- 1Department of Head Neck and Thyroid, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 2Department of Radiation Oncology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
Objectives: To analyze the diagnostic benefit of fine needle aspiration biopsy cytology (FNAB-C) and core needle biopsy tissue (CNB-T) with the addition of thyroglobulin (Tg) in the washout of the needle or BRAF V600E mutation assessment in assessing cervical lymph node metastasis (LNM) in papillary thyroid carcinoma.
Materials and Methods: A total of 186 lymph nodes were punctured by fine or core needle. The diagnostic performance of FNAB-C and CNB-T with Tg in the washout or BRAF V600E mutation assessment was compared.
Results: The optimal cutoff value of FNAB-Tg was 1.0 ng/ml, with an AUC of 0.976. The sensitivity and specificity of FNAB-C in predicting cervical LNM were 97.4% and 71.4%, respectively, and the addition of FNAB-Tg could contribute to a sensitivity of 100% and a specificity of 95%, but the introduction of BRAF V600E mutation assessment was associated with a decreased sensitivity of 96.3% and a decreased specificity of 50.0%. The FNAB-Tg level showed a comparable distribution in malignant lymph nodes with different TgAb statuses, serum TSH levels, and serum Tg levels. The sensitivity and specificity of CNB-T in predicting cervical LNM were 98.9% and 100%, respectively. The addition of CNB-Tg did not alter the diagnostic ability, but the introduction of BRAF V600E mutation assessment obtained the best performance, with a sensitivity of 100% and specificity of 100%.
Conclusion: The sensitivity and specificity of FNAB-C could be increased if combined with FNAB-Tg. CNB-T alone could provide satisfactory diagnostic reliability.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy and accounts for more than 90% of all thyroid cancers (1). Unilateral or bilateral thyroidectomy combined with central neck dissection is the routine method of operation according to our guidelines (2). Lateral neck dissection is performed if cervical lymph node metastasis (LNM) is pathologically confirmed preoperatively or intraoperatively. Even if the operation is successful, a number of patients suffer from regional recurrence (3, 4); for these patients, it is important for the surgeon to accurately assess the lesion status preoperatively to avoid unnecessary surgery.
Ultrasound-guided fine needle aspiration biopsy cytology (FNAB-C) and core needle biopsy tissue (CNB-T) play an important role in evaluating cervical lymph node status in thyroid cancer and head and neck squamous cell carcinoma (SCC), respectively (5–21). However, the value of FNAB-C is greatly limited if the number of specimens is small or the specimens are obtained from cystic lymph nodes. Since it was first described by Pacini et al. (11), the measurement of thyroglobulin (Tg) in the washout of the needle (FNAB-Tg) has attracted increasing attention because of its higher reliability than FNAB-C (12–15). However, there is still great controversy regarding the best cutoff value of FNAB-Tg and whether serum thyroid stimulating hormone (TSH), thyroglobulin antibody (TgAb), serum Tg, and thyroid tissue interfere with the accuracy of FNAB-Tg (16, 17). Some researchers have reported that the clinical performance of FNAB-Tg was not affected by TgAb and TSH (10, 12), but others have asserted that the diagnostic value of FNAB-Tg could be greatly influenced by the presence of thyroid tissue, serum Tg, and TgAb (7, 14, 15, 18).
No researchers have yet analyzed the association between cervical LNM and CNB-T in thyroid cancer, so it remains unclear whether CNB-T is superior to FNAB-C in indicating LNM. In addition, the BRAF V600E mutation is the most common genetic alteration in thyroid cancer and occurs in nearly 80% of primary tumors and 50% of metastatic lymph nodes (22, 23). It therefore remains unknown whether the introduction of BRAF V600E mutation assessment could increase the diagnostic reliability of CNB-T and FNAB-C.
Thus, in the current study, we aimed to determine the answer to optimal value of Tg in the washout and compare the diagnostic performance between CNB-T and FNAB-C combined with/without Tg or BRAF 600E mutation assessment.
Patients and Methods
Ethics Statement
The Our Hospital Institutional Research Committee approved our study, and all participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient Selection
From January 2018 to October 2020, medical records of patients with surgically treated primary or recurrent/metastatic PTC were retrospectively reviewed. Enrolled patients must meet the following criteria: the patient underwent preoperative fine or core needle biopsy for suspicious lymph nodes and the punctured lymph nodes had been surgically excised. Then, 306 patients who had lymph node biopsy for suspicious metastasis from PTC were selected. A total of 143 patients had negative CNB-T or FNAB-C results and did not receive surgical treatment for the lymph nodes, and 8 patients had positive CNB-T or FNAB-C results but did not receive surgical treatment for the lymph nodes. These 151 patients were excluded, therefore, a total of 155 patients were enrolled for analysis.
Treatment Principles
Based on our guidelines for thyroid cancer (2), central neck dissection was routinely performed on patients treated for the first time, and lateral lymph node status was first assessed by ultrasound if there was suspicion of lateral LNM, FNAB-C or CNB-T combined with or without Tg in the washout or BRAF 600E mutation assessment.
For patients suspected of having regional recurrence, FNAB-C or CNB-T combined with or without Tg in the washout or BRAF 600E mutation assessment was performed for lateral neck lymph nodes, and FNAB-C or CNB-T combined with or without BRAF 600E mutation assessment was performed for central neck lymph nodes.
Cervical LNM was suggested if there were the following features, for ultrasound: absence of an echogenic hilum, round shape, microcalcification, peripheral blood flow on color Doppler images, or cystic changes (7–18); for CT: area with clear evidence of nonfat, low-density, or liquid components; largest diameter >15 mm at level II and >10 mm at other levels; and ratio of the longest to smallest diameter ≤2.
The FNAB-C and CNB-T results were both classified into three groups: inadequate or nondiagnosis, negative, or positive for metastatic PTC. Immunohistochemistry was performed if there was a need for accurate diagnosis.
For patients treated for the first time, lateral neck dissection was performed if there was a positive FNAB-C or CNB-T result regardless of the value of Tg in the washout or BRAF 600E status; if there was a negative or nondiagnosis FNAB-C or CNB-T result but high Tg in the washout or BRAF 600E mutation assessment, frozen sectioning of the suspicious lymph nodes was also performed.
For patients suspected of having regional recurrence, an operation was performed if there was a positive FNAB-C or CNB-T result regardless of the Tg value in the washout or BRAF 600E status; if there was a negative or nondiagnosis FNAB-C or CNB-T result but high Tg in the washout or BRAF 600E mutation assessment, different procedures, including repeated aspiration, close monitoring, direct surgery, etc., were selected accordingly.
Important Variable Definition
The normal ranges for serum TSH, Tg, and TgAb were from 0.27 mIU/L to 4.2 mIU/L, from 3.5 ng/ml to 77 ng/ml, and from 0 to 115 IU/ml, respectively. TgAb(+) was indicated if its value was higher than 115 IU/ml.
Fine and Core Needle Aspiration Technique and BRAF 600E Mutation Assessment
Fine needle aspiration biopsy was performed using a 22-gauge, and core needle biopsy was performed using an 18-gauge needle. Each aspiration was repeated at least three times to allow cytological or tissue examination and Tg in the washout determination or BRAF 600E mutation assessment. Immediately after aspiration, the needle and syringe were washed with 1 ml of normal saline and then sent to the laboratory if the Tg assay was required. A sufficient sample with an adequate number of tumor cells was used for real-time PCR analysis if BRAF 600E mutation assessment was required.
Statistical Analysis
The final outcome was confirmed by postoperative pathology, and the diagnostic abilities of FNAB-C and CNB-T were assessed with respect to their sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The Mann-Whitney U test was used to compare the FNAB-Tg values between different groups. All statistical analyses were performed with SPSS 20.0, and p<0.05 was considered to be significant.
Results
Characteristics of the Patients and Lymph Nodes
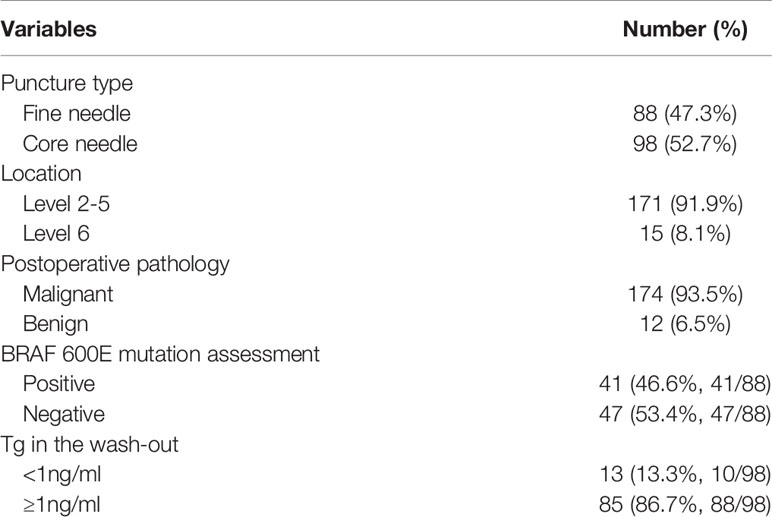
Finally, 155 patients (50 male and 105 female) were enrolled, with a mean age of 43.7 (range: 19-78) years. Ninety-five (61.3%) patients had received no prior treatment, 49 (31.6%) patients had previously undergone total thyroidectomy, and 11 (7.1%) patients had previously received unilateral thyroid lobectomy (Table 1). A total of 186 solid lymph nodes were punctured and surgically treated; among them, 88 (47.3%) lymph nodes underwent fine needle aspiration and 98 (52.7%) lymph nodes underwent core needle aspiration. Fifteen (8.1%) lymph nodes were located at level 6, and 171 (91.9%) lymph nodes were located at the lateral neck (levels 2-5). Postoperative pathology confirmed metastatic disease in 174 (92.6%) lymph nodes. BRAF 600E mutation assessment was performed in 88 lymph nodes, and a mutation was identified 41 (46.6%, 41/88) patients. Tg in the washout was evaluated in 98 lymph nodes, and its value was less than 1 ng/ml in 13 (13.3%, 10/98) patients and greater than 1 ng/ml in 85 (86.7%, 88/98) patients (Table 2).
ROC Curve of the Optimal Cutoff Value of FNAB-Tg
According to the ROC analysis (Figure 1), the optimal cutoff value of FNAB-Tg was 1.0 ng/ml, with an AUC of 0.976, and its sensitivity and specificity in predicting cervical LNM were 94.4% and 100%, respectively.
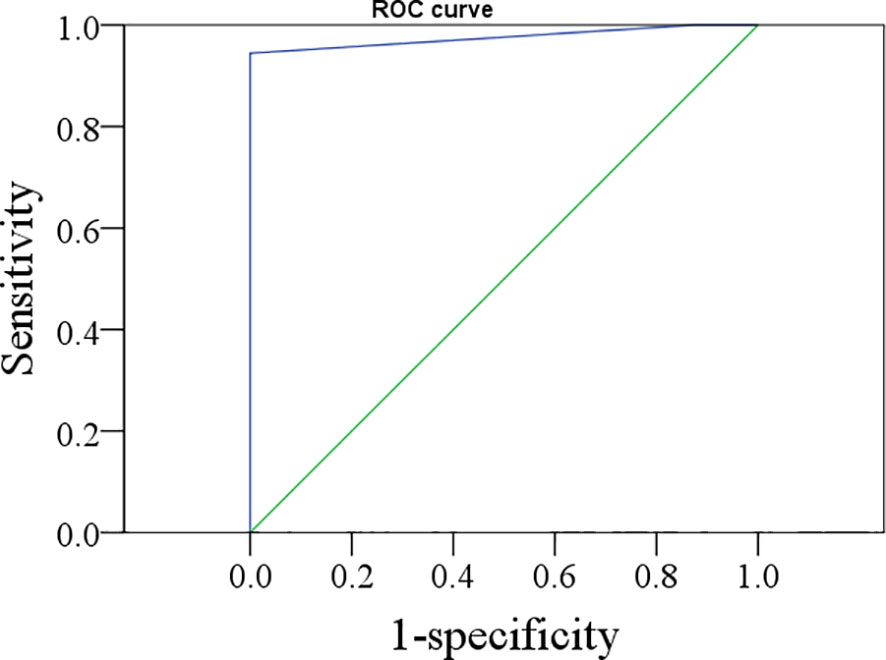
Figure 1 The ROC curve of thyroglobulin in the washout of the needle for indicating lymph node metastasis.
Diagnostic Performance Between FNAB-C and CNB-T
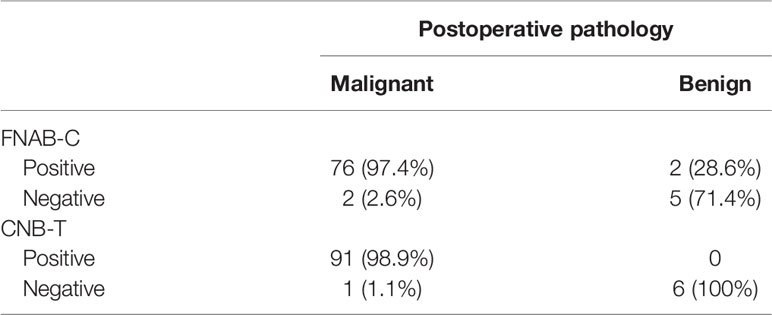
In 78 lymph nodes with positive FNAB-C results, 76 lymph nodes were confirmed to be metastatic by positive pathology; 7 lymph nodes had negative results, and 2 lymph nodes were confirmed to be metastatic by positive pathology. The sensitivity and specificity of FNAB-C in predicting cervical LNM were 97.4% and 71.4%, respectively (Table 3).
In 91 lymph nodes with positive CNB-T results, all lymph nodes were confirmed to be metastatic by positive pathology; in 7 lymph nodes had negative results, and 1 lymph node was confirmed to be metastatic by positive pathology. The sensitivity and specificity of CNB-T in predicting cervical LNM were 98.9% and 100%, respectively (Table 3).
Effect of BRAF 600E Mutation Assessment on the Diagnostic Performance of FNAB-C and CNB-T
In 32 lymph nodes, both FNAB-C and BRAF 600E mutation assessments were performed; in 28 lymph nodes with positive FNAB-C results or BRAF 600E mutation assessments, 27 lymph nodes were found to be metastatic. The sensitivity and specificity of FNAB-C combined with BRAF 600E mutation assessment in predicting cervical LNM were 96.4% and 50%, respectively (Table 4).
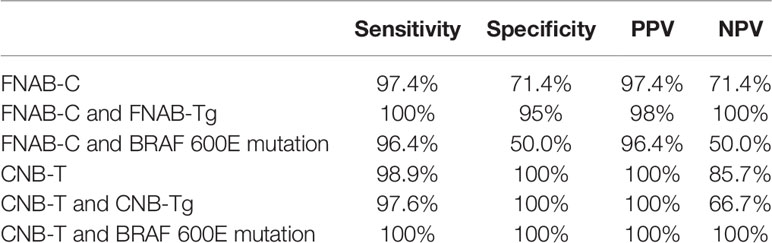
Table 4 Diagnostic performance in FNAB-C and CNB-T combined with or without Tg in the wash-out or BRAF 600E mutation assessment.
In 56 lymph nodes, both CNB-T and BRAF 600E mutation assessments were performed; in 52 lymph nodes with positive CNB-T results or BRAF 600E mutation assessments, all lymph nodes were found to be metastatic. The sensitivity and specificity of CNB-T combined with BRAF 600E mutation assessment in predicting cervical LNM were 100% and 100%, respectively (Table 4).
Effect of Tg on the Diagnostic Performance of FNAB-C and CNB-T in the Washout
In 55 lymph nodes, both FNAB-Tg and FNAB-C were performed, and all 49 lymph nodes with positive FNAB-C results or high FNAB-Tg (≥1 ng/ml) were confirmed to be metastatic postoperatively. The sensitivity and specificity of FNAB-C combined with FNAB-Tg in predicting cervical LNM were 100% and 95%, respectively (Table 4).
In 43 lymph nodes, both CNB-Tg and CNB-T were performed, and all 40 lymph nodes with positive CNB-T results or high CNB-Tg (≥1 ng/ml) were confirmed to be metastatic postoperatively. The sensitivity and specificity of CNB-T combined with CNB-Tg in predicting cervical LNM were 97.6% and 100%, respectively (Table 4).
Association Between TgAb, Serum TSH, Serum Tg, Thyroid Tissue and FNAB-Tg
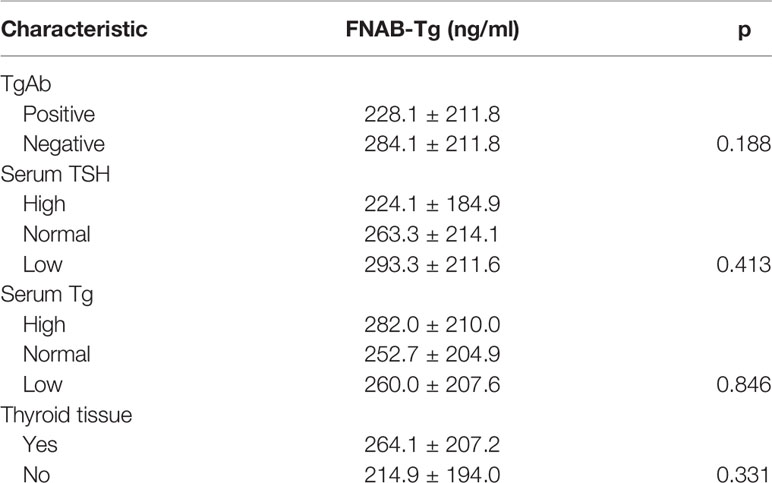
In 40 positive lymph nodes from TgAb(+) patients, the mean FNAB-Tg value was 228.1 ± 211.8 ng/ml, and in 58 positive lymph nodes from TgAb(-) patients, the mean FNAB-Tg value was 284.1 ± 211.8 ng/ml; the difference was not significant (p=0.188). (Table 5)
In 36 positive lymph nodes from low TSH patients, the mean FNAB-Tg value was 293.3 ± 211.6 ng/ml, in 35 lymph nodes from normal TSH patients, the mean FNAB-Tg value was 263.3 ± 214.1 ng/ml, and in 27 lymph nodes from high TSH patients, the mean FNAB-Tg value was 224.1 ± 184.9 ng/ml. However, this difference was not significant (p=0.413) (Table 5).
In 33 positive lymph nodes from low serum Tg patients, the mean FNAB-Tg value was 260.0 ± 207.6 ng/ml, in 35 lymph nodes from normal serum Tg patients, the mean FNAB-Tg value was 252.7 ± 204.9 ng/ml, and in 30 lymph nodes from high serum Tg patients, the mean FNAB-Tg value was 282.0 ± 210.0 ng/ml. However, this difference was not significant (p=0.846) (Table 5).
In 23 positive lymph nodes from patients without thyroid tissue presence, the mean FNAB-Tg value was 214.9 ± 194.0 ng/ml, and in 75 positive lymph nodes from patients with thyroid tissue presence, the mean FNAB-Tg value was 264.1 ± 207.2 ng/ml. However, this difference was not significant (p=0.331) (Table 5).
Association Between TgAb, Serum TSH, Serum Tg, Thyroid Tissue and the Diagnostic Performance of FNAB-Tg
As described in Table 6, both the sensitivity and NPV were 100% in positive lymph nodes with different characteristics, and both the specificity and PPV had a variation range of less than 5.0%.
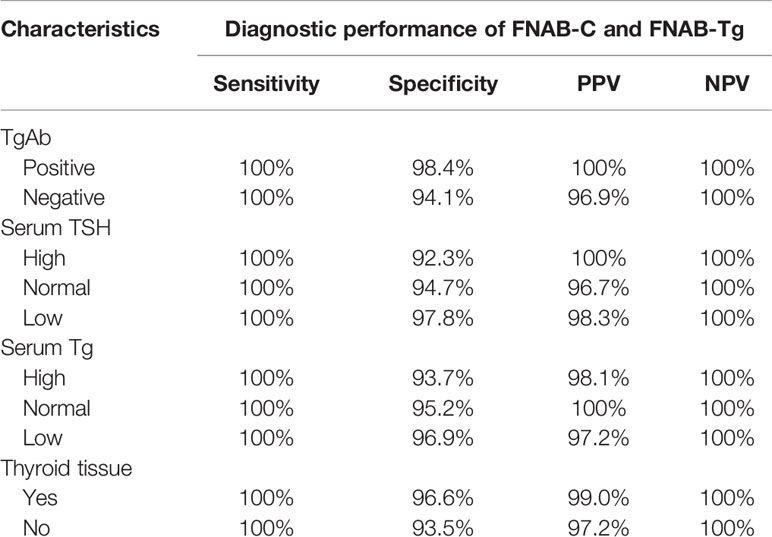
Table 6 Association between TgAb, serum TSH, serum Tg, thyroid tissue and the diagnostic performance of FNAB-C and FNAB-Tg in positive lymph nodes.
Discussion
The most important finding in the current study was that the optimal cutoff value of FNAB-Tg was 1.0 ng/ml, and its addition increased the sensitivity and specificity of FNAB-C. The diagnostic performance was not affected by TgAb, serum TSH, serum Tg, or thyroid tissue. CNB-T alone could provide satisfactory diagnostic reliability, and the additional benefit was greater with BRAF 600E mutation assessment than with FNAB-Tg. In all groups, CNB-T combined with BRAF 600E mutation assessment had the best diagnostic ability.
The optimal cutoff of FNAB-Tg value had been studied previously and reported to be in the range of 0.9 ng/ml to 50 ng/ml (17). A meta-analysis by Grani et al. (17) indicated a pooled sensitivity of 94.8% and a specificity of 91.2% with a cutoff of 0.9-1.1 ng/ml, a pooled sensitivity of 87.7% and a specificity of 94.2% with a cutoff of 10 ng/ml, and a pooled sensitivity of 97.3% and specificity of 94.9% with a cutoff of serum Tg in predicting cervical NLM. Uruno et al. (5) analyzed 129 fine needle punctured lymph nodes, of which 105 lymph nodes were positive by FNAB-Tg (range 6.2-8000 ng/ml) and 24 lymph nodes were negative (range 0.6-88.8 ng/ml). FNAB-Tg and FNAB-C can compensate for the loss of the other based on the assumption that the lymph node is positive if the FNAB-Tg value is higher than the serum Tg level, taking blood contamination into consideration. However, in practice, the sample for FNAB-Tg is diluted with 1 ml normal saline, with a typical dilution between 50 and 200. In addition, Borel et al. (24) reported that the maximum blood contamination of the needle washout fluid in theory was approximately 20% and contamination was less than 5% in experiments evaluating albumin concentration in FNAB washout fluid; therefore, even if blood contamination did occur, the FNAB-Tg value was still lower in a negative lymph node than in a positive lymph node. This finding was supported by our ROC results. Pathology results are typically the gold standard for decision making in thyroid cancer; therefore, the main contribution of FNAB-Tg assessment is in determining the best balance between necessary and unnecessary surgery in metastatic patients with a negative or nondiagnostic pathological result. Konca Degertekin et al. (12) analyzed the diagnostic accuracy of FNAB-C and FNAB-Tg in 51 lymph nodes and found that FNAB-Tg≥1 ng/ml was associated with similar accuracy and even a higher specificity and PPV compared to FNAB-C alone, and the authors concluded that the best cutoff value was 1.0 ng/ml. Moon et al. (25) reported that the median FNAB-Tg was 521.2 ng/ml in malignant lymph nodes, and the optimal cutoff value of FNAB-Tg was 1.0 ng/ml, with a sensitivity of 93.2% and a specificity of 95.9%. Combining FNAB-Tg and FNAB-C showed superior diagnostic power compared with the diagnostic strategy of using either FNAB-C or FNAB-Tg alone. The finding was consistent with our own results: a cutoff value of 1.0 ng/ml is related to high sensitivity and specificity.
However, factors that could affect FNAB-Tg need to be analyzed further. Li et al. (18) described 6 cases with very elevated FNAB-Tg levels that were confirmed to be normal thyroid tissue, in which 3 cases were labeled as metastatic lymph nodes at level 6. It has been accepted that residual thyroid tissue might be mistaken for suspicious lymph nodes in patients with a history of thyroid surgery, and this viewpoint was confirmed by Jeon et al. (7) and Tang et al. (15): some cases with high FNAB-Tg levels were ultimately explained by remnant thyroid tissue. Therefore, caution must be taken when a negative FNAB-C result but a high FNAB-Tg level is observed in samples collected from the central neck. However, the situation in lateral cervical lymph nodes is more difficult to discern. Usually, the needle path does not pass through the thyroid region during puncture for levels 2-5, and our results also confirmed that there was no thyroid tissue to be considered for a negative FNAB-C but positive FNAB-Tg.
The interference effect of TgAb is most frequently evaluated in the literature but remains unclear. Jo et al. (14) demonstrated that the FNAB-Tg level was significantly higher in TgAb- patients than in TgAb+ patients, and its sensitivity and PPV were increased by >10% in TgAb- patients compared to TgAb+ patients when using the same cutoff value. However, Konca Degertekin et al. (12) reported that TgAb+ and TgAb- patients had comparable FNAB-Tg levels in malignant lymph nodes, and the diagnostic performance of FNAB-Tg was independent of TgAb status. Similar findings were also published by Boi et al. (10) and Duval et al. (26). There were at least two explanations for this finding: on the one hand, TgAb was negative in FNAB from patients with positive serum TgAb (27), and on the other hand, an elevated FNAB-Tg level can easily override the effect of TgAb caused by blood contamination.
The role of TSH and serum Tg has only been analyzed occasionally. One study commented that TSH increased the possibility of false-negative FNAB-Tg in malignant cases (7), but there was no strong evidence to support this viewpoint. In our opinion, blood contamination is a direct or indirect cause that could explain the effect of TSH and serum Tg. In the current study, FNAB-Tg was at the same level in patients with positive lymph nodes who had different TSH and serum Tg levels, and the diagnostic performance of FNAB-C had little relationship with TSH and serum Tg. This finding might be partially due to the abilities of our experienced thyroidologists and cytopathologists.
BRAF 600E mutation was the most common genetic change in PTC patients with geographical differences and occurred in most of the primary tumors and metastatic lymph nodes. Chen et al. (23) reported that 78% of positive lymph nodes had BRAF 600E gene changes, but the mutation was not associated with the number, extranodal extension, or stage of the positive lymph nodes. A slightly lower rate of 47.1% was reported by Kurtulmus et al. (22), but the authors found that the presence of the BRAF 600E mutation in lymph nodes was not affected by mutation in the primary tumor. This finding introduced the possibility of BRAF 600E mutation assessment in predicting cervical LNM. Li et al. (28) might be the only author to evaluate the utilization of BRAF 600E mutation assessment in diagnosing cervical LNM; in their study, 27 positive lymph nodes had a negative FNAB-C result, but 17 of the 27 cases were positive for BRAF 600E mutation. The authors concluded that BRAF 600E mutation assessment could provide diagnostic support in PTC patients, but the benefit extent was small. In our study, it was also noted that compared to FNAB-C alone, the diagnostic performance of FNAB-C combined with BRAF 600E mutation assessment was reduced. One possible explanation is that the samples collected from fine needle puncture ready for BRAF 600E mutation assessment were very limited, increasing the misdiagnosis rate.
Core needle biopsy is another method for disease diagnosis. Novoa et al. (21) confirmed its reliability based on a systemic analysis revealing that CNB provided a correct specific diagnosis in 87% of cases without major complications; however, no other authors have tried to evaluate the feasibility of CNB in predicting cervical LNM in PTC. We were the first to report the high sensitivity and specificity of CNB alone, which could be explained by the fact that only clinically suspicious lymph nodes were punctured, and all the procedures were performed by experienced clinicians. It is interesting to note that the diagnostic ability of CNB-T is decreased when combined with CNB-Tg, and one possible explanation is that blood contamination is very frequent during CNB. However, the relationship between CNB-T and BRAF 600E mutation assessment can allow for the best diagnostic performance and prevent all unnecessary surgeries.
The current study has some limitations that must be acknowledged. First, our sample size was relatively small, which might have decreased our statistical power. Second, there is inherent bias in all retrospective studies. Third, we need to stay awake that the practicality of core biopsy on suspicious lymph nodes is limited in selective patients, especially the pediatric population, because core biopsy is more invasive than an fine needle aspiration.
In summary, the sensitivity and specificity of FNAB-C could be increased when combined with FNAB-Tg, with an optimal cutoff value of 1.0 ng/ml. CNB-T alone can provide satisfactory diagnostic reliability, and the additional benefit is greater with BRAF 600E mutation assessment than with FNAB-Tg.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Our Hospital Institutional Research Committee, and all participants provided written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All the authors made the contribution in study design, manuscript writing, studies selecting, data analysis, study quality evaluating, and manuscript revising. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
2. Liu ST, Zhang X. [Epidemiology and management guidelines of thyroid cancer]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2016) 51:146–9.
3. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
4. Brito JP, Hay ID. Management of Papillary Thyroid Microcarcinoma. Endocrinol Metab Clin North Am (2019) 48:199–213. doi: 10.1016/j.ecl.2018.10.006
5. Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg (2005) 29:483–5. doi: 10.1007/s00268-004-7701-0
6. Achille G, Garrisi VM, Russo S, Guastamacchia E, Giagulli VA, Schirosi L, et al. Thyroglobulin Determination in Fine Needle Aspiration Biopsy Washout of Suspicious Lymph Nodes in Thyroid Carcinoma Follow up. Endocr Metab Immune Disord Drug Targets (2017) 17:213–8. doi: 10.2174/1871530317666170531092501
7. Jeon SJ, Kim E, Park JS, Son KR, Baek JH, Kim YS, et al. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol (2009) 10:106–11. doi: 10.3348/kjr.2009.10.2.106
8. Cunha N, Rodrigues F, Curado F, Ilhéu O, Cruz C, Naidenov P, et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol (2007) 157:101–7. doi: 10.1530/EJE-07-0088
9. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid (1999) 9:105–11. doi: 10.1089/thy.1999.9.105
10. Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab (2006) 91:1364–9. doi: 10.1210/jc.2005-1705
11. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab (1992) 74:1401–4. doi: 10.1210/jcem.74.6.1592886
12. Konca Degertekin C, Yalcin MM, Cerit T, Ozkan C, Kalan I, Iyidir OT, et al. Lymph node fine-needle aspiration washout thyroglobulin in papillary thyroid cancer: Diagnostic value and the effect of thyroglobulin antibodies. Endocr Res (2016) 41:281–9. doi: 10.3109/07435800.2016.1141936
13. Al-Hilli Z, Strajina V, McKenzie TJ, Thompson GB, Farley DR, Regina Castro M, et al. Thyroglobulin Measurement in Fine-Needle Aspiration Improves the Diagnosis of Cervical Lymph Node Metastases in Papillary Thyroid Carcinoma. Ann Surg Oncol (2017) 24:739–44. doi: 10.1245/s10434-016-5625-1
14. Jo K, Kim MH, Lim Y, Jung SL, Bae JS, Jung CK, et al. Lowered cutoff of lymph node fine-needle aspiration thyroglobulin in thyroid cancer patients with serum anti-thyroglobulin antibody. Eur J Endocrinol (2015) 173:489–97. doi: 10.1530/EJE-15-0344
15. Tang S, Buck A, Jones C, Sara Jiang X. The utility of thyroglobulin washout studies in predicting cervical lymph node metastases: One academic medical center’s experience. Diagn Cytopathol (2016) 44:964–8. doi: 10.1002/dc.23554
16. Zhu XH, Zhou JN, Qian YY, Yang K, Wen QL, Zhang QH, et al. Diagnostic values of thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis diagnostic values of FNA-Tg. Endocr J (2020) 67:113–23. doi: 10.1507/endocrj.EJ18-0558
17. Grani G, Fumarola A. Thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis of diagnostic accuracy. J Clin Endocrinol Metab (2014) 99:1970–82. doi: 10.1210/jc.2014-1098
18. Li QK, Nugent SL, Straseski J, Cooper D, Riedel S, Askin FB, et al. Thyroglobulin measurements in fine-needle aspiration cytology of lymph nodes for the detection of metastatic papillary thyroid carcinoma. Cancer Cytopathol (2013) 121:440–8. doi: 10.1002/cncy.21285
19. Ryu KH, Lee JH, Jang SW, Kim HJ, Lee JY, Chung SR, et al. US-guided core-needle biopsy versus US-guided fine-needle aspiration of suspicious cervical lymph nodes for staging workup of non-head and neck malignancies: A propensity score matching study. J Surg Oncol (2017) 116:870–6. doi: 10.1002/jso.24747
20. Wagner JM, Monfore N, McCullough AJ, Zhao L, Conrad RD, Krempl GA, et al. Ultrasound-Guided Fine-Needle Aspiration With Optional Core Needle Biopsy of Head and Neck Lymph Nodes and Masses: Comparison of Diagnostic Performance in Treated Squamous Cell Cancer Versus All Other Lesions. J Ultrasound Med (2019) 38:2275–84. doi: 10.1002/jum.14918
21. Novoa E, Gürtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck (2012) 34:1497–503. doi: 10.1002/hed.21821
22. Kurtulmus N, Ertas B, Saglican Y, Kaya H, Ince U, Duren M. BRAFV600E Mutation: Has It a Role in Cervical Lymph Node Metastasis of Papillary Thyroid Cancer? Eur Thyroid J (2016) 5:195–200. doi: 10.1159/000448112
23. Chen P, Pan L, Huang W, Feng H, Ouyang W, Wu J, et al. BRAF V600E and lymph node metastases in papillary thyroid cancer. Endocr Connect (2020) 9:999–1008. doi: 10.1530/EC-20-0420
24. Borel AL, Boizel R, Faure P, Barbe G, Boutonnat J, Sturm N, et al. Significance of low levels of thyroglobulin in fine needle aspirates from cervical lymph nodes of patients with a history of differentiated thyroid cancer. Eur J Endocrinol (2008) 158:691–8. doi: 10.1530/EJE-07-0749
25. Moon JH, Kim YI, Lim JA, Choi HS, Cho SW, Kim KW, et al. Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large-scale validation of the cutoff value to determine malignancy and evaluation of discrepant results. J Clin Endocrinol Metab (2013) 98:1061–8. doi: 10.1210/jc.2012-3291
26. Duval MADS, Zanella AB, Cristo AP, Faccin CS, Graudenz MS, Maia AL. Impact of Serum TSH and Anti-Thyroglobulin Antibody Levels on Lymph Node Fine-Needle Aspiration Thyroglobulin Measurements in Differentiated Thyroid Cancer Patients. Eur Thyroid J (2017) 6:292–7. doi: 10.1159/000479682
27. Sigstad E, Heilo A, Paus E, Holgersen K, Grøholt KK, Jørgensen LH, et al. The usefulness of detecting thyroglobulin in fine-needle aspirates from patients with neck lesions using a sensitive thyroglobulin assay. Diagn Cytopathol (2007) 35:761–7. doi: 10.1002/dc.20726
Keywords: papillary thyroid carcinoma, lymph node metastasis, FNAB-C, FNAB-Tg, BRAF V600E
Citation: Zhang X, Zhang X, Du W, Dai L, Luo R, Fang Q and Ge H (2021) Fine Needle Biopsy Versus Core Needle Biopsy Combined With/Without Thyroglobulin or BRAF 600E Mutation Assessment for Detecting Cervical Nodal Metastasis of Papillary Thyroid Carcinoma. Front. Endocrinol. 12:663720. doi: 10.3389/fendo.2021.663720
Received: 03 February 2021; Accepted: 10 March 2021;
Published: 12 April 2021.
Edited by:
Christoph Reiners, University Hospital Würzburg, GermanyReviewed by:
Aime Franco, Children’s Hospital of Philadelphia, United StatesAkira Sugawara, Tohoku University, Japan
Copyright © 2021 Zhang, Zhang, Du, Dai, Luo, Fang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Du, duweitj@126.com; Hong Ge, gehong616@126.com
 Xiaojun Zhang1
Xiaojun Zhang1 Qigen Fang
Qigen Fang