- 1Department of Endocrinology, Affiliated Hospital of Yanan Medical University, Shaanxi, China
- 2Department of Endocrinology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
- 3Department of Cardiology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
Background: Patients with subclinical hypothyroidism (SCH) have elevated blood pressure, but the effect of levothyroxine (LT4) therapy on blood pressure among those patients is still unclear. This study aimed to assess whether LT4 therapy could reduce blood pressure in SCH patients through a systematic review and meta-analysis.
Methods: PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science were searched. Randomized controlled trials (RCTs) assessing the effect of LT4 therapy on blood pressure or prospective follow-up studies comparing the blood pressure level before and after LT4 treatment were included, and the mean difference of systolic blood pressure (SBP) or diastolic blood pressure (DBP) was pooled using random-effect meta-analysis.
Results: Twenty-nine studies including 10 RCTs and 19 prospective follow-up studies were eligible for the analysis. Meta-analysis of 10 RCTs suggested that LT4 therapy could significantly reduce SBP in SCH patients by 2.48 mmHg (95% CI −4.63 to −0.33, P = 0.024). No heterogeneity was observed among these 10 RCTs (I2 = 0%). Meta-analysis of the 19 prospective follow-up studies found that LT4 therapy significantly decreased SBP and DBP by 4.80 mmHg (95%CI −6.50 to −3.09, P < 0.001) and 2.74 mmHg (95%CI −4.06 to −1.43, P < 0.001), respectively.
Conclusion: The findings suggest that LT4 replacement therapy can reduce blood pressure in SCH patients, which needs to be validated in more clinical trials with larger samples.
Introduction
Subclinical hypothyroidism (SCH) is a state with elevated thyrotropin (thyroid stimulating hormone, TSH) level and normal free thyroxine (fT4) level (1). Compared with SCH, overt hypothyroidism is diagnosed by elevated TSH level with decreased fT4 level, and TSH level more than 10 mU/L is generally associated with decreased fT4 level (2). SCH is becoming increasingly prevalent in recent years especially among the elderly, and its prevalence ranges approximately from 5 to 20% in the general population (1, 3, 4). Clinical manifestations of SCH range from obvious symptoms to no signs or symptoms, and the most common symptoms of SCH include fatigue, mild depression, muscle weakness, cold intolerance, and weight gain (1, 2, 5). However, despite the clear biochemical pattern of mild thyroid failure, few patients with SCH have typical hypothyroid symptoms (1, 2, 5). SCH can be classified into two categories according to the elevation in TSH level: mild SCH (from the upper limit to 10 mIU/L) and severe SCH (>10 mIU/L) (6–8). Severe SCH or mild SCH with symptoms are generally recommended to be treated with levothyroxine (LT4), which requires monitoring of TSH level over several months and adjusting LT4 dosage accordingly (6–8). Oral LT4 administered daily is the treatment of choice for SCH, and the goal of LT4 treatment for SCH is to restore the TSH level within the reference range (6–8).
Many studies have revealed that both overt hypothyroidism and SCH can increase the risk of cardiovascular disease (9–13). Some observational studies have shown a difference in blood pressure between SCH patients and euthyroid individuals, and SCH patients have higher blood pressure than euthyroid controls (14, 15). Since blood pressure is an independent risk factor for cardiovascular diseases, the elevated blood pressure may mediate or further aggravate the harm of SCH on cardiovascular health (9, 10, 14). Some observational studies including one our study have also suggested an obviously positive relationship between TSH level and hypertension risk among euthyroid individuals, which supports a possibly causal role of elevated TSH level in the development of hypertension (16–18). However, the relationship between thyroid dysfunction and hypertension is still controversial, and the causal relationship between SCH and hypertension has not been well established (9, 19, 20).
Some studies suggested that LT4 therapy could offer benefits for SCH patients through decreasing TSH level, such as the lipid-lowering effect (21, 22). Findings from several studies revealed that LT4 therapy reduced blood pressure in SCH patients (23–25), but other studies did not support it (26, 27). Therefore, there is still no consensus regarding the effect of LT4 therapy on blood pressure in SCH patients. We thus performed a systematic review and meta-analysis to clarify this question.
Methods
Literature Search
This systematic review was done by the Preferred Reporting Items for Systematic Review and Meta-analysis statement (PRISMA) (28) and was registered at International Prospective Register of Systematic Reviews (CRD42018093138). Four bibliographic databases including PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science were searched to identify relevant studies assessing the effect of LT4 therapy on blood pressure in SCH patients using the following search strategy: (levothyroxine OR L-T4 OR L-thyroxine OR Thyroid hormone replacement OR thyroxine) AND (Subclinical hypothyroidism OR SCH OR mild thyroid failure OR mild thyroid hormone deficiency OR mild hypothyroidism). The literature search was performed on April 10, 2018, and was updated on June 26, 2018. No language restriction was applied. The references of identified reviews or included articles were retrieved to find more eligible studies.
Selection Criteria
The systematic review question was whether LT4 replacement therapy could reduce blood pressure among SCH patients. Studies eligible in this meta-analysis must meet the following inclusion criteria which were formulated by the five terms of systematic review question, namely population, interventions, comparators, outcomes, and study designs (PICOS). The inclusion criteria were showed as follows: (1) they were randomized controlled trials (RCTs) assessing the effect of LT4 treatment on blood pressure or prospective observational studies comparing the blood pressure levels before and after LT4 treatment (S); (2) participants were adult patients with SCH (P); (3) the intervention was thyroid hormone replacement alone with LT4 (I); (4) the comparator for RCTs was placebo or no LT4 treatment, while the comparator for prospective observational studies was the blood pressure before LT4 treatment (C); (5) they reported the difference in systolic blood pressure (SBP) or diastolic blood pressure (DBP) between LT4-treated groups and controls without LT4 treatment, or the changes in the levels of SBP or DBP before and after LT4 treatment (O). Studies recruiting adolescents (under 18 years of age) or pregnant women or using combination of LT4 with other drugs as interventions were excluded. Studies without usable data on the effect of LT4 treatment on blood pressure or containing overlapping data were all excluded.
Data Extraction and Quality Assessment
The following information was extracted independently by two investigators: first author, year of publication, study design, country, sample size, ethnicity, definition of SCH, treatment dosage of LT4, mean TSH level at baseline, change in TSH during treatment, TSH measurement methods, hypertension status of SCH patients, treatment duration as well as blood pressure before and after treatment. Two authors independently assessed the risk of bias of included RCTs using Cochrane risk of bias tool, which mainly included randomized sequence generation, treatment allocation concealment, blinding, outcome data completeness, and selective outcome reporting (29). The risk of bias in prospective studies comparing the blood pressure level before and after LT4 treatment was assessed using the Newcastle-Ottawa Scale (NOS) (30). Studies with scores <6 were defined to have suboptimal quality.
Statistical Analysis
To assess the effect of LT4 treatment on blood pressure, the mean differences of SBP or DBP were pooled using meta-analysis. Heterogeneity in the meta-analysis was assessed using both the Cochran's Q-test and the I2 statistic, and heterogeneity was considered to be substantial when I2 was more than 50% (31, 32). Random-effects meta-analysis allows for obvious differences in the intervention effect and can encompass the heterogeneity among included studies when compared with the fixed-effect meta-analysis (33–36). To cope with the difference among those included studies, data were thus all pooled using random-effects meta-analysis (33). Sensitivity analyses were conducted by excluding low-quality study or through sequential omission of single study. Meta-regression analysis was used to identify factors related to the effect of LT4 treatment on blood pressure, and candidate factors included sample size, mean age, baseline blood pressure, baseline TSH level, final TSH level, changes in TSH during follow-up, LT4 dosage, ethnicity, hypertension status of patients, TSH cut-off values to diagnose SCH, and treatment duration. Subgroup analyses were stratified by the mean age of patients (Mean age ≥60 years vs. Mean age <60 years), ethnicity (Caucasians vs. Asians), TSH cut-off values to diagnose SCH (TSH upper limit ≥4.5 mIU/L vs. TSH upper limit <4.5 mIU/L), the mean TSH level at baseline (Mean TSH ≥7.0 mIU/L vs. Mean TSH <7.0 mIU/L), TSH measurement methods, TSH changes after treatment (TSH change ≥4.5 mIU/L vs. TSH change <4.5 mIU/L), types of SCH patients (Only mild SCH patients vs. Mild SCH and severe SCH together), hypertension status of patients (Normotensive SCH patients vs. Normotensive and hypertensive patients together), baseline mean SBP level (Baseline mean SBP ≥130 mmHg vs. Baseline mean SBP <130 mmHg), baseline mean DBP level (Baseline mean DBP ≥80 mmHg vs. Baseline mean DBP <80 mmHg), initial LT4 doses (Initial LT4 dose ≥50 μg daily vs. Initial LT4 dose <50 μg daily), mean LT dosages during treatment (Mean LT4 dose ≥60 μg daily vs. Mean LT4 dose <60 μg daily), and treatment duration (Treatment duration >6 months vs. Treatment duration ≤6 months). To investigate the difference between subgroups, a test proposed by Borenstein et al. was used and it could detect the heterogeneity across subgroup results due to genuine subgroup differences rather than sampling error (36, 37). The evidence of publication bias was evaluated using funnel plot and Egger's test (38). Trim and fill method was used to assess the stability of the pooled estimates for the existence of publication bias (39). Statistical analyses were performed using STATA 12.0 (Stata Corp, Texas, USA) and Review Manager 5.2 (The Cochrane Collaboration, Copenhagen). P < 0.05 was considered as statistically significant.
Results
Study Selection and Characteristics
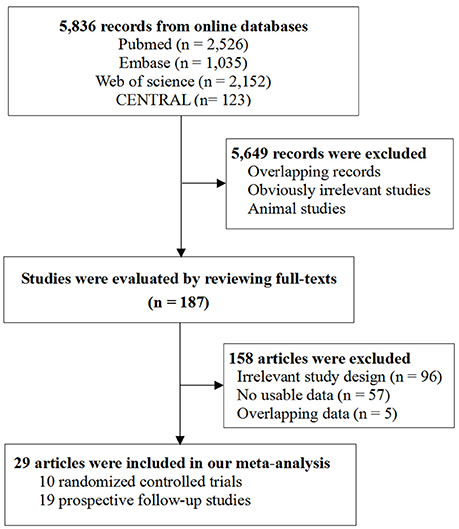
Figure 1 shows the flowchart of study selection (Figure 1). Literature search of online databases identified a total of 5,836 records. Further screening titles and abstracts found 187 potentially relevant articles. Full-text evaluation excluded 158 articles. Thus, 29 articles were included in the final meta-analysis (23–27, 40–63). Among them, 10 studies were RCTs including 1,637 participants (40–49) and the other 19 studies were prospective follow-up studies including 571 participants (23–27, 50–63).
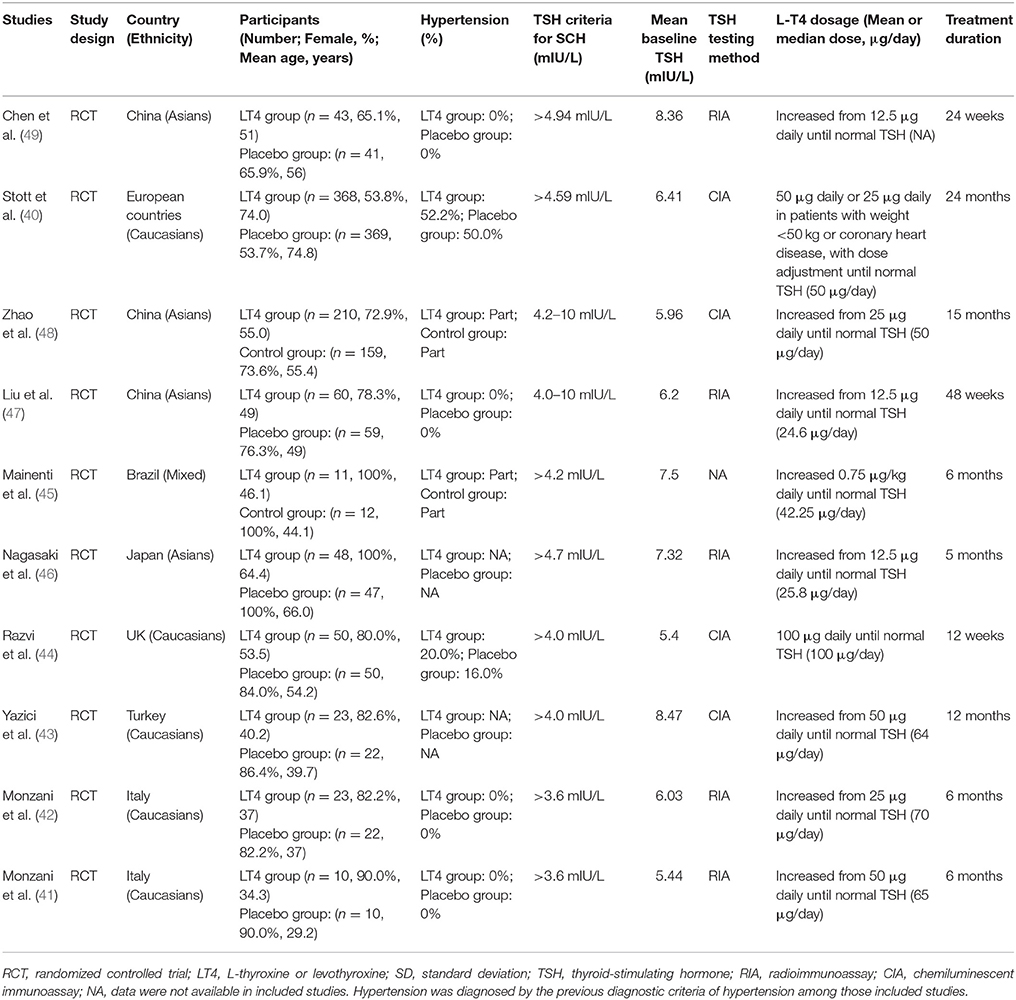
Tables 1, 2 listed the general characteristics of these eligible studies. The sample size ranged from 10 to 369 for LT4 treatment or placebo groups in RCTs and from 9 to 100 for prospective follow-up studies (Tables 1, 2). All studies enrolled adults with mean age ranging from 29.2 to 74.8 years old. These studies varied tremendously in baseline TSH level, LT4 dosage, and treatment duration. Though LT4 dosages used in studies varied substantially, most studies typically followed current guidelines for treating SCH, progressively increased to a target dose, and succeeded in restoring TSH level within the reference range. The TSH cut-off values to diagnose SCH ranged from 3.6 to 6.0 mIU/L. Most studies used either chemiluminescent immunoassay (CIA) or radioimmunoassay (RIA) to measure TSH, both of which were well-developed and widely-used measurement methods for TSH. The duration ranged from 12 weeks to 24 months for RCTs and 3–18 months for prospective follow-up studies. Among those 10 RCTs, eight used placebo-controlled design (40–49), and one used open-label design (48) (Table 1). Supplementary Figure 1 showed the results of bias risk assessment in RCTs, indicating no obvious risk of bias for most studies. Table 2 showed the results of quality assessment for prospective follow-up studies. The scores of quality assessment ranged from 4 to 7. Overall, eight prospective follow-up studies had good quality while the other 11 had suboptimal quality (Table 2).
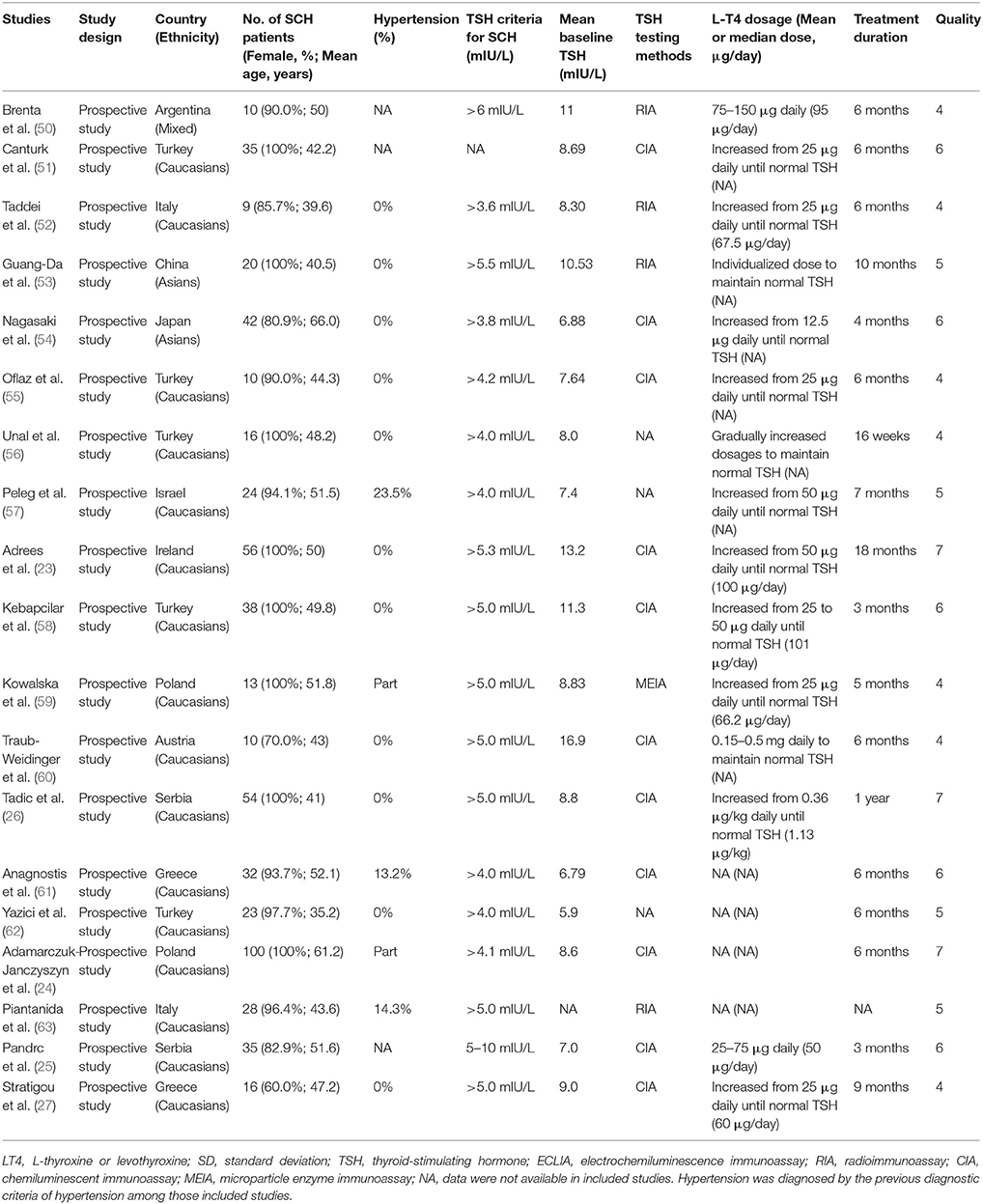
Table 2. Characteristics of 19 prospective follow-up studies evaluating blood pressure levels before and after LT4 treatment in SCH patients.
Because all included studies finished their work before 2018, they thus all used the previous diagnostic criteria for diagnosing hypertension (64). The proportion of SCH patients with hypertension among those included studies was shown in Tables 1, 2. Among those 10 RCTs, three RCTs reported that only normotensive SCH patients were recruited and hypertensive SCH patients were excluded (Table 1). Four RCTs reported that some SCH patients were hypertensive with the proportion of hypertensive SCH patients ranging from 20.0 to 52.2%, but few studies reported the information on the use of antihypertensive drugs (Table 1). Three RCTs did not provide information on the hypertension status of patients (Table 1). Among those 19 prospective follow-up studies, 11 studies reported that only normotensive SCH patients were recruited and those with hypertension were excluded, five studies reported that some patients were hypertensive (Table 2).
Meta-Analysis of RCTs
No heterogeneity was observed among those 10 RCTs (I2 = 0%). Meta-analysis of the 10 RCTs suggested that LT4 therapy could significantly reduce SBP (Mean difference: −2.48 mmHg, 95%CI −4.63 to −0.33, P = 0.024), but not DBP (Mean difference: −0.85 mmHg, 95%CI −2.27 to 0.58, P = 0.245; Figure 2). After excluding two studies without using placebo-controlled design, LT4 therapy still significantly reduced SBP (Mean difference: −2.64 mmHg, 95%CI −4.95 to −0.32, P = 0.03), but not DBP (Mean difference: −1.28 mmHg, 95%CI −2.90 to 0.34, P = 0.12). Sensitivity analysis with sequential omission of single trial found obvious changes in the pooled mean difference of DBP, but not in the pooled mean difference of SBP (Supplementary Figure 2). Meta-regression analysis of RCTs showed that no factor was related to the effect of LT4 therapy on SBP (P > 0.05).
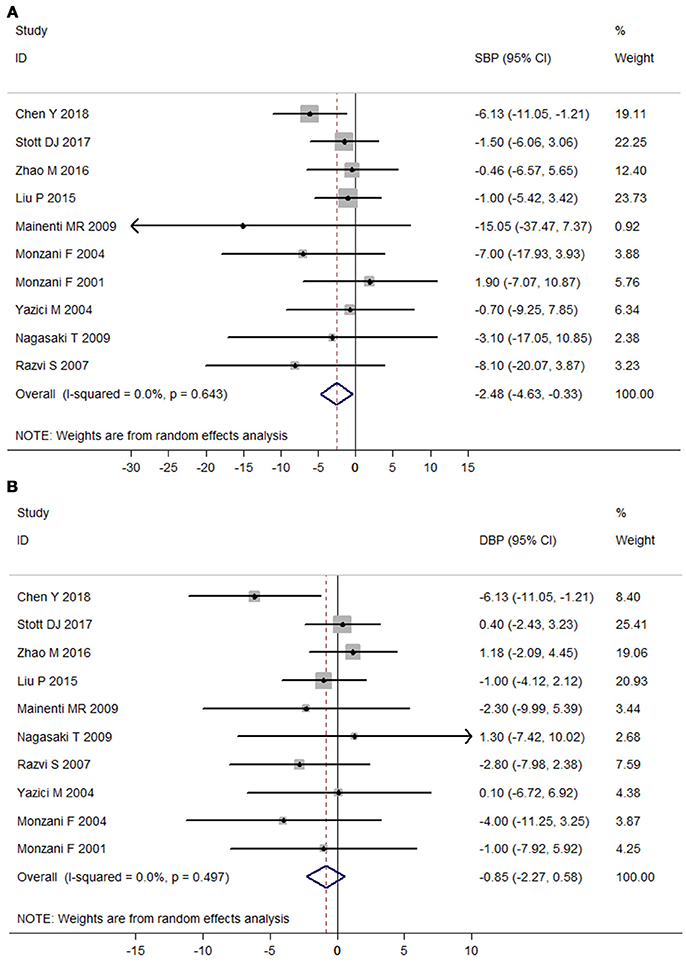
Figure 2. Meta-analysis of randomized controlled trials assessing the effect of LT4 therapy on blood pressure in SCH patients. (A) Effect of LT4 therapy on SBP in SCH patients. (B) Effect of LT4 therapy on DBP in SCH patients.
Subgroup analyses in the meta-analysis of RCTs suggested that pooled mean differences of SBP were statistically significant in most stratified analyses, but were not significant in the left analyses (Figure 3; Supplementary Table 1). For instance, subgroup analyses by the mean TSH level at baseline suggested that the SBP-lowering effect of LT4 therapy was more profound in those patients with higher mean TSH level (≥7.0 mIU/L; Pooled mean difference: −4.97 mmHg, 95%CI −8.98 to −0.95, P = 0.015), and it was not significant among those with lower mean TSH level (<7.0 mIU/L; Pooled mean difference: −1.48 mmHg, 95%CI −4.03 to 1.07, P = 0.256; Supplementary Table 1). In addition, there was no obvious difference in the outcomes of those stratified analyses in the meta-analysis of RCTs assessing the effect of LT4 therapy on SBP (P > 0.05, Supplementary Table 1).
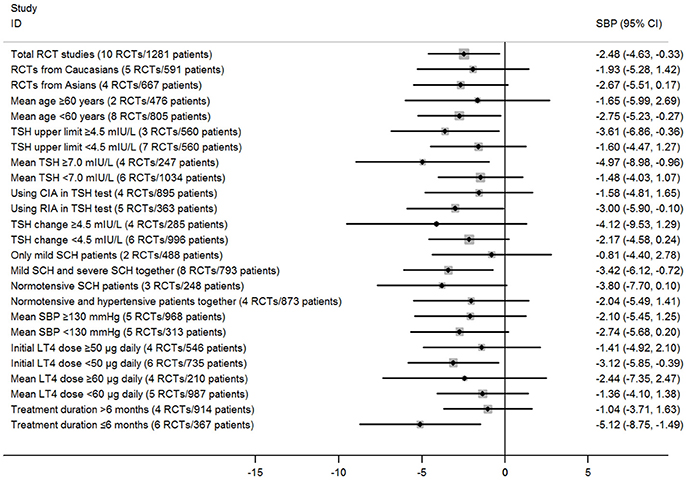
Figure 3. Main findings of subgroup analyses in the meta-analysis of randomized controlled trials assessing the effect of LT4 therapy on SBP in SCH patients.
Subgroup analyses in the meta-analysis of RCTs assessing the effect of LT4 therapy on DBP suggested that pooled mean differences of DBP were not significant in most stratified analyses (Supplementary Figure 3; Supplementary Table 2). Moreover, there was also no obvious difference in the outcomes of those stratified analyses in the meta-analysis of RCTs assessing the effect of LT4 therapy on DBP (P > 0.05, Supplementary Table 2).
Meta-regression analyses also suggested those stratified factors were not sources of heterogeneity in the effect of LT4 therapy on SBP or DBP. For example, there was no obvious difference in the pooled mean differences for SBP between subgroups stratified by hypertension status (−3.80 vs. −2.04 mmHg, P = 0.59), and meta-regression analyses also suggested that it had little possibility to cause substantial influence on the treatment effect (P > 0.05; Supplementary Tables 1, 2).
Meta-Analysis of Prospective Follow-Up Studies
Heterogeneity assessment of 19 prospective follow-up studies showed moderate heterogeneity in data for DBP (I2 = 47.0%), but not for SBP (I2 = 13.1%). Meta-analysis of these 19 studies found that LT4 therapy significantly decreased SBP (Mean difference: −4.80 mmHg, 95%CI −6.50 to −3.09, P < 0.001) and DBP of SCH patients (Mean difference: −2.74 mmHg, 95%CI −4.06 to −1.43, P < 0.001; Figure 4). In addition, meta-analysis of eight prospective follow-up studies with good quality verified that LT4 therapy could significantly reduce both SBP (Mean difference: −5.93 mmHg, 95%CI −9.11 to −2.74, P < 0.001) and DBP (Mean difference: −3.04 mmHg, 95%CI −5.13 to −0.95, P = 0.004; Figure 4C). Sensitivity analysis through sequential omission of single study showed no obvious change in both SBP change and DBP change (Supplementary Figures 2C,D). Moreover, meta-regression analysis suggested baseline SBP was an important factor influencing the SBP-lowering effect of LT4 therapy (β = −0.23, P = 0.038), and LT4 therapy was more effective in lowering SBP of SCH patients with higher baseline SBP than those with lower SBP (Supplementary Figure 4A). Besides, change in TSH of SCH patients during treatment was an important predictor of SBP-lowering effect of LT4 therapy (β = −0.97, P = 0.05), suggesting that SCH patients with more TSH change had more reduction in SBP after LT4 therapy than those with less TSH change (Supplementary Figure 4B). Furthermore, both baseline TSH level (β = −0.68, P = 0.025) and TSH change during treatment (β = −0.67, P = 0.014) were important influential factors of DBP-lowering effect of LT4 therapy (Supplementary Figures 4C,D).
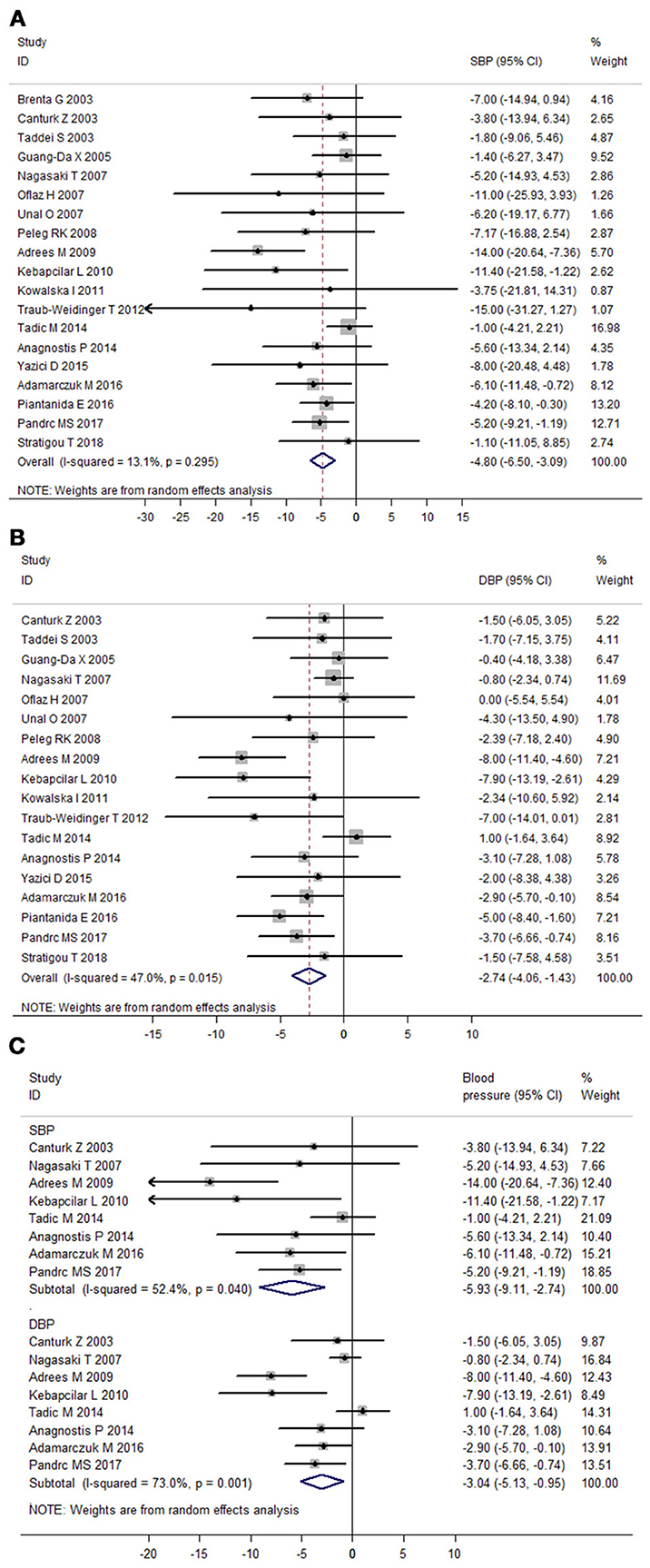
Figure 4. Meta-analysis of prospective follow-up studies assessing the effect of LT4 therapy on blood pressure in SCH patients. (A) Effect of LT4 therapy on SBP in SCH patients. (B) Effect of LT4 therapy on DBP in SCH patients. (C) Meta-analysis of eight prospective follow-up studies with good quality.
Subgroup analyses in the meta-analysis of prospective follow-up studies suggested that the pooled mean differences for both SBP and DBP were statistically significant in most stratified analyses despite of some difference in the magnitude of the treatment effect (Figure 5; Supplementary Figure 5; Supplementary Tables 3, 4). For instance, subgroup analyses by the mean TSH level at baseline suggested that the effect of LT4 therapy on blood pressure was more profound in those SCH patients with higher mean TSH level (≥10.0 mIU/L; Pooled mean difference for SBP: −8.66 mmHg, 95%CI −14.66 to −2.65, P = 0.005; Pooled mean difference for SBP: −5.63 mmHg, 95%CI −9.84 to −1.41, P = 0.009), and the effect was modest in those with lower mean TSH level (<10.0 mIU/L; Supplementary Tables 3, 4). In addition, there was no obvious difference in the treatment effects in most stratified analyses (P > 0.05, Supplementary Tables 3, 4). However, patients with higher blood pressure at baseline had more reduction in SBP (−7.74 vs. −3.27 mmHg, P = 0.025) and DBP (−4.52 vs. −1.03 mmHg, P = 0.004) than those with lower blood pressure (Supplementary Tables 3, 4). Additionally, patients receiving initial LT4 dose ≥50 μg daily had more reduction in SBP (−12.15 vs. −4.90 mmHg, P = 0.04) than those receiving initial LT4 dose <50 μg daily (Supplementary Tables 3, 4). Meta-regression analyses further suggested these three stratified factors (baseline SBP, baseline DBP, and initial LT4 doses) were sources of heterogeneity in the treatment effect of LT4 therapy (P < 0.05).
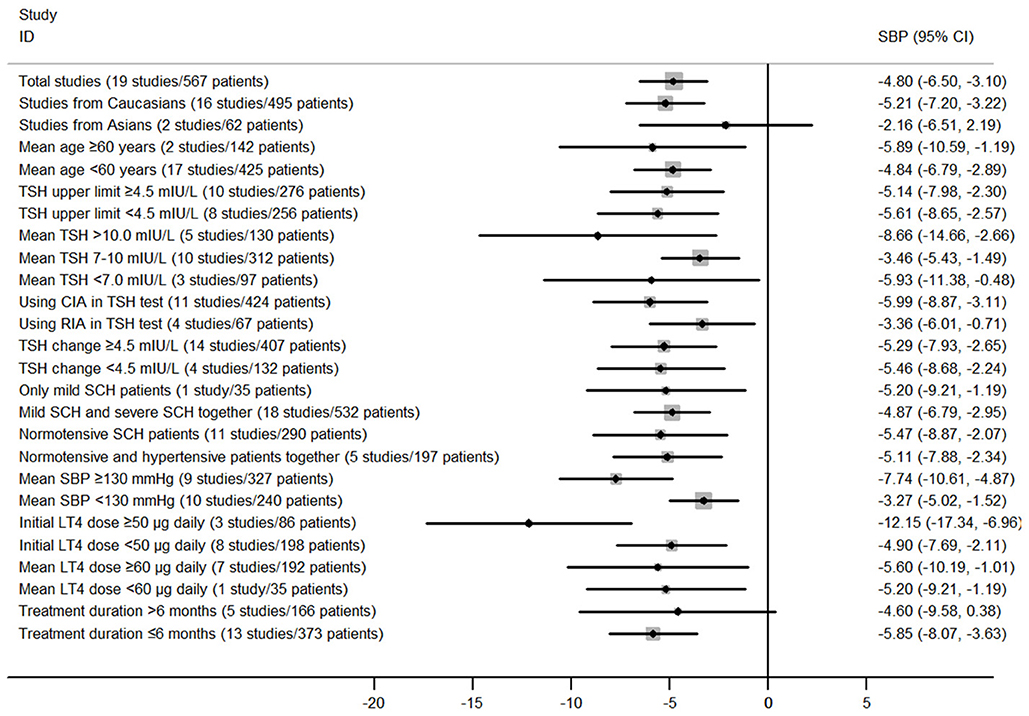
Figure 5. Main findings of subgroup analyses in the meta-analysis of prospective follow-up studies assessing the effect of LT4 therapy on SBP in SCH patients.
Publication Bias Evaluation
Both funnel plot and Egger's test found no publication bias in the meta-analysis of RCTs (Figures 6A,B). The P-values of Egger's test for SBP and DBP were 0.30 and 0.26, respectively. In addition, possible risk of publication bias was found in the meta-analysis of prospective follow-up studies on SBP (P = 0.03; Supplementary Figures 6A,B), but not on DBP (P = 0.15). Moreover, analysis using trim and fill method found no obvious change in the pooled mean difference of SBP.
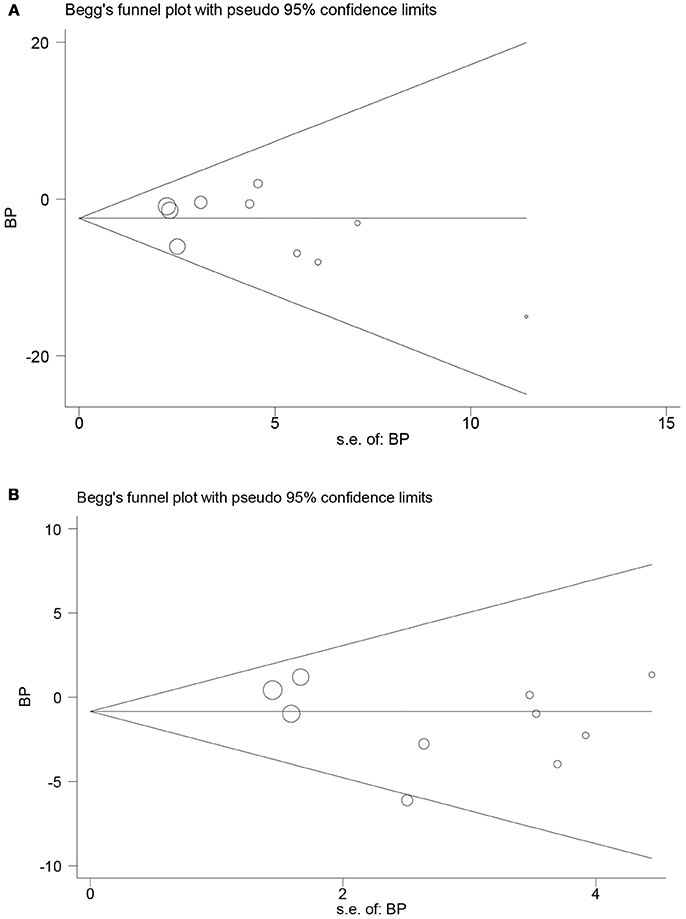
Figure 6. Funnel plots in the meta-analysis of randomized controlled trials assessing the effect of LT4 therapy on blood pressure in SCH patients. (A) Funnel plot in the meta-analysis of randomized controlled trials assessing the effect of LT4 therapy on SBP in SCH patients. (B) Funnel plot in the meta-analysis of randomized controlled trials assessing the effect of LT4 therapy on DBP in SCH patients.
Discussion
Although many trials have been published to evaluate the possible benefits of LT4 therapy in SCH patients (5, 65, 66), the impact of LT4 therapy on blood pressure in SCH patients has not been defined yet. To our best knowledge, this meta-analysis is unique and it is the first systematic review and meta-analysis aiming to study the effect of LT4 therapy on blood pressure in SCH patients. Meta-analysis of RCTs showed that LT4 therapy could reduce SBP in SCH patients. Moreover, meta-analysis of prospective follow-up studies revealed that LT4 therapy could reduce both SBP and DBP in SCH patients. Therefore, the present meta-analysis suggested that LT4 replacement therapy could help to reduce blood pressure in SCH patients.
In the present meta-analysis, all included RCTs showed no statistically significant effect of LT4 therapy on blood pressure except for the study by Chen et al. (49). However, our meta-analysis of RCTs confirmed that LT4 therapy could reduce SBP in SCH patients. This could be explained as follows. Firstly, the lack of statistically significant effect of LT4 therapy in single trial may result from the limited sample size. Our meta-analysis enrolled 10 RCTs with 1,637 SCH participants, which increased the sample size and could provide a credible evaluation of the real effect of LT4 therapy on blood pressure. Secondly, the modest impact of TSH on blood pressure may conceal the effect of LT4 therapy. Previous studies including one our study have shown a statistically significant relationship between TSH and blood pressure although with less dramatic coefficient, suggesting that decreasing TSH level through LT4 therapy may only have a modest effect on blood pressure (16–18). At last, many SCH participants enrolled in these RCTs were normotensive, which could interfere the blood pressure-lowering effect of LT4 therapy. Our meta-regression analysis showed that baseline SBP level was an important predictor of the blood pressure-lowering effect of LT4 therapy (P = 0.038), suggesting that LT4 therapy was more effective in reducing SBP in patients with higher baseline SBP than those with lower SBP.
Because SCH has an obviously adverse impact on cardiovascular function, it has been proposed that restoring TSH level to the normal range through LT4 therapy may reverse its negative impact on cardiovascular function (5, 9, 66). Some studies have shown that certain risk factors of cardiovascular disease can be reversed by LT4 therapy among SCH patients (65, 67). Though a meta-analysis published in 2007 concluded that LT4 therapy was not effective in decreasing cardiovascular morbidity in SCH patients (68), several recent studies found that LT4 therapy could reverse some detrimental impacts caused by SCH (21, 22, 69, 70). Two recent meta-analyses confirmed that LT4 therapy could reduce lipid level in SCH patients (21, 22). Moreover, another two meta-analyses found that LT4 therapy could reduce carotid intima-media thickness of SCH patients, suggesting that LT4 therapy could have a preventive effect against atherosclerosis (69, 70). The findings from our meta-analysis confirm the blood pressure-lowering effect of LT4 therapy in SCH patients, which adds new insights to the debated question of whether LT4 therapy is required in SCH patients. The findings above suggest that reducing TSH level through LT4 therapy may be a promising strategy to prevent cardiovascular events of SCH patients through controlling cardiovascular risk factors. However, there is still lack of clinical trials determining the long-term effect of LT4 therapy.
Several cross-sectional studies suggested an obviously positive relationship between TSH level and hypertension among euthyroid individuals (16–18). Two prospective cohort studies also supported an association between TSH level and hypertension risk among euthyroid individuals (71, 72). One cohort study by Jiang et al. revealed that changes in TSH level during follow-up was positively related to the changes in SBP (71). Another large cohort study by Asvold et al. also supported that high TSH level within the reference range was associated with higher blood pressure at follow-up (72). Apart from the significant relationship between TSH level and hypertension risk in euthyroid individuals, some studies have found that higher TSH level is associated with impaired endothelial function, renal vascular resistance and arterial stiffness, which may further result in elevated blood pressure (73–75). These findings support a possible causal role of TSH in the development of hypertension. Our meta-analysis confirms the blood pressure-lowering effect of restoring TSH level to the normal range through LT4 therapy in SCH patients, providing an argument for the causal role of increased TSH level in the development of hypertension.
Since the new ACC/AHA guidelines have lowered the definition of high blood pressure to 130/80 mmHg, more SCH patients will have concurrent hypertension (76, 77). Because both hypertension and SCH are important risk factors for cardiovascular diseases, earlier intervention with either non-drug approaches or medications may be necessary for those SCH patients with concurrent hypertension, which need to be determined in future research. Currently, the optimal way to employ LT4 replacement therapy in SCH patients is still not fully determined in large and well-designed RCTs (78, 79). To further clarify the benefits of LT4 therapy and identify those SCH patients for whom LT4 treatment will provide the most benefit, more RCTs and well-controlled studies are needed (78, 79).
Those studies included in our meta-analysis recruited patients with distinct degrees of SCH (Tables 1, 2), which may result in the conflicting effect of LT4 therapy on blood pressure among SCH patients. Subgroup analyses in our meta-analysis indicated generally less benefit of LT4 treatment among SCH patients with lower TSH level, and more benefit of LT4 treatment in patients with higher TSH level (Supplementary Tables). However, most included studies did not analyze the outcomes in mild SCH patients and severe SCH patients separately, making it impossible to draw conclusions by the types of SCH separately. Therefore, more RCTs or controlled studies are needed to define the optimal way to employ LT4 therapy in patients with distinct degrees of SCH.
One important limitation in this meta-analysis was the inconsistency across included studies, such as differences in the extent of elevated TSH level, baseline blood pressure, races and mean ages of patients, which could possibly result in the distinct findings in those included studies. However, the inconsistent characteristics across included studies are very common in meta-analyses of clinical trials, and meta-analyses of RCTs with different drug dosages, different ages of patients, different races and different treatment duration are both reasonable and feasible (80–83). Therefore, the inconsistent characteristics of included studies in our meta-analysis paralleled that in most published meta-analyses (80–83). Moreover, there are also a number of published meta-analyses assessing the effect of LT4 treatment for SCH patients, and there was also obvious inconsistency across included studies (21, 22, 68–70, 84, 85). Compared with those published meta-analyses, our meta-analysis adopted similar methods and protocol, which also proved that the methods used in our meta-analysis were adequate and feasible.
Despite of the obvious differences across those studies included in the meta-analysis, subgroup analyses and meta-regression analyses suggested that most of those baseline characteristics had little influence on the blood pressure-lowering effect of LT4 therapy. For instance, there were obvious differences in both the mean LT4 dosages and treatment duration across those included studies (Tables 1, 2), but subgroup analyses revealed that both the mean dosages of LT4 and treatment duration had little influence on the blood pressure-lowering effect of LT4 therapy (P > 0.05, Supplementary Tables). However, there was obvious difference in the effect of LT4 therapy on SBP between the subgroups stratified by initial LT4 doses (P = 0.04, Supplementary Table 3), which suggested that different initial LT4 doses may provide distinct effects in reducing blood pressure. Nevertheless, the findings in the subgroup analyses may result from the bias caused by the low statistical power and the limited number of included studies. Therefore, more RCTs or prospective follow-up studies with larger number of participants are necessary to further explore the possible impact of L-T4 dosages, severity of SCH, treatment duration, ages and races on the effect of LT4 therapy for SCH. Moreover, to investigate the effect of LT4 treatment on blood pressure among SCH patients with different characteristics, a meta-analysis of individual patient data stratified by patient characteristics is recommended to be done in the future, which may help to provide the reliable evidence required to fully evaluate the effect of LT4 therapy among SCH patients (86, 87).
Several other limitations should be noted in interpreting our findings. Firstly, obvious difference existed in the hypertension status of patients across those included studies. However, subgroup analysis and meta-regression analysis suggested that it was unlikely to result in distinct treatment effects across those included studies. Secondly, it was possible that misclassification of participants may have occurred owing to variations in TSH measurement methods and TSH cut-off values to diagnose SCH across those included studies. Though subgroup analyses by TSH cut-off values to diagnose SCH suggested that it had no obvious influence on the blood pressure-lowering effect of LT4 therapy (Supplementary Tables), the definition and clinical significance of SCH are still confounded by controversies on the correct reference range for TSH (1, 5, 88). Currently, the reference range for TSH is still a subject of debate and is still not consistently used in practice, which may result in misclassification of SCH patients or differences in study results. Finally, our study only included 10 eligible RCTs, some of which had small sample size. Therefore, our findings need to be further validated using more RCTs with larger sample size.
In conclusion, our meta-analysis provided some evidence for the effect of LT4 therapy on blood pressure among SCH patients. However, owing to the limited number of included RCTs, the evidence for the effect of LT4 therapy on blood pressure is still not strong, which highlights a clear need for more high-quality RCTs with larger sample size.
Author Contributions
WH and XL designed the study. WH, SL, J-aZ, JZ, KM, and XL contributed to the literature search, interpretation, writing, and proofreading of the manuscript. WH, SL, J-aZ, and KM extracted data and performed data analyses.
Funding
The present work was supported by Foundation of Key Disciplines in Pudong New Area, Shanghai (PWZxq2017–01); Cardiovascular Key Discipline Found of Shanghai Health and Family Planning Commission (ZK2015A13); grants from the National Natural Science Foundation of China (Grant No.81670722 and 81471004).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2018.00454/full#supplementary-material
References
1. Peeters RP. Subclinical hypothyroidism. N Engl J Med. (2017) 376:2556–65. doi: 10.1056/NEJMcp1611144
2. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet (2017) 390:1550–62. doi: 10.1016/S0140-6736(17)30703-1
3. Nakajima Y, Yamada M, Akuzawa M, Ishii S, Masamura Y, Satoh T, et al. Subclinical hypothyroidism and indices for metabolic syndrome in Japanese women: one-year follow-up study. J Clin Endocrinol Metab. (2013) 98:3280–7. doi: 10.1210/jc.2013-1353
4. Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. (2011) 164:943–50. doi: 10.1530/EJE-10-1041
5. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet (2012) 379:1142–54. doi: 10.1016/S0140-6736(11)60276-6
6. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. (2013) 2:215–28. doi: 10.1159/000356507
7. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid (2014) 24:1670–751. doi: 10.1089/thy.2014.0028
8. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. (2014) 35:433–512. doi: 10.1210/er.2013-1083
9. Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. (2018) 39:503–7. doi: 10.1093/eurheartj/ehx050
10. Silva N, Santos O, Morais F, Gottlieb I, Hadlich M, Rothstein T, et al. Subclinical hypothyroidism represents an additional risk factor for coronary artery calcification, especially in subjects with intermediate and high cardiovascular risk scores. Eur J Endocrinol. (2014) 171:327–34. doi: 10.1530/EJE-14-0031
11. Bano A, Chaker L, Mattace-Raso FUS, van der Lugt A, Ikram MA, Franco OH, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam study. Circ Res. (2017) 121:1392–400. doi: 10.1161/CIRCRESAHA.117.311603
12. Langen VL, Niiranen TJ, Puukka P, Lehtonen AO, Hernesniemi JA, Sundvall J, et al. Thyroid-stimulating hormone and risk of sudden cardiac death, total mortality and cardiovascular morbidity. Clin Endocrinol. (2018) 88:105–13. doi: 10.1111/cen.13472
13. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA (2010) 304:1365–74. doi: 10.1001/jama.2010.1361
14. Cai Y, Ren Y, Shi J. Blood pressure levels in patients with subclinical thyroid dysfunction: a meta-analysis of cross-sectional data. Hypertens Res. (2011) 34:1098–105. doi: 10.1038/hr.2011.91
15. Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid (2002) 12:421–5. doi: 10.1089/105072502760043512
16. Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab. (2007) 92:841–5. doi: 10.1210/jc.2006-2208
17. Ittermann T, Thamm M, Wallaschofski H, Rettig R, Volzke H. Serum thyroid-stimulating hormone levels are associated with blood pressure in children and adolescents. J Clin Endocrinol Metab. (2012) 97:828–34. doi: 10.1210/jc.2011-2768
18. He W, Li S, Wang B, Mu K, Shao X, Yao Q, et al. Dose-response relationship between thyroid stimulating hormone and hypertension risk in euthyroid individuals. J Hypertens. 2018. doi: 10.1097/HJH.0000000000001826. [Epub ahead of print].
19. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism and hypertension: fact or myth?—Authors' reply. Lancet (2018) 391:30. doi: 10.1016/S0140-6736(17)33321-4
20. Hofstetter L, Messerli FH. Hypothyroidism and hypertension: fact or myth? Lancet (2018) 391:29–30. doi: 10.1016/S0140-6736(17)33320-2
21. Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect. (2017) 6:188–99. doi: 10.1530/EC-17-0028
22. Li X, Wang Y, Guan Q, Zhao J, Gao L. The lipid-lowering effect of levothyroxine in patients with subclinical hypothyroidism: a systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol. (2017) 87:1–9. doi: 10.1111/cen.13338
23. Adrees M, Gibney J, El-Saeity N, Boran G. Effects of 18 months of L-T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol. (2009) 71:298–303. doi: 10.1111/j.1365-2265.2008.03509.x
24. Adamarczuk-Janczyszyn M, Zdrojowy-Welna A, Rogala N, Zatonska K, Bednarek-Tupikowska G. Evaluation of selected atherosclerosis risk factors in women with subclinical hypothyroidism treated with L-thyroxine. Adv Clin Exp Med. (2016) 25:457–63. doi: 10.17219/acem/38555
25. Pandrc MS, Ristic A, Kostovski V, Stankovic M, Antic V, Milin-Lazovic J, et al. The effect of early substitution of subclinical hypothyroidism on biochemical blood parameters and the quality of life. J Med Biochem. (2017) 36:127–36. doi: 10.1515/jomb-2017-0007
26. Tadic M, Ilic S, Kostic N, Caparevic Z, Celic V. Subclinical hypothyroidism and left ventricular mechanics: a three-dimensional speckle tracking study. J Clin Endocrinol Metab. (2014) 99:307–14. doi: 10.1210/jc.2013-3107
27. Stratigou T, Dalamaga M, Antonakos G, Marinou I, Vogiatzakis E, Christodoulatos GS, et al. Hyperirisinemia is independently associated with subclinical hypothyroidism: correlations with cardiometabolic biomarkers and risk factors. Endocrine (2018) 61:83–93. doi: 10.1007/s12020-018-1550-3
28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
29. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
30. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Cochran W. The combination of estimates from different experiments. Biometrics (1954) 10:101–29.
34. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48.
35. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (2011) 342:d549. doi: 10.1136/bmj.d549
36. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011). Available online at: www.handbook.cochrane.org
37. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. Chichester: John Wiley & Sons (2008).
38. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34.
39. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. (2007) 26:4544–62. doi: 10.1002/sim.2889
40. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. (2017) 376:2534–44. doi: 10.1056/NEJMoa1603825
41. Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. (2001) 86:1110–5. doi: 10.1210/jcem.86.3.7291
42. Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. (2004) 89:2099–106. doi: 10.1210/jc.2003-031669
43. Yazici M, Gorgulu S, Sertbas Y, Erbilen E, Albayrak S, Yildiz O, et al. Effects of thyroxin therapy on cardiac function in patients with subclinical hypothyroidism: index of myocardial performance in the evaluation of left ventricular function. Int J Cardiol. (2004) 95:135–43. doi: 10.1016/j.ijcard.2003.05.015
44. Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. (2007) 92:1715–23. doi: 10.1210/jc.2006-1869
45. Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest. (2009) 32:470–3. doi: 10.3275/6106
46. Nagasaki T, Inaba M, Yamada S, Shirakawa K, Nagata Y, Kumeda Y, et al. Decrease of brachial-ankle pulse wave velocity in female subclinical hypothyroid patients during normalization of thyroid function: a double-blind, placebo-controlled study. Eur J Endocrinol. (2009) 160:409–15. doi: 10.1530/EJE-08-0742
47. Liu P, Liu R, Chen X, Chen Y, Wang D, Zhang F, et al. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Curr Med Res Opin. (2015) 31:2233–40. doi: 10.1185/03007995.2015.1094044
48. Zhao M, Liu L, Wang F, Yuan Z, Zhang X, Xu C, et al. A worthy finding: decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid (2016) 26:1019–29. doi: 10.1089/thy.2016.0010
49. Chen Y, Wu G, Xu M. The effect of L-thyroxine substitution on oxidative stress in early-stage diabetic nephropathy patients with subclinical hypothyroidism: a randomized double-blind and placebo-controlled study. Int Urol Nephrol. (2018) 50:97–103. doi: 10.1007/s11255-017-1756-y
50. Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol. (2003) 91:1327–30. doi: 10.1016/S0002-9149(03)00322-9
51. Canturk Z, Cetinarslan B, Tarkun I, Canturk NZ, Ozden M, Duman C. Hemostatic system as a risk factor for cardiovascular disease in women with subclinical hypothyroidism. Thyroid (2003) 13:971–7. doi: 10.1089/105072503322511382
52. Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab. (2003) 88:3731–7. doi: 10.1210/jc.2003-030039
53. Guang-Da X, Hui-Ling S, Zhi-Song C, Lin-Shuang Z. Alteration of plasma concentrations of OPG before and after levothyroxine replacement therapy in hypothyroid patients. J Endocrinol Invest. (2005) 28:965–72.
54. Nagasaki T, Inaba M, Yamada S, Kumeda Y, Hiura Y, Nishizawa Y. Changes in brachial-ankle pulse wave velocity in subclinical hypothyroidism during normalization of thyroid function. Biomed Pharmacother. (2007) 61:482–7. doi: 10.1016/j.biopha.2007.04.004
55. Oflaz H, Kurt R, Sen F, Onur I, Cimen AO, Elitok A, et al. Coronary flow reserve after L-thyroxine therapy in Hashimoto's thyroiditis patients with subclinical and overt hypothyroidism. Endocrine (2007) 32:264–70. doi: 10.1007/s12020-008-9037-2
56. Unal O, Erturk E, Ozkan H, Kiyici S, Guclu M, Ersoy C, et al. Effect of levothyroxine treatment on QT dispersion in patients with subclinical hypothyroidism. Endocr Pract. (2007) 13:711–5. doi: 10.4158/EP.13.7.711
57. Peleg RK, Efrati S, Benbassat C, Fygenzo M, Golik A. The effect of levothyroxine on arterial stiffness and lipid profile in patients with subclinical hypothyroidism. Thyroid (2008) 18:825–30. doi: 10.1089/thy.2007.0359
58. Kebapcilar L, Comlekci A, Tuncel P, Solak A, Secil M, Gencel O, et al. Effect of levothyroxine replacement therapy on paraoxonase-1 and carotid intima-media thickness in subclinical hypothyroidism. Med Sci Monit. (2010) 16:CR41–7.
59. Kowalska I, Borawski J, Nikolajuk A, Budlewski T, Otziomek E, Gorska M, et al. Insulin sensitivity, plasma adiponectin and sICAM-1 concentrations in patients with subclinical hypothyroidism: response to levothyroxine therapy. Endocrine (2011) 40:95–101. doi: 10.1007/s12020-011-9446-5
60. Traub-Weidinger T, Graf S, Beheshti M, Ofluoglu S, Zettinig G, Khorsand A, et al. Coronary vasoreactivity in subjects with thyroid autoimmunity and subclinical hypothyroidism before and after supplementation with thyroxine. Thyroid (2012) 22:245–51. doi: 10.1089/thy.2011.0183
61. Anagnostis P, Efstathiadou ZA, Slavakis A, Selalmatzidou D, Poulasouchidou M, Katergari S, et al. The effect of L-thyroxine substitution on lipid profile, glucose homeostasis, inflammation and coagulation in patients with subclinical hypothyroidism. Int J Clin Pract. (2014) 68:857–63. doi: 10.1111/ijcp.12394
62. Yazici D, Ozben B, Toprak A, Yavuz D, Aydin H, Tarcin O, et al. Effects of restoration of the euthyroid state on epicardial adipose tissue and carotid intima media thickness in subclinical hypothyroid patients. Endocrine (2015) 48:909–15. doi: 10.1007/s12020-014-0372-1
63. Piantanida E, Gallo D, Veronesi G, Pariani N, Masiello E, Premoli P, et al. Masked hypertension in newly diagnosed hypothyroidism: a pilot study. J Endocrinol Invest. (2016) 39:1131–8. doi: 10.1007/s40618-016-0488-7
64. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet (2015) 386:801–12. doi: 10.1016/S0140-6736(14)61468-9
65. Ruggeri RM, Trimarchi F, Biondi B. Management of endocrine disease: L-thyroxine replacement therapy in the frail elderly: a challenge in clinical practice. Eur J Endocrinol. (2017) 177:R199–217. doi: 10.1530/EJE-17-0321
66. Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. (2013) 98:2256–66. doi: 10.1210/jc.2012-3818
67. Hennessey JV, Espaillat R. Reversible morbidity markers in subclinical hypothyroidism. Postgrad Med. (2015) 127:78–91. doi: 10.1080/00325481.2015.998158
68. Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. (2007) 3:CD003419. doi: 10.1002/14651858.CD003419.pub2
69. Aziz M, Kandimalla Y, Machavarapu A, Saxena A, Das S, Younus A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. (2017) 24:643–59. doi: 10.5551/jat.39917
70. Zhao T, Chen B, Zhou Y, Wang X, Zhang Y, Wang H, et al. Effect of levothyroxine on the progression of carotid intima-media thickness in subclinical hypothyroidism patients: a meta-analysis. BMJ Open (2017) 7:e016053. doi: 10.1136/bmjopen-2017-016053
71. Jiang F, Liu A, Lai Y, Yu X, Li C, Han C, et al. Change in serum TSH levels within the reference range was associated with variation of future blood pressure: a 5-year follow-up study. J Hum Hypertens. (2017) 31:244–7. doi: 10.1038/jhh.2016.59.
72. Asvold BO, Bjøro T, Vatten LJ. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur J Endocrinol. (2013) 169:73–82. doi: 10.1530/EJE-13-0087.
73. Volzke H, Robinson DM, Spielhagen T, Nauck M, Obst A, Ewert R, et al. Are serum thyrotropin levels within the reference range associated with endothelial function? Eur Heart J. (2009) 30:217–24. doi: 10.1093/eurheartj/ehn508
74. Tsuda A, Inaba M, Ichii M, Ochi A, Ohno Y, Nakatani S, et al. Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur J Endocrinol. (2013) 169:45–50. doi: 10.1530/EJE-13-0026
75. Lambrinoudaki I, Armeni E, Rizos D, Georgiopoulos G, Kazani M, Alexandrou A, et al. High normal thyroid-stimulating hormone is associated with arterial stiffness in healthy postmenopausal women. J Hypertens. (2012) 30:592–9. doi: 10.1097/HJH.0b013e32834f5076
76. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation (2018) 137:109–18. doi: 10.1161/CIRCULATIONAHA.117.032582
77. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. (2018) 71:2199–269. doi: 10.1016/j.jacc.2017.11.005
78. Franklyn JA, Boelaert K. Subclinical thyroid disease: where is the evidence? Lancet Diabetes Endocrinol. (2013) 1:172–3. doi: 10.1016/S2213-8587(13)70030-5
79. Waring AC, Arnold AM, Newman AB, Bùzková P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. (2012) 97:3944–50. doi: 10.1210/jc.2012-2481
80. Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet (2009) 373:1009–15. doi: 10.1016/S0140-6736(09)60212-9
81. Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet (2005) 366:2026–33. doi: 10.1016/S0140-6736(05)67814-2
82. Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA (2011) 305:913–22. doi: 10.1001/jama.2011.250
83. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA (2015) 313:603–15. doi: 10.1001/jama.2014.18574
84. Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab. (2000) 85:2993–3001. doi: 10.1210/jcem.85.9.6841
85. Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum Reprod Update (2013) 19:251–8. doi: 10.1093/humupd/dms052
86. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. (2015) 175:745–54. doi: 10.1001/jamainternmed.2015.0237
87. Blood Pressure Lowering Treatment Trialists' Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet (2014) 384:591–8. doi: 10.1016/S0140-6736(14)61212-5
Keywords: subclinical hypothyroidism, blood pressure, levothyroxine treatment, meta-analysis, systematic review
Citation: He W, Li S, Zhang J, Zhang J, Mu K and Li X (2018) Effect of Levothyroxine on Blood Pressure in Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-Analysis. Front. Endocrinol. 9:454. doi: 10.3389/fendo.2018.00454
Received: 11 May 2018; Accepted: 24 July 2018;
Published: 14 August 2018.
Edited by:
Bernadette Biondi, Università degli Studi di Napoli Federico II, ItalyReviewed by:
Cheng Han, Albert Einstein College of Medicine, United StatesRoberto Vita, Università degli Studi di Messina, Italy
Copyright © 2018 He, Li, Zhang, Zhang, Mu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-ming Li, shfushan001@126.com
 Weiwei He
Weiwei He Sheli Li1
Sheli Li1