Understanding Urogenital Schistosomiasis-Related Bladder Cancer: An Update
- 1Bladder Immunology Group, Biomedical Research Institute, Rockville, MD, United States
- 2Department of Urology, The George Washington University, Washington, DC, United States
- 3Division of Urology, Children's National Medical Center, Washington, DC, United States
Infection with Schistosoma haematobium leads to urogenital schistosomiasis, which has been correlated with the occurrence of bladder cancer. However, mechanisms responsible for this association have not yet been clearly identified. In this short review, we provide an update, highlighting the most recent studies on schistosome-associated bladder cancer, including those that focus on identifying changes in host biology during S. haematobium infection, as well as studies for the identification of potentially pro-carcinogenic parasite molecules, and we offer a discussion on some possible mechanisms driving schistosomal bladder cancer.
Schistosoma haematobium and Bladder Cancer
As with other members of the Schistosoma genus, infection with S. haematobium leads to schistosomiasis, a debilitating disease affecting more than 200 million people (1). Treatment relies primarily on one drug, praziquantel, and diagnosis methods include egg identification in stool or urine and serum tests for schistosome antigens. Interestingly, and perhaps worryingly, prevalence estimates based on egg identification may underrepresent the actual prevalence, given that such parasitological tests have high specificity but low sensitivity when compared to serum tests (2).
Unlike the more commonly studied S. mansoni and S. japonicum, which cause hepatosplenic and intestinal pathology, S. haematobium causes bladder pathology. While limited evidence exists for the carcinogenic potential of the other schistosome species, the association between S. haematobium infection and bladder cancer has been known and studied for many years (3–6). A study conducted in Egypt including nearly 10,000 patients over the period of 1970 to 2007 found a decrease in the frequency of S. haematobium infection concomitant with a decrease in squamous cell carcinoma, increase in urothelial cell carcinoma, and increase in the median age of patients (7). In addition to S. haematobium infection, other studies have also indicated tobacco smoking as a major risk factor (8, 9). S. haematobium joins two other helminth species, Opisthorchis viverrini and Clonorchis sinensis, as Group 1 definitive biological carcinogenic agents (5, 10), and other parasites from several genera, including Echinococcus, Strongyloides, Fasciola, Heterakis, Platynosomum, and Trichuris have also been found to be associated with cancer (11).
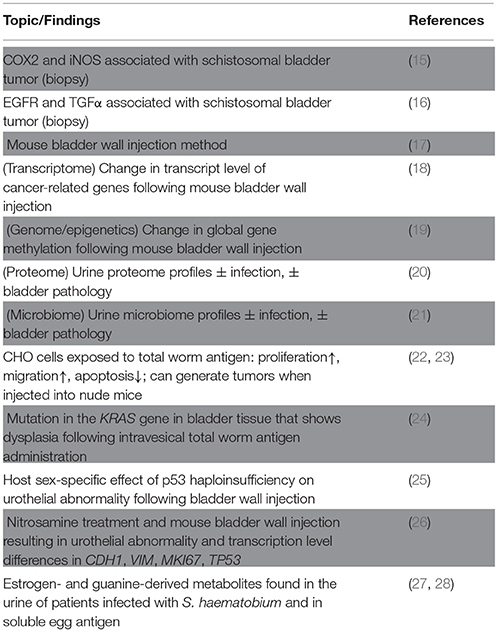
Regarding the cost of treatment, a recent cost analysis estimated about 18 million USD for praziquantel administration (0.16 USD per person for approximately 112 million people infected with S. haematobium), compared to an annual incrementing cost of 20 million USD for the treatment of schistosomal bladder cancer (an estimated 3-4 patients out of 100,000 people infected with S. haematobium develop bladder cancer each year) (12). These findings not only emphasize the importance of treating urinary schistosomiasis but they also inspire questions regarding the mechanisms by which S. haematobium contributes to the development of bladder cancer [reviewed in (10, 13, 14)]. Here, we highlight recent studies that examine host-side changes in the context of S. haematobium infection, as well as studies on potentially carcinogenic parasite molecules (some key studies shown in Table 1), and we also discuss possible mechanisms and future research directions for schistosomal bladder cancer.
Changes in Host Biology During S. haematobium Infection
In response to infection with S. haematobium, the host bladder undergoes changes at the gross morphological level, as well as at the molecular level. A major goal in the study of the link between S. haematobium infection and bladder cancer involves the identification of genes and proteins perturbed following (and during) parasite infection. As a starting point, clinical studies help to identify correlations and possible methods for diagnosis, and they also direct further experiments in animal and in vitro models that can validate the clinical findings, allowing us to delve deeper into the molecular pathways involved in schistosomal bladder cancer. For example, some recent studies have used bladder tumor biopsy samples, with and without schistosome eggs, to identify differential levels of host proteins, such as COX2, iNOS, EGFR, and TGFα, that could serve as unique markers for schistosomal bladder cancer (15, 16, 29, 30). Another study examined T cell protein marker expression for STAT4 (Th1), GATA3 (Th2), FOXP3 (T regulatory), and CD8 (T cytotoxic) using bladder tumor biopsy samples containing schistosome eggs, and found a Th2-skewed environment with elevated levels of FOXP3, suggesting that the downregulation of Th1 response through the action of FOXP3 on STAT4 may favor carcinogenesis (31). A meta-analysis for molecular markers related to schistosomal bladder cancer has also been performed (32).
Compared to clinical studies, studies using animal models allow for more experimental control; however, we face the challenge that a suitable animal infection model that completely recapitulates human infection does not exist. Specifically, mice or hamsters exposed to S. haematobium cercariae (the infective stage for humans) exhibit egg accumulation in the liver and intestines, rather than in the bladder, and while primate infections show similar pathophysiology to human infection, high costs, ethical concerns, and a dearth of species-specific reagents for the primate model collectively preclude extensive experimentation (14). To address this issue, our group developed a method to inject eggs directly into the bladder wall of mice, allowing us to replicate key pathological features of the human disease (17, 33). Here, we introduce recent studies that use this egg injection method and other methods to reveal important changes in host bladder biology related to S. haematobium infection, especially as it pertains to bladder carcinogenesis.
Mouse bladder wall injection with S. haematobium eggs leads to urothelial hyperplasia, a potentially pre-cancerous lesion (33). Microarray analysis comparing egg- and vehicle-injected bladders revealed decreased transcript levels of uroplakins in egg-injected organs, recapitulating other models of bladder damage that result in urothelial hyperplasia (18). The analysis also showed differential expression of genes belonging to cancer-associated and vascular endothelial growth factor (VEGF)-associated pathways, which is consistent with the observation that exposure to S. mansoni soluble egg antigen induces increased production of VEGF in human umbilical vein endothelial cells (34), as well as the report of elevated serum and urine VEGF protein levels in bladder cancer patients infected with S. haematobium (18, 35). These pathways provide an important starting point in further investigations to identify parasite and host molecules that give rise to schistosomal bladder cancer.
As with modulation of transcript expression, epigenetic changes in the host genome may also play a part in schistosomal bladder cancer. Inspired by the observation that DNA from the urine of patients infected with S. haematobium features hypermethylation of several genes correlated to degree of bladder damage (as evaluated by ultrasound) (36), our group conducted a similar study in egg-injected bladders and found a significant increase in DNA methylation in urothelial cells from parasite egg-injected bladders relative to vehicle-injected controls (19). Furthermore, drug-induced inhibition of DNA methylation resulted in decreased hyperplasia in the urothelium of the egg-injected bladders when compared to control animals that underwent egg-injection (19). These findings suggest a use for methylation-specific DNA sequencing as a potential method to detect schistosomal bladder cancer, although further study is necessary to help us identify methylation signatures specific for S. haematobium infection with bladder cancer (S+/C+), infection alone (S+/C-), cancer alone (S-/C+), uninfected individuals (S-/C-), as well as individuals with other sources of bladder damage (S-/C-/*).
Another “-omics” approach to deepen our understanding of schistosomal bladder carcinogenesis is proteomics. With a goal of identifying protein markers associated with schistosomal bladder cancer, Bernardo et al. characterized the urine proteomes from patients with or without S. haematobium infection and/or bladder cancer (S+/C+, S+/C–, S–/C+, S–/C–) and found different abundances of several proteins for each patient group (20). A similar study of the urine proteome used a larger patient pool but with less stringent inclusion criteria for bladder pathology (i.e., non-bladder cancer conditions were included), and it revealed, for each group, unique and overlapping host proteome profiles, as well as proteomic signatures characteristic of schistosomes (37).
Omics-relevant techniques to define the biology of schistosomal bladder cancer have also recently included microbiome experiments. A study of the urine microbiome from urogenital schistosomiasis patients showed differences in microbial communities between S. haematobium-infected and uninfected groups, as well as between advanced (infection with bladder pathology) and other groups (21). These findings demonstrate another possible method for the characterization and the diagnosis of schistosomal bladder cancer. Microbiome approaches could be combined with proteomics methods, allowing for the identification of bacterial proteins that may facilitate schistosomal bladder carcinogenesis.
Complementing “big data” studies, other work has focused on the specific host genes and pathways involved in the promotion of S. haematobium-associated bladder cancer. Chronic schistosome infection involves paired adult worms and their constant release of eggs. Both adult and egg stage parasites secrete molecules, inducing changes in the host microenvironment that may be pro-carcinogenic. Exposure to S. haematobium worm antigens increases proliferation and migration, and decreases apoptosis of Chinese hamster ovary (CHO) cells (22). Further experimentation demonstrated that the worm antigen-exposed CHO cells (but not the unexposed control cells) could generate tumors when injected subcutaneously into nude mice (23). The relevance of these findings, which are based on the use of ovarian rather than bladder-derived tissues, as well as a xenogeneic model, remains unclear. Moving to a more bladder-specific model, Botelho and coworkers introduced total adult antigen intravesically in mice, which produced urothelial dysplasia and inflammation (38). Inspection of the bladders with dysplasia revealed a mutation in the KRAS gene, a member of the RAS proto-oncogene family, suggesting a possible link between the occurrence of schistosomal bladder cancer and mutation in KRAS (24). We note that this study exposed the luminal side of the bladder urothelium to total worm antigen, whereas S. haematobium-infected hosts are exposed on the basal side of the bladder urothelium to egg antigens, as eggs are deposited in vesical blood vessels. Additionally, a single intravesical administration of the parasite extract is unlikely to recapitulate the location and degree of the host's exposure to parasite molecules found in a natural infection. We speculate that repeated intravenous administration of these products, perhaps intravenously, could produce more biologically relevant results.
Dysregulation of genes that control cell proliferation and death represents a core characteristic of cancer. In many cancers, dysfunction of the tumor suppressor gene TP53 (and its protein product p53) allows the inappropriate survival of cells that would otherwise undergo apoptosis. Increased nuclear accumulation of p53 protein has been shown to correlate with mutations in the gene and with tumor grade in non-schistosomal bladder cancer (39), while in S. haematobium-associated bladder cancer, elevated p53 protein levels were found in both malignant and pre-malignant lesions, suggesting the potential use of p53 in the detection of schistosomal bladder cancer (40). Given the function of p53 as a tumor suppressor, we reasoned that its decreased expression would contribute to neoplastic changes in the S. haematobium egg-exposed bladder. We performed bladder wall egg injections on transgenic mice haploinsufficient for p53 in the bladder and found a lack of ulceration in the urothelium for p53-intact female mice compared to p53-haploinsufficient female mice and male mice (whether p53-sufficient or haploinsufficient). Since urothelial ulceration is a potential pathologic indicator of preneoplasia, these findings suggest the presence of host sex-specific factors that affect the pathogenesis of schistosomal bladder preneoplasia (25, 41). The study also served to establish a new method to examine the effect of disrupting a gene of interest in this animal model of S. haematobium infection. However, it remains unclear how to reconcile the findings of Santos et al. that p53 protein levels are increased in preneoplastic and neoplastic schistosomal bladder lesions (40) with our observation that p53-haploinsufficiency (which would result in decreased p53 protein levels) contributes to development of S. haematobium egg-induced, bladder neoplasia-associated lesions. While increased p53 levels in these lesions likely resulted from mutations in the p53 protein, determining whether the specific mutations leading to p53 accumulation directly contribute to neoplasia in schistosomal bladder lesions, e.g., ascertaining whether the mutation is a “passenger” vs. “driver” mutation [described in (42)], will require further investigation.
In another study using the mouse bladder wall egg injection method, Chala and others co-administered S. haematobium eggs and the cancer-inducing nitrosamine compounds N-nitrosodimethylamine (NDMA) and N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) (26). This work was inspired by a prior baboon S. haematobium infection model which demonstrated that sub-carcinogenic levels of nitrosamines become carcinogenic when combined with S. haematobium exposure (43). This complementation model lends credence to Knudson's multi-hit hypothesis as applied to schistosomal bladder carcinogenesis (44). The approach used by Chala et al. is also corroborated by the observation of elevated levels of nitrosamines in the urine of patients infected with S. haematobium compared to uninfected individuals (45), as well as by the recent finding that the BBN-induced mouse bladder cancer model and some human muscle-invasive bladder cancers feature similar mutation frequencies in key cancer-related genes (46). When Chala and co-authors combined nitrosamine exposure with mouse bladder wall egg injection, they noted abnormal histology and altered expression levels of cell adhesion genes CDH1 (E-cadherin) and VIM (vimentin), the proliferation marker MKI67 (Ki-67), and TP53 (p53) (26). Interpretation of findings from this study is potentially confounded by several considerations: (1) presumably unblinded histological analyses, (2) use of a suboptimal egg dose injection (acknowledged by the authors), (3) use of eggs from frozen urine samples (e.g, dead eggs unable to secrete proteins), (4) lack of a time course sufficiently long enough to observe frankly neoplastic lesions, and (5) use of a high BBN concentration that, in our hands, results in a potent BBN-mediated procarcinogenic effect that may mask any procarcinogenic influence of S. haematobium eggs. Chala and co-authors are to be applauded for their efforts, but deeper studies will be mandatory to better elucidate the pathophysiology of schistosomal bladder carcinogenesis.
In the mouse bladder wall injection model, the preneoplastic changes and alteration in the described genes and pathways arise as a response to the damage associated with the retention and/or traversal of the eggs across the urothelium, as well as to the immunomodulatory egg-secreted molecules. Importantly, we note that the model delivers one installment of parasite eggs, giving only an acute view of their effect, while in contrast, infection with the adult parasites results in the continuous deposition of eggs. In turn, this chronic egg-induced bladder damage leads to increased inflammation and cell turnover, eventually giving rise to carcinoma. Improvement of the mouse model to facilitate chronic infection with egg-laying adult S. haematobium worms would allow for further investigation and identification of host and parasite molecules, genes, and pathways uniquely affected in schistosomal bladder cancer. Key comparisons would include parasite-infected mice with bladder cancer (S+/C+) and mice with only bladder cancer (S-/C+), as studied in patients (29, 47).
Identification of Key Parasite Molecules
The mouse bladder wall egg-injection studies discussed above have focused on changes in the biology of the host in response to the presence of S. haematobium eggs. Given that these host-side changes arise from a host-parasite interaction, a related line of investigation follows: determination of the identity of specific schistosome molecules that induce the host-side changes. Very few studies have focused on the parasite side of the host-parasite interaction, likely owing to the difficulty of isolating and analyzing parasite components. In contrast to the studies on host-side changes, schistosome molecular studies have mainly used in vitro methods, which, because of the remoteness from the original biological context, come with the caveat that the results may have limited relevance, but allow us to finely probe the function of such molecules and their effects on host cells.
Parasite-derived estrogen-related compounds and metabolites, found during a study of hypogonadism in schistosomiasis patients (48), have been proposed to play a role in the development of schistosomal bladder cancer. Proteomic analysis of human urine containing S. haematobium eggs revealed the presence of several products not found in urine from uninfected individuals (49), including estrogen-like metabolites and guanine-derived oxidation products, which can both give rise to genetic mutation and carcinogenesis (27, 50). Estrogen-like metabolites were also found in S. haematobium soluble egg antigen (SEA), which, when administered to urothelial cells, induced key cancer phenotypes, including increased cell proliferation and decreased apoptosis (28). These findings suggest that parasite-derived estrogen-related compounds may contribute to the development of schistosomal bladder cancer and that they may be useful as markers for schistosome infection, if not for schistosomal bladder cancer (27, 51). However, the relationship between these estrogen-related compounds and the observation that bladder cancer is more common in men (who have higher androgen:estrogen ratios) remains to be reconciled.
Like the eggs of other schistosome species, S. haematobium eggs secrete a variety of proteins, which modulate the host immune response and may facilitate egg escape from the host. Among the most abundant of these proteins is H-IPSE, the S. haematobium ortholog to S. mansoni IL-4 inducing principle of S. mansoni eggs (IPSE/alpha-1). Our studies have identified many interesting features of H-IPSE, including its ability to infiltrate host cells and translocate to the nucleus (52). Akin to the S. mansoni ortholog, H-IPSE can induce IL-4 production, bind immunoglobulins, cytokines, and chemokines, skew cell cycle state toward the S phase, and increase cell proliferation (unpublished observations). During chronic infection with S. haematobium, the continuous deposition of eggs in the bladder and subsequent retention and traversal of the eggs through the urothelium likely results in damage to the tissue. At the same time, however, H-IPSE released from the eggs may help to counteract the damage to the urothelium by increasing cell proliferation. We speculate that this increased cell proliferation in the urothelium contributes to malignancy associated with S. haematobium infection.
These studies represent some promising initial steps in identifying links between parasite molecules and host genes that contribute to bladder cancer. Further investigations could test the carcinogenic potential of other schistosome molecules, starting with those found in proteomic profiling studies of eggs and adults. Additionally, administration of such molecules in an adaptation of the current mouse bladder wall injection model of S. haematobium infection would help corroborate the findings of in vitro studies.
Possible Mechanisms of schistosomal Bladder Cancer
We generally accept the idea that urogenital schistosomiasis contributes to, but alone is probably insufficient for oncogenesis. The concurrent presence of additional carcinogens, including environmental exposures (intake of nitrosamine compounds through smoking or diet), genetic predisposition (loss-of-function mutation in tumor suppressor genes or gain-of-function mutation in oncogenes), and other infections (uropathogenic E. coli, HPV), could help accelerate the development of schistosomal bladder cancer (14). However, the understanding of the specific components of S. haematobium infection that lead to bladder cancer is in its infancy. Here, we discuss possible mechanisms through which urogenital schistosomiasis can give rise to bladder cancer.
S. haematobium infection leads to egg deposition in the bladder wall, with some eggs presumably causing damage by their traversal through the urothelium to the bladder lumen. The remaining eggs (that fail to traverse) cause damage through chronic inflammation by their interaction with host immune cells and subsequent granuloma formation (53). The bladder urothelium consists of multiple layers of different cell types, which, in response to injury, dramatically increase their otherwise slow turnover rate [reviewed in (54)]. Combining these observations for S. haematobium infection and urothelial regeneration leads to the hypothesis that infection-induced damage and chronic inflammation in the urothelium contribute heavily to the development of bladder cancer (55, 56). Indeed, the idea that sites of inflammation can give rise to cancer has been proposed in the mid-1800s by Virchow [reviewed in (57)], and, more recently, dysbiosis in the microbiome has also been suggested as a risk factor (58, 59). While current studies on the urine microbiome of patients (with different binary combinations of schistosome infection and bladder pathology) have identified the abundance and distribution of microbiota species in each case, future studies could delve into detailed associations by introducing more parameters, such as the degree of bladder pathology and parasite infection burden.
Upregulation of cell proliferation and injury repair mechanisms in response to egg traversal-associated urothelial damage also likely contributes to the development of schistosomal bladder cancer. The concurrent inflammation and presence of possibly mutagenic parasite molecules could explain the observation of increased chromosomal damage found in urogenital schistosomiasis patients (55). As discussed earlier, estrogen-like metabolites of schistosome origin may also drive an increase in the rate of gene mutation by forming adducts with DNA (50). Accumulation of these potentially mutagenic events over many cell generations could eventually give rise to dysfunctional or even cancerous cells.
Aside from the response to physical damage, the increase in urothelial cell proliferation can result from the action of S. haematobium egg secretions. Soluble egg antigen and IPSE, a highly abundant constituent of soluble egg antigen, have both shown the ability to increase cell proliferation in endothelial cells, corroborating the association of angiogenesis with egg granuloma formation, and reinforcing the idea that angiogenesis may be another link between schistosome infection and bladder cancer (60). The treatment of human umbilical vein endothelial cells with S. mansoni soluble egg antigen resulted in upregulation of VEGF (34), consistent with the observation of perturbation in the VEGF pathway following mouse bladder wall injection of S. haematobium eggs, as well as the observation of increased vascularization in the genital mucosa of women infected with S. haematobium compared to uninfected individuals (61). Given that IPSE increases cell proliferation, upcoming studies identifying its host gene targets may find that it modulates VEGF expression and genes in other pathways, such as Hedgehog (Hh) and Wingless (Wnt) pathways, which also play a role in cell proliferation (54).
Future Directions in Schistosomal Bladder Cancer Research
The introduction of the mouse bladder wall injection model has helped to characterize the pathologic, and possibly carcinogenic, changes in the host bladder in the presence of S. haematobium eggs. The ability to recapitulate natural bladder oviposition in the mouse represents the next challenge in improving the mouse model and would allow us to avoid artifacts associated with the egg injection procedure, as well as observe the effect of chronic oviposition in contrast to one-time egg administration. Similarly, with the identification of potentially carcinogenic parasite molecules, such as the estrogen-like metabolites and IPSE, methods for chronic local in vivo administration of these factors would help to strengthen the evidence for their carcinogenicity. More ambitiously, the development of a method to generate transgenic schistosomes that carry stable genomic modifications, such as gene disruptions and reporter gene insertions, would further the study of not only carcinogenic schistosome molecules but also schistosome genes and their role in the development of the parasite within the host, eventually leading to the identification of novel drug targets and therapeutics for the prevention and treatment of schistosomiasis.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
We gratefully acknowledge our funding sources (NIH DK113504 [MH] and the Margaret A. Stirewalt Endowment [MH]).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
2. Colley DG, Andros TS, Campbell CH. schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty (2017) 6:63 doi: 10.1186/s40249-017-0275-5
3. Mostafa MH, Sheweita SA, O'Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. (1999) 12:97–111.
4. Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA, Barocas DA. Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU Int. (2011) 107:206–11. doi: 10.1111/j.1464-410X.2010.09622.x
5. IARC Working group on the evaluation of carcinogenic risks to humans. biological agents. volume 100 B. a review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. (2012) 100:1–441. ISBN 978 92 832 1319 2
6. Khaled H. Schistosomiasis and cancer in Egypt: review. J Adv Res. (2013) 4:461–6. doi: 10.1016/j.jare.2013.06.007
7. Gouda I, Mokhtar N, Bilal D, El-Bolkainy T, El-Bolkainy NM. Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Canc Inst. (2007) 19:158–62.
8. Bedwani R, Renganathan E, El Kwhsky F, Braga C, Abu Seif HH, Abul Azm T, et al. Schistosomiasis and the risk of bladder cancer in Alexandria, Egypt. Br J Cancer (1998) 77:1186–9. doi: 10.1038/bjc.1998.197
9. Zheng YL, Amr S, Saleh DA, Dash C, Ezzat S, Mikhail NN, et al. Urinary bladder cancer risk factors in egypt: a multicenter case-control study. Cancer Epidemiol Biomarkers Prev. (2012) 21:537–46. doi: 10.1158/1055-9965.EPI-11-0589
10. Brindley PJ, Costa JMC Da, Sripa B. Why does infection with some helminths cause cancer? Trends Cancer (2015) 1:174–82. doi: 10.1016/j.trecan.2015.08.011
11. MacHicado C, Marcos LA. Carcinogenesis associated with parasites other than schistosoma, opisthorchis and clonorchis: a systematic review. Int J Cancer (2016) 138:2915–21. doi: 10.1002/ijc.30028
12. Botelho MC, Alves H, Richter J. Halting Schistosoma haematobium - associated bladder cancer. Int J Cancer Manag. (2017) 10:4–9. doi: 10.5812/ijcm.9430
13. Botelho MC, Machado JC, Brindley PJ, Correia da Costa JM. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence (2011) 2:267–79. doi: 10.4161/viru.2.4.16734
14. Honeycutt J, Hammam O, Fu CL, Hsieh MH. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. (2014) 30:324–32. doi: 10.1016/j.pt.2014.05.004
15. Hassan HE, Mohamed AAB, Bakhiet AO, Ahmed HG. Immunohistochemical expression of COX2 and iNOS in bladder cancer and its association with urinary schistosomiasis among Sudanese patients. Infect Agent Cancer (2013) 8:9. doi: 10.1186/1750-9378-8-9
16. Badawy AA, El-Hindawi A, Hammam O, Moussa M, Helal NS, Kamel A. Expression of epidermal growth factor receptor and transforming growth factor alpha in cancer bladder: Schistosomal and non-schistosomal. Curr Urol. (2017) 9:192–201. doi: 10.1159/000447140
17. Fu CL, Apelo CA, Torres B, Thai KH, Hsieh MH. Mouse bladder wall injection. J Vis Exp. (2011) 53:e2523. doi: 10.3791/2523
18. Ray D, Nelson TA, Fu CL, Patel S, Gong DN, Odegaard JI, et al. Transcriptional profiling of the bladder in urogenital schistosomiasis reveals pathways of inflammatory fibrosis and urothelial compromise. PLoS Negl Trop Dis (2012) 6:e1912. doi: 10.1371/journal.pntd.0001912
19. Conti SL, Honeycutt J, Odegaard JI, Gonzalgo ML, Hsieh MH. Alterations in DNA methylation may be the key to early detection and treatment of schistosomal bladder cancer. PLoS Negl Trop Dis. (2015) 9:e0003696. doi: 10.1371/journal.pntd.0003696
20. Bernardo C, Cunha MC, Santos JH, da Costa JMC, Brindley PJ, Lopes C, et al. Insight into the molecular basis of Schistosoma haematobium-induced bladder cancer through urine proteomics. Tumor Biol. (2016) 37:11279–87. doi: 10.1007/s13277-016-4997-y
21. Adebayo AS, Survayanshi M, Bhute S, Agunloye AM, Isokpehi RD, Anumudu CI, et al. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Negl Trop Dis (2017) 11:e0006067. doi: 10.1371/journal.pntd.0005826
22. Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, da Costa JMC. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol. (2009) 39:1083–91. doi: 10.1016/j.ijpara.2009.02.016
23. Botelho M, Oliveira P, Gomes J, Gartner F, Lopes C, Correia Da Costa JM, et al. Tumourigenic effect of Schistosoma haematobium total antigen in mammalian cells. Int J Exp Pathol. (2009) 90:448–53. doi: 10.1111/j.1365-2613.2009.00650.x
24. Botelho MC, Veiga I, Oliveira PA, Lopes C, Teixeira M, da Costa JMC, et al. Carcinogenic ability of Schistosoma haematobium possibly through oncogenic mutation of KRAS gene. Adv cancer Res Treat. (2013) 2013:876585. doi: 10.5171/2013.876585
25. Honeycutt J, Hammam O, Hsieh MH. Schistosoma haematobium egg-induced bladder urothelial abnormalities dependent on p53 are modulated by host sex. Exp Parasitol. (2015) 158:55–60. doi: 10.1016/j.exppara.2015.07.002
26. Chala B, Choi MH, Moon KC, Kim HS, Kwak C, Hong ST. Development of urinary bladder pre-neoplasia by schistosoma haematobium eggs and chemical carcinogen in mice. Korean J Parasitol. (2017) 55:21–9. doi: 10.3347/kjp.2017.55.1.21
27. Gouveia MJ, Santos J, Brindley PJ, Rinaldi G, Lopes C, Santos LL, et al. Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. (2015) 359:226–32. doi: 10.1016/j.canlet.2015.01.018
28. Botelho MC, Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL, et al. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: an oestrogen-DNA adducts mediated pathway? Int J Parasitol. (2013) 43:17–26. doi: 10.1016/j.ijpara.2012.10.023
29. Shaker OG, Hammam OA, El Leithy TR, El Ganzoury H, Wishahi MM, Mikhailidis DP. Molecular markers and bladder carcinoma: Schistosomal and non-schistosomal. Clin Biochem. (2011) 44:237–44. doi: 10.1016/j.clinbiochem.2010.09.028
30. Metwally NS, Ali SA, Mohamed AM, Khaled HM, Ahmed SA. Levels of certain tumor markers as differential factors between bilharzial and non-biharzial bladder cancer among Egyptian patients. Cancer Cell Int. (2011) 11:8. doi: 10.1186/1475-2867-11-8
31. El-Aal AAA, Emran AM, Al-Antably AS, El Saftawy EA, Bayoumy IR, Hassan NS, et al. Immunohistochemical pattern of T lymphocytes population within bilharzial-associated bladder neoplasm microenvironment. Int J Immunopathol Pharmacol. (2015) 28:209–17. doi: 10.1177/0394632015584733
32. Koonrungsesomboon N, Wadagni AC, Mbanefo EC. Molecular markers and Schistosoma-associated bladder carcinoma: a systematic review and meta-analysis. Cancer Epidemiol. (2015) 39:487–96. doi: 10.1016/j.canep.2015.06.004
33. Fu CL, Odegaard JI, Herbert DR, Hsieh MH. A novel mouse model of schistosoma haematobium egg-induced immunopathology. PLoS Pathog. (2012) 8:e1002605. doi: 10.1371/journal.ppat.1002605
34. Loeffler DA, Lundy SK, Singh KP, Gerard HC, Hudson AP, Boros DL. Soluble egg antigens from Schistosoma mansoni induce angiogenesis-related processes by up-regulating vascular endothelial growth factor in human endothelial cells. J Infect Dis. (2002) 185:1650–6. doi: 10.1086/340416
35. Salem HK, Ragab H, Abd El Maksoud N. 1068 vascular endothelial growth factor expression in schistosomiasis-associated bladder cancer, correlation with histopathological features and schistosomiasis. J Urol. (2012) 187:e433–4. doi: 10.1016/j.juro.2012.02.1174
36. Zhong X, Isharwal S, Naples JM, Shiff C, Veltri RW, Shao C, et al. Hypermethylation of genes detected in urine from ghanaian adults with bladder pathology associated with schistosoma haematobium infection. PLoS ONE (2013) 8:e59089.: doi: 10.1371/journal.pone.0059089
37. Onile OS, Calder B, Soares NC, Anumudu CI, Blackburn JM. Quantitative label-free proteomic analysis of human urine to identify novel candidate protein biomarkers for schistosomiasis. PLoS Negl Trop Dis. (2017) 11:e0006067. doi: 10.1371/journal.pntd.0006045
38. Botelho MC, Oliveira PA, Lopes C, Correia da Costa JM, Machado JC. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol Oncol Semin Orig Invest. (2011) 29:809–14. doi: 10.1016/j.urolonc.2009.09.017
39. Esrig D, Spruck CH, Nichols PW, Chaiwun B, Steven K, Groshen S, et al. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol. (1993) 143:1389–97.
40. Santos J, Fernandes E, Ferreira JA, Lima L, Tavares A, Peixoto A, et al. P53 and Cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with schistosoma haematobium infection. PLoS Negl Trop Dis. (2014) 8:e3329. doi: 10.1371/journal.pntd.0003329
41. Mbanefo EC, Hsieh MH. Defining the pathways of urogenital schistosomiasis-associated urothelial carcinogenesis through transgenic and bladder wall egg injection models. Methods Mol Biol. (2018) 1655:67–76. doi: 10.1007/978-1-4939-7234-0_6
42. Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature (2007) 446:153–8. doi: 10.1038/nature05610
43. Hicks RM, James C, Webbe G. Effect of schistosoma haematobium and N-butyl-N-(4-hydroxybutyl)nitrosamine on the development of urothelial neoplasia in the baboon. Br J Cancer (1980) 42:730–55. doi: 10.1038/bjc.1980.308
44. Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. (1971) 68:820–3. doi: 10.1073/pnas.68.4.820
45. Mostafa MH, Helmi S, Badawi AF, Tricker AR, Spiegelhalder B, Preussmann R. Nitrate, nitrite and volatile N-nitroso compounds in the urine of schistosoma haematobium and schistosoma mansoni infected patients. Carcinogenesis (1994) 15:619–25. doi: 10.1093/carcin/15.4.619
46. Fantini D, Glaser AP, Rimar KJ, Wang Y, Schipma M, Varghese N, et al. A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene (2018) 37:1911–25. doi: 10.1038/s41388-017-0099-6
47. Abdulamir AS, Hafidh RR, Kadhim HS, Abubakar F. Tumor markers of bladder cancer: the schistosomal bladder tumors versus non-schistosomal bladder tumors. J Exp Clin Cancer Res. (2009) 28:27. doi: 10.1186/1756-9966-28-27
48. Botelho MC, Crespo M, Almeida A, Vieira P, Delgado ML, Araujo L, et al. Schistosoma haematobium and Schistosomiasis mansoni: production of an estradiol-related compound detected by elisa. Exp Parasitol. (2009) 122:250–3. doi: 10.1016/j.exppara.2009.04.001
49. Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PLoS ONE (2013) 8:e73076. doi: 10.1371/journal.pone.0073076
50. Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL, Brindley PJ, et al. The role of estradiol metabolism in urogenital schistosomiasis-induced bladder cancer. Tumor Biol. (2017) 39. doi: 10.1177/1010428317692247
51. C. Botelho M, Alves H, Richter J. Estrogen catechols detection as biomarkers in schistosomiasis induced cancer and infertility. Lett Drug Des Discov. (2017) 14:135–8. doi: 10.2174/1570180813666160720165057
52. Pennington LF, Alouffi A, Mbanefo EC, Ray D, Heery DM, Jardetzky TS, et al. H-IPSE is a pathogen-secreted host nucleus-infiltrating protein (infiltrin) expressed exclusively by the Schistosoma haematobium egg stage. Infect Immun. (2017) 85. doi: 10.1128/IAI.00301-17
53. Barsoum RS. Urinary schistosomiasis: review. J Adv Res. (2013) 4:453–9. doi: 10.1016/j.jare.2012.08.004
54. Wang C, Ross WT, Mysorekar IU. Urothelial generation and regeneration in development, injury, and cancer. Dev Dyn. (2017) 246:336–43. doi: 10.1002/dvdy.24487
55. Rosin MP, El Din Zaki SS, Ward AJ, Anwar WA. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res Fundam Mol Mech Mutagen. (1994) 305:283–92. doi: 10.1016/0027-5107(94)90248-8
56. Nesi G, Nobili S, Cai T, Caini S, Santi R. Chronic inflammation in urothelial bladder cancer. Virchows Arch. (2015) 467:623–33. doi: 10.1007/s00428-015-1820-x
57. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420:860–7. doi: 10.1038/nature01322.Inflammation
58. Francescone R, Hou V, Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J. (2014) 20:181–9. doi: 10.1097/PPO.0000000000000048
59. Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. (2015) 45:17–31. doi: 10.1002/eji.201444972
60. Dematei A, Fernandes R, Soares R, Alves H, Richter J, Botelho MC. Angiogenesis in Schistosoma haematobium-associated urinary bladder cancer. APMIS (2017) 125:1056–62. doi: 10.1111/apm.12756
Keywords: cancer, mouse models, schistosomiasis, schistosomiasis haematobia, bladder cancer, bladder
Citation: Ishida K and Hsieh MH (2018) Understanding Urogenital Schistosomiasis-Related Bladder Cancer: An Update. Front. Med. 5:223. doi: 10.3389/fmed.2018.00223
Received: 11 March 2018; Accepted: 20 July 2018;
Published: 10 August 2018.
Edited by:
Joachim Richter, Heinrich Heine Universität Düsseldorf, GermanyReviewed by:
Ana Afonso, Universidade de São Paulo, BrazilAleksandra Barac, University of Belgrade, Serbia
Copyright © 2018 Ishida and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael H. Hsieh, mhsieh@childrensnational.org
 Kenji Ishida
Kenji Ishida Michael H. Hsieh
Michael H. Hsieh