- Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Currently, the graft-versus-host disease (GVHD) prophylaxis consists of an immunosuppressive therapy mainly based on antithymocyte globulin (ATG) or post-transplant cyclophosphamide (PTCy). GVHD remains a major complication and limitation to successful allogeneic haploidentical hematopoietic stem cell transplantation (haplo‐HSCT). We modified the ATG-based GVHD prophylaxis with the addition of basiliximab in the setting of haplo-HSCT and attempted to explore the appropriate dosages. We conducted a retrospective analysis of 239 patients with intermediate- or high-risk hematologic malignancies who received haplo-HSCT with unmanipulated peripheral blood stem cells combined or not with bone marrow. All patients received the same GVHD prophylaxis consisting of the combination of methotrexate, cyclosporine or tacrolimus, mycofenolate-mofetil, and basiliximab with different doses of ATG (5-9mg/kg). With a median time of 11 days (range, 7-40 days), the rate of neutrophil engraftment was 96.65%. The 100-day cumulative incidences (CIs) of grade II–IV and III–IV aGVHD were 15.8 ± 2.5% and 5.0 ± 1.5%, while the 2-year CIs of total cGVHD and extensive cGVHD were 9.8 ± 2.2% and 4.1 ± 1.5%, respectively. The 3-year CIs of treatment-related mortality (TRM), relapse, overall survival (OS), and disease-free survival (DFS) were 14.6 ± 2.6%, 28.1 ± 3.4%, 60.9 ± 3.4%, 57.3 ± 3.4%, respectively. Furthermore, the impact of the reduction of the ATG dose to 6 mg/kg or less in combination with basiliximab on GVHD prevention and transplant outcomes among patients was analyzed. Compared to higher dose of ATG(>6mg/kg), lower dose of ATG (≤6mg/kg) was associated with a significant reduced risk of CMV viremia (52.38% vs 79.35%, P<0.001), while the incidences of aGVHD and cGVHD were similar between the two dose levels. No significant effect was found with regard to the risk of relapse, TRM, and OS. ATG combined with basiliximab could prevent GVHD efficiently and safely. The optimal scheme of using this combined regimen of ATG and basiliximab is that administration of lower dose ATG (≤6mg/kg), which seems to be more appropriate for balancing infection control and GVHD prophylaxis.
Introduction
Haploidentical hematopoietic stem cell transplant (haplo-HSCT) has been applied with promising results for patients diagnosed with hematological malignancies lacking an appropriately matched sibling or unrelated donor and urgently requiring transplantation (1–5). Although considerable progress has been made to overcome the challenging human leukocyte antigen (HLA)-barriers in the setting of haplo-HSCT through various graft-versus-host disease (GVHD) prevention approaches, GVHD remains a major factor contributing to variable degrees of transplantation-related morbidity and mortality, as well as quality of life compromise. Currently, T-replete strategies using unmanipulated allografts have been the dominant procedures for haplo-HSCT, in which the GVHD prophylaxis consists of an immunosuppressive therapy mainly based on antithymocyte globulin (ATG) or post-transplant cyclophosphamide (PTCy) (5–12). Both ATG and PTCy have consistently demonstrated efficacies in GVHD prophylaxis, but each strategy uniquely affects post-transplant immune reconstitution resulting in concordant increased incidences of opportunistic infections and malignancy relapse (9, 11, 13–15). The optimal approach to manipulate the delicate balance between controlling GVHD and timely T-cell immune reconstitution remains uncertain and has become a main focus of attention.
Basiliximab is a chimeric murine-human monoclonal antibody that is directed against the IL-2Rα chain (CD25) to inhibit T lymphocyte activation. Compared with ATG, basiliximab has similar immunosuppressive efficacies but leads to a lower incidence of infection and infection-related mortality in renal transplantation and heart transplantation (16–18). In terms of HSCT, basiliximab has been used to treat acute steroid-refractory GVHD with satisfactory responses (19–23). Considering that basiliximab only selectively eliminates the donor-specific alloreactive T cells without affecting the resting T cells present in the graft (24), basiliximab may prevent GVHD without compromising immune function. In fact, the protective effect of CD25 blockade on GVHD is controversial. It has been reported that prophylactic administration of daclizumab (another CD25 blockade) does not prevent acute GVHD (aGVHD) but may increase the risk of chronic GVHD (cGVHD) (25). On the contrary, we and others have indicated that basiliximab alone or with the combination of ATG contributes to GVHD prophylaxis in HSCT without increasing infections (26–32). Nevertheless, these studies contained a relatively small number of patients; and a larger study with more patients and longer follow-up is warranted. Therefore, we modified the ATG-based GVHD prophylaxis with the addition of basiliximab in the setting of haplo-HSCT. Furthermore, in order to reduce the risk of GVHD without increasing the incidence of infection events or compromising overall survival, and simultaneously decrease expense related to ATG, we attempted to explore the appropriate dose of ATG combined with basiliximab.
In this study, we retrospectively analyzed a large series of patients with hematological malignancies who underwent haplo-HSCT using GVHD immunosuppressive prophylaxis with different dosages of ATG incorporated with basiliximab. On the one hand, we investigated the efficacy and safety of this combined GVHD prophylaxis option. On the other hand, we performed a preliminary assessment of the appropriate lower dose of ATG coupled with basiliximab in our haplo-HSCT system.
Methods
Patients
A cohort of 239 consecutive patients were included in this retrospective study. These patients received their haplo-HSCT from February 2013 to January 2019 at the Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China. The study was reviewed and approved by the Ethical Committee of Huazhong University of Science and Technology, and informed consents were provided by all patients or legal guardians. The eligibility criteria included patients diagnosed with intermediate- or high-risk hematologic malignancies who received haplo-HSCT. Patients with HLA matching rates of 10/10, 9/10 and 6/6 were excluded. Patients were stratified at intermediate or high risk as we described previously (28, 33, 34). High-risk ALL was defined as beyond first complete remission (CR1) and at least one of the following criteria at diagnosis: age >35 years; high white blood cell count (>30 × 109/L for B-lineage ALL, >100 × 109/L for T-lineage ALL); poor-risk cytogenetics (ph+, t(4;11), t(8;14), complex karyotype, or low hypodiploidy near triploidy); delayed CR1 (>28 days of induction therapy). The definition of high-risk AML was conformed to the following criteria at diagnosis: hyperleukocytosis at diagnosis; no response to induction chemotherapy; relapse within 6 months after induction or consolidation therapy; ≥2 relapses or relapse after auto-HSCT; secondary AML; ≥CR2 or in no remission; poor cytogenetics according to the National Comprehensive Cancer Network 2018 guidelines (www.nccn.org).
HLA matching and stem cell source
As previously defined (28), HLA class I and class II were detected in all donor/recipient pairs. For donor/recipient pairs in 6 loci (HLA-A, -B, -DRB1), HLA-A and HLA-B were typed by intermediate-resolution DNA techniques, and HLA-DRB1 was performed through high resolution DNA techniques. For donor/recipient pairs in 10 loci (HLA-A, -B, -C, -DRB1, -DQB1), HLA typing was performed in high-resolution DNA techniques. Donors were ranked according to HLA matching sites, young age, male, negative donor-specific antibodies (DSA), good physical condition, and blood type matching. Bone marrow (BM)stem cells and/or peripheral blood stem cells (PBSCs) were collected from donors according to standard mobilization protocols. Donors were administrated rhG-CSF(8-10μg/kg/day) by continuous subcutaneous injections for six days (from day -3 to day 0 during transplantation). For HLA 4-5/6 or 6-7/10 matched related donor transplantation, G-CSF-mobilized PBSCs were infused into the recipient after collection on the fourth to sixth day after subcutaneous administration. For the donor-recipient HLA 3/6 or 5/10-matched setting, G-CSF mobilized BM was harvested on the fourth day and G-CSF primed PBSCs were collected on the fifth and the sixth day after subcutaneous administration. If the target count for CD34+cells was not above 4×106/kg of the recipient weight, the mobilization period would be extended by one day. BM stem cells were extracted from the donor under general anesthesia in the operation room.
Conditioning regimen and GVHD prophylaxis
Conditioning regimens included in this study were divided into two categories: intensified myeloablative conditioning regimens (IMC) and myeloablative conditioning regimens (MAC). The details were described in our previous studies (28, 33, 34). All patients were administrated with a combination of cyclosporine (CsA) or tacrolimus, short-term methotrexate (MTX), mycofenolate-mofetil (MMF), ATG, and basiliximab for GVHD prophylaxis. The GVHD prophylaxis was described as follows: CsA 5mg/kg or tacrolimus 0.5mg/kg (twice daily) was given from day -1 until day +180. Individualized dosage adjustment of CsA or tacrolimus was based on plasma concentration to maintain a target dose (CsA:150–250 ng/mL, tacrolimous:10-15ng/ml). MTX was administered intravenously at dosages of 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6 and +11. MMF (7.5 mg/kg, orally twice daily) was administered from day +7, which was tapered to half until day +60 and was discontinued based on the presence or absence of severe GVHD, infectious diseases, and relapse risk. Basiliximab was given intravenously at a dose of 20 mg by 30-minute IV infusion on day 0 (2 hours before graft infusion) and day +4. ATG (rabbit anti-human thymocyte immunoglobulin, Sanofi Aventis, Paris, France) was given with a median total dose of 6mg/kg (range, 5 to 9) from day -3 until day -1. For donor-recipient HLA 4-5/6matched or HLA 6-7/10matched setting, patients received ATG at a total dose of 6 mg/kg. For the HLA 3/6 or 5/10 matched transplant, a total dose of 9 mg/kg ATG was used (28). If the patient was unable to tolerate a predetermined dose of ATG during the transplant procedure, the dose of ATG was reduced. Diagnosis and clinical grading of aGVHD and cGVHD were established according to the standard criteria (35, 36). GVHD prophylaxis was summarized in Supplementary Figure 1.
Supportive care
All patients were hospitalized in laminar airflow rooms and given standard antimicrobial prophylaxis covering fungal and bacteria agents in our institution. Cytomegalovirus (CMV) DNA copies in plasma specimen were monitored weekly from day -7 until day +100, once every 2 weeks until day+180, and once per month until one year after HSCT. CMV DNA copies were monitored by polymerase chain reaction for all patients. The threshold for CMV-DNA copies were no more than 500 copies/mL in plasma specimen (33, 37). Pre-emptive ganciclovir or foscarnet therapy was initiated with the evidence of two consecutive positive tests without a sign of viral diseases and was continued until the CMV DNA monitoring was negative on two occasions.
Definitions and endpoints
Overall survival (OS) was defined as the period from transplantation to the date of last follow-up or death due to any cause. Disease-free survival (DFS) was defined as the time from transplantation to either last follow-up or disease recurrence or death due to any causes. Data for patients who were alive or lost to follow-up were censored at the time of last contact. The time of neutrophil implantation was defined as from day 0 to the day when neutrophil count exceeded 0.5×109/L for 3 consecutive days. The time of platelet implantation was defined as from day 0 to the day when platelet count exceeded 20×109/L for 7 consecutive days without platelet transfusion. Treatment-related mortality (TRM) was defined as the time from transplantation to death due to any causes except disease recurrence/progression, considering relapse as the competing risk. The incidences of aGVHD and cGVHD were evaluated in all patients as described in the literature (30). GVHD/relapse-free survival (GRFS) was defined as the time from transplantation to last follow-up without grade III-IV aGVHD and/or cGVHD requiring immunosuppressive treatment and without relapse (38).
Statistical analysis
Continuous variables were expressed as median with range, whereas categorical variables were presented as frequency and percentages. Statistical comparisons were made with the χ2 or Fisher exact test for categorical variables. If assumption of normality was not met for continuous variables, the Mann–Whitney U test was used as nonparametric test. Relapse, OS, DFS, TRM, and GRFS were estimated using the Kaplan–Meier methods and were compared by the log-rank tests. The Cox regression model was used for analyzing prognostic factors for aGVHD, cGVHD, relapse, OS, DFS, and TRM. TRM and relapse were the competing risks. For aGVHD and cGVHD, the competing events were relapse and death. A multivariate analysis was performed using Cox proportional hazards model. A two-tailed p value < 0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS 25.0 and R version 4.1.2.
Results
Patient characteristics
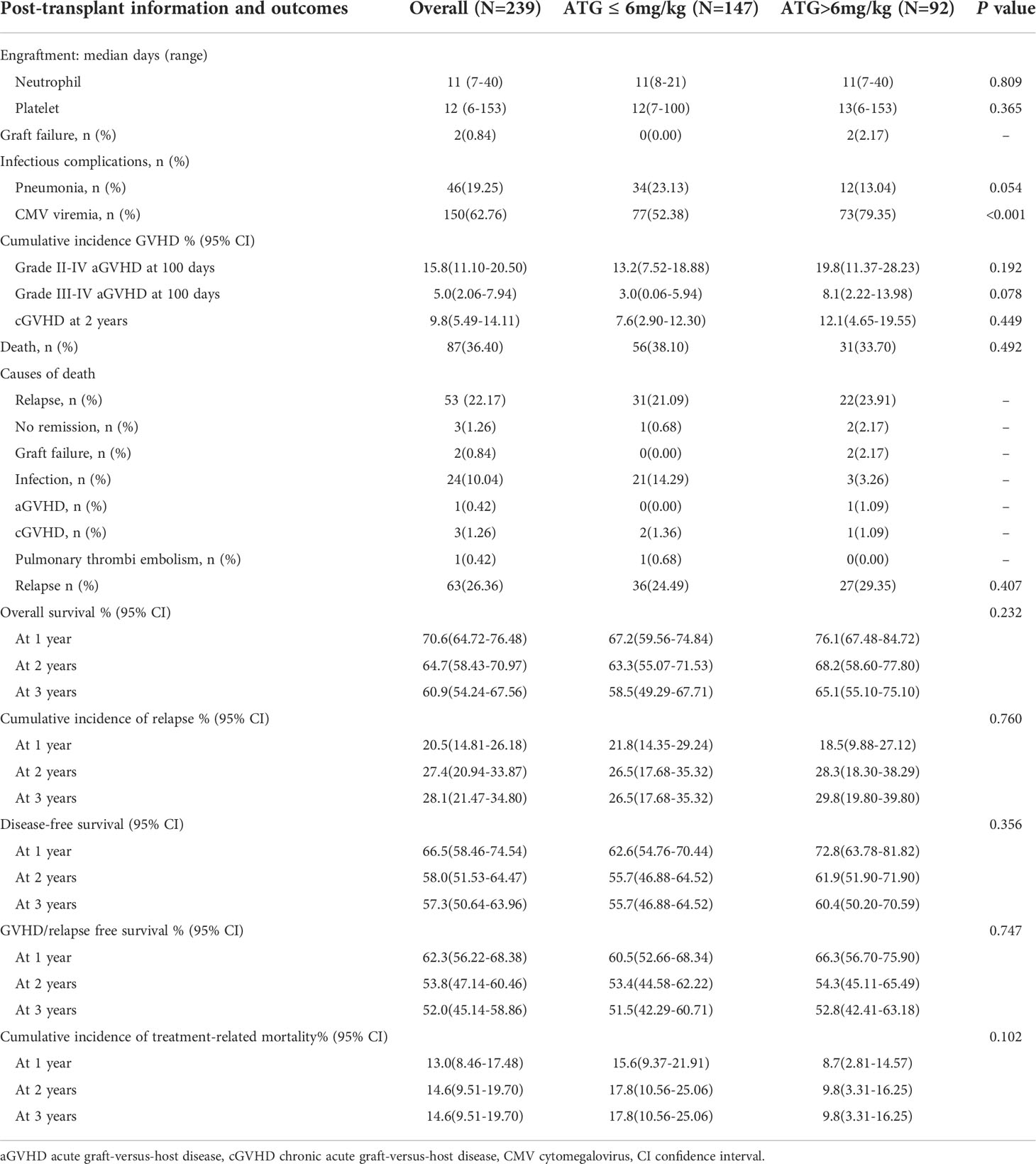
Two hundred and thirty-nine patients with hematologic malignancies were included from February 2013 to January 2019. The overall characteristics of the patients and donors were summarized in Table 1. The study was divided into two groups according to the doses of ATG, including lower-dose group (ATG ≤ 6mg/kg) and higher-dose group (ATG >6mg/kg). Detailed ATG doses for the two groups were shown in the Supplementary Table 1. There were no significant differences between the groups in terms of patients’ age, gender, diagnosis, disease status at HSCT, risk stratification, donor/recipient gender match, ABO match, conditioning regimen, and median number of CD34+ cells in two groups. The median doses of infused nucleated and CD34+cells for the whole study were 15.40×108/kg (7.08-48.35) and 6.06×106/kg (1.65-25.1), respectively. The median follow-up for survivors in the lower-dose ATG group and higher-dose ATG group was 21.8 months (range, 9.6-74.1) and 36.3 months (range, 20.9-79.2), respectively.
Engraftment
Eight patients died without neutrophil and platelet recovery. Two hundred and thirty-one (96.65%) patients achieved neutrophil engraftment with a median time of 11 days (range, 7 to 40). Two hundred and twenty-seven (94.98%) patients achieved platelet engraftment with a median time of 12 days (range, 6 to 153). The median time to achieve neutrophil and platelet engraftment showed no statistical difference in different ATG doses groups (neutrophil: 11 days (8-21) vs 11 days (7-40), P=0.809; platelet: 12 days (7-100) vs 13 days (6-153), P=0.635, Table 2). Multivariate analysis showed that no remission at transplantation was the only independent risk factor for platelet engraftment (HR: 6.374, 95% confidence interval (CI): 1.821–22.315, P=0.004).
Infection and complications
One hundred and fifty patients (62.76%) developed CMV viremia after transplantation. The CIs of CMV viremia by day +180 and +360 were 64.2 ± 3.2% and 64.8 ± 3.2%, respectively. Compared with lower-dose ATG group, higher-dose ATG group significantly increased the incidence of CMV viremia (52.38% vs 79.35%, P<0.001, Table 2). Three patients developed CMV-associated diseases (2 with pneumonia and 1 with enteritis) in the lower-dose ATG group and five patients (3 with pneumonia and 2 with enteritis) in the higher-dose ATG group. One patient died of CMV-associated pneumonia in the higher-dose ATG group. Forty-six patients (19.25%) suffered from severe pneumonia (bacterial pneumonia in 40 patients, fungal pneumonia in 6 patients, shown in Table 2).
Acute and chronic GVHD
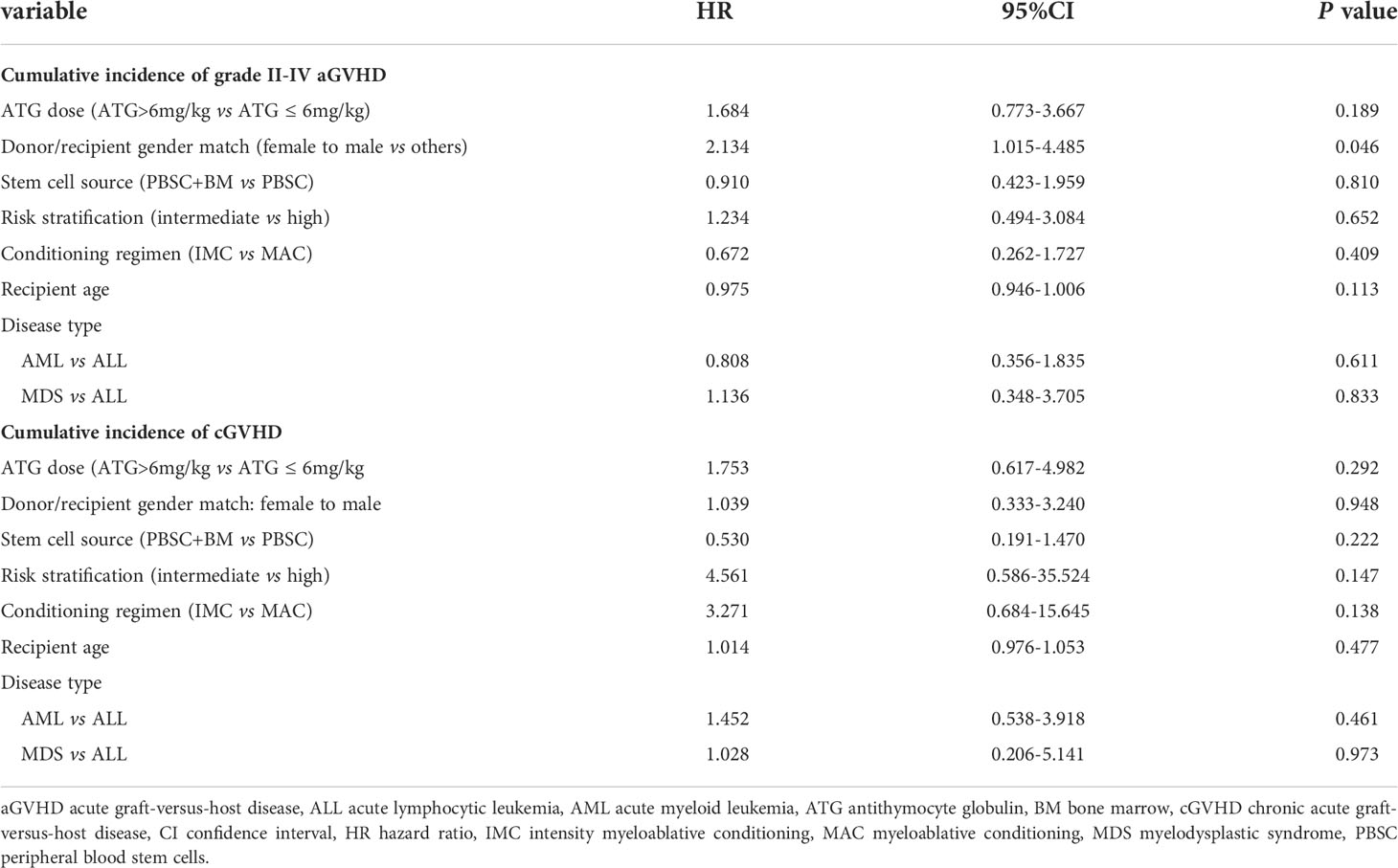
In this study, 231 patients were evaluated for aGVHD at 100 days. The total incidence of aGVHD was 21.21%. The incidences of grade I and II-IV aGVHD were 7.79% and 13.42%, respectively. The numbers of patients who occurred grade II aGVHD involvement of isolated skin, skin and gut, liver, and only gut were 14, 2, 1, and 3, respectively. Three patients developed grade III aGVHD and eight patients occurred grade IV aGVHD. The 100-day CIs of grade I-IV aGVHD, II-IV aGVHD, and III-IV aGVHD were 22.0 ± 2.8%, 15.8 ± 2.4%, and 5.0 ± 1.5%, respectively (Figure 1A). In this study, cGVHD was observed in 20 of 209 evaluable patients, including 12 patients with limited cGVHD and 8 patients with extensive cGVHD. The overall CIs of cGVHD at one year, two years, and three years were 7.7 ± 1.9%, 9.8 ± 2.2%, and 12.3 ± 2.7%, respectively. The CIs of extensive cGVHD at one year, two years, and three years were 2.6 ± 1.1%, 4.1 ± 1.5%, and 5.2 ± 1.9%, respectively (Figure 1B). There was no difference between PBSC and a mixture of BM and PBSC as graft in CI of grade II-IV aGVHD (15.5 ± 3.4%vs 16.2 ± 3.6%, P=0.878, Supplementary Figure 2A). Different conditioning regimens had no significant effect on CI of grade II-IV aGVHD (MAC:15.4 ± 4.7% vs IMC:16.0 ± 2.9%, P=0.958, Supplementary Figure 2B). Higher CI of grade II-IV aGVHD was observed in female to male than in others in donor/recipient gender match (26.2 ± 6.8% vs 13.4 ± 2.6%, P=0.035, Supplementary Figure 2C). Stem cell source, different conditioning regimens, and donor/recipient gender match didn’t show significantly statistical effect on cGVHD (Supplementary Figures 2D–F). Comparable CIs of grade II-IV aGVHD (ATG ≤ 6mg/kg: 13.2 ± 2.9%, ATG>6mg/kg:19.8 ± 4.3%, P=0.192) and cGVHD at two years (ATG ≤ 6mg/kg:7.6 ± 2.4%, ATG>6mg/kg:12.1 ± 3.8%, P= 0.449) were observed in different ATG groups (Table 2). Donor/recipient gender match (female to male) was the only risk factor for aGVHD in multivariate analysis. No risk factors were found in multivariate analysis of cGVHD (Table 3).

Figure 1 CIs of aGVHD and cGVHD. (A) The 100-day CI of grade I-IV aGVHD, II-IV aGVHD and III-IV aGVHD. (B)The CI of cGVHD and extensive cGVHD.
Relapse
Sixty-three patients relapsed after transplantation, with a median time of 168 days (range,13 to 811). In relapsed patients, 26 AML, 34 ALL, and 3 MDS were included. In all leukemia-relapsed patients, all the other patients relapsed in the bone marrow, except for one patient who relapsed in the central nervous system. By the time of follow-up, 53 patients died of leukemia or tumor progression or chemotherapy-related complications and 10 patients were still alive, with a median survival time of 343 days (range,170 to 811). The overall CI of relapse was 20.5 ± 2.9% at one year, 27.4 ± 3.3% at two years, and 28.1 ± 3.4% at three years, respectively (Figure 2A and Table 2). The overall CI of relapse did not show statistical difference between patients in the intermediate-risk group and those in the high-risk group (34.0 ± 7.1% vs 31.0 ± 3.9%, P=0.766). In all patients, ALL patients had higher overall CI of relapse than AML and MDS patients (42.5% ± 5.7% vs 26.7 ± 4.6% vs 15.9 ± 8.6%, P=0.012, Figure 2B). For all patients, there was no statistical difference between PBSC and the combination of BM and PBSC as graft in relapse (36.8 ± 4.9% vs 25.9 ± 4.5%, P=0.103). However, in subgroup analysis, for ALL patients, result showed that the CI of relapse had significant difference in different graft sources (PBSC: 45.1 ± 8%, PBSC+BM: 27.6 ± 7.1%, P=0.032, Figure 2C). The results showed that patients in MRD positive had higher recurrence rate than patients in MRD negative (MRD+: 52.9 ± 12.1%, MRD-: 29.4 ± 3.7%, P=0.003, Figure 2D). The comparable probabilities occurred in CI of relapse between lower ATG(≤6mg/kg) and higher ATG(>6mg/kg) (P=0.760, Figure 3A and Table 2). In multivariate analysis, the combination of BM and PBSC as graft had a beneficial influence on relapse. Patients with MRD positive or diagnosed with ALL before transplantation had higher risk of relapse (Supplementary Table 2).
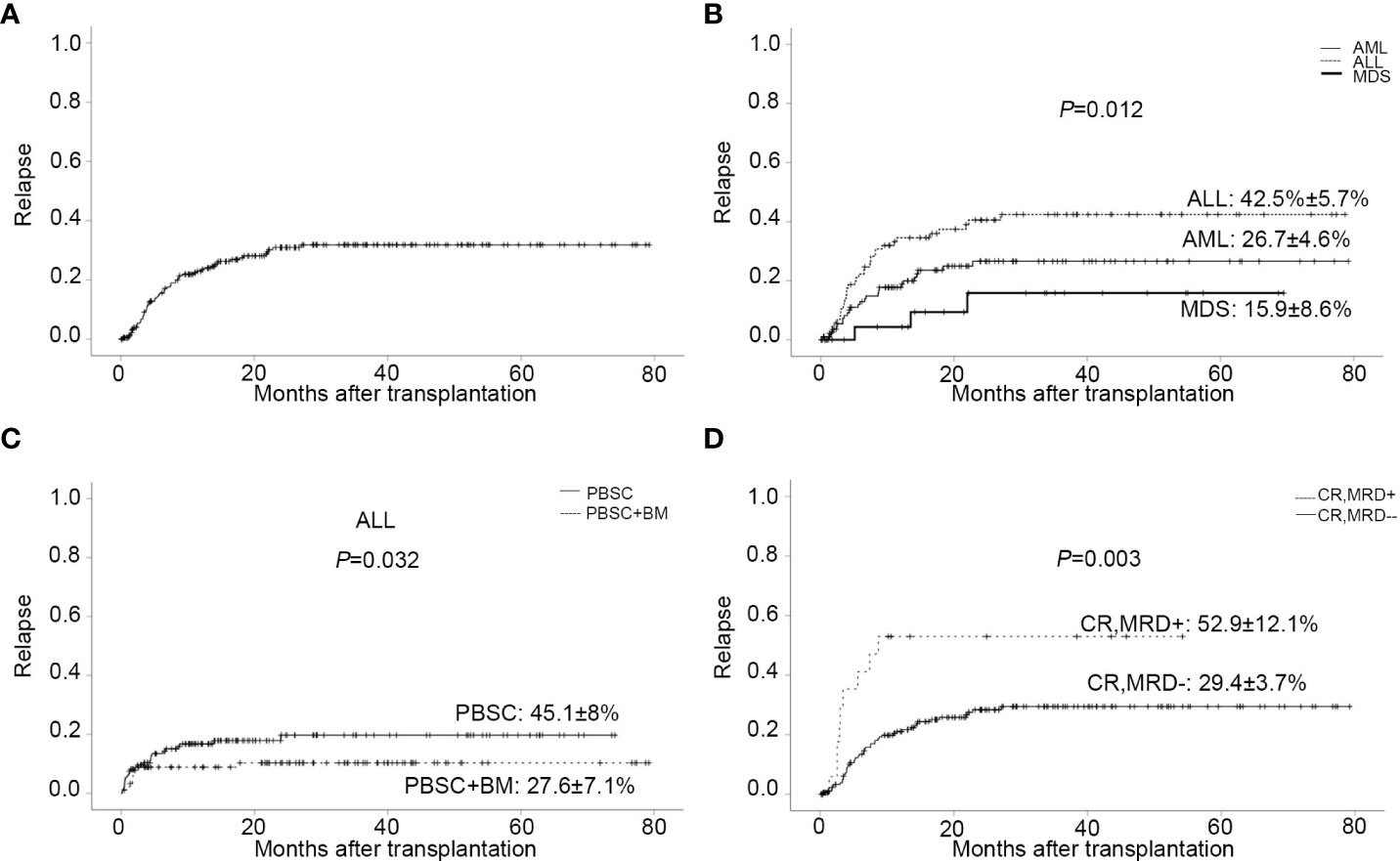
Figure 2 Relapse. (A) CI of relapse. (B) CI of relapse in patients with different disease types. (C) CI of relapse in ALL patients with different stem cell source. (D) CI of relapse in patients with MRD negative/positive before transplantation.
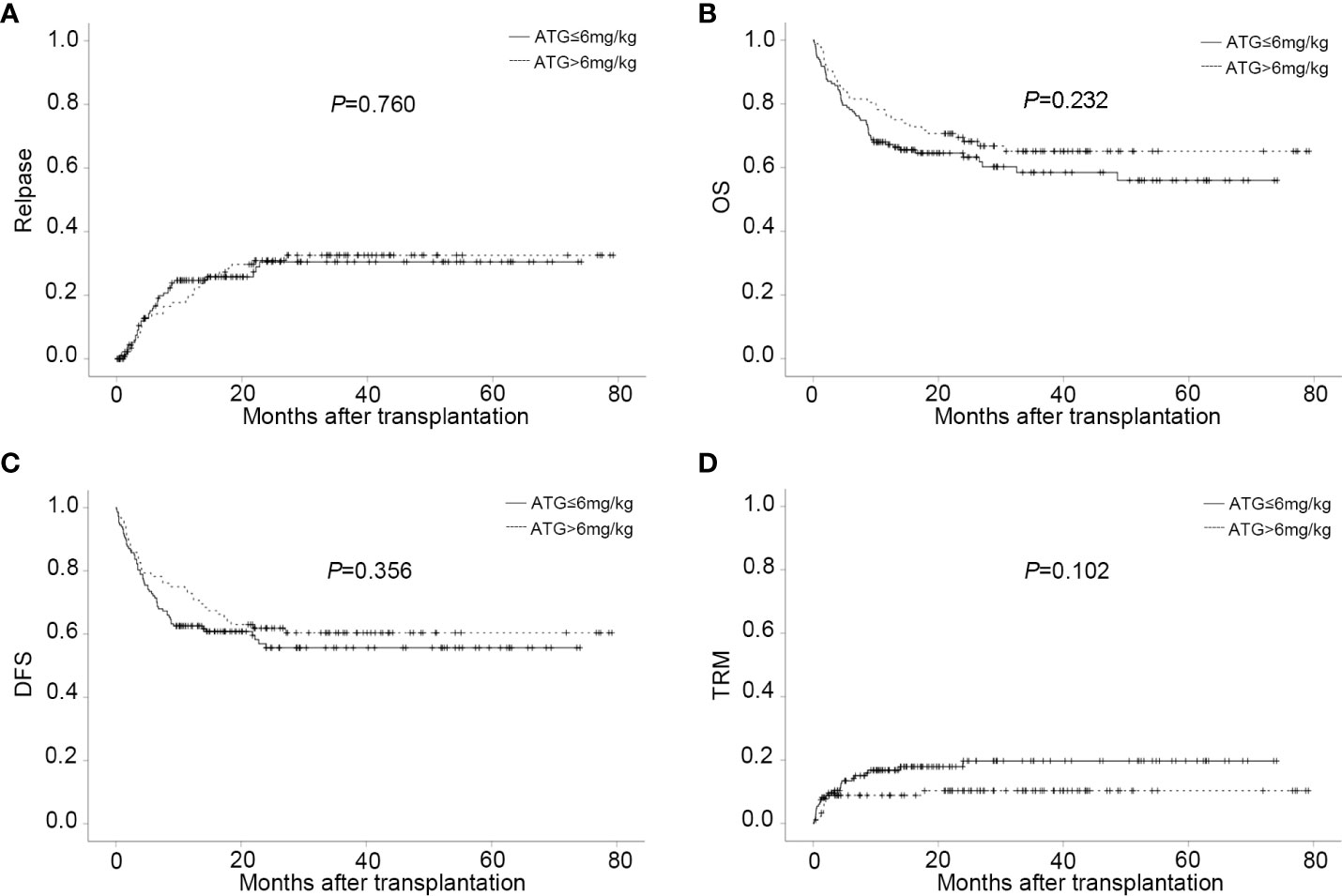
Figure 3 CIs of post-transplant outcome in different ATG doses groups. (A) CI of relapse in different ATG doses groups. (B) CI of OS in different ATG doses groups. (C) CI of DFS in different ATG doses groups. (D) CI of TRM in different ATG doses groups.
OS, DFS, TRM, and GRFS
Eighty-seven patients died after transplantation with a median time of 139 days (range,3 to 1453). The main causes of death were relapse (22.17%) and infection (10.04%) shown in Table 2. The CI of OS for all patients at one year, two years, and three years were 70.6 ± 3.0%, 64.7 ± 3.2%, and 60.9 ± 3.4%, respectively (Table 2). The 3-year probability of OS was significantly lower for patients in transfusion of PBSC as graft than those in transfusion of BM combined with PBSC (54.0 ± 4.8% vs 69.3 ± 4.7%, P= 0.023). There was no statistically different between the intermediate-risk group and high-risk group in the 3-year of OS (64.7 ± 7.6% vs 59.8 ± 3.9%, P=0.256).
The probability of DFS for all patients at one year, two years, and three years were 66.5 ± 3.1%, 58.0 ± 3.3%, and 57.3 ± 3.4%%, respectively. Thirty-four patients died of TRM in this study with a median time of 51 days (range, 3 to 719). Pneumonia was the main reason of death. The overall CI of TRM was 13.0 ± 2.3% at one year, 14.6 ± 2.6% at two years, and 14.6 ± 2.6% at three years. The CI of GRFS was 62.3% ± 3.1% at one year, 53.8% ± 3.4% at two years, and 52.0% ± 3.5% at three years (Table 2). Regardless of lower-dose or higher-dose ATG group, no significant statistical difference occurred in OS, DFS, GRFS and TRM (Figures 3B–D and Table 2).
In multivariate analysis, patient had a poor prognosis on OS (HR=2.025,95% CI:1.050-3.904, P=0.035), DFS (HR=1.893, 95% CI:1.010-3.546, P=0.046) and TRM (HR=3.131, 95% CI:1.172-8.369, P=0.023) when age was over 50 years at transplantation. The combination of BM and PBSC as graft had a positive impact on OS (HR=0.575, 95% CI: 0.369-0.896, P=0.014) and DFS (HR=0.566, 95% CI: 0.374-0.855, P=0.007). Days of neutrophil engraftment (≥16 days) was associated with lower DFS (HR=0.566,95% CI: 1.141-3.409, P=0.015) and higher TRM (HR=2.217,95% CI: 0.989-4.968, P=0.053). Grade III-IV aGVHD was associated with lower OS (HR=2.488,95% CI:1.185-5.222, P=0.016) and higher TRM (HR=3.638, 95% CI: 1.368-9.671, P=0.010). The conditioning regimen IMC had an adverse impact on DFS (HR=1.861,95% CI: 1.111-3.119, P=0.018) and TRM (HR=3.721, 95% CI:1.282-10.800, P=0.016). No remission before transplantation was a common risk factor for OS, DFS, and TRM. Comparing with ALL patients, AML and MDS patients had better OS (AML vs ALL : HR=0.605, 95% CI:0.385-0.951, P=0.029; MDS vs ALL : HR=0.293,95% CI: 0.116-0.739, P=0.009) (Supplementary Table 2).
Discussion
In the present retrospective study, basiliximab was administrated in addition to the ATG-based GVHD prophylaxis regimen in 239 patients who were diagnosed with intermediate- or high-risk hematologic malignancies and had received haplo-HSCT with a median follow-up of 2.4 years (range, 0.8-6.6). The efficacy of this similar protocol in high-risk hematologic malignancies has been explored (28–30). However, with respect to the experience of these groups, the higher number of patients, the longer follow-up of our study, the use of more intensive conditioning regimens, the different graft sources as well as the different dosages and types of ATG provide relevant novelty to the present report. Herein, this combined GVHD prophylaxis strategy could result in very favorable rates of acute and chronic GVHD and achieve satisfactory results of 3-year OS 60.9% with acceptable relapse rates and TRM. Remarkably, lower dose of ATG (≤6mg/kg) combined with basiliximab was associated with less CMV viremia but comparable GVHD preventive effect and survival rates.
Haploidentical donors provide the benefits of rapid and near universal donor availability. However, immunological barriers resulting from the high degree of HLA-mismatch in the haplo-HSCT settings were formidable (39, 40). In vivo T-cell depletion or modulation with ATG (the Beijing protocol) or PTCy (the Baltimore protocol) has been the basis for the development of multiple novel GVHD prophylaxis approaches used in haplo-HSCT. The clinical benefit of incorporating ATG or PTCy as GVHD prophylaxis in haplo-HSCT varied among studies, which could be related to the dose and formulation of ATG or PTCy administration, the conditioning regimen, the donor type, the stem cell source, the concomitant immunosuppressive medications and the background hematological malignancy (15, 41–50). We summarized several studies on the effects of ATG-based, PTCy-based, and two schemes combined GVHD prophylaxis (4–7, 12, 42, 43, 45, 47, 48, 51–54) (Supplementary Table 3). Comparable incidences of grade II-IV aGVHD (18%-42%) and severe cGVHD (10%-23%) were shown in ATG-based and PTCy-based prophylaxis. Meanwhile, PTCy-based regimen appears to be more effective in the incidence of grade III-IV aGVHD (5%-14%). Additionally, a novel regimen of combining ATG and PTCy after haplo-HSCT for hematological malignancies showed promising activity with grade II–IV aGVHD of 17%-26% and grade III–IV aGVHD of 3.2%-6.9%. It is worth noting that in our study, we observed that GVHD prophylaxis using the described ATG plus basiliximab combination results in very low rates of both acute and chronic GVHD, with estimated incidences of grade II-IV aGVHD, grade III-IV aGVHD and 3-year extensive cGVHD of 15.8%, 5% and 5.2%, respectively. The rates of clinically significant grade III–IV aGVHD and extensive cGVHD were significantly decreased, a benefit that has not been achieved in most of the studies using standard ATG regimen and PBSC. Accordingly, the effect of the use of ATG and basiliximab on the incidence of GVHD converted into the reduced risk of TRM and improved GRFS. Compared with PTCy-based studies, although our study showed lower severe aGVHD, lower TRM, and higher GRFS, this could be due to the younger baseline characteristics of the patients (47, 48, 51, 55). Older patients who receive reduced conditioning are often associated with more severe infections, GVHD, and recurrence, which results in lower GRFS. Therefore, clinical studies with larger sample sizes and older patients are needed to confirm the effectiveness.
Furthermore, our results observed that the female to male combination results in a higher incidence of severe acute GVHD than other donor-recipient sex combinations, which coincides with the results of others (5, 56, 57), but it did not translate into any higher TRM. Nevertheless, the incidence of GVHD was not influenced by the stem cell source, the types of hematological malignancy or intensity of conditioning regimens in multivariate analysis. Importantly, the incidence of GVHD in our study using unmanipulated PBSC combined or not with BM with intensified myeloablative or myeloablative conditioning was comparable to that in previous studies reported for similar GVHD prophylaxis, using unmanipulated BM haplo-HSCT with myeloablative or reduced intensity conditioning (29, 30). Thus, we concluded that the effect of this enhancing immunosuppression strategy may be independent of stem cell source and the intensity of conditioning regimen.
Infectious complications remain the major factors affecting overall survival and are central to advances seeking to improve haplo-HSCT. As far as we know, in vivo T-cell depletion with ATG results in delayed immune reconstitution, leaving patients vulnerable to severe infections, including viral reactivations with CMV or Epstein-Barr virus (EBV), bacterial infection, as well as infection-related deaths (13, 15, 58). The incidence of CMV viremia was 62.76% in our study, which was comparable with ATG-based regimens (60-78.6%) (5, 28, 30, 43, 59–61). Despite pre-emptive therapy of ganciclovir or foscarnet, 3% patients still progress to CMV disease. Antiviral drug resistance and treatment-limiting side effects remain major challenges. Letermovir was approved for prophylaxis of CMV-infection and -disease in adult CMV-seropositive recipients after allogeneic HSCT and demonstrated lower risk of CMV-infection than placebo without apparent safety concerns (62). Letermovir has a novel mechanism of action that inhibits CMV DNA synthesis at a late step by targeting the pUL56 subunit of the terminase enzyme complex (63–66). Because of a unique mechanism of action that is distinct from ganciclovir and other CMV DNA polymerase inhibitors, letermovir has the potential for treating multidrug-resistant CMV. We believe that with additional clinical efficacy data, this medication could emerge as a primary option for the prevention and treatment of CMV in the immunocompromised patient population.
As for the use of PTCy in haplo-HSCT for hematologic malignancies, a major concern is the high relapse rate, which is up to 50% (9, 11, 67–69). While the relapse rate at 1-year and 3-year were 20.5% and 28.1%, respectively, which was comparable to those in previous studies, ranging from 15% to 34.5% depending on the diagnosis and the disease risk (4, 5, 55, 70). In line with the published data, patients suffering from ALL showed a higher risk of relapse (51, 55). Different from ATG, basiliximab binds specifically to the IL-2R of activated T cells and only selectively eliminates the alloreactive donor lymphocytes, without affecting the resting T cells (24), which is important for preventing infectious complications. It was also reported that antibody mediated CD25 blockade may be useful to promote anti-tumor immunity (25). This may account for the comparable incidences of CMV viremia and relapse between our haplo-HSCT setting using ATG plus basiliximab and other regimens using ATG alone to prevent GVHD. Thus, we could conclude that ATG combined with basiliximab was complementary integration to reinforce GVHD prophylaxis without increasing infections and significantly compromising disease control.
The limitations associated with the use of ATG as a protocol for GVHD prophylaxis include the profound immune deficiency and higher risk of infectious complications, depending on the dose of ATG administered (15, 61, 71–73). To date, the optimal dose of ATG balancing the efficacy of GVHD prophylaxis and the risk of severe infections has not been established for haplo-HSCT. Hang et al. has documented that 6 mg/kg ATG applied in haplo-HSCT was related to a faster recovery of T cell reconstitution and lower incidence of EBV infection but a higher rate of severe GVHD and GVHD related death than 10 mg/kg ATG (15, 61, 74). Recently, a multicenter randomized study comparing two different doses of ATG (7.5 and 10 mg/kg) as GVHD prophylaxis for haplo-HSCT, showed that patients receiving 7.5 mg/kg ATG had a lower incidence of EBV and CMV DNAemia and a similar incidence of aGVHD and cGVHD compared with those receiving 10.0 mg/kg (75). In our haplo-HSCT system, we also made an initial attempt to investigate whether low doses of ATG, 6mg/kg and below, were more beneficial in the outcome of haplo-HSCT. Consistently with the mentioned results, the reduced incidence of CMV DNAemia was found in the lower dose arm(≤6mg/kg), compared to the higher dose arm (>6mg/kg). Surprisingly, there was no difference noted with regard to the occurrence of acute and chronic GVHD between the lower dose and higher dose groups. In addition, in the context of haplo-HSCT with similar GVHD prophylaxis, the incidence of GVHD in the lower dose arm seems to be better or equivalent to that in previous studies using higher dose of ATG, ranging from 6 to 9 mg/kg, or using different source of ATG (ATG-Fresenius, 20 mg/kg) (28–30). These findings suggest that lower dose ATG has sufficient efficacy for GVHD prophylaxis and minimizes the risk of infection when used in combination with basiliximab. Furthermore, long-term transplant outcomes did not show any significant differences between the two groups concerning disease recurrence, TRM, OS and DFS. It’s worth noting that a beneficial effect of TRM was not seen in the lower dose arm despite the lower incidences of CMV viremia and the comparable morbidity and mortality associated with GVHD. This may attribute to the effective preemptive treatment for CMV DNAemia at our center. Although survival benefit has not been achieved, it might bring a potential economic benefit as well as less side effects of preemptive therapies in the patients receiving lower dose ATG. In general, in our study, a lower dose ATG(≤6mg/kg) in combination with basiliximab was as effective as higher doses for GVHD prevention after haplo-HSCT. A higher dose did not confer any additional benefit; conversely it appeared to be associated with increased CMV DNAemia. Based on these results, a lower dose ATG(≤6mg/kg) seems to be more appropriate for balancing infection control and GVHD prophylaxis when used in combination with basiliximab. A prospective randomized trial would be required to reach a definite conclusion on the superior efficacy of lower dose ATG plus basiliximab regimen in haplo-HSCT.
Indeed, our study was limited by its retrospective nature and the heterogeneity of the sample size. To some extent, the dose of ATG was related to HLA mismatch and the impact of ATG dose on the incidence of GVHD may be confounded by HLA mismatch. Although several studies have shown that HLA mismatch has little effect on GVHD in haplo-HSCT, this issue cannot be ignored (5, 28, 76, 77). Thus, further prospective and randomized control studies are needed. In addition, more detailed analyses are needed to get information on other virus infections including EBV and immune reconstitution in this haplo-HSCT circumstance. However, we included a relatively large patient number with a rather long follow-up, which allowed us to reliably estimate the impact of ATG in conjunction with basiliximab on long-term clinical outcomes at our center.
In conclusion, the evidence reported in this paper indicates that the combination of ATG with basiliximab is a feasible and effective protocol to promote protection against GVHD and improve haploidentical transplant outcomes in the context of both intensified myeloablative and myeloablative conditioning regimens. Moreover, the combination with a lower dose of ATG has shown to be safer and equally effective than the original one with a higher dose, in preventing GVHD. We conclude that the optimal scheme of using a combined regimen of ATG and basiliximab is that administration of lower dose ATG(≤6mg/kg), which could exert a synergistic activity to reduce the risk of GVHD without increasing severe infection, particularly CMV viremia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Huazhong University of Science and Technology. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
LX and WS designed the study and enrolled patients. HY and ZH analyzed the data and wrote the paper. ZH and YT were responsible for the data collection. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No.81974003, No. 81500168, No. 81974007), the National Key R&D Program of China (Grant No 2019YFC1316204), and Collaborative Innovation Center of Hematology of China.
Acknowledgments
We are very grateful to all the participants at the institute who provided information in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1017850/full#supplementary-material
Abbreviations
aGVHD, acute GVHD; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BM, bone marrow; cGVHD, chronic GVHD; CI, cumulative incidence; CIs, cumulative incidences; CI, confidence interval; CsA, cyclosporin A; CMV cytomegalovirus; CR, complete remission; DSA, donor-specific antibodies; DFS, disease-free survival; EBV epstein-Barr virus; GRFS, GVHD/relapse-free survival; GVHD, graft versus host disease; HLA, human leukocyte antigen; HR, hazard ratio; Haplo-HSCT, haploidentical hematopoietic stem cell transplant; IMC, intensity myeloablative conditioning; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMF, mycofenolate mofetil; MRD, minimal residual disease; MRD+, minimal residual disease positive; MRD-, minimal residual disease negative; MTX, methotrexate; NR, no remission; OS, overall survival; PBSC, peripheral blood stem cells; PTCy, post-transplant cyclophosphamide; TRM, transplant-related mortality.
References
1. Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematol Am Soc Hematol Educ Program (2012) 2012:230–6. doi: 10.1182/asheducation-2012.1.230
2. Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant (2015) 50(4):476–82. doi: 10.1038/bmt.2014.312
3. Ciurea SO, Bayraktar UD. "No donor"? consider a haploidentical transplant. Blood Rev (2015) 29(2):63–70. doi: 10.1016/j.blre.2014.09.009
4. Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood (2015) 125(25):3956–62. doi: 10.1182/blood-2015-02-627786
5. Wang Y, Liu D-H, Liu K-Y, Xu L-P, Zhang X-H, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer (2013) 119(5):978–85. doi: 10.1002/cncr.27761
6. Law AD, Salas MQ, Lam W, Michelis FV, Thyagu S, Kim DDH, et al. Reduced-intensity conditioning and dual T lymphocyte suppression with antithymocyte globulin and post-transplant cyclophosphamide as graft-versus-Host disease prophylaxis in haploidentical hematopoietic stem cell transplants for hematological malignancies. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2018) 24(11):2259–64. doi: 10.1016/j.bbmt.2018.07.008
7. Huang WR, Li HH, Gao CJ, Bo J, Li F, Dou LP, et al. Haploidentical, unmanipulated G-CSF-primed peripheral blood stem cell transplantation for high-risk hematologic malignancies: an update. Bone Marrow Transplant (2016) 51(11):1464–9. doi: 10.1038/bmt.2016.166
8. Chang Y-J, Huang X-J. Haploidentical stem cell transplantation: anti-thymocyte globulin-based experience. Semin Hematol (2016) 53(2):82–9. doi: 10.1053/j.seminhematol.2016.01.004
9. Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2008) 14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005
10. Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol (2012) 39(6):683–93. doi: 10.1053/j.seminoncol.2012.09.005
11. Robinson TM, O'Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide. Semin Hematol (2016) 53(2):90–7. doi: 10.1053/j.seminhematol.2016.01.005
12. Ruggeri A, Sun Y, Labopin M, Bacigalupo A, Lorentino F, Arcese W, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin as graft- versus-host disease prophylaxis in haploidentical transplant. Haematologica (2017) 102(2):401–10. doi: 10.3324/haematol.2016.151779
13. Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-Host disease. New Engl J Med (2016) 374(1):43–53. doi: 10.1056/NEJMoa1506002
14. Kalra A, Williamson T, Daly A, Savoie ML, Stewart DA, Khan F, et al. Impact of donor and recipient cytomegalovirus serostatus on outcomes of antithymocyte globulin-conditioned hematopoietic cell transplantation. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2016) 22(9):1654–63. doi: 10.1016/j.bbmt.2016.05.020
15. Chang Y-J, Wang Y, Mo X-D, Zhang X-H, Xu L-P, Yan C-H, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: Long-term outcomes of a prospective randomized trial. Cancer (2017) 123(15):2881–92. doi: 10.1002/cncr.30540
16. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. New Engl J Med (2006) 355(19):1967–77.
17. Sageshima J, Ciancio G, Chen L, Burke GW. Anti-interleukin-2 receptor antibodies-basiliximab and daclizumab-for the prevention of acute rejection in renal transplantation. Biologics (2009) 3:319–36.
18. Mattei MF, Redonnet M, Gandjbakhch I, Bandini AM, Billes A, Epailly E, et al. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti-thymocyte globulin as induction therapy. J Heart Lung Transplant Off Publ Int Soc Heart Transplant (2007) 26(7):693–9.
19. Tan Y, Xiao H, Wu D, Luo Y, Lan J, Liu Q, et al. Combining therapeutic antibodies using basiliximab and etanercept for severe steroid-refractory acute graft-versus-host disease: A multi-center prospective study. Oncoimmunology (2017) 6(3):e1277307. doi: 10.1080/2162402X.2016.1277307
20. Shen M-Z, Li J-X, Zhang X-H, Xu L-P, Wang Y, Liu K-Y, et al. Meta-analysis of interleukin-2 receptor antagonists as the treatment for steroid-refractory acute graft–host disease. Front Immunol (2021) 12:749266. doi: 10.3389/fimmu.2021.749266
21. Deng D-X, Fan S, Zhang X-H, Xu L-P, Wang Y, Yan C-H, et al. Immune reconstitution of patients who recovered from steroid-refractory acute graft-Versus-Host disease after basiliximab treatment. Front Oncol (2022) 12:916442. doi: 10.3389/fonc.2022.916442
22. Freyer CW, Gier S, Carulli A, Gill SI, Hexner EO, Loren AW, et al. Salvage therapy with basiliximab and etanercept for severe steroid-refractory acute graft-versus-host disease. Am J Hematol (2022) 97(7):E273–6. doi: 10.1002/ajh.26568
23. Mo X-D, Hong S-D, Zhao Y-L, Jiang E-L, Chen J, Xu Y, et al. Basiliximab for steroid-refractory acute graft-versus-host disease: A real-world analysis. Am J Hematol (2022) 97(4):458–69. doi: 10.1002/ajh.26475
24. Campara M, Tzvetanov IG, Oberholzer J. Interleukin-2 receptor blockade with humanized monoclonal antibody for solid organ transplantation. Expert Opin Biol Ther (2010) 10(6):959–69. doi: 10.1517/14712598.2010.485187
25. Locke FL, Pidala J, Storer B, Martin PJ, Pulsipher MA, Chauncey TR, et al. CD25 blockade delays regulatory T cell reconstitution and does not prevent graft-versus-Host disease after allogeneic hematopoietic cell transplantation. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2017) 23(3):405–11. doi: 10.1016/j.bbmt.2016.12.624
26. Fang J, Hu C, Hong M, Wu Q, You Y, Zhong Z, et al. Prophylactic effects of interleukin-2 receptor antagonists against graft-versus-host disease following unrelated donor peripheral blood stem cell transplantation. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2012) 18(5):754–62. doi: 10.1016/j.bbmt.2011.09.005
27. Ji SQ, Chen HR, Yan HM, Wang HX, Liu J, Zhu Py, et al. Anti-CD25 monoclonal antibody (basiliximab) for prevention of graft-versus-host disease after haploidentical bone marrow transplantation for hematological malignancies. Bone Marrow Transplant (2005) 36(4):349–54.
28. Zhang R, Shi W, Wang HF, You Y, Zhong ZD, Li WM, et al. Idarubicin-intensified haploidentical HSCT with GvHD prophylaxis of ATG and basiliximab provides comparable results to sibling donors in high-risk acute leukemia. Bone Marrow Transplant (2017) 52(9):1253–60. doi: 10.1038/bmt.2017.100
29. Arcese W, Picardi A, Santarone S, De Angelis G, Cerretti R, Cudillo L, et al. Haploidentical, G-CSF-primed, unmanipulated bone marrow transplantation for patients with high-risk hematological malignancies: an update. Bone Marrow Transplant (2015) 50 Suppl 2:S24–30. doi: 10.1038/bmt.2015.91
30. Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood (2013) 121(5):849–57. doi: 10.1182/blood-2012-08-453399
31. Wang H-X, Yan H-M, Wang Z-D, Xue M, Liu J, Guo Z-K. Haploidentical hematopoietic stem cell transplantation in hematologic malignancies with G-CSF mobilized bone marrow plus peripheral blood stem cells grafts without T cell depletion: a single center report of 29 cases. Leukemia lymphoma (2012) 53(4):654–9. doi: 10.3109/10428194.2011.624225
32. Lu R-N, Miao K-R, Zhang R, Hong M, Xu J, Zhu Y, et al. Haploidentical hematopoietic stem cell transplantation following myeloablative conditioning regimens in hematologic diseases with G-CSF-mobilized peripheral blood stem cells grafts without T cell depletion: a single center report of 38 cases. Med Oncol (2014) 31(8):81. doi: 10.1007/s12032-014-0081-x
33. Wu Q, Zhang R, Wang H, You Y, Zhong Z, Hong M, et al. Comparison of outcomes of idarubicin intensified TBI-CY and traditional TBI-CY conditioning regimen for high-risk acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: A single center experience. Leukemia Res (2015) 39:1192–200. doi: 10.1016/j.leukres.2015.08.015
34. Fang J, Zhang R, Wang H, Hong M, Wu Q, Nie D, et al. Idarubicin-intensified BUCY2 conditioning regimen improved survival in high-risk acute myeloid, but not lymphocytic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation: A retrospective comparative study. Leukemia Res (2016) 46:61–8. doi: 10.1016/j.leukres.2016.04.014
35. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant (1995) 15(6):825–8.
36. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2005) 11(12):945–56.
37. Zhang R, Lu X, Wang H, You Y, Zhong Z, Zang S, et al. Idarubicin-intensified hematopoietic cell transplantation improves relapse and survival of high-risk acute leukemia patients with minimal residual disease. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2019) 25(1):47–55. doi: 10.1016/j.bbmt.2018.07.021
38. Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant (2016) 51(4):610–1. doi: 10.1038/bmt.2015.305
39. Al Malki MM, Jones R, Ma Q, Lee D, Reisner Y, Miller JS, et al. Proceedings from the fourth haploidentical stem cell transplantation symposium (HAPLO2016), San Diego, California, December 1, 2016. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2018) 24(5):895–908. doi: 10.1016/j.bbmt.2018.01.008
40. Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol (2016) 13(1):10–24. doi: 10.1038/nrclinonc.2015.128
41. Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica (2017) 102(2):224–34. doi: 10.3324/haematol.2016.148510
42. Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant (2019) 54(7):1049–57. doi: 10.1038/s41409-018-0382-3
43. Wang Y, Wu D-P, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol (2019) 12(1):88. doi: 10.1186/s13045-019-0781-y
44. Fuji S, Hirakawa T, Takano K, Doki N, Sawa M, Kanda Y, et al. Disease-specific impact of anti-thymocyte globulin in allogeneic hematopoietic cell transplantation: a nationwide retrospective study on behalf of the JSTCT, transplant complications working group. Bone Marrow Transplant (2022) 57(3):479–86. doi: 10.1038/s41409-022-01569-x
45. Bejanyan N, Pidala JA, Wang X, Thapa R, Nishihori T, Elmariah H, et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv (2021) 5(5):1154–63. doi: 10.1182/bloodadvances.2020003779
46. Li Z, Shi W, Lu X, Lu H, Cao X, Tang L, et al. Decitabine-intensified modified Busulfan/Cyclophosphamide conditioning regimen improves survival in acute myeloid leukemia patients undergoing related donor hematopoietic stem cell transplantation: A propensity score matched analysis. Front Oncol (2022) 12:844937. doi: 10.3389/fonc.2022.844937
47. Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer (2018) 124(7):1428–37. doi: 10.1002/cncr.31228
48. Bashey A, Zhang M-J, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-Cell-Replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(26):3002–9. doi: 10.1200/JCO.2017.72.8428
49. Zhang Yy, Liu Dh, Liu Ky, Xu Lp, Chen H, Han W, et al. HLA-haploidentical hematopoietic SCT from collateral related donors without in vitro T-cell depletion for hematological malignancies. Bone Marrow Transplant (2014) 49(4):496–501. doi: 10.1038/bmt.2013.223
50. Rubio MT, Savani BN, Labopin M, Piemontese S, Polge E, Ciceri F, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol (2016) 9:25. doi: 10.1186/s13045-016-0248-3
51. Lorentino F, Labopin M, Fleischhauer K, Ciceri F, Mueller CR, Ruggeri A, et al. The impact of HLA matching on outcomes of unmanipulated haploidentical HSCT is modulated by GVHD prophylaxis. Blood Adv (2017) 1(11):669–80. doi: 10.1182/bloodadvances.2017006429
52. Tang F, Xu Y, Chen H, Xu L, Zhang X, Wang Y, et al. Comparison of the clinical outcomes of hematologic malignancies after myeloablative haploidentical transplantation with G-CSF/ATG and posttransplant cyclophosphamide: results from the Chinese bone marrow transplantation registry group (CBMTRG). Sci China Life Sci (2020) 63(4):571–81. doi: 10.1007/s11427-019-9594-7
53. Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant (2015) 50 Suppl 2:S37–9. doi: 10.1038/bmt.2015.93
54. Salas MQ, Law AD, Lam W, Al-Shaibani Z, Loach D, Kim DDH, et al. Safety and efficacy of haploidentical peripheral blood stem cell transplantation for myeloid malignancies using post-transplantation cyclophosphamide and anti-thymocyte globulin as graft–host disease prophylaxis. Clin Hematol Int (2019) 1(2):105–13. doi: 10.2991/chi.d.190316.003
55. Nagler A, Kanate AS, Labopin M, Ciceri F, Angelucci E, Koc Y, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin for graft-versus-host disease prevention in haploidentical transplantation for adult acute lymphoblastic leukemia. Haematologica (2021) 106(6):1591–8. doi: 10.3324/haematol.2020.247296
56. Kim HJ, Park SJ, Im HW, Kim DW, Min WS, Kim HK, et al. The association of HLA antigen and GVHD in allogeneic hemopoietic stem cell transplantation with histocompatible sibling donor: a single-center experience in Korea. Int J Hematol (2002) 76(3):267–71.
57. Greinix HT, Eikema D-J, Koster L, Penack O, Yakoub-Agha I, Montoto S, et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega-file study. Haematologica (2022) 107(5):1054–63. doi: 10.3324/haematol.2020.265769
58. Lee K-H, Lee J-H, Lee J-H, Kim D-Y, Park H-S, Choi E-J, et al. Reduced-intensity conditioning with busulfan, fludarabine, and antithymocyte globulin for hematopoietic cell transplantation from unrelated or haploidentical family donors in patients with acute myeloid leukemia in remission. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2017) 23(9):1555–66. doi: 10.1016/j.bbmt.2017.05.025
59. Xu X, Yang J, Cai Y, Li S, Niu J, Zhou K, et al. Low dose anti-thymocyte globulin with low dose posttransplant cyclophosphamide (low dose ATG/PTCy) can reduce the risk of graft-versus-host disease as compared with standard-dose anti-thymocyte globulin in haploidentical peripheral hematopoietic stem cell transplantation combined with unrelated cord blood. Bone Marrow Transplant (2021) 56(3):705–8. doi: 10.1038/s41409-020-01047-2
60. Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol (2015) 94(10):1677–88. doi: 10.1007/s00277-015-2423-y
61. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant (2014) 49(3):426–33. doi: 10.1038/bmt.2013.191
62. Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhäuser M, Groth C, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. New Engl J Med (2014) 370(19):1781–9. doi: 10.1056/NEJMoa1309533
63. Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, et al. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother (2010) 54(3):1290–7. doi: 10.1128/AAC.01596-09
64. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. New Engl J Med (2017) 377(25):2433–44. doi: 10.1056/NEJMoa1706640
65. Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol (2011) 85(20):10884–93. doi: 10.1128/JVI.05265-11
66. Melendez DP, Razonable RR. Letermovir and inhibitors of the terminase complex: a promising new class of investigational antiviral drugs against human cytomegalovirus. Infect Drug Resist (2015) 8:269–77. doi: 10.2147/IDR.S79131
67. McCurdy SR, Kanakry JA, Showel MM, Tsai H-L, Bolaños-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood (2015) 125(19):3024–31. doi: 10.1182/blood-2015-01-623991
68. Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood (2015) 126(8):1033–40. doi: 10.1182/blood-2015-04-639831
69. Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Post-transplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan-based myeloablative conditioning and peripheral blood stem cells. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2015) 21(8):1506–14. doi: 10.1016/j.bbmt.2015.04.025
70. Tsai XC-H, Chen T-T, Gau J-P, Wang P-N, Liu Y-C, Lien M-Y, et al. Outcomes of different haploidentical transplantation strategies from the Taiwan blood and marrow transplantation registry. Cancers (Basel) (2022) 14(4):1097. doi: 10.3390/cancers14041097
71. Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TCG, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol (2015) 2(5):e194–203. doi: 10.1016/S2352-3026(15)00045-9
72. Nishihori T, Al-Kadhimi Z, Hamadani M, Kharfan-Dabaja MA. Antithymocyte globulin in allogeneic hematopoietic cell transplantation: benefits and limitations. Immunotherapy (2016) 8(4):435–47. doi: 10.2217/imt.15.128
73. Binkert L, Medinger M, Halter JP, Heim D, Gerull S, Holbro A, et al. Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transplant (2015) 50(10):1331–6. doi: 10.1038/bmt.2015.148
74. Liu J, Xu L-P, Bian Z, Chang Y-J, Wang Y, Zhang X-H, et al. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med (2015) 13:391. doi: 10.1186/s12967-015-0748-x
75. Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two dose levels of rabbit antithymocyte globulin as graft-versus-host disease prophylaxis in haploidentical stem cell transplantation: a multicenter randomized study. BMC Med (2019) 17(1):156. doi: 10.1186/s12916-019-1393-7
76. Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia (2015) 29(5):1069–75. doi: 10.1038/leu.2014.336
Keywords: ATG, basiliximab, GVHD prophylaxis, haploidentical HSCT, hematologic malignancies
Citation: Huang Z, Yan H, Teng Y, Shi W and Xia L (2022) Lower dose of ATG combined with basiliximab for haploidentical hematopoietic stem cell transplantation is associated with effective control of GVHD and less CMV viremia. Front. Immunol. 13:1017850. doi: 10.3389/fimmu.2022.1017850
Received: 12 August 2022; Accepted: 31 October 2022;
Published: 15 November 2022.
Edited by:
Jacopo Mariotti, Humanitas Research Hospital, ItalyReviewed by:
Sarita Jaiswal, Narayana Health, IndiaSaurabh Chhabra, Mayo Clinic Arizona, United States
Francesca Patriarca, University of Udine, Italy
Copyright © 2022 Huang, Yan, Teng, Shi and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linghui Xia, Linghuixia@hust.edu.cn; Wei Shi, shiwei076@hust.edu.cn
†These authors have contributed equally to this work
 Zhenli Huang
Zhenli Huang Han Yan
Han Yan Yao Teng
Yao Teng Wei Shi
Wei Shi Linghui Xia
Linghui Xia