Association Between Left Ventricular Geometry and Renal Outcomes in Patients With Chronic Kidney Disease: Findings From Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease Study
- 1Department of Internal Medicine, Chonnam National University Medical School and Chonnam National University Hospital, Gwangju, South Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, South Korea
- 3Department of Prevention and Management, School of Medicine, Inha University, Incheon, South Korea
- 4Division of Nephrology, Department of Internal Medicine, Gachon University of Gil Medical Center, Incheon, South Korea
- 5Department of Internal Medicine, School of Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University Seoul, Seoul, South Korea
Background: The impact of left ventricular (LV) geometry on the renal outcomes in patients with chronic kidney disease (CKD) has not been established yet. We aimed to investigate the association of LV geometry with renal outcomes and all-cause mortality in patients with pre-dialysis CKD.
Methods: A total of 2,144 subjects from the Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) were categorized by LV geometry, which was defined by LV mass index and relative wall thickness [normal geometry, concentric remodeling, eccentric hypertrophy (eLVH), and concentric hypertrophy (cLVH)]. Study outcomes were composite renal events [decline of kidney function (the first occurrence of > 50% decline of eGFR or doubling of serum creatinine from the baseline) and onset of ESRD (initiation of dialysis or kidney transplantation) during follow-up periods)] and all-cause mortality.
Results: Cox regression analysis revealed that eLVH [adjusted hazard ratio (HR) 1.498, 95% confidence interval (CI) 1.197–1.873] and cLVH (adjusted HR 1.289, 95% CI 1.011–1.643) were associated with increased risk of composite renal events, whereas concentric remodeling (adjusted HR 1.881, 95% CI 1.135–3.118) and cLVH (adjusted HR 2.216, 95% CI 1.341–3.664) were associated with increased risk of all-cause mortality. Sensitivity analyses confirmed that concentric remodeling (adjusted HR 1.993, 95% CI 1.197–3.368) and eLVH (adjusted HR 1.588, 95% CI 1.261–2.001) are independently associated with all-cause mortality and composite renal events, respectively.
Conclusion: In conclusion, we report that LV geometry is significantly associated with adverse renal outcomes and all-cause mortality in patients with pre-dialysis CKD. Echocardiographic determination of LV geometry may help the early identification for the patients with high risk of CKD progression.
Introduction
Structural remodeling of heart predicts adverse cardiovascular (CV) outcomes, which is best illustrated by left ventricular hypertrophy (LVH). LVH is a surrogate of CV events, such as heart failure, ischemic heart disease, and stroke (1), as the reversal of LVH by antihypertensive treatment has been associated with the reduction of the risk for subsequent CV events (2–4). Relative wall thickness (RWT), together with left ventricular (LV) mass, is commonly obtained echocardiographic parameter to describe the shape of heart (5), and also provides additional and independent prognostic impacts (6, 7). Accordingly, LV geometry has been classified into four categories by left ventricular mass index (LVMI) and RWT (8): normal geometry, concentric remodeling, eccentric hypertrophy (eLVH), and concentric hypertrophy (cLVH).
Kidney targets heart, as cardiac remodeling begins from the early stages of chronic kidney disease (CKD) (9, 10). The multifactorial mechanism of LVH involves increased afterload (11, 12), intravascular volume expansion (13), and anemia (14), all of which are closely related to CKD. The presence of arteriovenous fistula further accelerates cardiac remodeling in patients with end-stage renal disease (ESRD) (15). The prevalence of LVH in this population is estimated to be up to about 30% in individuals with an estimated glomerular filtration rate (eGFR) > 30 ml/min/1.73 m2, and it increases to 60–75% prior to initiation of dialysis (16). A previous study reported that the prevalence of normal LV geometry was less than 10% among the subjects with eGFR < 30 ml/min/1.73 m2 (9). Inversely, LVH increased the risk of ESRD among hypertensive patients (17), and is significantly associated with adverse CV events and all-cause mortality especially in patients on dialysis (18–20). Indeed, a study reported that LVH was associated with almost twofold increase of the risk for sudden cardiac death in patients on hemodialysis (20). Yet, the prognostic significance of LVH in patients with pre-dialysis CKD has been less clearly documented (21). Moreover, the independent association of RWT on renal outcomes in patients with pre-dialysis CKD has been never reported.
Focusing on the impact of cardiac remodeling on CKD progression, we here investigated the association of LV geometry with renal outcomes and all-cause mortality in patients with pre-dialysis CKD. In addition, the association of LVH or RWT with the outcomes was separately analyzed to address their independent role as a prognostic predictor. Finally, we conducted a series of subgroup analyses to examine whether the association of LV geometry with the outcomes might be modified by clinical contexts.
Materials and Methods
Study Designs
The Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) is a nationwide prospective cohort study involving 9 tertiary-care general hospitals in Korea (NCT01630486)1 (22). Korean patients with CKD from stage 1 to pre-dialysis stage 5, who voluntarily provided informed consent were enrolled from 2011 through 2016. The study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the institutional review boards of participating centers, including at Seoul National University Hospital, Yonsei University Severance Hospital, Kangbuk Samsung Medical Center, Seoul St. Mary’s Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital, and Busan Paik Hospital. All participants had been under close observation, and participants who experienced study outcomes were reported by each participating center. Among 2,238 who were longitudinally followed up, excluding those lacking the baseline determination of LV geometry, a total of 2,144 subjects were finally included for the analyses (Figure 1A). The study observation period ended on March 31, 2020. The median follow-up duration was 5.999 years.
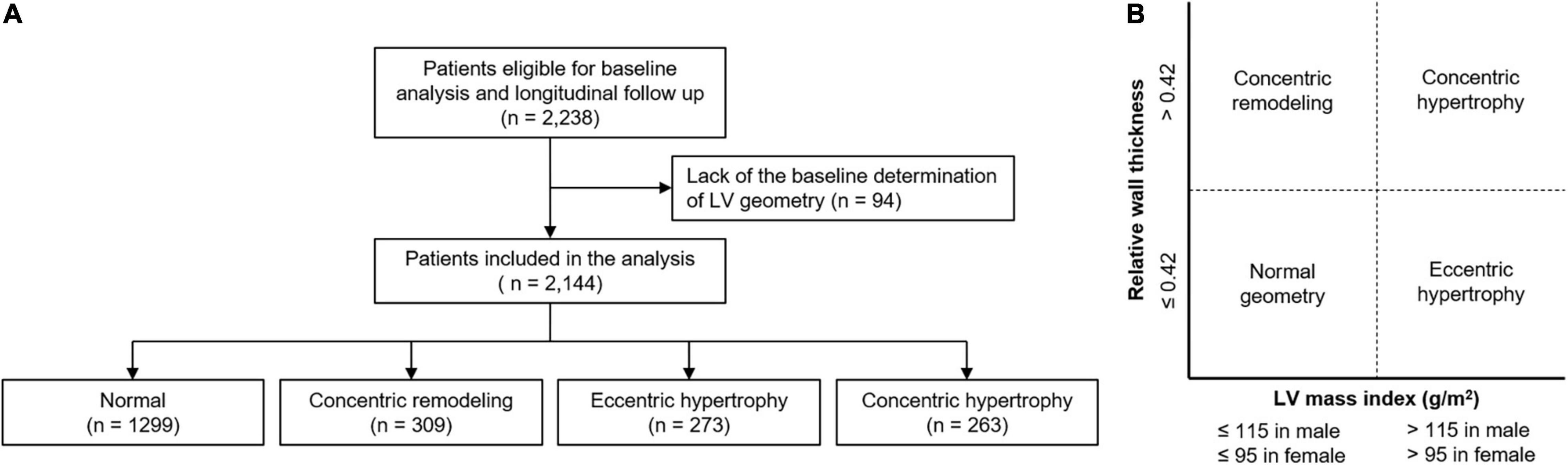
Figure 1. Flow diagram of the study participants. Flow diagram of the study participants (A) and definition of abnormal LV geometry (B) are depicted. LV, left ventricle.
Data Collection From Participants
Demographic information was collected from all eligible participants, including age, gender, comorbid conditions, primary renal disease, smoking history, and medication history (angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers (ACEi/ARBs), diuretics, number of anti-HTN drugs, statins). Trained staff members measured the height, weight, and waist circumference (WC) of study participants. Body mass index (BMI) was calculated as weight divided by the height squared. Systolic and diastolic blood pressures (SBP and DBP) were measured by an electronic sphygmomanometer after seated rest for 5 min. Venous samples were collected following overnight fasting, to determine hemoglobin, albumin, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol (HDL-C), triglyceride (TG), fasting glucose, high-sensitivity C-reactive protein (hs-CRP), 25-hydroxyvitamin D [25(OH) vitamin D] and creatinine levels at the baseline. eGFR was calculated by CKD Epidemiology Collaboration equation (23). Urine albumin-to-creatinine ratio (ACR) was measured in random, preferably second-voided, spot urine samples.
Echocardiographic Data Collection
Complete two-dimensional M-mode and Doppler studies were performed via standard approaches by cardiologists at the participating hospitals who were blinded to the clinical data. M-mode examination was performed according to American Society of Echocardiography guidelines (8). The recorded echocardiographic data were the ratio of the early transmitral blood flow velocity to early diastolic velocity of the mitral annulus, left ventricular ejection fraction (LVEF), left atrial diameter, regional wall motion abnormality, valve calcification, LV posterior wall thickness, inter-ventricular septum thickness, LV end diastolic diameter, and LV end systolic diameter. LV mass was determined using the Devereux formula (8). LVMI was calculated by normalizing LV mass to height2 (g/m2). LVH was defined as LVMI > 115 g/m2 in men and > 95 g/m2 in women (7, 8). RWT was calculated as (2 × posterior wall thickness)/LV end diastolic diameter. RWT > 0.42 was defined as increased (7, 8, 10). LV geometry was determined by LVH and RWT: normal (no LVH and normal RWT), concentric remodeling (no LVH and increased RWT), eLVH (LVH and normal RWT), and cLVH (LVH and increased RWT) (Figure 1B).
Study Outcomes
The primary outcomes of interest were composite renal events and all-cause mortality. Composite renal events included decline of kidney function (the first occurrence of > 50% decline of eGFR or doubling of serum creatinine from the baseline) and onset of ESRD (initiation of dialysis or kidney transplantation) during follow-up periods. The secondary outcomes were decline of kidney function and onset of ESRD.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median [interquartile range]. Categorical variables were expressed as number of participants and percentage. To compare the baseline characteristics according to LV geometry, one-way analysis of variance and χ2-test were used for continuous and categorical variates, respectively. The participants with any missing data were excluded for further analyses. To evaluate the association between LV diastolic dysfunction and study outcomes, Cox proportional hazard regression models were analyzed. Patients lost to follow-up were censored at the date of the last visit. Models were constructed after adjusting for the following variables. Model 1 represents crude hazard ratios (HRs). Model 2 was adjusted for age, sex, Charlson comorbidity index, primary renal disease, smoking history, medication (ACEi/ARBs, diuretics, number of antihypertensive drugs, statins), BMI, and SBP. Model 3 was further adjusted for hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hs-CRP, eGFR and spot urine ACR. Model 4 was additionally adjusted for LVEF at the baseline. The results of Cox proportional hazard models were presented as HRs and 95% confidence intervals (CIs). Restricted cubic splines were used to visualize the association between LVMI or RWT as a continuous variable and HRs for study outcomes. To validate our findings, we performed sensitivity analyses. First, we excluded the subjects with LEVE < 50% to demonstrate that the association between LV geometry and study outcomes is independent of LV systolic dysfunction. Second, we excluded the subjects with increased RWT to figure out an independent role of LVH in the study outcomes. Third, we conversely excluded the subjects with LVH to unveil an independent role of RWT in the study outcomes. To examine whether the association of LV geometry with the outcomes might be modified by clinical contexts, we conducted pre-specified subgroup analyses. Subgroups were defined by age (< 60 vs. (vs.) ≥ 60 years), sex (male vs. female), BMI (< 23 vs. ≥ 23 kg/m2), eGFR (< 45 vs. ≥ 45 mL/min/1.73 m2), and spot urine ACR (< 300 vs. ≥ 300 mg/gCr). Two-sided P-values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY) and R (version 4.1.1; R project for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
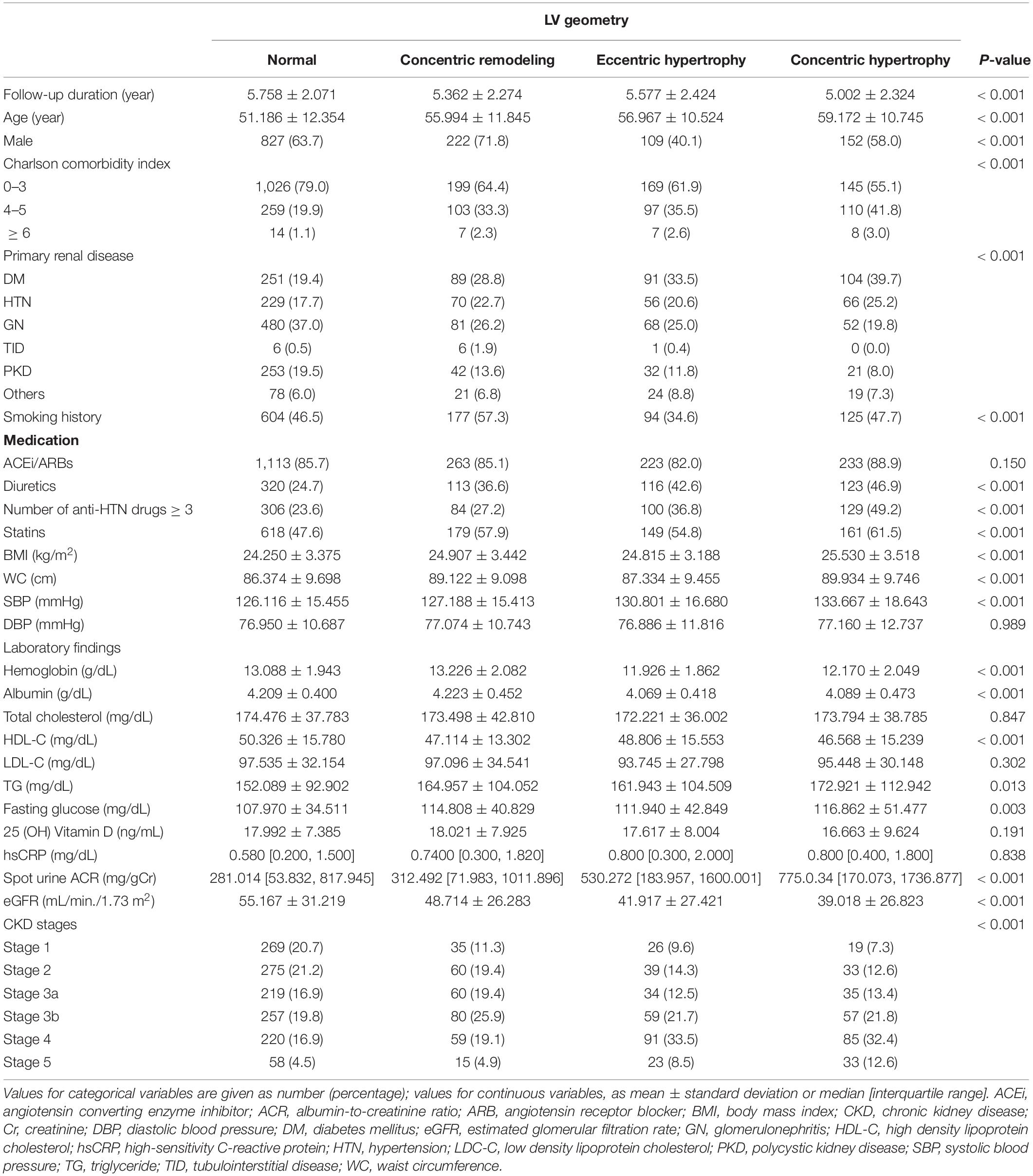
To describe the baseline characteristics, study participants were categorized by LV geometry (Table 1). Whereas the follow-up duration was significantly shortened in the subjects with cLVH, the mean age was highest in the subjects with cLVH. The proportion of male sex was relatively lower in the subjects with eLVH and cLVH, than in those with normal geometry and concentric remodeling. The proportion of Charlson comorbidity index ≥ 4 was highest in the subjects with cLVH. The proportion of DM and HTN as primary renal diseases was relatively higher in the subjects with eLVH and cLVH, than in those with normal geometry and concentric remodeling. The proportion of smoking history was lower in the subjects with eLVH and cLVH. The proportions of diuretic use, medication of no less than three anti-HTN drugs, statin medication were higher in the subjects with eLVH and cLVH. BMI, WC, SBP were highest in the subjects with cLVH. While hemoglobin level was lowest in the subjects with eLVH, albumin, HDL-C, 25(OH) vitamin D levels were lowest in the subjects with cLVH. Fasting glucose and TG levels were highest in the subjects with cLVH. Spot urine ACR and eGFR were highest and lowest in the subjects with cLVH, respectively. Accordingly, the proportion of relatively advance CKD was higher in the subjects with cLVH. The echocardiographic findings of study participants by LV geometry is summarized in Supplementary Table 1. Besides the structural measurement directly related to LV geometry, other parameters demonstrated significant differences by LV geometry. The ratio of the early transmitral blood flow velocity to early diastolic velocity of the mitral annulus and left atrial diameter were significantly higher in the subjects with eLVH and cLVH. The proportions of regional wall motion abnormality and valve calcification were also significantly higher in the subjects with eLVH and cLVH. LVEF was lowest in the subjects with eLVH.
Association of Left Ventricular Geometry With Adverse Renal Outcome and All-Cause Mortality in Chronic Kidney Disease
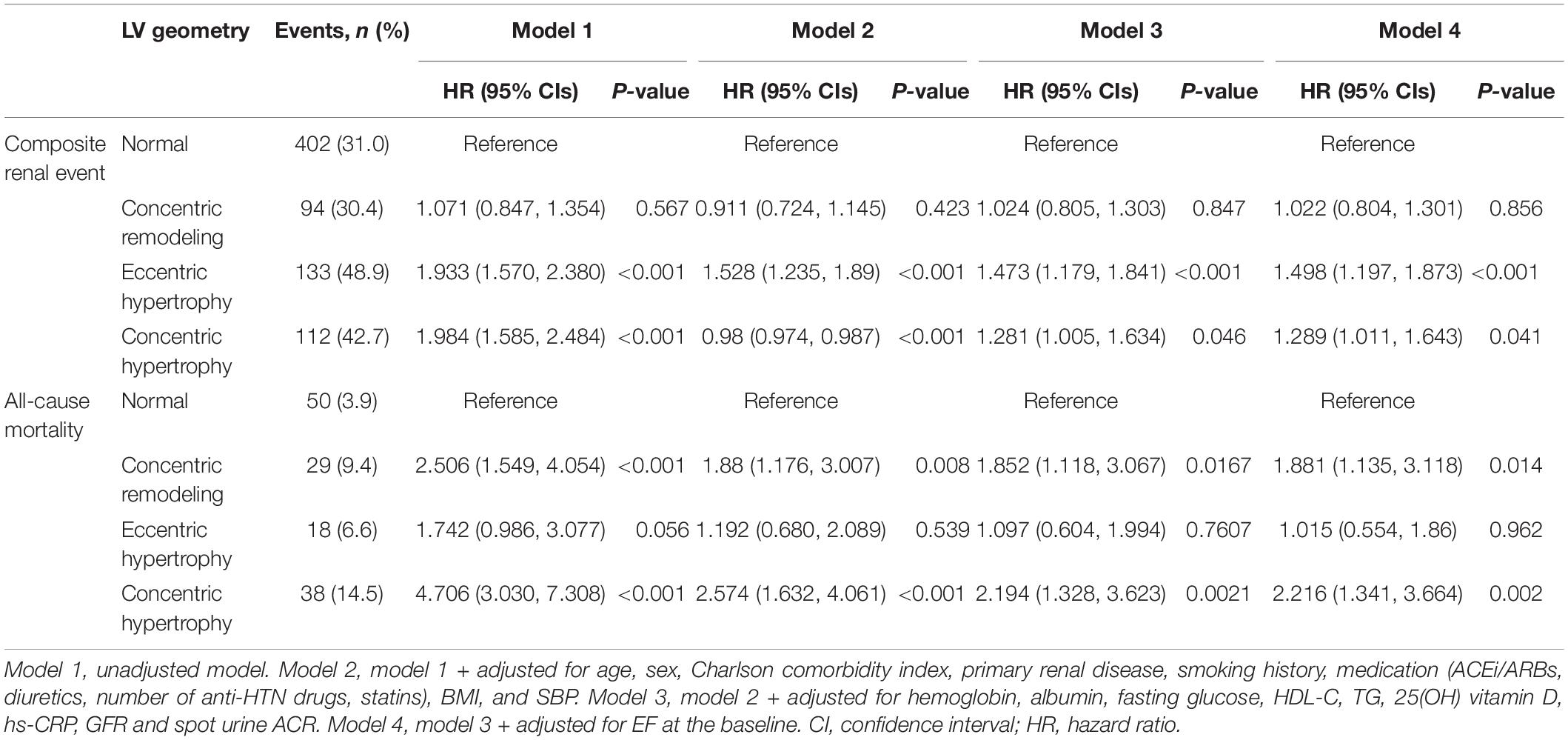
To define the association of LV geometry with study outcomes, Cox regression models were analyzed (Table 2). Compared to normal geometry, eLVH (adjusted HR 1.498, 95% CI 1.197–1.873) and cLVH (adjusted HR 1.289, 95% CI 1.011–1.643) were associated with increased risk of composite renal events, whereas concentric remodeling was not significantly associated with the risk of composite renal events. In contrast, concentric remodeling (adjusted HR 1.881, 95% CI 1.135–3.118) and cLVH (adjusted HR 2.216, 95% CI 1.341–3.664) were associated with increased risk of all-cause mortality, whereas eLVH was not significantly associated with the risk of all-cause mortality. In the analysis of secondary outcomes, only eLVH was significantly associated with both decline of kidney function (adjusted HR 1.535, 95% CI 1.171–2.013) and onset of ESRD (adjusted HR 1.390, 95% CI 1.072–1.802) (Supplementary Table 2). As the results suggested that LVH and concentric geometry (i.e., increased RWT, including concentric remodeling and cLVH) were associated with adverse renal outcome and all-cause mortality, respectively, we separately analyzed the association of LVH or RWT with the outcomes to address their independent role as a prognostic predictor. LVH was associated with increased risk of composite renal events (adjusted HR 1.391, 95% CI 1.161–1.667), but was not significantly associated with all-cause mortality (Supplementary Table 3). Restricted cubic splines visualized stringent linear correlation of LVMI with the risk of composite renal events, but not with all-cause mortality (Figure 2). On the other hand, concentric geometry was associated with all-cause mortality (adjusted HR 2.038, 95% CI 1.391–2.984), but was not significantly associated with the risk of composite renal events (Table 3). Restricted cubic splines demonstrated stringent linear correlation of RWT with all-cause mortality, while the correlation between RWT and the risk of composite renal events was evident only among the subjects with very high RWT (Figure 3).
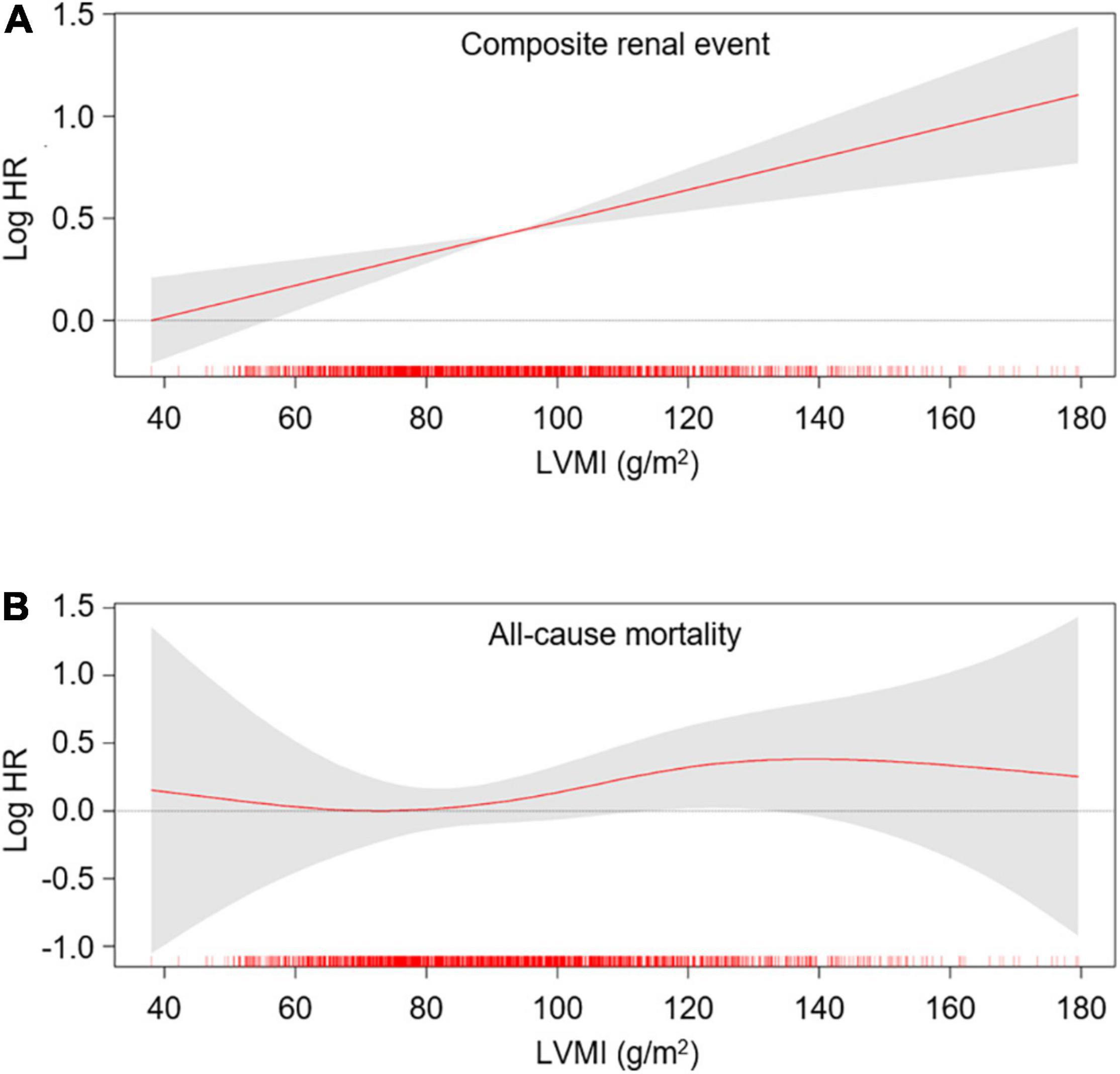
Figure 2. Restricted cubic spline of LVMI on primary outcomes. Adjusted HRs of LVMI as a continuous variable for composite renal events (A) and all-cause mortality (B) is depicted. The model was adjusted for age, sex, Charlson comorbidity index, primary renal disease, smoking history, medication (ACEi/ARBs, diuretics, number of anti-HTN drugs, statins), BMI, SBP, hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hs-CRP, GFR, spot urine ACR and EF at the baseline. HR, hazard ratio; LVMI, left ventricular mass index.
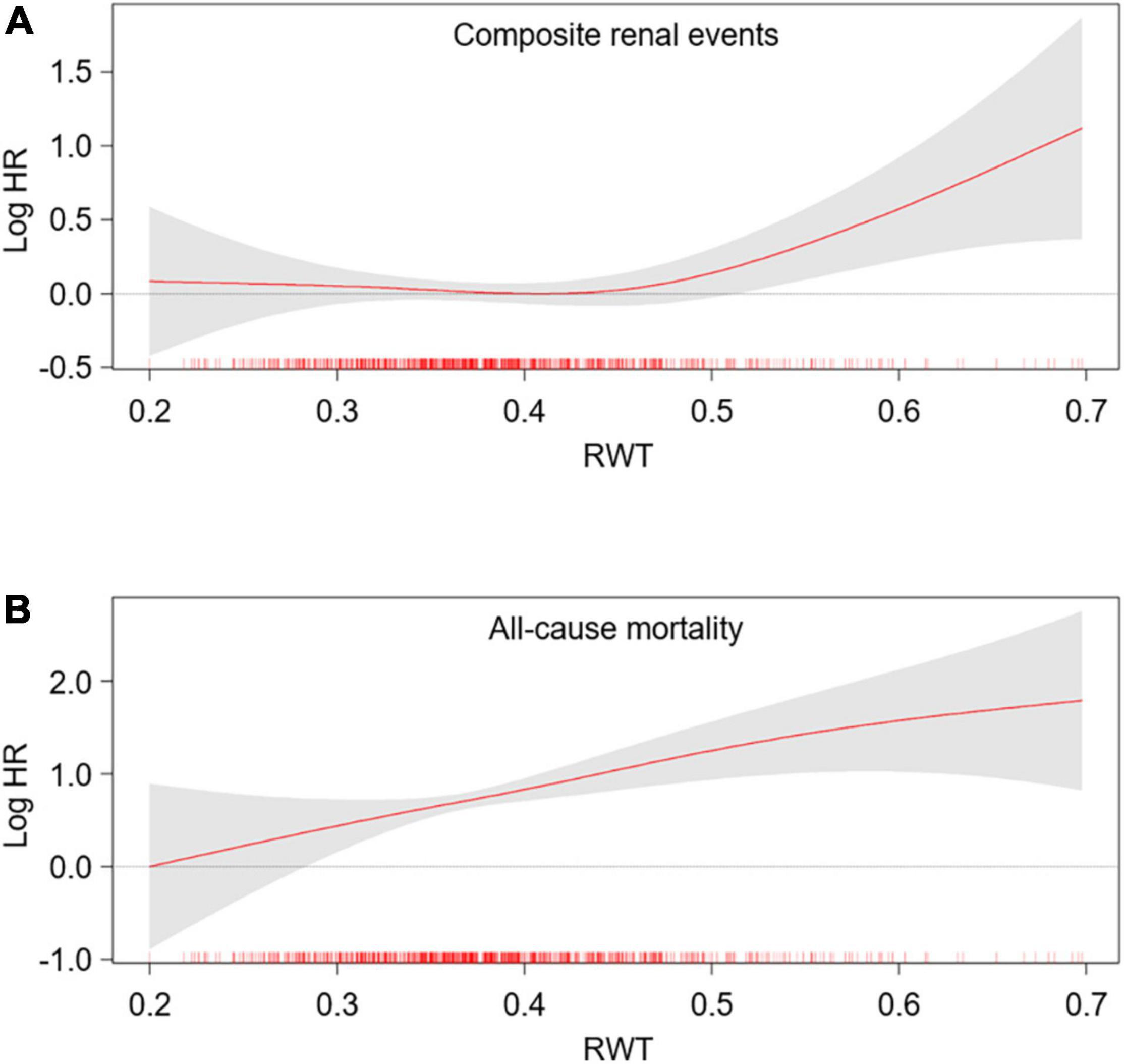
Figure 3. Restricted cubic spline of RWT on primary outcomes. Adjusted HRs of RWT as a continuous variable for composite renal events (A) and all-cause mortality (B) is depicted. The model was adjusted for age, sex, Charlson comorbidity index, primary renal disease, smoking history, medication (ACEi/ARBs, diuretics, number of anti-HTN drugs, statins), BMI, SBP, hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hs-CRP, GFR, spot urine ACR and EF at the baseline. HR, hazard ratio; RWT, relative wall thickness.
Sensitivity Analysis
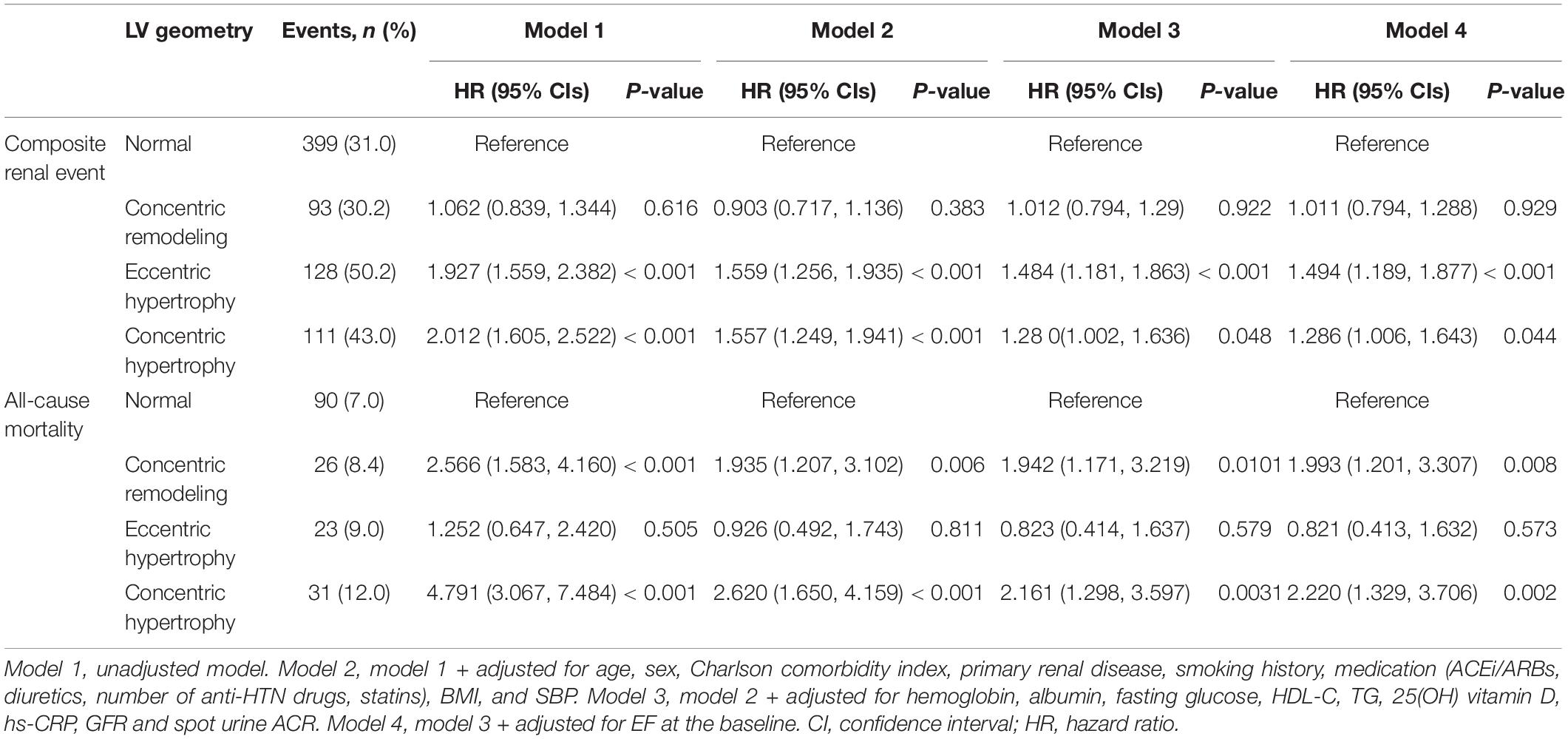
After excluding the subjects with LEVE < 50%, both eLVH (adjusted HR 1.494, 95% CI 1.189–1.877) and cLVH (adjusted HR 1.286, 95% CI 1.006–1.643) were associated with increased risk of composite renal events, while concentric remodeling (adjusted HR 1.993, 95% CI 1.201–3.307) and cLVH (adjusted HR 2.220, 95% CI 1.329–3.706) were associated with all-cause mortality, demonstrating the association between LV geometry and study outcomes that is independent of LV systolic dysfunction (Table 4). After excluding the subjects with increased RWT, increased LVMI (i.e., eLVH) was robustly associated with increased risk of composite renal events (adjusted HR 1.588, 95% CI 1.261–2.001), but was not significant associated with all-cause mortality (Supplementary Table 4). After excluding the subjects with LVH, increased RWT (i.e., concentric remodeling) was still significantly associated with all-cause mortality (adjusted HR 1.993, 95% CI 1.197–3.368), but was not significant associated with increased risk of composite renal events (Supplementary Table 5).
Subgroup Analysis
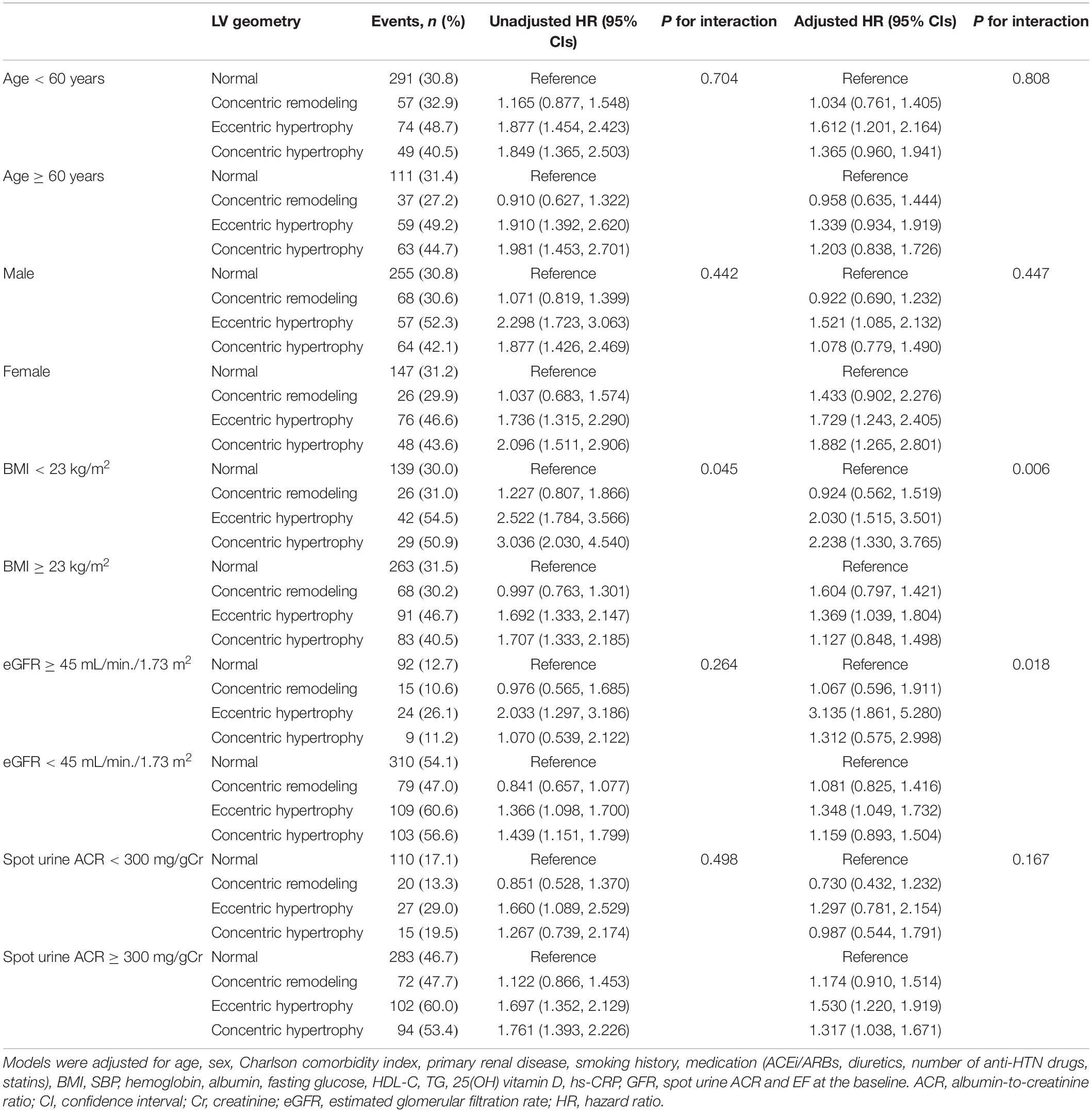
Subgroup analyses revealed that the association between LV geometry, especially eLVH, and the risk of composite renal events was more prominent in the subjects with BMI < 23 kg/m2 (P for interaction = 0.006) and eGFR ≥ 45 mL/min/1.73 m2 (P for interaction = 0.018) than in the subjects with BMI ≥ 23 kg/m2 and eGFR < 45 mL/min/1.73 m2 (Table 5), while age, sex, and spot urine ACR did not modify the association between LV geometry and the risk of composite renal events. None of age, sex, BMI, eGFR, and spot urine ACR modified the association between LV geometry and all-cause mortality (Supplementary Table 6).
Discussion
In the present study, we unveiled that LV geometry is associated with adverse renal outcome and all-cause mortality in patients with pre-dialysis CKD. More specifically, concentric geometry and LVH were independently associated with all-cause mortality and adverse renal outcome, respectively. The association between LV geometry and adverse renal outcome was more prominent in the subjects with BMI < 23 kg/m2 and eGFR ≥ 45 mL/min/1.73 m2 than in the subjects with BMI ≥ 23 kg/m2 and eGFR < 45 mL/min/1.73 m2.
Only a few studies reported the association of LV geometry and outcomes in patients with pre-dialysis CKD (21, 24, 25). Although cLVH has been consistently associated with adverse CV (21, 24) or renal outcomes (21, 25), the prognostic significance of eLVH and concentric remodeling was less clear yet. A previous study reported that cLVH, but not eLVH, was associated with dialysis-free survival (25), where the analysis of concentric remodeling was even omitted. Another study reported both cLVH and eLVH are associated with adverse CV and renal outcomes in patients with CKD (21), though independent role of concentric remodeling or RWT was not proven. In contrast, to our best knowledge, the current study is the first report to demonstrate that eLVH is strongly associated with adverse renal outcome, and that concentric geometry or concentric remodeling alone is also independently associated with all-cause mortality, presenting more specific association of LV geometry patterns with study outcomes. This could be primarily attributed to substantially larger number of subjects included in the analysis and relatively longer duration of follow-up periods, rather than analytic bias. Therefore, the findings in the current study expand our understanding of the prognostic value of LV geometry in patients with pre-dialysis CKD.
In the present study, cLVH was associated with both adverse renal outcomes and all-cause mortality, which could be partially attributed to impaired microvascular function associated with high prevalence of DM among the subjects with cLVH (26, 27). On the other hand, one of the intriguing findings in the present study is a prominent contribution of eLVH to adverse renal outcomes, as eLVH, but not cLVH, was significantly associated with secondary outcomes (Supplementary Table 2), even though the subjects with cLVH were mostly associated with unfavorable baseline characteristics. It seems that the role of LV geometry pattern largely depends on the clinical contexts, as previous studied reported a significant association of eLVH with CV outcomes or all-cause mortality that is distinguished from cLVH (28, 29). Among the patients who underwent transcatheter aortic valve replacement, cLVH, especially mild cLVH, was independently associated with a decreased risk for mortality compared to normal geometry, while eLVH was associated with a 33% increased risk for mortality compared to cLVH (29). Similarly, eLVH was less responsive to ACEi treatment and was associated with a greater risk of adverse CV events compared with cLVH in patients on chronic hemodialysis (28). Moreover, complete correction of anemia was associated with reduced CV-event free survival specifically in patients with eLVH (24). Provided that cardiac remodeling begins as an adaptive process (16), it is speculated that a pathophysiology involved in pre-dialysis CKD may confer a distinct prognostic value on eLVH, although further studies are warranted to reveal the precise mechanism.
Currently, although losartan, an ARB, has shown a superiority to atenolol, a beta blocker, in the reversal of LVH, both agents were not different in the efficacy to reverse RWT (30). Most studies (31, 32) so far on pharmacologic interventions to reverse LV geometry are focusing on blood pressure lowering effect, not the class effect of antihypertensive agents. It should be further investigated, therefore, to determine the optimal regimen for antihypertensive drugs that leads to normalization of LV geometry.
It is of note that the association between LV geometry and the risk of composite renal events was more prominent in the subjects with eGFR ≥ 45 mL/min/1.73 m2 than in those with eGFR < 45 mL/min/1.73 m2. The early identification for the patients with high risk of CKD progression by echocardiographic determination of LV geometry may confer additional therapeutic benefits, as LVH is a potentially modifiable CV risk factor (2–4). Several trials have shown that intensive hemodialysis alleviates LVH among the patient with ESRD (33–35), while observational studies suggested that intensive hemodialysis may confer CV benefits (36). Accordingly, despite the lack of direct evidence that the regression of LVH improves the clinical outcomes in pre-dialysis CKD, it is expected that echocardiographic examination at early stages of CKD may help guide the intensive medical treatment to prevent or reverse LVH.
Limitations
Some limitations are to be acknowledged in the present study. First, we are not able to confirm the casual relation between LV geometry and the study outcomes, because of the observational study design. Similarly, further studies are required to determine whether normalization of LV geometry improves renal outcome in patients with pre-dialysis CKD. Second, echocardiographic measurements were obtained from individual participating centers, and were not centralized with significant inter-observer variabilities in the measured parameters (Supplementary Table 7), whereas the multicenter nature of the current study is a strength. Third, as we did not measure right-side parameters, we are not able to assess the prognostic impact of pulmonary hypertension on adverse outcomes (37, 38). Fourth, as only ethnic Koreans were enrolled this cohort study, an extrapolation of the data to other populations requires precaution.
Conclusion
In conclusion, we report that LV geometry is associated with adverse renal outcome and all-cause mortality in patients with pre-dialysis CKD. More specifically, concentric geometry and LVH are independently associated with all-cause mortality and adverse renal outcome, respectively. These results suggest that echocardiographic determination of LV geometry may help the early identification for the patients with high risk of CKD progression.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the institutional review boards of participating centers, including at Seoul National University Hospital, Yonsei University Severance Hospital, Kangbuk Samsung Medical Center, Seoul St. Mary’s Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital, and Pusan Paik Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SS designed, helped in the data analysis and manuscript writing. SS, TO, and HC contributed to the conception of the study. SS and CK performed the data analyses and wrote the manuscript. EB, K-HO, JL, JJ, and K-BL collected the data. SM and SK helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (grant nos. 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, and 2019E320100) and the National Research Foundation of Korea (NRF) funded by the Korea Government (MSIT) (NRF-2019R1A2C2086276).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
KNOW-CKD: Study Group Clinical Centers. Seoul National University, Curie Ahn, Kook-Hwan Oh, Dong Wan Chae, Ho Jun Chin, Hayne Cho Park, Seungmi Lee, Hyun Hwa Jang and Hyun Jin Cho. Yonsei University, Severance Hospital, Kyu Hun Choi, Seung Hyeok Han, Tae Hyun Yoo and Mi Hyun Yu. Kangbuk Samsung Medical Center, Kyubeck Lee and Sooyeon Jin. The Catholic University of Korea, Seoul St. Mary’s Hospital, Yong-Soo Kim and Sol Ji Kim. Gachon University, Gil Hospital, Wookyung Chung, Youkyoung Jang and Ji Hye Park. Eulji University, Eulji General Hospital. Young-Hwan Hwang, Su-Ah Sung and Jeong Ok So. Chonnam University, Soo Wan Kim and Ji Seon Lee. Inje University, Pusan Paik Hospital, Yeong Hoon Kim, Sun Woo Kang and Yun Jin Kim. Epidemiology and Biostatistics. Department of Preventive Medicine, Seoul National University College of Medicine, Byung-Joo Park, Sue Kyung Park and Juyeon Lee. Coordinating Center. Medical Research Collaborating Center, Seoul National University Hospital and Seoul National University College of Medicine, Joongyub Lee, Dayeon Nam, Soohee Kang and Heejung Ahn. Central Laboratory, Donghee Seo, Lab Genomics, Korea and Dae Yeon Cho, Lab Genomics, Korea. Biobank. Korea Biobank, Korea Centers for Disease Control and Prevention, Osong, Korea. Korea Center for Disease Control and Prevention, Dukhyoung Lee, Hyekyung Park (Project Officer), Eunkyeong Jung (Project Officer), Suyeon Jeong, Eunmi Ahn and Sil-Hea Sung.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.848692/full#supplementary-material
Footnotes
References
1. Levy D, Salomon M, D’agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. (1994) 90:1786–93. doi: 10.1161/01.cir.90.4.1786
2. Muiesan ML, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E. Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens. (1995) 13:1091–5. doi: 10.1097/00004872-199510000-00003
3. Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. (2003) 115:41–6. doi: 10.1016/s0002-9343(03)00158-x
4. Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. (2004) 43:731–8. doi: 10.1161/01.HYP.0000121223.44837.de
5. Ganau A, Devereux RB, Roman MJ, De Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. (1992) 19:1550–8. doi: 10.1016/0735-1097(92)90617-v
6. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. (1991) 114:345–52. doi: 10.7326/0003-4819-114-5-345
7. Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan in acute myocardial iNfarcTion) echocardiographic study. JACC Cardiovasc Imaging. (2008) 1:582–91. doi: 10.1016/j.jcmg.2008.05.012
8. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. (2005) 18:1440–63. doi: 10.1016/j.echo.2005.10.005
9. Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. (2012) 23:1725–34. doi: 10.1681/ASN.2012020145
10. Park SK, Jung JY, Kang JG, Chung PW, Ryoo JH. Mildly decreased renal function and its relation to left ventricular geometry change. Circ J. (2019) 83:2236–41. doi: 10.1253/circj.CJ-19-0353
11. Mominadam S, Ozkahya M, Kayikcioglu M, Toz H, Asci G, Duman S, et al. Interdialytic blood pressure obtained by ambulatory blood pressure measurement and left ventricular structure in hypertensive hemodialysis patients. Hemodial Int. (2008) 12:322–7. doi: 10.1111/j.1542-4758.2008.00275.x
12. Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. (2009) 75:771–3. doi: 10.1038/ki.2009.35
13. Martin LC, Franco RJ, Gavras I, Matsubara BB, Garcia S, Caramori JT, et al. Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am J Hypertens. (2004) 17:1163–9. doi: 10.1016/j.amjhyper.2004.07.017
14. Ibernon M, Moreso F, Ruiz-Majoral A, Sarrias X, Sarrias M, Grinyó JM, et al. Contribution of anemia and hypertension to left ventricular hypertrophy during the initial 2 years after renal transplantation. Transplant Proc. (2011) 43:2199–204. doi: 10.1016/j.transproceed.2011.05.006
15. MacRae JM, Levin A, Belenkie I. The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: a cause for concern? Semin Dial. (2006) 19:349–52. doi: 10.1111/j.1525-139X.2006.00185.x
16. Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. (2015) 5:254–66. doi: 10.1159/000435838
17. Ravera M, Noberasco G, Signori A, Re M, Filippi A, Cannavo R, et al. Left-ventricular hypertrophy and renal outcome in hypertensive patients in primary-care. Am J Hypertens. (2013) 26:700–7. doi: 10.1093/ajh/hps100
18. London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. (2001) 12:2759–67. doi: 10.1681/ASN.V12122759
19. Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. (2004) 65:1492–8. doi: 10.1111/j.1523-1755.2004.00530.x
20. Krane V, Winkler K, Drechsler C, Lilienthal J, März W, Wanner C. Effect of atorvastatin on inflammation and outcome in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. (2008) 74:1461–7. doi: 10.1038/ki.2008.484
21. Paoletti E, De Nicola L, Gabbai FB, Chiodini P, Ravera M, Pieracci L, et al. Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin J Am Soc Nephrol. (2016) 11:271–9. doi: 10.2215/CJN.06980615
22. Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. (2014) 15:80. doi: 10.1186/1471-2369-15-80
23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12.
24. Eckardt KU, Scherhag A, Macdougall IC, Tsakiris D, Clyne N, Locatelli F, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol. (2009) 20:2651–60. doi: 10.1681/ASN.2009060631
25. Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. (2011) 6:2750–8. doi: 10.2215/CJN.04660511
26. Nakanishi K, Fukuda S, Shimada K, Miyazaki C, Otsuka K, Kawarabayashi T, et al. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. (2013) 112:928–32. doi: 10.1016/j.amjcard.2013.05.025
27. Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation. (2020) 141:21–33. doi: 10.1161/CIRCULATIONAHA.119.043916
28. Paoletti E, Cassottana P, Bellino D, Specchia C, Messa P, Cannella G. Left ventricular geometry and adverse cardiovascular events in chronic hemodialysis patients on prolonged therapy with ACE inhibitors. Am J Kidney Dis. (2002) 40:728–36. doi: 10.1053/ajkd.2002.35680
29. Rozenbaum Z, Finkelstein A, Zhitomirsky S, Topilsky Y, Halkin A, Banai S, et al. Impact of preprocedural left ventricle hypertrophy and geometrical patterns on mortality following TAVR. Am Heart J. (2020) 220:184–91. doi: 10.1016/j.ahj.2019.11.013
30. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) trial. Circulation. (2004) 110:1456–62. doi: 10.1161/01.cir.0000141573.44737.5a
31. Wachtell K, Dahlof B, Rokkedal J, Papademetriou V, Nieminen MS, Smith G, et al. Change of left ventricular geometric pattern after 1 year of antihypertensive treatment: the losartan intervention for endpoint reduction in hypertension (LIFE) study. Am Heart J. (2002) 144:1057–64. doi: 10.1067/mhj.2002.126113
32. Bertoluci C, Foppa M, Santos ABS, Branchi TV, Fuchs SC, Fuchs FD. Echocardiographic left ventricular reverse remodeling after 18 months of antihypertensive treatment in stage I hypertension. Results from the prever-treatment study. Am J Hypertens. (2018) 31:321–8. doi: 10.1093/ajh/hpx171
33. Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. (2002) 61:2235–9. doi: 10.1046/j.1523-1755.2002.00362.x
34. Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. (2005) 16:2778–88. doi: 10.1681/ASN.2005040392
35. Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. Jama. (2007) 298:1291–9. doi: 10.1001/jama.298.11.1291
36. McCullough PA, Chan CT, Weinhandl ED, Burkart JM, Bakris GL. Intensive hemodialysis. Left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis. (2016) 68:S5–14. doi: 10.1053/j.ajkd.2016.05.025
37. Navaneethan SD, Wehbe E, Heresi GA, Gaur V, Minai OA, Arrigain S, et al. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol. (2014) 9:855–63. doi: 10.2215/CJN.10191013
Keywords: chronic kidney disease, left ventricular geometry, left ventricular hypertrophy, relative wall thickness, renal outcome, all-cause mortality
Citation: Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Oh K-H, Lee J, Jung JY, Lee K-B, Ma SK and Kim SW (2022) Association Between Left Ventricular Geometry and Renal Outcomes in Patients With Chronic Kidney Disease: Findings From Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease Study. Front. Cardiovasc. Med. 9:848692. doi: 10.3389/fcvm.2022.848692
Received: 04 January 2022; Accepted: 30 March 2022;
Published: 18 April 2022.
Edited by:
Alberto Palazzuoli, University of Siena, ItalyReviewed by:
Kenichiro Otsuka, Osaka City University Graduate School of Medicine, JapanDaniele Masarone, Azienda Ospedaliera dei Colli, Italy
Copyright © 2022 Suh, Oh, Choi, Kim, Bae, Oh, Lee, Jung, Lee, Ma and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo Wan Kim, skimw@chonnam.ac.kr
 Sang Heon Suh
Sang Heon Suh Tae Ryom Oh1
Tae Ryom Oh1  Hong Sang Choi
Hong Sang Choi Chang Seong Kim
Chang Seong Kim Eun Hui Bae
Eun Hui Bae Ji Yong Jung
Ji Yong Jung