- 1Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 2Department of Pathology and Forensic Medicine, Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil
- 3Department of Biomaterials, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 4Faculty of Medicine, Institute for Infection Prevention and Hospital Epidemiology, Medical Centre, University of Freiburg, Freiburg, Germany
- 5Department of Biochemistry and Genetics, La Trobe Institute of Molecular Science, La Trobe University, Bundoora, VIC, Australia
- 6Department of Molecular Biotechnology and Health Sciences, University of Turin, Torino, Italy
Extracellular vesicles (EVs) are heterogeneous populations of nano- and micro-sized vesicles secreted by various cell types. There is mounting evidence that EVs have widespread roles in transporting proteins, lipids, and nucleic acids between cells and serve as mediators of intercellular communication. EVs secreted from stem cells could function as paracrine factors, and appear to mimic and recapitulate several features of their secreting cells. EV-mediated transport of regulatory RNAs provides a novel source of trans-regulation between cells. As such, stem cells have evolved unique forms of paracrine mechanisms for recapitulating their potencies with specialized functions by transporting non-coding RNAs (ncRNAs) via EVs. This includes the dissemination of stem cell-derived EV-ncRNAs and their regulatory effects elicited in differentiation, self-renewal, pluripotency, and the induction of reparative programs. Here, we summarize and discuss the therapeutic effects of mesenchymal stem cell-derived EV-ncRNAs in the induction of intrinsic regenerative programs elicited through regulating several mechanisms. Among them, most noticeable are the EV-mediated enrichment of ncRNAs at the injury sites contributing the regulation of matrix remodeling, epithelial mesenchymal transitions, and attraction of fibroblasts. Additionally, we emphasize EV-mediated transmission of anti-inflammatory RNAs from stem cells to injury site that potentially orchestrate the resolution of the inflammatory responses and immune alleviation to better facilitate healing processes. Collectively, this knowledge indicates a high value and potential of EV-mediated RNA-based therapeutic approaches in regenerative medicine.
Introduction
Extracellular vesicles (EVs) comprise a heterogeneous population of nano- and micro-sized vesicles secreted by virtually all cell types studied so far (Yáñez-Mó et al., 2015; Mateescu et al., 2017). The best-described EVs are the exosomes and microvesicles, which differ in their respective sizes, shapes, and origins. Exosomes are produced through the endocytic pathway, followed by the fusion of multivesicular endosomes with the plasma membrane where they are then released into the extracellular space (for detailed mechanisms see Nawaz et al., 2014). During the process of their biogenesis, EVs acquire repertoire of bioactive cargo such as proteins and lipids (Keerthikumar et al., 2016), coding- and non-coding RNAs (ncRNAs) including both microRNAs and long noncoding RNAs (miRNAs, lncRNAs) (reviewed by Fatima and Nawaz, 2017c), and presumably DNA (Thakur et al., 2014). The secreted EVs serve as mediators of intercellular communication (Ratajczak et al., 2006b; Mathivanan et al., 2010; Nawaz and Fatima, 2017), could disseminate biological information between cells and contribute as paracrine factors in health and disease (Bellingham et al., 2012; Buzas et al., 2014; Hoshino et al., 2015). EVs not only exchange biological material between neighboring cells but can also travel long distances, allowing the dissemination of genetic content between distal organs and regulate gene expression of host tissues (Fatima and Nawaz, 2017a; Thomou et al., 2017).
A complete understanding of stem cell biology is a prerequisite for gaining mechanistic insights into human diseases. The current applications of stem cells in translational medicine take advantage of their potency for regeneration and repairing tissue damage. The best studied in this context are the mesenchymal stem cells (MSCs). According to the International Society for Cellular Therapy, MSCs are defined as plastic adherent cells with the capacity to differentiate into osteoblasts, chondrocytes, myocytes, and adipocytes (Dominici et al., 2006). MSCs express markers such as CD73, CD90, and CD105, and lack the expression of several markers including CD14, CD34, CD45, or CD11b, CD79-α, or CD19 and HLA-DR surface molecules (Dominici et al., 2006).
The commonly considered sources of MSCs are bone marrow (BM), adipose tissue, the umbilical cord, nervous tissue, dental pulp, amniotic fluid, the placenta, and menstrual blood (Hass et al., 2011; Eirin et al., 2014). MSCs derived from these sources represent remarkable differences in morphology, proliferation, self-renewal ability, and differentiation potential (Dominici et al., 2006). Interestingly, their capacity to differentiate toward osteoblasts, chondrocytes, myocytes, and adipocytes, coupled with their ability to stay activated during injury and colonization to injury site offer a promising source in tissue regeneration. Although, the differentiation potential of MSCs is considerably less than that of embryonic stem cells (ESCs) as well as from induced pluripotent stem cells (iPS), they nevertheless hold greater promise for cell-based clinical applications (Uccelli et al., 2008). The benefits of MSC-based therapies are evident from their success in ameliorating the symptoms of many diseases including, diabetes, osteoarthritis, spinal cord injury, myocardial injury, graft vs. host disease, and bone repair shown in many clinical and preclinical models (Wei et al., 2013). The emerging biology of stem cells suggests that colonizing activity at the injury site is not always required and stem cells can extend their therapeutic effects in part via secreted paracrine factors at the site of injury (Nagaishi et al., 2016).
Recent studies propose that at least a part of MSC effects are mediated by MCS-derived EVs (Deregibus et al., 2007; Lai et al., 2016). MSC-derived EVs were tested in human patients with therapy-refractory graft-vs.-host disease. In this small study, using MSC-derived EVs from bone marrow donors to treat these patients, it was concluded that the MSC EVs were safe as well as effective in treating the disease (Kordelas et al., 2014). This study partly confirmed the direct application of MSC-derived EVs as an effective immune-suppressive factor. However, such therapeutic effects need to be confirmed in more patients.
NcRNAs are non-protein coding RNAs, which represent part of the genome that does not encode genetic information into proteins. In principle, ncRNAs are broadly categorized into short ncRNAs and long ncRNAs (lncRNAs) or long intergenic ncRNA (lincRNA). Several ncRNA species exist within the genome such as Piwi-interacting RNAs (piRNAs), small nuclear, and nucleolar RNAs (snRNAs, snoRNAs), and short interfering RNAs (siRNAs) among others described elsewhere (Fatima and Nawaz, 2017c). It is well-established that about 90% of the genome sequence is actively transcribed, but the translated proportion is <2% of the whole genome, which has been considered as “junk DNA.” But, the Encyclopedia of DNA Elements (ENCODE) project has revealed that more than 90% of the human genome contains functional ncRNAs (ENCODE Project Consortium, 2004, 2007), and thus untranslated fraction of the genome is no longer considered to be entirely without function (Lee, 2012).
The frequently studied class of ncRNAs are the miRNAs, which are precisely regulated during developmental processes. It is estimated that miRNAs regulate ~30% of all protein-coding genes and are fundamental in shaping the global transcriptome of eukaryotes (Filipowicz et al., 2008; Grosshans and Filipowicz, 2008). The miRNAs are customarily known to regulate gene expression at post-transcriptional level governing several key cellular pathways related to development, differentiation and cellular fates (Pasquinelli and Ruvkun, 2002; Ambros, 2003, 2004; Ivey and Srivastava, 2010). The contribution of ncRNAs in regulating healing and repair process of stem cells is now well-accepted and is thought to be more sophisticated than earlier studies suggested (Ounzain et al., 2013; Ounzain and Pedrazzini, 2015; Zhou et al., 2016). There is evolving evidence implicating stem cell-derived EVs in the maintenance of stem cell characteristics such as self-renewal, differentiation, maturation and cell fate determination (reviewed in Nawaz et al., 2016b). However, the roles of EV-derived ncRNA are only recently beginning to be explored. An increasing body of evidence has clarified that EV-ncRNAs could serve as potential mediators of the extended paracrine effects of stem cells. Since ncRNAs are central to gene regulation and cellular fates, it can be speculated that most of the EV-mediated regulatory roles elicited in cells/organs are mediated through ncRNAs. The ncRNAs are expressed in a tissue-specific manner, precisely regulated and actively involved in variety of developmental processes (Pasquinelli and Ruvkun, 2002; Ambros, 2003; Carrington and Ambros, 2003; Marson et al., 2008; Gangaraju and Lin, 2009; Pauli et al., 2011; Fatica and Bozzoni, 2014; Perry and Ulitsky, 2016). Lineage specific commitments of stem cells and the maintenance of their characteristic features such as pluripotency, self-renewal, differentiation, and efficiency of cellular reprogramming are largely regulated by ncRNAs (Dinger et al., 2008; Judson et al., 2009; Loewer et al., 2010; Shenoy and Blelloch, 2014; Xu et al., 2016; Deng et al., 2017; Hou et al., 2017; Wei et al., 2017; Zhang W. et al., 2017). Thus, the ncRNAs may govern the equilibrium between pluripotency and differentiation in the embryo and embryonic stem cells, and lineage specific fate decisions (Ivey and Srivastava, 2010; Flynn and Chang, 2014). Recent studies have shown that MSC-derived EVs are enriched in distinct ncRNA species such as miRNAs, tRNA, and Piwi-interacting RNA (piRNAs) which contribute to maintaining stem cell potency (Baglio et al., 2015), induction of cell survival and inhibition of cell differentiation of cord blood hematopoietic stem cells (De Luca et al., 2016). The comparison of transcriptomic (RNA-Seq) and proteomic profiles of ESC-derived EVs and EVs from human bone-marrow (BM-MSC) revealed distinctly different RNA profiles between EVs of two stem cell populations (Billing et al., 2016).
Stem Cell-Derived ncRNAs and EVs: Allies in Stem Cell Potency
Evidence now exists to support that EVs mimic several features of their parent cells and profoundly contribute to stem cell fate decisions (Nawaz et al., 2016b). Ratajczak and colleagues provided the first evidence that stem cell-derived EVs contain mRNA transcripts for pluripotent transcriptional factors such as HoxB4, Nanog, Oct-4, and Rex-1, which can be horizontally transferred to recipient cells, supporting hematopoietic progenitor cells expansion (Ratajczak et al., 2006a). EV-mediated transfer of miRNAs downregulate vascular cell adhesion molecule (VCAM1) expression, contributing to hematopoietic progenitor cell mobilization (Salvucci et al., 2012).
Quesenberry and colleagues proposed that EV-mediated communication and exchange of genetic material is the continuum model of stem cell biology, where the differentiation decision of stem cells is conditioned by the cell cycle transit and environmental stimuli (Quesenberry et al., 2010). It is tempting to speculate that the dependency of progenitor cell differentiation and lineage commitment could be reprogrammed by continuous flow of genetic material bidirectionally between progenitors and differentiated cells (Nawaz et al., 2016b). Stem cells preferably keep population equilibrium between progenitors and the differentiated mature cells. Thus, a deficiency of mature cells in a particular tissue could be sensed by progenitors, which produce more progenies to be differentiated into mature cells. As such, this equilibrium could be facilitated by EV-mediated bidirectional exchange of genetic material, which favors stem cell populations to maintain a stable co-existence (Nawaz et al., 2016b).
The secretion of a selective pattern of miRNAs from stem cells and their transfer to target cells via EVs raises enormous potential for stem cells to recapitulate lineage specific characteristics (Collino et al., 2010; Guo et al., 2011). Additional roles for EV-miRNAs in differentiation are observed where EV-miR-486 delivery confers a rapid response to hypoxia in erythroleukemia cells by targeting Sirt1 gene, and modulates hypoxia-induced erythroid differentiation (Shi et al., 2017). Likewise, ESC-derived EVs could transport selective subset of miRNA and transcriptional factor related mRNAs which may induce pluripotency in their target cells and turn on early retinogenic program of differentiation (Katsman et al., 2012). It is increasingly being recognized that stem cells have evolved mechanisms for maintaining stem cell specific features at least, in part through EV-mediated dissemination of ncRNAs (Figure 1).
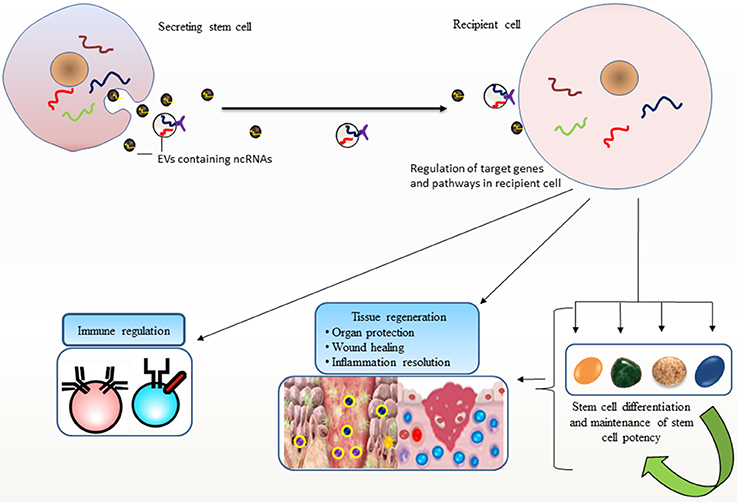
Figure 1. Stem cell potency and differentiation: Stem cells secrete extracellular vesicles (EVs) carrying non-coding RNAs (ncRNAs) that are transported to other cells. Such horizontal transfer is implicated in recapitulating variety of stem cell features in recipient cells, such as pluripotency, differentiation, and stem cell maintenance and their ability to facilitate regenerative processes. EV-mediated transport of ncRNAs elicits regulatory programs in recipient cells; maintain tissue homeostasis and immune regulation that may favor repair processes.
Tissue Regeneration and Organ Protection
The secretion of EVs from biologically active cells may be context dependent i.e., relating to disease progression or induction of regenerative programs (Fatima and Nawaz, 2015). As such, EV-mediated transportation of stem cell-derived ncRNA to the injured sites is considered one of the versatile regulatory routes of tissue regeneration and organ protection. This section will discuss roles of EVs in mediation of paracrine effects and the mechanisms in the context of tissue remodeling and repairing injuries.
Matrix Remodeling and Inhibition of Epithelial-Mesenchymal-Transition
MSC-derived EVs are demonstrated to optimize the matrix elements by activation of collagen regulation synthesis by stromal fibroblasts, which further support the healing processes (Zhang et al., 2015; Hu et al., 2016). MSCs transfer miR-125a to endothelial cells via EVs, which promotes the formation of endothelial tip cells and angiogenesis by repressing angiogenic inhibitor delta-like 4 (DLL4; Liang et al., 2016). Additionally, MSC-derived EVs containing miRNAs could inhibit the TGF-β/SMAD2 pathway and suppress myofibroblast differentiation during wound healing (Fang et al., 2016). The wound healing process is mainly facilitated by endothelial cell proliferation and fibroblast activation for which growth factors play a central role. Notably, the platelet-rich plasma (PRP) is rich source of growth factors and has a widespread role in repairing chronic wounds mainly through endothelial cell activation and angiogenesis. The role of PRP-derived EVs bearing the cargo of growth factors is much appreciated for the induction of fibroblast and endothelial cell proliferation and migration which favor angiogenesis and re-epithelialization in chronic wounds (Guo et al., 2017).
While the proliferation of fibroblasts facilitates matrix remodeling in favor of tissue repair, the excess number of fibroblasts may cause the thickening of the tissue and hinder the repair process. Epithelial-mesenchymal-transition (EMT) holds a central role in fibroblast functionality. In fact, EMT promotes the genesis of fibroblasts where the excess of fibroblasts may exhibit the phenomenon of organ fibrosis with deleterious effects in adult tissues (Kalluri and Neilson, 2003). Therefore, fibroblast optimization is essential for repairing defects, whereby inhibition of EMT potentially supports tissue repair (Câmara and Jarai, 2010; Xi et al., 2014). Recent studies show that MSC-derived EVs influence the inhibition of EMT during injuries in order to favor the healing process. In two concordant studies it was shown that the proximal tubular epithelial cells (PTEC) treated with TGF-β1 may repress E-cadherin and exhibit EMT associated morphological changes, whereas the cells administered with MSC-derived EVs may reverse the morphological changes by resuming the E-cadherin expression; allowing the protection of mice against renal failure (He et al., 2015; Wang et al., 2015a).
Notably, EVs from BM-MSCs demonstrate inhibitory effects on TGF-β1-mediated EMT in renal PTEC cells in-vivo (Wang et al., 2015a). This is interesting to consider that EMT inhibition was stronger in younger rats than older rats, indicating that renal protection is more active during young age and less in older age, and may play a role in the fibrosis of aging renal tissues (Wang et al., 2015a). In principle, such roles of EVs are projected by transferring selective patterns of miRNAs from MSCs to the injured renal PTEC cells, which inhibit EMT (He et al., 2015; Wang et al., 2015a). As stated earlier, EMT inhibition is potential choice for supporting tissue regenerative reprograms; EVs may serve new vehicle for inhibition of EMT and might be a useful therapeutic strategy in regenerative processes.
Transcriptional Repression of Apoptotic Genes: Role of Anti-Apoptotic miRNAs
The contribution of stem cell-derived EVs is increasingly being recognized in ameliorating organ functions through preventing cell death, promoting cell survival, and progenitor's self-renewal. MSC-derived EVs transfer anti-apoptotic miRNAs to injured cells, which not only promote cell survival but may also elicit transcriptional modulation of target genes in injured renal epithelial cells as noticed in in-vitro as wells in-vivo models of kidney injury (Lindoso et al., 2014; Cantaluppi et al., 2015). More evidence describe that the MSC-derived EVs could transfer anti-apoptotic miRNAs to cardiomyocytes, which transcriptionally repress apoptotic genes in cardiomyocytes and facilitate cell survival with enhanced angiogenesis, ultimately ameliorating the cardiomyocytes functions (Yu et al., 2013; Wang et al., 2015b, 2017). Similarly, EVs from cardiac progenitor cells (CPCs) transport anti-apoptotic miRNAs, which target apoptotic genes and inhibit apoptosis in cardiomyocytes, stimulate tube formation in endothelial cells, which favor angiogenesis, and thus improve heart function (Barile et al., 2014; Xiao et al., 2016). Agarwal and colleagues reported miRNA-mediated reparative potential of CPC-derived EVs from pediatric patients (Agarwal et al., 2017). This study demonstrates that the functional improvements are associated with increased angiogenesis, reduced fibrosis, and improved hypertrophy, resulting in improved cardiac function and such outcomes are linked to miRNA functions. Shao et al. (2017) recently reported that MSCs and MSC-derived EVs exhibit similar miRNA expression profile, which could be one of the reasons that MSC-derived EVs can replace MSCs for cardiac repair. This indicates that that MSC-derived EVs could be used alone to promote cardiac repair and are superior to MSCs in repairing injured myocardium. Wang and colleagues argue that endometrium MSCs (EnMSCs) confer superior cardioprotection as compared to BM-MSCs or adipose tissue derived MSCs (AD-MSCs) showing miR-21 as a potential mediator of EnMSC therapy (Wang et al., 2017).
Recently a study has demonstrated that EVs secreted from cardiosphere-derived cells are highly enriched in Y RNA fragment (EV-YF1) (Cambier et al., 2017). EVs transfer YF1 to macrophages (YF1 transfection of macrophages) thereby inducing the IL-10 transcription and secretion of IL-10 which ameliorates cardiomyocyte functions. Interestingly, EV-YF1-primed macrophages co-cultured with rat cardiomyocytes were potently protective against oxidatively stressed cardiomyocytes through induction of IL-10 expression. Additionally, the in-vivo intracoronary injection of EV-YF1 following ischemia/reperfusion reduced the infarct size (Cambier et al., 2017). Interestingly, a profound regenerative potential of cardiac stem cells (CSCs)-derived EVs has been demonstrated in a mice model of cardiomyopathy (Vandergriff et al., 2015). This study demonstrates the double advantage of EVs; firstly, the mice used in experiment was immunocompetent, but showed no adverse immune reaction against therapeutic EVs. Secondly, although mice received heterologous source of CSC-derived EVs (i.e., from human CSCs) but no cross reactivity was observed between EVs of two different sources.
Cellular Differentiation, Reprogramming, and Induction of Repair Programs
EVs derived from cells relay the stem cell messages to induce regeneration, resistance to apoptosis, and the induction of intrinsic repair cascades of injured cells. It has been shown that EVs secreted by MSCs undergoing osteogenic differentiation differs in the content of miRNAs compared to undifferentiated MSCs (Xu et al., 2014). These EVs are osteoinductive and are involved in RNA surveillance pathway, Wnt signaling, and RNA transport contributing to regulation of osteogenic differentiation (Xu et al., 2014). EVs carrying miR-196a from BM-MSCs govern the expression of osteogenic genes during osteoblasts differentiation (Qin et al., 2016). Additionally, EVs mediate a dialogue between osteoblasts and adipocytes during adipocyte and osteoblast differentiation. It has recently been suggested that the adipocyte/osteoblast balance is profoundly regulated at transcriptional level aided by EV-mediated transmission of miRNAs (Martin et al., 2015). EVs derived from miR-140-5p overexpressing human synovial MSCs could enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model by regulating Wnt signaling and by blocking the side effects of ECM secretion (Tao et al., 2017b).
EV-mediated transfer of miRNAs to CPCs could induce glycolytic switch within CPCs, which supports adaptation to hypoxic stress in cardiovascular tissues (Ong et al., 2014). Interestingly, EVs released from glucose-treated ECs contained significantly lower amounts of miR-126 and elicited reduced endothelial repair capacity in-vitro and in-vivo. This indicates that under diabetic or hyperglycemic conditions EVs may exhibit poor capacity to vascular endothelial repair. Moreover, the expression analysis of miR-126 in circulating EVs from patients with stable coronary artery disease with and without diabetes mellitus revealed significantly reduced miR-126 expression in EVs from diabetic patients (Jansen et al., 2013). In fact, EVs released from apoptotic endothelial cells (ECs) transfer miR-126 and influence the repair of recipient human coronary artery ECs (Jansen et al., 2013). Endothelial progenitor cells have also been shown to exhibit cellular reprograming by transferring EV-miRNAs to renal cells which protect kidney against ischemia reperfusion injury (Cantaluppi et al., 2012). EVs from pericardial fluid (secreted from heart) could mediate vascular repair responses in ECs by transferring pericardial fluid-miRNAs and inhibiting TGF-BR1 (Beltrami et al., 2017). This improves the survival, proliferation, and networking of ECs and could restore the angiogenic capacity of ECs, which promotes post-ischemic blood flow recovery in-vivo (Beltrami et al., 2017).
A recent study demonstrates that aging in mice initiates with a substantial loss of hypothalamic stem/progenitor cells (Zhang Y. et al., 2017). Conversely, aging retardation and lifespan extension were achieved in mid-aged mice that were locally implanted with healthy hypothalamic stem cells. This effect (i.e., slowing of aging) is mediated by stem cell-derived exosomal miRNAs in the cerebrospinal fluid and concomitant regulation of aging factors in the brain microenvironment (Zhang Y. et al., 2017). Additionally, EV-miRNAs from MSCs are transferred to neural cells where they could regulate nerve growth and exhibit neuro-protective effects in in-vivo animal models (Xin et al., 2012; Cui et al., 2016). Stem cell-derived EVs have also been implicated in the protection of eye functions during/and after laser injuries. In fact, MSC-derived EVs ameliorate retinal laser injury partially by downregulating monocyte chemotactic protein in the retina (Yu et al., 2016). Additionally, MSC-derived EVs transfer miRNAs and promote the survival of retinal ganglion cells and regeneration of axons in an in-vivo rat model (Mead and Tomarev, 2017). This suggests that MSC-derived EVs could be used as a tool for cell-free therapy for traumatic and degenerative ocular disease. EVs secreted from AD-MSCs contain MALAT1-ncRNA which promotes neural regeneration and enhanced neuronal survival through regulating PKCδII splicing (El Bassit et al., 2016). miR-181-5p-modified AD-MSCs are selectively transferred to damaged liver cells via EVs, and prevent liver fibrosis by activating autophagy and down-regulating Stat3, Bcl-2, fibronectin, collagen I, vimentin, and α-SMA in the hepatic cells (Qu et al., 2017). Several other EV-miRNAs, which are potential therapeutic source to organ protection and amelioration of injuries, are listed in Table 1.
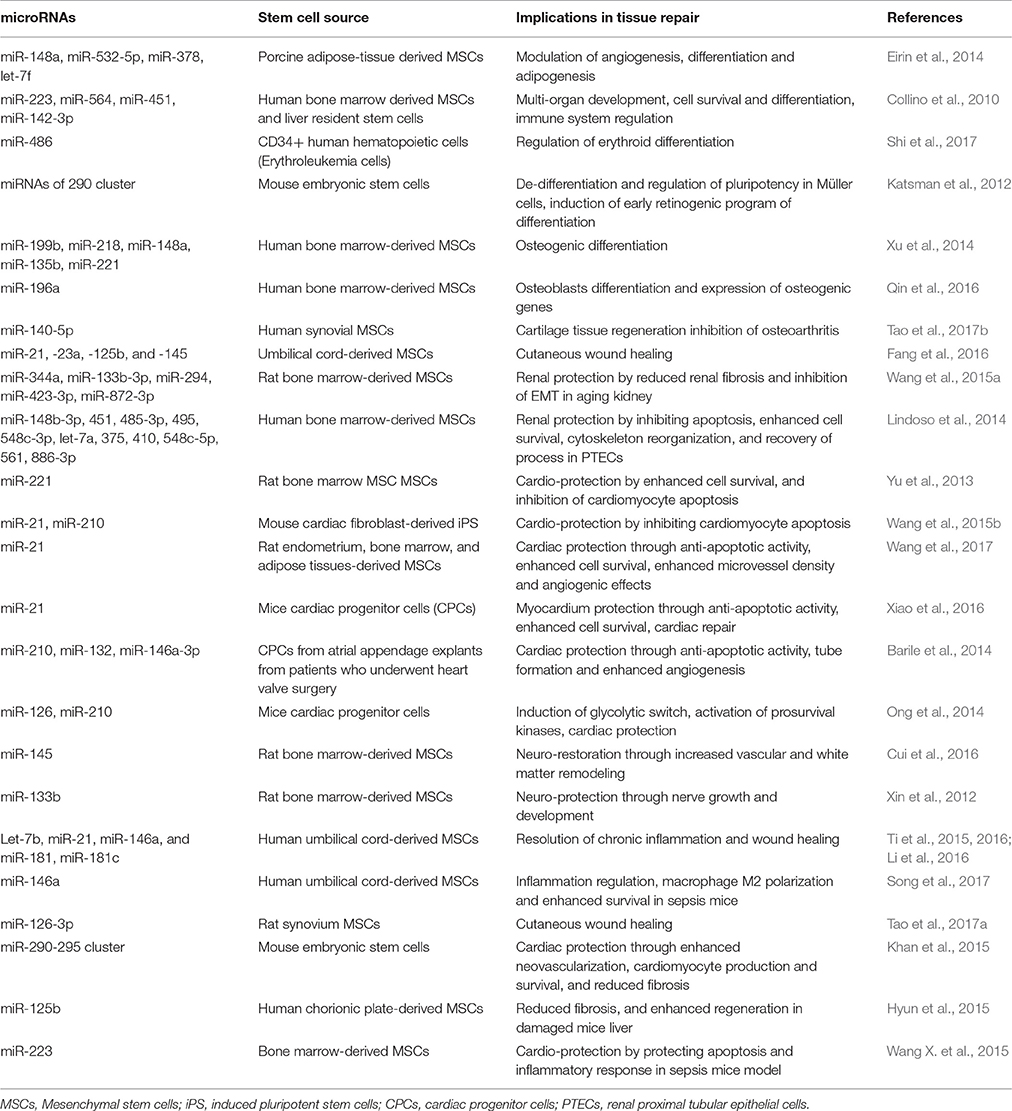
Table 1. List of extracellular vesicle-microRNAs form stem cells and their roles in regulation of stem cell maintenance and healing processes.
NcRNA-Mediated Inflammation Regulation and Healing
The successful healing process would require the alleviation of inflammatory insults during the course of tissue injury (White and Mantovani, 2013; Koniusz et al., 2016). In fact, the pro-inflammatory environment could modify the composition of EVs and the biological activities of immune effector cells; therefore, tissue regeneration therapy requires alleviation of inflammatory responses and immunosuppressive environment at the site of injury (Fatima and Nawaz, 2017b). This is possible through EV-assisted transfer/attraction of stem cell-derived ncRNAs at injury site, which efficiently govern inflammatory pathways.
The role of EVs have been well demonstrated in regulating inflammation resolution and immune regulation (Alexander et al., 2015; Nawaz et al., 2016a; Robbins et al., 2016; Silva et al., 2017), however, the contribution of EVs through ncRNAs in regulating inflammation resolution in the context of tissue repair is only more recently beginning to be explored. For instance, EVs carrying let-7b from preconditioned MSCs are shown to have avid effects on the regulation of macrophage plasticity and transition from inflammatory phase toward the proliferative phase (macrophage polarization), which favors the resolution of chronic inflammation (Ti et al., 2015). The umbilical cord MSC-derived EVs containing miR-21, miR-146a, and miR-181 have been demonstrated to regulate inflammation during the course of tissue repair (Ti et al., 2016).
Pretreatment with pro-inflammatory cytokines could improve the immunomodulatory efficacy of MSCs. Recent data suggest that the inflammatory effects of cytokines are regulated by MSC-derived EVs carrying miRNAs. For instance, IL-1β pre-treated MSCs (βMSCs) are shown to demonstrate upregulation of anti-inflammatory miRNAs such as miR-146a in response to IL-1β stimulation. In fact, IL-1β is transferred to macrophages via EVs and results in M2 polarization (characterized by anti-inflammatory phase), and increased survival in a septic mice model (Song et al., 2017). This is somewhat concordant with a study where bone marrow-derived macrophages efficiently take up EVs, which confer their switch from M1 to M2 phenotype enabling them to exhibit anti-inflammatory properties (Lo Sicco et al., 2017). In contrast, the inhibition of miR-146a may partially negate the immunomodulatory properties of βMSC-EVs. This indicates that IL-1β pre-treatment of MSCs could effectively enhance the immunomodulatory properties of MSCs through EV-mediated transfer of miR-146a. The educated βMSCs-EVs may contribute to enhanced immunomodulatory properties of βMSCs both in-vitro and in-vivo and may extend improved therapeutic application of MSCs in inflammatory disorders (Song et al., 2017).
Epithelial lining is thought to serve a direct exposure to macrophages and inflammatory responses. Therefore, EVs from stimulated epithelial cells could promote macrophage activation in-vitro and facilitate the re-colonization of immunomodulatory cells in-vivo as noticed in bronchoalveolar lavage fluid (Lee et al., 2016). The macrophage-mediated pro-inflammatory effects are reliant on delivery of proinflammatory miRNAs from epithelial cells, indicating that the epithelial cell-EV-miRNAs are potential stimulators of macrophage-regulated lung inflammatory responses (Lee et al., 2016). Recently, in-vivo assays have demonstrated the profound effects of MSC-derived EVs in inflammation resolution favored toward enhanced diabetic cutaneous wound healing (Tao et al., 2017a). MiR-181c expression in human umbilical cord MSC-derived EVs could reduce burn-induced inflammation by downregulating the Toll-like receptor 4 (TLR4) signaling pathway (Li et al., 2016). Interestingly, EVs from human iPS could be engineered for siRNA delivery to human primary pulmonary microvascular endothelial cells that alleviate inflammatory responses in recipient cells by selective gene silencing of inflammatory genes (Ju et al., 2017). Collectively, such features of EVs makes them potential tools for immune/inflammatory resolve that is prerequisite for repairing processes (Figure 2).
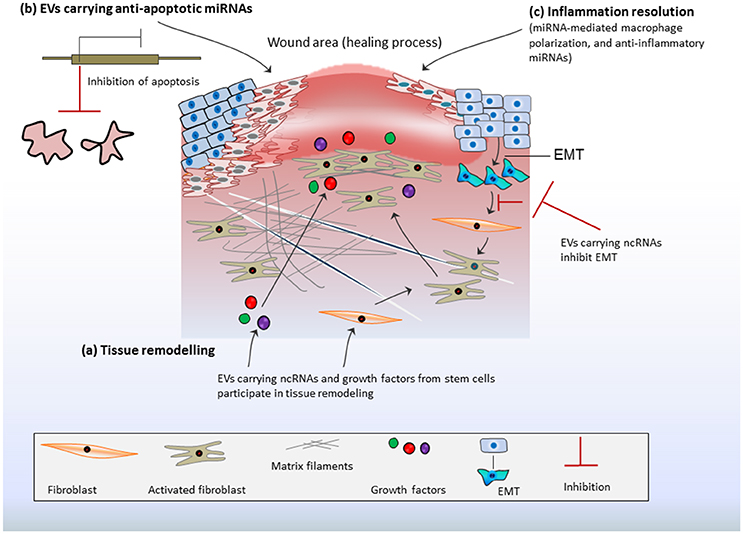
Figure 2. Stem cell-derived EV-ncRNAs and tissue repair: (A) Extracellular vesicles (EVs) carrying non-coding RNAs (ncRNAs) and growth factors may activate fibroblasts or/and endothelial cells. This promotes matrix reorganization and rapid responses in tissue regeneration. Fibroblasts proliferation is further enhanced by epithelial mesenchymal transition (EMT). This may contribute excess of fibroblasts at injury site or the formation of excess fibrous connective tissue. EV-ncRNAs regulate/inhibit EMT and ensure fibroblast optimization, which favors the repair process. (B) EVs transport anti-apoptotic miRNAs at the site of injury, which transcriptionally repress the expression of apoptotic genes and inhibit apoptosis thereby promoting cell survival during healing process. (C) EV-ncRNAs resolve inflammation by inducing macrophage polarization and transition from inflammatory phase to proliferative phase, whereas anti-inflammatory miRNAs from MSCs foster anti-inflammatory actions.
Concluding Remarks
Since ncRNAs are expressed endogenously and regulate several cellular process through orchestrating gene expression organization of cells in cis; the secretion of ncRNAs via EVs could be envisaged for the purpose that cells might have evolved mechanism of trans-regulation between cells (Fatima and Nawaz, 2017c). EV-assisted transportation of ncRNAs between long distance organs is a newly recognized mechanism of gene expression regulation and extending the physiological and pathological communications between organs (Thomou et al., 2017). In this context, stem cells may secrete ncRNAs via EVs and deliver them to injured sites for inducing and regulating tissues' intrinsic programs. The precise knowledge of such mechanisms could help developing strategies for engineering EVs with therapeutic RNAs and delivering them to injured sites. Keeping in view the proposition that ncRNAs exhibit heterogeneous regulatory mechanisms; it would be essential to determine the functional readouts arising from ncRNA regulatory effects in injured tissues.
Despite improvements in the approaches applied to tissue repair and organ transplantation over the last decade, cell-based therapies still have potential risks such as, the increased risk of infection, toxicity, tumorigenicity, and immunogenicity. Moreover, cell-based therapies need to consider additional complications such as off-target effects after transplant such as genetic instability, loss of functional properties or induction of senescence, immune-mediated rejection (graft vs. host disease), and the transformation of resident cells into malignant phenotypes, which collectively could limit the therapeutic applications of stem cells. More recently, MSC-derived EVs, which include both exosomes and microvesicles, are being examined for their therapeutic role in MSC-based cellular therapy since these vesicles are biological entities and are not associated with potential risk factors. In this context, EV-based cell-free therapies are considered promising tools, which improve patients' outcomes considerably with reduced complications in comparison to cell-based therapies (Lai et al., 2013; Fatima and Nawaz, 2015; Armstrong et al., 2017; Toh et al., 2017). Since MSC-derived EVs are non-immunological and the intriguing advantages of stem cell-derived EVs in therapy are largely due to the ability of EVs to stimulate endogenous repair processes within the injured tissue as well as their efficient regulation of immune tolerance and inflammation resolve. Furthermore, EVs could be reproduced in large quantities, easier to handle, are stable off shelf treatment material, less expensive, and do not raise potential ethical and legal issues. However, steering traditional stem cell-based therapies toward EV-based therapies need advance research and rigorous validation in vitro and in vivo models and in clinical trials. It could be of great interest to applying combination of EV-based therapies with existing approaches in order to improve the therapeutic benefits. Due to their therapeutic potentials, there is a natural desire to test MSC-derived EVs in many diverse clinical indications and there have been proposed best practice to use EVs as therapeutics (Fais et al., 2016; Reiner et al., 2017).
In addition to considering the regulatory effects and functional readouts of EVs, it is important also to consider the type of donor cells and implementation of more sensitive methods for obtaining EVs. To the extent that MSC-derived EVs can be used for cell-free regenerative medicine, much will depend on the quality, reproducibility, and potency of their production, in the same manner that these parameters dictate the development of cell-based MSC therapies (Phinney and Pittenger, 2017). Therefore, a development of highly sensitive platforms and standard operating procedures (SOPs) for obtaining the Good Manufacturing Practice (GMP) grade EVs, and developing best practice in animal models are highly recommended (Fais et al., 2016; Gimona et al., 2017; Reiner et al., 2017).
Author Contributions
FF, KE, IN, MM, HV, AH, GC, and MN participated in writing, reviewing and critical analysis of the manuscript. MN prepared the illustrations, and coordinated the manuscript. All authors agreed and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Jeremy A. Squire for critically reading the manuscript and valuable suggestions. MN acknowledges FAPESP (Sao Paulo Research Foundation, Proc. No. 12/24574-3), and CAPES (Coordination for the Improvement of Higher Education Personnel Brazil, Proc. No. 99999.007057/2015-06). FF acknowledges CAPES, Proc. No. 99999.006332/2015-03.
References
Agarwal, U., George, A., Bhutani, S., Ghosh-Choudhary, S., Maxwell, J. T., Brown, M. E., et al. (2017). Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ. Res. 120, 701–712. doi: 10.1161/CIRCRESAHA.116.309935
Alexander, M., Hu, R., Runtsch, M. C., Kagele, D. A., Mosbruger, T. L., Tolmachova, T., et al. (2015). Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 6:7321. doi: 10.1038/ncomms8321
Ambros, V. (2003). MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113, 673–676. doi: 10.1016/S0092-8674(03)00428-8
Armstrong, J. P., Holme, M. N., and Stevens, M. M. (2017). Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11, 69–83. doi: 10.1021/acsnano.6b07607
Baglio, S. R., Rooijers, K., Koppers-Lalic, D., Verweij, F. J., Perez Lanzon, M., Zini, N., et al. (2015). Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 6:127. doi: 10.1186/s13287-015-0116-z
Barile, L., Lionetti, V., Cervio, E., Matteucci, M., Gherghiceanu, M., Popescu, L. M., et al. (2014). Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 103, 530–541. doi: 10.1093/cvr/cvu167
Bellingham, S. A., Guo, B. B., Coleman, B. M., and Hill, A. F. (2012). Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front. Physiol. 3:124. doi: 10.3389/fphys.2012.00124
Beltrami, C., Besnier, M., Shantikumar, S., Shearn, A. I., Rajakaruna, C., Laftah, A., et al. (2017). Human pericardial fluid contains exosomes enriched with cardiovascular-expressed micrornas and promotes therapeutic angiogenesis. Mol. Ther. 25, 679–693. doi: 10.1016/j.ymthe.2016.12.022
Billing, A. M., Ben Hamidane, H., Dib, S. S., Cotton, R. J., Bhagwat, A. M., Kumar, P., et al. (2016). Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci. Rep. 6:21507. doi: 10.1038/srep21507
Buzas, E. I., Gyorgy, B., Nagy, G., Falus, A., and Gay, S. (2014). Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364. doi: 10.1038/nrrheum.2014.19
Câmara, J., and Jarai, G. (2010). Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair 3:2. doi: 10.1186/1755-1536-3-2
Cambier, L., de Couto, G., Ibrahim, A., Echavez, A. K., Valle, J., Liu, W., et al. (2017). Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 9, 337–352. doi: 10.15252/emmm.201606924
Cantaluppi, V., Gatti, S., Medica, D., Figliolini, F., Bruno, S., Deregibus, M. C., et al. (2012). Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427. doi: 10.1038/ki.2012.105
Cantaluppi, V., Medica, D., Mannari, C., Stiaccini, G., Figliolini, F., Dellepiane, S., et al. (2015). Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol. Dial. Transplant. 30, 410–422. doi: 10.1093/ndt/gfu364
Carrington, J. C., and Ambros, V. (2003). Role of microRNAs in plant and animal development. Science 301, 336–338. doi: 10.1126/science.1085242
Collino, F., Deregibus, M. C., Bruno, S., Sterpone, L., Aghemo, G., Viltono, L., et al. (2010). Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE 5:e11803. doi: 10.1371/journal.pone.0011803
Cui, C., Ye, X., Chopp, M., Venkat, P., Zacharek, A., Yan, T., et al. (2016). miR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl. Med. 5, 1656–1667. doi: 10.5966/sctm.2015-0349
De Luca, L., Trino, S., Laurenzana, I., Simeon, V., Calice, G., Raimondo, S., et al. (2016). MiRNAs and piRNAs from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: a new insight in transplantation. Oncotarget 7, 6676–6692. doi: 10.18632/oncotarget.6791
Deng, J., Yang, M., Jiang, R., An, N., Wang, X., and Liu, B. (2017). Long non-coding RNA HOTAIR regulates the proliferation, self-renewal capacity, tumor formation and migration of the cancer stem-like cell (CSC) subpopulation enriched from breast cancer cells. PLoS ONE 12:e0170860. doi: 10.1371/journal.pone.0170860
Deregibus, M. C., Cantaluppi, V., Calogero, R., Lo Iacono, M., Tetta, C., Biancone, L., et al. (2007). Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110, 2440–2448. doi: 10.1182/blood-2007-03-078709
Dinger, M. E., Amaral, P. P., Mercer, T. R., Pang, K. C., Bruce, S. J., Gardiner, B. B., et al. (2008). Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 18, 1433–1445. doi: 10.1101/gr.078378.108
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Eirin, A., Riester, S. M., Zhu, X. Y., Tang, H., Evans, J. M., O'Brien, D., et al. (2014). MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551, 55–64. doi: 10.1016/j.gene.2014.08.041
El Bassit, G., Patel, R. S., Carter, G., Shibu, V., Patel, A., Song, S., et al. (2016). MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCdeltaII in HT22 cells. Endocrinology 158, 183–195. doi: 10.1210/en.2016-1819
ENCODE Project Consortium (2004). The ENCODE (ENCyclopedia Of DNA Elements) Project. Science 306, 636–640. doi: 10.1126/science.1105136
ENCODE Project Consortium, Birney, E., Stamatoyannopoulos, J. A., Dutta, A., Guigo, R., Gingeras, T. R., et al. (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816. doi: 10.1038/nature05874
Fais, S., O'Driscoll, L., Borras, F. E., Buzas, E., Camussi, G., Cappello, F., et al. (2016). Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 10, 3886–3899. doi: 10.1021/acsnano.5b08015
Fang, S., Xu, C., Zhang, Y., Xue, C., Yang, C., Bi, H., et al. (2016). Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 5, 1425–1439. doi: 10.5966/sctm.2015-0367
Fatica, A., and Bozzoni, I. (2014). Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 15, 7–21. doi: 10.1038/nrg3606
Fatima, F., and Nawaz, M. (2015). Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin. J. Cancer 34:46. doi: 10.1186/s40880-015-0051-5
Fatima, F., and Nawaz, M. (2017a). Long distance metabolic regulation through adipose-derived circulating exosomal miRNAs: a trail for RNA-based therapies? Front. Physiol. 8:545. doi: 10.3389/fphys.2017.00545
Fatima, F., and Nawaz, M. (2017b). Nexus between extracellular vesicles, immunomodulation and tissue remodeling: for good or for bad? Ann. Transl. Med. 5:139. doi: 10.21037/atm.2017.03.71
Fatima, F., and Nawaz, M. (2017c). Vesiculated long non-coding RNAs: offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Non-Coding RNA 3:10. doi: 10.3390/ncrna3010010
Filipowicz, W., Bhattacharyya, S. N., and Sonenberg, N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. doi: 10.1038/nrg2290
Flynn, R. A., and Chang, H. Y. (2014). Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell 14, 752–761. doi: 10.1016/j.stem.2014.05.014
Gangaraju, V. K., and Lin, H. (2009). MicroRNAs: key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 10, 116–125. doi: 10.1038/nrm2621
Gimona, M., Pachler, K., Laner-Plamberger, S., Schallmoser, K., and Rohde, E. (2017). Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 18:E1190. doi: 10.3390/ijms18061190
Grosshans, H., and Filipowicz, W. (2008). Proteomics joins the search for microRNA targets. Cell 134, 560–562. doi: 10.1016/j.cell.2008.08.008
Guo, L., Zhao, R. C., and Wu, Y. (2011). The role of microRNAs in self-renewal and differentiation of mesenchymal stem cells. Exp. Hematol. 39, 608–616. doi: 10.1016/j.exphem.2011.01.011
Guo, S. C., Tao, S. C., Yin, W. J., Qi, X., Yuan, T., and Zhang, C. Q. (2017). Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 7, 81–96. doi: 10.7150/thno.16803
Hass, R., Kasper, C., Bohm, S., and Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 9:12. doi: 10.1186/1478-811X-9-12
He, J., Wang, Y., Lu, X., Zhu, B., Pei, X., Wu, J., et al. (2015). Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 20, 591–600. doi: 10.1111/nep.12490
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. doi: 10.1038/nature15756
Hou, J., Long, H., Zhou, C., Zheng, S., Wu, H., Guo, T., et al. (2017). Long noncoding RNA Braveheart promotes cardiogenic differentiation of mesenchymal stem cells in vitro. Stem Cell Res. Ther. 8:4. doi: 10.1186/s13287-016-0454-5
Hu, L., Wang, J., Zhou, X., Xiong, Z., Zhao, J., Yu, R., et al. (2016). Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 6:32993. doi: 10.1038/srep32993
Hyun, J., Wang, S., Kim, J., Kim, G. J., and Jung, Y. (2015). MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci. Rep. 5:14135. doi: 10.1038/srep14135
Ivey, K. N., and Srivastava, D. (2010). MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7, 36–41. doi: 10.1016/j.stem.2010.06.012
Jansen, F., Yang, X., Hoelscher, M., Cattelan, A., Schmitz, T., Proebsting, S., et al. (2013). Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 128, 2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720
Ju, Z., Ma, J., Wang, C., Yu, J., Qiao, Y., and Hei, F. (2017). Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation 40, 486–496. doi: 10.1007/s10753-016-0494-0
Judson, R. L., Babiarz, J. E., Venere, M., and Blelloch, R. (2009). Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat. Biotechnol. 27, 459–461. doi: 10.1038/nbt.1535
Kalluri, R., and Neilson, E. G. (2003). Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784. doi: 10.1172/JCI200320530
Katsman, D., Stackpole, E. J., Domin, D. R., and Farber, D. B. (2012). Embryonic stem cell-derived microvesicles induce gene expression changes in Muller cells of the retina. PLoS ONE 7:e50417. doi: 10.1371/journal.pone.0050417
Keerthikumar, S., Chisanga, D., Ariyaratne, D., Al Saffar, H., Anand, S., Zhao, K., et al. (2016). ExoCarta: a web-based compendium of exosomal cargo. J. Mol. Biol. 428, 688–692. doi: 10.1016/j.jmb.2015.09.019
Khan, M., Nickoloff, E., Abramova, T., Johnson, J., Verma, S. K., Krishnamurthy, P., et al. (2015). Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117, 52–64. doi: 10.1161/CIRCRESAHA.117.305990
Koniusz, S., Andrzejewska, A., Muraca, M., Srivastava, A. K., Janowski, M., and Lukomska, B. (2016). Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front. Cell. Neurosci. 10:109. doi: 10.3389/fncel.2016.00109
Kordelas, L., Rebmann, V., Ludwig, A. K., Radtke, S., Ruesing, J., Doeppner, T. R., et al. (2014). MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970–973. doi: 10.1038/leu.2014.41
Lai, R. C., Tan, S. S., Yeo, R. W., Choo, A. B., Reiner, A. T., Su, Y., et al. (2016). MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 5:29828. doi: 10.3402/jev.v5.29828
Lai, R. C., Yeo, R. W., Tan, K. H., and Lim, S. K. (2013). Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol. Adv. 31, 543–551. doi: 10.1016/j.biotechadv.2012.08.008
Lee, H., Zhang, D., Zhu, Z., Dela Cruz, C. S., and Jin, Y. (2016). Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 6:35250. doi: 10.1038/srep35250
Lee, J. T. (2012). Epigenetic regulation by long noncoding RNAs. Science 338, 1435–1439. doi: 10.1126/science.1231776
Li, X., Liu, L., Yang, J., Yu, Y., Chai, J., Wang, L., et al. (2016). Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBiomedicine 8, 72–82. doi: 10.1016/j.ebiom.2016.04.030
Liang, X., Zhang, L., Wang, S., Han, Q., and Zhao, R. C. (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell. Sci. 129, 2182–2189. doi: 10.1242/jcs.170373
Lindoso, R. S., Collino, F., Bruno, S., Araujo, D. S., Sant'Anna, J. F., Tetta, C., et al. (2014). Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 23, 1809–1819. doi: 10.1089/scd.2013.0618
Loewer, S., Cabili, M. N., Guttman, M., Loh, Y. H., Thomas, K., Park, I. H., et al. (2010). Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 42, 1113–1117. doi: 10.1038/ng.710
Lo Sicco, C., Reverberi, D., Balbi, C., Ulivi, V., Principi, E., Pascucci, L., et al. (2017). Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl. Med. 6, 1018–1028. doi: 10.1002/sctm.16-0363
Marson, A., Levine, S. S., Cole, M. F., Frampton, G. M., Brambrink, T., Johnstone, S., et al. (2008). Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134, 521–533. doi: 10.1016/j.cell.2008.07.020
Martin, P. J., Haren, N., Ghali, O., Clabaut, A., Chauveau, C., Hardouin, P., et al. (2015). Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 16:10. doi: 10.1186/s12860-015-0057-5
Mateescu, B., Kowal, E. J., van Balkom, B. W., Bartel, S., Bhattacharyya, S. N., Buzas, E. I., et al. (2017). Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J. Extracell. Vesicles 6:1286095. doi: 10.1080/20013078.2017.1286095
Mathivanan, S., Ji, H., and Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920. doi: 10.1016/j.jprot.2010.06.006
Mead, B., and Tomarev, S. (2017). Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl. Med. 6, 1273–1285. doi: 10.1002/sctm.16-0428
Nagaishi, K., Mizue, Y., Chikenji, T., Otani, M., Nakano, M., Konari, N., et al. (2016). Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 6:34842. doi: 10.1038/srep34842
Nawaz, M., Camussi, G., Valadi, H., Nazarenko, I., Ekstrom, K., Wang, X., et al. (2014). The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 11, 688–701. doi: 10.1038/nrurol.2014.301
Nawaz, M., and Fatima, F. (2017). Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links. Front. Mol. Biosci. 4:50. doi: 10.3389/fmolb.2017.00050
Nawaz, M., Fatima, F., Nazarenko, I., Ekstrom, K., Murtaza, I., Anees, M., et al. (2016a). Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev. Proteomics 13, 395–409. doi: 10.1586/14789450.2016.1165613
Nawaz, M., Fatima, F., Vallabhaneni, K. C., Penfornis, P., Valadi, H., Ekstrom, K., et al. (2016b). Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016:1073140. doi: 10.1155/2016/1073140
Ong, S. G., Lee, W. H., Huang, M., Dey, D., Kodo, K., Sanchez-Freire, V., et al. (2014). Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation 130(11 Suppl. 1), S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917
Ounzain, S., Crippa, S., and Pedrazzini, T. (2013). Small and long non-coding RNAs in cardiac homeostasis and regeneration. Biochim. Biophys. Acta 1833, 923–933. doi: 10.1016/j.bbamcr.2012.08.010
Ounzain, S., and Pedrazzini, T. (2015). The promise of enhancer-associated long noncoding RNAs in cardiac regeneration. Trends Cardiovasc. Med. 25, 592–602. doi: 10.1016/j.tcm.2015.01.014
Pasquinelli, A. E., and Ruvkun, G. (2002). Control of developmental timing by micrornas and their targets. Annu. Rev. Cell. Dev. Biol. 18, 495–513. doi: 10.1146/annurev.cellbio.18.012502.105832
Pauli, A., Rinn, J. L., and Schier, A. F. (2011). Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 12, 136–149. doi: 10.1038/nrg2904
Perry, R. B., and Ulitsky, I. (2016). The functions of long noncoding RNAs in development and stem cells. Development 143, 3882–3894. doi: 10.1242/dev.140962
Phinney, D. G., and Pittenger, M. F. (2017). Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35, 851–858. doi: 10.1002/stem.2575
Qin, Y., Wang, L., Gao, Z., Chen, G., and Zhang, C. (2016). Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 6:21961. doi: 10.1038/srep21961
Qu, Y., Zhang, Q., Cai, X., Li, F., Ma, Z., Xu, M., et al. (2017). Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 21, 2491–2502. doi: 10.1111/jcmm.13170
Quesenberry, P. J., Dooner, M. S., and Aliotta, J. M. (2010). Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp. Hematol. 38, 581–592. doi: 10.1016/j.exphem.2010.03.021
Ratajczak, J., Miekus, K., Kucia, M., Zhang, J., Reca, R., Dvorak, P., et al. (2006a). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856. doi: 10.1038/sj.leu.2404132
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A., and Ratajczak, M. Z. (2006b). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495. doi: 10.1038/sj.leu.2404296
Reiner, A. T., Witwer, K. W., van Balkom, B., de Beer, W. M., Brodie, J., Corteling, C., et al. (2017). Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 6, 1730–1739. doi: 10.1002/sctm.17-0055
Robbins, P. D., Dorronsoro, A., and Booker, C. N. (2016). Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest. 126, 1173–1180. doi: 10.1172/JCI81131
Salvucci, O., Jiang, K., Gasperini, P., Maric, D., Zhu, J., Sakakibara, S., et al. (2012). MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica 97, 818–826. doi: 10.3324/haematol.2011.056945
Shao, L., Zhang, Y., Lan, B., Wang, J., Zhang, Z., Zhang, L., et al. (2017). MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed. Res. Int. 2017:4150705. doi: 10.1155/2017/4150705
Shenoy, A., and Blelloch, R. H. (2014). Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat. Rev. Mol. Cell. Biol. 15, 565–576. doi: 10.1038/nrm3854
Shi, X. F., Wang, H., Kong, F. X., Xu, Q. Q., Xiao, F. J., Yang, Y. F., et al. (2017). Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp. Cell Res. 351, 74–81. doi: 10.1016/j.yexcr.2016.12.023
Silva, A. M., Teixeira, J. H., Almeida, M. I., Goncalves, R. M., Barbosa, M. A., and Santos, S. G. (2017). Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. Eur. J. Pharm. Sci. 98, 86–95. doi: 10.1016/j.ejps.2016.09.017
Song, Y., Dou, H., Li, X., Zhao, X., Li, Y., Liu, D., et al. (2017). Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells 35, 1208–1221. doi: 10.1002/stem.2564
Tao, S. C., Guo, S. C., Li, M., Ke, Q. F., Guo, Y. P., and Zhang, C. Q. (2017a). Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl. Med. 6, 736–747. doi: 10.5966/sctm.2016-0275
Tao, S. C., Yuan, T., Zhang, Y. L., Yin, W. J., Guo, S. C., and Zhang, C. Q. (2017b). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195. doi: 10.7150/thno.17133
Thakur, B. K., Zhang, H., Becker, A., Matei, I., Huang, Y., Costa-Silva, B., et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769. doi: 10.1038/cr.2014.44
Thomou, T., Mori, M. A., Dreyfuss, J. M., Konishi, M., Sakaguchi, M., Wolfrum, C., et al. (2017). Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455. doi: 10.1038/nature21365
Ti, D., Hao, H., Fu, X., and Han, W. (2016). Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 59, 1305–1312. doi: 10.1007/s11427-016-0240-4
Ti, D., Hao, H., Tong, C., Liu, J., Dong, L., Zheng, J., et al. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 13:308. doi: 10.1186/s12967-015-0642-6
Toh, W. S., Lai, R. C., Hui, J. H. P., and Lim, S. K. (2017). MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 67, 56–64. doi: 10.1016/j.semcdb.2016.11.008
Uccelli, A., Moretta, L., and Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736. doi: 10.1038/nri2395
Vandergriff, A. C., de Andrade, J. B., Tang, J., Hensley, M. T., Piedrahita, J. A., Caranasos, T. G., et al. (2015). Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015:960926. doi: 10.1155/2015/960926
Wang, K., Jiang, Z., Webster, K. A., Chen, J., Hu, H., Zhou, Y., et al. (2017). Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl. Med. 6, 209–222. doi: 10.5966/sctm.2015-0386
Wang, X., Gu, H., Qin, D., Yang, L., Huang, W., Essandoh, K., et al. (2015). Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci. Rep. 5:13721. doi: 10.1038/srep13721
Wang, Y., Fu, B., Sun, X., Li, D., Huang, Q., Zhao, W., et al. (2015a). Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-beta1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res. Ther. 6:185. doi: 10.1186/s13287-015-0179-x
Wang, Y., Zhang, L., Li, Y., Chen, L., Wang, X., Guo, W., et al. (2015b). Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 192, 61–69. doi: 10.1016/j.ijcard.2015.05.020
Wei, B., Wei, W., Zhao, B., Guo, X., and Liu, S. (2017). Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS ONE 12:e0169097. doi: 10.1371/journal.pone.0169097
Wei, X., Yang, X., Han, Z. P., Qu, F. F., Shao, L., and Shi, Y. F. (2013). Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin. 34, 747–754. doi: 10.1038/aps.2013.50
White, E. S., and Mantovani, A. R. (2013). Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J. Pathol. 229, 141–144. doi: 10.1002/path.4126
Xi, Y., Tan, K., Brumwell, A. N., Chen, S. C., Kim, Y. H., Kim, T. J., et al. (2014). Inhibition of epithelial-to-mesenchymal transition and pulmonary fibrosis by methacycline. Am. J. Respir. Cell Mol. Biol. 50, 51–60. doi: 10.1165/rcmb.2013-0099OC
Xiao, J., Pan, Y., Li, X. H., Yang, X. Y., Feng, Y. L., Tan, H. H., et al. (2016). Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 7, e2277. doi: 10.1038/cddis.2016.181
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., et al. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556–1564. doi: 10.1002/stem.1129
Xu, C., Zhang, Y., Wang, Q., Xu, Z., Jiang, J., Gao, Y., et al. (2016). Long non-coding RNA GAS5 controls human embryonic stem cell self-renewal by maintaining NODAL signalling. Nat. Commun. 7:13287. doi: 10.1038/ncomms13287
Xu, J. F., Yang, G. H., Pan, X. H., Zhang, S. J., Zhao, C., Qiu, B. S., et al. (2014). Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 9:e114627. doi: 10.1371/journal.pone.0114627
Yáñez-Mó, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borras, F. E., Buzas, E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4:27066. doi: 10.3402/jev.v4.27066
Yu, B., Gong, M., Wang, Y., Millard, R. W., Pasha, Z., Yang, Y., et al. (2013). Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS ONE 8:e73304. doi: 10.1371/journal.pone.0073304
Yu, B., Shao, H., Su, C., Jiang, Y., Chen, X., Bai, L., et al. (2016). Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 6:34562. doi: 10.1038/srep34562
Zhang, J., Guan, J., Niu, X., Hu, G., Guo, S., Li, Q., et al. (2015). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 13:49. doi: 10.1186/s12967-015-0417-0
Zhang, W., Dong, R., Diao, S., Du, J., Fan, Z., and Wang, F. (2017). Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 8:30. doi: 10.1186/s13287-017-0485-6
Zhang, Y., Kim, M. S., Jia, B., Yan, J., Zuniga-Hertz, J. P., Han, C., et al. (2017). Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548, 52–57. doi: 10.1038/nature23282
Keywords: extracellular vesicles, exosomes, mesenchymal stem cells, non-coding RNA, gene expression regulation, matrix remodeling, inflammatory resolve, tissue repair
Citation: Fatima F, Ekstrom K, Nazarenko I, Maugeri M, Valadi H, Hill AF, Camussi G and Nawaz M (2017) Non-coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Front. Genet. 8:161. doi: 10.3389/fgene.2017.00161
Received: 09 August 2017; Accepted: 12 October 2017;
Published: 25 October 2017.
Edited by:
Karthikeyan Narayanan, Institute of Bioengineering and Nanotechnology (A*STAR), SingaporeReviewed by:
Sita Somara, Wake Forest School of Medicine, United StatesSriram Ravindran, University of Illinois at Chicago, United States
Copyright © 2017 Fatima, Ekstrom, Nazarenko, Maugeri, Valadi, Hill, Camussi and Nawaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Nawaz, nawazm.edu@gmail.com
 Farah Fatima
Farah Fatima Karin Ekstrom
Karin Ekstrom Irina Nazarenko
Irina Nazarenko Marco Maugeri
Marco Maugeri Hadi Valadi
Hadi Valadi Andrew F. Hill
Andrew F. Hill Giovanni Camussi
Giovanni Camussi Muhammad Nawaz
Muhammad Nawaz