- 1College of Animal Science, Guizhou University, Guiyang, China
- 2College of Life Sciences, Baicheng Normal University, Jilin, China
- 3Shibing County Agricultural and Rural Bureau, Shibing, China
- 4College of Grassland, Resources and Environment, Key Laboratory of Forage Cultivation, Processing and High Efficient Utilization of Ministry of Agriculture, and Key Laboratory of Grassland Resources, Inner Mongolia Agricultural University, Ministry of Education, Hohhot, China
There is little information regarding the dynamics of fermentation products and the bacterial community in silage prepared with alfalfa (MS), perennial ryegrass (LP), and their mixture in the karst region. In this study, we explored the effects of combining MS with LP in different ratios (100% MS, 70% MS + 30% LP, 50% MS + 50% LP, 30% MS + 70% LP and 100% LP; fresh matter basis) on silage chemical composition, fermentation quality, bacterial communities and predicted functions during the ensiling process. Each treatment was prepared in triplicate and stored at room temperature (22–25°C) for 7, 15, and 45 days. The dry matter (DM) and water-soluble carbohydrate content of the silages increased as the LP proportion in the mixed silage increased; at 45 days, the 70% MS + 30% LP, 50% MS + 50% LP and 30% MS + 70% LP silages contained higher (p < 0.05) CP content than the 100% MS and 100% LP silages. The 30% MS + 70% LP and 100% LP silages exhibited lower (p < 0.05) pH and higher (p < 0.05) LA content than the other silages; at 45 days, none of the silages contained PA or BA. As fermentation proceeded, the abundance of harmful (Enterobacteriaceae and Sphingomonas) and beneficial (Lentilactobacillus, Lactiplantibacillus, Secundilactobacillus, and Levilactobacillus) microorganisms decreased and increased, respectively, as the LP proportion in the mixed silage increased. The predicted functional distribution of microbial communities and metabolic pathways revealed that the 30% MS + 70% LP and 100% LP silages had a stronger capacity for fermentation and a weaker capacity for nitrate reduction than the other silages. Moreover, as the fermentation proceeded, the 30% MS + 70% LP and 100% LP treatments enhanced the functions of “Metabolism,” “Genetic information processing” and “Organismal systems” at level 1, the functions of “Amino acid metabolism” and “Nucleotide metabolism” at level 2, and the functions of “Metabolic pathways,” “Biosynthesis of secondary metabolites,” “Biosynthesis of antibiotics” and “Purine metabolism” at level 3. Thus, adding LP could improve the fermentation quality of MS silage by changing the composition and metabolic function of microbes; furthermore, ensiling 30% alfalfa with 70% ryegrass can produce high-quality silage in the karst region.
Introduction
The region of Southwest China is a fragile area with grassland ecosystems and encompasses the most typical, complex, and impeccable development of karst worldwide (Ying et al., 2021). Due to the extreme environment, which involves rocky desertification, soil erosion, and soil depletion, herbivores often lack a food supply in winter and early spring; thus, the development of animal husbandry in this region is often restricted (Zhang et al., 2012; Yuan et al., 2021). Farmers have long stored forage as hay for supplementary feed in winter (Chen et al., 2017). However, dry matter (DM) and losses in nutritive value often occur during the haymaking stage. Furthermore, the karst area typically incurs high temperatures and precipitation at the same time, and compared to traditional hay, silage may be more suitable for the development of local animal husbandry (Zhang et al., 2015a).
Alfalfa (Medicago sativa L., MS), as a typical legume forage, is rich in crude protein and widely used as animal feed (Zhao et al., 2020). However, successfully ensiling MS is difficult due to its high buffering capacity, low water-soluble carbohydrate (WSC) content, and low epiphytic lactic acid bacteria (LAB) populations (Wang et al., 2020a). Ensiling MS in a mixture containing gramineous forage with high WSC content would be a good method of conservation (Wang et al., 2019). Numerous studies have shown that the fermentation quality of MS can be significantly improved by ensiling it in a mixture with gramineous forages (whole-plant corn or sweet sorghum; Zhang et al., 2015b; Wang et al., 2020a). Perennial ryegrass (Lolium perenn L., LP) is distributed widely in Asia, Europe, and northern Africa and exhibits several characteristics, including a long growing period, strong adaptability, and high forage yield (Su et al., 2021). LP can be preserved as ruminant feed through ensiling (Romero, 2010). However, the crude protein (CP) content in fresh LP and silage (96.1 g/kg; Dong et al., 2022) cannot meet the growth requirements of ruminants (NRC, 2007). Previous research demonstrated that to meet these requirements, legume forage with a high CP content can be mixed with low CP gramineous forages for ensiling (Zhang et al., 2015a). Therefore, ensiling MS with LP may be a good choice for improving the nutritional and fermentation quality of MS. However, few studies have investigated in detail the feasibility of mixing MS and LP for producing silage.
Ensiling is a fermentation process driven by microorganisms, especially LAB (Wang et al., 2022). In the fermentation process, lactic acid bacteria use WSC to produce lactic acid and other substances that lower the pH, thereby inhibiting other harmful microorganisms (such as Enterobacter and Escherichia coli; Zhao et al., 2020). Therefore, characterizing the microbial community is critical for further improving the quality of silage. To our knowledge, little information is available regarding the dynamics of fermentation products, bacterial communities and predicted functional profiles of silage prepared with MS, LP, and their mixture in the karst region. Therefore, this study was performed with the purpose of probing the effect of different ratios of MS and LP combinations on fermentation quality, bacterial communities and predicted functional profiles throughout the ensiling process. We hypothesized that (i) ensiling MS in a mixture with LP would enhance the nutritional and fermentation quality of silage, and (ii) increasing LP could improve the fermentation quality of MS silage by inhibiting harmful bacteria.
Materials and methods
Silage preparation
Alfalfa and perennial ryegrass were cultivated in Guanling County, Guiyang city, Guizhou Province. This region has a typical karst landscape, mainly with a humid mid-subtropical monsoon climate, with an average annual temperature of 16.2°C, an average annual precipitation of 1205.1–1656.8 mm, and an average altitude of 1,025 m. Fresh alfalfa (MS, Victoria varieties) was harvested at the early flowering stage, while perennial ryegrass (LP, Guicao NO.1 varieties) was harvested at the boot stage. Without wilting, each of the two forages was separately chopped to a length of 2–3 cm using a hand hay cutter. Approximately 300 g of chopped MS was mixed homogenously with LP, packed manually into polyethylene bags (25 cm × 30 cm), and then vacuum packed using a vacuum packing machine (SJ-400, Shanghai Precision Machinery Manufacturing Co. Ltd). The treatments were as follows: (1) 100% MS: 100% alfalfa+0% perennial ryegrass; (2) 70% MS + 30% LP: 70% alfalfa+30% perennial ryegrass; (3) 50% MS + 50% LP: 50% alfalfa+50% perennial ryegrass; (4) 30% MS + 70% LP: 30% alfalfa+70% perennial ryegrass; and (5) 100% LP: 0% alfalfa+100% perennial ryegrass. Three polyethylene bags of silage with the same treatment were stored at room temperature (22–25°C) and were opened in triplicate for each treatment after 7 and 45 days of ensiling to analyze microbial diversity, which was achieved by examining the chemical compositions and fermentation quality after 7, 15, and 45 days of ensiling.
Analysis of microbial populations, chemical compositions, and fermentation quality
As performed by Cai et al. (1999), the plate count method was used to determine the microbial populations of fresh materials. Briefly, 10 g of MS or LP was homogenized with 90 ml autoclaved 0.85% sodium chloride solution, shaken at 120 rpm and 37°C for 2 h, and serially diluted from 10−1 to 10−7. The LAB, yeasts and coliform bacteria were counted by culture-based methods using deMan, Rogosa, and Sharpe (MRS) agar plates (GCM188, Land Bridge Technology Co., Ltd., Beijing, China), malt extract agar (CM173, Land Bridge Technology Co., Ltd., Beijing, China) and blue light broth agar (Nissur Ltd., Tokyo, Japan), respectively. The microbial number was enumerated in colony-forming units (cfu), converted to logarithmic form, and expressed on a fresh material (FM) basis.
Each sample was dried at 65°C, crushed, and sieved through a 1 mm sieve to estimate the dry matter (DM) content. The content of water-soluble carbohydrate (WSC) was determined by the anthrone method (Owens et al., 1999). In brief, a dried sample of 0.2 g was ground with a mill (1 mm screen), mixed with 10 ml of sterile distilled water in a glass tube and incubated at 100°C for 30 min. The filtrate was transferred to a volumetric flask, and diluted with sterile distilled water to 100 ml. One microliter of the solution was mixed well with 5 ml of anthrone-sulfuric acid solution (0.4 g anthrone+100 ml 88% sulfuric acid) and incubated at 100°C for 10 min. The absorbance of the mixture was measured at 620 nm. Glucose was used as a reference substance, and the standard curve was as follows: y = 0.117x + 0.008, R2 = 0.9996, where x is the concentration of the filtrate (mg/mL) and y is the absorbance value at 620 nm. The minimum detection concentration was 1.0 mg/ml. When the absorbance value was >1.2 and <0.2, the filtrate was diluted to achieve highly accurate determination. Crude protein (CP) was analyzed using a Kjeldahl analyzer (Kjeldahl 2300 Automatic analyzer, FOSS Analysis AB, Hoganas, Sweden) according to the methods of the official Society of Analytical Chemists (AOAC, 1990). Both the neutral detergent fiber (NDF) and acid detergent fiber (ADF) levels were analyzed using the methods of Van Soest et al. (1991).
Each 10 g silage sample was mixed homogeneously with 90 ml of sterile water and stored at 4°C for 6 h, followed by filtration through four layers of cheesecloth. The filtrates were finally stored in 50 ml centrifuge tubes at −20°C for subsequent analyzes. The filtrate was tested for organic acids, ammoniacal nitrogen (AN) and pH. The pH was measured by a pH metre. The AN content was determined by the sodium hypochlorite-phenol method (Broderick and Kang, 1980). The filtrate was centrifuged at 10000 r at 4°C for 10 min and analyzed with a 0.22 μm dialyzer. Lactic acid (LA), acetic acid (AA), propionic acid (PA) and butyric acid (BA) levels were determined by high-pressure liquid chromatography (HPLC; KC-811 column, Shodex; Shimadzu Co. Ltd., Tokyo, Japan; oven temperature, 50°C; mobile phase, 3 mmol/L perchlorate solution; flow rate, 1.0 ml/min; flame photometric detection wavelength, 210 nm; and sample size 5.0 μl; Jia et al., 2019).
Bacterial community analysis
Microbial DNA was extracted from the silage sample according to the method described by Wang et al. (2020b). In brief, the preserved liquid for extracting DNA was centrifuged (rate, 12,000 × g; time, 10 min), and the pellet was collected and used to extract DNA by a Power Soil DNA Isolation Kit (MO BIO Laboratories) according to the manufacturer’s protocol. After purification, the DNA was diluted to 1 ng/ml using sterile water. All microbial DNA samples were immediately sent to Biomarker Technologies Corporation (Beijing, China) for PCR amplification and bioinformatic analysis. The 16S rDNA V3–V4 regions were amplified using a forward primer (50-ACTCCTACGGGAGGCAGCA-30) and reverse primer (50GGACTACHVGGGTWTCTAAT-30) combined with specific barcode sequences. FLASH software was applied to check the raw reads, and the high-quality sequences (scores >80) were saved based on the QIIME quality control process. The operational taxonomic units (OTUs) with 97% similarity were clustered by UPARSE pipeline software. Then, UCHIME software was used to identify and remove the chimeric sequences. The alpha diversity indices including the OTU, Ace, Chao, Shannon, Simpson and coverage indices, were determined using Mothur software. The metabolic potential of the bacterial community and the composition of functional genes were predicted by assigning 16S rRNA marker gene sequences to functional annotations of sequenced metagenomic sequences based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway Orthology (KO) classification, using Tax4Fun (version 0.3.1). The comparisons of the KEGG pathways were graphically presented via Graphpad Prism (version 8, IBM, Armonk, NY, United States).
Statistical analysis
Data for the chemical composition, fermentation quality and the bacterial community were analyzed via two-way analysis of variance to evaluate the effects of ratios (R), ensiling period (P), and their interaction (R × P). All statistical analyses were performed using the general linear model procedure with SPSS 26 software (IBM Crop., Armonk, NY, United States). The data on the relative abundances of the KEGG pathways were subjected to one-way ANOVA. Tukey’s multiple comparison test was applied to analyse the statistical differences. Differences were considered as significant at p < 0.05. All the figures were created using Graphpad Prism (version 8, IBM, Armonk, NY, United States).
Results and discussion
Characteristics of raw materials
The chemical parameters and microbial populations of fresh samples are shown in Table 1. The DM contents of fresh MS and LP were 23.48% DM and 30.6% DM, respectively. The CP content of MS was 23.3% DM, which was similar to the result (24.6% DM) of Luo et al. (2021). The NDF (47.43% DM) and ADF (24.22% DM) contents of LP in our study were comparable to those reported by Li et al. (2019), who reported that the NDF and ADF contents of annual ryegrass were 48.39% DM and 28.16% DM, respectively. The NDF (50.49% DM) and ADF (26.41% DM) contents of MS were higher than those reported by Yuan et al. (2020), who reported that the NDF and ADF contents of MS were 44.00% DM and 20.80% DM, respectively. This shows that environmental conditions and varieties are the main factors affecting the nutritional quality of the material (Li et al., 2019). Silage quality depends on many factors, including the WSC content and epiphytic LAB count of raw material (Cheng et al., 2021). During ensiling, LAB convert WSC to organic acids, mainly LA, under anaerobic conditions, thereby lowering the pH to protect forage from undesirable microorganisms (Li et al., 2019). Therefore, ensiling is a LAB-driven fermentation process and the minimum requirement for the LAB count in raw materials is >105 cfu/g FW (Wang et al., 2017). WSC acts as fermentation substrate, and the WSC content should be >50 g/kg of DM was shown to be crucial for ensuring acceptable fermentation quality (Seale et al., 1986). In our study, low numbers of attached LAB (< 105 cfu/g FM) and high numbers of yeasts (103.52 cfu/g FM) in fresh MS made it difficult to perform silage and necessitated the addition of LAB additives or high-sugar gram forages to improve silage quality (Zhang et al., 2017). Compared to MS, LP contained higher levels of LAB (104.97 cfu/g FM) and WSC (19.59% DM) and lower levels of yeasts (102.31 cfu/g FM). Therefore, we hypothesized that the addition of LP could promote the fermentation of MS silage.
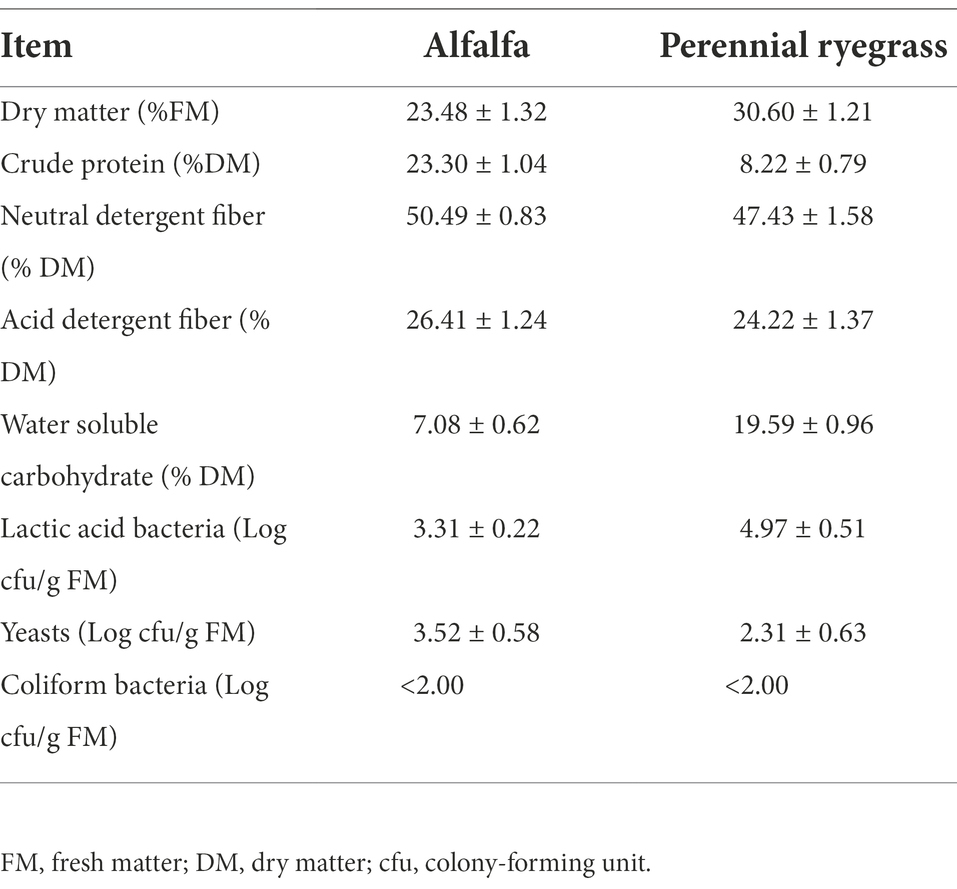
Table 1. Chemical compositions and microbial numbers of alfalfa and perennial ryegrass prior to ensiling.
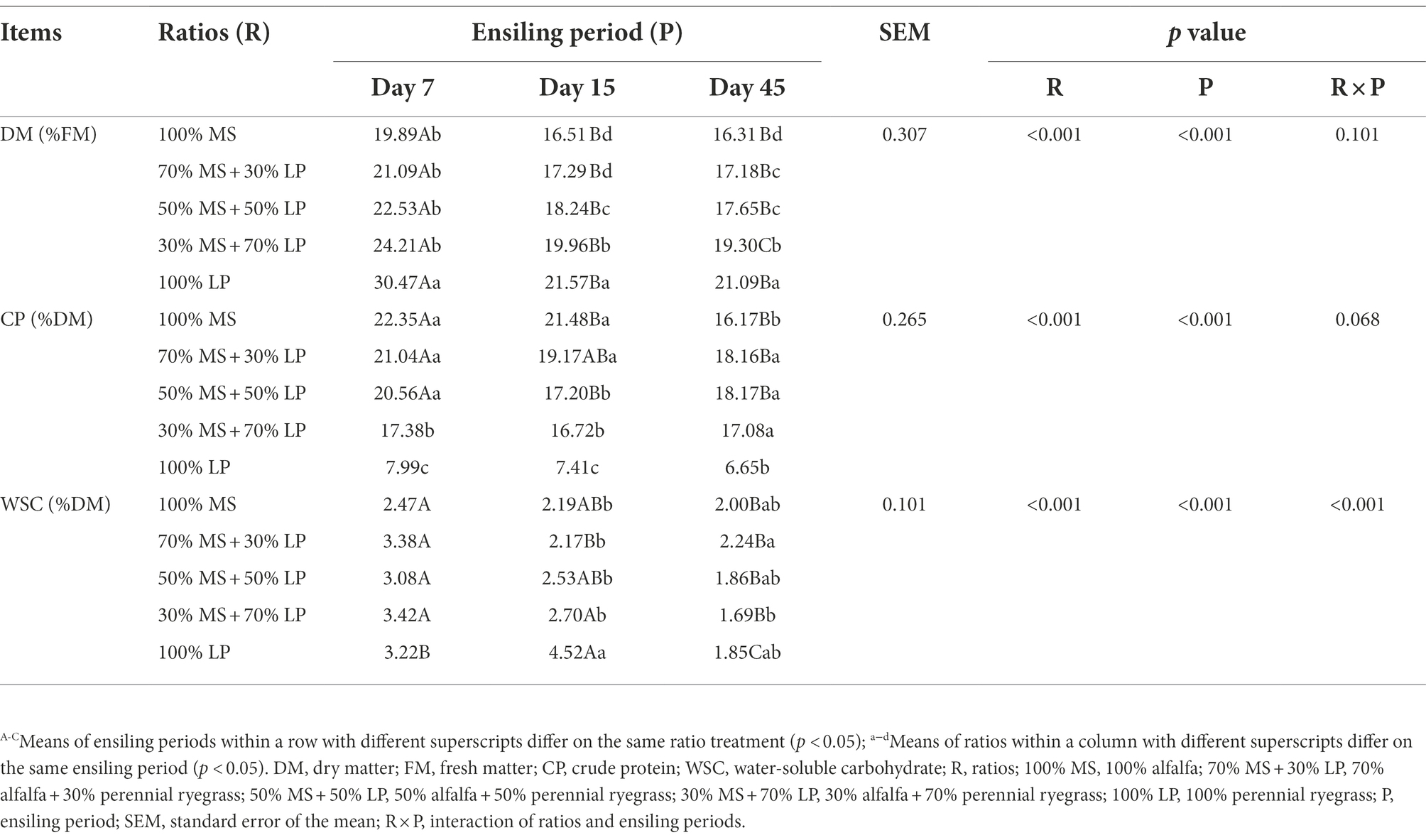
Dynamic changes In nutrient composition during ensiling
The chemical compositions of silages are shown in Table 2. The ratios (p < 0.01) and ensiling period (p < 0.01) significantly influenced the DM, CP and WSC contents of silages. The DM content is an important indicator in silage, as LAB need moisture for growth and reproduction (Oladosu et al., 2016). As fermentation proceeded, the DM content of silages gradually decreased. This reduction could be attributed to the metabolism of soluble substrates (e.g., WSC) by fermentative microbes (Oladosu et al., 2016). The DM content of silages increased as the proportion of LP in the mixed silage increased because the content of DM was higher in fresh LP than in fresh MS. In our study, as fermentation proceeded, the CP and WSC contents of silages gradually decreased because the nutrients were consumed by microorganisms (Dong et al., 2020). The CP content of silages at 7 and 15 days decreased as the proportion of LP in the mixed silage increased because the content of CP was lower in fresh LP than in fresh MS. Interestingly, at 45 days of ensiling, the CP content of MS and LP mixed silages was higher (p < 0.05) than that of MS or LP silage alone, which indicated that ensiling MS with LP may reduce the loss of CP. This may be because adding LP increased the abundance of beneficial microorganisms (such as LAB) in alfalfa silage, thereby inhibiting the degradation of CP by harmful microorganisms.
Dynamic change in fermentation characteristics during ensiling
The fermentation characteristics of the silages are shown in Table 3. The ratios (p < 0.01), ensiling period (p < 0.05) and their interactions (p < 0.01) significantly influenced the pH, LA and PA contents of silages. The key to limiting the growth of harmful microorganisms (e.g., enterobacteria or clostridia) is achieving quick early acidification (Pahlow et al., 2003). In our study, during 7–45 days of ensiling, the pH value of alfalfa silage (100% MS) first decreased and then increased slowly, which was consistent with the trend observed by Wang et al. (2020a). At 45 days of ensiling, the pH value of alfalfa silage was 5.82, which was higher than the study result (4.69) obtained by Wang et al. (2020a). This may be because the LAB count (103.31 cfu/g FM) of fresh MS in our study was lower than that (106.69 cfu/g FM) of Wang et al. (2020a). As expected, the fermentation quality of alfalfa silage was very poor, mainly manifested in high pH (5.82) and AN (5.50% DM) values and low LA content (0.68% DM). The pH value of silages decreased as the proportion of LP in the mixed silage increased, which was consistent with the result of Zhang et al. (2015c), who found that adding sweet sorghum could reduce the pH of alfalfa silage. This is due to the high WSC content of fresh LP, which provides a fermentation substrate for LAB growth and helps to reduce pH and increase LA content during the ensiling process (McDonald et al., 2002). At the early and late stages of ensiling (7 and 45 days, respectively), the LA content of silages increased as the proportion of LP in the mixed silage increased, which was consistent with the result of Dong et al. (2020), who reported that more organic acids, especially LA, could be generated by LAB when high WSC content forages were added to the silage. It is noteworthy that in the mixed silages, the LA content (8.37% DM) of the 30% MS + 70% LP treatment was significantly higher (p < 0.05) than that of the other treatments, while the pH value (4.63) was significantly lower (p < 0.05). This indicated that 30% MS + 70% LP treatment could better promote the growth of beneficial bacteria (e.g., LAB) and inhibit the growth of harmful bacteria. This result was confirmed by our microbial results (Figure 1B), in which the abundance of LAB (Lentilactobacillus and Lactiplantibacillus) in the 30% MS + 70% LP treatment was higher than that in the other treatments, while the abundance of Enterobacteriaceae was lower than that in the other treatments. It is well known that the AA, PA and BA contents of silage are undesirable (McDonald et al., 1991). The majority of AA is produced when heterofermentative LAB, propionibacteria, and enterobacteria interact with carbohydrates (McDonald et al., 1991). Propionibacterial activity partially contributes to the synthesis of silage PA (Woolford, 1975). The hallmark of a clostridial fermentation is that a significant amount of BA is generated in the silage (Pahlow et al., 2003). In our study, at 45 days of ensiling, the AA content was low in all silages, and PA and BA were not detected. The CP content was easily degraded and caused AN to accumulate during ensiling. As fermentation proceeded, the AN content of mixed silage in the late ensiling stage was lower than that in the early ensiling stage. This may be due to the dominance of LAB in the late ensiling stage, which inhibited the growth of AN-producing bacteria (Li et al., 2021). The AN content of silages decreased as the proportion of LP in the mixed silage increased in our study. This indicates that adding LP with a high sugar content can reduce the CP hydrolysis of MS silage (Wang et al., 2020a). At 45 days of ensiling, the AN content (1.48% DM) of the 30% MS + 70% LP treatment was significantly lower (p < 0.05) than that of the other treatments. In general, adding LP could improve the fermentation quality of MS silage, among which the silage quality of 30% MS + 70% LP treatment was the best; this improvement was mainly observed in a low pH (4.63), low AN content (1.48% DM) and high LA content (8.37% DM), and no PA or BA were detected.
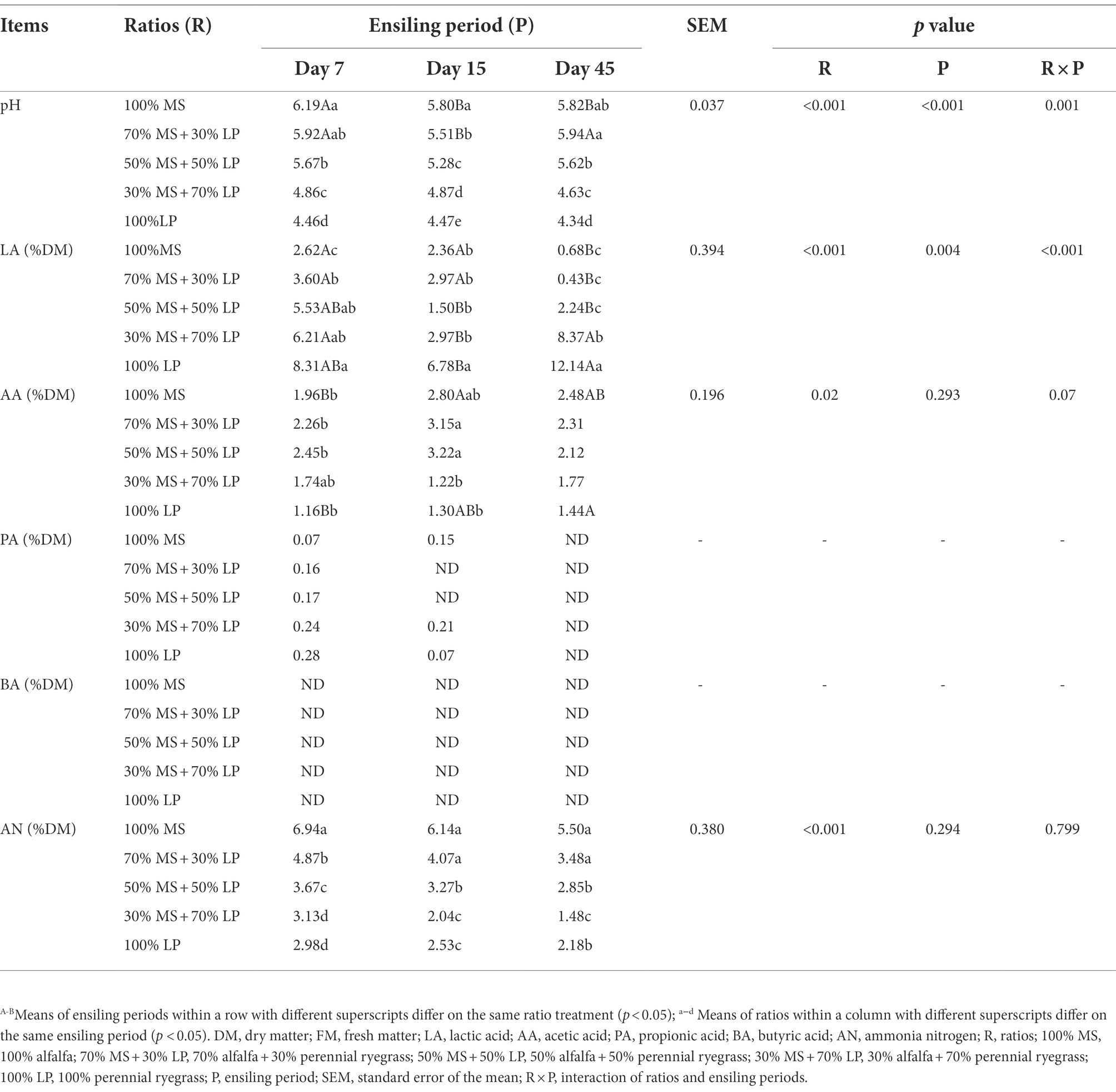
Table 3. Dynamic changes in the fermentation quality of perennial ryegrass and alfalfa mixed silage.
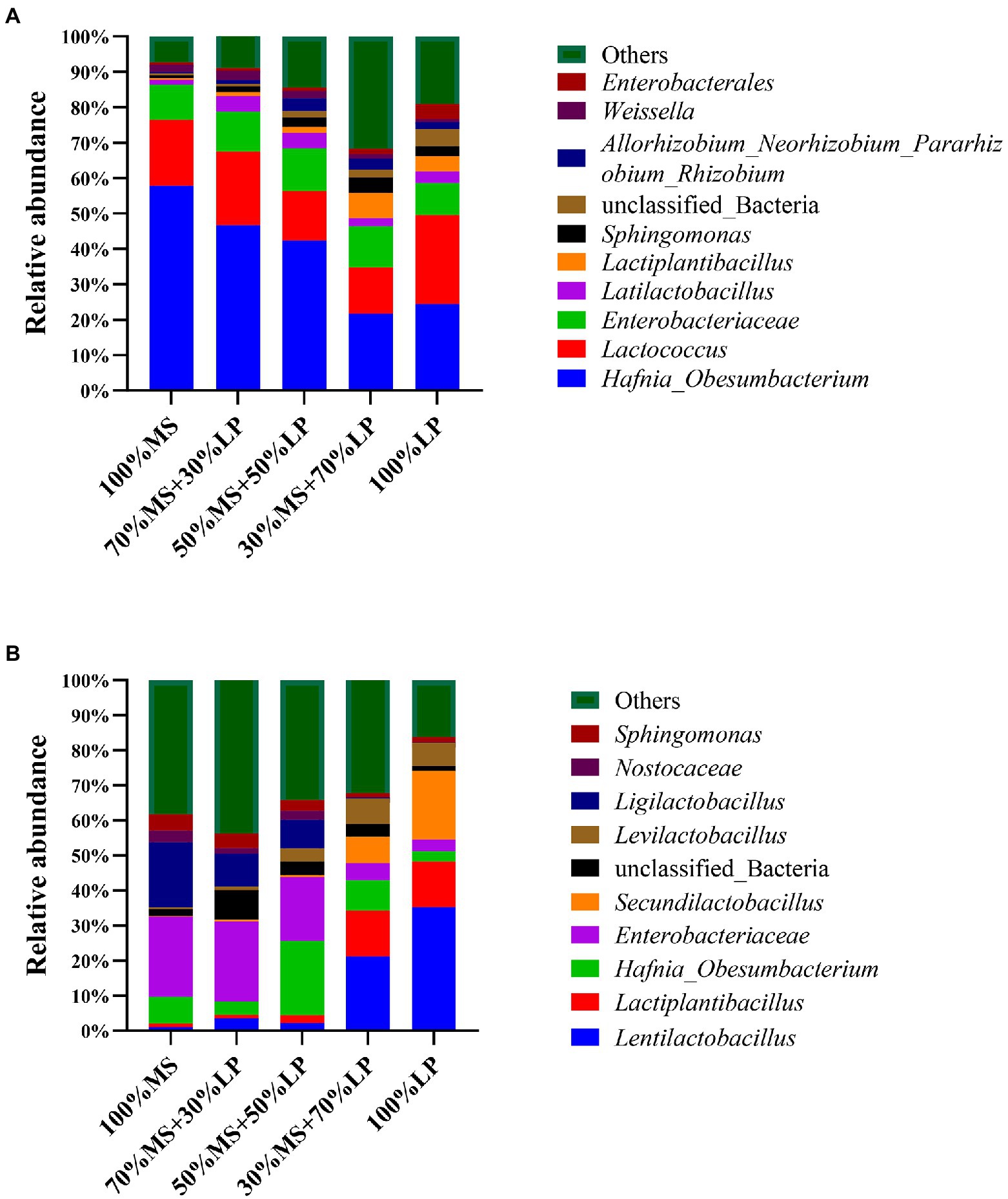
Figure 1. The relative abundance of bacterial communities at the genus level of alfalfa (MS) ensiled with perennial ryegrass (LP) silage for 7 (A) and 45 (B) days. 100% MS, 100% alfalfa; 70 MS + 30% LP, 70% alfalfa+30% perennial ryegrass; 50% MS + 50% LP, 50% alfalfa+50% perennial ryegrass; 30% MS + 70% LP, 30% alfalfa+70% perennial ryegrass; 100% LP, 100% perennial ryegrass.
Bacterial diversity and abundance during ensiling
The bacterial diversity of the silage mixtures is shown in Table 4. The inconsistent pattern of changes in alpha diversities (mainly the OTU, Ace, Chao, Shannon and Simpson indices) of silages with different percentages of LP added indicated that adding LP significantly affected the species and abundances of microbes in MS silage. The average good coverage for all samples was over 0.99, indicating that the sequencing process was adequate to characterize the dynamic change in the bacterial community (Cheng et al., 2022). In our study, the values of the OTU, Ace, Chao, Shannon and Simpson indices in 30% MS + 70% LP and 100% LP silages decreased sharply as the fermentation proceeded, which was in accordance with the results of Dong et al. (2020). This may be due to the presence of fresh material in an aerobic and neutral environment, which favors the reproduction of epiphytic aerobic microorganisms. In the early ensiling stage (7 days), the internal environment in silage had not yet reached a strictly anaerobic state. However, the acidic and anaerobic environments in silages were greatly developed after 45 days of ensiling, leading to a significant decrease in the bacterial diversity. This also suggests that the acidic and anaerobic environmental stresses had a significant effect on the reproduction and growth of microorganisms in silages, and this effect was more pronounced in the late ensiling stage.
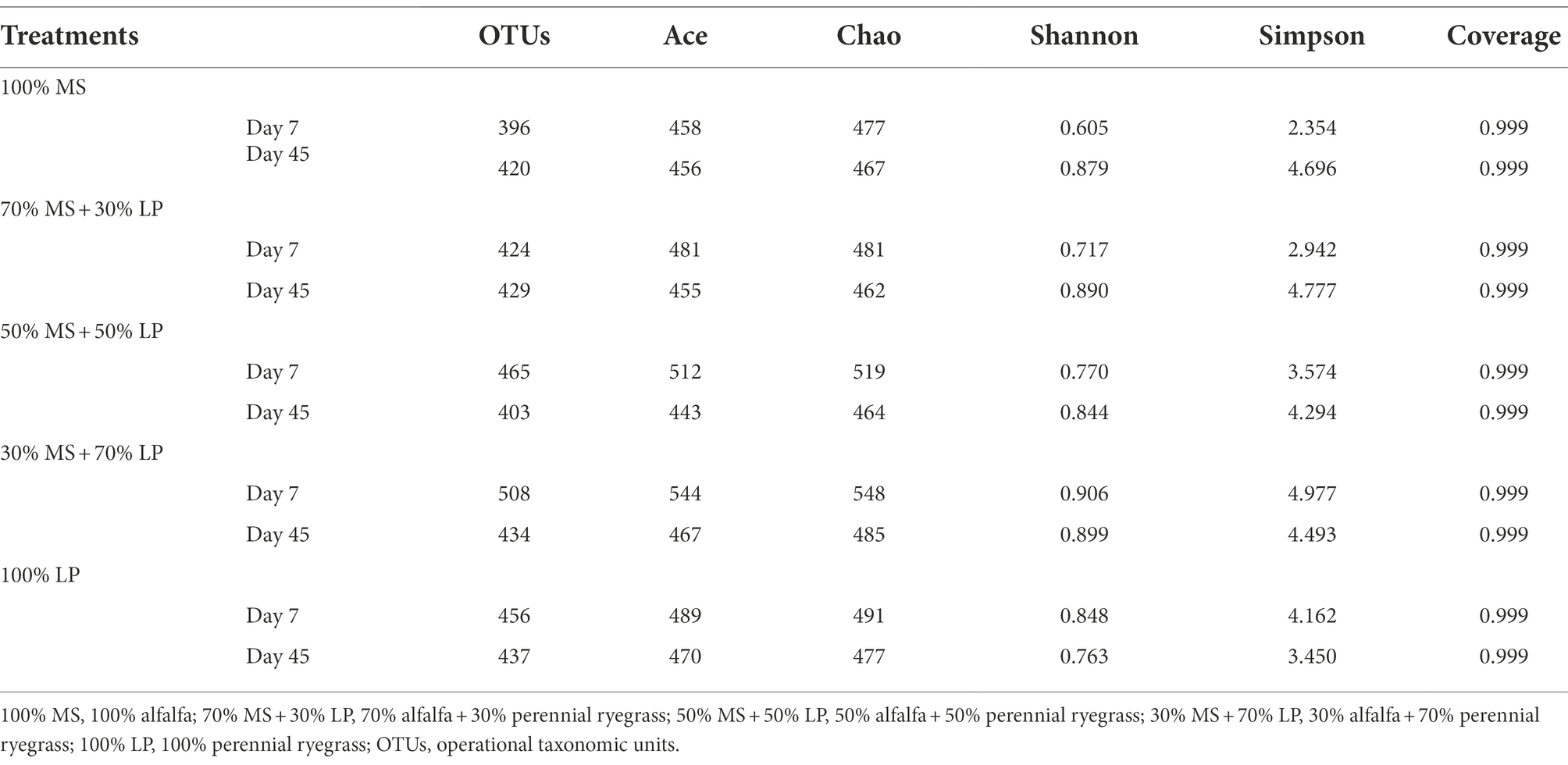
Table 4. Diversity and richness of the bacterial microbiota of alfalfa ensiled with perennial ryegrass.
Changes in the bacterial community composition during the fermentation process in silage mixtures at the genus level are shown in Figure 1A (7 days) and Figure 1B (45 days). In the early ensiling stage (7 days, Figure 1A), Hafnia_Obesumbacterium (21.64–57.75%) was the predominant genus in all silages, and the abundance of Enterobacteriaceae (9.10–12.21%) was relatively high. The abundance of harmful bacteria (Hafnia_Obesumbacterium and Enterobacteriaceae) in the 30% MS + 70% LP and 100% LP treatments was lower than that in the other treatments. According to a prior study, the presence of Enterobacter in silage increases the pH, which encourages the growth of other aerobic microorganisms (Zhang et al., 2015c). This was the main reason why the pH of the 30% MS + 70% LP and 100% LP treatments (< 4.9) was significantly lower than that of the other treatments (>5.6; Table 3). In the early ensiling stage, the main LAB genus was Lactococcus (13.11–25.21%), while the abundance of Latilactobacillus and Lactiplantibacillus was low. This was consistent with the study by Cai et al. (1998), who believed that lactic acid-producing cocci initiate lactic fermentation at the early ensiling stage, while lactic acid-rod is critical for pH reduction at the late ensiling stage. This also confirmed that the predominant LAB genus in the late ensiling stage was lactic acid-rod (including Lentilactobacillus, Ligilactobacillus, Lactiplantibacillus, Secundilactobacillus, and Levilactobacillus), and by this time, the Lactococcus had disappeared completely (Figure 1B).
In the late ensiling stage (45 days, Figure 1B), Enterobacteriaceae (21.13–35.26%) was the predominant genus in the 100% MS, 70% MS + 30% LP and 50% MS + 50% LP silages, and Lentilactobacillus (21.13–35.26%), Lactiplantibacillus (12.98–13.185%) and Secundilactobacillus (7.54–19.66%) were the predominant genera in the 30% MS + 70% LP and 100% LP silages. We speculated that the high number of Enterobacteriaceae and low number of LAB genera in 100% MS, 70% MS + 30% LP and 50% MS + 50% LP silages were responsible for the poor fermentation quality (Table 3). It is noteworthy that the abundance of Ligilactobacillus (18.59%) in alfalfa silage alone was higher than in other treatments, but this did not improve its fermentation quality. This result indicates that ensiling is a microbial-driven process, and the synergistic effect of various microorganisms is the key to improving the fermentation quality of silage (Ni et al., 2018). In our study, the abundance of harmful microorganisms (including Enterobacteriaceae and Sphingomonas) decreased and the abundance of beneficial microorganisms (including Lentilactobacillus, Lactiplantibacillus, Secundilactobacillus, and Levilactobacillus) increased as the proportion of LP in the mixed silage increased. These results indicated that adding LP could improve the fermentation quality of alfalfa silage by changing the microbial composition, and the 30% MS + 70% LP and 100% LP treatments provided the best effect.
In the present study, a Spearman correlation between fermentation parameters and the top 15 genera during silage ensiling was established, as illustrated in Figure 2A (7 days) and 2b (45 days). Abundant microorganisms helped to enhance the fermentation quality of silage, and numerous metabolites impacted the microbial process (Li et al., 2022a). In our study, as fermentation proceeded, Levilactobacillus and Lactiplantibacillus were positively correlated with the LA concentration but negatively correlated with the pH value, which further confirmed that these two genera exhibited strong resistance to acids; in addition, these genera played a crucial role in the decreasing pH during the late stage of ensiling (Yuan et al., 2019). In the early ensiling stage (7 days), Leuconostoc was positively correlated with the AA concentration and disappeared at the late stage of ensiling. This is because Leuconostoc is a heteromorphic LAB that can produce AA, is less acid-resistant than other bacteria and is replaced by other LAB (e.g., Loigolactobacillus, Lentilactobacillus, Paucilactobacillus, Secundilactobacillus, Ligilactobacillus, and Lacticaseibacillus) as the silage pH continues to decrease (Cai et al., 1998). In our study, some harmful microorganisms (including Falsirhodobacter, Pseudomonas, Rhodopseudomonas, Serratia, and Terrimicrobium) were positively correlated with the PA concentration, suggesting that these genera might be mainly responsible for the high level of PA in silages at the early ensiling stage.
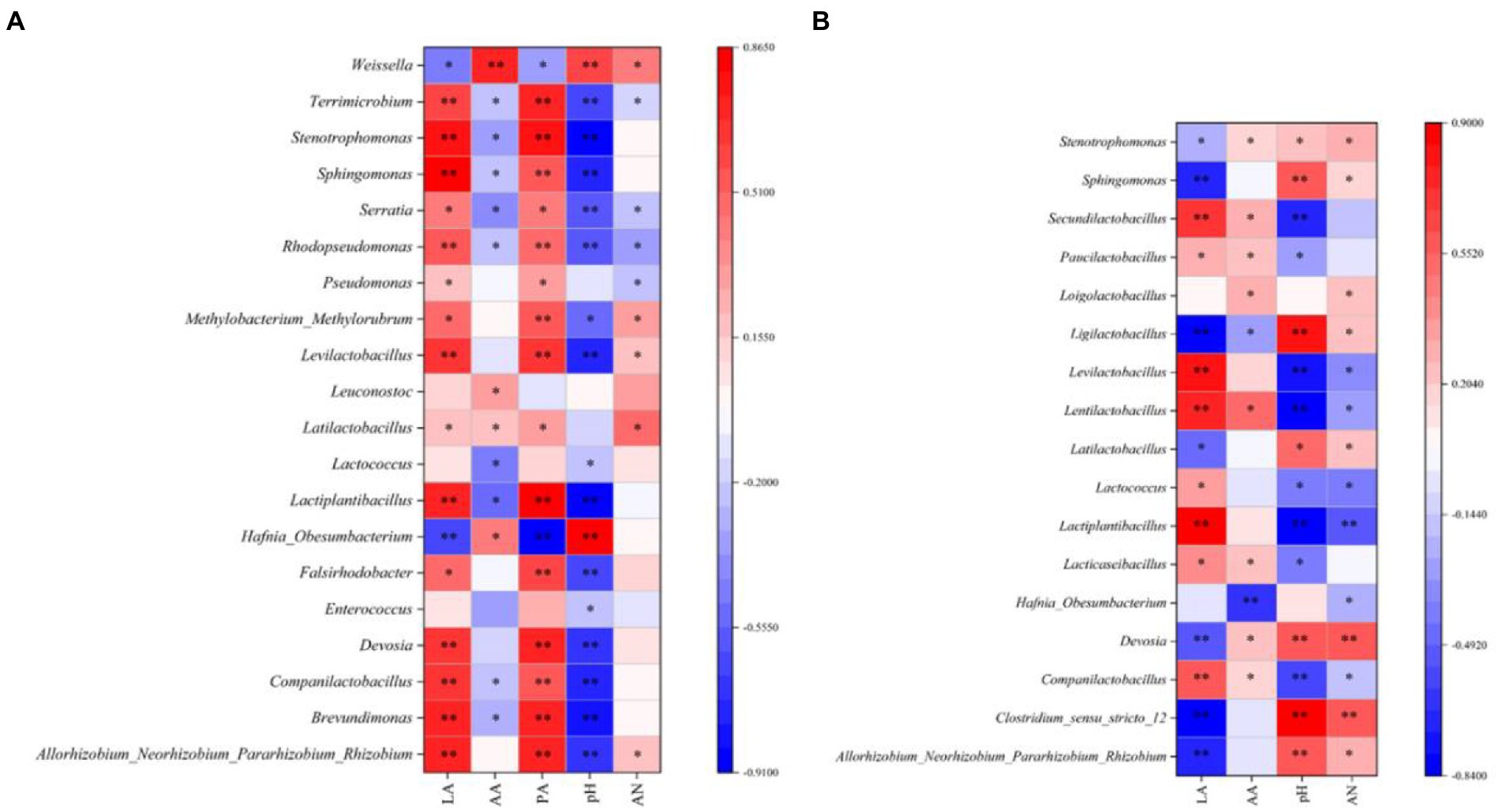
Figure 2. Spearman correlations between the silage fermentation parameters and the top 20 genera at 7 days (A) and 45 days (B). LA, lactic acid; AA, acetic acid; PA, propionic acid; AN, ammonia nitrogen; p values are shown as * 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01; 100% MS, 100% alfalfa; 70% MS + 30% LP, 70% alfalfa+30% perennial ryegrass; 50% MS + 50% LP, 50% alfalfa+50% perennial ryegrass; 30% MS + 70% LP, 30% alfalfa+70% perennial ryegrass; 100% LP, 100% perennial ryegrass.
Predicted functions of the microbial community in silages
Predicting the function profiles and metabolic pathways of the microbial community contributes to evaluating the influence of microbes on silage quality (Wang et al., 2020b). The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a bioinformatics resource that is used to understand the function and utility of cells and organisms from a high level and genomic perspective (Wang et al., 2020b). Therefore, the effect of adding LP on the metabolic pathways of the microbial community in MS silage was determined using a Tax4Fun-based KEGG pathway. The results of functional prediction for the microbial community of silages during ensiling are shown in Figure 3. Chemoheterotrophy was the primary functional component of the microbial community in all silages, followed by fermentation, nitrate reduction, aerobic chemoheterotrophy and nitrogen respiration, which was similar to the results of Li et al. (2022b), who found that chemoheterotrophs were the primary functional components of the bacterial population in paper mulberry silage, followed by fermenters. In the early ensiling stage (7 days, Figure 3A), the functions of chemoheterotrophy (34.56–36.05%) and fermentation (27.57–35.02%) of all silages showed little change. Interestingly, 30% MS + 70% LP and 100% LP silages had a weaker capacity for nitrate reduction than other silages, and as the fermentation time proceeded, the nitrate reduction ability of the late ensiling stage (45 days) was weaker than that of the early ensiling stage (7 days). Previous studies have found that some bacteria reduce nitrate to nitrite, ammonia or nitrogen through the function of nitrate reduction, which was the main reason why the AN content of the 30% MS + 70% LP and 100% LP treatments was lower than that of the other treatments and the AN content gradually decreased as the fermentation time proceeded (Table 3; Xiang et al., 2022). In the late ensiling stage (7 days, Figure 3B), the 30% MS + 70% LP and 100% LP silages had a stronger capacity for fermentation than the other silages. This was because the 30% MS + 70% LP and 100% LP treatments showed increased abundances of LAB, such as Lentilactobacillus, Lactiplantibacillus, and Secundilactobacillus, and decreased abundances of Enterobacteriaceae (Figure 1B); the degree of fermentation was greater in silage (Li et al., 2022b). Furthermore, the 30% MS + 70% LP and 100% LP treatments had a weaker capacity for chloroplasts, animal parasites or symbionts and ureolysis than the other treatments. This may be because the abundance of beneficial microorganisms in the 30% MS + 70% LP and 100% LP treatments was higher than that in the other treatments, and the fermentation capacity was stronger, resulting in a low pH environment, which inhibited the activity of other harmful microorganisms (Ni et al., 2018).
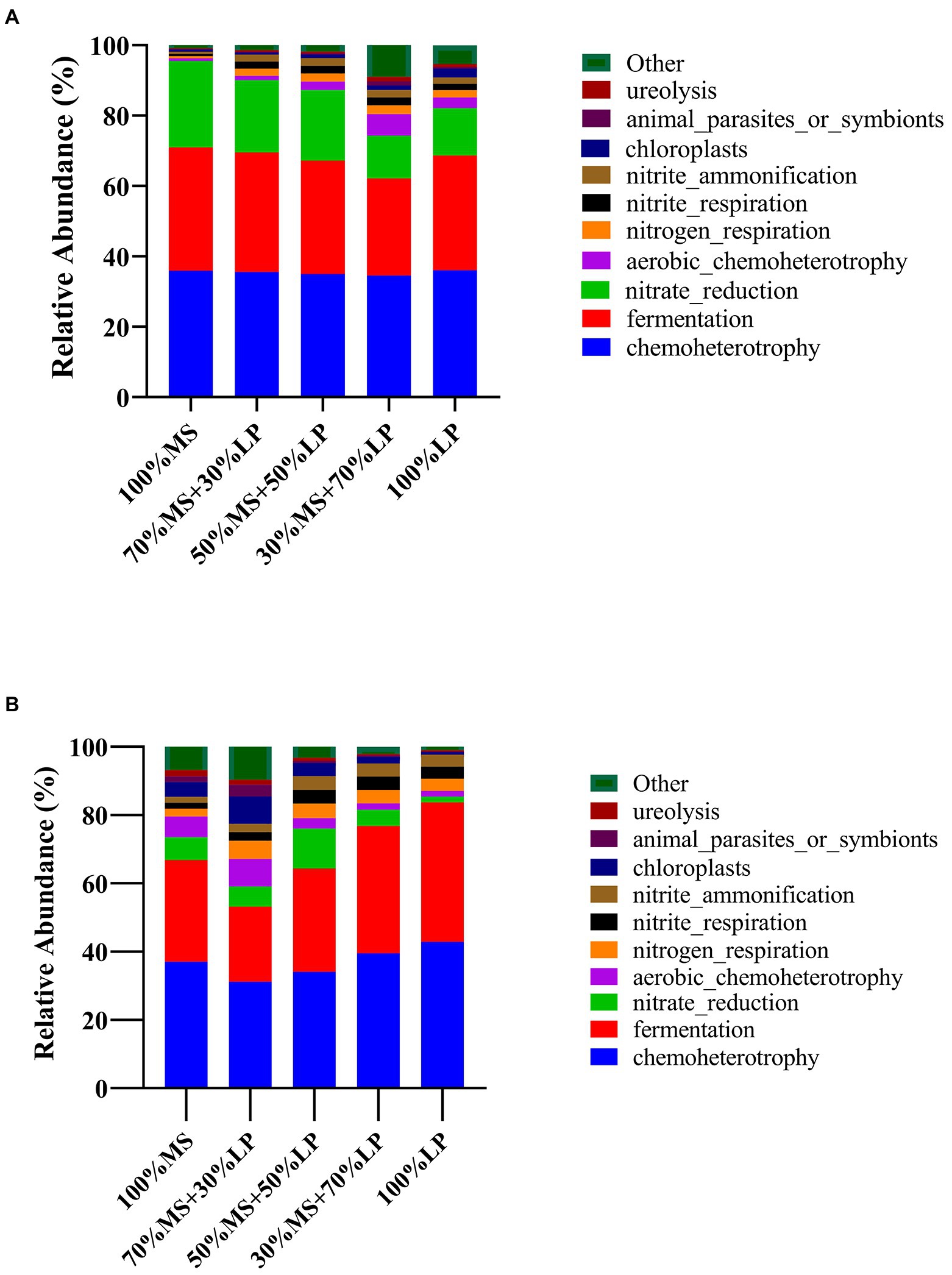
Figure 3. Dynamics of predicted microbial functional profiles of the relative abundances of the top 10 functions in alfalfa (MS) ensiled with perennial ryegrass (LP) silage for 7 (A) and 45 (B) days analyzed by Tax4Fun. 100% MS, 100% alfalfa; 70% MS + 30% LP, 70% alfalfa+30% perennial ryegrass; 50% MS + 50% LP, 50% alfalfa+50% perennial ryegrass; 30% MS + 70% LP, 30% alfalfa+70% perennial ryegrass; 100% LP, 100% perennial ryegrass.
The results of the metabolic pathways of the microbial community of silages ensiling for 45 days are shown in Figure 4. As shown in Figure 4A, a total of six different metabolic pathways were observed in all the silages. The main predicted genes were assigned to metabolism, accounting for >76% in all the silages, followed by environmental information processing, genetic information processing and cellular processes, which is in accordance with a previous report (Hussain et al., 2021). After 7 days of ensiling, the 30% MS + 70% LP and 100% LP treatments had lower abundances of “Environmental information processing,” “Cellular processes” and “Human diseases” and higher abundances of “Metabolism,” “Genetic information processing” and “Organismal systems” than the other treatments. Based on the fermentation quality of the different treatments, the main reason for the better fermentation quality of the 30% MS + 70% LP and 100% LP treatments was the altered cellular characteristics, inhibition of the membrane transport and signal transduction of undesirable bacteria, and acceleration of the proliferation rate and metabolic level of beneficial bacteria (such as LAB strains; Wang et al., 2020b). After 60 days of ensiling, a low variation in metabolic pathways (except “Organismal systems”) at the first pathway level was found among the different treatments. This may be due to the fact that a stable internal environment had developed during the final stage of fermentation (Wang et al., 2020b). The carbohydrate metabolism mainly contained gluconeogenesis and glycolysis metabolism (Kanehisa and Goto, 2000). In our study (Figure 4B), as the fermentation proceeded, the changes in the abundance of “Carbohydrate metabolism” were not significantly different among all the treatments. This confirmed our results (Table 2), and there was little difference in WSC content among the treatments during fermentation. After 7 and 45 days of ensiling, the 30% MS + 70% LP and 100% LP treatments had lower abundances of “Membrane transport” and “Signal transduction” than the other treatments. This is consistent with the results of Keshri et al. (2018), who observed a higher abundance of transporters in the silage with poor fermentation quality, which may be related to the symbiotic relationship in the bacterial community. Furthermore, as the fermentation proceeded, the 30% MS + 70% LP and 100% LP treatments had higher abundances of “Nucleotide metabolism” and “Replication and repair” than the other treatments. According to Kilstrup et al. (2005), nucleotides may be used to synthesize and replicate RNA and DNA as well as serve as the primary source of energy for cellular processes. This demonstrated that LAB strains in the 30% MS + 70% LP and 100% LP silages multiplied rapidly during the early stage of the ensiling, which was in accordance with the highest abundances of LAB (including Lentilactobacillus, Lactiplantibacillus, Secundilactobacillus, and Levilactobacillus) in the 30% MS + 70% LP and 100% LP silages (Figure 1B). Amino acids, as essential substances in plants, are essential to promote primary metabolism and plant protein synthesis. In this study, as the fermentation proceeded, “amino acid metabolism” in the 30% MS + 70% LP and 100% LP treatments was promoted, while it was inhibited in the other treatments. Previous studies have found that the level of amino acid metabolism might reflect the ability of bacterial populations in silage to de novo synthesize amino acids (Keshri et al., 2018). Therefore, the observed dynamics of amino acid metabolism in silage may reflect the metabolism of the dominant populations throughout the ensiling process. The top 6 metabolic pathways at level 3 are shown in Figure 4C, and “Metabolic pathways” and “Biosynthesis of secondary metabolites” were the critical metabolic pathways at level 3. Interestingly, as the fermentation proceeded, the 30% MS + 70% LP and 100% LP treatments had higher abundances of “Metabolic pathways,” “Biosynthesis of secondary metabolites,” “Biosynthesis of antibiotics” and “Purine metabolism” and lower abundances of “ABC transporters” and “Two-component system” than the other treatments. This indicated that the 30% MS + 70% LP and 100% LP treatments could inhibit the transport of harmful bacteria in the membrane system by promoting the synthesis of metabolites in the silages for LAB strain propagation and reducing the pH of the silages (Wang et al., 2020b).
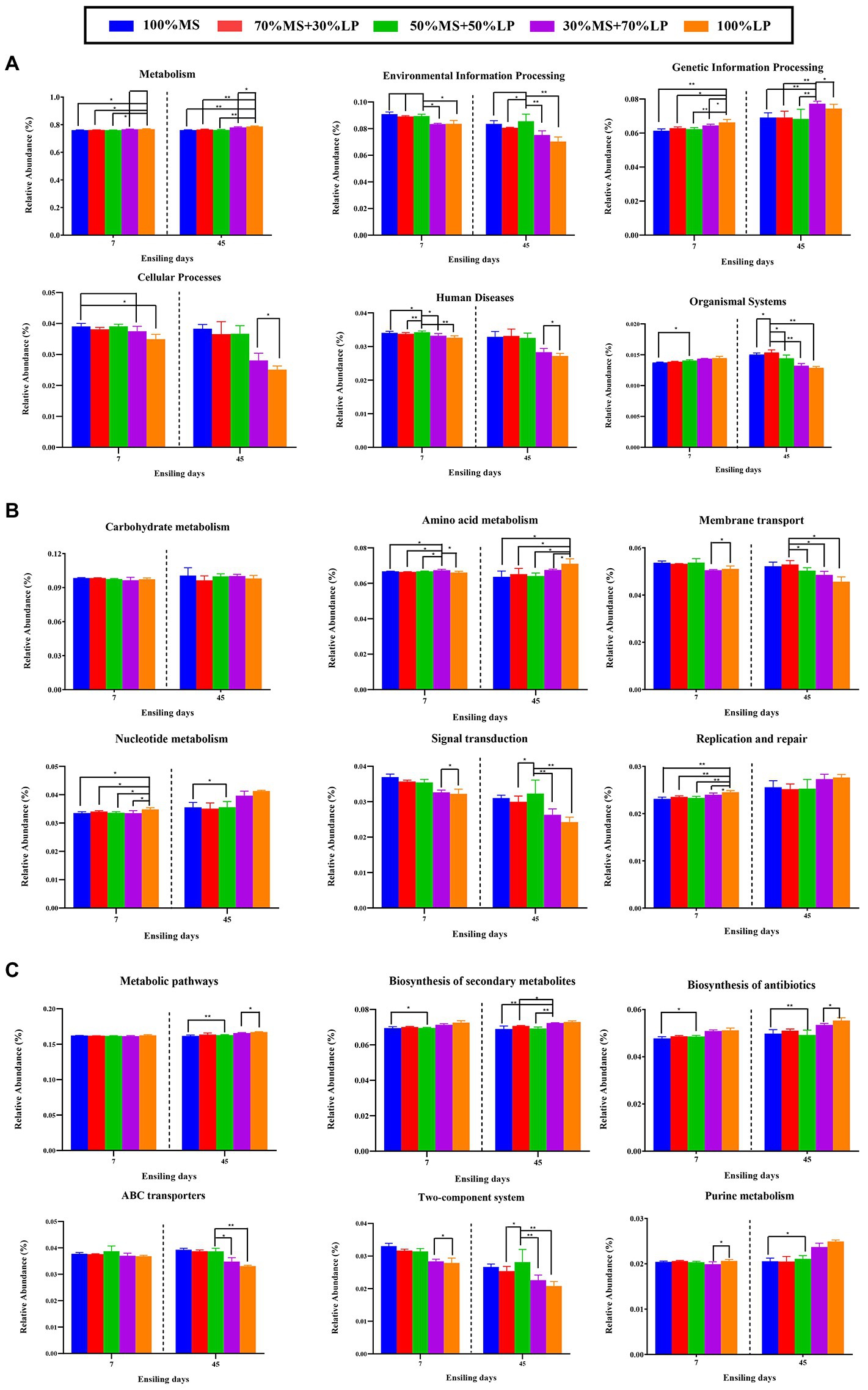
Figure 4. Bar graphs showing 16S rRNA gene-predicted functional profiles on the level 1 metabolic pathways (A), level 2 metabolic pathways (B) and level 3 metabolic pathways (C) of silages for 45 days obtained with Tax4Fun. 100% MS, 100% alfalfa; 70% MS + 30% LP, 70% alfalfa+30% perennial ryegrass; 50% MS + 50% LP, 50% alfalfa+50% perennial ryegrass; 30% MS + 70% LP, 30% alfalfa+70% perennial ryegrass; 100% LP, 100% perennial ryegrass. ∗, 0.01 < p < 0.05; ∗∗, 0.001 < p < 0.01.
Conclusion
In this study, we showed that the addition of perennial ryegrass with high WSC could affect the fermentation quality and microbial community of alfalfa silage by different metabolic pathways. Adding perennial ryegrass significantly improved the fermentation quality of alfalfa silage by increasing the abundance of beneficial microorganisms (e.g., Lentilactobacillus, Lactiplantibacillus, and Secundilactobacillus) and decreasing the abundance of harmful microorganisms (e.g., Enterobacteriaceae) in silages. By combining nutritional quality, fermentation quality and microbial community analyses, it was determined that ensiling 30% alfalfa with 70% ryegrass can result in high-quality silage in the karst region.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI project PRJNA778048 with accession number SRP344966.
Author contributions
ZX, QC, YJ, CC, and ZW designed the experiments and revised the manuscript. ML, JL, XF, and YL performed the experiments. QC, XF, and YC wrote the manuscript. ZX, ZW, and QC carried out the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Subject (2021YFD1300302), Guizhou University Introduced Talents Scientific Research Project [Guida Renji Hezi (2020)71], Guizhou University Cultivation Project [Guida Renji Hezi (2020)9], and Jilin Province Science and Technology Development Plan Project (20190303075SF).
Acknowledgments
We thank Biomarker Technologies Company (Beijing, China) for providing technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Cai, Y., Benno, Y., Ogawa, M., and Kumai, S. (1999). Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 82, 520–526. doi: 10.3168/jds.S0022-0302(99)75263-X
Cai, Y., Benno, Y., Ogawa, M., Ohmomo, S., Kumai, S., and Nakase, T. (1998). Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 64, 2982–2987. doi: 10.1128/AEM.64.8.2982-2987.1998
Chen, L., Guo, G., Yuan, X. J., Zhang, J., Wen, A. Y., Sun, X. H., et al. (2017). Effect of ensiling whole crop oat with lucerne in different ratios on fermentation quality, aerobic stability and in vitro digestibility on the Tibetan plateau. J. Anim. Physiol. An. N. 101, e144–e153. doi: 10.1111/jpn.12577
Cheng, Q., Chen, Y., Bai, S., Chen, L., You, M., Zhang, K., et al. (2021). Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim. Sci. J. 92:e13656. doi: 10.1111/asj.13656
Cheng, Q., Li, M., Fan, X., Chen, Y., Sun, H., Xie, Y., et al. (2022). Effects of epiphytic and exogenous lactic acid bacteria on fermentation quality and microbial community compositions of paper mulberry silage. Front. Microbiol. 13:973500. doi: 10.3389/fmicb.2022.973500
Dong, M. Y., Li, Q. Q., Xu, F. Q., Wang, S. Y., Chen, J. H., and Li, W. J. (2020). Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet sorghum bagasse silage. Sci. Rep. 10:837. doi: 10.1038/s41598-020-57628-0
Dong, D., Xu, G., Dai, T., Zong, C., Yin, X., Bao, Y., et al. (2022). Effect of molasses on fermentation quality of wheat straw ensiled with perennial ryegrass. Anim. Prod. Sci. 62, 1471–1479. doi: 10.1071/AN22047
Hussain, B., Chen, J. S., Hsu, B. M., Chu, I. T., Koner, S., Chen, T. H., et al. (2021). Deciphering bacterial community structure, functional prediction and food safety assessment in fermented fruits using next-generation 16S rRNA amplicon sequencing. Microorganisms 9:1574. doi: 10.3390/microorganisms9081574
Jia, T., Sun, Z., Gao, R., and Yu, Z. (2019). Lactic acid bacterial inoculant effects on the vitamin content of alfalfa and Chinese leymus silage. Asian. Austral. J. Anim. 32, 1873–1881. doi: 10.5713/ajas.19.0135
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Keshri, J., Chen, Y., Pinto, R., Kroupitski, Y., Weinberg, Z. G., and Sela Saldinger, S. (2018). Microbiome dynamics during ensiling of corn with and without lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 102, 4025–4037. doi: 10.1007/s00253-018-8903-y
Kilstrup, M., Hammer, K., Jensen, P. R., and Martinussen, J. (2005). Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol. Rev. 29, 555–590. doi: 10.1016/j.femsre.2005.04.006
Li, M., Fan, X., Cheng, Q., Chen, Y., Long, J., Lei, Y., et al. (2022b). Effect of Amomum villosum essential oil as an additive on the chemical composition, fermentation quality, and bacterial community of paper mulberry silage. Front. Microbiol. 13:951958. doi: 10.3389/fmicb.2022.951958
Li, P., Lu, Y., Zhao, M., Chen, L., Zhang, C., Cheng, Q., et al. (2021). Effects of phenyllactic acid, lactic acid bacteria, and their mixture on fermentation characteristics and microbial community composition of timothy silage. Front. Microbiol. 12:743433. doi: 10.3389/fmicb.2021.743433
Li, P., Zhang, Y., Gou, W. L., Cheng, Q. M., Bai, S. Q., and Cai, Y. M. (2019). Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 247, 285–293. doi: 10.1016/j.anifeedsci.2018.11.009
Li, P., Zhao, W., Yan, L., Chen, L., Chen, Y., Gou, W., et al. (2022a). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 347:126347. doi: 10.1016/j.biortech.2021.126347
Luo, R., Zhang, Y., Wang, F., Liu, K., Huang, G., Zheng, N., et al. (2021). Effects of sugar cane molasses addition on the fermentation quality, microbial community, and tastes of alfalfa silage. Animals 11:355. doi: 10.3390/ani11020355
McDonald, P., Edwards, R. A., Greenhalgh, J. F. D., and Morgan, C. A. (2002). Animal Nutrition, 6th ed. John Willey & Sons, New York.
Ni, K. K., Zhao, J. Y., Zhu, B. G., Su, R. N., Pan, Y., Ma, J. K., et al. (2018). Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 265, 563–567. doi: 10.1016/j.biortech.2018.05.097
NRC (2007). Nutrient requirements of small ruminants: Sheep, goats, Cervids and New World camelids. National Research Council, National Academies Press, Washington, DC, USA.
Oladosu, Y., Rafii, M. Y., Abdullah, N., Magaji, U., Hussin, G., Ramli, A., et al. (2016). Fermentation quality and additives: a case of rice straw silage. Biomed. Res. Int. 2016:14. doi: 10.1155/2016/7985167
Owens, V. N., Albrecht, K. A., Muck, R. E., and Duke, S. H. (1999). Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total nonstructural carbohydrates. Crop Sci. 39, 1873–1880. doi: 10.2135/cropsci1999.3961873x
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S., and Spoelstra, S. F. (2003). “Microbiology of ensiling” in Silage science and technology. eds. D. R. Buxton, R. E. Muck, and J. H. Harrison (Wisconsin: American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.), 31–93.
Romero, F. (2010). “Revista Veterinaria” in Proceedings of the 9th World Buffalo Congress, Buenos Aires, Argentina. vol.21, pp. 679–681. (Argentina: Facultad de Ciencias Veterinarias de la Universidad Nacional del Nordeste (UNNE)). Available at: http://www.vet.unne.edu.ar/revista/21 (Accessed April 25-28, 2010).
Seale, D. R., Henderson, A. R., Pettersson, K. O., and Lowe, J. F. (1986). The effect of addition of sugar and inoculation with two commercial inoculants on the fermentation of lucerne silage in laboratory silos. Grass Forage Sci. 41, 61–70. doi: 10.1111/j.1365-2494.1986.tb01793.x
Su, Y., Huang, Y., Dong, X., Wang, R., Tang, M., Cai, J., et al. (2021). Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Sci. 12:664519. doi: 10.3389/fpls.2021.664519
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, M., Franco, M., Cai, Y., and Yu, Z. (2020a). Dynamics of fermentation profile and bacterial community of silage prepared with alfalfa, whole-plant corn and their mixture. Anim. Feed Sci. Technol. 270:114702. doi: 10.1016/j.anifeedsci.2020.114702
Wang, C., He, L. W., Xing, Y. Q., Zhou, W., Yang, F. Y., Chen, X. Y., et al. (2019). Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 284, 240–247. doi: 10.1016/j.biortech.2019.03.129
Wang, S., Li, J., Zhao, J., Dong, Z., Dong, D., and Shao, T. (2022). Effect of epiphytic microbiota from napiergrass and Sudan grass on fermentation characteristics and bacterial community in oat silage. J. Appl. Microbiol. 132, 919–932. doi: 10.1111/jam.15293
Wang, S., Yuan, X., Dong, Z., Li, J., and Shao, T. (2017). Effect of ensiling corn Stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the tibetan plateau. Grassl. Sci. 63, 236–244. doi: 10.1111/grs.12173
Wang, S., Zhao, J., Dong, Z., Li, J., and Shao, T. (2020b). Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 309:123371. doi: 10.1016/j.biortech.2020.123371
Woolford, M. K. (1975). The significance of Propionibacterium spp. and Micrococcus lactilyticus to the ensiling process. J. Appl. Bact. 39, 301–306. doi: 10.1111/j.1365-2672.1975.tb00576.x
Xiang, Q., Zhang, J., Huang, X., Ma, M., Zhao, K., Yu, X., et al. (2022). Changes in the taxonomic and functional structures of microbial communities during vegetable waste mixed silage fermentation. Can. J. Microbiol. 68, 281–293. doi: 10.1139/cjm-2021-0059
Ying, B., Xiong, K., Wang, Q., and Wu, Q. (2021). Can agricultural biomass energy provide an alternative energy source for karst rocky desertification areas in southwestern China? Investigating Guizhou Province as example. Environ. Sci. Pollut. R. 28, 44315–44331. doi: 10.1007/s11356-021-12537-1
Yuan, X., Dong, Z., Li, J., and Shao, T. (2019). Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 75, 37–44. doi: 10.1111/gfs.12455
Yuan, X., Li, J., Dong, Z., and Shao, T. (2020). The reconstitution mechanism of napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 297:122391. doi: 10.1016/j.biortech.2019.122391
Yuan, L., Yue, K., Gu, Z., Chen, H., and Chi, Y. (2021). Analysis of rainfall factors and soil erosion in different soil and water conservation measures in the karst Plateau-Mountain. Pol. J. Environ. Stud. 30, 5343–5349. doi: 10.15244/pjoes/135824
Zhang, S. J., Chaudhry, A. S., Osman, A., Shi, C. Q., Edwards, G. R., Dewhurst, R. J., et al. (2015b). Associative effects of ensiling mixtures of sweet sorghum and alfalfa on nutritive value, fermentation and methane characteristics. Anim. Feed Sci. Technol. 206, 29–38. doi: 10.1016/j.anifeedsci.2015.05.006
Zhang, J., Guo, G., Chen, L., Li, J., Yuan, X., Yu, C., et al. (2015a). Effect of applying lactic acid bacteria and propionic acid on fermentation quality and aerobic stability of oats-common vetch mixed silage on the Tibetan plateau. Anim. Sci. J. 86, 595–602. doi: 10.1111/asj.12340
Zhang, Q., Li, X., Zhao, M., and Yu, Z. (2015c). Lactic bacteria for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 71, 472–481. doi: 10.1111/gfs.12190
Zhang, Q., Zhao, M., Wang, X., Yu, Z., and Na, R. (2017). Ensiling alfalfa with whole crop corn improves the silage quality and in vitro digestibility of the silage mixtures. Grassl. Sci. 63, 211–217. doi: 10.1111/grs.12168
Zhang, Y., Zhou, J. W., Guo, X. S., Cui, G. X., Ding, L. M., Wang, H. C., et al. (2012). Influences of dietary nitrogen and non-fiber carbohydrate levels on apparent digestibility, rumen fermentation and nitrogen utilization in growing yaks fed low quality forage based-diet. Livest. Sci. 147, 139–147. doi: 10.1016/j.livsci.2012.04.013
Keywords: alfalfa, perennial ryegrass, fermentation quality, bacterial community, predicted functional, karst region
Citation: Fan X, Xie Z, Cheng Q, Li M, Long J, Lei Y, Jia Y, Chen Y, Chen C and Wang Z (2022) Fermentation quality, bacterial community, and predicted functional profiles in silage prepared with alfalfa, perennial ryegrass and their mixture in the karst region. Front. Microbiol. 13:1062515. doi: 10.3389/fmicb.2022.1062515
Edited by:
Siran Wang, Nanjing Agricultural University, ChinaReviewed by:
Mao Li, Chinese Academy of Tropical Agricultural Sciences, ChinaJie Zhao, Nanjing Agricultural University, China
Copyright © 2022 Fan, Xie, Cheng, Li, Long, Lei, Jia, Chen, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiming Cheng, 429845801@qq.com; Zhijun Wang, zhijunwang321@126.com
†These authors have contributed equally to this work
 Xueying Fan
Xueying Fan Zhiming Xie2†
Zhiming Xie2† Qiming Cheng
Qiming Cheng Maoya Li
Maoya Li Yushan Jia
Yushan Jia