Association of Carotid Intima Media Thickness With Metabolic Syndrome Among Low-Income Middle-Aged and Elderly Chinese: A Population-Based Cross-Sectional Study
- 1Department of Cardiology, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
- 3Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, China
- 4Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Tianjin Neurological Institute, Ministry of Education and Tianjin City, Tianjin, China
- 5Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
- 6Center of Clinical Epidemiology & Evidence-Based Medicine, Tianjin Jizhou People's Hospital, Tianjin, China
Background: We aimed to evaluate the relationship between metabolic syndrome (MetS) including its components and carotid intima media thickness (CIMT) in a low-income Chinese population aged ≥45 years.
Methods: The participants underwent a general health screening and B-mode carotid ultrasonography that measured CIMT. The diagnosis of MetS and its components was based on the modified International Diabetes Federation Criteria for the Asian Population. The univariate and multivariable linear regression analyses were used to evaluate the relationship between MetS and CIMT.
Results: A total of 3,583 participants (mean age, 60 years) was included in the analyses (41.4% male and 58.6% female); more than 50% of the participants were diagnosed with MetS. In the multivariable linear regression analysis, the mean CIMT was 0.009 mm greater in the participants with MetS than in those without MetS (β = 0.009; 95% CI, 0.003–0.014; P < 0.05). Moreover, a high number of MetS components was associated with greater CIMT values; for example, CIMT increased by 0.007 and 0.015 mm for the individuals diagnosed with 3–4 and 5 MetS components, respectively. Among the MetS components, elevated blood pressure (β = 0.022; 95% CI, 0.015–0.029; P < 0.001) and abdominal obesity (β = 0.008; 95% CI, 0.001–0.015; P < 0.001) were positively correlated with CIMT. However, the increased triglyceride levels were negatively associated with CIMT (β = −0.008; 95% CI: −0.015 to −0.002; P = 0.012), especially among the elderly population.
Conclusions: The risk of carotid atherosclerosis increased in the presence of multiple MetS components in a low-income, middle-aged, and elderly population. Accordingly, more detailed management strategies are essential for the early prevention and intervention of atherosclerosis in this low-income population with MetS, in China.
Introduction
The tremendous burden of cardiovascular diseases (CVDs) (such as stroke) has become a major global public health problem. The CVDs and strokes are the leading causes of death not only in the developed world but also in the underdeveloped countries (1). These diseases are responsible for an estimated 12.1 million deaths in 1990, reaching 18.6 million in 2019, and the number of cardiovascular-related deaths is increasing rapidly (1). A major cause of CVDs and stroke is atherosclerosis (2). Accordingly, the early recognition and intervention of the progress of atherosclerosis are of great significance for reducing the burden of CVDs and stroke in the general population. The ultrasound measurements of the carotid intima-media thickness (CIMT) provide a valuable index of early atherosclerosis, independent of the traditional risk factors, and the CIMT is considered useful for predicting the cardiovascular events and ischemic strokes (3).
Metabolic syndrome (MetS) plays an essential role in the atherosclerotic process as it comprises a cluster of interrelated cardiometabolic risk factors that can increase the risk of atherogenic damage disorder (4). The individuals with MetS were more likely to have new plaques (5, 6), increased CIMT (5, 7, 8), and restenosis after carotid endarterectomy or stenting compared with the non-affected individuals (9). In addition, when larger numbers of MetS components were present, the risk of carotid atherosclerosis also increased. However, most of the previous studies were conducted in developed countries or in higher income, urban areas in China (7, 10–12); conversely, low-income populations have not been well-studied, particularly among individuals aged ≥ 45 years.
Therefore, we performed a population-based, cross-sectional study to evaluate the relationship between MetS and CIMT in a low-income population, aged ≥ 45 years, in China.
Methods
Participants and Study Design
This population-based, cross-sectional survey was conducted in the rural areas of Tianjin, China, between April 2014 and January 2015. The study was based on a subset of the population involved in the previously described Tianjin Brain Study (13). Briefly, the Tianjin Brain Study is a population-based stroke surveillance project that includes 14,251 participants from the 18 administrative villages in rural Tianjin, China. The low-income farmers account for approximately 95% of the population, with a per capita disposable income of <1,600 USD, in 2014 (14). The stroke burden in those rural areas was particularly severe among middle-aged adults, with an upward trend in the incidence of first-ever strokes observed between 1992 and 2012 (15).
The current study recruited all the local permanent residents aged ≥ 45 years using the clustering sampling method. Those individuals with histories of coronary heart disease or stroke were excluded because they might have received multiple interventions, such as lifestyle changes or surgical treatments. A total of 5,380 permanent residents were eligible for inclusion in 2014.
All the participants in this study underwent a health screening that included physical examinations, biochemical tests, and carotid ultrasonography.
Information Collection
The collected sociodemographic and clinical characteristics data included participant name, sex, age, educational level, lifestyle, history of diabetes and hypertension, history of hyperlipidemia, and current use of antihypertensive, antidiabetic, or lipid-lowering agents. The well-trained epidemiology researchers collected these data using face-to-face interviews that were based on a pre-designed standardized questionnaire. The participants were categorized into three age groups: 45–54, 55–64, and ≥ 65 years. Furthermore, the participants were categorized into three groups based on their education level, according to the length of formal education: illiterate (no formal education), primary school (1–6 years), or middle school and above (>6 years). Lifestyle information included smoking status (never, former, or current smoker) and drinking status (never, former, or current drinker).
Physical Examination and Biochemical Tests
In addition, each participant received a detailed physical examination, such as weight, height, waist circumference (WC), and blood pressure (BP). Body mass index (BMI) was calculated as weight (kg) divided by the square of height in meters (m2); the participants were categorized as underweight (BMI <18.50 kg/m2), normal (18.50 kg/m2 ≤ BMI <24.00 kg/m2), overweight (24.00 kg/m2 ≤ BMI <28.00 kg/m2), or obese (BMI ≥ 28.00 kg/m2), according to the Chinese-specific criteria (16). BP was measured with the participant in the sitting position and was calculated using the average of two measurements, at 1-min intervals, after 5 min of rest. To minimize white-coat hypertension, the researchers performed the BP measurements in a quiet room using the standard method described by the American Hypertension Association.
The fasting venous blood samples were collected and sent to the Ji County People's Hospital, within 2 h of collection, for routine examinations; the fasting plasma glucose (FPG) measurements and lipid profiling were performed to determine the levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
Ultrasonography Measurements
All ultrasound examinations and measurements were performed by one trained technician, blinded to the details of the participants. The participants were examined while they were in a supine position using B-mode ultrasonography (Terason 3000, Burlington, MA, USA) with a 5–12 MHz linear array transducer. The bilateral, extracranial carotid artery trees (including the common carotid artery, carotid sinus, and internal and external carotid arteries) were screened for CIMT. CIMT of the near and far walls of the common carotid artery was measured on the left and the right sides. The maximum, minimum, and average CIMT values for each side were obtained. The average CIMT was calculated using the means for the sum of the CIMTs on both the left and right sides. The images were obtained and digitally stored according to a standard protocol (17). All the scans were recorded on Vascular Research Tools 6 (MIA, LLC, IA, USA) for subsequent off-line analysis. The inter-observer and intra-observer correlation coefficients ranged from 0.88 to 0.94 and 0.80 to 0.95 for both sides of the CIMT measurement, respectively.
Criteria for Metabolic Syndrome
Metabolic syndrome was defined as the presence of three or more of the following according to the modified International Diabetes Federation criteria for the Asian Population, published in 2009(18): (1) abdominal obesity: WC ≥ 90 cm for men and ≥ 80 cm for women; (2) increased TG levels: TG ≥ 1.70 mmol/L (150 mg/dl) or using medications to treat the increased levels of TG; (3) reduced HDL-C: HDL-C <1.0 mmol/L (40 mg/dl) for men and <1.3 mmol/L (50 mg/dL) for women, or on drug treatment for low HDL-C; (4) elevated BP: systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg, and/or use of antihypertensive medication; (5) elevated FPG: FPG ≥ 5.6 mmol/L (100 mg/dl) and/or taking medication(s) for diabetes.
The five criteria described above were designated as the MetS components. The participants were divided into three groups according to the number of MetS components present upon enrollment: 0–2, 3–4, and 5 components.
Statistical Analysis
The continuous variables (age, BMI, education, WC, systolic BP, diastolic BP, FPG, TC, TG, HDL-C, LDL-C, and CIMT) are presented as means (SDs) or medians (interquartile ranges [IQRs]). The comparisons between the groups were performed using Student' s t-test, the Mann–Whitney test, or the Kruskal–Wallis test if appropriate. The categorical variables (sex, age group, education level, BMI group, smoking status, alcohol consumption, and presence of MetS and the number of its components) are presented as the numbers and frequencies. Fisher' s exact test was used for comparisons among the categorical variables. The univariate and multivariable linear regression analyses were applied to determine the association among the CIMT and MetS, MetS components, and the number of MetS components; the results are presented as β-coefficients and 95% CIs. The independent variables comprised variables for which P < 0.05 in the univariate linear regression analysis. The statistical significance was defined as a two-tailed value of P < 0.05. All the statistical analyses were performed using the SPSS software (version 22.0; SPSS, Chicago, IL, USA).
Results
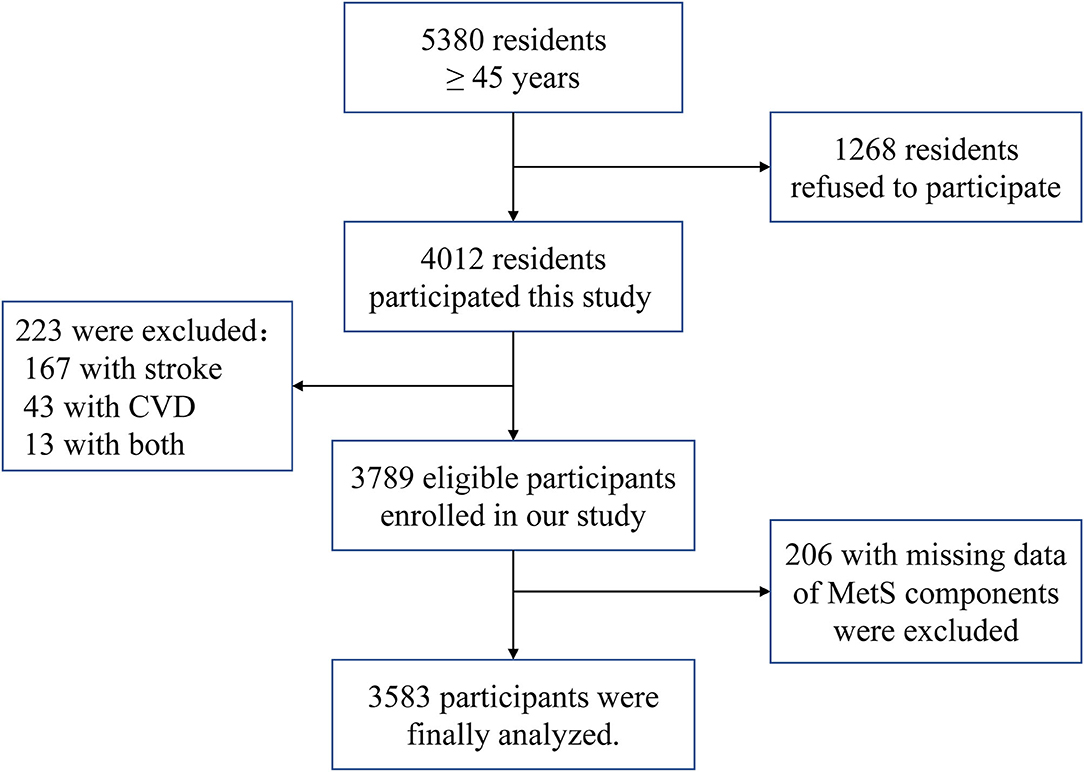
Among the 5,380 qualified residents, we initially excluded 1,268 residents who did not provide informed consent and an additional 223 participants with histories of coronary heart disease or stroke. Additionally, we excluded 206 participants with missing data regarding the MetS components. Therefore, the final sample consisted of 3,583 participants (Figure 1).
Baseline Characteristics
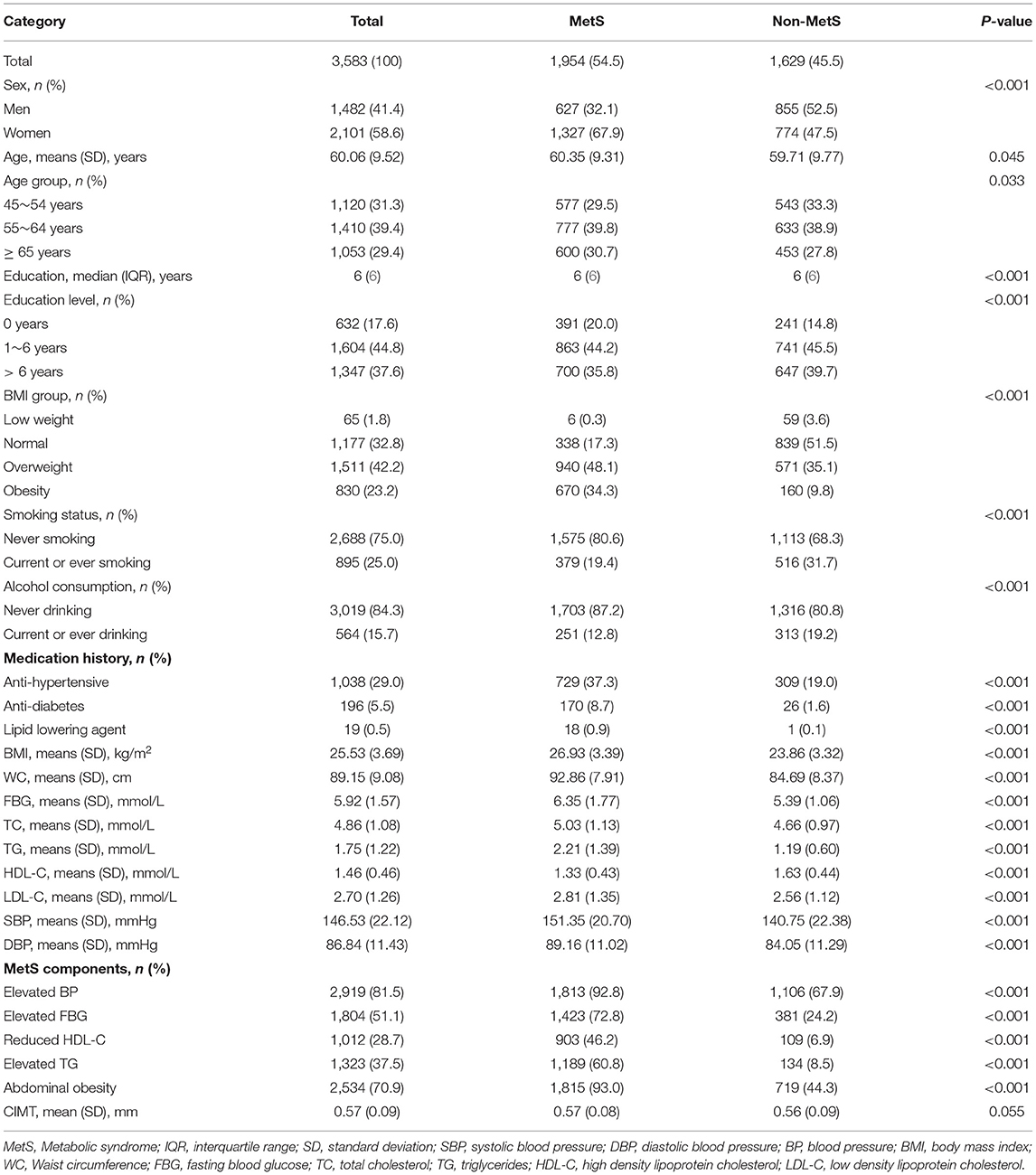
Of the 3,583 participants (41.4% men; mean age 60 years) included in the analysis, 54.5% were diagnosed with MetS. More than 60% of the participants had received a primary level of education or less, with a median education level of 6 years in both the MetS and non-MetS groups. A quarter of the participants were current or ever smokers, and 15.7% consumed alcohol. Moreover, 42.2% of participants were overweight, and nearly 25% were obese. Furthermore, 29.0% of the participants were taking at least one antihypertensive drug, whereas only 0.5% were taking a lipid-lowering agent. The average values for BMI (25.53 kg/m2), WC (89.15 cm), FBG (5.92 mmol/L), TC (4.86 mmol/L), TG (1.75 mmol/L), HDL-C (1.46 mmol/L), LDL-C (2.70 mmol/L), systolic BP (146.53 mmHg), diastolic BP (86.84 mmHg), and CIMT (0.57 mm) were determined (Table 1).
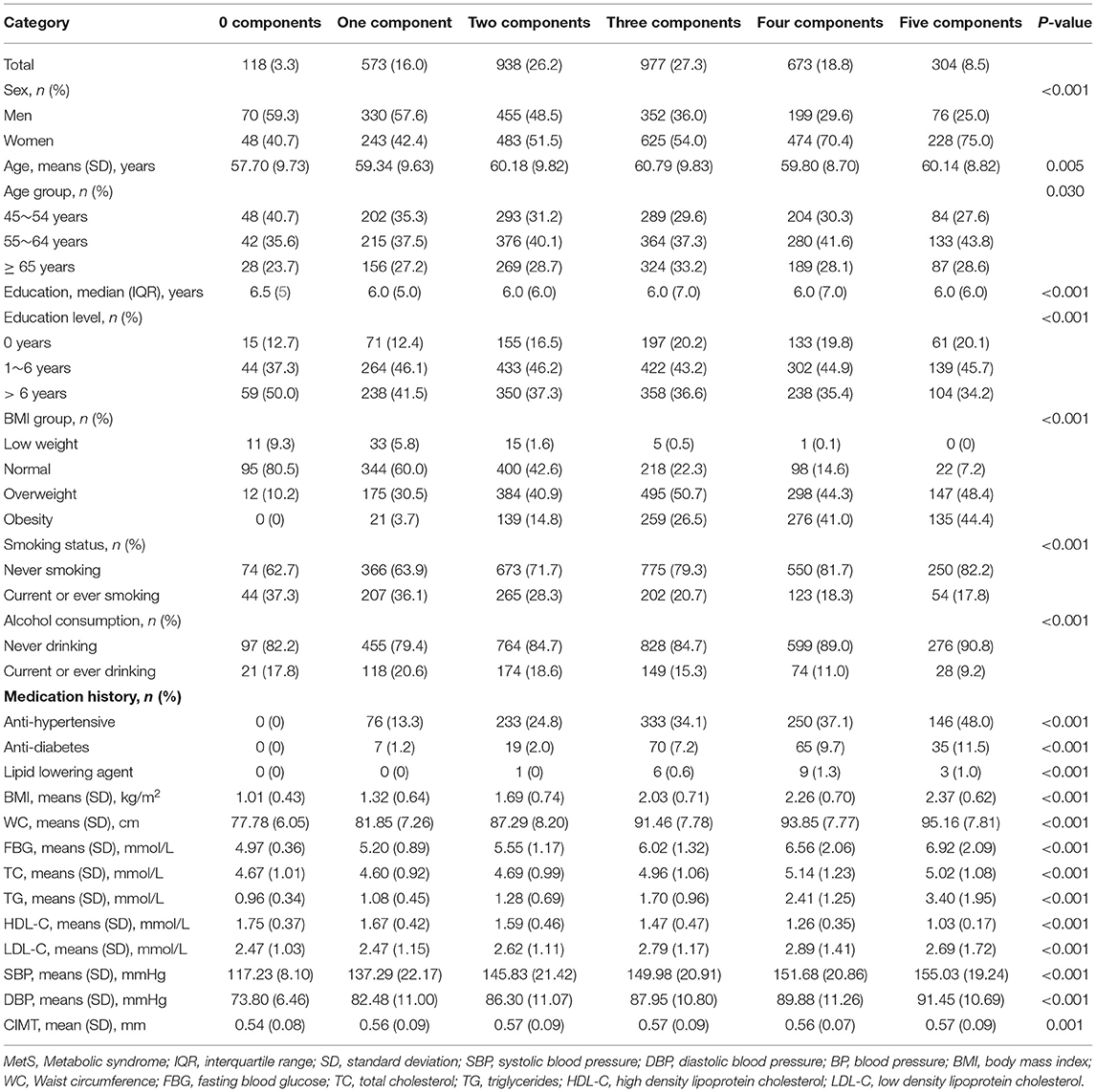
The total prevalence of MetS, in this study, was 54.5%. Among the MetS components, the most prevalent components were increased BP (81.5%) and abdominal obesity (70.9%); reduced HDL-C (28.7%) was the least prevalent of the five MetS components. The individuals with 0, 1, 2, 3, 4, and 5 MetS components accounted for 3.3, 16.0, 26.2, 27.3, 18.8, and 8.5%, respectively, of the participants (Table 2).
Relationship Between MetS and CIMT in the Univariable Linear Regression Analysis
Table 3 shows the results of the univariable analysis to estimate the relationship between MetS and CIMT. The participants with MetS exhibited a positive association between MetS and CIMT. Significant differences were found among the four MetS components: increased BP, increased FBG, reduced HDL-C, and increased TG (all P < 0.05). The TC and LDL-C levels were positively associated with increased CIMT (β = 0.003 and 0.007, respectively; both P < 0.05). Moreover, sex, age, education, smoking, and alcohol consumption were associated with the CIMT values.
Relationship Between MetS and CIMT in Multivariable Linear Regression Analysis
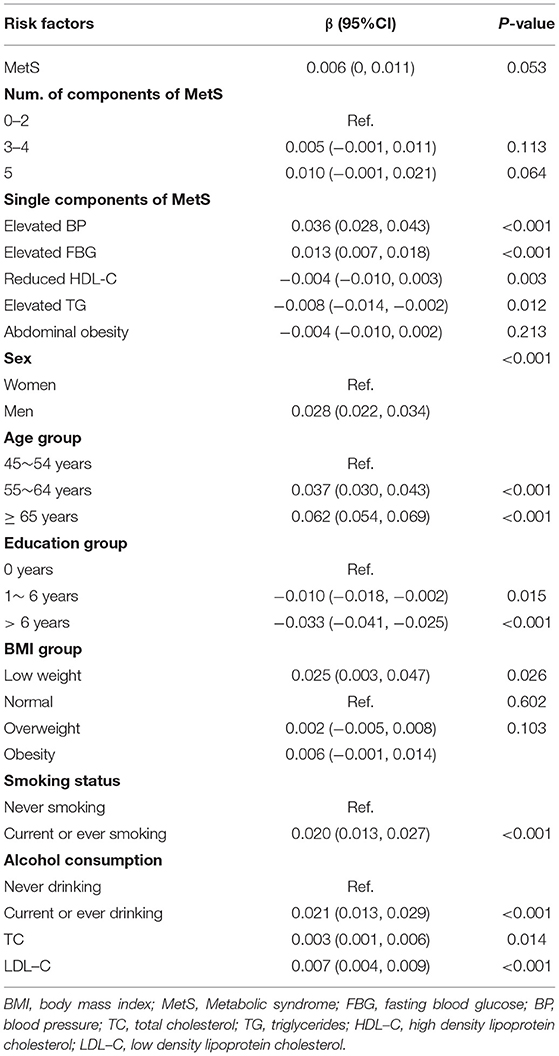
As shown in Table 4, after adjusting for age, sex, education, smoking, alcohol consumption, TC, and LDL-C, the mean participant CIMT increased 0.009 mm in the participants with MetS compared with those without MetS (β = 0.009; 95% CI, 0.003–0.014; P = 0.003). Relative to the number of MetS components present in the participants, the CIMT values increased by 0.007 and 0.015 mm in the participants with 3–4 and 5 components, respectively, compared with the participants with <3 components. Among the MetS components, the increased BP and abdominal obesity were positively correlated with CIMT. CIMT increased by 0.022 mm (β = 0.022; 95% CI, 0.015–0.029; P < 0.001) in the participants with increased BP and 0.008 mm (β = 0.008; 95% CI, 0.001–0.015; P < 0.001) in those with abdominal obesity. However, the increased triglycerides levels were negatively associated with CIMT value, with the mean CIMT decreasing by 0.008 mm (β = −0.008; 95% CI, −0.015 to −0.002; P = 0.012) compared with those with normal triglyceride level. The subgroup analysis revealed that the association between raised TG and CIMT was more pronounced among the participants ≥ 60 years (β = −0.014; 95% CI, −0.024 to −0.004), compared with those with <60 years (β = −0.002; 95% CI, −0.010 to 0.006) at baseline (P interaction = 0.002) (Supplementary Table).
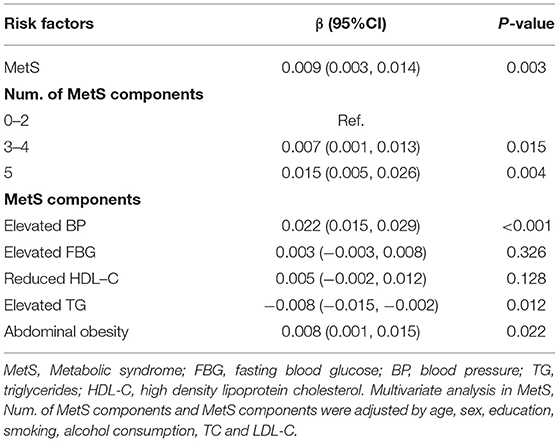
Table 4. Multivariable liner regression analysis of relationship between MetS, MetS components, and number of MetS components and CIMT.
Discussion
In the present study, we demonstrated a high prevalence (54.5%) of MetS among the low-income residents (aged ≥ 45 years) in an area of China. Further, the individuals with MetS were more likely to have the increased CIMT values than the non-affected individuals. Among the MetS components, increased BP and abdominal obesity were positively correlated with CIMT, whereas increased TG levels were negatively associated with CIMT. Moreover, CIMT increased significantly when more components of MetS were present. Our results provide new information and evidence for the relationship between the MetS, including its components, and CIMT in a low-income, rural, middle-aged, and elderly population with a high incidence of strokes.
The previous cross-sectional studies demonstrated that the individuals with MetS have increased CIMTs (7, 19). In addition, an increasing trend in the CIMT values was observed with an increasing number of MetS components in both genders (20, 21). Moreover, a cross-sectional study from northern China found that the average CIMT was higher in the participants with MetS (β = 0.020; 95% CI, 0.014–0.027; P < 0.001) and that CIMT increased by 0.022, 0.042, and 0.035 mm for those with 3, 4, and 5 MetS components, respectively (7). Another study, from Hong Kong, reported results consistent with those in the present study, showing that the MetS components were independently related to CIMT and that the risk of increased CIMT was significantly higher when the participants had increased numbers of MetS components, compared with those without MetS components (11). Similar to these previous studies, the current results suggest that the mean CIMT is higher in the participants with MetS than in those without MetS, and that there is a greater risk of increased CIMT when an individual has more MetS components present.
Individual abnormalities in the MetS components are traditional risk factors for CVD and stroke. For example, hypertension is a major risk factor for the development of atherosclerosis (22). Citing more than 13 years of a follow-up study, the Tromsø study, from Norway, reported that MetS, especially the hypertension component, was associated with increased CIMT (6). Additionally, a cross-sectional study of 8,144 apparently healthy Japanese individuals reported that hypertension was the most common MetS component and the greatest contributor to carotid arteriosclerosis (12). The present results are also in accordance with these previous studies, showing that, among all the MetS components, increased levels of BP contributed the greatest risk for increased CIMT. Compared with the participants without MetS, the mean CIMT for individuals with higher BP increased by 0.022 mm; another component, abdominal obesity, was associated with a 0.008-mm increase in CIMT. These data re-emphasize the need for clinicians to pay more attention to the impact of lowering BP for reducing the risk of both MetS and carotid arteriosclerosis.
Obesity involves a chronic inflammatory process characterized by an increase in various proinflammatory biomarkers, which may mediate all the stages of atherogenesis, such as the initiation, acceleration, and progression of atherosclerotic lesions (23). The Brazilian Longitudinal Study of Adult Health reported that, based on multivariate linear analysis, with each 1-cm increase in WC, CIMT increased 0.08 mm (β = 0.08; 95% CI, 0.04–0.11; P < 0.0001) (24). Another study conducted in Shanghai, China showed that WC was an independent risk factor for increased CIMT, with WC ≥ 80, 81–84, and ≥ 85 cm exhibiting odds ratios (ORs) of 1.632, 1.501, and 1.878, respectively (25). Moreover, a meta-analysis found that obesity has a long-term effect on CIMT, with childhood obesity significantly (2.5-fold) increasing the risk of a large CIMT in adulthood (OR = 3.5; 95% CI, 1.1–11.1; P = 0.034) (26). In the present study, WC was measured as an indicator of abdominal obesity, and we found that WC was positively associated with carotid atherosclerosis. The CIMT value in the individuals with abdominal obesity was 0.008-mm greater than in the individuals without abdominal obesity.
A 12-year longitudinal study revealed that abnormal FPG levels were also a risk factor for atherosclerosis (27). The Jackson Heart Study of 4,303 community-dwelling blacks revealed that each 10-mg/dl increase in FPG was associated with higher odds of subclinical CVD, such as left ventricular hypertrophy, coronary artery calcification, increased CIMT (all P < 0.005) after being adjusted for traditional CVD risk factors (28). However, a 10-year follow-up of the Hoorn Study showed no significant correlation between FPG and an increased risk of non-fatal CVD (29). Additionally, some studies have reported that the low HDL-C levels are protective against the high CIMT values (30, 31). However, among the patients with MetS, no association was found between the reduced HDL-C levels and carotid arteriosclerosis, after age stratification (12). In contrast, the present results showed that the increased FPG levels and reduced HDL-C levels had no significant bearing on CIMT. Nonetheless, since a cross-sectional study is based on data collected at a certain time point, the present data may not be able to verify a correlation between the HDL-C or FPG levels and CIMT.
The relationship between the TG levels and carotid atherosclerosis remains controversial. A previous study reported that the high TG levels were a risk factor for atherosclerosis (hazard ratio = 1.003; 95% CI, 1.00–1.006; P = 0.027) (32). However, a Mendelian randomization analysis reported no causal association between the TG levels and CIMT (33). Furthermore, another study of 6,142 Chinese individuals reported that hypertriglyceridemia was associated with a 20% reduction in risk of carotid atherosclerosis, but only in men (10). The present results showed that the TG levels protected against increased CIMT values; the CIMT value in individuals with the increased TG level decreased by 0.008 mm compared with the participants with the normal TG levels. The underlying reason for the negative correlation between elevated TG and CIMT remains unclear. However, the result in our study may be partially explained by the different distribution of TG and CIMT by age. The previous studies have shown that the age-related TG distribution was gradually declined after midlife due to the reduction of lipids absorption, hormonal changes, and decline of health status (10, 34). On the contrary, the CIMT was increasing with age (35, 36). The subgroup analysis in our study showed that the negative association between raised TG and CIMT was more pronounced among the participants ≥ 60 years. With the increasing of age, TG and CIMT show the opposite trend, which leads to higher TGs having the protect effect in CIMT, and the effect is more obvious in elderly patients. However, it still needs more studies to explore the relationship between TG and CIMT in different populations.
Atherosclerosis is the underlying process of the majority of CVDs and CVD-associated mortality. Non-invasive ultrasonographic assessment of CIMT is suitable for evaluating the early burden of atherosclerosis and predicting future CVD risks. Moreover, the burden of stroke among the low-income populations in rural areas remains severe; previous research illustrated that the incidence of stroke in one such area increased by 6.5%, annually, between 1992 and 2012 (15). MetS has been highlighted as a major, global socioeconomic problem owing to the high prevalence of MetS in aging societies and it is being significantly associated with adverse cardiovascular events. Therefore, the relationship between MetS and CIMT may indicate that MetS is associated with the initiation of the atherosclerotic process. This observation is of great significance for the early recognition and prevention of atherosclerosis progression and more detailed management strategies in individuals with MetS.
Limitations
The present study has several limitations. First, as observed in many other cross-sectional studies, the present results cannot validate the causal links between MetS and CIMT. Further cohort studies, with follow-up data, are needed to verify this possibility. Second, this is a population-based study from a single township in a low-income, rural population in Tianjin, China; therefore, this study is not representative of the entire population. Third, the study population included only participants aged ≥ 45 years, meaning that these findings cannot be generalized to broader age groups. However, with the advancing age of many societies and the poor lifestyles in those societies, there will likely be an increase in the age-specific prevalence of MetS among older adults (37). Hence, exploring MetS in individuals aged ≥ 45 years is both necessary and meaningful. Finally, the research failed to capture some potential confounding factors, such as dietary habits, which may affect the relationship between the raised TG level and CIMT. Despite part of the explanation being the different distribution of TG and CIMT by age, the underlying mechanisms remain unclear. Subsequent studies are needed to provide additional explanations.
Conclusion
In conclusion, our study showed that the individuals with MetS, particularly those with greater numbers of MetS components, had a substantial risk of having elevated CIMT values. The increased levels of BP and abdominal obesity had a positive correlation with CIMT. While the increased TG levels were negatively associated with CIMT, especially among the elderly population. These findings suggested that detailed and strict MetS management strategies should be encouraged to identify and intervene in the atherosclerosis process among the low-income populations in China.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tianjin Medical University General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XN, JW, and QinY were involved in the conception and design and data interpretation for this article. QiaY, QL, DG, HW, JL, XZ, and JT were involved in data collection, case diagnosis, and confirmation for this article. QiaY, QL, and DG were involved in manuscript drafting. JW was involved in data analysis for this article. XN, JW, and QinY were involved the critical review of this article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers XY and YG declared a shared affiliation with all of the authors to the handling editor at the time of the review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants of the Tianjin Brain Study and local medical care professionals for their valuable contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.669245/full#supplementary-material
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Zhong S, Li L, Shen X, Li Q, Xu W, Wang X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. (2019) 144:266–78. doi: 10.1016/j.freeradbiomed.2019.03.036
3. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142:621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
4. Aboonabi A, Meyer RR, Singh I. The association between metabolic syndrome components and the development of atherosclerosis. J Hum Hypertens. (2019) 33:844–55. doi: 10.1038/s41371-019-0273-0
5. Cuspidi C, Sala C, Tadic M, Gherbesi E, Grassi G, Mancia G. Association of metabolic syndrome with carotid thickening and plaque in the general population: a meta-analysis. J Clin Hypertens. (2018) 20:4–10. doi: 10.1111/jch.13138
6. Herder M, Arntzen KA, Johnsen SH, Mathiesen EB. The metabolic syndrome and progression of carotid atherosclerosis over 13 years. the Tromsø study. Cardiovasc Diabetol. (2012) 11:77. doi: 10.1186/1475-2840-11-77
7. Zhou PA, Zhang CH, Chen YR, Li D, Song DY, Liu HM, et al. Association between metabolic syndrome and carotid atherosclerosis: a cross-sectional study in Northern China. Biomed Environ Sci. (2019) 32:914–21. doi: 10.3967/bes2019.114
8. Novo S, Peritore A, Trovato RL, Guarneri FP, Di Lisi D, Muratori I, et al. Preclinical atherosclerosis and metabolic syndrome increase cardio- and cerebrovascular events rate: a 20-year follow up. Cardiovasc Diabetol. (2013) 12:155. doi: 10.1186/1475-2840-12-155
9. Casana R, Malloggi C, Tolva VS, Odero A Jr, Bulbulia R, Halliday A, et al. Does metabolic syndrome influence short and long term durability of carotid endarterectomy and stenting. Diabetes Metab Res Rev. (2019) 35:e3084. doi: 10.1002/dmrr.3084
10. Yin JH, Song ZY, Shan PF, Xu J, Ye ZM, Xu XH, et al. Age- and gender-specific prevalence of carotid atherosclerosis and its association with metabolic syndrome in Hangzhou, China. Clin Endocrinol. (2012) 76:802–9. doi: 10.1111/j.1365-2265.2011.04198.x
11. Leng XY, Chen XY, Chook P, Xiong L, Lin WH, Liu JY, et al. Association between metabolic syndrome and carotid atherosclerosis: a community-based study in Hong Kong. Metab Syndr Relat Disord. (2013) 11:109–14. doi: 10.1089/met.2012.0099
12. Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Hypertension is the most common component of metabolic syndrome and the greatest contributor to carotid arteriosclerosis in apparently healthy Japanese individuals. Hypertens Res. (2005) 28:27–34. doi: 10.1291/hypres.28.27
13. Zhan C, Shi M, Yang Y, Pang H, Fei S, Bai L, et al. Prevalence and risk factors of carotid plaque among middle-aged and elderly adults in rural Tianjin, China. Sci Rep. (2016) 6:23870. doi: 10.1038/srep23870
14. National Bureau of Statistics of China. China Statistical Yearbook. Beijing: China Statistics Press (2015).
15. Wang J, An Z, Li B, Yang L, Tu J, Gu H, et al. Increasing stroke incidence and prevalence of risk factors in a low-income Chinese population. Neurology. (2015) 84:374–81. doi: 10.1212/WNL.0000000000001175
16. Chen C, Lu FC, Department of Disease Control Ministry of Health PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17(Suppl):1–36. doi: 10.1111/j.1365-2028.2008.00991.x
17. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). an update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th european stroke conferences, mannheim, germany, 2004, brussels, belgium, 2006, and hamburg, germany, 2011. Cerebrovasc Dis. (2012) 34:290–6. doi: 10.1159/000343145
18. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
19. Zhao M, Caserta CA, Medeiros C, López-Bermejo A, Kollias A, Zhang Q, et al. Metabolic syndrome, clustering of cardiovascular risk factors and high carotid intima-media thickness in children and adolescents. J Hypertens. (2020) 38:618–24. doi: 10.1097/HJH.0000000000002318
20. Lee YH, Shin MH, Kweon SS, Nam HS, Park KS, Choi JS, et al. Normative and mean carotid intima-media thickness values according to metabolic syndrome in Koreans: the Namwon study. Atherosclerosis. (2014) 234:230–6. doi: 10.1016/j.atherosclerosis.2014.02.023
21. Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Chen W, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol. (2005) 46:457–63. doi: 10.1016/j.accreview.2005.10.020
22. Ning B, Chen Y, Waqar AB, Yan H, Shiomi M, Zhang J, et al. Hypertension enhances advanced atherosclerosis and induces cardiac death in watanabe heritable hyperlipidemic rabbits. Am J Pathol. (2018) 188:2936–47. doi: 10.1016/j.ajpath.2018.08.007
23. Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial dysfunction in obesity-induced inflammation: molecular mechanisms and clinical implications. Biomolecules. (2020) 10:291. doi: 10.3390/biom10020291
24. Raele R, Lotufo PA, Bittencourt MS, de Jesus M, Fonseca M, Goulart AC, et al. The association of waist-to-height ratio and other anthropometric measurements with subclinical atherosclerosis: Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutr Metab Cardiovasc Dis. (2020) 30:1989–98. doi: 10.1016/j.numecd.2020.05.025
25. Shen Y, Zhang L, Zong WH, Wang Z, Zhang Y, Yang MJ, et al. Correlation between waist circumference and carotid intima-media thickness in women from Shanghai, China. Biomed Environ Sci. (2013) 26:531–8. doi: 10.3967/0895-3988.2013.07.003
26. Ajala O, Mold F, Boughton C, Cooke D, Whyte M. Childhood predictors of cardiovascular disease in adulthood. a systematic review and meta-analysis. Obes Rev. (2017) 18:1061–70. doi: 10.1111/obr.12561
27. Sitnik D, Santos IS, Goulart AC, Staniak HL, Manson JE, Lotufo PA. Fasting glucose levels, incident diabetes, subclinical atherosclerosis and cardiovascular events in apparently healthy adults: a 12-year longitudinal study. Diab Vasc Dis Res. (2016) 13:429–37. doi: 10.1177/1479164116653356
28. Echouffo-Tcheugui JB, Chen H, Kalyani RR, Sims M, Simpson S, Effoe VS, et al. Glycemic markers and subclinical cardiovascular disease: the jackson heart study. Circ Cardiovasc Imaging. (2019) 12:e008641. doi: 10.1161/CIRCIMAGING.118.008641
29. van 't Riet E, Rijkelijkhuizen JM, Alssema M, Nijpels G, Stehouwer CD, Heine RJ, et al. HbA1c is an independent predictor of non-fatal cardiovascular disease in a Caucasian population without diabetes: a 10-year follow-up of the Hoorn Study. Eur J Prev Cardiol. (2012) 19:23–31. doi: 10.1097/HJR.0b013e32833b0932
30. Pirro M, Vaudo G, Lupattelli G, Pasqualini L, Mannarino MR, Schillaci G, et al. On-treatment C-reactive protein and HDL cholesterol levels in patients at intermediate cardiovascular risk: impact on carotid intima-media thickness. Life Sci. (2013) 93:338–43. doi: 10.1016/j.lfs.2013.07.008
31. Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. (2012) 60:508–16. doi: 10.1016/j.jacc.2012.03.060
32. Jung JM, Young K, won D, Han C, Park MH. Metabolic syndrome and early carotid atherosclerosis in the elderly. J Atheroscler Thromb. (2014) 21:435–44. doi: 10.5551/jat.18655
33. Shah S, Casas JP, Drenos F, Whittaker J, Deanfield J, Swerdlow DI, et al. Causal relevance of blood lipid fractions in the development of carotid atherosclerosis: mendelian randomization analysis. Circ Cardiovasc Genet. (2013) 6:63–72. doi: 10.1161/CIRCGENETICS.112.963140
34. Park YM, Sui X, Liu J, Zhou H, Kokkinos PF, Lavie CJ, et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol. (2015) 65:2091–100. doi: 10.1016/j.jacc.2015.03.517
35. Olmastroni E, Baragetti A, Casula M, Grigore L, Pellegatta F, Pirillo A, et al. Multilevel models to estimate carotid intima-media thickness curves for individual cardiovascular risk evaluation. Stroke. (2019) 50:1758–65. doi: 10.1161/STROKEAHA.118.024692
36. van den Munckhof I, Jones H, Hopman M, de Graaf J, Nyakayiru J, van Dijk B, et al. Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol. (2018) 41:698–704. doi: 10.1002/clc.22934
Keywords: carotid atherosclerosis, intima-media thickness, metabolic syndrome, components, epidemiology
Citation: Yang Q, Lin Q, Guo D, Wang H, Liu J, Zhang X, Tu J, Ning X, Yang Q and Wang J (2021) Association of Carotid Intima Media Thickness With Metabolic Syndrome Among Low-Income Middle-Aged and Elderly Chinese: A Population-Based Cross-Sectional Study. Front. Cardiovasc. Med. 8:669245. doi: 10.3389/fcvm.2021.669245
Received: 19 February 2021; Accepted: 18 October 2021;
Published: 19 November 2021.
Edited by:
Benjamin D. Pollock, Mayo Clinic Florida, United StatesReviewed by:
Xilin Yang, Tianjin Medical University, ChinaAlina Yu Babenko, Almazov National Medical Research Centre, Russia
Yalin Guan, Tianjin Huanhu Hospital, China
Copyright © 2021 Yang, Lin, Guo, Wang, Liu, Zhang, Tu, Ning, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianjia Ning, xning@tmu.edu.cn; Qing Yang, yq1963884@sina.com; Jinghua Wang, jwang3@tmu.edu.cn
†These authors have contributed equally to this work
 Qiaoxia Yang
Qiaoxia Yang Qiuxing Lin
Qiuxing Lin Dandan Guo
Dandan Guo Hanhua Wang
Hanhua Wang Jie Liu
Jie Liu Xin Zhang
Xin Zhang Jun Tu2,3,4,6
Jun Tu2,3,4,6  Xianjia Ning
Xianjia Ning Jinghua Wang
Jinghua Wang