Evaluation of PIK3CA mutations in advanced ER+/HER2-breast cancer in Portugal – U-PIK Project
- 1Serviço de Genética Laboratorial, Instituto Português de Oncologia do Porto Francisco Gentil (IPO Porto), Porto, Portugal
- 2IPATIMUP - Instituto de Patologia e Imunologia da Universidade do Porto, Porto, Portugal
- 3Serviço de Anatomia Patológica, Instituto Português de Oncologia de Lisboa Francisco Gentil (IPOLFG), Lisboa, Portugal
- 4Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
- 5Unidade de Mama, Centro Clínico Champalimaud, Fundação Champalimaud, Lisboa, Portugal
- 6Serviço de Anatomia Patológica, Hospital CUF Descobertas, Lisboa, Portugal
- 7Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal
- 8Serviço de Anatomia Patológica, Instituto Português de Oncologia do Porto Francisco Gentil (IPO Porto), Porto, Portugal
- 9Novartis Farma - Produtos Farmacêuticos, S.A., Porto Salvo, Portugal
- 10Serviço de Anatomia Patológica, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
- 11Departamento de Genética Molecular, SYNLAB Genética Médica, S.A., Porto, Portugal
- 12Instituto de Anatomia Patológica, Lisboa, Portugal
- 13Serviço de Anatomia Patológica, IPO Coimbra, Coimbra, Portugal
- 14Faculdade de Medicina da Universidade do Porto, Porto, Portugal
- 15Escola Superior de Tecnologia da Saúde de Coimbra, Coimbra, Portugal
- 16Associação Portuguesa de Técnicas de Anatomia Patológica, Porto, Portugal
- 17GenoMed – Diagnósticos de Medicina Molecular, S.A., Lisboa, Portugal
- 18Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal
Background: Around 40% of ER+/HER2-breast carcinomas (BC) present mutations in the PIK3CA gene. Assessment of PIK3CA mutational status is required to identify patients eligible for treatment with PI3Kα inhibitors, with alpelisib currently the only approved tyrosine kinase inhibitor in this setting. U-PIK project aimed to conduct a ring trial to validate and implement the PIK3CA mutation testing in several Portuguese centers, decentralizing it and optimizing its quality at national level.
Methods: Eight Tester centers selected two samples of patients with advanced ER+/HER2- BC and generated eight replicates of each (n = 16). PIK3CA mutational status was assessed in two rounds. Six centers used the cobas® PIK3CA mutation test, and two used PCR and Sanger sequencing. In parallel, two reference centers (IPATIMUP and the Portuguese Institute of Oncology [IPO]-Porto) performed PIK3CA mutation testing by NGS in the two rounds. The quality of molecular reports describing the results was also assessed. Testing results and molecular reports were received and analyzed by U-PIK coordinators: IPATIMUP, IPO-Porto, and IPO-Lisboa.
Results: Overall, five centers achieved a concordance rate with NGS results (allele frequency [AF] ≥5%) of 100%, one of 94%, one of 93%, and one of 87.5%, considering the overall performance in the two testing rounds. NGS reassessment of discrepancies in the results of the methods used by the Tester centers and the reference centers identified one probable false positive and two mutations with low AF (1–3%, at the analytical sensitivity threshold), interpreted as subclonal variants with heterogeneous representation in the tissue sections processed by the respective centers. The analysis of molecular reports revealed the need to implement the use of appropriate sequence variant nomenclature with the identification of reference sequences (HGVS-nomenclature) and to state the tumor cell content in each sample.
Conclusion: The concordance rates between the method used by each tester center and NGS validate the use of the PIK3CA mutational status test performed at these centers in clinical practice in patients with advanced ER+/HER2- BC.
Introduction
Female breast carcinoma surpassed lung carcinoma as the most commonly diagnosed cancer in 2020, with an estimated 2.3 million new cases (11.7%), and it was the fifth leading cause of cancer death (6.9%), according to GLOBOCAN (Sung et al., 2021). Over 70% of breast carcinomas are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-), collectively designated as HR+/HER2-breast carcinomas (Setiawan et al., 2009; Howlader et al., 2014). Approximately 40% of estrogen receptor-positive (ER+)/HER2-breast carcinomas have mutations in the PIK3CA gene, leading to hyperactivation of the alpha isoform (p110α) of phosphatidylinositol-3-kinase (PI3K) (Cancer Genome Atlas Network, 2012; Goncalves et al., 2018; Mollon et al., 2018). The PI3K-protein kinase B (AKT)-mammalian target of rapamycin (mTOR) cascade is one of the major downstream signaling pathways in human cells (Fruman et al., 2017), and its deregulation has been implicated in breast cancer development and progression (Lee et al., 2015).
PIK3CA activating tumor mutations may occur in several domains of the p110α catalytic subunit, but mostly (≈80%) arise in four hotspots of the helical and kinase domains: E542K, E545K, H1047R, and H1047L (Zhao and Vogt, 2008; Kalinsky et al., 2009; Dogruluk et al., 2015). According to the Cancer Genome Atlas Network, PIK3CA mutations are more frequent in luminal A (45%) compared to luminal B (29%) subtypes, also occurring in basal-like (9%) and HER2-enriched (39%) subtypes (Cancer Genome Atlas Network, 2012). A recent study including a large number of metastatic breast carcinomas (n = 3871) confirmed these data, reporting the presence of PIK3CA mutations in 39% of HR+/HER2- and 37% of HER2-amplified tumors, besides 21% of triple-negative carcinomas (Albanell et al., 2019). The presence of PIK3CA mutations represents an independent adverse prognostic factor in breast carcinoma (Sobhani et al., 2018) and has been associated with more aggressive disease and poor outcomes in metastatic disease (Fitzgerald et al., 2019).
Despite the high incidence of PIK3CA mutations in breast carcinoma and their putative prognostic role, the therapeutic targeting of the PIK3CA gene has fallen short of expectations, with results from clinical trials with pan-PI3K inhibitors in solid tumors largely disappointing, namely due to unfavorable toxicity versus clinical benefit ratio (Hanker et al., 2019). The α-selective PI3K inhibitor alpelisib was the first PI3K inhibitor to demonstrate a progression-free survival (PFS) benefit in HR+/HER2-metastatic breast carcinoma with activating PIK3CA mutations. In the SOLAR-1 phase III clinical trial, the addition of alpelisib to fulvestrant in patients with disease recurrence/progression on or after prior aromatase inhibitor therapy resulted in an almost double median PFS compared to fulvestrant alone in the PIK3CA-mutated cohort (11.0 vs. 5.7 months, HR 0.65; 95% CI 0.50–0.85; p < 0.001), with an acceptable safety profile (André et al., 2019). Results from the SOLAR-1 trial led to the approval by the U.S. Food and Drug Administration (FDA) in May 2019 of alpelisib in combination with fulvestrant for postmenopausal women and men with HR+/HER2-, PIK3CA-mutated advanced or metastatic breast carcinoma, as detected by an FDA-approved test, following progression on or after an endocrine-based regimen (US Food & Drug Administration (FDA), 2019), and subsequently also to the approval by the European Medicines Agency (EMA) in July 2020 of alpelisib in combination with fulvestrant for postmenopausal women and men with HR+/HER2-, locally advanced or metastatic breast cancer with a PIK3CA mutation, after disease progression following endocrine therapy as monotherapy (European Medicines Agency, 2021). The application of Therascreen® companion diagnostic test allowed the detection of 11 PIK3CA mutations in patients included in the SOLAR-1 trial: C420R, E542K, E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R, H1047L, H1047R, and H1047Y (QIAGEN; André et al., 2019).
In Europe, companion diagnostic tests are not required for market authorization of targeted Oncology drugs, and the prescription of approved targeted therapeutic options relies on evidence of the required mutational status assessed through validated methods. The use of Therascreen® is not widespread in Portugal, but multiple technologies and platforms are available for molecular testing, which can potentially be used to detect PIK3CA mutational status in HR+/HER2-breast carcinoma samples. These require local validation and implementation for clinical diagnostic purposes, similar to what has been previously done for several other actionable mutations in different tumor types (e.g., EGFR in lung cancer, BRAF in melanoma and lung cancer, etc.). Therefore, to enable local assessment of PIK3CA mutational status, hospitals and diagnostic centers should select, implement, and validate diagnostic methodologies that allow the molecular detection of all currently but also potentially actionable PIK3CA mutations.
Due to the absence of local validation and implementation, PIK3CA mutation testing was not routinely performed in HR+/HER2-breast carcinoma and remained largely unavailable in Portugal. This represents an unmet need since the assessment of PIK3CA mutational status is crucial for identifying patients eligible for treatment with PI3Kα inhibitors, particularly alpelisib, which is currently FDA- and EMA-approved in this clinical setting. The U-PIK project aimed to conduct a ring trial to validate and implement the PIK3CA mutation testing in multiple Portuguese centers, decentralizing the test and optimizing its quality at national level.
Materials and methods
U-PIK was a research collaboration between 11 partners ─ 10 centers (seven hospitals and three private laboratories) from the North, Central, and Lisbon regions of Portugal, and Novartis ─ that consisted of a multicenter, inter-laboratory ring trial conducted between December 2020 and December 2021.
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical approval for the study was obtained from multiple boards: Ethics Committee of IPOLFG and Research Council, Unidade de Investigação Clínica, Instituto Português de Oncologia de Lisboa Francisco Gentil, EPE; Ethics Committee of Centro Hospitalar e Universitário de Coimbra; Ethics Committee of Centro Hospitalar e Universitário de Lisboa Norte and of Centro Académico de Medicina de Lisboa; Ethics Committee of Hospital CUF Descobertas; and Ethics Council and Ethics Council for Health of Fundação Champalimaud. Written informed consent for study participation or research purposes in the scope of the study was provided by participants or participants’ legal guardians/next of kin. In addition, ethical approval from other institutions was waived, as the study is in accordance with Article 19 ("DNA Banks and Other Biological Products") of Portuguese Law No. 12/2005 of 26 January ("Personal genetic information and health information"), which states that in the case of using retrospective samples of human origin or in special situations where the consent of subjects involved cannot be obtained due to the amount of data or subjects, their age, or another similar reason, the material and data can be processed but only for purposes of scientific research or epidemiological and statistical data collection (Law No. 12/2005; Kalokairinou et al., 2018).
Eight centers selected two samples of patients with advanced ER+/HER2-breast carcinoma from routine clinical practice and generated eight replicates of each, in a total of 16 samples. All samples were duly anonymized, and each sample was processed through an initial section stained with hematoxylin and eosin (H&E) followed by 24 sequential sections of 6 µm and a final section stained with H&E with the tumor area demarcated.
All samples were centralized at Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP), which generated replicates of each, originating two sample sets with identical sample content comprising the overall group of Test samples. These two sample sets were sent to eight Tester centers, which assessed the PIK3CA mutational status in two rounds, 8 weeks apart, using their in-house methodology. Tables 1, 2 depict the characteristics of the ER+/HER2-breast carcinoma samples included in the analysis.
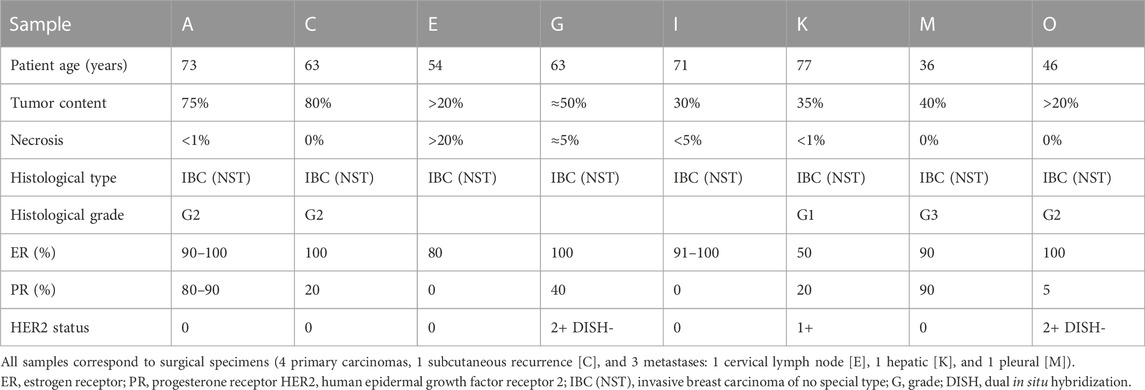
Table 1. Characteristics of the ER+/HER2-breast carcinoma samples included in the first PIK3CA testing round.
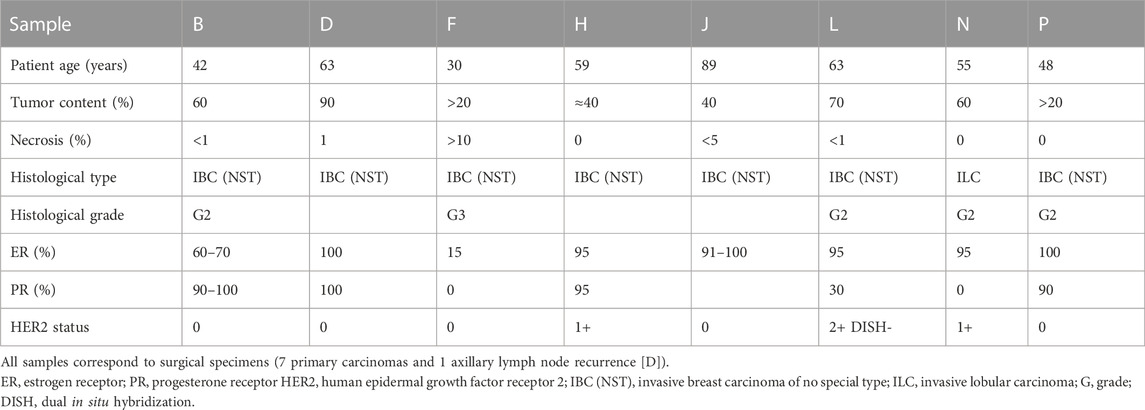
Table 2. Characteristics of ER+/HER2-breast carcinoma samples included in the second PIK3CA testing round.
Tester centers were given 15 running days from the date of sample set reception to test PIK3CA mutational status and report back on results. As a study requirement, the in-house methodology of each Tester center had to allow the detection of mutations in at least exons 7, 9, and 20 of PIK3CA (corresponding to exons 8, 10, and 21 using the MANE Select transcript NM_006218.4), as per SOLAR-1 clinical trial criteria (ClinicalTrials.gov). Six Tester centers used the cobas® PIK3CA Mutation Test, and two used PCR and Sanger sequencing (see Supplementary Material). In parallel, two reference centers (IPATIMUP and the Portuguese Institute of Oncology [IPO]-Porto) performed PIK3CA mutation testing by NGS in the two rounds (see Supplementary Material).
The concordance rate between the results of PIK3CA mutational status obtained by each Tester center and NGS was reported for both testing rounds. The concordance rate was also reported globally, considering all samples tested in both rounds at each Tester center. In addition, the quality of molecular reports describing PIK3CA mutation testing results was also assessed. The testing results from Tester sites and the molecular reports were received and analyzed by U-PIK coordinators: IPATIMUP, IPO-Porto, and IPO-Lisboa.
The original data presented in the study will be publicly available. The NGS data was submitted to the European Nucleotide Archive (ENA) at EMBL-EBI, under the project accession number:PRJEB58369 (https://www.ebi.ac.uk/ena).
Results
Results of the PIK3CA testing rounds
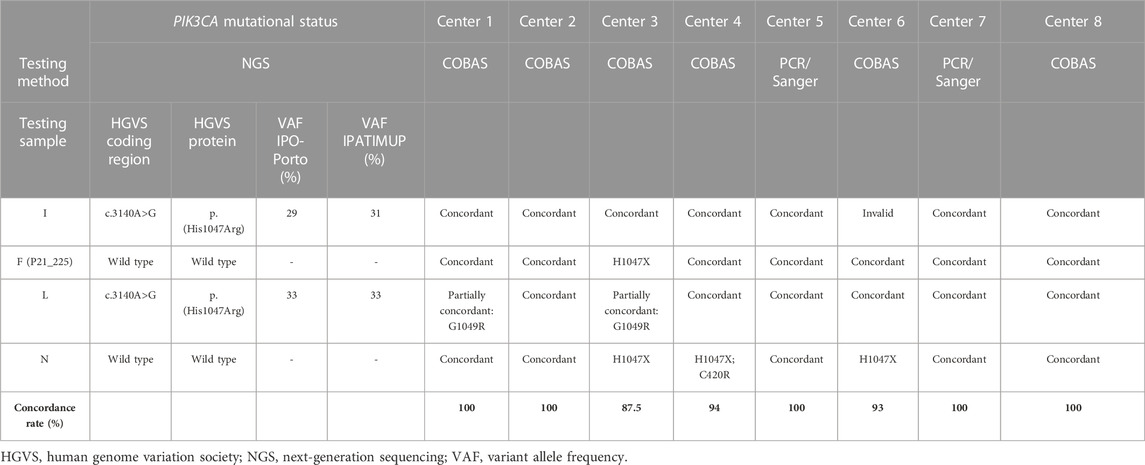
Of the eight participating Tester centers, six assessed the PIK3CA mutational status by cobas® PIK3CA Mutation Test and two by PCR and Sanger sequencing. In the first testing round, all Testers obtained a 100% concordance between the results of PIK3CA mutational status retrieved by their in-house methodology and NGS. Only one sample processed by one Tester center gave an invalid result due to damage of the slide and unavailability of adequate DNA yield, which precluded reanalysis. In the second testing round, four Tester centers achieved 100% concordance, three 88% concordance, and one 63% concordance in the PIK3CA mutational status results obtained by their in-house methodology and NGS. Overall, considering the two testing rounds, five centers achieved a concordance rate with NGS results (AF ≥5%) of 100%, one of 94%, one of 93%, and one of 87.5%.
Table 3 shows the discordant results obtained in the two PIK3CA testing rounds.
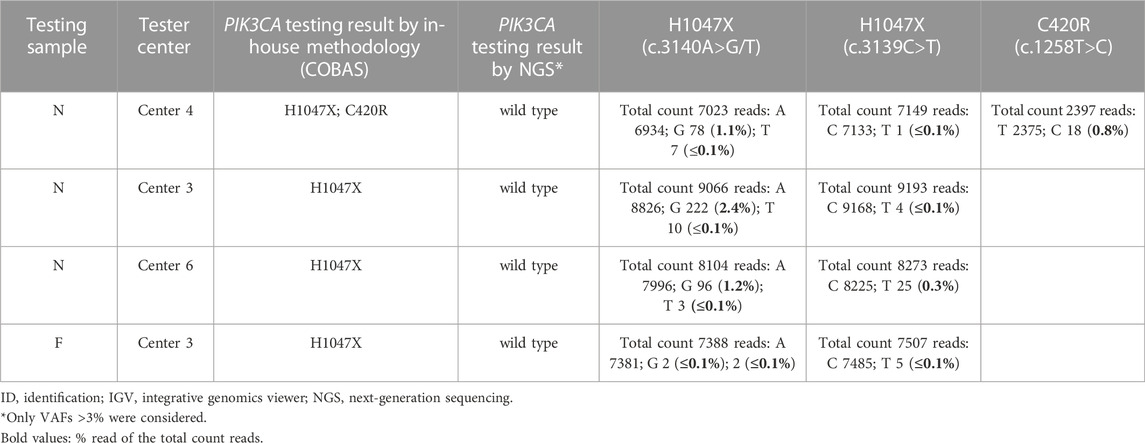
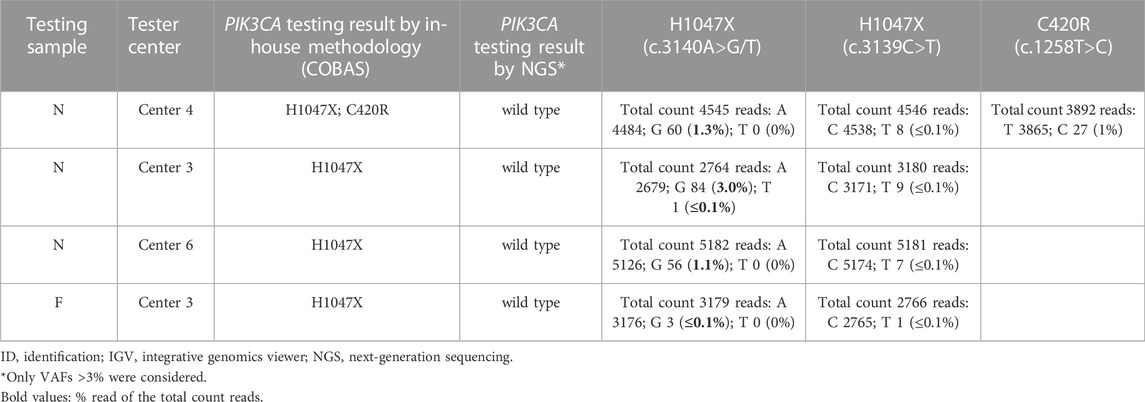
Discordant results were due to discrepancies in the output of three samples (samples F, N, and L), two of which had remaining DNA available and were reanalyzed by NGS (including allele frequencies [AFs] <5%) at IPO-Porto and IPATIMUP reference centers (Tables 4, 5). In one sample (sample F), the H1047X mutation was identified by the Tester center but not by NGS (either in the initial or repeated analysis), being considered a false positive probably resulting from sample contamination. In sample N, the H1047X mutation was detected by three Tester centers but not by NGS analysis using a 5% AF cut-off. However, in NGS reanalysis and integrative genomics viewer (IGV) visualization, the c.3140A>G variant was identified with low AF (1–3%) in all three samples of the three Tester centers, suggesting that this is a low-frequency subclonal variant with heterogeneous representation in the tissue sections processed by the respective centers. Furthermore, in this sample, the c.1258T>C variant (C420R) was detected by one of the Tester centers in addition to the H1047X mutation. This variant was also detected in NGS reanalysis with an AF of 1% and may also correspond to a low-frequency subclonal variant with higher representation in the tissue section processed by that center. The third sample (sample L) for which discrepancies were found could not be reassessed by NGS due to DNA unavailability. In this sample, although the result obtained by the methodology of the Tester center was concordant with NGS for the presence of the H1047X mutation, a second mutation (G1049R) was detected by the cobas® method used by that center (Tables 4, 5), which however was not validated by the Tester center using Sanger sequencing and might represent a known issue of cross-reactivity of the cobas® kit for some variants.
Quality of molecular reports
The analysis of the molecular reports revealed inconsistencies in the description of sequence variants, highlighting the need to foster the use of the appropriate sequence variant nomenclature (HGVS-nomenclature), the international standard for reporting and exchanging information of DNA, RNA, and protein sequence variants in a consistent and unambiguous way (den Dunnen et al., 2016). It also showed the need to include the identification of reference sequences and state the tumor cell content of each sample in the report.
Discussion
Despite significant accomplishments in the diagnosis and treatment of advanced breast cancer, it remains largely incurable. In recent years, several clinical studies have sought to identify novel molecular targets and predictive biomarkers that enable tailored management of these patients and improve outcomes. Within this approach, the pharmacologic targeting of PIK3CA mutations in ER+/HER2-advanced breast cancer has recently shown significant benefits after the development of endocrine therapy resistance. The U-PIK project aimed to evaluate the analytical performance of PIK3CA mutational status testing and contribute to its decentralized implementation in Portuguese centers. Within U-PIK, both testing results and molecular reports were analyzed by U-PIK coordinators IPATIMUP, IPO-Porto, and IPO-Lisboa.
The study found high concordance rates between the results obtained with the methodologies used in each tester center and NGS performed at the two reference centers (AF ≥5%):100% in five centers, and 94%, 93%, and 87.5% in one center each. These results validate the use of the PIK3CA mutational status test performed at those centers in the clinical diagnostics of patients with advanced ER+/HER2-breast carcinoma and enable these patients to be selected for an additional treatment option with PI3Kα inhibitors. The selective PI3Kα inhibitor alpelisib brought a renewed interest in PIK3CA as a predictive biomarker in HR+/HER2-disease, and the widespread use of the PIK3CA mutational status testing in the clinical practice will allow these patients to be identified and potentially benefit from this targeted agent, now available in their treatment armamentarium.
According to the literature, around 90% of PIK3CA mutations cluster in exons 9 and 20, and only ≈5–10% are found in other exons (Campbell et al., 2004; Samuels et al., 2004; Harlé et al., 2013; Arsenic et al., 2015; Martínez-Sáez et al., 2020). Exons 9 and 20 encode the helical and kinase domains of the PIK3CA gene, respectively, providing auto-inhibition of the tyrosine kinases, with mutations within these regions triggering the process of constant auto-phosphorylation and resulting in gain of function (Zhao and Vogt, 2008). Exon 4 has also been reported to be often altered in breast cancer, with the p.N345K mutation described with a 5.5% frequency in a recent analysis including 6338 breast cancer patients across 10 publicly available studies (Martínez-Sáez et al., 2020). Although this is a likely pathogenic variant according to the COSMIC and OncoKB databases (Chakravarty et al., 2017; Tate et al., 2019), its sensitivity to PI3K inhibitors has only been demonstrated in preclinical studies so far (Gymnopoulos et al., 2007; Dogruluk et al., 2015). In the present study, from the 16 specimens analyzed, 10 (62.5%) harbored PIK3CA mutations, predominantly found in exon 20 (n = 6, 37.5%) and exon 9 (n = 4, 25%), in agreement with most frequently reported PIK3CA mutations. Published studies show disparate results regarding the frequency of mutations in exon 9 and 20, with some also reporting that exon 20 is more frequently mutated than exon 9 (Li et al., 2006; Castaneda et al., 2014; Dirican et al., 2014; Loibl et al., 2014; Arsenic et al., 2015; Ahmad et al., 2016), and others showing otherwise (Campbell et al., 2004; Samuels et al., 2004). Nevertheless, the small sample size in this study should always be noted as a caveat and a limitation in the interpretation of the relative PIK3CA mutation frequency results in this study.
Four common mutations were identified in this small sample set, at codons 545 and 546 (E545K and Q546K) in the helical domain and at codon 1047 (H1047R and H1047L) in the kinase domain of the PIK3CA gene. All these alterations have been shown to respond to alpelisib (Verret et al., 2019; Anderson et al., 2020; Copur, 2020; Martínez-Sáez et al., 2020), although Q546K only in preclinical studies, where it has been associated with increased sensitivity to this agent (Mayer et al., 2017; Martínez-Sáez et al., 2020). In agreement with other reports, the four mutations observed in both exons were of missense type, with a single nucleotide change resulting in a different amino acid at the respective codons: nucleotide change c.1633G>A resulted in amino acid change p.E545K and nucleotide change c.1636C>A resulted in amino acid change p.Q546K at exon 9; nucleotide change c.3140A>G resulted in amino acid change p.H1047R and nucleotide change c.3140A>T resulted in amino acid change p.H1047L at exon 20 (Levine et al., 2005; Nosho et al., 2008; Ross et al., 2015; Ahmad et al., 2016). Both missense mutations E545K and Q546K were equally prevalent in exon 9 (50% each), while the H1047R missense mutation was more prevalent (83.3%) in exon 20 compared to the H1047L mutation (16.7%). These mutations were clustered within the mutational hotspot regions covering nucleotides 1633–1636 (n = 4) and 3140 (n = 6), in agreement with what has been reported by others (Nosho et al., 2008; Wang et al., 2015; Ahmad et al., 2016).
In addition to E545K, Q546K, H1047R, and H1047L, other less common PIK3CA mutations have been identified in the samples analyzed in this study. The exon 7 p.C420R mutation was found both in the assessment conducted by one Tester center and by NGS with an AF of 1%. This variant had also been previously reported by Corné et al. and Martínez-Sáez et al. with a frequency of 1.4% and 1.9%, respectively (Martínez-Sáez et al., 2020; Corné et al., 2021), and shown responsiveness to alpelisib in the SOLAR-1 trial (André et al., 2019). On the other hand, the PIK3CA exon 20 p.G1049R mutation was detected by the cobas® method used by one Tester center, and had been previously reported in 0.7% of the samples analyzed in the study by Martínez-Sáez and colleagues (Martínez-Sáez et al., 2020). Although rare, this variant is likely pathogenic, with preclinical studies suggesting an increased sensitivity to alpelisib, similar to p.Q546K (Mayer et al., 2017) and p.E542K (Dogruluk et al., 2015) alterations. However, whenever this mutation is found in a tumor also presenting the H1047X mutation, the possibility of a false positive due to a known issue of cross-reactivity of the quantitative PCR technology should be considered.
The detection of PIK3CA mutations in liquid biopsy, although not in the scope of the U-PIK study, is also being explored in metastatic breast cancer setting with encouraging results (Corné et al., 2021; Galvano et al., 2022), and may play a role in identifying patients harboring PIK3CA and possibly additional mutations. An individual patient data meta-analysis recently ascertained the accuracy of circulating tumor DNA (ctDNA) for the detection of PIK3CA mutations, raising the possibility of replacing tissue for ctDNA tumor sampling in the future as a preferred strategy for metastatic breast cancer patients with low clinical compliance or inaccessible metastatic sites (Galvano et al., 2022). The latest ESO-ESMO guidelines have also acknowledged the assessment of plasma circulating free DNA as a good alternative to metastatic tumor analysis, as it may overcome the challenges (both for clinicians and patients) of obtaining metastatic tissue biopsies, and as an option for the selection of patients eligible for alpelisib (Cardoso et al., 2020). Furthermore, and further attesting to the relevance of identifying actionable PIK3CA mutations in breast cancer, major hot spot-activating missense PIK3CA mutations (E542K, E545K/A, H1047R/L) have a level IA of evidence for actionability according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT; (Condorelli et al., 2019).
Overall, the results obtained in the U-PIK study validate the PIK3CA mutational status testing performed through cobas® and PCR/Sanger sequencing (and NGS) at the eight Portuguese centers participating in this study and show the feasibility of adopting these methodologies as part of the clinical routine practice of patients with advanced ER+/HER2-breast carcinoma, decentralizing the analysis from reference laboratories.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be accessed at: https://www.ebi.ac.uk/ena, PRJEB58369.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical approval for the study was obtained from multiple boards: Ethics Committee of IPOLFG and Research Council, Unidade de Investigação Clínica, Instituto Português de Oncologia de Lisboa Francisco Gentil, EPE; Ethics Committee of Centro Hospitalar e Universitário de Coimbra; Ethics Committee of Centro Hospitalar e Universitário de Lisboa Norte and of Centro Académico de Medicina de Lisboa; Ethics Committee of Hospital CUF Descobertas; and Ethics Council and Ethics Council for Health of Fundação Champalimaud. Written informed consent for study participation or research purposes in the scope of the study was provided by participants or participants’ legal guardians/next of kin. In addition, ethical approval from other institutions was waived, as the study is in accordance with Article 19 ("DNA Banks and Other Biological Products") of Portuguese Law No. 12/2005 of 26 January ("Personal genetic information and health information"), which states that in the case of using retrospective samples of human origin or in special situations where the consent of subjects involved cannot be obtained due to the amount of data or subjects, their age, or another similar reason, the material and data can be processed but only for purposes of scientific research or epidemiological and statistical data collection (Law No. 12/2005; Kalokairinou et al., 2018). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JCM, SA, MRT, and PMB conceived the U-PIK Project. JCM, SA, MRT, PMB, FCS, MJA, MJB, PB, MRD, HP, NS, ACS, JAT participated in the study design. AP, LCi, ALC, MJA, MJB, PB, NC, ASC, LCa, LCo, CF, PF, AG, JP, MP, HP, JR, NS, FCS, FS, ARS, ACS, JAT performed the experimental work involved in sample preparation, DNA extraction and PIK3CA testing and reporting. JCM, SA and MRT were responsible for analyzing PIK3CA testing data from U-PIK testers, for reporting anonymized PIK3CA testing performance back to U-PIK testers, and also for the analysis of molecular reports. JCM, SA, MRT, PMB and AP edited the manuscript, drafted and written with editorial assistance of JCS (Acknowledgements). All authors read and approved the final manuscript.
Funding
The funding for U-PIK project Research Collaboration was provided by Novartis, covering PIK3CA testing reagents, sample preparation and shipping, and also medical writing and study publication support.
Acknowledgments
The authors acknowledge Joana Cavaco-Silva (jo.cvsilva@gmail.com) for editorial assistance in the drafting and writing of the manuscript.
Conflict of interest
Author PB and MRD are employed by Novartis, authors LC and NS are employed by Synlab, and author ACS is employed by GenoMed, as described in “author affiliations”. All the remaining authors in this research collaboration and consortium are employed either by public or private hospitals or research institutions, as described in the “Author affiliations section”. This study received funding from Novartis “funder”, covering PIK3CA testing reagents, sample preparation, and shipping, and also medical writing and article publication support, as described in detail in the “Funding” section. The authors employed by the “funder”, together with the investigators, were involved in study conception and design and in manuscript editing, as described in detail under “Author Contributions”.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2023.1082915/full#supplementary-material
References
Ahmad, F., Badwe, A., Verma, G., Bhatia, S., and Das, B. R. (2016). Molecular evaluation of PIK3CA gene mutation in breast cancer: Determination of frequency, distribution pattern and its association with clinicopathological findings in Indian patients. Med. Oncol. 33, 74. doi:10.1007/s12032-016-0788-y
Albanell, J., Casadevall, D., Sokol, E. S., Albacker, L. A., Elvin, J. A., Vergilio, J.-A., et al. (2019). PIK3CA alterations in metastatic breast cancer (mBC). Ann. Oncol. 30, v104–v105. doi:10.1093/annonc/mdz242.001
Anderson, E. J., Mollon, L. E., Dean, J. L., Warholak, T. L., Aizer, A., Platt, E. A., et al. (2020). A systematic review of the prevalence and diagnostic workup of PIK3CA mutations in HR+/HER2– metastatic breast cancer. Int. J. Breast Cancer 2020, 3759179–3759216. doi:10.1155/2020/3759179
André, F., Ciruelos, E., Rubovszky, G., Campone, M., Loibl, S., Rugo, H. S., et al. (2019). Alpelisib for PIK3CA -mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940. doi:10.1056/NEJMoa1813904
Arsenic, R., Treue, D., Lehmann, A., Hummel, M., Dietel, M., Denkert, C., et al. (2015). Comparison of targeted next-generation sequencing and Sanger sequencing for the detection of PIK3CA mutations in breast cancer. BMC Clin. Pathol. 15, 20. doi:10.1186/s12907-015-0020-6
Campbell, I. G., Russell, S. E., Choong, D. Y. H., Montgomery, K. G., Ciavarella, M. L., Hooi, C. S. F., et al. (2004). Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 64, 7678–7681. doi:10.1158/0008-5472.CAN-04-2933
Cancer Genome Atlas Network (2012). Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70. doi:10.1038/nature11412
Cardoso, F., Paluch-Shimon, S., Senkus, E., Curigliano, G., Aapro, M. S., André, F., et al. (2020). 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649. doi:10.1016/j.annonc.2020.09.010
Castaneda, C. A., Lopez-Ilasaca, M., Pinto, J. A., Chirinos-Arias, M., Doimi, F., Neciosup, S. P., et al. (2014). PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastatic breast cancers. Hematology/Oncology Stem Cell. Ther. 7, 142–148. doi:10.1016/j.hemonc.2014.09.007
Chakravarty, D., Gao, J., Phillips, S., Kundra, R., Zhang, H., Wang, J., et al. (2017). OncoKB: A precision Oncology knowledge base. JCO Precis. Oncol. 2017, 1–16. doi:10.1200/PO.17.00011
ClinicalTrials.gov Study (2015). Assessing the efficacy and safety of alpelisib plus fulvestrant in men and postmenopausal women with advanced breast cancer which progressed on or after aromatase inhibitor treatment (SOLAR-1). United States: ClinicalTrials.gov Identifier.
Condorelli, R., Mosele, F., Verret, B., Bachelot, T., Bedard, P. L., Cortes, J., et al. (2019). Genomic alterations in breast cancer: Level of evidence for actionability according to ESMO Scale for clinical actionability of molecular targets (ESCAT). Ann. Oncol. 30, 365–373. doi:10.1093/annonc/mdz036
Copur, M. S. (2020). Alpelisib to treat breast cancer. Drugs Today 56, 357–363. doi:10.1358/dot.2020.56.6.3137526
Corné, J., Le Du, F., Quillien, V., Godey, F., Robert, L., Bourien, H., et al. (2021). Development of multiplex digital PCR assays for the detection of PIK3CA mutations in the plasma of metastatic breast cancer patients. Sci. Rep. 11, 17316. doi:10.1038/s41598-021-96644-6
den Dunnen, J. T., Dalgleish, R., Maglott, D. R., Hart, R. K., Greenblatt, M. S., McGowan-Jordan, J., et al. (2016). HGVS recommendations for the description of sequence variants: 2016 update. Hum. Mutat. 37, 564–569. doi:10.1002/humu.22981
Dirican, E., Kaya, Z., Gullu, G., Peker, I., Ozmen, T., Gulluoglu, B. M., et al. (2014). Detection of PIK3CA gene mutations with HRM analysis and association with IGFBP-5 expression levels in breast cancer. Asian Pac. J. Cancer Prev. 15, 9327–9333. doi:10.7314/APJCP.2014.15.21.9327
Dogruluk, T., Tsang, Y. H., Espitia, M., Chen, F., Chen, T., Chong, Z., et al. (2015). Identification of variant-specific functions of PIK3CA by rapid phenotyping of rare mutations. Cancer Res. 75, 5341–5354. doi:10.1158/0008-5472.CAN-15-1654
European Medicines Agency (2021). Piqray (alpelisib). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/piqray#authorisation-details-section.
Fitzgerald, D., Muzikansky, A., Pinto, C., Henderson, L., Walmsley, C., Allen, R., et al. (2019). Association between PIK3CA mutation status and development of brain metastases in HR+/HER2-metastatic breast cancer. Ann. Oncol. 30, v110. v104–v142. doi:10.1093/annonc/mdz242.013
Fruman, D. A., Chiu, H., Hopkins, B. D., Bagrodia, S., Cantley, L. C., and Abraham, R. T. (2017). The PI3K pathway in human disease. Cell. 170, 605–635. doi:10.1016/j.cell.2017.07.029
Galvano, A., Castellana, L., Gristina, V., La Mantia, M., Insalaco, L., Barraco, N., et al. (2022). The diagnostic accuracy of PIK3CAmutations by circulating tumor DNA in breast cancer: An individual patient data meta-analysis. Ther. Adv. Med. Oncol. 14, 17588359221110162. doi:10.1177/17588359221110162
Goncalves, M. D., Hopkins, B. D., and Cantley, L. C. (2018). Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 379, 2052–2062. doi:10.1056/NEJMra1704560
Gymnopoulos, M., Elsliger, M.-A., and Vogt, P. K. (2007). Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. 104, 5569–5574. doi:10.1073/pnas.0701005104
Hanker, A. B., Kaklamani, V., and Arteaga, C. L. (2019). Challenges for the clinical development of PI3K inhibitors: Strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491. doi:10.1158/2159-8290.CD-18-1175
Harlé, A., Lion, M., Lozano, N., Husson, M., Harter, V., Genin, P., et al. (2013). Analysis of PIK3CA exon 9 and 20 mutations in breast cancers using PCR-HRM and PCR-ARMS: Correlation with clinicopathological criteria. Oncol. Rep. 29, 1043–1052. doi:10.3892/or.2013.2229
Howlader, N., Altekruse, S. F., Li, C. I., Chen, V. W., Clarke, C. A., Ries, L. A. G., et al. (2014). US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. JNCI J. Natl. Cancer Inst. 106, dju055. doi:10.1093/jnci/dju055
Kalinsky, K., Jacks, L. M., Heguy, A., Patil, S., Drobnjak, M., Bhanot, U. K., et al. (2009). PIK3CA mutation associates with improved outcome in breast cancer. Clin. Cancer Res. 15, 5049–5059. doi:10.1158/1078-0432.CCR-09-0632
Kalokairinou, L., Howard, H. C., Slokenberga, S., Fisher, E., Flatscher-Thöni, M., Hartlev, M., et al. (2018). Legislation of direct-to-consumer genetic testing in Europe: A fragmented regulatory landscape. J. Community Genet. 9, 117–132. doi:10.1007/s12687-017-0344-2
Lee, J. J., Loh, K., and Yap, Y.-S. (2015). PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 12, 342–354. doi:10.7497/j.issn.2095-3941.2015.0089
Law No. 12/2005 (2005). Informação genética pessoal e informação de saúde [Personal genetic information and health information law]. Retrieved from: http://dre.pt/pdf1sdip/2005/01/018A00/06060611.pdf.
Levine, D. A., Bogomolniy, F., Yee, C. J., Lash, A., Barakat, R. R., Borgen, P. I., et al. (2005). Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 11, 2875–2878. doi:10.1158/1078-0432.CCR-04-2142
Li, S. Y., Rong, M., Grieu, F., and Iacopetta, B. (2006). PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res. Treat. 96, 91–95. doi:10.1007/s10549-005-9048-0
Loibl, S., von Minckwitz, G., Schneeweiss, A., Paepke, S., Lehmann, A., Rezai, M., et al. (2014). PIK3CA mutations are associated with lower rates of pathologic complete response to anti–human epidermal growth factor receptor 2 (HER2) therapy in primary HER2-overexpressing breast cancer. J. Clin. Oncol. 32, 3212–3220. doi:10.1200/JCO.2014.55.7876
Martínez-Sáez, O., Chic, N., Pascual, T., Adamo, B., Vidal, M., González-Farré, B., et al. (2020). Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 22, 45. doi:10.1186/s13058-020-01284-9
Mayer, I. A., Abramson, V. G., Formisano, L., Balko, J. M., Estrada, M. V., Sanders, M. E., et al. (2017). A phase ib study of alpelisib (BYL719), a PI3kα-specific inhibitor, with letrozole in ER+/HER2− metastatic breast cancer. Clin. Cancer Res. 23, 26–34. doi:10.1158/1078-0432.CCR-16-0134
Mollon, L., Aguilar, A., Anderson, E., Dean, J., Davis, L., Warholak, T., et al. (2018). “Abstract 1207: A systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in hr+/HER2-metastatic breast cancer,” in Epidemiology (United States: American Association for Cancer Research), 1207. doi:10.1158/1538-7445.AM2018-1207
Nosho, K., Kawasaki, T., Longtine, J. A., Fuchs, C. S., Ohnishi, M., Suemoto, Y., et al. (2008). PIK3CA mutation in colorectal cancer: Relationship with genetic and epigenetic alterations. Neoplasia 10, 534–541. doi:10.1593/neo.08336
Qiagen (2019). Qiagen. Available at: https://www.qiagen.com/us/cmp/mdx/pik3ca-rgq-pcr-kit-us.aspx.
Ross, J. S., Ali, S. M., Wang, K., Khaira, D., Palma, N. A., Chmielecki, J., et al. (2015). Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res. Treat. 154, 155–162. doi:10.1007/s10549-015-3592-z
Samuels, Y., Wang, Z., Bardelli, A., Silliman, N., Ptak, J., Szabo, S., et al. (2004). High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554. doi:10.1126/science.1096502
Setiawan, V. W., Monroe, K. R., Wilkens, L. R., Kolonel, L. N., Pike, M. C., and Henderson, B. E. (2009). Breast cancer risk factors defined by estrogen and progesterone receptor status: The multiethnic cohort study. Am. J. Epidemiol. 169, 1251–1259. doi:10.1093/aje/kwp036
Sobhani, N., Roviello, G., Corona, S. P., Scaltriti, M., Ianza, A., Bortul, M., et al. (2018). The prognostic value of PI3K mutational status in breast cancer: A meta-analysis. J. Cell. Biochem. 119, 4287–4292. doi:10.1002/jcb.26687
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tate, J. G., Bamford, S., Jubb, H. C., Sondka, Z., Beare, D. M., Bindal, N., et al. (2019). Cosmic: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947. doi:10.1093/nar/gky1015
US Food and Drug Administration (FDA) (2019). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alpelisib-metastatic-breast-cancer.
Verret, B., Cortes, J., Bachelot, T., Andre, F., and Arnedos, M. (2019). Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. 30, x12–x20. doi:10.1093/annonc/mdz381
Wang, Y. L., Dai, X., Li, Y. D., Cheng, R. X., Deng, B., Geng, X. X., et al. (2015). Study of PIK3CA, BRAF, and KRAS mutations in breast carcinomas among Chinese women in Qinghai. Genet. Mol. Res. 14, 14840–14846. doi:10.4238/2015.November.18.49
Keywords: advanced breast cancer, ER+/HER2−, PIK3CA mutations, ring trial, validation, molecular pathology
Citation: Peixoto A, Cirnes L, Carvalho AL, Andrade MJ, Brito MJ, Borralho P, Coimbra N, Borralho PM, Carneiro AS, Castro L, Correia L, Dionísio MR, Faria C, Figueiredo P, Gomes A, Paixão J, Pinheiro M, Prazeres H, Ribeiro J, Salgueiro N, Schmitt FC, Silva F, Silvestre AR, Sousa AC, Almeida-Tavares J, Teixeira MR, André S and Machado JC (2023) Evaluation of PIK3CA mutations in advanced ER+/HER2-breast cancer in Portugal – U-PIK Project. Front. Mol. Biosci. 10:1082915. doi: 10.3389/fmolb.2023.1082915
Received: 28 October 2022; Accepted: 05 January 2023;
Published: 07 February 2023.
Edited by:
Yunpeng Hua, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Maria La Mantia, University of Palermo, ItalyPathmanathan Rajadurai, Subang Jaya Medical Centre, Malaysia
Copyright © 2023 Peixoto, Cirnes, Carvalho, Andrade, Brito, Borralho, Coimbra, Borralho, Carneiro, Castro, Correia, Dionísio, Faria, Figueiredo, Gomes, Paixão, Pinheiro, Prazeres, Ribeiro, Salgueiro, Schmitt, Silva, Silvestre, Sousa, Almeida-Tavares, Teixeira, André and Machado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel R. Teixeira, manuel.teixeira@ipoporto.min-saude.pt; Saudade André, sandre@ipolisboa.min-saude.pt; José Carlos Machado, josem@i3s.up.pt
 Ana Peixoto
Ana Peixoto Luís Cirnes2
Luís Cirnes2  Maria José Brito
Maria José Brito Pedro M. Borralho
Pedro M. Borralho Carlos Faria
Carlos Faria Manuela Pinheiro
Manuela Pinheiro Fernando C. Schmitt
Fernando C. Schmitt Joana Almeida-Tavares
Joana Almeida-Tavares Manuel R. Teixeira
Manuel R. Teixeira José Carlos Machado
José Carlos Machado