- 1School of Public Health, Shandong First Medical University and Shandong Academy of Medical Sciences, Taian, China
- 2Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China
- 3The Second Affiliated Hospital of Shandong First Medical University, Taian, China
- 4Centre for Precision Health, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia
- 5Department of Endocrinology, Taian City Central Hospital, Taian, China
Evidence from observational studies for the effect of tea consumption on obesity is inconclusive. This study aimed to verify the causal association between tea consumption and obesity through a two-sample Mendelian randomization (MR) analysis in general population-based datasets. The genetic instruments, single nucleotide polymorphisms (SNPs) associated with tea consumption habits, were obtained from genome-wide association studies (GWAS): UK Biobank, Nurses’ Health Study, Health Professionals Follow-up Study, and Women’s Genome Health Study. The effect of the genetic instruments on obesity was analyzed using the UK Biobank dataset (among ∼500,000 participants). The causal relationship between tea consumption and obesity was analyzed by five methods of MR analyses: inverse variance weighted (IVW) method, MR-Egger regression method, weighted median estimator (WME), weighted mode, and simple mode. Ninety-one SNPs were identified as genetic instruments in our study. A mild causation was found by IVW (odds ratio [OR] = 0.998, 95% confidence interval [CI] = 0.996 to 1.000, p = 0.049]), which is commonly used in two-sample MR analysis, indicating that tea consumption has a statistically significant but medically weak effect on obesity control. However, the other four approaches did not show significance. Since there was no heterogeneity and pleiotropy in this study, the IVW approach has the priority of recommendation. Further studies are needed to clarify the effects of tea consumption on obesity-related health problems in detail.
Introduction
Obesity is a nutrition-related metabolic disorder caused by genetic and environmental determinants (González-Muniesa et al., 2017; Blüher, 2019). Obesity and obesity-related diseases have been becoming major public health burdens worldwide. In the United States, the healthcare expense was about $1,901 per year for each obese person, which extrapolated to about $149.4 billion at the national level (Kim and Basu, 2016). Due to the continuous rise of incidence in the past 50 years, obesity has now reached pandemic proportion (Blüher, 2019; Chooi et al., 2019), and is predicted to be 20% by 2025 ((NCD Risk Factor Collaboration (NCD-RisC), 2016). Moreover, obesity increases the risk of various diseases, such as type 2 diabetes, cardiovascular disease, dementia and cancers (Blüher, 2019). In spite of the crucial role of diet and exercise in the treatment of obesity, supportive herbal remedies are of increasing concern (Liang et al., 2019).
Tea is one of the popular beverages globally, which is consumed up to 2 billion cups per day (Drew, 2019). Tea is considered an anti-obesity beverage attributed to three main components: tea polyphenols, tea polysaccharides, and caffeine (Wang et al., 2014; Xu et al., 2015; Chen et al., 2018b). Although growing researches have focused on the relationship between tea and anti-obesity, the findings are inconsistent (Jurgens et al., 2012; Baladia et al., 2014; Li X. et al., 2020; Lin et al., 2020).
Conventional epidemiological studies are susceptible to the potential confounders and inverse causality, which over-or under-estimate the causal relationship between determinants and outcomes. Mendelian randomization (MR) analysis is able to control the biases by introducing instrumental variables (Cao et al., 2019). In MR studies, genetic variants that are closely associated with exposure factors are defined as instrumental variables, by which the causations between exposures and disease outcomes are measured by genetic variants as substitution (Zhang et al., 2020). Since the formation of gametes follows the Mendelian law of “parental alleles randomly assigned to offspring”, genetic variation is not affected by traditional confounding factors and is associated with outcomes in a time-sequential manner (Emdin et al., 2017). In the current study, a two-sample MR analysis was used to assess the causal relationship between tea consumption and obesity in general population-based databases.
Materials and Methods
Datasets
Obesity is a chronic disorder featured by excessive adiposity and defined by body mass index (BMI) ≥ 30 kg/m2 (ICD10: E66) for adults (WHO, 2004). For the datasets of exposure, significant single nucleotide polymorphisms (SNPs) related to tea consumption (p < 5 × 10–6) were obtained from a genome-wide association study (GWAS) among ∼120,000 participants of European ancestry, which included participants from the UK Biobank, Nurses’ Health Study, Health Professionals Follow-up Study and Women’s Genome Health Study (Supplementary Table S1). The summary statistics associated with tea consumption from the largest GWAS consortium were used to control the bias of participant overlap as well as to increase the statistical power (Wang et al., 2020; Zhang et al., 2021b). Exposure data were collected using a 24 h recall questionnaire (Oxford WebQ), where tea consumption refers to drinking any type of tea without other substances (e.g., sugary). (Zhong et al., 2019). The linkage disequilibrium (LD) of significant SNPs linked to tea consumption was set to meet r2 < 0.001 to avoid the effect of strong LD on the results. The outcome datasets of obesity were obtained from the UK Biobank study which recruited about 500,000 European participants aged 37–73 years from 2006 to 2010. The relevant data were extracted from two datasets respectively, including SNP sites, alleles, effect estimates for exposure and outcome (BETA), standard error (SE), and p values.
Statistical Analysis
There are three premises for two-sample MR (Emdin et al., 2017; Greenland, 2018): 1) Genetic variation as an instrumental variable must be closely related to exposure. 2) Instrumental variables are not associated with any known confounders. 3) The instrumental variables are not directly related to the outcome, that is, the instrumental variables cannot affect the outcome in other ways except through the exposure factors (Supplementary Figure S1).
Prior to two-sample MR analysis, there is a need to unify the effect-value directions of exposure data and outcome data. Exposure and outcome data are unified into a dataset by removing the intermediate allele frequencies of SNPs containing palindromes (Hemani et al., 2018). In addition, SNPs with A/T or G/C alleles are defined as palindromic SNPs, “intermediate allele frequencies” referred to 0.01 < allele frequency < 0.30 (Hartwig et al., 2016).
Inverse variance weighted (IVW) method, MR-Egger regression method, weighted median estimator (WME), weighted mode, and simple mode were used to evaluate the causal effect between tea consumption and obesity, and subsequently checked the stability and reliability of the results. The IVW model is a weighted linear regression model, which is based on the premise that all genetic variants are valid instrumental variables (Yuan et al., 2020). MR-Egger regression method can obtain unbiased estimation when there is pleiotropy in instrumental variables, measure average pleiotropy through intercept term, and perform sensitivity analysis (Bowden et al., 2015). WME can still calculate the causal association effect when the genetic variation below 50% violates the core assumptions of MR (Bowden et al., 2016). The weighted mode is effective when the majority of instrumental variables are valid, even though other instrumental variables in the method do not meet the requirements of MR causal inference (Hartwig et al., 2017). The simple mode is a model-based estimation method that provides robustness for pleiotropy, although it is not as powerful as IVW (Milne et al., 2017).
Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) global test was used to appraise the pleiotropy and to identify the outliers (Verbanck et al., 2018). The intercept of the MR-Egger regression line illustrated the magnitude of the genetic pleiotropy. It was considered that there was no pleiotropic effect if no significant difference between intercept and 0 (p > 0.05) (Bowden et al., 2015). Cochran’s Q statistic and I2 statistic were used to assess the heterogeneity among the estimates from included SNPs. The funnel plot showed the relationship between the individual Wald ratio of each SNP and its accuracy, and whether its symmetry indicated whether the results had directional horizontal pleiotropy (Choi et al., 2020). The “leave-one-out” method was used for sensitivity analysis. By gradually eliminating each SNP and calculating the combined effect of the remaining SNPs, the influence of individual SNP on results and the stability of the results were evaluated (Mokry et al., 2016). With regard to the overlap between the participants from which these summary statistics were generated and the outcome dataset, the analyses of Bias and Type 1 Error Rate for Mendelian Randomization with Sample Overlap were carried out to assess the potential bias caused by population overlap (https://sb452.shinyapps.io/overlap/) (Burgess et al., 2016). Finally, F statistics (Eq. (1)) were used to test the strength of the association between the SNPs as instrumental variables and tea consumption, and an F statistic >10 indicates a lower risk of weak instrumental variable bias (Zhang Q. et al., 2021; Chen et al., 2021).
N represents the sample size; eaf represents effect allele frequency.
All data analyses were performed by the “TwoSampleMR” package in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). A power calculation was conducted using mRnd (https://cnsgenomics.com/shiny/mRnd/) (Brion et al., 2013). Statistical significance was set as two-tailed p < 0.05 unless otherwise specified.
Results
Instrumental Variable Selection
A total of 108 significant SNPs (p < 5 × 10–6, LD r2 < 0.001) were obtained from the GWAS about tea consumption (Zhong et al., 2019). Among them, 16 SNPs were removed for being palindromic with intermediate allele frequencies, and one SNP was removed because of no corresponding outcome data. Finally, 91 SNPs were selected to perform the following MR analysis.
The detailed information of these SNPs was shown in Supplementary Table S2, mainly including effect allele (EA), other allele (OA) and summary statistics (beta coefficient, SE, and p-value).
MR Analysis
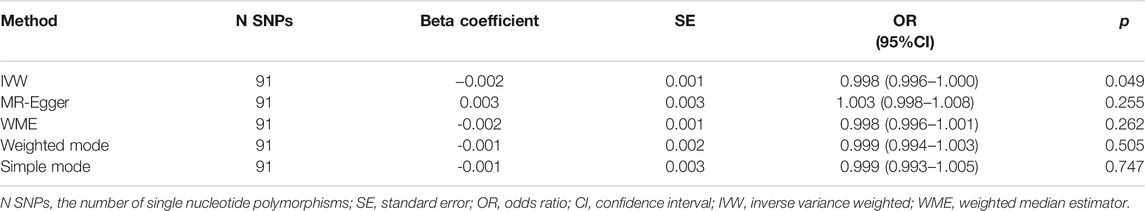
The causation between tea consumption and obesity was analyzed using the methods of IVW, MR Egger, WME, weighted mode, and simple mode, independently. As shown in Table 1 and Figure 1, a statistical significance was observed in IVW method analysis [odds ratio (OR) = 0.998, 95% confidence interval (CI) = 0.996 to 1.000, p = 0.049] (Table 1; Figures 1, 2). No significant causal relationships were observed in the analyses of MR Egger (OR = 1.003, 95% CI = 0.998 to 1.008, p = 0.255), WME (OR = 0.998, 95% CI = 0.996 to 1.001, p = 0.262), weighted mode (OR = 0.999, 95% CI = 0.994 to 1.003, p = 0.505) or simple mode (OR = 0.999, 95% CI = 0.993 to 1.005, p = 0.747) (Table 1; Figures 1, 2). In addition, a high statistical power (>85%) was identified in our study.
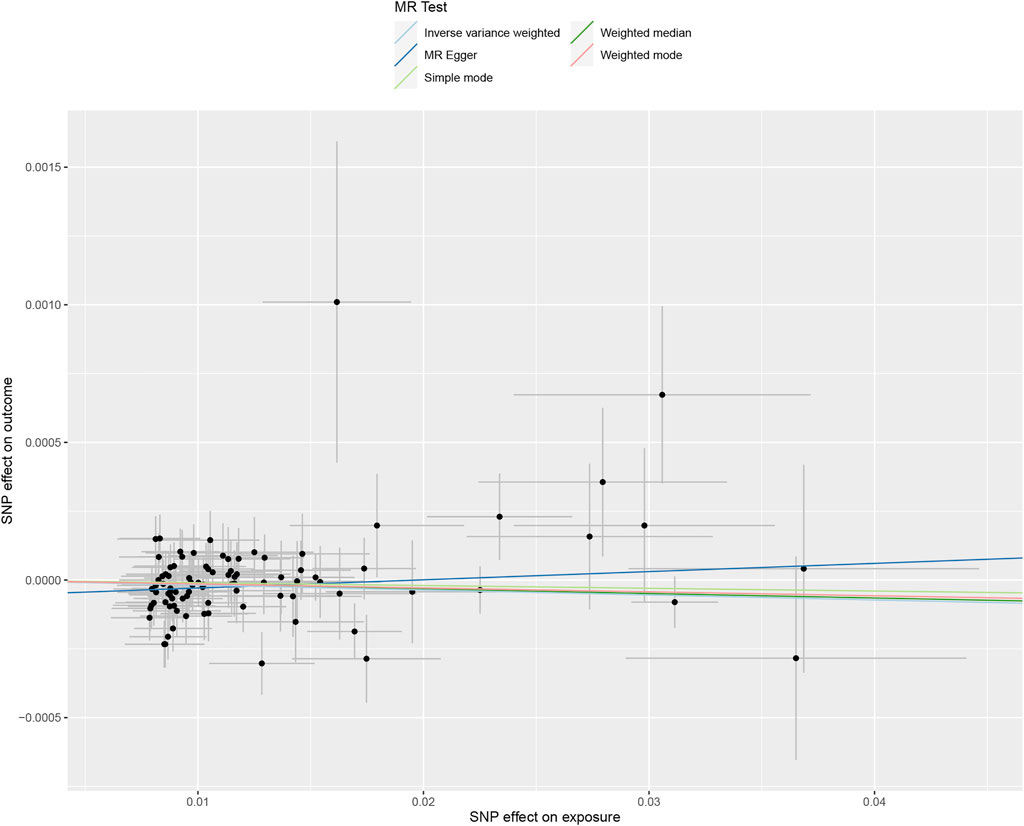
FIGURE 1. Scatter plot to visualize the causal effect between tea consumption and obesity. The slope of the straight line indicates the magnitude of the causal association, scatter plot of inverse variance weighted (IVW) method, MR-Egger regression method, weighted median estimator (WME), weighted mode and simple mode. MR, Mendelian randomization; SNP, single nucleotide polymorphism.
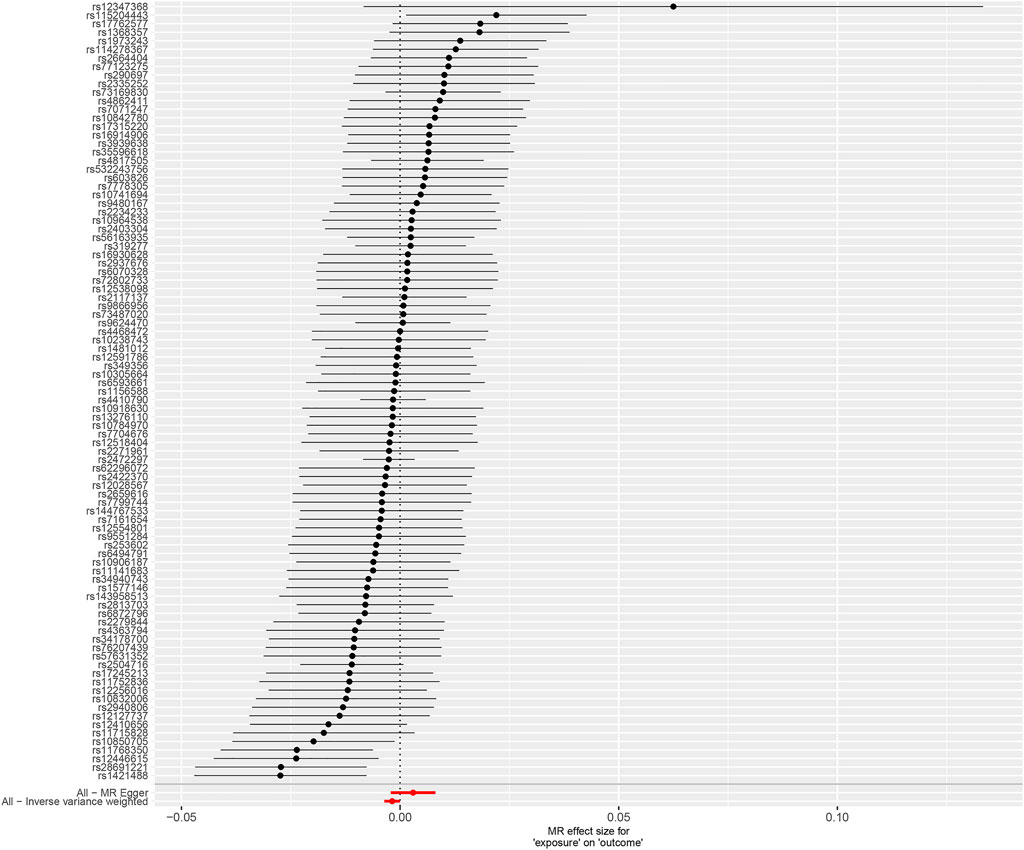
FIGURE 2. Forest plot to show the causal effect of tea consumption on obesity. Forest plot of IVW and MR-Egger regression method. MR, Mendelian randomization.
Sensitivity Analysis
The leave-one-out method displayed that the results of the current two-sample MR analysis were strong (Figure 3), indicating that no instrumental variables influenced the causal inference. Our Cochrane Q-test showed no significant heterogeneity across the estimates of included SNPs (p = 0.464, I2 = 0.61%). The funnel plot analysis illustrated a symmetry result (Figure 4), by which non-significance in directional and horizontal multipolarity was observed. And the genetic pleiotropy was not identified by the MR-Egger regression analysis (p = 0.053) or MR-PRESSO global test (p = 0.654), indicating that our findings were not influenced by the polymorphisms. As for population overlap, the overlap percentage was 17.17% (85852/500000). In addition, the bias and Type 1 error rate with sample overlap were <0.001 and 0.05 respectively, demonstrating that our results were less likely affected by sample overlap bias. Finally, no significant instrumental variable bias was observed by the F statistics (F > 10, ranging from 11.23 to 140.36).
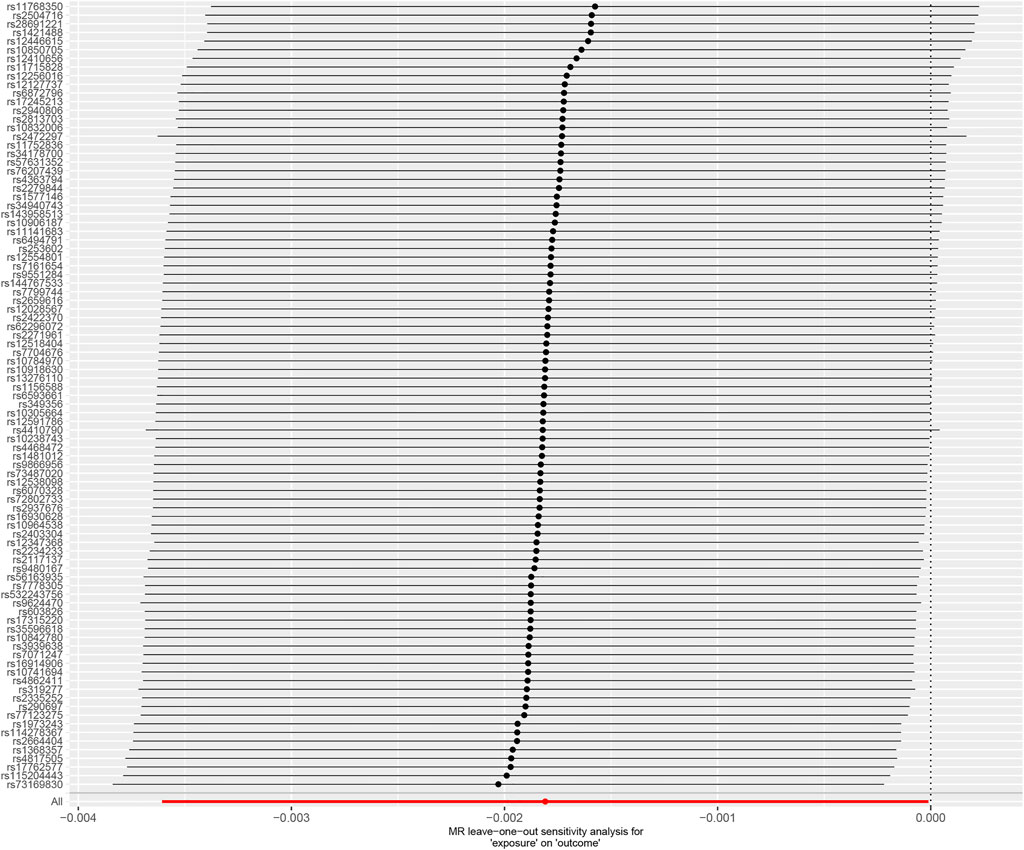
FIGURE 3. Forest plot of “leave-one-out” sensitivity analysis method to show the influence of individual SNP on the results. MR, Mendelian randomization.
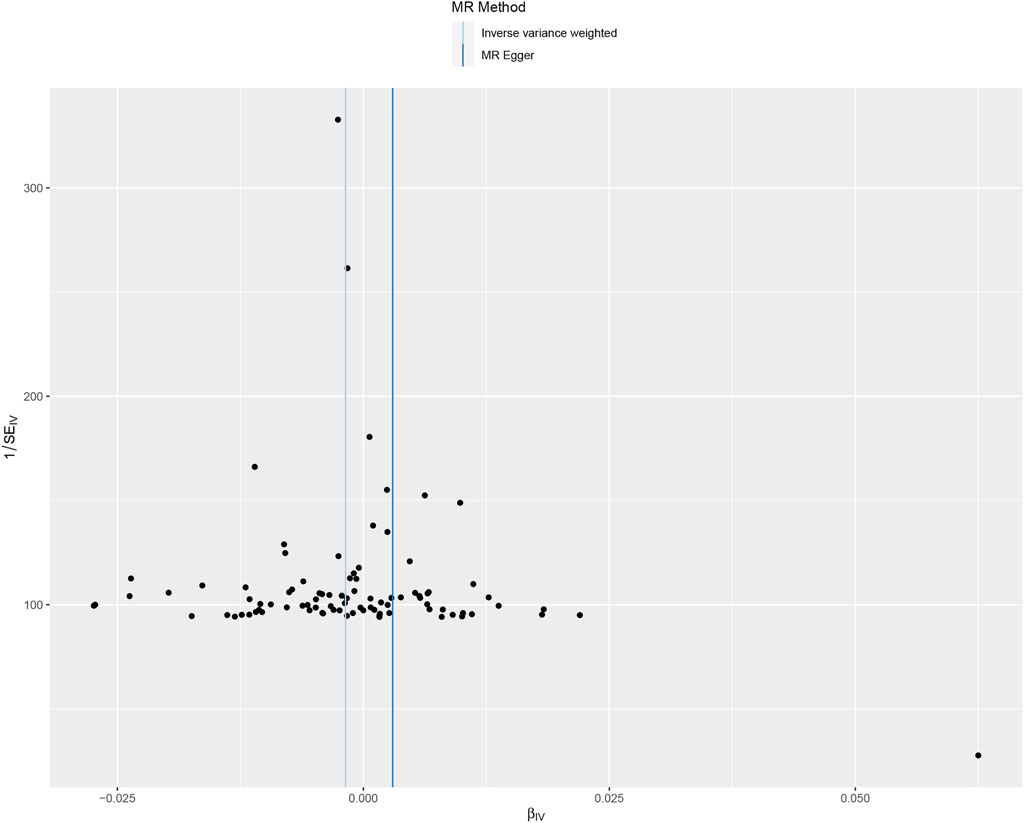
FIGURE 4. Funnel plot to visualize overall heterogeneity of Mendelian randomization assessment for the effect of tea consumption on obesity. MR, Mendelian randomization; SE, standard error; IV, instrument variable.
Discussion
Our two-sample MR analyses are conducted in five independent approaches. The result of IVW analysis evidenced that individuals drinking tea might have a 0.2% decrease in risk for obesity compared to those who do not. This mild causal relationship between tea consumption and obesity indicated that tea consumption has a statistically significant but medically weak effect on obesity control. The results of other approaches, including MR Egger, WME, weighted mode, and simple mode analyses, did not show causation between tea consumption and obesity. Since there was no heterogeneity and pleiotropy in this study, IVW results had the priority of recommendation. Besides, IVW is the most widely used and usually provides predominant results (Dan et al., 2020; Yuan et al., 2020).
It should be concerned whether instrumental variables in this study are associated with potential confounders. Firstly, the included SNPs were reported in the GWAS using statistical models on 24 h recall data, which were adjusted for age, sex, BMI, and top 20 principal components of population sub-structure (Zhong et al., 2019). Secondly, the GWAS studies for potential confounders (i.e., energy intake and expenditure, exercise, physical activity, and sleep duration) have been examined, consequently, there were no overlap SNPs between tea consumption and potential confounders (De Moor et al., 2009; Doherty et al., 2018; Jiang et al., 2018; Williams et al., 2021). Although there might be other confounder-associated SNPs that have not been reported in these studies, we did not observe a significant association between potential confounders and the instrumental variables selected in our study.
As a popular, economical, and safe drink, the effect of tea on obesity is widely understood, especially among overweight and obese individuals (Wang et al., 2014; Ahmad et al., 2015; Xu et al., 2015; Chen et al., 2018b). The potential mechanisms of tea on obesity are as follows: 1) reducing food intake and energy absorption (Yang et al., 2016), 2) regulating the expression of lipid metabolism genes and inhibiting fat accumulation (Chen et al., 2017), 3) enhancing the activity of antioxidant defense enzymes (Ren et al., 2015; Chen et al., 2018a), 4) regulating intestinal microflora disturbance and attenuating intestinal inflammation (Li Y. et al., 2020; Zhou et al., 2020), and 5) maintaining intestinal barrier integrity (Lu et al., 2019).
White adipose tissue (WAT) is one of the body’s adipose tissues, and its browning can increase the body’s energy expenditure. The consumption of tea could induce the browning of WAT through the activation of the AMP-activated protein kinase (AMPK) signaling pathway via the upregulation of uncoupling protein-1 (UCP-1) expression (Yamashita et al., 2014; Wu et al., 2018). In addition, tea can reduce fat synthesis by inhibiting fat synthases [fatty acid synthase (FAS) and stearoyl-CoA desaturase (SCD)] and fat synthesis transcription factor [sterol regulatory element-binding protein-1c (SrebP-1C)] (Li et al., 2016; Sun et al., 2019).
In recent years, a growing number of studies have begun to explore the association between tea consumption and obesity. For example, one RCT study found that the intake of black Chinese tea extract (BTE) (333 mg/day) before meals for 12 weeks induced a decrease in both BMI and weight (Kubota et al., 2011). Another study found that drinking 8 g of oolong tea per day could decrease body fat content (He et al., 2009). In addition, one cohort study published in 2021 [relative risk (RR) = 0.767, 95% CI = 0.738 to 0.796, p < 0.05] and two meta-analyses published in 2020 came up with the positive results of the effect of tea on obesity (Li X. et al., 2020; Lin et al., 2020; Zhang et al., 2021c). Our study, a two-sample MR analysis based on general population-based datasets, verified the causal relationship between tea consumption and obesity.
Traditional epidemiological studies, consisting of case-control studies and cohort studies, provide representative findings on the relationship between exposures and outcomes. However, these studies are usually biased by confounding factors and adverse causal effects (He et al., 2009; Vernarelli and Lambert, 2013; Cai et al., 2021). MR analysis can control the biases by introducing instrumental variables (Gage et al., 2018; Choi et al., 2020). MR analysis on general population-based datasets is a novel approach to provide evidence on causation. Our MR study based on the UK Biobank, Nurses’ Health Study, Health Professionals Follow-up Study and Women’s Genome Health Study validated that tea consumption has a mild causal relationship with obesity.
Limitations
There are inevitable limitations that should be notified. First, potential horizontal pleiotropy could not be comprehensively assessed even though multiple sensitivity analyses were performed. However, Cochran’s Q statistic, I2 statistics, MR-Egger intercept test, and MR-PRESSO global test found that there were no heterogeneity or pleiotropy in this MR analysis. Second, our study only checked the SNPs that were reported in the GWAS; the unpublished SNPs that are potentially associated with the confounders (i.e., energy intake and expenditure, exercise, physical activity, and sleep duration) might bias our findings. Third, we did not carry out subgroup analysis due to the lack of demographic information in detail. In the end, this study was on the basis of a European database. The differences in habit of tea drinking between Europeans and other races, as well as the variance within European populations living in different countries, may limit the generalizability of our results.
Conclusion
Our findings evidenced that tea consumption has a mild causal relationship with obesity in general population. More studies are needed to clarify the effects of tea and its components on obesity-related health problems.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
CL, MN, and ZG performed the acquisition of data. DL, SY, and ZG performed the data analysis and interpretation of data. CL, PL, YZ, and ZG drafted the article. WW, HH, MN, and YL performed the conception and design of the study. HH, MN, and YL performed the critical revision and final approval. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Shandong Provincial Natural Science Foundation, China (ZR2017MH100, ZR2017MH097), and Australia-China Collaborative Grant (NHMRC APP1112767-NSFC 81561128020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all researchers for sharing the GWAS pooled data on tea consumption and obesity.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.795049/full#supplementary-material
References
Ahmad, R. S., Butt, M. S., Sultan, M. T., Mushtaq, Z., Ahmad, S., Dewanjee, S., et al. (2015). Preventive Role of green tea Catechins from Obesity and Related Disorders Especially Hypercholesterolemia and Hyperglycemia. J. Transl Med. 13, 79. doi:10.1186/s12967-015-0436-x
Baladia, E., Basulto, J., Manera, M., Martínez, R., and Calbet, D. (2014). Effect of green tea or green tea Extract Consumption on Body Weight and Body Composition; Systematic Review and Meta-Analysis. Nutr. Hosp. 29 (3), 479–490. doi:10.3305/nh.2014.29.3.7118
Blüher, M. (2019). Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 15 (5), 288–298. doi:10.1038/s41574-019-0176-8
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 44 (2), 512–525. doi:10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 40 (4), 304–314. doi:10.1002/gepi.21965
Brion, M.-J. A., Shakhbazov, K., and Visscher, P. M. (2013). Calculating Statistical Power in Mendelian Randomization Studies. Int. J. Epidemiol. 42 (5), 1497–1501. doi:10.1093/ije/dyt179
Burgess, S., Davies, N. M., and Thompson, S. G. (2016). Bias Due to Participant Overlap in Two‐sample Mendelian Randomization. Genet. Epidemiol. 40 (7), 597–608. doi:10.1002/gepi.21998
Cai, J., Liu, S., Li, Y., Liu, Q., Xu, M., Mo, C., et al. (2021). Effects of Oil Tea on Obesity and Dyslipidemia: A Cross-Sectional Study in China. Dmso Vol. 14, 3173–3185. doi:10.2147/dmso.S312280
Cao, W., Li, X., Zhang, X., Zhang, J., Sun, Q., Xu, X., et al. (2019). No Causal Effect of Telomere Length on Ischemic Stroke and its Subtypes: A Mendelian Randomization Study. Cells 8 (2), 159. doi:10.3390/cells8020159
Chen, G., Wang, M., Xie, M., Wan, P., Chen, D., Hu, B., et al. (2018a). Evaluation of Chemical Property, Cytotoxicity and Antioxidant Activity In Vitro and In Vivo of Polysaccharides from Fuzhuan brick Teas. Int. J. Biol. Macromolecules 116, 120–127. doi:10.1016/j.ijbiomac.2018.04.184
Chen, G., Xie, M., Wan, P., Chen, D., Dai, Z., Ye, H., et al. (2018b). Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 66 (11), 2783–2795. doi:10.1021/acs.jafc.8b00296
Chen, L.-H., Chien, Y.-W., Liang, C.-T., Chan, C.-H., Fan, M.-H., and Huang, H.-Y. (2017). Green tea Extract Induces Genes Related to browning of white Adipose Tissue and Limits Weight-Gain in High Energy Diet-Fed Rat. Food Nutr. Res. 61 (1), 1347480. doi:10.1080/16546628.2017.1347480
Chen, L., Sun, X., Wang, Z., Lu, Y., Chen, M., He, Y., et al. (2021). The Impact of Plasma Vitamin C Levels on the Risk of Cardiovascular Diseases and Alzheimer's Disease: A Mendelian Randomization Study. Clin. Nutr. 40 (10), 5327–5334. doi:10.1016/j.clnu.2021.08.020
Choi, Y., Lee, S. J., Spiller, W., Jung, K. J., Lee, J.-Y., Kimm, H., et al. (2020). Causal Associations between Serum Bilirubin Levels and Decreased Stroke Risk. Atvb 40 (2), 437–445. doi:10.1161/atvbaha.119.313055
Chooi, Y. C., Ding, C., and Magkos, F. (2019). The Epidemiology of Obesity. Metabolism 92, 6–10. doi:10.1016/j.metabol.2018.09.005
Dan, Y.-L., Wang, P., Cheng, Z., Wu, Q., Wang, X.-R., Wang, D.-G., et al. (2020). Circulating Adiponectin Levels and Systemic Lupus Erythematosus: a Two-Sample Mendelian Randomization Study. Rheumatology (Oxford) 60, 940–946. doi:10.1093/rheumatology/keaa506
De Moor, M. H. M., Liu, Y.-J., Boomsma, D. I., Li, J., Hamilton, J. J., Hottenga, J.-J., et al. (2009). Genome-wide Association Study of Exercise Behavior in Dutch and American Adults. Med. Sci. Sports Exerc. 41 (10), 1887–1895. doi:10.1249/MSS.0b013e3181a2f646
Doherty, A., Smith-Byrne, K., Ferreira, T., Holmes, M. V., Holmes, C., Pulit, S. L., et al. (2018). GWAS Identifies 14 Loci for Device-Measured Physical Activity and Sleep Duration. Nat. Commun. 9 (1), 5257. doi:10.1038/s41467-018-07743-4
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian Randomization. Jama 318 (19), 1925–1926. doi:10.1001/jama.2017.17219
Gage, S. H., Bowden, J., Davey Smith, G., and Munafò, M. R. (2018). Investigating Causality in Associations between Education and Smoking: a Two-Sample Mendelian Randomization Study. Int. J. Epidemiol. 47 (4), 1131–1140. doi:10.1093/ije/dyy131
González-Muniesa, P., Mártinez-González, M.-A., Hu, F. B., Després, J.-P., Matsuzawa, Y., Loos, R. J. F., et al. (2017). Obesity. Nat. Rev. Dis. Primers 3, 17034. doi:10.1038/nrdp.2017.34
Greenland, S. (2018). An Introduction to Instrumental Variables for Epidemiologists. Int. J. Epidemiol. 47 (1), 358. doi:10.1093/ije/dyx275
Hartwig, F. P., Davey Smith, G., and Bowden, J. (2017). Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy assumption. Int. J. Epidemiol. 46 (6), 1985–1998. doi:10.1093/ije/dyx102
Hartwig, F. P., Davies, N. M., Hemani, G., and Davey Smith, G. (2016). Two-sample Mendelian Randomization: Avoiding the Downsides of a Powerful, Widely Applicable but Potentially Fallible Technique. Int. J. Epidemiol. 45 (6), 1717–1726. doi:10.1093/ije/dyx028
He, R.-r., Chen, L., Lin, B.-h., Matsui, Y., Yao, X.-s., and Kurihara, H. (2009). Beneficial Effects of Oolong tea Consumption on Diet-Induced Overweight and Obese Subjects. Chin. J. Integr. Med. 15 (1), 34–41. doi:10.1007/s11655-009-0034-8
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. Elife 7. doi:10.7554/eLife.34408
Jiang, L., Penney, K. L., Giovannucci, E., Kraft, P., and Wilson, K. M. (2018). A Genome-wide Association Study of Energy Intake and Expenditure. PLoS One 13 (8), e0201555. doi:10.1371/journal.pone.0201555
Jurgens, T. M., Whelan, A. M., Killian, L., Doucette, S., Kirk, S., and Foy, E. (2012). Green tea for Weight Loss and Weight Maintenance in Overweight or Obese Adults. Cochrane Database Syst. Rev. 2012, Cd008650. doi:10.1002/14651858.CD008650.pub2
Kim, D. D., and Basu, A. (2016). Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value in Health 19 (5), 602–613. doi:10.1016/j.jval.2016.02.008
Kubota, K., Sumi, S., Tojo, H., Sumi-Inoue, Y., I-chin, H., Oi, Y., et al. (2011). Improvements of Mean Body Mass index and Body Weight in Preobese and Overweight Japanese Adults with Black Chinese tea (Pu-Erh) Water Extract. Nutr. Res. 31 (6), 421–428. doi:10.1016/j.nutres.2011.05.004
Li, H., Kek, H. C., Lim, J., Gelling, R. W., and Han, W. (2016). Green tea (-)-Epigallocatechin-3-Gallate Counteracts Daytime Overeating Induced by High-Fat Diet in Mice. Mol. Nutr. Food Res. 60 (12), 2565–2575. doi:10.1002/mnfr.201600162
Li, X., Wang, W., Hou, L., Wu, H., Wu, Y., Xu, R., et al. (2020a). Does tea Extract Supplementation Benefit Metabolic Syndrome and Obesity? A Systematic Review and Meta-Analysis. Clin. Nutr. 39 (4), 1049–1058. doi:10.1016/j.clnu.2019.05.019
Li, Y., Rahman, S. U., Huang, Y., Zhang, Y., Ming, P., Zhu, L., et al. (2020b). Green tea Polyphenols Decrease Weight Gain, Ameliorate Alteration of Gut Microbiota, and Mitigate Intestinal Inflammation in Canines with High-Fat-Diet-Induced Obesity. J. Nutr. Biochem. 78, 108324. doi:10.1016/j.jnutbio.2019.108324
Liang, Y., Lin, C., Huang, S., and Xu, Y. (2019). Traditional Chinese Medicine and Intestinal Microbiota. Holist. Nurs. Pract. 33 (5), 259–265. doi:10.1097/hnp.0000000000000311
Lin, Y., Shi, D., Su, B., Wei, J., Găman, M. A., Sedanur Macit, M., et al. (2020). The Effect of green tea Supplementation on Obesity: A Systematic Review and Dose-Response Meta‐analysis of Randomized Controlled Trials. Phytotherapy Res. 34 (10), 2459–2470. doi:10.1002/ptr.6697
Lu, X., Liu, J., Zhang, N., Fu, Y., Zhang, Z., Li, Y., et al. (2019). Ripened Pu-Erh Tea Extract Protects Mice from Obesity by Modulating Gut Microbiota Composition. J. Agric. Food Chem. 67 (25), 6978–6994. doi:10.1021/acs.jafc.8b04909
Milne, R. L., Kuchenbaecker, K. B., Michailidou, K., Beesley, J., Kar, S., Lindström, S., et al. (2017). Identification of Ten Variants Associated with Risk of Estrogen-Receptor-Negative Breast Cancer. Nat. Genet. 49 (12), 1767–1778. doi:10.1038/ng.3785
Mokry, L. E., Ross, S., Timpson, N. J., Sawcer, S., Davey Smith, G., and Richards, J. B. (2016). Obesity and Multiple Sclerosis: A Mendelian Randomization Study. Plos Med. 13 (6), e1002053. doi:10.1371/journal.pmed.1002053
NCD Risk Factor Collaboration (NCD-RisC) (2016). Trends in Adult Body-Mass index in 200 Countries from 1975 to 2014: a Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. The Lancet 387 (10026), 1377–1396. doi:10.1016/s-0140-6736(16)30054-x10.1016/s0140-6736(16)30054-x
Ren, D., Hu, Y., Luo, Y., and Yang, X. (2015). Selenium-containing Polysaccharides from Ziyang green tea Ameliorate High-Fructose Diet Induced Insulin Resistance and Hepatic Oxidative Stress in Mice. Food Funct. 6 (10), 3342–3350. doi:10.1039/c5fo00557d
Sun, Y., Wang, Y., Song, P., Wang, H., Xu, N., Wang, Y., et al. (2019). Anti-obesity Effects of Instant Fermented Teas In Vitro and in Mice with High-Fat-Diet-Induced Obesity. Food Funct. 10 (6), 3502–3513. doi:10.1039/c9fo00162j
Verbanck, M., Chen, C.-Y., Neale, B., and Do, R. (2018). Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 50 (5), 693–698. doi:10.1038/s41588-018-0099-7
Vernarelli, J. A., and Lambert, J. D. (2013). Tea Consumption Is Inversely Associated with Weight Status and Other Markers for Metabolic Syndrome in US Adults. Eur. J. Nutr. 52 (3), 1039–1048. doi:10.1007/s00394-012-0410-9
Wang, S., Moustaid-Moussa, N., Chen, L., Mo, H., Shastri, A., Su, R., et al. (2014). Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 25 (1), 1–18. doi:10.1016/j.jnutbio.2013.09.001
Wang, X., Liu, Z., and Liu, W. (2020). Does Cannabis Intake Protect against Non-alcoholic Fatty Liver Disease? A Two-Sample Mendelian Randomization Study. Front. Genet. 11, 949. doi:10.3389/fgene.2020.00949
WHO (2004). ICD-10 : International Statistical Classification of Diseases and Related Health Problems : Tenth Revision, 2nd ed [Online]. World Health Organization. Available: https://apps.who.int/iris/handle/10665/42980.
Williams, C. J., Li, Z., Harvey, N., Lea, R. A., Gurd, B. J., Bonafiglia, J. T., et al. (2021). Genome Wide Association Study of Response to Interval and Continuous Exercise Training: the Predict-HIIT Study. J. Biomed. Sci. 28 (1), 37. doi:10.1186/s12929-021-00733-7
Wu, L., Zhang, L., Li, B., Jiang, H., Duan, Y., Xie, Z., et al. (2018). AMP-activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 9, 122. doi:10.3389/fphys.2018.00122
Xu, Y., Zhang, M., Wu, T., Dai, S., Xu, J., and Zhou, Z. (2015). The Anti-obesity Effect of green tea Polysaccharides, Polyphenols and Caffeine in Rats Fed with a High-Fat Diet. Food Funct. 6 (1), 296–303. doi:10.1039/c4fo00970c
Yamashita, Y., Wang, L., Wang, L., Tanaka, Y., Zhang, T., and Ashida, H. (2014). Oolong, Black and Pu-Erh tea Suppresses Adiposity in Mice via Activation of AMP-Activated Protein Kinase. Food Funct. 5 (10), 2420–2429. doi:10.1039/c4fo00095a
Yang, C. S., Zhang, J., Zhang, L., Huang, J., and Wang, Y. (2016). Mechanisms of Body Weight Reduction and Metabolic Syndrome Alleviation by tea. Mol. Nutr. Food Res. 60 (1), 160–174. doi:10.1002/mnfr.201500428
Yuan, S., Kar, S., Vithayathil, M., Carter, P., Mason, A. M., Burgess, S., et al. (2020). Causal Associations of Thyroid Function and Dysfunction with Overall, Breast and Thyroid Cancer: A Two‐sample Mendelian Randomization Study. Int. J. Cancer 147 (7), 1895–1903. doi:10.1002/ijc.32988
Zhang, Q., Zhang, X., Zhang, J., Wang, B., Meng, X., Tian, Q., et al. (2021a). Causal Relationship between Lung Function and Atrial Fibrillation: A Two Sample Univariable and Multivariable, Bidirectional Mendelian Randomization Study. Front. Cardiovasc. Med. 8, 769198. doi:10.3389/fcvm.2021.769198
Zhang, X., Tian, Q., Liu, D., Geng, T., Xu, X., Ge, S., et al. (2020). Causal Association of Circulating Cholesterol Levels with Dementia: a Mendelian Randomization Meta-Analysis. Transl Psychiatry 10 (1), 145. doi:10.1038/s41398-020-0822-x
Zhang, Y., Liu, Z., Choudhury, T., Cornelis, M. C., and Liu, W. (2021b). Habitual Coffee Intake and Risk for Nonalcoholic Fatty Liver Disease: a Two-Sample Mendelian Randomization Study. Eur. J. Nutr. 60 (4), 1761–1767. doi:10.1007/s00394-020-02369-z
Zhang, Y., Yang, H., Li, S., Li, W.-d., and Wang, Y. (2021c). Consumption of Coffee and tea and Risk of Developing Stroke, Dementia, and Poststroke Dementia: A Cohort Study in the UK Biobank. Plos Med. 18 (11), e1003830. doi:10.1371/journal.pmed.1003830
Zhong, V. W., Kuang, A., Danning, R. D., Kraft, P., van Dam, R. M., Chasman, D. I., et al. (2019). A Genome-wide Association Study of Bitter and Sweet Beverage Consumption. Hum. Mol. Genet. 28 (14), 2449–2457. doi:10.1093/hmg/ddz061
Keywords: tea consumption, obesity, mendelian randomization analysis, causal association, single nucleotide polymorphism
Citation: Li C, Niu M, Guo Z, Liu P, Zheng Y, Liu D, Yang S, Wang W, Li Y and Hou H (2022) A Mild Causal Relationship Between Tea Consumption and Obesity in General Population: A Two-Sample Mendelian Randomization Study. Front. Genet. 13:795049. doi: 10.3389/fgene.2022.795049
Received: 14 October 2021; Accepted: 25 January 2022;
Published: 24 February 2022.
Edited by:
Ayo Priscille Doumatey, National Institutes of Health (NIH), United StatesReviewed by:
Martha Guevara-Cruz, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoKei Hang Katie Chan, City University of Hong Kong, Hong Kong SAR, China
Karlijn A. C. Meeks, National Human Genome Research Institute (NHGRI), United States
Copyright © 2022 Li, Niu, Guo, Liu, Zheng, Liu, Yang, Wang, Li and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyun Niu, fyniumingyun@126.com; Haifeng Hou, hfhou@163.com; Yuanmin Li, liym575@126.com
†These authors have contributed equally to this work
 Cancan Li
Cancan Li Mingyun Niu3†*
Mingyun Niu3†* Zheng Guo
Zheng Guo Pengcheng Liu
Pengcheng Liu Yulu Zheng
Yulu Zheng Di Liu
Di Liu Wei Wang
Wei Wang Yuanmin Li
Yuanmin Li Haifeng Hou
Haifeng Hou