- 1Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Graduate School, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Clinical Laboratory, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 4School of Biomedical Sciences, Charles Sturt University, Orange, NSW, Australia
- 5NSW Health Pathology, Regional and Rural, Orange Hospital, Orange, NSW, Australia
Objective: The objective of the study was to investigate the antimicrobial susceptibility and extended-spectrum beta-lactamase (ESBL) positive rates of Escherichia coli from community-acquired urinary tract infections (CA-UTIs) in Chinese hospitals.
Materials and Methods: A total of 809 E. coli isolates from CA-UTIs in 10 hospitals (5 tertiary and 5 secondary hospitals) from different regions in China were collected during the period 2016–2017 according to the strict inclusion criteria. Antimicrobial susceptibility testing was carried out by standard broth microdilution method. Isolates were categorized as ESBL-positive, ESBL-negative, and ESBL-uncertain groups according to the CLSI recommended phenotypic screening method. ESBL and AmpC genes were amplified and sequenced on ESBL-positive and ESBL-uncertain isolates.
Results: The antimicrobial agents with susceptibility rates of greater than 95% included imipenem (99.9%), colistin (99.6%), ertapenem (98.9%), amikacin (98.3%), cefmetazole (97.9%), nitrofurantoin (96%), and fosfomycin (95.4%). However, susceptibilities to cephalosporins (varying from 58.6% to 74.9%) and levofloxacin (48.8%) were relatively low. In the phenotypic detection of ESBLs, ESBL-positive isolates made up 38.07% of E. coli strains isolated from CA-UTIs, while 2.97% were ESBL-uncertain. Antimicrobial susceptibilities of imipenem, cefmetazole, colistin, ertapenem, amikacin, and nitrofurantoin against ESBL-producing E. coli strains were greater than 90%. The percentage of ESBL-producing strains was higher in male (53.6%) than in female patients (35.2%) (p < 0.001). CTX-M-14 (31.8%) was the major CTX-M variant in the ESBL-producing E. coli, followed by CTX-M-55 (23.4%), CTX-M-15 (17.5%), and CTX-M-27 (13.3%). The prevalence of carbapenem-resistant E. coli among CA-UTI isolates was 0.25% (2/809).
Conclusion: Our study indicated high prevalence of ESBL in E. coli strains from strictly defined community-acquired urinary tract infections in adults in China. Imipenem, colistin, ertapenem, amikacin, and nitrofurantoin were the most active antimicrobials against ESBL-positive E. coli isolates. blaCTX–M–14 is the predominant esbl gene in ESBL-producing and ESBL-uncertain strains. Our study indicated that the use of cephalosporins and fluoroquinolone needs to be restricted for empirical treatment of CA-UTIs in China.
Introduction
Escherichia coli (E. coli) is a common pathogen of community-acquired infections such as intra-abdominal infection, urinary tract infection (UTI), and pelvic inflammatory disease. UTI is one of the most common bacterial infectious diseases of humans. Over 85% of community-acquired urinary tract infections (CA-UTIs) have been attributed to E. coli infection (Espínola et al., 2011). Increasing trend in extended-spectrum beta-lactamase (ESBL) rates was seen among isolates from CA-UTIs in many regions; the antimicrobial resistance surveillance program in China showed that the proportion of ESBL-producing E. coli in community-acquired infections ranges from 45.2 to 68.2% (Yun et al., 2014; Fupin et al., 2017), in Canada, from 9.1 to 14.1%, and in the United States, from 6.5% in 2010 to 16.0% in 2014 (Lob et al., 2016). In some European countries, the prevalence of ESBL-producing E. coli isolated from CA-UTIs is currently lower than 5% (van Driel et al., 2019; Richelsen et al., 2020; Larramendy et al., 2021), but can reach up to 23.6% in Spanish, 38.2% in Turkey, and 34.6% in Iran (Arana et al., 2017; Koksal et al., 2017; Naziri et al., 2020). Most of the discrepancy in ESBL prevalence rates between studies might be related to geographical difference. However, the inclusion and exclusion criteria of isolates is also an important factor, which may over- or underestimate the ESBL rates or antimicrobial resistance in community-acquired infections. Studying the antimicrobial resistance patterns of E. coli in real community-acquired infections is important not only for understanding the resistance status but also for choosing the most appropriate empirical antimicrobial therapy for CA-UTIs (Lob et al., 2015). The real ESBL rate and molecular epidemiology in CA-UTIs in China is unclear.
The aim of this study was to investigate the real ESBL status and antimicrobial susceptibility of E. coli isolates strictly collected from CA-UTIs in China.
Materials and Methods
Clinical Isolates
During the period 2016–2017, a total of 809 E. coli isolates from CA-UTIs were consecutively collected from 10 hospitals located in the following regions of China: northeastern (121 isolates), northern (179 isolates), central (172 isolates), western (159 isolates), and eastern (178 isolates). The specific geographical distribution is shown in Figure 1. The 10 hospitals included 5 tertiary hospitals (Peking Union Medical College Hospital; Sichuan Provincial People’s Hospital; Tongji Hospital, Tongji Medical College Huazhong University of Science and Technology; The First Affiliated Hospital, College of Medicine, Zhejiang University; and Shengjing Hospital of China Medical University) and 5 secondary hospitals located in the same provinces as the 5 tertiary hospitals (Beijing Pinggu Hospital; Sichuan Science City Hospital; Zaoyang First People’s Hospital; Zhuji People’s Hospital of Zhejiang Province; and Dalian Hospital, Shengjing Hospital of China Medical University).
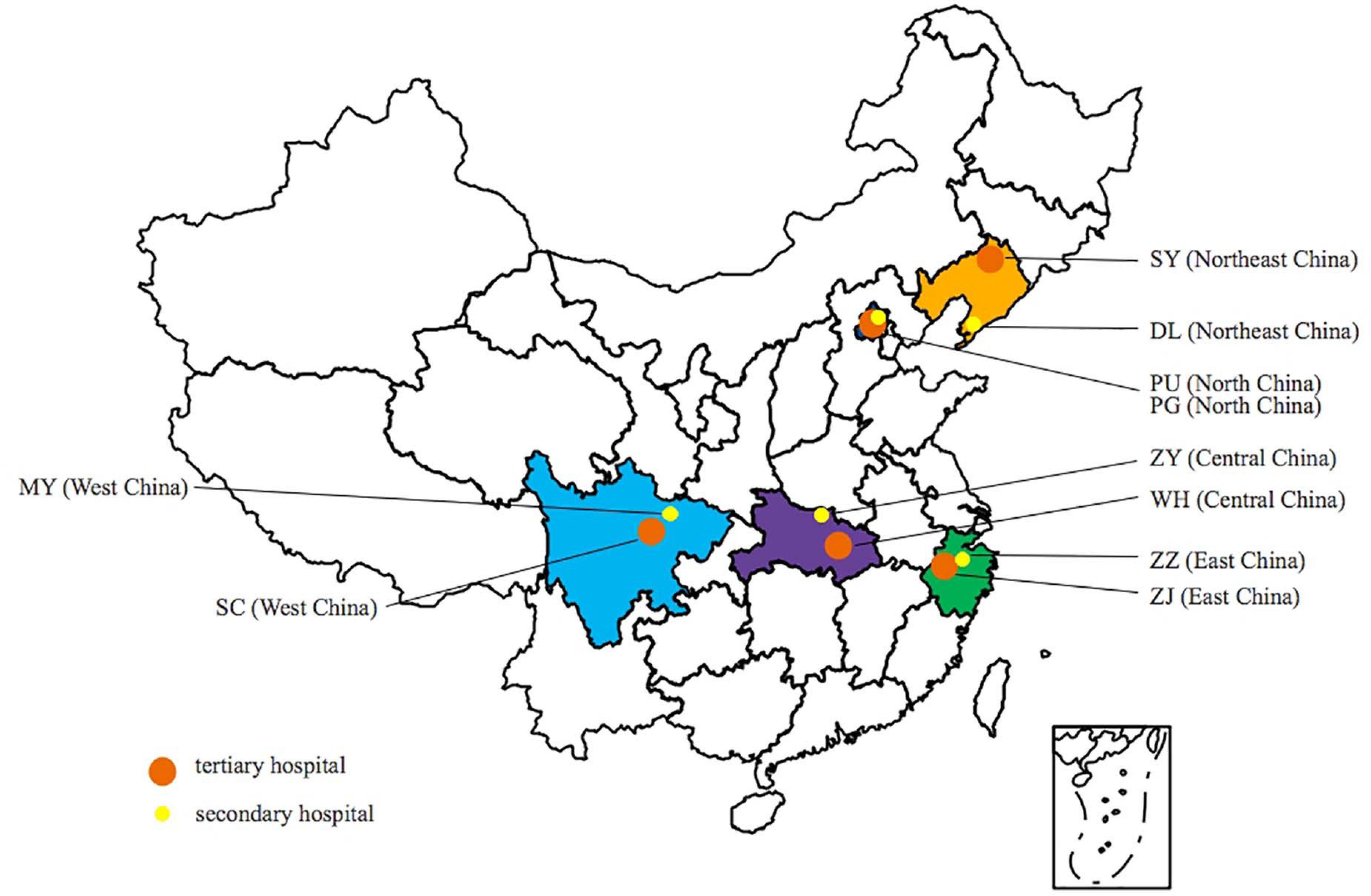
Figure 1. Geographical distribution of hospitals participating in the study. PU, Peking Union Medical College Hospital; PG, Beijing Pinggu Hospital; SY, Shengjing Hospital of China Medical University; DL, Dalian Hospital; ZZ, Zhuji People’s Hospital of Zhejiang Province; ZJ, The First Affiliated Hospital College of Medical Zhejiang University; WH, Tongji Hospital; ZY, First Peoples Hospital of Zaoyang; SC, Sichuan Provincial People’s Hospital; and MY, Sichuan Science City Hospital.
Isolates were strictly chosen by using the following inclusion and exclusion criteria to ensure all cases were community-acquired patients.
Inclusion Criteria
(1) All E. coli isolates were cultured from urines of adult UTI patients (>18 years old) from outpatient clinic/emergency department or admitted to a hospital in less than 48 h. (2) Isolates were cultured from uncomplicated UTI, such as acute cystitis, acute pyelonephritis, with evidence support of clinical symptoms, urine routine test, and/or imaging examination. (3) Isolates were infection-related pathogens cultured from qualified urine specimens with bacteria quantification of > 105 CFU/ml.
Exclusion Criteria
Isolates from patients with the following conditions were excluded; (1) patients with invasive devices (such as various venous catheters, urethral catheters, intubations, and artificial implants, etc.); (2) immunocompromised patients (e.g., patients who had received glucocorticoid, radiotherapy, and chemotherapy within 6 months); (3) patients with a history of surgery, hemodialysis/abdominal dialysis, hospitalization, and community clinic/health center hospitalization within the previous 1 year; (4) using broad-spectrum antibiotics within 3 months prior to infection; (5) patients with chronic urinary tract infections (previously isolated from the same type of specimen); (6) isolates implicated in healthcare-associated infections and recurrent UTIs (recurrences of uncomplicated and/or complicated UTIs, with a frequency of at least three UTIs/year or two UTIs in the last 6 months); (7) environmental samples or cultures for infection control purposes; and (8) duplicate isolates (the same species from the same patient).
Antimicrobial Susceptibility Test Method
Minimum inhibitory concentration determination for all antimicrobial agents, except fosfomycin, was performed using the microdilution broth method as per Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2020). MICs of fosfomycin were determined by agar dilution method (25 μg/ml of glucose-6-phosphate was added in Mueller–Hinton agar). Seventeen antimicrobial agents were analyzed, including cefazolin (CZO), ceftriaxone (CRO), ceftazidime (CAZ), cefepime (FEP), cefoperazone/sulbactam (CSL, 2:1), imipenem (IPM), ertapenem (ETP), amikacin (AMK), levofloxacin (LVX), cefmetazole (CMZ), trimethoprim/sulfamethoxazole (SXT, 1:19), colistin (COL), fosfomycin (FOS), cefotaxime (CTX), cefotaxime/clavulanic acid (CTC), ceftazidime/clavulanic acid (CCV), and nitrofurantoin (NIT). For each batch of MIC testing, reference strains E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control organisms.
Phenotypic Detection of Extended-Spectrum β-Lactamases
Phenotypic identification of ESBL in E. coli was carried out by CLSI-recommended methods (CLSI, 2020). If cefotaxime or ceftazidime MIC of an isolate was ≥ 2 μg/ml each, the MICs of cefotaxime + clavulanic acid (4 μg/ml) or ceftazidime + clavulanic acid (4 μg/ml) were comparatively determined. ESBL production was defined as a greater than or equal to eightfold decrease in MICs for cefotaxime or ceftazidime when tested in combination with clavulanic acid, compared with their MICs without clavulanic acid. ESBL-negative isolates were defined as isolates with cefotaxime or ceftazidime MICs of ≤ 1 μg/ml. ESBL-uncertain isolates were defined as isolates with cefotaxime or ceftazidime MICs of ≥ 2 μg/ml, but did not exhibit a greater than or equal to eightfold decrease in MICs for cefotaxime or ceftazidime after a combination with clavulanic acid, compared with their MICs alone.
Characterization of Antibiotic Resistance Genes
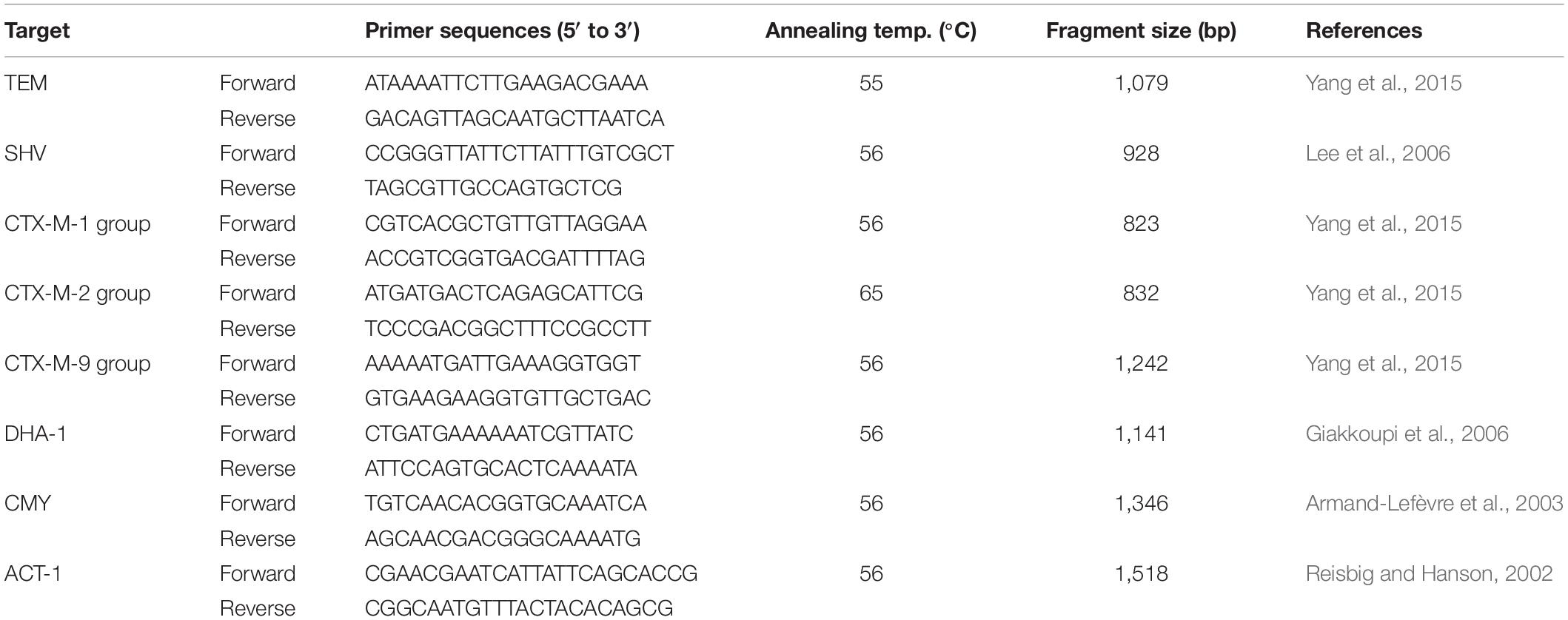
The main esbl and ampC genes, including blaTEM, blaSHV, blaCTX–M–1 group, blaCTX–M–2 group, blaCTX–M–9 group, blaDHA, blaCMY, and blaACT, were determined using polymerase chain (PCR) reaction method on the strains with ESBL-producing and ESBL-uncertain phenotype. The positive amplicons were sequenced and aligned by blastn web1. Primers used in this study are listed in Table 1.
Statistical Analysis
The results of antimicrobial susceptibility testing were analyzed by the WHONET5.6 program. Ninety-five percent confidence intervals were calculated using the adjusted Wald method; comparison of ESBL rates between tertiary and secondary hospital, in demographic characteristics and clinical features, was assessed using Chi-square test. Analyses were performed using SPSS version 25.0 (IBM Corporation), and p-values < 0.05 were considered statistically significant.
Results
Features of the Isolates
A total of 809 E. coli isolates from community-acquired adult urinary tract infections (CA-UTIs) were collected during the period 2016–2017. Most infections were lower UTIs (96.8%, 783/809), which included 5 cases of urethritis and 778 cases of cystitis. Upper UTIs accounted for 3.2% (26/809) including eight acute pyelonephritis, four hydronephrosis, seven kidney stones, and seven ureteral calculus. Isolates from female patients accounted for 85% of the total isolates. The ages of patients were as follows: 18–45 years, 31.8%; 46–65 years, 38.8%, and ≥ 66 years, 29.4%.
In vitro Susceptibility of Escherichia coli Isolates From Community-Acquired Adult Urinary Tract Infections
Among the 809 E. coli isolates studied, 308 (38.07%) were ESBL-producing strains, 477 (58.96%) were ESBL-negative, and 24 (2.97%) were ESBL-uncertain. The antimicrobial agents with susceptibility rates of greater than 95% included imipenem (99.9%), colistin (99.6%), ertapenem (98.9%), amikacin (98.3%), cefmetazole (97.9%), nitrofurantoin (96%), and fosfomycin (95.4%). However, the susceptibility rates to cephalosporins were relatively low, ranging from 58.6 to 74.9%. The antimicrobial susceptibility for all isolates is summarized in Table 2.
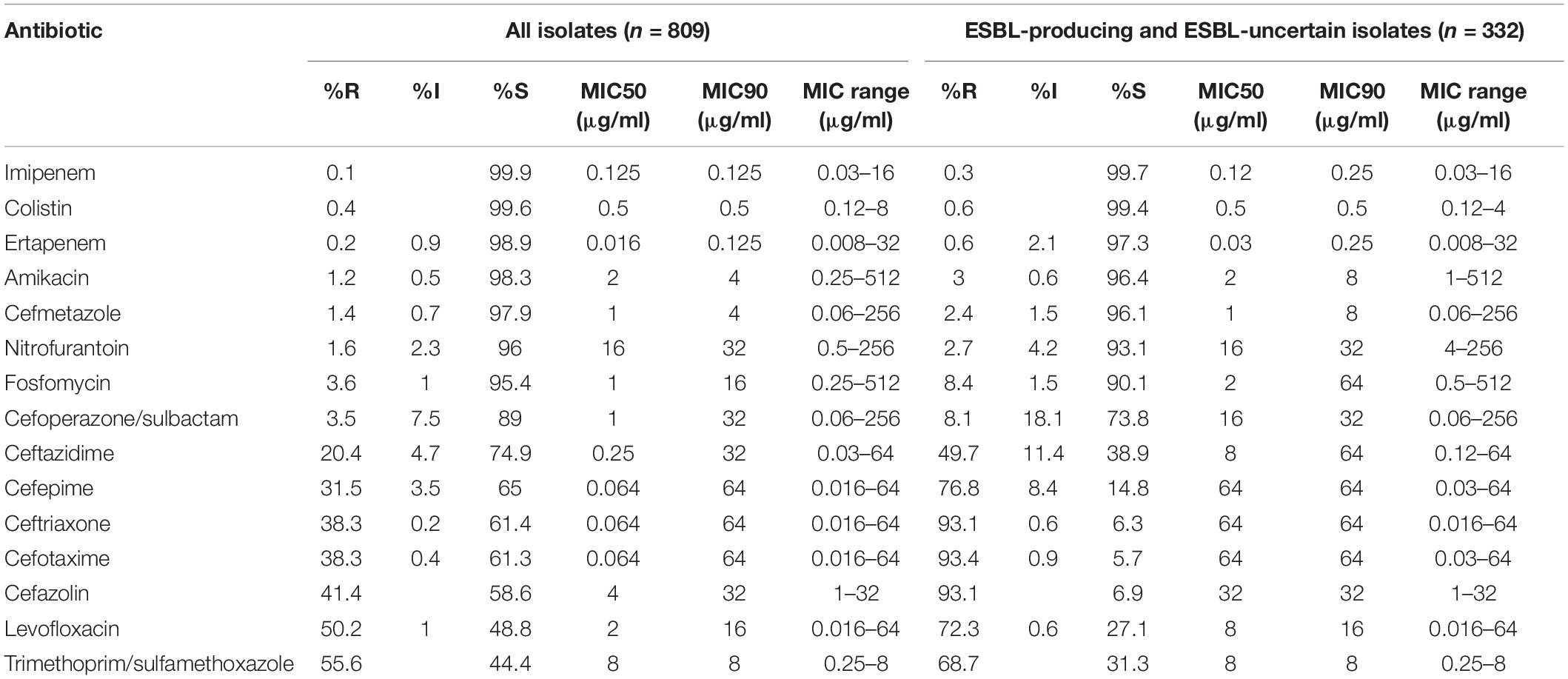
Table 2. The antimicrobial susceptibilities of 809 Escherichia coli strains isolated from community-acquired urinary tract infection (CA-UTI) in China.
We investigated ESBL differences between isolates from secondary hospitals and tertiary hospitals. Overall, there was no major difference in the proportion of ESBL-positive isolates (37.66% in secondary hospitals vs. 38.44% in tertiary hospitals) or ESBL-uncertain strains (3.12% in secondary hospitals vs. 2.83% in tertiary hospitals) (Figure 2). However, we found some differences, although very small, in the distribution of ESBLs by geographic region. Relatively higher percentages of ESBL rate were found in the northeast (46.3%), central (43.6%), and west (43.4%) China, compared with the sites in the north (30.2%) and east (30.3%) of China (Figure 2).
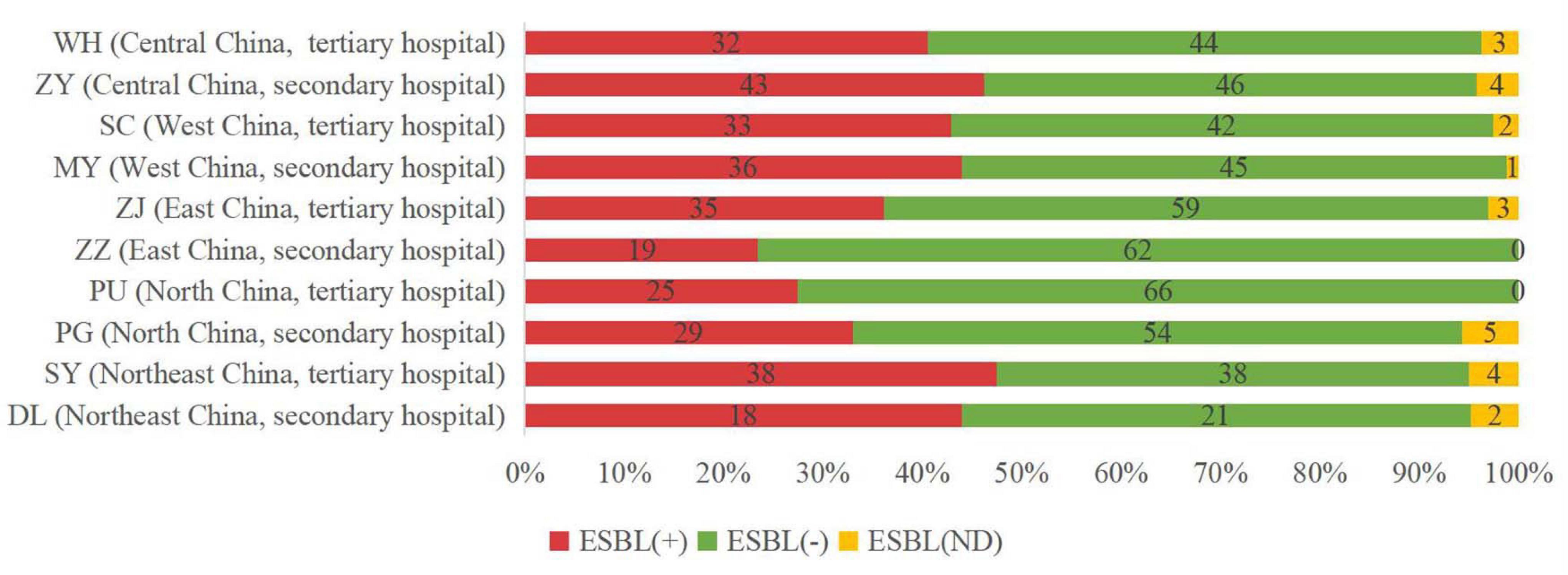
Figure 2. Incidence of extended-spectrum beta-lactamase (ESBL)-producing strains in different sites of China. ESBL (+), ESBL-positive isolates; ESBL (−), ESBL-negative isolates; and ESBL (ND), ESBL-uncertain isolates.
In general, E. coli isolates collected from secondary and tertiary hospitals showed high susceptibility rates to most antibiotics as follows: imipenem (100% vs. 99.8%), colistin (100% vs. 99.3%), ertapenem (99.5% vs. 98.3%), amikacin (98.4% vs. 98.1%), cefmetazole (97.9% vs. 97.9%), fosfomycin (95.8% vs. 95.0%), and nitrofurantoin (94% vs. 97.9%). Furthermore, cefoperazone/sulbactam showed high activity against strains isolated from secondary and tertiary hospitals, with a susceptibility rate of 90.4% and 87.7%. The susceptibilities to cephalosporins in isolates collected from tertiary hospitals varied from 58.0 to 75.5%.
Characterization of Extended-Spectrum Beta-Lactamase and AmpC Genes
Polymerase chain was performed on 332 ESBL-producing and ESBL-uncertain isolates to determine the presence of EBSL and AmpC. The results are shown in Figure 3. The major β-lactamase family detected in the ESBL-producing E. coli strains was CTX-M-9 group (149/308, 48.4%), followed by the CTX-M-1 group (136/308, 44.2%). The CTX-M-2 group was not detected. blaCMY–2 was detected in one isolate. Overall, 7 blaCTX–M subtypes were detected: blaCTX–M–14 (98 isolates, 31.8%), blaCTX–M–55 (72 isolates, 23.4%), blaCTX–M–15 (54 isolates, 17.5%), blaCTX–M–27 (41 isolates, 13.3%), blaCTX–M–65 (10 isolates, 3.2%), blaCTX–M–3 (5 isolates, 1.6%), blaCTX–M–64 (4 isolates, 1.3%), and blaCTX–M–79 (1 isolates, 0.3%). Among the blaCTX–M subtypes, two different variants were detected in 20/332 isolates (6.0%), most of which were blaCTX–M–14 and blaCTX–M–15 (7 isolates), blaCTX–M–14 and blaCTX–M–55 (7 isolates).
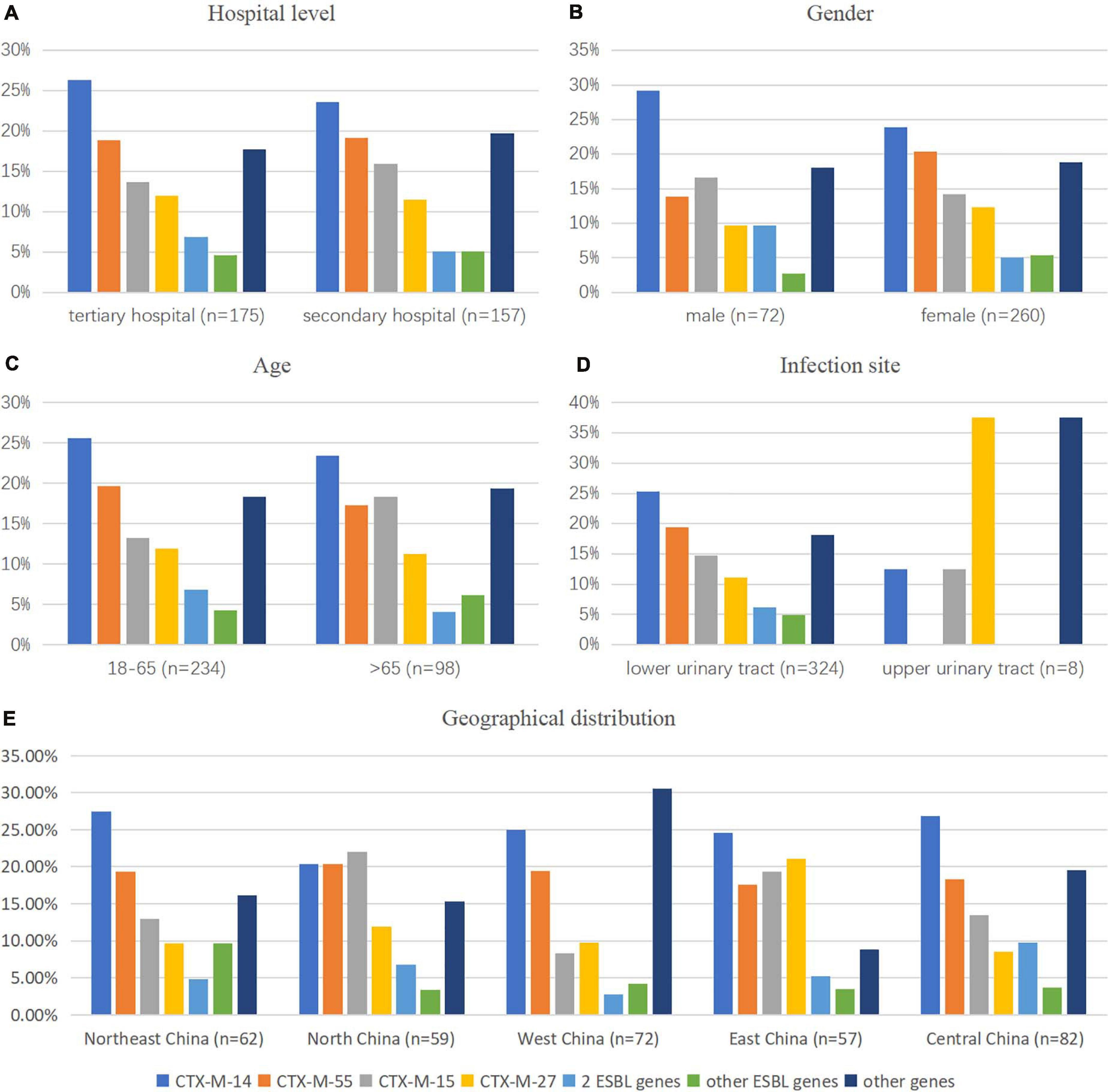
Figure 3. Comparison of the CTX-M gene pattern of E. coli isolates by (A) hospital category, (B,C,E) demographic characteristics, and (D) clinical features. 2 ESBL genes: CTX-M-1 coexisted with CTX-M-9 groups; other ESBL genes: genes that existed in less than 10 isolates in this study, such as CTX-M-3 (n = 5), CTX-M-64 (n = 3), CTX-M-65 (n = 9), and CTX-M-79 (n = 1); other genes: isolates with genes except ESBL genes detected in this study.
Among ESBL-uncertain isolates, blaCMY was the most common AmpC gene (18/24, 75%), consisting of blaCMY–2 (15 isolates, including 2 isolates coexisting with blaCTX–M–14), blaCMY–42 (2 isolates coexisted with blaCTX–M–15), and blaCMY–34 (1 isolate). blaTEM–1 was determined in eight strains (33.3%, 1 isolate coexisted with blaCTX–M–55). In five strains (20.8%), no ESBL genes were determined.
In vitro Susceptibility of Escherichia coli Strains With Different Extended-Spectrum Beta-Lactamase Phenotypes
Extended-spectrum beta-lactamase producing E. coli strains exhibited susceptibility rates of over 92% to imipenem (100%), cefmetazole (99.4%), colistin (99.4%), ertapenem (98.4%), amikacin (97.1%), and nitrofurantoin (92.9%). The susceptibility rates of ESBL-negative isolates against all the antimicrobial agents were higher than 90%, except levofloxacin (63.9%) and trimethoprim/sulfamethoxazole (53.5%). On the other hand, ESBL-uncertain isolates showed high susceptibility rates to colistin (100%)], imipenem (95.8%), fosfomycin (95.8%), and nitrofurantoin (95.8%). The susceptibility rate differences between ESBL-producing and ESBL-negative isolates were greater for cephalosporins (Figure 4), including cefotaxime (6.2% vs. 100%), ceftriaxone (6.5% vs. 99.8%), cefazolin (7.1% vs. 94.5%), and cefepime (9.7% vs. 100%).
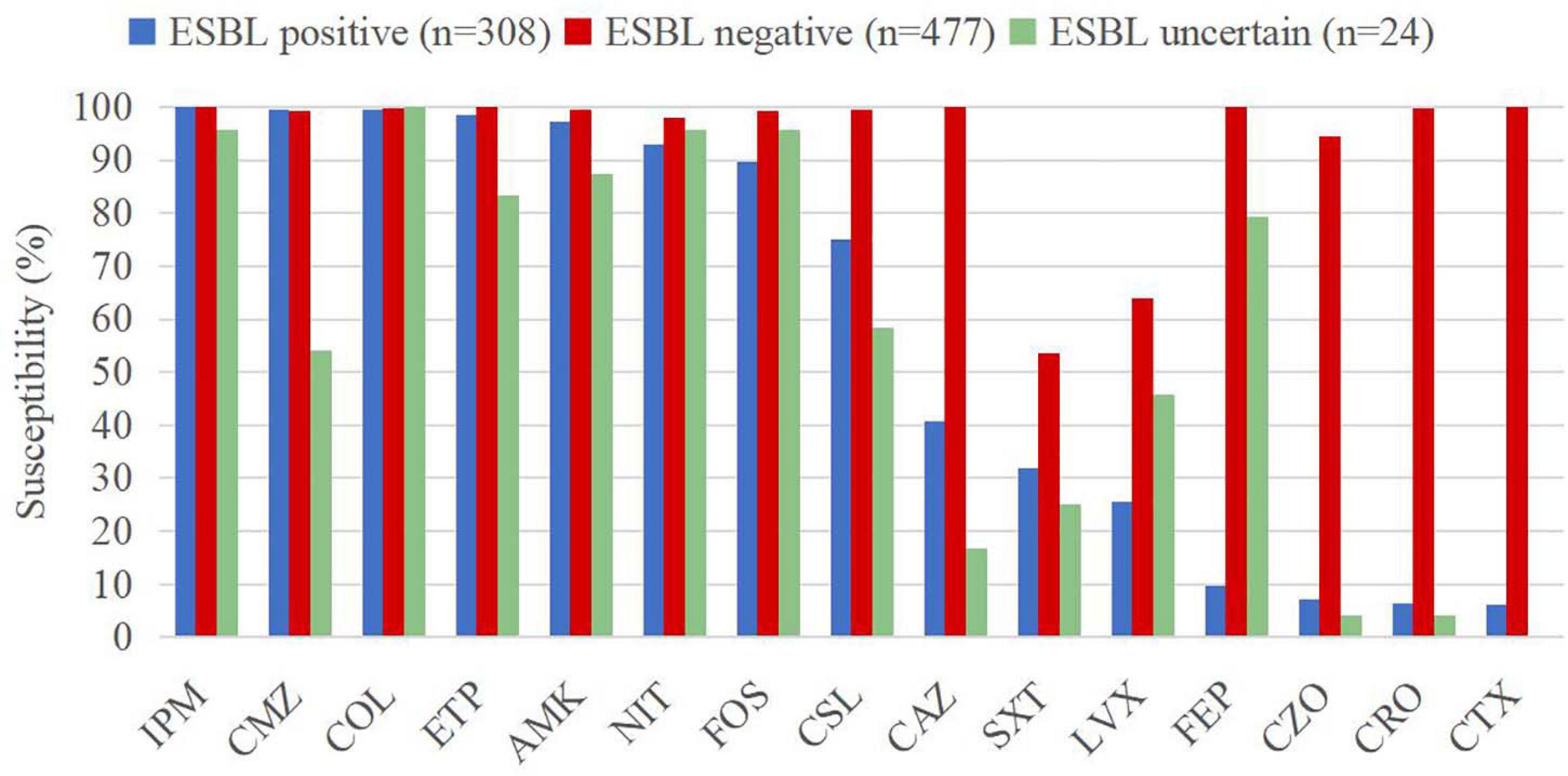
Figure 4. Susceptibility rates of CA-UTI Escherichia coli strains with different ESBL phenotypes. IMP, imipenem; ETP, ertapenem; COL, colistin; AMK, amikacin; CMZ, cefmetazole; NIT, nitrofurantoin; FOS, fosfomycin; CSL, cefoperazone/sulbactam; CAZ, ceftazidime; FEP, cefepime; CRO, ceftriaxone; CTX, cefotaxime; CZO, cefazolin; LVX, levofloxacin; and SXT, trimethoprim/sulfamethoxazole.
Comparison of the Antimicrobial Susceptibility Rates of Escherichia coli Isolates by Hospital Level, Demographic Characteristics, and Clinical Features
We observed that gender was a significant factor influencing antimicrobial susceptibility, with a significantly higher rate of ESBL-producing strains in male (53.6%) than in female patients (35.2%) (p < 0.001). Cephalosporins exhibited higher rates of in vitro activity against E. coli strains from female than from male patients (p < 0.05), including cefazolin, ceftazidime, ceftriaxone, cefotaxime, cefoperazone/sulbactam, and cefepime (Figure 5 and Table 3). There was no significant difference in antimicrobial susceptibility and esbl genes between E. coli strains from tertiary and secondary hospitals, between the age groups of 18 and 65 and over 65 years, or between upper and lower UTIs. The major CTX-M variant in northeast China, west China, and central China was CTX-M-14, followed by CTX-M-15, CTX-M-55, and CTX-M-27. While in north China, the major variant was CTX-M-15 (13/59, 22.03%), in east China, the secondary variant was CTX-M-27 (12/57, 21.05%). The CTX-M genotypes included high rates of CTX-M-14 followed by CTX-M-55, CTX-M-15, and CTX-M-27, which was similar between isolates from tertiary hospitals and from secondary hospitals. There existed a difference in the distribution of CTX-M variants; CTX-M-15 had a larger proportion than CTX-M-55 among male patients and patients over 65 years of age (Figure 3).
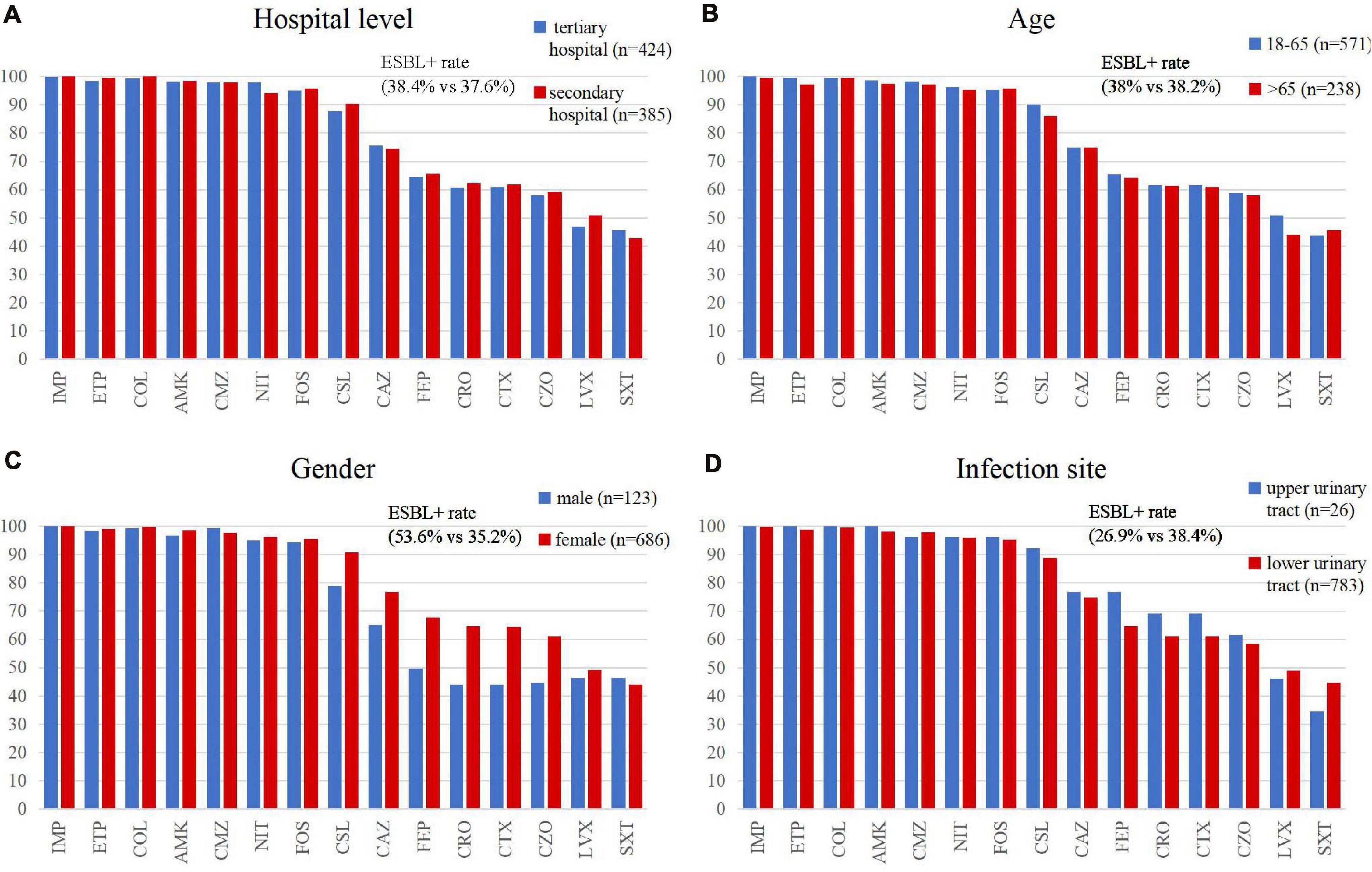
Figure 5. Comparison of the antimicrobial susceptibility rates of E. coli isolates by (A) hospital category, (B,C) demographic characteristics, and (D) clinical features. IMP, imipenem; ETP, ertapenem; COL, colistin; AMK, amikacin; CMZ, cefmetazole; NIT, nitrofurantoin; FOS, fosfomycin; CSL, cefoperazone/sulbactam; CAZ, ceftazidime; FEP, cefepime; CRO, ceftriaxone; CTX, cefotaxime; CZO, cefazolin; LVX, levofloxacin; and SXT, trimethoprim/sulfamethoxazole.
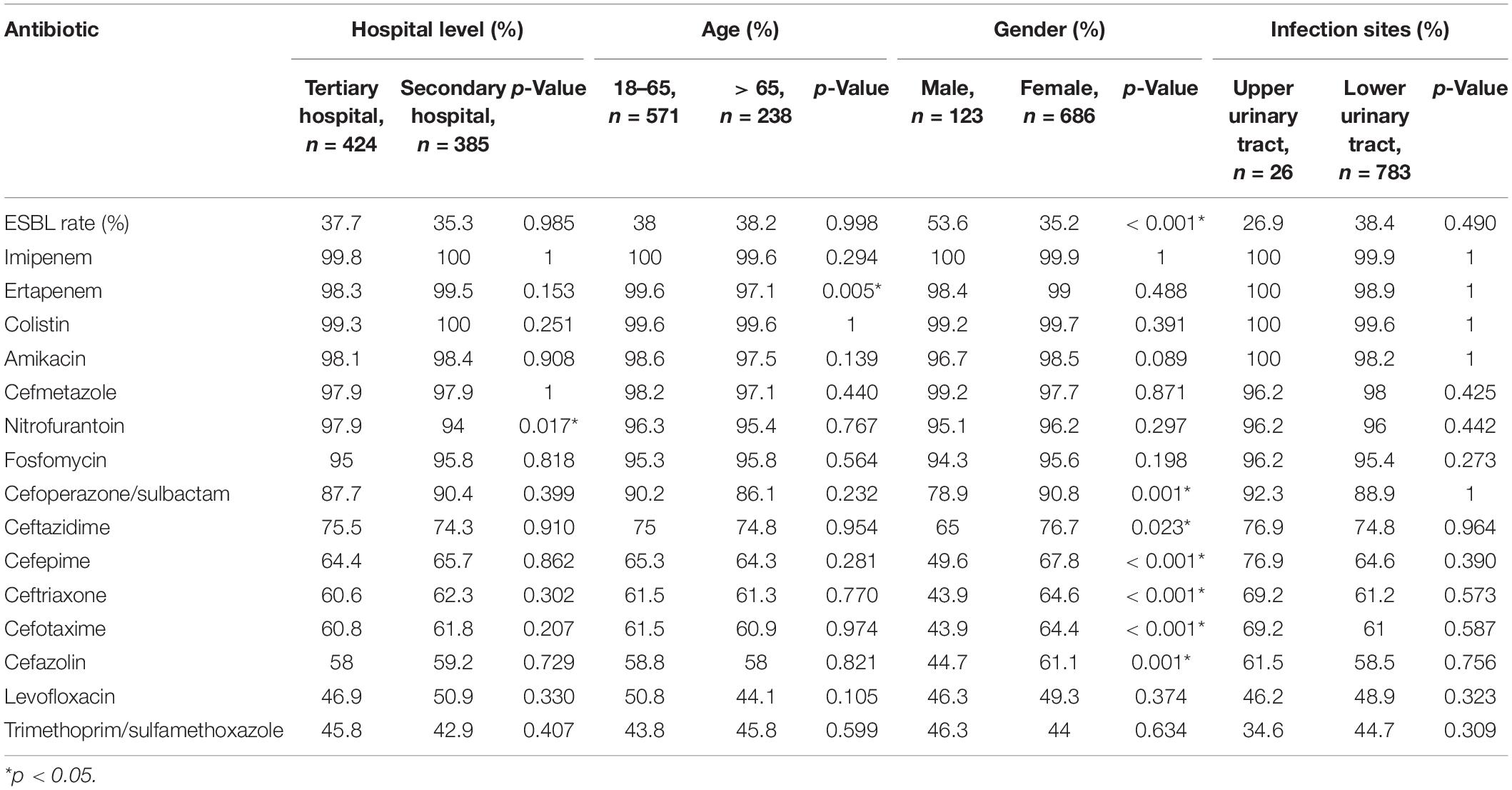
Table 3. Comparison of the antimicrobial susceptibility rates of E. coli isolates in hospital level, demographic characteristics, and clinical features.
Community-Acquired Carbapenem-Resistant Strains
In this study, two strains were identified as community-acquired carbapenem resistant. The two carbapenem-resistant Enterobacteriaceae (CRE) strains (MYU26 and SYU04) were resistant to almost all β-lactam antibiotics tested, with only three antimicrobial agents exhibiting potent activity against them, including colistin (MYU26: MIC ≤ 0.12, SYU04: MIC = 0.5), fosfomycin (MYU26: MIC = 1, SYU04: MIC = 4), and tigecycline (MYU26 and SYU04: MICs ≤ 0.06). MYU26 carried the blaCTX–M–15 and blaCMY–42 genes and SYU04 carried the blaCMY–2 gene. No carbapenemase genes were detected.
Discussion
Our analysis of stringently selected E. coli isolates from CA-UTIs, collected in China during the period 2016–2017, revealed a relatively lower ESBL rate (38.07%) than previously reported in 2012 (68.6%) and 2014 (59.1%) in a study from The Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program (Yang et al., 2017). However, another study in China (Zhang et al., 2019) found no significant difference in the rates of ESBLs among E. coli isolates from HA and CA UTIs, and the ESBL rate in that study was 48.8% among CA-UTI E. coli isolates during the period 2016–2017, which is higher than in the present study. In Asia, the proportion of ESBL-producing E. coli isolates was 4.1% in patients with acute uncomplicated cystitis in Japan (Hayami et al., 2019), with 10.8% rates in CA-UTI E. coli isolates in Korea (Park et al., 2017). In Europe, the frequency of ESBL-producing CA-UTI E. coli strains ranged from 2.2 to 24% (Yılmaz et al., 2016; Chervet et al., 2018; van Driel et al., 2019). In North America, the rates varied from 14.1% in Canada and 16.0% in the UnitedStates to 31.3% in Mexico (Lob et al., 2016; Galindo-Méndez, 2018). The difference in the prevalence of ESBLs among different studies may be due to population source of the isolates.
Our results show that CTX-M-14, CTX-M-55, and CTX-M-15 are the most dominant CTX-M variants in the ESBL-producing E. coli, followed by CTX-M-27, CTX-M-3, CTX-M-65, CTX-M-64, and CTX-M-79. CMY-2 is the major β-lactamase in the ESBL-uncertain E. coli. In different regions, the CTX-M variant pattern is different. In north China, CTX-M-15 (22.03%), CTX-M-14, and CTX-M-55 (20.34%) were distributed equally in the ESBL-producing and uncertain (phenotype) isolates, while in other regions, CTX-M-14 was the major variant with over 24% rate, and the rates of CTX-M-15 and CTX-M-55 were less than 20%. In east China, CTX-M-27 (21.05%) was the secondary variant that exceeded CTX-M-15 (19.3%) and CTX-M-55 (17.54%). In addition, another report also shows that CTX-M-27 has become more prevalent in East and Southeast Asia (Chong et al., 2018). Among the isolates from lower urinary tract infections, blaCTX–M–14 was the most common ESBL gene type, which was different from upper urinary tract infections (the main gene was blaCTX–M–27), but it may not be comprehensive since the number of isolates was extremely low in upper urinary tract infections (n = 8).
Most of CTX-Ms, such as CTX-M-14, CTX-M-3, and CTX-M-65, exhibit powerful activity against cefotaxime and ceftriaxone but not ceftazidime. Some CTX-Ms, such as CTX-M-15, CTX-M-27, CTX-M-55, and CTX-M-64, exhibit enhanced catalytic efficiencies against ceftazidime (Zhao and Hu, 2013). In our study, the rate of ceftazidime-resistant isolates (49.7%) corresponds to the rate of isolates that carried CTX-M-15/55/27 (48.8%). In addition, among the ESBL-producing and uncertain (phenotype) isolates, the rates of ceftriaxone/cefotaxime/cefazolin-resistant were 93.1%, 93.4%, and 93.1%, respectively. Although CTX-M can be inhibited by β-lactamase inhibitors as sulbactam, clavulanate, and tazobactam, the susceptibility rate of cefoperazone/sulbactam was only 73.8%. Moreover, most of ESBL-uncertain isolates carried CMY-2, which cannot be inhibited by β-lactamase inhibitors. For these isolates, fosfomycin would be useful for the empirical treatment of acute cystitis since it had high rates of activity, with a susceptibility rate of over 90%.
The common antimicrobial drugs for treating acute uncomplicated cystitis include fosfomycin, nitrofurantoin, trimethoprim/sulfamethoxazole, and β-lactams, including cephalexin, cefaclor, and amoxicillin/clavulanate. Besides, β-lactams and quinolones are recommended as renal excretion-type antibiotics for acute uncomplicated pyelonephritis (Choe et al., 2018). However, the two old antibiotics, fosfomycin and nitrofurantoin, which achieve high urinary concentrations and minimal toxicity, are not often used in China (Qiao et al., 2013), yet E. coli accounts for the majority of pathogens causing CA-UTIs (Qiao et al., 2013; Yang et al., 2017). It is important to understand the activity of β-lactams and quinolones against E. coli strains to guide empirical antimicrobial therapy decision making.
In the European Association of Urology guidelines updated in 2020, cephalosporins are recommended for oral empirical treatment of uncomplicated pyelonephritis and as alternative antimicrobials for therapy in uncomplicated cystitis (Bonkat et al., 2020). However, in the present study, cephalosporins had poor activity against ESBL-producing E. coli strains. Susceptibility rates of E. coli to the first-, third-, and fourth-generation cephalosporins were lower than 80%, among which, the susceptibility rates were 76.9% (for strains that caused pyelonephritis) and 74.8% (for strains that caused lower urinary tract infections) for ceftazidime, 69.2% and 61% for cefotaxime, 69.2% and 61.2% for ceftriaxone, and 76.9% and 64.6% for cefepime, respectively, which indicate that these agents might not be the optimum medications for empirical UTI therapies.
In the present study, the proportion of ESBL-non-producing E. coli isolates was 73.1% in pyelonephritis and 61.6% in lower UTIs, which is consistent with the cephalosporin susceptibility rates. Genes that encode for ESBLs are usually found on large plasmids accompanied by genetic determinants of resistance against multiple classes of antibiotics, such as aminoglycosides, sulfonamides, and fluoroquinolones (Lee et al., 2012; Bader et al., 2017). Our study also investigated the differences in ESBL carriage rates and cephalosporin susceptibility rates between males and females. Significantly higher ESBL and cephalosporin resistance rates were found in E. coli isolates from men. ESBL rates range from 30.2% to 46.3% in different regions of China, with northeast, central, and west China having higher rates in E. coli. The choice of cephalosporins for treatment of UTIs should be based on local prevalence of ESBL-producing isolate data.
Fluoroquinolones and cephalosporins are antimicrobial agents that can be recommended for oral empirical treatment of uncomplicated pyelonephritis. Meanwhile, ciprofloxacin, levofloxacin, and ofloxacin are not recommended in the treatment of uncomplicated cystitis. However, quinolone use has been compromised by the high resistance rates to most bacterial pathogens (Kim and Hooper, 2014), with a 50.2% resistance rate reported in this study higher than the rates reported in patients with acute uncomplicated cystitis in Japan (6.4%) (Yılmaz et al., 2016). Quinolone resistance mechanisms are multiple and complicated. Chromosomal gene mutation including gyrA, gyrB, parC, and parE genes, reduce binding of the drug to the enzyme–DNA complex. Furthermore, overexpression of native efflux pumps localized in the bacterial membrane may cause resistance to quinolones and other antimicrobials. Additionally, plasmid-mediated quinolone resistance determinants can encode additional antimicrobial resistances and transfer multidrug resistance to a variety of antimicrobials, including quinolones (Hooper and Jacoby, 2015). Through these resistance mechanisms, the number of quinolone-resistant bacterial strains has grown steadily over the years. A previous study reported that S83L/D87N in gyrA and S80I in parC were the most common topoisomerase mutations in ESBL-producing E. coli isolates from the community (Ni et al., 2016). In addition, the majority of UTIs in the present study were uncomplicated cystitis (96%, 778/809) for which fluoroquinolones are not recommended for treatment. Thus, in this case, the use of fluoroquinolones for empiric treatment of UTIs should be restricted.
Although carbapenems are not recommended as the first-line treatment for uncomplicated cystitis and pyelonephritis (Bonkat et al., 2020), the carbapenems exhibited high in vitro activity against ESBL-producing E. coli in the present study, with a susceptibility rate of 100% for imipenem and 98.4% for ertapenem. Given the relatively high ESBL rates in CA-UTIs in the present study, and the low susceptibility rates to beta-lactam and fluoroquinolones, carbapenems can be considered for empiric therapy in patients with suspected ESBL-producing and multidrug-resistant (MDR) bacterial strains (Essack, 2000; Paterson, 2000; Livermore et al., 2001). On the other hand, in order to maintain the activity of carbapenems, it is necessary to replace carbapenems by other antimicrobials once the susceptibilities to antimicrobial agents have been confirmed. Carbapenem-resistant E. coli (CREc) constituted 0.25% (2/809) of the CA-UTI E. coli isolates studied, which is consistent with previous reports. Findings from previous studies indicate an increase in the prevalence of community-acquired CRE in Taiwan (Lai et al., 2013; Tang et al., 2016). CA-CREs have also been described in the southeastern part of the United States (Thaden et al., 2014), suggesting widespread distribution of the organism in the community, and patients infected with CA-CRE have more urinary tract infections (Tang et al., 2016). The two patients with CREc were both elderly and female, which is in agreement with previous findings (Tang et al., 2016). In this study, amikacin colistin, fosfomycin, and tigecycline exhibited potent activity against CREs, suggesting that colistin, fosfomycin, tigecycline, and aminoglycosides could be treatment choices for UTIs caused by CRE (Bader et al., 2017).
As with majority of the studies, a limitation in this study was the lack of genomic analysis such as multilocus sequence typing (MLST). Since all the isolates were collected from 10 different regions in China and the number per site was no more than 100, there was a high possibility that these isolates were sporadic and rarely clonal outbreak. From other studies, we learned that ST131, ST69, ST95, and ST73 were the dominant sequence types (STs) in E. coli isolated from urinary tract infections (Riley, 2014; Yamaji et al., 2018; Kot, 2019). Among multidrug-resistance E. coli isolates associated with CA-UTIs, ST131 and ST69 were predominant in Australia and Saudi Arabia (Alghoribi et al., 2015; Rogers et al., 2015); however, ST648, ST224, ST38, and ST405 also occurred in China (Cao et al., 2011, 2014).
This study also found that two of three colistin-resistant E. coli isolates carried blaCTX–M–14 and blaTEM–1 genes but were susceptible to carbapenems, which is consistent with a previous study (Jiang et al., 2020). The results suggest we need to attach great importance to the management of MDR Gram-negative bacteria. This study showed the real ESBL rates and genotype distribution in community-acquired UTIs through strict selection criteria, since the strategies for treatment of hospital- and community-acquired UTIs are different. Besides, some UTIs might not represent genuine community acquisition if the patients were admitted to a hospital before infection. Hence, this strictly defined clinical epidemiological study in CA-UTIs will help the clinicians to better understand the antimicrobial resistance status and select empiric antimicrobial agents.
Conclusion
Our findings show that ESBLs are still a significant issue in E. coli isolates from CA-UTI in China, with an average prevalence of 38.07%. Higher rates of ESBL among the E. coli strains were confined to the northeast, central, and west parts of China. The choice of antimicrobial agents for the treatment of CA-UTIs should be based on local surveillance data. Use of fluoroquinolones for empiric treatment of UTIs should be restricted due to high resistance rate. Carbapenems can be used empirically for highly suspected ESBL-producing and MDR strains. However, the occurrence of CRE in the community is a cause for concern.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The protocol has been reviewed by the human research Ethics Committee of the Institutional Review Board (IRB) of the Peking Union Medical College Hospital (Ethics Approval Number: S-K136). This project did not involve any patient information nor did it affect the normal diagnosis and treatment of patients, and after consultation with the IRB, formal ethical approval was reviewed and waived, and written patient consent was not required.
Author Contributions
QY conceived and designed the experiments. YY, GZ, and JZ performed the experiments. PJ, XL, and YZ analyzed the data and wrote the manuscript. TK, YX, and QY reviewed the manuscript and polished the language. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82072318) and National Key Research and Development Program of China (2018YFE0101800 and 2018YFC1200105). This study was also supported by the China Pharmacists Association, Beijing Key Clinical Specialty for Laboratory Medicine—Excellent Project (No. ZK201000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Alghoribi, M. F., Gibreel, T. M., Farnham, G., Al Johani, S. M., Balkhy, H. H., and Upton, M. (2015). Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J. Antimicrob. Chemother. 70, 2757–2762. doi: 10.1093/jac/dkv188
Arana, D. M., Rubio, M., and Alós, J. I. (2017). Evolution of antibiotic multiresistance in Escherichia coli and Klebsiella pneumoniae isolates from urinary tract infections: a 12-year analysis (2003-2014). Enferm. Infecc. Microbiol. Clin. 35, 293–298. doi: 10.1016/j.eimc.2016.02.018
Armand-Lefèvre, L., Leflon-Guibout, V., Bredin, J., Barguellil, F., Amor, A., Pagès, J. M., et al. (2003). Imipenem resistance in Salmonella enterica serovar Wien related to porin loss and CMY-4 beta-lactamase production. Antimicrob. Agents Chemother. 47, 1165–1168. doi: 10.1128/aac.47.3.1165-1168.2003
Bader, M. S., Loeb, M., and Brooks, A. A. (2017). An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 129, 242–258. doi: 10.1080/00325481.2017.1246055
Bonkat, G., Bartoletti, R., Bruy, F., Cai, T., Geerlings, S. E., Köves, B., et al. (2020). EAU Guidelines on Urological Infections 2020 [Online]. (Arnhem: European Association of Urology Guidelines Office). Available online at: http://uroweb.org/guideline/urological-infections/
Cao, X., Cavaco, L. M., Lv, Y., Li, Y., Zheng, B., Wang, P., et al. (2011). Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J. Clin. Microbiol. 49, 2496–2501. doi: 10.1128/jcm.02503-10
Cao, X., Zhang, Z., Shen, H., Ning, M., Chen, J., Wei, H., et al. (2014). Genotypic characteristics of multidrug-resistant Escherichia coli isolates associated with urinary tract infections. APMIS 122, 1088–1095. doi: 10.1111/apm.12260
Chervet, D., Lortholary, O., Zahar, J. R., Dufougeray, A., Pilmis, B., and Partouche, H. (2018). Antimicrobial resistance in community-acquired urinary tract infections in Paris in 2015. Med. Mal. Infect. 48, 188–192. doi: 10.1016/j.medmal.2017.09.013
Choe, H. S., Lee, S. J., Yang, S. S., Hamasuna, R., Yamamoto, S., Cho, Y. H., et al. (2018). Summary of the UAA-AAUS guidelines for urinary tract infections. Int. J. Urol. 25, 175–185. doi: 10.1111/iju.13493
Chong, Y., Shimoda, S., and Shimono, N. (2018). Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 61, 185–188. doi: 10.1016/j.meegid.2018.04.005
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing, Document M100, 30th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Espínola, M., García, A., Somodevilla, Á, Martínez, M., Guiu, A., Correa, A., et al. (2011). Antibiotic susceptibility of Escherichia coli strains causing community-acquired urinary tract infection. Clin. Microbiol. Infect. 17:S690. doi: 10.1111/j.1469-0691.2011.03559.x
Essack, S. Y. (2000). Treatment options for extended-spectrum beta-lactamase-producers. FEMS Microbiol. Lett. 190, 181–184. doi: 10.1111/j.1574-6968.2000.tb09283.x
Fupin, H. U., Yan, G. U. O., Demei, Z. H. U., Fu, W., Xiaofei, J., Yingchun, X. U., et al. (2017). CHINET surveillance of bacterial resistance across China: report of the results in 2016. Chin. J. Infect. Chemother. 17, 481–491. doi: 10.16718/j.1009-7708.2017.05.001
Galindo-Méndez, M. (2018). Molecular characterization and antimicrobial susceptibility pattern of extended-spectrum β-lactamase-producing Escherichia coli as cause of community acquired urinary tract infection. Rev. Chilena Infectol. 35, 29–35. doi: 10.4067/s0716-10182018000100029
Giakkoupi, P., Tambic-Andrasevic, A., Vourli, S., Skrlin, J., Sestan-Crnek, S., Tzouvelekis, L. S., et al. (2006). Transferable DHA-1 cephalosporinase in Escherichia coli. Int. J. Antimicrob. Agents 27, 77–80. doi: 10.1016/j.ijantimicag.2005.09.013
Hayami, H., Takahashi, S., Ishikawa, K., Yasuda, M., Yamamoto, S., Wada, K., et al. (2019). Second nationwide surveillance of bacterial pathogens in patients with acute uncomplicated cystitis conducted by Japanese Surveillance Committee from 2015 to 2016: antimicrobial susceptibility of Escherichia coli, Klebsiella pneumoniae, and Staphylococcus saprophyticus. J. Infect. Chemother. 25, 413–422. doi: 10.1016/j.jiac.2019.02.021
Hooper, D. C., and Jacoby, G. A. (2015). Mechanisms of drug resistance: quinolone resistance. Ann. N. Y. Acad. Sci. 1354, 12–31. doi: 10.1111/nyas.12830
Jiang, B., Du, P., Jia, P., Liu, E., Kudinha, T., Zhang, H., et al. (2020). Antimicrobial susceptibility and virulence of mcr-1-positive Enterobacteriaceae in China, a multicenter longitudinal epidemiological study. Front. Microbiol. 11:1611. doi: 10.3389/fmicb.2020.01611
Kim, E. S., and Hooper, D. C. (2014). Clinical importance and epidemiology of quinolone resistance. Infect. Chemother. 46, 226–238. doi: 10.3947/ic.2014.46.4.226
Koksal, I., Yilmaz, G., Unal, S., Zarakolu, P., Korten, V., Mulazimoglu, L., et al. (2017). Epidemiology and susceptibility of pathogens from SMART 2011-12 Turkey: evaluation of hospital-acquired versus community-acquired urinary tract infections and ICU- versus non-ICU-associated intra-abdominal infections. J. Antimicrob. Chemother. 72, 1364–1372. doi: 10.1093/jac/dkw574
Kot, B. (2019). Antibiotic resistance among uropathogenic Escherichia coli. Pol. J. Microbiol. 68, 403–415. doi: 10.33073/pjm-2019-048
Lai, C. C., Wu, U. I., Wang, J. T., and Chang, S. C. (2013). Prevalence of carbapenemase-producing Enterobacteriaceae and its impact on clinical outcomes at a teaching hospital in Taiwan. J. Formos. Med. Assoc. 112, 492–496. doi: 10.1016/j.jfma.2012.09.021
Larramendy, S., Gaultier, A., Fournier, J. P., Caillon, J., Moret, L., and Beaudeau, F. (2021). Local characteristics associated with higher prevalence of ESBL-producing Escherichia coli in community-acquired urinary tract infections: an observational, cross-sectional study. J. Antimicrob. Chemother. 76, 789–795. doi: 10.1093/jac/dkaa514
Lee, J. H., Bae, I. K., and Lee, S. H. (2012). New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med. Res. Rev. 32, 216–232. doi: 10.1002/med.20210
Lee, Y. H., Cho, B., Bae, I. K., Chang, C. L., and Jeong, S. H. (2006). Klebsiella pneumoniae strains carrying the chromosomal SHV-11 beta-lactamase gene produce the plasmid-mediated SHV-12 extended-spectrum beta-lactamase more frequently than those carrying the chromosomal SHV-1 beta-lactamase gene. J. Antimicrob. Chemother. 57, 1259–1261. doi: 10.1093/jac/dkl115
Livermore, D. M., Oakton, K. J., Carter, M. W., and Warner, M. (2001). Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob. Agents Chemother. 45, 2831–2837. doi: 10.1128/aac.45.10.2831-2837.2001
Lob, S. H., Kazmierczak, K. M., Badal, R. E., Hackel, M. A., Bouchillon, S. K., Biedenbach, D. J., et al. (2015). Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART program, 2009 to 2013. Antimicrob. Agents Chemother. 59, 3606–3610. doi: 10.1128/aac.05186-14
Lob, S. H., Nicolle, L. E., Hoban, D. J., Kazmierczak, K. M., Badal, R. E., and Sahm, D. F. (2016). Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010-2014. Diagn Microbiol. Infect. Dis. 85, 459–465. doi: 10.1016/j.diagmicrobio.2016.04.022
Naziri, Z., Derakhshandeh, A., Soltani Borchaloee, A., Poormaleknia, M., and Azimzadeh, N. (2020). Treatment failure in urinary tract infections: a warning witness for virulent multi-drug resistant ESBL- producing Escherichia coli. Infect. Drug Resist. 13, 1839–1850. doi: 10.2147/idr.S256131
Ni, Q., Tian, Y., Zhang, L., Jiang, C., Dong, D., Li, Z., et al. (2016). Prevalence and quinolone resistance of fecal carriage of extended-spectrum β-lactamase-producing Escherichia coli in 6 communities and 2 physical examination center populations in Shanghai, China. Diagn Microbiol. Infect. Dis. 86, 428–433. doi: 10.1016/j.diagmicrobio.2016.07.010
Park, J. J., Seo, Y. B., and Lee, J. (2017). Antimicrobial susceptibilities of Enterobacteriaceae in community-acquired urinary tract infections during a 5-year period: a single hospital study in Korea. Infect. Chemother. 49, 184–193. doi: 10.3947/ic.2017.49.3.184
Paterson, D. L. (2000). Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin. Microbiol. Infect. 6, 460–463. doi: 10.1046/j.1469-0691.2000.00107.x
Qiao, L. D., Chen, S., Yang, Y., Zhang, K., Zheng, B., Guo, H. F., et al. (2013). Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open 3:e004152. doi: 10.1136/bmjopen-2013-004152
Reisbig, M. D., and Hanson, N. D. (2002). The ACT-1 plasmid-encoded AmpC beta-lactamase is inducible: detection in a complex beta-lactamase background. J. Antimicrob. Chemother. 49, 557–560. doi: 10.1093/jac/49.3.557
Richelsen, R., Smit, J., Anru, P. L., Schønheyder, H. C., and Nielsen, H. (2020). Incidence of community-onset extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae infections: an 11-year population-based study in Denmark. Infect. Dis. (Lond.) 52, 547–556. doi: 10.1080/23744235.2020.1763452
Riley, L. W. (2014). Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 20, 380–390. doi: 10.1111/1469-0691.12646
Rogers, B. A., Ingram, P. R., Runnegar, N., Pitman, M. C., Freeman, J. T., Athan, E., et al. (2015). Sequence type 131 fimH30 and fimH41 subclones amongst Escherichia coli isolates in Australia and New Zealand. Int. J. Antimicrob. Agents 45, 351–358. doi: 10.1016/j.ijantimicag.2014.11.015
Tang, H. J., Hsieh, C. F., Chang, P. C., Chen, J. J., Lin, Y. H., Lai, C. C., et al. (2016). Clinical significance of community- and healthcare-acquired carbapenem-resistant Enterobacteriaceae isolates. PLoS One 11:e0151897. doi: 10.1371/journal.pone.0151897
Thaden, J. T., Lewis, S. S., Hazen, K. C., Huslage, K., Fowler, V. G. Jr., Moehring, R. W., et al. (2014). Rising rates of carbapenem-resistant enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect. Control Hosp. Epidemiol. 35, 978–983. doi: 10.1086/677157
van Driel, A. A., Notermans, D. W., Meima, A., Mulder, M., Donker, G. A., Stobberingh, E. E., et al. (2019). Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur. J. Clin. Microbiol. Infect. Dis. 38, 2151–2158. doi: 10.1007/s10096-019-03655-3
Yamaji, R., Rubin, J., Thys, E., Friedman, C. R., and Riley, L. W. (2018). Persistent pandemic lineages of uropathogenic Escherichia coli in a college community from 1999 to 2017. J. Clin. Microbiol. 56:e01834-17. doi: 10.1128/jcm.01834-17
Yang, Q., Zhang, H., Cheng, J., Xu, Z., Xu, Y., Cao, B., et al. (2015). In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum β-lactamases in China. Int. J. Antimicrob. Agents 45, 485–490. doi: 10.1016/j.ijantimicag.2014.11.012
Yang, Q., Zhang, H., Wang, Y., Xu, Z., Zhang, G., Chen, X., et al. (2017). Antimicrobial susceptibilities of aerobic and facultative gram-negative bacilli isolated from Chinese patients with urinary tract infections between 2010 and 2014. BMC Infect. Dis. 17:192. doi: 10.1186/s12879-017-2296-x
Yılmaz, N., Ağuş, N., Bayram, A., Şamlıoğlu, P., Şirin, M. C., Derici, Y. K., et al. (2016). Antimicrobial susceptibilities of Escherichia coli isolates as agents of community-acquired urinary tract infection (2008-2014). Turk J. Urol. 42, 32–36. doi: 10.5152/tud.2016.90836
Yun, L., Yuan, L., Feng, X., Xiu-zhen, Z., Yun-jian, H., Ting, Y., et al. (2014). Antimicrobial susceptibility surveillance of gram-negative bacterial from Mohnarin 2011-20112. Chin. J. Clin. Pharmacol. 30, 260–277.
Zhang, H., Johnson, A., Zhang, G., Yang, Y., Zhang, J., Li, D., et al. (2019). Susceptibilities of Gram-negative bacilli from hospital- and community-acquired intra-abdominal and urinary tract infections: a 2016-2017 update of the Chinese SMART study. Infect. Drug Resist. 12, 905–914. doi: 10.2147/idr.S203572
Keywords: community-acquired urinary tract infections, Escherichia coli, extended-spectrum beta-lactamase, antibiotic resistance, empirical treatment, CTX-M
Citation: Jia P, Zhu Y, Li X, Kudinha T, Yang Y, Zhang G, Zhang J, Xu Y and Yang Q (2021) High Prevalence of Extended-Spectrum Beta-Lactamases in Escherichia coli Strains Collected From Strictly Defined Community-Acquired Urinary Tract Infections in Adults in China: A Multicenter Prospective Clinical Microbiological and Molecular Study. Front. Microbiol. 12:663033. doi: 10.3389/fmicb.2021.663033
Received: 02 February 2021; Accepted: 26 May 2021;
Published: 07 July 2021.
Edited by:
Mary Marquart, University of Mississippi Medical Center, United StatesReviewed by:
Leila Vali, Kuwait University, KuwaitMariana Carmen Chifiriuc, University of Bucharest, Romania
Copyright © 2021 Jia, Zhu, Li, Kudinha, Yang, Zhang, Zhang, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiwen Yang, yangqiwen81@vip.163.com
†These authors have contributed equally to this work
 Peiyao Jia
Peiyao Jia Ying Zhu
Ying Zhu Xue Li1,3†
Xue Li1,3† Timothy Kudinha
Timothy Kudinha Ge Zhang
Ge Zhang Jingjia Zhang
Jingjia Zhang Yingchun Xu
Yingchun Xu Qiwen Yang
Qiwen Yang