Polypyrrole/Metal Oxides-Based Composites/Nanocomposites for Corrosion Protection
- Department of Coatings and Polymeric Materials, North Dakota State University, Fargo, ND, United States
Corrosion is of paramount interest to researchers all around the world owing to its disastrous impact on both the economy and people’s safety. New strategies based on novel materials have been employed to mitigate corrosion. Conducting polymer-based nanocomposites are a class of materials which have shown promise for corrosion protection. This paper will focus on the specific case of the Conducting polymer-based nanocomposites for corrosion protection, and one important case will be discussed in detail. Corrosion protection offered by Polypyrrole (PPy)/Fe2O3 based composite coating for cold rolled substrate is characterized in this paper by electrochemical impedance spectroscopy and open circuit potential measurements. It was found that PPy/Fe2O3 based composite coating provides better corrosion performance after 40 days exposure to ASTM B117 salt fog test conditions. This improved performance was attributed to the increased surface area of PPy and passivating nature of the composite resulting from the redox activity provided by PPy.
Introduction
Corrosion is an electrochemical process by which metals or metal alloys go back to their oxide form. Generally, products of corrosion reaction are the most thermodynamically stable forms of the metals and metal alloys. It is in that stable form (oxide) of metal in which it exists in the ores. Huge amounts of energy are required to bring the metal oxide to metal or metal alloy form. The same amount of energy is released once the metal comes into contact with a conducive environment for corrosion (water, oxygen, salts, etc.) resulting in the formation of metal oxide (corrosion product). Microscopic strain, faults, defects, and impurities in the metal surface are responsible for its thermodynamic instability. Corrosion can be aqueous or atmospheric depending on the environment to which the metal is exposed.
Corrosion cannot be stopped as it is a thermodynamically favorable process. However, it can be mitigated by using several strategies. Design control, judicious alloy selection, overdesign, cathodic protection, organic coatings, metallic coatings (inorganic coatings), or plating are the avenues used for corrosion protection. Use of organic coatings is widely employed for the corrosion protection of the metals and their alloys owing to the application flexibility, cost effectiveness, ability to protect in different environments, and other performance attributes of this method. Traditional coating technologies protect by different mechanisms such as barrier, sacrificial, and active (corrosion inhibitors). These coatings have their own advantages and disadvantages. With the changing environment of the 21st century and an increasing demand for ecofriendly materials, researchers have been encouraged to work on innovative materials for corrosion protection. A combination of the protection mechanism is one of the ways to improve the corrosion resistance of the organic coating. In this paper we will be discussing the active corrosion protection offered by conducting polymers in combination with the passive protection imparted by the inorganic oxide pigments. This combination ultimately results in the conducting polymer composite coatings.
Conducting polymers are an important class of materials which have shown promise in areas such as batteries, sensors, actuators, electromagnetic shielding, semiconductors, and corrosion protection (Das and Prusty, 2012; Ratautaite et al., 2013; Deshpande et al., 2014). Their conductivity, ease of synthesis, and pigment-like nature make them a suitable candidate to be used in coatings for corrosion protection. Polyaniline (PANI), polypyrrole (PPy), and polythiophene (PTh) belong to the family of intrinsically conducting polymers. Polypyrrole is one of the most important members of this class of polymers. Polypyrrole possesses the necessary electrical conductivity and environmental stability for corrosion protection application for metals and their alloys. Significant progress has been made in the last 30 years toward the application of PPy for the protection of metal alloys. There are papers which discuss the milestones in the development of several avenues based on PPy for corrosion protection of metal substrates (Jadhav et al., 2013; Deshpande et al., 2014).
Composites combine unique properties of two or more materials to impart beneficial functionalities. Nanocomposites involve one phase in nanometer dimension. A high surface to volume ratio of nanoparticles render properties which are not attainable in the case of regular particle sizes of the same material. This paper will detail the various composites/nanocomposites of PPy with several materials.
Among many conductive polymer composites/nanocomposites, the composites/nanocomposites prepared using PPy as the conductive polymer have been extensively investigated for the corrosion protection of various metals and their alloys. Recent studies have demonstrated that the incorporation of nanoparticles into conductive polymers such as PPy can increase th surface area of the conductive polymers and thereby provide improved electrochemical interactions with corrosive species and metal substrates. Although PPy composites/nanocomposites can be prepared via several synthetic methods, the most commonly used ones are electrochemical and chemical oxidation methods. Some more information about these preparation methods is provided in Table 1.
Iron oxide is an anticorrosive pigment and is a workhorse of red oxide primers which provide decent corrosion protection to mild steel. Polypyrrole/Fe2O3 and PPy/Fe3O4 composites were synthesized on iron electrochemically and were found to provide corrosion protection by maintaining substrate in a passive state (Garcia et al., 2002). Core and shell PPy/Fe2O3 pigment composites were synthesized by chemical oxidative polymerization (Jadhav and Gelling, 2012). The layer of PPy on Fe2O3 was in nanometer range, rendering PPy/Fe2O3 as a nanocomposite. The corrosion performance testing of these core and shell particles is described in this paper below. Shell of PPy in nanometer thickness range on Fe2O3 effectively increases the redox active surface area in the nanocomposite.
Materials and Methods
Coatings were prepared on sandblasted and hexane degreased cold rolled steel substrate. Coatings were formulated at 10% Pigment Volume Concentration (PVC) for Fe2O3 pigment and Fe2O3/PPy composite pigment and 1:1 stoichiometric ratio of EPON 830 and Epicure 3015 were used. MEK was used to reach application viscosity for proper drawdown application on the cold rolled steel surface. The coatings were applied with a drawdown bar on cleaned cold rolled steel substrate. Coatings were cured at an ambient temperature for 8 days for full development of the protective properties. Final dry film thickness of the coatings was 75–85 μm.
The coatings were exposed to salt spray test conditions according to ASTM B 117. Corrosion performance was monitored by electrochemical impedance spectroscopy (EIS) with Gamry Reference 600 Potentiostats with Gamry Framework Version 5.58/EIS 300 software. For EIS, AC perturbation of 10 mV over a frequency range of 100,000–0.01 Hz at 10 points/decade was applied. For EIS experiments 5% NaCl was used as electrolyte. Experiments were performed in triplicates and data is collected from representative samples. The cell area for the EIS measurements was 7.1 cm2. Open circuit potential (OCP) measurements were also performed with the same set up used in EIS experiments.
Results and Discussion
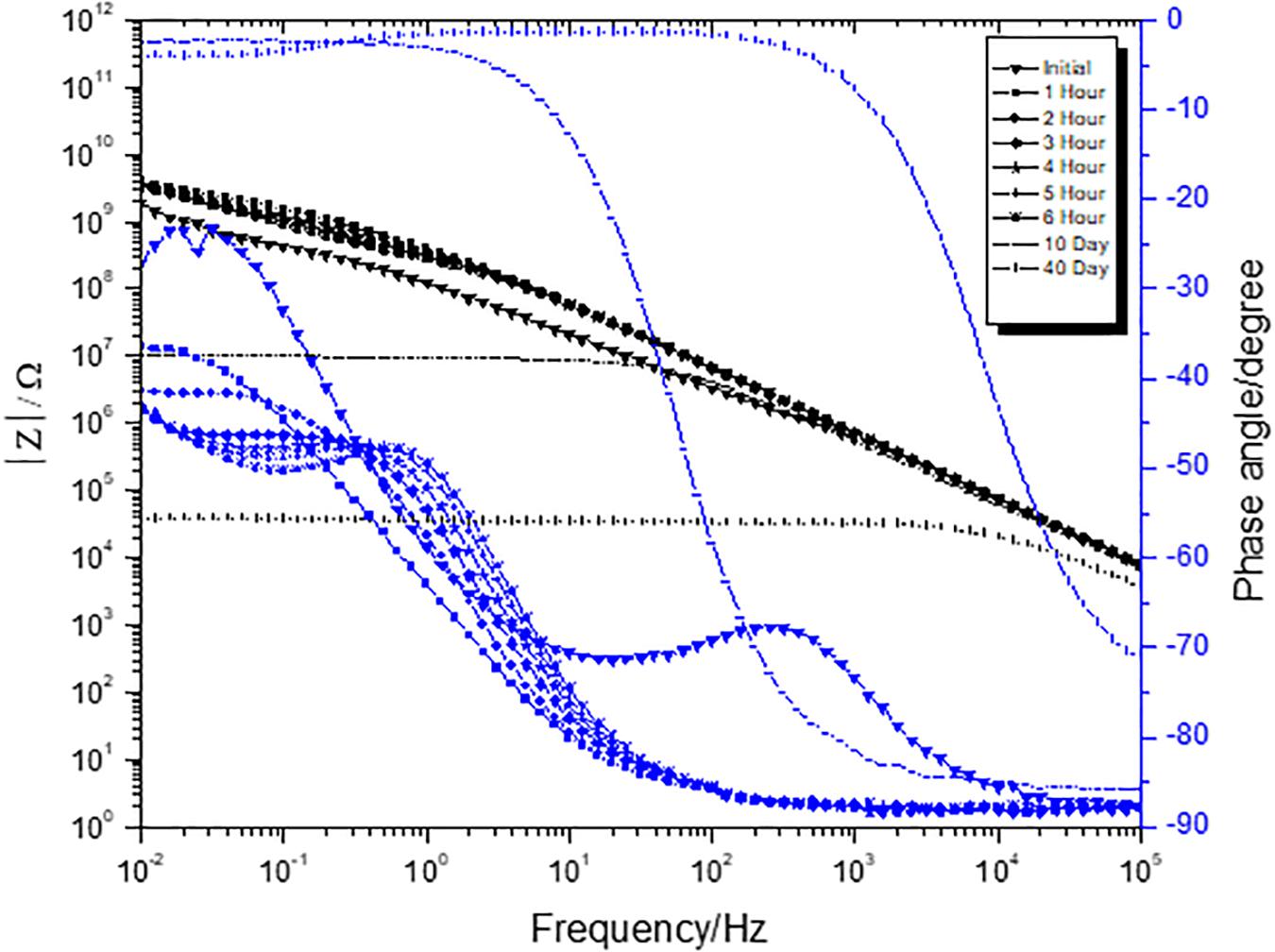
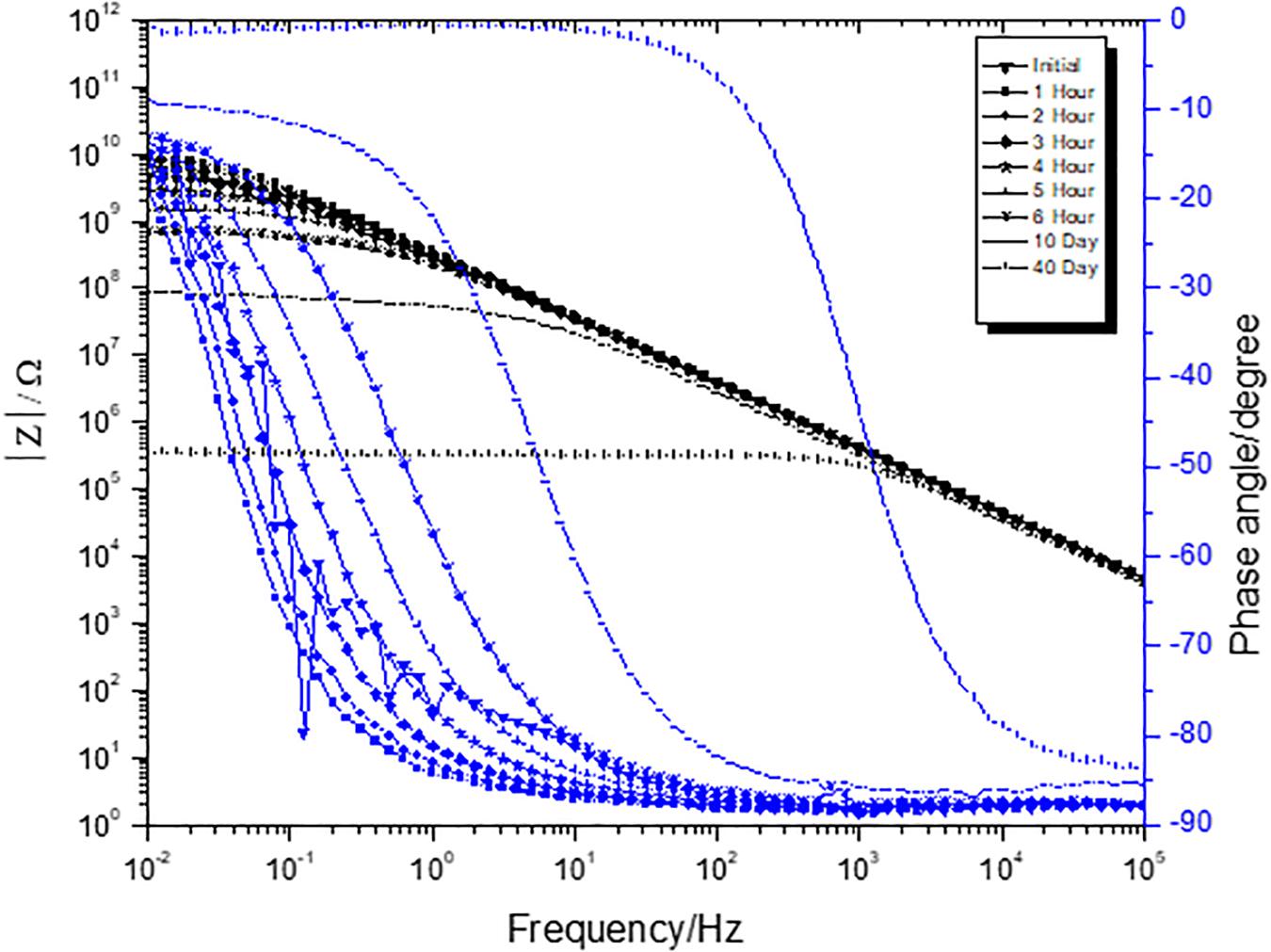
A three-electrode cell consisting of substrates with coating as working electrode, saturated calomel as reference electrode, and platinum mesh as a counter electrode was employed for performing EIS. As observed in Figures 1, 2, low frequency (0.01 Hz) impedance was very high initially for both Fe2O3 and Fe2O3/PPy coatings. As the duration of the salt spray exposure increased, a drop in low frequency impedance was observed for both Fe2O3 and Fe2O3/PPy coatings. The drop-in impedance in this case represents a decrease in protection offered by the coating to the corrosion (Le Thu et al., 2001). It represents the electrolyte ingress through the developed pores in the coating (Amirudin and Thieny, 1995). An appearance of a second time constant in Fe2O3 coating indicates the start of the corrosion reaction at coating and cold rolled steel interface.
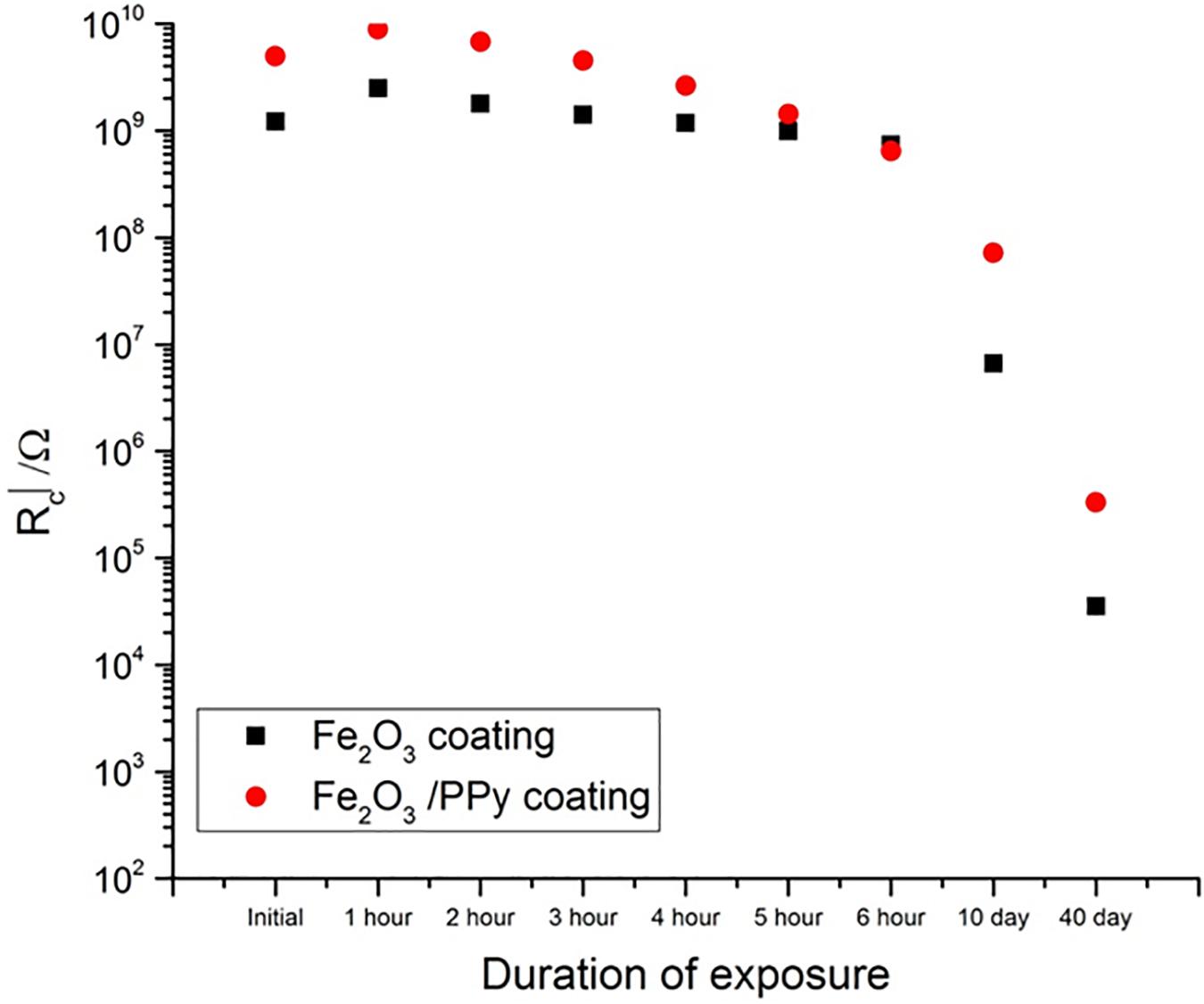
The EIS data was modeled by using ZView 2 software from Scribner Associates Inc. EIS data was fitted to Randles circuit model equivalent circuit. Randles circuit model consists of solution resistance and a constant phase element which is in parallel with coatings resistance (Rc). Resistance for both Fe2O3 and Fe2O3/PPy coatings was plotted against the duration of the exposure in salt spray (Figure 3). Initially Rc was higher for Fe2O3/PPy coating as compared with both Fe2O3 coating. Even though the value of Rc decreased with the duration of exposure to salt spray, it increased for Fe2O3/PPy coating and was still higher than that of Fe2O3 coating for the same duration of exposure. This suggested better corrosion performance offered by Fe2O3/PPy coating.
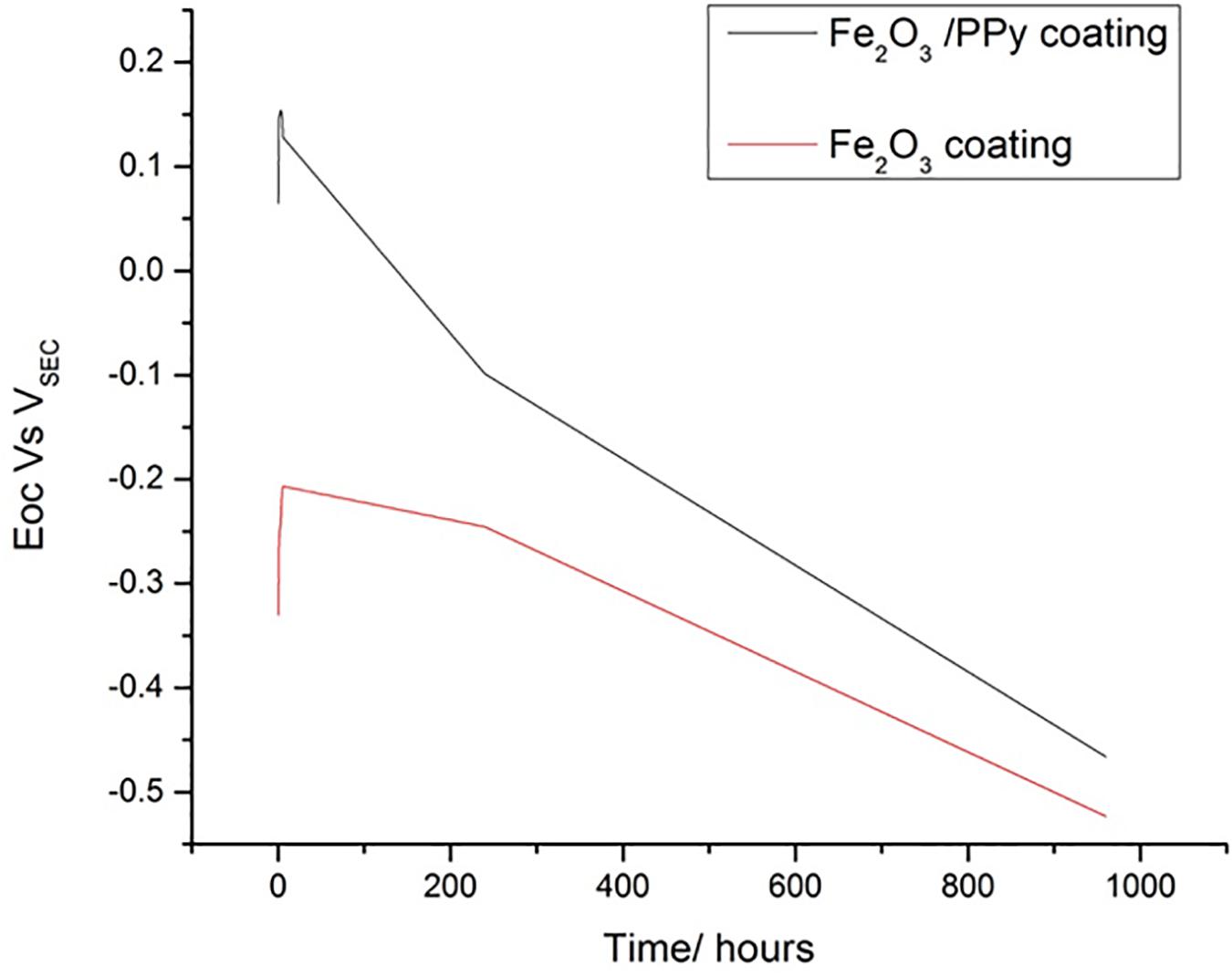
Simultaneously with EIS experiments, OCP values were also collected. As shown in Figure 4, OCP of the Fe2O3 coating was more toward the OCP of the cold rolled steel. For Fe2O3/PPy coating, OCP was more positive initially whereas it decreased over time as the duration to the salt spray exposure increased. Similar behavior was observed earlier in the case of PPy doped with sulfate anions. The more positive values of the OCP were attributed to the doped nature of the PPy (Ocón et al., 2005). Polypyrrole tends to be reduced and also serve as an oxidant to passivate underlying iron substrate, thereby further increasing corrosion protection (Spinks et al., 2002). The increased corrosion protection offered in the case of Fe2O3/PPy coating can also be attributed to the increased surface area of PPy for redox reactions and interactions with metal substrates and corrosive ions (Mahmoudian et al., 2011). The reduced PPy can also scavenge oxygen and get oxidized, thereby decreasing the rate of cathodic reaction and corrosion (Yan et al., 2010).
The efficiencies of the nanocomposites of inorganic pigments with PPy have been well documented in literature. In order to provide more evidence of the efficiency of the Fe2O3/PPy composite coating, we are providing a brief account of research. Polypyrrole/ZnO nanocomposites/composites can be synthesized by electrochemical or chemical oxidative polymerization (Moghaddam et al., 2009; Batool et al., 2012). Polypyrrole/ZnO nanocomposite was electrochemically prepared on mild steel with 10% loading of ZnO and was compared for corrosion protection with just PPy. Nanorod morphology was obtained for ZnO in the nanocomposite. It was found that the OCP of nanocomposite was more positive than just PPy and EIS measurements confirmed lower water uptake and lower capacitance suggesting better corrosion protection by PPy/ZnO nanocomposite (Hosseini et al., 2011). Polypyrrole/ZnO nanocomposites have also been obtained by chemical oxidative polymerization (Valença et al., 2015). Prepared PPy/ZnO nanocomposites were incorporated in epoxy coating at 0.2 w/w% loading and applied on SAE 1020 carbon steel. Electrochemical impedance spectroscopy and OCP measurements exhibited improved corrosion protection at lower loading of PPy/ZnO nanocomposites.
Polypyrrole/SiO2 composite was synthesized by chemical oxidative polymerization using FeCl3 as an oxidizer (Ruhi et al., 2014). The composite particles were added to epoxy powder coatings with the help of ball milling process. The composite particle concentrations added to epoxy powder coatings were 1–4 wt% with increments of 1 wt%. The addition of PPy/SiO2 composite at 3 wt% was found to provide optimum and superior corrosion performance on low carbon steel as observed in EIS and ASTM B117 salt fog exposures. The passivating ability of PPy in the defect/scribe was also noticed in Tafel experiments. Polypyrrole/SiO2 composite also exhibited improved corrosion protection for 316 stainless steel (Qun, 2017). In another attempt, multilayered coatings of PPy (obtained electrochemically) and SiO2 (deposited electrophoretically) were applied on AISI 304 stainless steel (Grari et al., 2015). The oxidizing ability of PPy leads to passivation along with added resistance of SiO2 results in improved corrosion protection. Oxalate ion doped PPy/SiO2 nanocomposite on carbon steel was also found to improve corrosion protection in polyvinylbutyral (PVB) binder matrix (Van et al., 2018). Multi-material composite of chitosan, PPy, and SiO2 was studied for the corrosion protection of mild steel (Ruhi et al., 2015). High cross linking is provided by chitosan for added barrier performance.
Polypyrrole/Al2O3 composite was electrochemically synthesized and was characterized for corrosion performance on 316 stainless steel (Yan et al., 2017). Improved barrier and increased charge transfer resistance, hence a lesser corrosion rate, was evident in 3.5 wt% NaCl constant immersion. Doped PPy composite films were prepared on stainless steel with alumina nanoparticles as the second phase (Ali, 2014). This composite was found to inhibit any increase in pH, thereby limiting the delamination process. Comparison of PPy/Al2O3, PPy/ZnO, PPy/TiO2, PPy/CeO2, and PPy/SnO2 nanocomposites was performed after they were synthesized by electrodeposition on the surface of mild steel (Babaei-Sati et al., 2019). The best corrosion protection was observed for PPy/Al2O3 nanocomposites. This was attributed to the lowest reduction potential (−2.33 V) exhibited by Al2O3 compared to other metal oxides, which ensures stability and less porosity under corroding situations. Hybrid composites of PPy deposited on alumina were incorporated in zinc rich epoxy primers found to improve corrosion performance on cold rolled steel by reducing the rate of zinc self-corrosion and blocking oxidizing entities (Gergely et al., 2011). Similar results were obtained in another study (Gergely et al., 2013). Nanocomposite of PPy and alumina was found to improve adhesion and corrosion resistance on Al 2024-T3 substrate (Tallman et al., 2008).
Polypyrrole containing composite pigments (PPy on aluminum flake surface) have been synthesized and used for the corrosion protection of metal substrates. Different morphologies of PPy on an aluminum flake surface resulted in improved corrosion performance on aluminum 2024-T3 surface (Jadhav et al., 2013). Incorporation of dopants (phosphates, nitrates, tungstate, and vanadate) on the backbone of PPy in these composites on an aluminum surface (as a single particle) resulted in better corrosion performance (Jadhav et al., 2012, 2015; Jensen et al., 2014). This ensured the intelligent release of corrosion inhibiting dopant at scribe in the event of corrosion as well as barrier properties provided by aluminum flakes, and the passivating nature of PPy in one composite particle for the overall corrosion protection of the substrate.
Mica provides improved corrosion protection by barrier mechanism because of its high aspect ratio and inert nature. Combing mica with PPy results in improved corrosion protection as there is the added advantage of active nature of PPy at coating metal interface and its dopant release ability in the event of defect or corrosion. Various organic surfactant dopants were incorporated on the backbone of PPy which was formed on mica flakes. The improved corrosion resistance on cold rolled steel was attributed to the decreased diffusion due to increased impediment for chloride ion ingress (Jadhav et al., 2018). Polypyrrole/mica composite with zinc as a pigment in epoxy coatings also exhibited improved corrosion protection (Qi and Gelling, 2009). Micaceous iron oxide (MIOx) is also another laminar flake pigment which provides barrier protection and passivation owing to its oxide nature. Micaceous iron oxide laminar flakes of different sizes (5, 10, and 30 μm) were used for preparation of PPy/MIOx composites pigments. Composite pigments with MIOx 30 μm size were selected for the corrosion resistance application on cold rolled surface. Improved corrosion protection was observed with PPy/MIOx due to the combination of impact from the passivating nature of PPy and the barrier properties exhibited by MIOx (Jadhav and Gelling, 2013).
TiO2 is a pigment which is widely used for hiding in paints and coatings. As it is already present in the protective coatings, combining it with an active corrosion provider such as PPy will improve overall corrosion performance. Polypyrrole/TiO2 composite on AISI 1010 mild steel exhibited improved corrosion protection as observed in weight loss and salt spray measurements (Lenz et al., 2003). Polypyrrole/TiO2 composites at 6.5 wt% concentration resulted in improved corrosion performance on AISI 1010 mild steel (Ferreira et al., 2001). Polypyrrole/TiO2 nanocomposite and PPy/co-doped TiO2 nanocomposite exhibited improved corrosion protection on AISI steel as observed in EIS, OCP, and Potentiodynamic polarization measurements. An insignificant decrease in the impedance was observed for 3.5 wt% NaCl immersion of the coatings (Ladan et al., 2017). The increased surface area of PPy on TiO2 particles was attributed to high chances on the surface of PPy for oxygen reduction (Mahmoudian et al., 2013). Passive film formation in case of PPy/V-doped TiO2 resulted in improved corrosion performance (Chen et al., 2019). Polypyrrole/TiO2 and PPy/tungstate doped TiO2 exhibited improved corrosion performance on cold rolled steel substrates due to effective PPy surface area increase and passivation after tungstate release in the event of corrosion (Jadhav and Gelling, 2015).
Conclusion
Polypyrrole/Metal oxides composites/nanocomposites have shown promise for corrosion protection. In this study, after exposure to salt spray test conditions, a lower drop in coating resistance in the case of Fe2O3/PPy coating as compared to Fe2O3 coating suggested better corrosion protection is offered by Fe2O3/PPy coating. An increased surface area for the interaction of ions with PPy and the passivating ability of PPy was responsible for the improved corrosion protection offered by Core and shell Fe2O3/PPy particles-based coatings.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
All authors contributed to the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the U.S. Army Research Laboratory under grant nos. W911 NF-04-2-0029, W911NF-09-2-0014, W911NF-10-2-0082, and W911NF-11-2-0027 for supporting this research.
References
Ali, E. (2014). Influence of electrosynthesis conditions and Al2O3 nanoparticles on corrosion protection effect of polypyrrole films. Anti-Corrosion Methods Mater. 61, 146–152. doi: 10.1108/ACMM-07-2012-1193
Amirudin, A., and Thieny, D. (1995). Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 26, 1–28. doi: 10.1016/0300-9440(95)00581-1
Babaei-Sati, R., Basiri Parsa, J., and Vakili-Azghandi, M. (2019). Electrodeposition of polypyrrole/metal oxide nanocomposites for corrosion protection of mild steel—A comparative study. Synth. Met. 247, 183–190. doi: 10.1016/j.synthmet.2018.12.009
Batool, A., Kanwal, F., Imran, M., Jamil, T., and Siddiqi, S. A. (2012). Synthesis of polypyrrole/zinc oxide composites and study of their structural, thermal and electrical properties. Synth. Met. 161, 2753–2758. doi: 10.1016/j.synthmet.2011.10.016
Chen, Z., Yang, W., Xu, B., Chen, Y., Qian, M., Su, X., et al. (2019). Corrosion protection of carbon steels by electrochemically synthesized V-TiO2/polypyrrole composite coatings in 0.1 M HCl solution. J. Alloys Compd. 771, 857–868. doi: 10.1016/j.jallcom.2018.09.003
Das, T. K., and Prusty, S. (2012). Review on conducting polymers and their applications. Polym. Plast. Technol. Eng. 51, 1487–1500. doi: 10.1080/03602559.2012.710697
Deshpande, P. P., Jadhav, N. G., Gelling, V. J., and Sazou, D. (2014). Conducting polymers for corrosion protection: a review. J. Coatings Technol. Res. 11, 473–494. doi: 10.1007/s11998-014-9586-7
Ferreira, C. A., Domenech, S. C., and Lacaze, P. C. (2001). Synthesis and characterization of polypyrrole/TiO2 composites on mild steel. J. Appl. Electrochem. 31, 49–56. doi: 10.1023/A:1004149421649
Garcia, B., Lamzoudi, A., Pillier, F., Nguyen, H., Le, T., and Deslouis, C. (2002). Oxide/polypyrrole composite films for corrosion protection of iron. J. Electrochem. Soc. 149, B560–B566.
Gergely, A., Bertóti, I., Török, T., Pfeifer, E., and Kálmán, E. (2013). Corrosion protection with zinc-rich epoxy paint coatings embedded with various amounts of highly dispersed polypyrrole-deposited alumina monohydrate particles. Prog. Org. Coatings 76, 17–32. doi: 10.1016/j.porgcoat.2012.08.005
Gergely, A., Pfeifer, E., Bertóti, I., Török, T., and Kálmán, E. (2011). Corrosion protection of cold-rolled steel by zinc-rich epoxy paint coatings loaded with nano-size alumina supported polypyrrole. Corros. Sci. 53, 3486–3499. doi: 10.1016/j.corsci.2011.06.014
Grari, O., Taouil, A. E., Dhouibi, L., Buron, C. C., and Lallemand, F. (2015). Multilayered polypyrrole–SiO2 composite coatings for functionalization of stainless steel: characterization and corrosion protection behavior. Prog. Org. Coatings 88, 48–53. doi: 10.1016/j.porgcoat.2015.06.019
Hosseini, M. G., Bagheri, R., and Najjar, R. (2011). Electropolymerization of polypyrrole and polypyrrole-ZnO nanocomposites on mild steel and its corrosion protection performance. J. Appl. Polym. Sci. 121, 3159–3166. doi: 10.1002/app.33952
Jadhav, N., and Gelling, V. (2015). Titanium dioxide/conducting polymers composite pigments for corrosion protection of cold rolled steel. J. Coatings Technol. Res. 12, 137–152. doi: 10.1007/s11998-014-9613-8
Jadhav, N., and Gelling, V. J. (2013). Synthesis and characterization of micaceous iron Oxide/Polypyrrole composite pigments and their application for corrosion protection of cold-rolled steel. Corrosion 70, 464–474. doi: 10.5006/0980
Jadhav, N., Jensen, M. B., and Gelling, V. (2015). Tungstate and vanadate-doped polypyrrole/aluminum flake composite coatings for the corrosion protection of aluminum 2024-T3. J. Coatings Technol. Res. 12, 259–276. doi: 10.1007/s11998-014-9633-4
Jadhav, N., Matsuda, T., and Gelling, V. (2018). Mica/polypyrrole (doped) composite containing coatings for the corrosion protection of cold rolled steel. J. Coatings Technol. Res. 15, 363–374. doi: 10.1007/s11998-017-9974-x
Jadhav, N., Vetter, C. A., and Gelling, V. J. (2012). Characterization and Electrochemical Investigations of Polypyrrole/Aluminum Flake Composite Pigments on AA 2024-T3 Substrate. ECS Trans. 41, 75–89.
Jadhav, N., Vetter, C. A., and Gelling, V. J. (2013). The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim. Acta 102, 28–43. doi: 10.1016/j.electacta.2013.03.128
Jadhav, N. G., and Gelling, V. J. (2012). Novel, facile method for the synthesis of iron oxide/polypyrrole core and shell particles, in: Polym. Mater. Sci. Eng. 2012:536.
Jensen, M. B., Peterson, M. J., Jadhav, N., and Gelling, V. J. (2014). SECM investigation of corrosion inhibition by tungstate- and vanadate-doped polypyrrole/aluminum flake composite coatings on AA2024-T3. Prog. Org. Coatings 77, 2116–2122. doi: 10.1016/j.porgcoat.2014.05.019
Ladan, M., Basirun, W. J., Kazi, S. N., and Rahman, F. A. (2017). Corrosion protection of AISI 1018 steel using Co-doped TiO2/polypyrrole nanocomposites in 3.5% NaCl solution. Mater. Chem. Phys. 192, 361–373. doi: 10.1016/j.matchemphys.2017.01.085
Le Thu, Q., Bierwagen, G. P., and Touzain, S. (2001). EIS and ENM measurements for three different organic coatings on aluminum. Prog. Org. Coatings 42, 179–187. doi: 10.1016/S0300-9440(01)00171-0
Lenz, D. M., Delamar, M., and Ferreira, C. A. (2003). Application of polypyrrole/TiO2 composite films as corrosion protection of mild steel. J. Electroanal. Chem. 540, 35–44. doi: 10.1016/S0022-0728(02)01272-X
Mahmoudian, M. R., Alias, Y., Basirun, W. J., and Ebadi, M. (2013). Effects of different polypyrrole/TiO2 nanocomposite morphologies in polyvinyl butyral coatings for preventing the corrosion of mild steel. Appl. Surf. Sci. 268, 302–311. doi: 10.1016/j.apsusc.2012.12.082
Mahmoudian, M. R., Basirun, W. J., Alias, Y., and Ebadi, M. (2011). Synthesis and characterization of polypyrrole/Sn-doped TiO2 nanocomposites (NCs) as a protective pigment. Appl. Surf. Sci. 257, 8317–8325. doi: 10.1016/j.apsusc.2011.03.075
Moghaddam, A. B., Nazari, T., Badraghi, J., and Kazemzad, M. (2009). Synthesis of ZnO nanoparticles and electrodeposition of Polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 4, 247–257.
Ocón, P., Cristobal, A. B., Herrasti, P., and Fatas, E. (2005). Corrosion performance of conducting polymer coatings applied on mild steel. Corros. Sci. 47, 649–662. doi: 10.1016/j.corsci.2004.07.005
Qi, X., and Gelling, V. (2009). “Polypyrrole coated mica flake as a pigment for corrosion protection,” in Proceedings of the DoD Corrosion Conference, Washington, DC, 2009.
Qun, Y. (2017). Electrochemical synthesis of polypyrrole-SiO2 composite coating on 316 stainless steel for corrosion protection. Anti-Corrosion Methods Mater. 64, 452–460. doi: 10.1108/ACMM-02-2016-1650
Ratautaite, V., Ramanaviciene, A., Oztekin, Y., Voronovic, J., Balevicius, Z., Mikoliunaite, L., et al. (2013). Electrochemical stability and repulsion of polypyrrole film. Colloids Surf. A Physicochem. Eng. Asp. 418, 16–21. doi: 10.1016/j.colsurfa.2012.10.052
Ruhi, G., Bhandari, H., and Dhawan, S. K. (2014). Designing of corrosion resistant epoxy coatings embedded with polypyrrole/SiO2 composite. Prog. Org. Coatings 77, 1484–1498. doi: 10.1016/j.porgcoat.2014.04.013
Ruhi, G., Modi, O. P., and Dhawan, S. K. (2015). Chitosan-polypyrrole-SiO2 composite coatings with advanced anticorrosive properties. Synth. Met. 200, 24–39. doi: 10.1016/j.synthmet.2014.12.019
Spinks, G. M., Dominis, A. J., Wallace, G. G., and Tallman, D. E. (2002). Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 6, 85–100. doi: 10.1007/s100080100211
Tallman, D. E., Levine, K. L., Siripirom, C., Gelling, V. G., Bierwagen, G. P., and Croll, S. G. (2008). Nanocomposite of polypyrrole and alumina nanoparticles as a coating filler for the corrosion protection of aluminium alloy 2024-T3. Appl. Surf. Sci. 254, 5452–5459. doi: 10.1016/j.apsusc.2008.02.099
Valença, A., Sa, D. P., Alves, K. G. B., de Melo, C. P., and Bouchonneau, N. (2015). Study of the Efficiency of Polypyrrole/ZnO Nanocomposites as additives in anticorrosion coatings. Mater. Res. 18, 273–278.
Van, V. T. H., Hang, T. T. X., Nam, P. T., Phuong, N. T., Thom, N. T., Devilliers, D., et al. (2018). Synthesis of Silica/Polypyrrole Nanocomposites and application in corrosion protection of carbon steel. J. Nanosci. Nanotechnol. 18, 4189–4195. doi: 10.1166/jnn.2018.15198
Yan, M., Vetter, C. A., and Gelling, V. J. (2010). Electrochemical investigations of polypyrrole aluminum flake coupling. Electrochim. Acta 55, 5576–5583. doi: 10.1016/j.electacta.2010.04.077
Keywords: polypyrrole, corrosion, coatings, composites, nanocomposites, electrochemical impedance spectroscopy, iron oxide
Citation: Jadhav N, Kasisomayajula S and Gelling VJ (2020) Polypyrrole/Metal Oxides-Based Composites/Nanocomposites for Corrosion Protection. Front. Mater. 7:95. doi: 10.3389/fmats.2020.00095
Received: 18 July 2019; Accepted: 30 March 2020;
Published: 05 May 2020.
Edited by:
Marco Salerno, Italian Institute of Technology (IIT), ItalyReviewed by:
Arunas Ramanavicius, Vilnius University, LithuaniaWan Jeffrey Basirun, University of Malaya, Malaysia
Copyright © 2020 Jadhav, Kasisomayajula and Gelling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niteen Jadhav, n3.uict@gmail.com
 Niteen Jadhav
Niteen Jadhav Subramanyam Kasisomayajula
Subramanyam Kasisomayajula  Victoria Johnston Gelling
Victoria Johnston Gelling