Tricking Parents: A Review of Mechanisms and Signals of Host Manipulation by Brood-Parasitic Young
- 1Departamento de Ecología, Genética y Evolución and IEGEBA-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina
- 2Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ, United States
Obligate avian brood parasites depend entirely on heterospecific hosts for rearing their offspring. From hatching until independence, the young parasites must deal with the challenge of obtaining sufficient parental care from foster parents that are attuned to provisioning their own offspring. Parent-offspring communication is mediated by complex begging displays in which nestlings and fledglings exhibit visual (e.g., gaping and postures) and vocal (e.g., begging calls) traits that serve as signals to parents to adjust and allocate parental effort. Parasites can manipulate host parental behavior by exploiting these stable parent-offspring communication systems in their favor. During the past 30 years, the study of host exploitation by parasitic chicks has yielded important insights into the function and evolution of manipulative signals in brood parasites. However, despite these major advances, there are still important gaps in our knowledge about how parasitic nestling and fledglings tune into the host’s communication channels and the adaptive value of the visual and acoustic signals they exhibit. Here we review the literature pertaining to host manipulation by parasitic young, focusing on four non-mutually exclusive mechanisms (i.e., host chick mimicry, begging exaggeration, host-attuned begging calls, and sensory exploitation) and the function and evolution of the signals involved, with the aim to summarize and discuss putative adaptations for stimulating parental feeding and escaping host discrimination. Finally, we bring some concluding remarks and suggest directions for future research on the ways in which brood parasites adapt to the communication systems of other birds to exploit the necessary parental care.
Introduction
To reproduce successfully, females of heterospecific brood parasites must locate nests of suitable host species and return to them at the appropriate time to lay their eggs. Once the eggs hatch, the parasitic offspring face the challenge of obtaining adequate levels of parental care from foster parents that are attuned to provision their own progeny. In parasite species that evict or kill all host eggs and nestlings soon after hatching (“nest mate evictors”), the chicks must deal with the problem of stimulating sufficient provisioning in the absence of any host nest mates. In species that do not eliminate their nest mates (“non-evictors”), chicks have the dual problem of eliciting parental feedings and competing for food within mixed broods. Given these selective pressures, it is not surprising that parasitic young have evolved behavioral and morphological traits that effectively serve to manipulate host parental behaviors in their favor (Davies, 2000; Soler, 2017). The well-known image of a tiny adult reed warbler (Acrocephalus scirpaceus) diligently feeding an enormous and completely alien common cuckoo (Cuculus canorus) chick depicts perfectly the kind of manipulative abilities that have intrigued naturalists since ancient times (Davies, 2000).
Host manipulation by parasitic chicks involves the exploitation of stable communication systems that play a role in solving parent-offspring conflict (Godfray, 1995). In birds, dependent young communicate their needs by means of complex begging displays that combine visual (e.g., gaping and stretching) and acoustic (i.e., begging calls) signals. In conjunction, the multiple components of begging displays convey honest information about offspring attributes, such as their need and condition, that care-giving adults can use to adjust their provisioning effort and to allocate food within the brood (Burford et al., 1998; Kilner et al., 1999; Leonard and Horn, 2001; Moreno-Rueda et al., 2009). Young of obligate brood parasites can “tune” their begging signals into these communication channels to secure the necessary parental care. Host exploitation can be achieved by means of two main kinds of adaptations, according to Davies (2011). Trickery adaptations are those that have coevolved with the host’s counter-defenses against brood parasitism and allow parasitic chicks to be accepted by hosts as if they were their own (Davies, 2011). Tuning adaptations refer to those that help ensure the success of parasitic offspring once they have been accepted by hosts (Davies, 2011). This distinction may become diffuse if mistuning in the parasites ultimately results in discrimination by hosts (Davies, 2011), yet it provides a useful conceptual framework to examine the tactics whereby parasite young deceive their hosts and tap into their provisioning rules.
Several non-mutually exclusive mechanisms have been suggested in evictor and non-evictor parasites to exploit host parental behavior to their own benefit (Soler, 2017). Here, we focus on those that serve parasites to evade host discrimination (trickery), or to attune their begging signals to the communication systems or sensory biases of their hosts (tuning). Within this framework, trickery involves host chick mimicry, either as a counter-defense against active host rejection of non-mimetic young, or as an adaptation to avoid discrimination from hosts that deliver food preferentially in response to conspecific signals (Grim, 2005; Wang et al., 2020). In turn, we consider begging exaggeration, host-attuned begging calls and sensory exploitation as the main tuning mechanisms. The distinction between them is not clear-cut, but we use this categorization because it reflects three different, though non-mutually exclusive tactics deployed by parasites to manipulate host behavior through their begging displays. Begging exaggeration is widespread among parasite species and likely serve to exploit host biases for non-specific visual and acoustic features that signal offspring need. For example, parasitic chicks can beg more rapidly or intensively than host young to gain more resources from their foster parents (Redondo, 1993; Kilner et al., 1999; Dearborn and Lichtenstein, 2002). Host-attuned begging calls imply the ability of parasite chicks to learn to modify certain acoustic features in a host-specific manner to make their begging signals more profitable in any given host environment (Madden and Davies, 2006; Langmore et al., 2008). Finally, in sensory exploitation parasitic chicks exhibit morphological traits that are not actually used in host parent-offspring communication, but effectively stimulate the sensory system of the host (Tanaka and Ueda, 2005b). Begging exaggeration and sensory exploitation tactics are similar in that both rely on exploiting host’s preexisting cognitive biases and can serve young parasites to compensate for deficient stimulation relative to a host’s own brood. However, following the suggestion of Tanaka and Ueda (2005b), we consider them as distinct mechanisms based on whether parasitic chicks exhibit and amplify communication signals already present in host chicks (begging exaggeration) or they display traits that are absent in host young to provide additional begging stimuli (sensory exploitation). The mechanisms outlined above are not mutually exclusive since, for example, chicks of host-generalist parasites can beg exaggeratedly overall while varying their call structure or call rate according to the particular host environment (Madden and Davies, 2006; Tuero et al., 2016). Likewise, trickery and tuning adaptations for host manipulation may occur within a single parasitic species (e.g., Jamie et al., 2020).
Our aim here was to provide an updated review of how parasitic young exploit the honest parent-offspring communication systems of their hosts, either to escape host discrimination or to tap into host provisioning decisions in response to begging signals. To achieve this, we searched the primary literature (journal articles and book chapters found in Scopus and Google Scholar databases) for information on behavioral and morphological traits that could play a role as manipulative signals in species currently recognized as obligate brood parasites, considering the visual and acoustic sensory modalities. Of the 101 parasite species (Feeney et al., 2014; Gill et al., 2017), we found data on 13, including observational and experimental studies on begging behavior, quantitative assessments of visual and acoustic mimicry between parasites and hosts, and comparative studies (Table 1). We first summarize and assess the available information about mechanisms and signals for host manipulation found in parasitic nestlings and fledglings, according to our proposed categorization within the trickery-tuning framework. The species for which more than one mechanism was reported were included in each of the corresponding sections. Finally, we bring some concluding remarks and suggest directions for future research.
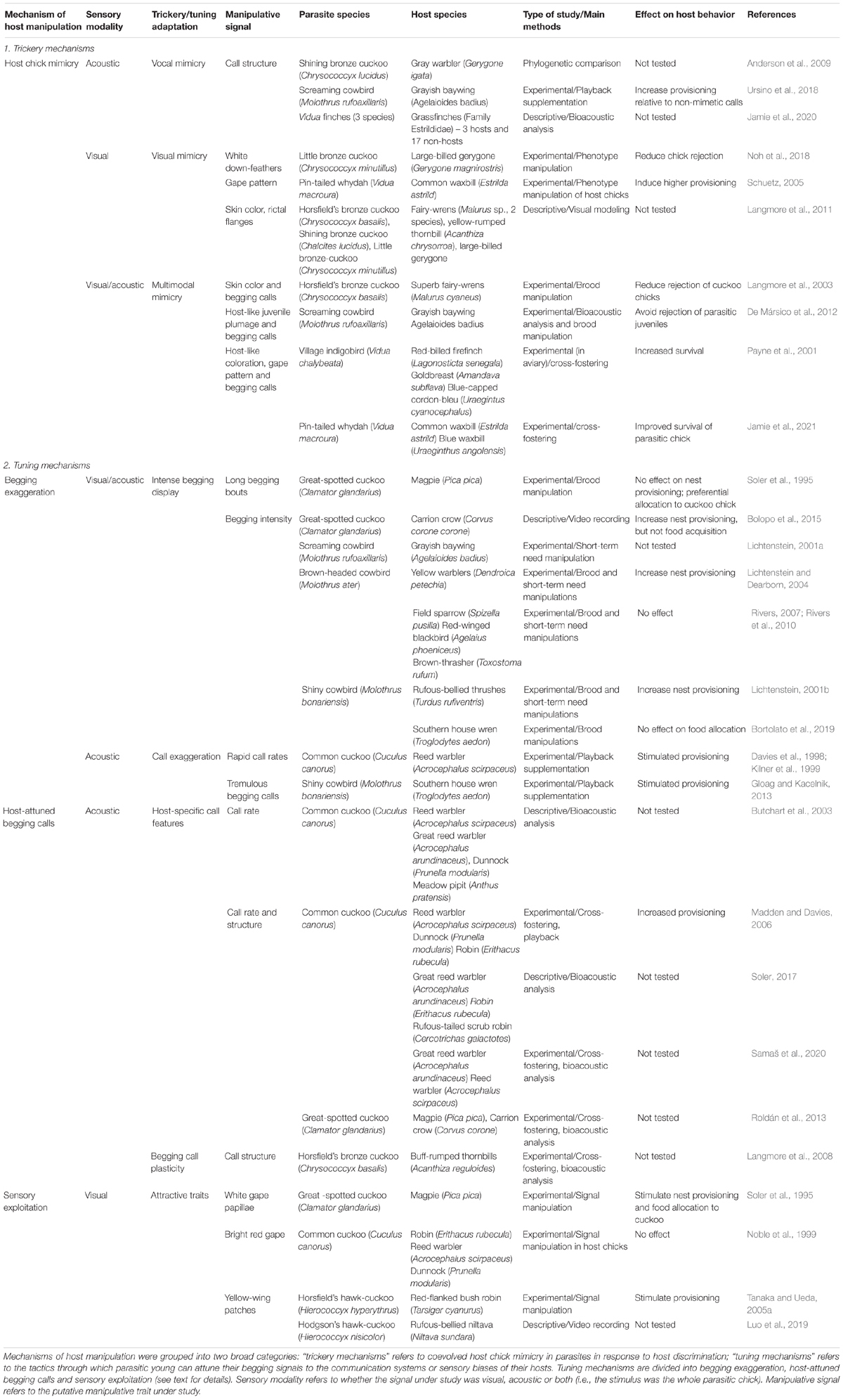
Table 1. Overview of relevant studies on mechanisms and signals for host manipulation by young of evictor and non-evictor brood parasites.
Trickery Adaptations in Parasitic Young: Host Chick Mimicry
Mimicry of host young has been suggested in several parasite species, but most available reports were based on anecdotal observations or subjective assessments of similarity by researchers (Grim, 2006; Jamie and Kilner, 2017). This is problematic in determining parasite trickery because humans and birds differ in their perceptual systems, especially regarding the visual modality (Hart et al., 2000a,b). In the past decades, it has been increasingly common to apply avian visual modeling techniques to quantify colors in birds (Vorobyev and Osorio, 1998). These techniques have been used to objectively assess the similarity between host and parasitic chicks as seen through a bird’s eye, thus providing important insights into the function and evolution of visual trickery adaptations driven by host discrimination (Langmore et al., 2008, 2011; Anderson et al., 2009; Tanaka et al., 2011; De Mársico et al., 2012; Attisano et al., 2018; Jamie et al., 2020). In addition to objective measurements of similarity, experimental tests of the function of host resemblance in parasites, for example using controlled cross-fostering or playback trials, are critical to properly assess the existence of mimicry. In this section we focus on the relatively few well-documented examples of visual and vocal mimicry in parasites that can be regarded as coevolved adaptations to evade host discrimination against young unlike their own (Grim, 2006), including results from our own studies on fledgling mimicry in the host-specialist screaming cowbird (Molothrus rufoaxillaris). We summarize and discuss examples of trickery on a species-by-species basis, instead of focusing on traits, to bring a more integrative perspective about how host behavior has driven host chick mimicry in each case and drive the attention to the fact that mimicry can occur in multiple sensory modalities within any single species.
Bronze Cuckoos
Active host rejection of parasitic chicks occurs in hosts of Australasian bronze cuckoos (Chrysococcyx spp.). Superb fairy-wrens are primary hosts of the Horsfield’s bronze cuckoo and known to reject cuckoo chicks by deserting them (Langmore et al., 2003). The main cue triggering this behavior is the presence of a single chick in the brood (Langmore et al., 2003), but fairy-wrens are less likely to abandon nests with Horsfield’s bronze cuckoo chicks than with shining bronze cuckoo (C. lucidus) chicks (Langmore et al., 2003). This suggests that Horsfield’s bronze cuckoos have evolved counter adaptations to evade host rejection. Consistent with this, chicks of this parasite species closely resemble the fairy-wren chicks in skin and rictal flange colors (Langmore et al., 2011), and they innately develop begging calls that match the acoustic structure of those of fairy-wren chicks as well (Langmore et al., 2008). Although it seems clear that host chick mimicry is adaptive for Horsfield’s bronze cuckoos, more experiments are needed to further determine the role played by visual and vocal signals of parasitic chicks in deceiving super fairy-wrens. Evidence from another study indicated that fairy-wren hosts would be able to discriminate between their own and alien cuckoo chicks based on parent-specific call signatures that are transmitted to its offspring during the embryonic stage (Colombelli-Négrel et al., 2012).
The little bronze cuckoo (C. minutillus) is another species faced with host defenses against alien chicks. Two primary hosts in Australia, the large-billed gerygone (Gerygone magnirostris) and the mangrove gerygone (G. laevigaster), are able to reject cuckoo chicks by dragging them out of the nest, sometimes within a few hours after hatching (Sato et al., 2010; Tokue and Ueda, 2010). Little bronze cuckoo chicks are striking visual mimics of gerygone chicks, closely matching their dark skin, multi-barbed white down-feathers and rictal flange color (Langmore et al., 2011; Figures 1A,B). Noh et al. (2018) showed that the number of down-feathers is a key trait used by gerygone hosts to discriminate between their own and alien chicks. Experimental trimming of down-feathers in cuckoo and gerygone chicks increased the likelihood of rejection relative to untrimmed chicks (Noh et al., 2018). This strongly suggests that host chick mimicry in little bronze cuckoos has evolved as a reciprocal adaptation against host recognition (Noh et al., 2018). However, the study also revealed that parasitic chicks do not fully match the recognition signals used by gerygones, since trimmed cuckoos were rejected at higher rates than trimmed host chicks (Noh et al., 2018). A recent study suggests that gerygone hosts could use the duration of the begging calls as a cue to spot and reject parasitic chicks and that cuckoo chicks more closely match the begging calls of host chicks at the age at which rejection typically occurs (Noh et al., 2021). More experiments that test if hosts cue also on acoustic or olfactory signals for making rejection decisions, and further examination of the similarity between little bronze cuckoos and host chicks in multiple sensory modalities, would provide new insights on this issue.

Figure 1. Three examples of host chick mimicry in parasitic birds. (A,B) Little bronze cuckoo (Chalcites minutillus) chicks (top) are visual mimics of large-billed gerygones (Gerygone magnirostris) chicks (bottom). (C) Parasitic pin-tailed whydah (Vidua macroura) chicks (left) bear a close resemblance in mouth ornamentation to its common waxbill (Estrilda astrild) host. (D) Screaming cowbird (Molothrus rufoaxillaris) juveniles (right) mimic the plumage coloration of its primary host, the grayish baywing (Agelaioides badius). Photo credits: (A,B) Naomi Langmore, (C) Justin Schuetz, (D) Alec Earnshaw.
The shining bronze-cuckoo and its primary host in New Caledonia, the fan-tailed gerygone (Gerygone flavolateralis), provide an interesting example of parasite chicks that have seemingly evolved visual mimicry driven by an ongoing co-evolutionary arms race with their hosts (Sato et al., 2015). Fan-tailed gerygones have chicks of two distinct morphs, namely bright and dark, which can occur in monomorphic or polymorphic broods (Sato et al., 2015; Attisano et al., 2018). Shining bronze cuckoo chicks from New Caledonia are, at present, of a single bright morph (Sato et al., 2015; Attisano et al., 2018), though distinct yellow and dark morphs are known to occur in the Australian subspecies (Langmore et al., 2011). The cuckoo bright morph match closely the bright gerygone morph from an avian perspective, but it is also more similar to the dark host morph than the bright and dark host morphs are to each other (Sato et al., 2015; Attisano et al., 2018). Despite this similarity, from a sample of 15 parasitized gerygone nests in which the cuckoo egg hatched, hosts always rejected the cuckoo chick, usually within 24 h after hatching (Attisano et al., 2018). These observations suggest a true-recognition mechanism based on multiple sensorial cues underlying chick rejection behavior in gerygones, although the precise recognition signals have not been identified (Attisano et al., 2018). On the other hand, there are a few observations of shining bronze cuckoo fledglings being fed by fan-tailed gerygones at other sites in New Caledonia, suggesting that parasitic chicks can sometimes evade the refined discrimination abilities of its primary host (Attisano et al., 2018). Clearly, more research is needed to understand how gerygones can spot the cuckoo chick in their nests so precisely, and how cuckoo chicks occasionally manage to surpass this host defense. Shining bronze cuckoos from New Zealand were reported to bear begging call similarity with their gray warbler (G. igata) host (McLean and Waas, 1987), and a posterior comparative study involving gray warblers and 17 other native forest species of New Zealand further supports a close matching of host begging calls in shining bronze cuckoos (Anderson et al., 2009). Nevertheless, the adaptive value of this call similarity has not been determined, nor is it known whether a similar begging call matching occurs in other shining bronze cuckoo populations.
Brood-Parasitic Finches
The Vidua whydahs and indigobirds comprise 19 non-evictor species specialized in parasitizing grassfinches (family Estrildidae; Davies, 2000). Young estrildid finches are unique in that they have species-specific mouth ornamentations that are exhibited in begging displays (Payne, 2005). Most Vidua species lay eggs in nests of a single host and parasitic chicks often match the mouth markings and begging calls of their respective hosts (Nicolai, 1974; Payne and Payne, 2002; Payne, 2005; Figure 1C). However, it was not until recently that the similarity in begging signals between Vidua chicks and their hosts was assessed with quantitative and objective methods. Jamie et al. (2020) tested whether parasitic chicks of three Vidua species matched more closely the mouth patterning, gape color, begging calls, and postural displays of their respective hosts than they do those of other co-occurring grassfinch species. The results showed a closer phenotypic similarity between parasitic chicks and their hosts, supporting the idea that Vidua finches have evolved host-specific mimicry (Jamie et al., 2020). Interestingly, the study also revealed that host resemblance was not 100% accurate, since some Vidua chicks presented exaggerated traits relative to their hosts, such as enlarged palatal spots, longer begging calls and increased wing-waving behavior (Jamie et al., 2020). The idea that this “imperfect mimicry” could be adaptive by, for example, providing a supernormal stimulus that enhances parental provisioning warrants further investigation (Jamie et al., 2020).
Regarding the adaptive value of chick mimicry in Vidua finches, there is some evidence from cross-fostering experiments that lacking the species-specific signals can result in reduced survival of alien chicks in estrildid nests (Payne et al., 2001; Jamie et al., 2021). Recently, a field experiment demonstrated that reduced survival of cross-fostered pin-tailed whydahs (V. macroura) was the result of foster parents delivering less food to non-mimetic parasitic chicks compared to their own (Jamie et al., 2021). To date, there is no evidence that estrildid hosts actively reject chicks unlike their own; indeed, non-mimetic Vidua chicks do sometimes fledge successfully from nests of grassfinches other than their host, which helps to explain the occasional colonization of new host species in this parasite lineage (Sorenson et al., 2003, 2004). The study by Jamie et al. (2021) also showed that parasitic chicks did not modify the acoustic structure of their begging calls when transferred to nests of a non-host species. Innate call mimicry is expected in specialist brood-parasites if failure in exhibiting the appropriate begging signals results in fitness costs for parasitic chicks (Jamie and Kilner, 2017). Nonetheless, more experimental work is needed to disentangle how estrildid hosts integrate visual and vocal cues in chick discrimination. In a field experiment, Schuetz (2005) manipulated the gape flanges of common waxbill (Estrilda astrild) chicks (i.e., the natural host of pin-tailed whydahs), to test host response toward chicks with dissimilar gape morphology. Host chicks that had their flanges painted black suffered only a slight reduction in mass and skeletal growth compared to unmanipulated or sham-painted chicks (Schuetz, 2005). Altogether, these findings suggest that host manipulation by Vidua chicks involve multiple sensory modalities and, possibly, some signal exaggeration in addition to host-specific mimicry (Jamie et al., 2020). Such remarkable fine-tuning with respect to host begging signals has more likely been driven by a preexisting parental feeding preference in estrildid hosts for chicks bearing the elaborate traits specific to each species, rather than by the existence of active host defenses against brood parasitism (Hauber and Kilner, 2007; Jamie et al., 2021).
Screaming Cowbird
In theory, the co-evolutionary arms race between brood parasites and their hosts can encompass all stages of the nesting cycle (Soler, 2017). However, co-evolved adaptations during the fledgling stage are much less known (De Mársico et al., 2017). In this regard, the studies on host-parasite interactions between the screaming cowbird and its primary host provide the most compelling evidence to date for the evolution of host fledging mimicry in parasitic juveniles. Screaming cowbirds are host-specialists that mainly parasitize grayish baywings (Agelaioides badius) in southern South America. The parasitic young bear a striking resemblance to baywing offspring that cannot be attributed to common ancestry (Lanyon, 1992) and lasts until the former attain nutritional independence (Hudson, 1874; Fraga, 1998; Ursino et al., 2012; Figure 1D). Quantitative analyses have indicated that screaming cowbird fledglings would be indistinguishable from host fledglings from an avian perspective, and that they also closely match baywing begging calls (De Mársico et al., 2012). The function of this close similarity was tested by cross-fostering non-mimetic shiny cowbird (Molothrus bonariensis) chicks to baywing nests and comparing their fate to that of host and screaming cowbird young (Fraga, 1998; De Mársico et al., 2012). Baywings accepted any chick in their nests but stopped providing parental care to shiny cowbirds as soon as they fledged, while they continued caring for screaming cowbird and their own fledglings for several weeks (Fraga, 1998; De Mársico et al., 2012). These results support the idea that the baywing-like appearance of screaming cowbird fledglings is a reciprocal adaptation in response to host rejection behavior (Fraga, 1998; De Mársico et al., 2012). A more recent study suggests that host discrimination against non-mimetic fledglings is context-dependent rather than based on an internal template of their own offspring’s appearance, since baywings accept shiny cowbird fledglings when they were reared in the absence of host nest mates (Rojas Ripari et al., 2019a).
Disentangling the role of visual and acoustic signals for fledgling recognition by baywings has proven to be difficult so far, but some advances have been made in understanding the function of begging call similarity in host manipulation. Playback experiments conducted at baywing nests during the nestling stage demonstrated that begging calls of screaming cowbird and host chicks were equally effective in eliciting parental provisioning, and more effective than non-mimetic shiny cowbird calls (Ursino et al., 2018). Indeed, shiny cowbird calls did not elicit any increase in provisioning rates from baywings compared to a silent control, despite being more exaggerated than those of baywing and screaming cowbird chicks (Gloag and Kacelnik, 2013; Ursino et al., 2018). Begging call similarity to host fledglings could play a key role in attracting the attention of baywing parents during the post-fledgling stage. This could be tested by using playback experiments to compare the response of adult baywings toward begging calls of conspecific, screaming cowbird (mimetic) and shiny cowbird (non-mimetic) fledglings. If baywings cue on acoustic signals to discriminate against alien fledglings, then they should be less responsive to non-mimetic begging calls than to own-species calls. And, if vocal similarity between screaming cowbird and baywing fledglings serve to avoid host discrimination, then baywings should respond similarly to conspecific and screaming cowbird begging calls. Cross-fostering experiments showed that baywing-like begging calls develop innately in screaming cowbirds. Despite slight variation in call structure with the host environment, screaming cowbird chicks reared in nests of another species retain the acoustic features that serve as recognition signals for baywings (Rojas Ripari et al., 2019b). These observations agree with the prediction of genetically fixed call similarity in host-specialist parasites for which modulating their calls in response to environmental cues could be maladaptive (Jamie and Kilner, 2017).
Screaming cowbirds and baywings have provided an excellent model to study visual and vocal mimicry at the last stage of the nesting cycle, but many questions are still unanswered. For example, it is yet to be determined how baywings integrate visual and vocal signals in fledgling recognition and what acoustic features of screaming cowbird begging calls are key to trick hosts during the post-fledgling stage. Also, the cognitive decision rules involved in fledgling discrimination by hosts are not well understood. Future studies that investigate the species-specific signals and cognitive mechanisms involved in fledgling recognition by baywings would help better illuminate the function and evolution of visual and acoustic manipulative signals in this parasitic cowbird.
Tuning Mechanisms to Exploit Host Parental Behavior Through Begging Displays
Begging Exaggeration
According to signaling models, begging behavior is modulated by the balance between the benefits of gaining extra resources through more vigorous displays and the potential costs that maintain signal honesty (Godfray, 1995; Kilner and Johnstone, 1997). The latter comprise physiological costs (Kilner, 2001; Soler et al., 2014), increased risk of nest predation (Haskell, 2002), or indirect costs due to competition with closely related nest mates (Trivers, 1974; Briskie et al., 1994; Caro et al., 2016, but see Bebbington and Kingma, 2017). Since obligate brood parasites are unrelated to their hosts, they are generally unconstrained by the inclusive fitness costs of begging (but see Rivers and Peer, 2016). Therefore, all things being equal, parasitic chicks are expected to beg more selfishly than those of non-parasitic species. Consistently with this, exaggerated begging displays are ubiquitous among evictor and non-evictor parasites (Redondo, 1993). Depending on the taxa, the exaggeration can manifest in traits such as rapid call rates (Davies et al., 1998; Kilner et al., 1999), long begging bouts (Redondo, 1993), tremulous or repetitive begging call structure (Gloag and Kacelnik, 2013), more vigorous displays (Redondo, 1993; Soler et al., 1995; Dearborn and Lichtenstein, 2002; Grim, 2008a) or brightly colored gapes (Álvarez, 2004; Tanaka et al., 2011).
The general view of parasites exhibiting increased levels of begging relative to host chicks is supported by quantitative studies conducted in cowbirds (Molothrus spp.; Lichtenstein and Sealy, 1998; Lichtenstein, 2001b; Bortolato et al., 2019; Figure 2A), great spotted cuckoo (Clamator glandarius; Redondo, 1993; Soler et al., 2012; Bolopo et al., 2015) and common cuckoo (Kilner and Davies, 1999; Kilner et al., 1999). Despite its exaggeration, however, empirical evidence suggests that begging in brood parasitic chicks is still informative regarding their level of need. Begging honesty in parasites has been tested experimentally by manipulating short-term need of parasitic chicks using food deprivation and hand-feeding treatments. In general, these experiments show that begging intensity increases with deprivation time and decreases after satiation, as predicted by honest signaling theory (Kilner and Davies, 1999; Lichtenstein, 2001b; Hauber and Ramsey, 2003; Lichtenstein and Dearborn, 2004; Soler et al., 2012; but see Rivers, 2007). In addition, begging levels can increase with age, as older chicks demand more food (Kilner and Davies, 1999; Butchart et al., 2003; Tuero et al., 2016). The observed effects of short-term need on begging behavior suggest that direct costs of begging could set a limit to begging exaggeration in brood parasites. However, data supporting this hypothesis are scarce. There is some experimental evidence that begging calls of parasitic chicks can increase nest predation risk (Dearborn, 1999; Ibáñez-Álamo et al., 2012), but the detection of physiological costs remains elusive (e.g., Martín-Gálvez et al., 2012).

Figure 2. (A) Shiny cowbird (Molothrus bonariensis) chicks (center) exhibit exaggerated begging displays in nests of a common host, the chalk-browed mockingbird (Mimus saturninus), where they compete strongly with host nest mates for parental feedings. (B) Whistling hawk-cuckoo (Hierococcyx nisicolor) chicks display a yellow wing-patch during begging that would serve to simulate an extra gape in host nests. (C) White palatal papillae of great-spotted cuckoo (Clamator glandarius) chicks (center) play a role in stimulating parental feedings from its magpie (Pica pica) host. Photo credits: (A) Vanina Fiorini, (B) Keita Tanaka, (C) Manuel Soler.
Exaggerated begging signals of parasitic chicks likely serve to gain resources from their hosts. However, few studies have clearly demonstrated a function of begging exaggeration in manipulating host parental behavior (Soler, 2017). The strongest evidence comes from the very rapid call rates of common cuckoo chicks that stimulate adult reed warblers to provision them at the same rate as an entire host brood (Davies et al., 1998; Kilner et al., 1999). This is because this host integrates visual (i.e., displayed gape area) and vocal (i.e., call rate) signals in a similar manner when provisioning unparasitized and parasitized nests, and cuckoo chicks exploit this rule in their favor by calling at a rate that compensates for the deficient visual stimuli provided by its single gape (Kilner et al., 1999). More recently, a study in the non-evictor shiny cowbird suggests that this species’ long and tremulous begging calls could act like a rapid call rate, stimulating higher provisioning rates from both common hosts and non-host species with shorter, monosyllabic begging calls (Gloag and Kacelnik, 2013). However, more studies are needed to better understand how cowbird hosts integrate visual and vocal begging signals, and the function of tremulous calls in host manipulation (Gloag and Kacelnik, 2013). In the closely related brown-headed cowbird (M. ater), parasitic chicks reared alone in nests of Bell’s vireo (Vireo bellii) were fed less than a host’s modal brood, suggesting that their faster and more repetitive calls did not fully compensate for deficient visual stimulation (Rivers et al., 2014).
Other studies that compared host provisioning rates between parasitized and unparasitized nests (e.g., Soler et al., 1995; Dearborn et al., 1998; Glassey and Forbes, 2003; Rivers et al., 2010; Ursino et al., 2011; Precioso et al., 2020), or food acquisition by parasitic and host chicks in mixed broods (e.g., Lichtenstein, 2001a; Lichtenstein and Dearborn, 2004; Rivers et al., 2010; Gloag et al., 2012; Bolopo et al., 2015; Bortolato et al., 2019) show conflicting results about the effect of exaggerated begging displays on host parental behavior. Accumulated data from non-evictor parasites indicate that begging exaggeration in these species would not be a key factor per se for securing sufficient provisioning (see Soler, 2017 for a recent review); rather, the success of parasitic chicks in mixed broods appears to be more dependent on their size relative to that of host nest mates and the ability to modulate begging effort according to the host environment (Lichtenstein and Sealy, 1998; Soler, 2002; Rivers, 2007; Rivers et al., 2010; Tuero et al., 2016; Bortolato et al., 2019). Disentangling how the multiple attributes of parasitic chicks (e.g., larger size relative to hosts, earlier hatching, and begging behavior) determine their competitive ability in mixed broods (Hauber, 2003) is important to better understand the function of begging exaggeration. Furthermore, it remains an open question whether begging exaggeration in parasitic chicks itself has evolved as an adaptation to parasitism. Two experimental studies have failed to find differences in the begging intensity and the effectiveness to stimulate parental feedings between brown-headed cowbird chicks and those of a related non-parasitic blackbird (Rivers et al., 2013; Li and Hauber, 2021). The lack of comparative studies represents a major gap in the study of begging evolution in brood parasites. Phylogenetic analyses or, at least, further comparisons between parasites and closely related non-parasitic species would be of great help to understand if exaggerated signals evolved specifically for the parasitic lifestyle.
The challenge of stimulating parental care may continue for several days or weeks after parasites fledge from host nests, until they attain nutritional independence. However, begging behavior in parasitic fledglings is poorly known (Hauber and Ramsey, 2003; Grim, 2008a; Tyller et al., 2018). It is possible that begging exaggeration is more relevant for attracting parental care and competing for parental feedings during the post-fledgling stage, but this idea needs to be examined.
Host-Attuned Begging Calls
Parasites that are host-generalists may benefit from varying their begging calls depending on the rearing host species if such fine-tuning allows them to better exploit the provisioning effort of any given host (McLean and Waas, 1987; Butchart et al., 2003; Jamie and Kilner, 2017). Plasticity in begging call development provides a way for parasitic chicks to rapidly attune call rate and/or call structure to different parent-offspring communication systems (Butchart et al., 2003; Jamie and Kilner, 2017). Evidence supporting this mechanism comes from cross-fostering experiments in the Horsfield’s bronze cuckoo, a parasite species that exhibit host-specific begging calls (Langmore et al., 2008). Parasitic females are host-generalist at individual level (Joseph et al., 2002). As mentioned in the previous section, they primarily parasitize fairy-wrens (Malurus sp.), but can use a variety of secondary hosts, including thornbills (Acanthiza spp.; Brooker and Brooker, 1989; Joseph et al., 2002). Langmore et al. (2008) cross-fostered cuckoo eggs from nests of superb fairy-wrens (M. cyaneus) to nests of buff-rumped thornbills (A. reguloides) to study begging call development in parasitic chicks. Their results revealed that cross-fostered chicks initially mimic the acoustic structure of fairy-wren calls, indicating that this vocal trickery is innate; however, within a few days after hatching, the chicks modified their call structure to match that of thornbill’s begging calls (Langmore et al., 2008). A plausible explanation is that changes in call structure were shaped by adult thornbills if, through the adjustment of food delivery rates, they reinforced the begging calls that more accurately matched their own species’ calls (Langmore et al., 2008). Experimental tests to see how thornbills respond toward mimetic and non-mimetic begging calls has not been conducted yet, and more studies are needed to better understand how Horsfield’s bronze cuckoo chicks learn to refine call structure in nests of thornbills and other secondary hosts. Nonetheless, these results suggest that both trickery and tuning adaptations can occur through chick development in host-generalist parasites.
The idea that parasitic chicks could learn to modify their begging calls to make them more profitable was first experimentally tested by Madden and Davies (2006) in common cuckoos. This species has distinct host-races each specializing in a single host (Gibbs et al., 2000). Cuckoo chicks do not mimic the begging calls of their respective hosts, but some differences in begging call features between host-races suggest that they could tune their calls in a host-specific manner to better stimulate provisioning (Butchart et al., 2003). Madden and Davies (2006) transferred cuckoo eggs or newly hatched chicks from reed warbler nests to nests of dunnocks (Prunella modularis) and robins (Erithacus rubecula). Cuckoo chicks cross-fostered to dunnock nests developed begging calls that were acoustically different from those of cuckoos reared by reed warblers, but similar to the begging calls of cuckoo chicks naturally reared in dunnock nests (Madden and Davies, 2006). The authors conducted an additional experiment in which they broadcast begging calls of 6–9 days old “dunnock-cuckoos” and “reed warbler-cuckoos” at nests of reed warblers, dunnocks and robins containing either a single blackbird (Turdus merula) or song thrush (T. philomelos) chick, similar in size to the cuckoo chick (Madden and Davies, 2006). Hosts responded differentially to each playback type, with dunnocks provisioning at higher rates in response to “dunnock-cuckoo” calls and the other host species showing the opposite trend (Madden and Davies, 2006). These results are consistent with a scenario in which begging call structure is not genetically fixed and parasitic chicks can modify their begging calls through their provisioning experience with a particular host (Madden and Davies, 2006). Jamie and Kilner (2017) termed this mode of begging call development as genetically polymorphic reaction norms in their proposed theoretical framework. According to it, parasitic chicks of distinct host-races attune their begging calls to the rearing host, while retaining certain call signatures of their own host-race (Madden and Davies, 2006).
The above-mentioned studies have provided important insights regarding the role of learning in begging call development and the ways in which parasitic cuckoos can tune their begging calls into different communication systems. However, two studies cast some doubts about the extent of polymorphism in begging call structure across common cuckoo host-races, and the ubiquity of host-attuned begging calls as a mechanism for host manipulation in common cuckoos. On the one hand, Samaš et al. (2020) failed to find differences in begging call rate and structure, after accounting for chick age and sex, between cuckoo chicks from nests of reed warblers and great reed warblers (A. arundinaceus), in contrast with a previous study that included these host-races (Butchart et al., 2003). The authors argued, based on these results, that begging development in common cuckoo chicks would better fit a genetically fixed bet-hedging strategy, rather than the proposed genetically polymorphic reaction norm (Jamie and Kilner, 2017). On the other hand, Soler (2017) reported original data on begging calls of cuckoo chicks recorded at nests of rufous-tailed scrub robin (Cercotrichas galactotes), robins and great reed warblers. Contrary to the expectation of host-attuned begging calls, begging call rates did not differ among host-races, despite substantial differences in this parameter between the respective hosts’ broods (Soler, 2017). Moreover, call rate of cuckoo chicks was more variable within than among host-races, which can be attributed to cuckoo chicks in the sample exhibiting three different call types, none of them exclusive to any particular host (Soler, 2017). Although sample sizes for these analyses were rather small, the results are consistent with the idea that common cuckoos could be more reliant on a bet-hedging strategy (based, for example, on call rate exaggeration) to elicit sufficient provisioning (Soler, 2017).
The role of experience in begging call development has also been examined in the great-spotted cuckoo. Roldán et al. (2013) quantified the begging calls of great-spotted cuckoo chicks from a reciprocal cross-fostering experiment between nests of its primary host, the magpie (Pica pica), and nests of carrion crows (Corvus corone corone). Contrary to earlier suggestions (Redondo et al., 1988), begging calls of parasitic chicks did not resemble those of host young, neither in magpie nor carrion crow nests (Roldán et al., 2013). Calls were acoustically similar between host species, but the number of notes per call was higher for chicks reared in magpie nests, consistent with the hypothesis that great-spotted cuckoo chicks modified their calls after hatching according to the rearing environment (Roldán et al., 2013). However, there are two important caveats to this conclusion. First, as the authors themselves point out, host-specific variation in call structure was largely restricted to the number of notes per call, which suggests that chicks could have been adjusting their begging effort rather than the acoustic properties of the begging calls (Roldán et al., 2013). Second, whether the observed variation in begging calls is adaptive and socially shaped by the foster parents cannot be established without playback experiments that test host response toward begging calls of cuckoo chicks from magpie and carrion crow nests.
Sensory Exploitation
Tuning through sensory exploitation, as considered here, involves the use of signals that are not part of the host’s parent-offspring communication system but serve parasites to effectively exploit pre-existing host’s sensory biases (Tanaka and Ueda, 2005b). An interesting example is found in Horsfield’s hawk-cuckoo (Hierococcyx hyperythrus). The chicks of this species pose a conspicuous yellow skin patch on the underside of each wing that is displayed during begging (Tanaka and Ueda, 2005a). By dying the wing-patch black, Tanaka and Ueda (2005a) demonstrated that it plays a role in stimulating provisioning from its host, the red-flanked bush robin (Tarsiger cyanurus). The authors proposed that wing-patches would serve to simulate additional gapes, based on the observation that hosts occasionally attempted to place food onto them when parasitic chicks flapped their wings (Tanaka and Ueda, 2005a). A similar wing-patch begging strategy has been recently reported in a closely related species, the whistling hawk-cuckoo (H. nisicolor), and it is possible that it occurs in two other species of the same clade (Luo et al., 2019; Figure 2B). The authors hypothesized that the evolution of exuberant begging calls in hawk-cuckoos like those of common cuckoo chicks might be constrained by high predation pressure on host nests (Tanaka and Ueda, 2005a; Luo et al., 2019). Under this scenario, wing-patch begging may pose an alternative evolutionary solution to the problem of having to compensate for a deficient gape area without incurring extra predation costs (Tanaka and Ueda, 2005a; Tanaka et al., 2011; Luo et al., 2019). Additional comparisons of visual signals using avian vision models suggest that the gape and colored wing-patches of cuckoos are more conspicuous than the gape of host chick from the host’s perspective (Tanaka et al., 2011), further supporting the idea that Horsfield’s hawk cuckoos would have evolved traits that act as supernormal stimuli (Dawkins, 1976; Noble et al., 1999). However, the hypothesis that gape-like wing-patches would play a role analogous to common cuckoo’s rapid call rates needs experimental testing. Wing-shaking, as performed by hawk-cuckoo chicks, is a widespread component of begging displays among parasitic and non-parasitic birds (Grim, 2008b). It would be useful in the future to examine if wing-shaking begging is already present in hosts of hawk-cuckoos and how it influences host provisioning behavior (Grim, 2008b). This would help better understand if wing-shaking could have served as a pre-adaptation for the evolution of colored wing-patches in these parasites (Grim, 2008b).
The bright red gape of common cuckoo chicks was formerly considered an irresistible stimulus acting upon the host’s nervous system (Dawkins, 1976). This idea received little support because, although there is some evidence that cuckoo chicks have redder gapes than host chicks (Kilner, 1999), experimental tests involving artificial dying of chick gapes in three host species failed to find the expected host preference for redder gapes (Noble et al., 1999). Nevertheless, since these studies were based on human color perception, it would be useful to re-evaluate the function of gape color in parasite chicks from an avian perspective. It is possible that colorful gapes in cuckoo chicks play at least some role in stimulating provisioning under certain situations, such as in host species that rely more on visual than auditory begging cues (Kilner and Davies, 1999; Álvarez, 2004) or in dark nests, where redder gapes may serve to increase chick detectability (Kilner, 1999). Alternatively, the red gape color in parasitic cuckoos could be maintained by phylogenetic constraints given that this trait is also found in some non-parasitic species within the Cuculidae family and there is no evidence that gape color in cuckoos had changed as a result of evolutionary interactions with their hosts (Kilner, 1999).
Sensory exploitation may play a role in host manipulation by great spotted cuckoos (Tanaka and Ueda, 2005b). The chicks of this species exhibit white palatal papillae, a trait that is absent in magpie chicks and influences food allocation within parasitized broods (Soler et al., 1995; Figure 2C). Using a repeated-measures design, Soler et al. (1995) showed that parasitic chicks were fed at lower rates when they had their papillae masked with red paint (i.e., the gape color of magpie chicks) than when they were left unpainted. Nest provisioning also decreased after masking the chick’s papillae, and painted chicks lost their competitive advantage relative to magpie chicks (Soler et al., 1995). These results suggest that palatal papillae in great spotted cuckoos would serve as a tuning adaptation that exploits preexisting host’s sensory biases (Soler et al., 1995). Future studies that disentangle the effects of this trait and other visual and acoustic begging features in stimulating parental provisioning are necessary to corroborate this idea.
Concluding Remarks and Future Directions of Research
Brood-parasitic young possess many morphological and behavioral traits that allow them to exploit the parental behavior of their hosts to their own benefit. However, the study of trickery and tuning adaptations during the nestling and fledgling stages have historically received less attention than those deployed during the egg stage. The discovery of chick rejection behaviors in hosts of bronze cuckoos nearly 20 years ago has led to renewed interest about co-evolved adaptations between parasitic chicks and their hosts. In recent years, new evidence has accumulated on host chick mimicry in evictor and non-evictor parasites driven by host discrimination against alien young. These studies highlight two aspects of this trickery adaptation that are important to consider in future research. First, host chick mimicry can occur in more than one sensory modality within a single parasite lineage. This observation begs for more research into how hosts integrate visual and acoustic signals in chick recognition to better understand the adaptive value of multimodal resemblance to host young in parasitic chicks. Second, even clearly mimetic parasites may show discrepancies with respect to the phenotype of host chicks. Such imperfect mimicry may be owing to additional adaptations in parasitic chicks for better tuning into the sensory preferences of host species (e.g., exaggerated begging traits), which could serve to compete for food with host nestmates or extract additional resources from their hosts. Alternatively, the discrepancies could be neutral or reflect evolutionary constraints on parasites to match more precisely the begging signals of host chicks. To tackle these questions, it is crucial to combine quantitative analyses of similarity that take into account the host’s perspective (e.g., avian vision models) with experimental manipulations (e.g., cross-fostering, playback experiments, and phenotype manipulation). Indeed, the application of objective methods for assessing the extent of visual or vocal resemblance to host chicks across more parasite species could certainly help unravel new cases of coevolved host chick mimicry. Phylogenetic studies are also necessary to disentangle the evolutionary pathways that gave rise to host chick mimicry across parasite lineages.
The study of begging behavior in the context of brood parasitism has received considerable attention over the past 30 years, from both empirical and theoretical perspectives. A pattern that has emerged from this body of knowledge is that begging in brood parasites is often exaggerated but informative about chick need, although the costs of maintaining signal honesty are not well understood. Exaggerated begging displays are widespread across parasite species and likely adaptive as a mechanism to exploit host’s response toward non-specific begging traits that signal offspring need. However, it has become increasingly clear that its role in securing sufficient provisioning from hosts depends on many other factors including the parasitism strategy (evictor or non-evictor), the relative size of parasitic chicks to their hosts, and how hosts integrate begging signals in making decisions about provisioning effort and food allocation within broods. More experimental and comparative studies on a broader range of parasite species are necessary to better understand the function and evolution of signal exaggeration in parasitic birds. This is especially true if we consider that research on this subject comprises only a minority (∼13%) of the parasite species and, even within those more extensively studied, data are limited to a narrow range of host-parasite associations. Likewise, the study of begging behavior and its role in host manipulation during the fledgling stage represents another major gap, often neglected in the literature on brood parasitism. For instance, little is known yet about host-parasite interactions beyond the nestling stage and the extent to which trickery and tuning adaptations similar to those observed in parasitic nestlings play a role after the young have left the nest.
In this review, we differentiate between begging exaggeration and sensory exploitation mechanisms based on whether parasitic manipulation is based on signals already used in host-parent offspring communication or not. This categorization becomes somewhat diffuse since parasites may exhibit traits that are actually absent in host chicks but imitate host begging signals, as it is the case of the colored wing-patches resembling yellow gapes in hawk cuckoos. Yet, we found this distinction useful to highlight alternative routes to the evolution of manipulative traits in parasitic chicks.
The role of learning in attuning the begging signals to the host environment provides another interesting venue for future research. The ability to modify call structure according to the host environment is a flexible mechanism for tuning into host’ acoustic communication in parasite species that are host-generalists at the population level, like the common cuckoo. However, more work is needed to solve discrepancies between studies and see if generalizations can be made regarding how begging calls develop across common cuckoo host-races. Varying levels of plasticity in begging call features have been observed in other parasite species, but in most cases, the specific function of begging call structure in host manipulation has not been assessed. Hence, it is difficult to say if the observed variation reflects an underlying tuning adaptation that makes begging signals more effective to stimulate provisioning in any given host. As it happens with the study of host chick mimicry, sound-spectrogram analyses must be combined with playback experiments to answer these questions. It is interesting to point out that the studies on begging call development have also revealed that parasitic chicks can use different mechanisms of host manipulation throughout their early life. This is clearly illustrated by Horsfield’s bronze cuckoos, which innately develop vocal mimicry of its primary host, but if reared by another host species, they can attune their begging calls to this new host within a few days of hatching.
How parasitic chicks tune into host communication channels to obtain sufficient food is a long-standing question that has promoted fruitful research. This review provides an overview of the advances in the study of how parasitic young evade host defenses and attune their begging signals to tap into host provisioning rules. It also outlines some unanswered questions and emphasizes the need that take into account the host’s perspective when assessing the existence of mimicry or sensory exploitation in parasitic chicks. In the future, an integrative approach that take into account the function, ecology, evolution and ontogeny of the manipulative signals displayed by parasitic chicks will increase our knowledge about the ways in which parasites are adapted to exploit the parental care of their hosts.
Author Contributions
JRR and MDM conducted the literature survey, organized the information, and wrote the first draft of the manuscript. All authors contributed to the conception and design of the review, and manuscript revision, read, and approved the submitted version.
Funding
CU was supported by a Presidential Postdoctoral Research Fellowship of Princeton University. JRR and CU have scholarships from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). JCR and MDM were research fellows of CONICET. This work was funded by grants from Agencia Nacional Científica y Tecnológica (PICT-2018-03622 and PICT-2019-00381).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful for the opportunity to contribute to this special topic and editorial team for their support in such difficult pandemic times. We thank Justin Schuetz, Naomi Langmore, Alec Earnshaw, Vanina Fiorini, Keita Tanaka, and Manuel Soler for kindly providing as pictures of parasitic chicks, and three reviewers for their constructive comments on earlier drafts of the manuscript.
References
Álvarez, F. (2004). The conspicuous gape of the nestling common cuckoo Cuculus canorus as a supernormal stimulus for rufous bush chat Cercotrichas galactotes hosts. Ardea 92, 63–68.
Anderson, M. G., Ross, H. A., Brunton, D. H., and Hauber, M. E. (2009). Begging call matching between a specialist brood parasite and its host: a comparative approach to detect coevolution. Biol. J. Linn. Soc. 98, 208–216. doi: 10.1111/j.1095-8312.2009.01256.x
Attisano, A., Sato, N. J., Tanaka, K. D., Okahisa, Y., Kuehn, R., Gula, R., et al. (2018). Visual discrimination of polymorphic nestlings in a cuckoo-host system. Sci. Rep. 8:10359. doi: 10.1038/s41598-018-28710-5
Bebbington, K., and Kingma, S. A. (2017). No evidence that kin selection increases the honesty of begging signals in birds. Evol. Lett. 1, 132–137. doi: 10.1002/evl3.18
Bolopo, D., Canestrari, D., Roldán, M., Baglione, V., and Soler, M. (2015). High begging intensity of great spotted cuckoo nestlings favours larger-size crow nest mates. Behav. Ecol. Sociobiol. 69, 873–882. doi: 10.1007/s00265-015-1895-z
Bortolato, T., Gloag, R., Reboreda, J. C., and Fiorini, V. D. (2019). Size matters: shiny cowbirds secure more food than host nestmates thanks to their larger size, not signal exaggeration. Anim. Behav. 57, 201–207. doi: 10.1016/j.anbehav.2019.09.009
Briskie, J. V., Naugler, C. T., and Leech, S. M. (1994). Begging intensity of nestling birds varies with sibling relatedness. Proc. R. Soc. B 258, 73–78. doi: 10.1098/rspb.1994.0144
Brooker, M. G., and Brooker, L. C. (1989). The comparative breeding behaviour of two sympatric cuckoos, Horsfield’s Bronze-Cuckoo Chrysococcyx basalis and the Shining Bronze-Cuckoo C. lucidus, in Western Australia: a new model for the evolution of egg morphology and host specificity in avian brood parasites. Ibis 131, 528–547. doi: 10.1111/j.1474-919X.1989.tb04789.x
Burford, J. E., Friedrich, T. J., and Yasukawa, K. E. N. (1998). Response to playback of nestling begging in the red-winged blackbird, Agelaius phoeniceus. Anim. Behav. 56, 555–561. doi: 10.1006/anbe.1998.0830
Butchart, S. H. M., Kilner, R. M., Fuisz, T., and Davies, N. B. (2003). Differences in the nestling begging calls of hosts and host-races of the common cuckoo, Cuculus canorus. Anim. Behav. 65, 345–354. doi: 10.1006/anbe.2003.2066
Caro, S. M., West, S. A., and Griffin, A. S. (2016). Sibling conflict and dishonest signaling in birds. Proc. Nat. Acad. Sci. U.S.A. 113, 13803–13808. doi: 10.1073/pnas.1606378113
Colombelli-Négrel, D., Hauber, M. E., Robertson, J., Sulloway, F. J., Hoi, H., Griggio, M., et al. (2012). Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. doi: 10.1016/j.cub.2012.09.025
Davies, N. B. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., Kilner, R. M., and Noble, D. G. (1998). Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B 265, 673–678. doi: 10.1098/rspb.1998.0346
De Mársico, M. C., Fiorini, V. D., Tuero, D. T., Gloag, R., Ursino, C. A., and Reboreda, J. C. (2017). “Parasite adaptations during the nestling and fledgling stages,” in Avian Brood Parasitism, ed. M. Soler (Cham: Springer), 557–574. doi: 10.1098/rspb.2012.0612
De Mársico, M. C., Gantchoff, M. G., and Reboreda, J. C. (2012). Host–parasite coevolution beyond the nestling stage? Mimicry of host fledglings by the specialist screaming cowbird. Proc. R. Soc. B 279, 3401–3408.
Dearborn, D. C. (1999). Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk 116, 448–457. doi: 10.2307/4089378
Dearborn, D. C., Anders, A. D., Thompson, F. R. III, and Faaborg, J. (1998). Effects of cowbird parasitism on parental provisioning and nestling food acquisition and growth. Condor 100, 326–334. doi: 10.2307/1370273
Dearborn, D. C., and Lichtenstein, G. (2002). “Begging behaviour and host exploitation in parasitic cowbirds,” in The Evolution of Begging, eds J. Wright and M. L. Leonard (Dordrecht: Springer), 361–387. doi: 10.1037/0735-7036.117.1.24
Feeney, W. E., Welbergen, J. A., and Langmore, N. E. (2014). Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246. doi: 10.1146/annurev-ecolsys-120213-091603
Fraga, R. M. (1998). “Interactions of the parasitic Screaming and Shiny Cowbirds (Molothrus rufoaxillaris and M. bonariensis) with a shared host, the Bay-winged Cowbird (M. Badius),” in Brood Parasites and Their Hosts: Studies in Coevolution, eds S. K. Robinson and Rothstein (Oxford: Oxford University Press), 173–193.
Gibbs, H. L., Sorenson, M. D., Marchetti, K., Brooke, M. D. L., Davies, N. B., and Nakamura, H. (2000). Genetic evidence for female host-specific races of the common cuckoo. Nature 407, 183–186. doi: 10.1038/35025058
Gill, F., Donsker, D., and Rasmussen, P. C. (2017). IOC World Bird List (version 11.2). Available online at: http://www.worldbirdnames.org/ (accessed October 30, 2021).
Glassey, B., and Forbes, S. (2003). Why brown-headed cowbirds do not influence red-winged blackbird parent behaviour. Anim. Behav. 65, 1235–1246. doi: 10.1006/anbe.2003.2168
Gloag, R., and Kacelnik, A. (2013). Host manipulation via begging call structure in the brood-parasitic shiny cowbird. Anim. Behav. 86, 101–109. doi: 10.1016/j.anbehav.2013.04.018
Gloag, R., Tuero, D. T., Fiorini, V. D., Reboreda, J. C., and Kacelnik, A. (2012). The economics of nestmate killing in avian brood parasites: a provisions trade-off. Behav. Ecol. 23, 132–140. doi: 10.1093/beheco/arr166
Godfray, H. C. J. (1995). Signaling of need between parents and young: parent-offspring conflict and sibling rivalry. Am. Nat. 146, 1–24.
Grim, T. (2005). Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 84, 69–78. doi: 10.1111/j.1095-8312.2005.00414.x
Grim, T. (2006). The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 8, 785–802.
Grim, T. (2008a). Begging behavior of fledgling rusty-breasted cuckoo (Cacomantis sepulcralis). Wilson J. Ornithol. 120, 887–890. doi: 10.1676/07-145.1
Grim, T. (2008b). Wing-shaking and wing-patch as nestling begging strategies: their importance and evolutionary origins. J. Ethol. 26, 9–15. doi: 10.1007/s10164-007-0037-0
Hart, N. S., Partridge, J. C., Bennett, A. T. D., and Cuthill, I. C. (2000a). Visual pigments, cone oil droplets and ocular media in four species of estrildid finch. J. Comp. Physiol. A 186, 681–694. doi: 10.1007/s003590000121
Hart, N. S., Partridge, J. C., Cuthill, I. C., and Bennett, A. T. (2000b). Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A 186, 375–387. doi: 10.1007/s003590050437
Haskell, D. G. (2002). “Begging behaviour and nest predation,” in The Evolution of Begging, eds J. Wright and M. L. Leonard (Dordrecht: Springer), 163–172. doi: 10.1007/0-306-47660-6_9
Hauber, M. E. (2003). Hatching asynchrony, nestling competition, and the cost of interspecific brood parasitism. Behav. Ecol. 14, 227–235. doi: 10.1093/beheco/14.2.227
Hauber, M. E., and Kilner, R. M. (2007). Coevolution, communication, and host chick mimicry in parasitic finches: Who mimics whom? Behav. Ecol. Sociobiol. 61, 497–503. doi: 10.1007/s00265-006-0291-0
Hauber, M. E., and Ramsey, C. K. (2003). Honesty in host-parasite communication signals: the case for begging by fledgling brown-headed cowbirds Molothrus ater. J. Avian Biol. 34, 339–344. doi: 10.1111/j.0908-8857.2003.03158.x
Hudson, W. H. (1874). Notes on the procreant instincts of the three species of Molothrus found in Buenos Ayres. Proc. Zool. Soc. Lond. 42, 153–174. doi: 10.1111/j.1096-3642.1874.tb02466.x
Ibáñez-Álamo, J. D., Arco, L., and Soler, M. (2012). Experimental evidence for a predation cost of begging using active nests and real chicks. J. Ornithol. 153, 801–807. doi: 10.1007/s10336-011-0797-8
Jamie, G. A., Hamama, S., Moya, C., Kilner, R. M., and Spottiswoode, C. N. (2021). Limits to host colonization and speciation in a radiation of parasitic finches. Behav. Ecol. 32, 529–538. doi: 10.1093/beheco/araa155
Jamie, G. A., and Kilner, R. M. (2017). “Begging call mimicry by brood parasite nestlings: adaptation, manipulation and development,” in Avian Brood Parasitism, ed. M. Soler (Cham: Springer), 517–538.
Jamie, G. A., Van Belleghem, S. M., Hogan, B. G., Hamama, S., Moya, C., Troscianko, J., et al. (2020). Multimodal mimicry of hosts in a radiation of parasitic finches. Evolution 74, 2526–2538. doi: 10.1111/evo.14057
Joseph, L., Wilke, T., and Alpers, D. (2002). Reconciling genetic expectations from host specificity with historical population dynamics in an avian brood parasite, Horsfield’s Bronze-Cuckoo Chalcites basalis of Australia. Mol. Ecol. 11, 829–837. doi: 10.1046/j.1365-294X.2002.01481.x
Kilner, R., and Johnstone, R. A. (1997). Begging the question: Are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 12, 11–15. doi: 10.1016/S0169-5347(96)10061-6
Kilner, R. M. (1999). Family conflicts and the evolution of nestling mouth colour. Behaviour 136, 779–804.
Kilner, R. M. (2001). A growth cost of begging in captive canary chicks. Proc. Nat. Acad. Sci. U.S.A. 98, 11394–11398. doi: 10.1073/pnas.191221798
Kilner, R. M., and Davies, N. B. (1999). How selfish is a cuckoo chick? Anim. Behav. 58, 797–808. doi: 10.1006/anbe.1999.1197
Kilner, R. M., Noble, D. G., and Davies, N. B. (1999). Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397, 667–672. doi: 10.1038/17746
Langmore, N. E., Hunt, S., and Kilner, R. M. (2003). Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160. doi: 10.1038/nature01460
Langmore, N. E., Maurer, G., Adcock, G. J., and Kilner, R. M. (2008). Socially acquired host-specific mimicry and the evolution of host races in Horsfield’s bronze-cuckoo Chalcites basalis. Evolution 62, 1689–1699. doi: 10.1111/j.1558-5646.2008.00405.x
Langmore, N. E., Stevens, M., Maurer, G., Heinsohn, R., Hall, M. L., Peters, A., et al. (2011). Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. doi: 10.1098/rspb.2010.2391
Lanyon, S. (1992). Interspecific brood parasitism in blackbirds (Icterinae): a phylogenetic perspective. Science 255, 77–79. doi: 10.1126/science.1553533
Leonard, M. L., and Horn, A. G. (2001). Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor). Behav. Ecol. Sociobiol. 49, 170–175. doi: 10.1007/s002650000290
Li, D., and Hauber, M. E. (2021). Parasitic begging calls of nestmate-evictor common cuckoos stimulate more parental provisions by red-winged blackbirds than calls of nest-sharing brown-headed cowbirds. Behav. Ecol. Sociobiol. 75, 1–9. doi: 10.1007/s00265-020-02955-5
Lichtenstein, G. (2001b). Selfish begging by screaming cowbirds, a mimetic brood parasite of the bay-winged cowbird. Anim. Behav. 61, 1151–1158. doi: 10.1006/anbe.2000.1688
Lichtenstein, G. (2001a). Low success of shiny cowbird chicks parasitizing rufous-bellied thrushes, chick-chick competition or parental discrimination? Anim. Behav. 61, 401–413. doi: 10.1006/anbe.2000.1595
Lichtenstein, G., and Dearborn, D. C. (2004). Begging and short-term need in cowbird nestlings, how different are brood parasites? Behav. Ecol. Sociobiol. 56, 352–359. doi: 10.1007/s00265-004-0795-4
Lichtenstein, G., and Sealy, S. G. (1998). Nestling competition, rather than supernormal stimulus, explains the success of parasitic brown-headed cowbird chicks in yellow warbler nests. Proc. R. Soc. B 265, 249–254. doi: 10.1098/rspb.1998.0289
Luo, K., Feng, L., Lu, Z., Li, D., and Quan, R. C. (2019). Novel instance of brood parasitic cuckoo nestlings using bright yellow patches to mimic gapes of host nestlings. Wilson J. Ornithol. 131, 686–693. doi: 10.1676/18-168
Madden, J. R., and Davies, N. B. (2006). A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. R. Soc. B 273, 2343–2351. doi: 10.1098/rspb.2006.3585
Martín-Gálvez, D., De Neve, L., Pérez-Contreras, T., Soler, M., Martínez, J. G., and Soler, J. J. (2012). Manipulation of hunger levels affects great spotted cuckoo and magpie host nestlings differently. J. Avian Biol. 43, 531–540. doi: 10.1111/j.1600-048X.2012.05597.x
McLean, I. G., and Waas, J. R. (1987). Do cuckoo chicks mimic the begging calls of their hosts? Anim. Behav. 35, 1896–1898. doi: 10.1016/S0003-3472(87)80083-0
Moreno-Rueda, G., Soler, M., Martín-Vivaldi, M., and Palomino, J. J. (2009). Brood provisioning rate and food allocation rules according to nestling begging in a clutch-adjusting species, the Rufous-tailed Scrub-robin Cercotrichas galactotes. Acta Ornithol. 44, 167–175. doi: 10.3161/000164509X482740
Noble, D. G., Davies, N. B., Hartley, I. R., and McRae, S. B. (1999). The red gape of the nestling Cuckoo (Cuculus canorus) is not a supernormal stimulus for three common cuckoo hosts. Behaviour 136, 759–777. doi: 10.1163/156853999501559
Noh, H. J., Gloag, R., and Langmore, N. E. (2018). True recognition of nestlings by hosts selects for mimetic cuckoo chicks. Proc. R. Soc. B 285:20180726. doi: 10.1098/rspb.2018.0726
Noh, H. J., Gloag, R., Leitão, A. V., and Langmore, N. E. (2021). Imperfect mimicry of host begging calls by a brood parasitic cuckoo: a cue for nestling rejection by hosts? Curr. Zool. [Epub ahead of print]. doi: 10.1093/cz/zoab056
Payne, R. B. (2005). Nestling Mouth Markings and Colors of Old World Finches Estrildidae: Mimicry and Coevolution of Nesting Finches and their Vidua Brood Parasites. (Ann Arbor, MI: University of Michigan), 194.
Payne, R. B., and Payne, L. L. (2002). “Begging for parental care from another species: specialization and generalization in brood-parasitic finches,” in The Evolution of Begging, eds J. Wright and M. L. Leonard (Dordrecht: Springer), 429–449. doi: 10.1007/0-306-47660-6_22
Payne, R. B., Woods, J. L., and Payne, L. L. (2001). Parental care in estrildid finches: experimental tests of a model of Vidua brood parasitism. Anim. Behav. 62, 473–483. doi: 10.1006/anbe.2001.1773
Precioso, M., Molina-Morales, M., Sánchez-Tójar, A., Avilés, J. M., and Martínez, J. G. (2020). Brood parasitism, provisioning rates and breeding phenology of male and female magpie hosts. J. Avian Biol. 51:e02522. doi: 10.1111/jav.02522
Redondo, T. (1993). Exploitation of host mechanism for parental care by avian brood parasites. Etología 3, 235–297. doi: 10.1007/978-3-319-73138-4_13
Redondo, T., and Arias, and de Reyna, L. (1988). Vocal mimicry of hosts by great spotted cuckoo Clamator glandarius: further evidence. Ibis 130, 540–544. doi: 10.1111/j.1474-919X.1988.tb02720.x
Rivers, J. W. (2007). Nest mate size, but not short-term need, influences begging behavior of a generalist brood parasite. Behav. Ecol. 18, 222–230. doi: 10.1093/beheco/arl068
Rivers, J. W., Blundell, M. A., Loughin, T. M., Peer, B. D., and Rothstein, S. I. (2013). The exaggerated begging behaviour of an obligate avian brood parasite is shared with a nonparasitic close relative. Anim. Behav. 86, 529–536. doi: 10.1016/j.anbehav.2013.06.004
Rivers, J. W., Blundell, M. A., and Rothstein, S. I. (2014). Mismatched begging displays between foreign and host offspring reduce brood parasite fitness. Behav. Ecol. 25, 785–793. doi: 10.1093/beheco/aru055
Rivers, J. W., Loughin, T. M., and Rothstein, S. I. (2010). Brown-headed cowbird nestlings influence nestmate begging, but not parental feeding, in hosts of three distinct sizes. Anim. Behav. 79, 107–116. doi: 10.1016/j.anbehav.2009.10.009
Rivers, J. W., and Peer, B. D. (2016). Relatedness constrains virulence in an obligate avian brood parasite. Ornithol. Sci. 15, 191–201. doi: 10.2326/osj.15.191
Rojas Ripari, J. M., Segura, L. N., Reboreda, J. C., and De Mársico, M. C. (2019a). Non-mimetic shiny cowbird nestlings escape discrimination by baywings in absence of host nest mates. Behav. Ecol. Sociobiol. 73, 1–9. doi: 10.1007/s00265-019-2749-x
Rojas Ripari, J. M., Ursino, C. A., Reboreda, J. C., and De Mársico, M. C. (2019b). Innate development of acoustic signals for host parent–offspring recognition in the brood-parasitic Screaming Cowbird Molothrus rufoaxillaris. Ibis 161, 717–729. doi: 10.1111/ibi.12672
Roldán, M., Soler, M., Márquez, R., and Soler, J. J. (2013). The vocal begging display of Great Spotted Cuckoo Clamator glandarius nestlings in nests of its two main host species: Genetic differences or developmental plasticity? Ibis 155, 867–876. doi: 10.1111/ibi.12088
Samaš, P., Žabková, K., Petrusková, T., Procházka, P., Požgayová, M., and Honza, M. (2020). Nestlings of the common cuckoo do not mimic begging calls of two closely related Acrocephalus hosts. Anim. Behav. 161, 89–94. doi: 10.1016/j.anbehav.2020.01.005
Sato, N. J., Tanaka, K. D., Okahisa, Y., Yamamichi, M., Kuehn, R., Gula, R., et al. (2015). Nestling polymorphism in a cuckoo-host system. Curr. Biol. 25, R1164–R1165. doi: 10.1016/j.cub.2015.11.028
Sato, N. J., Tokue, K., Noske, R. A., Mikami, O. K., and Ueda, K. (2010). Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. doi: 10.1098/rsbl.2009.0540
Schuetz, J. G. (2005). Reduced growth but not survival of chicks with altered gape patterns: implications for the evolution of nestling similarity in a parasitic finch. Anim. Behav. 70, 839–848. doi: 10.1016/j.anbehav.2005.01.007
Soler, M. (2002). “Breeding strategy and begging intensity: influences on food delivery by parents and host selection by parasitic cuckoos,” in The Evolution of Begging, eds J. Wright and M. L. Leonard (Dordrecht: Springer), 413–427. doi: 10.1007/0-306-47660-6_21
Soler, M. (2017). “Begging behaviour, food delivery and food acquisition in nests with brood parasitic nestlings,” in Avian Brood Parasitism, ed. M. Soler (Cham: Springer), 493–515.
Soler, M., De Neve, L., Roldán, M., Macías-Sánchez, E., and Martín-Gálvez, D. (2012). Do great spotted cuckoo nestlings beg dishonestly? Anim. Behav. 83, 163–169. doi: 10.1016/j.anbehav.2011.10.022
Soler, M., Martinez, J. G., Soler, J. J., and Møller, A. P. (1995). Preferential allocation of food by magpies Pica pica to great spotted cuckoo Clamator glandarius chicks. Behav. Ecol. Sociobiol. 37, 7–13. doi: 10.1007/BF00173893
Soler, M., Ruiz-Raya, F., Carra, L. G., Medina-Molina, E., Ibáñez-Álamo, J. D., and Martín-Gálvez, D. (2014). A long-term experimental study demonstrates the costs of begging that were not found over the short term. PLoS One 9:e111929. doi: 10.1371/journal.pone.0111929
Sorenson, M. D., Balakrishnan, C. N., and Payne, R. B. (2004). Clade-limited colonization in brood parasitic finches (Vidua spp.). Syst. Biol. 53, 140–153. doi: 10.1080/10635150490265021
Sorenson, M. D., Sefc, K. M., and Payne, R. B. (2003). Speciation by host switch in brood parasitic indigobirds. Nature 424, 928–931. doi: 10.1038/nature01863
Tanaka, K. D., Morimoto, G., Stevens, M., and Ueda, K. (2011). Rethinking visual supernormal stimuli in cuckoos, visual modeling of host and parasite signals. Behav. Ecol. 22, 1012–1019. doi: 10.1093/beheco/arr084
Tanaka, K. D., and Ueda, K. (2005b). Signal exploitation by parasitic young in birds, a new categorization of manipulative signals. Ornithol. Sci. 4, 49–54. doi: 10.2326/osj.4.49
Tanaka, K. D., and Ueda, K. (2005a). Horsfield’s hawk-cuckoo nestlings simulate multiple gapes for begging. Science 308, 653–653. doi: 10.1126/science.1109957
Tokue, K., and Ueda, K. (2010). Mangrove Gerygones Gerygone laevigaster eject Little Bronze-cuckoo Chalcites minutillus hatchlings from parasitized nests. Ibis 152, 835–839. doi: 10.1111/j.1474-919X.2010.01056.x
Tuero, D. T., Gloag, R., and Reboreda, J. C. (2016). Nest environment modulates begging behavior of a generalist brood parasite. Behav. Ecol. 27, 204–210. doi: 10.1093/beheco/arv140
Tyller, Z., Kysučan, M., and Grim, T. (2018). Postfledging behavior of the Common Cuckoo (Cuculus canorus) attended by the Chaffinch (Fringilla coelebs): a comprehensive approach to study the least-known stage of brood parasite–host coevolution. Wilson J. Ornithol. 130, 536–542. doi: 10.1676/16-223.1
Ursino, C. A., De Mársico, M. C., Sued, M., Farall, A., and Reboreda, J. C. (2011). Brood parasitism disproportionately increases nest provisioning and helper recruitment in a cooperatively breeding bird. Behav. Ecol. Sociobiol. 65, 2279–2286. doi: 10.1007/s00265-011-1238-7
Ursino, C. A., Facchinetti, C., and Reboreda, J. C. (2012). Preformative molt in brood parasitic screaming (Molothrus rufoaxillaris) and Shiny (M. bonariensis) Cowbirds. Ornitol. Neotrop. 23, 163–171.
Ursino, C. A., Gloag, R., Reboreda, J. C., and De Mársico, M. C. (2018). Host provisioning behavior favors mimetic begging calls in a brood-parasitic cowbird. Behav. Ecol. 29, 328–332. doi: 10.1093/beheco/arx167
Vorobyev, M., and Osorio, D. (1998). Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. doi: 10.1098/rspb.1998.0302
Keywords: brood parasitism, parent-offspring communication, begging, mimicry, host manipulation, sensory exploitation
Citation: Rojas Ripari JM, Ursino CA, Reboreda JC and De Mársico MC (2021) Tricking Parents: A Review of Mechanisms and Signals of Host Manipulation by Brood-Parasitic Young. Front. Ecol. Evol. 9:725792. doi: 10.3389/fevo.2021.725792
Received: 15 June 2021; Accepted: 08 November 2021;
Published: 03 December 2021.
Edited by:
Kevin R. Theis, Wayne State University, United StatesReviewed by:
Rose Thorogood, University of Helsinki, FinlandLuke Redmond, Penn State Schuylkill, United States
Samuel Slowinski, University of Maryland, College Park, United States
Copyright © 2021 Rojas Ripari, Ursino, Reboreda and De Mársico. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María C. De Mársico, de_marsico@ege.fcen.uba.ar
 Juan M. Rojas Ripari
Juan M. Rojas Ripari Cynthia A. Ursino
Cynthia A. Ursino Juan C. Reboreda
Juan C. Reboreda María C. De Mársico
María C. De Mársico