The Clinical Characteristics of Other HLA-B Types in Chinese Ankylosing Spondylitis Patients
- 1Department of Rheumatology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 2Department of Pediatrics, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
HLA-B27 has an established relationship with the development of ankylosing spondylitis (AS). After reviewing the HLA-B genotype from 407 Chinese subjects (318 patients and 89 sex-matched controls), we found that 252 patients and 32 controls were HLA-B27(+) and that HLA-B*27:04 was the dominant HLA-B27 subtype (N = 224). In all participants, HLA*27:04 homozygous were only detected in two patients. In the HLA-B27(+) group, HLA-B40 was observed in 51 cases and one control (p < 0.05, OR = 7.87, 95% CI 1.05–59.0); of these, the most genotype was HLA-B*27:04/B*40:01(N = 38). Two hundred thirty-nine patients' clinical information was recorded. Cases with HLA-B27/B46 had more peripheral joint involvement (OR = 3.95, 95% CI 1.77–8.79) in HLA-B27(+) AS. HLA-B*15:02 may be a significant risk element to peripheral joint involvement (p < 0.05) in HLA-B27(−) patients. Therefore, we believe HLA-B*40:01, HLA-B*46:01, and HLA-B*15:02 can be the test indicators for AS diagnostic value.
Introduction
Human leukocyte antigen (HLA)-B27 is the most critical gene in ankylosing spondylitis (AS). About 90–95% of AS cases were HLA-B27 positive, while only 1–2% of HLA-B27 positive persons can develop to AS (1, 2). Results showed that the occurrence of AS with HLA-B27 appeared in family aggregation. Among the first-degree relatives of HLA-B27 positive AS, the prevalence is 10–30% (3). Above 45 HLA-B27 subtypes, like B*27:02, B*27:10, and B*27:15, were found to be associated with AS, and their distribution varied in different populations (4, 5). B*27:04 is the primary subtype in the Chinese Han population (6), whereas the Caucasian people are dominated by the B*27:05 (4). On the contrary, B*27:06 and B*27:09 are unrelated to AS. Previous research found homozygous B*27:04 can affect AS susceptibility but not its clinical manifestations and functional disability (7, 8). How about HLA-B27 heterozygote with other HLA-B alleles in AS? Our studies aimed to evaluate the influence of heterozygous HLA-B27 on the clinical manifestations of AS patients.
Methods
Study Subjects
Three hundred eighteen Chinese Han patients and 89 sex-matched controls were recruited from the hospitals in Guangdong Province of China. All patients were older than 18 years old and met the 1984 modified New York criteria for AS (9). Two hundred thirty-nine patients had their clinical information collected by two trained rheumatologists during a face-to-face interview at the study visit. Clinical information included peripheral manifestations (uveitis, peripheral joint involvement, dactylitis, and enthesitis), onset age, body mass index (BMI), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and Bath Ankylosing Spondylitis Functional Index (BASFI). We also collected past and current medications, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and biologic agents. The general information included age, gender, and smoking (current) and drinking history (current). According to the 2009 ASAS classification criteria (either axial or peripheral) (10), patients without any peripheral manifestations (uveitis, peripheral joint involvement, dactylitis, and enthesitis) were classified as the axial AS (axAS). Controls were free of any history of rheumatic disease. Written informed consent was obtained from all the subjects. The ethics committee of the Third Affiliated Hospital of Sun Yat-Sen University approved our study. All participants gave written informed consent before enrollment.
HLA-B Genotype
Genomic DNA was extracted from peripheral blood using a standard salting-out method. All of the individuals were genotyped for HLA-B loci using the polymerase chain reaction sequence-based typing (PCRS-BT) method. Briefly, we performed locus-specific PCR amplification and bidirectional Sanger sequencing of HLA-B exons 2, 3, and 4. Amplification and sequencing of relevant exons was performed using “in-house” primers. Sequencing was performed on a 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). The typing results were accomplished using uTYPE v6.0 software (One Lambda, Canoga Park, CA, USA) against the IMGT/HLA database. When encountering ambiguous genotyping results (several genotypic combinations perform identically on sequencing results), alleles were assigned by referring to the most common alleles in the Chinese population (11).
Statistical Analysis
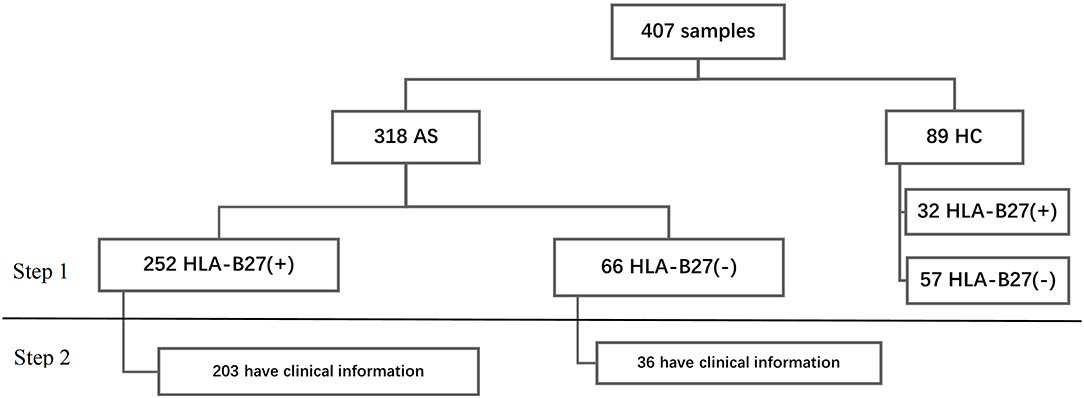
We analyzed the data in two steps. In step 1, we analyzed the HLA-B types in all participants. Then we researched the relationship with HLA-B types and clinical phenotype (Figure 1). For continuous variables, we calculated mean ± standard deviation (SD) and percentage for categorical variables. We performed Student's t-test or rank-sum test to make group comparisons for continuous data and chi-squared tests for categorical variables (Fisher's exact test where appropriate). All contrasts were bilateral and considered significant when p < 0.05. Data were collected, processed, and analyzed using the Statistical Package for the Social Sciences (SPSS) software v.19. The heatmaps were drawn by R software v3.6.1.
Results
Step 1
The HLA-B Genotypes Distribution in all Samples
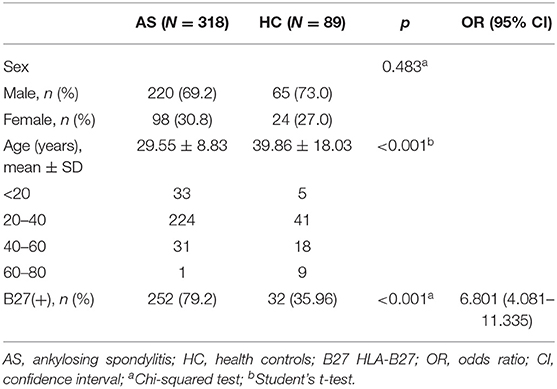
A total of 407 subjects were analyzed using HLA-B typing including 318 AS and 89 sex-matched controls (Figure 1) from January 2016 to September 2020. As shown in Table 1, the mean age of AS patients was 29.55 ± 8.83 years old and controls was 39.86 ± 18.03 years old. The AS patients were younger than controls (p < 0.05). The main age group of patients was under 40 years old. After HLA-B typing, we found 24 low-resolution HLA-B types and 55 high-resolution HLA-B subtypes in all participants, including eight homozygous and 399 heterozygous. The major HLA-B type was HLA-B27. Two hundred fifty-two (79.25%) cases and 32 (35.96%) controls (p < 0.05) were HLA-B27(+). Other HLA-B-type distributions are shown in Figure 2.
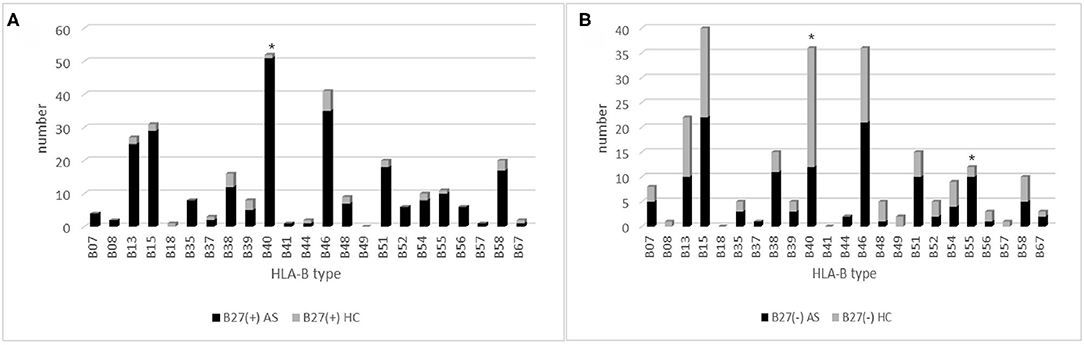
Figure 2. The HLA-B type distribution in HLA-B27(+) and HLA-B27(−) group: (A) HLA-B type distribution in HLA-B27(+) patients and HLA-B27(+) controls, and (B) HLA-B type distribution in HLA-B27(−) patients and HLA-B(−) controls. Chi-squared test were used to determine significance (*p < 0.05). AS, ankylosing spondylitis; HC, health control.
In 252 B27(+) patients and 32 B27(+) controls, HLA-B*27:04 was found in 224 cases (88.89%) and in all 32 controls (100%), respectively (p > 0.05). HLA-B*27:05 was detected in 21 cases (8.3%) but not found in controls. We also observed another HLA-B subtype (one HLA-B*27:07, one HLA-B*27:06, two HLA-B*27:15, three HLA-B*27:02, and one HLA-B*27:14) in patients (Table 2). The one with HLA-B*27:14 was a HLA-B*27:04 heterozygote (HLA-B*27:04/B*27:14). Fifty-one cases (15.87%) and one control (3.13%) carried B27/B40 (p = 0.018); the majority genotype was HLA-B*27:04/B*40:01 (N = 38, Figure 3). In HLA-B*27:04 carriers, the HLA-B*40:01 was also associated with AS (p = 0.024). In 21 HLA-B*27:05 patients, HLA-B*27:05/B*46:01 was the most HLA-B genotype (N = 6, 28.6%) (Figure 3). Between HLA-B*27:04 and HLA-B*27:05 patients, the distribution of the HLA-B*40:01 and HLA-B*46:01 had no significant difference. Another HLA-B27 subtype genotype is shown in Figure 3.
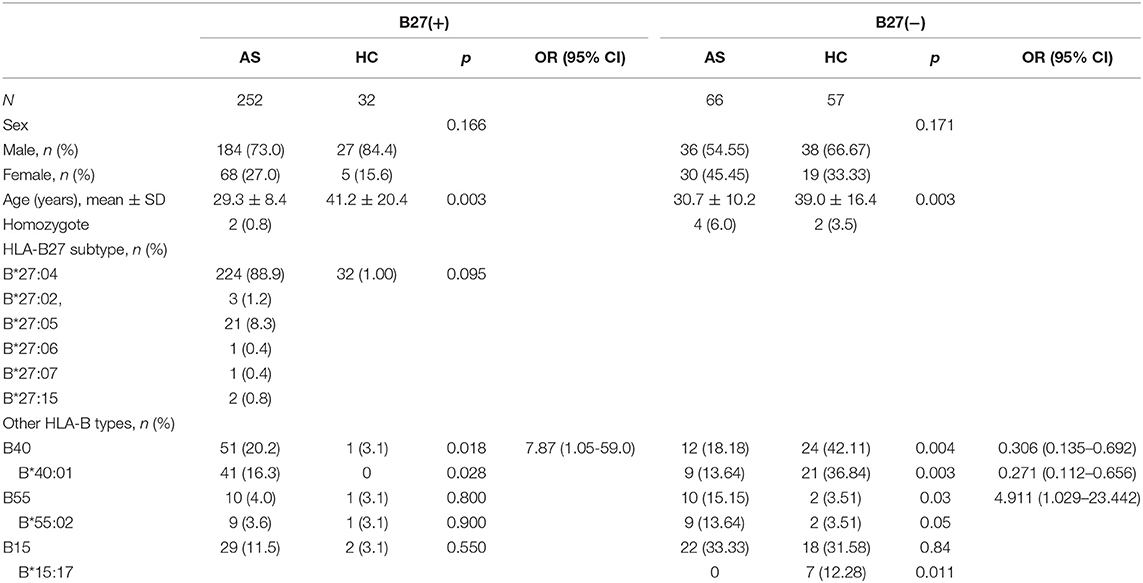
Table 2. The HLA-B27 subtypes in all B27(+) groups and the associated HLA-B types in B27(+) and B27(−) groups (step 1).
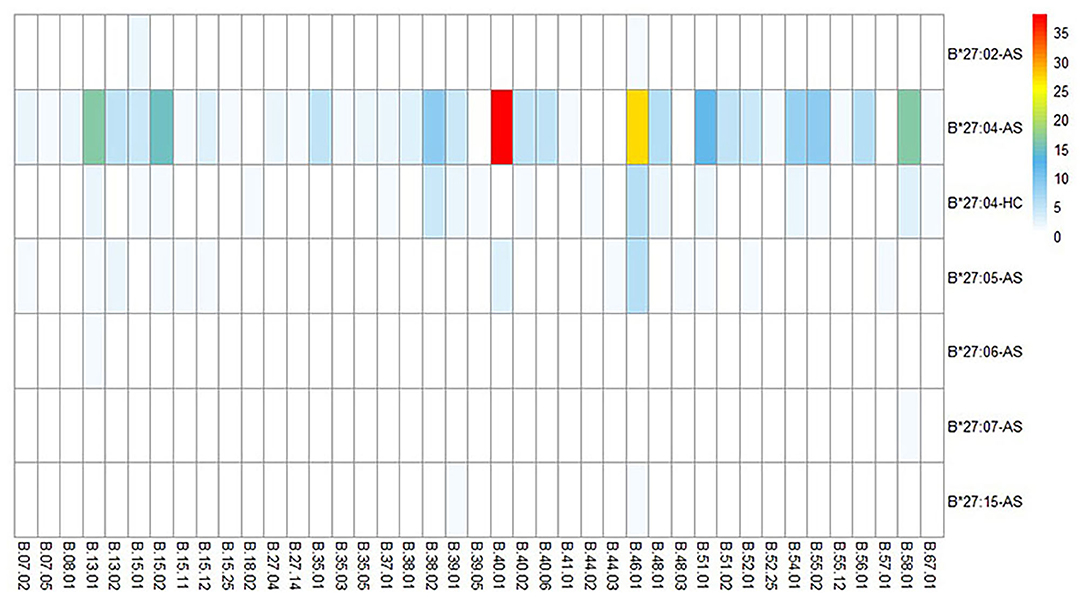
Figure 3. HLA-B alleles heatmap of different HLA-B27 subtypes combined with other HLA-B subtypes. Each square represents a genotype, like HLA-B*27:04/B*40:01. AS, ankylosing spondylitis; HC, health control.
Between 66 B27(−) patients and 57 B27(−) controls (Table 2), HLA-B40 was detected in 12 cases (18.18%) and 24 controls (42.11%) (p = 0.004). There was also a significant difference in HLA-B55 between the two groups (p = 0.03). At the high-resolution level, we found the number of HLA-B*40:01 and HLA-B*15:17 was significantly higher in the control group as compared to the case group (p < 0.05). The number range of every HLA-B heterozygous genotypes was 1 to 3 (Figure 4).
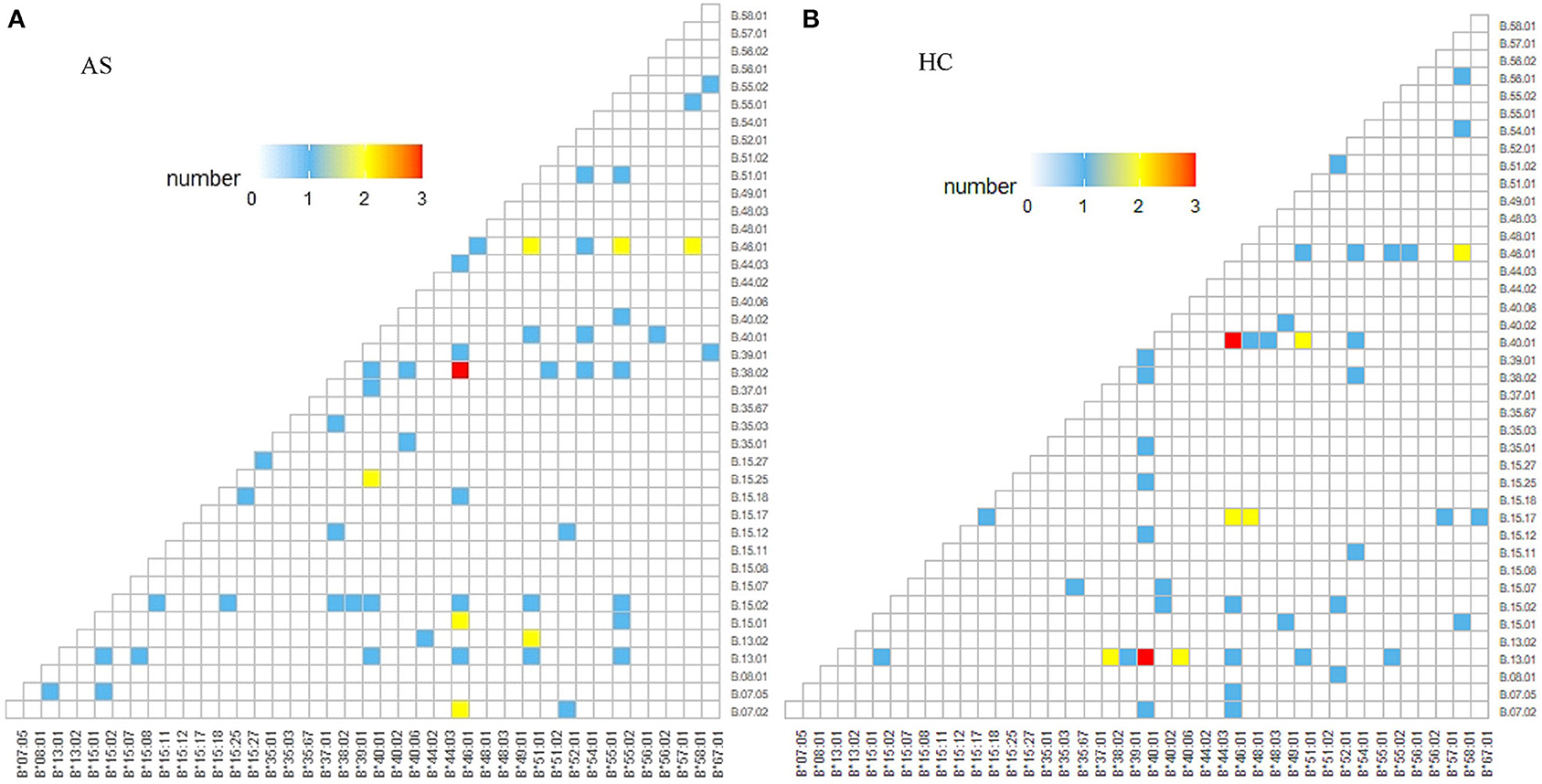
Figure 4. HLA-B alleles heatmap of HLA-B27(−) group. Each square represents a genotype, like HLA-B*40:01/B*46:01: (A) HLA-B alleles heatmap of HLA-B27(−) AS patients, and (B) HLA-B alleles heatmap of HLA-B27(−) controls. AS, ankylosing spondylitis; HC, health control.
Step 2
Comparisons of the Clinical Characteristics Between B27(+) and B27(−) AS Patients
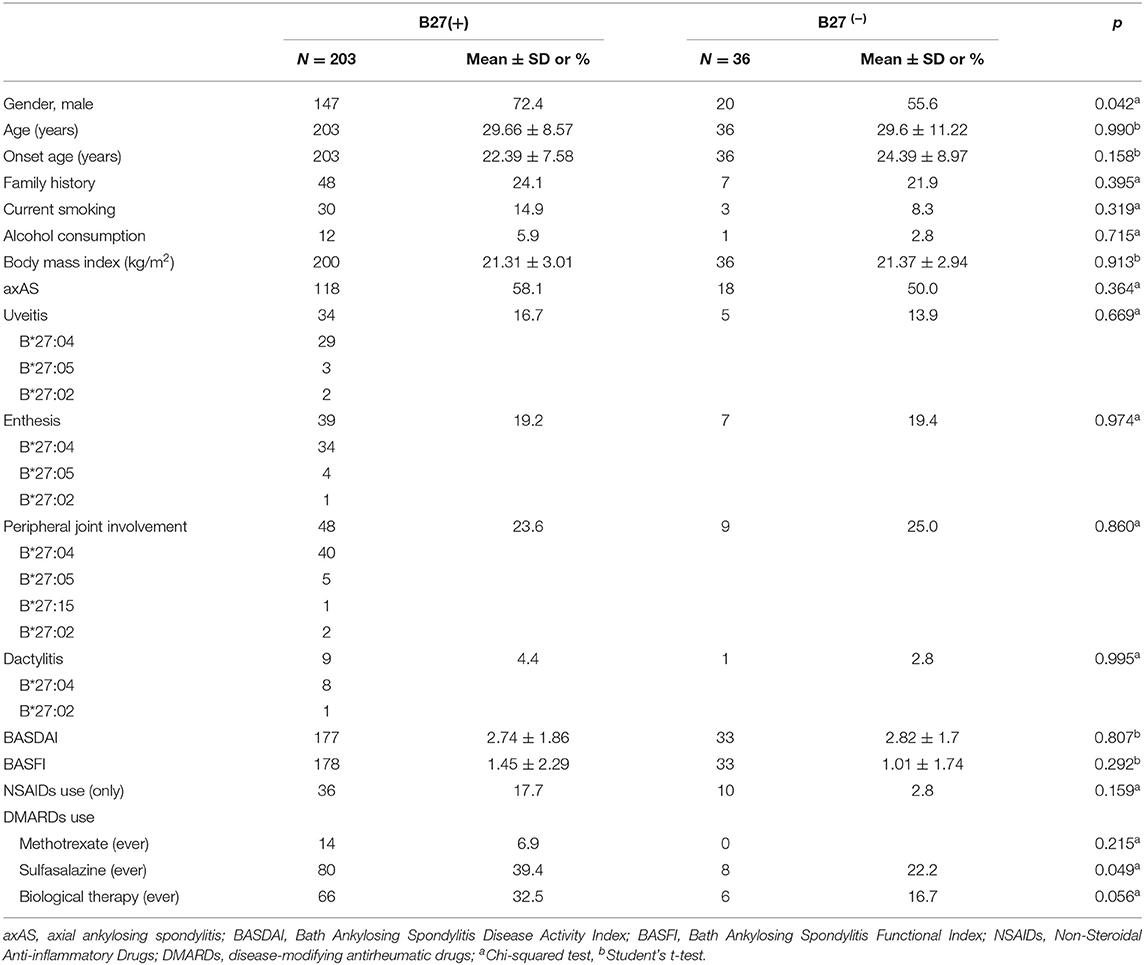
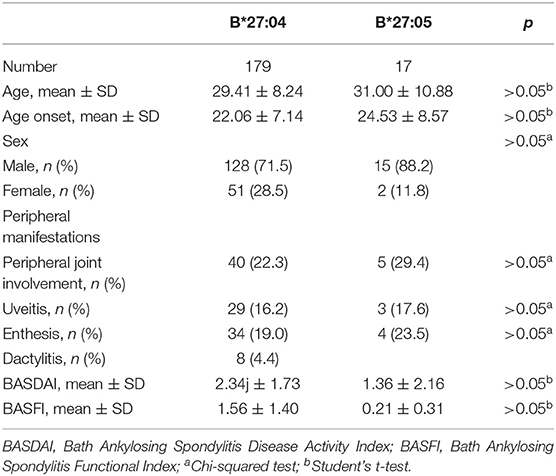
Two hundred thirty-nine patients had detailed clinical information, including 203 B27(+) and 36 B27(−) patients. As observed in Table 3, a significant difference was found in sex between two groups (p = 0.042), with more male participants in B27(+) patients. However, there was no statistical significance in the current age, age at symptom onset, family aggregation, smoking status, alcohol consumption, BMI, BASDAI, BASFI, peripheral manifestations (uveitis, peripheral joint involvement, dactylitis, and enthesitis), and medications. In B27(+) patients, we also compared clinical characteristics between HLA-B*27:04 and HLA-B*27:05 cases. We did not find any significant difference (Table 4).
The Low-Resolution HLA-B Genotypes Distribution in Different Peripheral Manifestations
We analyzed the low-resolution HLA-B genotypes of B27(+) patients. In 48 peripheral joint involvement patients with B27(+), 15 cases (31.25%) carried HLA-B46. HLA-B40 was observed in seven cases (14.58%). HLA-B58, HLA-B51, and HLA-B13 were detected in four cases (8%), respectively for each type (Figure 5A). In 39 B27(+) patients with enthesis (Figure 5D), HLA-B46, HLA-B40, and HLA-B15 were found in 11 cases (28.21%), nine cases (23.08%), and four cases (10.26%), respectively. As shown in Figure 5C, in 34 B27(+) patients with uveitis, eight cases (23.53%) carried HLA-B46. HLA-B40 and HLA-B15 were detected in seven cases (20.59%) and five cases (14.71%), respectively. There were nine B27(+) patients with dactylitis (Figure 5B), and HLA-B46 was observed in three cases (33.33%). Compared with HLA-B40 and other HLA-B types in B27(+) patients, HLA-B46 had relationships with peripheral joint involvement and enthesis, respectively (Table 5).
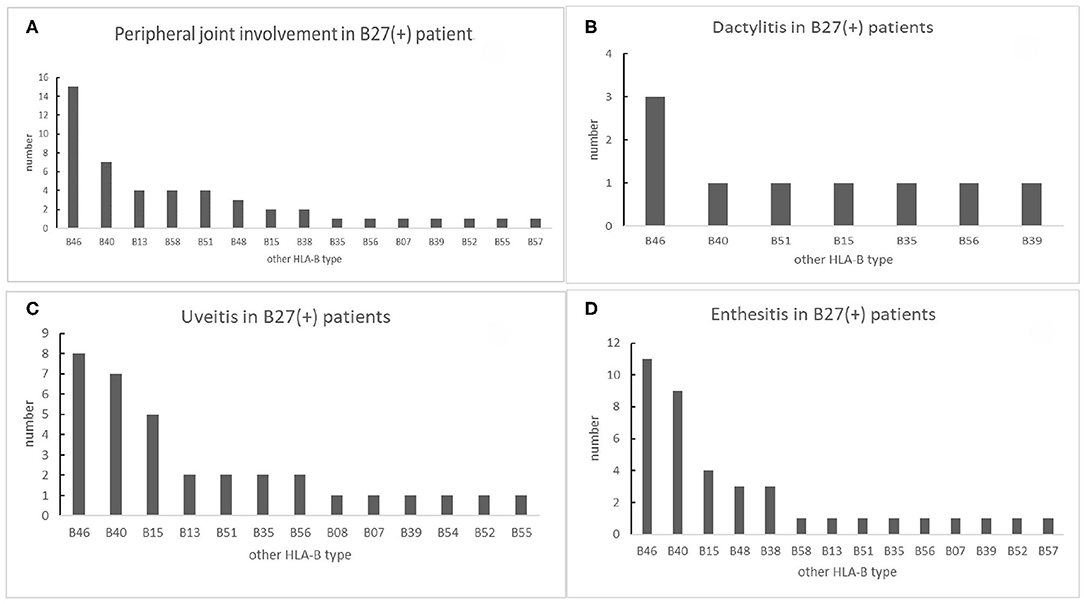
Figure 5. The number of other HLA-B types shown in B27(+) patients with different peripheral manifestations: (A) number of other HLA-B types shown in B27(+) patients with peripheral joint involvement, (B) number of other HLA-B types shown in B27(+) patients with dactylitis, (C) number of other HLA-B types shown in B27(+) patients with uveitis, and (D) number of other HLA-B types shown in B27(+) patients with enthesis.
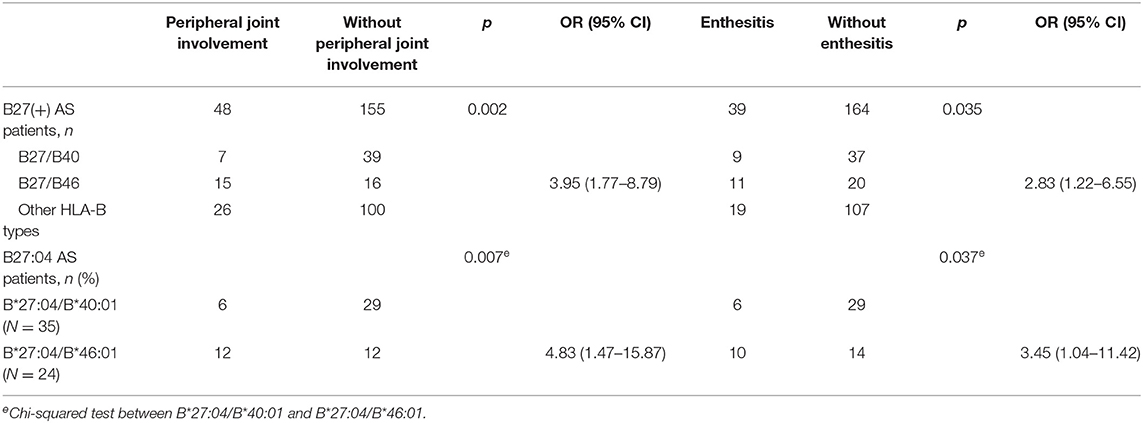
Table 5. Association of HLA-B40 and HLA-B46 with peripheral joint involvement in B27(+) AS patients (step 2).
In nine B27(−) patients with peripheral joint involvement patients, seven cases (77.78%) carried HLA-B15. There was a significant difference in HLA-B15 (p = 0.014) (Table 6). For a small number of HLA-B27(−) patients with other manifestations (uveitis and enthesitis), the HLA-B type distributions are shown in Figure 6. One patient with dactylitis has a HLA-B46/B58 in his HLA-B allele.
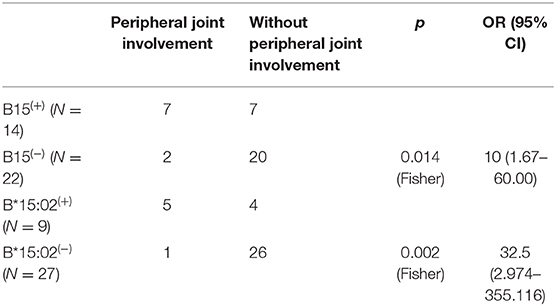
Table 6. HLA-B15 and HLA-B*15:02 had association with peripheral joint involvement in HLA-B27(−) AS patients (step 2).
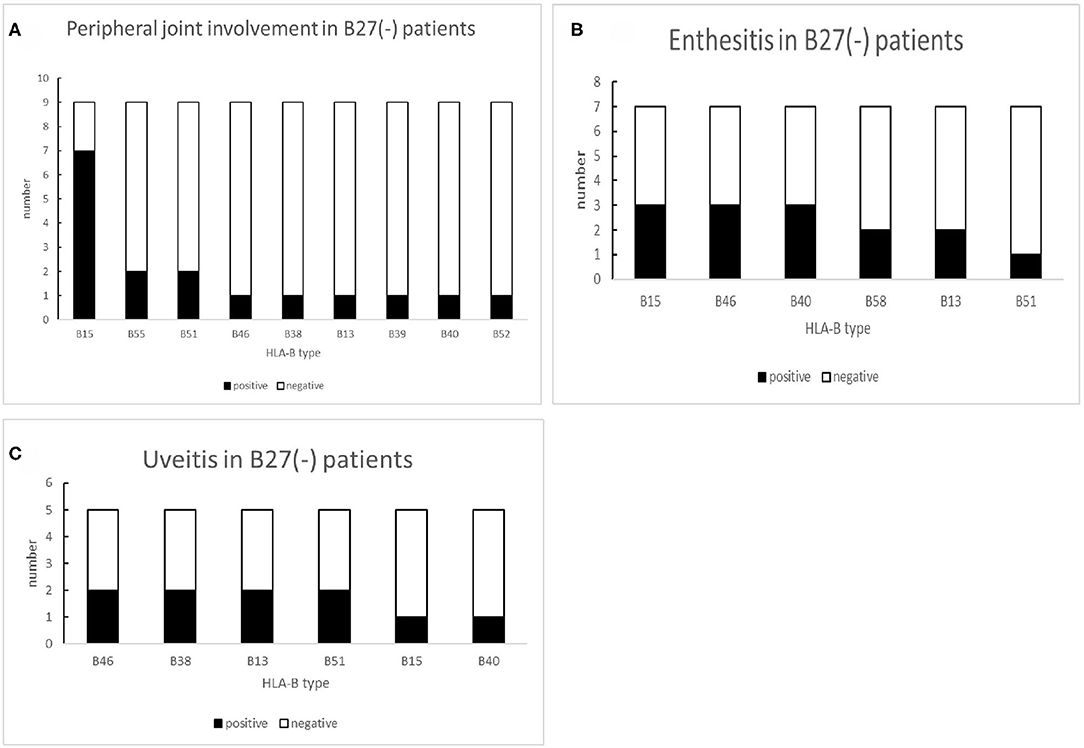
Figure 6. The number of other HLA-B types shown in B27(−) patients with different peripheral manifestations: (A) number of other HLA-B types shown in B27(−) patients with peripheral joint involvement, (B) number of other HLA-B types shown in B27(−) patients with uveitis, and (C) number of other HLA-B types shown in B27(−) patients with enthesis.
The Clinical Manifestations in HLA-B27 Subtypes Homozygote and Heterozygote AS Patients
The most frequent subtype was HLA-B*27:04 in the B27(+) group, so we further analyzed the clinical manifestations of HLA-B*27:04 homozygous and heterozygous AS patients. One of the HLA-B*27:04/B*27:04 patients was a woman who only had the axial phenotype. An X-ray showed that the front edge of each vertebra of the spine was straight, the facet joints of the thoracolumbar and lumbar vertebra were blurred, that there was bony ankylosing, scoliosis, atlantoaxial subluxation, bilateral sacroiliac joint fusion, narrowed hip space, osteoid destruction, and that the surface of the left ischial tuberosity was rough.
In HLA-B*27:04 heterozygous AS patients, HLA-B*27:04/B*40:01 was the most frequent genotype (N = 35), and the second was HLA-B*27:04/B*46:01(N = 24). But HLA-B*27:04/B*46:01 was significantly more frequent in patients with peripheral joint involvement compared to HLA-B*27:04/B*40:01 (p = 0.007, OR=4.833, 95% CI 1.472–15.867). The same to patients with enthesis (p = 0.037, OR=3.452, 95% CI 1.044–11.420) (Table 5). We did not find other significant peripheral manifestations. One patient with HLA-B*27:04/B*35:05 had all peripheral manifestations (uveitis, peripheral joint involvement, dactylitis, and enthesitis).
All HLA-B*27:05 samples were heterozygote patients. Seventeen patients had clinical information. Among five cases (29.4%) with arthritis, two patients were HLA-B*27:05/B*46:01, and one patient was HLA-B*27:05/B*40:01. There were no different clinical manifestations between B*27:04 and B*27:05 (Table 4). Other HLA-B27 subtypes were also heterozygous, and their clinical peripheral phenotypes are shown in Table 3. We found one HLA-B*27:02/B*15:01 patient with all the peripheral manifestations.
The Clinical Manifestations in HLA-B27(−) Homozygote and Heterozygote AS Patients
According to high-resolution HLA-B genotypes, in the 36 B27(−) cases with clinical information, the highest HLA-B type was HLA-B*46:01 (N = 11, 30.56%). HLA-B*15:02, HLA-B*13:01, HLA-B*38:02, and HLA-B*40:01 were tied for second (N = 6). As mentioned earlier, patients with peripheral joint involvement carried HLA-B15 more frequently, especially HLA-B*15:02, which also has an association with the phenotype in B27(−) patients [p = 0.002Fisher, OR=32.5, 95% CI (2.974–355.116)] (Table 6). However, the patient with HLA-B*15:02 homozygote was a man who only had axial manifestations in the same way as the HLA-B*27:04 homozygote patient.
Discussion
HLA-B27 as the major gene was closely related to the development of AS. The most prevalent subtypes are HLA-B*27:04 and HLA-B*27:05 in different populations. Other subtypes associated with the disease are B27:02, B*27:15, and so on. In our data, there were B*27:04, B*27:05, B*27:02, and B*27:15 in the patient group, and 88.9% of the patients were HLA-B*27:04. HLA-B27 positive patients had an earlier disease onset and higher family aggregation (12). HLA-B27 negative patients had a higher frequency of extra-spine manifestations (12). Research about Korean AS patients found that HLA-B27 homozygosity has no significant difference with heterozygosity on the clinical manifestations and radiographic progression (7, 8). Some research found only four homozygous of B*27:04 in 245 HLA-B27-positive AS patients (13). In our study, we found two HLA-B*27:04 homozygous, one HLA-B*27:04/B*27:14, and no homozygote of HLA-B*27:05, 27:02, or 27:15. Only two patients had axial symptoms. Perhaps other factors were associated with peripheral manifestations in AS patients.
In HLA-B27(+) patients, 20.2% of alleles showed as HLA-B40, and the primary subtype was HLA-B*40:01. And 16.9% of HLA-B*27:04 cases were HLA-B*27:04/B*40:01, which were not found in B27(+) controls. Samples with HLA-B*40:01 in HLA-B27(−) controls were more than B27(−) cases—perhaps as a result of the sample size. Other research found that 18.2% of AS patients carried B27/B40 and only 0.4% in healthy controls (14). HLA-B40 can increase HLA-B27 susceptibility to AS (15, 16). The different subtypes had different peripheral manifestations.
HLA-B46 can increase the risk of severe sacroiliitis development related to Japanese psoriatic arthritis (PsA) patients (17). The HLA-A2-B46-DR8 haplotype has a relationship with the levels of complement components (18). HLA-B*46:01 was the only subtype of HLA-B46 found in our data. In HLA-B27 AS, the frequency was second to that of HLA-B40. Relative to other HLA-B alleles, patients with HLA-B*27:04/B*46:01 had a higher prevalence of peripheral joint involvement. HLA-B*46:01 was associated with peripheral joint involvement in HLA-B*27:04 AS patients.
In our data, 11.5% of HLA-B27 patients combined with HLA-B15 and 33.33% in HLA-B27(−) patients. The major subtype was HLA-B*15:02. HLA-B*15:17 was found in seven controls, not AS patients. In undifferentiated SpA, HLA-B15 was increased (19). HLA-B15 can be an independent factor of peripheral SpA (20). In HLA-B27 negative patients, HLA-B15, especially HLA-B*15:02, had a relationship with peripheral joint involvement in patients. HLA-B15 may increase the risk of peripheral joint involvement in HLA-B27 negative patients.
HLA-B35 was associated with AS (21, 22). Previous research found that seven HLA-B27(−) AS families with idiopathic inflammatory bowel disease have a higher frequency of HLA-B15 (21). All five HLA-B*27:04/B*35:01 were patients. Three HLA-B27(−) patients carried HLA-B*35:03. One heterozygous patient with B*35:05/B*27:04 had multiple peripheral symptoms of uveitis, enthesitis, peripheral joint involvement, and dactylitis.
Allele HLA-B51 is associated with Behcet's disease (23), especially in ocular involvement (24). But some results showed that HLA-B27(+)B51(+) is a good factor of Behcet uveitis (25). HLA-B51 was present in autoimmune diseases other than Behcet's disease with high prevalence (26). Eighteen cases showed HLA-B27/B51 (including B*51:01 and B*51:02). Only one patient with HLA-B27:04/B*51:01 had uveitis and dactylitis.
HLA-B38 was associated with clozapine-induced agranulocytosis (27). In the Argentine and Israeli population, the HLA-B38 was associated with PsA (28, 29). But psoriatic arthritis patients with HLA-B38 had less back pain (30). In our data, no patients with HLA-B27/B38 showed psoriatic arthritis.
Seventeen B27(+) patients showed B58. There was no difference between patients and controls. As we all know, Allopurinol-induced severe cutaneous adverse drug reactions (SCAR) is strongly associated with the presence of HLA-B*58:01 (31). But no article has yet reported the relationship between B*58:01 and AS. Further study is necessary to explore the association.
In the present study, we evaluated the HLA-B genotype in AS patients compared to the control group. As a result, we found that more than 98% of the samples were heterozygous in the HLA-B region. HLA-B27 homozygous patients were rare and only had axial manifestations. Based on our study and other reports, for B27(+) people, HLA-B40 can increase the risk of AS. HLA-B40 was the second most common HLA-B subtype in all of the AS patients besides HLA-B27. Then the genotype HLA-B27:04/B*40:01 can improve diagnostic accuracy, and patients with HLA-B*27:04/B*46:01 had a high risk of arthritis and enthesitis. In the HLA-B27(−) group, HLA-B*15:02 was a risk maker of peripheral joint involvement. Perhaps HLA-B*40:01, HLA-B*46:01, and HLA-B*15:02 should be markers included in AS diagnosis value. Due to the limited information in this field and a small number of patients, our results did not show statistical significance in other HLA-B subtypes with peripheral clinical manifestations. There is a need for more samples and further workup on the relationship of the HLA-B heterozygous in AS patients.
In conclusion, our research shows that, besides HLA-B27, other HLA-B types also may impact the AS patient phenotype. It is critical to systematically screen and evaluate the HLA-B genotype in the patients with AS, which may result in an improved accurate diagnosis of the patients.
Data Availability Statement
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of Third Affiliated Hospital of Sun Yat-Sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JG conceived the study and critically revised the manuscript and provided final approval of the manuscript. JW, PZ, and XL were in charge of the experiment. XW performed the analysis. XZ, ZC, QL, LT, QW, and SC were in charge of collecting sample and data. XW wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by 5010 Subject (2016) of Sun Yat-Sen University. The Municipal Healthcare Joint-Innovation Major Project of Guangzhou (201604120013), Science and Technology Planning Project of Guangdong Province, China (2016A020216013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.568790/full#supplementary-material
References
1. Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors, Arthritis Rheum. (1998) 41:58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
2. Ng SC, Liao Z, Yu DT, Chan ES, Zhao L, Gu J. Epidemiology of spondyloarthritis in the People's Republic of China: review of the literature and commentary. Semin Arthritis Rheum. (2007) 37:39–47. doi: 10.1016/j.semarthrit.2007.01.003
3. Thomas GP, Brown MA. Genomics of ankylosing spondylitis. Discov Med. (2010) 10:263–71. doi: 10.1111/j.0105-2896.2009.00852.x
4. MacLean IL, Iqball S, Woo P, Keat AC, Hughes RA, Kingsley GH, et al. HLA-B27 subtypes in the spondarthropathies. Clin Exp Immunol. (1993) 91:214–9. doi: 10.1111/j.1365-2249.1993.tb05885.x
5. García-Fernández S, Gonzalez S, Miña BA, Martinez-Borra J, Blanco-Gelaz M, López-Vazquez A, et al. New insights regarding HLA-B27 diversity in the Asian population. Tissue Antigens. (2001) 58:259–62. doi: 10.1034/j.1399-0039.2001.580407.x
6. López-Larrea C, Sujirachato K, Mehra NK, Chiewsilp P, Isarangkura D, Kanga U, et al. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens. (1995) 45:169–76. doi: 10.1111/j.1399-0039.1995.tb02436.x
7. Kim TJ, Na KS, Lee HJ, Lee B, Kim TH. HLA-B27 homozygosity has no influence on clinical manifestations and functional disability in ankylosing spondylitis. Clin Exp Rheumatol. (2009) 27:574–9.
8. Kim TJ, Sung IH, Lee S, Joo KB, Choi JH, Park DJ, et al. HLA-B27 homozygosity has no influence on radiographic damage in ankylosing spondylitis: observation Study of Korean spondyloArthropathy Registry (OSKAR) data. Joint Bone Spine. (2013) 80:488–91. doi: 10.1016/j.jbspin.2012.12.003
9. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. (1984) 27:361–8. doi: 10.1002/art.1780270401
10. Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. (2011) 70:25–31. doi: 10.1136/ard.2010.133645
11. He Y, Li J, Mao W, Zhang D, Liu M, Shan X, et al. HLA common and well-documented alleles in China. HLA. (2018) 92:199–205. doi: 10.1111/tan.13358
12. Arévalo M, Gratacós MJ, Moreno M, Calvet J, Orellana C, Ruiz D, et al. Influence of HLA-B27 on the Ankylosing Spondylitis phenotype: results from the REGISPONSER database. Arthritis Res Ther. (2018) 20:221. doi: 10.1186/s13075-018-1724-7
13. Yi L, Wang J, Guo X, Espitia MG, Chen E, Assassi S, et al. Profiling of hla-B alleles for association studies with ankylosing spondylitis in the chinese population. Open Rheumatol J. (2013) 7:51–4. doi: 10.2174/1874312920130628001
14. van Gaalen FA, Verduijn W, Roelen DL, Böhringer S, Huizinga TW, van der Heijde DM, et al. Epistasis between two HLA antigens defines a subset of individuals at a very high risk for ankylosing spondylitis. Ann Rheum Dis. (2013) 72:974–8. doi: 10.1136/annrheumdis-2012-201774
15. Robinson WP, van der Linden SM, Khan MA, Rentsch HU, Cats A, Russell A, et al. HLA-Bw60 increases susceptibility to ankylosing spondylitis in HLA-B27+ patients. Arthritis Rheum. (1989) 32:1135–41. doi: 10.1002/anr.1780320912
16. Cortes A, Pulit SL, Leo PJ, Pointon JJ, Robinson PC, Weisman MH, et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun. (2015) 6:7146. doi: 10.1038/ncomms8146
17. Ikumi K, Kobayashi S, Tamura N, Tada K, Inoue H, Osaga S, et al. HLA-B46 is associated with severe sacroiliitis in Japanese patients with psoriatic arthritis. Mod Rheumatol. (2019) 29:1017–22. doi: 10.1080/14397595.2018.1538590
18. Muto M, Mashimo M, Urabe K, Suzuki T, Sasazuki T. Correlation between HLA-A2-Bw46-DRw8 haplotype and increased levels of complement components (C4 and C4a) in patients with psoriatic arthritis. Arch Dermatol Res. (1991) 283:347–9. doi: 10.1007/bf00376626
19. Vargas-Alarcón G, Londoño JD, Hernández-Pacheco G, Pacheco-Tena C, Castillo E, Cardiel MH, et al. Effect of HLA-B and HLA-DR genes on susceptibility to and severity of spondyloarthropathies in Mexican patients. Ann Rheum Dis. (2002) 61:714–7. doi: 10.1136/ard.61.8.714
20. Londono J, Santos AM, Peña P, Calvo E, Espinosa LR, Reveille JD, et al. Analysis of HLA-B15 and HLA-B27 in spondyloarthritis with peripheral and axial clinical patterns. BMJ Open. (2015) 5:e009092. doi: 10.1136/bmjopen-2015-009092
21. Said-Nahal R, Miceli-Richard C, Berthelot JM, Duché A, Dernis-Labous E, Le BG, et al. The familial form of spondylarthropathy: a clinical study of 115 multiplex families. Groupe Français d'Etude Génétique des Spondylarthropathies. Arthritis Rheum. (2000) 43:1356–65. doi: 10.1002/1529-0131(200006)43:6<1356::AID-ANR20>3.0.CO;2-Y
22. Wagener P, Zeidler H, Eckert G, Deicher H. Increased frequency of HLA-Bw62 and Bw35 CREG antigens in HLA-B27 negative ankylosing spondylitis. Z Rheumatol. (1984) 43:253–7.
23. de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behçet's disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. (2009) 61:1287–96. doi: 10.1002/art.24642
24. Shenavandeh S, Jahanshahi KA, Aflaki E, Tavassoli A. Frequency of HLA-B5, HLA-B51 and HLA-B27 in patients with idiopathic uveitis and Behçet's disease: a case-control study. Reumatologia. (2018) 56:67–72. doi: 10.5114/reum.2018.75516
25. Ahn JK, Park YG. Human leukocyte antigen B27 and B51 double-positive Behçet uveitis. Arch Ophthalmol. (2007) 125:1375–80. doi: 10.1001/archopht.125.10.1375
26. Salayandia L, Sibbitt W Jr., Bankhurst A, Fields R, Cook G, Konstantinov K, et al. HLA B51 and possible associated autoimmune disorders other than behcets disease: a retrospective cohort study [abstract]. Arthritis Rheumatol. (2015) 67 (Suppl. 10). Available online at: https://acrabstracts.org/abstract/hla-b51-and-possible-associated-autoimmunedisorders-other-than-behcets-disease-a-retrospective-cohort-study/
27. Lieberman JA, Yunis J, Egea E, Canoso RT, Kane JM, Yunis EJ. HLA-B38, DR4, DQw3 and clozapine-induced agranulocytosis in Jewish patients with schizophrenia. Arch Gen Psychiatry. (1990) 47:945–8. doi: 10.1001/archpsyc.1990.01810220061007
28. Schneeberger EE, Citera G, Rodríguez GG, Granel A, Arturi A, Rosemffet GM, et al. Clinical and immunogenetic characterization in psoriatic arthritis patients. Clin Rheumatol. (2015) 34:1413–8. doi: 10.1007/s10067-014-2739-3
29. Elkayam O, Segal R, Caspi D. Human leukocyte antigen distribution in Israeli patients with psoriatic arthritis. Rheumatol Int. (2004) 24:93–7. doi: 10.1007/s00296-003-0325-0
30. Yap KS, Ye JY, Li S, Gladman DD, Chandran V. Back pain in psoriatic arthritis: defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis. (2018) 77:1573–7. doi: 10.1136/annrheumdis-2018-213334
Keywords: ankylosing spondylitis, HLA-B40, HLA-B46, HLA-B genotype, peripheral joint involvement
Citation: Wu X, Wu J, Li X, Wei Q, Lv Q, Zhang P, Zheng X, Chen Z, Cao S, Tu L and Gu J (2021) The Clinical Characteristics of Other HLA-B Types in Chinese Ankylosing Spondylitis Patients. Front. Med. 7:568790. doi: 10.3389/fmed.2020.568790
Received: 02 June 2020; Accepted: 19 November 2020;
Published: 08 January 2021.
Edited by:
Dario Roccatello, University of Turin, ItalyReviewed by:
Cong-Qiu Chu, Oregon Health and Science University, United StatesRuben Burgos-Vargas, General Hospital of Mexico, Mexico
Copyright © 2021 Wu, Wu, Li, Wei, Lv, Zhang, Zheng, Chen, Cao, Tu and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, gujieruo@163.com
 Xinyu Wu
Xinyu Wu Jialing Wu
Jialing Wu Xiaomin Li
Xiaomin Li Qiujing Wei
Qiujing Wei Qing Lv1
Qing Lv1  Xuqi Zheng
Xuqi Zheng Zena Chen
Zena Chen Liudan Tu
Liudan Tu Jieruo Gu
Jieruo Gu