- 1Central New York Research Corporation, Syracuse, NY, United States
- 2Veterans Affairs Medical Center, Syracuse, NY, United States
The current standard of care therapy for pulmonary Mycobacterium kansasii infection is isoniazid (300 mg/day), rifampin (600 mg/day), and ethambutol (15 mg/kg/day) for 12 months after achieving sputum culture negativity. Rifampin is the key drug in this regimen. The contribution of isoniazid is unclear since its in vitro MICs against M. kansasii are near the peak achievable serum levels and more than 100-fold greater than the MICs for Mycobacterium tuberculosis. Ethambutol likely decreases the emergence of rifampin resistant organisms. There are several new drug classes (e.g., quinolones, macrolides, nitroimidazoles, diarylquinolines, and clofazimine) that exhibit antimycobacterial activities against M. tuberculosis but have not yet been adequately studied against M. kansasii infections. The evaluation of in vitro activities of these agents as well as their study in new regimens in comparison to the standard of care regimen in mouse infection models should be undertaken. This knowledge will inform development of human clinical trials of new regimens in comparison to the current standard of care regimen. It is likely that shorter and more effective therapy is achievable with currently available drugs.
Introduction
Mycobacterium kansasii (M. kansasii) is a group I non-tuberculous mycobacterium (NTM) (Buhler and Pollack, 1953). These organisms are ubiquitous in the environment and are often found in aquatic settings (Amha et al., 2017). Seven genotypes, or subtypes have been identified, along with an intermediate (I/II) and atypical (IIb) subtype (Bakuła et al., 2018). Types I and II are the most common clinical subtypes found while types III-VII have generally only been recovered from environmental samples (Taillard et al., 2003).
Mycobacterium kansasii causes infection in both immunocompetent and immunosuppressed individuals. Additionally, it is the non-tuberculous mycobacterium most frequently found in immunocompetent patients. It is also the second most frequent mycobacterium found in HIV-infected patients and is only surpassed by Mycobacterium avium complex (Canueto-Quintero et al., 2003). The number of NTM infections is increasing and are geographically wide-spread. M. kansasii is the second most prevalent cause of NTM disease in the United States, China, South American countries, and some European countries such as Poland, Slovakia, and the United Kingdom (Hoefsloot et al., 2013).
Mycobacterium kansasii causes lung disease that clinically and radiologically resembles tuberculosis. The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) recommend a combination of three anti-tuberculosis drugs, isoniazid (INH) at 300 mg/day, rifampin (RIF) at 600 mg/day, and ethambutol (EMB) at 15 mg/kg/day for treatment of M. kansasii pulmonary disease in HIV negative individuals. Treatment should be continued for 1 year after culture negativity (Griffith et al., 2007).
Mycobacterium kansasii infection is challenging to treat since the course of therapy requires multiple drugs and treatment periods of up to 2 years. Long periods of treatment often give rise to additional problems including patient non-adherence to the treatment plan, expense, and potential drug interactions and/or adverse events (Larsson et al., 2017). There are several antimycobacterial drugs that are effective against tuberculosis and other NTMs that might be better alternatives to the current three drug combination. The British Thoracic Society recommends the inclusion of a macrolide, such as azithromycin (AZI) at 250 mg/day or 500 mg of clarithromycin (CLA) twice a day as an alternative to INH (Haworth et al., 2017).
The focus of this review is to discuss in vitro susceptibility testing, in vivo evaluation in animal models, and clinical reports or studies utilizing these antimycobacterial agents. We will also discuss future efforts that should be undertaken to improve the clinical outcome of M. kansasii pulmonary infection.
In vitro Susceptibility Testing
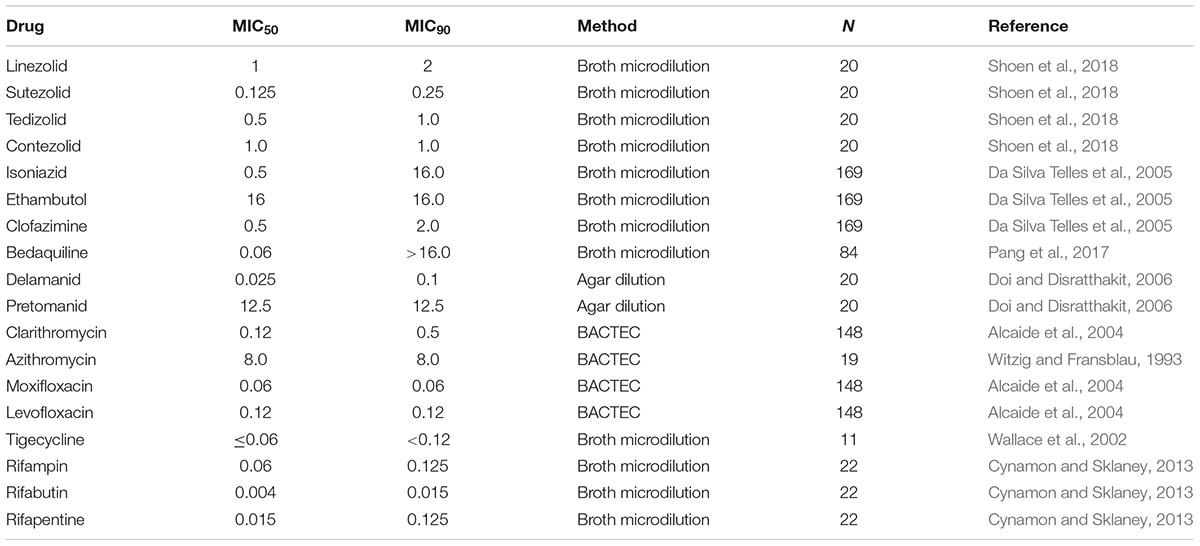
In vitro susceptibility testing of M. kansasii to antimycobacterial agents has been accomplished using several different methodologies, including agar dilution, broth macrodilution or microdilution method, or BACTEC. Investigations to determine the minimal inhibitory concentration (MIC) of antimicrobials against M. kansasii have been performed on multiple clinical isolates. Table 1 contains the MIC50 and MIC90 (defined as the lowest concentration at which 50 and 90% of the clinical isolates tested were inhibited, respectively) of drugs currently used to treat M. kansasii infection, as well as other drugs used to treat tuberculosis and some NTM infections. The list includes oxazolidinones, quinolones, macrolides, riminophenazines, diarylquinolines, nitroimidazoles, glycylcyclines, and rifamycins.
Throughout the literature there is a divergence in the MIC values for CLA and AZI. The reported MIC values of susceptible strains of M. kansasii for CLA are generally consistent with those listed in Table 1 with the MIC50 0.12 and MIC90 0.5 μg/mL. Other MIC values reported are: 0.125–0.25 μg/mL (Griffith et al., 2003), MIC50 ≤ 0.06 MIC90 0.125 μg/mL (Guna et al., 2005), MIC50 0.25 and MIC90 0.5 μg/mL (Witzig and Fransblau, 1993), and MIC50 0.125, MIC90 0.25 μg/mL (Brown et al., 1992). Although a more recent study (Li et al., 2016) suggests that CLA resistance is related to the Subtype I phenotype of M. kansasii and reports much higher MIC90 values (MIC50 0.25, MIC90 128 μg/mL). Subtype 1 is the most prevalent type of M. kansasii worldwide. In the same study the MIC values of AZI (MIC50 16 MIC90 32 μg/mL) are similar to those reported in Table 1 (MIC50 8 MIC90 8 μg/mL). It is possible that these higher values are due to cross resistance with CLA as there are CLA resistant isolates identified in the same study. Other studies report MIC values of >8 μg/mL (Yew et al., 1994). Conversely, there are also reports of MIC values of AZI being similar to those seen with clarithromycin (Brown et al., 1992; Klemens and Cynamon, 1992).
The clinical MIC cutoff for EMB to define susceptibility/resistance against M. kansasii is 4 μg/ml, however, there is growing evidence that many M. kansasii isolates are resistant to EMB by this definition (Table 1). Patients diagnosed with this infection are not routinely evaluated for EMB susceptibility, therefore, resistance may be more prevalent than previously thought. It has been suggested that resistance to EMB (and INH) in M. kansasii occurs when a patient develops RIF resistance while on the standard regimen (Hjelm et al., 1992; Griffith et al., 2003). In a 2016 study, isolates belonging to M. kansasii Subtype I were found to exhibit greater resistance to EMB than the other subtypes (Li, 2016) although the differences were not statistically significant perhaps due to their small sample size. In a study looking at isolates from Poland, Germany, and Netherlands, 97.9% of the isolates were EMB resistant (all subtypes with the exception of Subtype V), however, published results from other countries indicates a resistance range for EMB of 0–94% as well as variable methods used to determine susceptibility (Bakula, 2018). This raises the question whether EMB and INH should be used as companion drugs to rifampin when newer, potentially more efficacious drugs are currently available.
A model of intracellular M. kansasii infection has been designed to mimic the pharmacodynamics and pharmacokinetics between infected macrophages and therapeutic agents (Srivastava et al., 2015; Srivastava and Gumbo, 2018). The hollow fiber model of M. kansasii has been adapted from similar models for Mycobacterium tuberculosis and M. avium complex (Deshpande et al., 2010; Srivastava and Gumbo, 2011). It has been reported that this system evaluates compound efficacy, rank orders kill rates compared to standard regimens, and assesses antibiotic tolerance by the use of efflux pump inhibitors. In the case where samples were available from moxifloxacin (MOX) treated patients, analysis of bronchial secretions, alveolar macrophages and lung epithelium lining fluid were used to simulate optimal doses and breakpoints for resistance with the aid of computer software (Srivastava et al., 2015). This preclinical disease model will provide valuable additional information to help select the appropriate agents for in vivo testing and perhaps subsequent evaluation in clinical studies.
The immune status of a patient determines the course of infection and the selection of an appropriate regimen. RIF is currently the cornerstone for the therapy of M. kansasii infection, but in patients co-infected with HIV, RIF presents a problem since it increases the hepatic metabolism of protease inhibitors, often used for the treatment of HIV infection (Brown-Elliott et al., 2012). Rifabutin (RBT) has less effect on the hepatic metabolism, therefore, it is often used as an alternative to RIF in HIV-infected patients. Rifapentine (RPT) is an alternative to RIF or RBT. All three rifamycins have demonstrated good in vitro activity against M. kansasii (Cynamon and Sklaney, 2013).
Linezolid (LZD), an oxazalidinone, is currently an option for multi-drug resistant tuberculosis infections and has been shown to have good in vitro activity against M. kansasii (Table 1), but there are significant toxicity issues with this drug. These toxicity issues make the use of this drug for M. kansasii therapy problematic. The newer oxazolidinones, tedizolid, and contezolid, have fewer side effects than LZD, and have good in vitro activity against M. kansasii (Shoen et al., 2018).
Four other drugs that are used to treat drug resistant tuberculosis have also shown good in vitro activity against M. kansasii (Table 1). They are clofazimine (CFZ) (riminophenazine), bedaquiline (diarylquinoline), delamanid (nitroimidazole), and pretomanid (nitroimidazopyran) although the MIC50 of pretomanid is significantly high and therefore may not be a viable candidate compound. Attempting to correlate in vitro efficacy with achievable serum levels is not always useful when selecting compounds for in vivo testing and treatment.
Several factors affecting serum levels and compound availability have to be considered. These include tissue/cell accumulation, interaction with other drugs, and length of treatment (single dosing versus multiple doses). CFZ illustrates the complexity of this issue. A single 200 mg dose in humans results in peak plasma levels of 0.41 μg/ml, which is lower than reported MIC values (Table 1), however, CFZ concentrates in macrophages and tissues long-term due to its highly lipophilic character (Cholo et al., 2012). INH treatment with CFZ increases the plasma concentration and reduces tissue concentration of CFZ (Haworth et al., 2017). Several studies with other antimycobacterial agents allude to a synergistic relationship with CFZ when evaluated in combination therapy against a variety of NTM species (McGuffin et al., 2017).
Since treatment for M. kansasii and other NTM infections consist of a multi-drug regimen, a comprehensive review of each compound’s known characteristics need to be considered to better predict potential efficacy. The established method for evaluation of in vitro combination therapy, “the checkerboard” has been used to define synergy or antagonism between two compounds. It is not clear whether results from this method correlate with those from in vivo modeling. New methods are being developed for prioritizing drug combinations, however, they have not yet been utilized for modeling regimens of M. kansasii therapy. Due to the lack of resources available for M. kansasii research it continues to be important to utilize available information on candidate compounds from in vitro combination studies focused on M. tuberculosis to advise our prioritization of regimens for M. kansasii.
Animal Studies
Relatively little research has been done utilizing animal models to develop new therapies for NTM generally (Soni et al., 2016) and M. kansasii infection specifically. Research into the efficacy of compounds has largely been limited to drugs currently in use to treat tuberculosis or licensed drugs such as quinolones and macrolides. Due to the emerging importance of this infection, and the challenges of treatment for patients with resistant infections, more research into the development of an appropriate animal model to test new therapeutics is needed.
A review of the available literature indicates that several different strains of mice, levels of inocula, and routes of infection have been used, all with the ability to sustain a measurable bioburden. Presently there is no standard model and the strains of mice and routes of infection used are varied. Described below is a summary of the available literature on animal studies evaluating antimycobacterial compounds against M. kansasii.
BALB/c athymic (nude) mice infected with 107 CFU M. kansasii intravenously resulted in disseminated infection (Graybill and Bocanegra, 2001). Untreated control mice succumbed to the infection after about 2 months. Therapy given daily for 21 days was started 1-week post-infection. Increasing doses of RPT (0.15, 0.3, and 0.6 mg/kg), AZI (10, 15, 30, 50, and 150 mg/kg) and EMB (10, 25, and 100 mg/kg) were evaluated in a survival study. RPT dosed at 0.6 mg/kg, AZI at 15 mg/kg, and EMB at 10 mg/kg or higher doses of each prolonged survival. There was no difference in survival between RPT and AZI. The investigators also evaluated bioburden reduction in the spleens and livers of mice treated with RPT and AZI alone and in combination. The results indicated that the combination was no more effective than RPT alone.
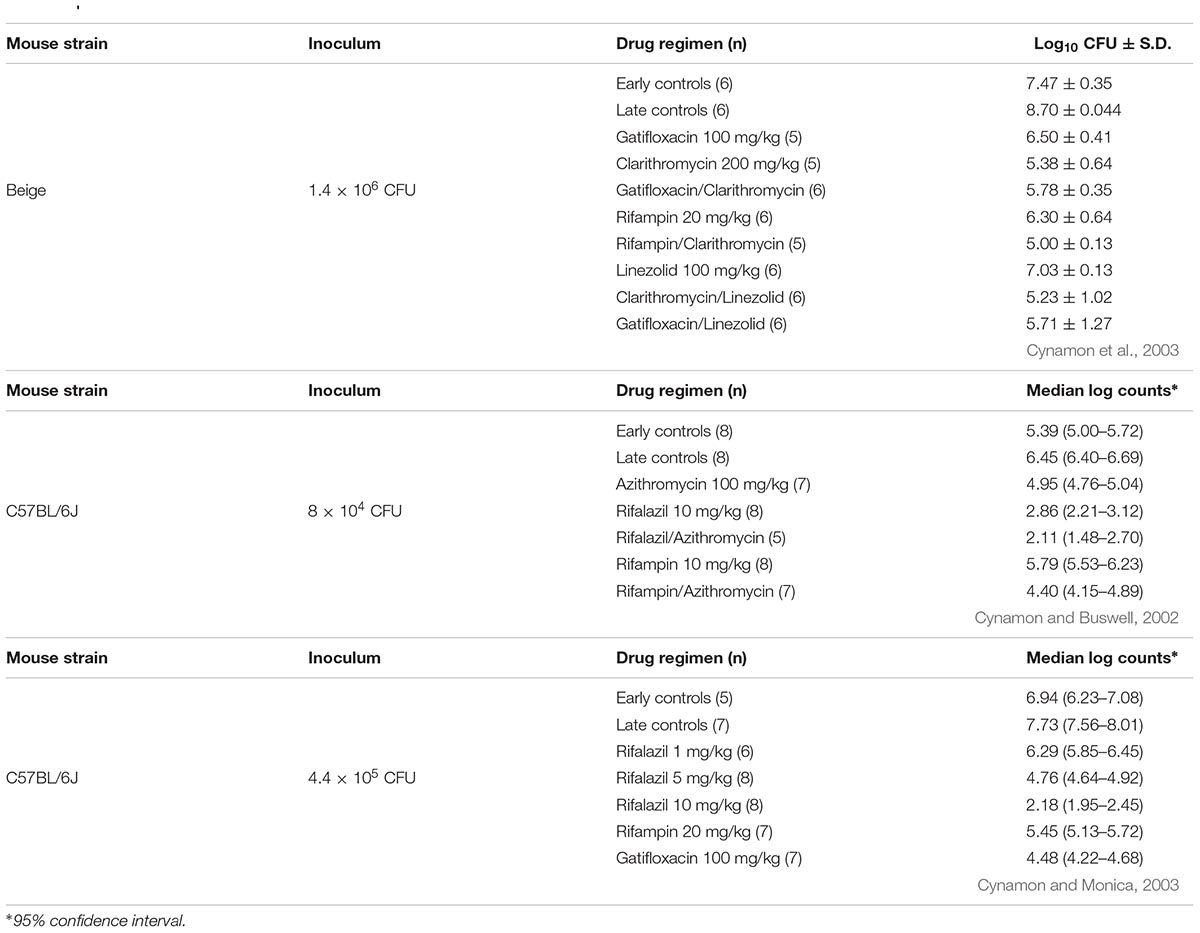
In another study, beige mice (C57BL/6J background) were infected intravenously with 107 CFU of M. kansasii and treated 1-week post-infection, 5 days/week for 4 weeks. AZI 200 mg/kg, CLA 200 mg/kg, EMB 125 mg/kg, RIF 20 mg/kg, and CFZ 20 mg/kg were evaluated for activity compared to untreated controls. Viable cell counts were enumerated from the spleens and lungs. RIF reduced lung CFUs by about 1 log compared to the early controls and yielded the greatest reduction in organisms in the lungs. AZI and CLA had similar, but modest activity (Klemens and Cynamon, 1994).
In a study using beige mice infected intranasally with approximately 106 CFU of M. kansasii, treatment with gatifloxacin (GAT) 100 mg/kg, CLA 200 mg/kg, LZD 100 mg/kg, RIF 20 mg/kg, GAT + CLA, CLA + LZD, GAT + LZD, and RIF + CLA was given for 4 weeks, 5 days/week. All of the treatment groups were significantly better than the early controls (evaluated at the initiation of therapy, 1-week post-infection). CLA was more effective than any other monotherapy. CLA combined with LZD, GAT, or RIF was better than any single drug therapy (Cynamon et al., 2003; Table 2).
More recent studies with immunocompetent C57BL/6J mice demonstrate that M. kansasii can also survive and replicate in these mice. Two studies were carried out where mice were infected with approximately 5 × 105 CFU/mouse via the intranasal route. In the first experiment, RIF 10 mg/kg, rifalazil (RZL) 10 mg/kg, AZI 100 mg/kg and combinations of RIF + AZI and RZL + AZI were evaluated. All treatment groups were significantly better than the early controls. The combination of RZL + AZI was the most effective treatment group (Cynamon and Buswell, 2002; Table 2). In the second experiment, 4 weeks (5 days/week) of GAT 100 mg/kg and RIF 20 mg/kg were compared to increasing doses of RZL (1, 5, and 10 mg/kg). The individual drugs were also compared to a combination of GAT + RZL10 mg/kg. All single agents and the combination regimen had significant activity compared to the early controls (evaluated 1-week post-infection). There was a dose response with RZL and the combination of RZL + GAT reduced lung CFUs to an undetectable level after 4 weeks of therapy (Cynamon and Monica, 2003; Table 2).
Although the published literature is limited regarding the use of M. kansasii infection models as a screening tool to evaluate recently licensed or new experimental compounds, the above studies suggest that these models should be used to determine if more effective therapy is achievable and whether their use may lead to a shorter duration of therapy compared to the current standard regimen.
Clinical Research
Rifampin, INH, and EMB (ATS/IDSA) or RIF, CLA (or AZI), EMB (British Thoracic Society) are suggested regimens for treatment of M. kansasii infection in humans taking between 12–18 months depending on the sputum culture time of conversion. The standard treatment regimen is effective in most instances; however, therapy is lengthy and it does not provide a cure for all patients (Santin et al., 2009). Treatment failure is almost always associated with resistance to RIF and/or CLA (Brown-Elliott et al., 2012). The Clinical and Laboratory Standards Institute (CLSI) currently recommends testing all initial patient isolates of M. kansasii for RIF and CLA in vitro susceptibility. Broth dilution is the suggested methodology utilizing cation-adjusted Mueller Hinton broth and 5% OADC (oleic acid, albumin, dextrose, catalase) enrichment as the media (Griffith et al., 2007). When isolates are RIF resistant (MIC > 1 μg/ml), the suggestion is to test secondary agents such as AMK, EMB, LZD, MOX, RBT, and trimethoprim-sulfamethoxazole for in vitro activity (Brown-Elliott et al., 2012).
The current method for testing and interpretation of the MIC for INH in the clinical laboratory utilizes the critical concentrations (0.2 μg/ml, 1 μg/ml) for M. tuberculosis susceptibility testing. This results in a false interpretation of INH resistance because the MIC values for M. kansasii usually range from 0.5 μg/ml to 5 μg/ml (Brown-Elliott et al., 2012). Heifets’ work illustrated this issue stating that of more than 100 M. kansasii isolates evaluated by agar dilution methodology almost all were completely resistant to 0.2 μg/ml and susceptible, or partially resistant to 1 μg/ml of INH (Heifets, 1991). MIC testing of INH is not recommended.
Although infection can occur in other organs or become disseminated, the focus of our literature review of clinical outcomes is pulmonary infection caused by M. kansasii. A systematic review of microbiological and clinical treatment outcomes for M. kansasii pulmonary infections highlighted the lack of randomized controlled clinical trials for this infection (Diel et al., 2017; Haworth et al., 2017). A small cohort of prospective and retrospective observational studies exist and a few representative studies are discussed below.
A prospective study by the British Thoracic Society evaluated 173 patients with pulmonary M. kansasii infection. Prior to species identification, 149 of these patients were treated with regimens of INH + RIF + EMB, INH + RIF + EMB + PZA, or INH + RIF + PZA + streptomycin. Once M. kansasii was identified patients were given RIF + EMB for an additional 9 months (Jenkins et al., 1994). The remaining 24 patients received 9 months of RIF + EMB. Their results suggested that the RIF + EMB was acceptable treatment for non-immune compromised patients. Only 11% of their patients had positive sputum cultures after 3 months of therapy. Fifteen patients (9.7%) of the 154 patients who entered the post-chemotherapy follow up period relapsed between 6 and 50 (median 23) months.
In a retrospective study of 111 patients diagnosed with M. kansasii lung disease and treated with RIF + EMB + INH supplemented with streptomycin during the initial 2–3 months, 75 patients completed 12 months of therapy (Santin et al., 2009). After a 41.5-month median follow-up, five (6.6%) patients relapsed. The authors concluded that a 12-month course is adequate for most, but not all patients.
A prospective trial by Griffith et al. (2003) evaluated a thrice-weekly regimen of CLA 1000 mg (two female patients who weighed <50 kg received 500 mg), EMB 25 mg/kg, and RIF 600 mg in 18 patients with M. kansasii lung disease. This regimen yielded a durable cure with no relapse after a follow-up of 46 ± 8 months (mean time ± standard deviation) for the 14 patients that completed therapy. Four patients were lost to follow-up. The mean time to sputum conversion was 1 ± 0.9 months and the mean duration of therapy was 13.4 ± 0.9 months (Griffith et al., 2003).
Future Efforts
The efficacy of INH when added to RIF + EMB should be measured in an appropriate mouse model to evaluate the contribution of INH to the standard regimen. New drug regimens should be tested in a mouse model of M. kansasii infection and compared to RIF + EMB ± INH to determine comparative efficacy. Similar to in vitro evaluation, multiple clinical isolates of M. kansasii should be studied in a mouse model to ensure that isolate differences do not affect efficacy in the mouse model. It is preferable to deliver the inoculum by the aerosol or intranasal route since this method most closely parallels the human pulmonary route of infection.
Initially, 4 weeks of treatment should be used to determine which regimens to evaluate in more detail (i.e., longer duration of therapy followed by a non-treatment observation period). It is yet to be determined whether one particular mouse strain is preferable to another. It would be of interest to evaluate C3HeB/FeJ mice as a potential model system for M. kansasii infection since they develop lesions similar to humans when infected with M. tuberculosis (Driver et al., 2012; Harper et al., 2012).
Several agents included in Table 1 (bedaquiline, delamanid, MOX, tedizolid, contezolid, CFZ, and CLA) should be studied in a mouse model of M. kansasii infection to determine whether they can improve outcomes with regard to efficacy and duration of therapy. CFZ added to INH + RIF + PZA in mice infected with tuberculosis was shown to decrease the time needed to achieve a durable cure (Tyagi et al., 2015; Ammerman et al., 2018). It should be determined whether CFZ has a similar effect with RIF + EMB or in the case of possible RIF resistance, a combination of CFZ with 2 other drugs in M. kansasii infections.
Several tuberculosis clinical trials with RIF suggest that doses of 20 and 35 mg/kg are both well tolerated and more efficacious than the standard 10 mg/kg dose (600 mg daily) (Tiberi et al., 2018). Higher doses of RIF should be evaluated to assess whether this treatment is also beneficial in reducing the lung bioburden of M. kansasii infected mice.
In order to accomplish randomized controlled clinical trials evaluating new regimens for therapy of pulmonary M. kansasii infection it is necessary to develop a clinical trials consortium, similar to the Mycoses Study Group1. This would enable multiple clinical trial sites to recruit and enter patients into clinical trials. In 2009 the European based MTB clinical trial consortium TBnet, formed the Non-tuberculous Mycobacteria Network European Trials Group (NTM-NET). NTM-NET is an international consortium of clinical NTM researchers that is primarily focused in Europe2 and is able to provide limited funding for NTM research. Perhaps now is an appropriate time for a Non-tuberculous Mycobacteria Study Group funded by NIAID to advance clinical trials for NTM infections by providing opportunities and resources for this important work.
Author Contributions
MD, CS, and MC have equally contributed to the research of the content, writing, and editing of this article. All authors have approved the final version of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript is dedicated to Dr. Leonid B. Heifets for his many contributions to expanding our understanding of susceptibility testing of mycobacteria.
Footnotes
References
Alcaide, F., Calatayud, L., Santín, M., and Martín, R. (2004). Comparative in vitro activities of linezolid, telithromycin, clarithromycin, levofloxacin, moxifloxacin, and four conventional antimycobacterial drugs against Mycobacterium kansasii. Antimicrob. Agents Chemother. 48, 4562–4565. doi: 10.1128/AAC.48.12.4562-4565
Amha, Y. M., Anwar, M. Z., Kumaraswamy, R., Henschel, A., and Ahmad, F. (2017). Mycobacteria in municipal wastewater treatment and reuse: microbial diversity for screening the occurrence of clinically and environmentally relevant species in arid regions. Environ. Sci. Technol. 51, 3048–3056. doi: 10.1021/acs.est.6b05580
Ammerman, N. C., Swanson, R. V., Bautista, E. M., Almeida, D. V., Saini, V., Omansen, T. F., et al. (2018). Impact of clofazimine dosing on treatment-shortening of the first-line regimen in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 62:e636-18. doi: 10.1128/AAC.00636-18
Bakuła, Z., Modrzejewska, M., Pennings, L., Proboszcz, M., Safianowska, A., Bielecki, J., et al. (2018). Drug susceptibility profiling and genetic determinants of drug resistance in Mycobacterium kansasii. Antimicrob. Agents Chemother. 62:e1788-17. doi: 10.1128/AAC.01788-17
Brown, B. A., Wallace, R. J. Jr., and Onyi, G. O. (1992). Activities of clarithromycin against eight slowly growing species of nontuberculous mycobacteria, determined by using a broth microdilution MIC system. Antimicrob. Agents Chemother. 36, 1987–1990.
Brown-Elliott, B. A., Nash, K. A., and Wallace, R. J. Jr. (2012). Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 25, 545–582. doi: 10.1128/CMR.05030-11
Buhler, V. B., and Pollack, A. (1953). Human infection with atypical acid-fast organisms. Am. J. Clin. Pathol. 23, 363–374.
Canueto-Quintero, J., Caballero-Granado, F. J., Herrero-Romero, M., Domínguez-Castellano, A., Martín-Rico, P., Verdú, V. E., et al. (2003). Epidemiological, clinical, and prognostic differences between the diseases caused by Mycobacterium kansasii and Mycobacterium tuberculosis in patients infected with human immunodeficiency virus: a multicenter study. Clin. Infect. Dis. 37, 584–590. doi: 10.1155/2017/4545721
Cholo, M. C., Steel, H. C., Fourie, P. B., Germishuizen, W. A., and Anderson, R. (2012). Clofazimine: current status and future prospects. J. Antimicrob. Chemother. 67, 290–298. doi: 10.1093/jac/dkr444
Cynamon, M., and Buswell, S. (2002). “Activities of rifampin, rifalazil and azithromycin in a mous model of Mycobacterium kansasii infection,” in Proceedings of the European Society of Mycobacteriology 23rd Annual Congress, Dubrovnik.
Cynamon, M., and Sklaney, M. (2013). “Comparative in vitro activities of rifampin, rifapentine and rifabutin against Mycobacterium kansasii,” in Proceedings of the European Society of Mycobacteriology 34th Annual Congress, Florence.
Cynamon, M. H., Elliott, S. A., DeStefano, M. S., and Yeo, A. E. T. (2003). Activity of clarithromycin alone and in combination in a murine model of M. kansasii infection. J. Antimicrob. Chemother. 52, 306–307.
Cynamon, M. H., and Monica, B. (2003). “Activities of rifalazil alone and in combination with gatifloxacin in a mouse model of Mycobacterium kansasii infection,” in Proceedings of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL.
Da Silva Telles, M. A., Chimara, E., Ferrazoli, L., and Riley, L. W. (2005). Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J. Med. Microbiol. 54, 975–979.
Deshpande, D., Srivastava, S., Meek, C., Leff, R., Hall, G. S., and Gumbo, T. (2010). Moxifloxacin pharmacokinetics/pharmacodynamics and optimal dose and susceptibility breakpoint identification for treatment of disseminated Mycobacterium avium infection. Antimicrob. Agents Chemother. 54, 2534–2539. doi: 10.1128/AAC.01761-09
Diel, R., Ringshausen, F., Richter, E., Welker, L., Schmitz, J., and Nienhaus, A. (2017). Microbiological and clinical outcomes of treating non-Mycobacterium avium complex nontuberculous mycobacterial pulmonary disease a systematic review and meta-analysis. Chest 152, 120–142. doi: 10.1016/j.chest.2017.04.166
Doi, N., and Disratthakit, A. (2006). “Characteristic antimycobacterial spectra of the novel anti-TB drug candidates OPC-67683 and PA-824,” in Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, F1–F1377.
Driver, E. R., Ryan, G. J., Hoff, D. R., Irwin, S. M., Basaraba, R. J., Kramnik, I., et al. (2012). Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56, 3181–3195. doi: 10.1128/AAC.00217-12
Graybill, J. R., and Bocanegra, R. (2001). Treatment alternatives for Mycobacterium kansasii. J. Antimicrob. Chemother. 47, 417–420.
Griffith, D. E., Aksamit, T., Brown-Elliott, B. A., Catanzaro, A., Daley, C., and Gordin, F. (2007). An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416.
Griffith, D. E., Brown-Elliott, B. A., and Wallace, R. J. Jr. (2003). Thrice-weekly clarithromycin-containing regimen for treatment of Mycobacterium kansasii infection. J. Antimicrob. Chemother. 37, 1178–1182. doi: 10.1086/378742
Guna, R., Munñoz, C., Domínguez, V., García-García, A., Gaálvez, G., de Juliaán-Ortiz, J. V., et al. (2005). Mycobacterium kansasii subtype I is associated with clarithromycin resistance in china. J. Antimicrob. Chemother. 55, 950–953. doi: 10.1093/jac/dki111
Harper, J., Sherry, C., Davis, S. L., Tasneen, R., Weir, M., Kramnik, I., et al. (2012). Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J. Infect. Dis. 205, 595–602. doi: 10.1093/infdis/jir786
Haworth, C. S., Banks, J., Capstick, T., Fisher, A. J., Gorsuch, T., Laurenson, I. F., et al. (2017). British thoracic society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72, ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927
Heifets, L. (1991). “Dilemmas and realities in drug susceptibility testing of M. avium-M. intracellulare and other slowly growing nontuberculous mycobacteria,” in Drug susceptibility in the chemotherapy of mycobacterial infections, ed. L. Heifets (Boca Raton, FL: CRC Press), 136.
Hjelm, U., Kaustová, J., Kubín, M., and Hoffner, S. E. (1992). Susceptibility of Mycobacterium kansasii to ethambutol and its combination with rifamycins, ciprofloxacin and isoniazid. Eur. J. Clin. Microbiol. Infect. Dis. 11, 51–54. doi: 10.1007/BF01971272
Hoefsloot, W., van Ingen, J., Andrejak, C., Angeby, K., Bauriaud, R., Bemer, P., et al. (2013). The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NETcollab. Eur. Respir. J. 42, 1604–1613. doi: 10.1183/09031936.00149212
Jenkins, P. A., Banks, J., Campbell, I. A., and Smith, A. P. (1994). Mycobacterium kansasii pulmonary infection: a prospective study of the results of nine months of treatment with rifampicin and ethambutol. research committee, British thoracic society. Thorax 49, 442–445. doi: 10.1136/thx.49.5.435
Klemens, S. P., and Cynamon, M. H. (1994). Activities of azithromycin and clarithromycin against nontuberculous mycobacteria in beige mice. Antimicrob. Agents Chemother. 38, 1455–1459.
Larsson, L. O., Polverino, E., Hoefsloot, W., Codecasa, L. R., Diel, R., Jenkins, S. G., et al. (2017). Pulmonary disease by non-tuberculous mycobacteria – clinical management, unmet needs and future perspectives. Expert Rev. Respir. Med. 11, 977–989. doi: 10.1080/17476348.2017.1386563
Li, Y., Pang, Y., Tong, X., Zheng, H., Zhao, Y., and Wang, C. (2016). Mycobacterium kansasii subtype I is associated with clarithromycin resistance in China. Front. Microbiol. 7:2097. doi: 10.3389/fmicb.2016.02097
McGuffin, S. A., Pottinger, P. S., and Harnisch, J. P. (2017). Clofazimine in nontuberculous mycobacterial infections: a growing niche. Open Forum Infect. Dis. 4:ofx147. doi: 10.1093/ofid/ofx147
Pang, Y., Zheng, H., Tan, Y., Song, Y., and Zhao, Y. (2017). In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob. Agents Chemother. 61:e2627-16. doi: 10.1128/AAC.02627-16
Santin, M., Dorca, J., Alcaide, F., Gonzalaz, F., Casas, S., Lopez, M., et al. (2009). Long-term relapses after 12-month treatment for Mycobacterium kansasii lung disease. Eur. Respir. J. 33, 148–152. doi: 10.1183/09031936.00024008
Shoen, C., Sklaney, M., and Cynamon, M. (2018). “Comparative in vitro activities of several oxazolidinones against Mycobacterium kansasii,” in Proceedings of the 18th International Congress on Infectious Diseases, Buenos Aires.
Soni, I., De Groote, M. A., Dasgupta, A., and Chopra, S. (2016). Challenges facing the drug discovery pipeline for non-tuberculous mycobacteria. J. Med. Microbiol. 65, 1–8. doi: 10.1099/jmm.0.000198
Srivastava, S., and Gumbo, T. (2011). In vitro and in vivo modeling of anti-tuberculosis drugs and its impact on optimization of doses and regimens. Curr. Pharm. Des. 17, 2881–2888. doi: 10.2174/138161211797470192
Srivastava, S., and Gumbo, T. (2018). Clofazimine for the treatment of Mycobacterium kansasii. Antimicrob. Agents Chemother. 62:e248-18. doi: 10.1128/AAC.00248-18
Srivastava, S., Pasipanodya, J., Sherman, C. M., Meek, C., Leff, R., and Gumbo, T. (2015). Rapid drug tolerance and dramatic sterilizing effect of moxifloxacin monotherapy in a novel hollow-fiber model of intracellular Mycobacterium kansasii disease. Antimicrob. Agents Chemother. 59, 2273–2279. doi: 10.1128/AAC.04441-14
Taillard, C., Greub, G., Weber, R., Pfyffer, G. E., Bodmer, T., Zimmerli, S., et al. (2003). Clinical implications of Mycobacterium kansasii species heterogeneity: swiss national survey. J. Clin. Microbiol. 41, 1240–1244. doi: 10.1128/JCM.41.3.1240-1244.2003
Tiberi, S., du Plessis, N., Walzl, G., Vjecha, M. J., Rao, M., and Ntoumi, F. (2018). Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 18, e183–e198. doi: 10.1016/S1473-3099(18)30110-5
Tyagi, S., Ammerman, N. C., Li, S. Y., Adamson, J., Converse, P. J., and Swanson, R. V. (2015). Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 112, 869–874. doi: 10.1073/pnas.1416951112
Wallace, R. J., Brown-Elliott, B. A., Crist, C. J., Mann, L., and Wilson, R. W. (2002). Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 46, 3164–3167.
Witzig, R. S., and Fransblau, S. G. (1993). Susceptibility of Mycobacterium kansasii to ofloxacin, sparfloxacin, clarithromycin, azithromycin, and fusidic acid. Antimicrob. Agents Chemother. 37, 1997–1999.
Keywords: M. kansasii, antimycobacterials, mouse models, non-tuberculous mycobacteria, pulmonary infection
Citation: DeStefano MS, Shoen CM and Cynamon MH (2018) Therapy for Mycobacterium kansasii Infection: Beyond 2018. Front. Microbiol. 9:2271. doi: 10.3389/fmicb.2018.02271
Received: 28 June 2018; Accepted: 05 September 2018;
Published: 24 September 2018.
Edited by:
Thomas Dick, Rutgers, The State University of New Jersey, Newark, United StatesReviewed by:
Amanda S. MacLeod, Duke University, United StatesTianyu Zhang, Guangzhou Institutes of Biomedicine and Health (CAS), China
Eric Nuermberger, Johns Hopkins University, United States
Copyright © 2018 DeStefano, Shoen and Cynamon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael H. Cynamon, Michael.Cynamon@va.gov
 Michelle S. DeStefano
Michelle S. DeStefano Carolyn M. Shoen1
Carolyn M. Shoen1 Michael H. Cynamon
Michael H. Cynamon