- Institute of Microbiology, Infectious Diseases and Immunology, Gastrointestinal Microbiology Research Group, Charité - University Medicine Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin Institute of Health, Berlin, Germany
Human campylobacteriosis constitutes a zoonotic food-borne disease and a progressively rising health burden of significant socioeconomic impact. We have recently shown that conventional mice are protected from Campylobacter jejuni infection, which was not the case for human microbiota associated (hma) mice indicating that the host-specific gut microbiota composition primarily determines susceptibility to or resistance against C. jejuni infection. In our present preclinical intervention study we addressed whether gut microbiota changes in stably C. jejuni infected hma mice following murine fecal microbiota transplantation (mFMT) could alleviate pathogen-induced immune responses. To accomplish this, secondary abiotic C57BL/6 mice were generated by broad-spectrum antibiotic treatment, perorally reassociated with a complex human gut microbiota and challenged with C. jejuni by gavage. Seven days later C. jejuni infected hma mice were subjected to peroral mFMT on 3 consecutive days. Within a week post-mFMT fecal pathogenic burdens had decreased by two orders of magnitude, whereas distinct changes in the gut microbiota composition with elevated numbers of lactobacilli and bifidobacteria could be assessed. In addition, mFMT resulted in less C. jejuni induced apoptotic responses in colonic epithelia, reduced numbers of macrophages and monocytes as well as of T lymphocytes in the large intestinal mucosa and lamina propria and in less distinct intestinal pro-inflammatory cytokine secretion as compared to mock challenge. Strikingly, inflammation dampening effects of mFMT were not restricted to the intestinal tract, but could also be observed systemically as indicated by elevated serum concentrations of pro-inflammatory cytokines such as TNF-α, IL-12p70, and IL-6 in C. jejuni infected hma mice of the mock, but not the mFMT cohort. In conclusion, our preclinical mFMT intervention study provides evidence that changes in the gut microbiota composition which might be achieved by pre- or probiotic formulations may effectively lower intestinal C. jejuni loads, dampen both, pathogen-induced intestinal and systemic inflammatory sequelae and may represent a useful tool to treat continuous shedding of C. jejuni by asymptomatic carriers which is critical in the context of food production, hospitalization and immunosuppression.
Introduction
Human campylobacteriosis is among the four most prevalent global causes of diarrheal morbidities, whereas Campylobacter jejuni even constitutes the most common bacterial etiologic agents of human gastroenteritis with increasing prevalences worldwide (1, 2). In 2016, more than 250,000 cases of campylobacteriosis were reported in the European Union and the European Economic Area with an incidence of 66 cases per 100,000 subjects (3). One needs to take into consideration, however, that the number of unreported cases might be much higher due to asymptomatic carriers and to difficulties in detection given the fastidious growth requirements of the bacteria (4). The zoonotic pathogens are part of the commensal gut microbiota of warm-blooded wild and domestic animals. Humans become infected by consumption of undercooked contaminated meat derived from Campylobacter colonized livestock animals such as poultry, but also swine and cattle or by ingestion of C. jejuni containing surface waters (5, 6). Infected patients are either asymptomatic, present with rather mild symptoms or suffer from abdominal cramps, fever, watery or even inflammatory and bloody diarrhea (7, 8). Severely affected intestinal tissues are histologically characterized by elevated immune cell counts, crypt abscesses and ulcerations (9, 10). In most cases, symptoms are self-limiting, resolve within 1 week and require symptomatic therapy only (11). Antibiotic treatment with macrolides or fluoroquinolones, however, might be indicated in severe cases mostly affecting immunocompromised patients (7, 8, 11). In rare cases, post-infectious sequelae such as the Guillain-Barré syndrome, Miller Fisher syndrome, Reiter's syndrome, or reactive polyarthritis might arise (12–14). The exact mechanisms underlying C. jejuni-host interactions are yet only incompletely understood. One of the reasons for this dilemma is that appropriate C. jejuni infection and inflammation models have been missing for many years. Mice are convenient in vivo vertebrate model organisms, but display a strong physiological colonization resistance against C. jejuni infection when bred and maintained under standard specific pathogen free (SPF) conditions (15–17). Whereas, conventionally colonized wildtype mice were protected from pathogenic colonization following peroral C. jejuni challenge even of high loads, the pathogen could stably establish alongside the gastrointesinal tract of mice in which the gut microbiota had been depleted following broad-spectrum antibiotic treatment (15, 16). Reassociation of microbiota-depleted (i.e., secondary abiotic) mice with conventional murine gut microbiota via peroral fecal microbiota transplantation (FMT), however, could restore the colonization resistance against the pathogen, which was not the case when microbiota depleted mice were reassociated with fecal microbiota derived from human donors (15, 16). Stable intestinal C. jejuni colonization of microbiota depleted as well as of human gut microbiota associated (hma) mice was further associated with pronounced pro-inflammatory immune responses mimicking key features of human campylobacteriosis (15). In our present preclinical intervention study we therefore addressed whether gut microbiota changes in stably infected C. jejuni mice harboring a human gut microbiota by peroral FMT derived from murine donors could lower intestinal pathogenic loads and dampen induced pro-inflammatory immune responses.
Materials and Methods
Ethics Statement
After approval by the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0097/12 and G0039/15) mouse experiments were conducted in accordance with the European Guidelines for animal welfare (2010/63/EU). Clinical conditions of mice were assessed once a day.
Introduction of Human Gut Microbiota Into Murine Microbiota Depleted Mice
C57BL/6j mice were reared under SPF conditions in the same unit of the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité - University Medicine Berlin). In order to override physiological colonization resistance and assure stable gastrointestinal C. jejuni colonization upon peroral challenge lateron (15), the murine gut microbiota was depleted by broad-spectrum antibiotic treatment as stated earlier (15, 18, 19). In brief, at the age of 6–8 weeks mice were treated with ampicillin plus sulbactam (1 g/L; Ratiopharm, Germany), vancomycin (500 mg/L; Cell Pharm, Germany), ciprofloxacin (200 mg/L; Bayer Vital, Germany), imipenem (250 mg/L; MSD, Germany), and metronidazole (1 g/L; Fresenius, Germany) via autoclaved drinking water for 8 weeks (ad libitum). Three days before human fecal microbiota transplantation, the antibiotic cocktail was replaced by autoclaved tap water (Figure 1).
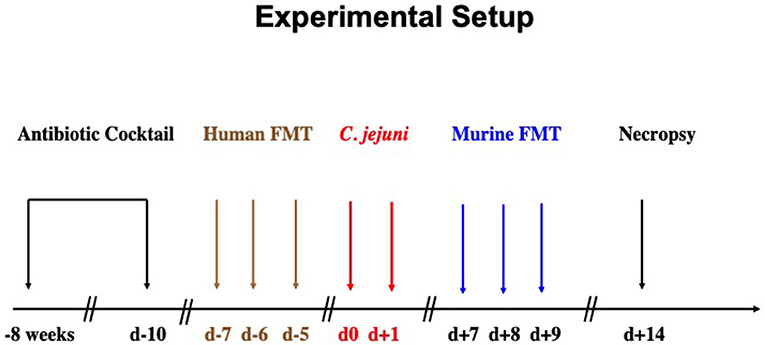
Figure 1. Experimental setup. Secondary abiotic mice were generated by broad-spectrum antibiotic treatment for 8 weeks. Three days before human fecal microbiota transplantation (FMT) the antibiotic cocktail was replaced by sterile tap water to guarantee antibiotic washout. Microbiota depleted mice were then subjected to human FMT on 3 consecutive days [i.e., day (d) −7, d−6, d−5]. To assure stable establishment of the human gut microbiota within the murine host, mice were kept for another 7 days before C. jejuni infection. On d0 and d+1, human microbiota associated (hma) mice were perorally subjected to C. jejuni by gavage. One week later, C. jejuni infected hma mice were treated with oral FMT derived from murine donors (d+7, d+8, d+9) and surveyed until necropsy on d+14.
To introduce a complex gut microbiota of human origin into the murine host, microbiota-depleted mice were subjected to peroral human FMT on 3 consecutive days as described earlier (20, 21). In brief, fresh fecal samples free of enteropathogenic bacteria, viruses and parasites were collected from five individual healthy human donors, dissolved in sterile phosphate buffered saline (PBS, Gibco, Life Technologies, UK), aliquoted and stored at −80°C as state elsewhere (15, 22–24). Immediately before FMT, individual fecal aliquots were thawed, pooled and applied to mice perorally by gavage in a total volume of 0.3 mL (15, 22–24). To assure proper establishment of the complex human microbiota in the murine host, mice were kept for 7 days after the initial human FMT until C. jejuni infection (Figure 1).
C. jejuni Infection and Fecal Loads
For infection, a stock solution of C. jejuni 81–176 strain that had been stored at −80°C was thawed, aliquots streaked onto karmali agar (Oxoid, Wesel, Germany) and incubated in a microaerophilic atmosphere at 37°C for 48 h. Immediately before peroral infection of mice, bacteria were harvested in sterile PBS to a final inoculum of 109 bacterial cells.
Female and male hma mice (4 months of age) were perorally infected by gavage (in a total volume of 0.3 mL PBS) on 2 consecutive days starting on day (d) 0 and d1 (Figure 1). C. jejuni loads were monitored in fecal samples over time post-infection as reported previously (15, 25). In brief, serial dilutions of fecal samples were dissolved in sterile PBS, streaked onto karmali agar and quantitatively assessed 48 h following incubation in a microaerophilic atmosphere at 37°C. The detection limit of viable pathogens was ≈100 CFU per g.
Murine Fecal Microbiota Transplantation
At days 7, 8, and 9 post-infection, C. jejuni infected hma mice were treated with FMT from murine donors (Figure 1) as described earlier (15, 22–24, 26). In brief, fresh murine fecal samples were collected from 10 age and sex matched conventionally colonized (i.e., SPF) mice, pooled, dissolved in 10 mL sterile PBS and the supernatant used as murine donor suspension. Sex and age matched C. jejuni infected hma mice were either perorally treated with 0.3 mL of murine donor suspension by gavage or received PBS only as mock control animals (15, 22–24, 26). Immediately before either FMT, aliquots from both, human and murine donor solutions were collected for quantitative molecular analyses of main intestinal bacterial communities as described elsewhere (15, 18, 27).
Gut Microbiota Composition
DNA was extracted from fecal samples or fecal donor suspensions as stated earlier (18, 28). In brief, DNA was quantified by using Quant-iT PicoGreen reagent (Invitrogen, UK) and adjusted to 1 ng per μL. Then, total eubacterial loads as well as the main bacterial groups abundant in the murine and human intestinal microbiota including enterobacteria, enterococci, lactobacilli, bifidobacteria, Bacteroides/Prevotella species, Clostridium coccoides group, Clostridium leptum group, and Mouse Intestinal Bacteroides were determined by quantitative real-time polymerase chain reaction (qRT-PCR) with species-, genera- or group-specific 16S rRNA gene primers (Tib MolBiol, Germany) as described previously (15, 27, 29) and numbers of 16S rRNA gene numbers per ng DNA of each sample assessed.
Sampling Procedures
At day 14 post-infection, mice were sacrificed (Figure 1) by isofluran inhalation (Abbott, Germany). Ex vivo biopsies from colon and mesenteric lymph nodes (MLN) were taken under sterile conditions. Large intestinal samples were collected from each mouse in parallel for microbiological, immunohistopathological, and immunological analyses.
Immunohistochemistry
Colonic ex vivo biopsies were immediately fixed in 5% formalin, embedded in paraffin, and subjected to in situ immunohistochemical analyses as reported previously (27, 30–32). In brief, for detection of apoptotic epithelial cells, macrophages/monocytes and T lymphocytes paraffin sections (5 μm) were stained with primary antibodies directed against cleaved caspase 3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), F4/80 (# 14-4801, clone BM8, eBioscience, San Diego, CA, USA, 1:50), and CD3 (#N1580, Dako, 1:10), respectively. Then, positively-stained cells were quantitatively examined by a blinded independent investigator (light microscopy, magnification 100x and 400x). The average number of respective positively-stained cells for each individual section was determined within at least six high power fields (HPF, 0.287 mm2, 400x magnification).
Pro-inflammatory Cytokine Secretion
Large intestinal ex vivo biopsies were cut longitudinally and washed in PBS. MLN (3 lymph nodes) or strips of approximately 1 cm2 colonic tissues were placed in 24-flat-bottom well culture plates (Nunc, Germany) with 500 μL serum-free RPMI 1640 medium (Gibco, life technologies, UK) and supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL; PAA Laboratories, Germany). After 18 h at 37°C, culture supernatants and serum samples were tested for TNF-α, IFN-γ, IL-6, and IL-12p70 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, Germany) on a BD FACSCanto II flow cytometer (BD Biosciences).
Statistical Analysis
Medians and levels of significance were determined using Mann-Whitney test (GraphPad Prism v7, USA) for pairwise comparisons of not normally distributed data and using the one-sided ANOVA with Tukey post-correction or the Kruskal-Wallis test with Dunn's post-correction for multiple comparisons as indicated. Two-sided probability p ≤ 0.05 were considered significant. Experiments were reproduced three times.
Results
Lower Intestinal Pathogenic Burdens Following Murine Fecal Microbiota Transplantation of C. jejuni Infected Mice Harboring a Human Gut Microbiota
Mice with a human gut microbiota were perorally infected with C. jejuni on days 0 and 1 and subjected to murine fecal microbiota transplantation (mFMT) or received vehicle only on 3 consecutive days starting at day 7 post-infection (Figure 1). Immediately before and after the mFMT we surveyed the intestinal pathogenic loads over time by cultural analyses of fecal samples. Following peroral infection, mice from the mock control group harbored more than 108 C. jejuni cells until the end of the observation period (Figure 2A). As early as 72 h following the latest mFMT (D+5), however, fecal C. jejuni burdens had declined (p < 0.001; Figure 2B), whereas 1 week following the initial mFMT (D+7), the median fecal pathogen loads were approximately two orders of magnitude lower as compared to those obtained before the intervention (p < 0.001; Figure 2B). Notably, single animals had even completely lost the pathogen from their intestines as early as 5 days following the initial mFMT (D+5; Figure 2B). Furthermore, in mice from the mFMT cohort fecal C. jejuni loads were lower at individual time points post- intervention as compared to mock counterparts (p < 0.001; Figure 2). Hence, mFMT could sufficiently lower pathogenic burdens in the intestines of hma mice.
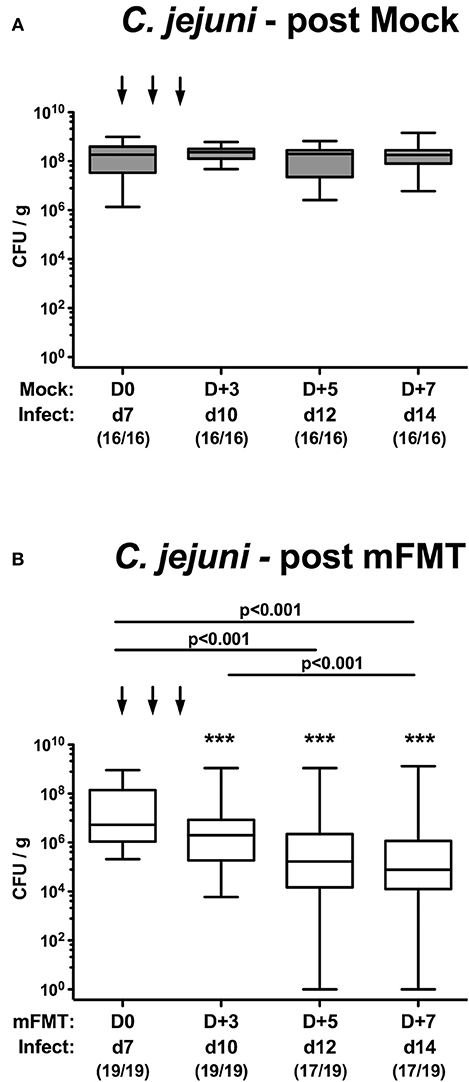
Figure 2. Kinetic survey of fecal C. jejuni shedding following murine fecal microbiota transplantation of infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 (Infect) and subjected to (A) vehicle (mock) treatment or to (B) murine fecal microbiota transplantation (mFMT) on d7, d8, and d9 post-infection (arrows; D0, D+1, D+2). Immediately before and after mFMT fecal samples were taken at defined time points as indicated to assess intestinal pathogenic loads by culture (expressed as colony forming units per gram, CFU/g). Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes). The total range, significance levels (p-values) determined by the Kruskal-Wallis test and Dunn's post-correction and the numbers of C. jejuni positive mice out of the total number of animals (in parentheses) are given. Data were pooled from four independent experiments. ***p < 0.001 comparing bacterial loads in mock vs. mFMT treated mice at identical time points (Mann Whitney U-test).
Gut Microbiota Changes Following Murine Fecal Microbiota Transplantation of C. jejuni Infected Mice Harboring a Human Gut Microbiota
We further addressed to what extent the commensal gut microbiota composition of mice harboring a human gut microbiota had changed upon the mFMT applying culture-independent molecular methods. Seven days after the start of the intervention (D+7), mice from the mFMT cohort displayed higher numbers of lactobacilli (p < 0.01), bifidobacteria (p < 0.05–0.001), and Mouse Intestinal Bacteroides (p < 0.01–0.001) as compared to mice from the mock group and naive control animals, whereas fecal loads of Bacteroides/Prevotella species (p < 0.001), and Clostridium coccoides (p < 0.001) were lower in the former as compared to the latter (Figure 3). Furthermore, mFMT treated mice displayed lower fecal Clostridium leptum loads as compared to naive mice (p < 0.001; Figure 3). As expected, marked quantitative differences in the main commensal bacterial group abundant in the intestinal tract could be assessed when comparing the fecal solutions from human vs. murine donors (Figure S1). Hence, within 1 week mFMT results in distinct changes of the intestinal microbiota composition of hma mice.
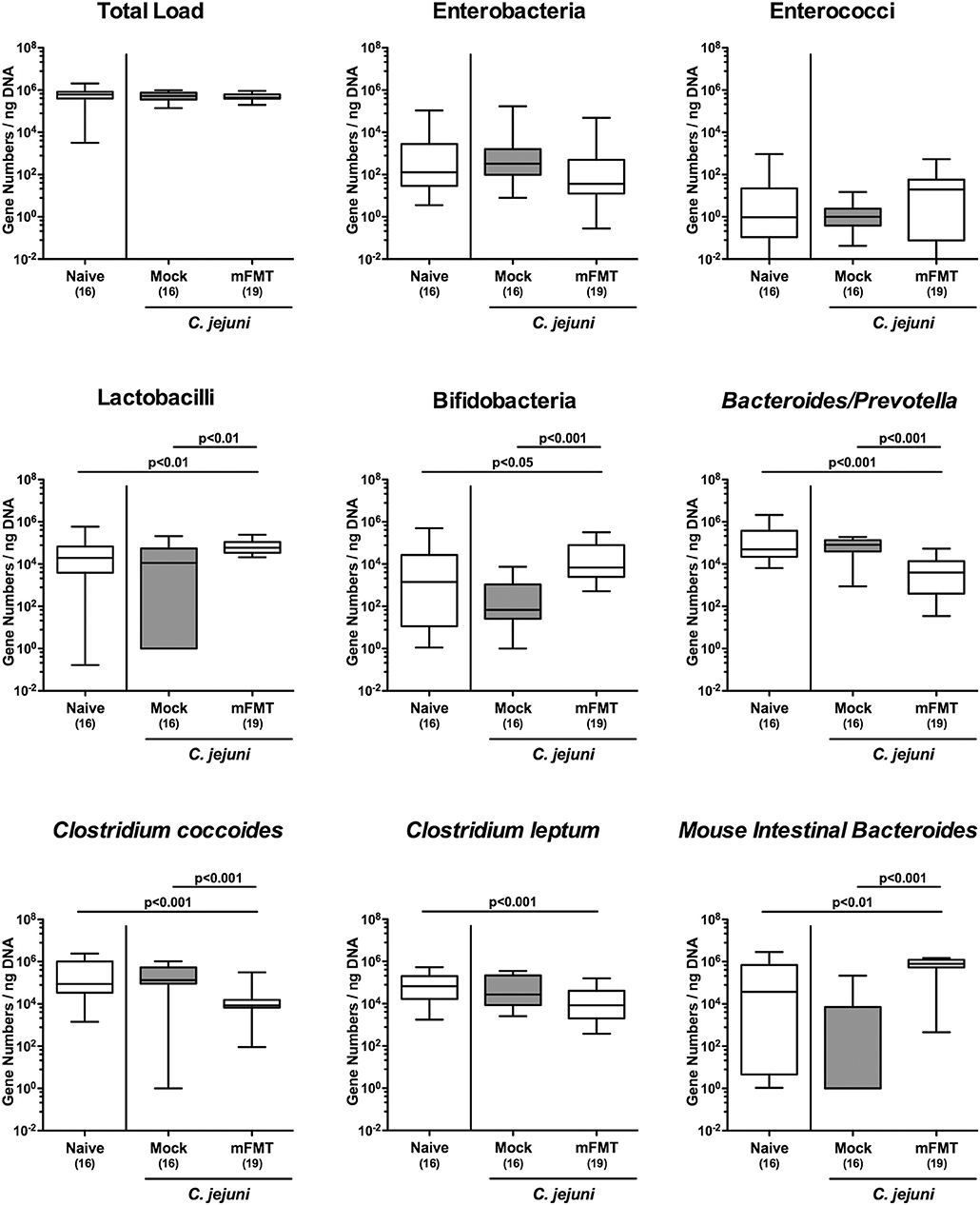
Figure 3. Changes in intestinal microbiota composition following murine fecal microbiota transplantation of C. jejuni infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 and subjected to murine fecal microbiota transplantation (mFMT) or to mock treatment on d7, d8, and d9 post-infection (i.e., D0, D+1, D+2) or received vehicle (mock). Seven days later (i.e., D+7), the fecal commensal microbiota composition was assessed applying culture-independent 16S rRNA methods quantitating the total eubacterial load and main bacterial groups as indicated (expressed as gene numbers per ng DNA). Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes). The total range and significance levels (p-values) determined by the Kruskal-Wallis test and Dunn's post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Less Pronounced Intestinal Apoptotic Cell and Immune Cell Responses Following Murine Fecal Microbiota Transplantation of C. jejuni Infected Mice Harboring a Human Gut Microbiota
We next addressed whether mFMT resulted in less pronounced C. jejuni induced inflammatory responses in the intestinal tract. C. jejuni infection was associated with multifold increased numbers of colonic apoptotic epithelial cells (p < 0.001), unless infected hma mice had been subjected to mFMT (n.s. vs. naive; Figure 4A; Figure S2A). At day 14 post-infection, mock control mice exhibited increased numbers of innate immune cell subsets such as macrophages and monocytes (p < 0.001; Figure 4B; Figure S2B) as well as of adaptive immune cells including T lymphocytes (p < 0.001; Figure 4C; Figure S2C) in their colonic mucosa and lamina propria, whereas respective immune cell populations were lower in infected mice after mFMT vs. mock application (p < 0.001; Figures 4B,C; Figures S2B,C). Hence, mFMT dampens C. jejuni induced apoptotic cell and immune cell responses in the large intestines of mice with a human gut microbiota.
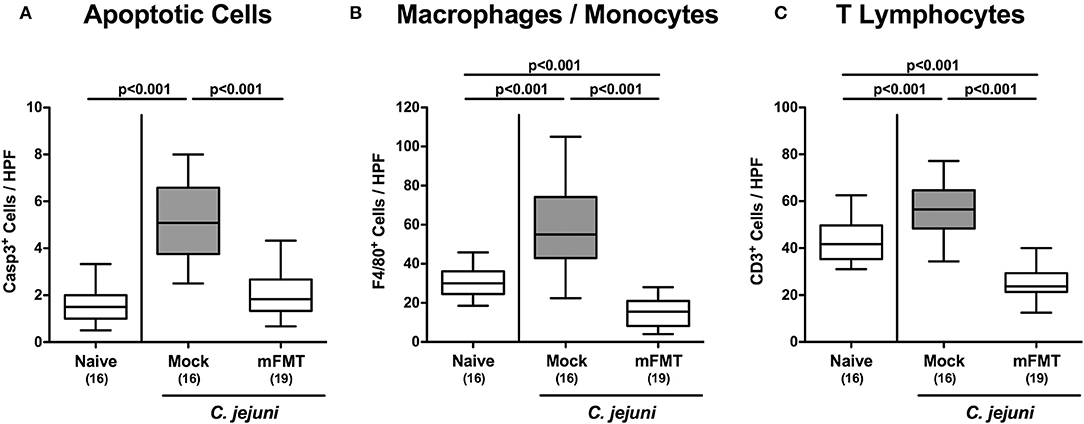
Figure 4. Colonic apoptotic epithelial and immune cell responses following murine fecal microbiota transplantation of C. jejuni infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 and subjected to murine fecal microbiota transplantation (mFMT) on d7, d8, and d9 post-infection (p.i.) or received vehicle (mock). On day 14 p.i., the average numbers of (A) caspase3+ (Casp3+) apoptotic epithelial cells, (B) F4/80+ macrophages and monocytes and of (C) CD3+ T lymphocytes were assessed from six high power fields (HPF, 400x magnification) per mouse in immunohistochemically stained colonic paraffin sections. Naive mice with a human gut microbiota served as uninfected and untreated controls. Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes). The total range, significance levels (p-values) determined by the one-sided ANOVA test with Tukey post-correction and the numbers of C. jejuni positive mice out of the total number of animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Less Intestinal Pro-inflammatory Cytokine Secretion Following Murine Fecal Microbiota Transplantation of C. jejuni Infected Mice Harboring a Human Gut Microbiota
We further addressed whether the dampened C. jejuni induced immune responses upon mFMT were associated with less pro-inflammatory cytokine secretion in the intestinal tract. In fact, elevated TNF-α concentrations could be measured in colonic ex vivo biopsies taken from mock controls at day 14 post-infection (p < 0.05 vs. naive), which was, however, not the case when mice had been challenged with mFMT (p < 0.001 vs. mock; Figure 5A). In support, multifold increased IFN-γ concentrations were determined in MLN when taken from mock animals (p < 0.001 vs. naive), but not from mice following mFMT at day 14 post-infection (p < 0.001 vs. mock; Figure 5B). Hence, mFMT resulted in less distinct pathogen induced pro-inflammatory cytokine secretion in the intestinal tract in C. jejuni mice with a human gut microbiota.
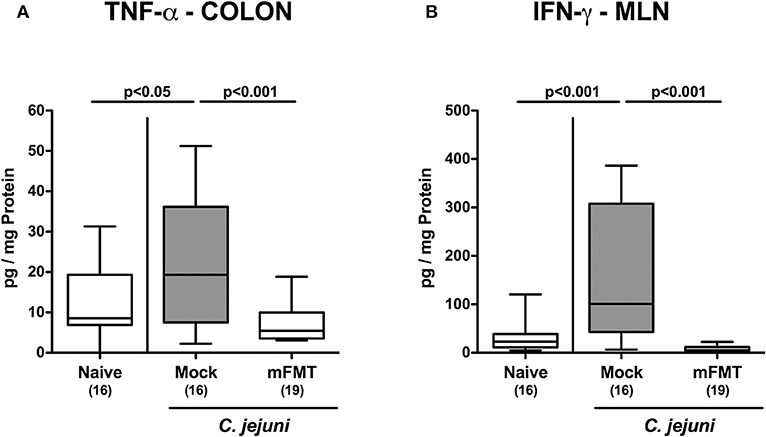
Figure 5. Intestinal pro-inflammatory cytokine secretion following murine fecal microbiota transplantation of C. jejuni infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 and subjected to murine fecal microbiota transplantation (mFMT) on d7, d8, and d9 post-infection (p.i.) or received vehicle (mock). On day 14 p.i., (A) TNF-α and (B) IFN-γ concentrations were measured in ex vivo biopsies derived from the colon and mesenteric lymph nodes (MLN), respectively. Naive mice with a human gut microbiota served as uninfected and untreated controls. Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes). The total range, significance levels (p-values) determined by the one-sided ANOVA test with Tukey post-correction and the numbers of C. jejuni positive mice out of the total number of animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Less Systemic Pro-inflammatory Cytokine Secretion Following Murine Fecal Microbiota Transplantation of C. jejuni Infected Mice Harboring a Human Gut Microbiota
We further addressed whether the anti-inflammatory effects of mFMT in C. jejuni infected mice was restricted to the intestinal tract or also effective in the systemic compartment. Remarkably, serum concentrations of TNF-α, IL-12p70, and IL-6 were all elevated at day 14 following C. jejuni infection of mice in the mock cohort (p < 0.01–0.001 vs. naive; Figure 6), but not in the mFMT intervention cohort (p < 0.05–0.01 vs. mock; Figure 6). Hence, the anti-inflammatory properties of mFMT in C. jejuni infected mice were not restricted to the intestinal tract, but were also effective systemically.
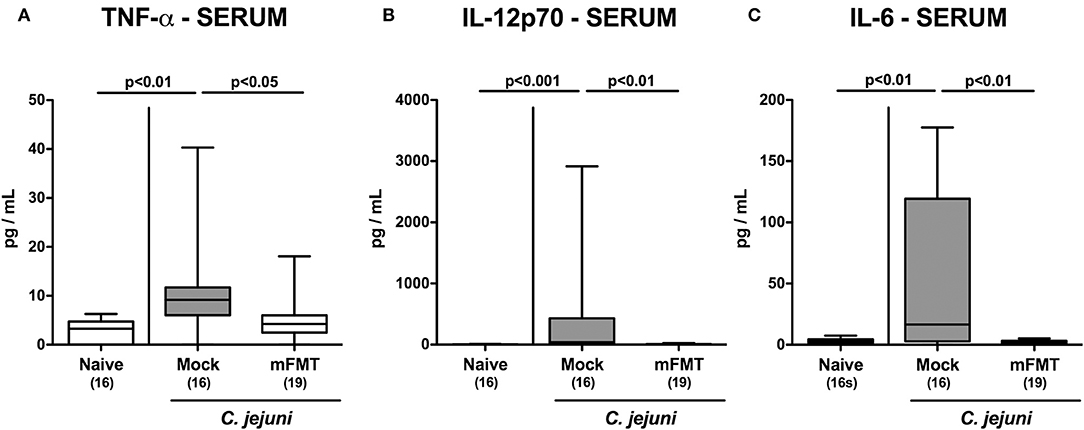
Figure 6. Systemic pro-inflammatory cytokine secretion following murine fecal microbiota transplantation of C. jejuni infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 and subjected to murine fecal microbiota transplantation (mFMT) on d7, d8, and d9 post-infection (p.i.) or received vehicle (mock). On day 14 p.i., (A) TNF-α, (B) IL-12p70, and (C) IL-6 concentrations were measured in serum samples. Naive mice with a human gut microbiota served as uninfected and untreated controls. Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes). The total range, significance levels (p-values) determined by the one-sided ANOVA test with Tukey post-correction or by the Kruskal-Wallis test and Dunn's post-correction and the numbers of C. jejuni positive mice out of the total number of animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Discussion
The specific gut microbiota composition of the vertebrate host primarily determines susceptibity to or resistance against infections with enteropathogens including C. jejuni (15–17). The FMT as therapeutic application dates back to the Chinese Dong-jin dynasty in the fourth century and has very recently experienced a renaissance for the treatment of recurrent and refractory infections with enterotoxin-producing Clostridioides difficile strains, of inflammatory bowel diseases and of the chronic fatigue syndrome, for instance (33–39). Furthermore, FMT constitutes a promising antibiotics-independent therapeutic approach to combat carriage with (opportunistic) pathogens, particularly with multi-drug resistant strains. In support, we were able to show very recently that mFMT in hma mice on 3 consecutive days could effectively reduce intestinal burdens of multi-drug resistant Pseudomonas aeruginosa by almost four orders of magnitude, whereas individual mice had even completely lost the opportunistic pathogen (40).
Given that conventionally colonized mice as well as microbiota depleted mice that had been reassociated with a complex murine gut microbiota were protected from even high-dose C. jejuni infection as opposed to hma counterparts (15), we here evaluated mFMT as a potential intervention strategy to combat C. jejuni infection and pathogen-induced inflammatory sequelae. In order to mimick human gut microbiota conditions we had subjected microbiota depleted mice to peroral FMT from human donors before C. jejuni infection. As any other experimental model also the here applied hma mouse model has its limitations. We cannot exclude, for instance, that some members of the human fecal donor samples, in particular obligate anaerobic and other fastidious bacterial species, might be reduced during asservation and processing including freezing, thawing and FMT and/or did not fully establish within the gastrointestinal ecosystem of the murine host (20, 24). Moreover, several host-related and environmental factors might further affect the fate of the human fecal transplant over time such as the genetic background, the anatomical and mucosal immunological repertoire within the respective gastrointestinal compartment, the intraluminal milieu, as well as the housing conditions including diet of the challenged mice (24, 41–43). Under consideration of both, the limitations and the strengths of the applied experimental model, hma mice constitute worthwhile measures to reliably unravel the interactions between pathogens, gut commensals and host immune responses in health and disease. In fact, with respect to their microbiota “humanized” mice have been successfully used as tools to investigate the interactions between the vertebrate host and enteropathogens including C. difficile, Salmonella, and C. jejuni (15, 44, 45).
In our actual preclinical intervention study applying C. jejuni infected hma mice we were able to show that mFMT (i) resulted in changes of the gut microbiota composition, (ii) could lower the intestinal pathogen burdens by two log orders of magnitude, (iii) dampen C. jejuni induced apoptotic cell and immune cell responses in the large intestine that were associated with (iv) less distinct pro-inflammatory cytokine secretion in both, the large intestines and MLN. Strikingly, (v) the anti-inflammatory properties of mFMT were not restricted to the intestinal tract, but could also be observed systemically.
Our molecular survey of the main abundant intestinal bacterial groups and species revealed that the mFMT on 3 consecutive days did in fact result in changes of the gut microbiota composition of hma mice within 1 week post-intervention. In line with the observed quantitative differences of bacterial taxa from fecal suspensions derived from human vs. murine donors, the fecal microbiota at day 7 post-mFMT of hma mice was characterized by higher numbers of lactobacilli, bifidobacteria and Mouse Intestinal Bacteroides, whereas Bacteroides/Prevotella species and clostridia were lower as compared to pre-mFMT conditions. Comparable gut microbiota shifts could be observed following mFMT of hma mice that were carrying multi-drug resistant Pseudomonas aeruginosa in their intestines (40). It is tempting to speculate that (yet to be identified) specific “health-beneficial,” immune-modulatory bacterial commensal species within the complex gut luminal ecosystem might make the difference and provide anti-inflammatory properties following infection with enteropathogens including C. jejuni. Lactobacilli and bifidobacteria may be potential candidates since they are well known for their probiotic effects due to production of bacteriocins and short chain fatty acids subsequently creating hostile conditions for invading pathogens (46, 47). In support, we were able to show recently that peroral application of a single Lactobacillus johnsonii strain that had been isolated from a fecal sample taken from a healthy C57BL/6 mouse could effectively alleviate intestinal, extra-intestinal and, remarkably, even systemic pro-inflammatory immune responses upon C. jejuni infection of secondary abiotic mice (48). Additional studies further revealed potent anti-inflammatory effects of L. johnsonii in enteric including infectious diseases (49) mounting in commercial probiotic application (e.g., Nestlé LC1).
Bifidobacteria are considered key players in maintaining intestinal homeostasis. In fact, a delay in intestinal colonization with bifidobacteria rendered individuals more susceptible for morbidities during infancy and later in life (50). Several intestinal immunopathological conditions including inflammatory bowel diseases, celiac disease and irritable bowel syndrome have been associated with a perturbed gut microbiota (i.e., dysbiosis) with decreased or even absent intestinal bifidobacteria (51). Due to their anti-inflammatory properties defined bifidobacteria as well as lactobacilli strains have been introduced into commercial probiotic formulations such as VSL#3 and have been shown to effectively alleviate clinical symptoms and maintain remission in inflammatory bowel diseases (51). Furthermore, and supporting our results obtained from our actual mFMT intervention study, we demonstrated recently that VSL#3 application did not only dampen pro-inflammatory immune responses in the intestinal tract, but also in extra-intestinal and even systemic compartments upon C. jejuni infection of microbiota depleted mice (52).
It is thus highly likely that synergistic effects between different commensal bacterial species are more sufficient to exert potent anti-inflammatory in the combat of enteropathogenic including C. jejuni infections than single strains alone. In support, we were able to show previously that mFMT in microbiota depleted mice could induce more prominent anti-inflammatory responses than reassociation with a commensal murine L. johnsonii strain alone as indicated by more pronounced anti-inflammatory CD25 expression in intestinal as well as systemic immunological compartments following the former as compared to the latter intervention (26). It would therefore be utmost appreciable to characterize the gut luminal milieu in more detail in order to define distinct bacterial strains and/or metabolites that might be promising candidate molecules for future prophylactic or therapeutic application in humans and food animals.
In conclusion, our preclinical mFMT intervention study provides evidence that changes in the gut microbiota composition which might be achieved by pre- or probiotic formulations may effectively lower intestinal C. jejuni loads and dampen both, pathogen-induced intestinal and systemic inflammatory sequelae. Furthermore, the applied infection model provides a valuable tool to identify luminal intestinal molecules mediating colonization resistance for future treatment and prevention of C. jejuni infection and colonization in the vertebrate host and may represent a promising option to treat continuous shedding of C. jejuni by asymptomatic carriers which is critical in the context of food production, hospitalization, and immunosuppression.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Ethics Statement
The animal study was reviewed and approved by Landesamt für Gesundheit und Soziales (LaGeSo, Berlin).
Author Contributions
MH designed and performed experiments, analyzed data, and wrote paper. KM performed experiments, analyzed data, and co-edited paper. SB provided advice in experimental design, critically discussed results, and co-edited paper.
Funding
This work was supported from the German Federal Ministries of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter to MH and SB (IP7/01KI1725D) and from the German Federal Ministries of Economy and Energy to SB and MH (ZIM; ZF4117904 AJ8). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Alexandra Bittroff-Leben, Ines Puschendorf, Ulrike Fiebiger, Ulrike Escher, Sigri Kløve, Gernot Reifenberger, and the staff of the animal research facility at Charité - University Medicine Berlin for excellent technical assistance and animal breeding. We further thank Dr. Anja A. Kühl (Department of Medicine I for Gastroenterology, Infectious Diseases and Rheumatology/Research Center ImmunoSciences (RCIS), Charité – Universitätsmedizin Berlin) for taking representative photomicropgraphs of immunohistochemically stained paraffin sections. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.02272/full#supplementary-material
Figure S1. Microbiota composition of suspensions derived from human and murine donors used for fecal microbiota transplantation. Immediately before fecal microbiota transplantation from human and murine donors, the commensal microbiota composition was assessed in respective fecal suspensions (n = 3 each) applying culture-independent 16S rRNA methods quantitating the total eubacterial load (TL) and main bacterial groups including enterobacteria (EB), enterococci (EC), lactobacilli (LB), bifidobacteria (BB), Bacteroides/Prevotella species (BP), Clostridium coccoides group (CC), Clostridium leptum group (CL), and Mouse Intestinal Bacteroides (MIB) and expressed as gene numbers per ng DNA. Box plots represent the 75th and 25th percentiles of medians (black bar inside the boxes) and the total range is indicated. Data were pooled from three independent suspensions.
Figure S2. Representative photomicrographs illustrating colonic apoptotic epithelial and immune cell responses following murine fecal microbiota transplantation in C. jejuni infected mice harboring a human gut microbiota. Mice with a human gut microbiota were perorally infected with C. jejuni on day (d) 0 and d1 and subjected to murine fecal microbiota transplantation (mFMT) on d7, d8, and d9 post-infection (p.i.) or remained untreated (mock). Photomicrographs representative for four independent experiments illustrate the average numbers of (A) apoptotic epithelial cells (caspase3+), (B) macrophages and monocytes (F4/80+), and (C) T lymphocytes (CD3+), in at least six high power fields (HPF) as quantitatively assessed in colonic paraffin sections applying in situ immunohistochemistry at day 14 p.i. (100x magnification, scale bar 100 μm).
Abbreviations
CFU, colony forming units; D, d, day; FMT, fecal microbiota transplantation; Hma, human microbiota associated; HPF, high power field; IFN, interferon; IL, interleukin; mFMT, murine fecal microbiota transplantation; MLN, mesenteric lymph nodes; n.s, not significant; PBS, phosphate buffered saline; qRT-PCR, quantitative real-time polymerase chain reaction; SPF, specific pathogen free; TNF, tumor necrosis factor.
References
1. Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. (2015) 12:e1001923. doi: 10.1371/journal.pmed.1001923
2. WHO (2018). Campylobacter. World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed May 27, 2019).
3. European Food Safety Authority European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. (2018) 16:e05500. doi: 10.2903/j.efsa.2018.5500
4. Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. (2015) 28:687–720. doi: 10.1128/CMR.00006-15
5. Alter T, Bereswill S, Glunder G, Haag LM, Hanel I, Heimesaat MM, et al. [Campylobacteriosis of man: livestock as reservoir for Campylobacter species]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2011) 54:728–34. doi: 10.1007/s00103-011-1289-y
6. Pielsticker C, Glunder G, Rautenschlein S. Colonization properties of Campylobacter jejuni in chickens. Eur J Microbiol Immunol. (2012) 2:61–5. doi: 10.1556/EuJMI.2.2012.1.9
7. Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter enteritis. Microbiol Rev. (1986) 50:81–94.
8. Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. (2001) 8:150–65. doi: 10.1159/000060405
9. van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. (1985) 26:945–51. doi: 10.1136/gut.26.9.945
10. Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. (2008) 21:505–18. doi: 10.1128/CMR.00055-07
11. Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, Gross U, et al. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol. (2009) 35:1–22. doi: 10.1080/10408410802636017
12. Allos BM. Association between Campylobacter infection and Guillain-Barre syndrome. J Infect Dis. (1997) 176(Suppl. 2):S125–8. doi: 10.1086/513783
13. Bereswill S, Kist M. Molecular microbiology and pathogenesis of Helicobacter and Campylobacter updated: a meeting report of the 11th conference on Campylobacter, Helicobacter and related organisms. Mol Microbiol. (2002) 45:255–62. doi: 10.1046/j.1365-2958.2002.03002.x
14. Backert S, Tegtmeyer N, Cróinín TÓ, Boehm M, Heimesaat MM. “Chapter 1: human campylobacteriosis”. In: Klein G, editor. Campylobacter. Academic Press (2017). p. 1–25.
15. Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE. (2011) 6:e20953. doi: 10.1371/annotation/5247af81-4595-44b7-9c3f-2e45ad85abfa
16. Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Gross U, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. (2013) 2013:526860. doi: 10.1155/2013/526860
17. Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol. (2016) 6:253–71. doi: 10.1556/1886.2016.00036
18. Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. (2006) 177:8785–95. doi: 10.4049/jimmunol.177.12.8785
19. Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, et al. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and−4 signaling. PLoS ONE. (2012) 7:e40761. doi: 10.1371/journal.pone.0040761
20. von Klitzing E, Ekmekciu I, Bereswill S, Heimesaat MM. Intestinal and systemic immune responses upon multi-drug resistant Pseudomonas aeruginosa colonization of mice harboring a human gut microbiota. Front Microbiol. (2017) 8:2590. doi: 10.3389/fmicb.2017.02590
21. Heimesaat MM, Escher U, Grunau A, Kuhl AA, Bereswill S. Multidrug-resistant Pseudomonas aeruginosa accelerate intestinal, extra-intestinal, and systemic inflammatory responses in human microbiota-associated mice with subacute ileitis. Front Immunol. (2019) 10:49. doi: 10.3389/fimmu.2019.00049
22. von Klitzing E, Bereswill S, Heimesaat MM. Multidrug-resistant Pseudomonas Aeruginosa induce systemic pro-inflammatory immune responses in colonized mice. Eur J Microbiol Immunol. (2017) 7:200–9. doi: 10.1556/1886.2017.00022
23. von Klitzing E, Ekmekciu I, Bereswill S, Heimesaat MM. Acute ileitis facilitates infection with multidrug resistant Pseudomonas aeruginosa in human microbiota-associated mice. Gut Pathog. (2017) 9:4. doi: 10.1186/s13099-017-0154-4
24. von Klitzing E, Oz F, Ekmekciu I, Escher U, Bereswill S, Heimesaat MM. Comprehensive survey of intestinal microbiota changes in offspring of human microbiota-associated mice. Eur J Microbiol Immunol. (2017) 7:65–75. doi: 10.1556/1886.2017.00002
25. Heimesaat MM, Plickert R, Fischer A, Gobel UB, Bereswill S. Can microbiota transplantation abrogate murine colonization resistance against Campylobacter jejuni? Eur J Microbiol Immunol. (2013) 3:36–43. doi: 10.1556/EuJMI.3.2013.1.5
26. Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P, et al. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front Immunol. (2017) 8:397. doi: 10.3389/fimmu.2017.00397
27. Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. (2010) 59:1079–87. doi: 10.1136/gut.2009.197434
28. Bereswill S, Kuhl AA, Alutis M, Fischer A, Mohle L, Struck D, et al. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. (2014) 6:19. doi: 10.1186/1757-4749-6-19
29. Rausch S, Held J, Fischer A, Heimesaat MM, Kuhl AA, Bereswill S, et al. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS ONE. (2013) 8:e74026. doi: 10.1371/journal.pone.0074026
30. Heimesaat MM, Lugert R, Fischer A, Alutis M, Kuhl AA, Zautner AE, et al. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS ONE. (2014) 9:e90148. doi: 10.1371/journal.pone.0090148
31. Alutis ME, Grundmann U, Fischer A, Hagen U, Kuhl AA, Gobel UB, et al. The role of gelatinases in Campylobacter jejuni infection of gnotobiotic mice. Eur J Microbiol Immunol. (2015) 5:256–67. doi: 10.1556/1886.2015.00033
32. Alutis ME, Grundmann U, Hagen U, Fischer A, Kuhl AA, Gobel UB, et al. Matrix metalloproteinase-2 mediates intestinal immunopathogenesis in Campylobacter jejuni-infected infant mice. Eur J Microbiol Immunol. (2015) 5:188–98. doi: 10.1556/1886.2015.00020
33. Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. (1958) 44:854–9.
34. Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. (2012) 107:1755. author reply p. 1755–1756. doi: 10.1038/ajg.2012.251
35. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. (2013) 368:407–15. doi: 10.1056/NEJMoa1205037
36. Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. (2014) 58:1515–22. doi: 10.1093/cid/ciu135
37. Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis. (2016) 48:587–92. doi: 10.1080/23744235.2016.1177199
38. Lewis BB, Pamer EG. Microbiota-based therapies for Clostridium difficile and antibiotic-resistant enteric infections. Ann Rev Microbiol. (2017) 71:157–78. doi: 10.1146/annurev-micro-090816-093549
39. Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. (2019) 7:e7502. doi: 10.7717/peerj.7502
40. Mrazek K, Bereswill S, Heimesaat MM. Fecal microbiota transplantation decreases intestinal loads of multi-drug resistant Pseudomonas aeruginosa in murine carriers. Eur J Microbiol Immunol. (2019) 9:14–22. doi: 10.1556/1886.2019.00002
41. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. (2009) 457:480–4. doi: 10.1038/nature07540
42. Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE. (2011) 6:e20944. doi: 10.1371/journal.pone.0020944
43. Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. (2015) 8:1–16. doi: 10.1242/dmm.017400
44. Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. (2012) 149:1578–93. doi: 10.1016/j.cell.2012.04.037
45. Collins J, Auchtung JM, Schaefer L, Eaton KA, Britton RA. Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome. (2015) 3:35. doi: 10.1186/s40168-015-0097-2
46. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. (2006) 40:235–43. doi: 10.1097/00004836-200603000-00015
47. Corr SC, Li Y, Riedel CU, Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. (2007) 104:7617–2. doi: 10.1073/pnas.0700440104
48. Bereswill S, Ekmekciu I, Escher U, Fiebiger U, Stingl K, Heimesaat MM. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci Rep. (2017) 7:2138. doi: 10.1038/s41598-017-02436-2
49. Lievin-Le Moal V, Servin AL. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev. (2014) 27:167–99. doi: 10.1128/CMR.00080-13
50. Sarkar A, Mandal S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res. (2016) 192:159–71. doi: 10.1016/j.micres.2016.07.001
51. Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. (2014) 20:15163–76. doi: 10.3748/wjg.v20.i41.15163
Keywords: Campylobacter jejuni infection, fecal microbiota transplantation, anti-inflammatory intervention strategies, human microbiota associated mice, host-pathogen-interactions
Citation: Heimesaat MM, Mrazek K and Bereswill S (2019) Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota. Front. Immunol. 10:2272. doi: 10.3389/fimmu.2019.02272
Received: 27 May 2019; Accepted: 09 September 2019;
Published: 24 September 2019.
Edited by:
Mats Bemark, University of Gothenburg, SwedenReviewed by:
Li Zhang, University of New South Wales, AustraliaDavid Tribble, Uniformed Services University of the Health Sciences, United States
Copyright © 2019 Heimesaat, Mrazek and Bereswill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus M. Heimesaat, markus.heimesaat@charite.de
 Markus M. Heimesaat
Markus M. Heimesaat Katharina Mrazek
Katharina Mrazek Stefan Bereswill
Stefan Bereswill