Case Report: Lyme Borreliosis and Pregnancy - Our Experience
- 1Department of Medical Sciences, University of Trieste, Trieste, Italy
- 2Azienda Sanitaria Universitaria Integrata Giuliano Isontina, Trieste, Italy
- 3Unità Operativa Semplice di Dermatologia, Centro Rif. Regionale Malattia di Hansen e Lyme, P.O. dei Pellegrini, ASL Napoli Centro, Naples, Italy
Lyme Borreliosis (LB) is an infection transmitted by Ixodes sp. ticks. Its early manifestation includes erythema migrans rash. Since the discovery of LB in 1975, the question arose as to whether this infection could be vertically transmitted from mother to fetus during pregnancy, as transplacental transmission has already been known for other spirochetoses, such as syphilis, relapsing fever and leptospirosis. The first confirmed case with positive Lyme serology was described in 1985 in a 28-year- old mother who had acquired Lyme in the first trimester and then developed an erythema migrans rash. Subsequently, transmission of Borrelia burgdorferi sl. in humans from mother to fetus has been documented through identification of Borrelia spirochetes in fetal tissues/and or placenta by various methods including culture, PCR and indirect immunofluorescence. Adverse birth outcomes, which are limited in case of prompt LB treatment, included spontaneous miscarriage, preterm birth and hyperbilirubinemia, but also cardiac involvement and cutaneous angiomas have been documented although rarely. No significant associations were found between adverse outcomes at birth and the trimester of infection. Patients treated for gestational LB had a lower frequency of miscarriages and premature births, as also the frequency of congenital malformations was similar to that observed in the normal population. The recommended treatment for LB in pregnancy is Amoxicillin, 1 g 3 times a day for 14–21 days. In the present study, we report our case series, which includes 11 pregnant women, 6 of which developed erythema migrans during pregnancy (between week 8 and 34), 3 had myoarticular or neurological symptoms and 2 had positive serology, but did not develop any clinical symptoms. Our data stress on the importance of early antibiotic treatment also in seropositive gestating women without symptoms in order to avoid any possible complication to fetus and newborns.
Introduction
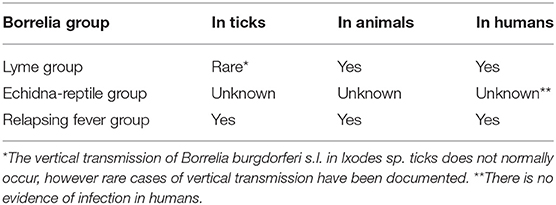
A wide range of infectious diseases can occur in pregnancy. Their acquisition, clinical presentation and course during gestation can be altered due to impaired maternal immunity. Not all, but some infectious diseases, of viral, fungal, bacterial origins, including Lyme disease and protozoal borreliosis, can lead to serious consequences to mother and/or fetus (1). Spirochaetes, both those transmitted by ticks and by other routes, usually have vertical transmission in pregnancy and cause fetal damage. Notably, for some Borreliae of the relapsing fever group (RF) transmitted by the soft ticks Ornithodoros sp., a vertical transmission is reported (2, 3). RF Borreliae are known to cause abortion in animals, in particular in cattle (Borrelia coriaceae is the agent of epizootic bovine abortion in the USA) and in horses (equine abortion) as documented in the USA (4) and in Iran and Israel (5).
Lyme borreliosis (LB) is an infection transmitted by Ixodes sp ticks, where vertical transmission of Borreliae seems to be negligible (6). The early manifestation of LB in humans is erythema migrans. Since its discovery in 1975, the question arose as to whether this infection could be transmitted to the fetus during pregnancy (7). Transplacental transmission in humans was indeed reported for other spirochaetosis, such as syphilis, RF and leptospirosis (8, 9) (Table 1).
Vertical transmission of LB was suspected in 1983 in a woman with febrile illness during pregnancy, but serological analyses for Lyme or syphilis were not carried out (10).
The first confirmed case of LB in a pregnant woman was described in 1985 in a 28-year- old mother who had acquired LB with erythema migrans in the first trimester and delivered at 35 weeks. The mother developed symptoms consistent with LB post-delivery as documented by positive immunofluorescence assay for LB. The child died of congenital heart disease and the autopsy revealed spirochetes infiltrating the spleen, kidneys, and bone marrow, but not cardiac tissue (11).
Lyme Group Borreliae can be transmitted vertically in specific animals which act as reservoirs and in humans (Table 1). Therefore, Borrelia burgdorferi sensu lato might be naturally maintained in an enzootic cycle by transplacental transmission (12).
In 1986 MacDonald described fetal LB, with cardiac involvement and fetal death (13). The different signs and symptoms in gestational Lyme borreliosis mirror the difference found in prenatal syphilis. The transplacental transmission of the spirochaete from mother to fetus is indeed possible. Clinical studies, including autopsy data have associated gestational Lyme borreliosis with different medical concerns including fetal death, hydrocephalus, cardiovascular abnormalities, neonatal respiratory distress, hyperbilirubinemia, intrauterine growth retardation, cortical blindness, sudden infant death syndrome and maternal toxemia in pregnancy (13).
The abovementioned birth outcomes would support a vertical transmission of B. burgdorferi, with possible negative consequences for the fetus (14, 15).
Overall, it seems that adverse birth outcomes in gestational LB are more likely if LB is not treated.
The initiation of antibiotic treatment in some spirochaetal infections can cause Jarisch-Herxeimer Reaction (JHR), which is a transient clinical phenomenon manifesting within 24 h of antibiotic therapy with fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of skin lesions (16). JHR can also occur in pregnancy, however in LB it is not frequent (17) and less severe than observed for other spirochaetosis (18).
In the present study we describe our experience regarding LB in pregnancy.
Clinical Cases
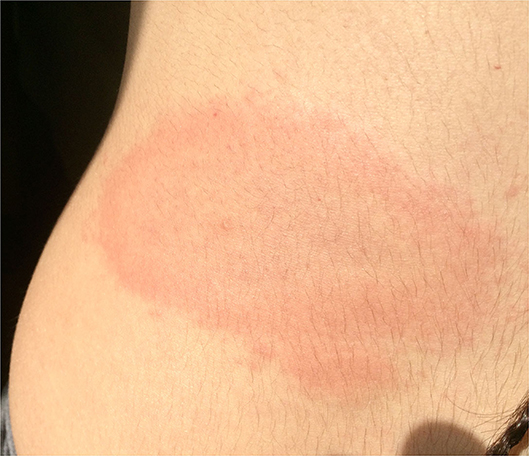
From 2008 to 2020, 11 pregnant women with LB were followed at Trieste University Hospital. Mean age of mothers at time of delivery was 30.5 years (range 21–40). The clinical data are summarized in Table 2. In short, 5 women were diagnosed and treated for LB in the first trimester, 3 in the second trimester and the last 3 women in the third trimester of pregnancy. Six patients developed erythema migrans during pregnancy (Figure 1), three had migratory myoarticular involvement and one of them also presented neurological manifestations. In two women serology tests showed high levels of IgG antibodies for LB despite patients not presenting any clinical symptom. Serological test was negative only in a 26-year-old patient with erythema migrans.
All women were immediately treated at the time of diagnosis with Amoxicillin 1g 3x/day for 14 days. In one case (case 7) the baby was born prematurely at 7 months by an emergency cesarean section, and in one case the newborn (case 9) presented angiomatoid patches (Figure 2), in the center of its forehead, on its left shoulder and on its back, which regressed spontaneously 18 months later. The other 8 babies were born at full term and healthy. At follow-up, after 1 year, most mothers and all babies were healthy and the weight and length of the premature baby were also appropriate for its age.
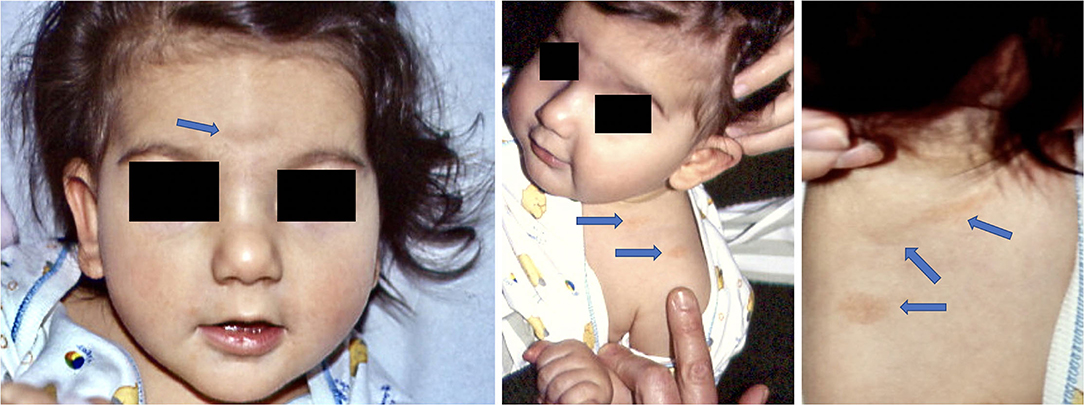
Figure 2. Child born with angiomatous patches at 7 months (Case 9). At 1 year examination angiomatous lesions were still visible, but not at 2 years follow-up.
At the time of diagnosis, the 21-year-old woman (case 2) with articular and neurological involvement had positive IgM antibodies against Borrelia burgorferi both in blood and CSF and positive PCR results for Borrelia in DNA isolated from her blood. The patient improved partially after treatment with Amoxicillin during pregnancy. Two years later, because of persistent symptoms, she was treated with Ceftriaxone 2 gr iv/day for 21 days. After one additional round of treatment 6 months later she was clinically healed and her serology was negative for Borrelia.
Discussion
Pregnant women are considered a vulnerable group due to a weakened immune system. Therefore, they are more susceptible to infection with an increased risk for severe illness. Furthermore, infections in pregnant women have the added gravity of potential infection in the developing fetus which may have important consequences including death in-utero or lifelong debilitation. Taking this evidence into consideration, women are at a higher risk of developing LB and coinfections during pregnancy (19). Complications of maternal Lyme borreliosis in pregnancy outcomes have been already reported (20), including stillbirth and possible congenital malformations (21). The vertical transmission of Lyme group Borreilae has been supported by the detection of spirochaetemia (11, 22) or erythema migrans rash (23) in newborns. Furthermore, also Borrelia specific antibodies have been detected in the cerebrospinal fluid of an infant with documented neurologic dysfunction (24). Therefore, gestating women with LB can transmit the disease to fetus with possible adverse outcomes, especially if antibiotic therapy is not carried out in women. Nevertheless, it is not yet well established if there is a greater risk for the fetus in pregnant women in endemic areas for LB. Some authors reported a higher incidence of abortions in the Italian region Friuli-Venezia Giulia, which is endemic for LB (25). In the LB endemic area of Westchester, New York, US, a higher incidence of cardiac abnormalities in newborn in comparison to the population of non-endemic areas was recorded (26). However, Strobino and co-workers did not observe any significant difference in fetal deaths, pre-term delivery, congenital anomalies and adverse outcomes in endemic vs. non-endemic population for LB (21).
Tick bite or suspected LB should be managed similarly in pregnant and non-pregnant adults, including antibiotic therapy for prophylaxis and treatment (27). Nonetheless, greater attention in pregnancy should be given to the possible symptoms of infection, which should be accompanied by a prompt investigation for Lyme Borrelia (7) and other infections transmitted by Ixodes ticks. Although in our case series we did not detect any other co-infecting bacteria or viruses, there are indeed scientific records reporting on Rickettsia (28), Babesia (29, 30) and Tick-Borne Encephalitis (TBE) virus (31, 32) in pregnant women. Co-infecting agents can be harmful per se to gestating women and fetuses. Among them, Babesia can be vertically transmitted (29, 33) and was diagnosed after antibiotic treatment for LB in a pregnant woman because of an acute hemolysis event, which resolved after proper antibiotic treatment without any consequence for mother and newborn (30). Transplacental transmission in mammals and humans has also been documented for Anaplasma phagocytophilum (34). Data are quite different for the TBE virus transmitted by Ixodes ticks and affecting the central nervous system. There are very few reports of TBE in pregnancy and clinical as well as virological data suggest that fetal TBE infection did not occur, despite severe manifestations in pregnant women (31, 32).
The rate of infected ticks varies in endemic areas from 10 to 18% with an average incidence of Borrelia in Ixodes scapularis in the US of around 11.2%; in Ixodes ricinus in Latvia of around 18%, in Germany of 12.8% and in Sweden of 10–15% (35–37). The incidence of LB or seroconversion after tick-bite in Northern European countries was 5% (78/1,546), of which 33 (2%) developed LB, while 45 subjects (3%) seroconverted only (38). Therefore, for Borrelia infected ticks the risk of infection is ~7–10 times higher, justifying preventive antibiotic treatment with Amoxicillin after a bite of a Borrelia positive tick, especially in pregnancy.
In addition to the Lyme group Borreliae, Ixodes ticks can also transmit another type of Borrelia, named Borrelia miyamotoi, which is a member of the relapsing fever (RF) group. For RF Borreliae the transmission in pregnancy as well as fetal damage and abortion in humans and animals are well known (39, 40). Infections by RF Borreliae in pregnant hosts can be also complicated by Jarisch-Herxheimer reaction, which has also been reported for other spirochaetes and may impair fetus life. However, JHR has a minor impact in LB infections (41).
In our case series, 8 patients treated with Amoxicillin did not have any complications neither for the mothers nor the newborns. We acknowledge however that our sample size is limited to conclusively ascertain the impact of treatment on the prevention of adverse fetal outcome, in larger studies comorbidity and maternal age should also be considered as possible factors impacting both on the course of LB and outcome. In our series patients did not report any comorbidity, despite a mild atopy in patient 1. Nevertheless, it is possible that coexistent comorbid conditions in pregnant women could alter the course of LB even if treated, as shown in immunocompromised patients (42). Nevertheless, at present there are no reports describing the course of LB and outcome in pregnant women with comorbidities. Although in our case series two mothers were over 35 years their clinical course and outcome were similar to those reported for younger women. However, we cannot exclude that advanced maternal age could influence the course of LB in pregnancy, because of the observed comorbidities as well as complications during pregnancy, delivery and outcome in women aged over 35 (43). In our series, an emergency cesarean section was performed and the child was born with erythematous angiomatous patches which disappeared spontaneously within 24 months, in line with the resolution of this manifestation. We acknowledge however that we cannot prove that those patches were caused by LB in the mother during pregnancy, although other authors reported angiomas in children born from mothers with LB (26, 44). In none of our cases JHR was observed after the initiation of treatment with Amoxicillin. A prompt antibiotic therapy with Amoxicillin during pregnancy prevented in most of our patients and newborns any type of complication in agreement with Waddel and co-authors who reported significantly fewer adverse birth outcomes in women treated for gestational LB (45). However, Maraspin et al. had similar results in 7 pregnant women treated with ceftriaxone for 14 days (46), recommending in addition to the above mentioned treatment in pregnant women with LB intravenous antibiotic therapy with penicillin, not only for early disseminated disease, but also for solitary erythema migrans (47).
In our case series no associations have been found between the trimester of pregnancy and LB manifestations, as already reported (45). Nevertheless, arthropod-borne bacterial diseases are difficult diagnoses in pregnancy, because they can mimic many other pathologic conditions and common pregnancy-specific conditions, such as typical and atypical preeclampsia, or symptoms of pregnancy itself (48). In addition, as shown in our case series, some women can have positive serology for LB, without evident clinical symptoms. This poses the question if in endemic areas for Lyme Borreliosis, serological tests for Borrelia should be included routinely for pregnant women to prevent possible adverse effect, as suggested for syphilis (49). Although, a positive serology does not prove an active LB without clinical symptoms, we should acknowledge that even in those cases a possible transmission to the fetus should be avoided with an antibiotic prophylaxis. Currently there is paucity of data surrounding tick-borne infections in pregnancy and long-term outcomes for mother and infant for conditions like LB and co-infections. At present there are no established international surveillance systems to identify and gain understanding of these infections in pregnancy (50) and international guidelines to manage gestational LB in endemic area are not available.
Taken that there is not increased risk to pregnant women who develop LB if they receive appropriate antimicrobial therapy (51) and that in treated gestating women the frequency of premature births and congenital malformations is similar to that observed in the general population (52), an antibiotic prophylaxis in seropositive gestating women without symptoms could be stressed or at least considered in endemic areas for LB. In pregnant women in endemic areas for Lyme Borreliosis, also testing antibodies against Borrelia should be routinely recommended (53), in case of positivity the same analysis could also be suggested on umbilical cord blood at delivery. Pregnant women with LB should be treated and clinically followed, with particular attention for cutaneous, myoarticular, neurological, ocular and cardiac manifestations, which could require a fetal ECO-cardiography follow-up. Newborns should be examined for possible clinical manifestations, as babies born from mothers with gestational LB have been documented in some cases to be small for dates, or presenting several manifestations including pyloric stenosis, cutaneous annular erythemato-papular eruption, cutaneous angiomas, neurological disorders, muscle hypotonia, hypospadias and skeletal abnormality (26, 44).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GT and SB: conceptualization and writing-original draft preparation. GT, MR, NM, KN, ST, and SB: methodology. GT, MR, NM, KN, and ST: data collection. GT, MR, NM, KN, ST, and PF: data analysis. ST, NM, KN, and PF: writing-review and editing. GT: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The APC to publish this article was funded by the Associazione Lyme Italia e Coinfezioni.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors would like to thank Erica Falkingham for the language revision of the manuscript and Associazione Lyme Italia and Coinfezioni for supporting Lyme Borreliosis studies and spreading awareness.
References
1. Mullegger RR, Haring NS, Glatz M. Skin infections in pregnancy. Clin Dermatol. (2016) 34:368–77. doi: 10.1016/j.clindermatol.2016.02.009
2. Trevisan G, Cinco M, Trevisini S, di Meo N, Chersi K, Ruscio M, et al. Borreliae part 1: borrelia lyme group and echidna-reptile group. Biology. (2021) 10:1036. doi: 10.3390/biology10101036
3. Trevisan G, Cinco M, Trevisini S, di Meo N, Ruscio M, Forgione P, et al. Borreliae part 2: borrelia relapsing fever group and unclassified borrelia. Biology. (2021) 10:1117. doi: 10.3390/biology10111117
4. Teglas MB, Mapes S, Hodzic E, Nieto NC. Co-infection of Ornithodoros coriaceus with the relapsing fever spirochete, Borrelia coriaceae, and the agent of epizootic bovine abortion. Med Vet Entomol. (2011) 25:337–43. doi: 10.1111/j.1365-2915.2011.00952.x
5. Walker RL, Read DH, Hayes DC, Nordhausen RW. Equine abortion associated with the Borrelia parkeri-B. turicatae tick-borne relapsing fever spirochete group. J Clin Microbiol. (2002) 40:1558–62. doi: 10.1128/JCM.40.4.1558-1562.2002
6. Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. (2013) 4:46–51. doi: 10.1016/j.ttbdis.2012.06.008
7. Trevisan G, Bonin S, Ruscio M. A practical approach to the diagnosis of lyme borreliosis: from clinical heterogeneity to laboratory methods. Front Med. (2020) 7:265. doi: 10.3389/fmed.2020.00265
8. Larsson C, Andersson M, Guo BP, Nordstrand A, Hagerstrand I, Carlsson S, et al. Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. J Infect Dis. (2006) 194:1367–74. doi: 10.1086/508425
9. Madjunkov M, Chaudhry S, Ito S. Listeriosis during pregnancy. Arch Gynecol Obstet. (2017) 296:143–52. doi: 10.1007/s00404-017-4401-1
10. Shirts SR, Brown MS, Bobitt JR. Listeriosis and borreliosis as causes of antepartum fever. Obstet Gynecol. (1983) 62:256–61.
11. Schlesinger PA, Duray PH, Burke BA, Steere AC, Stillman MT. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann Intern Med. (1985) 103:67–8. doi: 10.7326/0003-4819-103-1-67
12. Wan K, Zhang Z, Wang H, Hou X. Preliminary investigation on reservoir hosts of Borrelia burgdorferi in China. Wei Sheng Yan Jiu. (1999) 28:7–9.
13. MacDonald AB. Gestational Lyme borreliosis. Implications for the fetus. Rheum Dis Clin North Am. (1989) 15:657–77. doi: 10.1016/S0889-857X(21)01021-8
14. Weber K, Neubert U. Clinical features of early erythema migrans disease and related disorders. Zentralbl Bakteriol Mikrobiol Hyg A. (1986) 263:209–28. doi: 10.1016/S0176-6724(86)80125-0
15. Weber K, Bratzke HJ, Neubert U, Wilske B, Duray PH. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr Infect Dis J. (1988) 7:286–9. doi: 10.1097/00006454-198804000-00010
17. Nykytyuk S, Boyarchuk O, Klymnyuk S, Levenets S. The Jarisch-Herxheimer reaction associated with doxycycline in a patient with Lyme arthritis. Reumatologia. (2020) 58:335–8. doi: 10.5114/reum.2020.99143
18. Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg. (2017) 96:46–52. doi: 10.4269/ajtmh.16-0434
19. Watson AK, Ellington S, Nelson C, Treadwell T, Jamieson DJ, Meaney-Delman DM. Preparing for biological threats: Addressing the needs of pregnant women. Birth Defects Res. (2017) 109:391–8. doi: 10.1002/bdr2.1016
20. Markowitz LE, Steere AC, Benach JL, Slade JD, Broome CV. Lyme disease during pregnancy. JAMA. (1986) 255:3394–6. doi: 10.1001/jama.1986.03370240064038
21. Strobino BA, Williams CL, Abid S, Chalson R, Spierling P. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. (1993) 169:367–74. doi: 10.1016/0002-9378(93)90088-Z
22. MacDonald AB, Benach JL, Burgdorfer W. Stillbirth following maternal Lyme disease. N Y State J Med. (1987) 87:615–6.
23. Trevisan G, Stinco G, Cinco M. Neonatal skin lesions due to a spirochetal infection: a case of congenital Lyme borreliosis? Int J Dermatol. (1997) 36:677–80.
24. Dattwyler RJ, Volkman DJ, Luft BJ. Immunologic aspects of Lyme borreliosis. Rev Infect Dis. (1989) 11 (Suppl. 6):S1494–8. doi: 10.1093/clinids/11.Supplement_6.S1494
25. Carlomagno G, Luksa V, Candussi G, Rizzi GM, Trevisan G. Lyme Borrelia positive serology associated with spontaneous abortion in an endemic Italian area. Acta Eur Fertil. (1988) 19:279–81.
26. Williams CL, Strobino B, Weinstein A, Spierling P, Medici F. Maternal Lyme disease and congenital malformations: a cord blood serosurvey in endemic and control areas. Paediatr Perinat Epidemiol. (1995) 9:320–30. doi: 10.1111/j.1365-3016.1995.tb00148.x
27. Smith GN, Moore KM, Hatchette TF, Nicholson J, Bowie W, Langley JM. Committee opinion no. 399: management of tick bites and lyme disease during pregnancy. J Obstet Gynaecol Can. (2020) 42:644–53. doi: 10.1016/j.jogc.2020.01.001
28. McGready R, Prakash JA, Benjamin SJ, Watthanaworawit W, Anantatat T, Tanganuchitcharnchai A, et al. Pregnancy outcome in relation to treatment of murine typhus and scrub typhus infection: a fever cohort and a case series analysis. PLoS Negl Trop Dis. (2014) 8:e3327. doi: 10.1371/journal.pntd.0003327
29. Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. (2012) 18:1318–21. doi: 10.3201/eid1808.110988
30. Luckett R, Rodriguez W, Katz D. Babesiosis in pregnancy. Obstet Gynecol. (2014) 124 (2 Pt 2 Suppl. 1): 419–22. doi: 10.1097/AOG.0000000000000222
31. Dive I, Veje M, Dobler G, Bergstrom T, Buxmann H, Paul B, et al. Tick-borne encephalitis virus (TBEV) infection in pregnancy: absence of virus transmission to the fetuses despite severe maternal disease - A case study. Ticks Tick Borne Dis. (2020) 11:101491. doi: 10.1016/j.ttbdis.2020.101491
32. Weinmayr LM, Kanz D, Eckenweiler M, Bormann T, Huzly D, Bardutzky J, et al. Acute tick-borne encephalitis during pregnancy - an alarming combination. Ticks Tick Borne Dis. (2020) 11:101512. doi: 10.1016/j.ttbdis.2020.101512
33. Bednarska M, Bajer A, Drozdowska A, Mierzejewska EJ, Tolkacz K, Welc-Faleciak R. Vertical transmission of Babesia microti in BALB/c mice: preliminary report. PLoS ONE. (2015) 10:e0137731. doi: 10.1371/journal.pone.0137731
34. Reppert E, Galindo RC, Breshears MA, Kocan KM, Blouin EF, de la Fuente J. Demonstration of transplacental transmission of a human isolate of Anaplasma phagocytophilum in an experimentally infected sheep. Transbound Emerg Dis. (2013) 60 (Suppl. 2):93–6. doi: 10.1111/tbed.12120
35. Gustafson R, Jaenson TG, Gardulf A, Mejlon H, Svenungsson B. Prevalence of Borrelia burgdorferi sensu lato infection in Ixodes ricinus in Sweden. Scand J Infect Dis. (1995) 27:597–601. doi: 10.3109/00365549509047074
36. Capligina V, Salmane I, Keiss O, Vilks K, Japina K, Baumanis V, et al. Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick Borne Dis. (2014) 5:75–81. doi: 10.1016/j.ttbdis.2013.08.007
37. Adalsteinsson SA, Shriver WG, Hojgaard A, Bowman JL, Brisson D, D'Amico V, et al. Multiflora rose invasion amplifies prevalence of Lyme disease pathogen, but not necessarily Lyme disease risk. Parasit Vectors. (2018) 11:54. doi: 10.1186/s13071-018-2623-0
38. Wilhelmsson P, Fryland L, Lindblom P, Sjowall J, Ahlm C, Berglund J, et al. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Aland Islands, Finland (2008-2009). Ticks Tick Borne Dis. (2016) 7:71–9. doi: 10.1016/j.ttbdis.2015.08.009
39. Centers for Disease and Prevention. Tickborne relapsing fever in a mother and newborn child–Colorado, 2011. MMWR Morb Mortal Wkly Rep. (2012) 61:174–6. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6110a3.htm
40. McCoy BN, Maiga O, Schwan TG. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick Borne Dis. (2014) 5:401–3. doi: 10.1016/j.ttbdis.2014.01.007
41. Lam JC, Larios OE, Parkins MD, Vaughan SD. A case of tick-borne relapsing fever in pregnancy. Can Commun Dis Rep. (2020) 46:362–4. doi: 10.14745/ccdr.v46i10a09
42. Maraspin V, Bogovic P, Rojko T, Ogrinc K, Ruzic-Sabljic E, Strle F. Early lyme borreliosis in patients treated with tumour necrosis factor-alfa inhibitors. J Clin Med. (2019) 8:1857. doi: 10.3390/jcm8111857
43. Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. (2021) 70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006
44. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcome. Int J Infect Dis. (2010) 14:e494–8. doi: 10.1016/j.ijid.2009.07.019
45. Waddell LA, Greig J, Lindsay LR, Hinckley AF, Ogden NH. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS ONE. (2018) 13:e0207067. doi: 10.1371/journal.pone.0207067
46. Maraspin V, Ruzic-Sabljic E, Pleterski-Rigler D, Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagn Microbiol Infect Dis. (2011) 71:446–8. doi: 10.1016/j.diagmicrobio.2011.07.017
47. Maraspin V, Strle F. How do I manage tick bites and Lyme borreliosis in pregnant women? Curr Probl Dermatol. (2009) 37:183–90. doi: 10.1159/000213076
48. Dotters-Katz SK, Kuller J, Heine RP. Arthropod-borne bacterial diseases in pregnancy. Obstet Gynecol Surv. (2013) 68:635–49. doi: 10.1097/OGX.0b013e3182a5ed46
49. Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. (2020) 135:1121–35. doi: 10.1097/AOG.0000000000003788
50. Lambert JS. An overview of tickborne infections in pregnancy and outcomes in the newborn: the need for prospective studies. Front Med. (2020) 7:72. doi: 10.3389/fmed.2020.00072
51. Walsh CA, Mayer EW, Baxi LV. Lyme disease in pregnancy: case report and review of the literature. Obstet Gynecol Surv. (2007) 62:41–50. doi: 10.1097/01.ogx.0000251024.43400.9a
52. Maraspin V, Cimperman J, Lotric-Furlan S, Pleterski-Rigler D, Strle F. Erythema migrans in pregnancy. Wien Klin Wochenschr. (1999) 111:933–40.
Keywords: pregnancy, Lyme borreliosis, antibiotic treatment, newborn, Borrelia, serology
Citation: Trevisan G, Ruscio M, di Meo N, Nan K, Cinco M, Trevisini S, Forgione P and Bonin S (2022) Case Report: Lyme Borreliosis and Pregnancy - Our Experience. Front. Med. 9:816868. doi: 10.3389/fmed.2022.816868
Received: 17 November 2021; Accepted: 22 February 2022;
Published: 28 March 2022.
Edited by:
Christian Perronne, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Rina Tilak, Armed Forces Medical College, IndiaJohn Shearer Lambert, University College Dublin, Ireland
Copyright © 2022 Trevisan, Ruscio, di Meo, Nan, Cinco, Trevisini, Forgione and Bonin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giusto Trevisan, trevisan@units.it
 Giusto Trevisan
Giusto Trevisan Maurizio Ruscio
Maurizio Ruscio Nicola di Meo
Nicola di Meo Katiuscia Nan2
Katiuscia Nan2  Serena Bonin
Serena Bonin