoconstructional (Snellgrove, 2005) and perceptual-motor function (Zhao and Marquez, 2013) skills required for NL puzzle games. The perceptual-motor skills decline with age and neurodegenerative diseases. For PD and HD patients, the UPDRS-Motor score quantifies the decline in motor skills. It would be advisable to quantify the manual dexterity of older and oldest adults so that they can be adjusted for their game-based motor performances. PD patients recruited in our study were in their early disease stage and had very low UPDRS-Motor scores, which is seen clearly in their motor performances.
However, our finding of age-group differences in MPT (i.e., subtracting the MET from the TST) replicates previous studies that showed age effects on maze solving ability (Koss et al., 1991; Krishnasamy and Unsworth, 2011; de Souza et al., 2013), even when controlling for motor processing speed. The age effect on maze solving ability has been suggested to reflect age-related difficulties in executive and visuoconstructive functioning (Krishnasamy and Unsworth, 2011; de Souza et al., 2013). Interestingly, studies have suggested that executive deficits with aging are not general, but specifically affect planning ability that is usually measured using maze tasks (Zhang et al., 2007; Brandt et al., 2009) and strongly associated with everyday functioning in older age (Lewis and Miller, 2007). Furthermore, solving the NL puzzle game requires sequencing of motor actions to organize and sequence multiple paths that need to be connected in order to solve NL puzzles (Laurentiz, 2018). Similar to planning ability, sequencing motor actions is increasingly dependent on executive function and declines with age (Niermeyer et al., 2017).
Compared to young and older adults, patients with HD were significantly slower in both total solving, mental planning, and MET. This finding is underscored by the fact that HD patients made significantly more errors and excess moves when solving NL puzzles. Taken together, the difficulties of HD patients with solving maze-like NL puzzles are consistent with previous findings and reflect declines in psychomotor, visuospatial and executive function that occur throughout HD (Fedio et al., 1979; Montoya et al., 2006). Contrary to a previous study (Mimura et al., 2006), this study found no difference between PD patients and older adults in total solving, mental planning, and MET. This suggests that the PD patients in this study suffered no deficits in psychomotor and planning aspects of executive function. This finding was mirrored in the assessments done using the standardized SMT. PD patients recruited in our study had a good cognitive status, as indicated in their MoCA score (26.25 ± 3.40). Their MDS-UPDRS-III scores (17.75 ± 5.38) were significantly lower than the HD patients and displayed very few motor deficits. This was reflected in the game performance results such as mental planning and motor execution. However, PD medications can affect both their cognitive and motor skill levels, which cannot be ruled out.
As the MPT is TST minus the execution time, we could rule out a confounding effect of motor performance (Ebaid et al., 2017). In addition, we found identical group differences for game-based motor performance measures (MET and velocity and direction changes of movements), only this time, Parkinson’s patients were not different from oldest adults. Interestingly, these findings parallel earlier studies that assessed touch interactions on mobile devices in older adults and Parkinson’s patients (Keates and Trewin, 2005; Montague et al., 2014) and are supported by a recent viewpoint paper claiming that game-based movement measures reflect psychomotor deficits in age-related neurodegenerative diseases such as AD, PD, and HD (Mandryk and Birk, 2019).
The concurrent validity findings in this study show that game performance measures from the NL puzzle game were significantly correlated with performance on tests for visuomotor, visuospatial, executive, and visuoconstructional function. Game performance was also associated with global cognitive ability. These findings confirm recent suggestions that video games incorporate elements and cognitive challenges shared with psychological tasks (Holmgard et al., 2016). Furthermore, results indicate that game-based measures of cognitive and motor performance can be used to assess and monitor cognitive function in normal aging and neurodegenerative diseases (Koo and Vizer, 2019; Mandryk and Birk, 2019). Significant correlations between NL-based time and movement performance with tests of psychomotor, attentional, visuoconstructional, and executive functions support our assumption that NL puzzles share cognitive components with classical maze tasks (Snellgrove, 2005; Kirsch et al., 2006). Thus, a digital maze test like the NL can be used as a nonverbal intelligence test to estimate planning and inhibition. Moreover, they can overcome inconsistencies in interrater reliability seen in standard paper-based maze tests and can be deployed in a familiar user environment. The continuous and automatic data recording with digital technology will allow easy data collection of errors, corrections, and attempts of each user.
Our results on the usability and perception of the NL puzzles showed that they are enjoyable, motivating, user-friendly, and easy to use for both the healthy age groups and the neurodegenerative groups. However, there was a slight trend in rating the game session as challenging and frustrating, which is driven by perception ratings of the PD and HD patients. On the one hand, this might simply reflects the disease-related motor challenges in performance. On the other hand, it might be that it was harder for PD and HD patients to adapt to the difficulty levels. Adapting the difficulty levels to a person’s performance might resolve a part of this problem and also ensure that the puzzle games are equally challenging for players with different levels of cognitive ability (Lankoski, 2015; Holmgard et al., 2016).
Some limitations of this study need to be addressed: first, the sample size of this study is small, particularly for the groups with neurodegenerative disorders. Moreover, the level of progression of disease within the different neurodegenerative disease was not controlled for in this study. Therefore, validation studies with larger sample size are definitely needed to further confirm these initial findings. Second, our findings are based on comparing the performance on a limited set of NL puzzle difficulty levels. A possible solution to this would be to use computerized adaptive cognitive testing that adapts the difficulty of the task to the individual’s level of cognitive ability by selecting test items from graded difficulty levels. Using the pre-generated NL puzzle difficulty levels from this study, this would allow to better detect subtle changes in executive and motor function across a wider range of maze-like NL puzzle difficulties and avoid both floor and ceiling effects. Third, this study is cross-sectional, and participants play the NL only once and for the first time.
To sum up, this study supports recent suggestions that game-based data from playing a commercial maze-like puzzle game provides potential “digital biomarkers” to assess cognitive function and cognitive decline over time. In particular, NL puzzle games seem promising for capturing changes in visuomotor, visuoconstructional, and executive function related to aging and neurodegeneration.
Data Availability Statement
Data pertaining to the obtained results may be provided upon request.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of Canton Bern and Ethics Committee of Northwest and Central Switzerland. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TN, RM, and PU contributed to the conception and organization of the research. AC, TN, TV, JM, J-MB, and PU participated in the execution and data collection. AC, NS, AB, and PU designed the data analysis and statistical methods. AC wrote the first draft of the manuscript. All authors participated in the review and critical review of the manuscript.
Funding
The study was partially supported by a Novartis–Freenovation grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the residents and staff at the Senevita Bornblick senior residence home in Olten, Switzerland and the Burgerspittel, Bern, Switzerland. The authors extend their gratitude to Luca Persello for data handling and to Julia Truttmann and Manuela Wüthrich for their valuable help with recruiting and testing participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00087/full#supplementary-material.
References
Adcock, A., Demaine, E. D., Demaine, M. L., O’Brien, M. P., Reidl, F., Villaamil, F. S., et al. (2015). Zig-Zag numberlink is NP-complete. J. Info. Process. 23, 239–245. doi: 10.2197/ipsjjip.23.239
Agrigoroaei, S., and Lachman, M. E. (2011). Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J. Gerontol. B Psychol. Sci. Soc. Sci. 66, i130–i140. doi: 10.1093/geronb/gbr017
Ahle, T. D. (2017). Program for Solving and Generating Correct Numberlink Puzzles. Available online at: https://github.com/thomasahle/numberlink.
Allard, M., Husky, M., Catheline, G., Pelletier, A., Dilharreguy, B., Amieva, H., et al. (2014). Mobile technologies in the early detection of cognitive decline. PLoS One 9:e112197. doi: 10.1371/journal.pone.0112197
Alviano, M., Dodaro, C., and Ricca, F. (2013). “Comparing alternative solutions for unfounded set propagation in ASP,” in AI*IA 2013: Advances in Artificial Intelligence, eds M. Baldoni, C. Baroglio, G. Boella, R. Micalizio, M. Baldoni, C. Baroglio, G. Boella and R. Micalizio (Cham: Springer), 1–12.
Antal, M., and Szabó, L. Z. (2016). Biometric authentication based on touchscreen swipe patterns. Procedia Technol. 22, 862–869. doi: 10.1016/j.protcy.2016.01.061
Areàn, P. A., Hoa Ly, K., and Andersson, G. (2016). Mobile technology for mental health assessment. Dialogues Clin. Neurosci. 18, 163–169.
Bagnall, A., and Zatuchna, Z. (2005). “On the classification of maze problems,” in Foundations of Learning Classifier Systems, eds L. Bull, T. Kovacs, L. Bull and T. Kovacs (Berlin, Heidelberg: Springer), 305–316.
Bangor, A., Kortum, P. T., and Miller, J. T. (2008). An empirical evaluation of the system usability scale. Int. J. Hum. Comp. Inter. 24, 574–594. doi: 10.1080/10447310802205776
Baniqued, P. L., Lee, H., Voss, M. W., Basak, C., Cosman, J. D., Desouza, S., et al. (2013). Selling points: what cognitive abilities are tapped by casual video games? Acta Psychol. 142, 74–86. doi: 10.1016/j.actpsy.2012.11.009
Blatter, K., Opwis, K., Munch, M., Wirz-Justice, A., and Cajochen, C. (2005). Sleep loss-related decrements in planning performance in healthy elderly depend on task difficulty. J. Sleep Res. 14, 409–417. doi: 10.1111/j.1365-2869.2005.00484.x
Bleier, S. (2011). The Ramer-Douglas-Peucker Algorithm Implemented in Python: Sebleier/RDP. Available online at: https://github.com/sebleier/RDP.
Boller, F., Forette, F., Khachaturian, Z. S., Poncet, M., and Christen, I. (2013). Heterogeneity of Alzheimer’s Disease. Berlin, Heidelberg: Springer.
Boot, W. R. (2015). Video games as tools to achieve insight into cognitive processes. Front. Psychol. 6:3. doi: 10.3389/fpsyg.2015.00003
Boot, W. R., Champion, M., Blakely, D. P., Wright, T., Souders, D. J., and Charness, N. (2013). Video games as a means to reduce age-related cognitive decline: attitudes, compliance and effectiveness. Front. Psychol. 4:31. doi: 10.3389/fpsyg.2013.00031
Brandt, J., Aretouli, E., Neijstrom, E., Samek, J., Manning, K., Albert, M. S., et al. (2009). Selectivity of executive function deficits in mild cognitive impairment. Neuropsychology 23, 607–618. doi: 10.1037/a0015851
Carlozzi, N. E. (2011). “Porteus maze,” in Encyclopedia of Clinical Neuropsychology, eds J. S. Kreutzer, J. DeLuca, B. Caplan, J. S. Kreutzer, J. DeLuca and B. Caplan (New York, NY: Springer), 1964–1966.
Chesham, A., Wyss, P., Müri, R. M., Mosimann, U. P., and Nef, T. (2017). What older people like to play: genre preferences and acceptance of casual games. JMIR Serious Games 5:e8. doi: 10.2196/games.7025
Cummings, J. L., and Pillai, J. A. (2016). Neurodegenerative Diseases. USA: Oxford University press.
Davis, R., Libon, D. J., Au, R., Pitman, D., and Penney, D. L. (2014). THink: inferring cognitive status from subtle behaviors. Proc. Conf. AAAI Artif. Intell. 2014, 2898–2905. doi: 10.1609/aimag.v36i3.2602
Deary, I. J., Corley, J., Gow, A. J., Harris, S. E., Houlihan, L. M., Marioni, R. E., et al. (2009). Age-associated cognitive decline. Br. Med. Bull. 92, 135–152. doi: 10.1093/bmb/ldp033
Deckel, A. W., and Duffy, J. D. (2000). Vasomotor hyporeactivity in the anterior cerebral artery during motor activation in Huntington’s disease patients. Brain Res. 872, 258–261. doi: 10.1016/s0006-8993(00)02506-3
Diesing, T. S., and Rizzo, M. (2018). “Medical assessment of the aging mind and brain,” in The Wiley Handbook on the Aging Mind and Brain, eds M. Rizzo, S. Anderson and B. Fritzsch (USA: Wiley-Blackwell), 101–129.
Ding, W., Ding, L. J., Li, F. F., Han, Y., and Mu, L. (2015). Neurodegeneration and cognition in Parkinson’s disease: a review. Eur. Rev. Med. Pharmacol. Sci. 19, 2275–2281.
Douglas, D. H., and Peucker, T. K. (1973). Algorithms for the reduction of the number of points required to represent a digitized line or its caricature. Acad. Emerg. Med. 10, 112–122.
Dudeney, H. E. (1917). “Problem 252—a puzzle for motorists,” in Amusements in Mathematics, (MI, USA: Thomas Nelson and Sons Ltd.).
Dumas, E. M., van den Bogaard, S. J. A., Middelkoop, H. A. M., and Roos, R. A. C. (2013). A review of cognition in Huntington’s disease. Front. Biosci. 5, 1–18. doi: 10.2741/s355
Ebaid, D., Crewther, S. G., MacCalman, K., Brown, A., and Crewther, D. P. (2017). Cognitive processing speed across the lifespan: beyond the influence of motor speed. Front. Aging Neurosci. 9:62. doi: 10.3389/fnagi.2017.00062
Elithorn, A. (1955). A preliminary report on a perceptual maze test sensitive to brain damage. J. Neurol. Neurosurg. Psychiatry 18, 287–292. doi: 10.1136/jnnp.18.4.287
Fedio, P., Cox, C. S., Neophytides, A., Canal-Frederick, G., and Chase, T. N. (1979). Neuropsychological profile of Huntington’s disease: patients and those at risk. Adv. Neurol. 23, 239–255.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Giraudoux, P. (2018). pgirmess: Spatial Analysis and Data Mining for Field Ecologists. Available online at: https://rdrr.io/cran/pgirmess/.
Greenland, J. C., Williams-Gray, C. H., and Barker, R. A. (2019). The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur. J. Neurosci. 49, 328–338. doi: 10.1111/ejn.14094
Hartmann, S. (2018). Puzzle—solving smartphone puzzle apps by mathematical programming. INFORMS Trans. Educ. 18, 127–141. doi: 10.1287/ited.2017.0182
Holmgard, C., Togelius, J., and Henriksen, L. (2016). “Computational intelligence and cognitive performance assessment games,” in Proceeding of the 2016 IEEE Conference on Computational Intelligence and Games (CIG), Santorini, Greece, 1–8. doi: 10.1109/CIG.2016.7860388
Howieson, D. (2019). Current limitations of neuropsychological tests and assessment procedures. Clin. Neuropsychol. 33, 200–208. doi: 10.1080/13854046.2018.1552762
Hussain, R., Zubair, H., Pursell, S., and Shahab, M. (2018). Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 8:177. doi: 10.3390/brainsci8090177
Keates, S., and Trewin, S. (2005). “Effect of age and Parkinson’s disease on cursor positioning using a mouse,” in Proceedings of the 7th International ACM SIGACCESS Conference on Computers and Accessibility—Assets ’05, Baltimore, NV, USA, 68–75. doi: 10.1145/1090785.1090800
Keates, S., Trewin, S., and Paradise, J. (2005). “Using pointing devices: quantifying differences across user groups,” in Proceedings of the 3rd International Conference on Universal Access in Human-Computer Interaction, Las Vegas, Nevada, USA, 68–75.
Kim, S. (2015). ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun. Stat. Appl. Methods 22, 665–674. doi: 10.5351/CSAM.2015.22.6.665
Kirsch, P., Lis, S., Esslinger, C., Gruppe, H., Danos, P., Broll, J., et al. (2006). Brain activation during mental maze solving. Neuropsychobiology 54, 51–58. doi: 10.1159/000095742
Koss, E., Haxby, J. V., DeCarli, C., Schapiro, M. B., Friedland, R. P., and Rapoport, S. I. (1991). Patterns of performance preservation and loss in healthy aging. Dev. Neuropsychol. 7, 99–113. doi: 10.1080/87565649109540479
Koo, B. M., and Vizer, L. M. (2019). Mobile technology for cognitive assessment of older adults: a scoping review. Innov. Aging 3:igy038. doi: 10.1093/geroni/igy038
Kovacs, G. G. (2018). Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 145, 301–307. doi: 10.1016/B978-0-12-802395-2.00021-3
Krishnasamy, C., and Unsworth, C. A. (2011). Normative data, preliminary inter-rater reliability and predictive validity of the Drive Home Maze Test. Clin. Rehabil. 25, 88–95. doi: 10.1177/0269215510382846
Laurentiz, S. (2018). “Conformed thought: consolidating traces of memories,” in Design, User Experience and Usability: Users, Contexts and Case Studies, eds A. Marcus, W. Wang, A. Marcus and W. Wang (Cham, Switzerland: Springer), 28–40.
Levy, L., Lambeth, A., Solomon, R., and Gandy, M. (2018). “Method in the madness: the design of games as valid and reliable scientific tools,” in Proceedings of the 13th International Conference on the Foundations of Digital Games—FDG ’18, Sweden, 1–10. doi: 10.1145/3235765.3235793
Lewis, M. S., and Miller, L. S. (2007). Executive control functioning and functional ability in older adults. Clin. Neuropsychol. 21, 274–285. doi: 10.1080/13854040500519752
Loe, B. S., and Rust, J. (2019). The perceptual maze test revisited: evaluating the difficulty of automatically generated mazes. Assessment 26, 1524–1539. doi: 10.1177/1073191117746501
Loyd, S. (1897). “The puzzled neighbors,” in Brooklyn Public Library, (Brooklyn, NewYork: The Brooklyn Daily Eagle), 17.
Lüdecke, D. (2019). sjstats: Statistical Functions for Regression Models (Version 0.17.4). doi: 10.5281/zenodo.1284472
Mack, J. L., and Patterson, M. B. (1995). Executive dysfunction and alzheimer’s disease: performance on a test of planning ability, the porteus maze test. Neuropsychology 9, 556–564. doi: 10.1037/0894-4105.9.4.556
MacKenzie, I. S., Kauppinen, T., and Silfverberg, M. (2001). “Accuracy measures for evaluating computer pointing devices,” in Proceedings of the SIGCHI Conference on Human Factors in Computing Systems—CHI ’01, Seattle, Washington, DC, United States, 9–16. doi: 10.1145/365024.365028
Mandryk, R. L., and Birk, M. V. (2019). The potential of game-based digital biomarkers for modeling mental health. JMIR Ment. Health 6:e13485. doi: 10.2196/13485
Marhasev, E., Hadad, M., Kaminka, G. A., and Feintuch, U. (2009). The use of hidden semi-Markov models in clinical diagnosis maze tasks. Intell. Data Anal. 13, 943–967. doi: 10.3233/IDA-2009-0402
Martinovic, D., Burgess, G. H., Pomerleau, C. M., and Marin, C. (2015). Comparison of children’s gaming scores to NEPSY-II scores: validation of computer games as cognitive tools. Comput. Hum. Behav. 49, 487–498. doi: 10.1016/j.chb.2015.03.039
Massaldjieva, R. I. (2018). “Differentiating normal cognitive aging from cognitive impairment no dementia: a focus on constructive and visuospatial abilities,” in Gerontology, eds G. D’Onofrio, A. Greco, D. Sancarlo, G. D’Onofrio, A. Greco and D. Sancarlo (IntechOpen).
Mertens, A., Hurtmanns, J., Wacharamanotham, C., Kronenbürger, M., Borchers, J., and Schlick, C. M. (2012). Swabbing: touchscreen-based input technique for people with hand tremor. Work 2405–2411. doi: 10.3233/wor-2012-0474-2405
McClendon, M. S. (2001). “The complexity and difficulty of a maze,” in Proceedings of the 4th Annual Conference of Bridges: Mathematical Connections in Art, Music and Science, Winfield, KS, USA, 213–222.
Mimura, M., Oeda, R., and Kawamura, M. (2006). Impaired decision-making in Parkinson’s disease. Parkinsonism Relat. Disord. 12, 169–175. doi: 10.1016/j.parkreldis.2005.12.003
Montague, K., Nicolau, H., and Hanson, V. L. (2014). “Motor-impaired touchscreen interactions in the wild,” in Proceedings of the 16th International ACM SIGACCESS Conference on Computers and Accessibility—ASSETS ’14, Rochester, New York, NY, USA, 123–130. doi: 10.1145/2661334.2661362
Montoya, A., Price, B. H., Menear, M., and Lepage, M. (2006). Brain imaging and cognitive dysfunctions in Huntington’s disease. J. Psychiatry Neurosci. 31, 21–29.
Mosti, C. B., Rog, L. A., and Fink, J. W. (2019). “Differentiating mild cognitive impairment and cognitive changes of normal aging,” in Handbook on the Neuropsychology of Aging and Dementia, eds L. D. Ravdin, H. L. Katzen, L. D. Ravdin and H. L. Katzen (Cham: Springer), 445–463.
Mutser, P. (2014). Automated Rating of Level Difficulty for Puzzle Games. Utrecht, Netherlands: Utrecht University Repository.
Nasreddine, Z. S., Phillips, N. A., Bé dirian, V. R., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Newman, M., and Newman, S. (2012). “Flow free app,” in Flow Free Wiki, (FL, USA: Big Duck Games LLC).
Niermeyer, M. A., Suchy, Y., and Ziemnik, R. E. (2017). Motor sequencing in older adulthood: relationships with executive functioning and effects of complexity. Clin. Neuropsychol. 31, 598–618. doi: 10.1080/13854046.2016.1257071
Oei, A. C., and Patterson, M. D. (2013). Enhancing cognition with video games: a multiple game training study. PLoS One 8:e58546. doi: 10.1371/journal.pone.0058546
Ott, B. R., Heindel, W. C., Whelihan, W. M., Caron, M. D., Piatt, A. L., and DiCarlo, M. A. (2003). Maze test performance and reported driving ability in early dementia. J. Geriatr. Psychiatry Neurol. 16, 151–155. doi: 10.1177/0891988703255688
Pal, A., Pegwal, N., Kaur, S., Mehta, N., Behari, M., and Sharma, R. (2018). Deficit in specific cognitive domains associated with dementia in Parkinson’s disease. J. Clin. Neurosci. 57, 116–120. doi: 10.1016/j.jocn.2018.08.016
Papatheodorou, N., Pino, A., Kouroupetroglou, G. T. H., Constantinides, V., Andreadou, E., and Papageorgiou, C. C. (2019). Upper limb motor skills performance evaluation based on point-and-click cursor trajectory analysis: application in early multiple sclerosis detection. IEEE Access 7, 28999–29013. doi: 10.1109/access.2019.2901926
Pasek, T. R. (2016). Hierarchical mazes in psychological research. Am. J. Psychol. 129, 443–459. doi: 10.5406/amerjpsyc.129.4.0443
Paulsen, J. S., and Long, J. D. (2014). Onset of Huntington’s disease: can it be purely cognitive? onset of huntington disease: can it be purely cognitive? Mov. Disord. 29, 1342–1350. doi: 10.1002/mds.25997
Polzer, N., and Gewald, H. (2019). Can cognitive assessments be fun? A review of playful mobile applications to assess cognitive health. PSAKU Int. J. Interdisciplinary Res. 8:7. doi: 10.2139/ssrn.3398109
Porteus, S. D. (1945). Porteus maze tests: applications in medical and allied fields. Br. J. Med. Psychol. 20, 267–270. doi: 10.1111/j.2044-8341.1945.tb00762.x
Ramer, U. (1972). An iterative procedure for the polygonal approximation of plane curves. Comput. Graph. Image Process. 1, 244–256. doi: 10.1016/s0146-664x(72)80017-0
R Core Team. (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Roalf, D. R., Moberg, P. J., Xie, S. X., Wolk, D. A., Moelter, S. T., and Arnold, S. E. (2013). Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 9, 529–537. doi: 10.1016/j.jalz.2012.10.001
Rycroft, S. S., and Giovannetti, T. (2017). “Alzheimer’s disease and other dementia disorders,” in Changes in the Brain, eds N. D. Chiaravalloti, Y. Goverover, N. D. Chiaravalloti and Y. Goverover (New York, NY: Springer-Verlag Publishing), 37–63.
Salthouse, T. A. (2000). Aging and measures of processing speed. Biol. Psychol. 54, 35–54. doi: 10.1016/s0301-0511(00)00052-1
Schretlen, D., Bobholz, J. H., and Brandt, J. (1996). Development and psychometric properties of the brief test of attention. Clin. Neuropsychol. 10, 80–89. doi: 10.1080/13854049608406666
Silva, J. B. S., Cristino, E. D., Almeida, N. L., Medeiros, P. C. B., and Santos, N. A. (2017). Effects of acute alcohol ingestion on eye movements and cognition: a double-blind, placebo-controlled study. PLoS One 12:e0186061. doi: 10.1371/journal.pone.0186061
Silverberg, N. B., Ryan, L. M., Carrillo, M. C., Sperling, R., Petersen, R. C., Posner, H. B., et al. (2011). Assessment of cognition in early dementia. Alzheimers Dement. 7, e60–e76. doi: 10.1016/j.jalz.2011.05.001
Snellgrove, C. A. (2005). Cognitive Screening for the Safe Driving Competence of Older People with Mild Cognitive Impairment or Early Dementia. Canberra: Australian Transport Safty Bureau.
de Souza, D. E., Oliveira, D. C., and Campos, T. F. (2013). Maze test: an instrument to evaluate age-related cognitive and motor changes in humans. Rev. Bras. Ciênc. Envelhecim. Hum. 10:1. doi: 10.5335/rbceh.2013.3015
Staplin, L., Gish, K. W., Lococo, K. H., Joyce, J. J., and Sifrit, K. J. (2013). The maze test: a significant predictor of older driver crash risk. Accid. Anal. Prev. 50, 483–489. doi: 10.1016/j.aap.2012.05.025
Strauss, E., Sherman, E. M. S., and Spreen, O. (2006). A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press.
Thompson, O., Barrett, S., Patterson, C., and Craig, D. (2012). Examining the neurocognitive validity of commercially available, smartphone-based puzzle games. Psychology 03, 525–526. doi: 10.4236/psych.2012.37076
Tong, T., Chignell, M., Tierney, M. C., and Lee, J. (2016). A serious game for clinical assessment of cognitive status: validation study. JMIR Serious Games 4:e7. doi: 10.2196/games.5006
Valdes, E. G., Sadeq, N. A., Harrison Bush, A. L., Morgan, D., and Andel, R. (2016). Regular cognitive self-monitoring in community-dwelling older adults using an internet-based tool. J. Clin. Exp. Neuropsychol. 38, 1026–1037. doi: 10.1080/13803395.2016.1186155
van Kreveld, M., Löffler, M., and Mutser, P. (2015). “Automated puzzle difficulty estimation,” in Proceedings of the 2015 IEEE Conference on Computational Intelligence and Games (CIG), Tainan, 415–422.
White, A., Li, Y., Kuan, W.-L., and Mason, S. (2018). F31 Understanding cognitive heterogeneity beyond disease burden in huntington’s disease. J. Neurol. Neurosurg. Psychiatry 89, A50–A51. doi: 10.1136/jnnp-2018-EHDN.135
Yew, N.-Z., Tiong, K.-M., and Yong, S. T. (2011). Recursive Path-finding in a dynamic maze with modified tremaux’s algorithm. J. Mathemat. Comput. Sci. 12, 2102–2104. doi: 10.5281/zenodo.1077030
Yew, N.-Z., Tiong, K.-M., and Yong, S. T. (2012). “Path-finding in a maze-like puzzle using multipartite graph algorithm,” in Proceedings of the Hong Kong International Conference on Engineering and Applied Science (HKICEAS), Hong Kong, China, 14–16.
Yoshinaka, R., Saitoh, T., Kawahara, J., Tsuruma, K., Iwashita, H., and Minato, S.-I. (2012). Finding all solutions and instances of numberlink and slitherlink by ZDDs. Algorithms 5, 176–213. doi: 10.3390/a5020176
Zhang, Y., Han, B., Verhaeghen, P., and Nilsson, L.-G. (2007). Executive functioning in older adults with mild cognitive impairment: MCI has effects on planning, but not on inhibition. Aging Neuropsychol. Cogn. 14, 557–570. doi: 10.1080/13825580600788118
Zhao, M., and Marquez, A. G. (2013). Understanding humans’ strategies in maze solving. arXiv:1307.5713 [Preprint].
Keywords: cognitive assessment, maze task, puzzle video games, aging, neurodegenerative diseases
Citation: Nef T, Chesham A, Schütz N, Botros AA, Vanbellingen T, Burgunder J-M, Müllner J, Müri RM and Urwyler P (2020) Development and Evaluation of Maze-Like Puzzle Games to Assess Cognitive and Motor Function in Aging and Neurodegenerative Diseases. Front. Aging Neurosci. 12:87. doi: 10.3389/fnagi.2020.00087
Received: 04 December 2019; Accepted: 16 March 2020;
Published: 21 April 2020.
Edited by:
Rommy Von Bernhardi, Pontifical Catholic University of Chile, ChileReviewed by:
Gabriella Santangelo, University of Campania ‘Luigi Vanvitelli, ItalyAinhoa Plaza-Zabala, Achucarro Basque Center for Neuroscience, Spain
Copyright © 2020 Nef, Chesham, Schütz, Botros, Vanbellingen, Burgunder, Müllner, Müri and Urwyler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prabitha Urwyler, prabitha.urwyler@artorg.unibe.ch
 Tobias Nef
Tobias Nef Alvin Chesham1
Alvin Chesham1  Narayan Schütz
Narayan Schütz Angela Amira Botros
Angela Amira Botros Tim Vanbellingen
Tim Vanbellingen Jean-Marc Burgunder
Jean-Marc Burgunder Julia Müllner
Julia Müllner René Martin Müri
René Martin Müri Prabitha Urwyler
Prabitha Urwyler