Quality of and Recommendations for Relevant Clinical Practice Guidelines for COVID-19 Management: A Systematic Review and Critical Appraisal
- 1Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Evidence-Based Medicine and Clinical Epidemiology, Second Clinical College, Wuhan University, Wuhan, China
- 3School of Health Sciences, Wuhan University, Wuhan, China
- 4Precision Medicine Center, Second People's Hospital of Huaihua, Huaihua, China
- 5Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Background: The morbidity and mortality of coronavirus disease 2019 (COVID-19) are still increasing. This study aimed to assess the quality of relevant COVID-19 clinical practice guidelines (CPGs) and to compare the similarities and differences between recommendations.
Methods: A comprehensive search was conducted using electronic databases (PubMed, Embase, and Web of Science) and representative guidelines repositories from December 1, 2019, to August 11, 2020 (updated to April 5, 2021), to obtain eligible CPGs. The Appraisal of Guidelines for Research and Evaluation (AGREE II) tool was used to evaluate the quality of CPGs. Four authors extracted relevant information and completed data extraction forms. All data were analyzed using R version 3.6.0 software.
Results: In total, 39 CPGs were identified and the quality was not encouragingly high. The median score (interquartile range, IQR) of every domain from AGREE II for evidence-based CPGs (EB-CPGs) versus (vs.) consensus-based CPG (CB-CPGs) was 81.94% (75.00–84.72) vs. 58.33% (52.78–68.06) in scope and purpose, 59.72% (38.89–75.00) vs. 36.11% (33.33–36.11) in stakeholder involvement, 64.58% (32.29–71.88) vs. 22.92% (16.67–26.56) in rigor of development, 75.00% (52.78–86.81) vs. 52.78% (50.00–63.89) in clarity of presentation, 40.63% (22.40–62.50) vs. 20.83% (13.54–25.00) in applicability, and 58.33% (50.00–100.00) vs. 50.00% (50.00–77.08) in editorial independence, respectively. The methodological quality of EB-CPGs were significantly superior to the CB-CPGs in the majority of domains (P < 0.05). There was no agreement on diagnosis criteria of COVID-19. But a few guidelines show Remdesivir may be beneficial for the patients, hydroxychloroquine +/– azithromycin may not, and there were more consistent suggestions regarding discharge management. For instance, after discharge, isolation management and health status monitoring may be continued.
Conclusions: In general, the methodological quality of EB-CPGs is greater than CB-CPGs. However, it is still required to be further improved. Besides, the consistency of COVID-19 recommendations on topics such as diagnosis criteria is different. Of them, hydroxychloroquine +/– azithromycin may be not beneficial to treat patients with COVID-19, but remdesivir may be a favorable risk-benefit in severe COVID-19 infection; isolation management and health status monitoring after discharge may be still necessary. Chemoprophylaxis, including SARS-CoV 2 vaccines and antiviral drugs of COVID-19, still require more trials to confirm this.
Introduction
The morbidity and mortality associated with coronavirus disease 2019 (COVID-19) are still increasing at present. According to the official website of World Health Organization (WHO), by 10 April 2021, there have been 134,308,070 confirmed cases of COVID-19, including 2,907,944 deaths worldwide (1). Containing the spread poses a challenge because of the rising number of infected people with high mortality and the highly contagious nature of COVID-19. Clinical practice guidelines (CPGs) have been defined as “statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and a risk-benefit assessment of alternative care options” (2), and they play an important role in guiding clinical decisions about prevention, diagnosis, treatment and care. Some professional association, guideline development groups have issued successively COVID-19 management guidelines.
Previous reviews have also concentrated on methodological quality and recommendations for COVID-19 guidelines, but these have covered a narrow range of topics (3–6). The methods and reporting quality of practice guidelines for five different viruses causing public health emergencies of international concern, including the severe acute respiratory syndrome coronavirus 2, tended to be low, particularly in stakeholder involvement and applicability. There was also poor quality of recommendations for the use of antiviral drugs such as lopinavir-ritonavir, convalescent plasma, and intravenous immunoglobulins. Reverse transcription-polymerase chain reaction (RT-PCR) and Computed tomography (CT) were the most common diagnostic methods for COVID-19. Besides, there was no effective treatment against COVID-19; supportive therapy (mainly rest in bed, ensuring adequate calories, maintaining water-electrolyte balance, oxygen therapy, etc.) is the most significant treatment plan. Live evidence related to COVID-19 is still appearing on a daily basis, and live recommendations on chemoprophylaxis, diagnosis, and antiviral therapy are also being continuously updated. As for discharged patients, a small proportion of patients experienced reappearance of a positive test for SARS-CoV-2 during convalescence (7–9). As the number of cured patients increases, criteria for discharge management is also an important issue.
Thus, this review, based on a comprehensive literature search, has been conducted to compare the variations in recommendations within prophylaxis, diagnosis, antiviral treatment, and discharge management of COVID-19 and to assess their methodological quality. We aim to provide relatively more reliable suggestions for decision-making bodies regarding possible health problems to satisfy the needs of the public, providing guidance for government departments and COVID-19 prevention and control institutions.
Methods
The review was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (10).
Search Strategy
We searched PubMed, Embase, and Web of Science. Additionally, eight representative guideline repositories were searched: World Health Organization (WHO), National Institute for Health and Care Excellence (NICE), Guidelines International Network (GIN), National Institutes of Health (NIH), Scottish Intercollegiate Guidelines Network (SIGN), Association of American Medical Colleges (AAMC), ECRI Guideline Trust, and Biochemical Genetic and Genomic (BIGG). A list of the websites with COVID-19 guidelines is presented in Supplementary Table 1. The search dates were from December 1, 2019, to August 11, 2020 (updated to April 5, 2021). The key words mainly included “severe acute respiratory syndrome coronavirus 2 or SARS-COV-2 or COVID-19 or COVID19 or 2019 coronavirus or 2019 novel coronavirus or 2019-nCoV or Novel coronavirus pneumonia or NCP or coronavirus disease-19 or coronavirus disease 2019” AND “guideline or guidance or recommendation or clinical practice guideline or consensus.” MeSH terms were used to search Title/Abstract. Furthermore, taking PubMed as an example, the retrieval strategy is shown in Supplementary Figure 1.
Guidelines Identification
All guidelines related to COVID-19 published in English were included if they met the following criteria: (1) explicit recommendations on COVID-19 management (Which kind of agent can prevent COVID-19? Which strategy can be used to diagnose COVID-19 and identify and risk stratify patients with suspected or confirmed COVID-19? Which drugs can be used to treat patients with COVID-19? What are the discharge criteria for COVID-19, and what indicators are there for follow-up attention after discharge?); (2) evidence-based clinical practice guidelines (EB-CPGs) or consensus-based guidelines (CB-CPGs); and (3) updated versions of CPGs if multiple versions of the guidelines exists. To determine the eligible guidelines, EB-CPGs were defined as having recommendations based on a systematic literature search and literature quality assessment or grade for evidence and recommendation; CB-CPGs were defined as having recommendations developed by multidisciplinary experts (such as frontline clinicians) based on their experience or the existing literature using a consensus method rather than a systematic review.
We excluded (1) translated versions, interpretations, and summaries of existing CPGs; (2) regional or hospital protocols for COVID-19; and (3) CPGs without full text access.
Data Extraction
Four reviewers independently extracted the details of the guidelines relevant to their characteristics using a standardized data collection form. Extracted data included guideline title, date of publication, publication country/region, guideline developers, target population, development method (evidence-based or consensus-based), topic, funding, and the related recommendations. Another reviewer examined the data extraction forms to make sure no errors had occurred. Disagreements were resolved by consensus.
Methodological Quality Appraisal
Two reviewers independently evaluated the quality of each included guideline using the widely accepted CPG assessment tool—AGREE II, which is composed of 23 items within 6 domains including “scope and purpose,” “stakeholder involvement,” “rigor of development,” “clarity of presentation,” “applicability,” and “editorial independence” (11, 12). Details of each domain are shown in Supplementary Table 2. Each item was scored from 1 (strongly disagree) to 7 (strongly agree). We calculated each domain score for every eligible CPG individually using the following formula provided by the AGREE II tool: (obtained score–minimal possible score)/(maximal possible score–minimal possible score) × 100% (11).
Guideline Recommendations Synthesis
We performed a textual descriptive synthesis to analyze eligible CPGs from four aspects: chemoprophylaxis; diagnosis; antiviral therapy; and discharge management.
Statistical Analysis
Descriptive statistical analyses were performed. Data for each AGREE II domain of every included CPG were presented as medians and interquartile ranges (IQRs). The scores of EB-CPGs and CB-CPGs in each domain were compared using Wilcoxon Rank-Sum Test. A P < 0.05 was regarded as significance. Intraclass correlation coefficients (ICCs) with a 95% confidence interval (CI) were calculated to evaluate the agreement among two assessors for each domain. The degree of agreement between 0.00 and 0.40 was considered poor, 0.41 to 0.75 was good, and 0.75 to 1.00 was excellent (13). All the data were analyzed using R version 3.6.0 software (The R Foundation for Statistical Computing, Vienna, Austria) for Windows.
Results
Guidelines Identification and Selection
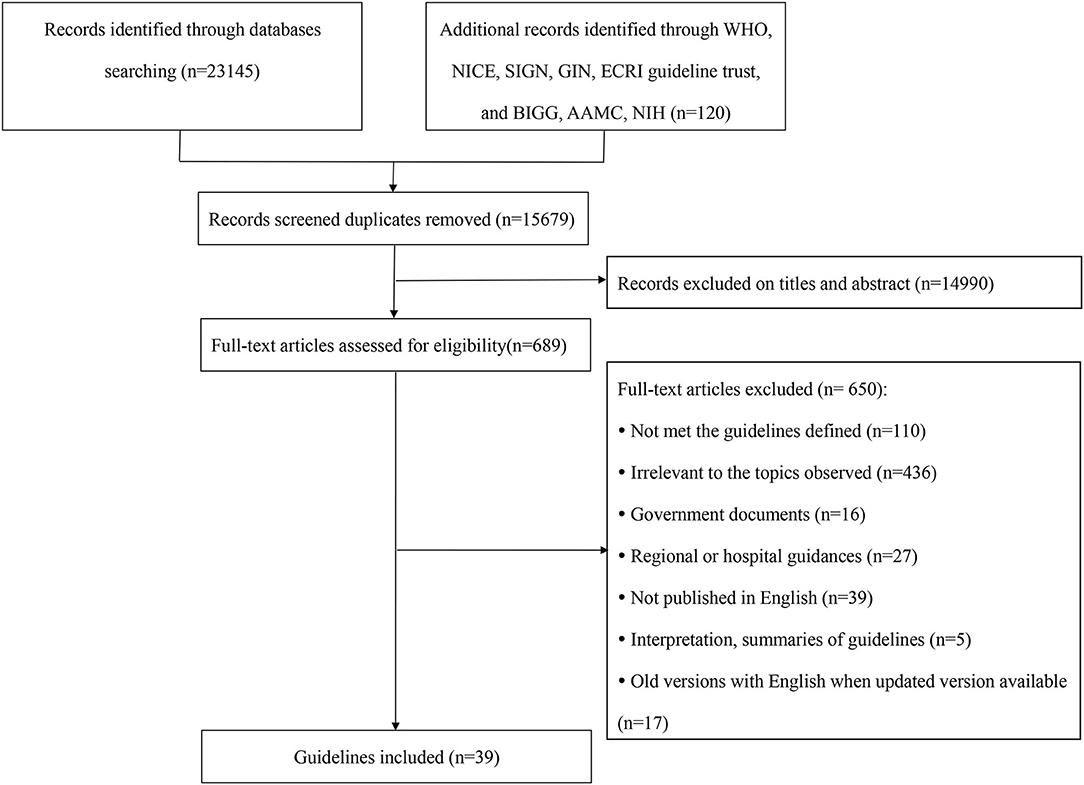
Figure 1 presents the flow chart of guidelines identification, and 39 CPGs were eventually included (14–52).
Characteristics of Included Guidelines
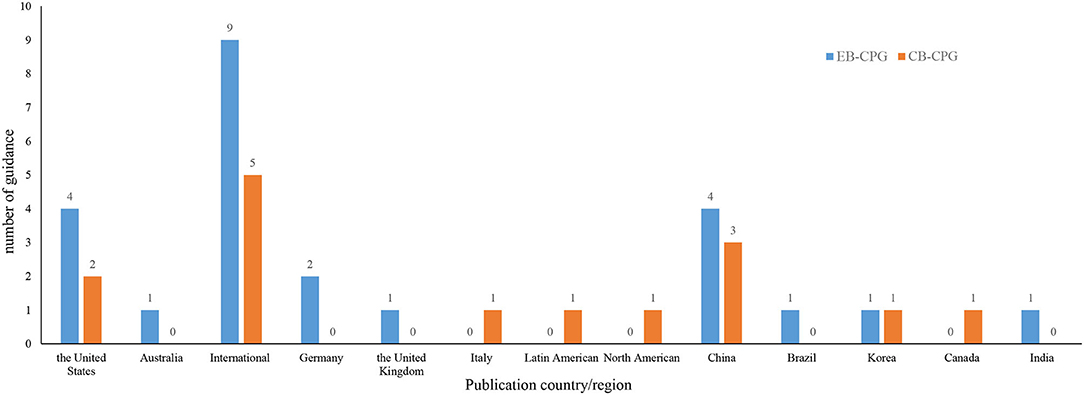
As Supplementary Table 3 shows, the guidelines were published from February 6, 2020, to April 5, 2021. Of them, 15 guidelines were CB-CPGs and 24 were EB-CPGs, and 15 received funding support. Among the recommendations in these 39 CPGs, 8 were on chemoprophylaxis, 18 on diagnosis, 1 on identification and triage of patients with COVID-19, 25 on antiviral drugs, and 6 on discharge. The guidelines were mainly developed by the United States, China, or other international organization or cooperation (See Figure 2).
Guidelines' Quality
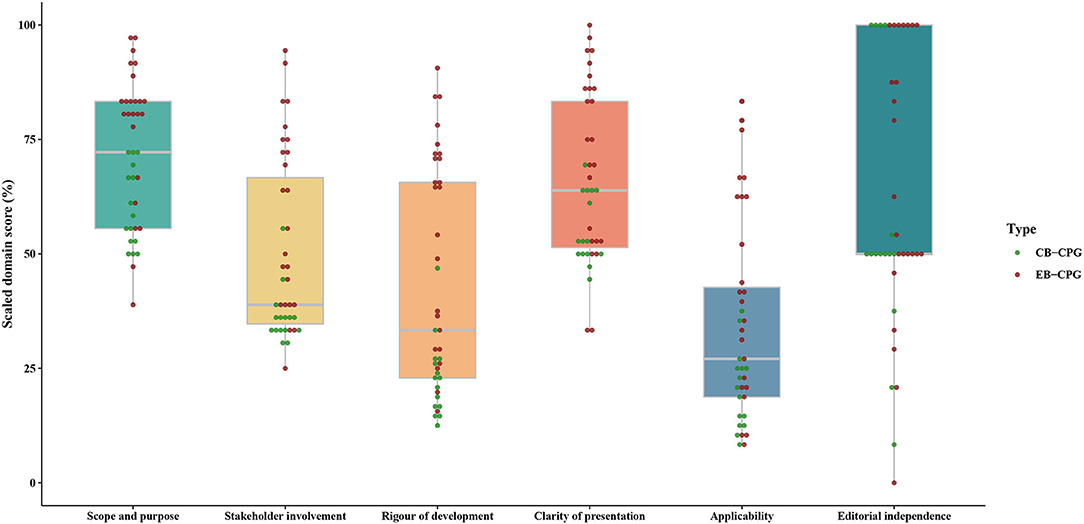
The ICC values for all six domains of AGREE II were over 0.75, indicating a high consistency on the scores between the two assessors. As shown in Supplementary Table 4, Table 1, Figure 3, the final domain score of every guideline across all domains ranged from 0% (Domain 6 of editorial independence in 1 guidelines) (51) to 100% (Domain 6 in 11 guidelines) (16, 19, 25, 29, 31, 32, 34, 44, 46–48). Regarding the score of each domain across all guidelines, for EB-CPGs, the score of Domain 5 (applicability) was the lowest with a median score of 40.63% (IQR 22.40–62.50), the median scores of Domains 1, 2, 3, 4, 6 (scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, editorial independence) were 81.94% (IQR 75.00–84.72), 59.72% (IQR 38.89–75.00), 64.58% (IQR 32.29–71.88), 75.00% (IQR 52.78–86.81), and 58.33% (IQR 50.00–100.00), respectively. For CB-CPGs, Domain 1 scored highest with a median score of 58.33% (IQR 52.78–68.06), Domain 5 scored lowest with median scores of 20.83% (13.54–25.00), and the median scores of Domains 2, 3, 4, 6 were 36.11% (33.33–36.11), 22.92% (16.67–26.56), 52.78% (50.00–63.89), and 50.00% (50.00–77.08), respectively. In addition, EB-CPGs were significantly superior to the CB-CPGs in the domain 1, 2, 3, 4, 5(P < 0.05).
Synthesis of Recommendations
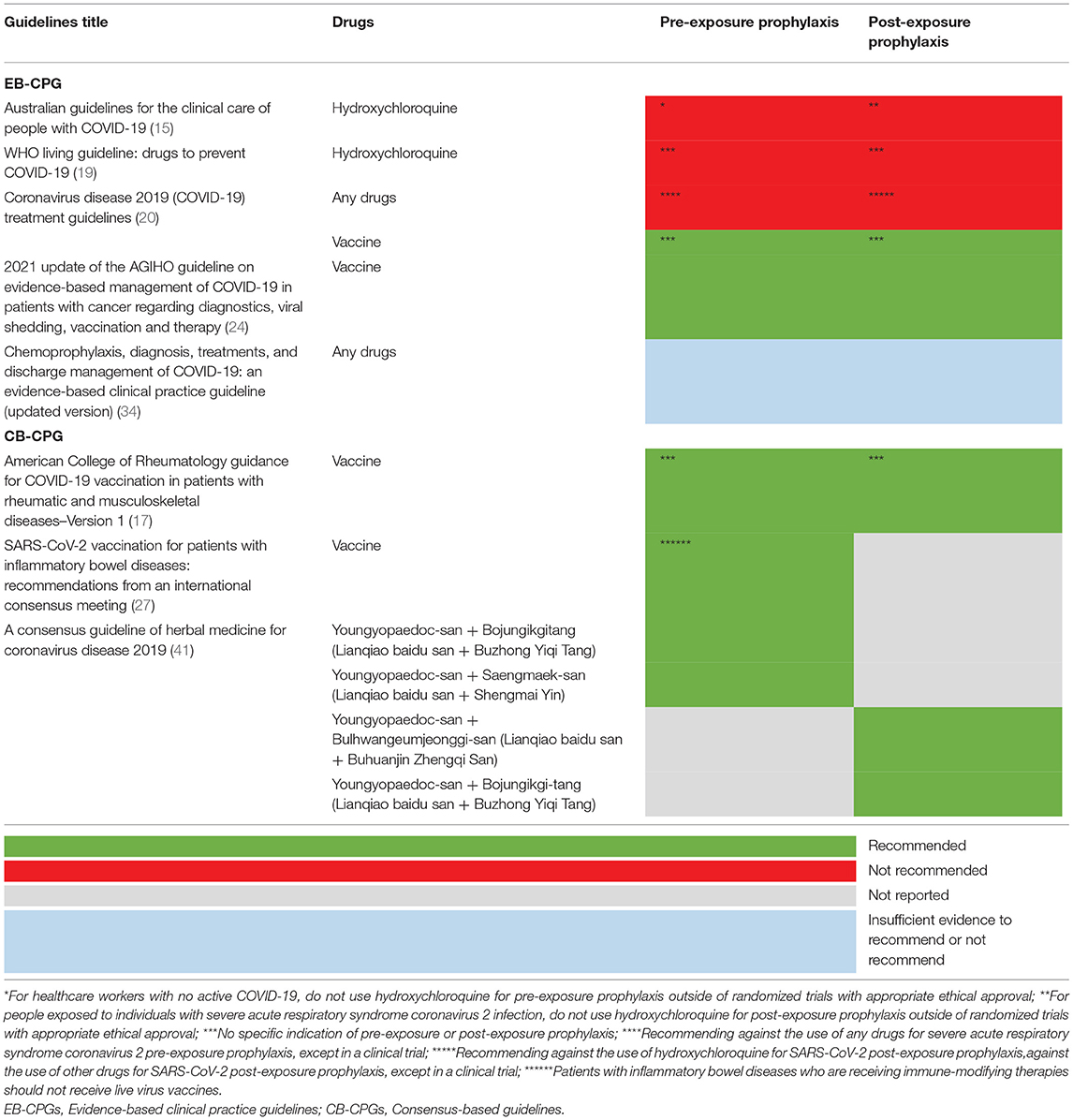
Five EB-CPGs (15, 19, 20, 24, 34) and three CB-CPGs (17, 27, 41) focused on the chemoprophylaxis of COVID-19. Two EB-CPGs of them recommended not to use hydroxychloroquine for COVID-19 pre-exposure prophylaxis or post-exposure prophylaxis outside the setting of a clinical trial (15, 19); two EB-CPG (20, 24) and two CB-CPGs (17, 27) recommended SARS-CoV vaccine for COVID-19 prevention; one CB-CPG (41) suggested that a few traditional Chinese medicine may be beneficial for COVID-19 prevention, for example, Youngyopaedoc-san plus Bojungikgitang, Youngyopaedoc-san plus Saengmaek-san (See Table 2).
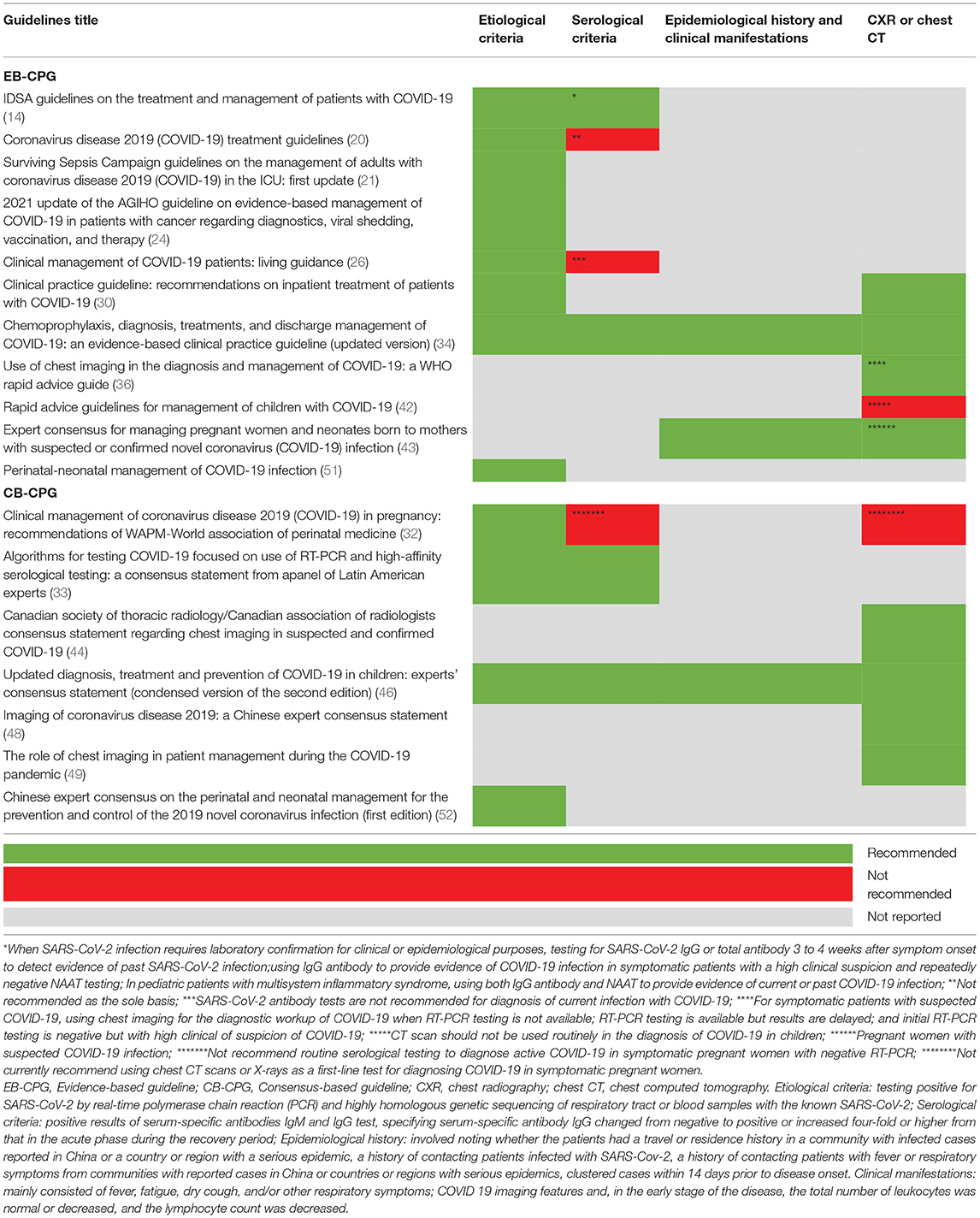
In total, 11 EB-CPGs (14, 20, 21, 24, 26, 30, 34, 36, 42, 43, 51) and 7 CB-CPGs (32, 33, 44, 46, 48, 49, 52) reported the diagnostic criteria for COVID-19 (See Table 3). The diagnosis of SARS-CoV-2 infection was mainly based on RT-PCR test, serum-specific antibodies IgM and IgG test, epidemiological history, and clinical manifestations in one EB-CPG (34) and one CB-CPG (46). However, nine EB-CPGs (14, 20, 21, 24, 26, 30, 36, 43, 51) and six CB-CPGs (32, 33, 44, 48, 49, 52) only focus on one or two of the above criteria. Three CPGs (two EB-CPGs, one CB-CPG) (20, 26, 32) did not suggest SARS-CoV-2 antibody tests for diagnosis of current infection with COVID-19 or as the sole basis or to routinely to diagnose active COVID-19 in symptomatic pregnant women with negative RT-PCR. Two CPGs (one EB-CPG, one CB-CPG) (32, 42) did not recommend that CT scan were used routinely in the diagnosis of COVID-19 in children or symptomatic pregnant women. In addition, one EB-CPG (31) provided some suggestions on how to predict whether a patient is COVID-19 positive, validated triage and severity of illness, risk stratify patients with suspected or confirmed COVID-19 in low- and middle-income countries.
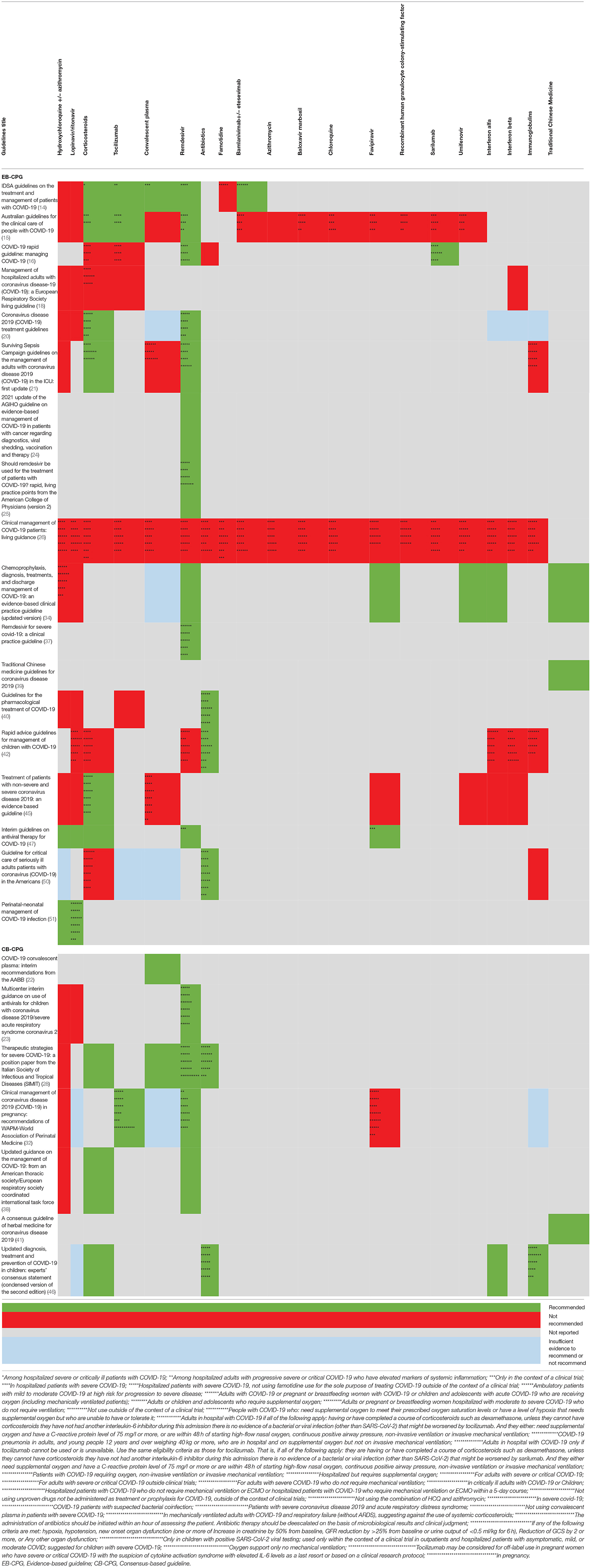
In total, 18 EB-CPGs (14–16, 18, 20, 21, 24–26, 34, 37, 39, 40, 42, 45, 47, 50, 51) and 7 CB-CPGs (22, 23, 28, 32, 38, 41, 46) provided suggestions on antivirals treatment for COVID-19. As shown in Table 4, there were no consistent views on effective and validated antiviral drugs such as hydroxychloroquine/chloroquine plus azithromycin, lopinavir/ritonavir, convalescent plasma for the treatment in clinical scenarios. The majority of guidelines agreed that some antiviral drugs such as Remdesivir can be used in the context of clinical trials or under special conditions such as severe and critical patients. Two EB-CPGs (34, 39) and one CB-CPG (41) provided a traditional Chinese medicine treatment plan for COVID-19.
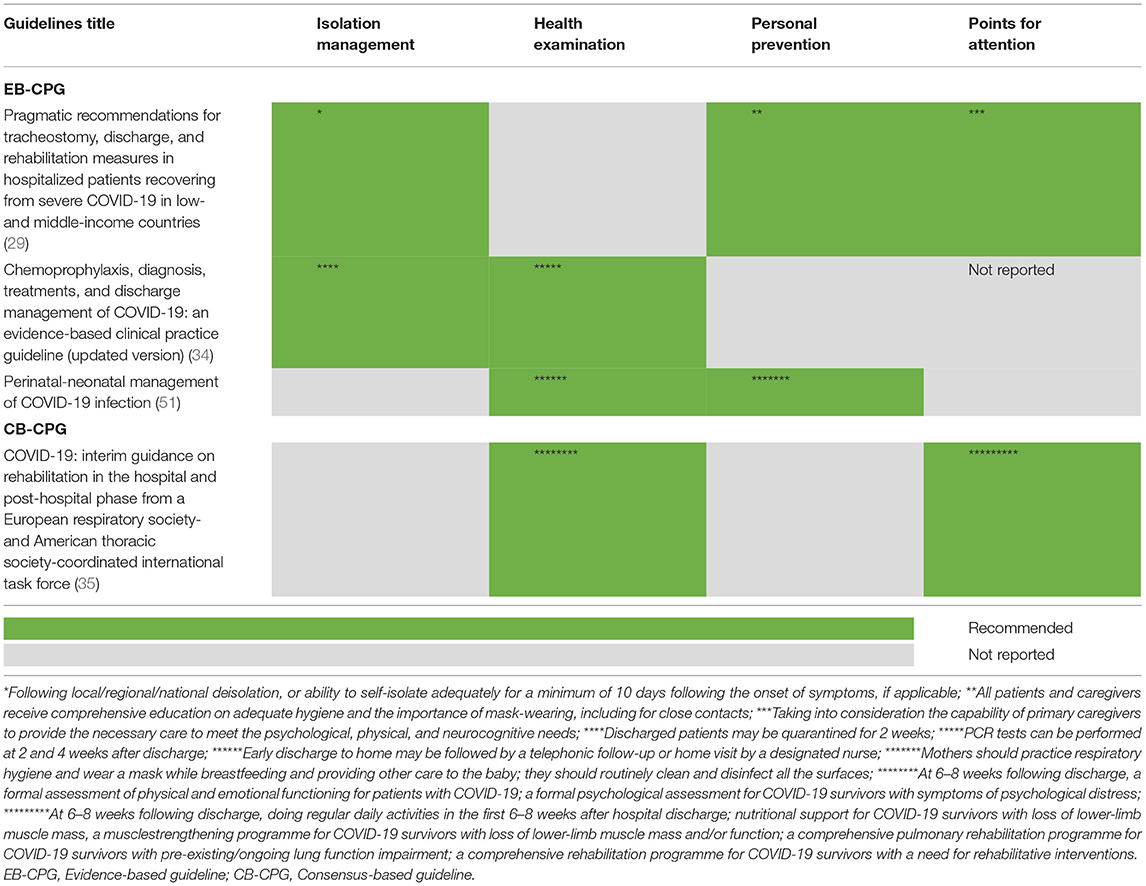
As presented in Tables 5A,B, four EB-CPGs (29, 34, 36, 51) and two CB-CPGs (35, 52) concentrated on the discharge management of COVID-19. The criteria were mainly based on temperature returning to normal more than 3 days, improvement in respiratory symptoms and negative results from two successive nucleic acid test of respiratory samples (with a sampling interval of at least 1 day). Besides, three EB-CPGs (29, 34, 51) and one CB-CPG (35) described the relevant precautions after discharge. For example, isolation management should be continued, and the patients should wear a mask if necessary.
Discussion
EB-CPGs and CB-CPGs play an important role in this pandemic, which is constantly being updated. The first EB-CPGs were published on Feb 6, 2020 (53); the first protocol of the updated EB-CPG was released on March 7, 2020 (54). Finally, 39 CPGs were included in this review. The methodological quality of EB-CPGs is better than CB-CPGs because the median score with IQR is statistically significantly higher in EB-CPGs for domains of the AGREE II assessment tool in general. However, they all still need to be further improved, especially in the areas of gathering and synthesizing reliable the latest up-to-date information, involving the target population in guideline development and improving the implementability of the recommendations. Recommendations relevant to chemoprophylaxis, diagnosis, antiviral drugs, and discharge management of COVID-19 showed small differences.
COVID-19 is a newly identified infectious disease, which poses a significant threat to both the general population and health care workers. In the early stage of the pandemic, the absolute lack of direct evidence is the biggest challenge for guideline development. A large number of CB-CPGs and EB-CPGs in accordance with experience of frontline health professionals, such as experts in infectious disease, medical imaging, and clinical immunology, have put forward valuable suggestions to guide clinical practice. Although the methodological quality of EB-CPGs is higher than CB-CPGs in general, they all have deficiencies in the following aspects, including obtaining the views and preferences of the target population, considering benefits and risks when formulating recommendations, introducing a detailed update plan, and providing implementation strategy for the recommendations or methods for managing potential conflicts of interest, similar to Dagens', Luo', and Zhao' studies (3, 4, 6). In view of the above topics, there are some examples of good practice, for example, conducting interviews and group surveys to collect information on treatment evidence from frontline experts fighting the disease (34); inviting patients recovering from COVID-19 to get involved in the guideline development panel (45); critically assessing new studies where these supersede previous outdated recommendations (14); providing available recommendation summaries in user-friendly and multilayered formats for clinicians and patients through the MAGIC app (55) or the provision of consultation decision aids to facilitate shared decision-making (45); and using the GRADEpro guideline development tool online software to conduct evidence-based CPGs (56). The methodology for guideline development to deal with public health emergencies is still a challenge, and methods for their development which ensure the rigor, timeliness and implementability of recommendations is a problem to be further explored by methodologists.
Recommendations relevant to chemoprophylaxis, diagnosis, antiviral treatments, and discharge management of COVID-19 varied in the guidelines. Chemoprophylaxis may be beneficial to reduce COVID-19 spread, which is important when lacking specific vaccines due to the high social and economic costs caused by social distancing of entire populations and blockade of entire cities. This method has been applied to other respiratory viruses; for example, healthcare workers who were exposed to high risk groups fought against the Middle East respiratory syndrome coronavirus using lopinavir-ritonavir plus ribavirin in South Korea (57). Unfortunately, there are still no effective and verified drugs for COVID-19 prophylaxis in the guidelines. However, a retrospective cohort study on family members and health care workers who were exposed to patients diagnosed with SARS-CoV-2 suggested that Arbidol could reduce risk of infection with the disease in hospital and family settings (58). SARS-CoV 2 vaccines may be beneficial for the prevention of COVID-19. The effectiveness and safety of them are still continuously ongoing trials. For instance, estimated BNT162b2 and mRNA-1273 COVID-19 vaccines effectiveness for prevention of infection was 90% for full immunization and 80% for partial immunization (59). Most commonly reported adverse effects of COVID-19 mRNA-1273 vaccine were localized pain, generalized weakness, headache, and myalgia (60). New evidence may inform decision making on chemoprophylaxis for healthcare personnel by policy makers in the future.
Diagnostic criteria for COVID-19 were not identical across the guidelines. What is more consistent is confirmation of diagnosis by testing positive for SARS-CoV-2 by real-time PCR. The main differences are the inclusion of other features, such as epidemiological history, serological tests, and clinical manifestations, as one of the bases for the diagnosis. Early studies have confirmed that 49–66% patients had contact with personnel in outbreak area (61). Up to now, asymptomatic infection of SARS-CoV-2 has become a worldwide concern. A recent study indicated that these cases may account for 60% of all infections and may trigger new outbreaks (62). Asymptomatic cases were significantly younger than those with symptomatic patients, had similar common incidence rate, and were more likely to come from high altitude and low mobility areas, with better history of epidemiology (63). Careful examination of the epidemiological history would help to identify asymptomatic patients that may have delayed symptoms after diagnosis. In addition, stability issues of RT-PCR testing of COVID-19 for hospitalized patients clinically diagnosed with SARS-CoV-2 are a problem. Li et al. reported a potentially high false negative rate of RT-PCR where results from several tests from the same patients at different points were inconsistent during the course of their diagnosis and treatment (64). Current systematic reviews have confirmed that the detection of anti-SARS-CoV-2 IgG and IgM had high diagnostic efficiency (2,282 patients with SARS-CoV-2 and 1,485 healthy persons or patients without SARS-CoV-2) (65) and a high sensitivity of chest CT for the detection of COVID-19 in regions with severe (3,186 patients) (66). The presentation of COVID-19 symptoms (such as fever, cough, myalgia/fatigue, leukocyte, and neutrophil counts) might be regarded as a surrogate marker for the disease' presence and severity (67, 68). Therefore, serological criteria, epidemiological history, clinical manifestations, and chest x-ray/CT should also be used for to assist diagnosis for COVID-19 infection during the current epidemic, counteracting possible false negative RT-PCR results if available.
Studies published after the deadline for analysis have been included here. Although there were no consistent recommendations on the usage of antiviral drugs, it does offer a few valuable suggestions, including antiviral drugs, such as hydroxychloroquine and remdesivir, for COVID-19. The majority of EB-CPGs did not recommend hydroxychloroquine +/– azithromycin to treat patients with COVID-19 because higher certainty benefits (e.g., mortality reduction) are now highly unlikely even if additional high quality randomized controlled trials would become available (14, 15, 18, 20, 21, 40, 45). Remdesivir is an antiviral drug with potent in vitro activity against a range of RNA viruses including MERS-CoV, SARS-CoV, there may be a favorable risk-benefit profile for remdesivir compared with no antiviral treatment in severe COVID-19 infection with limited safety data currently available for the drug (14–16, 20, 21, 24, 25, 34, 37, 47). In addition, Traditional Chinese medicine treatment may be beneficial for the treatment of COVID-19, including Lianhua Qingwen granules/capsules and Huashi Baidu granules. More new evidence concentrating on antiviral therapy continuously emerges. For example, early application of lopinavir / ritonavir+interferon-α can reduce the shedding time of sars-cov-2 (69); Early initiation with interferon) β- 1b, lopinavir, ribavirin combination therapy were more safe and effective than lopinavir alone in relieving symptoms, shortening length of stay in patients with mild to moderate COVID-19 (70); Lianhua Qingwen combined with Western medicine may have a significant effect and fewer side effects in the treatment of common patients with new coronavirus pneumonia (71). The new evidence above will help to update the recommendations of the guidelines.
The phenomenon that some discharge patients have tested positive for COVID-19 again after recovery has attracted a lot of attention. The included guidelines provided different suggestions on discharge criteria and precautions after discharge. As previously stated, Chest CT and X ray can be beneficial for COVID-19 diagnosis. Viral RNA was detected in 48.1% of patients' feces, even in the feces who have been diagnosed with negative results in respiratory tract samples (72). Thus, a nucleic acid test of upper airway specimens (nasopharyngeal and pharyngeal swabs) and fecal stool can be considered along with other criteria. Additionally, it may be necessary to continue isolation management and health status monitoring. A follow-up study for 651 patients recovered from COVID-19 revealed that 3% of the patients were positive for SARS-CoV-2 by RTqPCR in routine physical examination and the median time from discharge to retest with postive results was 15.0 days (73). Thus, the COVID-19 pandemic is a rapidly changing situation. The recommendations in the guidelines are also continuously changing. The evidence-based living guidelines are pursed (74, 75).
A strength of this review lies in the updated study (up to April 5, 2021) concentrating on hot topics, including chemoprophylaxis, diagnosis, antiviral therapy and discharge management of COVID-19 guidelines, at the same time and summarizing the recommendations. In addition, we defined the CPGs, distinguished EB-CPGs and CB-CPGs in order to gain the valuable recommendations developed by multidisciplinary experts and based on best evidence. However, there are several inevitable limitations in this current study. First, we did not compare the evidence and recommendation levels or different grade systems used in EB-CPGs. With new evidence emerging over time, some CPGs will be updated and evidence and recommendation levels may be changed or improved later. Second, we only searched the three medical databases and eight representative guidelines repositories, and some eligible EB-CPGs and CB-CPGs will thus have been missed.
Conclusion
In general, the methodological quality of EB-CPGs is greater than CB-CPGs. But we still need to pay attention to gathering and synthesizing reliable the latest up-to-date information, involving the target population in guideline development and improving the implementability of the recommendations. As for the recommendations of COVID-19, SARS-CoV 2 vaccines are still going through ongoing trials; various diagnosis strategies, including serological criteria and CT for COVID-19, may be more effective if available; hydroxychloroquine +/– azithromycin may be not beneficial to treat patients with COVID-19, but remdesivir may be a favorable risk-benefit in severe COVID-19 infection; and isolation management and health status monitoring after discharge may be still necessary. Thus, chemoprophylaxis and antiviral drugs of COVID-19 still need more trials for confirmation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Y-YW and X-TZ designed the study and formulated inclusion criteria. Y-YW, QS, HZ, QH, and B-HL searched and identified eligible guidelines. HZ, B-HL, M-ZL, and S-HH extracted important information. M-ZL and QS evaluated the quality of each included guideline using AGREE II. X-TZ and Y-HJ examined the data extraction forms. Y-YW, QH, and S-HH analyzed the data. Y-YW, X-TZ, XY, and Y-HJ contributed to discussed the findings. Y-YW, X-TZ, XY, and Y-HJ developed the final manuscript. All authors have read and approved the manuscript.
Funding
This work was supported (in part) by the Emergency Special Project for COVID-19 of Wuhan Municipal Health Commission (EG20A02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.630765/full#supplementary-material
References
1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. WHO (2021). Available online at: https://covid19.who.int/ (accessed April 10, 2021)
2. Graham R, Mancher M, Miller W, Greenfield S, Steinberg E. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press (2011). doi: 10.17226/13058
3. Zhao S, Cao J, Shi Q, Wang Z, Estill J, Lu S, et al. A quality evaluation of guidelines on five different viruses causing public health emergencies of international concern. Ann Transl Med. (2020) 8:500. doi: 10.21037/atm.2020.03.130
4. Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, Harris E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. (2020) 369:m1936. doi: 10.1136/bmj.m1936
5. Xu X, Ong YK, Wang Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil Med Res. (2020) 7:22. doi: 10.1186/s40779-020-00251-x
6. Luo X, Liu Y, Ren M, Zhang X, Janne E, Lv M, et al. Consistency of recommendations and methodological quality of guidelines for the diagnosis and treatment of COVID-19. J Evid Based Med. (2021) 14:40–55. doi: 10.1111/jebm.12419
7. Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. (2020) 323:1502–03. doi: 10.1001/jama.2020.2783
8. Qu YM, Kang EM, Cong HY. Positive result of Sars-Cov-2 in sputum from a cured patient with COVID-19. Travel Med Infect Dis. (2020) 34:101619. doi: 10.1016/j.tmaid.2020.101619
9. Zhao W, Wang Y, Tang Y, Zhao W, Fan Y, Liu G, et al. Characteristics of children with reactivation of SARS-CoV-2 infection after hospital discharge. Clin Pediatr. (2020) 59:929–932. doi: 10.1177/0009922820928057
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. AGREE Next Steps Consortium. The AGREE II Instrument. Available online at: http://www.agreecollaboration.org (accessed August 20, 2020).
12. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
14. Infectious Diseases Society of America. IDSA Guidelines on the Treatment and Management of Patients With COVID-19. Available online at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed April 5, 2020).
15. Australian National COVID-19 Clinical Evidence Taskforce. Australian Guidelines for the Clinical Care of People With COVID-19. Available online at: https://covid19evidence.net.au/#living-guidelines (accessed April 5, 2020).
16. National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing COVID-19. Available online at: www.nice.org.uk/guidance/ng191 (accessed April 5, 2020).
17. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American college of rheumatology guidance for covid-19 vaccination in patients with rheumatic and musculoskeletal diseases - version 1. Arthritis Rheumatol. (2021). doi: 10.1002/art.41734. [Epub ahead of print].
18. Chalmers JD, Crichton ML, Goeminne PC, Cao B, Humbert M, Shteinberg M, et al. Management of hospitalised adults with coronavirus disease-19 (COVID-19): a European respiratory society living guideline. Eur Respir J. (2021) 57:2100048. doi: 10.1183/13993003.00048-2021
19. World Health Organization. WHO Living Guideline: Drugs to Prevent COVID-19. Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-prophylaxes-2021-1 (accessed April 2, 2021).
20. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online at: https://www.covid19treatmentguidelines.nih.gov/ (accessed April 5, 2020).
21. Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving Sepsis campaign guidelines on the management of adults with Coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. (2021) 49:e219–34. doi: 10.1097/CCM.0000000000004899
22. Cohn CS, Estcourt L, Grossman BJ, Pagano MB, Allen ES, Bloch EM, et al. COVID-19 convalescent plasma: interim recommendations from the AABB. Transfusion. (2021) 61:1313–23. doi: 10.1111/trf.16328
23. Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. (2021) 10:34–48. doi: 10.1093/jpids/piaa115
24. Giesen N, Sprute R, Rüthrich M, Khodamoradi Y, Mellinghoff SC, Beutel G, et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer. (2021) 147:154–60. doi: 10.1016/j.ejca.2021.01.033
25. Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, Abraham GM, Jokela JA, Forciea MA, et al. Should remdesivir be used for the treatment of patients with COVID-19? Rapid, living practice points from the American college of physicians (version 2). Ann Intern Med. (2021) 174:M20–8101. doi: 10.7326/M20-8101
26. World Health Organization. Clinical management of COVID-19 patients: living guidance. Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed April 1, 2021).
27. Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. (2021) 70:635–40. doi: 10.1136/gutjnl-2020-324000
28. Mussini C, Falcone M, Nozza S, Sagnelli C, Parrella R, Meschiari M, et al. Therapeutic strategies for severe COVID-19: a position paper from the Italian society of infectious and tropical diseases (SIMIT). Clin Microbiol Infect. (2021) 27:389–95. doi: 10.1016/j.cmi.2020.12.011
29. Schultz MJ, Ahmed HY, Shrestha GS, West TE, Papali A, ‘COVID–LMIC Task Force’ the ‘Mahidol–Oxford Research Unit’ (MORU). Pragmatic recommendations for tracheostomy, discharge, and rehabilitation measures in hospitalized patients recovering from severe COVID-19 in low- and middle-income countries. Am J Trop Med Hyg. (2021) 104 (3 Suppl):110–9. doi: 10.4269/ajtmh.20-1173
30. Kluge S, Janssens U, Spinner CD, Pfeifer M, Marx G, Karagiannidis C. Clinical practice guideline: recommendations on inpatient treatment of patients with COVID-19. Dtsch Arztebl Int. (2021) 118. doi: 10.3238/arztebl.m2021.0110. [Epub ahead of print].
31. Barros LM, Pigoga JL, Chea S, Hansoti B, Hirner S, Papali A, et al. Pragmatic recommendations for identification and triage of patients with COVID-19 disease in low- and middle-income countries. Am J Trop Med Hyg. (2021) 104:3–11. doi: 10.4269/ajtmh.20-1064
32. Api O, Sen C, Debska M, Saccone G, D'Antonio F, Volpe N, et al. Clinical management of coronavirus disease 2019 (COVID-19) in pregnancy: recommendations of WAPM-world association of perinatal medicine. J Perinat Med. (2020) 48:857–66. doi: 10.1515/jpm-2020-0265
33. Ferreira CE, Bonvehi PE, de la Torre JCG, Sáenz-Flor KV, Condino-Neto A. Algorithms for testing COVID-19 focused on use of RT-PCR and high-affinity serological testing: a consensus statement from a panel of Latin American experts. Int J Infect Dis. (2021) 103:260–7. doi: 10.1016/j.ijid.2020.11.173
34. Jin YH, Zhan QY, Peng ZY, Ren XQ, Yin XT, Cai L, et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: an evidence-based clinical practice guideline (updated version). Mil Med Res. (2020) 7:41. doi: 10.1186/s40779-020-00270-8
35. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European respiratory society and American thoracic society-coordinated international task force. Eur Respir J. (2020) 56:2002197. doi: 10.1183/13993003.02197-2020
36. Akl EA, BlaŽić I, Yaacoub S, Frija G, Chou R, Appiah JA, et al. Use of chest imaging in the diagnosis and management of COVID-19: a WHO rapid advice guide. Radiology. (2021) 298:E63–9. doi: 10.1148/radiol.2020203173
37. Rochwerg B, Agarwal A, Zeng L, Leo Y, Appiah J, Agoritsas T, et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. (2020) 370:m2924. doi: 10.1136/bmj.m2924
38. Bai C, Chotirmall SH, Rello J, Alba GA, Ginns LC, Krishnan JA, et al. Updated guidance on the management of COVID-19: from an American thoracic society/European respiratory society coordinated international task force (29 July 2020). Eur Respir Rev. (2020) 29:200287. doi: 10.1183/16000617.0287-2020
39. Liang N, Ma Y, Wang J, Li H, Wang X, Jiao L, et al. Traditional Chinese medicine guidelines for coronavirus disease 2019. J Tradit Chin Med. (2020) 40:891–6. doi: 10.19852/j.cnki.jtcm.20200902.001
40. Falavigna M, Colpani V, Stein C, Azevedo L, Bagattini A, Brito G, et al. Guidelines for the pharmacological treatment of COVID-19. Rev Bras Ter Intensiva. (2020) 32:166–96. doi: 10.5935/0103-507x.20200039
41. Lee B, Lee J, Kim K, Choi J, Jung H. A consensus guideline of herbal medicine for coronavirus disease 2019. Integr Med Res. (2020) 9:100470. doi: 10.1016/j.imr.2020.100470
42. Liu E, Smyth R, Luo Z, Qaseem A, Mathew J, Lu Q. Rapid advice guidelines for management of children with COVID-19. Ann Transl Med. (2020) 8:617. doi: 10.21037/atm-20-3754
43. Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. (2020) 149:130–6. doi: 10.1002/ijgo.13146
44. Dennie C, Hague C, Lim R, Manos D, Memauri B, Nguyen E, et al. Canadian society of thoracic radiology/Canadian association of radiologists consensus statement regarding chest imaging in suspected and confirmed COVID-19. Can Assoc Radiol J. (2020) 71:470–81. doi: 10.1177/0846537120924606
45. Ye Z, Rochwerg B, Wang Y, Adhikari NK, Murthy S, Lamontagne F, et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ. (2020) 192:E536–45. doi: 10.1503/cmaj.200648
46. Shen KL, Yang YH, Jiang RM, Wang TY, Zhao DC, Jiang Y, et al. Updated diagnosis, treatment and prevention of COVID-19 in children: experts' consensus statement (condensed version of the second edition). World J Pediatr. (2020) 16:232–9. doi: 10.1007/s12519-020-00362-4
47. Kim S, Huh K, Heo J, Joo E, Kim Y, Choi W, et al. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. (2020) 52:281–304. doi: 10.3947/ic.2020.52.2.281
48. Yang Q, Liu Q, Xu H, Lu H, Liu S, Li H. Imaging of coronavirus disease 2019: a Chinese expert consensus statement. Eur J Radiol. (2020) 127:109008. doi: 10.1016/j.ejrad.2020.109008
49. Rubin G, Ryerson C, Haramati L, Sverzellati N, Kanne J, Raoof S, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Chest. (2020) 158:106–16. doi: 10.1016/j.chest.2020.04.003
50. Pan American Health organization and World Health Organization. Guideline for Critical Care of Seriously Ill Adults Patients With Coronavirus (COVID-19) in the Americans. Available online at: https://www.paho.org/en/documents/guidelines-critical-care-seriously-ill-adult-patients-coronavirus-covid-19-americas-short (accessed August 23, 2020)
51. Federation of Obstetric and Gynecological Societies of India National Neonatology Forum, India Indian Academy of Pediatrics. Perinatal-Neonatal Management of COVID-19 Infection. Available online at: http://www.iapindia.org (accessed August 15, 2020).
52. Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (first edition). Ann Transl Med. (2020) 8:47 doi: 10.21037/atm.2020.02.20
53. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. (2020) 7:4. doi: 10.1186/s40779-020-0233-6
54. Jin YH, Li HJ, Zhan QY, Peng ZY, Yuan YF, Cai L, et al. Evidence-based Chinese expert recommendations on drug prevention, diagnosis, treatment, and discharge management of covid-19 a protocol. Yixue Xinzhi Zazhi. (2020) 30:209–26. doi: 10.12173/j.issn.1004-5511.2020.03.07
55. MAGIC. COVID-19: MAGIC Making a Difference and MAGICapp Now Available to Develop Living Guidelines. Available online at: http://magicproject.org/magicapp/ (accessed March 28, 2021).
56. GRADEpro GDT. GRADE Your Evidence and Improve Your Guideline Development in Health Care. Available online at: https://gradepro.org/ (accessed April 2, 2020).
57. Gentile I, Maraolo AE, Piscitelli P, Colao A. COVID-19: time for post-exposure prophylaxis? Int J Environ Res Public Health. (2020) 17:3997. doi: 10.3390/ijerph17113997
58. Zhang JN, Wang WJ, Peng B, Peng W, Zhang YS, Wang YL, et al. Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission: a preliminary report of a retrospective cohort study. Curr Med Sci. (2020) 40:480–5. doi: 10.1007/s11596-020-2203-3
59. Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:495–500. doi: 10.15585/mmwr.mm7013e3
60. Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, et al. Adverse effects of COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. (2021) 93: 4420–9. doi: 10.1002/jmv.26996
61. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
62. Qiu J. Covert coronavirus infections could be seeding new outbreaks. Nature. (2020). doi: 10.1038/d41586-020-00822-x. [Epub ahead of print].
63. Kong W, Wang Y, Hu J, Chughtai A, Pu H. Comparison of clinical and epidemiological characteristics of asymptomatic and symptomatic SARS-CoV-2 infection: a multi-center study in Sichuan Province, China. Travel Med Infect Dis. (2020) 37:101754. doi: 10.1016/j.tmaid.2020.101754
64. Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. (2020) 92:903–8. doi: 10.1002/jmv.25786
65. Zhang ZL, Hou YL, Li DT, Li FZ. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: a meta-analysis. J Med Virol. (2021) 93:366–74. doi: 10.1002/jmv.26211
66. Xu B, Xing Y, Peng J, Zheng Z, Tang W, Sun Y, et al. Chest CT for detecting COVID-19: a systematic review and meta-analysis of diagnostic accuracy. Eur Radiol. (2020) 30:5720–7. doi: 10.1007/s00330-020-06934-2
67. Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: A systematic review of case reports and case series. Front Med. (2020) 7:231. doi: 10.3389/fmed.2020.00231
68. Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Med Clin. (2020) 155:143–51. doi: 10.1016/j.medcli.2020.05.017
69. Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol. (2020) 92:2666–74. doi: 10.1002/jmv.26127
70. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. (2020) 395:1695–704. doi: 10.1016/S0140-6736(20)31042
71. Zhang WB, Liu LN, Wang Z, Liu Y. Meta-analysis of the efficacy and safety of Lianhua Qingwen combined with western medicine in the treatment of common patients with new coronary pneumonia. J Hainan Med Univ. (2020) 26:1045–50. doi: 10.13210/j.cnki.jhmu.20200528.001
72. Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. (2020) 159:81–95. doi: 10.1053/j.gastro.2020.03.065
73. Mei Q, Li J, Du R, Yuan X, Li M, Li J. Assessment of patients who tested positive for COVID-19 after recovery. Lancet Infect Dis. (2020) 20:1004–5. doi: 10.1016/S1473-3099(20)30433-3
74. Jin YH, Yao XM, Zeng XT. Development of rapid advice guideline and standard and continuous updating guideline: experiences and practice. Mil Med Res. (2021) 8:10. doi: 10.1186/s40779-021-00298-4
Keywords: COVID-19, SARS-CoV-2, guideline, AGREE II, prophylaxis, diagnosis, treatments, discharge management
Citation: Wang Y-Y, Huang Q, Shen Q, Zi H, Li B-H, Li M-Z, He S-H, Zeng X-T, Yao X and Jin Y-H (2021) Quality of and Recommendations for Relevant Clinical Practice Guidelines for COVID-19 Management: A Systematic Review and Critical Appraisal. Front. Med. 8:630765. doi: 10.3389/fmed.2021.630765
Received: 18 November 2020; Accepted: 26 April 2021;
Published: 10 June 2021.
Edited by:
Longxiang Su, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Daniela Oliveira De Melo, Federal University of São Paulo, BrazilMorteza Arab-Zozani, Birjand University of Medical Sciences, Iran
Copyright © 2021 Wang, Huang, Shen, Zi, Li, Li, He, Zeng, Yao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Tao Zeng, zengxiantao1128@whu.edu.cn; Xiaomei Yao, yaoxia@mcmaster.ca; Ying-Hui Jin, jinyinghui0301@163.com
 Yun-Yun Wang
Yun-Yun Wang Qiao Huang
Qiao Huang Quan Shen2,3
Quan Shen2,3  Hao Zi
Hao Zi Bing-Hui Li
Bing-Hui Li Xian-Tao Zeng
Xian-Tao Zeng Xiaomei Yao
Xiaomei Yao