A U-shaped association of tracheostomy timing with all-cause mortality in mechanically ventilated patients admitted to the intensive care unit: A retrospective cohort study
- 1Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Critical Care Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 3Department of Critical Care Medicine, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Objectives: To evaluate the association of tracheostomy timing with all-cause mortality in patients with mechanical ventilation (MV).
Method: It’s a retrospective cohort study. Adult patients undergoing invasive MV who received tracheostomy during the same hospitalization based on the Medical Information Mart for Intensive Care-III (MIMIC-III) database, were selected. The primary outcome was the relationship between tracheostomy timing and 90-day all-cause mortality. A restricted cubic spline was used to analyze the potential non-linear correlation between tracheostomy timing and 90-day all-cause mortality. The secondary outcomes included free days of MV, incidence of ventilator-associated pneumonia (VAP), free days of analgesia/sedation in the intensive care unit (ICU), length of stay (LOS) in the ICU, LOS in hospital, in-ICU mortality, and 30-day all-cause mortality.
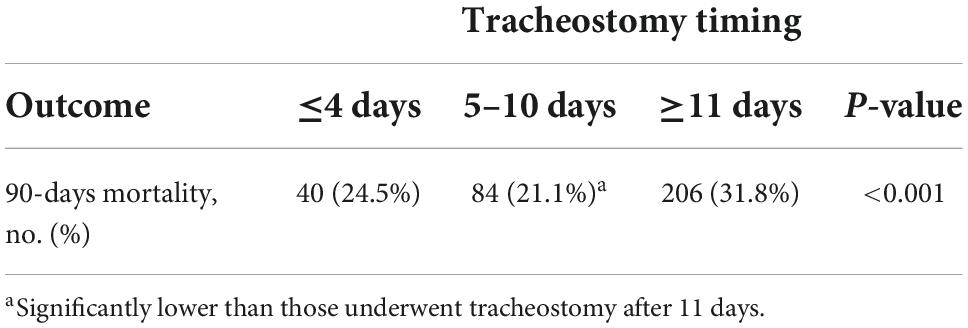
Results: A total of 1,209 patients were included in this study, of these, 163 (13.5%) patients underwent tracheostomy within 4 days after intubation, while 647 (53.5%) patients underwent tracheostomy more than 11 days after intubation. The tracheotomy timing showed a U-shaped relationship with all-cause mortality, patients who underwent tracheostomy between 5 and 10 days had the lowest 90-day mortality rate compared with patients who underwent tracheostomy within 4 days and after 11 days [84 (21.1%) vs. 40 (24.5%) and 206 (31.8%), P < 0.001].
Conclusion: The tracheotomy timing showed a U-shaped relationship with all-cause mortality, and the risk of mortality was lowest on day 8, but a causal relationship has not been demonstrated.
Introduction
Tracheostomy is one of the most used procedures in mechanically ventilated patients during the intensive care unit (ICU) stay (1, 2). The expected benefits of tracheostomy include enhanced comfort, improved pulmonary drainage, and decreased sedation depth. However, tracheostomy may also raise the risk of complications such as bleeding, incision infection, and tracheal stenosis. The optimal timing of tracheostomy remains unclear because uncertainty exists in the balance between the benefits and risks of tracheostomy (3, 4). Given the influence of tracheostomy timing on clinical outcomes, including the incidence of ventilator-associated pneumonia (VAP), duration of mechanical ventilation, length of stay (LOS) in the ICU and hospital, and mortality, observational studies with large sample sizes (5–12) and recent systematic reviews of randomized controlled trials (13–18) have yielded conflicting results, which might be due mainly to the diversity in patient populations and definitions of early versus late tracheostomy among different investigations.
Mechanically ventilated patients admitted to the ICU represent a heterogeneous group of illnesses. Certain categories of critically ill patients, such as acute brain injury, might benefit from early tracheostomy (19). Regarding the timing of tracheostomy, the definition of “early” in previous studies was also different, ranging from 3 to 21 days after mechanical ventilation (5–18). The timing of tracheostomy is a continuous variable. Binary classification to “early” and “late” might be unsuitable for exploring the effect of tracheostomy timing on clinical outcomes. Therefore, we constructed a non-linear regression model with adjustment of confounders between tracheostomy timing and 90-day all-cause mortality in different classifications of critically ill patients receiving mechanical ventilation.
Materials and methods
Data source
We performed a retrospective observational study using the Medical Information Mart for Intensive Care-III (MIMIC-III) database, containing de-identified datasets of 53,423 adult patients admitted to the ICUs in the Beth Israel Deaconess Medical Center in Boston (BIDMC) between 2001 and 2012 (20). MIMIC-III is an open-source database derived from two different information systems, the Philips CareVue Clinical Information System (hereinafter referred to as CareVue) and the iMDsoft MetaVision ICU (hereinafter referred to as MetaVision). CareVue and MetaVision recorded patients admitted to the hospital from 2001 to 2008 and after 2008, respectively. Data in MIMIC-III were downloaded from three sources, including archives from critical care information systems and hospital electronic health record databases for in-hospital data and the Social Security Administration Death Master File, which contained the follow-up time and out-of-hospital mortality. The establishment of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA), and consent was obtained for the original data collection. Therefore, the ethical approval statement and the need for informed consent were waived for this research by the Institutional Review Board of Beijing Tiantan hospital. The study was designed and conducted in accordance with relevant guidelines and regulations (Declaration of Helsinki).
Study cohort
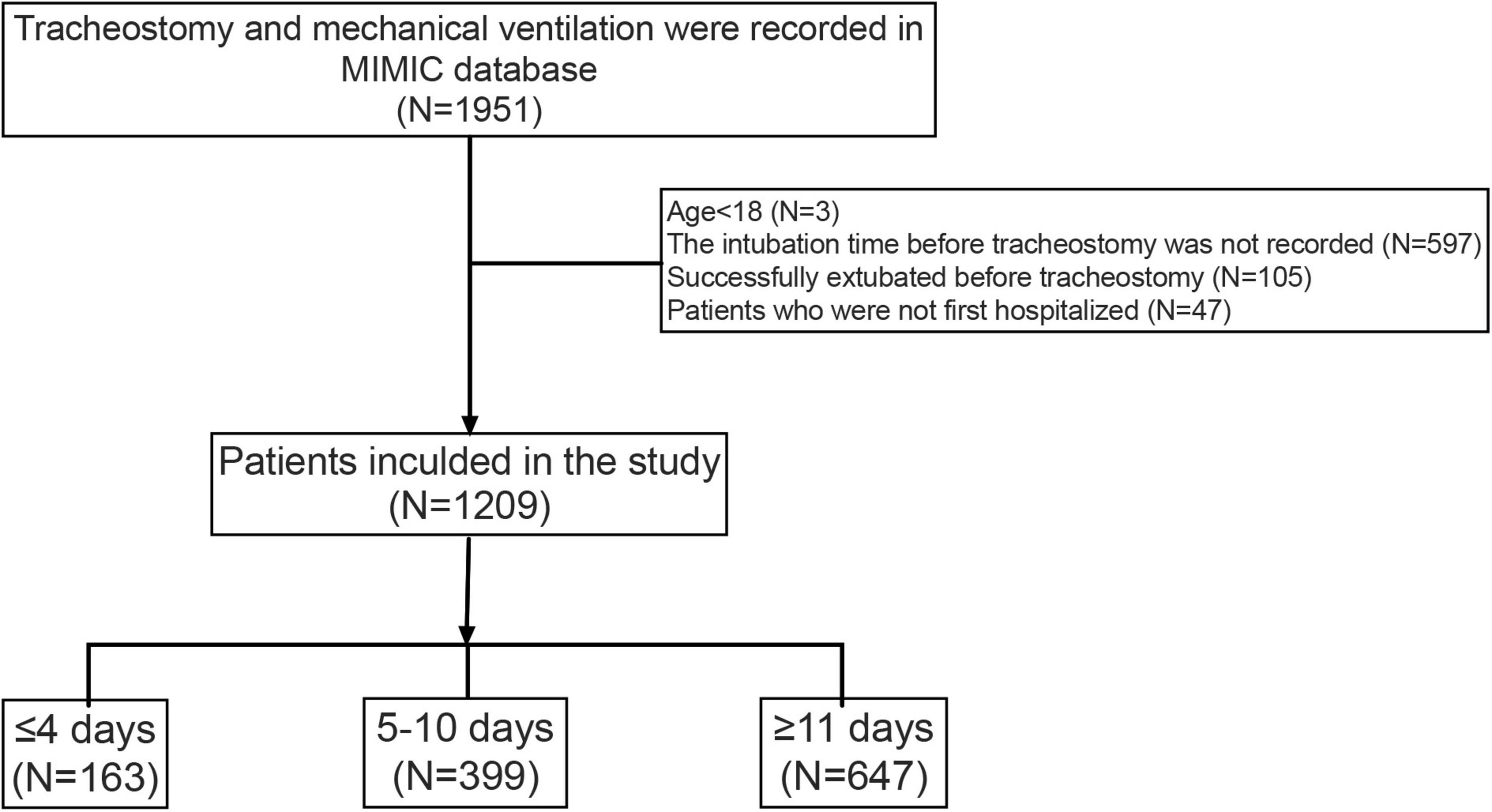
Data were extracted from all adult patients who were documented with mechanical ventilation and tracheostomy during the same hospitalization. For patients with multiple admissions to the ICU and those with multiple tracheostomy procedures, we collected only the first admission and the first tracheotomy data. Our inclusion criteria were: (1) adult patient aged 18 years or older; (2) accepted mechanical ventilation and tracheostomy in the same hospitalization; and (3) the time of tracheostomy could be obtained. Our exclusion criteria were: (1) the intubation time before tracheostomy was not recorded; (2) successfully extubated before tracheostomy was performed; and (3) patients who were not first hospitalized. To obtain our exposure of interest, which is the timing of tracheostomy, the following search strategy was developed. And all data were extracted using the Structured Query Language (SQL).
The MIMIC-III database is derived from the CareVue and MetaVision clinical information systems. Tracheostomy is documented as an operational event in the MetaVision system. The type of tracheostomy is also recorded. However, tracheostomy is not recorded as an operational event in the CareVue system. We retrieved the CareVue system using keywords including tracheostomy cuff, tracheostomy type, and tracheostomy care. The first record relating to tracheostomy was defined as the time of tracheostomy. The timing of tracheostomy was defined as the time interval between endotracheal intubation and tracheostomy. Six possible situations that existed in the collected datasets were shown in Figure 1 based on the criteria in the guideline (21).
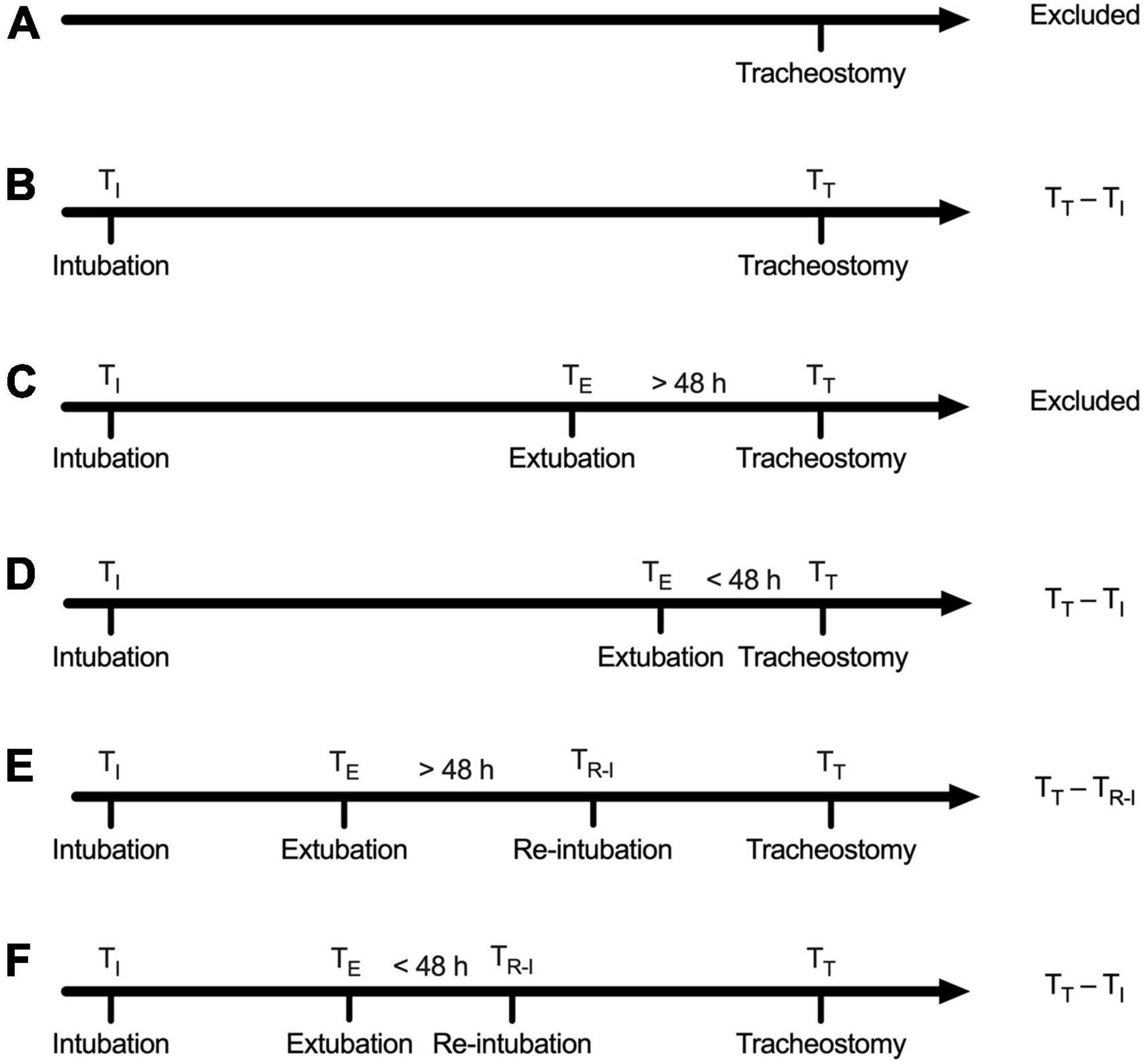
Figure 1. Definition of tracheostomy timing. (A) Patients with no record of endotracheal intubation regardless of the record of extubation were excluded. (B) Cases with only one record of intubation and without the record of extubation: Tracheostomy timing was calculated as the time interval between intubation and tracheostomy. (C) Tracheostomy was performed beyond 48 h after extubation: Defined as successful extubation and excluded. (D) Tracheostomy was performed within 48 h after extubation, defined as extubation failure, and tracheostomy timing was calculated as the time interval between tracheostomy and intubation prior to failed extubation. (E) Reintubation was performed beyond 48 h after extubation, defined as successful extubation, and tracheostomy timing was calculated as the time interval between reintubation and tracheostomy. (F) Reintubation was performed within 48 h after extubation, defined as extubation failure, and tracheostomy timing was calculated as the time interval between the first intubation and the tracheostomy.
Outcomes
The primary clinical outcome was 90-day all-cause mortality. Cases recorded in the CareVue system were followed for at least 4 years, whereas those recorded in the MetaVision system were followed for at least 90 days.
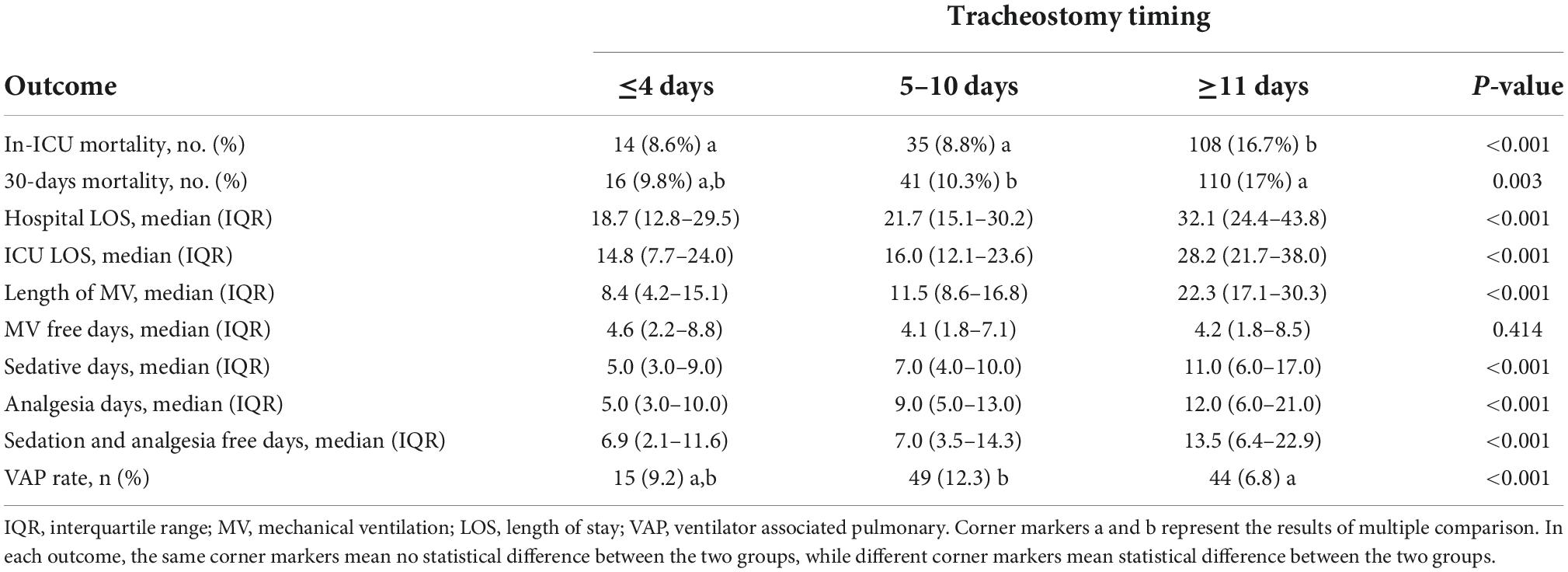
The secondary outcomes included duration of mechanical ventilation, free days of MV, incidence of ventilator-associated pneumonia (VAP), free days of analgesia/sedation in the intensive care unit (ICU), length of stay (LOS) in the ICU, LOS in hospital, in-ICU mortality, and 30-day all-cause mortality.
Possible confounding factors
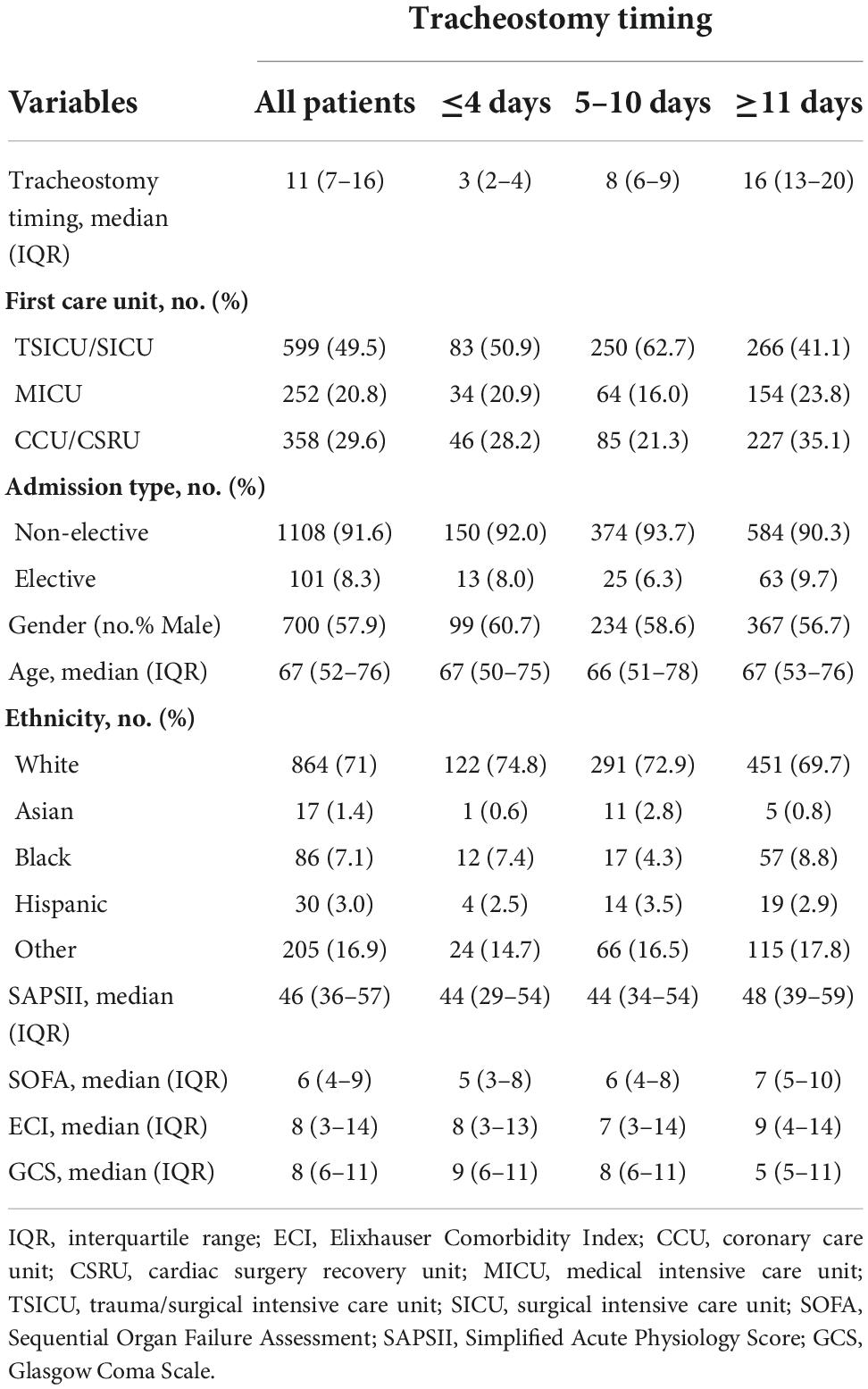
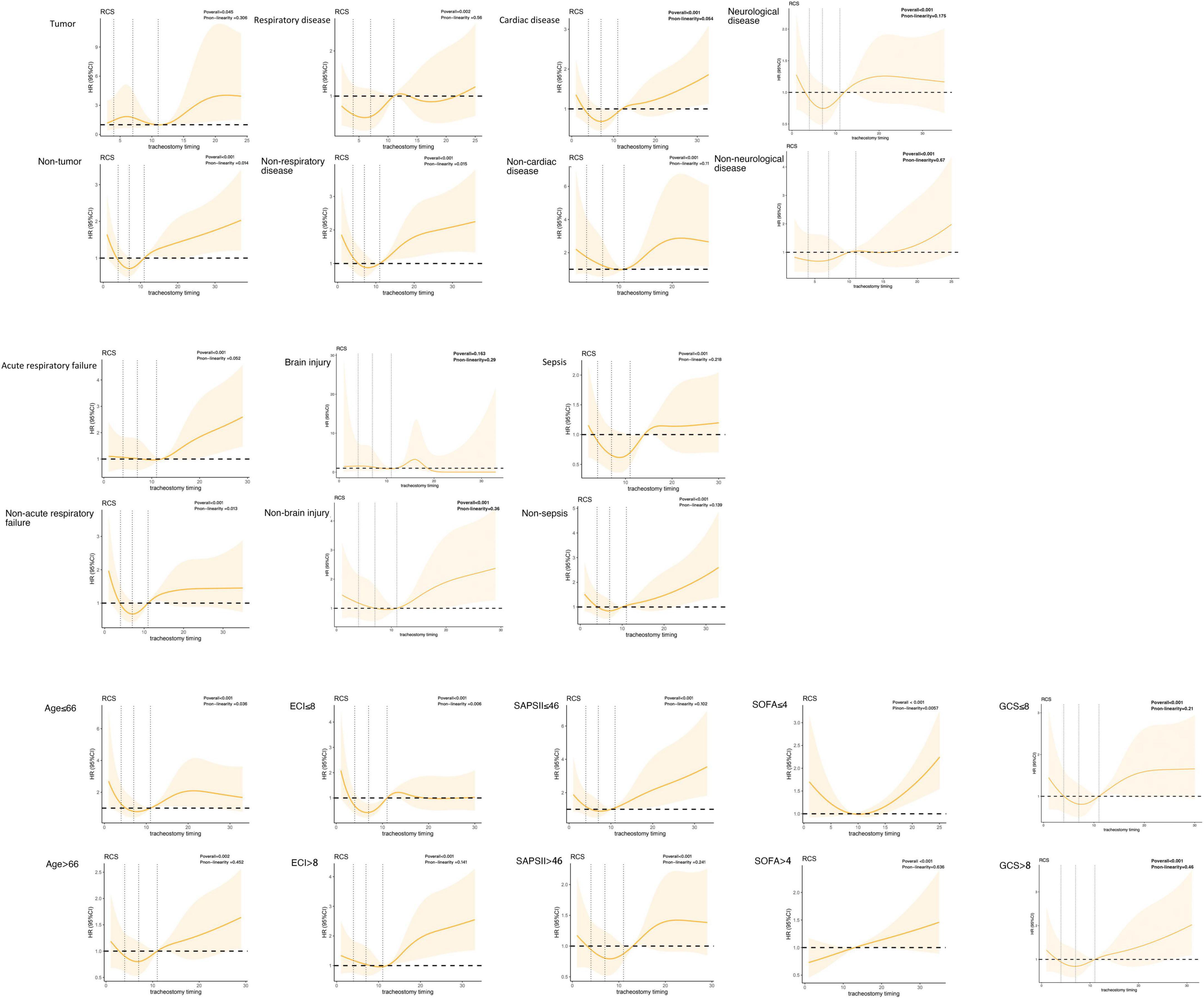
We collected possibly confounding factors that may affect 90-day mortality, including demographics, the severity of illness, and admission type. The severity of illness was represented by the Simplified Acute Physiology Score (SAPS) II (22) and Sequential Organ Failure Assessment (SOFA) (23) in the first 24 h of ICU admission. The Elixhauser Comorbidity Index (ECI) was used to assess the burden of comorbidities (24, 25) according to an algorithm provided by the authors of the MIMIC-III database, we quote the one named “elixhauser_score_quan.sql” (26). And Glasgow Coma Scale (GCS) in the first 24 h of ICU admission is also extracted. In order to further study the effect of tracheostomy timing on mortality in different comorbidities and diseases, the following four types of comorbidities were mainly concerned: neurological disease, tumor, respiratory disease, and cardiac disease. Neurological disease includes paralysis and other neurological disorders; tumor includes metastatic cancer, lymphoma, and solid tumor without metastasis; respiratory disease includes chronic pulmonary disease; cardiac disease includes congestive heart failure, cardiac arrhythmias, and valvular disease. Considering that different levels of consciousness may also influence the timing of tracheostomy, we also performed a subgroup analysis of patients according to different GCS scores on admission. We also identified three particular diseases: acute respiratory failure, brain injury and sepsis. Acute respiratory failure was identified from the International Classification of Diseases, Ninth Revision (ICD-9). Brain injury including traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), other intracerebral hemorrhage (ICH), intracranial space occupying lesion, and intracranial infections were identified from ICD-9 as well. Patients with sepsis were identified based on established definitions (27). Based on the small fraction of missing data, casewise deletion was used to deal with it.
Statistical analysis
We aimed primarily to determine the dose-response relationship between tracheostomy timing and all-cause mortality in ICU patients. Univariate and multivariate COX proportional hazard regression analyses with stepwise elimination (variables presenting P < 0.05 and were included in multivariate regression analyses) were used to identify the association between tracheostomy timing and 90-day all-cause mortality. Then, we used a restricted cubic spline Cox regression model to further analyze the non-linear association between tracheostomy timing and the risk of 90-day all-cause mortality. The restricted cubic splines we used had five knots at the 5th, 27.5th, 50th, 72.5th, and 95th centiles to flexibly model the association of tracheostomy timing with mortality (28, 29), and the reference point was the median tracheostomy timing. Then, we did a subgroup analysis tended to find the difference in tracheostomy timing in different classifications of critically ill patients receiving mechanical ventilation.
For continuous variables including age, data were reported as the median and quartile. Ranked data, such as SOFA score, SAPS II score, ECI score, and GCS score are reported as the median quartile. Two-tailed T-test or Mann–Whitney U test was used to compare differences between the two groups. Classified data such as tracheotomy method, type of admission, and type of ICU admission for the first time were described in terms of rate or percentage and compared by the chi-square test or Fisher’s exact test. P-value was adjusted with Bonferroni’s method in multiple comparisons. The R software program, version 4.0.5,1 and the SPSS program, version 26.0 (IBM Corporation, Armonk, NY, USA) were used for statistical analyses. A two-tailed P-value of 0.05 was considered significant for all analyses.
Results
Patient characteristics
A total of 1,951 hospitalizations with tracheostomy and MV records were retrieved. Based on our exclusion criteria, 1,209 patients were selected for the study. Of these, 163 (13.5%) patients underwent tracheostomy within 4 days after intubation, while 647 (53.5%) patients underwent tracheostomy more than 11 days after intubation. Figure 2 shows details of cohort selection. Table 1 describes the baseline and clinical characteristics of the cohort.
Primary outcome
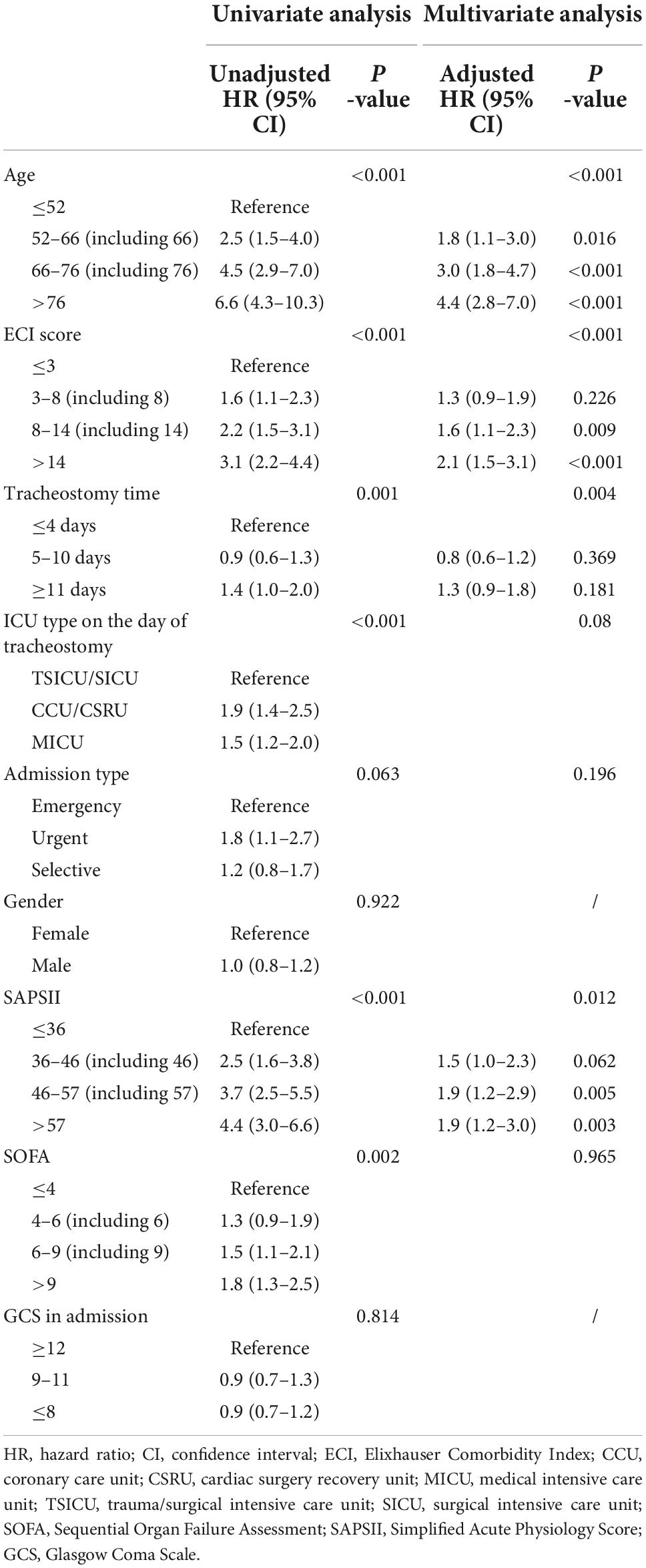
Patients who underwent tracheostomy between 5 and 10 days had lower 90-day mortality comparing to patients underwent tracheostomy after 11 days. While there was no statistical difference in patients who underwent tracheostomy between 5 and 10 days and patients who underwent tracheostomy within 4 days. Detailed multiple comparison results are shown in Table 2. To analyze factors that might affect 90-day mortality, univariate and multivariate COX proportional hazard regression analyses were used. Factors that might influence mortality were included in the analysis, including age, gender, tracheostomy timing, ICU type on the day of tracheostomy, admission type, GCS score on admission, SOFA score, SAPS-II score and ECI score. Detailed results are shown in Table 3. After adjusting age, SAPSII score and ECI score, tracheostomy timing was still an independent risk factor of death.
To further analyze the non-linear association between tracheostomy timing and the risk of 90-day all-cause mortality, we constructed restricted cubic spline model. In this model, the association between tracheostomy timing and all-cause 90-day mortality was U-shaped, as shown in Figure 3 (Poverall < 0.001), adjusted variables including age, SAPSII score, and ECI score. The risk of 90-day all-cause mortality decreased with the extension of tracheostomy timing until approximately 8 days after intubation and then started to increase afterward (P for non-linearity < 0.001). Before Day 8, the hazard ratio delayed each day was 0.888 (0.805–0.980), while after Day 8, the hazard ratio delayed each day was 1.031 (1.014–1.049). Meanwhile, around Day 4 to Day 11 after intubation, the hazard ratio was less than 1.
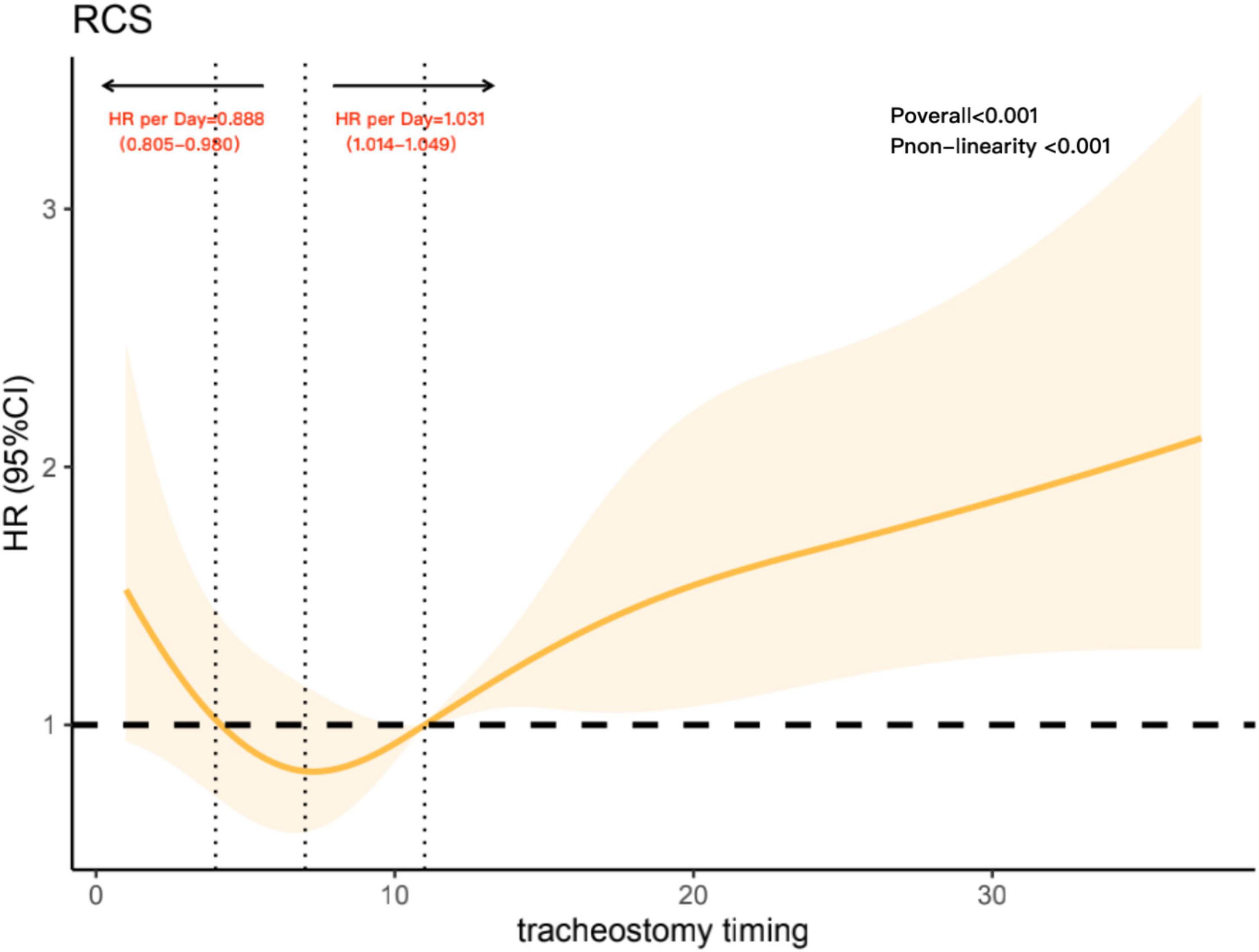
Figure 3. Association with tracheostomy timing and mortality, adjusted variables including age, Simplified Acute Physiology Score (SAPS) II score, and Elixhauser Comorbidity Index (ECI) score. The reference point is the median tracheostomy timing and knots at the 5th, 27.5th, 50th, 72.5th, and 95th centiles of tracheostomy timing. The vertical line on the left shows tracheostomy timing around Day 4, while the vertical line in the middle shows tracheostomy timing around Day 8. The vertical line on the right shows tracheostomy timing on Day 11; it intersects the horizontal dotted line, and the intersection point represents the reference point.
Then, we discussed the relationship between the tracheostomy timing and 90-day all-cause mortality for patients with different comorbidities to further refine the possible causes of the U-shaped curve. Comorbidities included tumor or non-tumor, respiratory disease or non-respiratory disease, cardiac disease or non-cardiac disease, neurological disease or non-neurological disease. According to the GCS scores on admission, we analyzed the association between tracheostomy timing and mortality in patients with severe consciousness disorders (GCS ≤ 8) and patients with mild to moderate consciousness conditions (GCS > 8). We also analyzed three particular diseases: sepsis, acute respiratory failure, and brain injury. Patients of different ages, ECI scores, SAPS II scores and SOFA scores were also analyzed. There was a strong linear relationship between SOFA ≥ 4 and 90-day mortality, which declared that in those patients, early tracheostomy might have more benefits. For patients younger than 66, ECI less than 8 and SOFA less than 4, there were a non-linear relationship between tracheostomy timing and 90-day mortality. While in the four kinds of comorbidities, different levels of consciousness and three kinds of diseases, we did not find such a non-linear relationship (Figure 4).
Secondary outcome
As showed in Table 4, patients who underwent tracheostomy within 4 days had less hospital and ICU LOS, less length of MV, and fewer sedative and analgesia days. Patients who underwent tracheostomy within 5–10 days were more likely to be diagnosed with VAP. Multiple comparisons were also performed and P-value after adjustment by Bonferroni’s method were showed in Supplementary Table 1.
Discussion
In this retrospective cohort study, we sought to explore the association between tracheotomy timing and mortality. We retrospectively analyzed MV patients with tracheostomy records in MIMIC-III database and found that compared to patients who underwent tracheostomy within 4 days and after 11 days, patients who underwent tracheostomy within 5–10 days had less 90-day mortality. Meanwhile, the association between tracheostomy timing and all-cause 90-day mortality was U-shaped, reaching the lowest risk around Day 8 and then increased thereafter.
In published clinical studies, researchers defined tracheostomy timing differently. Some RCTs limited early tracheostomy to within 5 days of MV and late tracheostomy to 10 days after MV (30, 31). Some studies also used a single time point as the time limit for distinguishing early and late tracheostomy (6, 8, 32, 33). However, regardless of the classification, the trajectory of change between continuous changes in tracheotomy timing and mortality risk in MV patients was ignored. Artificially segmenting the timing of tracheotomy may not only result in loss of information but also lead to low statistical efficiency, resulting in bias of results (34, 35). Additionally, guidelines have suggested that tracheostomy in intensive care should not be performed before the fourth day of MV, since early tracheostomy before the fourth day of mechanical ventilation is not associated with decreases in mortality (36). Therefore, we believe that there may be a non-linear relationship between tracheostomy timing and the risk of death. In this study, we used restricted cubic spline analysis to explore this relationship. After adjusting other influencing factors, the non-linear dose-response relationship was presented objectively and cleanly (37). We chose Day 11 as the reference point for restricted cubic spline analysis, not only because Day 11 is the median tracheostomy timing, conforming with the analysis of restrictive cubic spline routine (29) but also because previous studies used Day 10 to distinguish tracheostomy timing and obtained a positive result (6, 8), suggesting that approximately Day 10 may be the boundary between the risk of death. In this research, results suggest that performing tracheostomy between day 4 and 11 after intubation may be a safer choice and that proceeding with tracheostomy too early or too late would significantly increase the risk of 90-day all-cause mortality.
We also tended to find the potential reason for the U-shaped curve by dividing patients into different disease groups and different severity groups. There was a strong linear relationship between SOFA ≥ 4 and 90-day mortality, while in patients with lower SOFA scores, the relationship was still non-linear. Which suggested in patients with less severe disease, tracheostomy should be delayed, while in severe patients, tracheostomy should be performed as soon as possible. Considering the influence of consciousness level on tracheostomy strategy, we also compared the differences in correlation between tracheostomy and mortality in patients with different consciousness levels according to GCS scores. However, no significant non-linear relationship was found in patients with different GCS scores. And in the four comorbidities and three diseases we focused on, no significant non-linear relationship was found either. To explore the non-linear relationship between the tracheostomy timing and mortality in different diseases, more studies are needed.
In our study, it is important to note that after Day 11, which is the median tracheostomy timing, the risk of mortality increased nearly linearly, thus supporting the idea that late tracheostomy may increase the risk of mortality in MV patients. For early tracheostomy, based on the U-shaped curve between the tracheostomy timing and 90-day all-cause mortality, studies focusing on the influence of early tracheostomy and ultra-early tracheostomy on mortality could be regarded as future research directions.
This study found that early tracheostomy could significantly shorten LOS in the ICU and hospital, shorten MV duration, and reduce the need for sedation and analgesia, which was consistent with previous research results (38–40). Although the free days of sedation and analgesia in the late group were longer than the free days of sedation and analgesia in the early group, considering the huge gap between the LOS of these two groups in the ICU, the result is explainable. In our study, patients who underwent tracheostomy within 5–10 days suffered a higher rate of VAP than late patients, which is inconsistent with previous research results (15) and clinical cognition. In this study, the diagnosis of VAP was based on the diagnosis clearly recorded in the ICD-9 code in the database. Unfortunately, since the time of diagnosis was not recorded in the MIMIC-III database, we were unable to determine the time relationship between the occurrence of VAP and tracheostomy, and we were also unable to determine whether the cause of tracheostomy was due to the occurrence of VAP.
This study provides a large sample size, real-world clinical study that focuses on factors influencing the outcome of different tracheostomy timings. To the best of our knowledge, this is the first study introducing the combination of restricted cubic splines and Cox regression into exploring the relationship between tracheostomy timing and mortality. We not only discussed the relationship between tracheostomy timing and mortality but also analyzed the trend between tracheostomy timing and mortality risk and focused on the non-linear relationship between tracheostomy timing and mortality risk. Meanwhile, previous studies excluded patients who failed extubation several times prior to tracheostomy, which may have caused selection bias artificially. This study makes up for the defects of this type of research. In addition, according to the relationship between intubation, extubation and tracheostomy time, a new definition of tracheostomy timing was adopted. Patients with repeated endotracheal intubation before tracheostomy were also included in the study, which was more in line with clinical practice. However, as a single-center retrospective study, this study has limitations. First, the database we used was the MIMIC-III database. Although there have been many high-quality studies based on this open-source database (41, 42), the source of the MIMIC-III database was only one hospital. The timing of tracheostomy depends on the experience of the physician and the standardized procedures of the hospital. Therefore, the applicability of applying the results of this study in other situations will be reduced. Second, we mainly focused on the relationship between tracheostomy timing and mortality in tracheostomized patients. Patients who died before tracheostomy were not included in this research. Therefore, we cannot determine whether the absence of tracheostomy was one of the risk factors for death in such patients, which may cause particular bias. Third, as a retrospective study, we explored the correlation between tracheostomy timing and mortality, but the causal relationship between the two needs to be confirmed by further RCTs.
Conclusion
The tracheotomy timing showed a U-shaped relationship with all-cause mortality, and the risk of mortality was lowest on Day 8, but a causal relationship has not been demonstrated.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Johnson A., Pollard T., and Mark R. (2016). MIMIC-III Clinical Database (version 1.4). PhysioNet (https://doi.org/10.13026/C2XW26).
Ethics statement
The establishment of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Therefore, the ethical approval statement and the need for informed consent were waived for this manuscript by the Institutional Review Board of Beijing Tiantan hospital. The study was designed and conducted in accordance with relevant guidelines and regulations (Declaration of Helsinki).
Author contributions
J-XZ and G-QC conceptualized the research aims and guided the literature review. J-RC and H-RG improved the design and extracted data from the MIMIC-III database. Y-MZ verified the data. Y-LY and YW participated in processing the data and doing the statistical analysis. J-RC wrote the first draft of the manuscript. LZ and J-XZ provided comments and approved the final manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1068569/full#supplementary-material
Footnotes
References
1. Freeman BD, Morris PE. Tracheostomy practice in adults with acute respiratory failure. Crit Care Med. (2012) 40:2890–6. doi: 10.1097/ccm.0b013e31825bc948
2. Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care. (2014) 59:895–919. doi: 10.4187/respcare.02971
3. McCredie VA, Adhikari NK. Early tracheostomy in critically ill patients: still too fast. Lancet Respir Med. (2015) 3:95–6. doi: 10.1016/S2213-2600(14)70141-9
4. Esperanza JA, Pelosi P, Blanch L. What’s new in intensive care: tracheostomy-what is known and what remains to be determined. Intensive Care Med. (2019) 45:1619–21. doi: 10.1007/s00134-019-05758-z
5. Freeman BD, Borecki IB, Coopersmith CM, Buchman TG. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. (2005) 33:2513–20. doi: 10.1097/01.ccm.0000186369.91799.44
6. Scales DC, Thiruchelvam D, Kiss A, Redelmeier DA. The effect of tracheostomy timing during critical illness on long-term survival. Crit Care Med. (2008) 36:2547–57. doi: 10.1097/ccm.0b013e31818444a5
7. Arabi YM, Alhashemi JA, Tamim HM, Esteban A, Haddad SH, Dawood A, et al. The impact of time to tracheostomy on mechanical ventilation duration, length of stay, and mortality in intensive care unit patients. J Crit Care. (2009) 24:435–40. doi: 10.1016/j.jcrc.2008.07.001
8. Villwock JA, Jones K. Outcomes of early versus late tracheostomy: 2008-2010. Laryngoscope. (2014) 124:1801–6. doi: 10.1002/lary.24702
9. Shaw JJ, Santry HP. Who gets early tracheostomy ? : Evidence of unequal treatment at 185 academic medical centers. Chest. (2015) 148:1242–50. doi: 10.1378/chest.15-0576
10. Mehta AB, Cooke CR, Wiener RS, Walkey AJ. Hospital variation in early tracheostomy in the United States: a population-based study. Crit Care Med. (2016) 44:1506–14. doi: 10.1097/ccm.0000000000001674
11. Abe T, Madotto F, Pham T, Nagata I, Uchida M, Tamiya N, et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. (2018) 22:195. doi: 10.1186/s13054-018-2126-6
12. Blecha S, Brandl M, Zeman F, Dodoo-Schittko F, Brandstetter S, Karagiannidis C, et al. Tracheostomy in patients with acute respiratory distress syndrome is not related to quality of life, symptoms of psychiatric disorders or return-to-work: the prospective DACAPO cohort study. Ann Intensive Care. (2020) 10:52. doi: 10.1186/s13613-020-00671-x
13. Andriolo B, Andriolo R, Saconato H, Atallah Á, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. (2015) 1:CD007271. doi: 10.1002/14651858.CD007271
14. Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. (2015) 19:424. doi: 10.1186/s13054-015-1138-8
15. Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. (2015) 3:150–8. doi: 10.1016/S2213-2600(15)00007-7
16. Szakmany T, Russell P, Wilkes AR, Hall JE. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: meta-analysis of randomized controlled trials. Br J Anaesth. (2015) 114:396–405. doi: 10.1093/bja/aeu440
17. Wang R, Pan C, Wang X, Xu F, Jiang S, Li M. The impact of tracheotomy timing in critically ill patients undergoing mechanical ventilation: a meta-analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung. (2019) 48:46–54. doi: 10.1016/j.hrtlng.2018.09.005
18. Deng H, Fang Q, Chen K, Zhang X. Early versus late tracheotomy in ICU patients: a meta-analysis of randomized controlled trials. Medicine. (2021) 100:24329.
19. McCredie VA, Alali AS, Scales DC, Adhikari NK, Rubenfeld GD, Cuthbertson BH, et al. Effect of early versus late tracheostomy or prolonged intubation in critically ill patients with acute brain injury: a systematic review and meta-analysis. Neurocrit Care. (2017) 26:14–25. doi: 10.1007/s12028-016-0297-z
20. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
21. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. (2007) 29:1033–56. doi: 10.1183/09031936.00010206
22. Le-Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, et al. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. JAMA. (1996) 276:802–10. doi: 10.1001/jama.276.10.802
23. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. (1996) 22:707–10.
24. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. (1998) 36:8–27. doi: 10.1097/00005650-199801000-00004
25. Walraven C v, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. (2009) 47:626–33. doi: 10.1097/MLR.0b013e31819432e5
26. GitHub. Mimic-Code/Mimic-iii/Concepts/Comorbidity/. (2022). Available online at: https://github.com/MIT-LCP/mimic-code/tree/main/mimic-iii/concepts/comorbidity (accessed October 11, 2022).
27. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
28. Roshani D, Ghaderi E. Comparing smoothing techniques for fitting the nonlinear effect of covariate in cox models. Acta Inform Med. (2016) 24:38–41. doi: 10.5455/aim.2016.24.38-41
29. Harrell FE. Multivariable modeling strategies. In: Fienberg S editor. The Regression Modeling Strategies. New York, NY: Springer (2001). p. 63–102.
30. Devarajan J, Vydyanathan A, Xu M, Murthy SM, McCurry KR, Sessler DI, et al. Early tracheostomy is associated with improved outcomes in patients who require prolonged mechanical ventilation after cardiac surgery. J Am Coll Surg. (2012) 214:1008–16. doi: 10.1016/j.jamcollsurg.2012.03.005
31. Sugerman HJ, Wolfe L, Pasquale MD, Rogers FB, O’Malley KF, Knudson M, et al. Multicenter, randomized, prospective trial early tracheostomy. J Trauma. (1997) 43:741–7. doi: 10.1097/00005373-199711000-00002
32. Brook AD, Sherma G, Malen J, Kollef MH. Early versus late tracheostomy in patients who require prolonged mechanical ventilation. Am J Crit Care. (2000) 9:352–9.
33. Mohamed KAE, Mousa AY, ElSawy AS, Saleem AM. Early versus late percutaneous tracheotomy in critically ill adult mechanically ventilated patients. Egypt J Chest Dis Tubercul. (2014) 63:443–8.
34. Zhao LP, Kolonel LN. Efficiency loss from categorizing quantitative exposures into qualitative exposures in case-control studies. Am J Epidemiol. (1992) 136:464–74. doi: 10.1093/oxfordjournals.aje.a116520
35. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. (2006) 332:1080. doi: 10.1136/bmj.332.7549.1080
36. Trouillet J, Collange O, Belafia F, Blot F, Capellier G, Cesareo E, et al. Tracheotomy in the intensive care unit: guidelines from a French expert panel. Ann Intensive Care. (2018) 8:37. doi: 10.1186/s13613-018-0381-y
37. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
38. Tong CC, Kleinberger AJ, Paolino J, Altman KW. Tracheotomy timing and outcomes in the critically ill. Otolaryngol Head Neck Surg. (2012) 147:44–51. doi: 10.1177/0194599812440262
39. Mahafza T, Batarseh S, Bsoul N, Massad E, Qudaisat I, Al-Layla AE. Early vs. late tracheostomy for the ICU patients: experience in a referral hospital. Saudi J Anaesth. (2012) 6:152–4. doi: 10.4103/1658-354X.97029
40. Bickenbach J, Fries M, Offermanns V, Von Stillfried R, Rossaint R, Marx G, et al. Impact of early vs. Late tracheostomy on weaning: a retrospective analysis. Minerva Anestesiol. (2011) 77:1176–83.
41. Fuchs L, Feng M, Novack V, Lee J, Taylor J, Scott D, et al. The effect of ARDS on survival: do patients die from ARDS or with ARDS? J Intensive Care Med. (2019) 34:374–82. doi: 10.1177/0885066617717659
Keywords: intensive care unit, tracheostomy, mechanical ventilation, clinical outcomes, extubation
Citation: Chen J-R, Gao H-R, Yang Y-L, Wang Y, Zhou Y-M, Chen G-Q, Li H-L, Zhang L and Zhou J-X (2022) A U-shaped association of tracheostomy timing with all-cause mortality in mechanically ventilated patients admitted to the intensive care unit: A retrospective cohort study. Front. Med. 9:1068569. doi: 10.3389/fmed.2022.1068569
Received: 01 November 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Penglin Ma, Guiqian International General Hospital, ChinaReviewed by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaFeng Shen, Affiliated Hospital of Guizhou Medical University, China
Yun Long, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Chen, Gao, Yang, Wang, Zhou, Chen, Li, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Xin Zhou, zhoujx.cn@icloud.com
 Jing-Ran Chen
Jing-Ran Chen Hao-Ran Gao2
Hao-Ran Gao2  Yan Wang
Yan Wang Guang-Qiang Chen
Guang-Qiang Chen Hong-Liang Li
Hong-Liang Li Linlin Zhang
Linlin Zhang Jian-Xin Zhou
Jian-Xin Zhou