Androgen Deprivation Therapy in Patients With Prostate Cancer Increases Serum Levels of Thromboxane A2: Cardiovascular Implications
- 1Servicio de Urología, Hospital Universitario La Paz, Madrid, Spain
- 2Grupo de Investigación en Urología, IdiPAZ, Madrid, Spain
- 3Servicio de Análisis Clínicos, Hospital Universitario La Paz, Madrid, Spain
- 4Departamento de Fisiología, Facultad de Medicina, UAM, Madrid, Spain
- 5Departamento de Urología, Hospital Clínico San Carlos, Madrid, Spain
- 6Grupo de Investigación en Neonatología, IdiPAZ, Madrid, Spain
Introduction: Androgens have been described as important players in the regulation of vascular function/structure through their action on the release and effect of vasoactive factors, such as prostanoids. Patients with prostate cancer (PCa) under androgen deprivation therapies (ADTs) present increased risk of cardiovascular mortality. Since thromboxane A2 (TXA2) is one of the most studied prostanoids and its involvement in different cardiovascular diseases has been described, the aim of this study was to investigate: (i) the effect of ADT on the serum levels of TXA2 in PCa patients and its possible link to the redox status and (ii) the effect of the non-hydrolyzable TXA2 analog U-46619 on the function of the aorta of male rats.
Methods: The levels of TXA2 and total antioxidant status in 50 healthy subjects, 54 PCa patients, and 57 PCa under ADT were evaluated. These determinations were accompanied by levels of testosterone and C-reactive protein as an inflammation marker. In aortic segments from male rats, the U46619-induced effects on: (i) the vasomotor responses to acetylcholine (ACh), to the NO donor sodium nitroprusside (SNP), to the carbon monoxide-releasing molecule-3 (CORM-3), and to noradrenaline (NA) and (ii) the expression of cyclooxygenase-2 (COX-2), heme oxygenase-1 (HO-1), and phosphorylated ERK1/2 were analyzed.
Results: The serum level of TXA2 in patients with PCa was increased with respect to healthy subjects, which was further increased by ADT. There was no modification in the total antioxidant status among the three experimental groups. In aortic segments from male rats, the TXA2 analog decreased the endothelium-dependent relaxation and the sensitivity of smooth muscle cells to NO, while it increased the vasoconstriction induced by NA; the expression of COX-2, HO-1, and pERK1/2 was also increased.
Conclusions: ADT increased, along with other inflammatory/oxidative markers, the serum levels of TXA2. The fact that TXA2 negatively impacts the vascular function of the aorta of healthy male rats suggests that inhibition of TXA2-mediated events could be considered a potential strategy to protect the cardiovascular system.
Introduction
Prostate cancer (PCa) is one of the most important leading causes of cancer deaths in men worldwide (1, 2). Androgen deprivation therapy (ADT) is the most widely used treatment for advanced PCa, which aims to reduce the levels and function of androgens to prevent PCa growth and spread (3). However, ADT is associated with several adverse side effects including osteoporosis, fatigue, depressive symptoms, sexual dysfunction, and metabolic modifications (4). The metabolic changes linked to ADT include altered lipid profile, insulin resistance, increase in adipose tissue, and adipokines (5), which favors a pro-inflammatory and pro-oxidant environment, giving rise to the so-called metabolic syndrome (6, 7), which is a cluster of risk factors for cardiovascular diseases. Indeed, several observational trials have reported an increased risk of cardiovascular diseases in men with PCa on ADT (8–10). Likewise, an association between lower plasma levels of testosterone and hypertension has been reported (11–15).
These clinical observations have been reinforced with experiments performed in different animal models demonstrating that decreased levels of testosterone alter vascular function and structure by modulating lipid profile (16); the release of endothelial factors such as nitric oxide (NO), prostanoids, and reactive oxygen species (ROS) (17–19); and different cell signaling pathways (20, 21). Among prostanoids, thromboxane A2 (TXA2) has been implicated in the development of cardiovascular diseases such as hypertension (22, 23) and thromboembolic events (24, 25). Since TXA2 is able to modulate the production of NO (26) and ROS (27, 28) as well as to activate vascular remodeling (29), mechanisms that, if maintained for a long time, can lead to different vascular pathologies, the first objective was to analyze the effect of ADT in PCa patients on the serum levels of TXA2 and its possible link to the redox status. The second objective was to explore the possible detrimental action of TXA2 on vascular function of aortic segments of male rats by analyzing the effect of the non-hydrolyzable TXA2 analog U-46619 on the vasodilator and vasoconstrictor responses.
Materials and Methods
Participants and Study Design
This is a prospective cohort study whose participants were patients in the Department of Urology of the La Paz University Hospital. All participants gave written informed consent. The study protocol was approved by the local Clinical Research Ethics Committee (Ref. HULP: PI-1204).
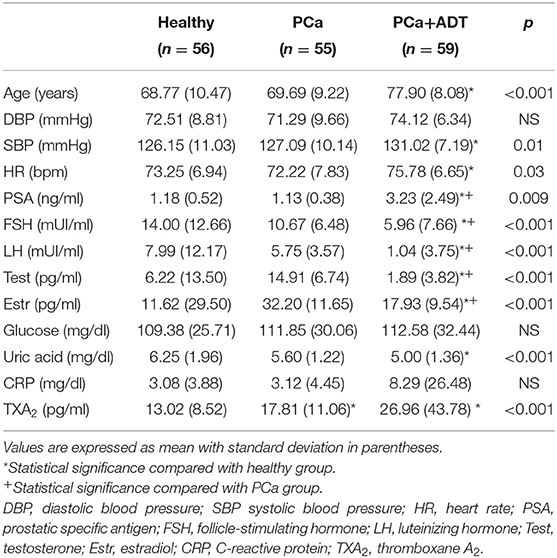
Participants were divided into the following three groups: healthy group (56 participants without PCa), PCa group (55 patients with localized PCa), and PCa+ADT (59 advanced PCa patients treated with ADT at least for 6 months and with testosterone concentration to castration levels during measurement defined by a serum testosterone concentration below 50 ng/dl). In the two groups of patients with PCa, the cancer was confirmed by standard prostate biopsy procedure. Systolic/diastolic blood pressure and heart rate were measured in all participants. Participants under medication for the treatment of hypertension, diabetes, or dyslipidemia were excluded.
Thromboxane A2, Total Antioxidant Capacity, and Other Biomarkers
Fasting blood was collected coinciding with a health-care blood extraction. Once the serum samples were obtained, they were stored at −80°C until used. The content of TXA2 was analyzed by measuring its stable metabolite TXB2 by enzyme immunoassay (Fine Test). The total antioxidant capacity in serum samples was analyzed by using the hydrophilic oxygen radical scavenging capacity (ORAC) assay (Randox Laboratories). Levels of prostate-specific antigen (PSA), follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, and estradiol were measured by chemiluminescence immunoassay in an Advia Centaur analyzer (Siemens Healthineers). The assays were carried out according to the manufacturer's protocols. Glucose and uric acid were measured by enzymatic-spectrophotometric methods in an Advia 2400 analyzer (Siemens Healthineers) and C-reactive protein (CRP) by immunoturbidimetric method in an Advia 2400 analyzer (Siemens Healthineers).
Animals and Vascular Tissue Preparation
Male Sprague–Dawley rats, 5 months old, were provided by the Animal Facility of the Universidad Autónoma de Madrid (UAM) (Registration number EX-021U). Systolic blood pressure was indirectly measured in awake animals by the tail-cuff method (Letica, Digital Pressure Meter, LE5000, Barcelona, Spain), and the animals were weighted before sacrifice. Rats were sacrificed by CO2 inhalation and subsequent decapitation, and the thoracic aorta was carefully dissected out, cleaned of connective tissue, and placed in Krebs–Henseleit solution (KHS) at 4°C. The composition of KHS is as follows (mM): NaCl 115, CaCl2 2.5, KCl 4.6, KH2PO4 1.2, MgSO4·7H2O 1.2, NaHCO3 25, glucose 11.1, and Na EDTA 0.03. All animal protocols were approved by the Research Ethics Committee of UAM according to directives 609/86 CEE and R.D. 233/88 of the Ministerio de Agricultura, Pesca y Alimentación of Spain (PROEX 202/16). The experiments were conducted in accordance with the published Guiding Principles in the Care and Use of Animals approved by the European Union directives 63/2010 UE and Spanish regulation RD53/2013.
Vascular Reactivity
The method used for isometric tension recording has been described in full elsewhere (30). Briefly, aortic segments were suspended in an organ bath containing 5 ml of KHS at 37°C, continuously bubbled with 95% O2-5% CO2 mixtures (pH 7.4). Two parallel stainless steel pins were introduced through the lumen of the vascular segment: one fixed to the bath wall and the other connected to a force transducer (Grass FTO3C; Grass Instruments Co., Quincy, MA, USA); this in turn was connected to a model 7D Grass polygraph. The aortic segments were subjected to a tension of 1 g, which was re-adjusted every 15 min during a 90-min equilibration period before drug administration. After this, the vessels were exposed to 75 mM of KCl to check the functional integrity. After a washout period, the viability of vascular endothelium was tested by the ability of 10 μM of ACh to relax precontracted segments with 0.1 μM of noradrenaline (NA). Vessels were then washed with KHS to recover the basal tension. To investigate the effect of the non-hydrolyzable TXA2 analog U-46619 on the vasomotor responses, separate aortic segments of SD rats were incubated with 1 nM of U-46619 for 1 h before performing cumulative concentration–response curves to ACh (0.1 nM−10 μM), to the NO donor sodium nitroprusside (SNP, 0.1 nM−10 μM), to the carbon monoxide-releasing molecule-3 (CORM-3, 1 μM−0.1 mM), and to NA (0.1 nM−10 μM). The concentration of the TXA2 analog (1 nM) was chosen because it was within the range of the intracrine concentration reached in the vascular wall that we had previously reported (26). Thus, we described that the release of TXA2 in the aorta from control and orchidectomized rats varied from 100 to 400 pg/ml/mg tissue.
Western Blotting Analysis
Arterial segments from the two experimental conditions (control and exposed to 1 nM of U-46619) were homogenized and processed to quantify protein concentration at 4°C in radioimmunoprecipitation assay (RIPA) buffer containing phosphatase inhibitors and a cocktail of protease inhibitors. Proteins (20 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio Rad Immun-Blot® overnight at 4°C, 230 mA, using a Bio-Rad Mini Protean III system; Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked with 5% (w/v) fat-free powdered milk or 5% (w/v) bovine serum albumin following the instructions of the antibody manufacturers and incubated overnight with mouse monoclonal antibody for anti-phospho-ERK1/2–Thr202/Tyr204 (1:2,000 dilution, Cell Signaling Technology) or with rabbit polyclonal antibody for cyclooxygenase-2 (COX-2) (1:200 dilution, Cayman Chemical) or heme oxygenase (HO-1) (1:2,000 dilution, Stressgen Bioreagents). After being washed, the membrane was incubated with the corresponding anti-immunoglobulin G conjugated to horseradish peroxidase (Amersham International Plc). The membrane was thoroughly washed, and the immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus, Amersham International Plc, Little Chalfont, UK) and subjected to autoradiography (Hyperfilm ECL, Amersham International Plc). Signals on the immunoblot were quantified using a computer program (NIH Image V1.56). The same membrane was used to determine GAPDH expression, and the content of the latter was used to correct COX-2 and HO-1 expression in each sample, by means of a monoclonal antibody anti GAPDH (1:5,000 dilution, Sigma). The total ERK1/2 was used as loading control to correct the phosphorylation level of ERK1/2.
Drugs and Chemicals
Drugs used were as follows: ACh chloride, potassium chloride, SNP, CORM, and L-NA hydrochloride (Sigma-Aldrich). Stock solutions (10 mM) of drugs were prepared in distilled water, except for NA, which was dissolved in NaCl (0.9%)–ascorbic acid (0.01% w/v) solution. These solutions were kept at −20°C, and appropriate dilutions were made in KHS on the day of the experiment.
Data Analysis
The results carried out on human participants are expressed as the mean with the standard deviation or by the mean value and the corresponding 25 and 75th percentiles. For comparison among the three groups, in the case of quantitative variables, with normal distribution, the one-way ANOVA test was used. Then, a post-hoc contrast was carried out to analyze the groups with different values. If the distribution of the variable is not normal, non-parametric tests as Kruskal–Wallis H Mann–Whitney U-tests were used. The normal distribution of quantitative variables was checked using the Shapiro–Wilk test. To analyze the associations between the levels of two variables, the Spearman coefficient (ρ) was used. A two-tailed p < 0.05 was considered statistically significant. The statistical analysis was performed using the statistical packages SPSS version 26.0 and Stata version 16.0.
The results of animal experiments are given as mean ± standard error of the mean (SEM). The relaxation induced by ACh, SNP, or CORM-3 was expressed as a percentage of initial contraction elicited by NA. The contraction induced by NA was expressed as percentage of the contraction induced by KCl (75 mM). Statistical analysis was performed by comparing the curves obtained in aortae of SD rats after U-46619 incubation with that obtained in the control condition by means of two-way analysis of variance (ANOVA). For protein expression, statistical analysis was done using Student's t-test for unpaired experiments. A p < 0.05 was considered significant.
Results
Effect of Androgen Deprivation Therapy in Prostate Cancer Patients on Serum Levels of Thromboxane A2, Total Antioxidant Activity, and Other Biomarkers
The characteristics of the three groups of subjects are summarized in Table 1. The patients of the ADT group were older than those of PCa or healthy groups (p < 0.001); patients belonging to PCa+ADT group showed the greatest values for systolic blood pressure (p < 0.02) and heart rate (p < 0.05) as compared with PCa or healthy groups.
The levels of PSA were similar between healthy and PCa groups, while they were increased in the PCa+ADT group (p < 0.01). The levels of FSH, LH, estradiol, and testosterone were reduced in the PCa+ADT group compared with PCa (p < 0.001) and healthy (p < 0.001) groups. The glucose concentration was similar in the three groups of the study. The concentration of uric acid was decreased in the PCa+ADT group with respect to the healthy group (p < 0.001). There were no statistical differences for CRP among groups.
The patients belonging to PCa group presented higher serum levels of TXA2 than those of the healthy group; those levels were further higher in PCa patients under ADT (Figure 1A and Table 1); the total antioxidant capacity was similar in the three groups of the study (Figure 1B).
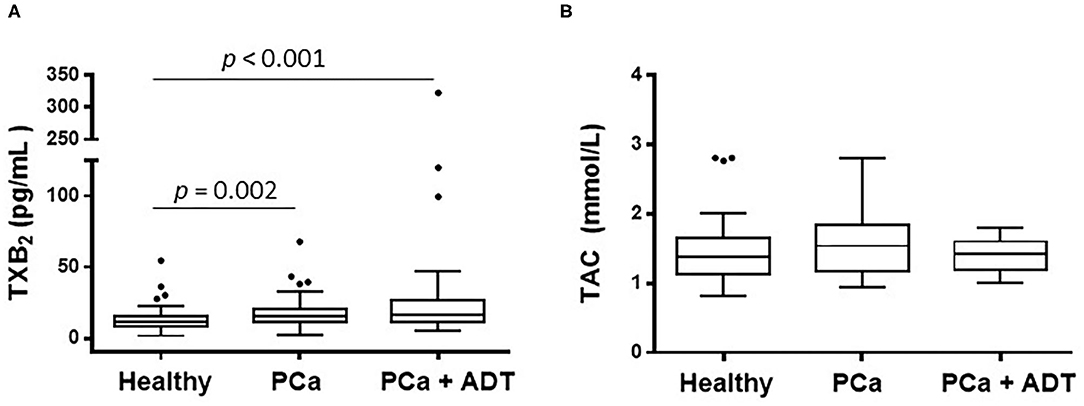
Figure 1. Effect of prostate cancer (PCa) and androgen deprivation therapy (ADT) in PCa patients on the serum concentration of thromboxane A2 (TXA2) (A) and on the total antioxidant capacity (TAC) (B). Results are shown as the median (solid line) with the top and bottom of box representing quartiles. The statistical significance, by means of Mann–Whitney U test, is indicated in the corresponding graphs.
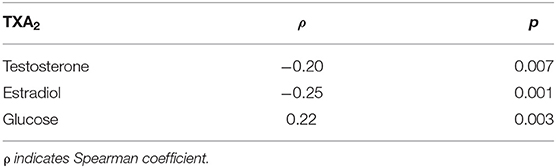
The concentration of TXA2 was inversely correlated with the concentration of testosterone or estradiol and directly correlated with glucose concentration (Table 2). In addition, a direct correlation between total antioxidant capacity and uric acid was observed (ρ: 0.185; p = 0.01).
Effect of the Non-hydrolyzable Thromboxane A2 Analog on Vascular Function of Rat Aorta
The effect of TXA2 mimetic on the endothelium-dependent vasodilator response was analyzed. Therefore, in NA-precontracted arterial segments, the vasodilator response induced by ACh (0.1 nM−10 μM) was decreased after U-46619 incubation (Figure 2A). Since NO is one of the most important factors released after endothelial stimulation, the possible action of the TXA2 mimetic on the sensitivity of smooth muscle cells to NO was also investigated by analyzing the vasodilator response induced by the NO donor, SNP. The results showed that in NA-precontracted arteries, the vasodilator response induced by SNP (0.1 nM−10 μM) was decreased after U-46619 incubation (Figure 2B).
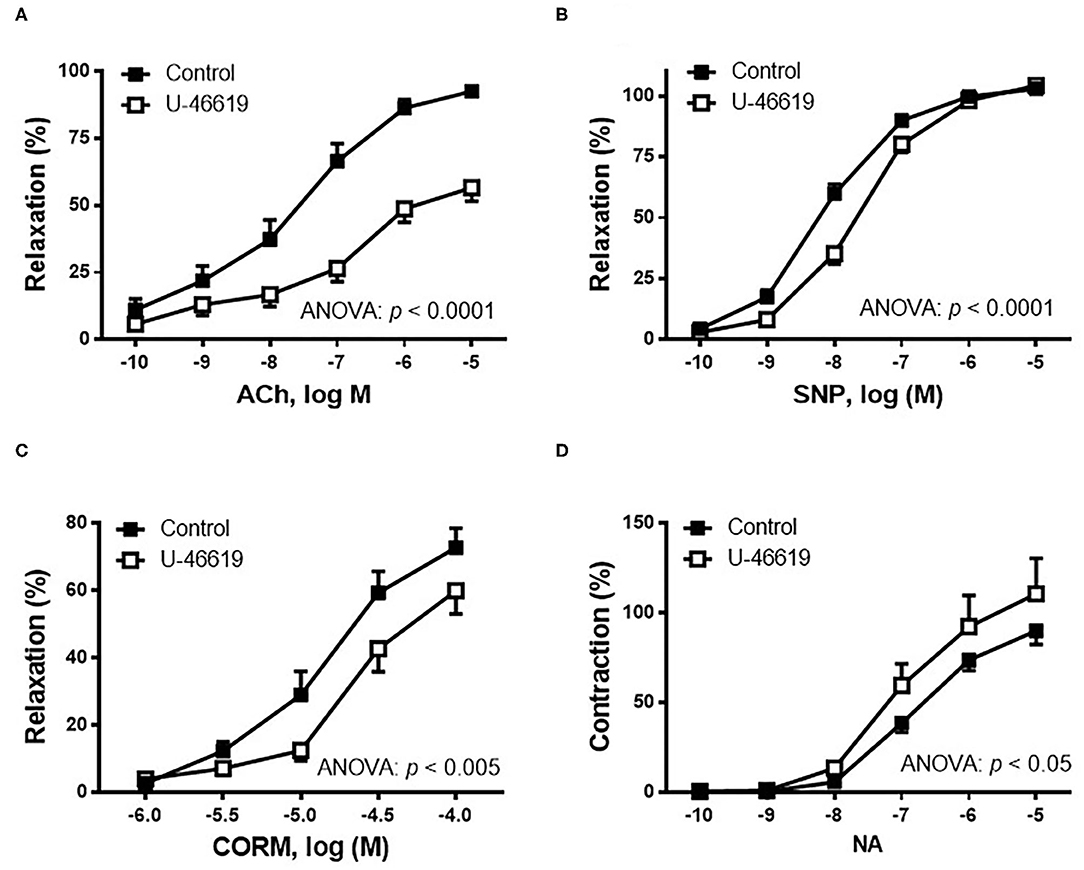
Figure 2. Effect of the non-hydrolyzable mimetic of thromboxane A2 (TXA2), U-46619 (1 nM), on the concentration–response curves to (A) acetylcholine (ACh), (B) sodium nitroprusside (SNP), (C) carbon monoxide-releasing molecule (CORM), and (D) noradrenaline (NA) in aortic segments of male rats. Results (mean ± SEM) are represented for the vasodilatory responses as percentage of the inhibition of the contraction elicited by 0.1 μM of noradrenaline; for the NA-induced vasoconstriction, the response was represented as percentage of the previous contraction induced by KCl (75 mM). Number of animals: 4–5. The statistical significance is indicated in the corresponding graphs.
Since carbon monoxide (CO) has been described to elicit cytoprotective actions in responses to cellular stress, the effect of U-46619 on the CORM-induced vasodilator response was also analyzed. The results showed that the vasodilator response to CORM (1–100 μM) was decreased after incubation with 1 nM of U-46619 (Figure 2C).
The contractile response elicited by 75 mM of KCl was not modified after incubation with then non-hydrolyzable mimetic of TXA2, U-46619 (control: 1,988 ± 184.5 mg; 1 nM of U-46619: 2,143 ± 139.0 mg; p > 0.05). The vasoconstrictor response induced by NA (0.1 nM−10 μM) was increased after incubation of vessels with U-46619 (Figure 2D).
The effect of U-46619 on the expression of COX-2, p-ERK1/2, and HO-1 was analyzed in homogenates of rat aorta by using western blot analysis. The results show that the expression of COX-2 was weakly increased (p > 0.05), while that of pERK1/2 and HO-1 was significantly increased after U-46619 incubation (Figure 3).
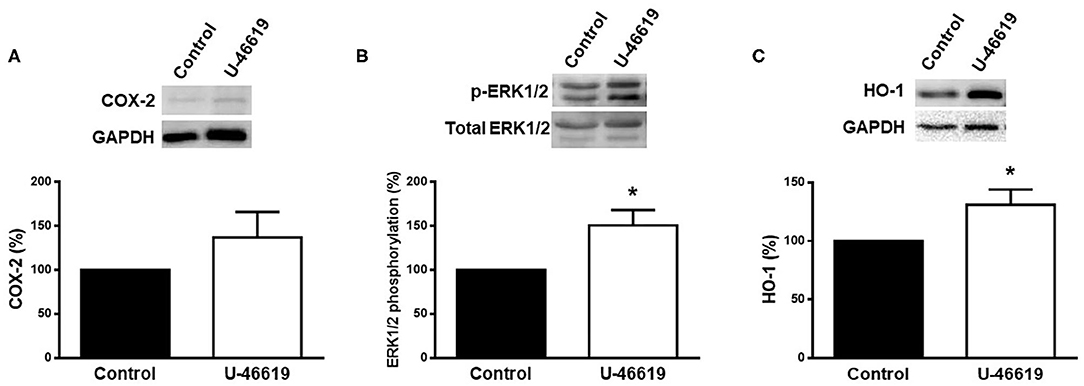
Figure 3. Representative western blot and densitometric analysis for the expression of COX-2, p-ERK1/2, and HO-1 protein in aortic segments of male rat in the absence (control) or presence of U-46619 (1 nM). Results (means ± SEM) are expressed as the relative percentage of the ratio between the signal for COX-2 or HO-1 protein and the signal for GAPDH; the signal for p-ERK1/2 was corrected to the corresponding total ERK1/2 signal. Number of animals: 3. *p < 0.05 compared with control condition.
Discussion
The present work describes for the first time that patients with PCa show increased serum levels of TXA2 and that ADT further increases those levels, which may account for the development of vascular dysfunction. In addition, the detrimental effect of the non-hydrolyzable TXA2 analog, U-46619, in an in vitro model is also demonstrated.
It is well-known that TXA2, through its T prostanoid receptor (TP), has been implicated in the progression of different cancers including PCa (31–33), which suggests the involvement of inflammatory pathways in PCa (34). Although higher circulating TXA2 levels have been associated with colorectal cancer progression (35), to our knowledge, there is no information on circulating TXA2 levels in PCa patients. The current study showed that serum TXA2 levels are increased in the PCa group with respect to healthy subjects. This finding is in agreement with that describing the involvement of prostanoids and chronic inflammation in carcinogenesis (36) and with the fact that aspirin intake reduced the PCa incidence (37). According to the literature (3, 38), administration of ADT achieves reduced levels of FSH, LH, and testosterone in PCa patients to avoid tumor growth and spread. Although ADT has been shown to improve survival, deleterious effects of ADT on cardiometabolic risk (4) and type 2 diabetes mellitus (39) have been reported. In addition, patients with these pathologies present chronic inflammation and increased synthesis of TXA2 (40). Despite that TXA2 has been implicated in the pathogenesis of a variety of cardiovascular diseases (41, 42), the impact of ADT on this prostanoid has not been still investigated. The results described in the current study showed that in PCa patients on ADT, the levels of TXA2 were strongly increased, showing an inverse correlation between the levels of testosterone and TXA2. This finding is consistent with data from other studies, which reported that administration of androgens to patients with coronary heart disease decreased the TXA2 level (43), while the loss of gonadal function of male rats increased the levels of TXA2 (18, 19, 26). Our results also showed that serum TXA2 concentration was directly correlated with glucose concentration, while it was not correlated with CRP, a chronic inflammation marker. It is important to mention that CaP patients on ADT showed higher values of CRP than did healthy and PCa groups, although the difference was not statistically significant. In this sense, long-term testosterone therapy of hypogonadal men decreased the level of CRP (15).
Since TXA2 is able to increase the synthesis of ROS (22, 27), which, in turn, decreases the bioavailability of NO and therefore may account for detrimental actions on cardiovascular function (16, 44), total antioxidant capacity was analyzed in the three experimental groups. Contrary to what was expected, the results showed that there were no statistical differences among the groups. A possible explanation is that the age of the participants is high, even in the healthy group; therefore, the antioxidant capacity could already be diminished according to previously reported data in aging (45). It is interesting to mention that total antioxidant capacity was directly correlated with the serum uric acid concentration, which was decreased in the PCa patients on ADT with respect to the healthy subjects. Still within the normal range, the increased levels of uric acid observed in the healthy group could be considered as a compensatory mechanism to counteract the oxidative stress related to aging because of its antioxidant property, as reported in a variety of pathophysiological conditions (46, 47). Despite the limitation of this study, which lacks healthy young participants and that precisely the PCa patients on ADT are significantly older than the other groups, undoubtedly, ADT increased thromboxane levels. Therefore, stratified studies—according to age and to ADT duration—analyzing in detail the potential association between TXA2 levels and different cardiovascular events should be of great interest to be performed in the future.
The current study revealed that TXA2 was able to induce dysfunction in rat aorta. The in vitro model consisted of incubating aortic rings of male rats with the non-hydrolyzable TXA2 analog, U-46619, according to previous investigations (27, 44). The results showed that 1 nM of U-46619 for 1 h decreased the endothelium-dependent response elicited by ACh. It is well-known that ACh induces the release of vasodilators factors including NO and hyperpolarizing factors (16, 48). This result could be compatible with a decreased release and/or bioavailability of NO induced by TXA2. In this sense, endogenous TXA2 was reported to negatively regulate the NO release in mesenteric artery of male rats (26). TXA2 is also able to decrease NO bioavailability through increasing the synthesis of ROS (28, 49). In addition, the possible modulation of TXA2 on the sensitivity of smooth muscle cells to NO was also analyzed. The results showed that the SNP-induced response was decreased by incubation with 1 nM of U-46619, which appears to agree with studies describing that endogenous TXA2 negatively modulates the vasodilator effect of NO (26). The underlying mechanism could be due to the inhibition of guanylate cyclase by U-46619 as reported in radial arteries (50).
Since NO and cGMP can hyperpolarize cell membranes by activating potassium channels (51, 52), possible modifications in the function of these channels should not be ruled out. Another important gas mediator activating potassium channels is CO, which is produced by heme oxygenases during the degradation of the heme group. The inducible isoform of HO-1 is one of the earliest expressed proteins in response to inflammation and oxidative stress, and its involvement in cardiovascular protection has been described (53, 54). In this regard, our results showed increased HO-1 expression in incubated arteries with U-46619, supporting the pro-inflammatory environment induced by the TXA2 analog. This response could be due, at least in part, to the action of products derived from COX-2—although COX-2 was slightly increased—and from the extracellular signal-regulated kinase (ERK1/2). Thus, reduction of TXA2 synthesis has been reported to prevent of ERK1/2 phosphorylation and prostaglandin E2 effect in human monocytes (55). Once the up-regulation of HO-1 was observed in arteries incubated with U-46619, the next step was to analyze the possible influence on the vasodilatory effect of CO. The fact that the CORM-induced relaxation was decreased by U-46619 incubation suggests a reduction of the potassium channels functionality. In this regard, the blockage of potassium channels by TP receptors activation in pulmonary arteries was described (27).
It is well-known that TP receptor activation results in the stimulation of intracellular pathways including phospholipase C with the subsequent production of 1,4,5-triphosphate and diacylglycerol and, therefore, increase in calcium release from sarcoplasmic reticulum and protein kinase activation (56). Activation of the above mentioned cell-signaling pathways potentiate the vasomotor response to several vasoconstrictor agents (57). This observation could explain the increased NA-induced response observed in the current study, which agrees with previous investigations describing that U-46619 facilitates sympathetic neurotransmission and potentiates constrictor effects of NA in human saphenous veins (58).
It is remarkable to note that most of published studies use higher concentrations than 1 nM of U-46619 used in the current investigation, which supports the relevance of the functional results observed in rat aorta. Although 1 nM of TXA2 analog is around 10-fold higher than the concentration observed in the serum of PCa patients under ADT, it is similar to the intracrine concentration reached in the vascular wall (26). The results obtained from the in vitro model showed a TXA2-induced detrimental effect on rat aorta, in which endothelium-dependent and endothelium-independent vasodilation was compromised. These results suggest a deleterious effect of TXA2 on vascular function as consequence of ADT, which could support the slight increase in blood pressure observed in the PCa+ADT group. Although our study did not measure the incidence of de novo cardiovascular events, ADT has shown increased cardiovascular risk (4), increase in diabetes mellitus (39), myocardial infarction, sudden cardiac death (59), and thromboembolic events (60, 61). Conducting stratified studies by age range of patients and by ADT duration would improve the analysis about the ADT effects on different biomarkers and cardiovascular events.
Conclusion
Overall, the current study showed that PCa patients on ADT increased the serum levels of TXA2, which could exert detrimental effects on the cardiovascular system. Our in vitro results showed that TXA2 negatively impacts the function of the aorta of healthy male rats. Therefore, inhibition of TXA2-mediated events could be considered a potential strategy to protect the cardiovascular system. Future investigations will be necessary to determine whether or not different biomarkers, in addition to TXA2, are modified during different periods of ADT.
Data Availability Statement
The original contributions generated for the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Clinical Research Ethics Committee of La Paz University Hospital (Ref. HULP: PI-1204). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Research Ethics Committee of Universidad Autónoma de Madrid according to directives 609/86 CEE and R.D. 233/88 of the Ministerio de Agricultura, Pesca y Alimentación of Spain (PROEX 202/16).
Author Contributions
MF and FdB contributed to the intellectual design of the study. MF, FdB, and AB-S supervised the work. MÁ-M, AE, PC, MK-M, and JG contributed to the development of the research in different proportions. MF wrote the article. All authors contributed to revising the manuscript and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by a grant from the Fondo de Investigaciones Sanitarias (PI19/01282) to MF. The authors thank David Muñoz and technical staff of the Animal Facility at the Facultad de Medicina for the care of animals.
References
1. Badal S, Aiken W, Morrison B, Valentine H, Bryan S, Gachii A, Ragin C. Disparities in prostate cancer incidence and mortality rates: solvable or not? Prostate. (2020) 80:3–16. doi: 10.1002/pros.23923
2. Gandaglia G, Albers P, Abrahamsson P-A, Briganti A, Catto JWF, Chapple CR, et al. Structured population-based prostate-specific antigen screening for prostate cancer: The European association of urology position in 2019. Eur Urol. (2019) 76:142–50. doi: 10.1016/j.eururo.2019.04.033
3. Owen PJ, Daly RM, Livingston PM, Fraser SF. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis. (2017) 20:137–45. doi: 10.1038/pcan.2016.69
4. Gupta D, Salmane C, Slovin S, Steingart RM. Cardiovascular complications of androgen deprivation therapy for prostate cancer. Curr Treat Options Cardiovasc Med. (2017) 19:61. doi: 10.1007/s11936-017-0563-1
5. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. (2013) 217:R47–71. doi: 10.1530/JOE-12-0582
6. Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. (2013) 189:S34–42. doi: 10.1016/j.juro.2012.11.017
7. Tzortzis V, Samarinas M, Zachos I, Oeconomou A, Pisters LL, Bargiota A. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones. (2017) 16:115–23. doi: 10.14310/horm.2002.1727
8. Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. (2015) 68:386–96. doi: 10.1016/j.eururo.2014.11.039
9. da Silva FC, da Silva FMC, Gonçalves F, Santos A, Kliment J, Whelan P, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European Uroncological Group. Eur Urol. (2014) 66:232–9. doi: 10.1016/j.eururo.2013.03.055
10. Zhao J, Zhu S, Sun L, Meng F, Zhao L, Zhao Y, et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS ONE. (2014) 9:e107516. doi: 10.1371/journal.pone.0107516
11. Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. (1988) 78:539–45. doi: 10.1161/01.CIR.78.3.539
12. Phillips GB, Jing TY, Resnick LM, Barbagallo M, Laragh JH, Sealey JE. Sex. hormones and hemostatic risk factors for coronary heart disease in men with hypertension, J. Hypertens. (1993) 11:699–702. doi: 10.1097/00004872-199307000-00003
13. Simon D, Charles MA, Nahoul K, Orssaud G, Kremski J, Hully V, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab. (1997) 82:682–5. doi: 10.1210/jc.82.2.682
14. Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. (2004) 150:65–71. doi: 10.1530/eje.0.1500065
15. Haider A, Yassin A, Haider KS, Doros G, Saad F, Rosano GM. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. (2016) 12:251–61. doi: 10.2147/VHRM.S108947
16. Villalpando DM, Navarro R, Del Campo L, Largo C, Muñoz D, Tabernero M, et al. Effect of dietary docosahexaenoic acid supplementation on the participation of vasodilator factors in aorta from orchidectomized rats. PLoS ONE. (2015) 10:e0142039. doi: 10.1371/journal.pone.0142039
17. Blanco-Rivero J, Sagredo A, Balfagón G, Ferrer M. Orchidectomy increases expression and activity of Cu/Zn-superoxide dismutase, while decreasing endothelial nitric oxide bioavailability. J Endocrinol. (2006) 190:771–8. doi: 10.1677/joe.1.06887
18. Blanco-Rivero J, Balfagón G, Ferrer M. Orchidectomy modulates alpha2-adrenoceptor reactivity in rat mesenteric artery through increased thromboxane A2 formation. J Vasc Res. (2006) 43:101–8. doi: 10.1159/000089791
19. Martorell A, Blanco-Rivero J, Aras-López R, Sagredo A, Balfagón G, Ferrer M. Orchidectomy increases the formation of prostanoids and modulates their role in the acetylcholine-induced relaxation in the rat aorta. Cardiovasc Res. (2008) 77:590–9. doi: 10.1093/cvr/cvm059
20. del Campo L, Guvenc Tuna B, Ferrer M, van Bavel E, Bakker EN. Testosterone and β-oestradiol prevent inward remodelling of rat small mesenteric arteries: role of NO and transglutaminase. Clin Sci. (2013) 124:719–28. doi: 10.1042/CS20120700
21. del Campo M, Sagredo A, del Campo L, Villalobo A, Ferrer M. Time-dependent effect of orchidectomy on vascular nitric oxide and thromboxane A2 release. Functional implications to control cell proliferation through activation of the epidermal growth factor receptor. PLoS ONE. (2014) 9:e102523. doi: 10.1371/journal.pone.0102523
22. García-Redondo AB, Briones AM, Beltrán AE, Alonso MJ, Simonsen U, Salaices M. Hypertension increases contractile responses to hydrogen peroxide in resistance arteries through increased thromboxane A2, Ca2+, and superoxide anion levels. J Pharmacol Exp Ther. (2009) 328:19–27. doi: 10.1124/jpet.108.144295
23. Chen H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins and Other Lipid Mediators. (2018) 134:32–7. doi: 10.1016/j.prostaglandins.2017.11.004
24. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. (2003) 110:255–8. doi: 10.1016/S0049-3848(03)00379-7
25. Cong Y, Wang L, Peng R, Zhao Y, Bai F, Yang C, et al. Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by the thromboxane A2 receptor. Sci Rep. (2016) 6: 38757. doi: 10.1038/srep38757
26. del Campo L, Sagredo A, Aras-López R, Balfagón G, Ferrer M. Orchidectomy increases the formation of non-endothelial thromboxane A2 and modulates its role in the electrical field stimulation-induced response in rat mesenteric artery. J Endocrinol. (2008) 197:371–9. doi: 10.1677/JOE-07-0647
27. Cogolludo A, Frazziano G, Cobeño L, Moreno L, Lodi F, Villamor E, et al. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann N Y Acad Sci. (2006) 1091:41–51. doi: 10.1196/annals.1378.053
28. Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GD, Newby AC, et al. Acute inhibition of superoxide formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analogue, U46619. Prostaglandins Leukot Essent Fatty Acids. (2008) 78:247–55. doi: 10.1016/j.plefa.2008.01.008
29. Gallet C, Blaie S, Levy-Toledano S, Habib A. Thromboxane-induced ERK phosphorylation in human aortic smooth muscle cells. Adv Exp Med Biol. (2003) 525:71–3. doi: 10.1007/978-1-4419-9194-2_14
30. Nielsen KC, Owman C. Contractile response and amine receptor mechanisms in isolated middle cerebral artery of the cat. Brain Res. (1971) 27:33–42. doi: 10.1016/0006-8993(71)90370-2
31. O'Sullivan AG, Mulvaney EP, Kinsella BT. Regulation of protein kinase C-related kinase (PRK) signalling by the TPalpha and TPbeta isoforms of the human thromboxane A(2) receptor: Implications for thromboxane- and androgen- dependent neoplastic and epigenetic responses in prostate cancer. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:838–56. doi: 10.1016/j.bbadis.2017.01.011
32. Ekambaram P, Lambiv W, Cazzolli R, Asthon AW, Honn KV. The thromboxane synthase and receptor signaling pathway in cancer: an emerging paradigm in cancer progression and metastasis. Cancer Metastasis Rev. (2011) 30:397–408. doi: 10.1007/s10555-011-9297-9
33. Dassesse T, de Leval X, de Leval L, Pirotte B, Castronovo V, Waltregny D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor gleason score and pathologic stage. Eur Urol. (2006) 50:1021–31. doi: 10.1016/j.eururo.2006.01.036
34. Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. (2004) 172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff
35. Li H, Liu K, Boardman LA, Zhao Y, Wang L, Sheng Y, et al. Circulating prostaglandin biosynthesis in colorectal cancer and potential clinical significance. EBioMed. (2015) 2:165–71. doi: 10.1016/j.ebiom.2014.12.004
36. Wan D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. (2010) 10:181–93. doi: 10.1038/nrc2809
37. Prause LW, Manka L, Millan C, Lang E, Wyler SF, Grobholz R, et al. Influence of regular aspirin intake on PSA values, prostate cancer incidence and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol. (2020) 38:2485–91. doi: 10.1007/s00345-019-03054-5
38. Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. (2020) 382:2187–96. doi: 10.1056/NEJMoa2004325
39. Haidar A, Yassin A, Saad F, Shabsigh R. Effect of androgen deprivation on glycaemic control and on cardiovascular risk factors in men with advanced prostate cancer with diabetes. Aging Male. (2007) 10:189–96. doi: 10.1080/13685530701653538
40. Novgorodtseva TP, Karaman YK, Zhukova NV, Lobanova EG, Antonyuk MV, Kantur TA. Composition of fatty acids in plasma and erythrocytes and eicosanoids level in patients with metabolic syndrome. Lipids Health Dis. (2011) 10:82. doi: 10.1186/1476-511X-10-82
41. Mousa AA, Strauss JF, Walsh SW. Reduced methylation of the thromboxane synthase gene is correlated with its increased vascular expression in preeclampsia. Hypertension. (2012) 59:1249–55. doi: 10.1161/HYPERTENSIONAHA.111.188730
42. Mehta JL, Lawson D, Mehta P, Saldeen T. Increased prostacyclin andthromboxane A2 biosynthesis in atherosclerosis. Proc Natl Acad Sci USA. (1988) 85:4511–4515. doi: 10.1073/pnas.85.12.4511
43. Wu S, Weng X. Regulation of atrial natriuretic peptide, thromboxane and prostaglandin production by androgen in elderly men with coronary heart disease. Chin Med Sci J. (1993) 8:207–9.
44. Villalpando DM, Gómez Rivas J, Flynn DR, de Bethencourt F, Ferrer M. Gonadal function protects against organ culture-induced vascular damage. involvement of prostanoids. Prostaglandins Other Lipid Mediat. (2020) 148:106406. doi: 10.1016/j.prostaglandins.2019.106406
45. Gawron-Skarbek A, Guligowska A, Prymont-Przymińska A, Nowak D, Kostka T. Plasma and salivary non-urate total antioxidant capacity does not depend on dietary vitamin C, E, or β-carotene intake in older subjects. Molecules. (2018) 23:983. doi: 10.3390/molecules23040983
46. Bagheri B, Zargari M, Meshkini F, Dinarvand K, Mokhberi V, Azizi S, et al. Uric acid and coronary artery disease, two sides of a single coin: a determinant of antioxidant system or a factor in metabolic syndrome. J Clin Diagn Res. (2016) 10:OC27–31. doi: 10.7860/JCDR/2016/16335.7281
47. Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. (2000) 148:131–9. doi: 10.1016/S0021-9150(99)00214-2
48. Sagredo A, del Campo L, Martorell A, Navarro R, Martín MC, Blanco-Rivero J, et al. Ovariectomy increases the participation of hyperpolarizing mechanisms in the relaxation of rat aorta. PLoS ONE. (2013) 8:e73474. doi: 10.1371/journal.pone.0073474
49. García-Redondo AB, Briones AM, Martínez-Revelles S, Palao T, Vila L, Alonso MJ, et al. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: r ole of TXA2, NAD(P)H oxidase and mitochondria. J Hypertens. (2015) 33:77–87. doi: 10.1097/HJH.0000000000000383
50. Arshad M, Vijay V, Floyd BC, Marks B, Sarabu MR, Wolin MS, et al. Thromboxane receptor stimulation suppresses guanylate cyclase-mediated relaxation of radial arteries. Ann Thorac Surg. (2006) 81:2147–54. doi: 10.1016/j.athoracsur.2006.01.024
51. Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. (1994) 368:850–3. doi: 10.1038/368850a0
52. Ferrer M, Marín J, Encabo A, Alonso MJ, Balfagón G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen Pharmacol. (1999) 33:35–41. doi: 10.1016/S0306-3623(98)00259-6
53. Kang L, Hillestad ML, Grande JP, Croatt AJ, Barry MA, Farrugia G, et al. Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo. Am J Physiol Heart Circ Physiol. (2015) 308:H1402–13. doi: 10.1152/ajpheart.00882.2014
54. Drummond GS, Baum J, Greenberg M, Lewis D, Abraham NG. HO-1 overexpression and underexpression: clinical implications. Arch Biochem Biophys. (2019) 673:108073. doi: 10.1016/j.abb.2019.108073
55. Eligini S, Violi F, Banfi C, Barbieri SS, Brambilla M, Saliola M, et al. Indobufen inhibits tissue factor in human monocytes through a thromboxane-mediated mechanism. Cardiovasc Res. (2006) 69:218–26. doi: 10.1016/j.cardiores.2005.07.013
56. Shenker A, Goldsmith P, Unson CG, Spiegel AM. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. (1991) 266:9309–13. doi: 10.1016/S0021-9258(18)31586-2
57. Mueed I, Zhang L, MacLeod KM. Role of the PKC/CPI-17 pathway in enhanced contractile responses of mesenteric arteries from diabetic rats to alpha-adrenoceptor stimulation. Br J Pharmacol. (2005) 146:972–82. doi: 10.1038/sj.bjp.0706398
58. Vila JM, Martínez-León JB, Medina P, Segarra G, Ballester RM, Otero E, et al. U-46619-induced potentiation of noradrenergic constriction in the human saphenous vein: antagonism by thromboxane receptor blockade. Cardiovasc Res. (2001) 52:462–77. doi: 10.1016/S0008-6363(01)00390-X
59. Melloni C, Roe MT. Androgen deprivation therapy and cardiovascular disease. Urol Oncol. (2020) 38:45–52. doi: 10.1016/j.urolonc.2019.02.010
60. Guo Z, Huang Y, Gong L, Gan S, Chan FL, Gu C, et al. Association of androgen deprivation therapy with thromboembolic events in patients with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. (2018) 21:451–60. doi: 10.1038/s41391-018-0059-4
Keywords: prostate cancer, androgen deprivation therapy (ADT), thromboxane A2 (TXA2), vascular function, endothelium
Citation: Álvarez-Maestro M, Eguibar A, Chanca P, Klett-Mingo M, Gómez Rivas J, Buño-Soto A, de Bethencourt FR and Ferrer M (2021) Androgen Deprivation Therapy in Patients With Prostate Cancer Increases Serum Levels of Thromboxane A2: Cardiovascular Implications. Front. Cardiovasc. Med. 8:653126. doi: 10.3389/fcvm.2021.653126
Received: 13 January 2021; Accepted: 15 March 2021;
Published: 13 April 2021.
Edited by:
Emma Louise Robinson, University of Colorado, United StatesReviewed by:
Ines Falcão-Pires, Universidade do Porto, PortugalBilge Güvenç Tuna, Yeditepe University, Turkey
Copyright © 2021 Álvarez-Maestro, Eguibar, Chanca, Klett-Mingo, Gómez Rivas, Buño-Soto, de Bethencourt and Ferrer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mercedes Ferrer, mercedes.ferrer@uam.es
†These authors have contributed equally to this work and share first authorship
 Mario Álvarez-Maestro1,2†
Mario Álvarez-Maestro1,2†  Aritz Eguibar
Aritz Eguibar Juan Gómez Rivas
Juan Gómez Rivas Mercedes Ferrer
Mercedes Ferrer