Insights Into Thiamine Supplementation in Patients With Septic Shock
- 1Faculty of Nutrition, Universidade Federal de Goiás (UFG), Goiânia, Brazil
- 2Department of Internal Medicine, Botucatu Medical School, São Paulo State University (UNESP), Botucatu, Brazil
Septic shock is associated with unacceptably high mortality rates, mainly in developing countries. New adjunctive therapies have been explored to reduce global mortality related to sepsis. Considering that metabolic changes, mitochondrial dysfunction and increased oxidative stress are specific disorders within the path of septic shock, several micronutrients that could act in cellular homeostasis have been studied in recent decades. Thiamine, also known as vitamin B1, plays critical roles in several biological processes, including the metabolism of glucose, synthesis of nucleic acids and reduction of oxidative stress. Thiamine deficiency could affect up to 70% of critically ill patients, and thiamine supplementation appears to increase lactate clearance and decrease the vasopressor dose. However, there is no evident improvement in the survival of septic patients. Other micronutrients such as vitamin C and D, selenium and zinc have been tested in the same context but have not been shown to improve the outcomes of these patients. Some problems related to the neutrality of these clinical trials are the study design, doses, route, timing, length of intervention and the choice of endpoints. Recently, the concept that multi-micronutrient administration may be better than single-micronutrient administration has gained strength. In general, clinical trials consider the administration of a single micronutrient as a drug. However, the antioxidant defense is a complex system of endogenous agents in which micronutrients act as cofactors, and the physiological interactions between micronutrients are little discussed. In this context, the association of thiamine, vitamin C and corticoids was tested as an adjunctive therapy in septic shock resulting in a significant decrease in mortality. However, after these initial results, no other study conducted with this combination could reproduce those benefits. In addition, the use of low-dose corticosteroids is recommended in patients with septic shock who do not respond to vasopressors, which can affect the action of thiamine. Therefore, given the excellent safety profile, good biologic rationale and promising clinical studies, this review aims to discuss the mechanisms behind and the evidence for single or combined thiamine supplementation improving the prognosis of patients with septic shock.
Introduction
Septic shock is a subset of sepsis characterized by profound hemodynamic alterations associated with organ dysfunction and is one of the most common causes of admission to intensive care units (ICUs) (1). Despite advances in management, rates of sepsis are still rising worldwide, and it is associated with high morbidity, disability and mortality (2).
In critical illness, and most notably in sepsis, the metabolic response to trauma, although necessary, can usually overwhelm the body's metabolism, leading to a wide range of clinical consequences. This response implies significant changes in intermediary metabolism, including increased glycogenolysis, inhibition of glycogenesis and increased lipolysis, producing glucose via gluconeogenesis of lactate, glycerol and amino acids (3). In this scenario, some vitamins and minerals are essential for energy metabolism and mitochondrial function; among these, thiamine deserves to be highlighted (4, 5). Currently, the role of thiamine in sepsis treatment has become of particular interest. Thiamine deficiency might be involved in the pathophysiology of septic shock because high serum lactate concentrations, metabolic acidosis and hypotension can occur in both conditions (6) (Figure 1).
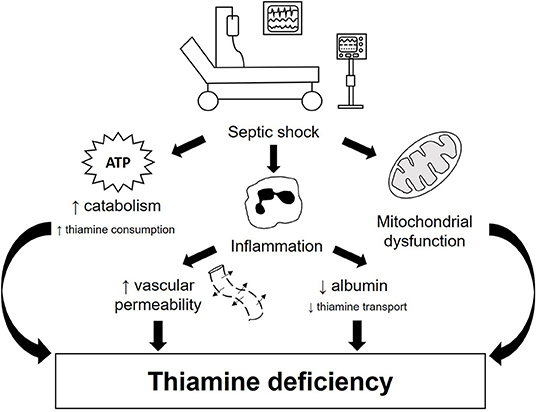
Figure 1. Pathophysiology of thiamine deficiency in septic shock patients. Thiamine deficiency in septic shock occurs both due to increased need and impaired transport. This condition can be triggered by three main mechanisms: hypercatabolism, exacerbated pro-inflammatory response and mitochondrial dysfunction. During septic shock, there is an increase in the metabolic demand for energy, resulting in increased glycogenolysis, inhibition of glycogenesis and increased production of glucose via gluconeogenesis, considerably increasing the need for thiamine for glucose metabolism. Inflammation, on the other hand, promotes increased vascular permeability and reduced albumin production, impairing thiamine transport. In addition, tissue hypoxia present in septic shock is one of the main triggers of mitochondrial dysfunction, which contributes to an imbalance in glucose homeostasis, including lower availability of ATP and increased serum lactate concentration. All these mechanisms together contribute to the development and/or worsening of thiamine deficiency in patients with septic shock.
The evidence of thiamine deficiency in critically ill patients was discovered in the 1980s in patients who developed classical clinical manifestations, such as cardiac disorders and neuropsychiatric syndromes, after ICU admission (7–9). Afterwards, thiamine body status levels were first assessed in critically ill patients by Cruickshank et al. (10), who reported deficiency in 20% of adult patients upon admission to the ICU. Also, higher thiamine body status levels were associated with lower mortality. Despite important limitations, this previous investigation brought to light a considerable concern that thiamine deficiency might be related to poorer outcomes or even could be a potentially life-threatening condition in critically ill patients.
Indeed, thiamine deficiency is relatively prevalent in septic shock patients, with rates as high as 71.3% (11). In addition, it is essential to note that in sepsis, there are some severe metabolic derangements, so improvement in organ failure is essential. In this way, antioxidant–enforcement and mitochondrial stress attenuation are specific targets for the rationale of thiamine treatment during septic shock states (12). However, the results of studies assessing thiamine supplementation effects on outcomes remain inconsistent (11, 13–15).
Thus, understanding thiamine's multiple functions in several biological processes by examining past and ongoing studies will lead to the further definition of potential targets for septic shock treatment. This review aims to discuss the mechanisms and the evidence for single or combined thiamine supplementation on the prognosis of patients with septic shock.
Thiamine: Functions and Mechanisms of Action
Thiamine or vitamin B1 is a water-soluble and thermosensitive vitamin that is not produced by our body and is indispensable in the human diet. It is considered an essential component of cell metabolism and is mainly involved in glucose metabolism (16).
In 9th-century appeared in Japan first descriptions of states associated with thiamine deficiency in the form of beriberi (17). Since then, knowledge about thiamine has increased along with the gradual recognition of diseases associated with its deficiency, which are divided into two classical clinical forms: encephalopathy (peripheral neuropathy and Wernicke-Korsakoff) and beriberi, which can be classified into dry beriberi (muscle weakness and anorexia), wet beriberi (high-output heart failure) and Shoshin beriberi (beriberi associated with shock) (18–20).
Thiamine is naturally present in several foods, such as meat (especially lean cuts of pork), cereals, yeasts, grains, fruits and other products of plant origin (21). Its biochemical structure consists of a thiazole ring and a pyrimidine group that together make up the sulfur-containing structure of two rings joined by a methylene group (16). Both fractions, pyrimidine and thiazole rings are necessary for thiamine biological activity (22). Its molecule can suffer the action of thiaminases, which are enzymes that cleave the thiamine in the methylene bridge, inactivating it. Thiaminases are produced by bacteria present in the small bowel and colon and can also be found in some raw foods such as fish and shellfish (22). Thus, frequent intake of these foods heightens the risk of thiamine deficiency.
Six thiamine compounds are known in metabolism: free thiamine; thiamine monophosphate (TMP); thiamine diphosphate, also referred to as thiamine pyrophosphate (TPP); adenosine thiamine diphosphate (ATDP); thiamine triphosphate and adenosine thiamine triphosphate (17). The most important biological active form is TPP, and it accounts for 80–90% of total body thiamine content (17, 23). Approximately 90% of the total thiamine in the blood is found in erythrocytes (75%) and leukocytes (15%) in the TPP form (24). About 5–15% of the total vitamin B1 is in the form of free thiamine, which is bound to albumin in the blood or as TMP form. The remaining forms of vitamin B1 account for only 1% of the total thiamine in humans (17, 24).
There are no significant stores of thiamine in any human tissue (21), and its concentrations are highest in the skeletal muscles, heart, kidney, liver and brain respectively (24, 25). Due to its short half-life of 9.5–18.5 days (24), necessary role in multiple metabolic processes and increased requirements in some pathological states, appropriate dietary intake is crucial for avoiding deficiency states (26, 27).
The Recommended Daily Allowance (RDA) of thiamine for healthy adults is 1.1–1.2 mg/day (21). However, in individuals at risk or with established thiamine deficiency (TD) there is no consensus on the appropriate dose, frequency, or duration of supplementation. In critically ill patients, prophylaxis or treatment for TD typically consists of parenteral administration of thiamine (25, 26). The intravenous route is most frequently used due to rapidly achieving high plasma concentrations, flexible rate of dosing, and better site tolerance as compared to intramuscular injection (28). Also, thiamine replacement by oral or enteral route is possible in situations of non-emergent deficiency, though it is important to note that the gastrointestinal microenvironment is often perturbed during sepsis, resulting in gut dysfunction and nutrient malabsorption (24, 29).
Although thiamine is considered to have a very good safety profile, the tolerable upper intake level (UL) is not established (30). Studies have shown that parenteral doses >500 mg have occasionally led to anaphylaxis and minimal adverse effects such as nausea, anorexia, lethargy, mild ataxia, and a diminution of gut tone (26, 28, 31).
Considering the availability and half-life, the most recommended methods to detect thiamine deficiency are the assessment of transketolase activity (which uses TPP as a cofactor) and the measurement of ATDP, both of which conducted in erythrocytes (24, 32). However, the lack of availability and high costs of these methods limit their use in clinical practice (33).
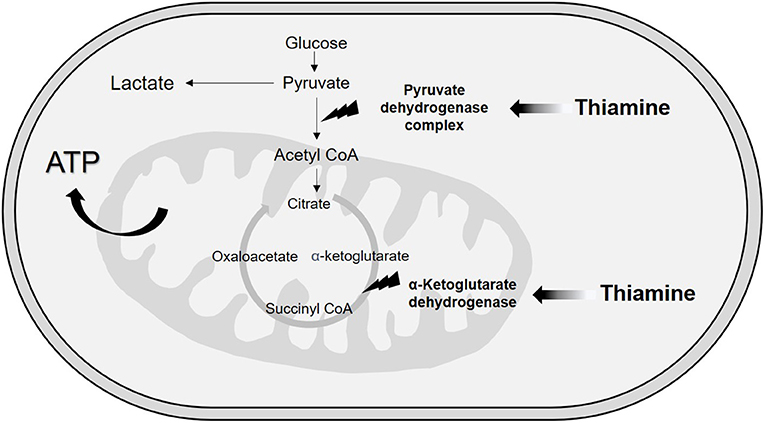
Although vitamin B1 is involved in several related and simultaneous biological processes, we can divide the functions of thiamine into metabolic or enzymatic and structural or nonenzymatic roles (34, 35). Concerning metabolic functions, thiamine plays an essential role in energy transformation because TPP is a cofactor of two enzymes related to the extraction of energy from carbohydrate sources (16, 24, 34). These mitochondrial enzymes are involved in decarboxylation reactions and dehydrogenation reactions (24). The first enzyme is the multienzyme complex pyruvate dehydrogenase (PDH), which is made up of TPP-dependent pyruvate decarboxylase, a lipoic acid-dependent dihydrolipoyl transacetylase and a flavin adenine dinucleotide (riboflavin)-dependent dihydrolipoyl dehydrogenase (24, 36). In the related PDH reactions, TPP receives electrons in the redox processes (16), resulting in the conversion of pyruvate to acetyl-CoA, which then takes place in the Krebs cycle (37). At this point, we can infer that in states of thiamine deficiency, pyruvate access to the mitochondria is impaired with cytosolic conversion to lactate via lactate dehydrogenase and further lactic acidosis (Figure 2).
TPP is also a cofactor for α-ketoglutarate dehydrogenase, a mitochondrial enzyme responsible for converting α-ketoglutarate to succinyl-CoA in the Krebs cycle (24) through a decarboxylation reaction (16). This function highlights crucial redox mechanisms taking place inside the mitochondria, which is responsible for over 95% of adenosine 5-triphosphate generation and, consequently, oxygen reactive species production (12, 16). Mitochondrial dysfunction is well known in sepsis (12), and potential targets for thiamine supplementation might be located inside this organelle.
In turn, TPP is also useful in non-oxidative carbohydrate metabolism. The cytosolic enzyme transketolase needs TPP as a cofactor (24). This reaction is found within the pentose phosphate pathway, where sugars are interconverted and are essential for pentoses generation to nucleic acids synthesis and nicotinamide adenine dinucleotide phosphate (NADPH) for the production of fatty acids, maintenance of myelin sheaths, nerve membrane function and signal transmission (16, 24). At this point, both functions of thiamine are interconnected. NADPH, in turn, is involved in glutathione cycling, an important antioxidant pathway and potential target of thiamine as a metabolic resuscitator in patients with septic shock (38). It is also important to emphasize that thiamine requires magnesium as a cofactor for conversion into its TPP active form, playing a key role in aerobic metabolism as well. Magnesium absence, therefore, may result in altered metabolism of glucose and increased lactate production (39). Hence, in clinical practice, the correction of concomitant magnesium deficiency is essential to thiamine utilization, although it is often overlooked (40, 41).
Regarding the nonenzymatic functions of vitamin B1, we can highlight its relevance in the nervous system and its interface with the immune system. In the nervous system, thiamine is related to the transmission of nerve impulses. Playing important roles in sodium permeability, TPP is also involved in maintaining negative charge on the inner surface of the cell membranes and facilitates neurotransmission by acting on the release of some neurotransmitters, notably acetylcholine (24). It was demonstrated that thiamine deficiency provoked a significant decrease in the voltage-dependent K+ membrane conductance of cerebellar neurons, mainly by suppressing an A-type K+ channel, which leads to important neuronal alterations (34) and a further reduction of nervous conduction velocity. In addition, TPP's role in fatty acid and NADPH synthesis is related to adequate neurologic functions (24).
In the immune system, thiamine has several functions in the regulation and activation of immune cells and proteins (16). The balance between glycolysis and Krebs cycle activities, where vitamin B1 is an important cofactor, is considered a determinant in controlling immune cell function, a concept referred to as immunometabolism (42). T-regulatory cells, resting macrophages and naïve T-cells generate energy mostly through the Krebs cycle, whereas activated macrophages, B-cells, Th1, Th2 and Th17 cells shift the balance toward aerobic glycolysis to complement energy from Krebs cycle (43).
Thiamine is also involved with hemin-dependent oxygenase, whose action affects the release of the specific members of the intracellular adhesion molecule (ICAM) proteins. ICAMs bind integrins during immunological reactions, affecting T-cell activity and other immune system cells (16). Vitamin B1 is important in immune system reactivity as well because it plays a pivotal part in the expression of immunoglobulins and, due to its antioxidative effects on neutrophils, by protecting the sulfhydryl groups on the cell surfaces from oxidation (16, 42). On macrophages, thiamine suppresses the oxidative stress-induced activation of the necrosis factor NF-kB, which induces the release of a variety of inflammatory markers such as cytokines, growth factors and immune-responsive proteins (44).
Given the crucial role of thiamine in the maintenance of metabolic functions and its supposed effect on the pathophysiology of mitochondrial dysfunction and microcirculatory changes that are characteristic of critical illness, it is essential to better understand the effectiveness of thiamine supplementation in patients with septic shock.
Single Thiamine Supplementation in Septic Shock
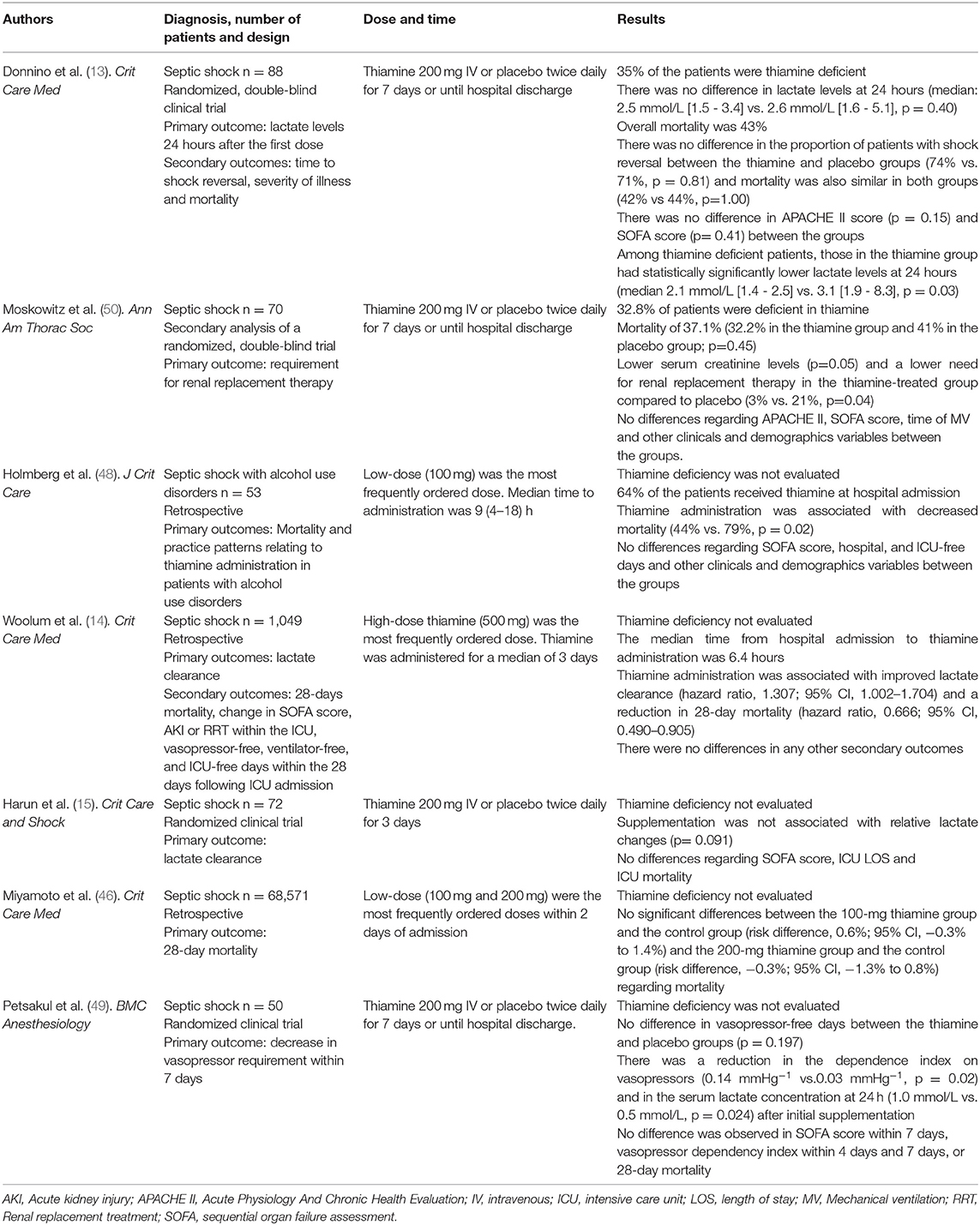
Several observational studies have explored the relationship between thiamine therapy and mortality rate in septic shock patients, with conflicting results (11, 14, 45–47). In a large retrospective matched cohort study, Woolum et al. (14) demonstrated that early intravenous (IV) thiamine supplementation (any dose between 100 and 500 mg) was associated with reduced 28-day mortality and improved lactate clearance. Additionally, lower mortality was also observed in septic shock patients with alcohol-use disorders who received thiamine within 48 h of sepsis onset (48).
In addition, a nationwide observational cohort study assessed the effect of thiamine administration on 28-day mortality in Japanese patients with septic shock over 7 years (46). The study included 68,571 patients, of which about 27% received 100 or 200 mg of IV thiamine within 2 days of admission. The findings of this large study did not support an association between early thiamine supplementation and a decrease in mortality. However, it is important to highlight the retrospective design of the study and the dose of thiamine used, which may be too low compared to the doses used in other studies (ranging from 200 to 500 mg daily).
To date, regardless of its relevance, only two randomized clinical trials have been published with IV thiamine supplementation as a single nutrient (13, 49). Donnino et al. (13) conducted the first randomized, double-blind, placebo-controlled trial. Patients with septic shock received 200 mg of IV thiamine twice daily or a matching placebo for 7 days or until hospital discharge. Contrary to expectations, supplementation was not effective in reducing serum lactate after the first 24 h of intervention. The prevalence of vitamin B1 deficiency among individuals was 35%, and supplementation was not associated with shock reversal time, disease severity or mortality. However, in the subset of patients with thiamine deficiency, lactate levels were lower after the first dose of supplementation (2.1 [1.4–2.5] mmol/L vs. 3.1 [1.9–8.3] mmol/L, p = 0.03), and a decrease in mortality was observed (2 [13%] vs. 6 [46%)], p = 0.047).
Despite this being a pioneering study, the results should be taken with caution. First, the effect of thiamine supplementation on lactate reduction and mortality was found in a small sample size (n = 28). Second, shock resuscitation treatment by itself would be able to reduce serum lactate. Third, some confounding variables such as illness severity and organ dysfunction scores among non-surviving patients who had baseline disabilities and did not receive supplementation are not known. Finally, lactate levels were assigned based on a predefined plan of patients who died before the 24-h period. Thus, an increase of 20%, 15% and 10% from baseline was imputed for patients who died before the 6-h, 6–12-h and 12–24-h point, respectively (6, 13).
Afterward, a secondary analysis of this trial was performed by Moskowitz et al. (50). The authors found significantly lower serum creatinine levels and a lower need for renal replacement therapy in the thiamine-treated group compared to the placebo group. As in the original trial (13) overall mortality was not different but raises the hypothesis of thiamine's role as a renal protective in septic shock.
More recently, another randomized clinical trial assessed the vasopressor-free days over 7 days in septic shock patients who received 200 mg of IV thiamine or placebo every 12 h for 7 days or until hospital discharge (49). On the one hand, thiamine supplementation was not associated with a reduction in the need for vasoactive drugs in the first week of ICU admission or with 28-day mortality. On the other hand, the study had interesting findings such as a reduction in vasopressor dependency index and in the serum lactate concentration 24 h after the initial supplementation. However, it is important to note that these findings are questionable due to the small sample size (n = 50) and the early stopping point, limiting the validity of the results. Table 1 describes the main studies that used single thiamine supplementation in patients with septic shock. Only two studies evaluated the concentration of vitamin B1 prior to its supplementation in patients with septic shock (13, 48). In addition, the studies involved patients with differences in sepsis severity, cause of infection, presence of risk factors and, when used, varied methods for assessing the vitamin concentration (11, 13–15, 46, 48–50). It is noteworthy that so far there are no reference values for detecting thiamine deficiency among septic patients. Therefore, it is essential to develop new studies that more assertively assess deficiency and establish specific reference values for septic patients to identify subgroups that can benefit more effectively from the treatment.
Studies with thiamine supplementation are still incipient, and therefore, it is essential to develop further clinical trials to conclusively determine the true role of thiamine in septic shock. Some problems related to the neutrality of these clinical trials are the study design, doses, route, timing, length of intervention and the choice of endpoints.
Recently, the concept that multi-micronutrient administration may be better than single-micronutrient administration has gained traction (12). In general, clinical trials consider the administration of a single micronutrient as a drug. However, the antioxidant defense is a complex system of endogenous agents in which micronutrients act as cofactors, and the physiological interactions between micronutrients are little discussed. Thus, current evidence does not support pharmacological use of single thiamine supplementation in septic shock patients, and future trials will probably focus on an early multi-micronutrient approach (12).
Combined Thiamine Supplementation on the Prognosis of Patients With Septic Shock
The combined supplementation of thiamine with ascorbic acid and corticosteroids was tested in a retrospective before–after clinical study developed by Marik et al. (51). Patients with severe sepsis or septic shock were treated with the administration of the combination of vitamin C, (1.5 g every 6 h), hydrocortisone (50 mg every 6 h), and thiamine (200 mg every 12 h) for 4 days. During the control period, patients with sepsis did not receive intravenous vitamin C or thiamine. Surprisingly, the treated group had a significantly lower mortality rate than the control group (8.5 vs. 40.4%, p < 0.001). Despite this tremendous potential as a treatment for sepsis, this study warrants caution in extrapolating its results because notable limitations were found. In sum, the study had a small sample size (n = 47 patients), had one single-center and lacked blinding and randomization.
The use of low-dose corticosteroids is recommended in patients with septic shock who do not respond to vasopressors. Its main benefit is its immune-stimulating effects, which may limit the anti-inflammatory immunosuppressive state (52). Although mortality reduction is seen mainly in patients receiving higher vasopressor doses, its supplementation improves other secondary outcomes, such as shock recovery and ICU length of stay (53, 54). In addition, glucocorticoids and vitamin C appear to act synergistically in protecting or reversing endothelial dysfunction (55, 56) and have become an extremely interesting target in this population. Vitamin C deficiency is highly prevalent among critically ill patients and is related to increased need for vasopressors, larger organ dysfunction, kidney injury and shorter survival (54, 57). Despite its potent antioxidant action, the IV administration of vitamin C in high doses and for a long period requires caution due to the risk of hyperoxaluria and pro-oxidant action (55, 58). Additionally, the correction of thiamine deficiency acts as a cofactor in the oxidation of glyoxylate by the enzyme glyoxylate aminotransferase (56, 59) and may help to attenuate oxidative stress and inflammation in animal models of sepsis (55, 60). Consequently, a deficiency of both vitamins concomitantly can aggravate the oxidative mitochondrial injury and bioenergetic failure present in septic shock (54).
Despite the rationale for combining these vitamins with corticosteroids, the improvement in mortality was not reproducible in recent trials (55, 61–63). Subsequently, in search of more promising outcomes, studies were carried out with the supplementation of thiamine in higher doses associated with ascorbic acid and hydrocortisone. Table 2 summarizes the main prospective and randomized studies published to date on the effect of combination therapy with ascorbic acid, hydrocortisone and thiamine in patients with septic shock. These combined therapy studies were also discussed in some systematic reviews (64–67).

Table 2. Randomized clinical trials evaluating thiamine supplementation as adjunctive therapy in septic shock.
To date, fewer than 10 randomized clinical studies have been published with combined thiamine supplementation in patients with sepsis and/or septic shock (38, 55, 61–63, 68, 69). The main outcomes involved organ dysfunction, time of need for vasopressor, ventilator-free days, development of acute kidney injury, lactate clearance, length of stay in the ICU and hospital mortality. Most studies did not observe any statistically significant difference regarding outcomes between treated individuals and the control group. Only in the study of Wani et al. (63) did the intervention group have shorter vasopressor use (96.13 ± 40.50 h vs. 75.72 ± 30.29 h, p = 0.010) and greater lactate clearance (41.81 vs. 56.83%, p = 0.031) compared to the control. It is noteworthy that the study in question has limitations, including being open-label, having a small sample size and having a high prevalence of antimicrobial resistance and mortality.
If the lack of beneficial effects of supplementation on the outcomes is evident, the assertiveness in the design of the work brings some questions. Between studies, doses are generally similar (vitamin C: 1,500 mg; hydrocortisone: 50 mg; thiamine: 100 mg, every 6 h); however, the administration time is highly variable (48 h, 4–10 days or until discharged from the ICU). Even with a more diverse design, the ATESS trial (68) did not include the administration of hydrocortisone in the treated group, and the interval for vitamin administration was longer (12 vs. 6 h), while the duration of treatment was shorter (48 vs. 96 h or more) compared to previous studies.
It is important to note that hydrocortisone monotherapy in patients with septic shock is associated with faster resolution of shock (70) and lower mortality (71) when compared with placebo groups. In this sense, the VITAMINS trial (61) compared IV thiamine combined with vitamin C and hydrocortisone with hydrocortisone administration alone and found no improvement on mortality or time free from vasopressors up to day 7, suggesting no synergic effect between them as previously postulated.
Still, for clinical practice, some questions still need to be clarified about the combined therapy for adjuvant management of septic shock. The current literature cannot sufficiently support use of single thiamine or combined administration outside of randomized controlled trials because most of the studies were interrupted early (69), a large number of patients were screened but not randomized (38), abdominal infections and tumors were highly prevalent (68) and the studies had mixed designs.
However, these efforts have unquestionably advanced care of septic shock patients. At the moment, it has been established that single thiamine administration or thiamine administration combined with vitamin C and hydrocortisone has a good safety profile with no adverse events, even at high doses. Consequently, studies are needed to fill the knowledge gaps regarding vitamin B1 supplementation as adjuvant therapy in septic shock.
Conclusion
Studies with thiamine supplementation in septic shock have notable differences in design, including dosage and time of supplementation; small sample sizes; and different septic phenotypes. Despite the excellent safety profile, good biologic rationale and promising clinical studies, no robust results support routine thiamine supplementation to improve outcomes. However, future trials should focus on combined multi-micronutrient therapy, higher doses, and early administration, which might be the key to improving mitochondrial function and reducing oxidative stress during hemodynamic resuscitation in patients with septic shock.
Author Contributions
NC, AP, and LG: conceptualization, methodology, and writing—original draft. CS and NV: revision of the original draft. PA, SP, and LZ: writing—review and editing. BP and MM: writing—original draft and writing—review and editing and supervision. All authors revised the article critically for important intellectual content and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlanet DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Preiser JC, Ichai C, Orban JC, Groeneveld ABJ. Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54. doi: 10.1093/bja/aeu187
4. Wesselink E, Koekkoek WAC, Grefte S, Witkamp RF, van Zanten ARH. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin Nutr. (2019) 38:982–95. doi: 10.1016/j.clnu.2018.08.032
5. Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. (2011) 107:57–64. doi: 10.1093/bja/aer093
6. Costa NA, Azevedo PS, Polegato BF, Zornoff LAM, Paiva SAR, Minicucci MF. Thiamine as a metabolic resuscitator in septic shock: one size does not fit all. J Thorac Dis. (2016) 8:E471–72. doi: 10.21037/jtd.2016.04.29
7. Williams AC, Sturman S, Kelsey S, Curran FT, Finnegan JA. The neuropathy of the critically ill. Br Med J (Clin Res Ed). (1986) 293:790–91. doi: 10.1136/bmj.293.6550.790-a
8. Anderson SH, Charles TJ, Nicol AD. Thiamine deficiency at a district general hospital: report of five cases. Q J Med. (1985) 55:15–32.
9. Chapman DH. The neuropathy of the critically ill. Br Med J (Clin Res Ed). (1986) 293:1102. doi: 10.1136/bmj.293.6554.1102-b
10. Cruickshank AM, Telfer AB, Shenkin A. Thiamine deficiency in the critically ill. Intensive Care Med. (1988) 14:384–87. doi: 10.1007/BF00262893
11. Costa NA, Gut AL, de Souza Dorna M, Pimentel JA, Cozzolino SM, Azevedo PS, et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care. (2014) 29:249–52. doi: 10.1016/j.jcrc.2013.12.004
12. Berger MM, Manzanares W. Micronutrients early in critical illness, selective or generous, enteral or intravenous? Curr Opin Clin Nutr Metab Care. (2021) 24:165–75. doi: 10.1097/MCO.0000000000000724
13. Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. (2016) 44:360–67. doi: 10.1097/CCM.0000000000001572
14. Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. (2018) 46:1747–52. doi: 10.1097/CCM.0000000000003311
15. Harun NF, Cheah SK, Yusof A, Lau C, Masdar A, Mahdi SNM, et al. Intravenous thiamine as an adjuvant therapy for hyperlactatemia in septic shock patients. Crit Care Shock. (2019) 22:288–98.
16. Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. (2014) 53:821–35. doi: 10.1021/bi401618y
17. Ott M, Werneke U. Wernicke's encephalopathy - from basic science to clinical practice. Part 1: Understanding the role of thiamine. Ther Adv Psychopharmacol. (2020) 10:1–24. doi: 10.1177/2045125320978106
18. Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, et al. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. (2007) 75:229–34. doi: 10.1016/j.resuscitation.2007.03.021
19. Minicucci MF, Zornoff LAM, Matsue M, Inoue RMT, Matsubara LS, Okoshi MP, et al. Generalized edema and hyperdynamic circulation. A possible case of beriberi. Arq Bras Cardiol. (2004) 83:176–78. doi: 10.1590/s0066-782x2004001400009
20. Coelho LS, Hueb JC, Minicucci MF, Azevedo PS, Paiva SAR, Zornoff LAM. Thiamin deficiency as a cause of reversible cor pulmonale. Arq Bras Cardiol. (2008) 91:e7–9. doi: 10.1590/s0066-782x2008001300013
21. Polegato BF, Pereira AG, Azevedo PS, Costa NA, Zornoff LAM, Paiva SAR, et al. Role of thiamin in health and disease. Nutr Clin Pract. (2019) 34:558–64. doi: 10.1002/ncp.10234
22. Jurgenson CT, Begley TP, Ealick SE. The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem. (2009) 78:569–603. doi: 10.1146/annurev.biochem.78.072407.102340
23. Sambon M, Wins P, Bettendorff L. Neuroprotective effects of thiamine and precursors with higher bioavailability: focus on benfotiamine and dibenzoylthiamine. Int J Mol Sci. (2021) 22:5418. doi: 10.3390/ijms22115418
24. Frank LL. Thiamin in clinical practice. JPEN J Parenter Enteral Nutr. (2015) 39:503–20. doi: 10.1177/0148607114565245
25. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. (2018) 356:382–90. doi: 10.1016/j.amjms.2018.06.015
26. Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care. (2011) 14:610–17. doi: 10.1097/MCO.0b013e32834b8911
27. Gomes F, Bergeron G, Bourassa MW, Fischer PR. Thiamine deficiency unrelated to alcohol consumption in high-income countries: a literature review. Ann N Y Acad Sci. (2021) 1498:46–56. doi: 10.1111/nyas.14569
28. McLaughlin K, Joyal K, Lee S, Corrado M, Marquis K, Anger K, et al. Safety of intravenous push thiamine administration at a tertiary academic medical center. J Am Pharm Assoc. (2020) 60:598–601. doi: 10.1016/j.japh.2019.12.005
29. Whitfield KC, Bourassa MW, Adamolekun B, Bergeron G, Bettendorff L, Brown KH, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. (2018) 1430:3–43. doi: 10.1111/nyas.13919
30. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US); 1998. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK114310/ (accessed December 4, 2021).
31. Juel J, Pareek M, Langfrits CS, Jensen SE. Anaphylactic shock and cardiac arrest caused by thiamine infusion. BMJ Case Rep. (2013) 2013:bcr2013009648. doi: 10.1136/bcr-2013-009648
32. Leite HP, de Lima LFP, Taddei JAAC, Paes ÂT. Effect of blood thiamine concentrations on mortality: Influence of nutritional status. Nutrition. (2018) 48:105–10. doi: 10.1016/j.nut.2017.11.020
33. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. (2014) 44:911–15. doi: 10.1111/imj.12522
34. Bâ A. Metabolic and structural role of thiamine in nervous tissues. Cell Mol Neurobiol. (2008) 28:923–31. doi: 10.1007/s10571-008-9297-7
35. Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, Mynak ML, et al. Thiamine deficiency disorders: a clinical perspective. Ann N Y Acad Sci. (2021) 1498:9–28. doi: 10.1111/nyas.14536
36. Starling-Soares B, Carrera-Bastos P, Bettendorff L. Role of the Synthetic B1 Vitamin Sulbutiamine on Health. J Nutr Metab. (2020) 2020:9349063. doi: 10.1155/2020/9349063
37. Aleshin VA, Mkrtchyan GV, Bunik VI. Mechanisms of non-coenzyme action of thiamine: protein targets and medical significance. Biochemistry (Mosc). (2019) 84:829–50. doi: 10.1134/S0006297919080017
38. Moskowitz A, Huang DT, Hou PC, Gong J, Doshi PB, Grossestreuer AV, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. (2020) 324:642–50. doi: 10.1001/jama.2020.11946
39. Maguire D, Catchpole A, Sheerins O, Talwar D, Burns A, Blythet M, et al. The relation between acute changes in the systemic inflammatory response and circulating thiamine and magnesium concentrations after elective knee arthroplasty. Sci Rep. (2021) 11:11271. doi: 10.1038/s41598-021-90591-y
40. Lonsdale D. Thiamine and magnesium deficiencies: keys to disease. Med Hypotheses. (2015) 84:129–34. doi: 10.1016/j.mehy.2014.12.004
41. Maguire D, McMillan D, Stefanowicz F, Burns A, Ross D, Talwar D. The effect of thiamine and/or magnesium supplementation on thiamine, magnesium, lactate and erythrocyte transketolase activity in patients presenting to the emergency department with alcohol withdrawal syndrome. Clin Nutr. (2018) 37:S18. doi: 10.1016/j.clnu.2018.06.1128
42. Peterson CT, Rodionov DA, Osterman AL, Peterson SN. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients. (2020) 12:E3380. doi: 10.3390/nu12113380
43. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic Instruction of Immunity. Cell. (2017) 169:570–86. doi: 10.1016/j.cell.2017.04.004
44. Yadav UCS, Kalariya NM, Srivastava SK, Ramana KV. Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic Biol Med. (2010) 48:1423–34. doi: 10.1016/j.freeradbiomed.2010.02.031
45. Kanchanasurakit S, Suthumpoung P, Santimaleeworagun W, Nakaranurack C, Huynh NS, Srisawat C, et al. Effectiveness of thiamine therapy in mortality rate in patients with septic shock: A systematic review and meta-analysis. Int J Crit Illn Inj Sci. (2021) 11:86–94. doi: 10.4103/IJCIIS.IJCIIS_159_20
46. Miyamoto Y, Aso S, Iwagami M, Yasunaga H, Matsui H, Fushimi K, et al. Association between IV thiamine and mortality in patients with septic shock: a nationwide observational study. Crit Care Med. (2020) 48:1135–39. doi: 10.1097/CCM.0000000000004394
47. Olanipekun TO, Bakinde N, Effoe VS, Snyder R. Further considerations on the benefits of thiamine administration in patients with septic shock. Crit Care Med. (2019) 47:e153–54. doi: 10.1097/CCM.0000000000003450
48. Holmberg MJ, Moskowitz A, Patel PV, Grossestreuer AV, Uber A, Stankovic N, et al. Thiamine in septic shock patients with alcohol use disorders: An observational pilot study. J Crit Care. (2018) 43:61–4. doi: 10.1016/j.jcrc.2017.08.022
49. Petsakul S, Morakul S, Tangsujaritvijit V, Kunawut P, Singhatas P, Sanguanwit P. Effects of thiamine on vasopressor requirements in patients with septic shock: a prospective randomized controlled trial. BMC Anesthesiol. (2020) 20:280. doi: 10.1186/s12871-020-01195-4
50. Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock. A secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc. (2017) 14:737–41. doi: 10.1513/AnnalsATS.201608-656BC
51. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. doi: 10.1016/j.chest.2016.11.036
52. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. (2017) 45:486–552. doi: 10.1097/CCM.0000000000002255
53. Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. (2003) 167:512–20. doi: 10.1164/rccm.200205-446OC
54. Marik PE. Hydrocortisone, ascorbic acid and thiamine (HAT Therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients. (2018) 10:E1762. doi: 10.3390/nu10111762
55. Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: The ORANGES trial. Chest. (2020) 158:164–73. doi: 10.1016/j.chest.2020.02.049
56. Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest. (2017) 152:954–62. doi: 10.1016/j.chest.2017.07.014
57. de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, Spoelstra-de Man AME, Girbes AR, Swart EL, et al. Vitamin C pharmacokinetics in critically ill patients: a randomized trial of four IV regimens. Chest. (2018) 153:1368–77. doi: 10.1016/j.chest.2018.02.025
58. Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther. (2018) 189:63–70. doi: 10.1016/j.pharmthera.2018.04.007
59. Dalimonte MA, DeGrado JR, Anger KE. Vasoactive agents for adult septic shock: an update and review. J Pharm Pract. (2020) 33:523–32. doi: 10.1177/0897190019844124
60. de Andrade JAA, Gayer CRM, Nogueira NPA, Paes MC, Bastos VLFC, Neto JDCB, et al. The effect of thiamine deficiency on inflammation, oxidative stress and cellular migration in an experimental model of sepsis. J Inflamm (Lond). (2014) 11:11. doi: 10.1186/1476-9255-11-11
61. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the vitamins randomized clinical trial. JAMA. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
62. Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, et al. Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. (2020) 158:174–82. doi: 10.1016/j.chest.2020.02.065
63. Wani SJ, Mufti SA, Jan RA, Shah SU, Qadri SM, Khan UH, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Lond). (2020) 52:271–78. doi: 10.1080/23744235.2020.1718200
64. Somagutta MKR, Pormento MKL, Khan MA, Hamdan A, Hange N, Kc M, et al. The Efficacy of vitamin C, thiamine, and corticosteroid therapy in adult sepsis patients: a systematic review and meta-analysis. Acute Crit Care. (2021) 36:185–200. doi: 10.4266/acc.2021.00108
65. Lee YR, Vo K, Varughese JT. Benefits of combination therapy of hydrocortisone, ascorbic acid and thiamine in sepsis and septic shock: A systematic review. Nutr Health. (2021) 26:2601060211018371. doi: 10.1177/02601060211018371
66. Ge Z, Huang J, Liu Y, Xiang J, Gao Y, Walline JH, et al. Thiamine combined with vitamin C in sepsis or septic shock: a systematic review and meta-analysis. Eur J Emerg Med. (2021) 28:189–95. doi: 10.1097/MEJ.0000000000000812
67. Zayed Y, Alzghoul BN, Banifadel M, Venigandla H, Hyde R, Sutchu S, et al. Vitamin C, thiamine, and hydrocortisone in the treatment of sepsis: a meta-analysis and trial sequential analysis of randomized controlled trials. J Intensive Care Med. (2021) 29:885066620987809. doi: 10.1177/0885066620987809
68. Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, Suh GJ, et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. (2020) 46:2015–25. doi: 10.1007/s00134-020-06191-3
69. Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA. (2021) 325:742–50. doi: 10.1001/jama.2020.24505
70. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. (2018) 378:797–808. doi: 10.1056/NEJMoa1705835
Keywords: thiamine supplementation, thiamine deficiency, septic shock, vitamin B1, mitochondrial dysfunction
Citation: Costa NA, Pereira AG, Sugizaki CSA, Vieira NM, Garcia LR, Paiva SARd, Zornoff LAM, Azevedo PS, Polegato BF and Minicucci MF (2022) Insights Into Thiamine Supplementation in Patients With Septic Shock. Front. Med. 8:805199. doi: 10.3389/fmed.2021.805199
Received: 30 October 2021; Accepted: 31 December 2021;
Published: 28 January 2022.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesCopyright © 2022 Costa, Pereira, Sugizaki, Vieira, Garcia, Paiva, Zornoff, Azevedo, Polegato and Minicucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcos Ferreira Minicucci, marcos.minicucci@unesp.br
 Nara Aline Costa
Nara Aline Costa Amanda Gomes Pereira
Amanda Gomes Pereira Clara Sandra Araujo Sugizaki
Clara Sandra Araujo Sugizaki Nayane Maria Vieira2
Nayane Maria Vieira2  Leonardo Antonio Mamede Zornoff
Leonardo Antonio Mamede Zornoff Paula Schmidt Azevedo
Paula Schmidt Azevedo Bertha Furlan Polegato
Bertha Furlan Polegato Marcos Ferreira Minicucci
Marcos Ferreira Minicucci