- 1Department of Entomology, Plant Pathology and Nematology, University of Idaho, Moscow, ID, United States
- 2Department of Biological Sciences, University of Idaho, Moscow, ID, United States
The larval environment of holometabolous insects determines many adult life history traits including, but not limited to, rate and success of development and adult lifespan and fecundity. The ancient stress signaling hormone abscisic acid (ABA), released by plants inundated with water and by leaf and root fragments in water, is likely ubiquitous in the mosquito larval environment and is well known for its wide ranging effects on invertebrate biology. Accordingly, ABA is a relevant stimulus and signal for mosquito development. In our studies, the addition of ABA at biologically relevant levels to larval rearing containers accelerated the time to pupation and increased death of A. stephensi pupae. We could not attribute these effects, however, to ABA-dependent changes in JH biosynthesis-associated gene expression, 20E titers or transcript patterns of insulin-like peptide genes. Adult females derived from ABA-treated larvae had reduced total protein content and significantly reduced post blood meal transcript expression of vitellogenin, effects that were consistent with variably reduced egg clutch sizes and oviposition success from the first through the third gonotrophic cycles. Adult female A. stephensi derived from ABA-treated larvae also exhibited reduced lifespans relative to controls. Collectively, these effects of ABA on A. stephensi life history traits are robust, durable and predictive of multiple impacts of an important malaria vector spreading to new malaria endemic regions.
Introduction
In 2017, mosquito transmission resulted in 219 million new cases of malaria, 435,000 of which were fatal (World Health Organization [WHO], 2019). A large effort has been made to estimate the burden of malaria in endemic areas using defined parameters including abiotic factors, human clearance of malaria parasites, and mosquito life history traits, such as survival, population density, reproductive output, biting rate, and parasite development (Smith et al., 2018). Many important mosquito species contribute to parasite transmission, increasing the complexity of both surveillance and control efforts. Our focus is Anopheles stephensi, the Indian malaria mosquito, an aggressive malaria vector mosquito that has invaded and become established in Sri Lanka, Djibouti, and Ethiopia, with significant risk for range expansion into Somalia, Eritrea and Sudan (Faulde et al., 2014; Surendran et al., 2018; Seyfarth et al., 2019; Takken and Lindsay, 2019). In Djibouti, A. stephensi has been linked to a resurgence of severe infection with the human malaria parasite Plasmodium falciparum (Seyfarth et al., 2019), so increased focus on this species is relevant and timely for control.
Larval development in A. stephensi is rapid and, under favorable conditions, may be completed within a week from egg hatching. On the other hand, less than ideal larval environments, including for example over-crowding, have been shown to alter life history traits, significantly reducing parasite transmission (Moller-Jacobs et al., 2014; Murdock et al., 2017). Insect development is largely controlled by the regulatory effects of juvenile hormone (JH) and 20-hydroxyecdysone (20E) and our understanding of these details is derived primarily from studies in Manduca sexta, Drosophila melanogaster and Aedes aegypti (Jindra et al., 2013). In particular, JH acts to suppress differentiation of imaginal disk into adult structures when levels of 20E rise, instead maintaining larval structures and inducing larval-larval molts (Riddiford, 2012). In M. sexta and D. melanogaster, titers of JH decline to undetectable levels in the final larval instar due to reduced JH biosynthesis, elevated JH esterase activity and 20E-dependent transcription that induces metamorphosis (Vince and Gilbert, 1977; Brutis et al., 1990; Riddiford et al., 2000, 2010; Yin and Thummel, 2005; Liu et al., 2009). To date there has been no comprehensive analysis of JH titers over the course mosquito larval development. However, a significant body of work has been published on JH biosynthesis rates and transcriptional regulation of enzymes in adult A. aegypti (Noriega, 2014; Van Ekert et al., 2014). In this species, JH acid methyl transferase (JHAMT) is the ultimate enzyme in the principal JH biosynthesis pathway, converting inactive JH acid to active JH (Van Ekert et al., 2014).
Proteins and lipids stored from the larval diet contribute to proper ovarian development in female A. aegypti, with approximately half of the lipid stores carried forward to the adult stage (Zhou et al., 2004), such that females with lower larval protein reserves develop smaller follicles (Caroci et al., 2004). In addition to the direct effects of diet, nutrient status of newly emerged adults modulates the effects of insulin/insulin-like growth factor signaling (IIS) on JH synthesis, which remodels the fat body in preparation for a bloodmeal (Raikhel and Lea, 1983; Perez-Hedo et al., 2014). During the first gonotrophic cycle in A. aegypti, 80% of the lipids within the ovaries are derived solely from sugar meals acquired as adults (Ziegler and Ibrahim, 2001). Following the blood meal, amino acids derived from blood proteins activate the target of rapamycin (TOR) signaling pathway and ovary ecdysteroidogenic hormone is released from the brain, triggering 20E synthesis and release in the ovaries (Dhara et al., 2013). Following release from the ovaries, 20E signaling is initiated by 20E binding to the heterodimeric receptor comprised of the ecdysone receptor (EcR) and ultraspiracle (USP) to upregulate fat body synthesis of vitellogenin (Vg) that is transported to the developing eggs (Martìn et al., 2001). In the context of these changes, 20E delivered from the male during mating in Anopheles gambiae can also interact with the EcR, together with a novel protein Mating-Dependent Regulator of Oogenesis or MISO, to regulate oogenesis and the post-mating switch to monandry and oviposition (Baldini et al., 2013; Gabrieli et al., 2014). While this physiology is presumably conserved in A. stephensi based on the presence of an orthologous miso gene, both post-feeding and post-mating physiology likely also modulate the switch to physiological sensitivity to oviposition site attractants (Davis and Takahashi, 1980).
Anopheles stephensi females show a breeding habitat preference for natural bodies of water, ranging in size from small puddles to larger calm riverbeds (Manouchehri et al., 1976). Flood and ditch irrigation can impact the ecology of mosquitoes by creating new breeding sites and larval habitats, which are more attractive to gravid female mosquitoes than natural habitats (Mwangangi et al., 2010; Wondwosen et al., 2016). Initial flooding and fast water currents are destructive to larval survival, causing physical harm and reducing critical oxygen tension (Soleimani-Ahmadi et al., 2014). However, following flooding there is an increase in mosquito density and diversity, due to increased temporary breeding habitats (Rodrigures et al., 2017). During flooding, inundated plants can experience high levels of stress, which can result in the release of plant stress hormones into aquatic environments. For example, research has shown flooding of tomato plants increases the concentration of the stress hormone abscisic acid (ABA) in soil water by approximately 2.5-fold over control levels to ∼1.7 μM (Else et al., 1995).
Abscisic acid was first identified in plants, however, it is now recognized as a universal signaling molecule which acts as an effective regulator of stress responses and pathogen biology in plants, parasitic protozoa, sponges, hydroids, insects, and mammals (reviewed in Olds et al., 2018). The interaction of ABA and insects has been studied in several contexts. In the big-headed grasshopper, Aulocara elliotti, ingested ABA increased the number of eggs per female, however, eggs derived from ABA-treated females exhibited decreased viability (Visscher, 1980). Injection of ABA reduced protein uptake and Vg concentrations following consumption of a liver meal in the flesh fly Sarcophaga bullata (De Man et al., 1981). In addition, ABA injection appeared to act negatively on 20E signaling by delaying the peak of 20E by 16 h (De Man et al., 1981). ABA from nectar and pollen ingested by honeybees (Apis mellifera) can be detected in honey (Lipp, 1990) and ingestion of ABA by honeybee larvae can increase cold tolerance and cellular immunity (Negri et al., 2017; Ramirez et al., 2017).
In our previous studies, we observed that ingestion of an ABA-supplemented blood meal by female A. stephensi induced signaling kinases associated with a transient metabolic shift in the midgut, fueling immune-mediated killing of P. falciparum prior to completion of oocyst development (Glennon et al., 2016, 2017). Interestingly, ingested ABA did not decrease A. stephensi fecundity in the first gonotrophic cycle in contrast to our predictions based on the effects of ABA in A. elliotti and in S. bullata (Glennon et al., 2017). However, given the effects of ABA on metabolism and homeostasis of the A. stephensi midgut, on nutrient stores and 20E levels in other insects, and the potential that ABA in water used for oviposition could impact larval growth, we sought to understand whether ABA, at levels consistent with those released by inundated plants, could affect A. stephensi larval development, pupation and fitness of adult females emerging from treated larvae.
Our data show that, at concentrations in water as low as 1 μM, ABA accelerated A. stephensi larval development with varying effects on larval 20E levels and increased mosquito death at the time of pupation. Adult females derived from ABA-treated larvae exhibited significantly reduced fecundity over multiple gonotrophic cycles and significantly reduced lifespan, which was not altered by additional treatment of adult females with ABA. Accordingly, the effects of exposure of larval A. stephensi to ABA were both striking and durable, suggesting that manipulation of ABA levels in breeding sites, perhaps through nanoparticle release of this natural compound, could be used to reduce mosquito development and reproduction as well as adult survival that is required for completion of the extrinsic incubation period of malaria parasite development.
Materials and Methods
Mosquito Rearing
Anopheles stephensi Liston (Indian wild-type strain) were reared and maintained 27°C and 80% humidity with a 16/8-hour light/dark cycle. Adult mosquitoes were housed in 1 ft3 wire mesh cages and provided continuous access to 10% sucrose-soaked cotton pads. Three days after eclosion, adult female mosquitoes were allowed to feed on live mice sedated with ketamine (50 mg/kg) and xylazine (5 mg/kg) in sterile saline. All animal procedures were approved by the University of Idaho Animal Care and Use Committee. Mosquitoes were provided shallow cups of water to oviposit at 48 h after blood feeding. Eggs were gently washed into 5 L Nalgene pans with shallow water. Larvae were maintained in 5 L Nalgene pans on a solution of 2% powdered fish food (Sera Micron) and baker’s yeast in a 2:1 ratio for the first 3 days followed by Game Fish Chow pellet food (Purina) until pupation. Adult mosquitoes were collected for experiments within 12 h post-eclosion and housed in screened, 500 mL polypropylene Nalgene containers.
Effects of ABA on A. stephensi Larval Development and Pupation
Larvae were collected at 36 h following transfer of eggs into Nalgene pans to reduce variability in the starting age among larvae used for these studies. For each treatment group, 100 larvae were placed in 500 mL polypropylene Nalgene containers with 200 mL water with or without 1, 10 or 100 μM ABA (Caisson Labs). Due to the light sensitivity of ABA, 50 mL of water from each container was removed daily and replaced with freshly made ABA-supplemented water to yield a final concentration of 1, 10 or 100 μM ABA. Low concentrations of ABA (1 and 10 μM) were based on published soil water concentrations of flooded tomatoes (Else et al., 1995) and on our data from submerged tomato leaves and roots in water (Supplementary Table S1); the highest concentration (100 μM) was based on ABA treatment of A. elliotti from previous studies (Visscher, 1980). Larvae were fed as above and maintained through pupation and eclosion to adults. After the first pupae were observed, pupae were collected every 12 h until no larvae remained. The numbers of pupae collected each day were recorded as “time to pupation” in days. Collected pupae were placed into cups with untreated water within cartons for adult eclosion; pupal survival was monitored until all adults had emerged and these data were recorded as the proportion of total pupae surviving through to adult eclosion. Based on this design, A. stephensi were exposed to ABA during the larval stage only. Five separate cohorts were used to complete biological replicates of this study.
Effects of ABA on Adult A. stephensi Lifespan
Female mosquitoes derived from untreated, control larvae or from larvae treated with 1, 10 or 100 μM ABA were maintained in separate cartons with 10% sucrose-soaked cotton pads. At 3–5 days following eclosion, each group of mosquitoes was offered a “human blood meal” of washed human type O + erythrocytes (Interstate Blood Bank) suspended 1:1 (vol:vol) in heat-inactivated human type A + serum (Interstate Blood Bank). A similarly prepared blood meal was offered once weekly via a Hemotek feeder (Hemotek Ltd) until no mosquitoes remained alive. For one lifespan study, emerged adult female A. stephensi from each larval control and treatment group were split into two groups and treated as follows. One group of adults received an unsupplemented human blood meal each week whereas the other group was provided a weekly human blood meal supplemented with 100 nM ABA, which approximates the concentration of ABA present in blood in mice and humans with malaria (Glennon et al., 2016, 2018). Two days following blood feeding, females were given the opportunity to oviposit in a shallow water dish. Dead females were counted and removed from each group every 48 h. Two separate cohorts were used to complete biological replicates of the lifespan studies.
Effects of ABA on A. stephensi Fecundity
At 3–5 days after adult eclosion from groups prepared as in section “Effects of ABA on Adult A. stephensi Lifespan,” female A. stephensi were allowed to feed on a human blood meal. Following feeding, engorged females were carefully removed and placed into individual 50 mL conical tubes with moist filter paper and allowed to oviposit. Following oviposition, the number of eggs were counted and the females were returned to their respective control or treatment cartons. All females were held until they were fed again the following week. This process of feeding followed by separation and oviposition was repeated until females were no longer receptive to blood feeding. Four separate cohorts of mosquitoes were used to complete biological replicates of these studies.
qRT-PCR Assays for Relative Gene Expression
Relative transcript levels of A. stephensi 3-hydroxy-3-methylglutaryl-coa reductase (hmg-r), juvenile hormone acid methyltransferase (jhamt), insulin-like peptides 1-5 (ilp1-5; Marquez et al., 2011), and Vg were determined by qRT-PCR (Supplementary Table S2). All data were normalized to transcript levels of A. stephensi housekeeping genes ribosomal protein s7 (rps7) and rps17. For larval gene expression analyses, five larvae from control and ABA-treated water were collected and pooled for RNA isolation at 1, 2, 4 and 6 h following daily replacement of rearing water as described in section “Effects of ABA on A. stephensi Larval Development and Pupation.” For Vg expression analyses, five adult female A. stephensi were collected from control and ABA-treated larvae and pooled at 6, 12, 24, and 48 h post-blood feeding for RNA isolation. Adults were killed by briefly freezing at −20°C and pools of larvae or adults were placed in 500 μL Trizol (Invitrogen). RNA was extracted using the phenol-chloroform method according to manufacturer’s instructions. cDNA was synthesized using the QuantiTect reverse transcriptase kit (Qiagen) according to manufacturer’s instructions. cDNA concentrations were adjusted to 500 ng/μL with molecular grade water. Data were normalized to housekeeping genes and reported as Log2(2–Δ Δ Ct). For each treatment group 4-5 replicates were completed, each with three technical replicates.
Effects of ABA on 20-Hydroxyecdysone (20E) Titer
20E titers were measured during larval and pupal development and in adult female A. stephensi derived from control and ABA-treated larvae. For these analyses, 40 larvae, 20 pupae or 20 adult female mosquitoes were collected and pooled for each time point from each group. Larval collections started at 3 days post-hatching and continued until pupae were detected at ∼7 days post-hatching. Larvae were collected once a day at 8 h following daily replacement of rearing water as described in section “Effects of ABA on A. stephensi Larval Development and Pupation.” The pupal stage of A. stephensi lasts ∼36 h; pupae were collected every 8 h for the duration of this stage. Adult female mosquitoes were collected within the first 8 h following eclosion. Samples were prepared for analysis by adding 500 μL of 100% chilled methanol to pooled insects, then sonicating on ice (Fisher Scientific Model 100) at level 4 for 5 s intervals. Samples were centrifuged at 5000 × g for 5 min and the resulting supernatant transferred to a new tube. Methanol extraction was performed a second time and supernatants were pooled. Pooled supernatants were dried under N2 stream and stored at −30°C until the 20E titers were measured using 20-hydroxyecdysone EIA kit (Arbor Assays), following manufacturer’s instructions. Three separate cohorts of mosquitoes were used to complete biological replicates of these studies.
Effects of ABA on Adult Female A. stephensi Protein Content
To measure total protein content of adult female A. stephensi derived from control or ABA-treated larvae, 2 mosquitoes within 8 h of eclosion were homogenized in 10 mM dithiothreitol with 1 mM protease inhibitor cocktail (Sigma) in 100 μL loading buffer (Bio-Rad). Samples were boiled for 5 min and then centrifuged at 10,000 × g for 10 min at 4°C. Supernatants were transferred to new tubes and stored at −80°C. Total protein content was determined using Bradford reagent (Alfa Aesar) using bovine albumin serum (BSA) as a standard. Three separate cohorts of mosquitoes were used to complete biological replicates of these studies, each with three technical replicates.
Statistical Analyses
All statistical analyses were performed using R statistical software version 3.5.3. Time to pupation and clutch sizes were analyzed by ANOVA and post hoc Tukey’s test. Proportions of A. stephensi laying eggs and dying as pupae were analyzed using likelihood ratio test of independence (GTest). 20E titers by day were analyzed by Student’s t-test. Data from qRT-PCR assays were normalized by 2–ΔΔCT, log2 transformed and analyzed by ANOVA. Lifespan data were analyzed by two stage hazard rate analysis, in which the first stage is a log-rank test, and the second stage is used in cases where the hazard rates are not proportional and cross each other (Qiu and Sheng, 2008). Data across biological replicates within treatments were analyzed by ANOVA; if differences across replicates were not significant, replicate data were combined for analysis. Differences were considered significant at α ≤ 0.05. All figures were created using the ggplot2 package within R.
Results
ABA Treatment of A. stephensi Larvae Reduced Time to Pupation, but Not in Association With Rising 20E Titers
Abscisic acid treatment of larval A. stephensi reduced mean time to pupation from 7.22 ± 0.77 days in untreated controls to 7.03 ± 0.67 days (1 μM ABA), 7.01 ± 0.76 days (10 μM ABA) and 7.11 ± 0.68 days (100 μM ABA) in treated larvae (Figure 1; ANOVA p < 0.001). Times to pupation in larvae treated with 1 and 10 μM ABA were not different (Tukey p = 0.877), but exhibited the shortest mean time to pupation relative to control. Larvae treated with 100 μM ABA had higher mean time to pupation relative to larvae treated with 1 μM ABA (Tukey p < 0.001) and 10 μM ABA (Tukey p < 0.001), but still pupated faster than untreated control larvae (Tukey p < 0.001). Although accelerated larval development would be expected to produce smaller adults (Lyimo et al., 1992), larval treatment with 1, 10, and 100 μM ABA was associated with increased size of emerged adult female A. stephensi relative to females derived from control untreated larvae (Supplementary Table S3).
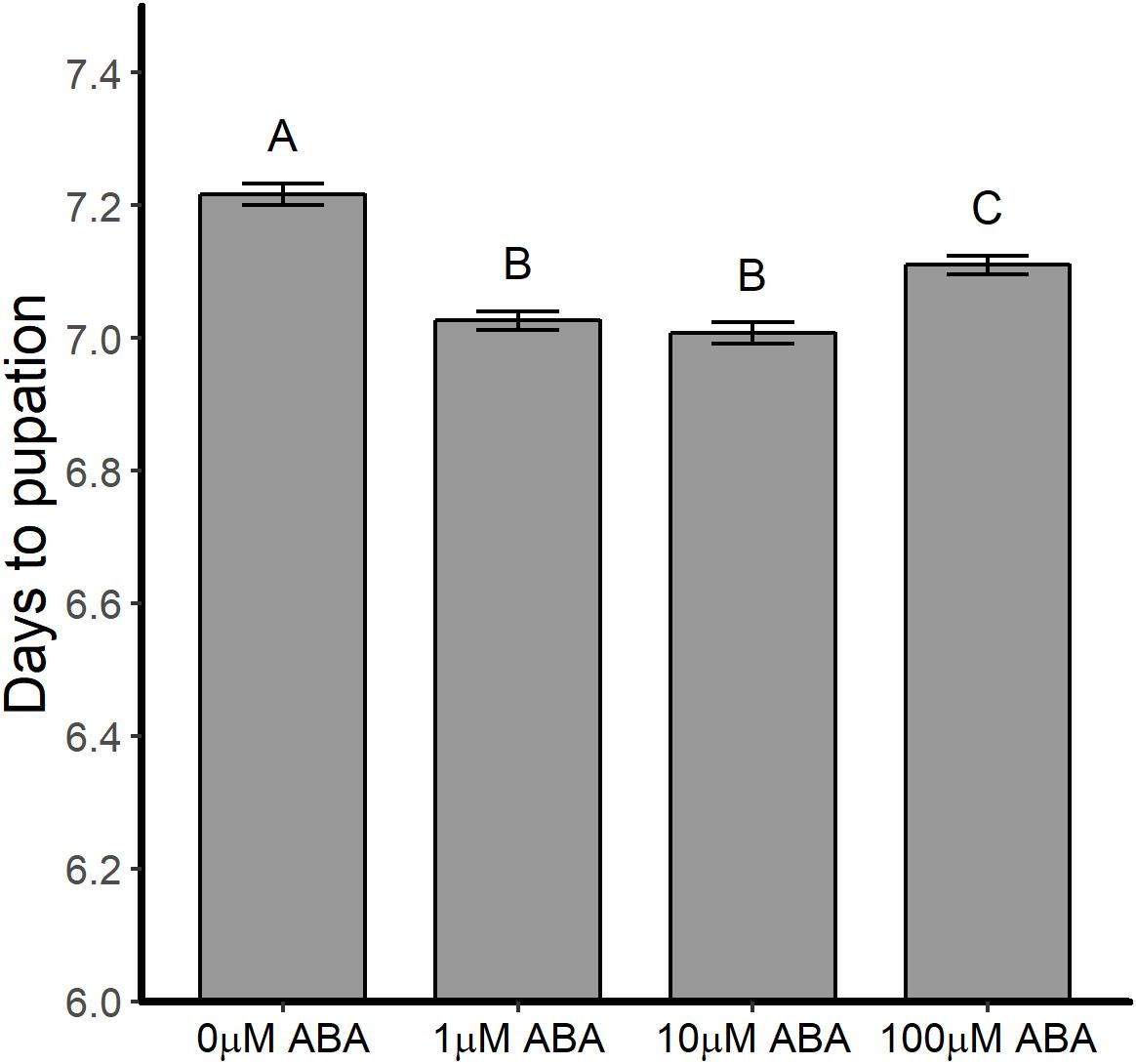
Figure 1. ABA treatment of larval A. stephensi reduced time to pupation. Larvae were exposed to 1, 10 or 100 μM ABA in rearing water as described in section “Effects of ABA on A. stephensi Larval Development and Pupation.” Time to pupation was reduced at all concentrations of ABA compared to control larvae (ANOVA p < 0.001). Data from five biological replicates were combined for analysis and are shown as mean ± SEM. Different capitalized letters indicate significant differences among controls and treatments. Control = 1456 larvae, 1 μM ABA = 1403 larvae, 10 μM ABA = 1382 larvae and 100 μM ABA = 1287 larvae.
In D. melanogaster, 20E regulates the timing of larval molts and, during the fourth and final instar, 20E titers rise to induce a cascade of transcriptional responses resulting in the physiological changes during the larval-pupal molt; studies in A. aegypti have shown similar 20E titers and expression of 20E-regulated genes (Margam et al., 2006; White and Ewer, 2014). Accordingly, we hypothesized that treatment of larvae with ABA might increase 20E titers earlier than in control larvae, resulting in reduced time to pupation in ABA-treated larvae. Based on the patterns observed in Figure 1, we analyzed 20E titers in larvae treated with the lowest (1 μM) and highest concentrations of ABA (100 μM) with untreated controls (Figure 2). Larvae exposed to 1 μM ABA showed no differences relative to controls in 20E titers over the course of larval development (days 1–7), during the pupal stage (days 8–9) or in newly emerged adult females (day 10). Larvae exposed to 100 μM ABA, however, had significantly reduced 20E titers on day 6 of larval development relative to controls (t-test p = 0.017), but no differences at any other timepoints. While there are no reports of 20E titers in A. stephensi larvae, our results are within the reported ranges for A. aegypti (Margam et al., 2006). These results suggested that the observed reduction in time to pupation in larvae exposed to ABA did not result from significantly elevated 20E titers in the final days of larval development.
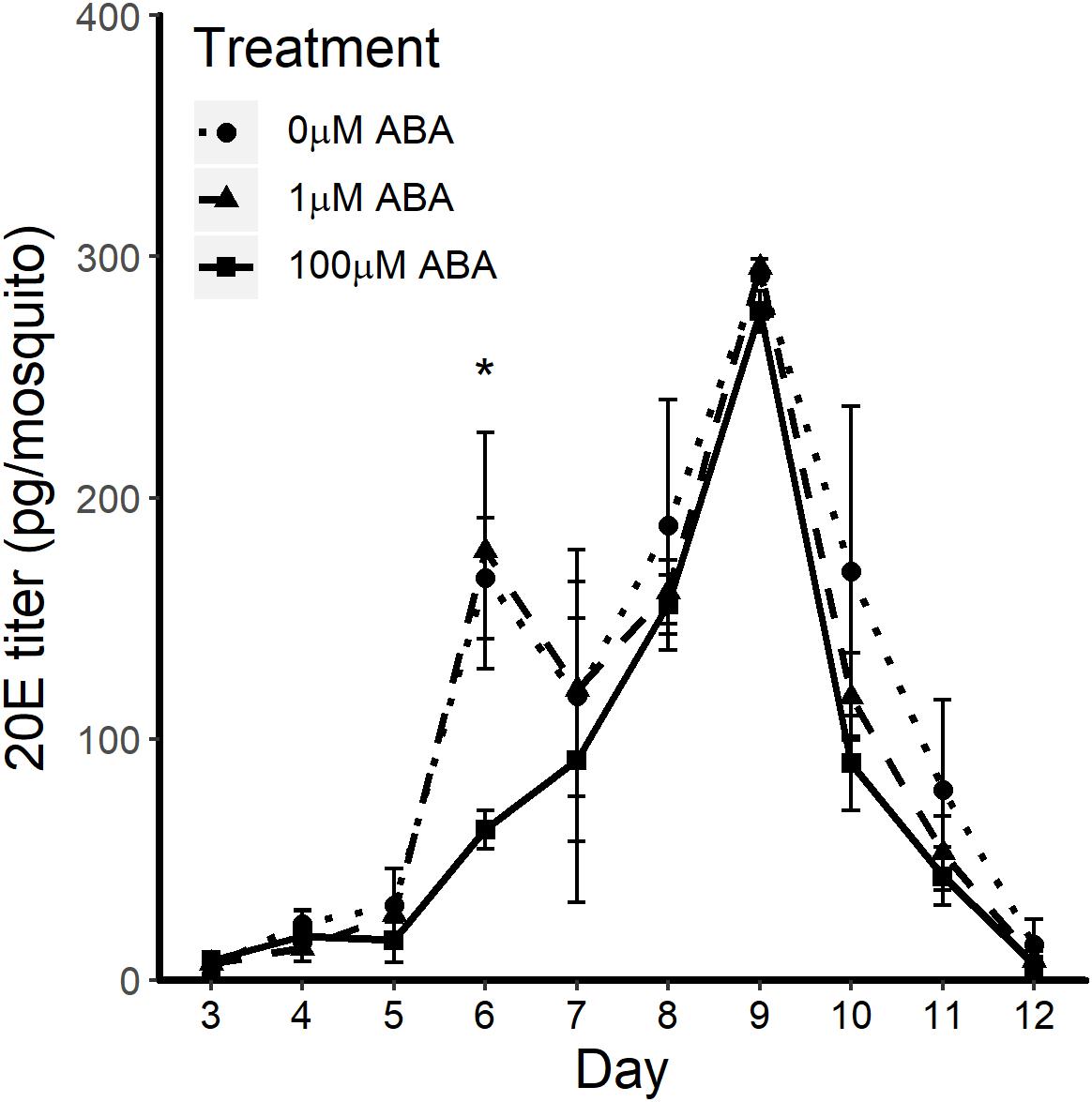
Figure 2. ABA treatment effects on 20E titers through larval development (days 1–7), during the pupal stage (days 8–9) and in newly emerged adult female A. stephensi (day 10) were minimal to undetectable. 20-hydroxyecdysone (20E) titers were measured from 20 to 40 individuals each day starting with second instar larvae from 3 days post-egg hatch through to 8 h post-eclosion of adult female mosquitoes. Larval exposure to 100 μM ABA reduced 20E titers on day 6 of larval development relative to controls (t-test p = 0.017), but no other differences were observed. Each biological sample was analyzed in triplicate to confirm assay reproducibility. Data from three biological replicates were combined for analysis and are shown as mean ± SEM.
ABA Did Not Alter Transcript Expression of JH-Associated Genes in Larval A. stephensi
Since ABA treatment was not associated with increased 20E titers, we examined the expression of key genes in the mevalonate pathway and the branch pathway that synthesizes juvenile hormone (JH). Studies in M. sexta during larval-larval molts have demonstrated that JH titers remain elevated to suppress expression of 20E target genes and, in the final instar, JH titers decrease to undetectable levels, at which point 20E gene targets are upregulated and the pupal molt is initiated (Boulan et al., 2015). In the insect mevalonate pathway, 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGCR) converts HMG-CoA to mevalonate, the precursor of farnesyl-pyrophosphate of the JH pathway. In the latter pathway, JH acid methyl transferase (JHAMT) methylates farnesoic acid to methylfarnesoate and is the terminal enzyme in JH biosynthesis in A. aegypti (Shinoda and Itoyama, 2003; Van Ekert et al., 2014). The gene expression profiles of hmgcr and jhamt were measured hourly for 6 h after daily replacement of rearing water as described in section “Effects of ABA on A. stephensi Larval Development and Pupation.” If expression levels of hmgcr and jhamt were down regulated in fourth instar larvae following exposure to ABA this could translate to reduced JH titers and earlier expression of 20E gene targets. However, we did not observe any significant changes in hmgcr expression relative to control in fourth instar larvae exposed to 1, 10 or with 100 μM ABA at 1 h (ANOVA p = 0.070), 2 h (ANOVA p = 0.275), 4 h (ANOVA p = 0.683) or 6 h post ABA treatment (ANOVA p = 0.239) relative to control (Supplementary Figure S1). There were also no significant changes in expression of jhamt in fourth instar larvae through the same 6 h period (ANOVA p = 0.144 for 1 h, p = 0.462 for 2 h, p = 0.054 for 4 h, p = 0.690 for 6 h post ABA treatment relative to control) (Supplementary Figure S1). Based on these data, it is unlikely that ABA treatment of A. stephensi fourth instar larvae alters JH titers in a pattern consistent with the effects of ABA on larval development.
ABA Did Not Alter Expression of ilp Genes in A. stephensi Larvae
We previously demonstrated that ABA supplementation in a blood meal reduced the expression of ilp3 and ilp4 in adult female A. stephensi (Glennon et al., 2017), suggesting that ABA might also alter ilp expression in the larval stage. In D. melanogaster larvae, decreased drosulfakinin (DSK) has been associated with increased feeding and reduced food selectivity, with decreased dsk mRNA levels detected in Dilp2,3,5 triple mutants but not Dilp5 mutants (Söderberg et al., 2012). Based on these observations and because we saw no increase in 20E titers or changes in transcript expression of hmgcr or jhamt, we reasoned that ABA might decrease ilp expression in A. stephensi larvae in the fourth instar, which could increase food intake and trigger earlier pupation. Exposure of A. stephensi fourth instar larvae to ABA, however, through 6 h after daily replacement of rearing water as described in section “Effects of ABA on A. stephensi Larval Development and Pupation” had no effect on transcript expression of ilp4 (ANOVA p = 0.362 for 1 h, p = 0.088 for 2 h, p = 0.276 for 4 h, p = 0.199 for 6 h post ABA treatment relative to control) or ilp5 (ANOVA p = 0.733 for 1 h, p = 0.876 for 2 h, p = 0.692 for 4 h, p = 0.397 for 6 h post ABA treatment relative to control) (Supplementary Figure S2). We also examined expression of ilp1-3 through the same 6 h period, but no significant differences relative to control were observed (not shown).
ABA Increased the Percentage of A. stephensi Pupae Dying Prior to Adult Eclosion
In addition to reducing the time to pupation, treatment of A. stephensi larvae with ABA resulted in a higher percentage of dead pupae (out of the total for each treatment group) relative to control. In the absence of ABA treatment, 3.4% of pupae died prior to adult eclosion (Figure 3). However, treatment with 10 and 100 μM ABA increased the percentage of dead pupae out of the total for each treatment group to 8.2 and 10.6% relative to control (Figure 3) (GTest p < 0.001 and p < 0.001, respectively). Accordingly, decreased time to pupation in ABA-treated larvae (Figure 1) was associated with increased pupal death prior to adult A. stephensi eclosion.
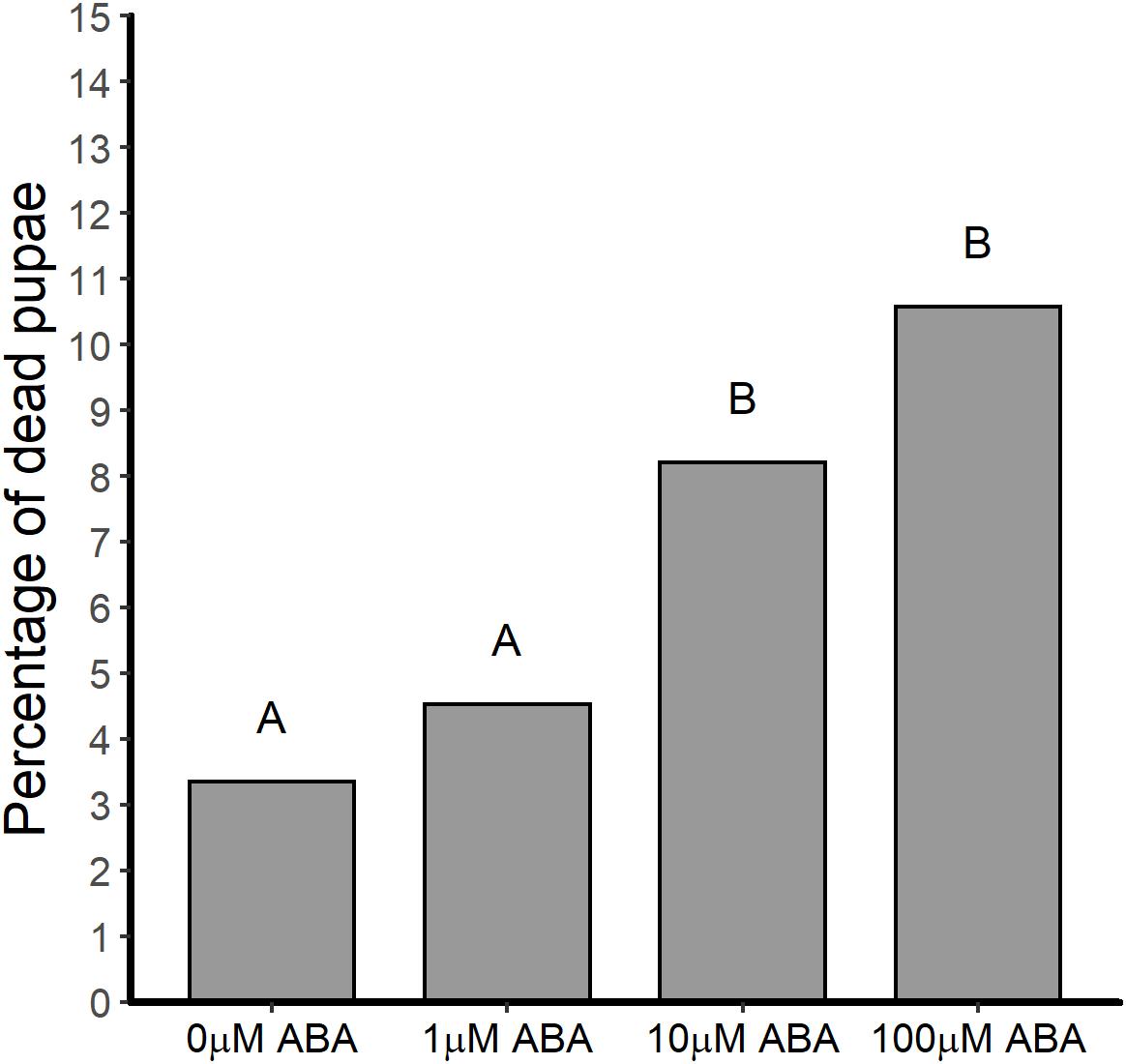
Figure 3. ABA treatment of larval A. stephensi increased the percentage of pupae dying prior to adult eclosion. While treatment of A. stephensi larvae with 1 μM ABA had no effect on pupae death relative to control (Gtest p = 0.249), larvae that were exposed to 10 and 100 μM ABA exhibited increased percentages of pupae death compared to control larvae (Gtest p < 0.001 and p < 0.001, respectively). Data are represented as percentages of pupae out of the total collected that died prior to adult eclosion. Data from three biological replicates were combined for analysis. Different capitalized letters indicate significant differences among controls and treatments. Control = 776 larvae, 1 μM ABA = 772 larvae, 10 μM ABA = 767 larvae and 100 μM = 700 larvae.
ABA Reduced the Protein Content in Newly Eclosed Female A. stephensi
Female mosquitoes break down proteins derived from blood meals to amino acids, activating the TOR pathway, which signals fat body 20E synthesis and the upregulation of yolk protein precursor (YPP) genes in this tissue (Hansen et al., 2005). The nutrients stored during the larval stage are essential for JH-regulated fat body competency to respond to TOR activation such that malnourished mosquitoes exhibit reduced and delayed Vg transcript expression (Shiao et al., 2008). Based on these observations, we sought to examine the protein content of newly eclosed female A. stephensi to better understand the potential effects of ABA larval treatment on adult life history traits. Protein content of adult female A. stephensi derived from untreated control larvae was 9.5 ± 0.92 μg/mosquito (Figure 4). In comparison, protein content of adult females derived from larvae treated with 1 μM ABA was 7.7 ± 0.54 μg/mosquito trended downward relative to control but was not significantly different (Figure 4). However, protein content of females derived from larvae treated with 100 μM ABA, 6.6 ± 0.38 μg/mosquito, was significantly lower than that in controls, but not significantly different from that of females derived from larvae treated with 1 μM ABA (Figure 4). Accordingly, increased body size in adult females derived from larvae treated with 10 and 100 μM ABA (Supplementary Table S3) was associated with a trend toward decreased protein content (10 μM ABA) and significantly reduced protein content (100 μM ABA) relative to controls.
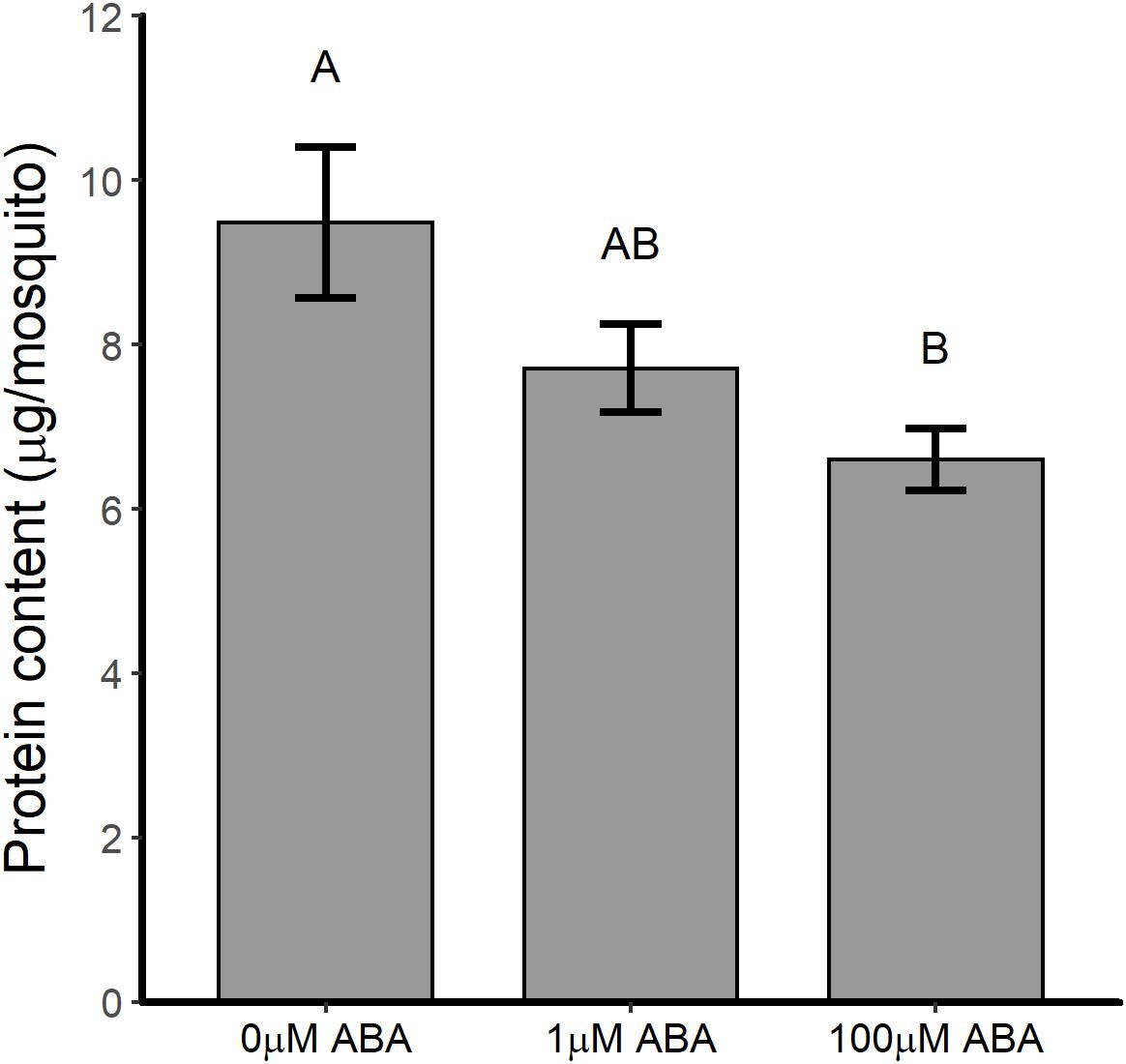
Figure 4. ABA treatment of larval A. stephensi reduced the protein content of newly eclosed adult females. Females emerged from larvae treated with 1 μM ABA exhibited a trend toward reduced protein content relative to controls (Tukey p = 0.214). Females emerged from larvae treated with 100 μM ABA exhibited reduced protein content relative to controls (Tukey p = 0.047), but were not significantly different from females emerged from larvae treated with 1 μM ABA (Tukey p = 0.493). Each biological sample was analyzed in triplicate to confirm assay reproducibility. Data from three biological replicates were combined for analysis and are shown as mean ± SEM. Different capitalized letters indicate significant differences among controls and treatments.
Adult Female A. stephensi Derived From ABA-Treated Larvae Exhibited Reduced Fecundity
Given the reduction in protein content of newly eclosed female A. stephensi derived from ABA-treated larvae and the impact of nutritional status on JH-regulated fat body competency, we examined the fecundity of female A. stephensi derived from ABA-treated control larvae. In replicated assays, the percentages of females ovipositing and clutch sizes of female A. stephensi derived from ABA-treated larvae compared to females derived from untreated control larvae were variably reduced across three gonotrophic cycles.
For the first gonotrophic cycle adult females derived from larvae treated with 1 and 100 μM ABA had reduced clutch sizes (ANOVA F = 15.195, p < 0.001; Tukey p < 0.001) relative to controls (Figure 5A). In addition to smaller clutch sizes in the first gonotrophic cycle, females derived from larvae treated with 100 μM ABA treatment were less likely to oviposit compared to controls (GTest p = 0.013) (Figure 5B). There was no effect of larval ABA treatment on clutch sizes in the second gonotrophic cycle (ANOVA F = 0.009, p = 0.99; Figure 5C) and an increase in the percentage of ovipositing females derived from larvae treated with 1 μM ABA mosquitoes compared to controls (GTest p = 0.038). As in the first gonotrophic cycle, however, there was a decrease in the percentage of ovipositing females derived from larvae treated with 100 μM ABA compared to controls (GTest p < 0.001; Figure 5D). By the third gonotrophic cycle, clutch sizes from females derived from larvae treated with ABA were again reduced relative to controls (ANOVA F = 4.49, p = 0.012; Tukey p = 0.017 for 1 μM ABA, Tukey p = 0.034 for 100 μM ABA; Figure 5E). However, in contrast to both prior gonotrophic cycles, there were no differences in the percentages of ovipositing females among the groups (GTest p = 0.62; Figure 5F).
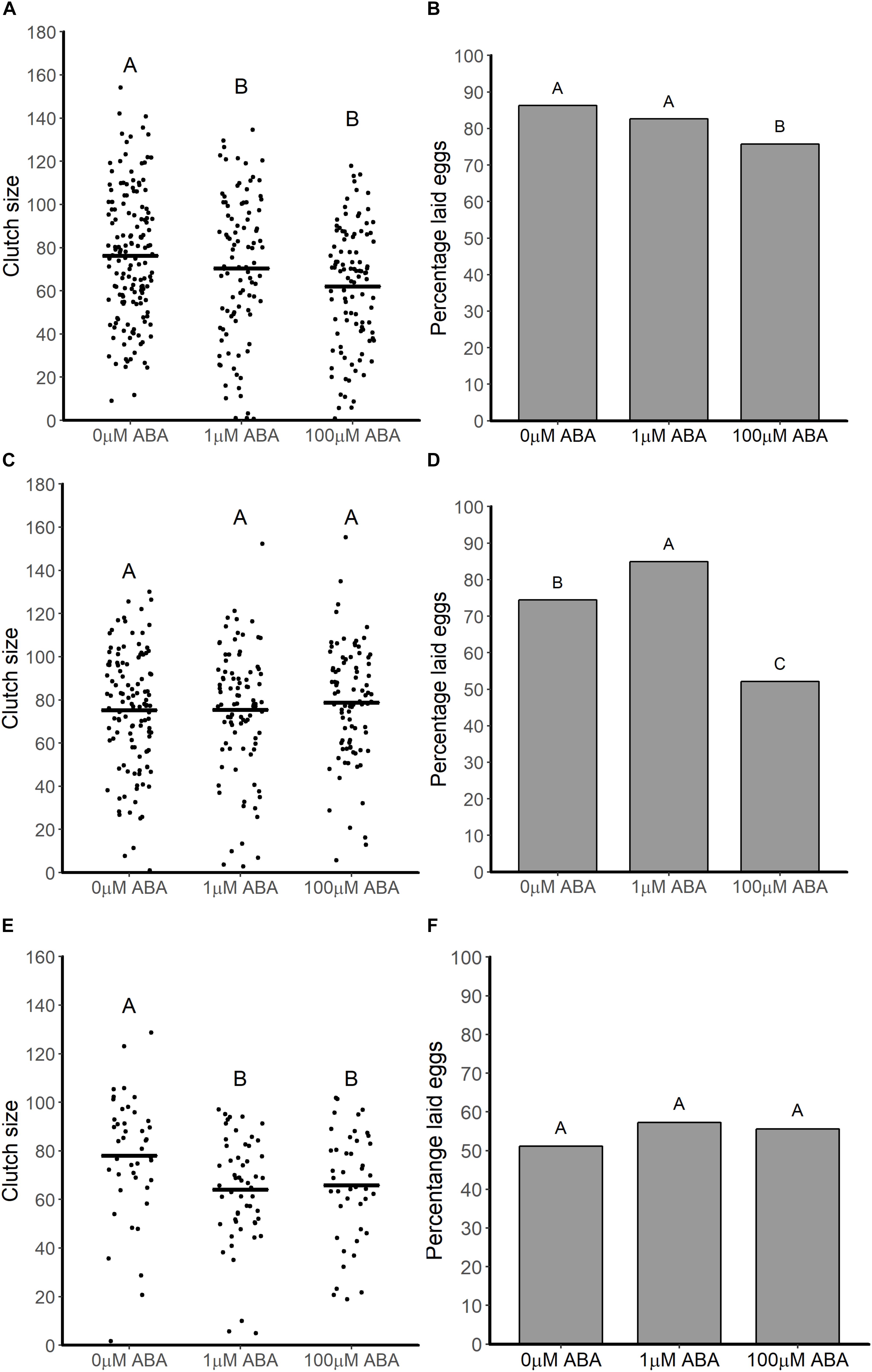
Figure 5. ABA treatment of larval A. stephensi variably reduced clutch sizes and percentages of ovipositing adult females across the first (A,B), second (C,D) and third (E,F) gonotrophic cycles. (A) Female mosquitoes derived from larvae treated with 1 and 100 μM ABA had reduced clutch sizes in the first gonotrophic cycle compared to controls (Tukey p < 0.001 and p < 0.001, respectively). Each dot represents the clutch size of a single female mosquito from four biological replicates; black bars represent means. (B) Treatment of larvae with 100 μM ABA reduced the percentage of ovipositing females relative to controls in the first gonotrophic cycle (Gtest p = 0.013). Data are shown as the means from three biological replicates, n = 389. (C) Larval treatment with ABA had no effect on clutch sizes of adult female A. stephensi (ANOVA F = 0.009, p = 0.99) in the second gonotrophic cycle. Each data point represents the clutch size of a single female mosquito from four biological replicates; black bars represents means. (D) Treatment of larvae with 1 μM ABA increased the percentage of ovipositing females mosquitoes compared to controls in the (GTest p = 0.038), while treatment of larvae with 100 μM ABA reduced the percentage of ovipositing females compared to controls (GTest p < 0.001). Data are shown as the means of three biological replicates, n = 298. (E) Larval treatment with ABA reduced clutch size in the third gonotrophic cycle (ANOVA p = 0.012; Tukey p = 0.017 for 1 μM ABA, Tukey p = 0.034 for 100 μM ABA). Each dot represents the clutch size of a single female mosquito from four biological replicates, black bars represent the mean of each treatment. (F) Percentage of fully engorged A. stephensi that laid eggs was not affected by larval treatment with ABA (GTest p = 0.62). Data are shown as the means of three biological replicates, n = 141.
Post-blood Meal Vg Transcript Expression in Adult Female A. stephensi Derived From ABA-Treated Larvae Was Delayed Relative to Controls
Based on the effects of ABA larval treatment on adult fecundity, we sought to quantify the expression of Vg, the major YPP gene, in adult female A. stephensi within the first 48 h following a bloodmeal. The pattern of Vg expression in control females derived from untreated larvae followed the expected pattern of expression rising to a peak at 24 h, then declining (Figure 6). Adult females derived from larvae treated with 1 and 100 μM ABA showed significantly reduced Vg expression at 24 h post-blood meal (ANOVA p < 0.001, Tukey p < 0.001 for 1 μM ABA; Tukey p < 0.001 for 100 μM ABA) (Figure 6), suggesting that the effects of ABA larval treatment on adult female fecundity are at least partially explained by a significant reduction in Vg expression post-blood meal.
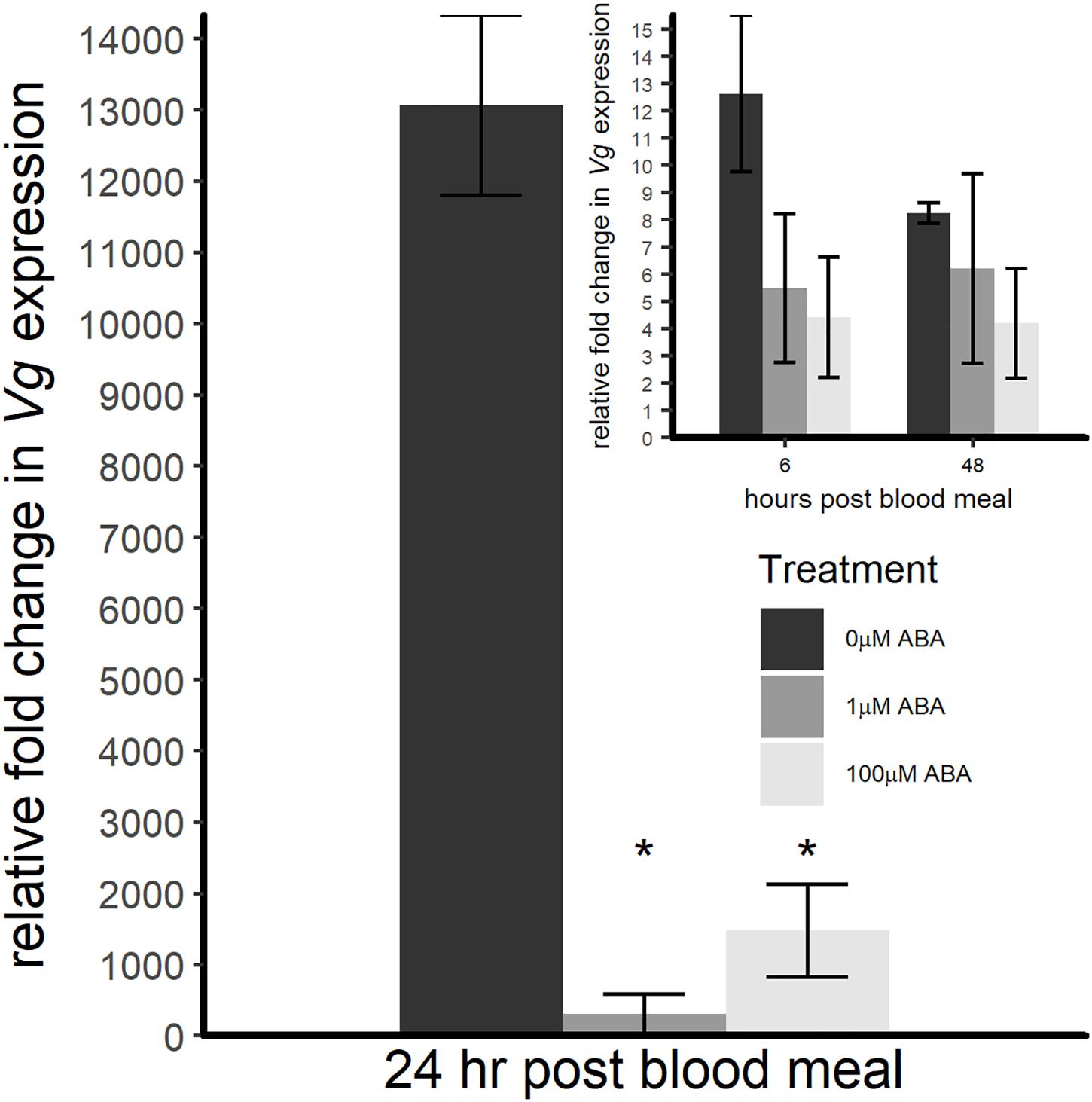
Figure 6. ABA treatment of larval A. stephensi reduced Vg transcript expression in adult female mosquitoes during the first 24 h following a blood meal. At 6 h post-blood meal (inset), there was no difference in Vg expression in females derived from larvae treated with 1 or 100 μM ABA compared to control (ANOVA F = 0.722, p = 0.519). At 24 h post-blood meal, however, Vg expression levels of females derived from larvae treated with 1 μM and 100 μM ABA were significantly reduced relative to controls (ANOVA F = 56.374, p < 0.001; Tukey ∗p < 0.001 for 1 μM ABA, Tukey ∗p < 0.001 for 100 μM ABA). At 48 h post-blood meal (inset), there was no difference in Vg expression in either ABA group compared to control (ANOVA F = 0.518, p = 0.616). Data are shown for three biological replicates as fold change normalized within treatment group to Vg expression before the blood meal.
Adult Female A. stephensi Derived From ABA-Treated Larvae Exhibited Reduced Lifespan
Based on our observations of reduced protein content (Figure 4) and reduced fecundity (Figure 5) in adult female A. stephensi derived from ABA-treated larvae and evidence for tradeoffs between mosquito lifespan and reproduction (Harshman and Zera, 2007; Faiman et al., 2017), we predicted that ABA treatment of larvae might extend the lifespan of adult female A. stephensi. For this study, females derived from untreated control larvae and from larvae treated with 1 and 100 μM ABA received a blood meal once a week with maintenance on 10% sucrose between blood meals. For an additional study, adult females derived from control and treated larvae were each split into two groups, with one group receiving no ABA in the weekly blood meals and the other group receiving 100 nM ABA in the weekly blood meals. In contrast to our prediction, median survival in adult females derived from larvae treated with ABA was reduced to 28 ± 10.78 days (1 μM ABA; log rank p = 0.028) and to 30 ± 14.22 days (100 μM ABA; two-stage p = 0.025) relative to the untreated control median lifespan of 34 ± 14.2 days (Figure 7). Consistent with previous observations of no effect of ABA in blood on adult female lifespan (Glennon et al., 2017), the addition of 100 nM ABA in the blood meal had no effect on adult lifespan nor did it alter the effects of larval treatment with ABA on adult lifespan (Supplementary Table S4).
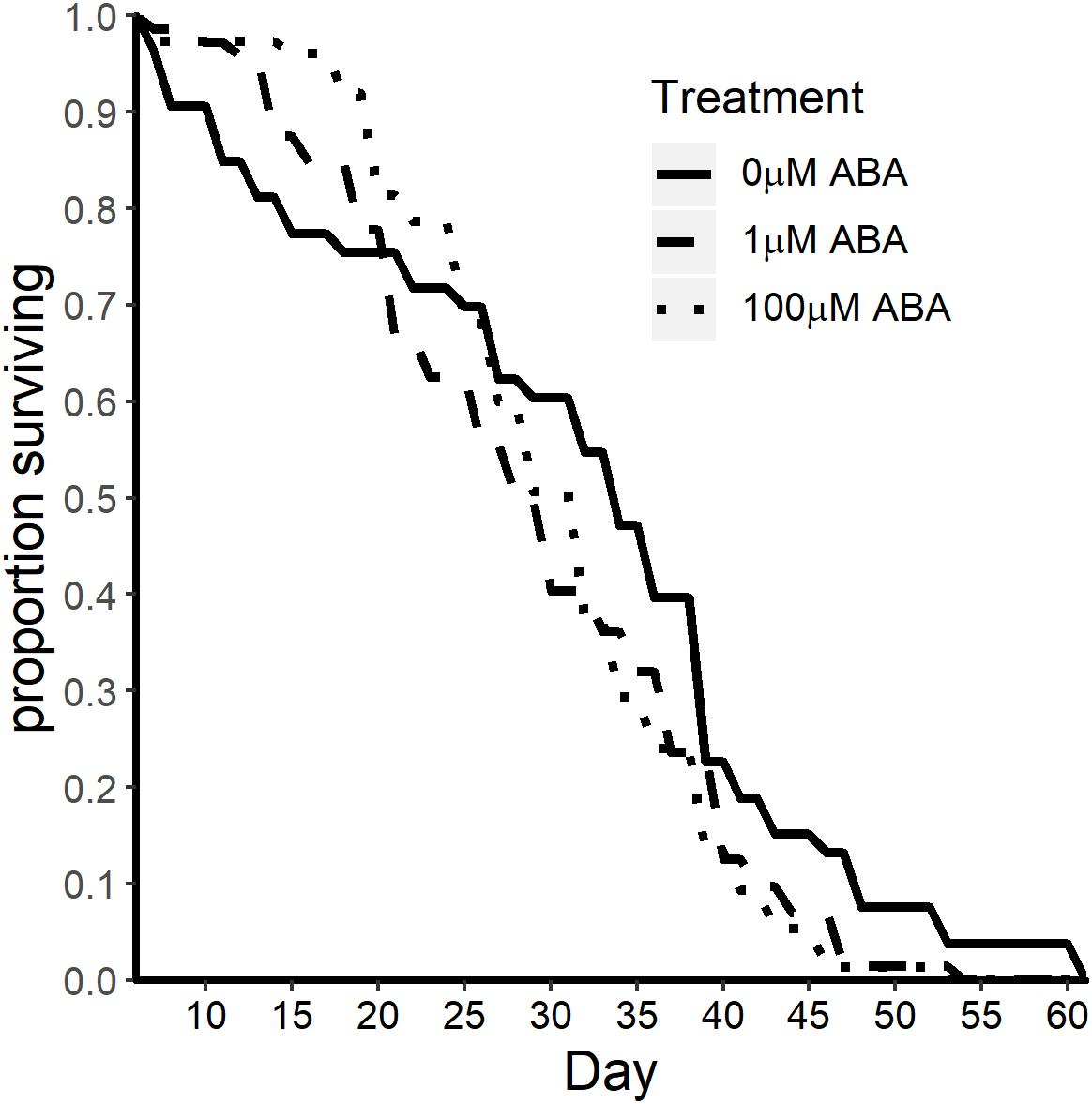
Figure 7. ABA treatment of larval A. stephensi reduced adult female survival. Survival curves of adult female mosquitoes derived from control untreated larvae and larvae treated with 1 μM ABA (log-rank p = 0.028) and 100 μM ABA (two-stage p = 0.025). Median survival was reduced from 34 ± 14.2 days in controls to 28 ± 10.78 days (1 μM ABA) and to 30 ± 14.22 days (100 μM ABA). Data are representative of two biological replicates.
Discussion
Taken together, our data demonstrate that the effects of treating A. stephensi larvae with ABA are durable, starting with accelerated pupation and increased pupal death and lasting through multiple gonotrophic cycles to reduce fecundity and adult female survivorship. These effects indicate the potential for multiple population level impacts on mosquito density, biting and pathogen transmission through combined reductions in immature stages, fecundity and lifespan at ABA concentrations that could occur under natural conditions (Else et al., 1995). In trying to understand these effects of ABA, we examined some obvious developmental cues in our studies. Accelerated pupation in holometabolous insects can result from blocking JH synthesis or activity. For example, removal of the corpora allata, which generates JH, results in precocious pupation in Manduca sexta due to a reduction in the critical weight threshold (Nijhout and Williams, 1974). Despite the obvious similarities in accelerated pupation, ABA had no effect on the expression of the JH synthesis-associated genes hmgcr and jhamt. Further, ABA affected 20E titers on only a single day of larval development (day 6) at the highest concentration of ABA (100 μM), affirming minimal to no effect of ABA on the interacting effects of JH and 20E. Our data contrast with the reported effects of ABA on 20E in adult S. bullata (De Man et al., 1981), suggesting the likely possibility that the effects of ABA vary to some degree across insect species. In D. melanogaster, reduced food seeking behavior is mediated by the overexpression and release of ILP2 and ILP4 (Wu et al., 2005), suggesting that reduced expression of ilps in our ABA treated larvae might be associated with increased food seeking behavior. However, expression of ilp genes in fourth instar A. stephensi larvae was not altered by ABA treatment. While the current state of technology is not sufficient to quantify ILPs in A. stephensi, we can reasonably conclude that JH, 20E and changes in ILP levels are not mediating the acceleration of development by ABA. Future work regarding potential effects of ABA on A. stephensi larval feeding behavior could focus on sulfakinin and neuropeptide F, both of which modulate feeding behavior in D. melanogaster larvae (Söderberg et al., 2012; Fadda et al., 2019). Both neuropeptides are also known to be conserved in A. gambiae (Strand et al., 2016) and are represented as orthologs in A. stephensi1.
The protein content of newly eclosed adult females derived from larvae treated with ABA was reduced, perhaps as a consequence of accelerated development and increased adult size. Following a blood meal, malnourished mosquitoes have both delayed and reduced Vg transcript abundance and may require a second blood meal for proper egg development (Shiao et al., 2008). The reduction in Vg expression that we observed is consistent with reduced Vg levels in S. bullata injected with ABA (De Man et al., 1981) and reduced Vg levels reported for malnourished A. aegypti (Shiao et al., 2008). Reduced protein content has been shown to result in smaller follicle size and increased resorption of oocytes (Caroci et al., 2004; Clifton and Noriega, 2011). Our observed association between reduced Vg mRNA expression and decreased fecundity is consistent with other studies showing 50% of Vg depleted mosquitoes still produced mature eggs (Rono et al., 2010). In our studies, reduced protein content of newly eclosed adult females likely contributed to lower clutch sizes and reduced oviposition in the first gonotrophic cycle (following a blood meal at 4 days in week 1). Patterns of oviposition and fecundity in the second and third gonotrophic cycles (weeks 2–3) could reflect the fact that nutrients for optimizing reproduction and preserving the soma are limited. In fact, the inflection points in our lifespan data – which occur at 21 days (Figure 7) for 1 μM ABA and 26 days for 100 μM ABA – are consistent with the possibility that the cost of reproduction, even at reduced levels, outweighs any further investment in the soma of females derived from treated larvae relative to controls, which have consistently higher survivorship after these timepoints.
While dietary restriction and the resulting impacts on nutrient stores have been associated with lifespan extension in Anopheles mosquitoes (Faiman et al., 2017), we observed reduced survivorship with reduced body protein levels in A. stephensi derived from larvae treated with ABA. Reduced survival of ABA-treated mosquitoes was unexpected as weekly supplementation of adults with ABA in blood meals did not change adult female survival (Glennon et al., 2017). Our data indicate that the substantial effect of ABA on adult lifespan carries over from the larval stage and across not one, but two developmental transitions.
Study of the role of plants and plant biology in regulating mosquito life history has focused on the characteristics of oviposition sites that are shaped by both wild and cultivated plant species (Omlin et al., 2007; Overgaard, 2007; Eneh et al., 2016; Wondwosen et al., 2016; Asmare et al., 2017; Wondwosen et al., 2017; Wondwosen et al., 2018), on nectar feeding and its effects on mosquito physiology (Nikbakhtzadeh et al., 2014; Nyasembe et al., 2014; Jacob et al., 2018), the potential role of invasive plants in promoting malaria parasite transmission (Stone et al., 2018), and the utility of plant-derived compounds as novel insecticides (Govindarajan et al., 2008; Nathan et al., 2008; Elango et al., 2009; Elimam et al., 2009). Here, we have taken the relationship between plant biology and mosquito biology a step further, connecting the effects of ABA, a universal signaling molecule first described in and well known from plants (Olds et al., 2018), at concentrations detected in water with submerged plant tissue that can alter substantial features of mosquito growth, development and survivorship across immature and adult stages. Together with our previous studies on the effects of ABA on P. falciparum development in A. stephensi (Glennon et al., 2017), the association of elevated blood levels of ABA with asymptomatic malaria in humans and reduced infection and disease pathology in our animal model of malaria (Glennon et al., 2018), the effects of ABA on the life cycle of malaria are both comprehensive and complex and will become undoubtedly more so with continued studies of mechanisms underlying this biology.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
All animal procedures were approved by the University of Idaho Animal Care and Use Committee.
Author Contributions
DT and SL designed the experiments and wrote the manuscript. RH designed and conducted the fecundity studies. BT assisted in completing various studies and provided input for experimental design. CO contributed to writing and editing the manuscript.
Funding
Funding for these studies was provided by the University of Idaho and the UI College of Agricultural and Life Sciences, Moscow, ID, United States.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Sarah M. Garrison and Jason Jackson for their assistance with mosquito rearing and colony maintenance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03024/full#supplementary-material
Footnotes
References
Asmare, Y., Hill, S. R., Hopkins, R. J., Tekie, H., and Ignell, R. (2017). The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar. J. 16:65. doi: 10.1186/s12936-017-1717-z
Baldini, F., Gabrieli, P., South, A., Valim, C., Mancini, F., and Catteruccia, F. (2013). The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 11:e1001695. doi: 10.1371/journal.pbio.1001695
Boulan, L., Milán, M., and Léopold, P. (2015). The systemic control of growth. Cold Spring Harb. Perspect. Biol. 7, 1–29. doi: 10.1101/cshperspect.a019117
Brutis, K. C., Thummel, C. S., James, C. W., Karim, F. D., and Hogness, D. S. (1990). The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell 61, 85–91. doi: 10.1016/0092-8674(90)90217-3
Caroci, A. S., Li, Y., and Noriega, F. (2004). Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. J. Exp. Biol. 207, 2685–2690. doi: 10.1242/jeb.01093
Clifton, M. E., and Noriega, F. G. (2011). Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 57, 1274–1281. doi: 10.1016/j.jinsphys.2011.06.002
Davis, E. E., and Takahashi, F. T. (1980). Humoral alteration of chemoreceptor sensitivity in the mosquito. Olfaction Taste 7, 139–142.
De Man, W., De Loof, A., Briers, T., and Huybrechts, R. (1981). Effect of abscisic acid on vitellogenesis in Sarcophaga bullata. Entomol. Exp. Appl. 29, 259–267. doi: 10.1111/j.1570-7458.1981.tb03068.x
Dhara, A., Eum, J., Robertson, A., Gulia-nuss, M., Vogel, K. J., Clark, K. D., et al. (2013). Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 43, 1100–1108. doi: 10.1016/j.ibmb.2013.09.004
Elango, G., Bagavan, A., Kamaraj, C., Zahir, A. A., and Rahuman, A. A. (2009). Oviposition-deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae). Parasitol. Res. 103, 691–695. doi: 10.1007/s00436-009-1593-8
Elimam, A. M., Elmalik, K. H., and Ali, F. S. (2009). Larvicidal, adult emergence inhibition and oviposition deterrent effects of foliage extract from Ricinus communis L. against Anopheles arabiensis and Culex quinquefasciatus in Sudan. Trop. Biomed. 26, 130–139.
Else, M. A., Hall, K. C., Arnold, G. M., Davies, W. J., and Jackson, M. B. (1995). Export of abscisic acid, 1-aminocyclopropane-1-carboxylic acid, phosphate, and nitrate from roots to shoots of flooded tomato plants (accounting for effects of xylem sap flow rate on concentration and delivery). Plant Physiol. 107, 377–384. doi: 10.1104/pp.107.2.377
Eneh, L. K., Saijo, H., Karin, A., Karlson, B., Lindh, J. M., and Rajarao, G. K. (2016). Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass Cyperus rotundus. Malar. J. 15:478. doi: 10.1186/s12936-016-1536-7
Fadda, M., Hasakiogullari, I., Temmerman, L., Beets, I., Zels, S., and Schoofs, L. (2019). Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front. Endocrinol. 10:64. doi: 10.3389/fendo.2019.00064
Faiman, R., Solon-biet, S., Sullivan, M., Huestis, D. L., and Lehmann, T. (2017). The contribution of dietary restriction to extended longevity in the malaria vector Anopheles coluzzii. Parasit. Vectors 10:156. doi: 10.1186/s13071-017-2088-6
Faulde, M. K., Rueda, L. M., and Khaireh, B. A. (2014). First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 139, 39–43. doi: 10.1016/j.actatropica.2014.06.016
Gabrieli, P., Kakani, E. G., Mitchell, S. N., Mameli, E., Want, E. J., Anton, A. M., et al. (2014). Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 111, 16353–16358. doi: 10.1073/pnas.1410488111
Glennon, E. K. K., Adams, L. G., Hicks, D. R., Dehesh, K., and Luckhart, S. (2016). Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. Am. J. Trop. Med. Hyg. 94, 1266–1275. doi: 10.4269/ajtmh.15-0904
Glennon, E. K. K., Megawati, D., Torrevillas, B. K., Ssewanyana, I., Huang, L., Aweeka, F., et al. (2018). Elevated plasma abscisic acid is associated with asymptomatic falciparum malaria and with IgG-/caspase-1-dependent immunity in Plasmodium yoelii-infected mice. Sci. Rep. 8:8896. doi: 10.1038/s41598-018-27073-1
Glennon, E. K. K., Torrevillas, B. K., Morrissey, S. F., Ejercito, J. M., and Luckhart, S. (2017). Abscisic acid induces a transient shift in signaling that enhances NF-κB-mediated parasite killing in the midgut of Anopheles stephensi without reducing lifespan or fecundity. Parasit. Vectors 10:333. doi: 10.1186/s13071-017-2276-4
Govindarajan, M., Jebanesan, A., Pushpanathan, T., and Samidurai, K. (2008). Studies on effect of Acalypha indica L. (Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 103, 691–695. doi: 10.1007/s00436-008-1032-2
Hansen, I. A., Attardo, G. M., Roy, S. G., and Raikhel, A. S. (2005). Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 280, 20565–20572. doi: 10.1074/jbc.M500712200
Harshman, L. G., and Zera, A. J. (2007). The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86. doi: 10.1016/j.tree.2006.10.008
Jacob, J. W., Tchouassi, D. P., Lagat, Z. O., Mathenge, E. M., Mweresa, C. K., and Torto, B. (2018). Independent and interactive effect of plant- and mammalian- based odors on the response of the malaria vector, Anopheles gambiae. Acta Trop. 185, 98–106. doi: 10.1016/j.actatropica.2018.04.027
Jindra, M., Palli, S. R., and Riddiford, L. M. (2013). The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204. doi: 10.1146/annurev-ento-120811-153700
Lipp, J. (1990). Nachweis und herkunft von abscisinsäure und prolin in honig. Apidiologie 21, 249–259. doi: 10.1051/apido:19900310
Liu, Y., Sheng, Z., Liu, H., Wen, D., He, Q., Wang, S., et al. (2009). Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 136, 2015–2025. doi: 10.1242/dev.033712
Lyimo, E. O., Takken, W., and Koella, J. C. (1992). Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol. Exp. Appl. 63, 265–271. doi: 10.1111/j.1570-7458.1992.tb01583.x
Manouchehri, A. V., Javadian, E., Eshghy, N., and Motabar, M. (1976). Ecology of Anopheles stephensi Liston in southern Iran. Trop. Geogr. Med. 28, 228–232.
Margam, V. M., Gelman, D. B., and Palli, S. R. (2006). Ecdysteroid titers and developmental expression of ecdysteroid-regulated genes during metamorphosis of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). J. Exp. Biol. 52, 558–568. doi: 10.1016/j.jinsphys.2006.02.003
Marquez, A. G., Pietri, J. E., Smithers, H. M., Nuss, A., Antonova, Y., Drexler, A. L., et al. (2011). Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 173, 303–312. doi: 10.1016/j.ygcen.2011.06.005
Martìn, D., Wang, S., and Raikhel, A. S. (2001). The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol. Cell. Endocrinol. 173, 75–86. doi: 10.1016/s0303-7207(00)00413-5
Moller-Jacobs, L. L., Murdock, C. C., and Thomas, M. B. (2014). Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit. Vectors 7:593. doi: 10.1186/s13071-014-0593-4
Murdock, C. C., Evans, M. V., McClanahan, T. D., Miazgowicz, K. L., and Tesla, B. (2017). Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 11:e0005640. doi: 10.1371/journal.pntd.0005640
Mwangangi, J. M., Muriu, S., Mbogo, C. M., Githure, J., Shililu, J., Muturi, E. J., et al. (2010). Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar. J. 9:228. doi: 10.1186/1475-2875-9-228
Nathan, S. S., Hisham, A., and Jayakumar, G. (2008). Larvicidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia 79, 106–111. doi: 10.1016/j.fitote.2007.07.013
Negri, P., Ramirez, L., Quintana, S., Szawarski, N., Maggi, M., Le Conte, Y., et al. (2017). Dietary supplementation of honey bee larvae with arginine and abscisic acid enhances nitric oxide and granulocyte immune responses after trauma. Insects 8:E85. doi: 10.3390/insects8030085
Nijhout, H. F., and Williams, C. M. (1974). Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta. J. Exp. Biol. 61, 493–501.
Nikbakhtzadeh, M. R., Terbot, J. W. II., Otienoburu, P. E., and Foster, W. A. (2014). Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J. Vector Ecol. 39, 372–383. doi: 10.1111/jvec.12113
Noriega, F. G. (2014). Juvenile hormone biosynthesis in insects: what is new, what do we know, and what questions remain? Int. Sch. Res. Notices 2014:967361. doi: 10.1155/2014/967361
Nyasembe, V. O., Teal, P. E. A., Sawa, P., Tumlinson, J. H., Borgemeister, C., and Baldwyn, T. (2014). Plasmodium falciparum-infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr. Biol. 24, 217–221. doi: 10.1016/j.cub.2013.12.022
Olds, C. L., Glennon, E. K. K., and Luckhart, S. (2018). Abscisic acid: new perspectives on an ancient universal stress signaling molecule. Microbes Infect. 20, 484–492. doi: 10.1016/j.micinf.2018.01.009
Omlin, F. X., Carlson, J. C., Ogbunugafor, C. B., and Hassanali, A. (2007). Anopheles gambiae exploits the treehole ecosystem in western Kenya: a new urban malaria risk? Am. J. Trop. Med. Hyg. 77, 264–269. doi: 10.4269/ajtmh.2007.77.264
Overgaard, H. J. (2007). Effect of plant structure on oviposition behavior of Anopheles minimus s.l. J. Vector Ecol. 32, 193–197.
Perez-Hedo, M., Rivera-Perez, C., and Noriega, F. G. (2014). Starvation increases insulin sensitivity and reduces juvenile hormone synthesis in mosquitoes. PLoS One 9:e86183. doi: 10.1371/journal.pone.0086183
Qiu, P., and Sheng, J. (2008). A two-stage procedure for comparing hazard rate. J. R. Stat. Soc. B 70, 191–208. doi: 10.1111/j.1467-9868.2007.00622.x
Raikhel, A. S., and Lea, A. O. (1983). Previtellogenic development and vitellogenin synthesis in the fat body of a mosquito: an ultrastructural and immunocytochemical study. Tissue Cell 15, 281–299. doi: 10.1016/0040-8166(83)90023-x
Ramirez, L., Negri, P., Sturla, L., Guida, L., Zocchi, E., Vigliarolo, T., et al. (2017). Abscisic acid enhances cold tolerance in honeybee larvae. Proc. Biol. Sci. 284:20162140. doi: 10.1098/rspb.2016.2140
Riddiford, L. M. (2012). How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 179, 477–484. doi: 10.1016/j.ygcen.2012.06.001
Riddiford, L. M., Cherbas, P., and Truman, J. W. (2000). Ecdysone receptors and their biological actions. Vitam. Horm. 60, 1–73. doi: 10.1016/s0083-6729(00)60016-x
Riddiford, L. M., Truman, J. W., Mirth, C. K., and Shen, Y. (2010). A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137, 1117–1126. doi: 10.1242/dev.037218
Rodrigures, M. S., Batista, E. P., Silva, A. A., Costa, F. M., Neto, V. A. S., and Gil, L. H. S. (2017). Change in Anopheles richness and composition in response to artificial flooding during the creation of the Jirau hydroelectric dam in Porto Velho, Brazil. Malar. J. 16, 87. doi: 10.1186/s12936-017-1738-7
Rono, M. K., Whitten, M. M. A., Oulad-abdelghani, M., and Elena, A. (2010). The major yolk protein Vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 8:e1000434. doi: 10.1371/journal.pbio.1000434
Seyfarth, M., Khaireh, B. A., Abdi, A. A., Bouh, S. M., and Faulde, M. K. (2019). Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established-malaria emerging. Parasitol. Res. 118, 725–732. doi: 10.1007/s00436-019-06213-0
Shiao, S.-H., Hansen, I. A., Zhu, J., Siegleff, D. H., and Raikhel, A. S. (2008). Juvenile hormone connects larval nutrition with target of rapamycin signaling in the mosquito Aedes aegypti. J. Insect Physiol. 54, 231–239. doi: 10.3390/insects9020060
Shinoda, T., and Itoyama, K. (2003). Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 100, 11986–11991. doi: 10.1073/pnas.2134232100
Smith, N. R., Trauer, J. M., Gambhir, M., Richards, J. S., Maude, R. J., Keith, J. M., et al. (2018). Agent-based models of malaria transmission? a systematic review. Malar. J. 17:299. doi: 10.1186/s12936-018-2442-y
Söderberg, J. A. E., Carlsson, M. A., and Nässel, D. R. (2012). Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front. Endocrinol. 3:109. doi: 10.3389/fendo.2012.00109
Soleimani-Ahmadi, M., Vatandoost, H., and Zare, M. (2014). Characterization of larval habitats for anopheline mosquitoes in a malarious area under elimination program in the southeast of Iran. Asian Pac. J. Trop. Biomed. 4, S73–S80. doi: 10.12980/apjtb.4.2014c899
Stone, C. M., Witt, A. B. R., Walsh, G. C., Foster, W. A., and Murphy, S. T. (2018). Would the control of invasive alien plants reduce malaria transmission? A review. Parasit. Vectors 11:76. doi: 10.1186/s13071-018-2644-8
Strand, M. R., Brown, M. R., and Vogel, K. J. (2016). Mosquito peptide hormones: diversity, production, and function. Adv. Insect Physiol. 51, 145–188. doi: 10.1016/bs.aiip.2016.05.003
Surendran, S. N., Sivabalakrishnan, K., Gajapathy, K., Arthiyan, S., Jayadas, T., Karvannan, K., et al. (2018). Genotype and biotype of invasive Anopheles stephensi in Mannar Island of Sri Lanka. Parasit. Vectors 11:3. doi: 10.1186/s13071-017-2601-y
Takken, W., and Lindsay, S. (2019). Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg. Infect. Dis. 25, 1431–1433. doi: 10.3201/eid2507.190301
Van Ekert, E., Powell, C. A., Shatters, R. G., and Borovsky, D. (2014). Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J. Insect Physiol. 70, 143–150. doi: 10.1016/j.jinsphys.2014.08.001
Vince, R. K., and Gilbert, L. I. (1977). Juvenile hormone esterase activity in precisely timed last instar larvae and pharate pupae of Manduca sexta. Insect Biochem. 7, 115–120. doi: 10.1016/0020-1790(77)90003-8
Visscher, S. N. (1980). Regulation of grasshopper fecundity, longevity and egg viability by plant growth hormones. Experientia 36, 130–131. doi: 10.1007/bf02004017
White, B. H., and Ewer, J. (2014). Neural and hormonal control of postecdysial behaviors in insects. Annu. Rev. Entomol. 59, 363–381. doi: 10.1146/annurev-ento-011613-162028
Wondwosen, B., Birgersson, G., Seyoum, E., Tekie, H., Torto, B., Fillinger, U., et al. (2016). Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci. Rep. 6:37930. doi: 10.1038/srep37930
Wondwosen, B., Birgersson, G., Tekie, H., Torto, B., Ignell, R., and Hill, S. R. (2018). Sweet attraction: sugarcane pollen-associated volatiles attract gravid Anopheles arabiensis. Malar. J. 17:90. doi: 10.1186/s12936-018-2245-1
Wondwosen, B., Hill, S. R., Birgersson, G., Seyoum, E., Tekie, H., and Ignell, R. (2017). A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar. J. 16:39. doi: 10.1186/s12936-016-1656-0
World Health Organization [WHO] (2019). World Malaria Report, ISBN: 978-92-4-156572-1. Geneva: World Health Organization.
Wu, Q., Zhang, Y., Xu, J., and Shen, P. (2005). Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 102, 13289–13294. doi: 10.1073/pnas.0501914102
Yin, V. P., and Thummel, C. S. (2005). Mechanisms of steroid-triggered programmed cell death in Drosophila. Semin. Cell Dev. Biol. 16, 237–243. doi: 10.1016/j.semcdb.2004.12.007
Zhou, G., Pennington, J. E., and Wells, M. A. (2004). Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochem. Mol. Biol. 34, 919–925. doi: 10.1016/j.ibmb.2004.05.009
Keywords: Anopheles, malaria, abscisic acid, ABA, lifespan, fecundity, development
Citation: Taylor DM, Olds CL, Haney RS, Torrevillas BK and Luckhart S (2020) Comprehensive and Durable Modulation of Growth, Development, Lifespan and Fecundity in Anopheles stephensi Following Larval Treatment With the Stress Signaling Molecule and Novel Antimalarial Abscisic Acid. Front. Microbiol. 10:3024. doi: 10.3389/fmicb.2019.03024
Received: 03 October 2019; Accepted: 17 December 2019;
Published: 17 January 2020.
Edited by:
Rhoel Dinglasan, University of Florida, United StatesReviewed by:
Guido Favia, University of Camerino, ItalyMark R. Brown, University of Georgia, United States
Copyright © 2020 Taylor, Olds, Haney, Torrevillas and Luckhart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shirley Luckhart, sluckhart@uidaho.edu
 Dean M. Taylor
Dean M. Taylor Cassandra L. Olds1
Cassandra L. Olds1 Shirley Luckhart
Shirley Luckhart