Combination of Isoflurane and Propofol as General Anesthesia During Orthopedic Surgery of Perioperative Cerebral Hypoperfusion Rats to Avoid Cognitive Impairment
- 1Department of Anesthesiology, The Third Central Clinical College of Tianjin Medical University, Tianjin, China
- 2Tianjin Key Laboratory of Extracorporeal Life Support for Critical Diseases, Artificial Cell Engineering Technology Research Center, Tianjin Institute of Hepatobiliary Disease, Tianjin, China
- 3The Third Central Hospital of Tianjin, Tianjin, China
- 4Tianjin Third Central Hospital, Nankai University, Tianjin, China
- 5Department of Anesthesiology, University of Hong Kong, Hong Kong, China
- 6State Key Laboratory of Medicinal Chemical Biology, Key Laboratory of Bioactive Materials for Ministry of Education, College of Medicine, Nankai University, Tianjin, China
- 7Tianjin Research Institute of Anesthesiology, Tianjin, China
Background: Perioperative cerebral hypoperfusion (CH) is common, although the underlying mechanism of cognitive impairment that results due to perioperative cerebral hypoperfusion remains to be determined. Isoflurane anesthesia induces neuronal injury via endoplasmic reticulum (ER) stress, whereas a sub-anesthetic dose of propofol improves postoperative cognitive function. However, the effects of the combination of isoflurane plus propofol, which is a common aesthetic combination administered to patients, on ER stress and cognition remain unknown.
Methods: We sought to determine the effects of isoflurane plus propofol on ER stress and cognitive function in rats insulted by cerebral hypoperfusion. Ligation of the bilateral common carotid arteries (CCA) was adopted to develop the cerebral hypoperfusion rat model. A second surgery, open reduction and internal fixation (ORIF), requiring general anesthesia, was performed 30 days later so that the effects of anesthetics on the cognitive function of CH rats could be assessed. Rats received isoflurane alone (1.9%), propofol alone (40 mg·kg−1·h−1) or a combination of isoflurane and propofol (1% and 20 mg·kg−1·h−1 or 1.4% and 10 mg·kg−1·h−1). Behavioral studies (contextual fear conditioning [FC] test), histological analyses (Nissl staining) and biochemical analyses (western blotting of the harvested rat brain tissues) were employed.
Results: Hippocampus-dependent memory of rats in group IP1 (1% isoflurane plus 20 mg·kg−1·h−1 propofol) was not impaired, and expression level of γ-aminobutyric acid A type receptor α1 subunit, a key cognition-related protein, remained normal. ER stress alleviator, binding immunoglobulin protein, increased extremely while ER stress transcription factor, C/EBP homologous protein, showed no statistical difference compared with the control group. Numbers of surviving neurons confirmed the substantial neuronal damage caused by propofol or isoflurane alone.
Conclusions: These data suggest that ER stress contributes to the underlying mechanism of cognitive impairment and that the combination of isoflurane and propofol did not aggravate cognitive impairment and ER stress in aging rats with CH that were further subjected to ORIF surgery.
Introduction
Perioperative neurocognitive disorders (PND) have become the most common complications after routine surgical procedures, particularly in the elderly (1, 2). Following surgery (e.g., common orthopedic procedures), up to 50% of patients experience cognitive disturbances that can lead to serious complications, including poorer prognosis and a higher 1-year mortality rate in subjects with pre-existing neurodegeneration (3). Carotid artery stenosis (CAS) can be detected in 75% of men and 62% of women aged ≥65, with a stenosis extent of ≥50% occurring in 7% of men and 5% of women in this age group (4). CAS is an independent risk factor for chronic cerebral hypoperfusion (CH) (5), which reduces tissue oxygen levels and leads to oxidative stress and endothelial injury (6). In rodents, experimental chronic CH can be initiated by occlusion of the major arterial supply. And chronic CH could lead to mitochondrial dysfunction and protein synthesis inhibition. These effects may destroy the balance of anti-oxidases and reactive oxygen species (ROS) and produce oxidative damage. Oxidative injury to vascular endothelial cells, glia, and neurons also impair vascular function and neurovascular coupling, which may result in a vicious cycle that further reduces cerebral perfusion (7). Taking all these factors into account, aging orthopedic patients with preoperative carotid stenosis make up a population that needs to be treated carefully. Special caution on the selection of anesthetic drugs is needed to protect cognitive function.
We and others (8–10) previously reported that two commonly used anesthetics, isoflurane, and propofol, have opposite effects on cognitive function at certain doses. Isoflurane induces neuronal injury upon prolonged exposure to high doses (11), with an underlying mechanism linked to endoplasmic reticulum (ER) stress. By contrast, propofol at a sub-anesthetic dosage protects against neuronal damage due to cerebral ischaemia reperfusion injury, and such protective effects were not observed at a higher dose (12). We, therefore, tested the effect of partially replacing isoflurane with a sub-anesthetic dose of propofol (combined use of isoflurane and propofol) on the cognitive function of rats with CH in the current study. Previous studies showed that isoflurane minimum alveolar concentration (MAC) value was 1.45 ± 0.17%. 1.9% isoflurane, equivalent to 1.3 MAC, was sufficient to induce general anesthesia in rats (13), while a minimal infusion rate at 40 mg·kg−1·h−1 was required using propofol alone to induce general anesthesia in rats (14). Therefore, in our study, doses were carefully selected combining isoflurane and propofol (1% and 20 mg·kg−1·h−1 or 1.4% and 10 mg·kg−1·h−1) to ensure the required depth of general anesthesia.
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter (15). The major subtype of GABAA receptor (GABAAR) contains the α1 subunit. According to a previous study, GABA receptors in the central nervous system are divided into three types: A, B, and C. Among the three receptors, GABAAR is the earliest expressed and most widely distributed, found mainly in the hippocampus, the prefrontal cortex and the striatum. Studies have confirmed that most GABAergic synaptic transmission in the mammalian brain is mediated by GABAAR. It is also an important target receptor for central nervous system (CNS) general anesthetics, such as propofol and isoflurane. GABAAR consists of five subunits embedded in the cell membrane of neurons. At the center, a 0.5 mm diameter GABA-gated Cl− channel is formed. When GABA binds to GABAAR, the Cl− channel of the postsynaptic membrane is opened, and Cl− enters the cell due to the concentration gradient. The potential increases to produce hyperpolarization, which in turn causes neuronal inhibition (16, 17). In 2014, Labrakakis et al. confirmed that the post-synaptic membrane GABAAR subunit composition determines the heterogeneity of inhibitory postsynaptic potential (IPSP), namely, GABAAR function (18). The native GABAARs present in the mammalian brain are mainly composed of α, β, and γ subunits. The most common configuration is a transmembrane pentamer composed of 2α12β2γ2, accounting for 43% of all GABAAR configurations and representing the most abundant configuration in the hippocampus and the cerebral cortex (18). The GABAAR α1 subunit, which is related to cognition, is the most widely distributed in the mammalian brain, (19, 20). Its main function is to maintain CNS arousal and the sensitivity of the receptor to sedative hypnotics (propofol, isoflurane, etc.). Mutation of the M2 domain Ser270 and the M3 domain Ala291 in the α1 subunit affects the potency of isoflurane and propofol on GABAARs (21). Kelley et al. confirmed that cerebral ischaemia can induce miniature inhibitory postsynaptic current (mIPSC) reduction and GABA-activated current inhibition (22). Further studies found that mIPSC frequency and kinetic parameters did not change, only amplitude decreased, while oxygen-glucose deprivation (OGD) inhibited neuronal GABAAR α1 subunit expression (22). This finding suggests that the change in GABAAR activity is triggered by a decrease in the expression of its functional subunit α1. Furthermore, our previous study showed that a sub-anesthetic dose (20 mg·kg−1·h−1) of propofol exerts post-treatment brain protection by activating the KCC2-GABAAR pathway. Propofol post-treatment can reverse the decrease in hippocampal IPSCs after OGD injury, promote KCC2 expression, and maintain the normal function of GABAAR. However, administration of KCC2 antagonists only partially reversed the effect of propofol on mIPSC (23). IT remains unknown whether or not cerebral ischaemia triggers the expression change and structural regulation of GABAAR functional subunit protein. Is there any upstream mechanism other than KCC2 that regulates the GABAAR structure, thereby affecting its function? To answer these questions, we chose the GABAAR α1 subunit as a target of research in this study.
GABAAR undergoes post-synthesis modification and folding in the ER. Prolonged ER stress has been well-known to be related with neurodegenerative diseases (24, 25). The unfolded protein response (UPR) triggered by ER stress is an important quality control system for maintaining protein homeostasis (Proteostasis). Proteostasis refers to an equilibrium state of specific protein synthesis, folding and unfolding, modification and degradation in the intracellular proteome at a specific time point. The ER of the cell is a site for the folding and post-translational processing of secreted proteins and membrane proteins (~1/3 of the human proteome). Binding immunoglobulin protein (BiP), also known as glucose-regulated protein 78 (GRP78), is an ER chaperone protein whose expression is part of the UPR and is required to alleviate ER stress (26). Once ER stress occurs, BiP binds to unfolded proteins and activates downstream receptor proteins, increasing molecular chaperone expression, reducing global protein translation, and increasing unfolded/misfolded proteins. It degrades and reduces ER stress and protects cells through endoplasmic reticulum-associated degradation (ERAD).
The expression of C/EBP homologous protein (CHOP) is acknowledged as a specific and transcription factor of ER stress (27). It expresses at a very low level in normal physiology, but cellular stress leads to high-level expression (28). During stress, UPR attempts to increase protein-folding capacity and remove misfolded and unfolded proteins. If homeostasis is inadequately restored under chronic ER stress, terminal UPR will trigger apoptosis through abundant signaling mechanisms, mainly mediated by CHOP, c-Jun N-terminal kinase (JNK), and caspase-12, with CHOP as the most widely studied (29).
Thus, the expression levels of BiP, CHOP and the GABAAR α1 subunit were used to evaluate the cellular mechanisms accounting for the neural substrate conditions that allow normal cognitive functions in this study.
The objective of the current study was to explore general anesthetics for rats with CH that are subjected to ORIF surgery to protect cognitive function. By using behavioral and biochemical analyses, we tested the hypothesis that a combination of isoflurane and propofol better protects cognitive function than isoflurane or propofol administered alone during ORIF surgery.
Materials and Methods
In our study, a ligation of bilateral CCA surgery (30) was adopted to prepare rats as CH animal model (31). A second surgery, ORIF (32), requiring general anesthesia, was operated 30 days later so that the effects of anesthetics on cognitive function of these CH rats could be assessed.
Animals
Male Wistar rats, 16–18 months of age and 450–570 g in weight, were purchased from the Academy of Military Medical Science of the Chinese People's Liberation Army and housed in groups of six per cage (545 mm in length, 395 mm in width, and 200 mm in hight) with ad libitum access to food and water. The housing environment was maintained at a temperature of 20–22°C and a humidity of 45–65% under a 12 h light/dark cycle. All animal experiments were carried out according to the Guide for the Care and Use of Laboratory Animals (33) and were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University. Rats were housed individually per cage (380 mm in length, 325 mm in width, and 180 mm in hight) 3 days before ligation of the CCA and fasted 12 h before surgery a normally supply of drinking water. After surgery, rats were also housed individually per cage for recovery.
Ligation of the CCA
Rats were first anesthetized with intraperitoneal (i.p.) injection of 10% thiobutabarbital (100 ml/kg). After disappearance of body motion and the righting reflex, the rat was fixed on the operation platform. The surgical field was maintained sterile throughout the entire procedure. The skin of the rat's neck was shaved and disinfected with iodine tincture. A median incision of ~2–3 cm was made in the neck. The muscles and surrounding tissues were separated to expose the CCA. The CCA and a blunt end syringe needle (0.45 mm in diameter, 1 cm in length) were ligated tightly at the proximal side 1.5 cm from the bifurcation of the internal and external carotid arteries. The slipknot was firmly fixed, and the needle was carefully removed. The wound was sutured and disinfected. During surgery, a heating lamp was used to help maintain the body temperature of anesthetized rats at 37 ± 0.5°C (30).
Anesthesia and ORIF Surgery
During ORIF surgery, rats were administered isoflurane via inhalation or propofol through tail vein injection. For the induction phase of anesthesia, the rat was placed in a transparent chamber (W 25 cm × D 15 cm × H 10 cm) connected to a vaporizer and anesthetized with 5% isoflurane and 40% oxygen. When the rat's righting reflex disappeared, the chamber was replaced by a mask. Each rat was then assigned to one of the following 5 groups (n = 32/group) and administered the respective anesthesia as maintenance: (1) Group C: local administration of anesthesia with 2% lidocaine and inhalation with air containing 40% oxygen via the mask for 3 h; (2) Group I: inhalation with air containing 40% oxygen and 1.9% isoflurane for 3 h; (3) Group P: venous transfusion with 40 mg·kg−1·h−1 propofol and inhalation with air containing 40% oxygen via the mask for 3 h; (4) Group IP1: venous transfusion with 20 mg·kg−1·h−1 propofol and inhalation with air containing 40% oxygen and 1% isoflurane for 3 h; and (5) Group IP2: venous transfusion with 10 mg·kg−1·h−1 propofol and inhalation with air containing 40% oxygen and 1.4% isoflurane for 3 h. The concentration of isoflurane was detected continuously by a gas monitor (Puritan-Bennett; Tewksbury, MA, USA) during the surgery.
ORIF surgical model: Under different modes of general anesthesia, the rats underwent an open tibial fracture of the left hind paw with intramedullary fixation. Supplemental analgesia was provided using <1 ml buprenorphine (0.3 mg/kg in saline) administered intraperitoneally (32). Surgery was carried out via aseptic techniques. The left hind paw of the rat was shaved and disinfected with iodine tincture. After the skin was incised, a 0.38 mm pin was inserted into the intramedullary canal. Once the tibia was internally fixated, the bone was fractured at the middiaphysis (tibial, midshaft) using surgical pliers. The skin was sutured with 8/0 Prolene sutures. In Group C, only the skin was incised and sutured. During surgery, a heating lamp was used to help maintain the body temperature of the anesthetized rats at 37 ± 0.5°C. Postintervention rats were moved to heated pads for recovery and then returned to their home cage supplied with sufficient food and water. For post-procedural pain relief, the rats were administered buprenorphine (0.05 mg/kg, subcutaneous) twice daily for 3 days (34).
Contextual Fear Conditioning Test
The contextual FC test was utilized to evaluate cognitive function (35). The contextual FC test consisted of a training phase at 24 h prior to ORIF surgery and an evaluation phase on days 1 and 7 after ORIF (36), when hippocampal-dependent memory was assessed (37).
During the training phase, rats were placed in a chamber (Ugo Basile, Italy) and allowed to adapt to the environment for 120 s. After adaption, a 20 s 70-dB tone (conditional stimulus) was delivered, followed by an interval of 25 s. After the interval, an 0.70 mA electrical foot shock was delivered to the rat for 2 s (unconditional stimulus). After six pairs of conditional-unconditional stimuli, the rats learned the association and had established long-term memory. The pairs of conditional-unconditional stimuli were separated by 60 s inter-training intervals. Each training chamber was cleaned with 75% ethyl alcohol before placement of the next rat and was illuminated only with a 10 W bulb in a dark experimental room.
During the evaluation phase, rats were placed again in the training chamber for 5 min without tone and foot shock (38). Each animal's freezing behavior (without any movements) was analyzed by using the ANY-Maze Video Tracking System (Stoelting, Illinois, USA). The percentage of time spent exhibiting freezing behavior was calculated using the formula of 100*f/5 min, where f was the total of freezing time within 5 min. The results in this experiment were used to assess hippocampus-dependent memory (35, 37).
Nissl Staining
On days 1 and 7 after ORIF, rats (n = 8/group) were first anesthetized with 10% thiobutabarbital (100 ml/kg, i.p.). Rats were perfused with saline before the heart stopped, followed by perfusion with 4% paraformaldehyde solution. Then, the brain was taken out and fixed in 4% paraformaldehyde for 24 h. Coronal slices (3.0-mm thick) from each brain containing the dorsal hippocampus and the medial dorsal prefrontal cortex were dehydrated and embedded in paraffin. A series of 10-μm-thick coronal sections was obtained from each slice, and the sections were stained with cresyl violet (39). For each brain, five sections at the dorsal hippocampus located at coordinates −3.14 from the bregma to −4.52 from bregma were analyzed for Ammon's horn pyramidal cell counts (40). Sections were examined by an observer who was blinded to the experimental conditions under light microscopy at a magnification of 200x. The number of surviving neurons in a 30,000 μm2 area of the CA1 was counted in each section. Only pyramidal neurons showing normal morphology with distinct cytoplasmic and nuclear outlines and a visible nucleolus were counted. Analysis of the data was performed by using Image Pro Plus 6.0 software (Media Cybernetics Co., USA).
Western Blotting
On days 1 and 7 after ORIF, rats (n = 8/group) were sacrificed with sodium pentobarbital (240 mg/ml, Department of Pharmacy, Tianjin Medical University General Hospital, i.p., 800 mg/kg) (41). After ensuring that the heart of the rat had stopped, the brain was removed, and the hippocampal tissue was separated. To obtain total cellular protein, the hippocampus was homogenized in RIPA solution (Biomart, Beijing, China) buffer and then centrifuged at 4°C at 12,000 r/min for 10 min (Sigma 3–30KS, Sigma Laboratory Centrifuges, Germany). Membrane protein fractions were obtained with a Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific, USA). The quantity of protein in the supernatant was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Beijing, China). Equal amounts of protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. Then, the membranes were blocked by 5% skim milk Tris-buffered saline containing 0.1% Tween (TBST) buffer for 90 min and washed with TBST buffer for 5 min. The membranes were incubated with the following primary antibodies: anti-GABAAR α1 (1:1,000, Abcam, Cambridge, UK), anti-BiP (1:1,000; Abcam), anti-pan-cadherin (1:2,000, Sigma, St. Louis, MO, USA), and anti-β-actin (1:10,000, Proteintech, Wuhan, China) overnight at 4°C. After washing with TBST 5 times (each for 5 min), the membranes were incubated with a secondary polyclonal antibody conjugated to horseradish peroxidase, anti-rabbit immunoglobulin G (IgG) (1:5,000, KPL, Gaithersburg, MD), and anti-mouse IgG (1:5,000, KPL) at room temperature for 1 h. The membranes were again washed 5 times (each for 5 min) and treated with an enhanced chemiluminescence detection kit (EMD Millipore, Billerica, MA, USA). The intensity of each band was quantified by densitometry using a gel image analysis software (Image Pro Plus, Media Cybernetics, USA). Relative expression was normalized to the expression of anti-pan-cadherin (1:2,000, Sigma) and anti-β-actin (1:10,000, Proteintech).
Statistical Analysis
The data were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation (SD). Behavioral data were tested using a two-way analysis of variance (ANOVA) with repeated measures. Other data were analyzed using a one-way ANOVA with Tukey post-hoc comparisons. P < 0.05 was the criterion for statistical significance.
Results
Combination Treatment With 1% Isoflurane and 20 mg·kg−1·h−1 Propofol Protected Cognitive Function in Aging Rats With CH and Being Subjected to an ORIF Surgery
To observe the effects of different dosages of isoflurane and propofol on cognitive function, a contextual FC test was performed on the first and seventh days after ORIF. The percentage of freezing time in Group C and Group IP1 was not significantly different on the first day (C vs. IP1: 44.23 ± 6.60 vs. 42.86 ± 7.12, P = 1.00) or the seventh day (C vs. IP1: 35.70 ± 5.21 vs. 34.85 ± 5.02, P = 1.000) after ORIF (Figure 1A). However, in Groups IP2, I, and P, the percentage of freezing time was significantly reduced compared with Group C on day 1 (C vs. IP2: 44.23 ± 6.60 vs. 31.55 ± 5.68; C vs. I: 44.23 ± 6.60 vs. 22.86 ± 3.53; C vs. I: 44.23 ± 6.60 vs. 21.32 ± 3.42; all P < 0.05) and day 7 (C vs. IP2: 35.70 ± 5.21 vs. 28.48 ± 2.54; C vs. I: 35.70 ± 5.21 vs. 21.34 ± 2.12; C vs. I: 35.70 ± 5.21 vs. 22.16 ± 2.74; all P < 0.05) (Figure 1A). The results suggest that the combination of 1% isoflurane and 20 mg·kg−1·h−1 propofol could protect cognitive function, while other dosages could not.
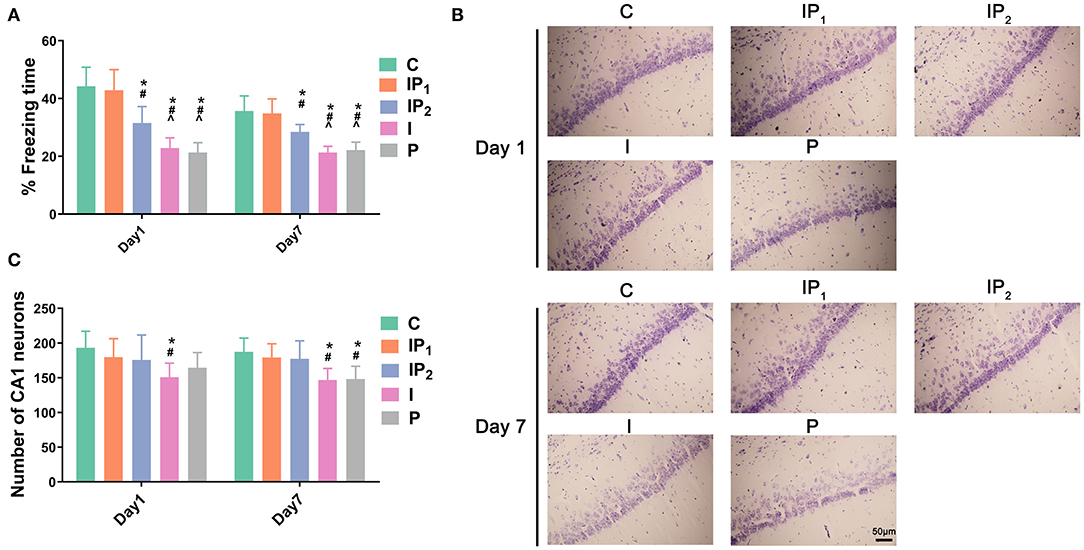
Figure 1. Combined treatment with 1% isoflurane and 20 mg·kg−1·h−1 propofol protected cognitive function and survival neurons in CH rats. (A) Hippocampus-dependent memory was evaluated as the percentage of freezing time on day 1 and day 7 after ORIF. Data are expressed as the mean ± SD (n = 8/group). Note that ORIF resulted in a significant reduction in the time of freezing behavior in the CA1 in Groups IP2, I, and P, which was prevented by the anesthetic schedule in Group IP1. (B) Nissl staining images of the hippocampal CA1 region were used to evaluate neuronal damage on day 1 and day 7 after ORIF. Note that ORIF resulted in a significant reduction in the number of remaining pyramidal neurons in the CA1 in Groups IP2, I, and P, which was prevented by the anesthetic schedule in Group IP1. (C) Quantification of surviving neurons in the CA1 on day 1 and day 7 after ORIF. Data are expressed as the mean ± SD (n = 8/group). *P < 0.05 compared with Group C; #P < 0.05 compared with Group IP1; ∧P < 0.05 compared with Group IP2. Scale bars = 50 μm.
Treatments With Isoflurane or Propofol Alone Were Not Able to Prevent CA1 Neuronal Death in Aging Rats With CH That Were Subjected to ORIF Surgery
Hippocampal slices were stained with cresyl violet (Nissl staining) to investigate potential neuronal damage caused by anesthetics on days 1 and 7 after ORIF. Compared with Group C, the number of surviving neurons decreased 1 day after ORIF only in Group I (C vs. I: 193.13 ± 23.94 vs. 150.88 ± 20.19, P = 0.039, Figures 1B,C). On the seventh day after ORIF, the number of surviving neurons in Groups I and P was significantly lower than that in Group C (C vs. I: 187.38 ± 19.86 vs. 146.75 ± 16.70, P = 0.008; C vs. P: 187.38 ± 19.86 vs. 148.13 ± 18.39, P = 0.011). No significant changes were found in Groups IP1 and IP2 on day 1 (C vs. IP1: 193.13 ± 23.94 vs. 179.75 ± 26.60, P = 0.923; C vs. IP2: 193.13 ± 23.94 vs. 175.75 ± 35.94, P = 0.799) or day 7 (C vs. IP1: 187.38 ± 19.86 vs. 179.13 ± 19.96, P = 0.975; C vs. IP2: 187.38 ± 19.86 vs. 177.25 ± 26.02, P = 0.940) (Figures 1B,C).
Combination Treatment With 1% Isoflurane and 20 mg·kg−1·h−1 Propofol Maintained the Expression Level of Cell GABAAR α1 in the Hippocampus
As described above, GABAAR α1 is a key functional component of the neural substrate involved in cognitive functions. Therefore, western blotting was performed on the first and seventh days after ORIF to evaluate the membrane expression of the GABAAR α1 subunit. Cadherin was used as positive control membrane marker (42, 43). There was no difference in the expression of GABAAR α1 between Group C and Group IP1 on day 1 (C vs. IP1: 100.00 ± 18.48 vs. 91.86 ± 15.45, P = 0.629) or day 7 (C vs. IP1: 100.00 ± 14.72 vs. 112.39 ± 20.17, P = 0.261) after ORIF. The expression of GABAAR α1 was downregulated after ORIF in Groups IP2, I, and P compared with Group C on day 1 (C vs. IP2: 100.00 ± 18.48 vs. 57.57 ± 8.39, P < 0.005; C vs. I: 100.00 ± 18.48 vs. 18.02± 3.07, P < 0.001; C vs. P: 100.00 ± 18.48 vs. 16.90 ±3.45, P < 0.001) and day 7 (C vs. IP2: 100.00 ± 14.72 vs. 56.23 ± 8.12, P < 0.001; C vs. I: 100.00 ± 14.72 vs. 27.92 ± 4.39, P < 0.001; C vs. P: 100.00 ± 14.72 vs. 24.71 ±4.01, P < 0.001) (Figure 2).
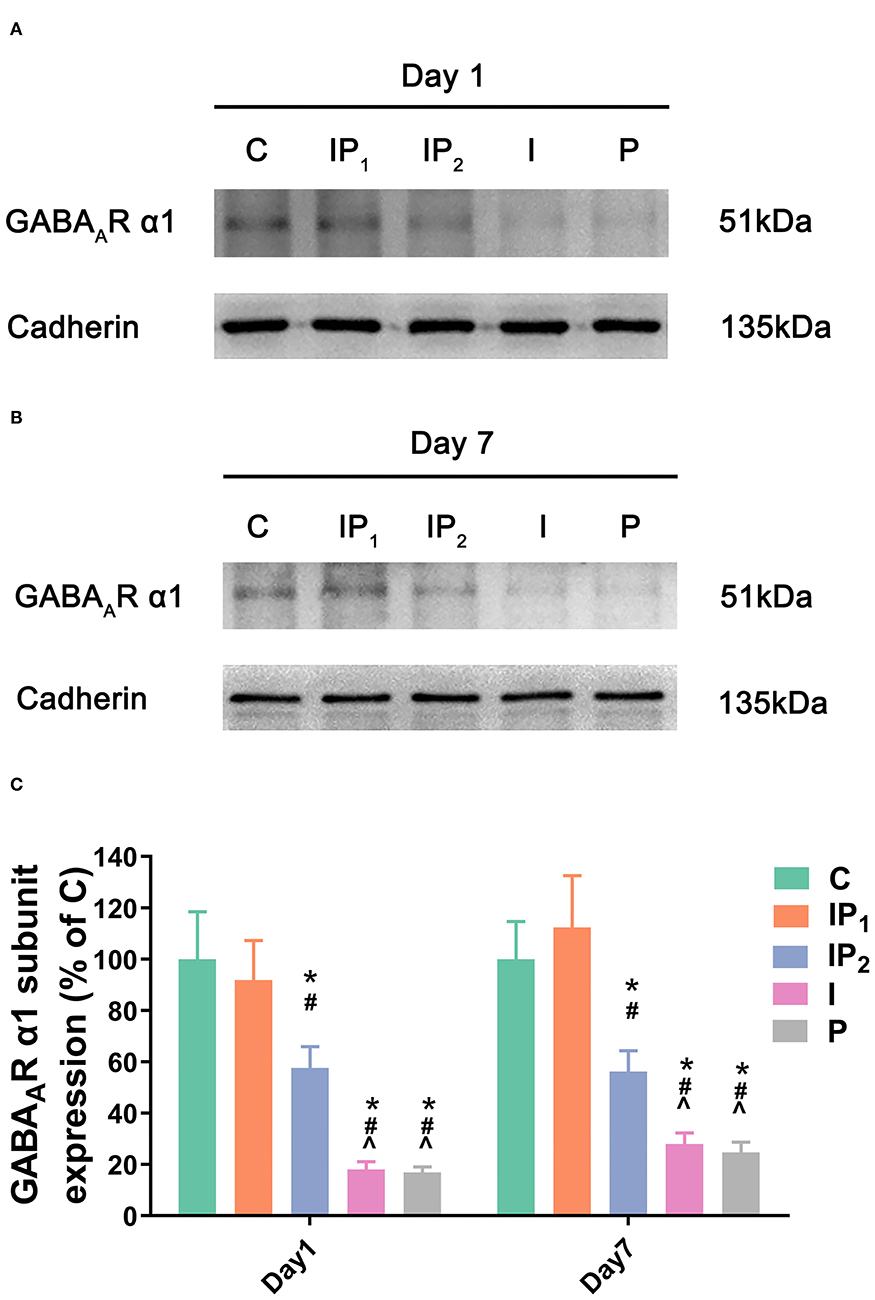
Figure 2. Combined treatment with 1% isoflurane and 20 mg·kg−1·h−1 propofol maintained the expression of the GABAAR α1 subunit. (A,B) The expression of the GABAAR α1 subunit in the hippocampus was determined by western blotting on day 1 and day 7 after ORIF. (C) Statistical graph of the expression of the GABAAR α1 subunit on day 1 and day 7 after ORIF. Data are expressed as the mean ± SD (n = 8/group). Note that ORIF resulted in a significant reduction in the expression of the GABAAR α1 subunit in the CA1 in Groups IP2, I, and P, which was prevented by the anesthetic schedule in the Group IP1. *P < 0.05 compared with Group C; #P < 0.05 compared with Group IP1; ∧P < 0.05 compared with Group IP2.
Combination Treatment With 1% Isoflurane and 20 mg·kg−1·h−1 Propofol Protected Neurons From ER Stress-Related Damage
To analyse ER stress-related damage, the expression of CHOP was evaluated by western blotting. There was no difference between Group C and Group IP1 on day 1 (C vs. IP1: 100.00 ± 13.63 vs. 76.93 ± 13.74, P = 0.409) or day 7 (C vs. IP1: 100.00 ± 20.70 vs. 82.77 ± 11.96, P = 0.876). Compared with Group C, the expression of CHOP in Group IP2 did not markedly change on the first day (C vs. IP2: 100.00 ± 13.63 vs. 136.70 ± 17.07, P = 0.058) but increased markedly on the seventh day after ORIF (C vs. IP2: 100.00 ± 20.70 vs. 191.85 ± 37.16, P < 0.001). The expression of CHOP was significantly upregulated in Groups I and P on day 1 (C vs. I: 100.00 ± 13.63 vs. 256.72 ± 33.15, P < 0.001; C vs. P: 100.00 ± 13.63 vs. 270.81 ± 40.61, P < 0.001) and day 7 (C vs. I: 100.00 ± 20.70 vs. 277.16 ± 50.77, P < 0.001; C vs. P: 100.00 ± 20.70 vs. 304.08 ± 45.71, P < 0.001) after ORIF (Figure 3).
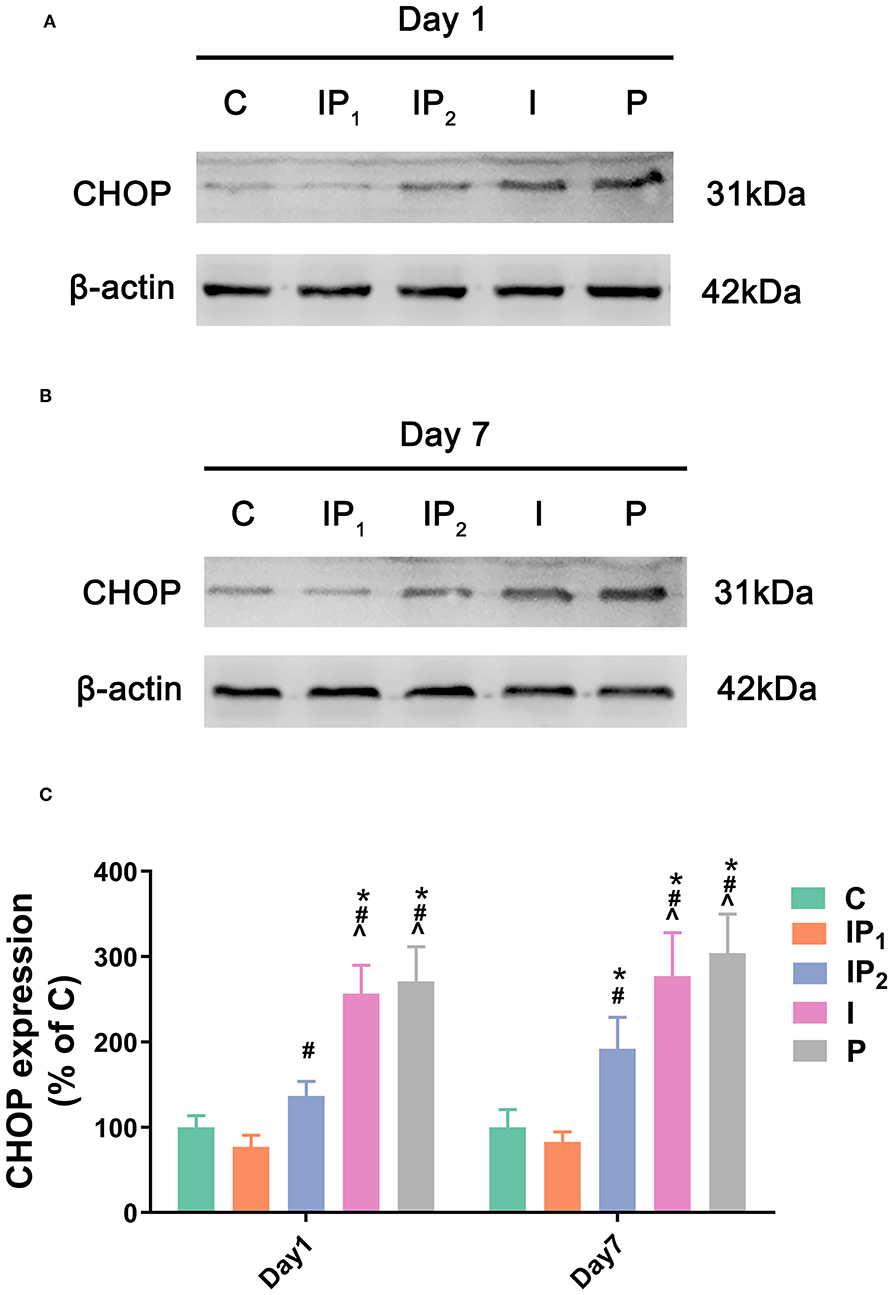
Figure 3. Combined treatment with 1% isoflurane and 20 mg·kg−1·h−1 propofol prevented ER stress-related damage. (A,B) The expression of CHOP in the hippocampus was determined by western blotting on day 1 and day 7 after ORIF. (C) Statistical graph of the expression of CHOP on day 1 and day 7 after ORIF. Data are expressed as the mean ± SD (n = 8/group). Note that ORIF resulted in a significant increase in the expression of CHOP in the CA1 in Groups IP2, I, and P, which was prevented by the anesthetic schedule in Group IP1. *P < 0.05 compared with Group C; #P < 0.05 compared with Group IP1; ∧P < 0.05 compared with Group IP2.
1% Isoflurane and 20 mg·kg−1·h−1 Propofol Protect Neurons by Elevating the Expression of BiP
The expression levels of BiP in Groups IP1, IP2, I, and P were all upregulated compared with that in Group C on day 1 (C vs. IP1: 100.00 ± 18.58 vs. 442.86 ± 69.09, C vs. IP2: 100.00 ± 18.58 vs. 248.02 ± 35.15, C vs. I: 100.00 ± 18.58 vs. 165.13 ± 25.53, C vs. P: 100.00 ± 18.58 vs. 188.54 ± 27.90, all P < 0.05). The highest expression level was found in Group IP1, and the lowest expression level was found in Group I. On day 7, the expression of BiP fell in all four groups, and there was no significant difference between Group I and Group C (C vs. I: 100.00 ± 13.91 vs. 142.57 ±18.70, P = 0.053). However, the expression of BiP in Groups IP1, IP2, and P was significantly higher than that in Group C (C vs. IP1: 100.00 ± 13.91 vs. 268.27 ± 46.51, C vs. IP2: 100.00 ± 13.91 vs. 199.47 ±31.66, C vs. P: 100.00 ± 13.91 vs. 154.64 ± 27.93, all P < 0.05, Figure 4). The highest expression was observed in Group IP1 (Figure 4).
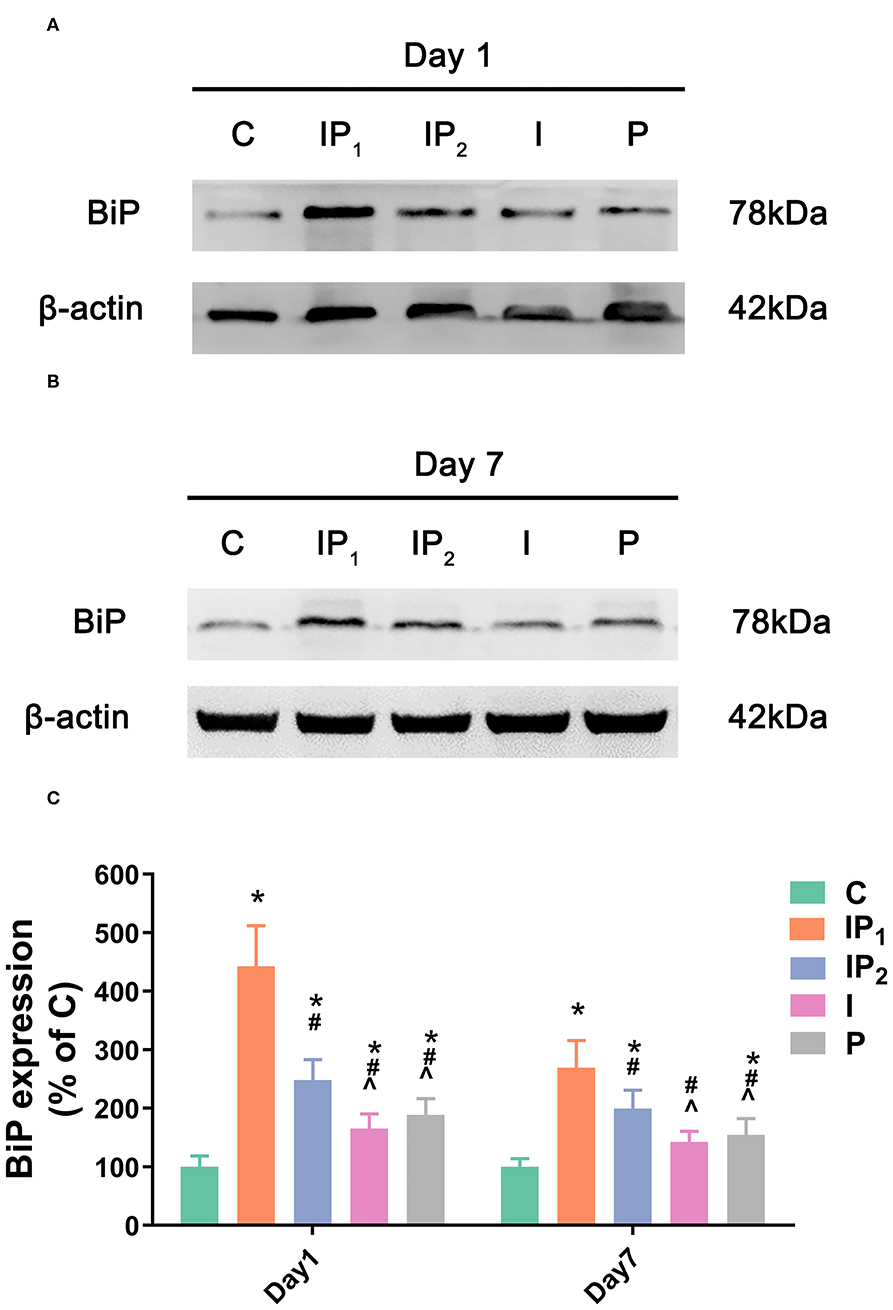
Figure 4. Combined treatment with 1% isoflurane and 20 mg·kg−1·h−1 propofol maintained the adaptive ability of neurons by increasing the expression of BiP. (A,B) The expression of BiP in the hippocampus was determined by western blotting on day 1 and day 7 after ORIF. (C) Statistical graph of the expression of BiP on day 1 and day 7 after ORIF. Data are expressed as the mean ± SD (n = 8/group). Note that ORIF resulted in a significant increase in the expression of BiP in the CA1 in Groups IP2, I, and P, which was prevented by the anesthetic schedule in Group IP1. *P < 0.05 compared with Group C; #P < 0.05 compared with Group IP1; ∧P < 0.05 compared with Group IP2.
Discussion
In our study, aging (16–18 month) rats were chosen as test subjects. All rats received ligation of the bilateral CCA to mimic the pathological process of CAS. Thirty days after ligation surgery, ORIF surgery was performed under different anesthesia regimes according to the group. After ORIF surgery, behavioral experiments (FC test) were carried out to evaluate the cognitive function of the rats. Histological analyses (Nissl staining) were performed to explore neuronal damage, and biochemical analyses (western blotting) of harvested rat brain tissues were performed to detect molecular changes.
The first consideration that must discussed is the selection and intervention of the test subject. The incidence of PND in orthopedic patients varies from 16 to 45%, although it can be as high as 72% (44), and it has been proven that aging is a risk factor (45). Therefore, we chose aging rats as test subjects. CH has been reported to be a key factor in the development of cognitive impairment (7). The underlying mechanism could be hypoxia-induced white matter damage, microvascular inflammation, and neuro-glio-vascular dysfunction (6). We deem that aging patients with perioperative CH require more attention to be paid to the selection of surgery and anesthesia. Moreover, CAS has been detected in 75% of men and 62% of women older than 65 years, with a prevalence of ≥50% stenosis of 7% in men and 5% in women (46). Taking incidence into account, we therefore used ligation of the CCA to induce CH in aging rats in this study. Moreover, our previous study has confirmed that ligation of the CCA contributes to cognitive impairment and histopathologic changes in aging rats (47). As it is difficult to separate clinical anesthesia and surgery, and our main purpose was to explore the combined effects of the two factors, no separate anesthesia group was used, which is consistent with most recent studies (48–50).
The FC test is a very sensitive and effort-independent test of learning and memory (51). To eliminate effects on motor ability caused by tibial fracture, the FC test was chosen to inspect cognitive function after ORIF surgery. Isoflurane has been reported to suppress learning in a dose-dependent fashion. Hence, we trained animals before surgery and anesthesia to remove the influence of the acquisition phase on the assessment of memory postoperatively (36). After ORIF surgery and anesthesia, the rats were placed in the same chamber that was used during the FC training phase. No tone or shock was delivered while the rats were in the chamber. In this circumstance, freezing behaviors rely on hippocampal memory (35, 37). It was demonstrated that medial temporal lobe regions, including the hippocampus, are most commonly affected in mild cognitive impairment and early AD (52–54). Thus, in our study, we focus on measurement of hippocampus-dependent memory. We found that the freezing time of rats was significantly shorter in Groups I, P, and IP2 than in Group C, while there was no obvious difference between Groups C and IP1. The only difference in the intervention among Groups I, P, IP2, and IP1 was the anesthesia method. Our results suggest that hippocampal-dependent memory impairment was not exhibited by the rats anesthetized with 1% isoflurane and 20 mg·kg−1·h−1 propofol, in contrast to all other groups of rats. Such an obvious difference aroused our interest in evaluating the state of related anatomic structures.
The hippocampal CA1 area is crucial for context-specific memory retrieval and spatial memory. After CA1 lesions, both recent and remote memory are impaired (55). Furthermore, this area is vulnerable to ischaemia injury (56). Thus, we chose the hippocampal CA1 area to measure the number of survival neurons and the expression of certain protein. On day 1, the number of neurons in Group I decreased obviously compared with Group C, and on day 7, the number of neurons in Groups I and P were markedly decreased compared with Group C. The difference between Group C and the combined anesthesia groups was not significant. Thus, we can draw the conclusion that, compared with the combination groups, the high dose of isoflurane or propofol alone can cause irreversible damage to the nervous system.
The GABAAR α1 subunit has also been linked to brain cognitive functions (57). More recently, the expression level of GABAAR α1 in the hippocampal CA1 region was found to be significantly downregulated in rats with chronic ischaemic encephalopathy (57). Proteostasis of GABAAR α1 subunit highly relies on ER function. Neuronal failure of the proteostasis network may cause protein aggregation that leads to neurodegeneration (58, 59). In our study, the expression of GABAAR containing the α1 subunit decreased in all but one group (the 1% isoflurane and 20 mg·kg−1·h−1 propofol group). It indicated that protein homeostasis was altered in all but not IP1 groups, which was coincident with the change in freezing time.
Accumulated evidence could support our study. Previous in vitro study showed that high dose isoflurane (treatment at a dose of 2% for 6 h) induced apoptosis by causing ER stress through ryanodine receptors but lower dose isoflurane (treatment at a dose of 1% for 1, 3, 6 h) did not (60). In vivo study suggested that ER stress-mediated apoptotic pathway was involved in isoflurane (treatment at a dose of 1.3% for 4 h) neurotoxicity in aged rats. Inhibition of ER stress overactivation contributed to the relief of isoflurane-induced histopathologic changes (61). Moreover, Coghlan et al. confirmed that the induction of ER stress by isoflurane (treatment at a dose of 1.1% for 4 h) occurred after the initiation of protein misfolding (62). These results indicate that cytotoxicity of isoflurane is in a dose-dependent way and related to ER stress. Analogously, propofol has a dose-dependent neuroprotective effect. Our previous study showed that propofol at doses of 10 or 20 mg·kg−1·h−1 infused at the onset of reperfusion for 30 min could provide neuroprotection to transient MCAO rats but 30 mg·kg−1·h−1 could not (12). Another study showed that infusion of propofol (36 or 72 mg·kg−1·h−1) resulted in aggravation of neurologic dysfunction, increased 28-day mortality rate, and impaired posttraumatic neurogenesis (63). In vitro study showed that the neuroprotective effect of propofol increased in a dose-dependent manner within 10 uM and decreased in a dose-dependent manner beyond 10 uM. Increase of endogenous BiP was the key of propofol's neuroprotection (64). In the present study, we only quantified CHOP and BiP. However, it is these two key factors that could confirm the neuroprotection of 1% isoflurane and 20 mg·kg−1·h−1 propofol. BiP, the key molecular chaperone in the ER, can help to alleviate ER stress and maintain calcium homeostasis (65), overexpression or induction of BiP possesses anti-apoptosis potential (66, 67). In our experiments, the expression of BiP was the highest in rats anesthetized with 1% isoflurane and 20 mg·kg−1·h−1 propofol among all four general anesthesia groups. CHOP is acknowledged as a specific transcription factor of ER stress (27). Unlike ER chaperones, CHOP is not generally synthesized under normal physiological conditions, or is present in the cytosol at very low levels under non-stressed conditions. Stress leads to the induction of CHOP and its accumulation in the nucleus (68). CHOP overactivation was closely related to neurodegenerative disease (28). In consistent with this, our study showed that elevated expression of CHOP in all but not IP1 groups. In group I and P, expression of CHOP was extremely high, while the mount of surviving neurons showed a distinguished decrease, which could provide a more intuitionistic result of cell damage.
In this study, hippocampus-dependent memory of rats in group IP1 was not impaired, and expression level of GABAAR α1, a key cognition-related protein, remained normal. ER stress alleviator, BiP, increased extremely while ER stress transcription factor, CHOP, showed no statistical difference compared with the control group. Numbers of surviving neurons confirmed the substantial neuronal damage caused by propofol or isoflurane alone.
Taking the above results into consideration, we consider that 1% isoflurane and 20 mg·kg−1·h−1 propofol is a more favorable aesthetic combination to avoid further damage to cognitive function of aging rats with CH during orthopedic surgery. The potential mechanism of this phenomenon may be related to alleviation of ER stress, but it remains to be verified.
With the advent of the aging society, clinical anesthesia is facing a variety of complex challenges, more exploration is needed to ensure the overall safety of patients. The harm caused by the application of large dose of a single anesthetic drug has been paid more and more attention. Therefore, this experiment explores the combined application of low-dose anesthetics to ensure the safety of anesthesia while minimizing the adverse effects of drugs, so as to provide new ideas for the practical clinical work. The experimental results show that the combination of low-dose anesthetics can enhance the protection of cognitive function. At the same time, more comprehensive and in-depth research is needed to explore related mechanisms and lay a solid foundation for personalized and precise anesthesia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Tianjin Medical University.
Author Contributions
HW helped with conception and design, acquisition, analysis and interpretation of the data, critical revision of the article, and giving final approval. XB and TL helped with conception and design, acquisition, analysis and interpretation of the data, drafting and critical revision of the article, and giving final approval. ZX, ZY, and GW helped with critical revision of the article and giving final approval. DG helped with analysis and interpretation of data, acquisition, critical revision of the article, and giving final approval. JW, YS, and CY helped with conception and design, analysis and interpretation of the data, critical revision of the article, and giving final approval. GL and JM helped with analysis and interpretation of the data, critical revision of the article, and giving final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82071220), Major Support Program of Tianjin Municipal Science and Technology (18YFZCSY00530), and Natural Science Foundation of Tianjin (20JCYBJC31000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at Research Square (69).
Abbreviations
BiP, binding immunoglobulin protein; CCA, common carotid arteries; CH, cerebral hypoperfusion; CHOP, C/EBP homologous protein; CNS, central nervous system; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated degradation; FC, fear conditioning; GABA, γ-aminobutyric acid; GABAAR, γ-aminobutyric acid A type receptor; MAC, minimum alveolar concentration; mIPSC, miniature inhibitory postsynaptic current; OGD, oxygen-glucose deprivation; ORIF, open reduction and internal fixation; PND, perioperative neurocognitive disorders; ROS, reactive oxygen species; UPR, unfolded protein response.
References
1. AGS/NIA Delirium Conference Writing Group Planning Committee and Faculty. The American Geriatrics Society/National Institute on Aging Bedside-to-Bench Conference: research agenda on delirium in older adults. J Am Geriatr Soc. (2015) 63:843–52. doi: 10.1111/jgs.13406
2. Evered L, Silbert BS, Knopman DS, Scott D, Dekosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesth Analg. (2018) 127:1189–95. doi: 10.1213/ANE.0000000000003634
3. Lee HB, Oldham MA, Sieber FE, Oh ES. Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: a case of effect modification. Am J Geriatr Psychiatry. (2017) 25:308–15. doi: 10.1016/j.jagp.2016.10.008
4. Chang X, Zhou H, Lei C, Wu B, Chen Y, Hao Z, et al. Association between asymptomatic carotid stenosis and cognitive function: a systematic review. Neurosci Biobehav Rev. (2013) 37:1493–9. doi: 10.1016/j.neubiorev.2013.05.011
5. Zhao Y, Gong C. From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cell Mol Neurobiol. (2015) 35:101–10. doi: 10.1007/s10571-014-0127-9
6. Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci. (2017) 131:2451–68. doi: 10.1042/CS20160727
7. Liu H, Zhang J. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci. (2012) 122:494–9. doi: 10.3109/00207454.2012.686543
8. Wei H, Liang G, Yang H, Wang Q, Hawkins BJ, Madesh M, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. (2008) 108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e
9. Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol. (2015) 10:179–89. doi: 10.1007/s11481-014-9580-y
10. Geng Y, Wu Q, Zhang R. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. J Clin Anesth. (2017) 38:165–71. doi: 10.1016/j.jclinane.2017.02.007
11. Cai X, Li X, Jin S, Liang D, Wen Z, Cao H, et al. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol. (2014) 257:148–56. doi: 10.1016/j.expneurol.2014.04.029
12. Wang HY, Wang GL, Yu YH, Wang Y. The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. (2009) 1297:177–84. doi: 10.1016/j.brainres.2009.08.054
13. Boruta DT, Sotgiu G, Golder FJ. Effects of intraperitoneal administration of gabapentin on the minimum alveolar concentration of isoflurane in adult male rats. Lab Anim. (2012) 46:108–13. doi: 10.1258/la.2011.011127
14. Logginidou HG, Li BH, Li DP, Lohmann JS, Schuler HG, Divittore NA, et al. Propofol suppresses the cortical somatosensory evoked potential in rats. Anesth Analg. (2003) 97:1784–8. doi: 10.1213/01.ANE.0000090318.16879.A8
15. Xu G, Broadbelt KG, Haynes RL, Folkerth RD, Borenstein NS, Belliveau RA, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. (2011) 70:841–58. doi: 10.1097/NEN.0b013e31822f471c
16. Willenbring D, Liu LT, Mowrey DD, Xu Y, Tang P. Isoflurane alters the structure and dynamics of GLIC. Biophys J. (2011) 101:1905–12. doi: 10.1016/j.bpj.2011.09.026
17. Ghosh B, Satyshur KA, Czajkowski C. Propofol binding to the resting state of the Gloeobacter violaceus ligand gated ion channel (GLIC) induces structural changes in the inter and intrasubunit transmembrane domain (TMD) cavities. J Biol Chem. (2013) 288:17420–31. doi: 10.1074/jbc.M113.464040
18. Labrakakis C, Rudolph U, de Koninck Y. The heterogeneity in GABAA receptor-mediated IPSC kinetics reflects heterogeneity of subunit composition among inhibitory and excitatory interneurons in spinal lamina II. Front Cell Neurosci. (2014) 8:424. doi: 10.3389/fncel.2014.00424
19. Mohler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. (2006) 326:505–16. doi: 10.1007/s00441-006-0284-3
20. Berry RB, Werner DF, Wang X, Jablonski MM, Homanics GE, Mittleman G, et al. Mice with targeted genetic reduction of GABA(A) receptor alpha1 subunits display performance differences in Morris water maze tasks. Neurobiol Learn Mem. (2008) 90:580–3. doi: 10.1016/j.nlm.2008.06.004
21. Williams DB, Akabas MH. Structural evidence that propofol stabilizes different GABA(A) receptor states at potentiating and activating concentrations. J Neurosci. (2002) 22:7417–24. doi: 10.1523/JNEUROSCI.22-17-07417.2002
22. Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Traystman RJ, et al. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and Allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J Neurochem. (2008) 107:668–78. doi: 10.1111/j.1471-4159.2008.05617.x
23. Wang H, Liu S, Wang H, Wang G, Zhu A. The effect of propofol postconditioning on the expression of K(+)-Cl(-)-co-transporter 2 in GABAergic inhibitory interneurons of acute ischemia/reperfusion injury rats. Brain Res. (2015) 1597:210–9. doi: 10.1016/j.brainres.2014.11.036
24. Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. (1999) 155:302–14. doi: 10.1006/exnr.1998.7002
25. Mota SI, Costa RO, Ferreira IL, Santana I, Caldeira GL, Padovano C, et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer's disease. Biochim Biophys Acta. (2015) 1852:1428–41. doi: 10.1016/j.bbadis.2015.03.015
26. Rao RV, Peel A, Logvinova A, Rio GD, Hermel E, Yokota T, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. (2002) 514:122–8. doi: 10.1016/S0014-5793(02)02289-5
27. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. (2004) 11:381–9. doi: 10.1038/sj.cdd.4401373
28. Yang Y, Liu L, Naik I, Braunstein Z, Zhong J, Ren B. Transcription factor C/EBP homologous protein in health and diseases. Front Immunol. (2017) 8:1612. doi: 10.3389/fimmu.2017.01612
29. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. (2012) 13:89–102. doi: 10.1038/nrm3270
30. Zhang Q, Zhang J, Han Z. Efficacy of Rho kinase inhibitor on cognitive impairment induced by chronic cerebral hypoperfusion in rats. Int J Clin Exp Med. (2015) 8:2435–40. doi: 10.1007/s11062-016-9548-z
31. Zhou Z, Zhang Y, Zhu C, Sui J, Wu G, Meng Z, et al. Cognitive functions of carotid artery stenosis in the aged rat. Neuroscience. (2012) 219:137–44. doi: 10.1016/j.neuroscience.2012.05.060
32. Hu N, Guo D, Wang H, Xie K, Wang C, Li Y, et al. Involvement of the blood–brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. (2014) 1551:13–24. doi: 10.1016/j.brainres.2014.01.015
33. Bayne K. Revised guide for the care and use of laboratory animals available. American physiological society. Physiologist. (1996) 39:199, 208–11.
34. Roughan JV, Flecknell PA. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Animals. (2002) 36:322–43.
35. Du Y, Gong XD, Fang X, Xing F, Xia TJ, Gu XP. Sevoflurane plays a reduced role in cognitive impairment compared with isoflurane: limited effect on fear memory retention. Neural Regen Res. (2020) 15:96–102. doi: 10.4103/1673-5374.264468
36. Vizcaychipi MP, Xu L, Barreto GE, Ma D, Maze M, Giffard RG. Heat shock protein 72 overexpression prevents early postoperative memory decline after orthopedic surgery under general anesthesia in mice. Anesthesiology. (2011) 114:891–900. doi: 10.1097/ALN.0b013e31820ad3ce
37. Glenn DE, Minor TR, Vervliet B, Craske MG. The effect of glucose on hippocampal-dependent contextual fear conditioning. Biol Psychiatry. (2014) 75:847–54. doi: 10.1016/j.biopsych.2013.09.022
38. Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. (2008) 105:5567–72. doi: 10.1073/pnas.0801853105
39. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. New York, NY: McGraw-Hill (1968).
40. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Edn. San Diego, CA: Academic Press (1986).
41. Zatroch KK, Knight CG, Reimer JN, Pang DSJ. Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res. (2016) 13:60. doi: 10.1186/s12917-017-0982-y
42. Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. (2008) 59:634–47. doi: 10.1016/j.neuron.2008.06.027
43. Deurloo MHS, Turlova E, Chen WL, Lin YW, Tam E, Tassew NG, et al. Transcription factor 2I regulates neuronal development via TRPC3 in 7q11.23 disorder models. Mol Neurobiol. (2019) 56:3313–25. doi: 10.1007/s12035-018-1290-7
44. Tomaszewski D. Biomarkers of brain damage and postoperative cognitive disorders in orthopedic patients: an update. Biomed Res Int. (2015) 2015:402959. doi: 10.1155/2015/402959
45. Mashour GA, Woodrum DT, Avidan MS. Neurological complications of surgery and anaesthesia. Br J Anaesth. (2015) 114:194–203. doi: 10.1093/bja/aeu296
46. Demarin V, Zavoreo I, Kes VB. Carotid artery disease and cognitive impairment. J Neurol Sci. (2012) 322:107–11. doi: 10.1016/j.jns.2012.07.008
47. Wang J, Yang C, Wang H, Li D, Li T, Sun Y, et al. A new rat model of chronic cerebral hypoperfusion resulting in early-stage vascular cognitive impairment. Front Aging Neurosci. (2020) 12:86. doi: 10.3389/fnagi.2020.00086
48. Terrando N, Monaco C, Ma D, Foxwell BMJ, Feldmann M, Maze M. Tumor necrosis factor-Alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. (2010) 107:20518–22. doi: 10.1073/pnas.1014557107
49. Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in Mice. Front Immunol. (2017) 8:902. doi: 10.3389/fimmu.2017.00902
50. Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F, et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun. (2019) 85:128–41. doi: 10.1016/j.bbi.2019.04.006
51. Quillfeldt JA. Behavioral methods to study learning and memory in rats. In: Andersen ML, Tufik S, editors. Rodent Model as Tools in Ethical Biomedical Research. Cham: Springer International Publishing (2016). p. 271–311.
52. Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. (1996) 94:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x
53. Kordower JH, Chu Y, Stebbins GT, Dekosky ST, Cochran EJ, Bennett DA, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. (2001) 49:202–13. doi: 10.1002/1531-8249(20010201)49:2<202::AID-ANA40>3.0.CO;2-3
54. Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. (2006) 27:1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012
55. Ocampo AC, Squire LR, Clark RE. Hippocampal area CA1 and remote memory in rats. Learn Mem. (2017) 24:563–8. doi: 10.1101/lm.045781.117
56. Liang H, Kurimoto S, Shima KR, Shimizu H, Ota T, Minabe Y, et al. Why is hippocampal CA1 especially vulnerable to ischemia? SOJ Biochem. (2016) 2:7. doi: 10.15226/2376-4589/2/2/00114
57. Liu L, Li CJ, Lu Y, Zong XG, Luo C, Sun J, et al. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Sci Rep. (2015) 5:14474. doi: 10.1038/srep14474
58. Ogen-Shtern N, Ben David T, Lederkremer GZ. Protein aggregation and ER stress. Brain Res. (2016) 1648:658–66. doi: 10.1016/j.brainres.2016.03.044
59. Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol. (2017) 13:477–91. doi: 10.1038/nrneurol.2017.99
60. Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA, et al. Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth. (2014) 113:695–707. doi: 10.1093/bja/aeu053
61. Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav. (2015) 151:16–23. doi: 10.1016/j.physbeh.2015.07.008
62. Coghlan M, Richards E, Shaik S, Rossi P, Vanama RB, Ahmadi S, et al. Inhalational anesthetics induce neuronal protein aggregation and affect ER trafficking. Sci Rep. (2018) 8:5275. doi: 10.1038/s41598-018-23335-0
63. Thal SC, Timaru-Kast R, Wilde F, Merk P, Johnson F, Frauenknecht K, et al. Propofol impairs neurogenesis and neurologic recovery and increases mortality rate in adult rats after traumatic brain injury. Crit Care Med. (2014) 42:129–41. doi: 10.1097/CCM.0b013e3182a639fd
64. Wang L, Tang W, Jiang T, Lu P, Li Y, Sun A, et al. Endoplasmic reticulum stress is involved in the neuroprotective effect of propofol. Neurochem Res. (2014) 39:1741–52. doi: 10.1007/s11064-014-1369-0
65. Ouyang Y-B, Xu L-J, Emery JF, Lee AS, Giffard RG. Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion. (2011) 11:279–86. doi: 10.1016/j.mito.2010.10.007
66. Zhang PL, Lun M, Teng J, Huang J, Blasick TM, Yin L, et al. Preinduced molecular chaperones in the endoplasmic reticulum protect cardiomyocytes from lethal injury. Ann Clin Lab Sci. (2004) 34:449–57. doi: 10.1097/00007691-200410000-00019
67. Xiao-Hong Y, Li L, Yan-Xia P, Hong L, Wei-Fang R, Yan L, et al. Salusins protect neonatal rat cardiomyocytes from serum deprivation-induced cell death through upregulation of GRP78. J Cardiovasc Pharmacol. (2006) 48:41–6. doi: 10.1097/01.fjc.0000242059.89430.ac
68. Zhang GG, Teng X, Liu Y, Cai Y, Zhou YB, Duan XH, et al. Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides. (2009) 30:1109–16. doi: 10.1016/j.peptides.2009.03.024
Keywords: cerebral hypoperfusion, cognitive function, isoflurane, propofol, GABAAR α1, BiP
Citation: Bu X, Li T, Wang H, Xia Z, Guo D, Wang J, Sun Y, Yang C, Liu G, Ma J, Yang Z and Wang G (2020) Combination of Isoflurane and Propofol as General Anesthesia During Orthopedic Surgery of Perioperative Cerebral Hypoperfusion Rats to Avoid Cognitive Impairment. Front. Med. 7:549081. doi: 10.3389/fmed.2020.549081
Received: 23 April 2020; Accepted: 08 September 2020;
Published: 20 October 2020.
Edited by:
Zhongcong Xie, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Wolfgang Weihs, Medical University of Vienna, AustriaCheng Ni, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2020 Bu, Li, Wang, Xia, Guo, Wang, Sun, Yang, Liu, Ma, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyun Wang, why819@126.com
†These authors have contributed equally to this work
 Xinyue Bu
Xinyue Bu Tang Li1,2†
Tang Li1,2†  Jinxin Wang
Jinxin Wang Chenyi Yang
Chenyi Yang