All-inclusive coral reef restoration: How the tourism sector can boost restoration efforts in the caribbean
- 1Wave of Change, Iberostar Hotels and Resorts, Bávaro, Dominican Republic
- 2Department of Biological Sciences, Old Dominion University, Norfolk, VA, United States
- 3Wave of Change, Iberostar Hotels and Resorts, Quintana Roo, Mexico
- 4Wave of Change, Iberostar Hotels and Resorts, Miami, FL, United States
Following a strong decline in the health of Caribbean coral reefs in the 1970s, disease outbreaks, overfishing, and warming events have continued to push these reefs towards a point of no return. As such, researchers and stakeholders have turned their attention to restoration practices to overcome coral recovery bottlenecks on Caribbean reefs. However, successful restoration faces many challenges, including economical and logistical feasibility, long-term stability, and biological and ecological factors yet to fully understand. The tourism sector has the potential to enhance and scale restoration efforts in the Caribbean, beyond simple financial contributions. Its strengths include long-term presence in several locations, logistical and human resources, and a business case focused on preserving the ecosystem services on which it depends. Here, we present the restoration program of Iberostar Hotels and Resorts which includes a scientific team that incorporates science-based solutions into resort operations to promote reef resilience in the face of climate change. We exemplify the potential of our program to scale up science-based reef restoration in collaboration with academia, local community, and government by presenting the first utilization of the Coral Bleaching Automated Stress System (CBASS) in Latin America and the Latin American Caribbean, with the aim of applying findings on coral thermotolerance directly to Iberostar’s reef restoration program across the Caribbean. This program presents a new model for tourism involvement in coral restoration and illustrates its capacity to scale up existing restoration practices by utilizing the strengths of the sector while maintaining science-based decision making.
Introduction
Reef degradation at global and regional scales
Global warming is impacting ecosystems at an increasingly alarming rate, with few ecosystems affected as heavily as coral reefs. Ocean warming and acidification are the primary drivers of reef decline on a global scale (Albright and Langdon, 2011; Arias-González et al., 2016; Hughes et al., 2018), while overfishing, destructive fishing practices, hurricane damage, nutrient and sediment pollution, and poor management of coastal development, among others, synergistically affect reefs at local scales (Hughes and Connell 1999; Gardner et al., 2005; Anthony et al., 2014; Hughes et al., 2017; Hughes et al., 2018). As a result, reefs are facing a rapid global decline with a bleak future that will compromise their services to marine ecosystems and society (Pratchett et al., 2014). Due to differences in local pressures, as well as contrasting evolutionary histories and environmental conditions, there is heterogeneity in the state of the reefs across geographic regions, with Caribbean reefs experiencing arguably the strongest negative shift in ecosystem state in recent decades (Cinner et al., 2016; Beyer et al., 2018; Cortés-Useche et al., 2019; Roff, 2021). The deterioration of Caribbean reefs has been documented since the 1970s, with a 50% decline in reef-building coral cover in recent decades and an increasing dominance by macroalgae, sponges, and the hydrozoan Millepora spp. (Mumby et al., 2007; Maliao et al., 2008; Cramer et al., 2021). This catastrophic decline in coral cover and diversity has largely been attributed to a loss of key herbivores shifting the competitive balance on the benthos in favor of macroalgae (Lessios et al., 1984; Hughes, 1994) and an increasing prevalence of coral diseases (Gladfelter, 1982; Precht et al., 2016). While these issues persist, a lower coral diversity and difference in evolutionary histories compared to their Indo-Pacific counterparts compromises sexual recruitment, which limits the recover potential of Caribbean coral communities (Roff, 2021). Furthermore, the frequency and intensity of mass bleaching events has increased in the region, since the first region-wide event in 1987 (McWilliams et al., 2005; Manzello, 2015), hampering recovery and resilience further. Coral bleaching is now one of the major threats to the region (Eakin et al., 2010). Yet, bleaching events can affect corals heterogeneously at different scales even at the level of species and colonies, promoting the adaptation to future conditions (Baker et al., 2008; Thomas et al., 2019; McClanahan et al., 2020). Nonetheless, since the current rate of environmental change is greater than that required for natural adaptation, active intervention through restoration efforts, in combination with efforts to reduce carbon emissions and localized pollution, are increasingly being developed as necessary approaches to aid the recovery of reef-building corals in this region (Rinkevich, 2015; Cortés-Useche et al., 2021; Suggett and van Oppen, 2022).
Reef restoration bottlenecks in the Caribbean
Global climate action, the establishment of marine protected areas, sustainable fishing practices, and effective management of water systems all are critical tools for reef recovery. Alongside these efforts, coral restoration has the potential to further help recovery of damaged or depleted reefs (Wilkinson and Souter, 2008; Young et al., 2012) and there is increasing recognition that it should play a strategic role in protecting critical ecosystem services (Abelson, 2006; Edwards, 2010; Chamberland et al., 2015; Schopmeyer et al., 2017; Calle-Triviño et al., 2018; Calle-Triviño et al., 2021). Numerous reef restoration projects have been developed in recent years to alleviate bottlenecks of recovery for Caribbean coral communities (Bayraktarov et al., 2020), leading to numerous advancements in techniques to growing and reproducing corals in aquarium settings. Still, there remain major challenges to successful reef restoration due to the slow growth rates of most foundational coral species, limiting the rate at which coral cover and abundance can be increased through outplanting, as well as the feasibility of restoring corals across large spatial scales. This is further constrained by the logistic (diving operations, permits) and economic (materials, equipment, labor) obstacles of underwater work (Boström-Einarsson et al., 2020), as well as a lack of long-term stakeholder engagement (Hein et al., 2020). Most restoration programs in the Caribbean are limited to the species Acropora cervicornis and A. palmata due to their fast growth rates compared to those of other mounding species, ease of fragmentation, historical and functional importance in the Caribbean, and endangered status (Aronson et al., 2008; Lirman et al., 2014; Calle-Triviño et al., 2020; Cramer et al., 2020). Low species diversity as well as often disregarded genetic diversity in these programs can limit functional diversity, sexual recruitment, and genetic exchange, which compromises the adaptive capacity and resiliency of these ecosystems for the future (Baums et al., 2019).
Scaling up the efforts strategically
In order to boost the efficiency and success of reef restoration, as well as build scalable solutions, major barriers to restoration need to be tackled by utilizing the strengths of the different sectors and stakeholders that benefit from coral reef ecosystem services. The tourism sector, especially in coastal tropical areas such as the Latin American Caribbean, constitutes a great benefactor of coral reefs (Spalding et al., 2017). Its potential role as a major contributor to reef restoration, beyond simple financial contributions, could help address many of the above-mentioned challenges (Hein et al., 2018). Here, we present the example of a private sector driven reef restoration program across the Caribbean as a solution to overcome some of the bottlenecks of scalability, economic feasibility, and long-term stability in coral restoration (Waltham et al., 2020; Cortés-Useche et al., 2021; Quigley et al., 2022). This program incorporates scientific research aimed at solving biological and ecological knowledge gaps that sets the base for restoration operation decisions. The research program is aligned with the current recommendations of the scientific community for reef restoration, such as the selection of resilient species and individuals to accelerate natural selection in the face of climate change, while maintaining the biodiversity and genetic diversity that will support long-term ecosystem resilience (Baums et al., 2019; Caruso et al., 2021; Vardi et al., 2021; Cunning et al., 2021). Moreover, it includes close collaboration with the local community, government, and academia to streamline legal and operational processes for restoration, as well as aligning with the needs and findings of the scientific community, increasing the scope of restoration benefits. By working together, we can utilize the strengths of each sector towards the common goal of restoring reefs to a healthy and productive state.
Tourism sector in reef restoration
Until recent years, the tourism sector has had a purely financial role in restoration projects, and while this is a major strength since the sector has longer-term economic stability than sectors and projects depending on grant cycles and external funding, its involvement can provide a boost to resolve other constraints. Here, we present a novel involvement of the coastal tourism industry in reef restoration (Figure 1) and exemplify it with the case of Iberostar Hotels and Resorts. Iberostar, as with other large networks of hotels and resorts, has an established foundation and resources in different destinations, as well as a network of existing suppliers and multidisciplinary teams that can help solve logistical and geographical bottlenecks. This is a key advantage in terms of scalability, and not only facilitates operating in different destinations, but also standardized and replicable approaches across locations, which can provide valuable information for restoration science. While research permit acquisition is a constraint for many projects to scale up, the tourism sector can advance it through already existing relations and collaboration with governments at each destination. The tourism sector has the capacity to form multidisciplinary teams that support their own restoration programs, such as scientists, operation coordinators, and restoration technicians. These can be strengthened through alliances with actors within the local community (i.e., NGOs, interns, volunteers, employees) and academia.
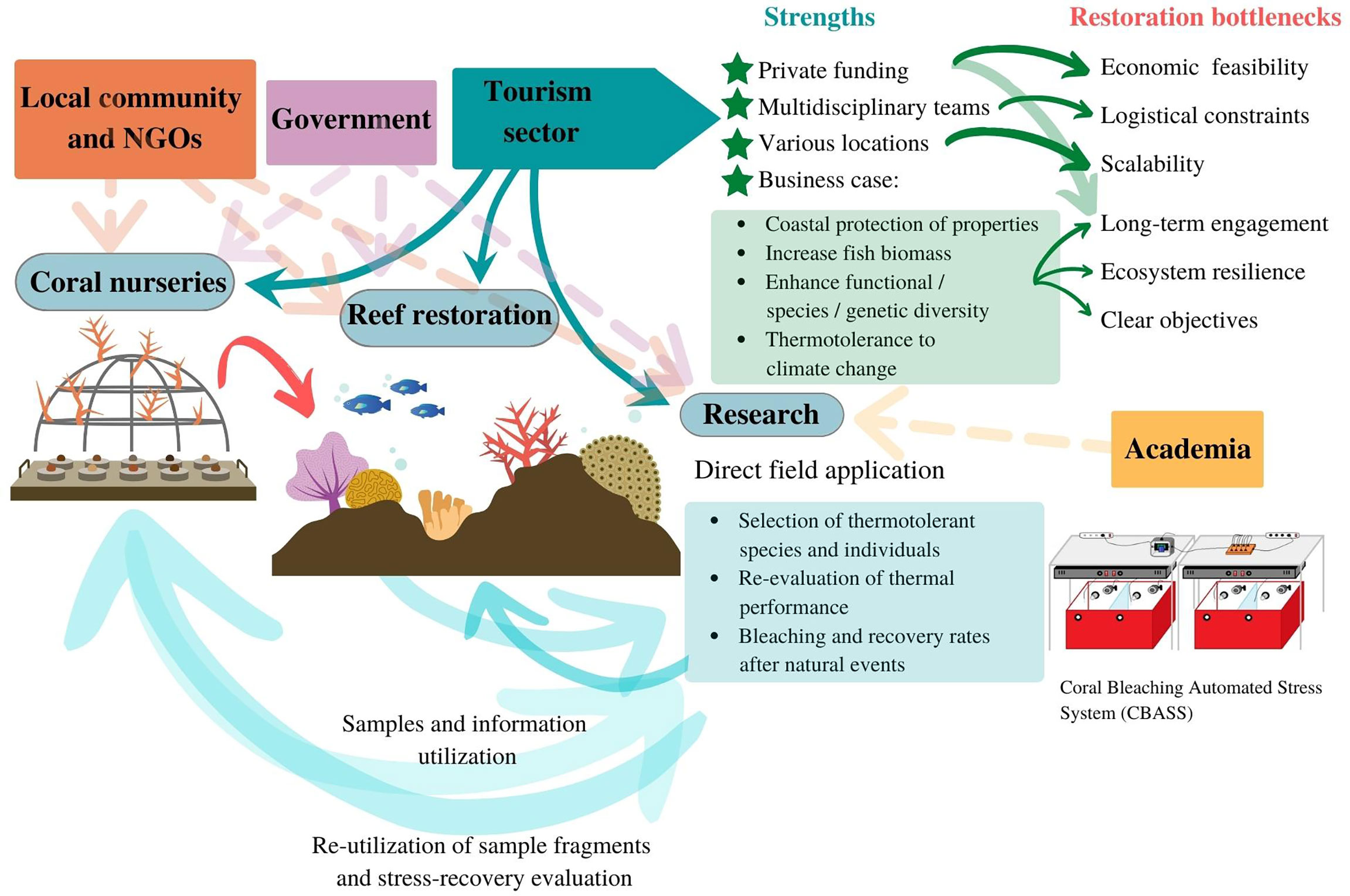
Figure 1 Reef restoration approach that utilizes the strengths of the tourism sector to overcome common restoration barriers, brings science into the core of restoration decisions and involves government, local community, and NGOs to ensure the resilience of restored reefs. Dashed lines indicate possible areas of collaboration, whereas thick lines indicate direct involvement of the tourism sector.
Approximately half of Iberostar Hotels and Resorts’ global operations are based on coastlines facing the Caribbean basin, and its presence in the region dates to 1993. As of 2020, Iberostar complexes create a combined total of 10.2 km of beachfront in this region. According to the World Resources Institute 500 m resolution map of tropical coral reefs of the world, 80% of Iberostar’s beachfront in this region has reefs within 5 km of their properties totaling almost 6 km2 of reefs that provide direct ecosystem services to Iberostar’s hotels, operations, and community. Thus, ensuring coral reef protection, resilience, and restoration are a major part of Iberostar’s strategy to improve ecosystem health and profitable tourism by 2030. This strategy is aligned with the objectives of the United Nations Decade of Ecological Restoration (2021-2030) and Sustainable Development Goals (Claudet et al., 2019; Schmidt-Roach et al., 2020), and is implemented globally through the Iberostar’s Wave of Change initiative that emerged in 2018, which intends to use the strengths of the tourism sector to tackle the ocean’s biggest challenges while leading sustainable tourism. The Wave of Change reef restoration strategy aims at restoring reefs that value coastal protection first through coral outplanting and propagation, then focusing on increasing fish biomass (important for local food security), and lastly on restoring biodiversity on reefs adjacent to Iberostar hotels.
In order to maximize the success of the conservation and restoration program, Iberostar’s internal team of scientists and coastal health managers collaborate closely with local NGOs to involve the community in their initiatives and support other marine conservation efforts, as well as with the government through collaboration agreements and the constant communication of results and projects, and its participation in sustainability events and activities. Additionally, success of the program relies on employees who are trained to follow the sustainability practices at the heart of the operations and are motivated to participate in environmental activities. Lastly, the program relies on a strong collaboration with academia to reinforce the legitimacy and scope of the scientific program and provide local students with internship opportunities and scholarships. The scientific program addresses lines of research that can inform and make restoration practices more efficient. These lines include asexual reproduction techniques such as reskinning and microfragmentation to address the limitation of slow coral growth rates (Page, 2013; Page and Vaughan, 2014); the creation of baselines through photomosaics (Lirman et al., 2007) and reef health assessments (Lang et al., 2011) to enable measuring the impact of the efforts, growing multiple species and genotyping coral individuals in nurseries to ensure species and genetic diversity (Baums et al., 2019), and the selection of thermotolerant coral species and colonies to accelerate natural selection (Morikawa and Palumbi, 2019; Caruso et al., 2021; Cunning et al., 2021), among other projects. Over four years, Iberostar’s restoration program has already been established in three countries (Dominican Republic, Mexico, and Jamaica), demonstrating the capacity for scalability (Figure 2), with concurrent scientific research also being conducted at these locations. As a demonstration of scalable and applicable reef restoration research with the involvement of the tourism sector, and its alignment with the current need of experimental standardization (Grottoli et al., 2021), we present a collaboration between the science team of Iberostar and academia. The collaboration focuses on identifying heat tolerant corals across Iberostar’s nurseries for inclusion in reef restoration efforts using standardized thermotolerance experiments across Iberostar’s restoration sites. Iberostar established an agreement with Old Dominion University (Virginia, USA) to acquire a portable lab system developed by Dr. Daniel Barshis and colleagues to conduct short-term heat stress experiments (Voolstra et al., 2020; Evensen et al., 2021). As part of the collaboration, Iberostar researchers were trained by Dr. Barshis and his team during an experiment conducted together at an Iberostar location. The collaboration also included participation by local partners, employees, internship students, and international clients, promoting knowledge exchange and education between multiple actors (Schmidt-Roach et al., 2020).
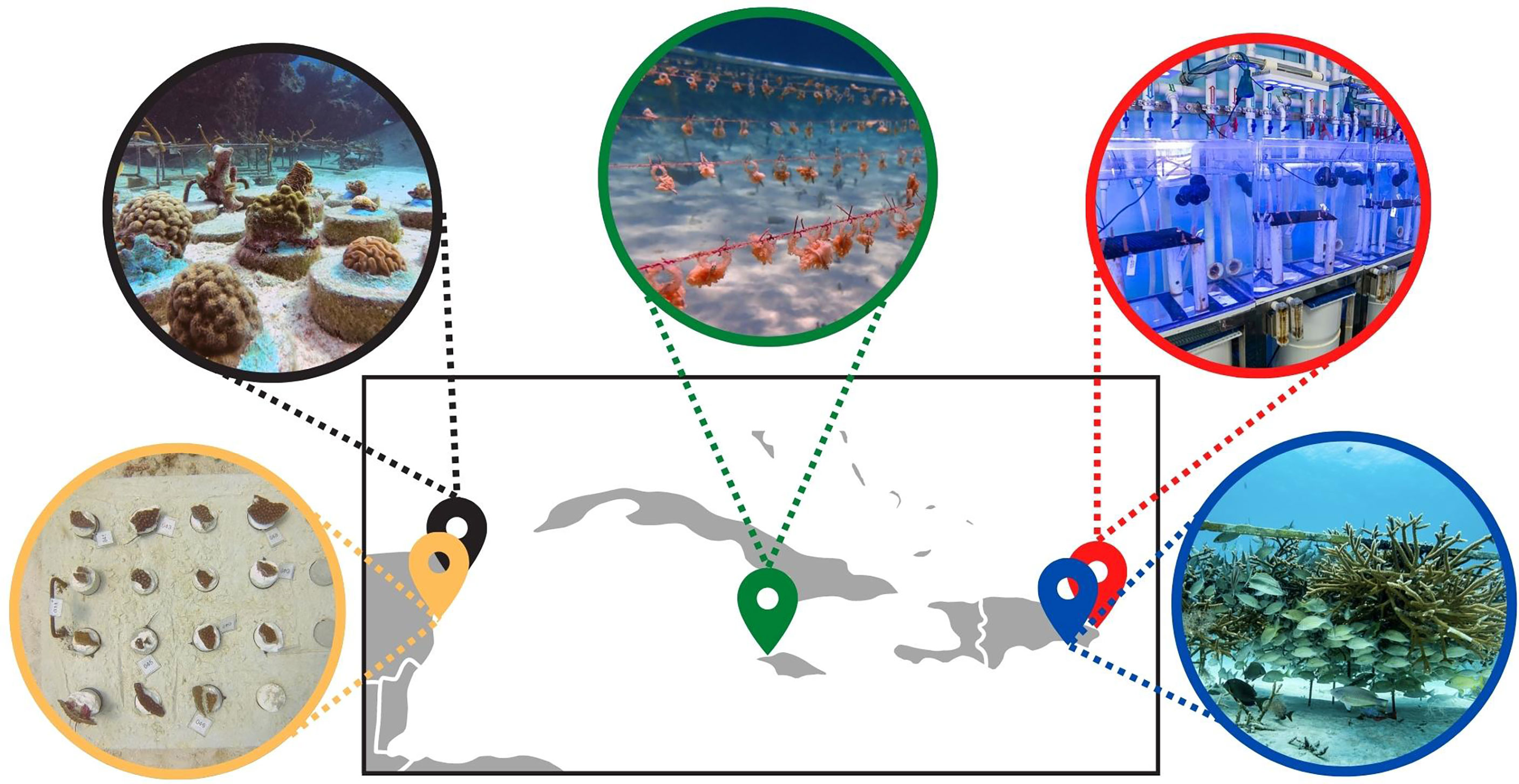
Figure 2 Iberostar coral nurseries’ locations across the Caribbean: Mexico (Paraiso Beach in yellow and Cozumel in black), Jamaica (Montego Bay in green) and Dominican Republic (Bavaro Coral Lab in red and Bayahibe in blue).
The use of a portable lab system and acute heat stress experiments for coral restoration
Background and experimental objectives
Experiments were conducted using the recently developed Coral Bleaching Automated Stress System (CBASS; Voolstra et al., 2020; Evensen et al., 2021). This highly portable experimental system is designed to conduct standardized thermal stress experiments in a variety of field settings, allowing for the direct comparison of thermal tolerance across coral species and populations. The comparability of heat stress assays is a priority that has recently been highlighted by the scientific community as a need for informing and facilitating reef conservation strategies (Grottoli et al., 2021). While Iberostar already has a coral laboratory in the Dominican Republic where similar thermal stress experiments are being conducted (Bayraktarov et al., 2020), this portable experimental system allows for highly reproducible and standardized experiments to be conducted across the Caribbean, at Iberostar locations where coral restoration programs are being implemented, with the aim of identifying heat tolerant coral individuals for their inclusion in restoration efforts at each location (Figure 1). To date, the CBASS has successfully been used to assay corals across a number of reefs, including American Samoa (Klepac and Barshis, 2020), numerous locations across the Red Sea and Gulf of Aden (Voolstra et al., 2020; Evensen et al., 2021; Voolstra et al., 2021; Evensen et al., 2022), the Great Barrier Reef, and the Galapagos (unpublished data). Notably, the CBASS has recently been used to compare heat tolerances of Acropora cervicornis colonies from six coral nurseries spanning the Florida Reef Tract (Cunning et al., 2021). The experiments presented herein, carried out in Playa Paraíso, Mexico, represent the first utilization of the CBASS in the Latin American Caribbean: a breakthrough for coral science and coral restoration in this region, which is often underrepresented in both fields globally due to language and resource barriers (Bayraktarov et al., 2020). The aim of the experiments was to evaluate the thermal tolerances of four common (yet underrepresented in restoration programs) species on Caribbean reefs: Montastraea cavernosa, Orbicella annularis, O. faveolata and Porites astreoides. For each species, 10 colonies were randomly sampled from Manchoncitos reef (20.759444°N, 86.95°W), located directly offshore from Iberostar’s Playa Paraíso resort, where one of the Iberostar coral nurseries is located. Acute heat stress assays, each lasting 18 h (detailed in Evensen et al., 2021), took place over 4 days, assaying one species per day. Response of the corals to thermal stress was assessed through pulse-amplitude modulated (PAM) fluorescence. Thermal thresholds of each species were calculated from PAM measurements (calculating the Fv/Fm ED50, sensu Evensen et al., 2021) and thresholds were compared to those from the Dominican Republic, previously obtained in the coral laboratory of Iberostar. Moving forward, we intend to continue assaying common coral species across Iberostar’s locations in the Caribbean to provide a region-wide sensus of coral thermotolerance, as well as conducting longer-term heat stress experiments at Iberostar’s nurseries to compare short- and long-term responses of corals to heat stress (Figure 2).
Field application
Despite consistency in heat stress susceptibility across coral taxa (Marshall and Baird, 2000; Loya et al., 2001; Grottoli et al., 2006; Guest et al., 2016; Singh et al., 2019), thermotolerance traits can also be influenced by the environment. Phenotypic plasticity itself can vary depending on the genotype, so the top performing genotype can change depending on the environment, which challenges the selection of heat tolerant individuals for reef restoration. As such, selecting individuals for reef restoration requires testing of locally adapted corals for thermotolerance. Additionally, re-evaluating thermal performance at regular intervals following outplanting could considerably improve our understanding of the mechanisms underpinning thermotolerance and how these are influenced by environmental changes (Kenkel et al., 2013; Palumbi et al., 2014; Kenkel et al., 2015; Drury et al., 2017; Kenkel and Matz, 2017; Morikawa and Palumbi, 2019; Caruso et al., 2021; Cunning et al., 2021; Drury and Lirman, 2021). The long-term presence of Iberostar at these sites will allow for an unrivaled opportunity to continually assess thermotolerance and bleaching recovery rates for previously assayed and outplanted individuals in the field, as well as their response to natural heat stress events. Bearing in mind the urgency of action to help reefs recover, and the time and resource constraints of conducting multiple experiments, short-term heat stress assays are an advantage for rapid thermotolerance tests with direct application to reef restoration (Ferse et al., 2021). With restoration efforts in the Caribbean to date focusing primarily on Acropora cervicornis or A. palmata, the capacity to rapidly test and select for a variety of species using these stress assays and include them in nurseries and restoration operations is key to securing biologically diverse and functional reefs (Baums et al., 2019). Moreover, thermotolerance research is often limited to specific areas with established laboratories and experimental systems, making it difficult to draw general, region-wide conclusions about thermal resilience, especially when methodologies differ between studies (Grottoli et al., 2021). Conducting these standardized studies across multiple locations where restoration programs are already in place will help to identify consistent tolerance mechanisms across species and populations, including those influenced by site-specific conditions. The established network of coral nurseries and restoration sites of Iberostar across the Caribbean, together with the internal team of scientists, restoration practitioners and operation managers, constitutes an ideal framework for restoration-applied thermotolerance research. Applying thermal stress assays information into a multinational restoration program in a cost-effective and logistically efficient manner is particularly promising for the advancement of restoration science and its application (Ferse et al., 2021), and could help accelerate and expand the scale of restoration efforts that are currently limited in scope considering the vast area of reefs requiring restoration across the Caribbean.
Conclusion
Caribbean reefs have a long history of environmental threats and degradation that are rapidly intensifying. Addressing the main causes of this degradation through the reduction of greenhouse gas emissions and the sustainable use of fisheries, among other solutions, is crucial to bring hope to these extremely important ecosystems. Corals have some ability to adapt to change, however, due to the severity and rate of climate change, intervention through science-based restoration may be necessary to accelerate this process and keep up with our rapidly changing environment. Ecosystem restoration at scale is still a great challenge due to the many economic, logistical, and scientific barriers that this practice presents. The tourism sector has the potential to scale up reef restoration with a new role that goes beyond being a financial investor, but rather takes advantage of other strengths of this sector, such as the long-term presence and logistics resources in different destinations. The tourism sector can also directly participate in the scientific and operational processes of restoration and include the conservation of these natural assets as part of the business case in the face of climate change. Iberostar Hotels and Resorts’ restoration program across the Caribbean is presented as an example of this novel role. It includes a scientific program that is aligned with research priorities to solve the primary knowledge and implementation bottlenecks for efficient and scalable reef restoration practices. Among these priorities is the selection of thermotolerant corals for restoration, which can help to optimize and accelerate ecosystem resilience in the face of increasingly rapid climate change. We present a cost-effective approach to identify and select heat tolerant corals for restoration through the first use of the Coral Bleaching Automated Stress System (CBASS; Voolstra et al., 2020; Evensen et al., 2021) in Latin America and the Latin American Caribbean. Novel solutions, collaborations, and the participation of diverse sectors and actors, such as the tourism sector presented herein, are crucial to push the boundaries of science and accelerate reef restoration efforts before Caribbean reefs are pushed past the point of no return.
Author contributions
MB-P, NRE, CC-U, JC-T, DJB, VG, EH and MKM conceived the manuscript. MB-P, NE, CC-U, JC-T wrote the initial draft. All authors reviewed and edited the article and approved the submitted version.
Funding
This work was funded by Iberostar Group through its Wave of Change initiative.
Acknowledgments
The authors would like to thank Iberostar Group and the Department of Biological Sciences at Old Dominion University for making possible the collaboration. Research work was possible thanks to the help of Iberostar hotel managers and maintenance team employees who arranged an optimal space to set up and conduct the experiments; the dive center Dressel Divers, who supported diving operations; and the assistance of both scientists from the National Polytechnical Institute Research and Advanced Studies Center (Cinvestav) and Iberostar marine biologist interns from the National Autonomous University of Mexico (UNAM). Handling of species and development of research activities were possible thanks to the alliance Iberostar-Cinvestav.
Conflict of interest
Authors MB-P, CC-U, JC-T, VG, EH, MKM were employed by the company Iberostar Hotels and Resorts.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abelson A. (2006). Artificial reefs vs coral transplantation as restoration tools for mitigating coral reef deterioration: benefits, concerns and proposed guidelines. B. Mar. Sci. 78 (1), 151–159.
Albright R., Langdon C. (2011). Ocean acidification impacts multiple early life history processes of the Caribbean coral porites astreoides. Glob. Change Biol. 17 (7), 2478–2487. doi: 10.1111/j.1365-2486.2011.02404.x
Anthony K. R. N., Marshall P. A., Abdullah A., Beeden R., Bergh C., Black R., et al. (2014). Operationalising resilience for adaptive coral reef management under global environmental change. Glob. Change Biol. 21, 48–61. doi: 10.1111/gcb.12700
Arias-González J. E., Rivera-Sosa A., Zaldívar-Rae J., Alva-Basurto C., Cortés-Useche C. (2016). “The animal forest and its socio-ecological connections to land and coastal ecosystems,” in Marine animal forests. Eds. Rossi S., Bramanti L., Gori A., Orejas C. (Springer Switzerland: Cham: Springer), 1209–1240. doi: 10.1007/978-3-319-17001-5_33-1
Aronson R., Bruckner A., Moore J., Precht B., Weil. E. (2008). Acropora cervicornis (United Kingdom: The IUCN Red List of Threatened Species 2008). doi: 10.2305/IUCN.UK.2008.RLTS.T133381A3716457.en
Baker A. C., Glynn P. W., Riegl B. (2008). Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf. Sci. 80 (4), 435–471. doi: 10.1016/j.ecss.2008.09.003
Baums I. B., Baker A. C., Davies S. W., Grottoli A. G., Kenkel C. D., Kitchen S. A., et al. (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Applic. 29 (8), 1–23. doi: 10.1002/eap.1978
Bayraktarov E., Banaszak A., Maya P. H. M., Kleypas J., Arias-Gonzalez J. E., Blanco M., et al. (2020). Coral reef restoration efforts in Latin American countries and territories. PloS One 15 (8), 1–19. doi: 10.1371/journal.pone.0228477
Beyer H. L., Kennedy E. V., Beger M., Chen C. A., Cinner J. E., Darling E. S., et al. (2018). Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 11 (6), 1–10. doi: 10.1111/conl.12587
Boström-Einarsson L., Babcock R. C., Bayraktarov E., Ceccarelli D., Cook N., Fersel S. C. A., et al. (2020). Coral restoration–a systematic review of current methods, successes, failures and future directions. PloS One 15 (1), 1–24. doi: 10.1371/journal.pone.0226631
Calle-Triviño J., Cortés-Useche C., Sellares R., Arias-González J. E. (2018). Assisted fertilization of threatened staghorn coral to complement the restoration of nurseries in southeastern Dominican Republic. Reg. Stud. Mar. Sci. 18, 129–134. doi: 10.1016/j.rsma.2018.02.002
Calle-Triviño J., Muñiz-Castillo A. I., Cortés-Useche C., Morikawa M., Sellares- Blasco R., Arias-González J. E. (2021). Approach to the functional importance of acropora cervicornis in outplanting sites in the Dominican Republic. Front. Mar. Sci. 8, 1–21. doi: 10.3389/fmars.2021.668325
Calle-Triviño J., Rivera-Madrid R., León-Pech M. G., Cortés-Useche C., Sellares-Blasco R. I., Aguilar-Espinosa M., et al. (2020). Assessing and genotyping threatened staghorn coral acropora cervicornis nurseries during restoration in southeast Dominican republic. PeerJ 8. doi: 10.7717/peerj.8863
Caruso C., Hughes K., Drury C. (2021). Selecting heat-tolerant corals for proactive reef restoration. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.632027
Chamberland V. F., Snowden S., Marhaver K. L., Petersen D., Vermeij M. J. A. (2015). Restoration of critically endangered elkhorn coral (Acropora palmata) populations using larvae reared from wild-caught gametes. Glob. Ecol. Conserv. 4, 526–537. doi: 10.1016/j.gecco.2015.10.005
Cinner J. E., Huchery C., MacNeil M. A., Graham N. A., McClanahan T. R., Maina J., et al. (2016). Bright spots among the world’s coral reefs. Nature 535 (7612), 416–419. doi: 10.1038/nature18607
Claudet J., Bopp L., Cheung W. W. L., Devillers R., Escobar-Briones E., Haugan P., et al. (2019). A roadmap for using the UN decade for ocean science for sustainable development in support of science, policy, and action. One Earth 2 (1), 34–42. doi: 10.1016/j.oneear.2019.10.012
Cortés-Useche C., Hernández-Delgado E. A., Calle-Triviño J., Sellares Blasco R., Galván V., Arias-González J. E. (2021). Conservation actions and ecological context: Optimizing coral reef local management in the Dominican Republic. PeerJ 9, e10925. doi: 10.7717/peerj.10925
Cortés-Useche C., Muñiz-Castillo A. I., Calle-Triviño J., Yathiraj R., Arias-González J. E. (2019). Reef condition and protection of coral diversity and evolutionary history in the marine protected areas of southeastern Dominican Republic. Reg. Stud. Mar. Sci. 32, 100893. doi: 10.1016/j.rsma.2019.100893
Cortés-Useche C., Reyes-Gamboa W., Cabrera-Pérez J. L., Calle-Triviño J., Cerón-Flores A., Raigoza-Figueras R., et al. (2021). Capture, culture and release of postlarvae fishes: proof-of-concept as a tool approach to support reef management. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.718526
Cramer K. L., Donovan M. K., Jackson J. B., Greenstein B. J., Korpanty C. A., Cook G. M., et al. (2021). The transformation of Caribbean coral communities since humans. Ecol. Evol. 11 (15), 10098–10118. doi: 10.1002/ece3.7808
Cramer K. L., Jackson J. B., Donovan M. K., Greenstein B. J., Korpanty C. A., Cook G. M., et al. (2020). Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6 (17), 1–11. doi: 10.1126/sciadv.aax9395
Cunning R., Parker K. E., Johnson-Sapp K., Karp R. F., Wen A. D., Williamson O. M., et al. (2021). Census of heat tolerance among florida’s threatened staghorn corals finds resilient individuals throughout existing nursery populations. Proc. R. Soc B. 288 (1961), 1–10. doi: 10.1098/rspb.2021.1613
Drury C., Lirman D. (2021). Genotype by environment interactions in coral bleaching. Proc. R. Soc B. 288 (1946), 1–8. doi: 10.1098/rspb.2021.0177
Drury C., Manzello D., Lirman D. (2017). Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, acropora cervicornis. PloS One 12 (3), 1–21. doi: 10.1371/journal.pone.0174000
Eakin C. M., Morgan J. A., Heron S. F., Smith T. B., Liu G., Alvarez-Filip L., et al (2010). Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PloS One 5 (11). doi: 10.1371/journal.pone.0013969.
Edwards A. (2010). Reef rehabilitation manual. coral reef targeted research and capacity building for management. St. Lucia. Aust., 166.
Evensen N. R., Fine M., Perna G., Voolstra C. R., Barshis D. J. (2021). Remarkably high and consistent tolerance of a red Sea coral to acute and chronic thermal stress exposures. Limnol. Oceanogr. 66 (5), 1718–1729. doi: 10.1002/lno.11715
Evensen N. R., Voolstra C. R., Fine M., Perna G., Buitrago-López C., Cárdenas A., et al. (2022). Empirically derived thermal thresholds of four coral species along the red Sea using a portable and standardized experimental approach. Coral. Reefs. 41 (2), 1–14. doi: 10.1007/s00338-022-02233-y
Ferse S. C., Hein M. Y., Rölfer L. (2021). A survey of current trends and suggested future directions in coral transplantation for reef restoration. PloS One 16 (5), 1–21. doi: 10.1371/journal.pone.0249966
Gladfelter W. B. (1982). White-band disease in acropora palmata: implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32, 639–643.
Grottoli A. G., Rodriguez L. J., Palardy J. E. (2006). Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. doi: 10.1038/nature04565
Grottoli A. G., Toonen R. J., van Woesik R., Vega Thurber R., Warner M. E., McLachlan R. H., et al. (2021). Increasing comparability among coral bleaching experiments. Ecol. Appl. 31 (4), 1–17. doi: 10.1002/eap.2262
Guest J. R., Low J., Tun K., Wilson B., Ng C., Raingeard D., et al. (2016). Coral community response to bleaching on a highly disturbed reef. Sci. Rep. 6 (1), 1–10. doi: 10.1038/srep20717
Hein M. Y., Couture F., Scott C. M. (2018). “Ecotourism and coral reef restoration: case studies from Thailand and the Maldives,” in Coral reefs: Tourism, conservation and management. Eds. Prideaux B., Pabel A.(Oxford: Routledge: Taylor & Francis), 137–150. doi: 10.4324/9781315537320-10
Hein M. Y., McLeod I. M., Shaver E. C., Vardi T., Pioch S., Boström-Einarsson L., et al. (2020). Coral reef restoration as a strategy to improve ecosystem services a guide to coral restoration methods (Nairobi: United Nations Environment Program).
Hughes T. P., Anderson K. D., Connolly S. R., Heron S. F., Kerry J. T., Lough J. M., et al. (2018). Spatial and temporal patterns of mass bleaching of corals in the anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes T. P., Kerry J. T., Alvarez-Noriega M., Alvarez-Romero J. G., Anderson K. D., Babcock R. C., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature22901
Hughes T. P., Connell J. H.. (1999). Multiple stressors on coral reefs: A long–term perspective. Limnol. Oceanogr. 44 (3part2), 932–940. doi: 10.4319/lo.1999.44.3_part_2.0932
Gardner T. A., Côté I. M., Gill J. A., Grant A., Watkinson A. R. (2005). Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long‐term decline. Ecology 86 (4), 174–184. doi: 10.1890/04-0141
Hughes T. P.. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265 (5178), 1547–1551. doi: 10.1126/science.265.5178.1547
Kenkel C. D., Almanza A. T., Matz M. V. (2015). Fine-scale environmental specialization of reef-building corals might be limiting reef recovery in the Florida keys. Ecol 96 (12), 3197–3212. doi: 10.1890/14-2297.1
Kenkel C. D., Goodbody-Gringley G., Caillaud D., Davies S. W., Bartels E., Matz M. V. (2013). Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol. Ecol. 22 (16), 4335–4348. doi: 10.1111/mec.12391
Kenkel C., Matz M. (2017). Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol. 1, 14. doi: 10.1038/s41559-016-0014
Klepac C. N., Barshis D. J. (2020). Reduced thermal tolerance of massive coral species in a highly variable environment. Proc. R. Soc B. 287, 1–9. doi: 10.1098/rspb.2020.1379
Lang J. C., Marks K. W., Kramer P. A., Kramer P. R., Ginsburg R. N. (2011). AGRRA Protocols. Version 5.5. The Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program.
Lessios H. A., Robertson D. R., Cubit J. D. (1984). Spread of diadema mass mortality through the caribbean. Science 226 (4672), 335–337. doi: 10.1126/science.226.4672.335
Lirman D., Gracias N. R., Gintert B. E., Gleason A. C.R., Reid R. P., Negahdaripour S., et al (2007). Development and application of a video-mosaic survey technology to document the status of coral reef communities. Environ. Monit. Assess. 125 (1) 59–73. doi: 10.1007/s10661-006-9239-0
Lirman D., Schopmeyer S., Galvan V., Drury C., Baker A. C. (2014). Growth dynamics of the threatened Caribbean staghorn coral acropora cervicornis: Influence of host genotype, symbiont identity, colony size, and environmental setting. PloS One 9 (9), 1–9. doi: 10.1371/journal.pone.0107253
Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., Van Woesik R. (2001). Coral bleaching: the winners and the losers. Ecol. Lett. 4 (2), 122–131. doi: 10.1046/j.1461-0248.2001.00203.x
Maliao R. J., Turingan R. G., Lin J. (2008). Phase-shift in coral reef communities in the florida keys national marine sanctuary (FKNMS), USA. Mar. Biol. 154 (5), 841–853. doi: 10.1007/s00227-008-0977-0
Manzello D. P. (2015). Rapid recent warming of coral reefs in the Florida keys. Sci. Rep. 5 (1), 1–10. doi: 10.1038/srep16762
Marshall P. A., Baird A. H. (2000). Bleaching of corals on the great barrier reef: differential susceptibilities among taxa. Coral. Reefs. 19 (2), 155–163. doi: 10.1007/s003380000086
McClanahan T. R., Darling E. S., Maina J. M., Muthiga N. A., Leblond J., Arthur R., et al. (2020). Highly variable taxa-specific coral bleaching responses to thermal stresses. Mar. Ecol. Prog. Ser. 648, 135–151. doi: 10.3354/meps13402
McWilliams J. P., Côté I. M., Gill J. A., Sutherland W. J., Watkinson A. R. (2005). Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology 86 (8), 2055–2060. doi: 10.1890/04-1657
Morikawa M. K., Palumbi S. R. (2019). Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. 116, 1–6. doi: 10.1073/pnas.1721415116
Mumby P. J., Hastings A., Edwards H. J. (2007). Thresholds and the resilience of Caribbean coral reefs. Nature 450 (7166), 98–101. doi: 10.1038/nature06252
Page C. (2013). Reskinning a reef: Mote Marine Lab scientists explore a new approach to reef restoration. In BBenthic Ecology Meeting.
Page C., Vaughan D. (2014). The cultivation of massive corals using “micro-fragmentaion” for the ‘reskinning’ of degraded coral reefs. Coral Magazine 10 (5), 72–80.
Palumbi S. R., Barshis D. J., Traylor-Knowles N., Bay R. A. (2014). Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. doi: 10.1126/science.1251336
Pratchett M. S., Hoey A. S., Wilson S. K. (2014). Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 7, 37–43. doi: 10.1016/j.cosust.2013.11.022
Precht W. F., Gintert B. E., Robbart M. L., Fura R., Van Woesik R. (2016). Unprecedented disease-related coral mortality in southeastern Florida. Sci. Rep. 6 (1), 1–11. doi: 10.1038/srep31374
Quigley K. M., Hein M., Suggett D. J. (2022). Translating the ten golden rules of reforestation for coral reef restoration. Cons. Biol 36 (4), 1–8. doi: 10.1111/cobi.13890
Rinkevich B. (2015). Climate change and active reef restoration–ways of constructing the “reefs of tomorrow. J. Mar. Sci. Eng. 3 (1), 111–127. doi: 10.3390/jmse3010111
Roff G. (2021). Evolutionary history drives biogeographic patterns of coral reef resilience. BioScience 71 (1), 26–39. doi: 10.1093/biosci/biaa145
Schmidt-Roach S., Duarte C., Hauser C. A., Aranda M. (2020). Beyond reef restoration: next-generation techniques for coral gardening, landscaping, and outreach. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00672
Schopmeyer S. A., Lirman D., Bertels E., Gilliem D. S., Goergen E. A., Griffin S. P., et al. (2017). Regional restoration benchmarks for acropora cervicornis. Coral. Reefs. 36, 1047–1057. doi: 10.1007/s00338-017-1596-3
Singh T., Iijima M., Yasumoto K., Sakai K. (2019). Effects of moderate thermal anomalies on acropora corals around sesoko island, Okinawa. PloS One 14 (1), 1–20. doi: 10.7717/peerj.8138
Spalding M., Burke L., Wood S. A., Ashpole J., Hutchison J., Zu Ermgassen P., et al (2017). Mapping the global value and distribution of coral reef tourism. Mar. Policy 82, 104–113. doi: 10.1016/j.marpol.2017.05.014
Suggett D. J., van Oppen M. J. (2022). Horizon scan of rapidly advancing coral restoration approaches for 21st century reef management. Emerg. Top. Life Sci. 6 (1), 125–136. doi: 10.1042/ETLS20210240
Thomas L., López E. H., Morikawa M. K., Palumbi S. R. (2022). Transcriptomic resilience, symbiont shuffling, and vulnerability to recurrent bleaching in reef–building corals. Mol. Ecol. 28 (14), 3371–3382. doi: 10.1111/mec.15143
Vardi T., Hoot W. C., Levy J., Shaver E., Winters R. S., Banaszak A. T., et al. (2021). Six priorities to advance the science and practice of coral reef restoration worldwide. Restor. Ecol. 29 (8), 1–7. doi: 10.1111/rec.13498
Voolstra C. R., Buitrago-López C., Perna G., Cárdenas A., Hume B. C., Rädecker N., et al. (2020). Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Change Biol. 26 (8), 4328–4343. doi: 10.1111/gcb.15148
Voolstra C. R., Valenzuela J. J., Turkarslan S., Cárdenas A., Hume B. C., Perna G., et al. (2021). Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol. Ecol. 30 (18), 4466–480. doi: 10.1111/mec.16064
Waltham N. J., Elliott M., Lee S. Y., Lovelock C., Duarte C. M., Buelow C., et al. (2020). UN Decade on ecosystem restoration 2021–2030—what chance for success in restoring coastal ecosystems? Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00071
Wilkinson C. R., Souter D. (2008). Status of caribbean coral reefs after bleaching and hurricanes in 2005. global coral reef monitoring network and reef and rainforest research center (Townsville: Global Coral Reef Monitoring Network).
Keywords: reef restoration, Caribbean, private sector, sustainable tourism, coral bleaching, thermal stress, coral bleaching automated stress system (CBASS)
Citation: Blanco-Pimentel M, Evensen NR, Cortés-Useche C, Calle-Triviño J, Barshis DJ, Galván V, Harms E and Morikawa MK (2022) All-inclusive coral reef restoration: How the tourism sector can boost restoration efforts in the caribbean. Front. Mar. Sci. 9:931302. doi: 10.3389/fmars.2022.931302
Received: 28 April 2022; Accepted: 01 August 2022;
Published: 24 August 2022.
Edited by:
Nina Yasuda, The University of Tokyo, JapanReviewed by:
Graham Forrester, University of Rhode Island, United StatesCopyright © 2022 Blanco-Pimentel, Evensen, Cortés-Useche, Calle-Triviño, Barshis, Galván, Harms and Morikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Macarena Blanco-Pimentel, macarena.blanco@iberostar.com
 Macarena Blanco-Pimentel
Macarena Blanco-Pimentel Nicolas R. Evensen
Nicolas R. Evensen Camilo Cortés-Useche
Camilo Cortés-Useche Johanna Calle-Triviño
Johanna Calle-Triviño Daniel J. Barshis
Daniel J. Barshis Victor Galván4
Victor Galván4