- 1Mycotoxicology and Chemoprevention Research Group, Institute of Biomedical and Microbial Biotechnology, Cape Peninsula University of Technology, Bellville, South Africa
- 2Microbiology Department, Stellenbosch University, Stellenbosch, South Africa
Infection by the fumonisin-producing Fusarium spp. and subsequent fumonisin contamination of maize adversely affect international trade and economy with deleterious effects on human and animal health. In developed countries high standards of the major food suppliers and retailers are upheld and regulatory controls deter the importation and local marketing of fumonisin-contaminated food products. In developing countries regulatory measures are either lacking or poorly enforced, due to food insecurity, resulting in an increased mycotoxin exposure. The lack and poor accessibility of effective and environmentally safe control methods have led to an increased interest in practical and biological alternatives to reduce fumonisin intake. These include the application of natural resources, including plants, microbial cultures, genetic material thereof, or clay minerals pre- and post-harvest. Pre-harvest approaches include breeding for resistant maize cultivars, introduction of biocontrol microorganisms, application of phenolic plant extracts, and expression of antifungal proteins and fumonisin degrading enzymes in transgenic maize cultivars. Post-harvest approaches include the removal of fumonisins by natural clay adsorbents and enzymatic degradation of fumonisins through decarboxylation and deamination by recombinant carboxylesterase and aminotransferase enzymes. Although, the knowledge base on biological control methods has expanded, only a limited number of authorized decontamination products and methods are commercially available. As many studies detailed the use of natural compounds in vitro, concepts in reducing fumonisin contamination should be developed further for application in planta and in the field pre-harvest, post-harvest, and during storage and food-processing. In developed countries an integrated approach, involving good agricultural management practices, hazard analysis and critical control point (HACCP) production, and storage management, together with selected biologically based treatments, mild chemical and physical treatments could reduce fumonisin contamination effectively. In rural subsistence farming communities, simple, practical, and culturally acceptable hand-sorting, maize kernel washing, and dehulling intervention methods proved to be effective as a last line of defense for reducing fumonisin exposure. Biologically based methods for control of fumonisin-producing Fusarium spp. and decontamination of the fumonisins could have potential commercial application, while simple and practical intervention strategies could also impact positively on food safety and security, especially in rural populations reliant on maize as a dietary staple.
Introduction
Fusarium spp. are agriculturally important plant pathogenic fungi associated with disease and mycotoxin contamination of grain crops (Wild and Hall, 2000; Picot et al., 2011). Fusarium ear rot in maize is one of the major diseases affecting maize production worldwide and poses an enormous threat to the international trade of foods and feeds. Fungal species of Fusarium Section Liseola, including Fusarium verticillioides, Fusarium proliferatum, and Fusarium subglutinans are some of the most important causative fungal agents of Fusarium ear or kernel rot as well as symptomless infection of maize crops, leading to contamination with the fumonisin mycotoxins (Munkvold et al., 1997).
Fifteen Fusarium spp. have been reported to produce fumonisins. Eight species are from the Section Liseola, i.e., F. verticilloides, Fusarium sacchari, Fusarium fujikuroi, F. proliferatum, F. subglutinans, Fusarium thapsinum, Fusarium anthophilum, and Fusarium globosum (Rheeder et al., 2002). Another five species fall within Section Dlaminia, i.e., Fusarium nygamai, Fusarium dlamini, and Fusarium napiforme. Trace amounts of fumonisin were detected in culture material of two species, i.e., Fusarium andiyazi and Fusarium pseudonygamai. The remaining two fumonisin-producing Fusarium spp. are one species in Section Elegans, i.e., Fusarium oxysporum and one in Section Arthrosporiella, i.e., Fusarium polyphialidicum. The fumonisins are associated with several diseases in humans, animals, poultry, and fish (Marasas, 2001; Marasas et al., 2004; Kimanya et al., 2010) and are classified as Group 2B carcinogens (IARC, 2002). Home-grown maize is a major dietary staple in southern Africa and known to be frequently contaminated with unacceptable levels of fumonisins, with fumonisin B1 (FB1) being the most prevalent natural occurring fumonisin (Marasas, 2001; Marasas et al., 2004; Shephard et al., 2007, 2013; Burger et al., 2010). The Eastern Cape Province of South Africa is one of the areas in the world where the highest levels of FB1 were recorded in home-grown maize. As a result exposure to FB1 in adults is more than four times above the provisional maximum tolerable daily intake (2 μg FB1/kg body weight/day) set by the Joint Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO) Expert Committee on Food Additives (Bolger et al., 2001).
The fumonisins comprise a group of 28 characterized analogs, which can be separated into four main groups: fumonisin A, B, C, and P (Rheeder et al., 2002). The fumonisin B (FB) analogs, which includes FB1, FB2, and FB3, are the most abundant naturally occurring fumonisins, with FB1 predominating and usually being found at the highest levels. Apart from FB, some of the other analogs may occur in naturally contaminated maize at relatively low levels. The complete fumonisin molecule plays an important role in toxic and cancer-initiating activities in vivo (Gelderblom et al., 1993). Studies evaluating the structure-activity relationship of fumonisin analogs, hydrolysis products and a monomethyl ester of FB1 in short-term carcinogenesis in rats and cytotoxicity assays in primary rat hepatocytes, indicated that the free amino group plays a pivotal role in the toxicological effects of the fumonsins in vitro and in vivo. It was suggested that the tricarballylic acid moiety is required for effective absorption of the fumonisins from the gut. The fumonisins disrupt sphingolipid biosynthesis by inhibiting the enzyme ceramide synthase (Wang et al., 1991), and the tricarballylic acid moiety is required for maximal effect (Van der Westhuizen et al., 1998).
Fusarium infect maize in the field with the highest levels of fumonisins present at harvest, concentrated in the pericarp and embryo of the maize kernel (Fandohan et al., 2006; Kimanya et al., 2008; Burger et al., 2013). Kinetics of Fusarium growth and mycotoxin production are mainly affected by water activity, temperature, and atmospheric composition, while nutritional factors such as kernel endosperm composition and nitrogen sources also play an important role (Chulze, 2010; Picot et al., 2011). Fumonisin production strongly depends on the kernel stage, and may be regulated by physicochemical factors that vary during ear ripening. Insect damage of maize by the European corn borer (Ostrinia nubilalis Hübner) and the corn earworm (Helicoverpa zea Boddie) further favors Fusarium infection (Betz et al., 2000).
Methods for reduction of fumonisins in maize are applied pre-harvest or during harvesting and processing (Wild and Gong, 2010). These include several existing strategies to reduce Fusarium growth and production of fumonisins in food sources, i.e., controlled agricultural practices, ensiling strategies, breeding for insect and fungal resistance in maize cultivars, various physical-, chemical-, and biological treatment methods and genetic engineering approaches. Good agricultural management and hazard analysis and critical control point (HACCP) practices promote the general condition of crops, reducing but not eliminating fungal growth, and mycotoxin contamination, while resistance breeding strives to achieve a balance between developing resistant crops and maintaining high quality crop yield (Cleveland et al., 2003; Wild and Gong, 2010). However, optimization of agricultural management practices is not always possible due to high production costs, the geographical location or nature of the production systems, and challenging environmental conditions.
Several physical and chemical control methods for mycotoxins have been commercialized involving sorting and flotation, solvent extraction, chemical detoxification by alkalization (e.g., ammonia, sodium hydroxide, and sulfur dioxide treatments), oxidation (e.g., ozone), and irradiation and pyrolysis (He and Zhou, 2010). There are, however, several limitations, challenges, and concerns with regards to physical and chemical control methods (Schatzmayr et al., 2006). Physical methods generally have low efficacy and less specificity, while chemical methods are not always effective, are considered expensive and may decrease the nutritional value of foods, affect the sensory quality, and could produce toxic derivatives (Alabouvette et al., 2009; He and Zhou, 2010). Furthermore, methods involving fungicides pose a potential health, safety, and environmental risk as certain antifungal chemical compounds are not biodegradable or have a long degradation period, could contaminate soil and water and their effect on food quality and human health is a concern (Larkin and Fravel, 1998; da Cruz Cabral et al., 2013). Prolonged chemical treatment of grains can lead to the development of resistance in fungal strains, a demand for higher concentrations, and an increase in toxic residues in food crops. Increasingly more stringent regulation is enforced with regards to the use of chemical control methods together with a strong consumer demand to reduce the use of potentially harmful chemicals in the food supply (Liu et al., 2013). There is also an ecological and societal movement toward safe and natural food, without chemical treatments and/or preservatives (Edlayne et al., 2009).
Research over the past 25 years indicates support for agricultural management practices and a renewed interest in practical and biological control methods as possible alternatives. In this regard several methods for controlling fungal growth and mycotoxin production pre- and post-harvest involving clay minerals, plant extracts and a variety of microbial taxa have been commercialized (He and Zhou, 2010). In rural subsistence farming communities a number of effective, practical, and culturally acceptable intervention methods have been developed (Kimanya et al., 2008; Van der Westhuizen et al., 2010). While the focus in the past was more on the most economically important mycotoxins, i.e., aflatoxin B1 (AFB1), much less information is available on other important mycotoxins such as FB1, trichothecenes, zearalenone, citrinin, and patulin (Kabak et al., 2006). This paper presents a comprehensive overview of recent research on biological- and practical-based approaches for control of fumonisin-producing Fusarium spp. and methods for reduction thereof during pre- and post-harvest conditions. Current information on the application of natural clay adsorbents, biocontrol organisms, antioxidants, essential oils, plant extracts, and molecular approaches are reviewed; as well as practical and culturally acceptable methods for reduction of fumonisin exposure in rural subsistence farming communities.
Pre-Harvest Biologically Based Control Methods for Fumonisin-Producing Fusarium Spp.
Biocontrol Microorganisms
This approach involves a three-way interaction between the host commodity, the pathogen and the antagonistic biocontrol microorganism together with dynamics such as competition for nutrients and space, parasitism of the pathogen, secretion of antifungal compounds, induction of systemic resistance (ISR), biofilm formation and involvement with reactive oxygen species in defense response (Larkin and Fravel, 1998; Alabouvette et al., 2009). Recent research also suggested that the aflatoxin biocontrol mechanism, employing atoxigenic strains of Aspergillus flavus, is triggered by physical contact or interaction between hyphae of the competing fungal strains (Damann, 2014). Essential criteria for effective biocontrol microorganisms include the ability to colonize the plant part infected by the pathogen organism, efficacy under the relevant environmental conditions and compatibility with other control methods that are applied (Bacon and Hinton, 2011; Liu et al., 2013). Niche overlap indices (NOIs) provide information on ecological similarity, coexistence, and competition between microorganisms in a specific niche and assists in identifying possible microbial antagonists against F. verticillioides colonization (Cavaglieri et al., 2004). Microorganisms naturally associated with and adapted to the vegetative parts of a specific plant, sharing the ecological niche with pathogen microorganisms, could hold advantages as biocontrol agents. One such a microorganism, Bacillus subtilis occupies the same ecological niche as F. verticillioides within the maize plant and effectively inhibits growth of the fungus, based on competitive exclusion (Bacon et al., 2001; Table 1). B. subtilis is considered generally regarded as safe (GRAS) by the United States Food and Drug Administration [US FDA, GRAS substances evaluated by the Select Committee on GRAS substances (SCOGS)], is easy to cultivate and manipulate genetically, and therefore suitable for industrial processes. A pre-harvest biological control system, involving B. subtilis RRC101, was developed on maize which reduces fumonisin accumulation during the endophytic growth phase of F. verticillioides (= F. moniliforme; Bacon et al., 2001). The endophytic phase of F. verticillioides is transferred vertically to the next generation through clonal infection of seeds. This phase is characterized by intercellular systemic infection of plants and seeds, which cannot be controlled with fungicides. Effective biocontrol has also been demonstrated with wild type and fusaric acid resistant mutant strains of the bacterial endophyte, Bacillus mojavensis, in vitro and in planta (Bacon and Hinton, 2011). Efficacy of these strains under field conditions could be influenced by fusaric acid produced by F. verticillioides. The mechanism of biocontrol by B. mojavensis is complex and still unclear, as indicated by broad differences in maize seedling protection by a range of strains evaluated.
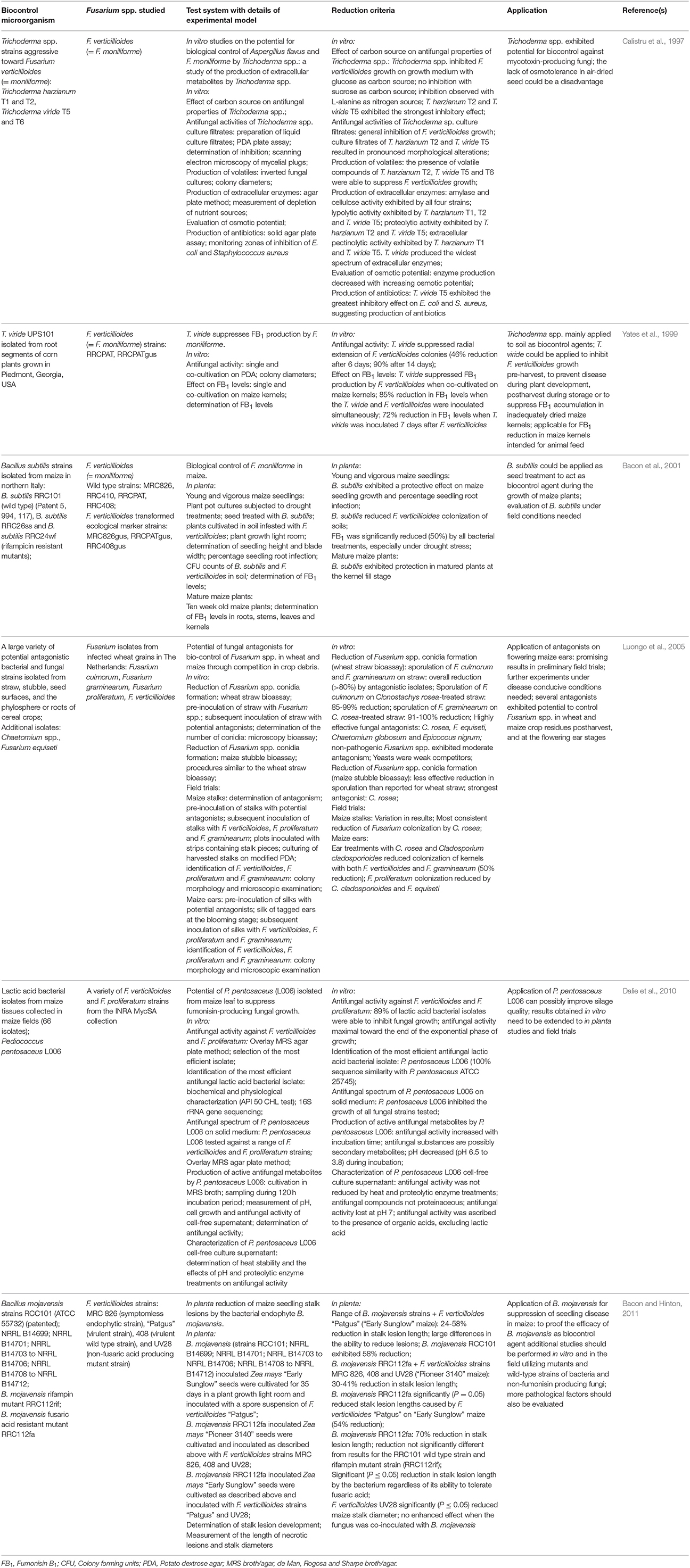
Table 1. Current information on reduction of fumonisin-producing Fusarium spp. by biocontrol microorganisms in vitro, in planta, and in field trials.
Pediococcus pentosaceus, a lactic acid bacterial isolate from maize, inhibits F. verticillioides and F. proliferatum growth in vitro (Dalie et al., 2010; Table 1). Antifungal activity in P. pentosaceus culture supernatant was observed toward the end of the exponential phase of growth and was pH dependent. The antifungal metabolites produced proved to be heat stable and resistant to proteolytic enzymes. Culture fractions exhibiting antifungal activity contained compounds with molecular masses ranging from 500 to 1400 Da. P. pentosaceus has GRAS status, has been widely used in the fermentation of a variety of foods and could be suitable as biocontrol organism to improve the quality of ensilage. Clonostachys rosae, a fungal isolate from straw, stubble, seed surfaces, and the phylosphere or roots of cereal crops, effectively reduced sporulation of F. verticillioides and F. proliferatum on maize stalks in vitro and in field trials (Luongo et al., 2005). C. rosae exhibited potential to control Fusarium spp. in maize at the flowering ear stages and in crop residues post-harvest. Food-grade yeasts are also considered ideal biocontrol microorganisms, as they are generally genetically stable, effective at low concentrations, easy to cultivate, capable to survive under adverse environmental conditions, compatible with commercial processing, and resistant to pesticides.
Trichoderma spp.
Trichoderma spp. are considered effective biocontrol agents because of their repertoire of extracellular lytic enzymes that cause necrotrophic action through lysis of fungal cell walls as well as the role they play in ISR in plants (Bacon et al., 2001; Hermosa et al., 2012). Trichoderma mainly colonizes the rhizosphere and intercellular root areas of plants, and maintains interactions by promoting plant growth and providing protection against infections, while utilizing plant sucrose to facilitate root colonization (Hermosa et al., 2012). Plant disease severity is reduced in the presence of Trichoderma by inhibition of a wide range of plant pathogens through antagonistic and mycoparasitic action; ISR or induction of localized resistance. Trichoderma is also able to withstand toxic metabolites that are produced by the plant in response to invasion. Plants are able to detect pathogen- or microbe associated molecular patterns (MAMPs), which leads to activation of defense mechanisms and eventually synthesis of antimicrobial compounds. Certain Trichoderma strains produce a variety of MAMPs, contributing to activation of plant defense responses. Salicylic acid, jasponic acid and ethylene play a key role in plant immunity and hormone-signaling pathways as well as defense response pathways of the hormones abscisic acid, indole-3-acetic acid, and gibberellin (Pieterse et al., 2009). Indole-3-acetic acid produced by Trichoderma contributes to ethylene biosynthesis, which in turn stimulates abscisic acid biosynthesis. Depending on Trichoderma stimuli, phytohormone homeostasis will control plant development and immune responses. Trichoderma chitinases also release fungal chitin oligosaccharides, and elicit ISR by jasmonic acid/ethylene dependent pathways, thereby triggering defense responses in plants. A polyketide synthase/non-ribosomal peptide synthetase hybrid enzyme of Trichoderma virens is involved in plant interactions and was shown to induce plant defense responses (Mukherjee et al., 2012). Several Trichoderma spp. with GRAS status, including Trichoderma viride and Trichoderma harzianum, are capable of effectively reducing F. verticillioides (= F. moniliforme) growth and fumonisin production in vitro and in planta (Calistru et al., 1997; Larkin and Fravel, 1998; Yates et al., 1999; Table 1). The inhibitory effect on F. verticillioides growth when co-cultured with Trichoderma spp. can be attributed to antibiosis through production of volatile compounds, extracellular enzymes and antibiotics. The antagonistic fungal species T. viride is widely used in bio-fertilizers for biological control of soil borne plant-pathogenic fungi in crops.
Non-Pathogenic Biocontrol Strains
Non-pathogenic strains of pathogenic species are often applied for biocontrol (Liu et al., 2013). In this regard, moderate suppression of toxigenic F. verticillioides and F. proliferatum strains by non-pathogenic Fusarium strains was demonstrated by Luongo et al. (2005; Table 1).
The development of Fusarium biocontrol strains with reduced mycotoxin production ability through RNA silencing technology may be a useful tool for reducing mycotoxin contamination in agricultural products (McDonald et al., 2005). Transformation of F. graminearum with inverted repeat transgenes (IRT) containing sequences of mycotoxin-specific regulatory genes results in suppression of mycotoxin production. Other gene silencing techniques involving deletion of ZFR1 of F. verticillioides, which regulates sugar transporter genes and in turn affect fumonisin biosynthesis during kernel colonization, resulted in significantly less growth on maize kernel endosperm tissue (Bluhm et al., 2008).
Rhizobacteria
Fusarium verticillioides is the most prevalent Fusarium spp. present in the rhizoplane and endorhizosphere areas of maize, while Arthrobacteria and Azotobacter are the predominant bacterial genera (Cavaglieri et al., 2005a). Pathogens germinate and colonize roots within a few days of planting, while biocontrol rhizobacteria could be metabolically active during this period. A number of rhizobacterial isolates of maize plants sampled from a commercial maize field and exhibiting high NOIs with F. verticillioides, including Arthrobacter globiformis, Azotobacter armeniacus, Pseudomonas solanacearum, B. subtilis, Enterobacter cloacae, and Microbacterium eoleovorans exhibited antifungal activity in vitro by effectively reducing F. verticillioides growth and FB1 production on maize meal extract agar (Cavaglieri et al., 2004, 2005a,b,c) (Table 2). Maize seeds pre-treated with A. armeniacus RC2, A. globiformis RC5, E. cloacae, M. eoleovorans, and Bacillus sp. CE1 and evaluated in planta, resulted in effective reduction of F. verticillioides growth in the rhizoplane and endorhizosphere areas. A good correlation was observed between results obtained from in vitro and in planta studies (Cavaglieri et al., 2005c). Enterobacter cloacae exhibited potential for biocontrol of root colonization by F. verticillioides. Inducible Type 1 fimbrae of E. cloacae may play a role in the colonization of roots (Hinton and Bacon, 1995). Rhizobacterial strains could have potential application as seed inoculants to reduce F. verticillioides colonization on root level, in the rhizoplane and endorhizosphere areas (Cavaglieri et al., 2005c). Effectiveness of a biocontrol organism to colonize the rhizosphere and its value as biocontrol agent could, however, be influenced by environmental conditions and the initial cell concentrations of the biocontrol organism and the pathogen.

Table 2. Current information on reduction of Fusarium verticillioides growth and fumonisin B1 production by rhizobacteria in vitro and in planta.
Antioxidants, Phenolic Compounds, and Essential Oils
Several natural phenolic compounds derived from plants are strong antioxidants and exhibit antimicrobial activity by inhibiting the activity of key fungal enzymes, and are applied as preservatives in the cosmetic, food and drug industries (Table 3). These compounds are also considered promising antifungal agents for controlling fungal growth and associated mycotoxin production in agricultural crops pre-harvest, post-harvest, and during storage.

Table 3. Current information on reduction of fumonisin-producing Fusarium spp. and fumonisin production in vitro by antioxidants/phenolic compounds and essential oils extracted from plants.
Antioxidants
The food-grade antioxidants butylated hydroxyanisole (BHA) and propylparaben (PP) have shown potential for controlling F. verticillioides and F. proliferatum growth and fumonisin production at a variety of water activities and incubation temperatures in vitro (Etcheverry et al., 2002; Table 3). Both fungal species were more sensitive to BHA and PP than the other antioxidants evaluated, i.e., trihydroxybutyrophenone (THBP) and butylated hydroxytoluene (BHT). In another study, combination treatments of BHA and PP resulted in further reduction of fumonisin production (Reynoso et al., 2002). BHA, PP, and BHT alone or in combination also resulted in a significant (P < 0.001) reduction in hydrolytic enzyme activity, which is required for early fungal growth. Similar results were reported by Torres et al. (2003). BHA is produced naturally by Botryococcus braunii, Cylindrospermopsis raciborskii, Microcystis aeruginosa, and Oscillatoria sp., while PP is a natural compound extracted from plants. Both antioxidants are also produced synthetically, are considered GRAS by the US FDA and frequently employed as preservatives in the food and cosmetic industries (Reynoso et al., 2002; Rawal et al., 2010; US FDA, GRAS substances evaluated by SCOGS).
Tetrahydrocurcuminoids (THC), a class of phenolic antioxidants extracted from the roots of the non-toxic herbaceous plant Curcoma longa L. (Turmeric), inhibits F. proliferatum growth and FB1 production in vitro (Coma et al., 2011; Table 3). THC1, a food-grade compound containing two guaiacyl phenolic subunits, exhibited high antifungal activity and inhibition of FB1 production in liquid cultures at low inhibitory concentrations. FB1 production was affected irrespective of the effect on fungal growth, indicating that fungal growth and FB1 biosynthesis are independently modified by THC1. Comparative studies on THCs and related molecules n-propylguaiacol, eugenol, acetylacetone, and ferulic acid indicated that the presence of the benzene rings and guaiacyl groups play an important role in fungal inhibition (Beekrum et al., 2003; Samapundo et al., 2007). It was further noticed that the presence of hydroxyl and methoxy groups in the ortho position of the benzene ring of THC molecules affects the degree of antifungal activity, while the enolic part of the non-phenolic THC3 molecule could play a role in bioactivity. It was suggested that the biochemical mechanisms involved during antioxidant and antifungal activities differ between the respective THC compounds, as the presence of a phenol group in the meta- or para-position of the linking chain and a phenol or a methoxy group adjacent to it is required for antioxidant activity.
Phenolic Compounds
Investigations into the effects of the natural phenolic compounds vanillic and caffeic acid on F. verticillioides and F. proliferatum growth and FB1 production at different water activities in maize in vitro indicated that an increase in phenolic compound concentration results in an increase in the lag phase of growth, and a decrease in fungal growth rate and FB1 production (Samapundo et al., 2007; Table 3). In general, complete inhibition of Fusarium growth was observed at relatively high phenolic concentrations and low water activities. F. proliferatum was more sensitive, exhibiting complete inhibition of growth in the presence of the compounds. Both compounds significantly reduced FB1 production by F. verticillioides and F. proliferatum, with vanillic acid being more effective. No FB1 was produced by F. verticillioides in the presence of vanillic acid at the lowest concentration tested.
F. verticillioides growth and FB1 production are inhibited by several other plant phenolic compounds in vitro (Table 3). Chlorophorin, iroko, maakianin, vanillic acid, and caffeic acid inhibits F. verticillioides growth, while FB1 production is inhibited by chlorophorin, iroko, vanillic acid, caffeic acid, and ferulic acid (Beekrum et al., 2003; Table 3). Flavonoids, phenolic acid, and terpine rich 70% ethanol extracts of the non-toxic food-grade plants Equisetum arvense (Horsetail) and Stevia rebaudiana (Candyleaf), effectively inhibited F. verticillioides growth, with S. rebaudiana being more effective (Garcia et al., 2012). However, fumonisin production was not affected. Extracts of the herbaceous climbing vine of the family Cucurbitaceae, Gynostemma pentaphyllum (Southern Ginseng), inhibited growth of F. verticillioides (Srichana et al., 2011). G. pentaphyllum is frequently applied as herbal medicine and exhibits high antioxidant activity. Fumigation by trans-2-hexanal (extracted from fruits and vegetables), carvacrol (extracted from oregano and thyme), and eugenol (extracted from cinnamon and clove) effectively inhibits F. verticillioides conidial germination and mycelial growth in maize kernels, with trans-2-hexanal the most effective (Menniti et al., 2010). Trans-2-hexanal fumigation was also effective in controlling the fungus in asymptomatic kernels. However, the treatment does not reduce fumonisin levels post-harvest, but reduces the germ-ability of maize kernels. The compound 6,7-dimethoxycoumarin, occurring in Penicillium digitatum infected Citrus sinensis cultivar Valencia fruit (Valencia orange), reduces F. verticillioides growth and FB1 production (Mohanlall and Odhav, 2006). Possible mechanisms of inhibition by phenolic plant extracts include disruption of the fumonisin biosynthetic pathway; effects on colony morphology; granulation of the cytoplasm; and rupture of the cytoplasmic membrane (Garcia et al., 2012).
Essential Oils
Essential oil and oleoresins extracted from Zingiber officinale (Ginger) rhizomes exhibit clear antimicrobial activity against F. verticillioides (= F. moniliforme) in vitro (Singh et al., 2008; Table 3). Ginger oil and carbon tetrachloride oleoresin extracts have shown highly effective inhibition of F. verticillioides growth. The antioxidative potential of the essential oil and oleoresins, in terms of peroxide content, anisidine and thiobarbituric acid values, 1,1-diphenyl-2-picrylhydrazyl free radical scavenging activity and total antioxidant activity was in general comparable to the antioxidants BHA and BHT, but not as effective as propyl gallate. The phenolic compound geranial is dominant in the essential oil component, while eugenol and singerone are dominant in the oleoresin extracts. The antioxidant activity could also be enhanced by a possible synergistic effect of the phenolic compounds.
Essential oils extracted from cinnamon, clove, oregano, palmarosa and lemongrass inhibit growth and FB1 production by F. verticillioides and F. proliferatum in vitro (Velluti et al., 2003; Table 3). The inhibitory effect of the essential oils was overall more pronounced at higher water activities, probably due to more effective penetration of oils into kernels in the presence of water. The antimicrobial activity of these oils could be attributed to the presence of aliphatic alcohols and phenols in their chemical composition. Oils of cinnamon and oregano were most promising for control of fungal growth and FB1 production by F. proliferatum, and cinnamon, oregano and lemongrass oils for F. verticillioides. These oils could be effective in controlling fungal growth and FB1 production in maize under pre-harvest conditions.
Developing Resistant Crops through Breeding and Genetic Engineering
Studies in breeding and genetic engineering for resistance in crops are mainly aimed at preventing invasion by insects, contamination by mycotoxigenic fungi and detoxification of mycotoxins in planta through various molecular strategies (Duvick, 2001; Cleveland et al., 2003). Selection of resistant genotypes is complex, it requires sufficient genotypic variation within the breeding material; is affected by climatic conditions; and should be tested across several locations and years (Löffler et al., 2010). Lower mycotoxin levels measured in United States and Canadian maize, where no fungicide was introduced, was attributed to successes with breeding resistant maize varieties.
Extensive genomic resources are essential for investigations into the biochemical and regulatory pathways of mycotoxin biosynthesis, pathogenesis of fungal–plant interactions, and the development of targeted and innovative approaches for breeding and engineering crops for resistance (Cleveland et al., 2003; Brown et al., 2006; Desjardins and Proctor, 2007). Whole genome sequences and expression sequence tags (ESTs) are important tools for understanding disease caused by fungi, fungal lifecycles and secondary metabolism. Available genomic resources include genetic maps, genome sequences, an EST library, and an integrated gene index. Next-generation RNA sequencing was used to study transcriptional changes associated with F. verticillioides inoculation in resistant and susceptible maize genotypes by including an extensive range of maize inbred lines (Lanubile et al., 2014). The technique generated extremely useful data on genetic markers involved in recognition, signaling, and controlling host resistance mechanisms. It also provided quantification of expression, thus enabling interpretation of defense responses. The data provides an important genomic resource for the development of disease resistant maize genotypes. Genetic markers identified through this technique could be added to existing information on single nucleotide polymorphism markers.
Natural Resistance in Crops
Comprehensive knowledge on the biochemical and molecular mechanisms involved in natural resistance of crops is imperative for the further development of resistance to Fusarium infection and insect infestation in crops (Cleveland et al., 2003). The whole genome sequence of maize is available (Schnable et al., 2009), permitting genome-wide expression analysis of the maize–Fusarium interaction. Studying maize varieties with varying degrees of resistance enables researchers to associate resistant crops with specific genetic, biochemical and anatomical traits. Regions on chromosomes associated with natural resistance to insect invasion, fungal contamination, or mycotoxin production are identified, resistant traits mapped and resistant lines crossed with commercially acceptable lines. Chromosomal regions could be associated with resistance to fungal growth; with mycotoxin production; or with both traits, indicating the possibility of separate genetic control (Cleveland et al., 2003). Comparison of kernel protein profiles between susceptible and resistant genotypes through proteomic analyses contributes to identifying resistance associated proteins. Resistant inbred lines are distinguished from susceptible lines and serve as sources of resistant germplasm.
Expression profiles for maize genes during infection with F. verticillioides indicated up-regulation of genes encoding a range of proteins related to cell rescue, defense, and virulence in both resistant and susceptible maize lines, including pathogenesis related (PR) proteins [e.g., chitinase (reducing chitin in fungal membrane); permatin (fungal hyphae leak and rupture)]; proteins involved in detoxification response (e.g., cytochrome P450 monoxygenase, peroxidases, and glutathione-S-transferases); heat-shock proteins (regulating folding of resistance proteins); and proteinase inhibitors (Lanubile et al., 2010). Resistance in maize lines could be due to constitutive defense mechanisms that resist fungal infection (Lanubile et al., 2010; Campos-Bermudez et al., 2013). In resistant maize lines defense-related genes, encoding constitutively expressed PR, detoxification enzymes, and β-glucosidases, were transcribed at high levels before infection, and provided defense against the fungus. In susceptible maize lines, defense genes are induced as a response to pathogen infection, though not sufficiently enough to prevent progress of the disease.
Host–pathogen recognition and interaction processes underlie resistance and susceptibility (Campos-Bermudez et al., 2013). Sucrose is one of the compounds that play an important role in host-pathogen recognition and in the outcome of interactions. During fungal infection plant carbohydrate metabolism is manipulated by induced invertase and sucrose synthase enzymes and the formation of hexoses required for fungal growth. Maize lipoxygenase (ZmLOX) derived oxylipins (e.g., jasmonic acid) are known for regulating plant defense against pathogens, and also play an important role in recognition during host-pathogen interactions, as indicated by up-regulation of LOX genes ZmLOX5 and ZmLOX12 in a response to F. verticillioides infection (Maschietto et al., 2015).
Mapping of chromosomal regions encoding Fusarium ear mold resistance as quantitative trait loci (QTL) and the employment of marker-assisted QTL in selection for Fusarium ear mold resistance are valuable tools being developed for maize hybrid development (Duvick, 2001). Ear mold resistance can be mapped as QTL using large segregating plant populations. Molecular markers linked to these QTL could be valuable during inbred development. Other factors that enhance the susceptibility of maize genotypes include: late-maturing cultivars where grain moisture content decreases slowly; upright cobs and thin grain pericarp which increase susceptibility to fungal infection; tightness of husks; and the competitive advantage of F. verticillioides by having a broader optimum temperature range than F. graminearum (Butròn et al., 2006).
Genetic Engineering for Resistance to Insect Infestation and Fusarium Infection in Crops
Natural fungal and insect resistance mechanisms could be further enhanced in commercially acceptable crops through genetic engineering (Cleveland et al., 2003). The role of hemicellulose, cysteine protease, peroxidase, α-amylase inhibitors, as well as maize ribosomal inactivating protein in insect resistance mechanisms are important focus areas. Genetically modified Bt maize expressing cry proteins from the bacterium Bacillus thuringiensis, has the potential to reduce insect damage and fumonisin levels compared to non-Bt hybrids. Furthermore, chitinase enzymes for digestion of chitin, an integral part of the exoskeleton of insects, have been applied for control of Sesamia cretica (corn borer; Osman et al., 2015). A chitinase gene from the cotton leaf worm, Spodoptera littoralis, was expressed in transgenic maize, and resulted in enhanced resistance against S. cretica. The development of transgene resistance to fungal disease appears to be more challenging than insect resistance (Duvick, 2001). Although, moderate resistance was demonstrated in model systems, no transgenic crops with effective resistance to fungal disease are commercially available. However, genetics of Fusarium infection of maize kernels, development of disease symptoms and biosynthesis of fumonisins is a rapid developing field and could provide more insights for developing transgenic resistance to Fusarium infection in the near future.
Genetic engineering approaches include the cloning and expression of genes encoding maize secondary metabolites with antifungal properties and the overexpression of pathway-limiting enzymes (Duvick, 2001). However, it should be kept in mind that diversion of metabolic pathways could compromise other vital biosynthetic routes. Expression of antifungal protein in tissue critical for fungal infection could be a strategy, while different types of resistance could be employed by pyramiding different types of resistance genes into commercial germplasm. Host plant–pathogen interactions are complex, involving multiple proteins and metabolites as well as competition for biomass and nutrients. Signaling pathway genes control a variety of cellular defense pathways involving protein-protein interactions. Engineering of the main signals controlling defense gene expression could result in more effective defense response including constitutive response or a chemically induced response and the development of enhanced disease resistance phenotypes.
Another approach involves the expression of catabolic enzymes to detoxify mycotoxins in situ before it accumulates in the plants (Duvick, 2001). Success depends on several factors: the extent to which the plant-produced enzyme reaches its target substrate and the stability of the detoxification step; enzyme localization in the seed in relation to mycotoxin accessibility; kinetic parameters of the enzyme in the context of its localization in the plant; stability and activity of the enzyme pre- and post-harvest; and the identity and toxicity of breakdown products.
Bt Maize
Genetic modification of maize plants to express insecticidal Cry proteins of Bacillus thuringiensis (called Bt maize) provides a safe and highly effective method for insect control and accompanying Fusarium infection and fumonisin production (Betz et al., 2000). Corn borers cause considerable damage to maize stalk and ear tissue, which in turn stimulates germination of F. verticillioides spores, leading to progressive ear and kernel rot and eventually production of increased levels of fumonisins. A significant correlation was reported between the extent of insect damage and total fumonisin levels in maize (Dowd, 2001). Cry1Ab protein in Bt protected maize reduces corn-borer damage in maize dramatically, resulting in considerable less Fusarium infection and reduced fumonisin levels (Betz et al., 2000). Cry proteins are selectively active against a specific range of insects including lepidopteron and coleopteran insect pests. Extensive field trials across the USA and Europe confirmed frequently lower fumonisin concentrations detected in maize using Bt maize hybrids (Hammond et al., 2004), thereby increasing the percentage maize grain suitable for human consumption. In South Africa, there has been a decrease over the last 20 years in the amount of chemical insecticides used, due to the cultivation of Bt crops (Kunert, 2011). In the US States the annual benefits that Bt maize provides in terms of lower fumonisin and aflatoxin contamination are estimated at about $23 million (Wu, 2006). Bt maize could especially be a useful tool in developing countries.
The insecticidal nature of the Cry proteins has led to the development of a variety of commercial Bt microbial pesticide products since 1961 (Betz et al., 2000). Extensive toxicological studies by the US Environmental Protection Agency (EPA) and the World Health Organisation (WHO) have proven the safety of Bt protected crops and products to humans, animals and the environment [US EPA, 1998a,b; International Programme on Chemical Safety (IPCS), 1999]. Food derived from Bt crops has also been fully approved by numerous regulatory agencies through-out the world. Safety considerations were further supported by the more than 50 years history of safe use of these products (McClintock et al., 1995). The potential for human and non-target exposure is extremely low, as Cry proteins exhibit a high degree of specificity toward the target insect species, should be ingested to activate in the target species and should have no contact activity (Betz et al., 2000). Bt products are considered to reduce the risks posed by insecticides, thereby impacting less on the environment. It also functions as a supplementary pest control by enhancing the presence of beneficial natural occurring non-target insects (Gianessi and Carpenter, 1999). The cultivation of Bt protected maize by growers increased rapidly throughout the world since its commercial introduction in 1996 (Betz et al., 2000). Grower approval could be ascribed to increased crop yields, reduced crop damage and input costs as a result of reduction in the use of chemical pesticides; and highly effective pest control. Cry proteins in the plant tissue are not affected by application timing, accuracy of application, concentration, rain or sunlight. Bt crops are entirely equivalent to non-recombinant plants, except for the presence of cry genes and proteins. Bt protected crops and products meet important standards for biological control agents regarding technical viability, need, safety and efficacy.
Recently, increasing insect resistance and accompanied occurrence of resistance alleles in insects against first generation Bt crops have been reported (Kunert, 2011; Abbas et al., 2013). Efforts to reduce the development of target insect resistance to Bt crops include introduction of a refuge strategy, which involves the cultivation of non-Bt crops nearby Bt crops to prevent domination of resistant insect species. The effectiveness of Bt crops is also influenced by fluctuation of the Bt protein concentrations produced in plants, which in turn is determined by factors such as plant maturation and photosynthesis. Possible structural changes of Bt proteins, including changes in micro-RNA and protein profiles were also reported. Bt maize genotype plays a determining role in the efficacy of insect damage control (Clements et al., 2003). Bt (Cry1Ab protein) protected plants could reduce fumonisin concentration in maize during seasons when the European corn borer (O. nubilalis Hübner) dominates, but not in seasons when the corn earworm (H. zea Boddie) dominates. Tende et al. (2010) evaluated sensitivity of the stalk borer species Chile partellus (Lepidoptera, Crambidae) and Busseola fusca (Lepidoptera, Noctuidae) toward endotoxins constitutively produced by two Bt maize inbred lines frequently cultivated in Kenya. The Bt maize inbred lines (Event 223 cry1AB::Ubiquitin and Event 10 cry1Ba::Ubiquitin) reduced C. partellus survival significantly and sensitivity remained constant through eight generations. However, B. fusca invasion could not be sufficiently controlled by these inbred lines and remained unchanged through five generations. More efficient transgenic Bt crops could be produced through gene pyramiding (Kunert, 2011).
Post-Harvest Biologically Based Control Methods for Reduction of the Fumonisins in Food and Feed
Natural Clay Adsorbents
Introduction of natural clay adsorbents during food processing leads to detoxification of contaminated food through adsorption of mycotoxins (Aly et al., 2004; Robinson et al., 2012). The bioavailability of mycotoxins in animal feed is also reduced in this manner, thereby preventing toxic interactions and absorption across the gastrointestinal tract.
Montmorillonites are a group of phyllosilicate clay minerals that have the ability to adsorb organic compounds through cation-exchange (Aly et al., 2004). The adsorption abilities of montmorillonite clays are higher than other clay minerals due to their large molecular structure and surface area that increases considerably when wet. Their chemical structures are characterized by alternating layers of tetrahedral silicon and octahedral aluminum coordinated with oxygen atoms. Montmorillonite clay minerals effectively reduce FB1 in aqueous solutions in vitro, and in human- and animal models in vivo through adsorption (Table 4). The adsorption is saturable and occurs largely within the interlaminar regions of the clay (Mitchell et al., 2013). Certain clay minerals, particularly naturally occurring aluminum oxides have structure-selective affinities for different mycotoxins and the degree of adsorption depends on the polarity of the molecules, while the particle size of clays could also influence binding affinity (He and Zhou, 2010). A correlation exists between the binding capacity of the clays and the ratio of their surface acidity to pore volume. In this regard, the slightly higher adsorption of AFB1 than FB1 to hydrated sodium calcium aluminum magnesium silicate hydroxide (Egyptian montmorillonite, EM) and hydrated sodium calcium aluminum silicate (HSCAS) in spiked malt extracts, could be ascribed to the difference in polarity between the molecules (Aly et al., 2004). The adsorption capacity of montmorillonite clays can be enhanced by addition of phosphate and polyphosphate salts, bentonite, or calcined attapulgite (He and Zhou, 2010). A combination of clay minerals (1–10%) and modified yeast cell wall extracts (90–99%) could be beneficial for adsorption of multiple mycotoxins, including the fumonisins (Howes and Newman, 2000).
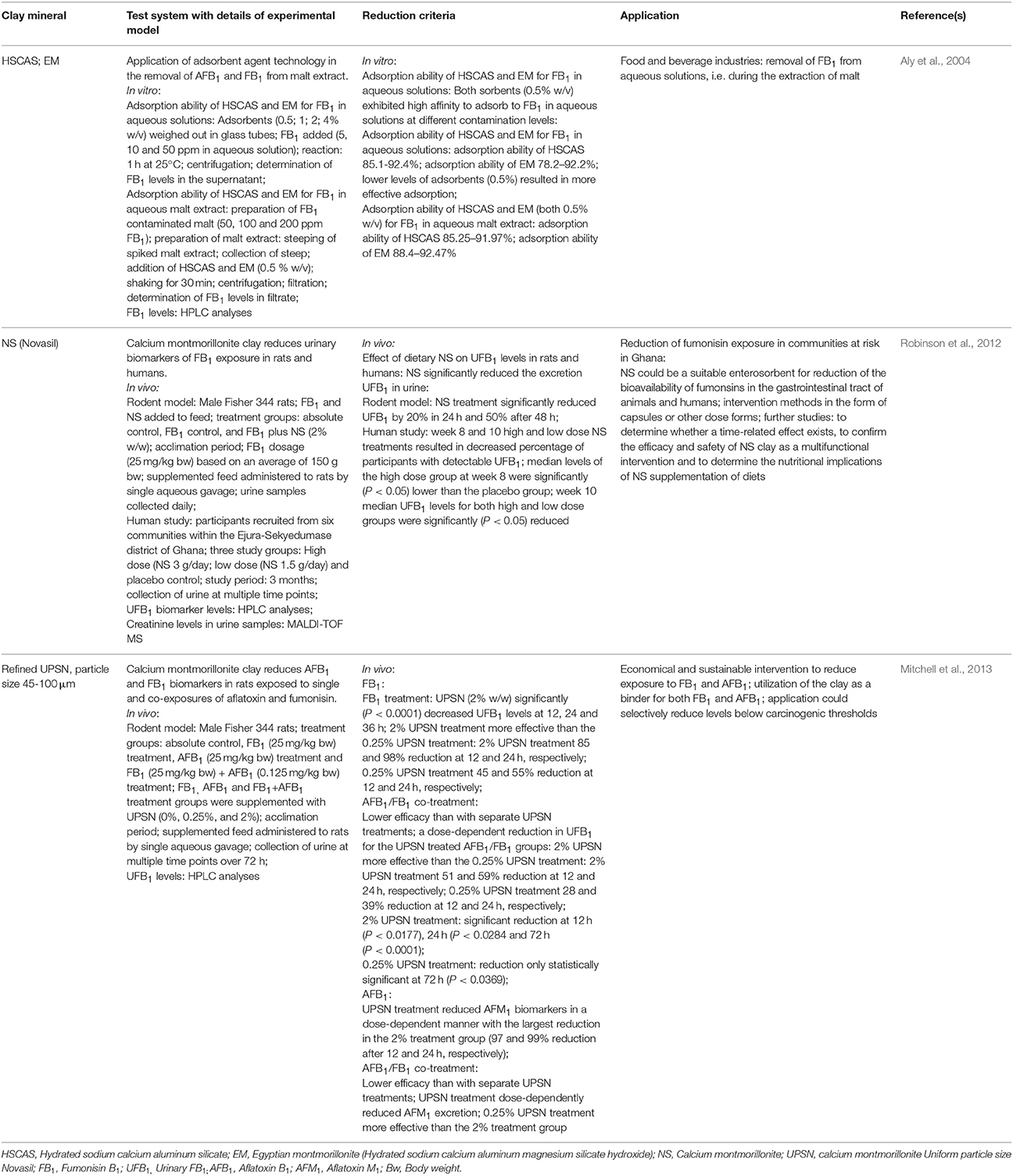
Table 4. Current information on reduction of fumonisin B1 in aqueous solutions (in vitro), and human and animal models (in vivo) through adsorption to clay minerals.
Because natural clay mineral adsorbents are considered GRAS by the US FDA (2015), they could be applied effectively and economically in the food and feed industries and several clay minerals have been proven to be acceptable for commercial uses [US FDA, GRAS substances evaluated by the Select Committee on GRAS substances (SCOGS); He and Zhou, 2010]. However, application of clay minerals often requires high levels to be included into animal feed; interaction of natural clays with food- and gut-based nutrients remains unclear; and the possibility of accumulation of dioxin (a toxic trace component in montmorillonite) in animals remains a concern.
Microbial Transformation of the Fumonisins
Development of control methods to detoxify the fumonisins through transformation should be directed toward deamination of the free amino group at C-2 and hydrolysis of the ester bonds at C-14 and C-15 (Gelderblom et al., 1993). Microorganisms capable of transforming FB1 to less toxic end products include Exophiala spinifera ATCC 74269, Rhinocladiella atrovirens ATCC 74270, Bacterium ATCC 55552, and Sphingopyxis macrogoltabida MTA144 (Duvick et al., 1998a,b; Blackwell et al., 1999; Heinl et al., 2010). Transformation of FB1 by the black-yeast E. spinifera was mainly achieved through decarboxylation by inducible extracellular esterase enzymes and amino oxidases converting hydrolysed fumonisin (HFB1) to unknown end products. Degradation by Bacterium ATCC 55552 and S. macrogoltabida MTA144 is achieved through de-esterification by carboxylesterases and subsequent deamination of HFB1 by aminotransferases, with the formation of 2-keto HFB1 (Heinl et al., 2010; Hartinger et al., 2011). The microbial gene sequences coding for these enzymes were determined by employing degenerate polymerase chain reaction (PCR) primers, inverse PCR and gene walking techniques. Carboxylesterase (FumD) and aminotransferase enzymes (FumI) of S. macrogoltabida MTA144 and Bacterium ATCC 55552 were expressed in Pichia pastoris and E. coli, respectively, by employing episomal pET-3a vectors. Production of the recombinant enzymes were induced in liquid cultures by isopropyl-beta-D-thiogalactopyranoside, where after degradation of FB1 and HFB1 was demonstrated with the recombinant culture supernatant as well as with purified enzyme preparations. HFB1 prepared through enzymatic transformation by FumD carboxylesterases exhibited considerable less toxicity than FB1 when evaluated in a pig intestine model as indicated by the modified sphinganine/sphingosine ratios in the liver and plasma, modified intestinal immune response, and absence of hepatotoxicity and impaired intestinal morphology (Oswald et al., 2012). Although, certain of these technologies are considered safe for humans, animals and the environment by the European Food Safety Authority (EFSA), applications of microbial enzymes are presently mainly directed toward the animal feed industry (Duvick et al., 1998b, 2003; Moll et al., 2011). Recombinant enzymes are mass produced in a bioreactor and are applied during storage and food-processing to incorporate into animal feed and act in the intestinal tract of animals, or for treatment of grains in the form of a wash, additive or spray. Other post-harvest methods involving microbial transformation include the engineering of ruminal organisms and supplementation to feed in the form of a probiotic inoculant.
Commercialization of Biological Methods of Control
The lack of effective and environmentally safe chemical control methods against fungal growth and mycotoxin production in food crops has led to investigations into biologically safe alternatives to prevent these contaminants from entering the food chain (Beekrum et al., 2003). Biological pesticides and methods involving natural resources such as plants, microorganisms, genetic factors thereof, and clay minerals are popular alternatives being evaluated for control of mycotoxigenic fungi in grains (Alabouvette et al., 2009). Fusarium growth and fumonisin production pre-harvest and post-harvest are effectively reduced by several natural and biological methods involving plant material, microorganisms and minerals, as evident by the extensive research done on this subject in recent years.
Several commercial products for biological control of Fusarium diseases and the fumonsins have been developed for application alone, in combination or as part of an integrated control strategy. Products containing biocontrol microorganisms are mainly aimed at application as seed and soil treatments as outlined by Fravel et al. (1998) and Kahn (2013):
• “Fusaclean” and “Biofox C” (non-pathogenic F. oxysporum for control of F. oxysporum and F. verticillioides in a variety of vegetables).
• “Epic” and “Kodiak” (B. subtilis for control of Fusarium in cotton and legumes).
• “Intercept” (Pseudomonas cepacia for control of Fusarium in maize, vegetables and cotton).
• “Mycostop” (Streptomyces griseoviridis for control of Fusarium in ornamental and vegetables crops).
• T-22G and T-22HB (Trichoderma harziatum for control of Fusarium in grains, soya, cotton and vegetables).
• “Biofungus” (Trichoderma spp. for control of Fusarium in citrus and pome fruit).
• “Blue circle” (Burkholderia cepacia) for control of Fusarium in vegetables).
• “Deny” (B. cepacia for control of Fusarium in a variety of grain crops).
• “Cedomon” and “Cerall” (Pseudomonas chlororaphis for control of Fusarium in wheat, rye and triticale).
• Commercial GRAS products developed from clay minerals include Novasil ® and Nevalite® (calcium montmorillonite) (Robinson et al., 2012).
• Fumzyme® (Biomin, Austria) was developed from the carboxylesterase enzyme of S. macrogoltabida (Heinl et al., 2010).
Although, there is an increased interest in biological control methods, much effort is put into details of natural compounds capable of controlling fungal growth and mycotoxins in vitro. However, the growing knowledge base on this subject should be further developed for application in planta and in the field pre-harvest, post-harvest, and during storage and food-processing. In order to develop the available information into appropriate methods for application in planta and in the field, there are many economic and technological hurdles to overcome. The effectiveness of antioxidants, essential oils, phenolic compounds and combinations for example, has been demonstrated at laboratory scale, and bioactivity in the vapor phase makes it promising as fumigant for protection of grains on the field immediately after harvest or during storage (Chulze, 2010). However, evaluation studies in grains are limited due to cost implications and the inhibitory effect in maize generally achieved with higher concentrations than in synthetic media, because of possible matrix interference and reduced bioavailability relating to distribution on kernel surfaces and penetration into the pericarp (Torres et al., 2003; Samapundo et al., 2007). In certain cases, high concentrations of phenolic compounds could also affect the sensory quality of the maize. Certain antioxidants such as BHA and PP, clay minerals, and plant extracts are considered GRAS, making it very promising for biocontrol purposes. Mixtures of antioxidants or combinations with other food preservatives (i.e., benzoic and sorbic acids) could further enhance the antifungal efficacy (Reynoso et al., 2002).
Even though biologically based treatments most likely will have a reduced effect than chemical methods on the desired nutritional value, quality, safety, or sensory attributes of foods and feed and impact on the environment, compliance to food safety assessment guidelines, such as those prescribed by the European Network on Safety Assessment of Genetically Modified Food Crops (ENTRANSFOOD) and the FAO/WHO, have to be met (He and Zhou, 2010). Assessments could include compositional analyses of key components of treated food including nutrients, micronutrients, and predictable secondary metabolites; assessment of possible toxicity, allergens; potential environmental impact; long-term nutritional impact; influence of food/feed processing; potential dietary intake and change in dietary pattern. While there are several opportunities for further exploring and developing biological control methods for Fusarium growth and fumonisins, each method has its own challenges. However, an integrated approach, involving good agricultural management practices, HACCP models and storage management, together with appropriately selected biologically based microbial treatments, mild chemical and physical treatments could reduce Fusarium diseases and fumonisins effectively pre- and post-harvest (da Cruz Cabral et al., 2013).
Practical and Culturally Acceptable Methods for Mycotoxin Reduction—Approaches in Sub-Saharan Countries
Methods for prevention of chronic exposure to the fumonisins, particularly in low socio-economic rural subsistence farming communities, remain critically important. In developed countries high standards of the major food suppliers and retailers are upheld and the regulatory controls deter the importation and marketing of seriously contaminated products. In developing countries only a limited number of countries have legislative maximum levels for fumonisins, and implementation thereof is often poor. In rural subsistence farming communities, legislation is not applicable and with continued pressure on food security, an increased mycotoxin exposure on a daily basis is the norm. In addition, due to the stringent mycotoxin standards in developed countries, the best-quality food products are normally exported resulting in highly contaminated foods being utilized domestically which increases the risk of mycotoxin exposure and the associated adverse health effects (Pitt et al., 2012). High risk population groups include rural communities and/or subsistence farmers heavily reliant on maize as their staple diet. Although, commercial maize is contaminated with lower levels, daily exposure could be a risk factor for disease development in impoverished communities.
In developing countries, where resources are limited and sophisticated technologies are lacking, the importance of cost-effective and simple intervention methods, predominantly at population level, has been emphasized. In this regard, culturally acceptable simple, practical and biologically based methods of reduction are relevant, as a last line of defense in rural subsistence farming communities exposed to high levels of the fumonisins in their staple diet. Effective reduction has been demonstrated with hand sorting, flotation, washing, dehulling of maize kernels and combinations thereof in vitro and in field studies (Table 5). Dehulling and shelling of maize are common practices in West-Africa (Fandohan et al., 2006), with the removal of the pericarp an effective way to reduce mycotoxin contamination (Sydenham et al., 1994; Bullerman and Bianchini, 2007; Burger et al., 2013). The effectiveness of hand-sorting of maize by removing visibly infected and damaged kernels, resulting in a significant reduction of fumonisins has been demonstrated in several African countries, including Benin (Fandohan et al., 2005), Nigeria (Afolabi et al., 2006), Tanzania (Kimanya et al., 2008), South Africa (Van der Westhuizen et al., 2010), and Malawi (Matumba et al., 2015). In South Africa a simple, practical and culturally acceptable hand-sorting and washing intervention method was developed and implemented for reduction of fumonisin exposure in a subsistence maize-farming community (Van der Westhuizen et al., 2010, 2011b). The efficacy of the maize kernel wash method could possibly be further enhanced by incorporating clay minerals or fumonisin detoxifying enzymes. Advantages of interventions involving practical methods usually take the form of improved health outcomes rather than market outcomes (Wu and Khlangwiset, 2010a,b). Public health interventions should be culturally acceptable; be implemented through educational campaigns; and must have financial and infrastructural support to be feasible in remote rural areas where they are most needed. Sustainability of these reduction strategies is, however, dependent on the available maize supply (food security), as well as the socio-economic status and education of a community.
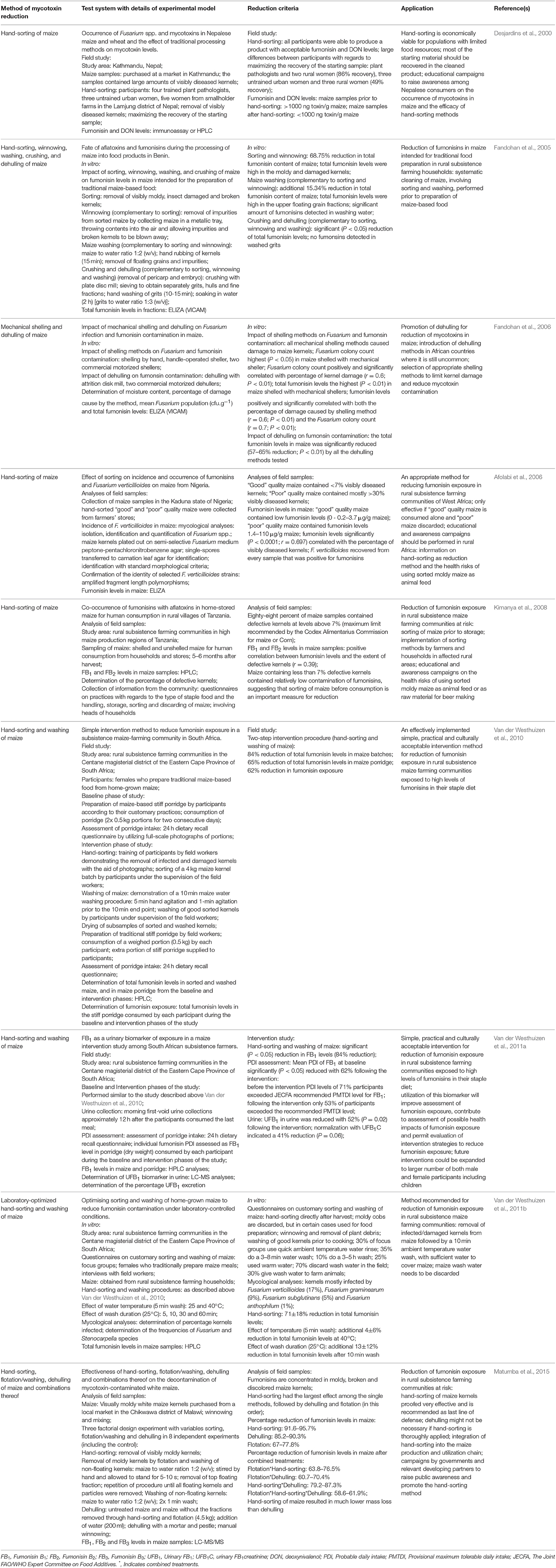
Table 5. Practical and culturally acceptable methods of mycotoxin reduction for rural subsistence farming communities exposed to high levels of fumonisins in their staple diet (in vitro, field- and intervention studies).
Author Contributions
Dr. JA, Wrote article; Prof. WG, Coordinated and assisted in writing article; Prof. WV, Assisted in writing article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the South African Maize Trust for their financial support of research on the use of biological methods for mycotoxin control.
References
Abbas, H. K., Zablotowicz, R. M., Weaver, M. A., Shier, W. T., Bruns, H. A., Bellaloui, N., et al. (2013). Implications of Bt traits on mycotoxin contamination in maize: overview and recent experimental results in southern United States. J. Agric. Food Chem. 61, 11759–11770. doi: 10.1021/jf400754g
Afolabi, C. G., Bandyopadhyay, R., Leslie, J. F., and Ekpo, E. J. A. (2006). Effect of sorting on incidence and occurrence of fumonisins and Fusarium verticillioides on maize from Nigeria. J. Food Prot. 69, 2019–2023.
Alabouvette, C., Olivain, C., Migheli, Q., and Steinberg, C. (2009). Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 184, 529–544. doi: 10.1111/j.1469-8137.2009.03014.x
Aly, S. E., Abdel-Galil, M. M., and Abdel-Wahhab, M. A. (2004). Application of adsorbent agents technology in the removal of aflatoxin B1 and fumonisin B1 from malt extract. Food Chem. Toxicol. 42, 1825–1831. doi: 10.1016/j.fct.2004.06.014
Bacon, C. W., and Hinton, D. M. (2011). In planta reduction of maize seedling stalk lesions by the bacterial endophyte Bacillus mojavensis. Can. J. Microbiol. 57, 485–492. doi: 10.1139/w11-031
Bacon, C. W., Yates, I. E., Hinton, D. M., and Meredith, F. (2001). Biological control of Fusarium moniliforme in maize. Environ. Health Persp. 109, 325–332. doi: 10.1289/ehp.01109s2325
Beekrum, S., Govinden, R., Padayachee, T., and Odhav, B. (2003). Naturally occurring phenols: a detoxification strategy for fumonisin B1. Food Addit. Contam. 20, 490–493. doi: 10.1080/0265203031000098678
Betz, F. S., Hammond, B. G., and Fuchs, R. L. (2000). Safety and advantages of Bacillus thuringiensis - protected plants to control insect pests. Regul. Toxicol. Pharm. 32, 156–173. doi: 10.1006/rtph.2000.1426
Blackwell, B. A., Gilliam, J. T., Savard, M. E., Miller, D., and Duvick, J. P. (1999). Oxidative deamination of hydrolysed fumonisin B1 (AP1) by cultures of Exophiala spinifera. Nat. Toxins 7, 31–38.
Bluhm, B. H., Kim, H., Bitchko, R. A. E., and Woloshuk, C. P. (2008). Involvement of ZFR1 of Fusarium verticillioides in kernel colonization and the regulation of FST1, a putative sugar transporter gene required for fumonisin biosynthesis on maize kernels. Mol. Plant Pathol. Online 9, 203–211. doi: 10.1111/j.1364-3703.2007.00458.x
Bolger, M., Coker, R. D., DiNovi, M., Gaylor, D., Gelderblom, W., and Olsen, M. (2001). “Fumonisins,” in Prepared by the 56th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Safety Evaluation of Certain Mycotoxins in Food. WHO Food Additives Series, Vol. 47, (Geneva: WHO, FAO Food and Nutrition Paper 74), 103–279.
Brown, D. W., Butchko, R. A. E., and Proctor, R. H. (2006). Fusarium genomic resources: tools to limit crop diseases and mycotoxin contamination. Mycopathologia 162, 191–199. doi: 10.1007/s11046-006-0053-6
Bullerman, L. B., and Bianchini, A. (2007). Stability of mycotoxins during food processing. Int. J. Food Microbiol. 119, 140–146. doi: 10.1016/j.ijfoodmicro.2007.07.035
Burger, H. M., Lombard, M. J., Shephard, G. S., Rheeder, J. R., van der Westhuizen, L., and Gelderblom, W. C. (2010). Dietary fumonisin exposure in a rural population of South Africa. Food Chem. Toxicol. 48, 2103–2108. doi: 10.1016/j.fct.2010.05.011
Burger, H. M., Shephard, G. S., Louw, W., Rheeder, J. P., and Gelderblom, W. C. A. (2013). The mycotoxin distribution in maize milling fractions under experimental conditions. Int. J. Food Microbiol. 165, 57–64. doi: 10.1016/j.ijfoodmicro.2013.03.028
Butròn, A., Santiago, R., Mansilla, P., Pintos-Varela, C., Ordás, A., and Malvar, R. A. (2006). Maize (Zea mays L.) Genetic factors for preventing fumonisin contamination. J. Agric. Food Chem. 54, 6113–6117. doi: 10.1021/jf0611163
Calistru, C., McLean, M., and Berjak, P. (1997). In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species: a study of the production of extracellular metabolites by Trichoderma species. Mycopathologia 137, 115–121. doi: 10.1023/A:1006802423729
Campos-Bermudez, V. A., Fauguel, C. M., Tronconi, M. A., Casati, P., Presello, D. A., and Andreo, C. S. (2013). Transcriptional and metabolic changes associated to the infection by Fusarium verticillioides in maize inbreds with contrasting ear rot resistance. PLoS ONE 8:e61580. doi: 10.1371/journal.pone.0061580
Cavaglieri, L., Orlando, J., and Etcheverry, M. (2005b). In vitro influence of bacterial mixtures on Fusarium verticillioides growth and fumonisin B1 production: effect of seeds treatment on maize root colonization. Lett. Appl. Microbiol. 41, 390–396. doi: 10.1111/j.1472-765X.2005.01785.x
Cavaglieri, L., Orlando, J., Rodríguez, M. I., Chulze, S., and Etcheverry, M. (2005c). Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 156, 748–754. doi: 10.1016/j.resmic.2005.03.001
Cavaglieri, L., Passone, A., and Etcheverry, M. (2004). Screening procedures for selecting rhizobacteria with biocontrol effects upon Fusarium verticillioides growth and fumonisin B1 production. Res. Microbiol. 155, 747–754. doi: 10.1016/j.resmic.2004.06.001
Cavaglieri, L. R., Andrés, L., Ibáñez, M., and Etcheverry, M. G. (2005a). Rhizobacteria and their potential to control Fusarium verticillioides: effect of maize bacterisation and inoculum density. Antonie van Leeuwenhoek. 87, 179–187. doi: 10.1007/s10482-004-3193-z
Chulze, S. N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Addit. Contam. 27, 651–657. doi: 10.1080/19440040903573032
Clements, M. J., Campbell, K. W., Maragos, C. M., Pilcher, C., Headrick, J. M., Pataky, J. K., et al. (2003). Influence of Cry1Ab protein and hybrid genotype on fumonisin contamination and Fusarium ear rot of corn. Crop Sci. 43, 1283–1293. doi: 10.2135/cropsci2003.1283
Cleveland, T. E., Dowd, P. F., Desjardins, A. E., Bhatnagar, D., and Cotty, P. J. (2003). United States Department of Agriculture-Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 59, 629–642. doi: 10.1002/ps.724
Coma, V., Portes, E., Gardrat, C., Richard-Forget, F., and Castellan, A. (2011). In vitro inhibitory effect of tetrahydrocurcuminoids on Fusarium proliferatum growth and fumonisin B1 biosynthesis. Food Addit. Contam. 28, 218–225. doi: 10.1080/19440049.2010.540721
da Cruz Cabral, L., Pinto, V. F., and Patriarca, A. (2013). Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 166, 1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026
Dalie, D. K., Deschamps, A. M., Atanasova-Penichon, V., and Richard-Forget, F. (2010). Potential of Pediococcus pentosaceus (L006) isolated from maize leaf to suppress fumonisin-producing fungal growth. J. Food Prot. 73, 1129–1137.
Damann, K. E. Jr. (2014). Atoxigenic Aspergillus flavus biological control of aflatoxin contamination: what is the mechanism? World Mycotoxin J. 8, 235–224. doi: 10.3920/WMJ2014.1719
Desjardins, A. E., Manandhar, G., Plattner, R. D., Maragos, C. M., Shrestha, K., and McCormick, S. P. (2000). Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J. Agric. Food Chem. 48, 1377–1383. doi: 10.1021/jf991022b
Desjardins, A. E., and Proctor, R. H. (2007). Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 119, 47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024
Dowd, P. F. (2001). Biotic and abiotic factors limiting efficacy of Bt corn in indirectly reducing mycotoxin levels in commercial fields. J. Econ. Entomol. 94, 1067–1074. doi: 10.1603/0022-0493-94.5.1067
Duvick, J. (2001). Prospects for reducing fumonisin contamination of maize through genetic modification. Environ. Health Persp. 109, 337–342. doi: 10.1289/ehp.01109s2337
Duvick, J., Maddox, J., and Gilliam, J. (2003). Composition and Methods for Fumonisin detoxification. U.S. Patent No 6,538,177 B1. Washington, DC: U.S. Patent and Trademark Office.
Duvick, J., Rood, T., Maddox, J., and Gilliam, J. (1998a). “Detoxification of mycotoxins in planta as a strategy for improving grain quality and disease resistance: identification of fumonisin-degrading microbes from maize,” in Molecular Genetics of Host-Specific Toxins in Plant Disease, Developments in Plant Pathology Vol. 13, eds K. Kohmoto and O. C. Yoder (Daisen; Tottori: Springer International Publishing AG), 369–381.
Duvick, J., Rood, T., and Wang, X. (1998b). Fumonisin Detoxification Enzymes. U.S. Patent No 5,716,820. Washington, DC: U.S. Patent and Trademark Office.
Edlayne, G., Simone, A., and Felicio, J. D. (2009). Chemical and biological approaches for mycotoxin control: a review. Recent Pat. Food Nutr. Agric. 2, 155–161. doi: 10.2174/2212798410901020155
Etcheverry, M., Torres, A., Ramirez, M. L., Chulze, S., and Magan, N. (2002). In vitro control of growth and fumonisin production by Fusarium verticillioides and F. proliferatum using antioxidants under different water availability and temperature regimes. J. Appl. Microbiol. 92, 624–632. doi: 10.1046/j.1365-2672.2002.01566.x
Fandohan, P., Ahouansou, R., Houssou, P., Hell, K., Marasas, W. F. O., and Wingfield, M. J. (2006). Impact of mechanical shelling and dehulling on Fusarium infection and fumonisin contamination in maize. Food Addit. Contam. 23, 415–421. doi: 10.1080/02652030500442516
Fandohan, P., Zoumenou, D., Hounhouigan, D. J., Marasas, W. F., Wingfield, M. J., and Hell, K. (2005). Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. Int. J. Food Microbiol. 98, 249–259. doi: 10.1016/j.ijfoodmicro.2004.07.007
Fravel, D. R., Connick, W. J., and Lewis, J. A. (1998). “Formulation of microorganisms to control plant diseases,” in Formulation of Microbial Biopesticies. Beneficial Microorganisms, Nematodes and Seed Treatments, ed H. D. Burges (Dordrecht: Springer Science and Business media, B.V.), 188–191.
Garcia, D., Ramos, A. J., Sanchis, V., and Marín, S. (2012). Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int. J. Food Microbiol. 153, 21–27. doi: 10.1016/j.ijfoodmicro.2011.10.010
Gelderblom, W. C. A., Cawood, M. E., Snyman, S. D., Vleggaar, R., and Marasas, W. F. O. (1993). Structure-activity relationships of fumonisins in short-term carcinogenesis and cytotoxicity assays. Food Chem. Toxicol. 31, 407–414. doi: 10.1016/0278-6915(93)90155-R
Gianessi, L. P., and Carpenter, J. E. (1999). Agricultural Biotechnology: Insect Control Benefits. National Center for Food and Agricultural Policy (NCFAP), USA.
Hammond, B. G., Campbell, K. W., Pilcher, C. D., Degooyer, T. A., Robinson, A. E., Mcmillen, B. L., et al. (2004). Lower fumonisin mycotoxin levels in the grain of Bt corn grown in the United States in 2000-2002. J. Agric. Food Chem. 52, 1390–1397. doi: 10.1021/jf030441c
Hartinger, D., Schwartz, H., Hametner, C., Schatzmayr, G., Haltrich, D., and Moll, W. D. (2011). Enzyme characteristics of aminotransferase FumI of Sphingopyxis sp. MTA144 for deamination of hydrolyzed fumonisin B1. Appl. Microbiol. Biotechnol. 91, 757–768. doi: 10.1007/s00253-011-3248-9
He, J., and Zhou, T. (2010). Patented techniques for detoxification of mycotoxins in feeds and food matrices. Recent Pat. Food Nutr. Agric. 2, 96–104. doi: 10.2174/1876142911002020096
Heinl, S., Hartinger, D., Thamhesl, M., Kunz-Vekiru, E., Krska, R., Schatzmayr, G., et al. (2010). Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 145, 120–129. doi: 10.1016/j.jbiotec.2009.11.004
Hermosa, R., Viterbo, A., Chet, I., and Monte, E. (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158, 17–25. doi: 10.1099/mic.0.052274-0
Hinton, D. M., and Bacon, C. W. (1995). Enterobacter cloacae is an endophytic symbiont of corn. Mycopathologia 129, 117–125. doi: 10.1007/BF01103471
Howes, A. D., and Newman, K. E. (2000). Compositions and Methods for Removal of Mycotoxins from Animal Feed. U.S. Patent No 6,045,834. Washington, DC: U.S. Patent and Trademark Office.
International Agency for Research on Cancer (IARC), World Health Organisation (WHO). (2002). “Fumonisin B1,” in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene Vol. 82 (Lyon: IARC Press), 301–366.
International Programme on Chemical Safety (IPCS). (1999). Environmental Health Criteria 217: Microbial Pest Control Agent Bacillus Thuringiensis. World Health Organisation (WHO).
Kabak, B., Dobson, A. D., and Var, I. (2006). Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit. Rev. Food Sci. 46, 593–619. doi: 10.1080/10408390500436185
Kahn, M. R. (2013). “Beneficial bacteria for biological control of fungal pathogens of cereals,” in Bacteria in Agrobiology: Disease management, ed D. K. Maheshwari (New York, NY: Springer), 153.
Kimanya, M. E., De Meulenaer, B., Roberfroid, D., Lachat, C., and Kolsteren, P. (2010). Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 54, 659–667. doi: 10.1002/mnfr.200900483
Kimanya, M. E., De Meulenaer, B., Tiisekwa, B., Ndomondo-Sigonda, M., Devlieghere, F., Van Camp, J., et al. (2008). Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. 25, 1353–1164. doi: 10.1080/02652030802112601
Kunert, K. J. (2011). How effective and safe is Bt-maize in South Africa? S. Afr. J. Sci. 107, 9–10. doi: 10.4102/sajs.v107i9/10.803
Lanubile, A., Ferrarini, A., Maschietto, V., Delledonne, M., Marocco, A., and Bellin, D. (2014). Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genomics 15:710. doi: 10.1186/1471-2164-15-710
Lanubile, A., Pasini, L., and Marocco, A. (2010). Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant Phys. 167, 1398–1406. doi: 10.1016/j.jplph.2010.05.015
Larkin, R. P., and Fravel, D. R. (1998). Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 82, 1022–1028. doi: 10.1094/PDIS.1998.82.9.1022
Liu, J., Sui, Y., Wisniewski, M., Droby, S., and Liu, Y. (2013). Review: utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 167, 153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004
Löffler, M., Kessel, B., Ouzunova, M., and Miedaner, T. (2010). Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays L.) inbred lines. Theor. Appl. Genet. 120, 1053–1062. doi: 10.1007/s00122-009-1233-9
Luongo, L., Galli, M., Corazza, L., Meekes, E., Haas, L., Plas, L. C., et al. (2005). Potential of fungal antagonists for bio-control of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci. Technol. 15, 229–242. doi: 10.1080/09583150400016852
Marasas, W. F., Riley, R. T., Hendricks, K. A., Stevens, V. L., Sadler, T. W., Gelineau-van Waes, J., et al. (2004). Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 134, 711–716.
Marasas, W. F. O. (2001). Discovery and occurrence of the fumonisins: a historical perspective. Environ. Health Persp. 109, 239–243. doi: 10.1289/ehp.01109s2239
Maschietto, V., Marocco, A., Malachova, A., and Lanubile, A. (2015). Resistance to Fusarium verticillioides and fumonisin accumulation in maize inbred lines involves an earlier and enhanced expression of lipoxygenase (LOX) genes. J. Plant Phys. 188, 9–18. doi: 10.1016/j.jplph.2015.09.003
Matumba, L., Van Poucke, C., Njumbe Ediage, E., Jacobs, B., and De Saeger, S. (2015). Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize. Food Addit. Contam. 32, 960–969. doi: 10.1080/19440049.2015.1029535
McClintock, J. T., Schaffer, C. R., and Sjoblad, R. D. (1995). A comparative review of the mammalian toxicity of Bacillus thuringiensis-based pesticides. Pestic. Sci. 45, 95–105. doi: 10.1002/ps.2780450202
McDonald, T., Brown, D., Keller, N. P., and Hammond, T. M. (2005). RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol. Plant Microbe Interact. 18, 539–545. doi: 10.1094/MPMI-18-0539
Menniti, A. M., Gregori, R., and Neri, F. (2010). Activity of natural compounds on Fusarium verticillioides and fumonisin production in stored maize kernels. Int. J. Food Microbiol. 136, 304–309. doi: 10.1016/j.ijfoodmicro.2009.10.008
Mitchell, N. J., Xue, K. S., Lin, S., Marroquin-Cardona, A., Brown, K. A., Elmore, S. E., et al. (2013). Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. J. Appl. Toxicol. 34, 795–804. doi: 10.1002/jat.2942
Mohanlall, V., and Odhav, B. (2006). Biocontrol of aflatoxins B1, B2, G1, G2, and fumonisin B1 with 6,7-dimethoxycoumarin, a phytoalexin from Citrus sinensis. J. Food Prot. 69, 2224–2229.
Moll, D., Hartinger, D., Grießler, K., Binder, E. M., and Schatzmayr, G. (2011). Method for the Production of an Additive for the Enzymatic Decomposition of Mycotoxins, Additive, and Use Thereof. US Patent No. 8703460 B2. Washington, DC: U.S. Patent and Trademark Office.
Mukherjee, P. K., Buensanteai, N., Moran-Diez, M. E., Druzhinina, I. S., and Kenerley, C. M. (2012). Functional analysis of non-ribosomal peptide synthetase (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NPRS hybrid enzyme involved in the induced systemic resistance response in maize. Microbiology 158, 155–165. doi: 10.1099/mic.0.052159-0
Munkvold, G. P., Hellmich, R. L., and Showers, W. B. (1997). Reduced Fusarium ear rot and symptomless infection in kernels of maize genetically engineered for European corn borer resistance. Phytopathology 87, 1071–1107. doi: 10.1094/PHYTO.1997.87.10.1071
Osman, G. H., Assem, S. K., Alreedy, R. M., El-Ghareeb, D. K., Basry, M. A., Rastogi, A., et al. (2015). Development of insect resistant maize plants expressing a chitinase gene from the cotton leaf worm, Spodoptera littoralis. Sci. Rep. 14, 18067. doi: 10.1038/srep18067
Oswald, I. P., Grenier, B., Schatzmayr, G., and Moll, W. (2012). “Enzymatic detoxification of mycotoxins: hydrolysis of fumonisin B1 strongly reduced the toxicity for piglets,” in World Nutrition Forum, NutriEconomics, Balancing Global Nutrition & Productivity, ed E. M. Binder (Leicestershire: Anytime Publishing Leicestershire), 263–271.
Picot, A., Barreau, C., Pinson-Gadais, L., Piraux, F., Caron, D., Lannou, C., et al. (2011). The dent stage of maize kernels is the most conducive for fumonisin biosynthesis under field conditions. Appl. Environ. Microbiol. 77, 8382–8390. doi: 10.1128/AEM.05216-11
Pieterse, C. M. J., Leon-Reyes, A., Van der Ent, S., and Van Wees, S. C. M. (2009). Networking by small-molecule hormones in plant immunity. Nat Chem. Biol. 5, 308–316. doi: 10.1038/nchembio.164
Pitt, J. I., Wild, C. P., Baan, R., Gelderblom, W. C. A., Miller, J. D., Riley, R., et al. (2012). Improving Public Health through Mycotoxin Control. International Agency for Research on Cancer (IARC) Scientific Publication no. 158. Lyon: IARC Press.
Rawal, S., Kim, J. E., and Coulombe, R. Jr. (2010). Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res. Vet. Sci. 89, 325–331. doi: 10.1016/j.rvsc.2010.04.011
Reynoso, M. M., Torres, A. M., Ramirez, M. L., Rodrigues, M. I., Chulze, S., and Magan, N. (2002). Efficacy of antioxidant mixtures on growth, fumonisin production and hydrolytic enzyme production by Fusarium verticillioides and F. proliferatum in vitro on maize-based media. Mycol. Res. 106, 1093–1099. doi: 10.1017/S0953756202006135
Rheeder, J. P., Marasas, W. F. O., and Vismer, H. F. (2002). Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 68, 2101–2105. doi: 10.1128/AEM.68.5.2101-2105.2002
Robinson, A., Johnson, N. M., Strey, A., Taylor, J. F., Marroquin-Cardona, A., Mitchell, N. J., et al. (2012). Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B1 exposure in rats and humans. Food Addit. Contam. 29, 809–818. doi: 10.1080/19440049.2011.651628
Samapundo, S., De Meulenaer, B., Osei-Nimoh, D., Lamboni, Y., Debevere, J., and Devlieghere, F. (2007). Can phenolic compounds be used for the protection of corn from fungal invasion and mycotoxin contamination during storage? Food Microbiol. 24, 465–473. doi: 10.1016/j.fm.2006.10.003
Schatzmayr, G., Zehner, F., Täubel, M., Schatzmayr, D., Klimitsch, A., Loibner, A. P., et al. (2006). Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 50, 543–551. doi: 10.1002/mnfr.200500181
Schnable, P. S., Ware, D., Fulton, R. S., Stein, J. C., Wie, F., Pasternak, S., et al. (2009). The B73 maize genome: complexity, diversity and dynamics. Science 326, 1112–1120. doi: 10.1126/science.1178534
Shephard, G. S., Burger, H. M., Gambacorta, L., Krska, R., Powers, S. P., Rheeder, J. P., et al. (2013). Mycological analysis and multimycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J. Agric. Food Chem. 61, 8232–8240. doi: 10.1021/jf4021762
Shephard, G. S., Marasas, W. F., Burger, H. M., Somdyala, N. I., Rheeder, J. P., Van der Westhuizen, L., et al. (2007). Exposure assessment for fumonisins in the former Transkei region of South Africa. Food Addit. Contam. 24, 621–629. doi: 10.1080/02652030601101136
Singh, G., Kapoor, I. P. S., Singh, P., de Heluani, C. S., de Lampasona, M. P., and Catalan, C. A. N. (2008). Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 46, 3295–3302. doi: 10.1016/j.fct.2008.07.017
Srichana, D., Taengtip, R., and Kondo, S. (2011). Antimicrobial activity of Gynostemma pentaphyllum extracts against fungi producing aflatoxin and fumonisin and bacteria causing diarrheal disease. Southeast Asian J. Trop. Med. Public Health 42, 704–710.
Sydenham, E. W., Van der Westhuizen, L., Stockenström, S., Shephard, G. S., and Thiel, P. G. (1994). Fumonisin-contaminated maize: physical treatment for the partial decontamination of bulk shipments. Food Addit. Contam. 11, 25–32. doi: 10.1080/02652039409374199
Tende, R. M., Mugo, S. N., Nderitu, J. H., Olubayo, F. M., Songa, J. M., and Bergvinson, D. J. (2010). Evaluation of Chilo partellus and Busseola fusca susceptibility to d-endotoxins in Bt maize. Crop Prot. 29, 115–120. doi: 10.1016/j.cropro.2009.11.008
Thembo, K. M., Vismer, H. F., Nyazema, N. Z., Gelderblom, W. C., and Katerere, D. R. (2010). Antifungal activity of four weedy plant extracts against selected mycotoxigenic fungi. J. Appl. Microbiol. 109, 1479–1486. doi: 10.1111/j.1365-2672.2010.04776.x
Torres, A. M., Ramirez, M. L., Arroyo, M., Chulze, S. N., and Magan, N. (2003). Potential use of antioxidants for control of growth and fumonisin production by Fusarium verticillioides and Fusarium proliferatum on whole maize grain. Int. J. Food Microbiol. 83, 319–324. doi: 10.1016/S0168-1605(02)00380-X
United States (US) Environmental Protection Agency (EPA) (1998a). EPA Registration Eligibility Decision (RED) Bacillus Thuringiensis. Washington, DC: EPA 738-R-98-004.
United States Environmental Protection Agency (EPA) (1998b). (RED Facts) Bacillus thuringiensis. Washington, DC: EPA 738-F-98-001.
United States Food Drug Administration (US FDA) (2015). RAS Substances Evaluated by Select Committee on GRAS Substances (SCOGS): CFSAN/Office of Food Additive Safety.
Van der Westhuizen, L., Shephard, G. S., Abel, S., Swanevelder, S., and Gelderblom, W. C. A. (1998). Inhibition of sphingolipid biosynthesis in rat primary hepatocyte cultures by fumonisin B1 and other structurally related compounds. Food Chem. Toxicol. 36, 497–503. doi: 10.1016/S0278-6915(98)00012-X
Van der Westhuizen, L., Shephard, G. S., Burger, H. M., Rheeder, J. P., Gelderblom, W. C., Wild, C. P., et al. (2011a). Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidem. Biomark. 20, 483–489. doi: 10.1158/1055-9965.EPI-10-1002
Van der Westhuizen, L., Shephard, G. S., Rheeder, J. P., Burger, H.-M., Gelderblom, W. C. A., Wild, C. P., et al. (2011b). Optimising sorting and washing of home-grown maize to reduce fumonisin contamination under laboratory-controlled conditions. Food Control 22, 396–400. doi: 10.1016/j.foodcont.2010.09.009
Van der Westhuizen, L., Shephard, G. S., Rheeder, J. P., Burger, H.-M., Gelderblom, W. C. A., Wild, C. P., et al. (2010). Simple intervention method to reduce fumonisin exposure in a subsistence maize-farming community in South Africa. Food Addit. Contam. 27, 1582–1588. doi: 10.1080/19440049.2010.508050
Velluti, A., Sanchis, V., Ramos, A. J., Egido, J., and Marin, S. (2003). Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 89, 145–154. doi: 10.1016/S0168-1605(03)00116-8
Wang, E., Norred, W. P., Bacon, C. W., Riley, R. T., and Merrill, A. H. Jr. (1991). Inhibition of sphingolipid bio-synthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 266, 14486–14490.
Wild, C. P., and Gong, Y. Y. (2010). Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 31, 71–82. doi: 10.1093/carcin/bgp264
Wild, C. P., and Hall, A. J. (2000). Primary prevention of hepatocellular carcinoma in developing countries. Mutat. Res. 462, 381–393. doi: 10.1016/S1383-5742(00)00027-2
Wu, F. (2006). Mycotoxin reduction in Bt corn: potential economic, health, and regulatory impacts. Transgenic Res. 15, 277–289. doi: 10.1007/s11248-005-5237-1
Wu, F., and Khlangwiset, P. (2010a). Evaluating the technical feasibility of aflatoxin risk reduction strategies in Africa. Food Addit. Contam. 27, 658–676. doi: 10.1080/19440041003639582
Wu, F., and Khlangwiset, P. (2010b). Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: case studies in biocontrol and post-harvest interventions. Food Addit. Contam. 27, 496–509. doi: 10.1080/19440040903437865
Keywords: Fusarium, fumonisins, prevention, biological control, reduction, sub-Saharan countries
Citation: Alberts JF, van Zyl WH and Gelderblom WCA (2016) Biologically Based Methods for Control of Fumonisin-Producing Fusarium Species and Reduction of the Fumonisins. Front. Microbiol. 7:548. doi: 10.3389/fmicb.2016.00548
Received: 28 January 2016; Accepted: 04 April 2016;
Published: 26 April 2016.
Edited by:
Daniela Gwiazdowska, Poznan University of Economics, PolandReviewed by:
Jose M. Diaz-Minguez, CIALE - Universidad de Salamanca, SpainJon Y. Takemoto, Utah State University, USA
Copyright © 2016 Alberts, van Zyl and Gelderblom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wentzel C. A. Gelderblom, gelderblomw@cput.ac.za
 Johanna F. Alberts
Johanna F. Alberts Willem H. van Zyl
Willem H. van Zyl Wentzel C. A. Gelderblom
Wentzel C. A. Gelderblom