- 1Department of Endocrinology & Rheumatology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
- 2Department of Endocrinology, Jinshan Hospital of Fudan University, Shanghai, China
Background: To help inform decision making in the clinical setting, we carried out a systematic review and meta-analysis to estimate the association of thyroid disease risks with obesity.
Methods: Pubmed, Embase, Web of Science, Cochrane database and Google Scholar electronic databases were searched from inception to October 31, 2018 without language restrictions to explore the relationship between thyroid disorders and obesity. The relative risk (RR) or odds risk (OR) for thyroid disorders were pooled using the SPSS and STATA software.
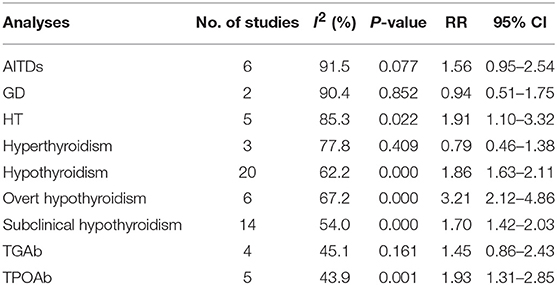
Results: A total of 22 studies were included in the study. (1) Meta-analysis showed that obesity was significantly associated with an increased risk of hypothyroidism (RR = 1.86, 95% CI 1.63–2.11, P < 0.001). Meta-analyses after stratification further showed that obese population had increased risks of overt hypothyroidism (RR = 3.21, 95% CI 2.12–4.86, P < 0.001) and subclinical hypothyroidism (RR = 1.70, 95% CI 1.42–2.03, P < 0.001). (2) Further meta-analysis also showed obesity was clearly associated with Hashimoto's thyroiditis (RR = 1.91, 95% CI 1.10–3.32, P = 0.022), but not with Graves' disease. (3) In the meta-analysis of antibodies, obesity was correlated with positive thyroid peroxidase antibody (TPOAb) (RR = 1.93, 95% CI 1.31–2.85, P = 0.001), but not with positive thyroglobulin antibody (TGAb).
Conclusions: Obesity was significantly related to hypothyroidism, HT, and TPOAb, implying that prevention of obesity is crucial for thyroid disorders.
Systematic Review Registration: PROSPERO: CRD42018096897.
Introduction
Since the rise of obesity epidemic worldwide, obesity has gained increasing attention and been regarded as a significant public health challenge globally for its wide-ranging adverse consequences on human health such as increased risks of diabetes (1), cardiovascular disease (2), and cancers (3).
The incidence of thyroid disorders, which mainly include thyroid dysfunctions and autoimmune thyroid diseases (AITDs), is increasing in these years. Thyroid dysfunctions include hyperthyroidism and hypothyroidism (4), both of which can be categorized into subclinical (only with changes in TSH) and overt stages (with changes in both TSH and thyroid hormones). AITDs, one of the most common autoimmune diseases, are characterized by autoantibodies against thyroid antigens, such as TSH receptor antibody (TRAb), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TGAb). They have two principal subtypes: Graves' disease (GD) and Hashimoto's thyroiditis (HT), which hold different clinical manifestations though have similar immunogenetic mechanisms (5). Patients with thyroid disorders also have a high risk of other non-thyroid diseases, such as cardiovascular diseases, cancer, obesity, and adverse pregnancy outcomes (6–9). Patients with thyroid dysfunctions or Graves' disease need long-term medical therapy or surveillance to optimize prognosis (10, 11). Identifying risk factors for thyroid disorders may help clinicians recognize individuals at risk for or with subclinical thyroid disorders and provide immediate treatment to improve patients' outcomes, and is crucial for elucidating the underlying pathophysiological mechanisms of these thyroid disorders.
Although previous studies have revealed that immune dysfunction, environmental elements and genetic factors all contribute to the pathogenesis of thyroid disorders, their pathology is not yet completely clear. It is well-known that obesity is associated with changes in hormones including thyroid-stimulating hormone (TSH) and thyroid hormones and is accompanied by several endocrine and metabolic diseases (12, 13). In clinic, it is well-known that hypothyroidism may induce obesity, so we propose a hypothesis that the relationship between obesity and thyroid disease may be bidirectional. Furthermore, if this relationship is bidirectional and if obesity indeed influences the risk of thyroid disorders, it is still incompletely elucidated how obesity influences the risk of thyroid dysfunctions and impacts the risk of thyroid autoimmunity. Although some studies have reported that obesity may be associated with dysfunctions of thyroid immunity and thyroid gland (14–16), these results are not entirely the same and even controversial. In addition, some of these studies have a relatively small sample size. Therefore, in this study, we conducted a systematic review and meta-analysis with aims to review the influence of obesity on thyroid diseases and uncover their association.
Methods
Registration
This systematic review and meta-analysis was conducted in accordance with the PRISMA guideline (17) and has been registered in the International Prospective Register of Systemic Reviews (PROSPERO, www.crd.york.ac.uk/PROSPERO, CRD42018096897).
Literature Search
Pubmed, Embase, Web of Science, Cochrane database, and Google Scholar were searched from inception to October 31, 2018. The search in Pubmed used the following criteria: (obese OR obesity OR overweight) AND (thyroid autoimmunity OR Hashimoto's thyroiditis OR Graves' disease OR Graves hyperthyroidism OR hyperthyroidism OR hypothyroidism OR TPOAb OR TGAb OR thyroid peroxidase antibodies OR thyroid peroxidase antibody OR thyroglobulin antibodies OR thyroglobulin antibody OR thyroiditis). No restrictions were applied on language or publication period. Reference lists of eligible studies and reviews were also screened to identify more details.
Eligibility Criteria
Inclusion criteria were as follows: (1) observational studies including cohort studies, cross-sectional studies and case-control studies; (2) studies comparing the risk of thyroid disorders of obese patients, who were defined as people with body mass index (BMI) ≥ 30 kg/m2 (in western population) or 28 kg/m2 (in eastern population), and normal controls, who were defined as people with 18.5 ≤ BMI < 24.9 kg/m2, or providing risk estimates for the associations of thyroid disorders with obesity; (3) studies analyzing thyroid disorders including hyperthyroidism, hypothyroidism, or AITDs; (4) studies providing risk estimates with 95% CI for the associations of thyroid disorders, such as relative risk (RR) and odds ratio (OR), or providing other data that could be transformed into risk estimates. Studies against any item of the eligibility criteria were excluded. Case reports and studies containing overlapping data were also excluded. Studies using overweight (24 ≤ BMI < 28 kg/m2), but not obesity (BMI ≥ 28 kg/m2) as the exposure were also excluded. The primary outcomes were the risk of thyroid autoimmunity, hypothyroidism or hyperthyroidism among obese patients and the secondary outcomes were the risk of AITDs, TPOAb positive, and TGAb positive among obese patients.
Data Extraction
Data extraction was conducted using the extraction form, which mainly included study characteristics (the first author, publication year, design, country), participant characteristics (number, subgroups), outcomes (types of thyroid diseases), and adjusted matched factors.
Quality Assessment
Quality assessment of included studies was conducted using Newcastle-Ottawa scale (NOS) based on participant selection (4 points), exposure evaluation (2 points), outcome evaluation, and confounders adjustment (3 points) (18). Studies scoring 5 or less were considered to have sub-optimal quality, and studies scoring 6 or higher were considered in good quality.
Data Analysis
The pooled relative risk (RR) with 95% CI was used to evaluate the impact of obesity on the risk of thyroid autoimmunity and dysfunctions. To account for heterogeneity among included studies, data were pooled using random-effect meta-analysis (19). Heterogeneity was assessed using the I2 statistic, and I2 > 40% was considered high heterogeneity (20). Subgroup analysis was conducted based on types of thyroid autoimmunity, hypothyroidism and hyperthyroidism. Sensitivity analysis was conducted by excluding low-quality studies. Publication bias was assessed by the Begg's funnel plot and Egger's test (21). Trim-and-fill method was utilized when publication bias existed (22). All analyses were conducted in SPSS (version 25, IBMCorp) and STATA (version 12.0, StataCorp), and P < 0.05 was considered statistically significant.
Results
Search Results
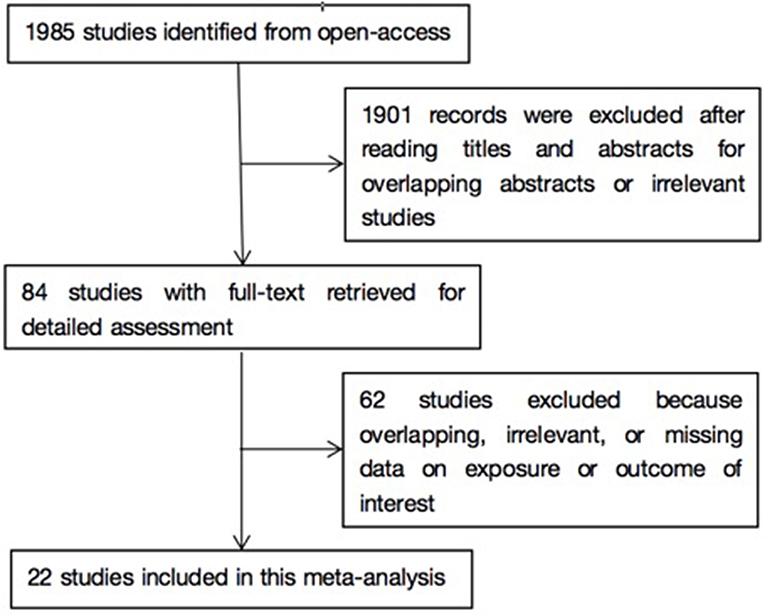
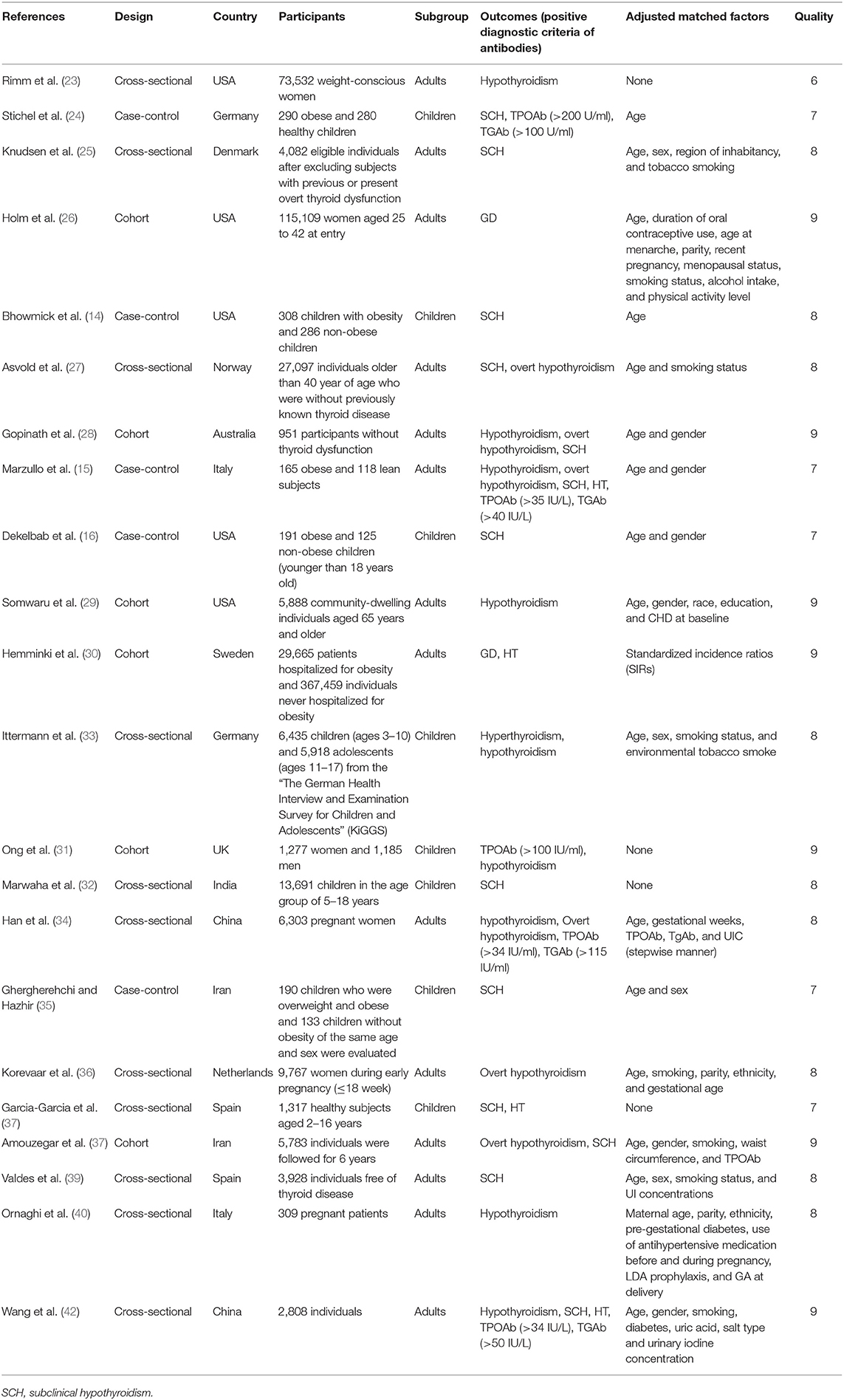
As shown in Figure 1, literature search yielded 1985 related papers. After further careful abstracts viewing, 84 studies with full-text publications were retrieved for detailed assessment. After eliminating 62 papers with unrelated or ambiguous results, 22 papers were further analyzed in detail (14–16, 23–41). Table 1 lists the abstract items of the final 22 papers, including publication year, design, country or region, sample size, source of study sample, outcomes, adjusted matched factors, and quality assessment score.
Obesity and Thyroid Dysfunctions
As shown in Figure 2, meta-analysis of the 22 studies indicated that obesity was significantly associated with the increased risk of hypothyroidism (OR = 1.86; 95% CI 1.63–2.11, P < 0.001). Further meta-analysis of 6 studies on hypothyroidism (shown in Figure 3) showed that patients with BMI ≥ 28 kg/m2 had an increased risk of overt hypothyroidism (OR = 3.21, 95% CI 2.12–4.86, P < 0.001). Likewise, meta-analysis of 14 studies on subclinical hypothyroidism (SCH) also showed that obese population had an 70% increased risk of subclinical hypothyroidism (OR = 1.70, 95% CI 1.42–2.03, P < 0.001). However, meta-analysis of studies on hyperthyroidism showed no significant association between obesity and an increased risk of hyperthyroidism (P > 0.05).
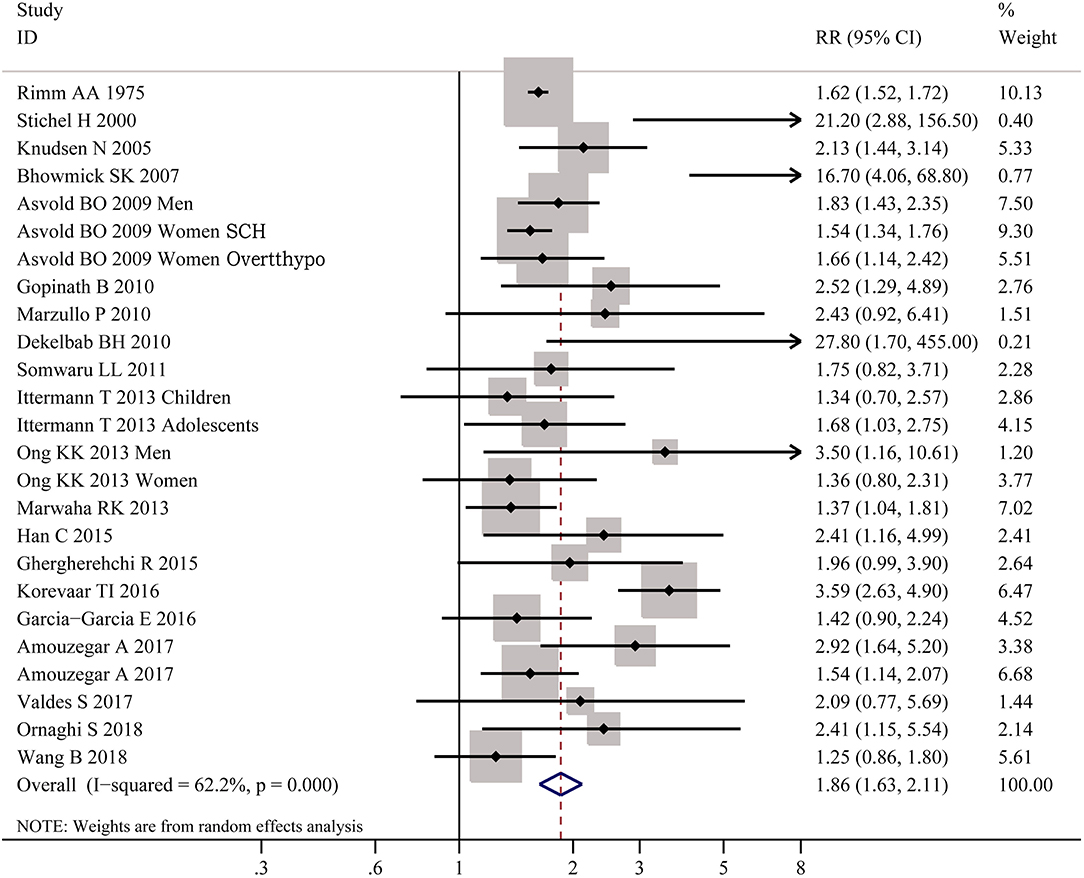
Figure 2. Forest plot for the risk of the whole hypothyroid disorders in obesity. SCH, subclinical hypothyroidism; Overtthypo, overt hypothyroidism.
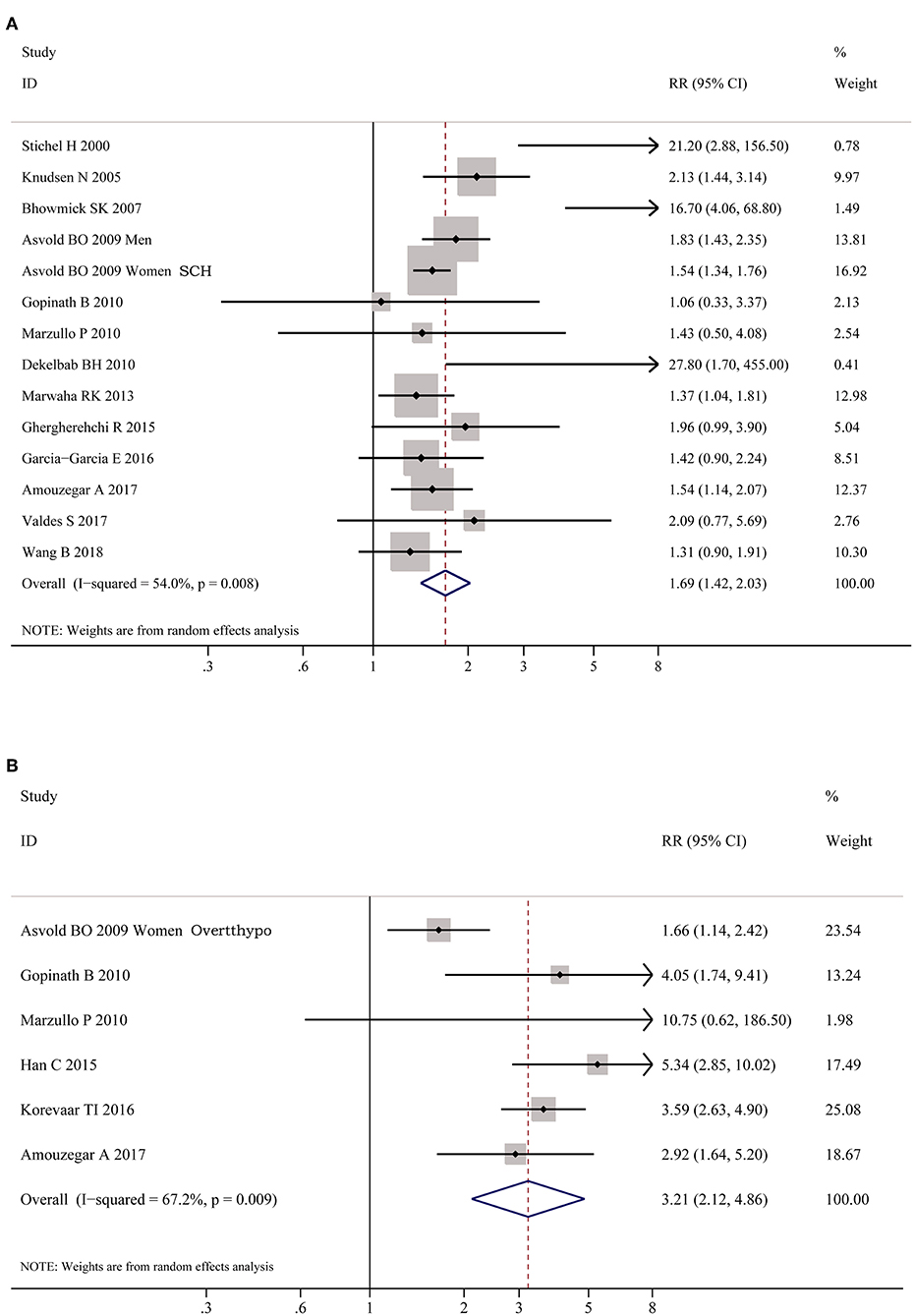
Figure 3. Forest plots for the risk of hypothyroid disorders in obesity. (A) Forest plot for the risk of overt hypothyroidism in obesity patients. (B) Forest plot for the risk of subclinical hypothyroidism in obesity patients. SCH, subclinical hypothyroidism; Overtthypo, overt hypothyroidism.
Obesity and Thyroid Autoimmunity
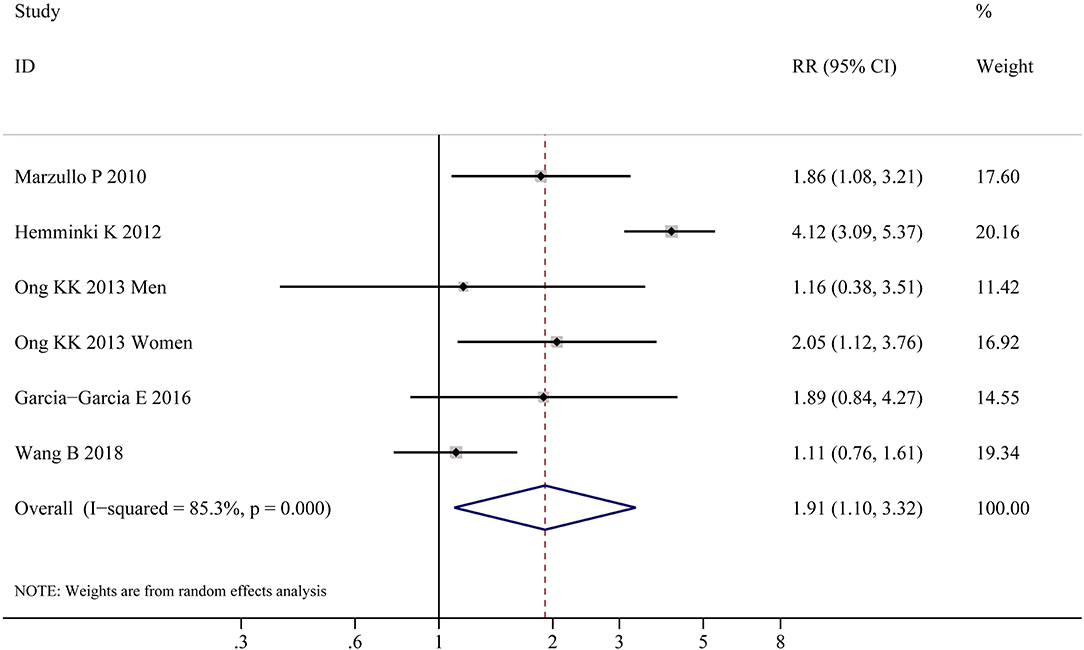
Table 2 shows the pooled estimates of AITDs risk in obese patients. Although obese patients had increased risk of AITDs, the difference was not statistically significant (P = 0.077). Similarly, meta-analysis of two studies on GD showed that obese population had no increased risk of GD (P = 0.852). But, there was a significant association between HT and obesity (OR = 1.91; 95% CI 1.10–3.32, P = 0.022), as shown in Figure 4. As shown in Table 2 and Figure 5, meta-analysis of thyroid antibodies (TGAb and TPOAb) revealed that there was a significant association between TPOAb positive and obesity (OR = 1.93; 95% CI 1.31–2.85, P = 0.001), but no such an association between TGAb positive and obesity. The risks of HT and TPOAb in obese population were increased by 91 and 93%, respectively.
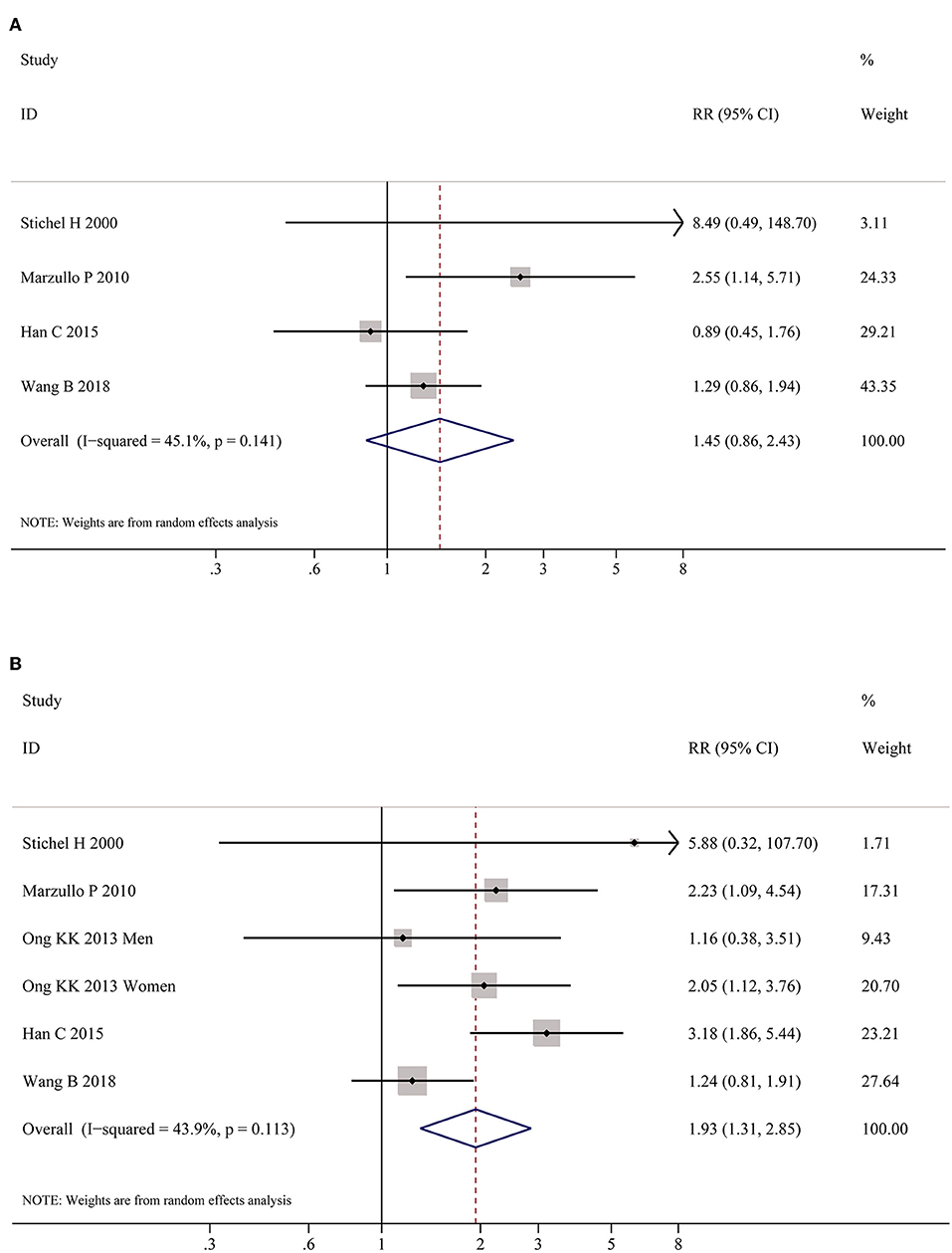
Figure 5. Meta-analysis of association between thyroid auto-antibodies and obesity. (A) Association between positive TGAb and obesity. (B) Association between positive TPOAb and obesity patients.
Discussion
Obesity and thyroid disorders are two common conditions and there is an intriguing relationship between these two entities. Although available data have uncovered the relationship between thyroid disorder and body weight status, their results are inconsistent. For example, researchers have previously found that obese individuals have higher serum TSH levels (42, 43), while others have found no significant differences (44, 45). The aim of our study is to analyze these results systemically and also to reveal casual relationship between obesity and thyroid disorders.
A total of 22 researches with a size large enough were included in the present study. Clinically, it is easy to find that patients with hyperthyroidism often lose a lot of weight and regain it after remission. In contrast, patients with hypothyroidism often gain some weight and lose modest weight after thyroid hormone replacement. Therefore, it is a common sense that obesity is often regarded to be secondary to hypothyroidism (8). And the mechanisms by which hypothyroidism causes weight increase is supposed to be achieved via altered energy expenditure and appetite (41, 46).
Until recently, there have been more novel views identifying that thyroid disorders could well be secondary to obesity (47). Our meta-analysis showed that obesity was significantly associated with increased risks of hypothyroidism, including overt hypothyroidism and subclinical hypothyroidism, and could be accompanied by at least 1.86-fold increase of developing hypothyroidism. These results are concordant with other studies showing that lower levels of FT3 and FT4 or higher level of TSH are associated with high body weight (15, 25, 32, 48). The parthenogenesis of this relation is not yet entirely revealed, but some explanations have been proposed. Obesity is a chronic low-grade inflammation process; thus the cytokines and other inflammatory markers produced by over-loading adipose tissue, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-alpha), will be increased (46). These increased inflammatory cytokines may inhibit the mRNA expression of symporter sodium/iodide, then influence iodide uptake activity of human thyroid cells (49). These cytokines can also induce vasodilation and elevated permeability of blood vessels of thyroid gland, thus bringing morphological and functional changes in thyroid (46, 49). Leptin, a factor produced by adipocytes, also plays a role in chronic inflammation may result in the morphological changes in thyroid, and may also restrain the expressions of soidium/iodide symporter and thyroglobulin, thus inducing the changes of thyroid hormone levels in obese people (50). Some other studies found that this chronic inflammation status in obesity may also affect thyroid function by modulating the expression and activity of deiodinases (51, 52). The above researches may partially explain the mechanisms by which obesity may induce hypothyroidism (13, 49–53). Nevertheless, the etiology for the correlation of obesity and hypothyroidism still needs to be further elucidated in more in-depth studies.
Moreover, our meta-analysis also found the obese population had increased odds for thyroid autoimmunity. It is well-known that autoimmune diseases are caused by both genetic and immune pathogenesis. Our results are in accordance with previous reports showing that adiposity is a risk factor for many autoimmune inflammatory diseases, such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and psoriatic arthritis (54–57). The mechanisms linking obesity and autoimmune disease are unclear. Some studies suggest that adipokines may play a vital role in immune disorders (58, 59). Adipokines, including leptin and interleukin-6, could mediate immune and inflammatory responses. Adipose tissue is crucial for maintaining normal immune function for humans (60, 61). Similarly, other observational researches also provide evidence that dysfunction in adipokines is associated with thyroid autoimmunity (62, 63). Meanwhile, meta-analysis of thyroid antibodies showed the correlation between TPOAb positive and obesity, and obesity is associated with a 93% increased risk of developing positive TPOAb. Leptin, which is mainly produced by adipocytes, is identified to mediate the immune system and contribute to increased production of TPOAb by shifting T helper balance toward to T helper 1 (Th1) cells phenotype and inhibiting the function of regulatory T (Treg) cells (64, 65). Autoimmune thyroiditis, mainly HT, is believed to be the main cause of hypothyroidism in iodine-sufficient regions, and thyroid auto-antibodies (TPOAb and TGAb) are the hallmarks of this disease (66). This may be another interpretation to explain the mechanism why obesity induces hypothyroidism.
Holm has reported that obesity may reduce the risk of hyperthyroidism (26). However, our meta-analysis including both Holm's study and another one showed no relationship between hyperthyroidism and GD with obesity. We speculate that this discrepancy is due to limited GD cases to reveal a fact in heterogeneous populations. In future, much larger and more ethnic researches are warranted.
In this study, we demonstrate the association between obesity and thyroid disorders, indicating that obesity may be a contributing factor for hypothyroidism, HT and positive TPOAb, and suggest that thyroid functions in obese population needs extra attention. So by synthesizing our present study and some other researches (13, 47, 49–53, 58–65), it seems reasonable to suggest that the relationship between obesity and thyroid disease is bidirectional; of course, it needs more studies to be elucidated. The present study still has some limitations. For instance, abnormal weight including overweight and underweight were barely explored. Additionally, most studies only explored the association between obesity and thyroid disorders, and barely investigated whether thyroid dysfunction is the cause or consequence of obesity, which needs further prospective cohort and causality studies to investigate.
In conclusion, obesity is significantly associated with hypothyroidism, HT and TPOAb, indicating that prevention of obesity is crucial for thyroid disorders.
Author Contributions
BW designed the study and generated the hypotheses. BW and RS extracted the data. RS analyzed the data and wrote the first draft of the report, with support from QY, QL, and XJ. JZ and BW both revised the manuscript. All authors participated in interpreting the data and critically reviewed the paper.
Funding
The present work has received fundings from the National Natural Science Foundation of China (Grant No. 81800696, 81873636 and 81670722) and Science and Technology Development Fund of Pudong New District Minsheng Scientific Research (Medical and Health) Project (No. PKJ2018-Y39).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RS and handling editor declared their shared affiliation.
References
1. Riobo Servan P. Obesity and diabetes. Nutr Hosp. (2013) 28(Suppl. 5):138–43. doi: 10.3305/nh.2013.28.sup5.6929
2. Mitchell AB, Cole JW, McArdle PF, Cheng YC, Ryan KA, Sparks MJ, et al. Obesity increases risk of ischemic stroke in young adults. Stroke. (2015) 46:1690–2. doi: 10.1161/STROKEAHA.115.008940
3. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 Countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
4. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. (2018) 14:301–16. doi: 10.1038/nrendo.2018.18
5. Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. (2014) 9:147–56. doi: 10.1146/annurev-pathol-012513-104713
6. Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. (2017) 136:2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753
7. Journy NMY, Bernier MO, Doody MM, Alexander BH, Linet MS, Kitahara CM. Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid. (2017) 27:1001–10. doi: 10.1089/thy.2017.0063
8. Biondi B. Thyroid and obesity: an intriguing relationship. J. Clin. Endocrinol. Metab. (2010) 95:3614–17. doi: 10.1210/jc.2010-1245
9. Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diab Endocrinol. (2013) 1:238–49. doi: 10.1016/S2213-8587(13)70086-X
10. Burch HB, Cooper DS. Management of graves disease: a review. JAMA. (2015) 314:2544–54. doi: 10.1001/jama.2015.16535
11. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. (2015) 14:174–80. doi: 10.1016/j.autrev.2014.10.016
12. Kopelman PG. Hormones and obesity. Baillieres Clin Endocrinol Metab. (1994) 8:549–75. doi: 10.1016/S0950-351X(05)80286-1
13. Pearce EN. Thyroid hormone and obesity. Curr Opin Endocrinol Diabetes Obes. (2012) 19:408–13. doi: 10.1097/MED.0b013e328355cd6c
14. Bhowmick SK, Dasari G, Levens KL, Rettig KR. The prevalence of elevated serum thyroid-stimulating hormone in childhood/adolescent obesity and of autoimmune thyroid diseases in a subgroup. J Natl Med Assoc. (2007) 99:773–6. doi: 10.1016/S0929-6646(07)60013-8
15. Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. (2010) 95:3965–72. doi: 10.1210/jc.2009-2798
16. Dekelbab BH, Abou Ouf HA, Jain I. Prevalence of elevated thyroid-stimulating hormone levels in obese children and adolescents. Endocr Pract. (2010) 16:187–90. doi: 10.4158/EP09176.OR
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
18. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. (2007) 26:4544–62. doi: 10.1002/sim.2889
23. Rimm AA, Werner LH, Yserloo BV, Bernstein RA. Relationship of ovesity and disease in 73,532 weight-conscious women. Public Health Rep. (1975) 90:44–51.
24. Stichel H, l'Allemand D, Gruters A. Thyroid function and obesity in children and adolescents. Horm Res. (2000) 54:14–9. doi: 10.1159/000063431
25. Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. (2005) 90:4019–24. doi: 10.1210/jc.2004-2225
26. Holm IA, Manson JE, Michels KB, Alexander EK, Willett WC, Utiger RD. Smoking and other lifestyle factors and the risk of Graves' hyperthyroidism. Arch Intern Med. (2005) 165:1606–11. doi: 10.1001/archinte.165.14.1606
27. Asvold BO, Bjoro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. (2009) 94:5023–7. doi: 10.1210/jc.2009-1180
28. Gopinath B, Wang JJ, Kifley A, Wall JR, Eastman CJ, Leeder SR, et al. Five-year incidence and progression of thyroid dysfunction in an older population. Intern Med J. (2010) 40:642–9. doi: 10.1111/j.1445-5994.2009.02156.x
29. Somwaru LL, Arnold AM, Cappola AR. Predictors of thyroid hormone initiation in older adults: results from the cardiovascular health study. J Gerontol A Biol Sci Med Sci. (2011) 66:809–14. doi: 10.1093/gerona/glr063
30. Hemminki K, Li X, Sundquist J, Sundquist K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann Med. (2012) 44:289–95. doi: 10.3109/07853890.2010.547515
31. Ong KK, Kuh D, Pierce M, Franklyn JA. Childhood weight gain and thyroid autoimmunity at age 60–64 years: the 1946 British birth cohort study. J Clin Endocrinol Metab. (2013) 98:1435–42. doi: 10.1210/jc.2012-3761
32. Marwaha RK, Tandon N, Garg MK, Ganie MA, Narang A, Mehan N, et al. Impact of body mass index on thyroid functions in Indian children. Clin Endocrinol. (2013) 79:424–8. doi: 10.1111/cen.12148
33. Ittermann T, Thamm M, Schipf S, John U, Rettig R, Volzke H. Relationship of smoking and/or passive exposure to tobacco smoke on the association between serum thyrotropin and body mass index in large groups of adolescents and children. Thyroid. (2013) 23:262–8. doi: 10.1089/thy.2012.0110
34. Han C, Li C, Mao J, Wang W, Xie X, Zhou W, et al. High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. Biomed Res Int. (2015) 2015:351831. doi: 10.1155/2015/351831
35. Ghergherehchi R, Hazhir N. Thyroid hormonal status among children with obesity. Ther Adv Endocrinol Metab. (2015) 6:51–5. doi: 10.1177/2042018815571892
36. Korevaar TI, Nieboer D, Bisschop PH, Goddijn M, Medici M, Chaker L, et al. Risk factors and a clinical prediction model for low maternal thyroid function during early pregnancy: two population-based prospective cohort studies. Clin Endocrinol. (2016) 85:902–9. doi: 10.1111/cen.13153
37. Garcia-Garcia E, Vazquez-Lopez MA, Garcia-Fuentes E, Galera-Martinez R, Gutierrez-Repiso C, Garcia-Escobar I, et al. Thyroid function and thyroid autoimmunity in relation to weight status and cardiovascular risk factors in children and adolescents: a population-based study. J Clin Res Pediatr Endocrinol. (2016) 8:157–62. doi: 10.4274/jcrpe.2687
38. Amouzegar A, Ghaemmaghami Z, Beigy M, Gharibzadeh S, Mehran L, Tohidi M, et al. Natural course of euthyroidism and clues for early diagnosis of thyroid dysfunction: tehran thyroid study. Thyroid. (2017) 27:616–25. doi: 10.1089/thy.2016.0409
39. Valdes S, Maldonado-Araque C, Lago-Sampedro A, Lillo-Munoz JA, Garcia-Fuentes E, Perez-Valero V, et al. Reference values for TSH may be inadequate to define hypothyroidism in persons with morbid obesity: Di@bet.es study. Obesity. (2017) 25:788–93. doi: 10.1002/oby.21796
40. Ornaghi S, Algeri P, Todyrenchuk L, Vertemati E, Vergani P. Impact of excessive pre-pregnancy body mass index and abnormal gestational weight gain on pregnancy outcomes in women with chronic hypertension. Pregnancy Hypertens. (2018) 12:90–5. doi: 10.1016/j.preghy.2018.04.005
41. Santini F, Marzullo P, Rotondi M, Ceccarini G, Pagano L, Ippolito S, et al. Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol. (2014) 171:R137–52. doi: 10.1530/EJE-14-0067
42. Wang B, Song R, He W, Yao Q, Li Q, Jia X, et al. Sex differences in the associations of obesity with hypothyroidism and thyroid autoimmunity among chinese adults. Front Physiol. (2018) 9:1397. doi: 10.3389/fphys.2018.01397
43. Matzen LE, Kvetny J, Pedersen KK. TSH, thyroid hormones and nuclear-binding of T3 in mononuclear blood cells from obese and non-obese women. Scand J Clin Lab Invest. (1989) 49:249–53. doi: 10.1080/00365518909089090
44. Strata A, Ugolotti G, Contini C, Magnati G, Pugnoli C, Tirelli F, et al. Thyroid and obesity: survey of some function tests in a large obese population. Int J Obes. (1978) 2:333–40.
45. Duntas L, Hauner H, Rosenthal J, Pfeiffer EF. Thyrotropin releasing hormone (TRH) immunoreactivity and thyroid function in obesity. Int J Obes. (1991) 15:83–7.
46. Fontenelle LC, Feitosa MM, Severo JS, Freitas TE, Morais JB, Torres-Leal FL, et al. Thyroid function in human obesity: underlying mechanisms. Horm Metab Res. (2016) 48:787–94. doi: 10.1055/s-0042-121421
47. Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab. (2016) 20:554–7. doi: 10.4103/2230-8210.183454
48. Rotondi M, Leporati P, La Manna A, Pirali B, Mondello T, Fonte R, et al. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. (2009) 160:403–8. doi: 10.1530/EJE-08-0734
49. Longhi S, Radetti G. Thyroid function and obesity. J Clin Res Pediatr Endocrinol. (2013) 5(Suppl. 1):40–4. doi: 10.4274/jcrpe.856
50. Isozaki O, Tsushima T, Nozoe Y, Miyakawa M, Takano K. Leptin regulation of the thyroids: negative regulation on thyroid hormone levels in euthy- roid subjects and inhibitory effects on iodide uptake and Na + /I- sym- porter mRNA expression in rat FRTL-5 cells. Endocr J. (2004) 51:415–23. doi: 10.1507/endocrj.51.415
51. Jakobs TC, Mentrup B, Schmutzler C, Dreher I, Köhrle J. Proinflamma- tory cytokines inhibit the expression and function of human type I 5′-deiodinase in HepG2 hepatocarcinoma cells. Eur J Endocrinol. (2002) 146:559–66. doi: 10.1530/eje.0.1460559
52. Kwakkel J, Surovtseva OV, Vries EM, Stap J, Fliers E, Boelen A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology. (2014) 155:2725–34. doi: 10.1210/en.2013-2066
53. Radetti G, Kleon W, Buzi F, Crivellaro C, Pappalardo L, diIorgi N, et al. Thyroid function and structure are affected in childhood obesity. J Clin Endocrinol Metab. (2008) 93:4749–54. doi: 10.1210/jc.2008-0823
54. Verbeeten KC, Elks CE, Daneman D, Ong KK. Association between childhood obesity and subsequent Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. (2011) 28:10–8. doi: 10.1111/j.1464-5491.2010.03160.x
55. Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. (2012) 18:1334–6. doi: 10.1177/1352458512436596
56. Gremese E, Tolusso B, Gigante MR, Ferraccioli G. Obesity as a risk and severity factor in rheumatic diseases (autoimmune chronic inflammatory diseases). Front Immunol. (2014) 5:576. doi: 10.3389/fimmu.2014.00576
57. Russolillo A, Iervolino S, Peluso R, Lupoli R, Di Minno A, Pappone N, et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology. (2013) 52:62–7. doi: 10.1093/rheumatology/kes242
58. Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. (2014) 16:1484–92. doi: 10.1111/cmi.12336
59. Hino J, Nakatani M, Arai Y, Tsuchida K, Shirai M, Miyazato M, et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int J Obes. (2017) 41:483–8. doi: 10.1038/ijo.2017.15
60. Lourenco EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci USA. (2016) 113:10637–42. doi: 10.1073/pnas.1607101113
61. Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gomez-Reino JJ, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. (2017) 13:100–9. doi: 10.1038/nrrheum.2016.209
62. Teixeira PF, Cabral MD, Silva NA, Soares DV, Braulio VB, Couto AP, et al. Serum leptin in overt and subclinical hypothyroidism: effect of levothyroxine treatment and relationship to menopausal status and body composition. Thyroid. (2009) 19:443–50. doi: 10.1089/thy.2007.0393
63. Drobniak A, Kanecki K, Grymowicz M, Radowicki S. Serum leptin concentration in women of reproductive age with euthyroid autoimmune thyroiditis. Gynecol Endocrinol. (2016) 32:128–31. doi: 10.3109/09513590.2015.1092512
64. Procaccini C, Carbone F, Galgani M. Obesity and susceptibility to autoimmune diseases. Expert Rev. Clin. Immunol. (2011) 7:287–94. doi: 10.1586/eci.11.18
65. Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. (2011) 117:151–64. doi: 10.3109/13813455.2011.562514
Keywords: obesity, thyroid disease, thyroid autoimmunity, thyroid dysfunction, hypothyroidism, systematic review, meta-analysis
Citation: Song R, Wang B, Yao Q, Li Q, Jia X and Zhang J (2019) The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 10:2349. doi: 10.3389/fimmu.2019.02349
Received: 16 December 2018; Accepted: 17 September 2019;
Published: 01 October 2019.
Edited by:
James J. Pestka, Michigan State University, United StatesReviewed by:
Weiping Teng, The First Hospital Affiliated of China Medical University, ChinaRita S. Strakovsky, Michigan State University, United States
Copyright © 2019 Song, Wang, Yao, Li, Jia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-an Zhang, zhangjinan@hotmail.com
 Rong-hua Song
Rong-hua Song Bin Wang
Bin Wang Qiu-ming Yao
Qiu-ming Yao Qian Li2
Qian Li2 Xi Jia
Xi Jia Jin-an Zhang
Jin-an Zhang