- 1Clinical Research Center for Mental Disorders, Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University, Shanghai, China
- 2Department of Developmental and Behavioral Pediatrics, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3State Key Laboratory of Subtropical Silviculture, School of Forestry and Biotechnology, Zhejiang A&F University, Hangzhou, China
Background: Clozapine is considered one of the most effective antipsychotic drugs, but it is most likely to cause metabolic abnormalities. Researchers have studied the causes of metabolic abnormalities caused by clozapine from multiple perspectives, but the reasons remain unclear.
Purpose: Characterize the gut microbiota of people with schizophrenia taking clozapine, exploring the association between gut microbiota and glucose lipid metabolic markers in schizophrenia patients taking clozapine.
Research design: Sixty-one long-term inpatients with schizophrenia in clozapine monotherapy were selected as study subjects. We got four subgroups by sex and the presence of metabolic syndrome.
Data analysis: 16s analysis technology was applied at the genus level to determine the classification of gut microbiota. Then we compared the characteristics of gut microbiota and the association of gut microbiota with glucose lipid metabolic markers in each group.
Findings: We found differences in the diversity of gut microbiota among groups. The association between gut microbiota and glucose lipid metabolic markers was complicated. Gender was an important differentiating factor. Oscillibacter has a low abundance. However, it was the only genus associated with glycemic or lipids in each group. Among metabolic syndromes, Gemmiger was positively correlated with most lipids in females but negatively correlated in males, showing gender differences. In female non-metabolic syndromes, Bifidobacterium lost its probiotic character; instead, showing pathogenicity, which has strong positive correlations with fasting blood glucose and low-density lipoprotein but negative correlations with Apolipoprotein A1. Maybe schizophrenia, taking clozapine, and gender factors influenced the gut microbiota, which complicated our findings. The significance of the results remains to be determined by in-depth studies.
1. Introduction
Second-generation antipsychotics are currently the mainstay of treatment for schizophrenia. Clozapine is considered the prototype and representative of second-generation antipsychotics (Stahl, 2013). Clozapine is significantly better than other antipsychotics in reducing psychotic symptoms, violence, and persistent aggression in patients with schizophrenia (Frogley et al., 2012). It is the only antipsychotic used as the standard of care for refractory schizophrenia in the treatment guidelines for schizophrenia developed in several countries (Stahl et al., 2013; Nice, 2014; Warnez and Alessi-Severini, 2014). The U.S. Food and Drug Administration (FDA) approved clozapine as the only drug to reduce suicidal behavior in patients with schizophrenia (Versacloz, 1989).
Patients on clozapine have the most significant increases in cholesterol, triglycerides, and blood glucose (Krakowski et al., 2009), which limits its use in clinical practice (Citrome et al., 2016). Up to 60% of people with schizophrenia taking clozapine develop metabolic disorders such as dyslipidemia, insulin resistance, and type 2 diabetes (Brunero et al., 2009; Meltzer, 2012; Mitchell et al., 2013). Some studies have suggested that the metabolic disorders in patients are related to neurotransmitters such as serotonin 5-HT2A/2C receptors and histamine H1 receptors (Kim et al., 2007; Esen-Danacı et al., 2008); others have suggested that it may be the result of clozapine affecting the production or the secretion mechanism of adipocytokines such as adiponectin, leptin, and resistin in the body (Yuen et al., 2021). Some other studies believe that clozapine induces the production of inflammatory cytokines such as interleukins and tumor necrosis factors in the body. The monocyte infiltration and inflammatory state lead to insulin resistance, subsequently interfering with the insulin’s antilipolytic effects, thus causing metabolic disorders (Leonard et al., 2012). Several studies have found that variants in certain genes (especially LEP, HTR2C, and SREBF2) may make patients taking clozapine genetically susceptible to metabolic disorders (Suetani et al., 2017). Overall, the current studies represent a variety of cellular, biochemical, molecular, and physiological pathways; The findings are complex and even contradictory. The mechanisms underlying clozapine-induced metabolic disorders are not well understood to date, which may result from a combination of factors; there may also be some mechanisms that have not yet been identified (Panariello et al., 2011).
More and more evidence shows that the gut microbiota is important in the body’s energy metabolism (Fan and Pedersen, 2021). Influenced by specific factors, changes in the abundance and composition of the gut microbiota associated with metabolic abnormalities may lead to metabolic disorders (Singh et al., 2015; DeGruttola et al., 2016). Type 2 diabetes were characterized by a moderate degree of gut microbial dysbiosis (Qin et al., 2012). A strong association exists between certain gut microbiota composition, body mass index (BMI) changes, and blood lipid levels (Fu et al., 2015). Specific gut microbiota may restrain the expression of fasting-induced adipose factor, promote cellular uptake of fatty acids and enhance the accumulation of triglycerides in adipose tissue (Backhed et al., 2007). Some gut microbiota also produces short-chain fatty acids (SCFA) via anaerobic fermentation of dietary fiber, which in turn affects cholesterol and fat synthesis through the gut microbiota-liver axis pathway (Singh et al., 2015).
Due to limitations in research methods, the important role of gut microbiota has not been considered in previous studies investigating the mechanisms underlying metabolic abnormalities in patients with schizophrenia induced by second-generation antipsychotics. In fact, with the application of high-throughput omics technologies in the field of gut microbiota, the comprehensive study of the structure and function of all microbial species in the gut microbiota has only been achieved. Studies have found that some gut microbiota were associated with the severity of symptoms (Li et al., 2020). Risperidone treatment induces significant changes in some gut microbiota (Bahr et al., 2015a,b; Yuan et al., 2018). The changes in gut microbiota composition may reflect the effects of metabolic disorders following risperidone treatment (Xue et al., 2021). Risperidone leads to significant changes in the gut microbiota, which are mechanistically related to weight gain by suppressing energy expenditure (Bahr et al., 2015b). The gut microbiota also has a role in the cycle of metabolic abnormality associated with olanzapine. Olanzapine has contributed to a shift in gut microbiota toward an “obesogenic” bacterial profile (Morgan et al., 2014); increased the abundance of Short Chain Fatty Acids (SCFA) producing gut microbiota increased the levels of SCFAs, which are strongly associated with the development of lipid accumulation (Chang et al., 2023). Chronic olanzapine treatment altered the gut microbiota of rats and caused severe metabolic side effects in rats. After killing the gut microbiota in rats with a combined antibiotic cocktail, indicators of metabolic disorders such as blood lipids, adipose tissue inflammation, and fat deposition were significantly improved (Davey et al., 2013). The abnormal lipid metabolism induced by olanzapine may be strongly associated with the vagus-mediated gut microbiota-brain axis (Zhu et al., 2022).
Although certain second-generation antipsychotics alter the abundance and composition of the gut microbiota and induce metabolic disturbances in people with schizophrenia (Chen et al., 2023), it is unclear whether gut microbiota is also involved in the mechanism of metabolic abnormalities caused by clozapine. Therefore, We analyzed the characterization of gut microbiota in people with schizophrenia with and without metabolic syndrome (MetS) after monotherapy with clozapine and the relationship between gut microbiota and Glycemic or lipids.
Despite its best efficacy among all antipsychotic drugs, its clinical application is restricted due to the greatest metabolic side effects. Therefore, further exploring the mechanisms of metabolic side effects induced by clozapine in people with schizophrenia would provide a theoretical basis for future targeted interventions.
2. Materials and methods
2.1. Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Clinical Research Center for Mental Disorders, Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University (protocol code: PDJWLL2019020 and date of approval: 5 July 2019). It was registered in the Chinese Clinical Trials Registry (Registration number: ChiCTR2300069814). All participants gave their informed consent for inclusion before participating in the study.
2.2. Study design and participants
The study was conducted among inpatients at the Pudong New Area Mental Health Center in Shanghai, China, and data were collected from October 2022 to April 2023. In order to minimize heterogeneity in the study population, long-term hospitalized patients with chronic schizophrenia were recruited into the study. The hospital restaurant supplies their daily food. They lived in the same environment for a long time, shared the same recipes as all inpatients, and had similar portion sizes. All this will minimize the differences in gut microbiota affected by different living environments and foods.
The participants recruited for this study were schizophrenia in clozapine monotherapy (SCM), with MetS (MA) and without MetS (MN). Study participants met the diagnostic criteria for schizophrenia in the International Classification of Diseases, Tenth Revision (ICD-10), were Han Chinese, aged >18 years, currently on single-agent oral clozapine therapy for more than six consecutive months, and volunteered to participate. Participants in the MA group met the diagnostic criteria for metabolic syndrome of the Chinese Diabetes Association (2021) and Chen et al. (2023). In contrast, participants in MN did not meet the diagnostic criteria for metabolic syndrome. Participants were excluded from this study if they (1) smoked; (2) had a history of bowel cancer; (3) had a history of inflammation; (4) had used antibiotics in the last 6 months, or (5) had received invasive medical interventions during the previous 6 months.
2.3. Clinical study parameters
We collected data on age, gender, blood pressure (BP), body mass index (BMI), and waist circumference (WC) of participants. We collected blood samples from participants under fasting conditions. Venipuncture was performed using vacuum blood collection tubes, and blood was stored in blood collection tubes, serum separator tubes, and EDTA Vacutainer (Becton Dickinson, Franklin Lakes, NJ, USA). Blood samples were centrifuged at 1,500 × g for 15 min at 4°C. Standard laboratory methods and certified biochemical and hematological tests were performed using an automated analyzer (Roche Diagnostics, Mannheim, Germany).
Participants’ fasting blood glucose (FBG), Glycated albumin (GA), triglycerides (TG), total cholesterol (TC), High-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Small dense low-density lipoprotein-cholesterol (sdLDL-C), Apolipoprotein A1 (ApoA1), Apolipoprotein B (ApoB), Apolipoprotein E (ApoE) and lipoprotein (a) [Lp (a)].
2.4. DNA extraction, PCR amplification, and 16S rRNA gene sequencing
Deoxyribonucleic acid was extracted from each fecal sample following the manufacturer’s protocols of the QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany). The V3-V4 region of the bacteria 16S ribosomal RNA genes was amplified by PCR (95°C for 3 min, followed by 30 cycles at 98°C for 20 s, 58°C for 15 s, and 72°C for 40 s and a final extension at 72°C for 5 min) using barcoded primers 341F (5′-CCTACGGGRSGCAGCAG-3′) and 806R (5′-GGACTACVVGGGTATCTAATC-3′). PCR reactions were performed in a 25 μL mixture containing 12.5 μL of KFX HiFi 2 × PCR Master Mix, 1 μL of each primer (10 μM), 50 ng of template DNA and dd H2O. Amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, US) and quantified using Qubit®2.0 (Invitrogen, US). All quantified amplicons were pooled to equalize concentrations for sequencing using Illumina NovaSeq (Illumina, Inc., San Diego, CA, USA). The total samples resulted in 2,181,677 tags, averaging 35,765 tags per sample. DNA extraction, library construction, and sequencing were conducted at Realbio Genomics Institute (Shanghai, China).
2.5. Taxonomy classification and statistical analysis
High-quality 16S tags were clustered into Operational Taxonomic Units (OTUs) with 97% similarity using Usearch (version 7.0.1090) in QIIME (version 1.9.1). Taxonomy was assigned using the RDP classifier (https://sourceforge.net/projects/rdp-classifier/) using a confidence threshold of 0.8. Student’s unpaired t-test and one-way analysis of variance (ANOVA) was performed using SPSS Statistics software (V22.0, IBM, Armonk, NY, USA), and a P-value of < 0.05 was considered statistically significant. Duncan’s test was performed to compare differences in gut bacterial diversity among different subgroups of schizophrenia patients receiving clozapine. Changes in the structure of gut bacterial communities were analyzed by beta-diversity based on Bray-Curtis distances and visualized by principal coordinate analysis (PCoA) of the “vegan” package. The significance of differences in composition between bacterial communities was calculated using the permutational multivariate analysis of variance (PERMANOVA). STAMP software was used to analyze differences in the composition of the gut microbiota community at the phylum and genus levels. Redundancy analysis was used to analyze the relationship between gut bacterial community structure and Clinical index.
3. Results
3.1. Baseline characteristics
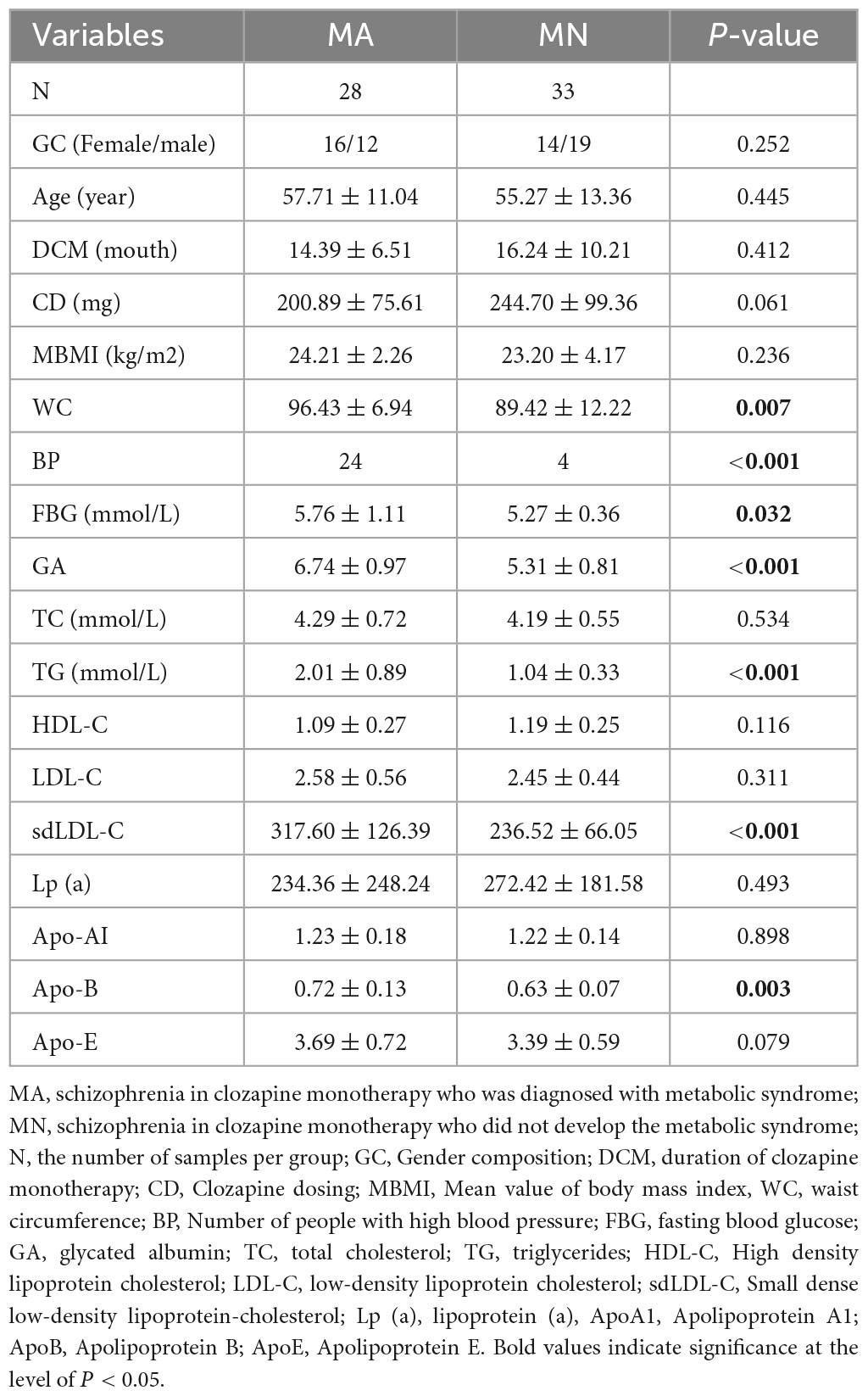
A total of 61 SCM were recruited, including 28 in the MA group (16 females and 12 males) and 33 in the MN group (14 females and 19 males). The two groups were comparable in age, gender, duration of clozapine monotherapy (DCM), and Clozapine dosing (CD). The FBG values were higher in the MA group than in the MN group (p < 0.05). The proportion of WC, BP, GA, TG, sdLDL-C, and ApoB was significantly higher in the MA group than in the MN group (p < 0.01) (Table 1).
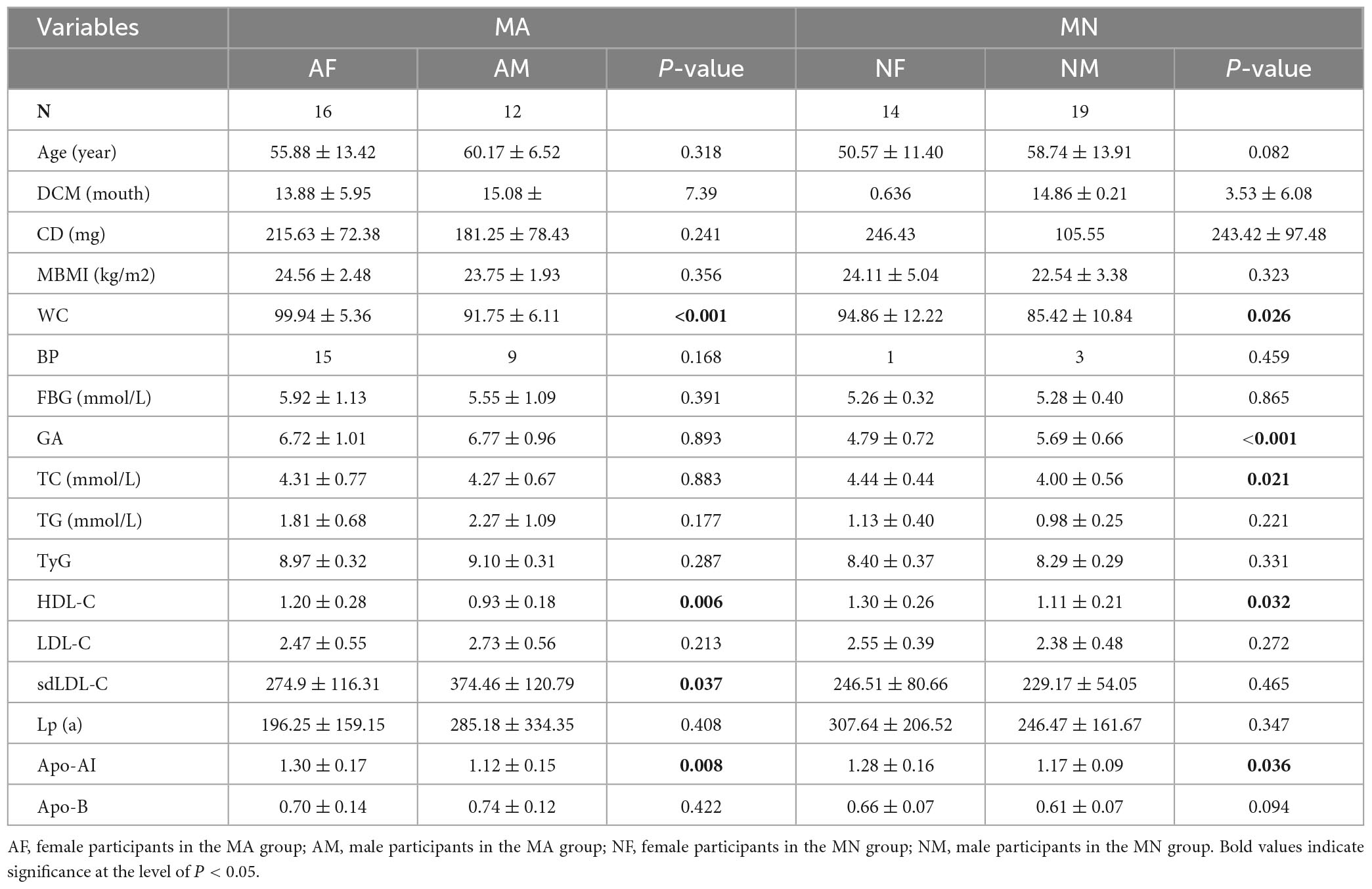
Female participants in the MA group (AF) had significantly higher WC, HDL-C, and Apo-AI than male participants in the MA group (AM) (P < 0.01). At the same time, sdLDL-C was higher in the AF than in the AM (P < 0.05). Female participants in the MN group (NF) had higher WC, TC, HDL-C, Apo-AI, and Apo-E than male participants in the MN group (NM) (P < 0.05). GA was significantly higher in the NM group than in the NF group (p < 0.01) (Table 2).
3.2. Gut microbiota status in study participants
3.2.1. Diversity of gut microbiota in each group
There were significant differences in the α-diversity of gut microbiota In the MA and MN groups. Observed_species, Chao1, and PD_whole_tree indices of gut microbiota were significantly higher in the MN group than in the MA group. In contrast, the opposite was true for the Goods_coverage index (Figure 1A). Within the MA or MN groups, the diversity of gut microbiota was higher in male participants than in female participants, i.e., the diversity of AM group was higher than that of the AF group, and NM was higher than that of the NF. In addition, there were no significant differences in the α-diversity of gut microbiota between the AF and NF groups. However, the α-diversity of gut microbiota in the NM group was significantly higher than that in the AM group (Figure 1B). For β-diversity, principal coordinates analysis (PCoA) of Bray-Curtis distances showed that the community composition of gut microbiota was significantly different between MA and MN (PERMANOVA, F = 1.119, P < 0.05) (Figure 1C). In addition, there were significant differences in gut microbiota community composition between the four subgroups (PERMANOVA, F = 1.743, P < 0.01) (Figure 1D).
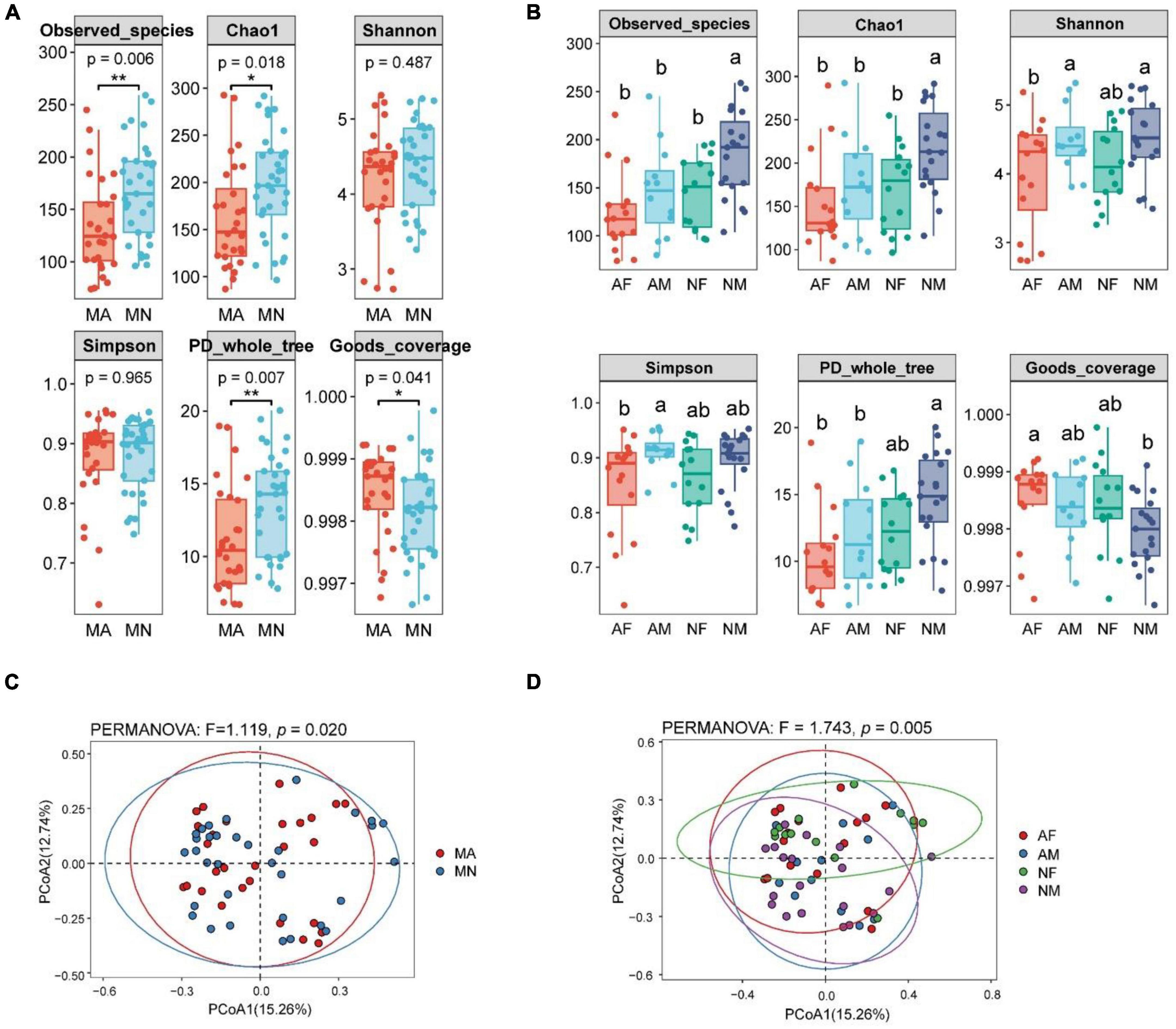
Figure 1. Differences in α-diversity and β-diversity of gut microbiota of participants in different groups. The lowercase letters represented significant differences at the 0.05 level. MA, schizophrenia in clozapine monotherapy who was diagnosed with metabolic syndrome; MN, schizophrenia in clozapine monotherapy who did not develop the metabolic syndrome; AF, female participants in MA; AM, male participants in MA; NF, female participants in the MN; NM, male participants in MN; (A), Differences in α-diversity between MA and MN groups; (B), Differences in α-diversity among AF, AM, NF, and NM groups; (C), Differences in β-diversity be-tween MA and MN groups; (D), Differences in β-diversity among AF, AM, NF, and NM groups. *P < 0.05 and ***P < 0.01.
3.2.2. Composition of gut microbiota among different groups
At the phylum level, The Firmicutes, Bacteroidetes, Actinobacteria, and Proteo-bacteria were the most abundant four phyla in MA, MN, AF, AM, and NM groups. Moreover, the NF group’s most abundant four phyla were Firmicutes, Actinobacteria, Bacteroidetes, and Verrucomicrobia, respectively (Figures 2A, B). No significant differences were found in the abundance of each phylum in both MA and MN groups. The abundance of proteobacteria in the NF group was significantly lower than that in the NM and AF groups. The abundance of Bacteroidetes in the NM group was higher than that in the NF group. The abundance of Actinobacteria in the NM group was lower than that in the NF group (Figure 2C).
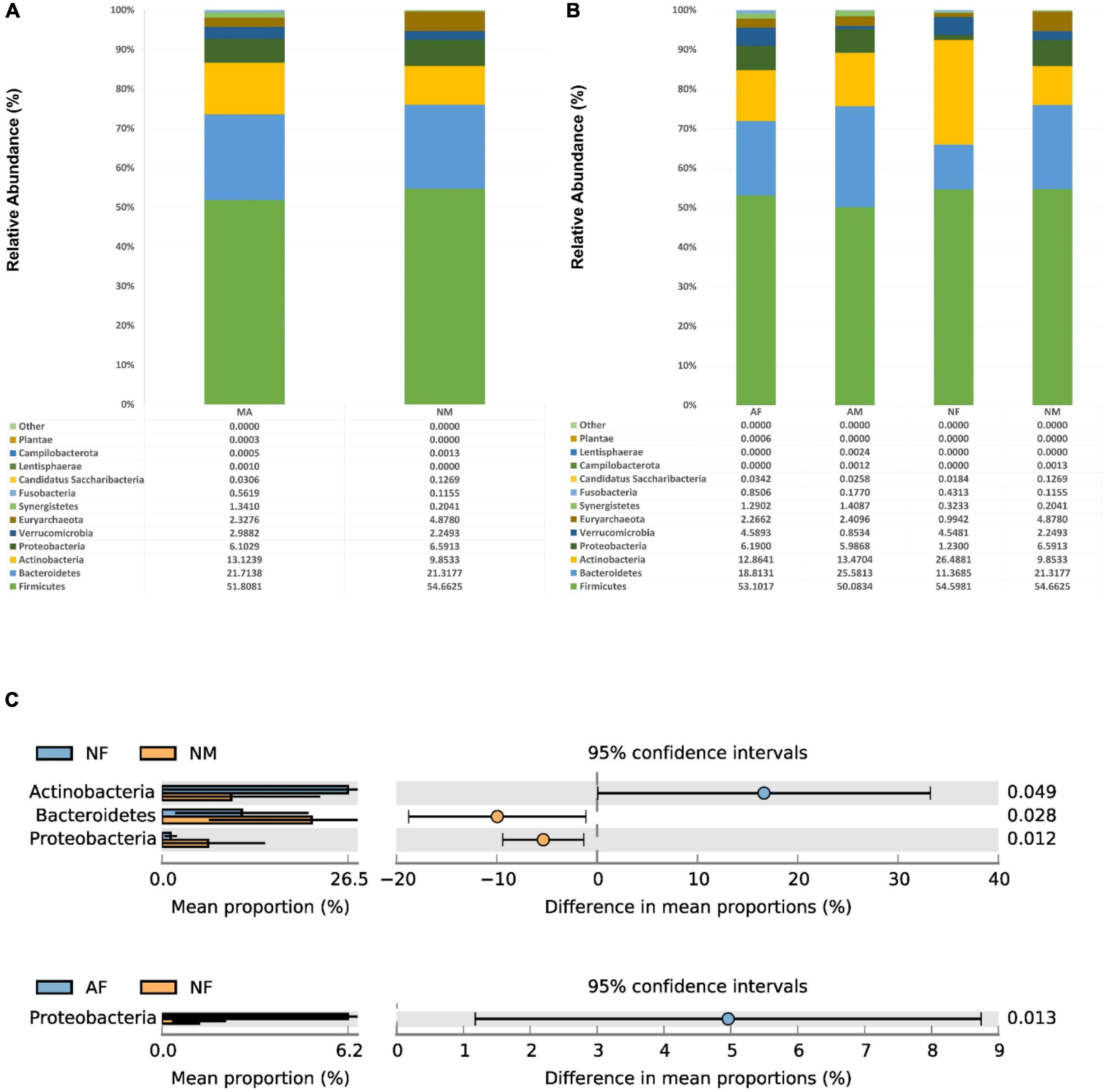
Figure 2. At the phylum level, the composition and the differences of gut microbiota in each group. (A), Composition of the gut microbiota in the MA and MN groups at the phylum level; (B), Composition of the gut microbiota in the AF, AM, NF, and NM groups at the phylum level; (C), At the phylum level, statistically significant groups comparisons are shown.
At the genus level, There were no significant differences among the genera in both MA and MN groups. Bifidobacterium was the most abundant in all four sub-groups, with a relative abundance of 23.23% in the NF group. In addition, Blautia, Bacteroides, Prevotella, Phocaeicola, Agathobacter, and Collinsella were also relatively abundant in each group (Figures 3A, B). The abundance of Faecalibacterium in the NM group (9.63%) was statistically different from that in the AM (3.63%) and NF (2.13%) groups (P < 0.01). The Escherichia/Shigella and Prevotella were also significantly more abundant in the NM group than in the NF group.
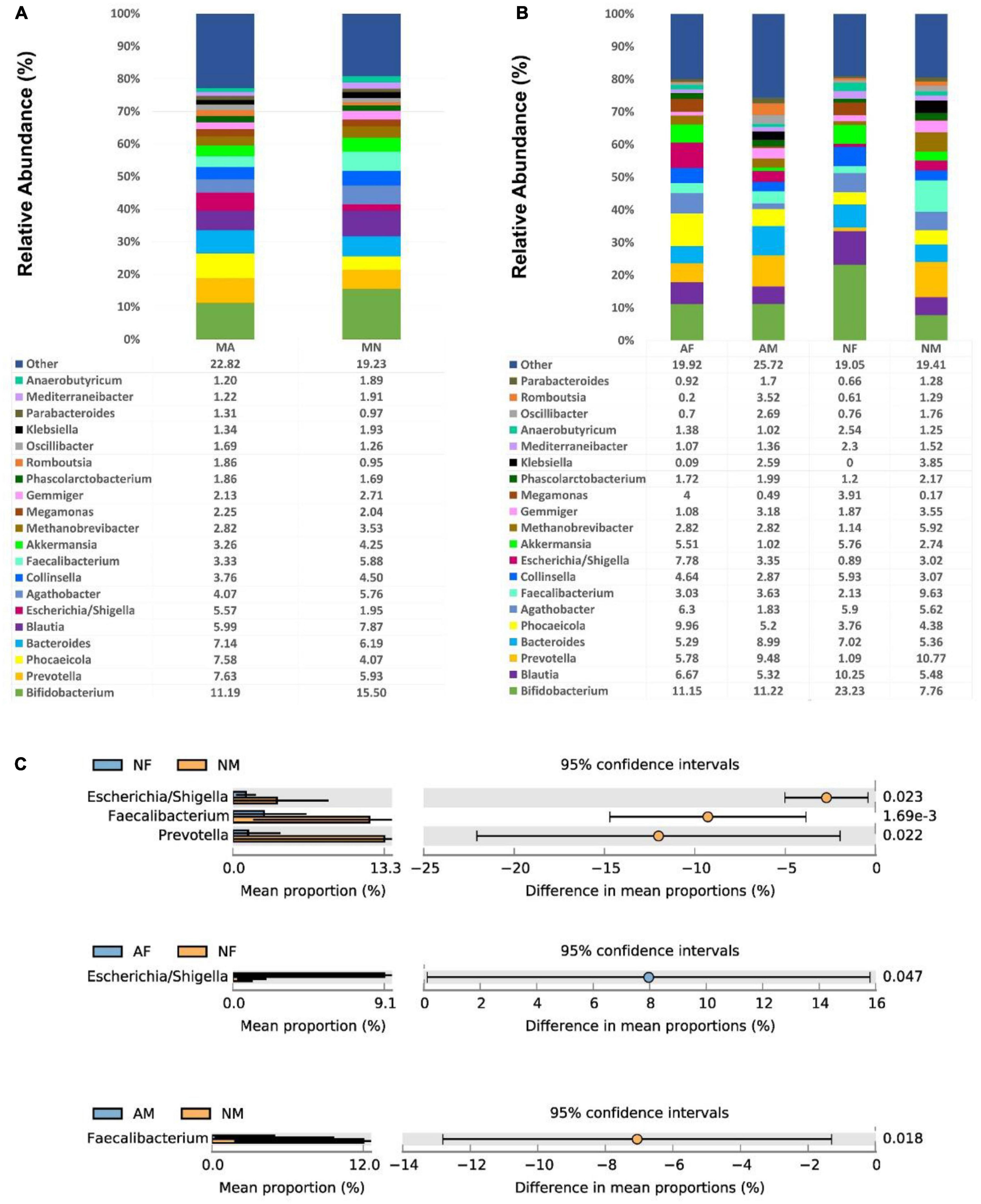
Figure 3. At the genus level, the composition and the differences of gut microbiota in each group. (A), Composition of the gut microbiota in the MA and MN groups at the genus level; (B), Composition of the gut microbiota in the AF, AM, NF, and NM groups at the genus level; (C), At the genus level, statistically significant groups comparisons are shown.
Among all female participants, there was a significantly higher abundance of Escherichia/Shigella in the AF group (7.78%) than in the NF group (0.89%) (P < 0.05). In contrast, among all male participants, there was a significantly higher abundance of Faecalibacterium in the NM group (9.63%) than in the AM group (3.63%) (P < 0.05) (Figure 3C).
3.2.3. Correlation analysis between gut microbiota abundance and clinical parameters
We performed a correlation analysis between the abundance of all gut microbial genera and glycemic or lipids in each group. Overall, the relationship between gut microbial genera and clinical indicators in MA and MN is quite different. Among all genera, only the abundance of Oscillibacter was positively correlated with TG of both MA and MN. Megamonas was positively associated with FBG in MA, but Romboutsia was positively associated with FBG in the MN group. In the MA group Methanobrevibacter, Oscillibacter, Parabacteroides, and Klebsiella were positively correlated with GA. In the MN group, it was Romboutsia and Faecalibacterium that were positively correlated with GA. Agathobacter showed a mostly positive correlation with the serum lipid profiles in the MA group. However, it showed a mostly negative correlation with the HDL-C and ApoE in the MN group (Figures 4A, B).
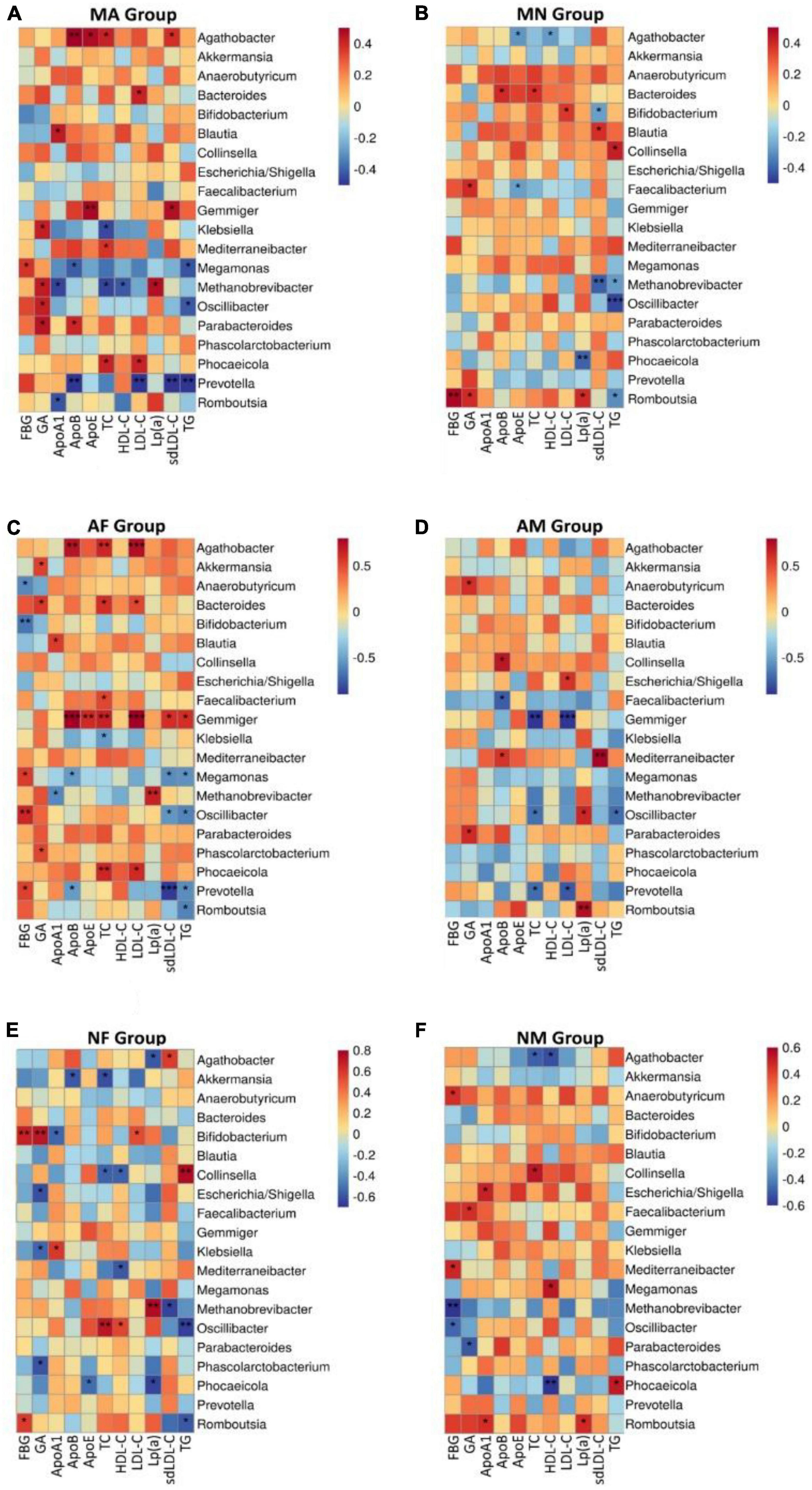
Figure 4. At the genus level, correlation analysis of the abundance of gut microbiota with glycemic or lipids in each group. (A), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in MA group at the genus level; (B), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in MN group at the genus level; (C), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in AF group at the genus level; (D), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in AM group at the genus level; (E), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in NF group at the genus level; (F), Correlation analysis of the abundance of gut microbiota with glycemic or lipids in NM group at the genus level. *P < 0.05 and **P < 0.01.
The association between the abundance of gut microbial genera and glycemic or lipids showed some gender differences also. We found that Gemmiger all showed positive correlations with serum lipid profiles except HDL-C and Lp (a) in the AF group (Figure 4C). In contrast, Gemmiger was predominantly negatively correlated with TC and LDL-C (Figure 4D). The association between gut microbiota and glycemic indicators also differed significantly in the AF and AM groups. Prevotella, Oscillibacter, and Megamonas were positively associated with FBG in the AF group, while Anaerobutyricum and Bifidobacterium were negatively associated with FBG. No genus was associated with FBG in the AM group, but Parabacteroides and Anaerobutyricum were positively associated with GA in the AM group. In the AF group, Agathobacter was positively correlated with TC, LHL-C, and Apo-B, while in the AM group, Agathobacter did not show any association with blood glucose indicators or serum lipid profiles (Figures 4C, D).
There was also a degree of difference between the NF and NM subgroups; for example, Phocaeicola was positively correlated with TG and negatively correlated with HDL-C in the NM group, while in the NF group, Phocaeicola was negatively correlated with Lp (a) and Apo-E. In the NF group, Collinsella was positively correlated with TG and negatively correlated with TC and HDL. In contrast, in the NM group, Collinsella was positively correlated with TC Bifidobacterium and was positively correlated with FBG and GA in the NF group, while not associated with them in the NM group (Figures 4E, F).
In addition, the association between their gut microbiota and glycemic or lipids was different when comparing the same-gender subgroups. In the two female groups, AF and NF, this difference was mainly shown in the association of Gemmiger, Collinsella, and Agathobacter with glycemic or lipids (Figures 4C, E). In the two male groups, AM and NM, this difference was mainly shown in the association of Agathobacter, Phocaeicola, Prevotella, and Romboutsia with glycemic or lipids (Figures 4D, F).
It is worth pointing out that, as a good lipoprotein (Qin et al., 2012), HDL-C was not associated with any of the genera in the AF and AM subgroups. In contrast, HDL-C was positively associated with Oscillibacter and negatively associated with Mediterraneibacter and Collinsella in the NF group. In the NM group, HDL-C was negatively correlated with Phocaeicola and Agathobacter and positively correlated with Megamonas (Figure 4).
Surprisingly, the Oscillibacter genus seems unaffected by the metabolic and gender grouping methods. It was negatively correlated with TG in all groups, which is noteworthy, although there were no statistical differences in the NM group.
4. Discussion
The mechanism of clozapine-induced metabolic abnormalities is unclear, and there might be other unknown mechanisms (Panariello et al., 2011). To date, relatively few studies have been conducted to analyze the gut microbiota of patients with antipsychotic-induced metabolic syndrome. To our knowledge, this is the first study to analyze the association between metabolic syndrome and gut microbiota in SCM.
Previous studies have confirmed that antipsychotics can induce changes in the diversity and composition of the gut microbiota (Chen et al., 2023). The present study also found that the α-diversity index was significantly lower in the MA group of SCM than that in the MN group of SCM, and there was also a significant difference in the β-diversity of the gut microbiota between the MA and MN groups of SCM.
Previous studies have not agreed on the gut microbiota characteristics of patients with metabolic syndrome in the general population (Armougom et al., 2009; Schwiertz et al., 2010). A He et al. (2018) survey of gut microbiota in patients with metabolic syndrome in the general population in Guangdong, China, found that Bacteroidetes, Proteobacteria, and Firmicutes ranked among the top three abundance of gut microbiota and account for 92.3% of the total abundance of gut microbiota in metabolic syndrome patients. In contrast, this study found that Firmicutes, Bacteroidetes, and Actinobacteria were ranked in the top three abundance in MA, MN, and the four subgroups classified by gender, which implies that the composition of the gut microbiota of SCM may differ from that of the general population.
There were no significant differences in the abundance of each phylum between the MA and MN groups in this study. In contrast, another study conducted in 2021 in Shanghai, China, analyzing the gut microbiota of males with schizophrenia with and without MetS, found that the abundance of Bacteroidetes, Proteobacteria, and Synergistetes was higher in the MetS group than in the non-MetS group; While the abundance of Lentisphaerae, Fusobacteria, and Firmicutes was higher in the non-MetS group (Xing et al., 2022).
This difference may be because the participants in that study were taking a wider variety of antipsychotics, unlike the present study, which included schizophrenia patients treated with clozapine monotherapy. Studies have found that taking different antipsychotic medications can have different effects on the composition and abundance of gut microbiota (Chen et al., 2023). We speculate that it is this reason that accounts for this discrepancy.
Due to the increased abundance of Proteobacteria in nutrition, metabolic disorders, and inflammation disorders, Proteobacteria is called the microbial signature of these diseases (Shin et al., 2015; Rizzatti et al., 2017). In this study, we found that the abundance of Proteobacteria in the AF and AM groups (which represented the MetS) was significantly higher than that in the NF group (which did not have the MetS), supporting the above theoretical hypothesis. The difference in the abundance of Proteobacteria in NF and NM groups may be due to the influence of gender.
The gender differences in the gut microbiota may determine the gender predisposition to disease (Santos-Marcos et al., 2019a). In the general population with and without MetS, there are many differences between males and females at the genus level. In men with MetS, the gut microbiota is dominated by Clostridium, SMB53, Coprococcus, Roseburia, and Faecalibacterium; in women with MetS, the gut microbiota is characterized by the predominance of Cyanobacteria phylum, Parabacteroides genus, and the less abundant Prevotella genus. In men without MetS, several genera, such as Clostridium, Coprococcus, Dorea, Lachnospira, Roseburia, and Veillonella, dominated the gut microbiota. However, the gut microbiota of women without MetS was characterized by several genera, such as Bacteroides, Barneciellaceae, Butyricimonas, Parabacteroides, and Rikenellaceae (Santos-Marcos et al., 2019a).
In contrast, the differences in gut microbiota among the groups in this study did not show such obvious, and there were only significant differences in Escherichia/Shigella, Faecalibacterium, and Prevotella between the NF and NM groups. There were no significant differences among genera in both AF and AM groups (Figure 3). Again, as mentioned above, this could be due to the better homogeneity of the samples in this study. A Korean study has demonstrated the influences of different dietary structures and environmental factors on gut microbiota (Noh et al., 2021). Therefore, our study design tried to remove the influence of dietary structure and environmental factors. We selected SCM from the same hospital with an identical dietary structure as the study participants, so there were little differences in the gut microbiota in different sub-groups in this study.
At the genus level, we found that the association between the gut microbiota and the clinical indicators was more complex in all groups. It is thought that some bacteria may significantly affect human metabolism, although their abundance is low (Quigley, 2013). As in the case of Oscillibacter, although it was not considered a dominant genus in any groups in this study, it is noteworthy that it was somehow associated with glycemic or lipid indicators in each group. Oscillibacter is an essential component of microbial regulation of host metabolism, which affects the release of gut hormones such as PYY and GLP-1 (Olivares et al., 2018; Zeng et al., 2021). PYY, GLP-1, and some other gut hormones regulate glucose and lipid metabolism, fat storage, and appetite in hosts, and atypical antipsychotics, in turn, can regulate these gut hormones. It is hypothesized that as well as regulating PYY and GLP-1, atypical antipsychotics also affect the abundance of Oscillibacter, which in turn affects the metabolism of clinical indicators such as blood glucose or lipids in the host (Olivares et al., 2018; Bretler et al., 2019; Martin et al., 2019; Zeng et al., 2021). Therefore, we should pay further attention to Oscillibacter in future studies on antipsychotic-mediated nutritional and metabolic disorders.
Gemmiger belongs to the phylum Bacillota, which had opposite correlations with the fat distribution of females and males (Min et al., 2019; Zhong et al., 2020), and it also positively correlated with insulin resistance in girls with Idiopathic central precocious puberty (Dong et al., 2020). However, in the present study, we had different findings. In the participants with MetS in this study, Gemmiger was positively associated with TG, sdLDL-C, lLDL-C, TC, ApoB, and ApoE in the AF group. In contrast, it showed a negative association with sdLDL-C and TC in the AM group. The finding suggests that there may be a gender difference in the association of Gemmiger with Lipid metabolism in MetS of SCM. These new findings of the present study remind us that, on the one hand, Gemmiger is extremely closely related to lipid metabolism in the MetA group of SCM. There may be a new mechanism that we do not understand.
On the other hand, we found that although Gemmiger was positively associated with six lipid indices in the AF group and negatively associated with two lipid indices in the AM group, in the MA group, which was composed of the AF and AM groups, only sdLDL-C and ApoE were associated with Gemmiger. This result was caused by the fact that the MA data were mixed from both AF and AM groups, which obscured the differences related to gender factors. The finding strongly suggests that we must not ignore the importance of gender classification in gut microbiota studies. Suppose we do not consider gender classification but simply mix data from females and males for analysis, once different genders have an opposite influence on the association between a gut microbiota and a clinical index. In that case, the results will lose important information, and the true conclusions will be blurred and diluted.
Bifidobacterium had the highest relative abundance in all groups in this study. As an essential probiotic, it is well established that Bifidobacterium has positive health benefits for human hosts (O’Callaghan and van Sinderen, 2016). The abundance of Bifidobacteria in gut microbiota is decreased in diabetic patients, and Bifidobacterium is negatively correlated with FBG and postprandial blood glucose (Fang et al., 2018). Bifidobacterium supplementation can significantly reduce FBG in elderly patients with type 2 diabetes (Moroti et al., 2012), TC, LDL-C, and TG in patients with moderate hypercholesterolemia (Xiao et al., 2003).
However, this characteristic of Bifidobacterium was not shown in the present study. In the female participants of this study, only FBG in the AF group was negatively associated with Bifidobacterium. What surprised us most was that Bifidobacterium lost its probiotic character and showed pathogenic behavior in the NF group of this study. It showed a relatively strong positive correlation with FBG, GA, and LDL-C and a negative correlation with Apo-A1. Since Apo-A1 is the main component of HDL-C, which is responsible for tissue lipid removal and anti-atherosclerosis protection in the human body, it means that the higher the level of Bifidobacterium in NF group, the more susceptible to dysglycemia and dyslipidemia in this group.
One reason behind this new finding could be that the Relationship between Bifidobacterium and glucose or lipids in SCM is influenced by gender factors, as mentioned before. Another reason could be that the genus Bifidobacterium includes many species, and they are not all probiotics. Maybe the superposition of gender factors with clozapine leads to a decrease of some Bifidobacterium species with beneficial effects to the host and or an increase of some other Bifidobacterium species with un-favorable glycolipid metabolism in the body causing this situation, and it is especially expressed specially and paradoxically in female SCM.
However, our finding differs from the current common belief about Bifidobacterium that it is so impressive. We should pay more attention to female SCM to explore the reasons behind this interesting phenomenon. This finding also suggests that the mechanism of metabolic abnormalities caused by clozapine is quite complex. In future in-depth studies, we should pay more attention to the significance of sex differences in gut microbiota for metabolic disorders mediated by clozapine (Mauvais-Jarvis, 2015).
For the first time, We explored the gut microbiota characteristics in SCM with and without MetS and the association between the gut microbiota and clinical glucose and lipid metabolic indices. Although the gut microbiota composition in SCM with and without MetS was essentially the same, there were significant differences in the diversity of their gut microbiota and the abundance of some gut microbiota. We found that at the genus level, the association between gut microbiota and glucose and lipid metabolism indicators was more complex in different gender SCM. On the one hand, the association between particular glycemic or lipid metabolic indicators and gut microbiota may vary among different genders of SCM. On the other hand, the association between a particular gut microbial genus and glucose and lipid metabolism indices may also differ among different genders of SCM. However, since the participants in this study were SCM, their gut microbiota may be affected by gender, disease, medication, and other factors that may complicate our findings. The implication of our findings remains to be determined.
Since the participants in this study were long-term inpatients with schizophrenia from the same hospital, treated with clozapine monotherapy, and having the same daily dietary structure, it was possible to minimize the influence of confounding factors. We tried to ensure that the sample was as homogeneous as possible, which is the most prominent strength of this study.
From a gender perspective, we analyzed the relationship between gut microbiota and glycemic or lipids and observed some new phenomena that may be related to gender factors. It is known that studies in the general population have found that glucose and lipid metabolic processes differ in men and women. Women tend to store adipose tissue in the subcutaneous region, while men tend to deposit visceral fat (Enzi et al., 1986). The prevalence of prediabetes syndrome varies by gender. Impaired fasting glucose is more common in men than women, while impaired glucose tolerance is more common in women (Mauvais-Jarvis, 2015). Women appear more likely than men to develop diseases such as metabolic syndrome and obesity (Kelly et al., 2008). Our new findings support and complement the theories mentioned above, which is another strength of this study.
However, the premise of satisfying sample homogeneity as much as possible led to a relatively insufficient sample size for this study. Therefore, the findings of this study need to be more representative, which is one of the shortcomings of this study. Each gut microbiota may express distinct nutritional and metabolic functions at the species level. Since 16S can only identify at the genus level, we could not identify gut microbiota at the species level. Each species expresses different functions in nutrition and metabolism, so we could not draw precise conclusions about the species, which is another shortcoming of this study. It will be improved by metabolomics and meta-genomic analysis in the future. Also, Sex hormones are associated with gut microbiota sex-dependent differences in humans (Santos-Marcos et al., 2019a). Due to objective constraints, we did not obtain data on the sex hormones of the study participants. No correlation analysis between sex hormones and gut microbiota was performed, which is a regret of this study.
Despite its excellent efficacy, due to the high side effects of clozapine, many psychiatrists waited until the end before having to choose clozapine for schizophrenia (Wimberley et al., 2017). Therefore, schizophrenia patients taking clozapine are generally older. Because schizophrenia most often begins in young adults and continues throughout life, our findings apply only to the age group of this study. They cannot be generalized to all ages.
Finally, although exercise interventions are known to benefit the physical health of patients with MetS, it has been found in recent years that altering the composition of the gut microbiota through dietary interventions can similarly promote nutritional and metabolic abnormalities in MetS patients (Santos-Marcos et al., 2019b), In the future, we can conduct such intervention studies in SCM with metabolic abnormalities.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI–PRJNA991641.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of Clinical Research Center for Mental Disorders, Shanghai Pudong New Area Mental Health Center, School of Medicine, Tongji University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZS: conceptualization, methodology, writing—review and editing, and funding acquisition. KD: software, formal analysis, data curation, and visualization. FY and ZS: investigation. XM: resources and supervision. FY: writing—original draft preparation. YZ and SM: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science and Technology Development Fund of Shanghai Pudong New Area, Grant number: PKJ2019-Y23. With additional support from Medical discipline Construction Project of Pudong Health Committee of Shanghai (Grant No: PWYgy2021-02) and General Program of Shanghai Pudong New Area Mental Health Center (Grant No: PDJWM-202004).
Acknowledgments
We thank Yanhua Ni for her help on this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Armougom, F., Henry, M., Vialettes, B., Raccah, D., and Raoult, D. (2009). Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One 4:e7125. doi: 10.1371/journal.pone.0007125
Backhed, F., Manchester, J., Semenkovich, C., and Gordon, J. (2007). Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. U. S. A. 104, 979–984. doi: 10.1073/pnas.0605374104
Bahr, S., Tyler, B., Wooldridge, N., Butcher, B., Burns, T., Teesch, L., et al. (2015a). Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl. Psychiatry 5:e652. doi: 10.1038/tp.2015.135
Bahr, S., Weidemann, B., Castro, A., Walsh, J., Deleon, O., Burnett, C., et al. (2015b). Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. Ebiomedicine 2, 1725–1734. doi: 10.1016/j.ebiom.2015.10.018
Bretler, T., Weisberg, H., Koren, O., and Neuman, H. (2019). The effects of antipsychotic medications on microbiome and weight gain in children and adolescents. BMC Med. 17:112. doi: 10.1186/s12916-019-1346-1
Brunero, S., Lamont, S., and Fairbrother, G. (2009). Prevalence and predictors of metabolic syndrome among patients attending an outpatient clozapine clinic in Australia. Arch. Psychiatr. Nurs. 23, 261–268. doi: 10.1016/j.apnu.2008.06.007
Chang, X., Shen, Y., Yun, L., Wang, X., Feng, J., Yang, G., et al. (2023). The antipsychotic drug olanzapine altered lipid metabolism in the common carp (Cyprinus carpio L.): Insight from the gut microbiota-SCFAs-liver axis. Sci. Total Environ. 856:159054. doi: 10.1016/j.scitotenv.2022.159054
Chen, H., Cao, T., Zhang, B., and Cai, H. (2023). The regulatory effects of second-generation antipsychotics on lipid metabolism: Potential mechanisms mediated by the gut microbiota and therapeutic implications. Front. Pharmacol. 14:1097284. doi: 10.3389/fphar.2023.1097284
Chinese Diabetes Association (2021). Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellilus 13, 315–409.
Citrome, L., McEvoy, J., and Saklad, S. (2016). Guide to the management of clozapine-related tolerability and safety concerns. Clin. Schizophr. Relat. Psych. 10, 163–177. doi: 10.3371/CSRP.SACI.070816
Davey, K., Cotter, P., O’Sullivan, O., Crispie, F., Dinan, T., Cryan, J., et al. (2013). Antipsychotics and the gut microbiome: Olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl. Psychiatry 3:e309. doi: 10.1038/tp.2013.83
DeGruttola, A., Low, D., Mizoguchi, A., and Mizoguchi, E. (2016). Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 22, 1137–1150. doi: 10.1097/MIB.0000000000000750
Dong, G., Zhang, J., Yang, Z., Feng, X., Li, J., Li, D., et al. (2020). The association of gut microbiota with idiopathic central precocious puberty in girls. Front. Endocrinol. 10:941. doi: 10.3389/fendo.2019.00941
Enzi, G., Gasparo, M., Biondetti, P., Fiore, D., Semisa, M., and Zurlo, F. (1986). Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am. J. Clin. Nutr. 44, 739–746.
Esen-Danacı, A., Sarandöl, A., Taneli, F., Yurtsever, F., and Özlen, N. (2008). Effects of second generation antipsychotics on leptin and ghrelin. Prog. Neuro Psychopharmacol. Biol. Psychiatry 32, 1434–1438. doi: 10.1016/j.pnpbp.2008.03.015
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fang, C., Shuqin, P., Yong, X., Li, G., Huiming, X., Liqun, Y., et al. (2018). Correlation between Bifidobacterium and blood sugar in elderly patients with type 2 diabetes. Chin. J. Microecol. 9, 1033–1036.
Frogley, C., Taylor, D., Dickens, G., and Picchioni, M. (2012). A systematic review of the evidence of clozapine’s anti-aggressive effects. Int. J. Neuropsychopharmacol. 15, 1351–1371. doi: 10.1017/S146114571100201X
Fu, J., Bonder, M., Cenit, M., Tigchelaar, E., Maatman, A., Dekens, J., et al. (2015). The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117, 817–824. doi: 10.1161/CIRCRESAHA.115.306807
He, Y., Wu, W., Wu, S., Zheng, H., Li, P., Sheng, H., et al. (2018). Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 6:172. doi: 10.1186/s40168-018-0557-6
Kelly, T., Yang, W., Chen, C., Reynolds, K., and He, J. (2008). Global burden of obesity in 2005 and projections to 2030. Int. J. Obesity 32, 1431–1437.
Kim, S., Huang, A., Snowman, A., Teuscher, C., and Snyder, S. (2007). Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc. Natl. Acad. Sci. U. S. A. 104, 3456–3459.
Krakowski, M., Czobor, P., and Citrome, L. (2009). Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr. Res. 110, 95–102. doi: 10.1016/j.schres.2009.02.006
Leonard, B., Schwarz, M., and Myint, A. (2012). The metabolic syndrome in schizophrenia: Is inflammation a contributing cause? J. Psychopharmacol. 26, 33–41. doi: 10.1177/0269881111431622
Li, S., Zhuo, M., Huang, X., Huang, Y., Zhou, J., Xiong, D., et al. (2020). Altered gut microbiota associated with symptom severity in schizophrenia. Peerj 8:e9574. doi: 10.7717/peerj.9574
Martin, A., Sun, E., Rogers, G., and Keating, D. (2019). The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Physiol. 10:428. doi: 10.3389/fphys.2019.00428
Mauvais-Jarvis, F. (2015). Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 6:14. doi: 10.1186/s13293-015-0033-y
Meltzer, H. (2012). Clozapine: Balancing safety with superior antipsychotic efficacy. Clin. Schizophr. Relat. Psych. 6, 134–144. doi: 10.3371/CSRP.6.3.5
Min, Y., Ma, X., Sankaran, K., Ru, Y., Chen, L., Baiocchi, M., et al. (2019). Sex-specific association between gut microbiome and fat distribution. Nat. Commun. 10:2408.
Mitchell, A., Vancampfort, D., Sweers, K., van Winkel, R., Yu, W., and De Hert, M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr. Bull. 39, 306–318. doi: 10.1093/schbul/sbr148
Morgan, A., Crowley, J., Nonneman, R., Quackenbush, C., Miller, C., Ryan, A., et al. (2014). The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9:e115225. doi: 10.1371/journal.pone.0115225
Moroti, C., Souza Magri, L., de Rezende Costa, M., Cavallini, D., and Sivieri, K. (2012). Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 11:29.
Nice (2014). Psychosis and schizophrenia in adults: Treatment and management: NICE clinical guideline 178. London: NICE.
Noh, H., Jang, H., Kim, G., Zouiouich, S., Cho, S., Kim, H., et al. (2021). Taxonomic composition and diversity of the gut microbiota in relation to habitual dietary intake in Korean adults. Nutrients 13:366. doi: 10.3390/nu13020366
O’Callaghan, A., and van Sinderen, D. (2016). Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7:925. doi: 10.3389/fmicb.2016.00925
Olivares, M., Neyrinck, A., Pötgens, S., Beaumont, M., Salazar, N., Cani, P., et al. (2018). The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia 61, 1838–1848.
Panariello, F., De Luca, V., and de Bartolomeis, A. (2011). Weight gain, schizophrenia and antipsychotics: New findings from animal model and pharmacogenomic studies. Schizophr. Res. Treat. 2011:459284. doi: 10.1155/2011/459284
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60.
Rizzatti, G., Lopetuso, L., Gibiino, G., Binda, C., Gasbarrini, A., and Canducci, F. (2017). Proteobacteria: A common factor in human diseases. Biomed. Res. Int. 2017:9351507. doi: 10.1155/2017/9351507
Santos-Marcos, J., Haro, C., Vega-Rojas, A., Alcala-Diaz, J., Molina-Abril, H., Leon-Acuña, A., et al. (2019a). Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol. Nutr. Food Res. 63:e1800870. doi: 10.1002/mnfr.201800870
Santos-Marcos, J., Perez-Jimenez, F., and Camargo, A. (2019b). The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 70, 1–27. doi: 10.1016/j.jnutbio.2019.03.017
Schwiertz, A., Taras, D., Schäfer, K., Beijer, S., Bos, N., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195.
Shin, N., Whon, T., and Bae, J. (2015). Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503. doi: 10.1016/j.tibtech.2015.06.011
Singh, V., Chassaing, B., Zhang, L., San, Y., Xiao, X., Kumar, M., et al. (2015). Microbiota-dependent hepatic lipogenesis mediated by stearoyl coa desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 22, 983–996. doi: 10.1016/j.cmet.2015.09.028
Stahl, S. (2013). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications, 4th Edn. New York, NY: Cambridge University Press, 608.
Stahl, S., Morrissette, D., Citrome, L., Saklad, S., Cummings, M., Meyer, J., et al. (2013). “Meta-guidelines” for the management of patients with schizophrenia. CNS Spectr. 18, 150–162. doi: 10.1017/S109285291300014X
Suetani, R., Siskind, D., Reichhold, H., and Kisely, S. (2017). Genetic variants impacting metabolic outcomes among people on clozapine: A systematic review and meta-analysis. Psychopharmacology 234, 2989–3008. doi: 10.1007/s00213-017-4728-0
Versacloz (1989). Versacloz (clozapine) oral suspension [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.
Warnez, S., and Alessi-Severini, S. (2014). Clozapine: A review of clinical practice guidelines and prescribing trends. BMC Psychiatry 14:102. doi: 10.1186/1471-244X-14-102
Wimberley, T., MacCabe, J., Laursen, T., Sorensen, H., Astrup, A., Horsdal, H., et al. (2017). Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am. J. Psychiatry 174, 990–998. doi: 10.1176/appi.ajp.2017.16091097
Xiao, J., Kondo, S., Takahashi, N., Miyaji, K., Oshida, K., Hiramatsu, A., et al. (2003). Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J. Dairy Sci. 86, 2452–2461. doi: 10.3168/jds.S0022-0302(03)73839-9
Xing, M., Sheng, J., Zhu, L., Lu, Y., Shi, K., and Lu, W. (2022). Analysis of intestinal flora diversity in patients with schizophrenia with metabolic syndrome. Chin. J. Nerv. Ment. Dis. 48, 730–736.
Xue, L., Xiuxia, Y., Lijuan, P., Yu, M., Shuying, W., Xiaoyun, Z., et al. (2021). Gut microbiota markers for antipsychotics induced metabolic disturbance in drug naïve patients with first episode schizophrenia – a 24 weeks follow-up study. Medrxiv [Preprint]. doi: 10.1101/2020.12.26.20248886
Yuan, X., Zhang, P., Wang, Y., Liu, Y., Li, X., Kumar, B., et al. (2018). Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr. Res. 201, 299–306. doi: 10.1016/j.schres.2018.05.017
Yuen, J., Kim, D., Procyshyn, R., Panenka, W., Honer, W., and Barr, A. (2021). A focused review of the metabolic side-effects of clozapine. Front. Endocrinol. 12:609240. doi: 10.3389/fendo.2021.609240
Zeng, C., Yang, P., Cao, T., Gu, Y., Li, N., Zhang, B., et al. (2021). Gut microbiota: An intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 106:110097.
Zhong, X., Harrington, J., Millar, S., Perry, I., O’Toole, P., and Phillips, C. (2020). Gut microbiota associations with metabolic health and obesity status in older adults. Nutrients 12:2364. doi: 10.3390/nu12082364
Keywords: metabolic syndrome, gut microbiota, schizophrenia, clozapine, monotherapy
Citation: Yin F, Shi Z, Ma X, Ding K, Zhang Y and Ma S (2023) Impact of clozapine monotherapy on gut microbiota and metabolism in people with schizophrenia. Front. Microbiol. 14:1253156. doi: 10.3389/fmicb.2023.1253156
Received: 04 July 2023; Accepted: 22 August 2023;
Published: 06 September 2023.
Edited by:
Stefania Schiavone, University of Foggia, ItalyReviewed by:
Kiran Veer Sandhu, University College Cork, IrelandGabriele Deidda, University of Padua, Italy
Copyright © 2023 Yin, Shi, Ma, Ding, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidao Shi, dr.szd@outlook.com
†These authors have contributed equally to this work and share first authorship
 Feiyan Yin1†
Feiyan Yin1† Zhidao Shi
Zhidao Shi Xiquan Ma
Xiquan Ma Kai Ding
Kai Ding