Spitting Seeds From the Cud: A Review of an Endozoochory Exclusive to Ruminants
- 1Estación Biológica de Doñana, Spanish National Research Council, Sevilla, Spain
- 2Centre d'Ecologie et des Sciences de la Conservation, Sorbonne Universités, MNHN, CNRS, UPMC, Paris, France
- 3Ecologie, Systématique et Evolution, Université Paris-Sud XI, Orsay, France
- 4Centre for Applied Ecology “Prof. Baeta Neves”/InBIO, Institute Superior of Agronomy, University of Lisbon, Lisbon, Portugal
- 5Centro de Investigaciones sobre Desertificación, Spanish National Research Council, The University of Valencia, Instituto Valenciano de Investigaciones Agrarias, Valencia, Spain
Given their strong masticatory system and the powerful microbial digestion inside their complex guts, mammalian ruminants have been frequently considered seed predators rather than seed dispersers. A number of studies, however, have observed that ruminants are able to transport many viable seeds long distances, either attached to the hair or hooves (i.e., epizoochory) or inside their body after ingesting them (i.e., endozoochory). However, very few studies have investigated a modality of endozoochory exclusive to ruminants: the spitting of usually large-sized seeds while chewing the cud. A systematic review of the published information about this type of endozoochory shows a marked scarcity of studies. Nonetheless, at least 48 plant species belonging to 21 families are dispersed by ruminants in this manner. Most of these plants are shrubs and trees, have fleshy or dry fruits with large-sized seeds, and are seldom dispersed via defecation. Many cases have been observed in tropical areas, where more frugivorous ruminant species occur, but other records are from temperate and dry areas, covering thus all continents except Antarctica. Twenty-one species of ruminants from 18 genera have been reported as endozoochore spitters. They involve domestic and wild species belonging to the families Tragulidae, Cervidae, and Bovidae. This suggests that almost any ruminant species could potentially eat fruits and regurgitate large hard seeds during rumination. Likely, this seed dispersal mechanism has been neglected due to the difficulty of observing rumination behavior and locating spat seeds. Further research on the potential of wild and domestic ruminant species as long-distance seed dispersers through spitting seeds from the cud appears particularly important given their increasing pervasiveness and abundance worldwide.
Introduction
Reproductive plants frequently benefit from moving their seeds away from their immediate vicinity. Such benefits include the increase of seed and seedling survival, enhanced germination, reduced sibling competition, increased gene flow, and the colonization of vacant habitats. Not surprisingly, plants use myriad mechanisms for seed dispersal, such as the transport outside and inside vertebrates' bodies (Herrera, 2002). To attract vertebrate seed dispersers, many plants have evolved edible fruits covering seeds, so that these nutritious fruits are ingested by animals that later eject seeds in suitable conditions to germinate (Herrera, 2002). However, fruits are attractive also for some other frugivores that kill seeds during ingestion and/or digestion, acting thus as seed predators. Given their strong masticatory system and the powerful microbial digestion inside their complex guts, mammalian ungulates, and particularly those that are ruminants, have been frequently considered predators of the large-sized seeds characteristic of many woody plant species. Indeed, many authors consider that ungulates maximize nutritional intake from fruits by digesting the entire resource, including seeds (e.g., Bodmer, 1991).
The antagonistic role of ungulates as plant consumers and seed predators has been frequently highlighted. As a result, the environmental risk of overgrazing derived from the recent increase in numbers of some wild ungulate species is receiving increasing research attention (e.g., Côté et al., 2004; Perea et al., 2014a; Lecomte et al., 2016). In the same way, global livestock production is an important cause of environmental concern, either for climate change, reactive nitrogen mobilization, or appropriation of plant biomass at planetary scales (Pelletier and Tyedmers, 2010). Nevertheless, the mutualistic role of ungulates as effective seed dispersers (sensu Schupp et al., 2010) has been also investigated, especially for grasses and some other plants whose small seeds often escape mastication and pass intact through the gut (i.e., endozoochory; e.g., Janzen, 1984; Myers et al., 2004; Mouissie et al., 2005). Also, ruminants can move many viable seeds at long distances attached to the fur and the hooves (i.e., epizoochory; e.g., Manzano and Malo, 2006). Recent reviews analyzing the main seed traits that facilitate dispersal by ungulates concluded that seeds having hooks or an elongated shape would be likely dispersed by epizoochory, while rounded and small seeds would be dispersed via endozoochory (Albert et al., 2015a,b).
Interestingly, a particular group of ungulates, the ruminants, can disperse large-sized seeds via a “less well-reported form of endozoochory specific to ruminants: large viable seeds are spat out after some time in the rumen” (Feer, 1995). In this case, seeds are released without completing the whole digestion process (i.e., by defecation), but ejected from the regurgitated bolus (the cud) while ruminating. Ruminants using this modality of seed dispersal were named by Forget et al. (2007) “endozoochore spitters” (a type of dispersal similar to the regurgitation of many bird species; Levey, 1987). Here we estimate for the first time the global importance of such a peculiar kind of endozoochory exclusive to ruminants.
Ruminants, i.e., the members of the mammalian Order Artiodactyla that include a rumen, reticulum, omasum (or some part homologous to the omasum) and abomasum in their digestive system, are abundant and frequently large body sized mammals that are able to severely influence ecosystem structure and functioning (e.g., Danell et al., 2006). According to Hackmann and Spain (2010), there are about 200 wild species of ruminants distributed in six families (Tragulidae, Moschidae, Bovidae, Giraffidae, Cervidae, and Antilocapridae), although most of them are Bovidae (140 species) and Cervidae (41). Besides, there are nine species of domestic ruminants (eight Bovidae and one Cervidae). Their estimated population numbers are impressive: about 75.3 million wild ruminants and 3.6 billion domestic ones (Hackmann and Spain, 2010). Importantly, only 10% of these domestic ruminants are raised as industrial livestock (i.e., detached from the land base of feed supply and waste disposal), with the remaining 90% being raised in mixed and grazing land-based systems (http://www.fao.org/ag/againfo/themes/en/meat/backgr_productions.html).
As ruminants are abundant and rather large body sized animals (up to 900 kg; median of extant wild species is 45 kg; Hackmann and Spain 2010), their effects on the diversity and dynamics of plant communities are usually very relevant (e.g., McNaughton et al., 1988), either directly affecting plant demography through grazing, browsing and seed dispersal, or indirectly by modifying the plant environment, such as soil, nutrient flows, and water cycle (Hobbs, 1996). Thus, attaining a comprehensive understanding of the patterns, mechanisms, and consequences of plant-ruminant interactions is an important challenge for ecologists, conservationists and managers (Danell et al., 2006; Foster et al., 2014; Bernes et al., 2018).
As is known, the rumen (or first chamber of the ruminant stomach) delays plant food at the gut for enough time to allow symbiotic microbes to ferment it. The delaying mechanism relies on the orifice between the rumen and the omasum, which limits the size of the food particles that can pass on to successive stomach chambers and intestines (Wenninger and Shipley, 2000). Ruminants ingest many whole fruits with scarce or no mastication. In their forestomach, sized particles are stratified into small and large, and the latter are regurgitated and then remasticated to smaller, easier-to-digest particles (Schwarm et al., 2008). In this way, hard small seeds can pass directly all along the gut and be defecated, relatively soft seeds are crushed or digested, and well-protected large ones must be regurgitated at the cud (one or several times), to be expelled from the mouth or swallowed again and destroyed by rumen microbes (Bodmer, 1991; Sridhara et al., 2016). In addition to the typically large size of regurgitated seeds, this modality of seed dispersal is likely characterized by other parameters, such as retention time, dispersal distance, condition of seed deposition (e.g., level of aggregation), seed survival, and germination success, which must differ from those of seeds dispersed through defecation (Sridhara et al., 2016).
Given the high and increasing pervasiveness and abundance of domestic and wild ruminants worldwide, their capacity to ingest daily a large number of fruits, and the large extent of their movements, their role as potential long-distance seed dispersers deserves particular attention. In this sense, if regurgitating viable seeds while chewing the cud is commonplace, ruminants would be much more important dispersers than previously considered. So far, however, research on this particular kind of endozoochory is largely lacking.
Here we present the results of a review including all available information about, exclusively, dispersal of seeds while chewing the cud (i.e., we do not consider the short-distance dispersal of seeds ejected during fruit mastication) from both wild and domestic ruminant species.
Methods
A literature review was carried out to know the state of knowledge about regurgitation and spitting of seeds during rumination, without considering a priori if the articles refer specifically to seed dispersal. We consulted reference databases such as Science Citation Index, Science Direct, Scopus, and Google Scholar, among others. Search terms included several combinations of “seeds,” “seed dispersal,” “endozoochory,” “rumination,” “spitting,” “regurgitation,” “ungulates,” “ruminants,” “livestock,” “cattle,” “goat,” “sheep,” “deer,” and “antelope.” We also searched for articles citing Feer (1995) and Prasad et al. (2006) and examined all references contained in each of the previously selected articles mentioning spitting of seeds during rumination. More than 1000 papers were considered, but only 40 (plus two communications in litt.) were useful for this review (Table 1). Some of them simply quoted other authors to say that seeds are regurgitated during rumination (e.g., Krefting and Roe, 1949; Corlett, 1998), others alluded to indirect observations of “apparent” seed regurgitation by some species of ruminant (e.g., Jordano, 1987), and finally some others assumed seed spitting, for instance because they found undamaged seeds at the rumen of dead animals but never at the dung (e.g., Slater and du Toit, 2002). Only 25 papers included direct assertions on original observations or experimental evidence of spitting seeds from the cud.
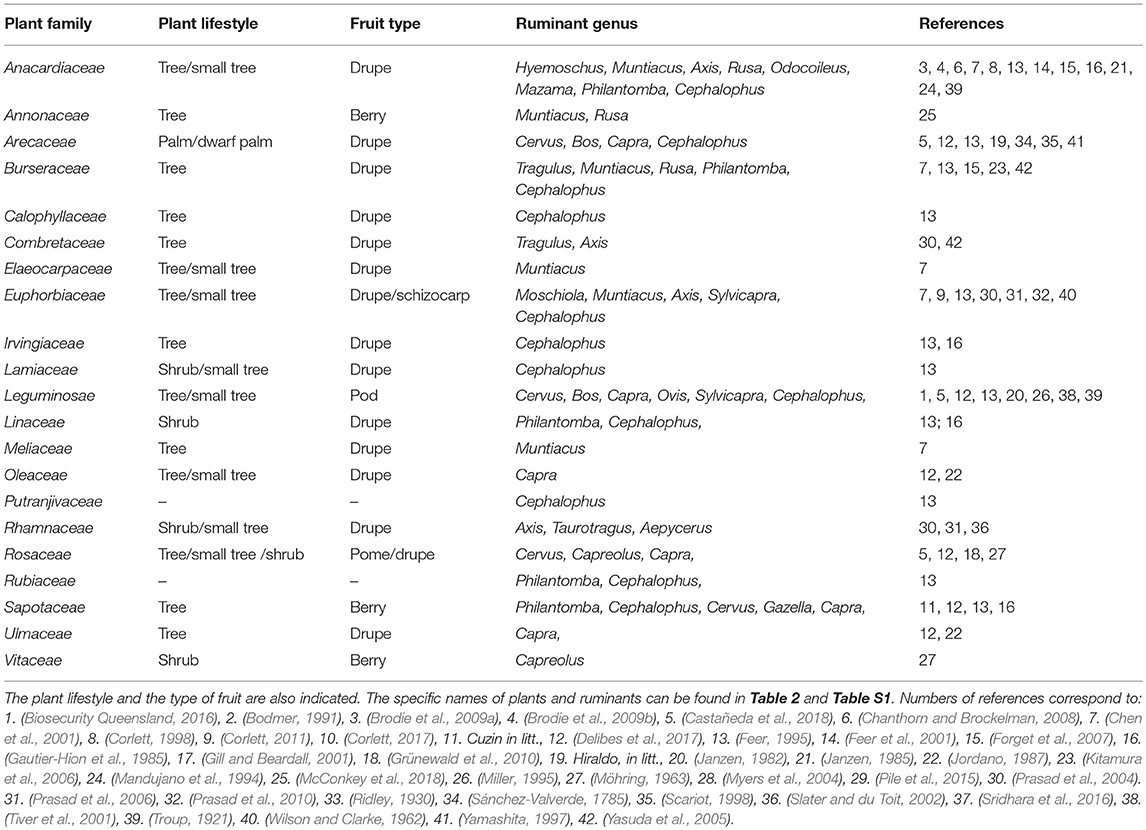
Table 1. Families of plants whose seeds are spat by different genus of ruminants while chewing their cud.
To unify scientific names, we used as reference for plants (Table S1) the Plant List (www.theplantlist.org) created by the Royal Botanic Gardens of Kew and the Missouri Botanical Garden, and the list of Mammal Species of the World of (Wilson and Reeder, 2005), third edition (www.departments.bucknell.edu/biology/resources/msw3/) for mammals. Seed mass was obtained from the original papers on seed dispersal, when available, or from the Seed Information Database of the Royal Botanic Gardens, Kew (http://data.kew.org/sid/). For ruminants' body weight and other characteristics we used Bodmer (1990) and the Handbook of the Mammals of the World, vol. 2 (Wilson and Mittermeier, 2011; Table 2).
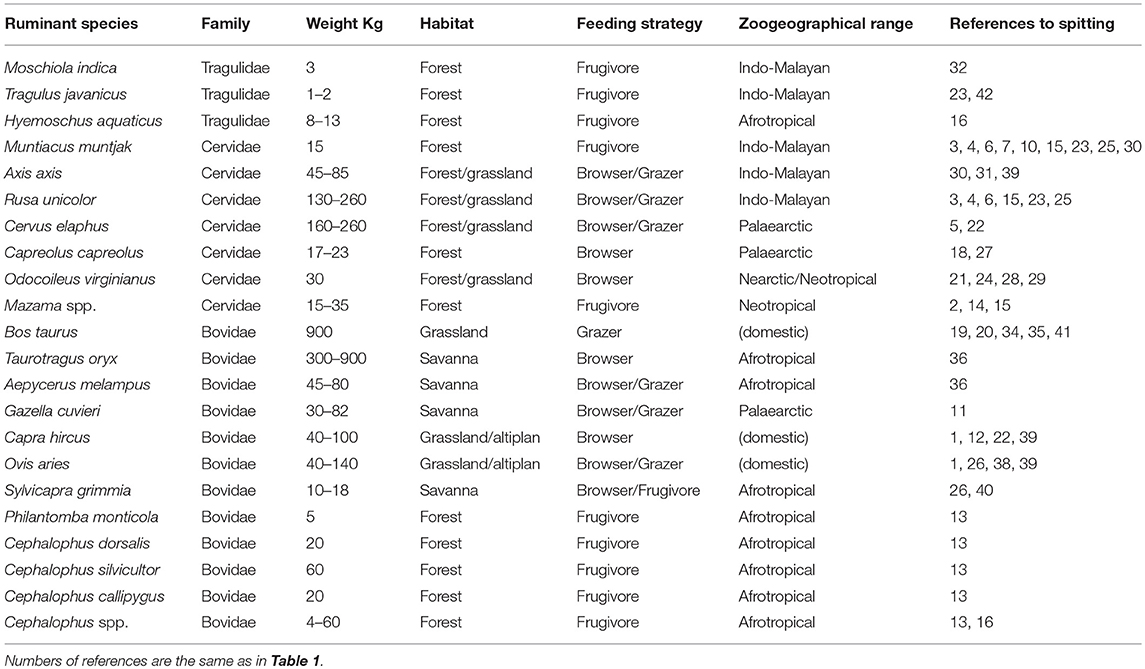
Table 2. Species of ruminants reported as spitters of seeds while ruminating, and some taxonomic, biological, and ecological information about them (from Bodmer, 1990, complemented with Wilson and Mittermeier, 2011).
Results and Discussion
A Brief History of Seed Spitting During Rumination
As a matter of fact, the places where domestic ruminants lay down for rumination do appear frequently covered by spat seeds mixed with dung. Then, likely human herders at least since the Neolithic knew that ruminants were able to spit undamaged seeds while ruminating. In the XVIII century, Antonio Sánchez-Valverde, a priest and lawyer in the Hispaniola Island (current Haiti and Dominican Republic), wrote (in Spanish) about corozo palm (Acrocomia aculeata) fruits: “Bovine livestock, that swallow these globes very barely chewed, digests the flesh and throw up the remains, i.e., the hard nut” (Sánchez-Valverde, 1785, p. 56). According to our knowledge, the first scientific report on this kind of endozoochory corresponds to Troup (1921) in a treatise on silviculture of Indian trees. He described that piles of stones (of Spondias mangifera, today Spondias pinnata) “are continually met with in place where deer have lain ruminating and bringing them up” (p. 247, vol. 1), and also that the seed of Acacia arabica (at present, Acacia nilotica) “seldom passes completely through sheep and goats, but is ejected by them from the mouth during rumination” (p. 427, vol.2). Besides, he added that “the fermentation and moistening which the seeds undergo before their ejection undoubtedly assists germination”. In his seminal review on seed dispersal, Ridley (1930, p. 372) quoted Troup mentioning briefly that seeds of some fruits consumed by deer in Asia were not defecated, but “disgorged during rumination.” The findings of Troup were also mentioned by Krefting and Roe (1949), but later they would be practically forgotten. Wilson and Clarke (1962) reported this type of endozoochory in captive duikers (Sylvicapra grimmia) in Africa and Möhring (1963) in a captive roe deer (Capreolus capreolus) in Europe. Later, Janzen (1982, 1985) experimentally investigated the passing of seeds of guanacaste (Enterolobium cyclocarpus) through cattle guts and of nuts of Spondias mombin through white-tailed deer (Odocoileus virginianus) guts. Most seeds of guanacaste were defecated by cattle and very few spat from the cud; contrarily, white-tailed deer “regurgitate the nuts (of Spondias) while chewing their cud” and these “nuts never pass into the lower digestive tract.” Since the middle of the 1980s, a few authors described this overlooked behavior in African ruminants (e.g., Gautier-Hion et al., 1985; Feer, 1995), and at the beginning of the XXI century in Australian sheep and goats (e.g., Tiver et al., 2001) and wild Asian ruminants (e.g., Chen et al., 2001; Prasad et al., 2006; Brodie et al., 2009a). More recently, endozoochorous spitters have received attention in Europe and North Africa (e.g., Grünewald et al., 2010; Delibes et al., 2017; Castañeda et al., 2018).
Some recent reviews on frugivory and seed dispersal in tropical areas have considered this type of endozoochory (e.g., Forget et al., 2007), especially in Asia (Corlett, 2011, 2017; Sridhara et al., 2016). However, other reviews refer only to seed regurgitation by birds, primates and fish (e.g., Parolin et al., 2013), mention ungulates mainly as seed predators (e.g., Stoner et al., 2007) or refer exclusively to plant species identified in dung, overlooking spat seeds (e.g., Miceli-Méndez et al., 2008, which introduced the term “bovinochory” to refer to seed dispersal by cattle in the Neotropics).
Plant Species Dispersed During Rumination
At least 48 plant species belonging to 21 families are likely dispersed from the cud by domestic and wild ruminants (Table 1 and Table S1). These include some plants spontaneously consumed and some others fed to domestic and captive animals in more or less controlled conditions. Curiously, only two of these species (Crataegus laevigata and Prunus avium), both European, are considered in the meta-analysis carried out by Albert et al. (2015a,b) to compare, at community level, the traits of seeds dispersed and not dispersed by ungulates, and both are cataloged in their study as “never dispersed in endozoochory, fur-epizoochory and hoof-epizoochory studies taken into account in the analysis.” At the same line, Albert et al. (2015a,b) reported the dispersal by ungulates of 278 plant species belonging to 42 families, but only two of these (Fabaceae or Leguminosae, and Rosaceae) are represented among the 21 families we reported as dispersed through endozoochore spitting. This supports the idea that plants dispersed by ruminants while chewing their cud are a different set of species than those usually dispersed by conventional endozoochory (i.e., defecated seeds). The contrast is evident when comparing traits of plants whose seeds are dispersed in ungulate dung and those of plants dispersed while ruminating. For instance, through the review of 52 studies, Albert et al. (2015a) concluded that plants dispersed by ungulates are mainly grasses typical of open habitats (93%), while most (but not all) species dispersed from the cud are forest trees (Figure 1A and Table S1). Also, most (85.2%) of the fruit consumed by wild ruminants in Asia, according to the review carried out by Sridhara et al. (2016), corresponded to trees.
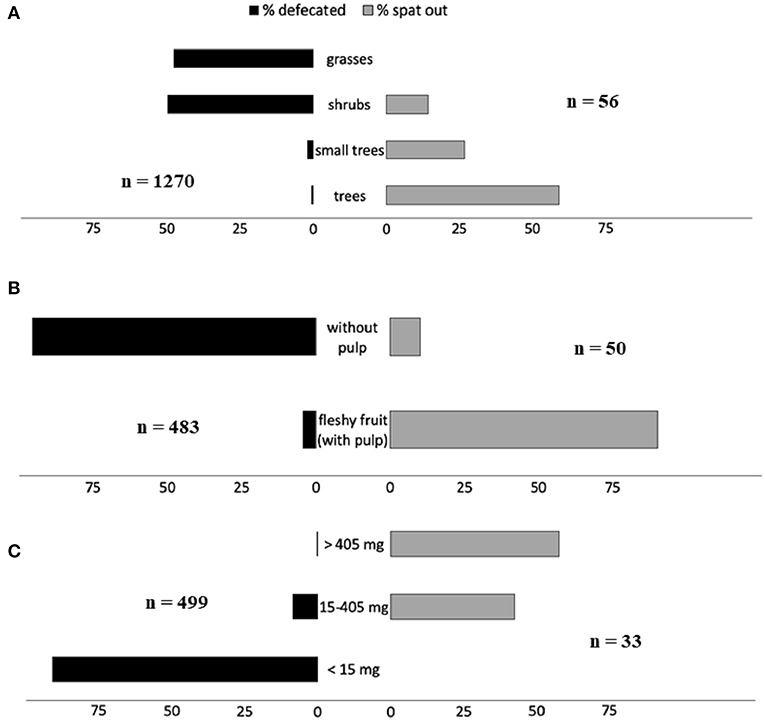
Figure 1. Distribution of frequencies (percentages) of (A) type of plants, (B) type of fruits, and (C) propagule weights, whose seeds are dispersed by ruminant defecation (black bars; data in Albert et al. 2015b, Appendix 5) and spitting from the cud (gray bars; data in this review). Plants under 0.4 m in Albert et al. (2015b) were associated with grasses, from 0.4 to 2 m to shrubs, from 2 to 5 m to small trees and above 5 m to trees. Some species were included in several categories (e.g., small trees and trees).
Fruit and Seed Characteristics of Plants Dispersed While Ruminating
Most plants dispersed by ungulates inside the dung respond to the “foliage is the fruit” hypothesis enunciated by Janzen (1984), i.e., they are grasses with small seeds which are ingested inadvertently while eating the foliage. In the case of plants dispersed by ruminants via regurgitation, the reward is the fruit pulp, as for other vertebrate seed dispersers (e.g., birds, carnivores). So, fruits must be attractive to ruminants and usually they include nutritious pulp or pod (Table 1 and Figure 1B).
According to Prasad et al. (2006), fruit traits of species dispersed while ruminating “appear to converge toward being green or brown, drupaceous, with fibrous pulp and strong seed protection.” Sridhara et al. (2016) found more variation in the fruits consumed by wild large ruminants in Asia, 36.7% being yellow and most of them drupes (50.3%) and berries (27.2%). In contrast, seeds dispersed through defecation usually correspond to fruit without pulp (Figure 1B).
Besides, dispersal units (i.e., individual seeds or cocci including a small number of them) must be large enough to avoid being passed out of the ruminant forestomach. The contrast between the sizes of the propagules dispersed by ruminants through defecation and those spat from the cud is very evident (Figure 1C). In spite of these differences, some plant species can be dispersed through both types of endozoochory by the same or different ruminant species. For example, cattle defecated most seeds of Enterolobium cyclocarpum, but spat from the cud a small portion of them (Janzen, 1982). Contrarily, deer spat most Chamaerops humilis seeds, but defecated a small percentage (Castañeda et al., 2018). In general, large ruminants defecate some seeds that are usually spat by small ruminants (e.g., Slater and du Toit, 2002; see below).
Frequently large seeds are held in large fruits, hence the plants dispersed from the cud often have rather large fruits (e.g., 50 mm diameter in Acrocomia aculeata). However, as expected, the size of regurgitated seeds tends to increase with the size of the ruminant consumer (Gautier-Hion et al., 1985). That is evident also in our sample (Figure 2) and contrasts markedly with the conclusion of the global analysis of Chen and Moles (2015) wherein ungulates showed a negative relationship between body mass and ingested seed size. Indeed, the fact that Chen and Moles (2015) did not include any information on seeds dispersed through regurgitation biased their results.
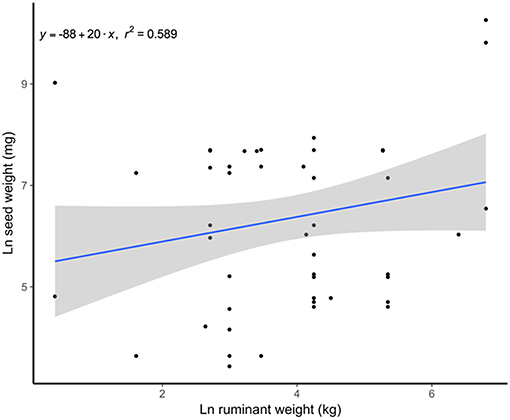
Figure 2. Positive relationship between the weight of spat seeds and the size of the spitter ruminants. Data from Table S1.
To some extent the relative sizes of seeds and ruminants will determine whether a hard seed is handled without swallowing or swallowed, and in the second case if it is later spat during rumination, defecated, or completely digested. For example, while cattle (900 kg) pass through the gut most Acacia nilotica seeds, the smaller sheep (around 50 kg) reject many of them during pod ingestion and mastication, spit some others during rumination, and scarcely 1% are delivered in the dung (Tiver et al., 2001).
This suggests strong context-dependency of these fruit-frugivore interactions, as seed fate (dispersal mechanism/predation) would vary with partner identities (Perea et al., 2014b). Nevertheless, relatively small seed size does not guarantee that seeds will be defecated. For instance, captive red deer (Cervus elaphus) spat from the cud 19% and defecated 25% of the ingested seeds of Ceratonia siliqua (seed weight = 0.18 g; Castañeda et al., 2018). Future studies should add information about the relationship between ruminant body size and spat seed size and the extent to which this relationship shapes seed fate.
Some other fruit characteristics of plants dispersed while ruminating seem to be shared with those of plants dispersed via endozoochory by mammalian carnivores (e.g., Herrera, 1989), such as having scented fruits that fall to ground when ripe, “possibly to attract terrestrial dispersers” (Brodie et al., 2009a). In addition, some of the fruits eaten by ruminants are also edible by people and have economic value, such as Choerospondias axillaris (Chen et al., 2001) or Phyllanthus emblica (Prasad et al., 2004).
Interestingly, some traits of fruits dispersed from the cud by ruminants match those of the so-called megafaunal fruits, i.e., those dispersed during the Pleistocene by now-extinct megafauna (Janzen and Martin, 1982). In particular, “ruminant fruits” resemble the type 1 megafaunal fruits described by Guimarães et al. (2008): usually brown or green large-sized fleshy fruits with either a single or few large and hard seeds. Janzen and Martin (1982) mention 14 plant families of Costa Rica, which include species “probably dispersed by extinct megafauna;” seven of these families (e.g., Anacardiaceae, Arecaeae, Sapotaceae) are quoted in our list of plants dispersed through spitting by ruminants (Table 1), and only one (Leguminosae) does appear in the list of families dispersed by ungulates in the dung according to Albert et al. (2015a,b). Frequently, studies of current Neotropical communities consider only the tapir (Tapirus terrestris) and exotic livestock (including feral pigs) to be capable of dispersing megafaunal fruits by endozoochory (e.g., Donatti et al., 2007), but the potential role of wild ruminants such as Mazama spp. and Odocoileus virginianus should not be ignored anymore (Janzen, 1985; Mandujano et al., 1994).
Disperser Ruminants
At least 21 species included in 18 genera of ruminants have been reported as endozoochorous spitters (Table 2). They include domestic (n = 3) and wild (n = 18) species, belonging to the families Tragulidae (three species, but the taxonomy of Tragulus is not clear; Wilson and Reeder, 2005), Cervidae (n = 7) and Bovidae (n = 11). They range in body size from 1 to 2 kg for the small mousedeer (Tragulus spp.) of Southeast Asia to near 900 kg for some domestic cows (Bos taurus). According to Bodmer (1990), ungulates could be distributed along a linear continuum ranging from fruit feeders to browsers and then grazers, with the ruminants included mainly in the browsers and grazers categories. Assuming the classification proposed by Bodmer (1990), 5–7 of the genera we identified as spitters of seeds (Table 2) would belong to the frugivores (Mazama, Tragulus, probably including Moschiola, Muntiacus, Hyemoschus, and Cephalophus, including Philantomba), one would be browser/frugivore (Sylvicapra), four browsers (Odocoileus, Taurotragus, Capreolus and Capra), 4–6 browser/grazers (Cervus, probably including Axis and Rusa, Aepyceros, Gazella, and Ovis) and 1 grazer (Bos). It seems that almost any ruminant species could consume fruits and eventually regurgitate their seeds during rumination. Thus, future studies should identify ruminant and fruit traits that play a major role in this mutualistic interaction.
It must be emphasized that seed dispersal while chewing the cud does appear in the Tragulidae, the most primitive family of extant ruminants, whose members lack a true omasum and have been considered “living fossils” (Hackmann and Spain, 2010). That means that this particular type of endozoochory is a rather ancestral behavior in the group.
Geographical Area and Habitat
Spitting seeds from the cud seems to be a universal seed dispersal mechanism among ruminants, as we recorded cases in all continents except Antarctica (Table S1). In particular, we found 10 plant species dispersed in Europe, 12 in Asia, 21 in Africa, nine in America and one in Oceania (some species were dispersed in more than one continent). Many plant species are from tropical forests, where a higher diversity of frugivorous ungulates exist (Bodmer, 1990), suggesting their feeding habits make them prone to regurgitate seeds (Table 2). In all, we found 40 dispersed plant species from tropical areas (forests and wooded savannas) and 13 species from non-tropical areas (Mediterranean scrubland, temperate forests, and subdeserts).
Apart from the number of involved plant species, it is very difficult to speculate about the relevance of this modality of endozoochory in different continents or ecosystems. A priori, tropical forests should be favored because of the high number of frugivores living there, but it can be suspected, for instance, that African ungulates could disperse during rumination many seeds of dry and fleshy fruits (e.g., Leguminosae, Arecaceae). Additionally, it must be considered that a given plant species is often dispersed by contrasting types of seed dispersers. For instance, some seeds regurgitated by ruminants can be dispersed also through defecation by other mammals, such as mammalian carnivores (Order Carnivora). So, in Europe Chamaerops humilis can be regurgitated by goats and deer (Table S1), but also defecated by foxes (Vulpes vulpes) and badgers (Meles meles) (Fedriani and Delibes, 2011); also, roe deer does spit out while ruminating viable seeds of Prunus avium, while foxes and badgers defecate them (Grünewald et al., 2010). In the same way, frugivores with different dispersal abilities (including several ruminants) can compete for the same fruit. The relative importance of seed dispersal of any plant species by ruminants and other mammals (or large birds) should be analyzed with more attention, emphasizing the context-dependence of fruit-animal interactions affecting seed fate.
Quantity of Seed Dispersal
Seed dispersal effectiveness has a quantitative (number of seeds dispersed) and a qualitative (probability that a dispersed seed produces a new adult) component (Schupp et al., 2010). For endozoochorous plants, the number of dispersed seeds largely depends of the amount of ingested fruit. Because ruminants cannot take most fruit directly from the trees, they often rely on either primates (or other arboreal vertebrates) or natural falling of the fruit onto the ground to consume them (see Prasad and Sukumar, 2010, for a discussion of the topic). Despite these limitations, they can ingest large quantities of fruit when available (e.g., Johnsingh, 1981; Bodmer, 1991; Brodie et al., 2009a). For instance, two species of ruminants accounted for over 95% of the total Phyllanthus emblica fruits removed by frugivores in India (Prasad et al., 2010).
However, in the case of ruminants, ingestion does not guarantee that seeds will be dispersed, because a particular seed can be ejected while foraging, spat from the cud, defecated or digested, as previously said (Castañeda et al., 2018). The fate relies on many factors, not only the relative size of seeds and consumers (Forget et al., 2007), but also on consumer satiation, foraging speed, and availability of alternative foods, among others. In addition, direct observation of seed regurgitation by ruminants is very difficult, even in captivity (Prasad et al., 2006; McConkey et al., 2018). Because of this, data about the individual rate of seed spitting often come from captive or semi-captive individuals (e.g., Möhring, 1963). Consequently, quantitative information about seed dispersal while ruminating is scarce.
The percentages of ingested seeds of different plants that were spat from the cud by several domestic and captive wild ruminants are summarized in Table 3. They are quite variable (ranging from 2 to 100%). Even if the per capita percentages of spat seeds are generally low, the high abundance of wild and domestic ruminants and the potential high number of consumed fruits per individual suggest that the quantity of dispersed seeds by this type of endozoochory is important. Thus, when overlooking spitting by ruminants, a relevant fraction (quantitative and qualitative) of seed dispersal by these animals is likely missed by researchers. Besides, several authors (e.g., Möhring, 1963; Janzen, 1985; Prasad et al., 2006) emphasized that the seeds of some species spat from the cud were never detected at the dung (see Figure 1).
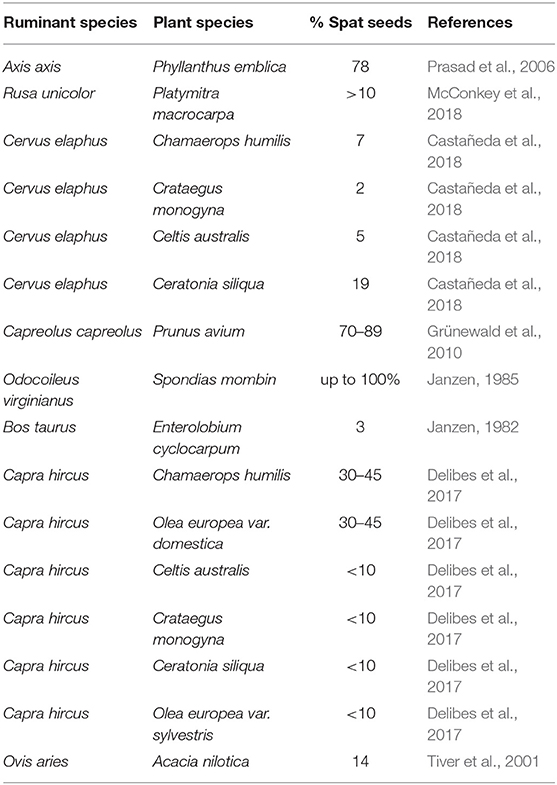
Table 3. Percentages of the ingested seeds of several plant species that were spat from the cud by domestic and wild captive ruminant species.
Quality of Seed Dispersal
Rumen Retention Time and Dispersal Distance
Usually, ruminants ingest fruits with limited or no mastication and eject clean seeds some time later while bedding and ruminating. Observations on the distance between the foraging and the ruminating points are logistically very difficult. Then, available direct information on dispersal distance is reduced, but several authors reported that seeds were spat far from the mother plant. Chen et al. (2001) stated that “the whole fruit (of Choerospondias axillaris) is eaten (by Muntiacus muntjak); then, the stone is regurgitated at a different location.” In a small plot of central Thailand, Rusa unicolor and Muntiacus muntjak dispersed while ruminating 83–98% (2 years) of the seeds of Choerospondias axillaris to distances up to 70 m from the nearest fruiting tree (Chanthorn and Brockelman, 2008), but the authors considered that “deer are capable of longer distance dispersal than was recorded in this study.” According to Feer (1995), dispersal by duikers (Cephalophinae) in Gabon is characterized by “scattered deposition sites and long distance.” Gautier-Hion et al. (1985) wrote that seed spitting during rumination “always occurs away from the fruit source.” In peninsular Malaysia, hard and large seeds of Canarium littorale and Terminalia citrina “are likely to be regurgitated (by Tragulus javanicus) from the mouth in rumination and dispersed at a distance of the mother tree” (Yasuda et al., 2005). In southern Spain, we have found many clean seeds of Chamaerops humilis at sheep and goat pens, very far (i.e., kilometers) from the places where the fruits were likely consumed, suggesting long retention times, and dispersal distances.
Alternatively, seed retention time at the rumen can provide a surrogate of dispersal distance, assuming a positive relationship between elapsed time and distance traveled. Feeding and ruminating rhythm of ungulates (hence, also retention time) were influenced by body size and feeding type, typically grazers having longer lapses than browsers and concentrate selectors (i.e., those with a mixed diet, tending to avoid fiber; Hofmann, 1989). Additionally, retention time can be affected by particle size, or in this case seed size (e.g., Clauss et al., 2009). However, most of these studies refer to retention time before defecation (e.g., Picard et al., 2015), while references to retention time at the rumen are scarce. Estimations about the time elapsed from fruit ingestion to seed regurgitation from the cud go from scarcely 35 min for a young roe deer eating cherries to ten days for a red deer eating hawthorn pomes, but typically range between 3 h and 2 days (Table 4). This rather long seed retention time has a strong potential to facilitate long-distance seed dispersal. As an indication, with average retention times of several hours, Couvreur et al. (2005) estimated that at least half of the epizoochorous seeds attached to the fur of some horses and cattle were released from 47 to 3,080 m from the source site. For defecated seeds and passage times of 48 h, maximal dispersal distances of 3.5 km by red deer and 2.0 km by roe deer were estimated by Pellerin et al. (2016). Obviously, higher distances should be expected for domestic ruminants driven hundreds of kilometers (transhumance; e.g., Manzano and Malo, 2006) and for migratory wild ruminants (e.g., Berger, 2004).
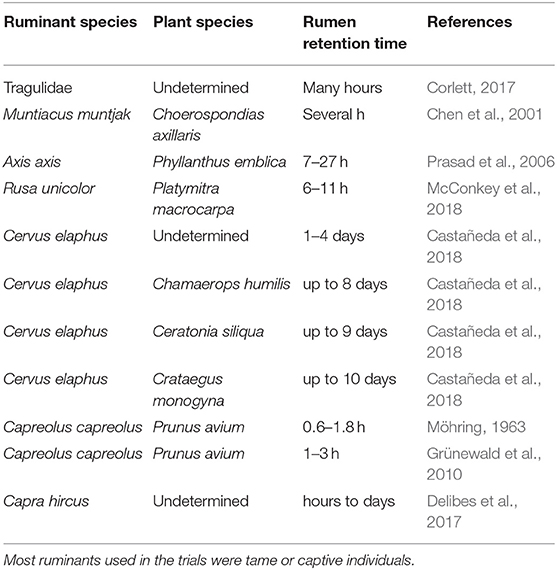
Table 4. Elapsed time between fruit ingestion and seed release from the cud (i.e., seed retention time at the rumen) according different authors.
Deposition Patterns and Seed Fate
There is very scarce information about the destination microhabitat of spat seeds. Domestic species that forage at day often ruminate at night in pens or farmyards (e.g., Troup, 1921; Yamashita, 1997), where there is little or no probability of seed germination. Brodie et al. (2009a) conclude that sambar deer regurgitated higher numbers of seeds of Choerospondias axillaris, but muntjak was the only disperser moving them to open microhabitats, where germination was enhanced. Feer (1995) suggested that nocturnal duikers (Cephalophinae) choose to ruminate (and to deposit regurgitated seeds) places favorable for plant species needing improvements in light conditions for their establishment.
Typically, regurgitated seeds are clumped. Different authors refer to “small piles” or “loose clusters” of clean seeds (e.g., Corlett, 2011), but others mention dense clusters and “very shiny piles” (Brodie et al., 2009a). Piles of five seeds of Platymitra macrocarpa regurgitated by sambar deer were found by McConkey et al. (2018) in Thailand. The same deer species, also in Thailand, deposited seeds of Choerospondias axillaris in piles containing “between 14 and 140 seeds, with the exception of one pile which was found to contain 750 seeds;” in the same area muntjak “tended to deposit seeds in smaller piles (of usually <100)” (Chanthorn and Brockelman, 2008). At chital deer bedding sites, clusters of 4–193 (median = 15, n = 23) cocci of Phyllanthus emblica can be found, along with cocci of other plant species (Prasad et al., 2004). Seeds of Acrocomia aculeata fruit consumed by cattle during the day are regurgitated at night in piles of up to 85 seeds (Scariot, 1998). In Morocco, we found at field piles of 15–30 clean seeds of Chamaerops humilis where goats were ruminating. White-tailed deer make piles of 15–62 seeds of Spondias purpurea, but as a consequence of deer sociality, large numbers of seeds can be concentrated in a few square meters (Mandujano et al., 1994). Similarly, Janzen (1985) reported the following: “A portion of a 2–4-nut-deep layer of Spondias mombin nuts on the forest floor beneath a parent tree; this accumulation was produced by fruit drop and regurgitation by white-tailed deer 8 months previously.” These observations introduce a new and often neglected factor to consider in a qualitative assessment of seed dispersal: social species of ruminants (e.g., sheep) and species or individuals using recurrent rumination sites will accumulate dispersed seeds, reducing the quality of dispersal.
As expected, groups of clean seeds can attract different seed-eaters, so post-dispersal predation (and likely secondary dispersal, e.g., by rodents; Vander Wall et al., 2005; Jansen et al., 2012) is usually high among endozoochorous spat plants. Brodie et al. (2009a) found that 30-40% of seeds of Choerospondias axillaris were removed from their primary local deposition, but seed pile size did not influence germination or first year seedling survivorship. More than 80% of seeds of Chrysophillum beguei regurgitated by duikers in Gabon were eaten or removed by rodents in the next 60 days (Feer, 1995). Most of the dispersed seeds of Spondias mombin in Costa Rica were killed by bruchid beetles (Janzen, 1985). In Thailand, bruchids attack the dispersed seeds of Platymitra macrocarpa in 6–22 days (McConkey et al., 2018). In Brazil, >50% of seeds of the palm Acrocomia aculeata chewed by cattle were infested by bruchids, and this proportion increased to 99% after 27 days; regurgitated seeds had a significant higher rate of insect predation than seeds of non-chewed fruit (Scariot, 1998). Additionally, piles of seeds of the palms Acrocomia aculeata, Attalea phalerata, and Syagrus coronata regurgitated by cattle were regularly visited by macaws (Anodorhynchus leari and A. hyacinthinus) to feed on them, cracking the nuts (Yamashita, 1997). This last author hypothesizes that macaws could track the movements of the extinct Pleistocene megaherbivores in order to collect the large seeds they dispersed.
Germinability of Seeds Spat From the Cud
Different studies have stated that seeds were intact and in a great proportion alive after being regurgitated from the rumen (e.g., Delibes et al., 2017). Some others indicated that spat seeds germinated under field conditions (e.g., Feer, 1995; Castañeda et al., 2018; McConkey et al., 2018). In his pioneer description of seed dispersal while chewing the cud, Troup (1921) indicated that retention at the rumen improved germination of Acacia nilotica from 7 to 35%, concluding: “The superiority of seed collected from goat and sheep pens is generally recognized and seed so collected is extensively used for artificial sowing.” Also, it was said that treatment by goats was necessary for the germination of Argania spinosa seeds (Morton and Voss, 1987). Seeds of Spondias purpurea spat by deer germinated better than those defecated by the iguana Ctenosaura pectinata (Mandujano et al., 1994). In other cases, treatment seemed to be unimportant, as it occurs with the seeds of Choerospondias axillaris that “germinate equally well whether they are defecated by gibbons, regurgitated by deer, or the fruits are uneaten” (Brodie et al., 2009a). Finally, other authors found that rumen retention influences negatively the germination; unconsumed seeds of Phyllanthus emblica germinated more (72%) than pulp-removed seeds (58%) and deer-regurgitated seeds (22%); latency period, however, was shorter for deer-regurgitated and depulped seeds than for those of unconsumed fruit (Prasad et al., 2006).
We can speculate that retention time at the rumen will affect the germination of spat seeds, either improving (by scarification) or decreasing (by damaging the embryos) it. Thus, future studies should quantify these effects.
Conclusions
Many species of ruminants are potential dispersers of numerous species and families of plants by spitting their seeds while chewing the cud (Tables 1, 2 and Table S1). Until now, this behavior had been described mainly in tropical habitats, but it has also been found in other ecosystems in all continents except Antarctica. Given the abundance of wild and free-living domestic ruminants and their high rate of food consumption, it can be suspected they are able to mobilize great numbers of seeds during rumination. Recent reviews on frugivory and seed dispersal by vertebrates in tropical Asia have recognized the importance of this type of endozoochory (Sridhara et al., 2016; e.g., Corlett, 2017), but in general it has been overlooked in many other reviews, even devoted to tropical areas (e.g., Parolin et al., 2013) or specifically to ungulates (e.g., Miceli-Méndez et al., 2008; Albert et al., 2015a). The difficulty in monitoring rumination, a process that usually occurs in sheltered and quiet places, may explain why this modality of endozoochory has been overlooked so far. In fact, a significant part of the data that we reviewed came from captive animals (Table S1). Besides, methods to study seed rain or deposition patterns are biased toward avian or arboreal dispersers (e.g., seed traps) or rely on fecal surveys, being unable to capture seeds spat from the cud by ruminants.
By neglecting the seeds spat from the cud, the quantity and quality of seed dispersal by ruminants could have been severely underestimated until the present. The dispersal of some plant species can persist undetected because they are exclusively spat while ruminating. In other cases, underestimation may result in considering only seeds contained in feces, ignoring that some others of the same species are being spat from the cud. For instance, Miller (1996) investigated the dispersal of seeds of Acacia tortilis and A. nilotica in the dung of South African ungulates: at least five species of ruminants consumed Acacia pods in her study parcel, but we can speculate that probably some of them will spit seeds during rumination, as sheep and goats in Australia do (Tiver et al., 2001). In the same way, by overlooking endozoochorous spitting, the dispersal by ruminants of large-sized seeds of fleshy-fruited plants, frequently shrub and trees, will be missed. This is the case of many comprehensive reviews and meta-analysis of seed dispersal by ungulates that consider exclusively seeds released inside the dung (e.g., Mouissie et al., 2005; Albert et al., 2015a,b) and conclude that tiny seeds of grasses are favored. As reported decades ago by Janzen (1985), some large seeds are solely ejected during rumination, never passing “into the lower digestive tract,” and are thus absent in the feces. As previously said, by ignoring the seeds dispersed from the cud, some studies about the size of seeds dispersed by ungulates arrive at wrong conclusions (e.g., Chen and Moles, 2015).
Another potential bias resulting from ignoring the spitting of seeds from the cud appear when ruminants in experimental cages are fed with different fruits, and the manure collected to investigate the proportion of released seeds and their potential germination (e.g., Grande et al., 2013). Already, Troup (1921) signaled that “the seeds are, it is true, found among their droppings (from sheep and goats), but this is because of the fact that rumination ordinarily takes place where the animals are herded.” This means that the manure of captive ruminants usually includes spat and defecated seeds, which should be considered in a different way in any analysis.
Indeed, the ability of ruminants to disperse large-sized seeds of fleshy and dry fruits must be kept in mind in multi-species mutualistic studies (e.g., Bascompte and Jordano, 2007), instead of treating them exclusively as plant antagonists. However, our review shows that the interaction of ruminants with seeds is very complex. On the one hand, seeds of the same species can be dispersed at short distance while eating, dispersed at long distance while ruminating, dispersed at long distance via defecation, or totally digested (i.e., predated). The proportions of seeds in each of these categories will depend on the relative sizes of both seeds and consumers (Gautier-Hion et al., 1985), but also on other plant- and animal-related factors, such as plant chemical and physical defenses, rate of ingestion, satiation and physical condition. On the other hand, the access of particular ruminants to fruits will be severely influenced by the spatiotemporal context (e.g., plant aggregation, crop size, alternative food availability, numbers of competitors, and predators; Prasad and Sukumar, 2010; Perea et al., 2014b).
Finally, high levels of post-dispersal predation on clumped spat seeds could reverse the sign of the plant-ruminant interaction from mutualistic to antagonistic. Thus, future studies should address the complexities of this fascinating type of plant-animal interaction and quantify the variable fate of seeds ingested by ruminants in different ecosystems.
Author Contributions
MD conceived the original idea and wrote the first draft. MD and IC did the literature review. MD, IC, and JF reviewed and edited the manuscript several times.
Funding
JF was funded by the Portuguese FCT (grant IF/00728/2013).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many herders in Morocco and Spain helped us to obtain information from their animals. F. Cuzin and F. Hiraldo offered us unpublished data on gazelles and cattle spitting seeds from the cud. G. Calvo and M. Jácome helped us in fieldwork and S. Conradi in the laboratory work. S. Sridhara and C. Baltzinger significantly improved a previous version of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00265/full#supplementary-material
References
Albert, A., Auffret, A. G., Cosyns, E., Cousins, S. A. O., D'Hondt, B., Eichberg, C., et al. (2015a). Seed dispersal by ungulates as an ecological filter: A trait-based meta-analysis. Oikos 124, 1109–1120. doi: 10.1111/oik.02512
Albert, A., Mårell, A., Picard, M., and Baltzinger, C. (2015b). Using basic plant traits to predict ungulate seed dispersal potential. Ecography 38, 440–449. doi: 10.1111/ecog.00709
Bascompte, J., and Jordano, P. (2007). Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593. doi: 10.1146/annurev.ecolsys.38.091206.095818
Berger, J. (2004). The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331. doi: 10.1111/j.1523-1739.2004.00548.x
Bernes, C., Macura, B., Jonsson, B. G., Junninen, K., Müller, J., Sandström, J., et al. (2018). Manipulating ungulate herbivory in temperate and boreal forests: effects on vegetation and invertebrates. A systematic review. Environ, Evid. 7:13. doi: 10.1186/s13750-018-0125-3
Biosecurity Queensland (2016). Prickly acacia Vachellia nilotica subsp. Indica (Benth) Kyal and Boatwr. Restricted Invasive Plants. Brisbane, QLD: The Dept of Agriculture and Fisheries; Queensland Government.
Bodmer, R. E. (1990). Ungulate frugivores and the browser-grazer continuum. Oikos 57, 319–325. doi: 10.2307/3565960
Bodmer, R. E. (1991). Strategies of seed dispersal and seed predation in Amazonian Ungulates. Biotropica 23, 255–261. doi: 10.2307/2388202
Brodie, J. F., Helmy, O. E., Brockelman, W. Y., and Maron, J. L. (2009a). Functional differences within a guild of tropical mammalian frugivores. Ecology 90, 688–698. doi: 10.1890/08-0111.1
Brodie, J. F., Helmy, O. E., Brockelman, W. Y., and Maron, J. L. (2009b). Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol. Applic. 19, 854–863. doi: 10.1890/08-0955.1
Côté, S. D., Rooney, T. P., Tremblay, J.-P., Dussault, C., and Waller, D. M. (2004). Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147. doi: 10.1146/annurev.ecolsys.35.021103.105725
Castañeda, I., Fedriani, J. M., and Delibes, M. (2018). Potential of red deer (Cervus elaphus) to disperse viable seeds by spitting them from the cud. Mamm. Biol. 90, 89–91. doi: 10.1016/j.mambio.2017.10.004
Chanthorn, W., and Brockelman, W. Y. (2008). Seed dispersal and seedling recruitment in the light-demanding tree Choerospondias axillaris in old-growth forest in Thailand. Sci. Asia 34, 129–135. doi: 10.2306/scienceasia1513-1874.2008.34.129
Chen, J., Deng, X.B., Bai, Z.L., Chen, G.Q., Liu, Y., and Liu, Z.Q. (2001). Fruit Characteristics and Muntiacus muntijak vaginalis (Muntjac) visits to individual plants of Choerospondias axillaris. Biotropica 33, 718–722. doi: 10.1111/j.1744-7429.2001.tb00231.x
Chen, S. C., and Moles, A. T. (2015). A mammoth mouthful? A test of the idea that larger animals ingest larger seeds. Glob. Ecol. Biogeogr. 24, 1269–1280. doi: 10.1111/geb.12346
Clauss, M., Nunn, C., Fritz, J., and Hummel, J. (2009). Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp. Biochem. Physiol. A Mol. Integrat. Physiol. 154, 376–382. doi: 10.1016/j.cbpa.2009.07.016
Corlett, R. T. (1998). Frugivory and seed dispersal by vertebrates in the Oriental (Hindomalayan) Region. Biol. Rev. 73, 413–448. doi: 10.1017/S0006323198005234
Corlett, R. T. (2011). Seed dispersal in Hong Kong, China: past, present and possible futures. Integrat. Zool. 6, 97–109. doi: 10.1111/j.1749-4877.2011.00235.x
Corlett, R. T. (2017). Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: An update. Glob. Ecol. Conserv. 11, 1–22. doi: 10.1016/j.gecco.2017.04.007
Couvreur, M., Verheyen, K., and Hermy, M. (2005). Experimental assessment of plant seed retention times in fur of cattle and horse. Flora 200, 136–147. doi: 10.1016/j.flora.2004.06.003
Danell, K., Bergström, R., Duncan, P., and Pastor, J. (2006). Large Herbivore Ecology, Ecosystem Dynamics and Conservation.Vol. 11. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511617461
Delibes, M., Castañeda, I., and Fedriani, J. M. (2017). Tree-climbing goats disperse seeds during rumination. Front. Ecol. Environ. 15, 222–223. doi: 10.1002/fee.1488
Donatti, C. I., Galetti, M., Pizo, M. A., Guimares Jr, P. R., and Jordano, P. (2007). “Living in the land of ghosts: fruit trait and the importance of large mammals as seed dispersers in the Pantanal Brazil,” in Seed Dispersal: Theory and Its Application in a Changing World, eds A. J. Dennis., E. W. Schupp., R. J. Green., D. A. Westcott (Wallingford: Commonwealth Agricultural Bureau International), 104–123. doi: 10.1079/9781845931650.0104
Fedriani, J. M., and Delibes, M. (2011). Dangerous liaisons disperse the Mediterranean dwarf palm : fleshy-pulp defensive role against seed predators. Ecology 92, 304–315. doi: 10.1890/09-2194.1
Feer, F. (1995). Seed dispersal in African forest ruminants. J. Trop. Ecol. 11, 683–689. doi: 10.1017/S0266467400009238
Feer, F., Henry, O., Forget, P. M., and Gayot, M. (2001). “Frugivory and seed dispersal by terrestrial mammals,” in Nouragues: Dynamics and Plant-Animal Interactions in a Neotropical Rainforest, eds F. Bongers, P. C. Dominique, P. M. Forget, and M. Théry (Dordrecht: Springer Sicence and Business Media), 227–232. doi: 10.1007/978-94-015-9821-7_21
Forget, P., Mazer, S. J., Barbara, S., Jansen, P. A., and Kitamura, S. (2007). “Seed allometry and disperser assemblages in tropical rainforests: a comparison of four floras on different continents” in Seed dispersal: Theory and its application in a changing world, ed A. J. Dennis (Wallingford: CAB International), 5–36. doi: 10.1079/9781845931650.0005
Foster, C. N., Barton, P. S., and Lindenmayer, D. B. (2014). Effects of large native herbivores on other animals. J. Appl. Ecol. 51, 929–938. doi: 10.1111/1365-2664.12268
Gautier-Hion, A., Duplantier, J.-M., Quris, R., Feer, F., Sourd, C., Decoux, J.-P., et al. (1985). Fruits characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 65, 324–337. doi: 10.1007/BF00378906
Gill, R. M. A., and Beardall, V. (2001). The impact of deer on woodlands: the effects of browsing and seed dispersal on vegetation structure and composition. Forestry 74, 209–218. doi: 10.1093/forestry/74.3.209
Grande, D., Mancilla Leytón, J. M., and Martín Vicente, A. (2013). Endozoochorus seed dispersal by goats: recovery, germinability and emergence of five Mediterranean shrub species. Spanish J. Agric. Res. 11, 347–355. doi: 10.5424/sjar/2013112-3673
Grünewald, C., Breitbach, N., and Böhning-Gaese, K. (2010). Tree visitation and seed dispersal of wild cherries by terrestrial mammals along a human land-use gradient. Basic Appl. Ecol. 11, 532–541. doi: 10.1016/j.baae.2010.07.007
Guimarães, P. R., Galetti, M., and Jordano, P. (2008). Seed dispersal anachronisms: Rethinking the fruits extinct megafauna ate. PLoS ONE 3:e1745. doi: 10.1371/journal.pone.0001745
Hackmann, T. J., and Spain, J. N. (2010). Ruminant ecology and evolution: Perspectives useful to ruminant livestock research and production. J. Dairy Sci. 93, 1320–1334. doi: 10.3168/jds.2009-2071
Herrera, C. M. (1989). Frugivory and seed dispersal by carnivorous mammals, and associated fruit characteristics, in undisturbed mediterranean habitats. Oikos 55, 250–262. doi: 10.2307/3565429
Herrera, C. M. (2002). “Seed dispersal by vertebrates,” in Plant–Animal Interactions: An Evolutionary Approach, eds C. M. Herrera and O. Pellmyr (Oxford: Blackwell, 185–208.
Hobbs, N. T. (1996). Modification of Ecosystems by Ungulates. J. Wildl. Manag. 60, 695–713. doi: 10.2307/3802368
Hofmann, R. R. (1989). Evolutionary steps of ecophysiologicl adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457. doi: 10.1007/BF00378733
Jansen, P. A., Hirsch, B. T., Emsens, W.-J., Zamora-Gutierrez, V., Wikelski, M., and Kays, R. (2012). Thieving rodents as substitute dispersers of megafaunal seeds. Proc. Natl. Acad. Sci. U.S.A. 109, 12610–12615. doi: 10.1073/pnas.1205184109
Janzen, D. H. (1982). Differential seed survival and passage rates in cows and horses, surrogate Pleistocene dispersal agents. Oikos 150–156. doi: 10.2307/3544014
Janzen, D. H. (1984). Dispersal of small seeds by big herbivores : foliage is the fruit. Am. Nat. 123, 338–353. doi: 10.1086/284208
Janzen, D. H. (1985). Spondias mombin is culturally deprived in megafauna-free forest. J. Trop. Ecol. 1, 131–155. doi: 10.1017/S0266467400000195
Janzen, D. H., and Martin, P. S. (1982). Neotropical anachronisms: the fruits the gomphothere ate. Science 215, 19–27. doi: 10.1126/science.215.4528.19
Johnsingh, A. J. T. (1981). Importance of fruit in the diet of chital in dry season. J. Bombay Nat. Hist. Soc. 78:594.
Jordano, P. (1987). Avian fruit removal: effects of fruit variation, crop size, and insect damage. Ecology 68, 1711–1723. doi: 10.2307/1939863
Kitamura, S., Suzuki, S., Yumoto, T., Poonswad, P., Chuailua, P., Plongmai, K., et al. (2006). Dispersal of Canarium euphyllum (Burseraceae), a large-seeded tree species, in a moist evergreen forest in Thailand. J. Trop. Ecol. 22, 137–146. doi: 10.1017/S0266467405002889
Krefting, L. W., and Roe, E. I. (1949). The role of some birds and mammals in seed germination. Ecol. Monogr. 19, 269–286. doi: 10.2307/1943538
Lecomte, X., Fedriani, J. M., Caldeira, M. C., Clemente, A. S., Olmi, A., and Bugalho, M. N. (2016). Too many is too bad: long-term net negative effects of high density ungulate populations on a dominant Mediterranean shrub. PLoS ONE 11:e0158139. doi: 10.1371/journal.pone.0158139
Levey, D. J. (1987). Seed size and fruit-handling techniques of avian frugivores. Am. Nat. 129, 471–485. doi: 10.1086/284652
Mandujano, S., Gallina, S., and Bullock, S. H. (1994). Frugivory and dispersal of Spondias purpurea (Anacardiaceae) in a tropical deciduous forest in México. Rev. Biol. Trop. 42, 107–114.
Manzano, P., and Malo, J. E. (2006). Extreme long-distance seed dispersal via sheep. Front. Ecol. Environ. 4, 244–248. doi: 10.1890/1540-9295(2006)004[0244:ELSDVS]2.0.CO;2
McConkey, K. R., Nathalang, A., Brockelman, W. Y., Saralamba, C., Santon, J., Matmoon, U., et al. (2018). Different megafauna vary in their seed dispersal effectiveness of the megafaunal fruit Platymitra macrocarpa (Annonaceae). PLoS ONE 13:0198960. doi: 10.1371/journal.pone.0198960
McNaughton, S. J., Ruess, R. W., and Seagle, S. W. (1988). Large mammals and process dynamics in African ecosystems. Bioscience 38, 794–800. doi: 10.2307/1310789
Miceli-Méndez, C. L., Ferguson, B. G., and Ramírez-Marcial, N. (2008). “Seed dispersal by cattle : natural history and applications to neotropical forest restoration and agroforestry,” in Post-Agricultural Succession in the Neotropics, ed R. W. Myster (New York, NY: Springer), 165–191. doi: 10.1007/978-0-387-33642-8_7
Miller, M. F. (1995). Acacia seed survival, seed germination and seedling growth following pod consumption by large herbivores and seed chewing by rodents. Afr. J. Ecol. 33, 194–210. doi: 10.1111/j.1365-2028.1995.tb00797.x
Miller, M. F. (1996). Dispersal of Acacia seeds by ungulates and ostriches in an African savanna. J. Trop. Ecol. 12, 345–356. doi: 10.1017/S0266467400009548
Morton, J. F., and Voss, G. L. (1987). The argan tree (Argania sideroxylon, Sapotaceae), a desert source of edible oil. Econ. Bot. 41, 221–233. doi: 10.1007/BF02858970
Mouissie, A. M., Vos, P., Verhagen, H. M. C., and Bakker, J. P. (2005). Endozoochory by free-ranging, large herbivores: Ecological correlates and perspectives for restoration. Basic Appl. Ecol. 6, 547–558. doi: 10.1016/j.baae.2005.03.004
Myers, J. A., Vellend, M., and Gardescu, S. (2004). Seed dispersal by white-tailed deer: Implications for long-distance dispersal, invasion, and migration of plants in eastern North America. Oecologia 139, 35–44. doi: 10.1007/s00442-003-1474-2
Parolin, P., Wittmann, F., and Ferreira, L. V. (2013). Fruit and seed dispersal in Amazonian floodplain trees - a review. Ecotropica 19, 15–32.
Pellerin, M., Picard, M., Saïd, S., Baubet, E., and Baltzinger, C. (2016). Complementary endozoochorous long-distance seed dispersal by three native herbivorous ungulates in Europe. Basic Appl. Ecol. 17, 321–332. doi: 10.1016/j.baae.2016.01.005
Pelletier, N., and Tyedmers, P. (2010). Forecasting potential global environmental costs of livestock production 2000-2050. Proc. Natl. Acad. Sci. U.S.A. 107, 18371–18374. doi: 10.1073/pnas.1004659107
Perea, R., Delibes, M., Polko, M., Suárez-Esteban, A., and Fedriani, J. M. (2014b). Context-dependent fruit-frugivore interactions: Partner identities and spatio-temporal variations. Oikos 122, 943–951. doi: 10.1111/j.1600-0706.2012.20940.x
Perea, R., Girardello, M., and San Miguel, A. (2014a). Big game or big loss? High deer densities are threatening woody plant diversity and vegetation dynamics. Biodivers. Conserv. 23, 1303–1318. doi: 10.1007/s10531-014-0666-x
Picard, M., Papaïx, J., Gosselin, F., Picot, D., Bideau, E., and Baltzinger, C. (2015). Temporal dynamics of seed excretion by wild ungulates: implications for plant dispersal. Ecol. Evol. 5, 2621–2632. doi: 10.1002/ece3.1512
Pile, L. S., Wang, G. G., Polomski, R., Yarrow, G., and Stuyck, C. M. (2015). Potential for nonnative endozoochorous seed dispersal by white-tailed deer in a southeastern maritime forest. Invas. Plant Sci. Manag. 8, 32–43. doi: 10.1614/IPSM-D-14-00027.1
Prasad, S., Chellam, R., Krishnaswamy, J., and Goyal, S. P. (2004). Frugivory of Phyllanthus emblica at Rajaji National Park, northwest India. Curr. Sci. 87, 1188–1190.
Prasad, S., Krishnaswamy, J., Chellam, R., and Goyal, S. P. (2006). Ruminant-mediated seed dispersal of an economically valuable tree in Indian Dry forests. Biotropica 38, 679–682. doi: 10.1111/j.1744-7429.2006.00182.x
Prasad, S., Pittet, A., and Sukumar, R. (2010). Who really ate the fruit? A novel approach to camera trapping for quantifying frugivory by ruminants. Ecol. Res. 25, 225–231. doi: 10.1007/s11284-009-0650-1
Prasad, S., and Sukumar, R. (2010). Context-dependency of a complex fruit–frugivore mutualism: temporal variation in crop size and neighborhood effects. Oikos 119, 514–523. doi: 10.1111/j.1600-0706.2009.17971.x
Ridley, H. N. (1930). The Dispersal of Plants Throughout the World. Ashford: Reeve and Company, Limited
Sánchez-Valverde, A. (1785). Idea del Valor de la Isla Española, y Utilidades, Que de Ella Puede Sacar su Monarquia. Madrid: Imprenta de Don Pedro Marín.
Scariot, A. (1998). Seed dispersal and predation of the palm Acrocomia aculeata. Principes, 42, 5–8.
Schupp, E. W., Jordano, P., and Gómez, J. M. (2010). Seed dispersal effectiveness: a conceptual review. New Phytol. 188, 333–353. doi: 10.1111/j.1469-8137.2010.03402.x
Schwarm, A., Ortmann, S., Wolf, C., Jürgen Streich, W., and Clauss, M. (2008). Excretion patterns of fluid and different sized particle passage markers in banteng (Bos javanicus) and pygmy hippopotamus (Hexaprotodon liberiensis): two functionally different foregut fermenters. Comp. Biochem. Physiol. 150, 32–39. doi: 10.1016/j.cbpa.2008.02.022
Slater, K., and du Toit, J. T. (2002). Seed dispersal by chacma baboons and syntopic ungulates in southern African savannas. South Afr. J. Wildl. Res. 32, 75–79.
Sridhara, S., Mcconkey, K., Prasad, S., and Corlett, R. T. (2016). “Frugivory and seed dispersal by large herbivores of Asia” in The Ecology of Large Herbivores in South and Southeast Asia, eds F. S. Ahrestani and M. Sankaran (Dordrecht, Holland: Springer), 121–150. doi: 10.1007/978-94-017-7570-0_5
Stoner, K. E., Riba-Hernández, P., Vulinec, K., and Lambert, J. E. (2007). The role of mammals in creating and modifying seedshadows in tropical forests and some possible consequences of their elimination. Biotropica 39, 316–327. doi: 10.1111/j.1744-7429.2007.00292.x
Tiver, F., Nicholas, M., Kriticos, D., and Brown, J. R. (2001). Low density of Prickly Acacia under sheep grazing in Queensland. J. Range Manag. 54, 382–389. doi: 10.2307/4003107
Vander Wall, S. B., Kuhn, K. M., and Beck, M. J. (2005). Seed removal, seed predation, and secondary dispersal. Ecology 86, 801–806. doi: 10.1890/04-0847
Wenninger, P. S., and Shipley, L. A. (2000). Harvesting, rumination, digestion and passage of fruit and leaf diets by a small ruminant, the blue duiker. Oecologia 123, 466–474. doi: 10.1007/s004420000338
Wilson, D. E., and Mittermeier, R. A. (eds.). (2011). Handbook of the Mammals of the World, Vol. 2: Hoofed Mammals (Barcelona: Lynx Editions), 886.
Wilson, D. E., and Reeder, D. M. (eds.) (2005). Mammal Species of the world: A Taxonomic and Geographic Reference. Baltimore, MD: JHU Press.
Wilson, V. J., and Clarke, J. E. (1962). Observations on the common duiker Sylvicapra grimmia Linn., based on material collected from a Tsetse control game elimination scheme. Proc. Zool. Soc. Lond. 138, 487–497. doi: 10.1111/j.1469-7998.1962.tb05711.x
Yamashita, C. (1997). Anodorhynchus macaws as followers of extinct megafauna: an hypothesis. Ararajuba 5, 176–182.
Yasuda, M., Miura, S., Ishii, N., Okuda, T., and Hussein, N. A. (2005). “Fallen fruits and terrestrial vertebrate frugivores: a case study in a lowland tropical rain forest in Peninsular Malaysia,” in Seed Fate: Predation, Dispersal and Seedling Establishment, eds P.-M. Forget, J. E. Lambert, P. E. Hulme, and S. B. Vander Wall (Wallingford: CABI Publishing), 151–174. doi: 10.1079/9780851998060.0151
Keywords: mutualism, plant-animal interactions, rumination, seed regurgitation, seed dispersal
Citation: Delibes M, Castañeda I and Fedriani JM (2019) Spitting Seeds From the Cud: A Review of an Endozoochory Exclusive to Ruminants. Front. Ecol. Evol. 7:265. doi: 10.3389/fevo.2019.00265
Received: 12 November 2018; Accepted: 24 June 2019;
Published: 17 July 2019.
Edited by:
Casper H. A. Van Leeuwen, Netherlands Institute of Ecology (NIOO-KNAW), NetherlandsReviewed by:
Christophe Baltzinger, National Research Institute of Science and Technology for Environment and Agriculture, FranceSachin Sridhara, National Center for Biological Science, India
Copyright © 2019 Delibes, Castañeda and Fedriani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose M. Fedriani, fedriani@csic.es
 Miguel Delibes
Miguel Delibes Irene Castañeda
Irene Castañeda Jose M. Fedriani
Jose M. Fedriani