- Genetics of Prokaryotes, Faculty of Biology, Center for Biotechnology, Bielefeld University, Bielefeld, Germany
Corynebacterium glutamicum is a natural producer of the C50 carotenoid decaprenoxanthin. The crtEcg0722crtBIYEb operon comprises most of its genes for terpenoid biosynthesis. The MarR-type regulator encoded upstream and in divergent orientation of the carotenoid biosynthesis operon has not yet been characterized. This regulator, named CrtR in this study, is encoded in many actinobacterial genomes co-occurring with terpenoid biosynthesis genes. CrtR was shown to repress the crt operon of C. glutamicum since DNA microarray experiments revealed that transcript levels of crt operon genes were increased 10 to 70-fold in its absence. Transcriptional fusions of a promoter-less gfp gene with the crt operon and crtR promoters confirmed that CrtR represses its own gene and the crt operon. Gel mobility shift assays with purified His-tagged CrtR showed that CrtR binds to a region overlapping with the −10 and −35 promoter sequences of the crt operon. Isoprenoid pyrophosphates interfered with binding of CrtR to its target DNA, a so far unknown mechanism for regulation of carotenogenesis. The molecular details of protein-ligand interactions remain to be studied. Decaprenoxanthin synthesis by C. glutamicum wild type was enhanced 10 to 30-fold upon deletion of crtR and was decreased 5 to 6-fold as result of crtR overexpression. Moreover, deletion of crtR was shown as metabolic engineering strategy to improve production of native and non-native carotenoids including lycopene, β-carotene, C.p. 450 and sarcinaxanthin.
Introduction
Carotenoids, natural yellow to red colored pigments, are the colorful representatives of the versatile and extensive group of terpenoids. Carotenoids have diverse biological functions serving for instance as photo protectors, light harvesting molecules or as membrane stabilizers (Sandoval et al., 1984; Lee and Schmidt-Dannert, 2002). They are synthesized by plants, fungi, algae and bacteria (Britton et al., 2004). Commercially, carotenoids and their derivatives are mainly applied in the food, beverage and cosmetics industries (Downham and Collins, 2000; Winterhalter and Rouseff, 2001; Dembitsky, 2005), but they are also receiving increasing attention due to their potential beneficial effects on human health (Armstrong, 1994; Sandmann, 2001; Umeno et al., 2005; Das et al., 2007). The total commercial market value of carotenoids was $1.5 billion in 2014 and it is expected to increase to $1.8 billion in 2019 (BBCResearch, 2015).
Corynebacterium glutamicum, a GRAS organism used in industrial biotechnology, is a natural producer of the C50 carotenoid decaprenoxanthin, responsible for its characteristic yellow pigmentation. C. glutamicum belongs to a rare group of bacteria producing long chain carotenoids. Carotenogenesis is not essential in C. glutamicum since transposon mutants (Krubasik et al., 2001a,b; Sandmann and Yukawa, 2005) as well as directed gene deletion mutants were shown to be deficient of carotenoid biosynthesis, but viable (Heider et al., 2012). C50 carotenoids have been mainly isolated from extremely halophilic archaea (Kelly and Jensen, 1967; Pfander, 1994) and from Gram-positive bacteria of the order Actinomycetales (Netzer et al., 2010). The pathway for decaprenoxanthin biosynthesis starting from the precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) and the respective carotenogenic genes of C. glutamicum are characterized (Krubasik et al., 2001a; Heider et al., 2012). Besides the biosynthesis of the ε-cyclic decaprenoxanthin in C. glutamicum only four other pathways toward C50 carotenoids have been elucidated: the pathway of the β-cyclic C.p.450, first isolated from C. poinsettiae (Norgård et al., 1970), in Dietzia sp. CQ4 (Tao et al., 2007), the γ-cyclic C50 carotenoid sarcinaxanthin in Micrococcus luteus NCTC2665 (Netzer et al., 2010) and recently the route to the acyclic bacterioruberin in the extremely halophilic archaeon Haloarcula japonica was described (Yang et al., 2015).
In many bacteria the carotenogenic genes are clustered. C. glutamicum possesses the crt operon with seven co-transcribed genes (crtE, cg0722, crtB, crtI, crtYe, crtYf, crtEb) necessary for the conversion of IPP and DMAPP to decaprenoxanthin, and one gene of unknown function (cg0722). A second functional phytoene synthase is encoded elsewhere in the genome (Heider et al., 2012). The major geranylgeranyl pyrophosphate (GGPP) synthase of C. glutamicum is not encoded by crtE, the first gene of the crt operon, but by idsA that is located elsewhere on the C. glutamicum chromosome (Heider et al., 2014a). It is not known whether synthesis of the redundant GGPP synthase and phytoene synthase enzymes is regulated.
Biosynthesis of terpenoids is commonly triggered by photo-oxidative stress factors in plant, fungi and bacteria (Bramley and Mackenzie, 1988), as they serve e.g., as light harvesting molecules or as protection against excess light energy or against the resulting reactive oxygen species (Johnson and Schroeder, 1996; Britton, 2008). Little is known about the regulatory mechanisms governing carotenoid biosynthesis in bacteria which may change in response to blue light, oxygen levels or growth phase (Johnson and Schroeder, 1996). The mechanism of light-induced carotenogenesis in the non-photosynthetic Gram-negative bacterium Myxococcus xanthus (Fontes et al., 2003), the Gram-positive bacteria Streptomyces coelicolor A3(2) (Takano et al., 2005), Thermus thermophilus (Takano, 2016) and Bacillus megaterium (Takano et al., 2015b) has been studied in more detail. MerR-type transcriptional regulators with a vitamin B12 binding domain are involved in regulation of carotenogenesis in those organisms. In addition, the RNA polymerase sigma factor SigF has been shown to be involved in regulation of carotenoid biosynthesis in Mycobacterium smegmatis (Provvedi et al., 2008).
Regulation of carotenoid biosynthesis in C. glutamicum has not been investigated so far. Gene expression analyses performed under different stress conditions revealed that mRNA levels of crtE, the first gene of the crt operon, altered in response to various stresses (Silberbach et al., 2005; Follmann et al., 2009; Jochmann et al., 2009). The putative multiple antibiotic resistance (MarR)-family transcriptional regulator gene cg0725, named crtR due to the results described in this study, is located upstream of the crt operon and is transcribed in divergent orientation to the crt operon. Previously, it has been shown that crtR was disrupted by transposon insertion in a mutant accumulating 3-fold more decaprenoxanthin (Krubasik et al., 2001a), but the regulatory mechanism has not been characterized. The majority of characterized MarR proteins are transcriptional repressors and their genes are generally found in the vicinity or are part of the regulated gene cluster (Wilkinson and Grove, 2006). This genetic organization and the finding that transposon insertions into this gene and the orthologuous gene in the related Mycobacterium marinum increased biosynthesis of decaprenoxanthin and β-carotene (Krubasik et al., 2001a; Gao et al., 2003), prompted us to characterize CrtR with respect to regulation of carotenogenesis in C. glutamicum.
Materials and Methods
Bacterial Strains, Media, and Growth Conditions
The strains and plasmids used in this work are listed in Table 1. C. glutamicum ATCC 13032 was used as wild type (WT) and the prophage cured C. glutamicum MB001 (Baumgart et al., 2013) as basal strain for genetic engineering. C. glutamicum cultivations were performed in CGXII medium with 100 mM glucose as carbon and energy source (Eggeling and Reyes, 2005) after inoculation to an initial OD600 of 1 at 30°C in either a volume of 50 ml in 500 ml flasks with two baffles shaking at 120 rpm or in 1 ml volume in microtiterplates at 1100 rpm at 30°C using Biolector® micro fermentation system (m2p-labs GmbH, Baesweiler, Germany). The growth was followed by measuring the OD600 using a Shimadzu UV-1202 spectrophotometer (Duisburg, Germany). Cloning was conducted with E. coli DH5α as host, cultivated in LB medium at 37°C. When appropriate, kanamycin (25 μg/ml), spectinomycin (100 μg/ml), or ampicillin (100 μg/ml) was added to the culture medium. If not stated otherwise 1 mM of IPTG was added for induction of gene expression at inoculation of the main culture.
Recombinant DNA Work
Plasmids were constructed in E. coli DH5α from PCR-generated fragments (KOD, Novagen, Darmstadt, Germany) and isolated with the NucleoSpin kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany). Oligonucleotides used in this study were obtained from Eurofins MWG Operon (Ebersberg, Germany) and Metabion (Planegg/Steinkirchen, Germany) and are listed in Table 1. Plasmid construction was executed by standard PCR, restriction and ligation reactions as described previously (Sambrook and Russell, 2001) as well as Gibson assembly (Gibson et al., 2009). The RbCl method was used for transformation of E. coli (Hanahan, 1983) and C. glutamicum was transformed via electroporation at 2.5 kV, 200 Ω, and 25 μF (Eggeling and Reyes, 2005). The correctness of the cloned DNA fragments was verified by sequencing.
Homologous Overexpression of Genes from C. glutamicum
Plasmids for inducible gene expression were constructed on the basis of pEKEx3 (Stansen et al., 2005) or pVWEx1 (Peters-Wendisch et al., 2001). The gene crtR (cg0725) was amplified from genomic DNA of C. glutamicum WT, which was prepared as described (Eikmanns et al., 1995). Amplification was carried out by PCR using the respective crtR-fw/rv primers (Table 1).
Deletion of crtR in C. glutamicum Strains
Deletion of the gene crtR (cg0725) was carried out using the suicide vector pK19mobsacB (Schäfer et al., 1994). The construction of pK19mobsacB-crtR has been described (Henke et al., 2016). The genomic regions flanking the gene to be deleted by homologous recombination were amplified from genomic DNA of C. glutamicum WT using the primer pairs crtR_A/B and crtR_C/D (Table 1). The PCR products were purified and assembled and simultaneously cloned into SmaI restricted pK19mobsacB by Gibson assembly, which resulted in the deletion vector pK19mobsacB-crtR (Table 1). Targeted gene deletion proceeds via two-step homologous recombination as described previously using the before mentioned deletion vector (Eggeling and Bott, 2005). The first recombination event, the integration of the vector into one of the gene flanking regions, was selected via kanamycin resistance. Integration of the deletion vector into the genome triggers sucrose sensitivity due to the expression of sacB, encoding a levansucrase. In a second recombination event the deletion vector is excised and clones can be selected upon sucrose-resistance. By PCR analysis of three selected mutants using the primer pair crtR_E/F, deletion of the respective gene was verified (Table 1). The PCR products were then sequenced and in-frame deletion of crtR was confirmed.
Extraction of Carotenoids from Bacterial Cell Cultures
The extraction of carotenoids from C. glutamicum was performed as described previously (Heider et al., 2014b) using 0.8 or 1 ml aliquots of the cell cultures. The pigments were extracted from the cell pellets with a methanol:acetone mixture (7:3) at 60°C for 30 min with thorough vortexing every 10 min. When necessary, extraction was repeated to remove all visible colors from the cell pellet. Subsequently, extraction mixtures were centrifuged for 5 min at 13,000 × g and the clear supernatant was used for analysis.
Analysis of Carotenoids
The carotenoid content of cell extracts was determined through absorbance at 470 nm by high performance liquid chromatography (HPLC) analysis as described previously (Heider et al., 2014b). HPLC analyses were performed on an Agilent 1200 series HPLC system (Agilent Technologies GmbH & Co. KG, Böblingen, Germany), including a diode array detector (DAD) for UV/visible (Vis) spectrum recording for detection. Separation of the carotenoids was accomplished by application of a column system (all columns from CS Chromatographie Service GmbH, Langerwehe, Germany) consisting of a pre-column (10 × 4 mm MultoHigh 100 RP18-5) and a main column (ProntoSIL 200-5 C30, 250 × 4 mm). Alternatively, 50 μL of the sample was separated with a column system consisting of a precolumn (LiChrospher 100 RP18 EC-5, 40 × 4 mm) and a main column (LiChrospher 100 RP18 EC-5, 125 × 4 mm) with methanol (A) and methanol/water (9:1) (B) as mobile phase. The following gradient was used at a flow rate of 1.5 mL/min; 0 min B: 0%, 10 min B: 100%, 32.5 min B: 100%. Quantification of lycopene and β-carotene was performed running a standard curve with HPLC grade standards (Sigma-Aldrich, Taufkirchen, Germany). Due to the lack of appropriate standards for decaprenoxanthin, sarcinaxanthin and C.p. 450 the quantification was calculated based on a β-carotene standard and reported as β-carotene equivalents (y = 79.7x; R2 = 0.96). The standards were dissolved in chloroform according to its solubility and diluted in methanol:acetone (7:3) for generating a standard curve.
Transcriptome Analysis
For transcriptome analysis triplicate cultures were grown in LB or CgXII medium. At an optical density of OD600 between 4 and 5 cells were harvested and total RNA was isolated with the RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. DNA was degraded in two reactions, in solution as well as on-column, using the RNase-Free DNase set (Qiagen, Hilden, Germany). Fluorescently labeled cDNA from 20 μg of total RNA was generated by indirect labeling as described by Hüser et al. (2003). cDNA probes labeled with Cy3 and Cy5 were combined for hybridization. Hybridization and scanning was performed as described earlier (Hüser et al., 2003). Signal-background segmentation, spot finding and intensity quantification were executed by ImaGene 6.0 software (BioDiscovery, Hawthorne, United States). Normalization (lowess) and t-test statistics were accomplished with EMMA 2.2, a software platform for consistent storage and efficient analysis of microarray data (Dondrup et al., 2003, 2009). Genes were regarded as differentially expressed when transcript abundance changed 2-fold or more (M ≥ 1 or M ≤ −1) with a statistical significance of p ≤ 0.05 in a student's t-test was determined.
In vivo Measurement of Promoter Activities of crtR and crtE
In vivo expression levels of crtR and crtE were assayed by transcriptional fusion of the intergenic region of crtR and crtE to the promoter-less GFPUV gene using the pEPR1 plasmid system (Knoppova et al., 2007). Therefore, the corresponding promoter sequences were amplified from genomic DNA of C. glutamicum WT with primer pairs PcrtE-pEPR-fw/PcrtE-pEPR-rv and PcrtR-pEPR-fw/PcrtR-pEPR-rv, respectively and cloned into the BamHI restricted pEPR1 vector upstream of the promoter-less gfpUV gene. Site-directed mutagenesis of putative motifs was performed on both constructed vectors using the primer pairs SDM-fw-nat/ SDM-rv-mut, SDM-fw-mut/ SDM-rv-nat and SDM-fw-mut/SDM-rv-mut. The GFPUV shows characteristic emission at 509 nm with an excitation wavelength of 385 nm. Measurements of fluorescence were performed on a FACS Gallios™ (Beckmann Coulter GmbH, Krefeld, Germany) with 405 nm excitation from a blue solid-state laser. Forward-scatter characteristics (FSC) and side-scatter characteristics (SSC) were detected as small- and large-angle scatters of the 405 nm laser. GFPUV fluorescence was detected using a 500/50 nm band-pass filter. C. glutamicum MB001ΔcrtR and MB001 harboring the plasmids pEPR1-PcrtR and pEPR1-PcrtE or the plasmid with the mutated promoter sequences, were harvested in mid-exponential growth washed once in phosphate-buffered saline and the optical density was adjusted to OD600 <1. Cells harboring the empty vector pEPR1 were used to determine background fluorescence.
Overproduction and Purification of the Transcriptional Regulator CrtR
The vector pET16b-crtR was constructed using PCR-amplified crtR using oligonucleotides crtR-NdeI-fw and crtR-BamHI-rv and ligating it into pET16b restricted with BamHI and NdeI. E. coli BL21(DE3) cells carrying the plasmid pET16b-crtR were grown at 37°C in 500 ml LB medium with 100 μg/ml ampicillin to an OD600 of 0.5 before adding IPTG for induction of the gene expression to a final concentration of 0.5 mM. After induction, cells were cultivated at 21°C for additional 5 h. Cells were harvested by centrifugation and stored at −20°C. For cell extract preparation, thawed cells were re-suspended in TNI buffer (20 mM Tris-HCl, pH 7.9, 300 mM NaCl, 5% (v/v) glycerol) with 5 mM imidazole containing a proteinase inhibitor cocktail tablet (Complete Mini, Roche, Basel, Switzerland). Cells were disrupted by ultrasonification using Hielscher UP200S2 (Teltow, Germany) with an amplitude of 60% and a pulsing cycle of 0.5 (power discharge 0.5 s, pause 0.5 s) for 2 min. After ultracentrifugation (1.5 h, 45,000 × g, 4°C) the supernatant was filtered through a 0.2 μm filter and purified by nickel affinity chromatography using nickel-activated nitrilotriacetic acid-agarose (NTA) (Novagen, San Diego, USA). TNI buffer containing 5 mM or 100 mM imidazole was used for sequentially washing of the column. The regulator protein was eluted with TNI buffer containing 400 mM imidazole and the fractions of highest protein concentrations were pooled. The elution buffer was exchanged against band shift (BS) buffer (50 mM Tris–HCl, 10% (v/v) glycerol, 50 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, pH 7.5) using PD10 columns (GE Healthcare, Chalfont St. Giles, Great Britain). Protein concentrations were determined with the Bradford assay kit (Bio-Rad Laboratories, Hercules, Canada) using bovine serum albumin as reference. Protein purification was ascertained by 12% SDS-PAGE (polyacrylamide gel electrophoresis). The purified protein was applied for band shift assay without removing the N-terminal His-tag.
Electrophoretic Mobility Shift Assay (EMSA)
For verification of a physical Protein-DNA interaction between the regulator CrtR and the promoter region of crtE and crtR, band shift assay was performed according to Krause et al. (2012). His-tagged CrtR in varying molar excess was mixed with 90 ng of purified promoter-fragments of the target genes in band shift (BS) buffer (50 mM Tris–HCl, 10% (v/v) glycerol, 50 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, pH 7.5) in a total volume of 20 μL. The 5′UTR of crtE was PCR-amplified with the oligonucleotides SFT8 and SFT3 (fragment A) listed in Table 1 and purified with NucleoSpin kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany). Truncated and mutated promoter fragments were amplified using oligonucleotide pairs SFT8/SFT10 (fragment B), SFT8/SFT12 (fragment C), SFT4/SFT27 (fragment D), SFT28/SFT27 (fragment E), SFT29/SFT27 (fragment F), SFT30/SFT27 (fragment G), SFT8/SFT23 (fragment H), SFT8/SFT26 (fragment I), SFT8/SFT25 (fragment K). A 78 bp-fragment of the upstream region of cg2228 was added in every sample as a negative control using oligonucleotides cg2228_fw and cg2228_rv. After 30 min of incubation at room temperature, gel shift samples were separated on a 6% DNA Retardation gel (Life Technologies GmbH, Darmstadt, Germany) at 100 V buffered in 44.5 mM Tris, 44.5 mM boric acid, and 1 mM EDTA at pH 8.3. Additionally, the binding affinity in presence of selected effectors was analyzed by incubation of the protein with the effector substance under buffered conditions for 15 min at room temperature prior to the addition of the promoter. Subsequently, the gel shift samples were separated on a 6% DNA Retardation gel (Life Technologies GmbH, Darmstadt, Germany) at 100 V buffered in 44.5 mM Tris, 44.5 mM boric acid, and 1 mM EDTA at pH 8.3. Staining of the DNA was achieved with ethidium bromide.
Results
Co-occurrence of the MarR-Type Regulator Gene crtR with Genes of Carotenoid Biosynthesis in Completely Sequenced Actinobacterial Genomes
In the genome of C. glutamicum the carotenogenic genes responsible for conversion of the building blocks IPP and DMAPP to decaprenoxanthin are organized in the crt operon (crtE-cg0722-crtBIYeYfEb) (Heider et al., 2012). The major GGPP synthase IdsA (Heider et al., 2014a) and a second phytoene synthase are encoded elsewhere on the chromosome (Heider et al., 2012). Divergent to the crt operon and 200 bp upstream of its first gene, crtE, one of nine MarR-type transcriptional regulator genes in the genome of C. glutamicum (Brune et al., 2005) is found. This gene cg0725 was named crtR based on the results described in this study. Previously, a transposon mutagenesis screen showed that insertion of a transposon into crtR enhanced carotenoid production (Krubasik et al., 2001a). A model of the protein secondary structure of the 195 aa containing crtR product revealed a pfam12802 domain with a centrally located winged HTH motif located from aa position 73 to 134 as is typical for MarR-type regulator proteins of C. glutamicum (Brune et al., 2005).
A sequence comparison by BLAST showed that C. glutamicum CrtR is distinct from MarR-type regulators of Bacillus subtilis, Escherichia coli, Mycobacterium, and Streptomyces species (data not shown), but showed highest similarities to MarR-type proteins of the high GC content family of Actinobacteria, primarily of species of the genera Corynebacterium, Microbacterium, Leucobacter, Arthrobacter, Propionibacterium, and Gulosibacter (Figure S1). A sequence alignment with the closest relatives of CrtR (CAF19334) from C. vitaeruminis, C. efficiens, C. callunae, Leucobacter sp. Ag1, M. mangrovi, Brevibacterium sp. VCM10, and L. chironomi shows high similarity especially in the central region of the proteins where the proposed HTH motif is located (Figure S2). Since crtR is localized in proximity to the crt operon in C. glutamicum, it was tested if this pattern is conserved among bacteria containing a CrtR ortholog. First, sequence comparisons of C. glutamicum CrtR to completely sequenced genomes identified bacteria possessing a CrtR homolog showing at least 25% amino acid identity and an e-value <10−10. Subsequently, the completely sequenced genomes of these bacteria were analysed for the presence of homologs (25% amino acid identity and e-value <10−10) of the C. glutamicum proteins encoded by crtEb (cg0717), crtYf (cg0718), crtYe (cg0719), crtI (cg0720), crtB (cg0721), cg0722, crtE (cg0723), crtX (cg0730), and idsA (cg2384). Although the low identity cut-off of 25% may have led to more false-positives than searches with higher identity cut-offs (Rost, 1999), this was compensated for by the high significance value of e−10 and the combinatory search for CrtR homologs and other carotenogenesis genes. If at least one homolog of the proteins in this list were encoded in a completely sequenced genome in addition to a homolog of C. glutamicum CrtR, the genome was considered further and the results depicted as heatmap (Figure S3). Opposite and in divergent orientation to the crtR homologous genes, individual genes or crt operons were found. For the most part, the individual genes or the first genes of the crt operons encoded GGPP synthase (crtE), IPP isomerase (idi), and putative multidrug efflux protein (mmpl), respectively (Figure 1).
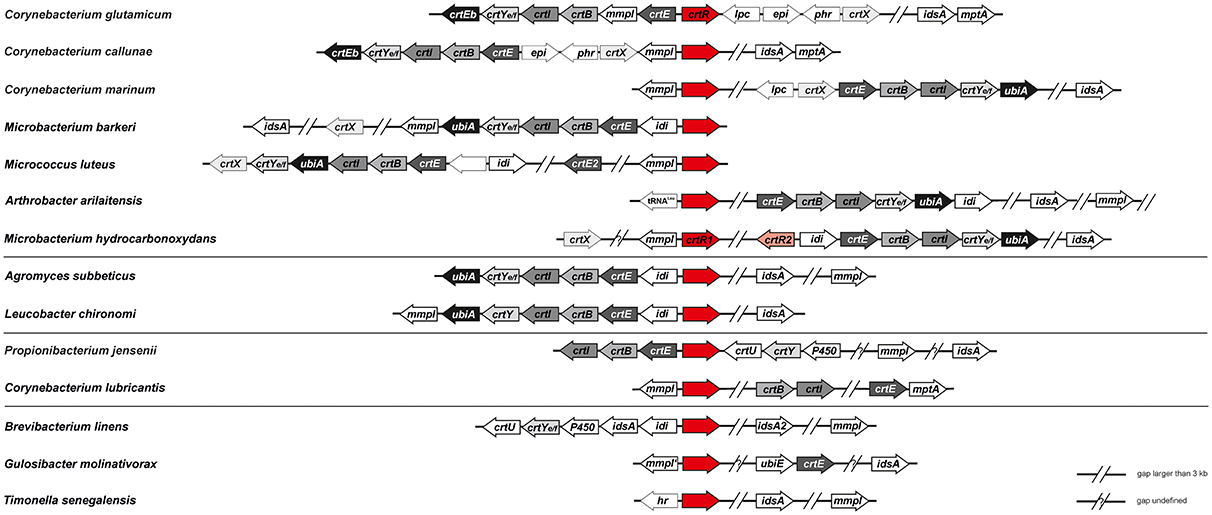
Figure 1. Co-occurrence of homologs of C. glutamicum crtR with genes of carotenogenesis in various completely sequenced genomes. The genetic organization of crtR homologs and genes relevant for carotenoid biosynthesis is depicted for representative genomes containing a crtR homologue and at least one gene of carotenogenesis (s. also Figures S1, S3). crtR, regulator of carotenoid biosynthesis and its homologs (in red); crtE/idsA, geranygeranyl diphosphate synthase; crtB, phytoene synthase; crtI, phytoene dehydratase; crtYe/f, C50 epsilon cyclase; crtEb, lycopene elongase, crtX, carotenoid glycosyl transferase, mmpl, putative RND drug exporter; mmpl', pseudogene with homology to mmpl; lpc, putative lipocalin; phr, putative deoxyribopyrimidine photolyase; epi, putative NDP sugar epimerase; idi, isopentenyl diphosphate isomerase; crtEb/ubiA, prenyltransferases; P450, cytochrome P450 monooxygenase; mptA, α(1→6) mannopyranosyltransferase; ubiE, ubiquinone biosynthesis methyltransferase; crtU, β-carotene desaturase/isorenieratene synthase; crtY, lycopene cyclase (no significant homology to CrtYe/f from C. glutamicum); hr, hemerythrin.
With respect to the genes encoding homologs of C. glutamicum CrtR and genes involved in carotenogenesis four distinct patterns emerged (Figure 1). First, a group of bacteria possessing a full complement of carotenogenesis genes including genes for carotenoid elongase (crtEb), carotenoid cyclase (crtY, crtYe/Yf) and a carotenoid glycosyltransferase (crtX). This group comprised bacteria known to produce glycosylated, cyclic C50 carotenoids (C. glutamicum, C. callunae, C. marinum, Micrococcus luteus), but also bacteria of which it is unknown which carotenoids they synthesize (Arthrobacter arilaitensis, Microbacterium barkeri, and Microbacterium hydrocarbonoxydans; Figure 1). A second group with Agromyces subbeticus and Leucobacter chironomi lacks crtX homologs likely synthesizing non-glucosylated carotenoids and a third group with Propionibacterium jensenii and Corynebacterium lubricantis lacks crtX and crtY homologs likely synthesizing noncyclic nonglycosylated carotenoids. Finally, a group with Brevibacterium linens, Gulosibacter molinativorax and Timonella senegalensis lacks crtX, crtY and crtE/ubiA homologs and these are likely unable to synthesize glycosylated or cyclic or elongated carotenoids. Taken together, homologs of C. glutamicum CrtR are conserved in various bacteria (mostly actinobacteria) that were shown to synthesize various carotenoids (cyclic and noncyclic, elongated or not, glycosylated or not) or do have the potential to synthesize these according to their gene repertoire. Moreover, the crtR homologs are found in diverse genomic locations, but mostly adjacent to crt operons or genes involved in carotenogenesis. These findings supported the hypothesis that CrtR homologs may have a conserved role in regulation of carotenogenesis.
CrtR Negatively Regulates Expression of the crt Operon and of Its Own Gene
To test if C. glutamicum CrtR plays a role in transcriptional regulation of genes of carotenogenesis a crtR deletion mutant was constructed and characterized with respect to global gene expression. Comparative transcriptome analyses of C. glutamicum MB001ΔcrtR and C. glutamicum MB001 were performed to identify genes directly or indirectly regulated by CrtR. The transcriptomes of cells cultured in triplicates either in glucose minimal medium or LB complex medium revealed only eight differentially expressed genes (Table 2). All of the differentially expressed genes showed 8 to 69-fold increased mRNA levels and they belonged to the crt operon or, as in the case of cg0726 that codes for a putative secreted lipoprotein, are localized adjacent to it. Deletion of crtR led to considerably higher mRNA ratios for cg0717 and cg0719 during growth in LB than in minimal medium, however, this does not pertain to the other genes of the crt operon. Currently, it remains unclear if these differences may be caused by technical issues or have a biological cause, however, it has to be noted that RNAseq analysis revealed expression of cg0717 and cg0718 independent of the other crt genes, thus, sub-operons may exist (Pfeifer-Sancar et al., 2013). As expected from the operon structure all transcripts were decreased from the first to the last genes in the crt operon. Taken together, the crt operon of C. glutamicum is directly or indirectly regulated by CrtR.
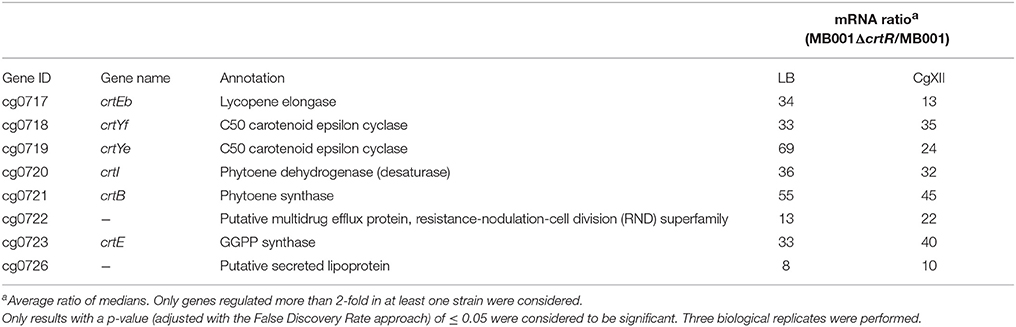
Table 2. Genes differentially expressed in C. glutamicum strains MB001 and MB001ΔcrtR during growth in minimal medium with 100 mM glucose as well as in complex LB medium.
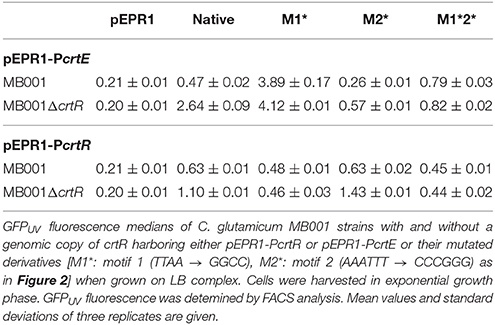
The pEPR1-promoter probe vector system for transcriptional fusion analysis (Knoppova et al., 2007) was used to determine if CrtR regulates transcription of its own gene and of the crt operon in vivo. The intergenic region between crtR and crtE, the first gene of the crt operon, was cloned upstream of the promoter-less gfpUV gene in both orientations (named PcrtE and PcrtR, respectively). Reporter gene expression in C. glutamicum MB001(pEPR1-PcrtE), MB001(pEPR1-PcrtR), MB001ΔcrtR(pEPR1-PcrtR), and MB001ΔcrtR(pEPR1-PcrtE) was determined by fluorescence cell scanning in LB medium in the early exponential growth phase (Table 3). About 2-fold higher expression of the PcrtR fusion in the absence of CrtR revealed that CrtR is subject to negative autoregulation (Table 3). Expression of the PcrtE fusion was about 5-fold higher when crtR was deleted indicating that CrtR represses transcription of the crt operon (Table 3).
Gel Mobility Shift and Transcriptional Fusion Analyses Revealed That CrtR Binds to the Intergenic Region between crtR and crtE
As CrtR was shown to negatively regulate transcription of the crt operon and of its own gene, gel mobility shift assays were performed to determine if CrtR directly interacts with the intergenic region between crtR and crtE. Therefore, CrtR protein was produced in recombinant E. coli BL21 (DE3) as N-terminal His-tagged protein and purified to apparent homogeneity by affinity chromatography (Figure S4). Gel mobility shift analysis revealed that His-tagged CrtR bound to the intergenic region between crtR and crtE (fragment A; Figures 2a,c), whereas BSA as a control did not bind to this DNA fragment (data not shown). At a 30 to 40-fold molar excess of CrtR a complete shift of the promoter fragment C was observed, whereas a negative control fragment comprising the sequence upstream of the unrelated gene cg2228 was not bound by CrtR (Figure 2b). To localize the CrtR binding site, truncated fragments of the intergenic region were used for further gel shift assays. In line with the observation that MarR-type regulators typically bind to palindromic sites of 16–20 bp which often overlap the −10 and −35 regions for steric inhibition of RNA polymerase (Wilkinson and Grove, 2006), two inverted repeats were found in the intergenic region between crtR and crtE (Figure 2a). CrtR bound to fragments A, C, D, E, and F that contained a TTAA sequence motif 25–28 bases upstream of the transcription start site of crtE (Figures 2c,d). Fragments B and G that lack the complete TTAA motif were not bound by CrtR (Figures 2c,d) indicating that this sequence motif might be essential for CrtR binding. Thus, this motif and a similar motif (AAATTT overlapping with the −10 hexamer of the crtE promoter; Figure 2a) were mutated. Mutating the latter motif (fragment H) did not influence binding of CrtR, whereas CrtR did not bind to fragments with a mutated TTAA motif (fragments I and K; Figure 2d). Thus, the TTAA motif was shown to be required for binding of CrtR in vitro.
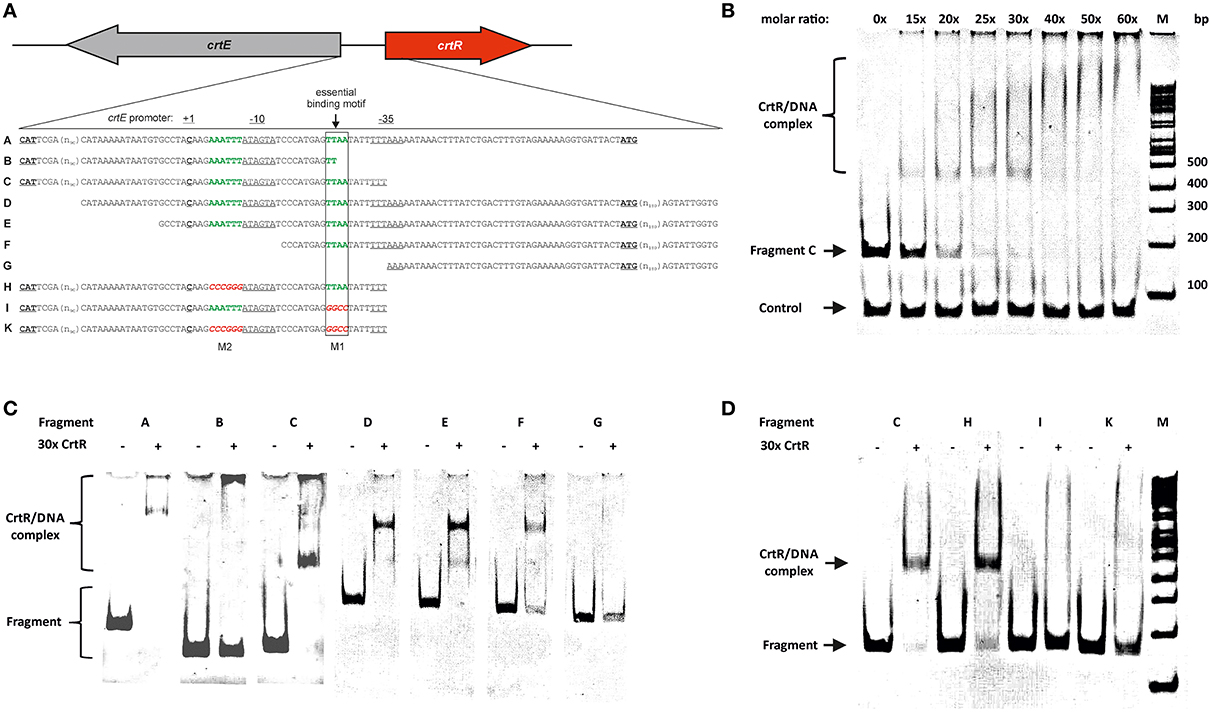
Figure 2. DNA-binding studies of purified CrtRHis6 protein with the 5′UTR of crtE. Promoter region of crtE/crtR and fragments used for electromobility shift assay (EMSA) (a). In green putative binding motifs (M1 and M2) are depicted and in red the respective mutations. The transcriptional start site of the crt-operon is depicted as C in bold (Heider et al., 2012). Fragment A represents the full length intergenic region of crtE and crtR including both translational starts (in bold letters). Fragment B,C,H,I, and K are shortened from the 3′ end, possessing either the original sequence (B, C) or mutations (H, I, K). Fragments D, E, F, and G were ‘successively shortened from the 5′ end and’ constructed with an extra 190 bp downstream of the crtR translational start codon. (b) EMSA with fragment C and different molar rations of purified CrtRHis6 from 0 (no protein added), 15, 20, 25, 30, 40, 50, and 60-fold molar excess. As a control the 5′UTR of cg2228 was used. (c) EMSA with 3′ (B, C) and 5′ (D, E, F, G) truncated fragments of the crtE-crtR intergenic region as depicted in a) and 30-fold molar excess of purified CrtRHis6. (d) EMSA with 3′ truncated fragment C and mutated fragments (H, I, K) and 30-fold molar excess of purified CrtRHis6.
In order to determine the role of the TTAA motif for regulation by CrtR in vivo, transcriptional fusions of the promoters PcrtR and PcrtE containing mutations in the TTAA and/or the AAATTT motifs were constructed. Mutation of the AAATTT motif changed the −10 hexamer of the PcrtE promoter from TATAAA to TATGGG (Figure 2a). Consequently, only very low reporter gene activities were measured (Table 3) and thus, the relevance of the AAATTT motif for regulation of the PcrtE promoter could not be determined. Reporter gene activities of a transcriptional fusion of PcrtR containing the mutated AAATTT motif were comparable to those obtained with the non-mutated PcrtR promoter and revealed that this motif is dispensable for negative autoregulation by CrtR (Table 3). Mutation of the TTAA motif led to very low reporter gene activity with PcrtR, which precluded deducing its involvement in crtR autoregulation. By contrast, mutation of the TTAA motif led to high reporter gene activities of PcrtE regardless of the absence or presence of CrtR (Table 3) which supports the notion that this motif is required for function of CrtR as transcriptional repressor of the crt operon in vivo.
Isoprenoid Pyrophosphates Prevent Binding of CrtR to Its DNA Target
Regulation of carotenogenesis in response to low-molecular-weight compounds has not yet been described. However, since CrtR belongs to the MarR family of transcriptional regulators and representatives of this family show regulation in response to low-molecular-weight compounds, such as hydrogen peroxide in the case of RosR of C. glutamicum (Bussmann et al., 2010), low-molecular-weight compounds were tested as effectors of CrtR in gel mobility shift assays. Intermediates of carotenogenesis (isoprenoid pyrophosphates), precursors of the MEP pathway (GAP and pyruvate), but also phosphorylated intermediates of glycolysis (DHAP, phosphoenolpyruvate and D-glucosamine 6-phosphate), organic acids (acetate, propionate) and compounds affecting other MarR-type regulators [vitamin B12, salicylic acid, urea, protocatechuic acid ‘(data not shown)’] were tested as effectors of CrtR with concentrations in the mM range. Neither DHAP (Figure 3) nor pyruvate (data not shown) had an effect on CrtR binding to its DNA target. In the contrary, in the presence of GAP CrtR binding was prevented (Figure 3). In addition, the isoprenoid pyrophosphates IPP, DMAPP, geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP) and GGPP perturbed binding of His-tagged CrtR to the intergenic region between crtR and crtE (Figure 3). Among these isoprenoid pyrophosphates, GGPP was effective at the lowest concentration (50 μM), whereas FPP, GPP, IPP and DMAPP exerted similar effects on CrtR binding in vitro at a 5-fold higher concentration (Figure 3). Thus, GGPP, shorter isoprenoid pyrophosphates and the MEP-pathway precursor GAP interfere with the in vitro binding of CrtR to the intergenic region between crtR and crtE and the strength of interference follows the order GGPP >> FPP, GPP, IPP, DMAPP >> GAP (Figure S5).
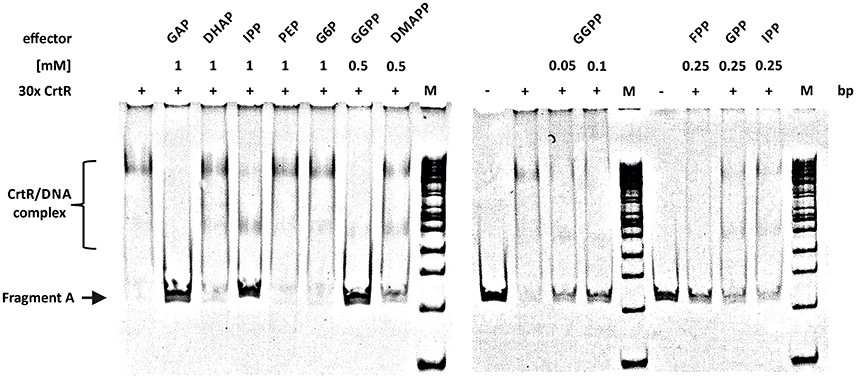
Figure 3. Electromobility shift assay of purified CrtRHis6 protein in the presence of different low molecular weight molecules. Analysis of phosphorylated intermediates from the carotenoid biosynthesis pathway as potential effector molecules on the interaction of CrtRHis6 with the full length intergenic region of crtE and crtR. The purified CrtRHis6 was used in 30 molar excess and the effector molecules were used in depicted concentrations.
Deletion of crtR Is a Metabolic Engineering Strategy to Increase Production of Native and Non-native Carotenoids
Since CrtR represses the crt operon the effect of deletion and overexpression of crtR on decaprenoxanthin accumulation in cells of C. glutamicum wild type and the phage-cured strain MB001 was determined. While growth was not significantly affected by the deletion or overexpression of crtR, neither in complex medium (data not shown) nor in minimal medium, the crtR deletion mutant showed more intense yellow pigmentation, while pigmentation of the overexpressing strain was slightly decreased (Figure S6). HPLC analyses of extracts from cells grown in CGXII medium revealed that the crtR deletion mutants accumulated decaprenoxanthin to 15 to 30-fold higher levels than the corresponding parental strains (Table 4). By contrast, as consequence of plasmid-borne overexpression of crtR decaprenoxanthin levels were significantly decreased (Table 4).
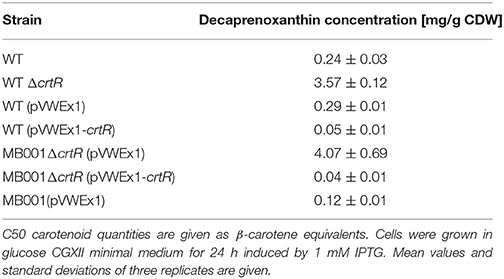
Table 4. Decaprenoxanthin accumulation and growth rates in the presence and absence of the crtR gene and its plasmid-borne overexpression in C. glutamicum WT and MB001 strains.
Since lycopene is a central intermediate of C40 and C50 carotenoid biosynthesis, the effect of deletion of crtR was elucidated in a metabolically engineered lycopene producing LYC5. Lycopene concentration increased about 5-fold (Figure 4). To test if this type of regulatory engineering can also be applied to the production of non-native carotenoids, crtR was deleted in the β-carotene producing strain BETA3, the sarcinaxanthin producing strain LYC5 (pEKEx3-crtE2Y), and the C.p.450 (2,2′-bis-(4-hydroxy-3-methybut-2-enyl)-β,β-carotene) producing strain LYC5 (pEKEx3-lbtABC). Indeed, all strains lacking CrtR produced 1.5 to 2 fold more carotenoid than the parental strains (Figure 4). Thus, deletion of crtR is a general strategy to boost production of native and non-native C40 and C50 carotenoids by C. glutamicum.
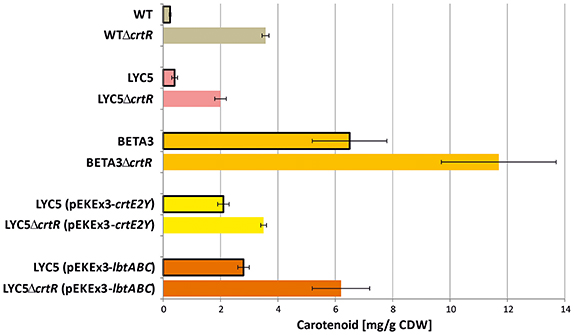
Figure 4. Application of regulator engineering on C40 and C50 carotenoid producing C. glutamicum strains. C50 carotenoid quantities are given as β-carotene equivalents. Cells were grown in glucose CGXII minimal medium for 24 h induced by 1 mM IPTG. Mean values and standard deviations of three biological triplicates are given [except LYC5ΔcrtR (pEKEx3-crtE2Y)]. Data from WT and WTΔcrtR are taken from Table 3. p < 0.05 between control and ΔcrtR strains in students t-test (two-sided, unpaired). Gray, decaprenoxanthin; pink; lycopene; light orange, β-carotene; yellow, sarcinaxanthin; red, C.p.450.
Discussion
In this study, the MarR-type transcriptional regulator CrtR was shown to repress the crt operon, its own gene and likely also the adjacent gene cg0726. GGPP and to lesser extent the isoprenoid pyrophosphates FPP, GPP, IPP, and DMAPP as well as the MEP pathway precursor GAP interfered with CrtR binding in vitro to a TTAA containing sequence in the intergenic region between crtR and the crt operon overlapping with the crt operon promoter.
CrtR is the first MarR-type regulator repressing carotenogenic genes in bacteria that is characterized to some detail. Commonly, RNA polymerase σ factors and transcriptional regulators of a different protein family (MerR-type, but not MarR-type) are involved in repression of carotenogenic operons in non-phototrophic bacteria. Unlike CrtR, these MerR-type regulators bind vitamin B12 as corepressor. By contrast, CrtR lacks a vitamin B12 binding domain (Figure S2) but EMSA experiments revealed that its capacity to bind target DNA is influenced by isoprenoid pyrophosphates (Figure 3). The MerR-type regulators CarH, CarA and LitR involved in regulation of carotenogenic operons in non-phototrophic bacteria have been characterized (Gorham et al., 1996; Whitworth and Hodgson, 2001; Fontes et al., 2003; Takano et al., 2005, 2006). In the complex regulation of carotenogenesis in M. xanthus, two MerR-type regulators (CarH and CarA), an anti-repressor (CarS), a RNA polymerase extracytoplasmic function (ECF)-σ factor (CarQ), anti-σ factor (CarR) and anti-anti-σ factor (CarF) are involved. The carotenogenic carB operon in M. xanthus is repressed in the dark by CarA and/or CarH, the latter of which requires binding of vitamin B12 as corepressor. Light induction is mediated by the SH3 domain-containing anti-repressor CarS, which binds to the DNA binding domains of CarA (Leon et al., 2010) and CarH/B12-complex and therefore counteracts repression of the carotenogenic carB operon (Galbis-Martinez et al., 2012). CarS itself is only synthesized in the light, since the carQRS operon is transcribed in the light by CarQ. The ECF-σ factor CarQ is only available when its anti-σ factor CarR is inactivated by anti-anti-σ factor CarF in response to light induced formation of singlet oxygen by heme precursor protoporphyrin IX interacting with molecular oxygen (Galbis-Martinez et al., 2012).
Light induction may also be regulated directly by the MerR-type regulators functioning as vitamin B12-dependent photoreceptors (Jost et al., 2015). Carotenoid production in Gram-negative T. thermophilus is characterized by litR and crtB, the first gene of the crt operon, both being repressed in the dark by the LitR with 5′-deoxyadenosylcobalamin (AdoB12) bound as corepressor (Jost et al., 2015; Takano, 2016). In the light vitamin B12, the chromophore of the tetrameric AdoB12-LitR-complex is photolysed to hydroxycobalamin, which causes a large-scale conformational change of the regulator. This leads to the loss of operator binding and therefore derepression of its own gene and the subsequent cAMP-CRP family activator gene ldrP (Ortiz-Guerrero et al., 2011). This LdrP protein activates transcription of the crt operon, DNA photolyase gene phrB, and some further genes (Takano et al., 2014). LitR of S. coelicolor also functions as vitamin B12-dependent photoreceptor and its own gene and the ECF-σ factor gene litS are derepressed upon illumination. As consequence, RNA polymerase holoenzyme containing σLitS transcribes the crt operons (Takano, 2016). B. megaterium possesses a LitR homologue as vitamin B12-dependent photoreceptor. The crt operon is relieved from repression by LitR in the light, however, a dedicated σ factor as in S. coelicolor is not involved since transcription occurs by RNA polymerase holoenzyme containing σA (Takano et al., 2015a). In C. glutamicum, however, the MarR-type transcriptional regulator CrtR has been shown here to repress the carotenoid biosynthesis operon. Based on the in vitro analysis it is hypothesized that isoprenoid pyrophosphates act as inducers. Hitherto, neither MarR-type regulators nor low-molecular-weight ligands, such as intermediates of the MEP-pathway or carotenogenesis have been reported to be involved in transcriptional regulation of microbial or plant carotenoid biosynthesis.
MarR-type transcriptional regulators are categorized according to their physiological function acting as regulators of (i) the stress response, (ii) virulence factors or (iii) aromatic catabolism (Wilkinson and Grove, 2006). Typically, MarR-type regulators are sensing environmental changes and some of them regulate drug efflux pump gene expression (Grove, 2013). C. glutamicum is equipped with nine MarR-type regulators (Brune et al., 2005) of which only the hydrogen peroxide-responsive activator of the nitrate/nitrite transporter and the dissimilatory nitrate reductase complex genes RosR (Bussmann et al., 2010), the repressor of the malic enzyme gene MalR (Krause et al., 2012), and PhdR, the repressor of the genes required for β-oxidation of phenylpropanoids (Alekshun et al., 2001), have been analyzed to date. MarR proteins are mostly encoded either as part of the regulated gene cluster or their genes are localized adjacent to and in divergent transcriptional organization to the regulated genes (Wilkinson and Grove, 2006). The latter case applies for CrtR, MalR, RosR, and PhdR of C. glutamicum WT. Although N-terminal HTH domains are most common for negative transcriptional regulators (Perez-Rueda et al., 1998), CrtR possesses a central HTH domain preceded by 72 amino acids (corresponding to aa 73–134). This is also true for MalR, RosR and PhdR, however, their HTH domains are only preceded by 36 to 47 N-terminal amino acids and these proteins are about 40 amino acids shorter than CrtR.
The regulon of CrtR is small, comprising only the crt operon, its own gene and possibly cg0726. CrtR may have only one DNA binding site on the C. glutamicum genome which is located between crtR and the crt operon and comprises a TTAA motif required for CrtR binding (Figure 2). This motif is located 25–28 bp upstream of the transcription start site of crtE, which was identified 114 nucleotides upstream of the start codon (Heider et al., 2012). This motif overlaps the −35 and/or −10 promoter elements of the crt operon, which for other MarR-type regulators was shown to indicate that repression is achieved by steric inhibition of RNA polymerase binding to the promoter (D'Souza et al., 1997). Although short, this motif is palindromic as generally observed for most MarR-type transcriptional regulators (Kelly et al., 1970; Ronnekleiv, 1995). When using promoter fusions of PcrtE and PcrtR, a more intense yellow pigmentation in comparison to the empty vector carrying control strains was observed (data not shown), which likely is due to a titrating effect by the CrtR binding site present on the medium copy promoter-probe vectors (Korshunov and Imlay, 2006). The more intense yellow pigmentation was not observed when the CrtR binding site in the promoter probe vectors was mutated. Deletion of crtR derepressed the crt operon by about 5-fold, but derepressed its own gene by about 2-fold, which might indicate that PcrtE is stronger than PcrtR. The −10 hexamer of PcrtE does not deviate from the consensus sequence TANNNT (Pfeifer-Sancar et al., 2013), while the −35 promoter hexamer TTTAAA consensus (TTGNCA) hexamer is less conserved. However, transcription of crtR and its autoregulation are less well understood since promoter motifs for crtR cannot easily be found (Figure 2a), although it has been reported that crtR is transcribed as a leaderless transcript (Pfeifer-Sancar et al., 2013). A surprisingly high fraction of transcripts in C. glutamicum (33%) lack an 5′ untranslated region (Pfeifer-Sancar et al., 2013) and it remains to be studied if leaderless transcripts are translated less efficient than transcripts containing leaders (Moll et al., 2004) and if they are resistant to inhibition by kasugamycin as is the case for E. coli (Lange et al., 2016).
MarR-type regulators are often involved in stress responses and conformational changes due to binding of an effector molecule impair their binding to their specific DNA targets, as it was described e.g., for MarR in its salicylated form (Alekshun et al., 2001). The MarR-type regulator of oxidative stress response RosR of C. glutamicum showed reduced binding to its target DNA when exposed to H2O2. Furthermore, H2O2 sensitivity was increased in the absence of the rosR adjacent gene cg1322, which is proposed to encode a secreted protein likely binding an octaprenyl pyrophosphate molecule. Since menaquinone contains an octaprenyl side chain and has an effect on the flux of superoxide to the periplasm of E. coli (Korshunov and Imlay, 2006), a correlation between oxidative stress and polyisoprenoid structures in the cytoplasmic membrane of C. glutamicum has been postulated (Bussmann et al., 2010). In this study, an influence of typical MarR effectors, such as acetate, propionate, salicylic acid, urea or protocatechuic acid could not be observed (data not shown). Vitamin B12 involved in LitR-mediated control of carotenogenesis in S. coelicolor (Takano et al., 2005), did not affect binding of CrtR to PcrtE. However, we cannot exclude that the CrtR preparations from E. coli are free from low molecular weight compounds.
Here, we have described that isoprenoid pyrophosphates interfered with binding of CrtR to its cognate DNA target. Consistently, many members of the MarR family bind similar, mostly anionic lipophilic or even phenolic effector molecules (Wilkinson and Grove, 2005). The interfering effect of isoprenoid pyrophosphates was specific as vitamin B12, phosphoenolpyruvate, D-glucosamine 6-phosphate, acetate, propionate, salicylic acid, urea or protocatechuic acid had no significant influence on CrtR binding (data not shown). Based on these in vitro results, it is tempting to speculate that CrtR may be used as a potential biosensor of IPP, DMAPP, GPP, FPP, and GGPP for strain engineering and optimizing terpenoid production by controlling gene expression on demand. This kind of strain development has been shown to work efficiently using photocaged-IPTG as applied to valencene production by C. glutamicum (Binder et al., 2016) or using riboswitches as applied to lysine production by C. glutamicum (Zhou and Zeng, 2015a,b).
CrtR is not only found in corynebacteria, but conserved in actinobacteria that, based on their genome content, have the potential to synthesize various carotenoids (Figure 1). It has to be noted that the genomic organization of crtR in most of these bacteria is linked to genes involved in carotenogenesis. Thus, it is not unlikely that CrtR plays a comparable role in transcriptional regulation of carotenogenic genes in these bacteria.
Author Contributions
All authors planned and designed the experiments. NAH, SAEH, and SH performed the experiments and analyzed the data. PPW and VFW analyzed data. NAH, SAEH, and PPW drafted the manuscript. VFW and PPW coordinated the study and finalized the manuscript. All authors read and approved the final manuscript.
Funding
We acknowledge support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BL and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We thank Boas Pucker, Ruben Hamann and Stefan Albaum for bioinformatic support.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00633/full#supplementary-material
References
Abe, S., Takayarna, K., and Kinoshita, S. (1967). Taxonomical studies on glutamic acid producing bacteria. J. Gen. Appl. Microbiol. 13, 279–301. doi: 10.2323/jgam.13.279
Alekshun, M. N., Levy, S. B., Mealy, T. R., Seaton, B. A., and Head, J. F. (2001). The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution. Nat. Struct. Biol. 8, 710–714. doi: 10.1038/90429
Armstrong, G. A. (1994). Eubacteria show their true colors: genetics of carotenoid pigment biosynthesis from microbes to plants. J. Bacteriol. 176, 4795–4802. doi: 10.1128/jb.176.16.4795-4802.1994
BBCResearch (2015). The Global Market for Carotenoids. Available online at: http://www.bccresearch.com/market-research/food-and-beverage/carotenoids-global-market-report-fod025e.html
Baumgart, M., Unthan, S., Ruckert, C., Sivalingam, J., Grunberger, A., Kalinowski, J., et al. (2013). Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl. Environ. Microbiol. 79, 6006–6015. doi: 10.1128/AEM.01634-13
Binder, D., Frohwitter, J., Mahr, R., Bier, C., Grunberger, A., Loeschcke, A., et al. (2016). Light-controlled cell factories: employing photocaged isopropyl-β-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl. Environ. Microbiol. 82, 6141–6149. doi: 10.1128/AEM.01457-16
Bramley, P. M., and Mackenzie, A. (1988). Regulation of carotenoid biosynthesis. Curr. Top. Cell. Regul. 29, 291–343. doi: 10.1016/B978-0-12-152829-4.50009-4
Britton, G. (2008). “Functions of intact carotenoids,” in Carotenoids: Natural Functions, eds G. Britton, S. Liaaen-Jensen, and H. Pfander (Basel: Birkhäuser Verlag), 189–212.
Britton, G., Liaaen-Jensen, S., and Pfander, H. (2004). Carotenoids Handbook. Basel: Birkhauser Verlag.
Brune, I., Brinkrolf, K., Kalinowski, J., Puehler, A., and Tauch, A. (2005). The individual and common repertoire of DNA-binding transcriptional regulators of Corynebacterium glutamicum, Corynebacterium efficiens, Corynebacterium diphtheriae and Corynebacterium jeikeium deduced from the complete genome sequences. BMC Genomics 6:86. doi: 10.1186/1471-2164-6-86
Bussmann, M., Baumgart, M., and Bott, M. (2010). RosR (Cg1324), a hydrogen peroxide-sensitive MarR-type transcriptional regulator of Corynebacterium glutamicum. J. Biol. Chem. 285, 29305–29318. doi: 10.1074/jbc.M110.156372
D'Souza, E. S., Altekar, W., and D'Souza, F. S. (1997). Adaptive response of Haloferax mediterranei to low concentrations of NaCl (<20%) in the growth medium. Arch. Microbiol. 168, 68–71.
Das, A., Yoon, S. H., Lee, S. H., Kim, J. Y., Oh, D. K., and Kim, S. W. (2007). An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 77, 505–512. doi: 10.1007/s00253-007-1206-3
Dembitsky, V. M. (2005). Astonishing diversity of natural surfactants: 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids 40, 535–557. doi: 10.1007/s11745-005-1415-z
Dondrup, M., Albaum, S. P., Griebel, T., Henckel, K., Jünemann, S., Kahlke, T., et al. (2009). EMMA 2–A MAGE-compliant system for the collaborative analysis and integration of microarray data. BMC Bioinformatics 10:50. doi: 10.1186/1471-2105-10-50
Dondrup, M., Goesmann, A., Bartels, D., Kalinowski, J., Krause, L., Linke, B., et al. (2003). EMMA: a platform for consistent storage and efficient analysis of microarray data. J. Biotechnol. 106, 135–146. doi: 10.1016/j.jbiotec.2003.08.010
Downham, A., and Collins, P. (2000). Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 35, 5–22. doi: 10.1046/j.1365-2621.2000.00373.x
Eggeling, L., and Bott, M. (2005). Handbook of Corynebacterium glutamicum. Boca Raton, FL: CRC Press Taylor and Francis Group.
Eggeling, L., and Reyes, O. (2005). “Experiments,” in Handbook of Corynebacterium glutamicum, eds L. Eggeling and M. Bott (Boca Raton, FL: CRC Press), 3535–566.
Eikmanns, B. J., Rittmann, D., and Sahm, H. (1995). Cloning, sequence analysis, expression, and inactivation of the Corynebacterium glutamicum icd gene encoding isocitrate dehydrogenase and biochemical characterization of the enzyme. J. Bacteriol. 177, 774–782. doi: 10.1128/jb.177.3.774-782.1995
Follmann, M., Ochrombel, I., Kramer, R., Trotschel, C., Poetsch, A., Ruckert, C., et al. (2009). Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genomics 10:621. doi: 10.1186/1471-2164-10-621
Fontes, M., Galbis-Martinez, L., and Murillo, F. J. (2003). A novel regulatory gene for light-induced carotenoid synthesis in the bacterium Myxococcus xanthus. Mol. Microbiol. 47, 561–571. doi: 10.1046/j.1365-2958.2003.03319.x
Galbis-Martinez, M., Padmanabhan, S., Murillo, F. J., and Elias-Arnanz, M. (2012). CarF mediates signaling by singlet oxygen, generated via photoexcited protoporphyrin IX, in Myxococcus xanthus light-induced carotenogenesis. J. Bacteriol. 194, 1427–1436. doi: 10.1128/JB.06662-11
Gao, L. Y., Groger, R., Cox, J. S., Beverley, S. M., Lawson, E. H., and Brown, E. J. (2003). Transposon mutagenesis of Mycobacterium marinum identifies a locus linking pigmentation and intracellular survival. Infect. Immun. 71, 922–929. doi: 10.1128/IAI.71.2.922-929.2003
Gibson, D. G., Young, L., Chuang, R. Y., Venter, J. C., Hutchison, C. A. III., and Smith, H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345. doi: 10.1038/nmeth.1318
Gorham, H. C., McGowan, S. J., Robson, P. R., and Hodgson, D. A. (1996). Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol. Microbiol. 19, 171–186. doi: 10.1046/j.1365-2958.1996.360888.x
Grove, A. (2013). MarR family transcription factors. Curr. Biol. 23, R142–R143. doi: 10.1016/j.cub.2013.01.013
Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. doi: 10.1016/S0022-2836(83)80284-8
Heider, S. A., Peters-Wendisch, P., Beekwilder, J., and Wendisch, V. F. (2014a). IdsA is the major geranylgeranyl pyrophosphate synthase involved in carotenogenesis in Corynebacterium glutamicum. FEBS J. 281, 4906–4920. doi: 10.1111/febs.13033. Epub 2014
Heider, S. A. E., Peters-Wendisch, P., Netzer, R., Stafnes, M., Brautaset, T., and Wendisch, V. F. (2014b). Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 98, 1223–1235. doi: 10.1007/s00253-013-5359-y
Heider, S. A., Peters-Wendisch, P., and Wendisch, V. F. (2012). Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol. 12:198. doi: 10.1186/1471-2180-12-198
Henke, N. A., Heider, S. A., Peters-Wendisch, P., and Wendisch, V. F. (2016). Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs 14:124. doi: 10.3390/md14070124
Hüser, A. T., Becker, A., Brune, I., Dondrup, M., Kalinowski, J., Plassmeier, J., et al. (2003). Development of a Corynebacterium glutamicum DNA microarray and validation by genome-wide expression profiling during growth with propionate as carbon source. J. Biotechnol. 106, 269–286. doi: 10.1016/j.jbiotec.2003.08.006
Jochmann, N., Kurze, A. K., Czaja, L. F., Brinkrolf, K., Brune, I., Huser, A. T., et al. (2009). Genetic makeup of the Corynebacterium glutamicum LexA regulon deduced from comparative transcriptomics and in vitro DNA band shift assays. Microbiology 155(Pt 5), 1459–1477. doi: 10.1099/mic.0.025841-0
Johnson, E. A., and Schroeder, W. A. (1996). Microbial carotenoids. Adv. Biochem. Eng. Biotechnol. 53, 119–178.
Jost, M., Fernandez-Zapata, J., Polanco, M. C., Ortiz-Guerrero, J. M., Chen, P. Y., Kang, G., et al. (2015). Structural basis for gene regulation by a B12-dependent photoreceptor. Nature 526, 536–541. doi: 10.1038/nature14950
Kelly, M., and Jensen, S. L. (1967). Bacterial Carotenoids.26. C50-Carotenoids.2. Bacterioruberin. Acta Chem. Scand. 21:2578.
Kelly, M., Norgard, S., and Liaaen-Jensen, S. (1970). Bacterial carotenoids. 31. C50-carotenoids 5. Carotenoids of Halobacterium salinarium, especially bacterioruberin. Acta Chem. Scand. 24, 2169–2182. doi: 10.3891/acta.chem.scand.24-2169
Knoppova, M., Phensaijai, M., Vesely, M., Zemanova, M., Nesvera, J., and Patek, M. (2007). Plasmid vectors for testing in vivo promoter activities in Corynebacterium glutamicum and Rhodococcus erythropolis. Curr. Microbiol. 55, 234–239. doi: 10.1007/s00284-007-0106-1
Korshunov, S., and Imlay, J. A. (2006). Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 188, 6326–6334. doi: 10.1128/JB.00554-06
Krause, J. P., Polen, T., Youn, J. W., Emer, D., Eikmanns, B. J., and Wendisch, V. F. (2012). Regulation of the malic enzyme gene malE by the transcriptional regulator MalR in Corynebacterium glutamicum. J. Biotechnol. 159, 204–215. doi: 10.1016/j.jbiotec.2012.01.003
Krubasik, P., Kobayashi, M., and Sandmann, G. (2001a). Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur. J. Biochem. 268, 3702–3708. doi: 10.1046/j.1432-1327.2001.02275.x
Krubasik, P., Takaichi, S., Maoka, T., Kobayashi, M., Masamoto, K., and Sandmann, G. (2001b). Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Arch. Microbiol. 176, 217–223. doi: 10.1007/s002030100315
Lange, C., Matthiass, L., Zerulla, K., Ludwig, P., Schweitzer, J., Polen, T., et al. (2016). Effects of Kasugamycin on the translatome of Escherichia coli. PLoS ONE 12:e068143. doi: 10.1371/journal.pone.0168143
Lee, P. C., and Schmidt-Dannert, C. (2002). Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 60, 1–11. doi: 10.1007/s00253-002-1101-x
Leon, E., Navarro-Aviles, G., Santiveri, C. M., Flores-Flores, C., Rico, M., Gonzalez, C., et al. (2010). A bacterial antirepressor with SH3 domain topology mimics operator DNA in sequestering the repressor DNA recognition helix. Nucleic Acids Res. 38, 5226–5241. doi: 10.1093/nar/gkq277
Moll, I., Hirokawa, G., Kiel, M. C., Kaji, A., and Blasi, U. (2004). Translation initiation with 70S ribosomes: an alternative pathway for leaderless mRNAs. Nucleic Acids Res. 32, 3354–3363. doi: 10.1093/nar/gkh663
Netzer, R., Stafsnes, M. H., Andreassen, T., Goksoyr, A., Bruheim, P., and Brautaset, T. (2010). Biosynthetic pathway for gamma-cyclic sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C carotenoid cyclases. J. Bacteriol. 192, 5688–5699. doi: 10.1128/JB.00724-10
Norgård, S., Aasen, A. J., and Liaaen-Jensen, S. (1970). Bacterial carotenoids. 32. C50-carotenoids 6. Carotenoids from Corynebacterium poinsettiae including four new C50-diols. Acta Chem. Scand. 24, 2183–2197. doi: 10.3891/acta.chem.scand.24-2183
Ortiz-Guerrero, J. M., Polanco, M. C., Murillo, F. J., Padmanabhan, S., and Elias-Arnanz, M. (2011). Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. U.S.A. 108, 7565–7570. doi: 10.1073/pnas.1018972108
Perez-Rueda, E., Gralla, J. D., and Collado-Vides, J. (1998). Genomic position analyses and the transcription machinery. J. Mol. Biol. 275, 165–170. doi: 10.1006/jmbi.1997.1465
Peters-Wendisch, P. G., Schiel, B., Wendisch, V. F., Katsoulidis, E., Mockel, B., Sahm, H., et al. (2001). Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 3, 295–300.
Pfeifer-Sancar, K., Mentz, A., Ruckert, C., and Kalinowski, J. (2013). Comprehensive analysis of the Corynebacterium glutamicum transcriptome using an improved RNAseq technique. BMC Genomics 14:888. doi: 10.1186/1471-2164-14-888
Provvedi, R., Kocincova, D., Dona, V., Euphrasie, D., Daffe, M., Etienne, G., et al. (2008). SigF controls carotenoid pigment production and affects transformation efficiency and hydrogen peroxide sensitivity in Mycobacterium smegmatis. J. Bacteriol. 190, 7859–7863. doi: 10.1128/JB.00714-08
Ronnekleiv, M. (1995). Bacterial carotenoids 53* C50-carotenoids 23; carotenoids of Haloferax volcanii versus other halophilic bacteria. Biochem. Syst. Ecol. 23, 627–634. doi: 10.1016/0305-1978(95)00047-X
Rost, B. (1999). Twilight zone of protein sequence alignments. Protein Eng. 12, 85–94. doi: 10.1093/protein/12.2.85
Sambrook, J., and Russell, D. (2001). Molecular Cloning. A Laboratory Manual, 3rd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratoy Press.
Sandmann, G. (2001). Carotenoid biosynthesis and biotechnological application. Arch. Biochem. Biophys. 385, 4–12. doi: 10.1006/abbi.2000.2170
Sandmann, G., and Yukawa, H. (2005). “Vitamin synthesis: carotenoids, biotin and pantothenate,” in Handbook of Corynebacterium glutamicum, eds L. Eggeling and M. Bott (Boca Raton, FL: CRC Press), 399–417.
Sandoval, H., Aguilar, A., Paniagua, C., and Martín, J. F. (1984). Isolation and physical characterization of plasmid pCCl from Corynebacterium callunae and construction of hybrid derivatives. Appl. Microbiol. Biotechnol. 19:409. doi: 10.1007/BF00454379
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., and Puhler, A. (1994). Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73. doi: 10.1016/0378-1119(94)90324-7
Schäfer, T., Selig, M., and Schönheit, P. (1993). Acetyl-CoA synthetase (ADP forming) in archaea, a novle enzyme involved in acetate formation and ATP synthesis. Arch. Microbiol. 159, 72–83.
Silberbach, M., Schafer, M., Huser, A. T., Kalinowski, J., Puhler, A., Kramer, R., et al. (2005). Adaptation of Corynebacterium glutamicum to ammonium limitation: a global analysis using transcriptome and proteome techniques. Appl. Environ. Microbiol. 71, 2391–2402. doi: 10.1128/AEM.71.5.2391-2402.2005
Stansen, C., Uy, D., Delaunay, S., Eggeling, L., Goergen, J. L., and Wendisch, V. F. (2005). Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl. Environ. Microbiol. 71, 5920–5928. doi: 10.1128/AEM.71.10.5920-5928.2005
Takano, H. (2016). The regulatory mechanism underlying light-inducible production of carotenoids in non-phototrophic bacteria. Biosci. Biotechnol. Biochem. 80, 1264–1273. doi: 10.1080/09168451.2016.1156478
Takano, H., Agari, Y., Hagiwara, K., Watanabe, R., Yamazaki, R., Beppu, T., et al. (2014). LdrP, a cAMP receptor protein/FNR family transcriptional regulator, serves as a positive regulator for the light-inducible gene cluster in the megaplasmid of Thermus thermophilus. Microbiology 160(Pt 12), 2650–2660. doi: 10.1099/mic.0.082263-0
Takano, H., Asker, D., Beppu, T., and Ueda, K. (2006). Genetic control for light-induced carotenoid production in non-phototrophic bacteria. J. Ind. Microbiol. Biotechnol. 33, 88–93. doi: 10.1007/s10295-005-0005-z
Takano, H., Mise, K., Hagiwara, K., Hirata, N., Watanabe, S., Toriyabe, M., et al. (2015a). Role and function of LitR, an Adenosyl B12-bound light-sensitive regulator of Bacillus megaterium QM B1551, in regulation of carotenoid production. J. Bacteriol. 197, 2301–2315. doi: 10.1128/JB.02528-14
Takano, H., Mise, K., Hagiwara, K., Hirata, N., Watanabe, S., Toriyabe, M., et al. (2015b). The role and function of LitR, an AdoB12-bound light-sensitive regulator of Bacillus megaterium QM B1551, in the regulation of carotenoid production. J. Bacteriol. 197, 2301–2315. doi: 10.1128/JB.02528-14
Takano, H., Obitsu, S., Beppu, T., and Ueda, K. (2005). Light-induced carotenogenesis in Streptomyces coelicolor A3: identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J. Bacteriol. 187, 1825–1832. doi: 10.1128/JB.187.5.1825-1832.2005
Tao, L., Yao, H., and Cheng, Q. (2007). Genes from a Dietzia sp. for synthesis of C40 and C50 β-cyclic carotenoids. Gene 386, 90–97. doi: 10.1016/j.gene.2006.08.006
Umeno, D., Tobias, A. V., and Arnold, F. H. (2005). Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiol. Mol. Biol. Rev. 69, 51–78. doi: 10.1128/MMBR.69.1.51-78.2005
Whitworth, D. E., and Hodgson, D. A. (2001). Light-induced carotenogenesis in Myxococcus xanthus: evidence that CarS acts as an anti-repressor of CarA. Mol. Microbiol. 42, 809–819. doi: 10.1046/j.1365-2958.2001.02679.x
Wilkinson, S. P., and Grove, A. (2005). Negative cooperativity of uric acid binding to the transcriptional regulator hucr from Deinococcus radiodurans. J. Mol. Biol. 350, 617–630. doi: 10.1016/j.jmb.2005.05.027
Wilkinson, S. P., and Grove, A. (2006). Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr. Issues Mol. Biol. 8, 51–62.
Winterhalter, P., and Rouseff, R. (2001). “Carotenoid-derived aroma compounds: an introduction,” in Carotenoid-Derived Aroma Compounds (Washington, DC: ACS Symposium Series), 1–17.
Yang, Y., Yatsunami, R., Ando, A., Miyoko, N., Fukui, T., Takaichi, S., et al. (2015). Complete biosynthetic pathway of the C50 carotenoid bacterioruberin from lycopene in the extremely halophilic archaeon Haloarcula japonica. J. Bacteriol. 197, 1614–1623. doi: 10.1128/JB.02523-14
Zhou, L. B., and Zeng, A. P. (2015a). Engineering a Lysine-ON Riboswitch for metabolic control of lysine production in Corynebacterium glutamicum. ACS Synth. Biol. 4, 1335–1340. doi: 10.1021/acssynbio.5b00075
Keywords: regulation of carotenogenesis, terpenoid biosynthesis, actinobacteria, isoprenoid pyrophosphates as inducers, CrtR, MarR-type regulators
Citation: Henke NA, Heider SAE, Hannibal S, Wendisch VF and Peters-Wendisch P (2017) Isoprenoid Pyrophosphate-Dependent Transcriptional Regulation of Carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 8:633. doi: 10.3389/fmicb.2017.00633
Received: 21 December 2016; Accepted: 28 March 2017;
Published: 24 April 2017.
Edited by:
Angel Angelov, Technische Universität München, GermanyReviewed by:
Benedikt Leis, Technische Universität München, GermanyKapil Tahlan, Memorial University of Newfoundland, Canada
Copyright © 2017 Henke, Heider, Hannibal, Wendisch and Peters-Wendisch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Peters-Wendisch, petra.peters-wendisch@uni-bielefeld.de
†Present Address: Sabine A. E. Heider, GlaxoSmithKline Vaccines S.r.l., Siena, Italy
 Nadja A. Henke
Nadja A. Henke Sabine A. E. Heider
Sabine A. E. Heider Silvin Hannibal
Silvin Hannibal Volker F. Wendisch
Volker F. Wendisch Petra Peters-Wendisch
Petra Peters-Wendisch