Long-Term Effect of Early Post-operative Transcutaneous Electrical Stimulation on Voiding Function After Radical Hysterectomy: A Multicenter, Randomized, Controlled Trial
- 1Department of OB/Gyn, Peking University People's Hospital, Beijing, China
- 2Beijing Key Laboratory of Female Pelvic Floor Disorders, Beijing, China
- 3Department of OB/Gyn, Beijing Hospital, Beijing, China
- 4Department of OB/Gyn, Sheng-Jing Hospital of China Medical University, Shenyang, China
- 5Department of OB/Gyn, Peking University First Hospital, Beijing, China
- 6Department of OB/Gyn, Peking University Third Hospital, Beijing, China
- 7Department of OB/Gyn, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China
- 8Department of OB/Gyn, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 9Department of OB/Gyn, Gansu Provincial Maternal and Child Health Hospital, Lanzhou, China
- 10Department of OB/Gyn, Peking University Shen-zhen Hospital, Beijing, China
- 11Department of Medicine, Peking University Clinical Research Institute, Beijing, China
- 12Department of Medicine, Peking University Medical Informatics Center, Beijing, China
Introduction: Post-radical-hysterectomy (RH) patients suffer from a series of problems resulting from neurovascular injury, such as bladder dysfunction, which reduce their quality of life. We have designed this study to evaluate the efficacy of transcutaneous electrical stimulation (TENS) on patient rehabilitation after RH for early cervical cancer.
Materials and methods: A total of 97 patients were enrolled in a randomized-controlled trial (from January 2015 to December 2019) involving 7 medical centers nationwide. Patients were assigned to either the intervention group (n = 46), or the control group (n = 51). TENS was given to patients in the intervention group from the 7th day after surgery for a total of 14–21 days. The control group received no TENS. Primary outcomes were measured for residual urine volume and recovery of urination function. Secondary outcomes were measures for urodynamics (UDS), pelvic floor electromyography function examination (PFEmF), and quality of life (QoL).
Results: Residual urine volume and improvement in the rate of urination were found to show no significant differences on the 14th, 21st, and 28th days after surgery. The maximum flow rate (Qmax) in the intervention group was significantly higher than that in the control group on the 28th day, but there were no significant differences in average flow rate, voiding time, time to Qmax, muscle fiber strength, muscle fiber fatigue, and the abnormal rate of A3 reflection on the 28th day and the 3rd mo., as well as in the QoL at 3rd mo., 6th mo., and 12th mo. after surgery.
Conclusion: Our study showed no sufficient evidence to prove that TENS under the trialed parameters could improve the subject's voiding function, PFEmF, and QOL after RH. This has provided valuable data for rehabilitation after RH.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT02492542.
Introduction
Cervical cancer, with almost 0.6 million new cases per year, is globally the 4th most common cancer among women. Approximately 106,000 cases of cervical cancer occurred in China in 2018 (1). Radical hysterectomy (RH), with bilateral pelvic lymphadenectomy, is currently the gold standard surgical treatment for early-stage cervical cancer. It is the definitive therapy, and associated with an excellent prognosis for most patients (2, 3).
However, many patients may suffer from decreased Qol due to the symptoms following RH. It is reported that 70%-85% of cervical cancer patients undergoing RH had De novo bladder symptoms (4, 5), 76% of them developed lower urinary tract symptoms (LUTS) in the 12 months after surgery (6). The main reason for post-treatment bladder dysfunction is neurovascular injuries including direct cuts, stretching, and thermal injury. Marloes's (7) study indicated that patients undergoing more radical surgery had more significantly urinary dysfunction.
In clinical practice, providers have tried many methods to prevent and manage urinary retention (UR) in patients undergoing RH, among which post-operative bladder training in the early stages was the most commonly applied. However, Fanfani's randomized trial showed that bladder training did not reduce the rate of UR or re-admission for bladder catheterization (8). Some Chinese doctors reported a potential efficacy when using traditional acupuncture to treat UR after RH (9), but due to limited sample size and poor study design in those studies, more studies are needed to prove its effects.
Electrical stimulation (ES) has been used to treat bladder disorders for many years, especially for UR and incontinence, and its efficacy is promising (10, 11). TENS was the most widely used type of ES. Our previous study (12) showed that TENS could cure acute RH in rats by angiogenesis and nerve fiber regeneration in the detrusor and urethral sphincter, and also increased the expression of collagen. It was reported that ES could promote post-operative recovery of bladder function in prostate cancer (13–17), though there have also been some unsupportive findings (18–21). As the complexity is similar to radical prostatectomy, the same questions also exist in regard to ES treatment for cervical cancer patients undergoing RH.
There are a few studies (22) focused on ES-related post-operative rehabilitation of cervical cancer patients that showed that TENS was effective in preventing UR after RH. Since those studies were not prospective randomized control, we designed this prospective, multi-center, randomized-controlled trial (RCT) to investigate if TENS can improve the voiding function after RH with early cervical cancer.
In this study, UDS was performed post-operatively to evaluate lower urinary tract dysfunction, this method being the gold standard to investigate that particular pathology, while the free flow study was essential in the evaluation of voiding patterns and the characteristics of the emptying phase (23), represented by the Qmax, average flow rate, voiding time, and the time to Qmax. The basic electrophysiological indicators of pelvic floor muscle, which mainly comprise strength, duration, and fatigue of the muscle contraction, were used as an evaluated index for pelvic floor dysfunction (24).
Materials and Methods
Having been registered at www.clinicaltrials.gov (Identifier: NCT02492542), the RCT was conducted from January 2015 to December 2019. The research protocol was designed based on a methodology published by Xiu-li Sun, etc. in 2017 (25) and reviewed and approved by the ethics boards of the following seven Hospitals: Peking University People's Hospital, Peking University First Hospital, Peking University Third Hospital, Beijing Obstetrics and Gynecology Hospital of Capital Medical University, Beijing Chao-yang Hospital of Capital Medical University, Beijing Hospital, Sheng-Jing Hospital of China Medical University, Peking University Shen-zhen Hospital and Gansu Provincial Maternal and Child Health Hospital.
Participants and Randomization
Participants of this study were recruited from the nine hospitals. Patients would be enrolled for analysis if they matched the following inclusive criteria: (1) 18–60 years of age; (2) clinically diagnosed as cervical squamous cell carcinoma and staged according to International Federation of Gynecology and Obstetrics Standards [FIGO 2009] as Ia2, Ib1, and IIa1; (3) had underwent RH according to Piver III classification, with a non-nerve-sparing technique; (4) did not have pelvic nodes, margins, and/or the involvement of any vascular/lymphatic spaces; (5) evidenced to have stromal invasion <1/2; (6) histologically graded as 1–2; and (7) willing to provide informed consent. Consenting patients would be excluded if they met any of the following exclusive criteria: (1) had undergone adjuvant chemotherapy/radiotherapy before or after their RH operation; (2) had had nerve-preserving surgery performed; (3) were suspected or confirmed to have urinary system injury; (4) were staged as POP-Q I or higher; (5) had evidenced moderate or severe SUI (Pad test ≥ 10 g); (6) had urinary retention before surgery; (7) experienced severe constipation or difficulty in defecation before surgery; and (8) had investigator-judged uncontrollable epilepsy/central nervous system disease/mental disorder.
Based on statistical calculations, 208 patients must be enrolled to make this study comprehensive enough to achieve a meaningful and representative conclusion. Eligible patients were randomized into the intervention and control groups with a ratio of 1:1 generated by a dynamic randomization system, which was conducted by Peking University Clinical Research Institute, an independent trial administration office. Randomization of the enrolled patients was also conducted with varying block sizes stratified by menopausal status (menopause vs. Non-menopause) and surgical modality (laparoscopic RH or abdominal RH). Participants and investigators were not blinded to the intervention assignments because of the device stimulation and blank control.
However, the study was terminated in June 2019 since the interim analysis on the data of 97 patients showed that the intervention group was not showing significant benefits over the control group, and this data was enough to demonstrate the study hypothesis. It was decided to terminate the study under consideration of the ethical perspective and for the well-being of the research subjects.
A total of 97 patients were enrolled and randomized (46 patients in the intervention group and 51 in the control group) by the termination of the study, of which three withdrew from the study immediately after signing informed consent (1 from the intervention group and 2 from the control group). According to the ITT principle, 94 subjects (45 in the intervention group and 49 in the control group) were included in the final statistical analysis.
Research Quality Control
Quality control on research procedures was conducted by assuring that: (1) All patients were recruited from gynecological oncology departments and all the principal practitioners conducting the operations were selected senior gynecological oncologists who had been trained for the uniform surgical procedures; (2) follow-up observation was carried out by specialist personnel, who was specifically assigned and trained on the standardized procedure; and (3) Peking University Clinical Research Institute conducted regular supervision.
Intervention and Control
Patients in the intervention group would receive 30 min TENS twice a day for a total of 14 days (28 sessions) from the 7th day after the operation. The urethral catheter would be removed on the 14th day post the operation. Those who failed to urinate by themselves or had difficulty in fully emptying their bladder (with residual urine more than 100 ml) would receive TENS for additional 7 days.
The TENS parameters used were 1/4/1 Hz of frequency and 270/230/270 μs of pulse width, which were proven to be effective for urinary retention in clinical practices. The intensity of stimulation would be adjusted on a session-by-session basis according to the individual, being applied at the highest threshold that patient could tolerate. The stimulatory electrode was placed on the S3 region and the neutral electrode was placed on the projected position of the bladder on the surface of the body.
Subjects in the control group would receive only routine clinical care during the trial stage. With the exception of the TENS, the patients in the control group would undergo the same study procedures as the intervention group throughout the entire study. The urethral catheter would be removed on the 14th d after the surgery. The catheter would be inserted again and kept for another 7 days if any patient failed to empty their bladder of residual urine. The same procedures would be repeated until the residual urine retention became <100 ml.
Data Collection and Follow-Up
Enrolled patients were followed up by the 21st d, 28th d, 3rd mo., 6th mo., 12th mo., 18th mo., and 24th mo. after the operations. Residual urine was evaluated via ultrasound by the 14th d, 21st d, and 28th d post the operation. UDS parameters were assessed by the 28th d and 3rd mo. post-operatively. QoL was evaluated by the European Quality of Life-5 Dimensions (EQ-5D-5L) (26), Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) (27), Pelvic Floor Distress Inventory (PFDI-20) (28), and International Consultations on Incontinence Questionnaire (ICIQ) (29) by the 3rd mo., 6th mo., 12th mo., and 24th mo. after the surgery, except PISQ-12 by the 3rd mo. During the follow-ups, data created from laboratory testing, pelvic examination, chest X-ray examination, and adverse event reports were collected and assessed. The trial profile was shown in Figure 1.
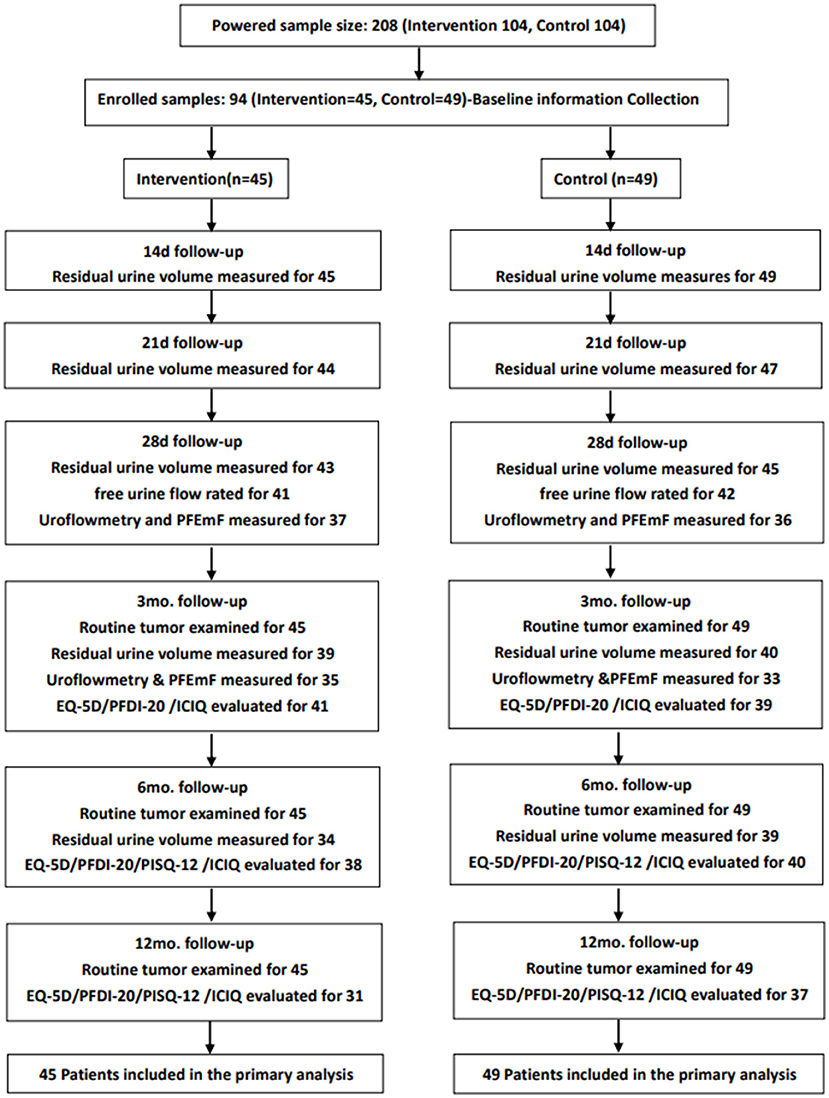
Figure 1. The trial profile of the study: Routine tumor examination, baseline information, gynecological examination (Human Papillomavirus and Thinprep Cytological Testing), tumor markers (squamous cell carcinoma antigen, CA125, CA199, carcinoembryonic antigen), CT or MRI examination, chest radiograph, etc.
Study Outcomes
Primary Outcomes
The primary outcomes were measured for residual urine volume and recovery of urination function. The recovery of urination function was classified into three levels as: (1) recovered, which refers to the achievement of patient's automatic micturition with residual urine ≤50 ml; (2) improved, which refers to the achievement of patient's automatic micturition with residual urine 50–100 ml; (3) invalidated, which refers to the patient's situation wherein automatic micturition is not achieved, or is achieved but residual urine is ≥100 ml.
Secondary Outcomes
The secondary outcomes were evaluated from the following aspects:
(1) UDS parameters: including the Qmax, average flow rate, voiding time, and the time to Qmax.
(2) PFEmF: The strengths and fatigue of the type I and II muscle fibers, the abnormal rate of A3 reflection.
(3) QoL: evaluated by EQ-5D-5L, PISQ-12, PFDI-20, and ICIQ.
(4) Adverse events: skin damage, skin allergies, local pain, etc.
Statistical Analyses
Statistical analysis was performed using SPSS 19.0. Data are expressed as means ± SD or median ± interquartile. Quantitative data were compared using group t-test or Wilcoxon rank-sum test, and qualitative data was analyzed by χ2 test or Fisher exact probability method.
The residual urine volume and the decrease in residual urine volume were evaluated using the Wilcoxon rank-sum test and repeated measures analysis of variance. Wilcoxon rank-sum test and the generalized estimation equation were used to compare the improvement rate of urination, considering the central effect, the Cochran-Mantel-Haenzsel (CMH) test was used to compare the improvement in rate of urination in the 28th d after the operation. UDS parameters, PFEmF, scores of PISQ-12, PFDI-20, and ICIQ were analyzed using the Mann-Whitney U test. Scores of EQ-5D-5L were analyzed by Mann-Whitney U test and χ2 test or Fisher exact probability method. The level of significance was set to 0.05.
Results
Participants Characteristics
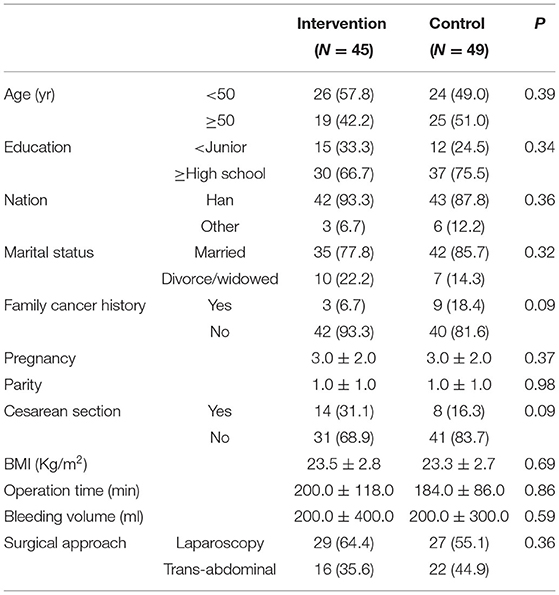
In analyzing the data of the 94 enrolled patients, we found no significant differences between the 2 groups in baseline and clinical characteristics (Table 1), indicating that the subjects in the two groups were comparable.
Lower Urinary Tract Function
The Residual Urine Volume
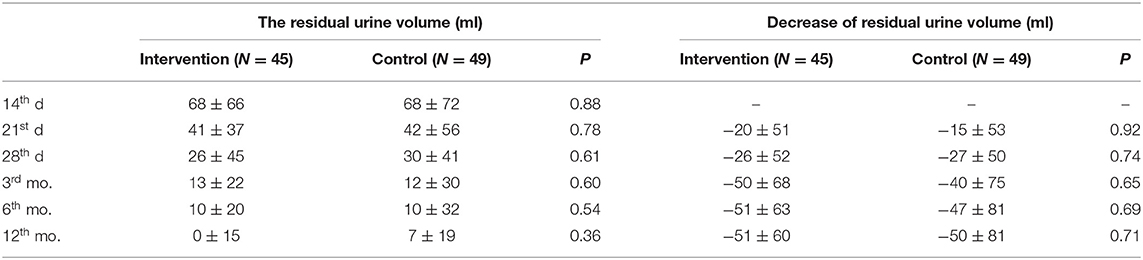
The results of the analysis showed that there was no significant difference in the residual urine volume at the time of each follow-up between the two groups, there was no significant difference between the two groups in the decrease of residual urine volume compared with the baseline either (Table 2). Repeated measures analysis of variance showed that there was no significant difference in the interaction between groups and time (P = 0.75).
The Recovery of Urination Function
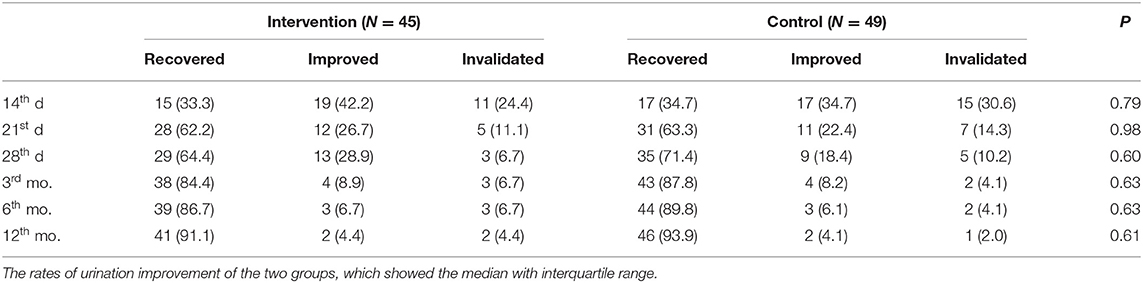
There was no significant difference between the two groups in the recovery of urination function as shown in Table 3. The generalized estimation equation showed that there was no significant difference in the interaction between groups and time (P = 0.57). There was no significant difference between the two groups in the 28th d by CMH either (P = 0.91, OR = 1.249, 95% CI: 0.650, 5.883).
UDS Parameters
Although the Qmax of the intervention group was significantly higher than that in the control group by the 28th d (15.7 ± 9.6 vs. 10.45 ± 9.6 ml/s, P = 0.02), there was no significant difference between the two groups in the Qmax by the 3rd mo. (19 ± 11.2 vs. 13 ± 8.2 ml/s, P = 0.02), as well as in average flow rates (8.1 ± 9.2 vs. 5.55 ± 5.6 ml/s, P = 0.07) (9.5 ± 6 vs. 7.7 ± 4.2 ml/s, P = 0.22), voiding time (34.05 ± 33 vs. 41 ± 44.9 s, P = 0.62) (34.9 ± 27.5 vs. 35.95 ± 30.85 s, P = 0.97), and time to Qmax (11.5 ± 13.1 vs. 14.1 ± 22.9 s, P = 0.71) (8.1 ± 9 vs. 10.95 ± 12.75 s, P = 0.15) by the 28th d and the 3rd mo.
Pelvic Floor Function on Electromyography
There were no significant differences between the two groups in the strengths of the type I muscle fibers (2 ± 3.5 vs. 3 ± 5, P = 1.00) (5 ± 2 vs. 5 ± 4, P = 0.99), the strengths of the type II muscle fibers (3 ± 3 vs. 1 ± 5, P = 0.16) (−1 ± 2 vs. −1 ± 2, P = 0.88), the fatigue of the type I muscle fibers (−1 ± 3.5 vs. −2 ± 3, P = 0.93) (−1 ± 2 vs. −1 ± 2, P = 0.88), the fatigue of the type II muscle fibers (0 ± 1 vs. 0 ± 1, P = 0.80) (0 ± 0 vs. 0 ± 1, P = 0.35), as well as the abnormal rate of A3 reflection (13.9 vs. 22.2%, P = 0.36) (20.7 vs. 8.3%, P = 0.15) by the 28th d and the 3rd mo.
Quality of Life Questionnaire
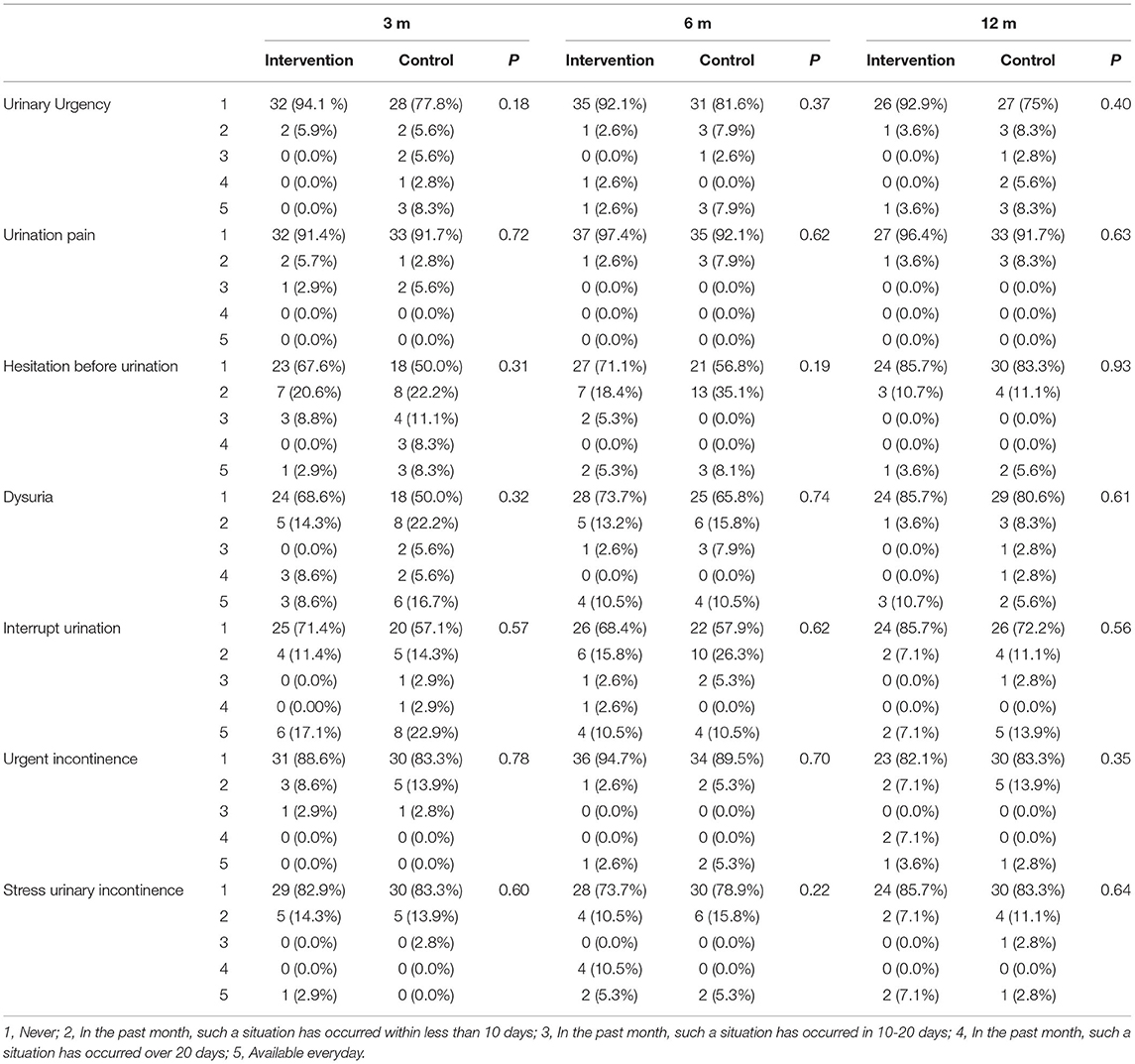
There were no statistically significant differences between the two groups in LUTS based on the ICIQ questionnaire (Table 4).
In EQ-5D-5L, state 1 represents no problem, whereas state 5 represents extreme problems. In all dimensions by the 3rd mo., 6th mo., and 12th mo. follow-ups, the percentage of state 1 in the intervention group was higher than that in the control group, except for pain/discomfort by the 3rd mo. and mobility by the 6th mo. The intervention group was significantly better than the control one in usual activities by the 3rd mo. (P = 0.02), anxiety/depression by the 12th mo. (P = 0.03), and EQ-5D score by the 12th mo. (P = 0.01). However, statistical analysis showed no significant difference in the other EQ-5D-5L scores. The PISQ-12 scores and PFDI-20 scores in the intervention group were lower than that in the control one but had no significant difference (Table 5).
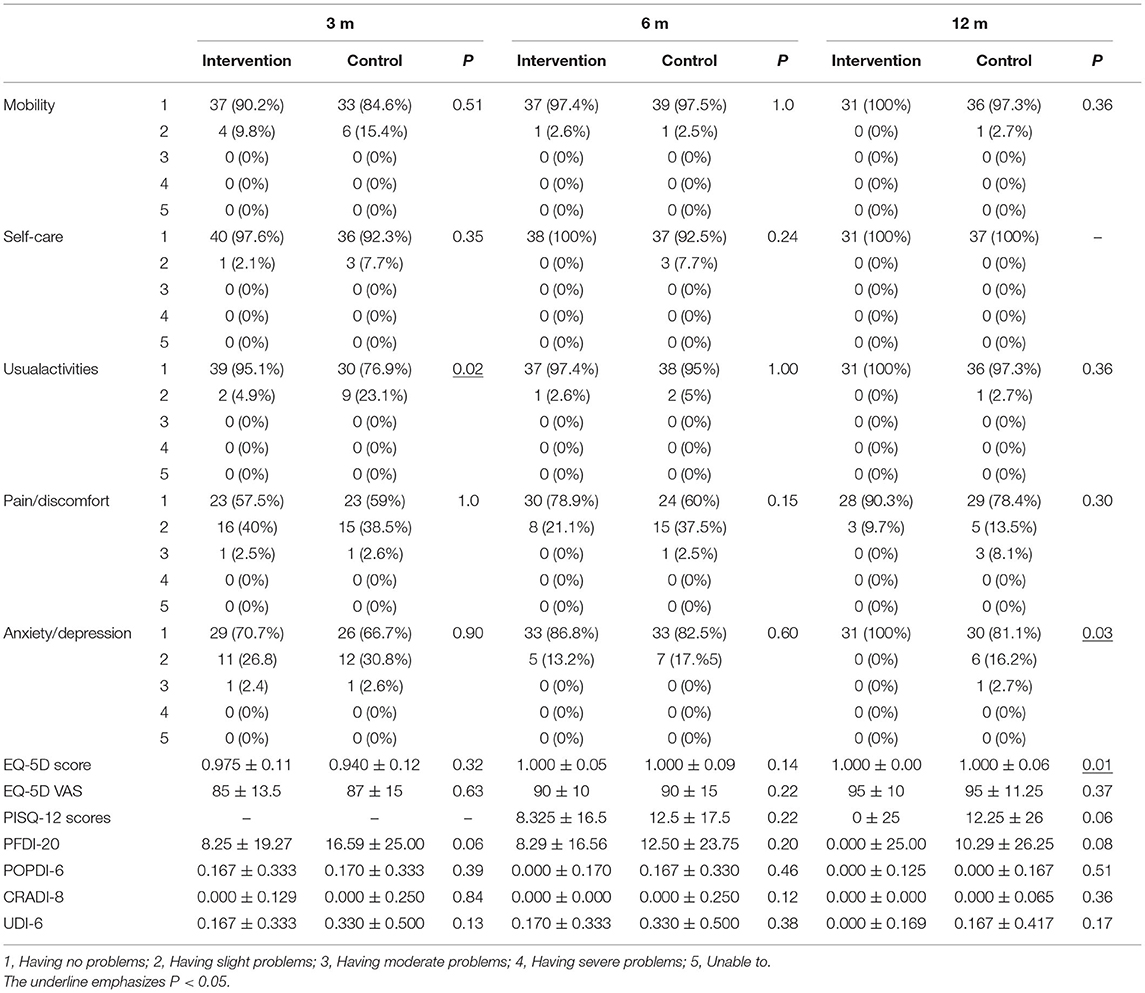
Table 5. The QoL-questionnaire scores between the two groups at 3rd mo., 6th mo., and 12th mo. after operation.
Discussion
Our results demonstrated that TENS could increase the Qmax in the early post-operative period. However, TENS did not make a significant difference in the improvement of residual urine volume and the urination function and did not have an effect on PFEmF or QoL, indicating that TENS cannot work to improve those post-operative problems.
The mean duration of post-operative catheterization varies in different literatures. In Roh's study, the median period for obtaining a PVR volume of <50 ml among patients from the conventional RH group was 18 days, while that in the nerve-sparing RH group was 11 days (30). In another study, 14 days on average was necessary to achieve a PVR volume of <50 ml after nerve-sparing RH (31). In China, the duration of catheterization is usually between 10 and 14 days after the surgery (32). In our research, we removed the catheter 14 days after the operations.
Chuang's research showed that the residual volume of urine tended to increase at 2 and 6 weeks after RH, the mean and maximal flow rates both showed reduction at 2 weeks, 6 weeks, and 3 mos. after surgery (33). This may be attributed to the surgery impairing the parasympathetic motor innervation in maintaining detrusor contractility. In a neuro-urological study, transient neurological changes were observed after RH: pudendal nerve motor latency was prolonged in the 2 and 6 week checks, but returned to baseline levels in the 3 mos (34). Our study also shows that the application of TENS made no significant difference in residual urine volume and the recovery of urination function, although it increased the Qmax by the 28th day. This result suggests that recovery of urination function may be related to the time that has passed allowing for post-operative recovery, which is a natural process, but not to the early TENS.
In Huan Li's study (22), 91 patients diagnosed with stage IA2–IB2 cervical cancer, and treated with RH were enrolled and randomized into two groups. The results showed that low-frequency electrical stimulation was more effective than conventional intervention in preventing urinary retention after RH, and it also intensified the recovery of pelvic muscle strength. A prospective random control trial by Yang (35) demonstrated that sacral and transcranial magnetic stimulation improved pelvic floor dysfunction and QoL of gynecological cancer patients. Hwang's (36) research suggested that transcutaneous electrical stimulation (TES) training resulted in a beneficial effect on sexual function in women with stress urinary incontinence, which was evidenced by the significant differences in pelvic floor muscle strength, power, endurance, and Female Sexual Function Index domain scores in both between-group analyses (TES vs. control group) and within-group analyses (pre-TES vs. post-TSE). Research by Yamanishi (16) showed that electrical stimulation could significantly improve the QOL of patients after radical prostatectomy.
However, Laurienzo (20) reported an unsupportive result based on their research, in which they investigated the effect of electrical stimulation and pelvic floor muscle training on muscle strength, urinary incontinence, and erectile function in men with prostate cancer treated by radical prostatectomy and concluded that the muscle strength recovery occurs independently of the therapy employed; electrical stimulation also did not have an impact on the recovery of urinary continence and erectile function. Marloes (7) found that, although some patients were undergoing more radical surgery, their QoL was not different, suggesting that the surgery itself had few effects on QoL, so the effect of TENS on QoL could not be important. In our study, TENS did not make a significant difference in improvement for residual urine volume and the urination function, and showed no effect on PFEmF or QoL.
Although the exact mechanism of action has yet to be fully understood, TENS was believed to restore the balance between excitation and inhibition in bladder function by modulating the signal traffic to and from the bladder through the sacral plexus. Electrical parameters such as the stimulation frequency, intensity, number, and duration of stimulation sessions were highly variable among studies, which reinforces that there was not a universally established regimen for it. Generally, the frequency of TENS ranges from 2 to 75 Hz (16, 17, 19–21, 37–39). Electrical stimulation with low frequency (2–50 HZ) evoked bladder contraction, resulting in increasing voiding efficiency (39). PFM can be activated with frequencies between 35 and 40 Hz, while the effects at 5–10 Hz spread also to the detrusor muscle (40). Huan Li's study compared the treatment frequency of 35 Hz and 1 Hz, and showed that 1 Hz is more effective (22). The stimulation sites of peripheral nerves were different, such as the sacral nerve roots, tibial nerve, pudendal nerve, and dorsal genital nerves. Electrodes could be placed adjacent to the sacral region (S3), tibial region, clitoral or penile region, or into the vagina or rectum. The sacral or tibial region was likely the most logical site since it directly or indirectly targets the medullar root S3, and the latter was often be preferred as a minimally invasive modality due to the poor tolerability of intra-vaginal and intra-anal electrodes resulting from pain or discomfort (37, 41–43). In this study, frequencies of 1/4/1 Hz was selected, and the electrode site was placed in the S3 region.
The indicators observed in this study are related to urination function, PFM, QoL, etc. While the frequency of TENS for different indicators may be different, so the results may be different if the treatment frequency was changed. The treatment period of this study was 2–3 weeks, also, the results may be changed when the treatment time was extended. TENS parameters need to be explored continuously.
Conclusions
In summary, our study showed no sufficient evidence to prove that TENS under the trialed parameters could improve the subject's voiding function, pelvic floor muscle strength, and QoL after RH, which has provided valuable data for rehabilitation after RH.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by 2015PHB050-04. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
X-lS and J-lW conceived the study and drafted the study design. X-wL, LG, and QW helped with implementation. X-wL wrote the manuscript. J-lW helped to revise the manuscript. H-bW designed the statistical analysis of the study and undertook power calculation. Others are responsible for recruiting patients, performing surgery, collecting data and completing follow-up. YL is responsible for the establishment of medical database. All authors approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (nos. 2018YFC2002204), Clinical Trials.gov (NCT02492542) on June 25, 2015 and Chinese Preventive Medicine Association (nos. 2020-Z-23).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks for the study participants' great effort and the funding body.
References
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 2:e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. Nama V, Angelopoulos G, Twigg J, Murdoch JB, Bailey J, Lawrie TA. Type II or type III radical hysterectomy compared to chemoradiotherapy as a primary intervention for stage IB2 cervical cancer. Cochrane Database Syst Rev. (2018) 10:CD011478. doi: 10.1002/14651858.CD011478.pub2
3. Leath CA III, Monk BJ. Twenty-first century cervical cancer management: a historical perspective of the gynecologic oncology group/NRG oncology over the past twenty years. Gynecol Oncol. (2018) 3:391–7. doi: 10.1016/j.ygyno.2018.06.023
4. Scotti RJ, Bergman A, Bhatia NN, Ostergard DR. Urodynamic changes in urethrovesical function after radical hysterectomy. Obstet Gynecol. (1986) 1:111–20.
5. Low JA, Mauger GM, Carmichael JA. The effect of Wertheim hysterectomy upon bladder and urethral function. Am J Obstet Gynecol. (1981) 7:826–34. doi: 10.1016/0002-9378(81)90551-2
6. Benedetti-Panici P, Zullo MA, Plotti F, Manci N, Muzii L, Angioli R. Long-term bladder function in patients with locally advanced cervical carcinoma treated with neoadjuvant chemotherapy and type 3-4 radical hysterectomy. Cancer. (2004) 10:2110–7. doi: 10.1002/cncr.20235
7. Derks M, van der Velden J, Frijstein MM, Vermeer WM, Stiggelbout AM, Roovers JP, et al. Long-term pelvic floor function and quality of life after radical surgery for cervical cancer: a multicenter comparison between different techniques for radical hysterectomy with pelvic lymphadenectomy. Int J Gynecol Cancer. (2016) 8:1538–43. doi: 10.1097/IGC.0000000000000776
8. Fanfani F, Costantini B, Mascilini F, Vizzielli G, Gallotta V, Vigliotta M, et al. Early postoperative bladder training in patients submitted to radical hysterectomy: is it still necessary? A randomized trial. Arch Gynecol Obstet. (2015) 4:883–8. doi: 10.1007/s00404-014-3500-5
9. Yi WM, Pan AZ, Li JJ, Luo DF, Huang QH. Clinical observation on the acupuncture treatment in patients with urinary retention after radical hysterectomy. Chin J Integr Med. (2011) 11:860–3. doi: 10.1007/s11655-011-0800-5
10. Monga AK, Tracey MR, Subbaroyan J. A systematic review of clinical studies of electrical stimulation for treatment of lower urinary tract dysfunction. Int Urogynecol J. (2012) 8:993–1005. doi: 10.1007/s00192-012-1691-5
11. McGee MJ, Grill WM. Selective co-stimulation of pudendal afferents enhances bladder activation and improves voiding efficiency. Neurourol Urodyn. (2014) 8:1272–8. doi: 10.1002/nau.22474
12. Cao T, Xie B, Yang S, Wang J, Yang X, Shen B, et al. Low-frequency intravesical electrical stimulation for the treatment of acute urinary retention: a promising therapeutic approach. Front Med. (2021) 8:572846. doi: 10.3389/fmed.2021.572846
13. Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled. Lancet. (2000) 8:98–102. doi: 10.1016/S0140-6736(99)03473-X
14. Mariotti G, Sciarra A, Gentilucci A, Salciccia S, Alfarone A, Di Pierro G, et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electrical stimulation and biofeedback associated treatment. J Urol. (2009) 4:1787–93. doi: 10.1016/j.juro.2008.11.104
15. Mariotti G, Salciccia S, Innocenzi M, Gentilucci A, Fasulo A, Gentile V, et al. Recovery of urinary continence after radical prostatectomy using early vs late pelvic floor electrical stimulation and biofeedback-associated treatment. Urology. (2015) 1:115–20. doi: 10.1016/j.urology.2015.02.064
16. Yamanishi T, Mizuno T, Watanabe M, Honda M, Yoshida K. Randomized, placebo controlled study of electrical stimulation with pelvic floor muscle training for severe urinary incontinence after radical prostatectomy. J Urol. (2010) 5:2007–12. doi: 10.1016/j.juro.2010.06.103
17. Radziszewski K. Outcomes of electrical stimulation of the neurogenic bladder: results of a two-year follow-up study. Neuro Rehabil. (2013) 4:867–73. doi: 10.3233/NRE-130911
18. Moore KN, Griffiths D, Hughton A. Urinary incontinence after radical prostatectomy: a randomized controlled trial comparing pelvic muscle exercises with or without electrical stimulation. BJU Int. (1999) 1:57–65. doi: 10.1046/j.1464-410x.1999.00894.x
19. Wille S, Sobottka A, Heidenreich A, Hofmann R. Pelvic floor exercises, electrical stimulation and biofeedback after radical prostatectomy: results of a prospective randomized trial. J Urol. (2003) 8:490–3. doi: 10.1097/01.ju.0000076141.33973.75
20. Laurienzo CE, Magnabosco WJ, Jabur F, Faria EF, Gameiro MO, Sarri AJ, et al. Pelvic floor muscle training and electrical stimulation as rehabilitation after radical prostatectomy: a randomized controlled trial. J Phys Ther Sci. (2018) 6:825–31. doi: 10.1589/jpts.30.825
21. Zhu YP, Yao XD, Zhang SL, Dai B, Ye DW. Pelvic floor electrical stimulation for post prostatectomy urinary incontinence: a meta-analysis. Urology. (2012) 3:552–5. doi: 10.1016/j.urology.2011.10.005
22. Li H, Zhou CK, Song J, Zhang WY, Wang SM, Gu YL, et al. Curative efficacy of low frequency electrical stimulation in preventing urinary retention after cervical cancer operation. World J Surg Oncol. (2019) 1:141. doi: 10.1186/s12957-019-1689-2
23. Writing group of the International Urogynecological Association. IUGA report on reporting urodynamics in women. Int Urogynecol J. (2021). doi: 10.1007/s00192-021-04742-w. [Epub ahead of print]
24. Wang S, Wang R, Wen H, Gao Y, Lv Q, Li H, et al. Association of pelvic floor function with postoperative urinary incontinence in cervical cancer patients after the radical hysterectomy. Neurourol Urodyn. (2021)1:483–92. doi: 10.1002/nau.24587
25. Sun XL, Wang HB, Wang ZQ, Cao TT, Yang X, Han JS, et al. Effect of transcutaneous electrical stimulation treatment on lower urinary tract symptoms after class III radical hysterectomy in cervical cancer patients: study protocol for a multicentre, randomized controlled trial. BMC Cancer. (2017) 1:416. doi: 10.1186/s12885-017-3387-1
26. Luo N, Liu G, Li M, Guan H, Jin X, Rand-Hendriksen K. Estimating an EQ-5D-5L value set for China. Value Health. (2017) 4:662–9. doi: 10.1016/j.jval.2016.11.016
27. Zhu L, Yu S, Xu T, Yang X, Lu Y, Lang J. Validation of the Chinese version of the pelvic organ prolapse/urinary incontinence sexual questionnaire short form (PISQ-12). Int J Gynecol Obstetr. (2012) 2:117–9. doi: 10.1016/j.ijgo.2011.08.021
28. Ma Y, Xu T, Zhang Y, Mao M, Kang J, Zhu L. Validation of the Chinese version of the Pelvic Floor Distress Inventory-20 (PFDI-20) according to the COSMIN checklist. Int Urogynecol J. (2019) 7:1127–39. doi: 10.1007/s00192-018-3847-4
29. Huang W, Wang Q, Chen J, Wu P. Development and validation of the International Consultation on Incontinence Modular Questionnaire for Male Lower Urinary Tract Symptoms (ICIQ-MLUTS) and the ICIQ-MLUTS Long Form in Chinese population. Low Urin Tract Symptoms. (2019)4:189–94. doi: 10.1111/luts.12260
30. Roh JW, Lee DO, Suh DH, Lim MC, Seo SS, Chung J, et al. Efficacy and oncologic safety of nerve-sparing radical hysterectomy for cervical cancer: a randomized controlled trial. J Gynecol Oncol. (2015) 2:90–9. doi: 10.3802/jgo.2015.26.2.90
31. Fujii S, Takakura K, Matsumura N, Higuchi T, Yura S, Mandai M, et al. Anatomic identification and functional outcomes of the nerve sparing Okabayashi radical hysterectomy. Gynecol Oncol. (2007) 1:4–13. doi: 10.1016/j.ygyno.2007.08.076
32. Gong Y, Zhao L, Wang L, Wang F. The effect of clamping the indwelling urinary catheter before removal in cervical cancer patients after radical hysterectomy. J Clin Nurs. (2017) 7–8:1131–6. doi: 10.1111/jocn.13579
33. Chuang TY, Yu KJ, Penn IW, Chang YC, Lin PH, Tsai YA. Neurourological changes before and after radical hysterectomy in patients with cervical cancer. Acta Obstet Gynecol Scand. (2003) 10:954–9. doi: 10.1034/j.1600-0412.2003.00177.x
34. Laterza RM, Sievert KD, de Ridder D, Vierhout ME, Haab F, Cardozo L, et al. Bladder function after radical hysterectomy for cervical cancer. Neurourol Urodyn. (2015) 4:309–15. doi: 10.1002/nau.22570
35. Yang EJ, Lim JY, Rah UW, Kim YB. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol. (2012) 3:705–11. doi: 10.1016/j.ygyno.2012.03.045
36. Hwang UJ, Lee MS, Jung SH, Ahn SH, Kwon OY. Pelvic floor muscle parameters affect sexual function after 8 weeks of transcutaneous electrical stimulation in women with stress urinary incontinence. Sex Med. (2019) 4:505–13. doi: 10.1016/j.esxm.2019.08.011
37. Gross T, Schneider MP, Bachmann LM, Blok BF, Groen J, Hoen LA, et al. Transcutaneous electrical nerve stimulation for treating neurogenic lower urinary tract dysfunction: a systematic review. Eur Urol. (2016) 6:1102–11. doi: 10.1016/j.eururo.2016.01.010
38. Tornic J, Liechti MD, Stalder SA, Birkhäuser V, van der Lely S, Leitner L, et al. Transcutaneous tibial nerve stimulation for treating neurogenic lower urinary tract dysfunction: a pilot study for an international multicenter randomized controlled trial. Eur Urol Focus. (2020) 5:909–15. doi: 10.1016/j.euf.2019.11.008
39. Grill WM. Electrical stimulation for control of bladder function. In: Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. Minneapolis, MN: IEEE (2009). p. 2369–70. doi: 10.1109/IEMBS.2009.5335001
40. La Rosa VL, Platania A, Ciebiera M, Garzon S, Jedra R, Ponta M, et al. A comparison of sacral neuromodulation vs. transvaginal electrical stimulation for the treatment of refractory overactive bladder: the impact on quality of life, body image, sexual function, and emotional well-being. Prz Menopauzalny. (2019) 2:89–93. doi: 10.5114/pm.2019.86834
41. Lee YH, Creasey GH. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyper reflexia in incomplete spinal cord injury: a case report. Arch Phys Med Rehabil. (2002) 2:273–7. doi: 10.1053/apmr.2002.28817
42. Padilha JF, Avila MA, Seidel EJ, Driusso P. Different electrode positioning for transcutaneous electrical nerve stimulation in the treatment of urgency in women: a study protocol for a randomized controlled clinical trial. Trials. (2020) 1:166. doi: 10.1186/s13063-020-4096-7
43. Shendy WS, El Semary MM, Battecha KH, Abdel-Azim MS, Mourad HS, El Gohary AM, et al. Efficacy of transcutaneous electrical nerve stimulation versus biofeedback training on bladder and erectile dysfunction in patients with spinal cord injury. Egypt J Neurol Psychiatry Neurosurg. (2015) 3:194–200. doi: 10.4103/1110-1083.162044
Keywords: pelvic floor electromyography function, examination, quality of life, radical hysterectomy, transcutaneous electrical stimulation, voiding function
Citation: Li X-w, Gao L, Wang Q, Lv Q-b, Xia Z-j, Wen H-w, Han J-s, Wu Y-m, Wang S-m, Liu Q, Li H, Wang H-b, Li Y, Wang S-y, Wang Z-q, Sun X-l and Wang J-l (2021) Long-Term Effect of Early Post-operative Transcutaneous Electrical Stimulation on Voiding Function After Radical Hysterectomy: A Multicenter, Randomized, Controlled Trial. Front. Med. 8:677029. doi: 10.3389/fmed.2021.677029
Received: 07 March 2021; Accepted: 12 August 2021;
Published: 30 September 2021.
Edited by:
Andrea Rosati, Agostino Gemelli University Polyclinic, ItalyReviewed by:
Matteo Pavone, Agostino Gemelli University Polyclinic, ItalyMatteo Loverro, Agostino Gemelli University Polyclinic, Italy
Riccardo Tudisco, Catholic University of the Sacred Heart, Rome, Italy
Copyright © 2021 Li, Gao, Wang, Lv, Xia, Wen, Han, Wu, Wang, Liu, Li, Wang, Li, Wang, Wang, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu-li Sun, sunxiuli918@126.com; Jian-liu Wang, wangjianliu1203@163.com
 Xiao-wei Li1,2
Xiao-wei Li1,2  Lei Gao
Lei Gao Yu-mei Wu
Yu-mei Wu Hai-bo Wang
Hai-bo Wang Zhi-qi Wang
Zhi-qi Wang Xiu-li Sun
Xiu-li Sun