Ecological Restoration of Abies religiosa Forests Using Nurse Plants and Assisted Migration in the Monarch Butterfly Biosphere Reserve, Mexico
- 1Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), Morelia, Mexico
- 2Instituto de Investigaciones Agropecuarias y Forestales (IIAF), UMSNH, Morelia, Mexico
- 3Escuela Nacional de Estudios Superiores (ENES), Universidad Nacional Autónoma de México (UNAM), Morelia, Mexico
- 4British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development, Vernon, BC, Canada
- 5Cátedras CONACyT, Facultad de Biología, UMSNH, Morelia, Mexico
- 6Instituto de Investigaciones en Ecosistemas y Sustentabilidad, UNAM, Morelia, Mexico
- 7Southern Research Station, USDA Forest Service, Asheville, NC, United States
- 8Instituto de Investigaciones Sobre los Recursos Naturales (INIRENA), UMSNH, Morelia, Mexico
Increasing disturbance events (forest fires, windstorms, pest outbreaks) associated with climate change are creating new ecological restoration challenges. Here, we examine the utility of assisted migration in combination with naturally established nurse plants in order to improve the success of afforestation with Abies religiosa (sacred fir), the overwintering host of the Monarch butterfly (Danaus plexippus). We established high-elevation assisted migration A. religiosa provenance field tests at two sites (Las Palomas and Los Ailes) in the core overwintering areas of the Monarch Butterfly Biosphere Reserve (MBBR), central Mexico. At each site, 2-year-old seedlings were planted either in the open or under existing nurse plants. Three and a half years after planting at the Las Palomas site (5.5 years from germination), A. religiosa seedling survival was 72% under the shade of nurse plants but only 18% in open areas (without shade). At the Los Ailes site, 1 year and a half after planting in the field (3.5 years from germination), survival was 94% and 10%, respectively. There were not significant differences in seedling height increment among populations at either site. The results of our study and those published elsewhere suggest that A. religiosa benefits from shade protection of nurse plants and that population transfer 400 m upward in elevation (i.e., assisted migration) to compensate for future warmer climates does not appear to have any negative impacts on the seedlings, while potentially conferring closer alignment to future climates. If absent in planting sites, we recommend growing nurse shrub species (such as Baccharis conferta) alongside tree seedlings in forest nurseries so that these shrubs can be transplanted to the reforestation site 2 years before planting the tree seedlings.
Introduction
Abies religiosa (Kunth) Schltdl. & Cham. (sacred fir) forests, which comprise the Monarch butterfly (Danaus plexippus) overwintering sites in the core area of the Monarch Butterfly Biosphere Reserve (MBBR) at the border between the Michoacán and Estado de México states in west-central Mexico, are occasionally subject to serious disturbances, likely related to climate change. For example, in a single night in March 2016, a windstorm felled approximately 20,000 trees and damaged many more (Brower et al., 2017; Fondo Monarca, 2017; Figure S1). Such disturbances undoubtedly reduce stand density (Figure S2) and, when combined with illegal cutting (Brower et al., 2016; Figure S3), are decreasing the umbrella and blanket effect (sensu Anderson and Brower, 1996) that protects overwintering Monarch butterfly colonies. There is an urgent need for ecological restoration in the face of ongoing climate change, as reflected in the declining Monarch butterfly populations (Semmens et al., 2016) and an agreement between the federal governments of Mexico, United States and Canada regarding conservation of habitat targets in the MBBR (Environment Canada, 2014). In this study, we explore the use of nurse plants as facilitators for the establishment of A. religiosa seedlings in “climate-smart” reforestation programs and consider the A. religiosa seed source as a tool with which to improve adaptation to climatic change.
Abies religiosa is a shade tolerant species (Sánchez-Velásquez et al., 1991; Rzedowski, 2006) distributed on moist and cold sites at high elevations with more affinity to northern aspects, mostly along the Trans-Mexican Volcanic Belt, at between 2,800 and 3,500 m above sea level (m a.s.l.) (Rzedowski and Rzedowski, 2005; Benavides-Meza et al., 2011).
The use of local seed sources of A. religiosa when reforesting the MBBR may no longer be appropriate. A. religiosa populations are genetically differentiated for adaptative quantitative traits (seedling height, seedling annual elongation, date of growth cessation, foliage dry weight and frost resistance) along environmental gradients. In particular, populations separated by as little as 350 m in elevation, or 1.2°C in mean temperature of the coldest month (MTCM), differ genetically; there is a very strong association between A. religiosa seedling frost damage and elevation of the provenance (and its corresponding MTCM), suggesting that climate is the selective driving force in terms of genetic differentiation among populations (Ortiz-Bibian et al., 2017). There is also evidence of genetic differentiation among populations of A. religiosa at the landscape scale, detected with molecular markers [amplified fragment length polymorphisms (AFLPs) and three chloroplast microsatellites (cpSSRs) (Méndez-González et al., 2017)]. There are consistent reports of genetic differentiation for adaptive quantitative traits among conifer populations along environmental gradients, in which a vital trade-off is expressed: higher growth potential and lower frost resistance for populations growing in mild environments, and vice versa in harsh colder environments (see for example: Rehfeldt et al., 1999, 2002, 2003, 2014a,b, 2017). Furthermore, ongoing climate change will act to decouple host (A. religiosa) populations from the relatively narrow climate interval to which they are adapted, with the suitable climatic habitat for A. religiosa inside the MBBR expected to disappear entirely by the end of the century (Sáenz-Romero et al., 2012).
Assisted migration is the practice of humans moving populations (in our case, tree populations by seeding or transplant) to a different habitat. Such practice could be used to alleviate the impacts of increased warming if species′ historic climatic envelope can be matched to the future climate at a transplant location (Rehfeldt et al., 2014c). Some authors do not distinguish between translocating genotypes or populations inside versus outside a species' historic natural distribution (Rehfeldt et al., 1999, 2002, 2012; O'Neill et al., 2008; Marris, 2009; Pedlar et al., 2012; Castellanos-Acuña et al., 2015; Prober et al., 2015; Sáenz-Romero et al., 2016). Other authors (e.g., Hewitt et al., 2011; Commander et al., 2018) consider assisted migration to involve only the translocation to locations outside the historic range of the species. By planting A. religiosa from seed sources collected in locations with historic (e.g., period 1961–1990) climates that match the MBBR climates anticipated for the near future (e.g., the decade centered on the years 2030 or 2050), populations could be realigned with their suitable climatic habitat (in this case, inside the historic range of the sacred fir) and misalignment may thus be mitigated (Sáenz-Romero et al., 2012).
The climate distance that seed sources are moved (i.e., the migration distance) may be a critical factor in the success of assisted migration efforts. If migration distances are too short (i.e., sites are established with populations whose contemporary climates are only slightly warmer than that of the planting site), planted trees may be maladapted to the prevailing climate by the time the stand reaches late maturity. However, if migration distances are too great (i.e., sites are established with populations whose contemporary climates are much warmer than that of the planting site), planted trees may be susceptible to frost damage during their period of establishment (Loya-Rebollar et al., 2013). Clearly, the target for optimum migration distance lies between the climates expected during these two stand phases (establishment and late maturity). Balancing the two risks, and considering that trees are most sensitive to climate extremes in the establishment phase, we propose a migration target of 2030 in order to maximize adaptation during establishment (Sáenz-Romero et al., 2010; Ortiz-Bibian et al., 2017). Similarly, in British Columbia, Canada, a commercial system of large-scale assisted migration was recently introduced using a migration distance that accounts for climate change in the last 70 years and for the change anticipated in the next 20 years (O'Neill et al., 2017). In Mexico, however, there are no experiences of assisted migration conducted on commercial or large scale conservation programs—only those at small experimental scale, where germplasm migration has generally been successful where it did not exceed 400 m upward elevation shift (e.g., Valle-Díaz et al., 2009; Castellanos-Acuña et al., 2015; García-Hernández et al., 2019; Gómez-Ruiz et al., 2019).
Shrubs can serve as nurse plants, facilitating the establishment of target (due to their economic or ecological value) species, where harsh environmental conditions act as barriers to restoration (Callaway et al., 2002; Filazzola and Lortie, 2014). Such ecological restoration using nurse plants as facilitators has been achieved under extremely harsh conditions, e.g. (a) growing as “krummholz mats” in cold and windy timberline sites, serving as nurse plants for Picea engelmanii and Abies lasiocarpa (Germino et al., 2002; Brodersen et al., 2019); (b) at abandoned coal mine sites in Spain, using native shrubs Genista florida and Cytisus scoparius as nurse plants to facilitate the establishment of Quercus petraea and Q. pyreniaca seedlings (Torroba-Balmori et al., 2015; Alday et al., 2016); (c) in grazing areas in dry Mediterranean environments in Spain (using Cytisus multiflorus as the nurse plant for Quercus pyrenaica and Q. ilex; Costa et al., 2017); and (d) at low altitude, sunny, dry Mediterranean slope sites (Gómez-Aparicio et al., 2004).
Similarly, in restoration ecology efforts in Michoacán state, western Mexico, survival of planted A. religiosa seedlings increases when planted in conjunction with nurse plants. For example, Lupinus elegans seeds were sown together with A. religiosa seedlings in an abandoned farm field at a site originally occupied by A. religiosa and Pinus pseudostrobus forest. When L. elegans covered the A. religiosa seedlings, the mortality of the latter essentially fell to zero (Blanco-García et al., 2011). These results indicate the potential utility of nurse plants to A. religiosa, especially in the context of forest management for climate change.
Assisted migration of A. religiosa genotypes inside the core area of the MBBR would require an upward shift of 350 m in elevation in order to restore populations to their 1961–1990 climatic origin and account for the climatic change projected for the decade centered on the year 2030 (Ortiz-Bibian et al., 2017). Since most of the Monarch butterfly overwintering sites are between 3,000 and 3,300 m asl (García-Serrano et al., 2004), nurse plants must be resistant to severe frost; however, L. elegans is sensitive to the frequent frosts that occur at those elevations (Díaz-Rodríguez et al., 2013). Lupinus montanus, a native species found at the over-wintering elevations, is more frost-tolerant but usually grows to only 40 cm in height, which is too short to provide shade to A. religiosa during the critical establishment phase. Thus, taller native plants, such as the shrub Baccharis conferta Kunt (Snook, 1993; Lara-González et al., 2009; Sánchez-Velásquez et al., 2011), should be explored as possible nurse plants for A. religiosa.
The objective of the present study was to test the feasibility of using naturally established nurse plants to moderate possible stress associated with population migration during seedling establishment in sites within the core area of the MBBR that are disturbed and increasingly hostile as a result of climate change. Our specific research question was: What are the differences in survival and growth for A. religiosa seedlings when planted with and without the protection of existing naturally established shrubs serving as nurse plants, when the A. religiosa seed originates from an elevation lower than that of the planting site?
Materials and Methods
Seed Origin and Design of Field Tests
We established two high-elevation A. religiosa provenance field tests: Las Palomas at 3,440 m and Los Ailes at 3,360 m elevation. Both sites are inside the core area of the MBBR, at Ejido La Mesa, Municipality of San José del Rincón, Estado de México (Table 1; Figure 1), and both were natural pure old A. religiosa stands that had been heavily disturbed: Las Palomas by a severe crown fire in 1989 and Los Ailes by deforestation and subsequent sheep grazing ~2,000. We use the term “population” to refer to a group of open-pollinated individuals represented in the tests by their seedlings and “provenance” as the geographic origin of a population. Our operational definition of seasons is illustrated in Figure S4.
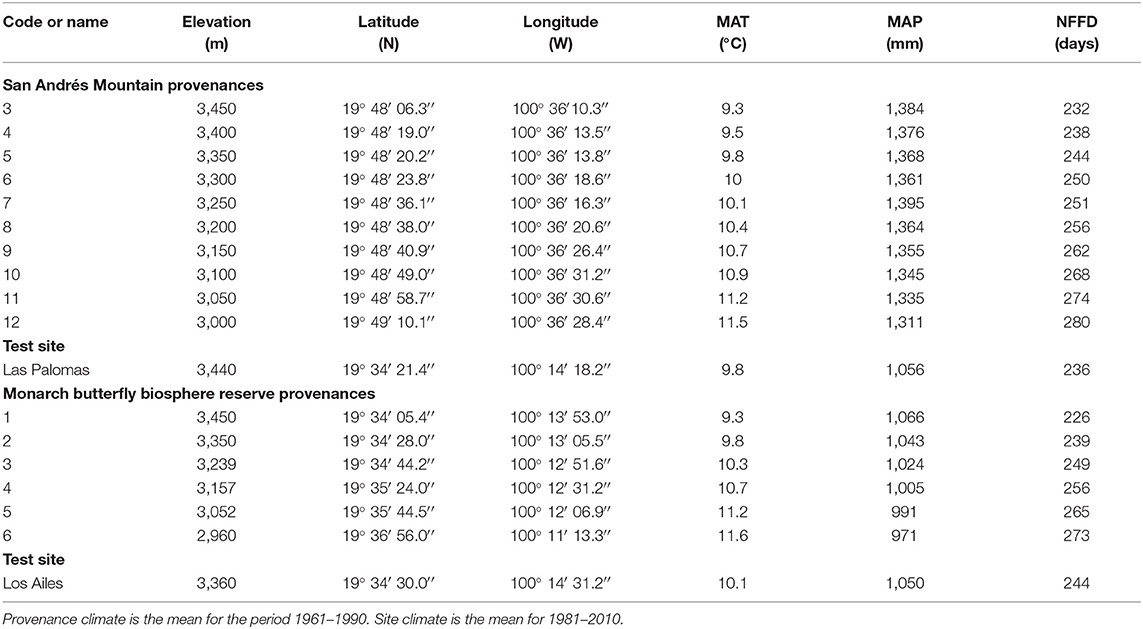
Table 1. Geographic coordinates, elevation, mean annual temperature (MAT), mean annual precipitation (MAP) and number of frost-free days per year (NFFD) for the Abies religiosa provenances and for the test sites at Las Palomas and Los Ailes, both within the core area of the MBBR, Mexico.
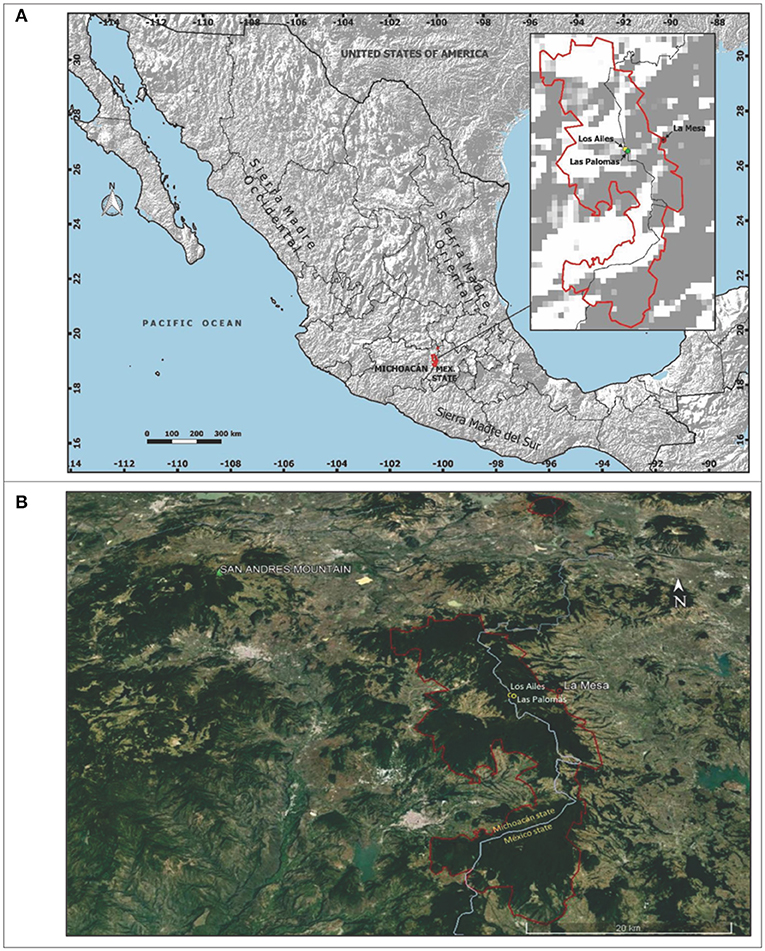
Figure 1. Geographic location of (A) the Monarch Butterfly Biosphere Reserve (MBBR; red contour), at the border of the Mexican states of Michoacán and Estado de México, and the field test sites (Las Palomas and Los Ailes). (B) San Andrés Mountain, MBBR (red contour), Ejido La Mesa village and the test sites.
For the Las Palomas test site, open-pollinated seeds were collected from 11 randomly selected trees (five to ten cones per tree), in each of 10 natural populations from between 3,000 and 3,450 m on the San Andrés mountain, located 35 km northwest of the provenance test site (Table 1; Figure 1). Seeds from each population were bulked (i.e., seeds from the 11 trees of each population were combined) and sown in a forest nursery in Morelia, Michoacán (1,830 m), then transferred after 1 year to the Ejido Los Remedios communal forest nursery (3,000 m, under a shade-mesh, with herbivores excluded) inside the MBBR for a full second year as a hardening treatment. Since germination was very poor (averaging 13%, but with large differences among populations, and extremely poor at both altitudinal extremes; Ortiz-Bibian et al., 2019) and there was high nursery mortality due to frost and other maintenance problems, the number of seedlings available for field testing was limited.
For the Los Ailes provenance trial, in October 2015, recently germinated seedlings from mossy microsites were collected under six natural stands of A. religiosa, between 2,960 and 3,450 m and up to 20 km from Los Ailes (Table 1; Figure 1) and transplanted to nursery containers (initially around 200 seedlings per population), where they were grown for 2 years at Ejido La Mesa, also as a hardening treatment (3,000 m, inside a shade-house, with herbivores excluded), before being out-planted in July 2017. The number of transplanted seedlings (henceforth, simply “seedlings”) that died at the nursery just after transplanting from the field also limited the availability of seedlings for the field tests.
Seedlings were planted at both field sites with two treatments: (a) under the shade of nurse shrubs already existing at the site—B. conferta (the most abundant), Ribes ciliolata, Juniperus monticola, Salix paradoxa, and Senecio cinerarioides—and (b) in open areas without the shade of nurse plants. The few existing shrubs on microsites chosen for the planting in the open at Las Palomas site were removed in order to achieve the condition of full absence of shrubs for the treatment of planting in open areas (Figures 2, 3).
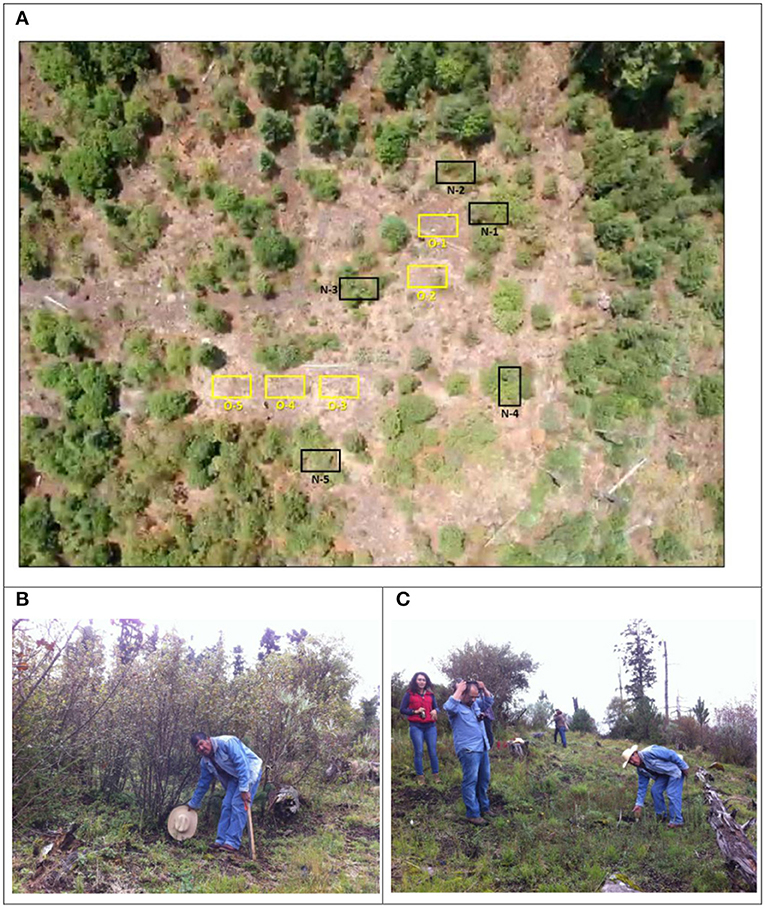
Figure 2. Treatments at the Las Palomas field site: (A) Aerial view of the site (taken by a drone); black squares are blocks of A. religiosa seedlings planted under nurse plants; yellow squares blocks planted on open areas. (B) Planting of Abies religiosa seedlings under the shade of nurse plants (Baccharis conferta in this case) and (C) the open treatment without nurse plants. Las Palomas site, 3,440 m, Ejido La Mesa, Municipality of San José del Rincón, Estado de México, core area of the Monarch Butterfly Biosphere Reserve. The site was heavily impacted by a crown fire in 1989. Prior to this fire, the site consisted of a dense Abies religiosa stand and an overwintering site for Monarch butterflies. Francisco Ramírez-Cruz, ejidatario of La Mesa, is pointing to recently planted A. religiosa seedlings. Appropriate informed consent was provided by Francisco Ramírez-Cruz in terms of appearing in this photograph indicating his personal identity.
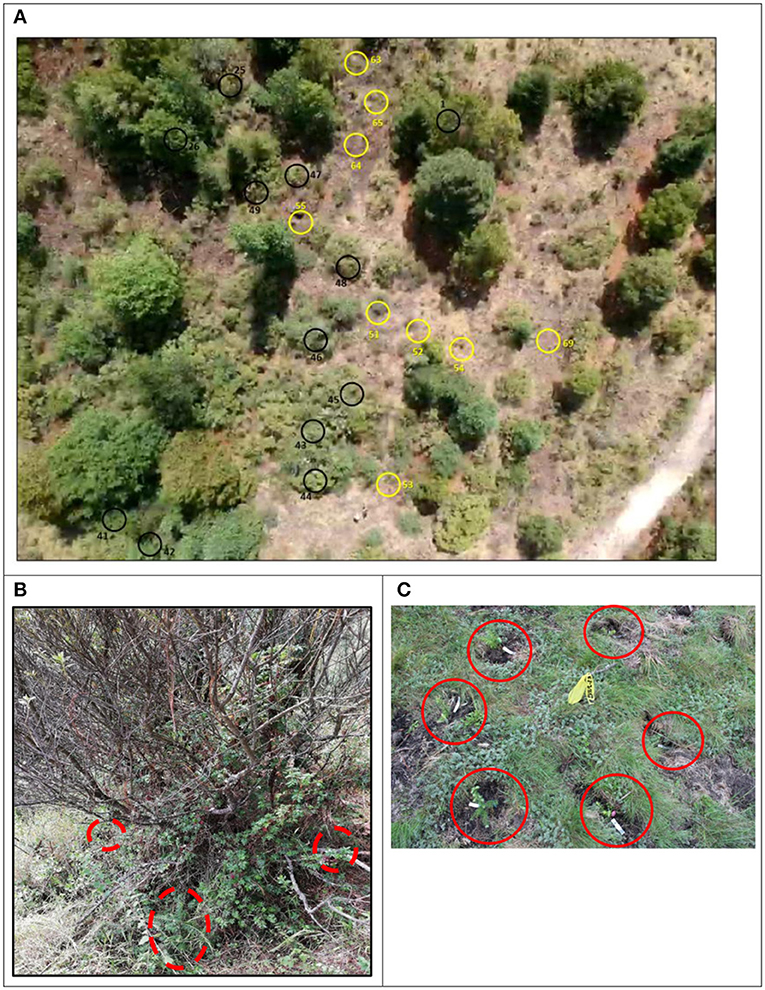
Figure 3. Treatments at the Los Ailes field site: (A) Aerial view of a fragment of the site (taken by a drone); black circles are blocks of A. religiosa seedlings planted under nurse plants; yellow circles blocks planted on open areas. (B) Planting of Abies religiosa seedlings under the shade of nurse plants (Baccharis conferta in this case), and (C) without nurse plants (red circles to highlight the seedlings). The Los Ailes site, Ejido La Mesa, Municipality of San José del Rincón, Estado de México, core area of the Monarch Butterfly Biosphere Reserve. The site was heavily disturbed ~20 years ago and reforested with Pinus pseudostrobus (with very irregular survival, since the site is too high in elevation for that particular species) and, very recently (2 years ago), with Pinus hartwegii. Prior to disturbance, this site was a dense Abies religiosa stand.
In July 2015, seedlings (aged 2 years from germination) were planted in the rainy season at the Las Palomas test site following a randomized complete block design, where five blocks each were assigned to nurse plant and open field treatments. In each block, the 10 populations were each randomly assigned to a single plot. Populations were represented by three seedlings in a row in each plot. Spacing was 1.5 × 1.5 m in a regular grid (Figure 2).
In July 2017, seedlings (aged 2 years from germination) were planted in the rainy season at the Los Ailes site. Seedlings from six provenances (see Table 1) were planted in 50 blocks with nurse plants and in 20 main plots without nurse plants (in open field treatment). With the experience gained in the Las Palomas site 2 years previously, the blocks in the Los Ailes site were composed of one seedling from each of the six populations, randomly selected and planted in a circle around the stem of the nurse plants, similar to the methodology used by Costa et al. (2017) (Figure 3B), and around an imaginary point for the blocks without nurse plants (open field; Figure 3C). This unorthodox arrangement of seedlings in the blocks helped ensure that all the seedlings under the nurse plant treatment received approximately equal levels of shade. Uniform application of shade was not well-achieved at the Las Palomas site, since a regular grid arrangement was used for the planted seedlings within the existing irregular location of naturally established nurse plants.
Field Measurements and Analysis
Seedling height and survival were assessed periodically (approximately every 2 months) for 3.5 years at Las Palomas (when they reached 5.5 years of age from germination) and 1.5 years at Los Ailes (3.5 years of age from germination). When we measured seedling height, the status of the seedling was examined; if it appeared to be alive, we recorded survival as “1”; if not alive, we recorded survival as “0” and did not record seedling height.
To characterize the amount of shade received by the plants, the percentage of plant cover was estimated at the end of the dry and warm season (May) with a digital image analyzer (Winscanopy, Regent Instruments, Canada), by taking a photograph with a hemispheric camera from the center of each block, at ground level. Images were processed with the Winscanopy program (2014).
Temperatures at 40 cm above ground surface (the approximate seedling height 2 years after planting) were monitored hourly at the Las Palomas site using dataloggers (Hobo® H01-001-01 Onset Computer Corporation, USA). One sensor was installed in each block for two periods: January 20th to May 31st, 2016, to record the dry season, and December 3rd, 2016 to February 13th, 2017, to record the coldest part of the dry and cold season.
In order to obtain a detailed record of the temperature fluctuations to which the seedlings were exposed, air temperature in the close environment of A. religiosa seedlings grown either in the open field or under a nurse plant was measured continuously during a day/night cycle. Simultaneously, the sky (or sky and nurse plant) brightness temperature (taken as a proxy for downwelling long wave radiation) and the soil brightness temperature (an approximation of soil surface temperature) were measured. For this purpose, a device was built using two infrared (IR) temperature sensors (Melexis® MLX 90614, 40° view angle) inserted within two horizontal polystyrene plates and installed in the field close to and at the same height as the seedlings. The sensors measured air temperature between the plates, while the IR sensors pointed both up and down to measure the sky and soil brightness temperatures, respectively. The system was connected to an Arduino® data-logger, which recorded measurements every 3 min. Two devices were installed and monitored over a 24-h period: one in the open field and the other under a nurse plant (B. conferta shrub) at the Los Ailes site in January (a month of the cold and dry season) and April (a month of the dry and warm season).
A visual stress index was created to evaluate the physiological status of the plants, starting the year after planting. The index values ranged from 1 (shiny dark-green needles, indicating a healthy non-stressed seedling) to 6 (brown decaying foliage, apparently dead seedling) (Figure S5). Stress assessments were conducted after planting at both sites from December to February, to represent most of the cold and dry season, and then also from March to early May, months of the warm and dry season, as well as during the rainy season (June to October).
Analysis of variance (ANOVA) was performed using Proc Mixed in SAS (SAS Institute Inc, 2004) to test the main effects of treatment, block and population, and their interactions, on final height increment (final seedling height minus seedling height at the time of field planting) and stress index. Treatment was considered a fixed effect; all remaining effects were considered random effects. Significance was tested using the COVTEST option for the random terms and the F-test for the fixed effects.
Survival data were examined with a General Linear Model, using a binomial distribution, logit link function, with the GLM module of the R program, and testing with Chi-square (Crawley, 2013).
Analyses were performed separately for each site due to the different experimental designs used at the two sites.
Results
Three and a half years after planting at the Las Palomas site (5.5 years old from germination), A. religiosa seedling survival was 72% under the shade of nurse plants, but only 18% when planted in open areas (Figure 4A). In the Los Ailes site, one and a half years after planting (3.5 years-old from germination), seedling survival was 94% and 10%, under nurse shrubs and in open areas, respectively (Figure 4B). Differences between treatments were highly significant at both sites (P < 0.0001; Table 2). The timing of the pulses of mortality for the seedlings planted in open areas differed between sites: for the Las Palomas site, the mortality pulse occurred during the dry and warm season (March to early May; Figure 4A), whereas in the Los Ailes site, it occurred during the second half of the cold and dry season (January to February; Figure 4B).
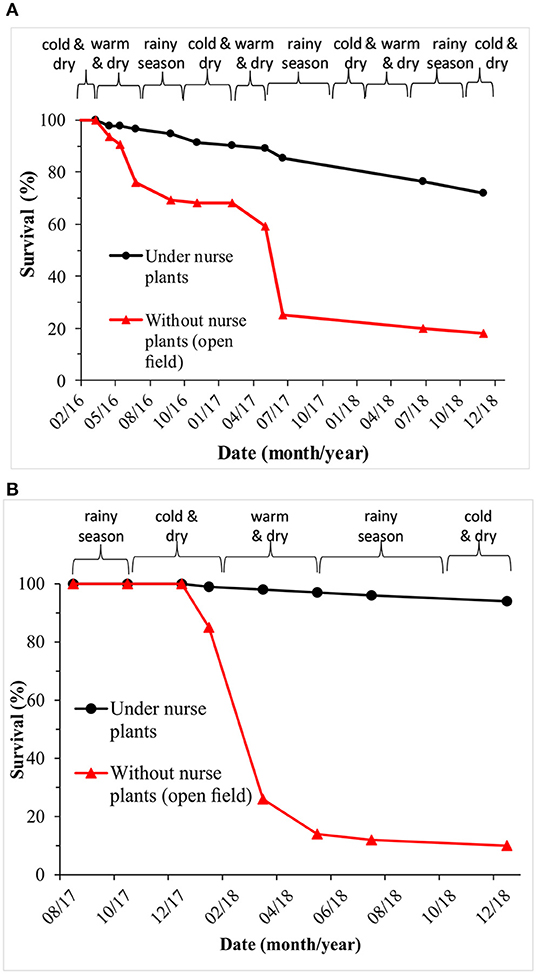
Figure 4. Survival per treatment and site. Average (across populations) survival per treatment (under nurse plants vs. open field) and per site. (A) Las Palomas site (5.5 years old from germination; 3.5 years after planting). (B) Los Ailes site (3.5 years old from germination; 1.5 years after planting). Square brackets indicate the season of the year (seasons defined as in Figure S4).
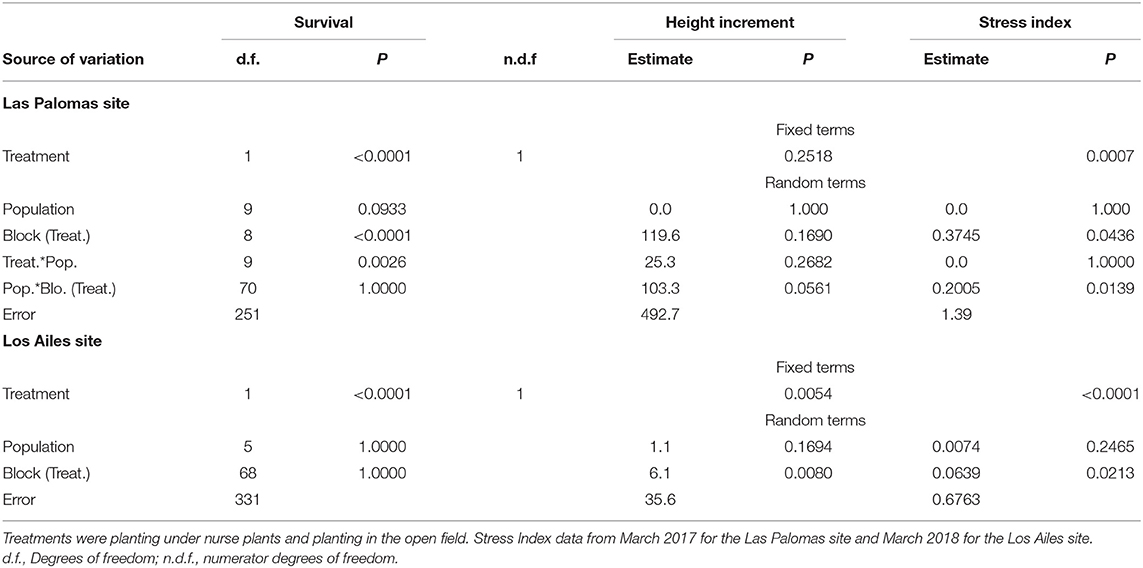
Table 2. Significance (P) of the sources of variation in the ANOVA for Abies religiosa seedlings for survival (using a General Linear Model analysis, binomial distribution), height increment and stress index (using a mixed model), at the Las Palomas and Los Ailes assisted migration provenance test sites, 5.5 and 3.5 years from germination (3.5 and 1.5 years after planting in the field), respectively.
Measurement of the coverage using a hemispheric camera indicated a highly significant difference between the treatments in open areas and under nurse plants, at both the Las Palomas (mean percent of coverage ± interval coefficient at 95%, in open areas: 48.9 ± 11.3; under nurse plants: 10.4 ± 3.76%; P < 0.001) and the Los Ailes (85.3 ± 1.7%; 36.2 ± 2.3%; P < 0.001; respectively) sites (Figure 5).
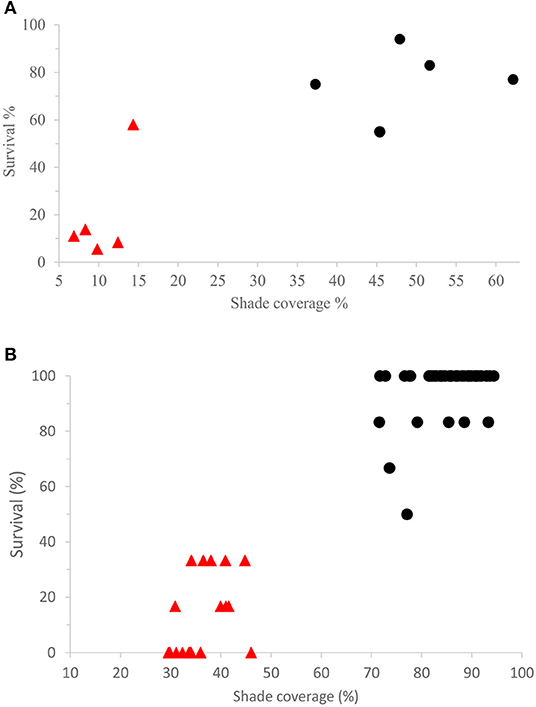
Figure 5. Survival per block, against shade coverage per block, for the treatments of nurse plant shade vs. open field. Survival was estimated per block [(number of seedlings alive/number of seedlings planted) × 100]. Percentage of shade coverage was measured at the center of the blocks, for blocks under the shade of nurse plants (black circle symbols) and for those with no nurse plants (red triangle symbols), for (A) the Las Palomas site and (B) the Los Ailes site.
The temperatures measured with Hobo dataloggers were cooler under the nurse plants than in open areas (Figure 6) and survival was positively related to shade (Figure 5).
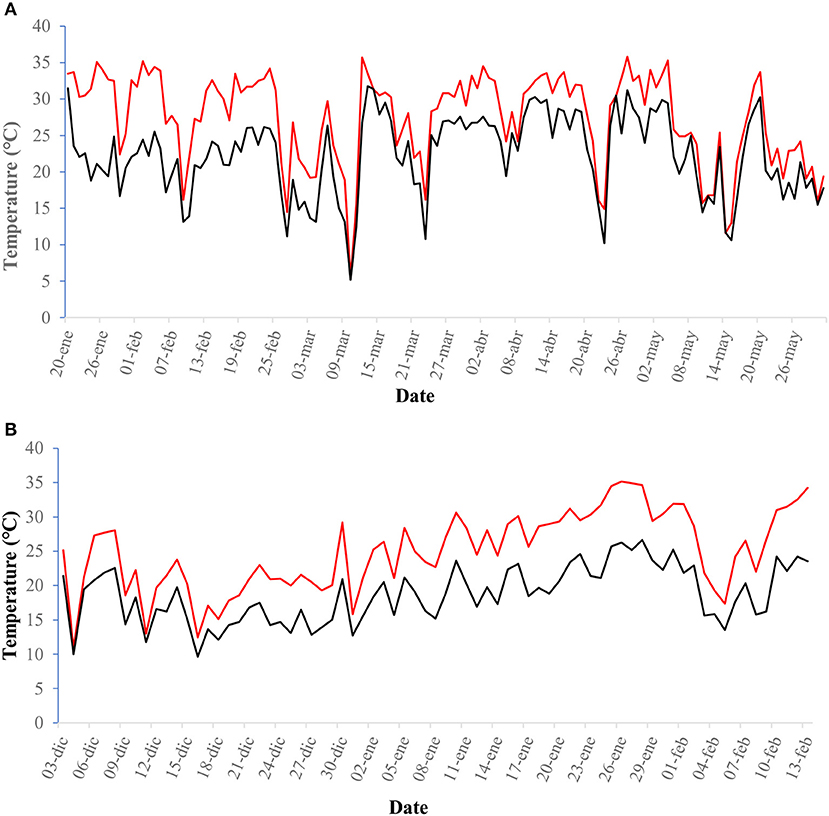
Figure 6. Temperatures (°C) for the nurse plant shade and open field treatments. Average daily temperatures at Las Palomas sites for the center of the blocks under the shade of nurse plants (black line) and for those with no nurse plants (red line), for the (A) period January-May 2015 (January to February are months part of the cold and dry season; March to May part of the warm and dry season), and (B) period December 2016–February 2017 (months part of the cold and dry season).
However, perhaps the greatest difference between the treatments in open areas vs. under nurse plants was not the average daily air temperatures (Figure 6), but rather the different patterns of temperature variation experienced by seedlings during the day. There was a very wide variation of temperatures in the open areas, while the temperature variations recorded below the nurse plants were much smaller (Figure 7).
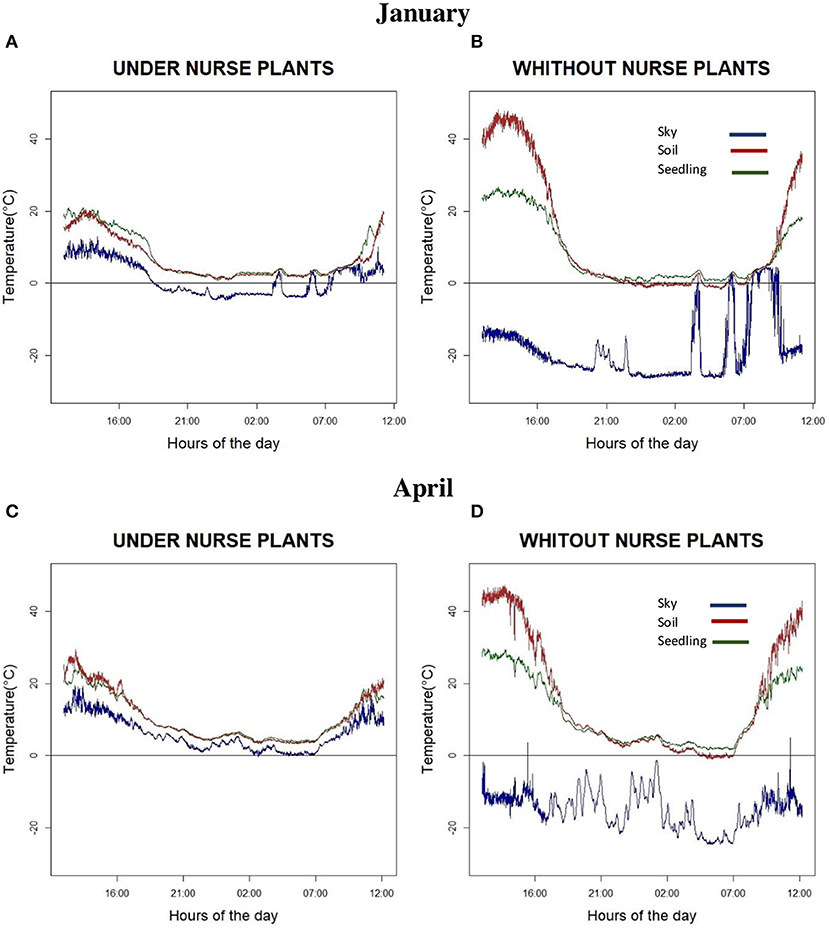
Figure 7. Temperatures measured during a 24-h observation period in January (cold and dry season; A,B) and in April (dry and warm season; C,D) at the Los Ailes site (3,360 m) with and without the shade of a nurse plant (a Baccharis conferta shrub). The green line represents air temperature (°C) measured at 40 cm above the ground (as a proxy of the temperatures to which an Abies religiosa seedling would be exposed), while the red and blue lines represent the brightness temperature of the soil and sky, measured by two IR sensors pointing downwards and upwards, respectively.
Survival differed little among populations in the shade treatment (Figures 8A,C). However, the differences in survival among populations in blocks without existing nurse plants appeared to be larger (Figures 8B,D), and differences among populations were found to be statistically significant only when the analysis was conducted separately per shade treatment, both at the Las Palomas site (Table 3) and at the Los Ailes site (Table 4). That is consistent with the significance of the interaction population by treatment at the Las Palomas site (P = 0.0026; Table 2). In the open area planting of the Los Ailes site (Figure 8D), greater and earlier mortality was found in the population from the second lowest elevation (i.e., transferred from 3,052 m at the seed source to 3,360 m at the test site); in contrast, the lowest mortality occurred in the population that originated at the highest elevation (3,450 m at the seed source, close to the test site). This pattern was not found under the shade of the nurse plants (Figure 8C). A similar pattern was found at the Las Palomas site: in the open area planting, the lowest survival was for the population that was migrated the furthest in elevation (from 3,000 m at the seed source to 3,440 m. at the field test site), whereas the highest survival was for the local population (3,450 m at the seed source) (Figure 8B). In contrast, these differences were buffered under the shade of the nurse plants (Figure 8A). When plotting the average final survival per population against elevation of the provenance (Figure S6), it becomes apparent that under the shade of nurse plants, there is not a pronounced patterning of differences among populations associated with the elevation of origin, whereas when planting on open areas, the best survival is of the populations originated at the highest altitudes, closer to the elevation of the planting sites.
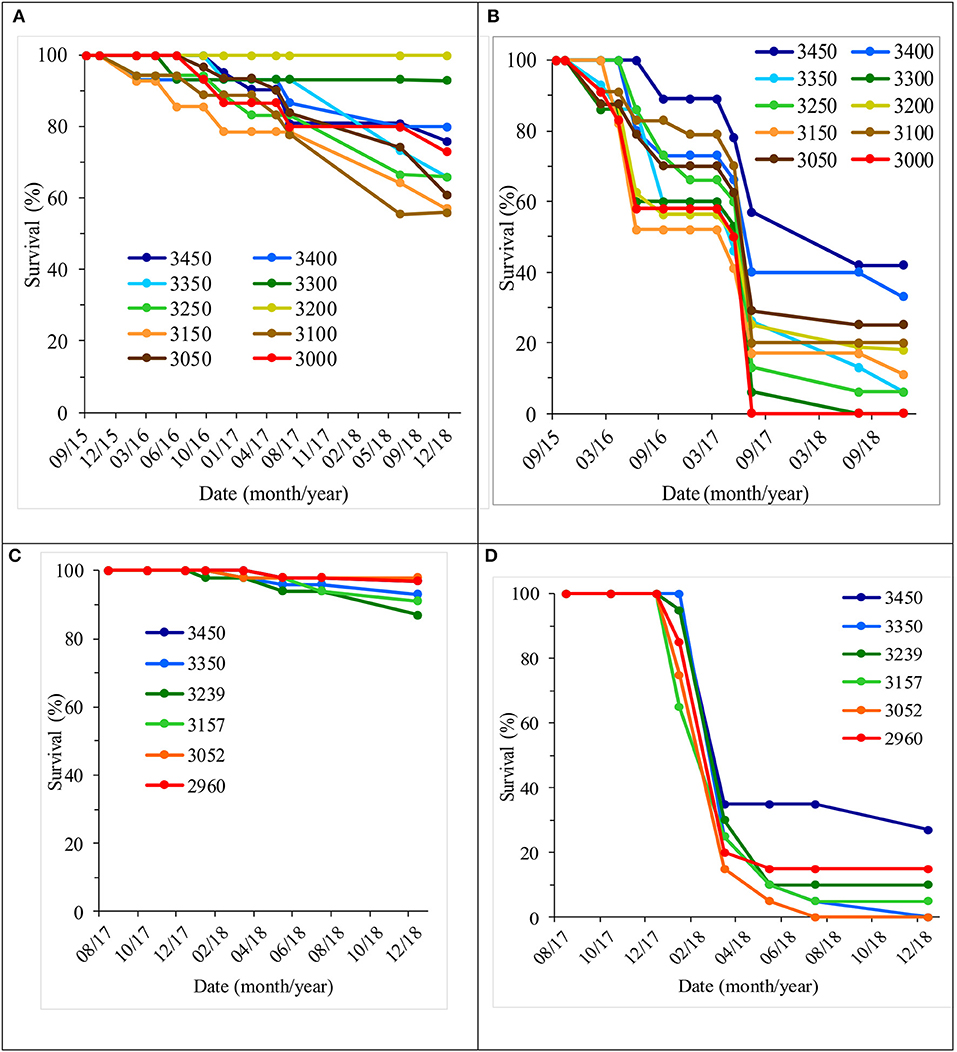
Figure 8. Average survival by population and treatment under nurse plants at the Las Palomas site (A) and the Los Ailes site (C), and in open field conditions at the Las Palomas site (B) and the Los Ailes site (D).
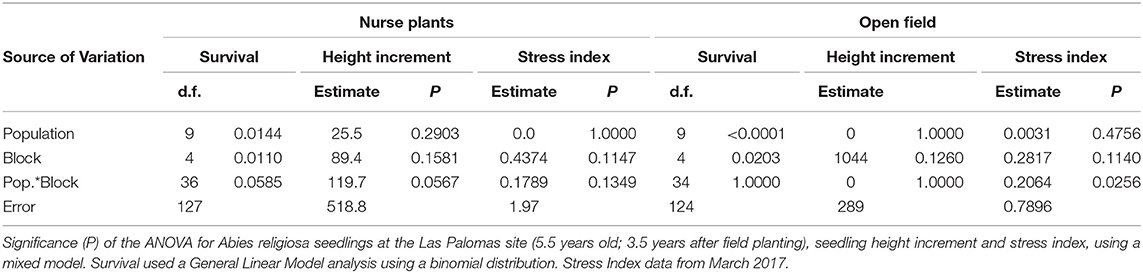
Table 3. ANOVA per treatment (planting under nurse plants and planting in the open field) at the Las Palomas site.
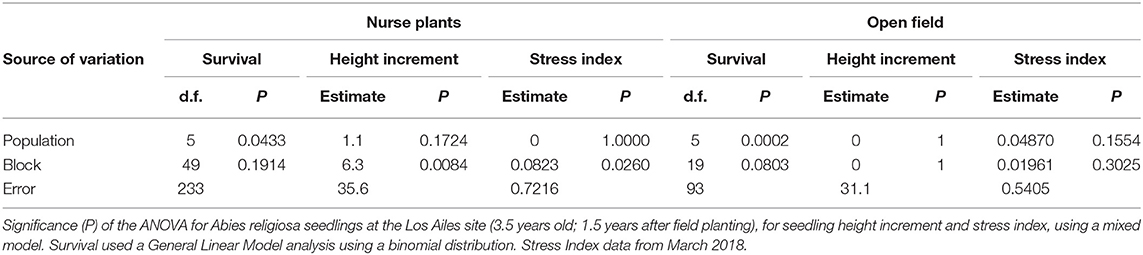
Table 4. ANOVA per treatment (planting under nurse plants and planting in the open field) at the Los Ailes test site.
There were no statistically significant differences in seedling height increment among treatments at the Las Palomas site, although there were significant differences at the Los Ailes site (Table 2). There were not significant differences among populations for seedling height increment at both sites (Table 2), even when the maximum upward shift in elevation was 440 m from seed source to planting site at the Las Palomas site, and of 400 m at the Los Ailes site.
The effect of the nurse plants was reflected in the stress status of the seedlings, as measured by the stress index. Seedlings under the shade of nurse plants had significantly lower stress index values (indicating reduced stress) than those without nurse plants (Figure 9; index values explained on Figure S5) in both sites (Las Palomas: P = 0.0007; Los Ailes: P < 0.0001; Table 2). There were no significant differences among populations for stress index in either test site (Table 2).
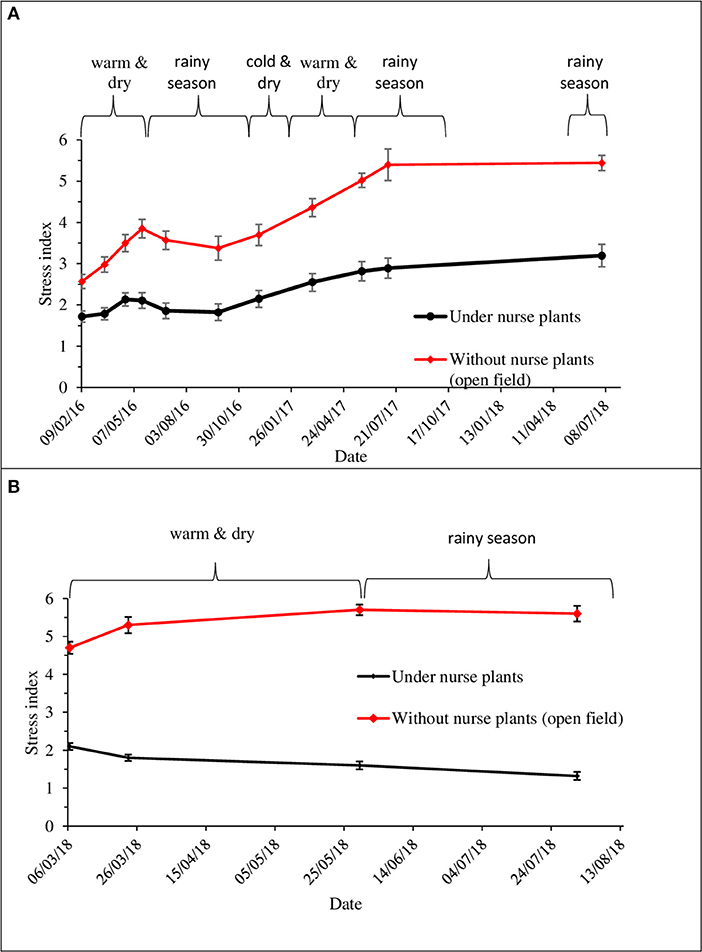
Figure 9. Stress status of seedlings. Stress index value, averaged across populations per shade treatment, for (A) Las Palomas site from February 2016 to July 2018; (B) Los Ailes site from February to August 2018. Higher values indicate more stress (see Figure S5 for explanation of values). Square brackets indicate the season of the year (seasons defined as in Figure S4). Vertical bars indicate 95% confidence interval.
Discussion
Our results strongly suggest that nurse plants provide shade protection to recently planted A. religiosa seedlings, significantly increasing their survival regardless of the seedling seed source. Thus, the use of nurse plants in the early stages of a reforestation program with A. religiosa is highly recommended, as was found with planted Lupinus elegans serving as nurse plants in a previous study (Blanco-García et al., 2011) and, in our case, with the existing nurse plants (mostly the shrub Baccaris conferta). Our results are very similar to the patterns of survival found when Quercus petraea, Q. pyrenaica, and Q. ilex were grown under the shade of native shrubs (Genista florida, Cytisus scoparius, and C. multiflorus), and without the nurse effect, in studies conducted in harsh environments (Torroba-Balmori et al., 2015; Alday et al., 2016; Costa et al., 2017). In recent decades, the determinant role of facilitation has been recognized as a positive interaction among species that directly affects their performance, distribution and metabolism (Bruno et al., 2003; Brooker et al., 2008). The role of shade as a modifier of microclimatic conditions is important because this mechanism of improving microclimatic conditions is one of the most recognized forms of facilitation (Callaway et al., 2002; Callaway, 2007; Walker et al., 2007; Torroba-Balmori et al., 2015; Alday et al., 2016; Costa et al., 2017). The effects of nurse plants are of particular importance to plants growing under severe climatic conditions, as predicted by the stress gradient hypothesis (Bertness and Callaway, 1994; Callaway et al., 2002).
At both sites, seedling survival was substantially lower in the open areas than under the nurse shrubs. The nurse plants significantly increased the amount of shade received by the A. religiosa seedlings. A. religiosa is a shade tolerant species (Rzedowski, 2006) and thus greater height increment and survival are to be expected under shade. Differences in survival between sites occurred at different times of the year and under different environmental site conditions. Consequently, this observation is difficult to explain in simple terms; however, a closer look at the data shows that at Las Palomas, the site containing the 5.5-year-old seedlings, survival began to decrease at the end of the warm and dry season of 2016, then declined steadily during the cold and dry season, before presenting a pronounced decrease at the end of the warm and dry season (Figure 4A).
The 3.5-year-old seedlings at the Los Ailes site began to present mortality during the cold and dry season (Figure 4B). The common trend between both sites is that initial drops in survival began during dry periods when either warm or cold. The reduction in survival of the 3.5-year-old seedlings was less gradual and more dramatic than that of the 5.5-year-old seedlings. The more rapid decline in survival at the Los Ailes site is also likely due to the fact that the site contained transplanted seedlings with disturbed root systems. This is probably due to the different rooting depths of the 3.5- and 5.5-year-old seedlings. Although no data were measured, it can be presumed that 5.5-year-old seedlings would have rooted further and deeper than the 3.5-year-old seedlings, allowing these plants to better buffer the drought (Padilla and Pugnaire, 2007). Figure 6 indicates temperatures that were frequently high enough to allow reasonably high rates of photosynthesis and stomatal conductance, so water loss could have been sufficient to kill the seedlings during the dry periods. The lower temperatures under nurse plants (Figure 6) would also confer a lower vapor pressure deficit, which acts to decrease transpiration (Will et al., 2013).
The detailed measurements of the temperatures experienced by the A. religiosa seedlings at Los Ailes site, with and without nurse plant protection, indicate that the nurse plants prevented the formation of significant temperature gradients within the bottom 40 cm layer of the atmosphere, resulting in considerably reduced temperature fluctuations in this location during the diurnal cycle. This is due both to the interception of solar radiation during the day and to the emission of IR radiation by the nurse plant canopy during the night. The consequence of this for the seedling is a reduction of heat stress during the day along with a 2.5°C temperature increase during the night, which probably acts to decrease the risk of frost.
Another factor that might contribute to the greater mortality of A. religiosa seedlings without nurse plant protection during the cold and dry (November-February) and the dry and warm (March to May) seasons (Figure 4; Figure S4), could be saturation of the photosynthetic apparatus, given that the plants experience drought in both seasons (Yin et al., 2006; Arena et al., 2008; Lambers et al., 2008). In the cold, dry season, sub-zero temperatures are common at night. Therefore, water is not available for plants for several hours each day. Likewise, insufficient water is available during the dry, warm season. In both cases, in the treatment with no nurse plants, photosynthesis might be water limited (as stated above), promoting oxidative stress. The detailed temperature measurements indicate that the shade provided by nurse plants prevents excess insolation during the cold and dry January-February period and also prevents excess insolation along with elevated temperatures during the late March to May period. In any case, in order to confirm any of the previous possible explanations, it would be necessary to conduct detailed ecophysiological measurements to pinpoint with certainty the mechanism that causes the mortality recorded.
The lack of differences among populations for seedling height increment suggests that upwards seed transfer of up to 440 m in elevation may be a feasible climate change adaptation strategy. In other words, A. religiosa seedlings seem to have sufficient phenotypic plasticity to survive and prosper under the nurse plant protection when exposed to colder temperatures at a higher elevation.
From our results, we strongly suggest that local communal forest nurseries in the MBBR begin producing local shrubs (such as B. conferta) for planting as nurse plants prior to planting A. religiosa seedlings in disturbed sites that lack naturally established shrubs. Nurse plants are needed because there are many seriously disturbed sites lacking adequate shrub cover inside the MBBR that would require identification of appropriate nurse plant species, and their field planting perhaps 2 years in advance of A. religiosa planting. This strategy, combined with use of seed sources from lower elevations, appears to be an effective approach to restoring disturbed sites for overwintering Monarch butterflies.
An alternative to produce shrubs in the nursery and then planting them as nurse plants, might be to use artificial shading over young recently planted A. religiosa seedlings. That would be placing a shade cloth, a practice that actually is used today to protect avocado young seedlings (the first year after planting a new orchard) in the region (at lower altitude). However, that has not been tested for A. religiosa.
The relatively current common practice of removing existing bushes as “site preparation” previous to reforestation with A. religiosa seedlings, seems to be a counterproductive practice that needs to be eradicated. There is the idea among some foresters that shrubs compete with conifer seedlings. That might be true for some shade-intolerant pine species, but not for the shade-tolerant A. religiosa. Also, in our field observations, when A. religiosa matures and becomes a large tree, understory bushes are naturally excluded by light competition when the A. religiosa stand forms a dense closed (and dark) canopy.
Limitations of This Study
The possibility that microsites with shrubs might have some sort of pre-condition that facilitates shrub establishment remains unexplored. This could also be beneficial for A. religiosa seedlings (independently of the shade provided by the nurse plants). Similarly, it might be possible that perturbed open areas might have a negative precondition that hampers the establishment of both shrubs and A. religiosa. It would therefore be desirable to conduct an experiment with two treatments: transplantation of shrubs in 1) open areas and 2) microsites where shrubs were naturally established but removed prior to the experiment. This would maximize the efficiency of the strategy proposed above regarding the planting of shrubs in open areas prior to any A. religiosa reforestation.
Conclusions
We conclude that A. religiosa seedlings require shade protection from nurse plants and that shifting populations upwards up to 440 m in elevation in order to compensate for future warmer climates does not appear to have any negative impacts on the seedlings and may confer adaptation to future climates. We recommend reforestation programs that include shrub species in their forest nursery production in order to provide nurse plants to accompany A. religiosa seedlings in those sites without shrubs. In the case of deforested sites with shrubs, it would not be necessary to remove these shrubs before planting the A. religiosa seedlings.
Author Contributions
CS-R, AB-G, and RL-C conceived the research project. AC-N, AC-V, EN-M, AB-G, and CS-R produced the seedlings. AC-N, AC-V, EN-M, EG-P, MG-R, AB-G, and CS-R planted the field experiments. AC-N, EN-M, AC-V, EG-P, CZ-S, AB-G, and CS-R took the measurements. FP-G, AC-N, and CS-R developed and standardized the stress index. RL-C, AC-N, and EN-M designed and conducted the plant cover measurements. AC-N, EN-M, CS-R, and AB-G conducted the statistical analysis. EG-P developed the maps and provided monthly precipitation data. FP-G, KJ, and PL contributed to the ecophysiology discussion. PL conceived, designed and constructed the devices for taking detailed temperature measurements on the field. GO'N provided climate estimates, helpful discussion about assisted migration, and improved the English writing. LL-T and YH-R provided helpful comments during the development of the project. All co-authors revised and contributed to the manuscript. CS-R led the writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper is an undertaking of the Forest Genetic Resources Working Group (FGRWG)/North American Forest Commission/Food and Agricultural Organization of the United Nations. Financial support was provided to CSR by The Monarch Butterfly Fund (Madison, Wisconsin, USA), the Mexican Fund for the Conservation of Nature and United States Forest Service USDA (funded by US Agency for International Development), the Forest Genetic Resources Working Group of the North American Forestry Commission (FAO), the Joint Fund (Fondo Mixto) CONACyT-Michoacán (FOMIX-2009-127128), and the UMSNH Coordinación de la Investigación Científica; the CONACYT Basic Research Fund (Ciencia Básica-2014-242985) to AB-G; the PAPIIT program (IN-116218) of UNAM to RALC; and the Southern Research Station of Department of Agriculture US Forest Service to KJ. We thank Francisco Ramírez Cruz and Doña Petra Cruz-Cruz for the provision of numerous facilities for seed collection, seedling nursery production, field test planting, maintenance and measurements at Ejido La Mesa; without their help, this study would not have been possible. Thanks also go to Gabriel Muñoz Montoya (Queréndaro, Michoacán) and Miguel Angel Silva-Farías (Servi-Ambiental El Bosque) for the facilities and assistance provided for seed collection at Cerro de San Andrés; Eduardo Rendón, World Wildlife Mexico, and Jaime and Rogelio Díaz, Ejido Los Remedios, for providing maintenance to the seedlings at the forest nursery at Sierra Chincua, RBMM; Felipe Martínez-Meza and Gerónimo Mondragón, Director and staff of the MBBR, respectively, provided the official permits and advice to establish the field tests at the core zone of the MBBR; Dante Castellanos-Acuña, Marisol Ortiz-Bibian, Gerardo Guzmán-Aguilar, Nancy Farías-Rivero, Miriam Linares-Rosas, Jorge Herrera-Franco, Rubí Contreras-Bailón, Francisco Loera-Padilla, Ana Beatriz Guerrero-Carmona, Nancy Izquierdo-Calderón and other UMSNH students for assistance in maintenance and measurements of the nursery and field experiments; Isabel Ramírez, CIGA-UNAM, Morelia, for providing data from weather stations within the MBBR; Eligio García-Serrano (Monarch Fund, Zitácuaro, Michoacán) and Xavier Madrigal-Sánchez (School of Biology, UMSNH) for providing helpful comments about the ecology and distribution of A. religiosa and the overwintering sites of the Monarch butterfly; I. Ramírez and E. García-Serrano also provided photographic evidence of the storm damage. Special thanks go to G. E. Rehfeldt (USDA-Forest Service, Moscow, Idaho) for advice on the experimental design and data analysis. An editor and three reviewers provided valuable comments that helped to significantly improve the manuscript. Keith MacMillan provided assistance with the English writing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00421/full#supplementary-material
References
Alday, J. G., Zaldívar, P., Torroba-Balmori, P., Fernández-Santos, B., and Martínez-Ruiz, C. (2016). Natural forest expansion on reclaimed coal mines in Northern Spain: the role of native shrubs as suitable microsites. Environ. Sci. Pollut. Res. 23, 13606–13616. doi: 10.1007/s11356-015-5681-2
Anderson, J. B., and Brower, L. P. (1996). Freeze-protection of overwintering monarch butterflies in Mexico: critical role of the forest as a blanket and an umbrella. Ecol. Entomol. 21, 107–116. doi: 10.1111/j.1365-2311.1996.tb01177.x
Arena, C., Vitale, L., and Virso de Santo, A. (2008). Photosynthesis and photoprotective strategies in Laurus nobilis L. and Quercus Ilex L. under summer drought and winter cold. Plant Biosyst. 142, 472–479. doi: 10.1080/11263500802410819
Benavides-Meza, H. M., Gazca Guzmán, M. O., López, S. F., Camacho Morfín, F., Fernández Grandizo, D. Y., de la Garza López de Lara, M. P., et al. (2011). Growth variability in seedlings of eight provenances of Abies religiosa (H.B.K.) Schlecht. et Cham., in nursery conditions. Madera Bosques 17, 83–102.
Bertness, M., and Callaway, R. (1994). Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. doi: 10.1016/0169-5347(94)90088-4
Blanco-García, A., Sáenz-Romero, C., Martorell, C., Alvarado-Sosa, P., and Lindig-Cisneros, R. A. (2011). Nurse plant and mulching effects on tree conifer species in a Mexican temperate forest. Ecol. Eng. 37, 994–998. doi: 10.1016/j.ecoleng.2011.01.012
Brodersen, C. R., Germino, M. J., Johnson, D. M., Reinhardt, K., Smith, W. K., Resler, L. M., et al. (2019).Seedling survival at timberline is critical to conifer mountain forest elevation and extent. Front. Forests Glob. Change 2:9. doi: 10.3389/ffgc.2019.00009
Brooker, R., Maestre, F., Callaway, R., Lortie, C., Cavieres, L., Kunstler, G., et al. (2008). Facilitation in plant communities: the past, the present and the future. J. Ecol. 96, 18–34. doi: 10.1111/j.1365-2745.2007.01295.x
Brower, L. P., Slayback, D. A., Jaramillo-López, P., Ramírez, I., Oberhauser, K. S., Williams, E. H., et al. (2016). Illegal logging of 10 hectares of forest in the Sierra Chincua Monarch Butterfly overwintering area in Mexico. Am. Entomol. 62, 92–97. doi: 10.1093/ae/tmw040
Brower, L. P., Williams, E. H., Jaramillo-López, P., Kust, D. R., Slayback, D. A., and Ramírez, M. I. (2017). Butterfly mortality and salvage logging from the March 2016 storm in the Monarch Butterfly Biosphere Reserve in Mexico. Am. Entomol. 63, 151–164. doi: 10.1093/ae/tmx052
Bruno, J., Stachowicz, J., and Bertness, M. (2003). Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125. doi: 10.1016/S0169-5347(02)00045-9
Callaway, R. (2007). Positive Interactions and Interdependence in Plant Communities. Missoula, MO: Springer.
Callaway, R., Brooker, R., Choler, P., Kikvidze, Z., Lortie, C., Michalet, R., et al. (2002). Positive interactions among alpine plants increase with stress. Nature 417, 844–848. doi: 10.1038/nature00812
Castellanos-Acuña, D., Lindig-Cisneros, R. A., and Sáenz-Romero, C. (2015). Altitudinal assisted migration of Mexican pines as an adaptation to climate change. Ecosphere 6, 1–16. doi: 10.1890/ES14-00375.1
Commander, L. E., Coates, D., Broadhurst, L., Offord, C. A., Makinson, R. O., and Matthes, M. (2018). Guidelines for the Translocation of Threatened Plants in Australia. Canberra, ACT: Australian Network for Plant Conservation.
Costa, A., Villa, S., Alonso, P., García-Rodríguez, J. A., Martín, F. J., Martínez-Ruiz, C., et al. (2017). Can native shrubs facilitate the early establishment of contrasted co-occurring oaks in Mediterranean grazed areas? J. Veg. Sci. 28, 1047–1056. doi: 10.1111/jvs.12550
Díaz-Rodríguez, B., del-Val, E., Gómez-Romero, M., Gómez-Ruiz, P. A., and Lindig-Cisneros, R. (2013). Conditions for establishment of a key restoration species, Lupinus elegans Kunth, in a Mexican temperate forest. Bot. Sci. 91, 225–232. doi: 10.17129/botsci.417
Environment Canada (2014). Management Plan for the Monarch (Danaus plexippus) in Canada [Proposed]. Species at Risk Act Management Plan Series. Ottawa, ON: Environment Canada.
Filazzola, A., and Lortie, C. J. (2014). A systematic review of nurse-plant mechanisms. Glob. Ecol. Biogeogr. 23, 1335–1345. doi: 10.1111/geb.12202
Fondo Monarca (2017). Degradación Forestal en la Zona Núcleo de la Reserva de la Biósfera de la Mariposa Monarca. Internal Report. Zitácuaro: Fondo Monarca.
García-Hernández, M. A., Toledo-Aceves, T., López-Barrera, F., Sosa, V. J., and Paz, H. (2019). Effects of environmental filters on early establishment of cloud forest trees along elevation gradients: Implications for assisted migration. Forest Ecol. Manage. 432, 427–435. doi: 10.1016/j.foreco.2018.09.042
García-Serrano, E., Lobato-Reyes, J., and Mora Álvarez, B. X. (2004). “Locations and area occupied by monarch butterflies overwintering in Mexico from 1993–2002,” in The Monarch Butterfly: Biology and Conservation, eds K. S. Oberhauser and M. J. Solensky (Ithaca, NY: Cornell University Press, 129–134.
Germino, M. J., Smith, W. K., and Resor, A. C. (2002). Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecol. 162,157–168. doi: 10.1023/A:1020385320738
Gómez-Aparicio, L., Zamora, R., Gómez, J. M., Hódar, J. A., Castro, J., and Baraza, E. (2004). Applying plant facilitation toforest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 14, 1128–1138. doi: 10.1890/03-5084
Gómez-Ruiz, P. A., Sáenz-Romero, C., and Lindig-Cisneros, R. (2019). Early performance of two tropical dry forest species transferred to different altitudes of pine-oak forest: an assisted migration attempt in response to climate change. J. Forestry Res. doi: 10.1007/s11676-019-00973-2. [Epub ahead of print].
Hewitt, N., Klenk, N., Smith, A. L., Bazely, D. R., Yan, N., Wood, S., et al. (2011). Taking stock of the assisted migration debate. Biol. Conserv. 144, 2560–2572. doi: 10.1016/j.biocon.2011.04.031
Lambers, H., Chapin, I. I. I. S., and Pons, T. (2008). Plant Physiological Ecology, 2nd Edn. Newyork, NY: Springer.
Lara-González, R., Sánchez-Velásquez, L. R., and Corral-Aguirre, J. (2009). Regeneration of Abies religiosa in canopy gaps versus understory, Cofre de Perote National Park, México (bilingüe). Agrociencia 43, 739–747.
Loya-Rebollar, E., Sáenz-Romero, C., Lindig-Cisneros, R. A., Lobit, P., Villegas-Moreno, J. A., and Sánchez-Vargas, N. M. (2013). Clinal variation in Pinus hartwegii populations and its application for adaptation to climate change. Silvae Genet. 62, 86–95. doi: 10.1515/sg-2013-0011
Marris, E. (2009). Forestry: planting the forest of the future. Nature 459, 906–908. doi: 10.1038/459906a
Méndez-González, I. D., Jardón-Barbolla, L., and Jaramillo-Correa, J. P. (2017). Differential landscape effects on the fine-scale genetic structure of populations of a montane conifer from central Mexico. Tree Genet. Genomes, 13:30. doi: 10.1007/s11295-017-1112-5
O'Neill, G., Wang, T., Ukrainetz, N., Charleson, L., McAuley, L., Yanchuk, A., et al. (2017). A Proposed Climate-Based Seed Transfer System for British Columbia. Technical Report. Availble online at: www.for.gov.bc.ca/hfd/pubs/Docs/Tr/Tr099.htm
O'Neill, G. A., Hamann, A., and Wang, T. (2008). Accounting for population variation improves estimates of the impact of climate change on species′ growth and distribution. J. Appl. Ecol. 45, 1040–1049. doi: 10.1111/j.1365-2664.2008.01472.x
Ortiz-Bibian, M. A., Blanco-García, A., Lindig-Cisneros, R. A., Gómez-Romero, M., Castellanos-Acuña, D., Herrerías-Diego, Y., et al. (2017). Genetic variation in Abies religiosa for quantitative traits and delineation of elevational and climatic zoning for maintaining Monarch Butterfly overwintering sites in Mexico, considering climatic change. Silvae Genet. 66, 14–23. doi: 10.1515/sg-2017-0003
Ortiz-Bibian, M. A., Castellanos-Acuña, D., Gómez-Romero, M., Lindig-Cisneros, R. A., Silva-Farías, M. A., and Sáenz-Romero, C. (2019). Variation among Abies religiosa (H.B.K.) Schl. et Cham populations along an altitudinal gradient. I. Seed germination capacity. Rev. Fitot. Mexic. 42, 301–308.
Padilla, F. M., and Pugnaire, F. I. (2007). Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 21, 489–495. doi: 10.1111/j.1365-2435.2007.01267.x
Pedlar, J. H., McKenney, D. W., Aubin, I., Beardmore, T., Beaulieu, J., Iverson, L., et al. (2012). Placing forestry in the assisted migration debate. BioScience 62, 835–842. doi: 10.1525/bio.2012.62.9.10
Prober, S., Byrne, M., McLean, E., Steane, D., Potts, B., Vaillancourt, R., and Stock, W. (2015). Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 3:65. doi: 10.3389/fevo.2015.00065
Rehfeldt, G. E., Crookston, N. L., Sáenz-Romero, C., and Campbell, E. (2012). North American vegetation model for land use planning in a changing climate: a statistical solution to large classification problems. Ecol. Appl. 22, 119–141. doi: 10.1890/11-0495.1
Rehfeldt, G. E., Jaquish, B. C., López-Upton, J., Sáenz-Romero, C., St. Clair, J. B., Leites, L. P., et al. (2014a). Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: realized climate niches. Forest Ecol. Manage. 324, 126–137. doi: 10.1016/j.foreco.2014.02.035
Rehfeldt, G. E., Jaquish, B. C., Sáenz-Romero, C., Joyce, D. G., Leites, L. P., St. Clair, J. B., et al. (2014c). Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: reforestation. Forest Ecol. Manage. 324, 147–157. doi: 10.1016/j.foreco.2014.02.040
Rehfeldt, G. E., Leites, L. P., Joyce, D. G., and Weiskittel, A. R. (2017). Role of population genetics in guiding ecological responses to climate. Glob. Change Biol. 2017, 1–11. doi: 10.1111/gcb.13883
Rehfeldt, G. E., Leites, L. P., St. Clair, J. B., Jaquish, B. C., Sáenz-Romero, C., López-Upton, J., et al. (2014b). Comparative genetic responses to climate in the varieties of Pinus ponderosa and Pseudotsuga menziesii: clines in growth potential. Forest Ecol. Manage. 324, 138–146. doi: 10.1016/j.foreco.2014.02.041
Rehfeldt, G. E., Tchebakova, N. M., Milyutin, L. I., Parfenova, Y. I., Wykoff, W. R., Kouzmina, N. A., et al. (2003). Assessing population responses to climate in Pinus sylvestris and Larix spp. of Eurasia with climate-transfer models. Eur. J. Forest Res. 6, 83–98.
Rehfeldt, G. E., Tchebakova, N. M., Parfenova, Y. I., Wykoff, W. R., Kuzmina, N. A., and Milyutin, L. I. (2002). Intraspecific responses to climate in Pinus sylvestris. Glob. Change Biol. 8, 912–929. doi: 10.1046/j.1365-2486.2002.00516.x
Rehfeldt, G. E., Ying, C. C., Spittlehouse, D. L., and Hamilton, D. A. Jr.. (1999). Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol. Monogr. 69, 375–407.
G. C.. Rzedowski, and J.. Rzedowski (Eds.). (2005). Flora fanerogámica del Valle de México, 2nd Edn. Pátzcuaro: Instituto de Ecología, A. C. and Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO).
Rzedowski, J. (2006). Vegetación de México. 1ra. Edición Digital. México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Sáenz-Romero, C., Lindig-Cisneros, R. A., Joyce, D. G., Beaulieu, J., St-Clair, J. B., and Jaquish, B. C. (2016). Assisted migration of forest populations for adapting trees to climate change. Revista Chapingo Serie Ciencias Forestales y del Ambiente 22, 303–323. doi: 10.5154/r.rchscfa.2014.10.052
Sáenz-Romero, C., Rehfeldt, G. E., Crookston, N. L., Pierre, D., St-Amant, R., Beaulieu, J., et al. (2010). Spline models of contemporary, 2030, 2060 and 2090 climates for Mexico and their use in understanding climate-plant impacts on vegetation. Clim. Change 102, 595–623. doi: 10.1007/s10584-009-9753-5
Sáenz-Romero, C., Rehfeldt, G. E., Duval, P., and Lindig-Cisneros, P. (2012). Abies religiosa habitat prediction in climatic change scenarios and implications for monarch butterfly conservation in Mexico. Forest Ecol. Manage. 275, 98–106. doi: 10.1016/j.foreco.2012.03.004
Sánchez-Velásquez, L. R., Domínguez-Hernández, D., Pineda-López, Ma., del, R., and Lara-González, R. (2011). Does Baccharis conferta shrub act as a nurse plant to the Abies religiosa seedling? Open For. Sci. J. 4, 67–70. doi: 10.2174/1874398601104010067
Sánchez-Velásquez, L. R., Pineda-López, M. R., and Hernández, A. (1991). Distribución y estructura de la población de Abies religiosa (H.B.K) Schl.et cham., en el Cofre de Perote, Edo. de Veracruz, México. Acta Botánica Mexicana 16, 45–55. doi: 10.21829/abm16.1991.625
SAS Institute Inc (2004) SAS/STAT Computer Software. Release 9.1 3th edition. Cary NC: SAS Institute Inc.
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Snook, L. (1993). “Conservation of the Monarch butterfly reserves in México: focus on the forest” in Biology and Conservation of the Monarch Butterfly, eds S. Malcolm and M. Zalucki (Los Angeles, CA: Natural History Museum of Los Angeles County: Science Series No. 38, 362–375.
Torroba-Balmori, P., Zaldívar, P., Alday, J. G., Fernández-Santos, B., and Martínez-Ruiz, C. (2015). Recovering Quercus species on reclaimed coal wastes using native shrubs as restoration nurse plants. Ecol. Eng. 77, 146–153. doi: 10.1016/j.ecoleng.2015.01.024
Valle-Díaz, O., Blanco-García, A., Bonfil, C., Paz, H., and Lindig-Cisneros, R. (2009). Altitudinal range shift detected through seedling survival of Ceiba aesculifolia in an area under the influence of an urban heat island. Forest Ecol. Manage. 258, 1511–1515. doi: 10.1016/j.foreco.2009.07.001
Walker, L., Walker, J., and del Moral, R. (2007). “Forging a new alliance between succession and restoration” in Linking Restoration and Ecological Succession, eds L. Walker, J. Walker, and R. Hobbs (New York, NY: Springer), 1–18. doi: 10.1007/978-0-387-35303-6_1
Will, R. E., Wilson, S. M., Zou, C. B., and Hennesey, T. C. (2013). Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol. 200, 366–374. doi: 10.1111/nph.12321
Keywords: Abies religiosa, assisted migration, climatic change, nurse plants, reforestation, Monarch butterfly, overwintering sites, ecological restoration
Citation: Carbajal-Navarro A, Navarro-Miranda E, Blanco-García A, Cruzado-Vargas AL, Gómez-Pineda E, Zamora-Sánchez C, Pineda-García F, O'Neill G, Gómez-Romero M, Lindig-Cisneros R, Johnsen KH, Lobit P, Lopez-Toledo L, Herrerías-Diego Y and Sáenz-Romero C (2019) Ecological Restoration of Abies religiosa Forests Using Nurse Plants and Assisted Migration in the Monarch Butterfly Biosphere Reserve, Mexico. Front. Ecol. Evol. 7:421. doi: 10.3389/fevo.2019.00421
Received: 22 December 2018; Accepted: 18 October 2019;
Published: 06 November 2019.
Edited by:
Jay E. Diffendorfer, United States Geological Survey (USGS), United StatesReviewed by:
Carolina Martínez-Ruiz, University of Valladolid, SpainDavid Gorchov, Miami University, United States
Copyright © 2019 Carbajal-Navarro, Navarro-Miranda, Blanco-García, Cruzado-Vargas, Gómez-Pineda, Zamora-Sánchez, Pineda-García, O'Neill, Gómez-Romero, Lindig-Cisneros, Johnsen, Lobit, Lopez-Toledo, Herrerías-Diego and Sáenz-Romero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuauhtémoc Sáenz-Romero, csaenzromero@gmail.com
 Aglaen Carbajal-Navarro
Aglaen Carbajal-Navarro Esmeralda Navarro-Miranda
Esmeralda Navarro-Miranda Arnulfo Blanco-García
Arnulfo Blanco-García Ana Laura Cruzado-Vargas
Ana Laura Cruzado-Vargas Erika Gómez-Pineda
Erika Gómez-Pineda Cecilia Zamora-Sánchez2
Cecilia Zamora-Sánchez2  Fernando Pineda-García
Fernando Pineda-García Roberto Lindig-Cisneros
Roberto Lindig-Cisneros Kurt H. Johnsen
Kurt H. Johnsen Leonel Lopez-Toledo
Leonel Lopez-Toledo Cuauhtémoc Sáenz-Romero
Cuauhtémoc Sáenz-Romero