Can Blood Biomarkers Help Predicting Outcome in Transcatheter Aortic Valve Implantation?
- 1Department of Cardiology, Heart Valve Clinic, University of Liège Hospital, GIGA Cardiovascular Sciences, CHU Sart Tilman, Liège, Belgium
- 2Gruppo Villa Maria Care and Research, Anthea Hospital, Bari, Italy
- 3Department of Cardiology, Medical University of Vienna, Vienna, Austria
Transcatheter aortic valve implantation (TAVI) has become the method of choice for patients with severe aortic valve stenosis, who are ineligible or at high risk for surgery. In this high risk patient population, early and late mortality and rehospitalization rates after TAVI are still relatively high. In spite of recent improvements in procedural TAVI, and establishment of risk models for poor outcome, determining individual risk remains challenging. In this context, current data from several small studies strongly suggest that blood biomarkers of myocardial injury, cardiac mechanical stretch, inflammation, and hemostasis imbalance might play an important role by providing informations on patient risk at baseline, and postprocedural progression of patient clinical conditions from days up to years post-TAVI. Although the role of biomarkers for predicting survival post-TAVI remains to be validated in large randomized studies, implementing biomarkers in clinical practice might improve risk stratification, thereby further reducing TAVI-associated morbidity and mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) has changed dramatically the treatment of severe aortic stenosis in inoperable patients or in patients at high risk for surgery. In the high risk population, particularly in the elderly, TAVI can offer a marked change in the life expectancy and quality of life of patients, and even nonagenarian patients can have successful valve replacement with acceptable periprocedural morbidity and mortality rates (1). However, early and late mortality after TAVI still remains relatively high. Results from registries and from the PARTNER trials reported 1 year all-cause mortalities between 22 and 30% (2–4). In order to improve patient evaluation and minimize futility, risk models for poor outcomes post-TAVI have been built and validated, providing Heart teams with important decision-making tools and informations (5–8). Since the prognosis of patients who benefit the most from TAVI is often not only determined by severe symptomatic aortic stenosis (AS), but also by multiple comorbidities, it would still be very useful to have parameters or biomarkers that would help to better predict the risk of major cardiovascular events for these patients.
Here, we present an overview of the role of most studied blood biomarkers for predicting poor outcome post-TAVI (Figure 1). Despite recent procedural advances that improved safety and flexibility of TAVI, these studies strongly suggest that biomarkers, in addition to risk scores, might help reducing further TAVI-associated morbidity and mortality, in a more personnalized manner.
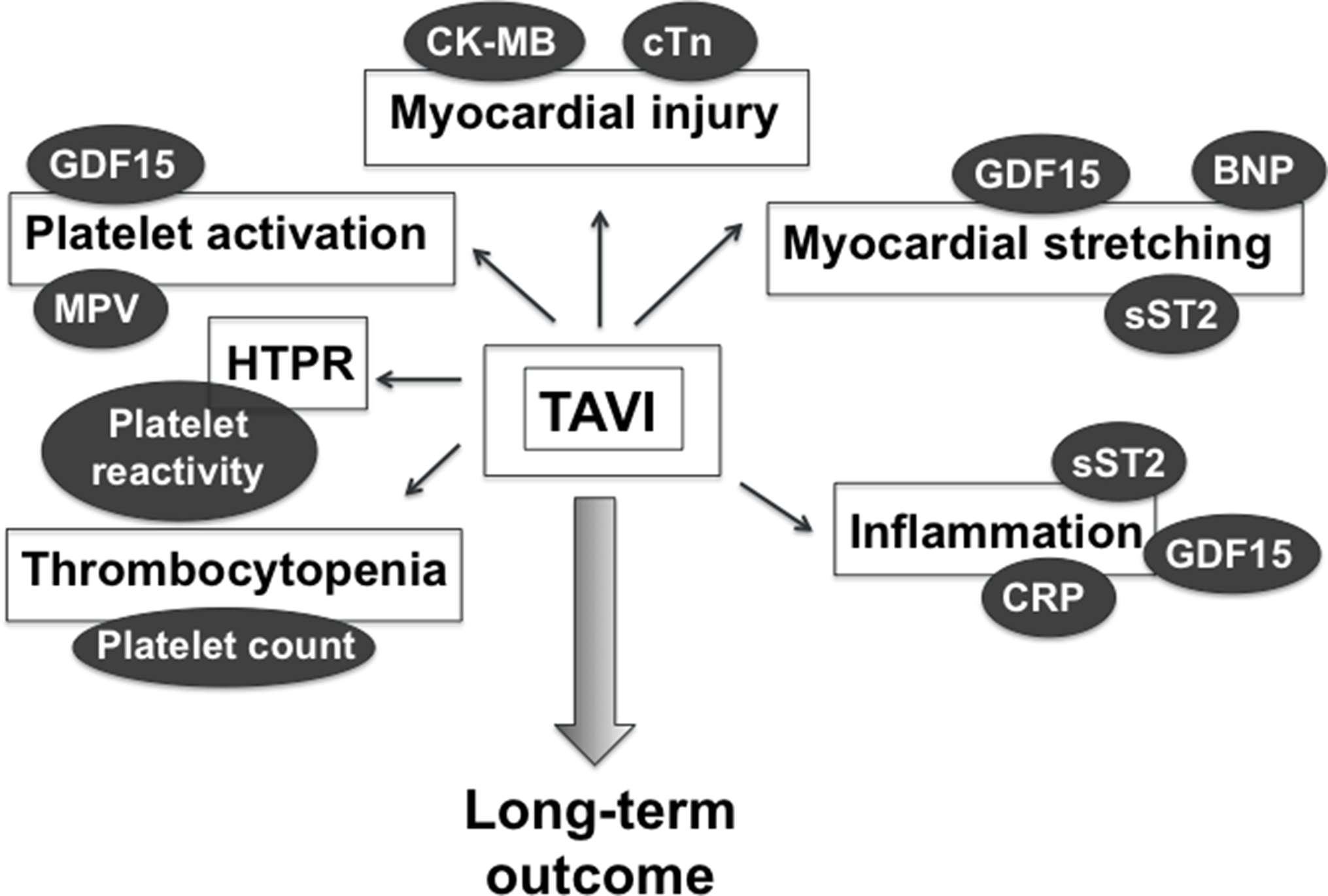
Figure 1. Blood biomarkers of TAVI-related myocardial injury, myocardial stretching, inflammation, and hemostasis imbalance that might provide postprocedural prognostic information. BNP, brain natriuretic peptide; CK-MB, creatinine kinase myocardial band; CRP, C-reactive protein; cTn, cardiac troponin; GDF-15, growth differentiation factor-15; HTPR, high on-treatment platelet reactivity; MPV, mean platelet volume.
Markers of Myocardial Injury: Creatine Kinase Myocardial Band, Cardiac Troponin
Periprocedural elevation of cardiac biomarkers of myocardial injury is common in TAVI, with greater values observed following transapical or transaortic approaches compared to transfemoral (TF) approach (9). Higher levels of myocardial injury have been associated with reduced early and midterm survival following uncomplicated TAVI (10–13). Transapical (TA) procedure significantly associates with left ventricular apical fibrosis, contributing to apical wall motion abnormalities, which may, in turn, impair myocardial recovery (14).
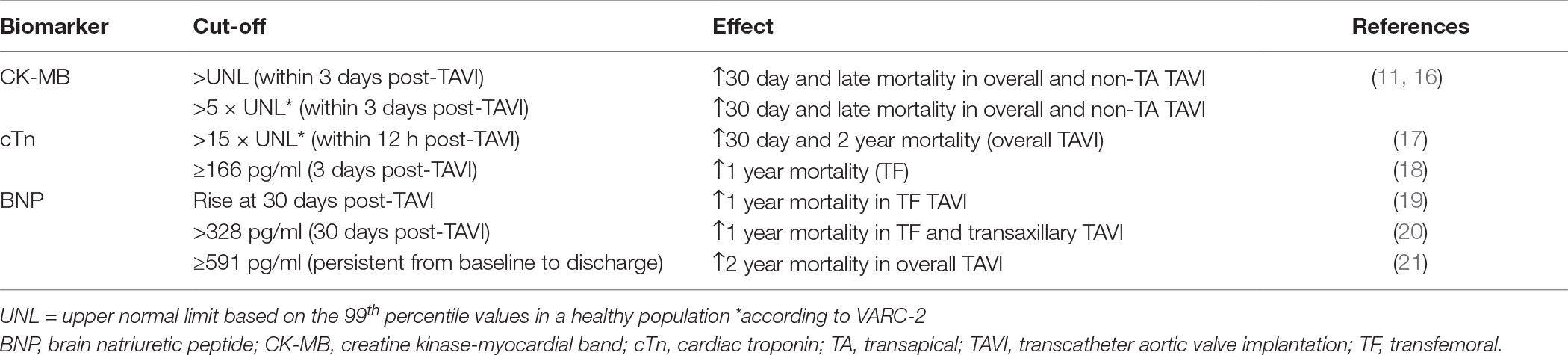
TAVI clinical endpoints have been revisited in the current Valve Academic Research Consortium (VARC) −2 document (15), defining specific biomarker cut-off values for clinically significant myocardial infarction post-TAVI. In a large multicenter study of patients undergoing TAVI with different valve types and approaches, myocardial injury, determined by postprocedural rise in levels of creatine kinase myocardial band (CK-MB), was detected in two-third of patients undergoing TAVI, especially through transapical approach (16). Higher peak of CK-MB post-TAVI translated into impaired systolic left ventricle function at 6 to 12 months follow-up, and were associated with greater acute and late mortality (Table 1). Regarding cardiac troponin (cTn), correlation with patient outcome is less clear. Two small prospective studies of TF TAVI patients showed that baseline high sensitive TnT (hs-TnT) independently predicted survival in symptomatic high-risk patients with severe AS (18, 22). Post-procedural hs-TnT rose significantly after TF TAVI until day 3, which had prognostic value for 1 year mortality. Determinants of post-procedural hs-TnT were baseline renal function, duration of intraprocedural rapid spacing, as well as pre-TAVI hs-TnT values (18). Despite hemodynamic relief, cTnT levels did not normalize even after months following successful TAVI, suggesting that the prognostic value of cTn for 1 year patient outcome may rely on long-term changes in myocardial texture. A larger study indicated that cTnT elevation above VARC-2 cut-off within 12 h post-procedure was a strong independent predictor of 30 day mortality, and remained significant at 2 years (17). In disagreement with these findings, a more recent study indicated that, in contrast to CK-MB, cTn elevation above normal limit defined by VARC-2 had no impact on late mortality of patients undergoing TF TAVI (23). Notably, VARC-2 cTnI cut-off values failed to distinguish myocardial injury from type 1 myocardial infarction (angiographically high-grade coronary artery stenoses or occlusions) in TF and TA TAVI patients, and therefore could not be used as a marker of periprocedural MI (24). Furthermore, different cut-offs may apply to TA and TF patients. These results should still be confirmed in larger randomized studies.
Markers of Myocardial Stretching: B-Type Natriuretic Peptides
Elevation of circulating B-type natriuretic peptides (BNP) that results from left ventricle myocardial stretching is commonly used in clinics to predict the onset of symptoms and adverse events in patients with severe AS (25–27).
Several studies performed on TAVI patients have assessed the value of preprocedural or serial BNP or of its biologically inactive N-terminal-proBNP (NT-proBNP) as predictors of postprocedural outcome. Initial studies found no association of baseline BNP or NT-proBNP levels and 2 month mortality after TF or TA TAVI (28, 29). A high BNP level in high-risk patients with severe AS was not an independent marker for higher mortality. These two studies showed a transient increase of BNP levels from baseline to discharge, followed by a stepwise decrease until 1 year. The authors related the transient increase in BNP to the transient left ventricle dysfunction with depression of both systolic and diastolic left ventricular (LV) function associated with TAVI (30).
In contrast, a more recent study indicated that a high preprocedural BNP, and a rise in BNP at 30 days independently predicted 1 year outcome post-TF or transaxillary TAVI (20). This result was confirmed in another study from the PARTNER trial (19) showing that an increase of BNP at 30 days was a predictor of 1 year mortality of transfemoral TAVI patients, as was moderate or severe aortic regurgitation over 1 year, and Society of Thoracic Surgeons (STS) score. Therefore, a rise in BNP at 30 days from baseline could provide prognostic information that should prompt careful clinical evaluation of these patients (Table 1).
Koskinas et al described an association between a high baseline BNP and a higher risk of all-cause death and cardiovascular death at 2 years, and a more frequent occurrence of VARC-2 clinical endpoints at 1 year (21). In this study, BNP levels increased or remained unchanged from baseline to discharge in 35% of patients, while these levels decreased in 65% of them. A baseline-to-discharge decrease was related to New York Heart Association functional improvement. Patients with persistently high BNP before intervention and at discharge had increased rates of death at 2 years. The same authors compared the prognostic values of BNP and NT-proBNP, revealing superiority of postprocedural NT-proBNP to BNP as a predictor of all-cause mortality at 2 years. Another study analyzed the prognostic value of preprocedural NT-proBNP ratio, defined as the ratio of measured NT-proBNP to maximal normal NT-proBNP values specific for age and gender, on short- and long-term mortality (31). The authors showed that baseline NT-proBNP ratio could predict all-cause mortality at 30 days and 1 year post-TAVI. Finally, in a later study, preinterventional levels of mid-regional (MR), pro-adrenomedullin (MR-proADM), and MR-pro-A-type natriuretic peptide (MR-proANP) and N-terminal pro-natriuretic peptide (NT-proBNP) were associated with 1 year cardiovascular events and all-cause mortality, while no association was found with 30 day outcome (32). Among most recently studied biomarkers, baseline levels of carbohydrate antigen 125 were reported to be superior to NT-proBNP to predict adverse outcome of TAVI (33).
Thus, altogether these studies depict some prognostic value of periprocedural BNP in TAVI that should be validated in larger multicenter studies in order to foster their implementation in current clinical practice.
Markers of Inflammation and Myocardial Stress
GDF-15
A prospective observational study was conducted that compared the prognostic value of risk scores (logistic European System for Cardiac Operative Risk Evaluation [EuroSCORE], EuroSCORE II, Society of Thoracic Surgeons predicted risk of mortality, and German aortic valve score) and circulating biomarkers (high-sensitivity C-reactive protein [hsCRP], growth differentiation factor [GDF]-15, interleukin-6, interleukin-8, and NT-proBNP) to predict all-cause mortality and rehospitalization during the first year after TAVI (34). Strikingly, GDF-15, a cytokine belonging to the family of transforming growth factor-β, appeared to be the best predictor of poor outcome when added to the logistic EuroSCORE and EuroSCORE II.
These results are in agreement with another study in which high preintervention GDF-15 levels were associated with reduced time survival post-TAVI, and were superior to NT-proBNP for patient risk stratification (35). Interestingly, high GDF-15 levels were significantly associated with several variables of poor outcome, such as reduced kidney function, diabetes, STS score, high creatinine and NT-proBNP levels, and VARC-2 criteria, suggesting that GDF-15 could integrate numerous complicating factors that could contribute to poor TAVI outcome.
Among eight biomarkers measured prior to valve replacement (GDF-15, soluble ST2 [sST2], NT-proBNP, galectin-3 [GAL-3], hs-cTnT, myeloperoxidase, hsCRP, and monocyte chemotactic protein-1 [MCP-1]), Lindman et al identified a combination of elevated levels of GDF-15, sST2 and NT-proBNP as the best predictors of 1 year mortality post-TAVI (36). However, since this study included both TAVI and patients who underwent surgical valve replacement, the utility of these three biomarkers should still be evaluated in specific populations of TAVI patients.
A recent study assessed the association of preprocedural BNP, hs-TnI, CRP, GDF-15, GAL-3, and cystatin-C with LV mycordial recovery with long-term all-cause mortality. Again, GDF-15 was strongly associated with all-cause mortality, as was CRP. GDF-15 improved the risk model when added to the STS score. Though frailty has been associated with worse 1 year outcome post-TAVR, in this study, frailty alone was not superior to GDF-15 and did not significantly improve net reclassification when added to STS score. The authors also found that a lower baseline level of GDF-15 predicted improvement of global longitudinal strain (GLS) at 1 year follow-up, which may partly explain the effect on survival. Notably, GLS at baseline was not as strongly related to outcome as GDF-15 and CRP. GLS at 1 month could, however, predict 1 year mortality. In addition, this study uncovered an intriguing correlation between GDF-15 and left ventricular mass index.
Thus, baseline GDF-15 appears as a promising biomarker that could improve current risk prediction models for patients undergoing TAVI. Furthermore, these findings indicate that inflammation may play a major role in ventricular remodeling and recovery post-TAVI. Performing serial measurements of GDF-15 and CRP would thus be interesting to determine the effect of the TAVI procedure on the progression of the inflammatory process, and its impact on patient outcome.
GDF-15 has been associated with multiple cardiovascular outcomes, possibly due to its pleiotropic effects on inflammation, oxidative stress, endothelial dysfunction, myocardial stress, and aging. Of particular interest, several studies reported an association of GDF-15 with a risk of major bleeding in acute coronary syndrome patients on dual antiplatelet therapy (37, 38). However, no studies have evaluated the possible role of GDF-15 in TAVI-related bleeding events (see below), so far.
Markers of Inflammation and Myocardial Stress
Soluble ST2
sST2 is an interleukin-1 receptor family member that acts as a decoy receptor for interleukin-33, and inhibits cardioprotective IL-33/ST2 signaling (39). Released following hemodynamic stress and cardiomyocyte strains (40), sST2 accurately predicts cardiovascular outcome of patients with acute and chronic heart failure. Consequently, sST2 was introduced in the ACC/AHA guidelines for risk stratification of patients (41). Our team showed an association of sST2 with outcome in aortic stenosis (42).
sST2 levels increase during the 24 h following TAVI, probably related to periprocedular myocardial dysfunction (30). Three studies recently indicated that preprocedural soluble ST2 might have long-term prognostic value after TAVI. The first study showed an association of baseline sST2 with 1 year mortality, with no effect at 1 month (43). sST2 correlated significantly with echocardiographic parameters, CRP, creatinine, and BNP. In a second study, sST2 was independently associated with 1 year mortality after TAVI, as were logistic EuroSCORE, chronic renal failure, and left ventricular ejection fraction (44). However, it was not superior to NT-proBNP or surgical risk scores (STS-PROM) for risk assessment, possibly due to confounding effect of inflammation on sST2 levels. In a third study, sST2 predicted mortality and the occurrence of major cardiovascular events post-TAVI (45). In contrast to the study of Stundl et al, adding sST2 to the STS score improved risk prediction of 2 year mortality.
Again, regarding sST2, future larger studies are awaited to validate these findings.
Markers of Hemostasis Imbalance
In aortic stenosis, high shear stress through aortic valve induces a loss of high molecular weight von Willebrand factor (vWF) multimers (HMWM), platelet activation and release of platelet granule content (46). Increased activation of coagulation with concurrent hypofibrinolysis is also observed (47), all this contributing to the dual clinical picture of AS, characterized by mild bleeding tendency (48), and high thrombotic risk.
Thromboembolic events, primarily stroke, are serious complications of TAVI procedures, occurring in up to 3–5% of patients. In addition, TAVI causes thrombocytopenia in one-third of patients. Importantly, while thrombocytopenia often resolves at discharge, persistent thrombocytopenia accurately predict 1 year mortality post-TAVI (49). Moreover, post-TAVI thrombocytopenia was found to be related to early post-procedural adverse events, including vascular complications, bleeding, and the need for multiple blood transfusions. To prevent TAVI-associated thromboembolic events and thrombocytopenia, a 3- to 6 month dual antiplatelet therapy (DAPT) is currently recommended for all approved balloon expandable and self-expandable transcatheter heart valve prostheses.
To determine which factors may explain the drop in platelet count that occurs after TAVI, Mitrosz et al (50) have prospectivelly analyzed changes in platelet count, along with markers of coagulation activation (F1 +2) and soluble markers of platelet activation (P-selectin, PF4) in a small cohort of severe AS, before TAVI and on the three postoperative days. While platelet reduction shortly after TAVI procedure was mostly influenced by the amount of contrast agent applied during the procedure, levels of PF4 and P-selectin positively correlated with the drop of platelet count, suggesting that thrombocytopenia is secondary to platelet activation. In-hospital major adverse cardiovascular events were observed more frequently in patients with more severe platelet count decrease (51). In another study, levels of thrombin-antithrombin complexes (TAT), plasmin-α₂-antiplasmin complex (PAP), and D-dimers significantly increased after TAVI, and D-dimer as well as PAP remained elevated until day 7, indicative of TAVI-induced increased thrombin formation and fibrinolysis (52). Post-TAVI thrombocytopenia occurred in one-fifth of patients and was associated with a significantly higher incidence of post-TAVI complications, e.g., acute kidney injury and vascular complications, whereas no impact of activated coagulation on thrombocytopenia was observed.
Thus, altogether these studies indicate that consumption of activated platelets might be the mechanism leading to thrombocytopenia after TAVI. Therefore, periprocedural platelet activation markers may potentially represent predictors of adverse outcome.
Bleeding is a more common complication of TAVI than thromboembolic events, as major and life-threatening bleeding (MLTB) according to VARC-2 can occur in up to 30% of patients (53, 54). Of note, periprocedural bleeding independently predicts all-cause mortality after TAVI (53). High mean platelet volume (MPV) and low platelet distribution width (PDW) were associated with increased risk of any bleeding and MLTB (55). Since larger platelets are more reactive and are believed to increase thromboembolic risk (56–58), this finding may be surprising. However, it is possible that high MPV could be a consequence of patient’s health state, making them more prone to bleeding. It has been shown that MPV progressively normalizes during the days following TAVI, in parallel with NT-proBNP and hemodynamic parameters (59), but its relation with patient outcome has not been investigated yet.
Importantly, a decrease of platelet reactivity is probably not the only determinant of bleeding post-TAVI. Acquired von Willebrand disease may also play a role. However, to date, evidence for a link between vWF deficiency and overt bleeding in TAVI is lacking. Indeed, the loss of HMWM does not always associate with bleeding events after valve replacement (48, 60). Though, it has recently been shown that recovery of HMWM levels post-TAVI could be used as a marker of postprocedural paravalvular regurgitation, with a positive effect on 1 year mortality (61).
Finally, a high on-treatment platelet reactivity (HTPR) to clopidogrel, due to impaired response to this antiplatelet medication, appears to be very frequent in TAVI patients (62, 63). Yet, no studies have evaluated the association of HTPR with post-TAVI outcomes. The ARTE randomized clinical trial showed a reduction of death, myocardial infarction, stroke, transient ischemic attack, or MLTB within the 3 months following TAVI with aspirin monotherapy versus DAPT (64). Thus, since there is currently no approved alternative to clopidogrel medication in >75 years TAVI patients (65), larger clinical trials aimed at defining the optimal antithrombotic regimen in these patients are awaited.
Strikingly, a recent study indicated that periprocedural changes in plasma markers of inflammation, interleukin-6 and S100A8/A9, could predict the decline in platelet count in the days following TAVI (66). A drop in platelet count and inhibition of agonist-induced platelet activation occurred in parallel with an increase of the inflammation markers following valve deployment. Thus, the inflammatory process elicited by TAVI may contribute to postprocedural thrombocytopenia. This is in line with a study showing that severe systemic inflammatory response syndrome (SIRS) was related to higher 6 month all-cause mortality after TAVI (67). This concept warrants further investigation.
Conclusion
In conclusion, blood biomarkers may enrich current risk scores in the future. BNP is readily available and easy to perform. Large studies will clarify the role of further markers.
Author Contributions
CO wrote the manuscript. PL, AN, and JB provided intellectual contributions and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
CO is a Senior Research Associate at the Belgian National Funds for Scientific Research (F.R.S-FNRS).
References
1. Murashita T, Greason KL, Suri RM, Nkomo VT, Holmes DR, Rihal CS, et al. Aortic valve replacement for severe aortic valve stenosis in the nonagenarian patient. Ann Thorac Surg (2014) 98(5):1593–7. doi: 10.1016/j.athoracsur.2014.06.015
2. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med (2010) 363(17):1597–607. doi: 10.1056/NEJMoa1008232
3. Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation (2011) 123(3):299–308. doi: 10.1161/CIRCULATIONAHA.110.946533
4. Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, et al. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol (2012) 60(19):1864–75. doi: 10.1016/j.jacc.2012.08.960
5. Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation (2014) 129(25):2682–90. doi: 10.1161/CIRCULATIONAHA.113.007477
6. Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol (2016) 68(17):1868–77. doi: 10.1016/j.jacc.2016.07.762
7. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, et al. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol (2016) 1(1):46–52. doi: 10.1001/jamacardio.2015.0326
8. Puri R, Iung B, Cohen DJ, Rodés-Cabau J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J (2016) 37(28):2217–25. doi: 10.1093/eurheartj/ehv756
9. Ribeiro HB, Dahou A, Urena M, Carrasco JL, Mohammadi S, Doyle D, et al. Myocardial injury after transaortic versus transapical transcatheter aortic valve replacement. Ann Thorac Surg (2015a) 99(6):2001–9. doi: 10.1016/j.athoracsur.2015.01.029
10. Rodés-Cabau J, Gutiérrez M, Bagur R, de Larochellière R, Doyle D, Côté M, et al. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol (2011) 57(20):1988–99. doi: 10.1016/j.jacc.2010.11.060
11. Yong ZY, Wiegerinck EM, Boerlage-van Dijk K, Koch KT, Vis MM, Bouma BJ, et al. Predictors and prognostic value of myocardial injury during transcatheter aortic valve implantation. Circ Cardiovasc Interv (2012) 5(3):415–23. doi: 10.1161/CIRCINTERVENTIONS.111.964882
12. Barbash IM, Dvir D, Ben-Dor I, Badr S, Okubagzi P, Torguson R, et al. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol (2013) 111(9):1337–43. doi: 10.1016/j.amjcard.2012.12.059
13. Paradis JM, Maniar HS, Lasala JM, Kodali S, Williams M, Lindman BR, et al. Clinical and functional outcomes associated with myocardial injury after transfemoral and transapical transcatheter aortic valve replacement: a subanalysis from the PARTNER trial (placement of aortic transcatheter valves). JACC Cardiovasc Interv (2015) 8(11):1468–79. doi: 10.1016/j.jcin.2015.06.018
14. Ribeiro HB, Larose É, de La Paz Ricapito M, Le Ven F, Nombela-Franco L, Urena M, et al. Myocardial injury following transcatheter aortic valve implantation: insights from delayed-enhancement cardiovascular magnetic resonance. EuroIntervention (2015b) 11(2):205–13. doi: 10.4244/EIJV11I2A39
15. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol (2012) 60(15):1438–54. doi: 10.1016/j.jacc.2012.09.001
16. Ribeiro HB, Nombela-Franco L, Muñoz-García AJ, Lemos PA, Amat-Santos I, Serra V, et al. Predictors and impact of myocardial injury after transcatheter aortic valve replacement: a multicenter registry. J Am Coll Cardiol (2015c) 66(19):2075–88. doi: 10.1016/j.jacc.2015.08.881
17. Koskinas KC, Stortecky S, Franzone A, O'Sullivan CJ, Praz F, Zuk K, et al. Post-procedural troponin elevation and clinical outcomes following transcatheter aortic valve implantation. J Am Heart Assoc (2016) 5(2):e002430. doi: 10.1161/JAHA.115.002430
18. Chorianopoulos E, Krumsdorf U, Geis N, Pleger ST, Giannitsis E, Katus HA, et al. Preserved prognostic value of preinterventional troponin T levels despite successful TAVI in patients with severe aortic stenosis. Clin Res Cardiol (2014) 103(1):65–72. doi: 10.1007/s00392-013-0624-8
19. O'Neill BP, Guerrero M, Thourani VH, Kodali S, Heldman A, Williams M, et al. Prognostic value of serial B-type natriuretic peptide measurement in transcatheter aortic valve replacement (from the PARTNER Trial). Am J Cardiol (2015) 115(9):1265–72. doi: 10.1016/j.amjcard.2015.01.561
20. Gotzmann M, Czauderna A, Aweimer A, Hehnen T, Bösche L, Lind A, et al. B-type natriuretic peptide is a strong independent predictor of long-term outcome after transcatheter aortic valve implantation. J Heart Valve Dis (2014) 23(5):537–44.
21. Koskinas KC, O'Sullivan CJ, Heg D, Praz F, Stortecky S, Pilgrim T, et al. Effect of B-type natriuretic peptides on long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol (2015) 116(10):1560–5. doi: 10.1016/j.amjcard.2015.08.016
22. Frank D, Stark S, Lutz M, Weissbrodt A, Freitag-Wolf S, Petzina R, et al. Preprocedural high-sensitive troponin predicts survival after transcatheter aortic valve implantation (TAVI). Int J Cardiol (2013) 169(3):e38–9. doi: 10.1016/j.ijcard.2013.08.108
23. Koifman E, Garcia-Garcia HM, Alraies MC, Buchanan K, Hideo-Kajita A, Steinvil A, et al. Correlates and significance of elevation of cardiac biomarkers elevation following transcatheter aortic valve implantation. Am J Cardiol (2017) 120(5):850–6. doi: 10.1016/j.amjcard.2017.05.059
24. Liebetrau C, Kim WK, Meyer A, Arsalan M, Gaede L, Blumenstein JM, et al. Identification of periprocedural myocardial infarction using a high-sensitivity troponin i assay in patients who underwent transcatheter aortic valve implantation. Am J Cardiol (2017) 120(7):1180–6. doi: 10.1016/j.amjcard.2017.06.069
25. Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: focus on natriuretic peptides. Can J Cardiol (2014) 30(9):1027–34. doi: 10.1016/j.cjca.2014.07.014
26. Henri C, Magne J, Dulgheru R, Davin L, Laaraibi S, Voilliot D, et al. Use fulness of serial B-type natriuretic peptide assessment in asymptomatic aortic stenosis. Am J Cardiol (2014) 114(3):441–8. doi: 10.1016/j.amjcard.2014.04.053
27. Shen M, Tastet L, Bergler-Klein J, Pibarot P, Clavel MA. Blood, tissue and imaging biomarkers in calcific aortic valve stenosis: past, present and future. Curr Opin Cardiol (2018) 33(2):125–33. doi: 10.1097/HCO.0000000000000487
28. Pfister R, Wahlers T, Baer FM, Scherner M, Strauch J, Erdmann E. Utility of NT-pro-BNP in patients undergoing transapical aortic valve replacement. Clin Res Cardiol (2010) 99(5):301–7. doi: 10.1007/s00392-010-0118-x
29. Ben-Dor I, Minha S, Barbash IM, Aly O, Dvir D, Deksissa T, et al. Correlation of brain natriuretic peptide levels in patients with severe aortic stenosis undergoing operative valve replacement or percutaneous transcatheter intervention with clinical, echocardiographic, and hemodynamic factors and prognosis. Am J Cardiol (2013) 112(4):574–9. doi: 10.1016/j.amjcard.2013.04.023
30. Dworakowski R, Wendler O, Bhan A, Smith L, Pearson P, Alcock E, et al. Successful transcatheter aortic valve implantation (TAVI) is associated with transient left ventricular dysfunction. Heart (2012) 98(22):1641–6. doi: 10.1136/heartjnl-2012-302505
31. Stähli BE, Gebhard C, Saleh L, Falk V, Landmesser U, Nietlispach F, et al. N-terminal pro-B-type natriuretic peptide-ratio predicts mortality after transcatheter aortic valve replacement. Catheter Cardiovasc Interv (2015) 85(7):1240–7. doi: 10.1002/ccd.25788
32. Baldenhofer G, Laule M, Mockel M, Sanad W, Knebel F, Dreger H, et al. Mid-regional pro-adrenomedullin (MR-proADM) and mid-regional pro-atrial natriuretic peptide (MR-proANP) in severe aortic valve stenosis: association with outcome after transcatheter aortic valve implantation (TAVI). Clin Chem Lab Med (2017) 55(2):275–83. doi: 10.1515/cclm-2015-0419
33. Rheude T, Pellegrini C, Schmid H, Trenkwalder T, Mayr NP, Joner M, et al. Comparison of carbohydrate antigen 125 and N-terminal pro-brain natriuretic peptide for risk prediction after transcatheter aortic valve implantation. Am J Cardiol (2018) 121(4):461–8. doi: 10.1016/j.amjcard.2017.11.020
34. Sinning JM, Wollert KC, Sedaghat A, Widera C, Radermacher MC, Descoups C, et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J (2015) 170(4):821–9. doi: 10.1016/j.ahj.2015.07.003
35. Krau NC, Lünstedt NS, Freitag-Wolf S, Brehm D, Petzina R, Lutter G, et al. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur J Heart Fail (2015) 17(9):945–55. doi: 10.1002/ejhf.318
36. Lindman BR, Breyley JG, Schilling JD, Vatterott AM, Zajarias A, Maniar HS, et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart (2015a) 101(17):1382–8. doi: 10.1136/heartjnl-2015-307742
37. Hagström E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, et al. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J (2016) 37(16):1325–33. doi: 10.1093/eurheartj/ehv491
38. Lindholm D, Hagström E, James SK, Becker RC, Cannon CP, Himmelmann A, et al. Growth differentiation factor 15 at 1 month after an acute coronary syndrome is associated with increased risk of major bleeding. J Am Heart Assoc (2017) 6(4):e005580. doi: 10.1161/JAHA.117.005580
39. Dieplinger B, Mueller T. Soluble ST2 in heart failure. Clin Chim Acta (2015) 443:57–70. doi: 10.1016/j.cca.2014.09.021
40. Weinberg EO, Shimpo M, de Keulenaer GW, Macgillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation (2002) 106(23):2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
41. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol (2013) 62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019
42. Lancellotti P, Dulgheru R, Magne J, Henri C, Servais L, Bouznad N, et al. Elevated plasma soluble ST2 Is associated with heart failure symptoms and outcome in aortic stenosis. PLoS ONE (2015) 10(9):e0138940. doi: 10.1371/journal.pone.0138940
43. Wernly B, Lichtenauer M, Jirak P, Eder S, Reiter C, Kammler J, et al. Soluble ST2 predicts 1-year outcome in patients undergoing transcatheter aortic valve implantation. Eur J Clin Invest (2017) 47(2):149–57. doi: 10.1111/eci.12719
44. Stundl A, Lünstedt NS, Courtz F, Freitag-Wolf S, Frey N, Holdenrieder S, et al. Soluble ST2 for risk stratification and the prediction of mortality in patients undergoing transcatheter aortic valve implantation. Am J Cardiol (2017) 120(6):986–93. doi: 10.1016/j.amjcard.2017.06.033
45. Schmid J, Stojakovic T, Zweiker D, Scharnagl H, Maderthaner RD, Scherr D, et al. ST2 predicts survival in patients undergoing transcatheter aortic valve implantation. Int J Cardiol (2017) 244:87–92. doi: 10.1016/j.ijcard.2017.06.066
46. Rouzaud-Laborde C, Delmas C, Pizzinat N, Tortosa F, Garcia C, Mialet-Perez J, et al. Platelet activation and arterial peripheral serotonin turnover in cardiac remodeling associated to aortic stenosis. Am J Hematol (2015) 90(1):15–19. doi: 10.1002/ajh.23855
47. Natorska J, Bykowska K, Hlawaty M, Marek G, Sadowski J, Undas A. Increased thrombin generation and platelet activation are associated with deficiency in high molecular weight multimers of von Willebrand factor in patients with moderate-to-severe aortic stenosis. Heart (2011) 97(24):2023–8. doi: 10.1136/hrt.2010.217273
48. Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med (2003) 349(4):343–9. doi: 10.1056/NEJMoa022831
49. Dvir D, Généreux P, Barbash IM, Kodali S, Ben-Dor I, Williams M, et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: clinical correlates and association with outcomes. Eur Heart J (2014) 35(38):2663–71. doi: 10.1093/eurheartj/ehu082
50. Mitrosz M, Kazimierczyk R, Sobkowicz B, Waszkiewicz E, Kralisz P, Frank M, et al. The causes of thrombocytopenia after transcatheter aortic valve implantation. Thromb Res (2017) 156:39–44. doi: 10.1016/j.thromres.2017.05.020
51. Gallet R, Seemann A, Yamamoto M, Hayat D, Mouillet G, Monin JL, et al. Effect of transcatheter (via femoral artery) aortic valve implantation on the platelet count and its consequences. Am J Cardiol (2013) 111(11):1619–24. doi: 10.1016/j.amjcard.2013.01.332
52. Sedaghat A, Falkenberg N, Sinning JM, Kulka H, Hammerstingl C, Nickenig G, et al. TAVI induces an elevation of hemostasis-related biomarkers, which is not causative for post-TAVI thrombocytopenia. Int J Cardiol (2016) 221:719–25. doi: 10.1016/j.ijcard.2016.07.094
53. Amabile N, Azmoun A, Ghostine S, Ramadan R, Haddouche Y, Raoux F, et al. Incidence, predictors and prognostic value of serious hemorrhagic complications following transcatheter aortic valve implantation. Int J Cardiol (2013) 168(1):151–6. doi: 10.1016/j.ijcard.2012.09.025
54. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg (2013) 145(1):6–23. doi: 10.1016/j.jtcvs.2012.09.002
55. Huczek Z, Kochman J, Kowara MK, Wilimski R, Scislo P, Scibisz A, et al. Baseline platelet indices and bleeding after transcatheter aortic valve implantation. Blood Coagul Fibrinolysis (2015) 26(5):527–32. doi: 10.1097/MBC.0000000000000283
56. Bath P, Algert C, Chapman N,Neal B PROGRESS Collaborative Group. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke (2004) 35(3):622–6. doi: 10.1161/01.STR.0000116105.26237.EC
57. Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol (2005) 46(2):284–90. doi: 10.1016/j.jacc.2005.03.065
58. Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost (2010) 8(1):148–56. doi: 10.1111/j.1538-7836.2009.03584.x
59. Gul M, Uyarel H, Akgul O, Uslu N, Yildirim A, Eksik A, et al. Hematologic and clinical parameters after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis. Clin Appl Thromb Hemost (2014) 20(3):304–10. doi: 10.1177/1076029612462762
60. Bolliger D, dell-Kuster S, Seeberger MD, Tanaka KA, Gregor M, Zenklusen U, et al. Impact of loss of high-molecular-weight von Willebrand factor multimers on blood loss after aortic valve replacement. Br J Anaesth (2012) 108(5):754–62. doi: 10.1093/bja/aer512
61. van Belle E, Rauch A, Vincent F, Robin E, Kibler M, Labreuche J, et al. Von willebrand factor multimers during transcatheter aortic-valve replacement. N Engl J Med (2016) 375(4):335–44. doi: 10.1056/NEJMoa1505643
62. Polzin A, Schleicher M, Seidel H, Scharf RE, Merx MW, Kelm M, et al. High on-treatment platelet reactivity in transcatheter aortic valve implantation patients. Eur J Pharmacol (2015) 751:24–7. doi: 10.1016/j.ejphar.2015.01.028
63. Orvin K, Eisen A, Perl L, Zemer-Wassercug N, Codner P, Assali A, et al. Platelet reactivity in patients undergoing transcatheter aortic valve implantation. J Thromb Thrombolysis (2016) 42(1):11–18. doi: 10.1007/s11239-015-1322-3
64. Rodés-Cabau J, Masson JB, Welsh RC, Garcia del Blanco B, Pelletier M, Webb JG, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (aspirin versus aspirin + clopidogrel following transcatheter aortic valve implantation) randomized clinical trial. JACC Cardiovasc Interv (2017) 10(13):1357–65. doi: 10.1016/j.jcin.2017.04.014
65. Roberts DI, Nawarskas JJ. Treatment options for patients with poor clopidogrel response. Cardiol Rev (2013) 21(6):309–17. doi: 10.1097/CRD.0b013e3182a72fab
66. Sexton TR, Wallace EL, Chen A, Charnigo RJ, Reda HK, Ziada KM, et al. Thromboinflammatory response and predictors of outcomes in patients undergoing transcatheter aortic valve replacement. J Thromb Thrombolysis (2016) 41(3):384–93. doi: 10.1007/s11239-015-1326-z
Keywords: TAVI, blood biomarkers, inflammation, myocardial stress, platelet, thrombocytopenia
Citation: Oury C, Nchimi A, Lancellotti P and Bergler-Klein J (2018). Can Blood Biomarkers Help Predicting Outcome in Transcatheter Aortic Valve Implantation? Front. Cardiovasc. Med. 5:31. doi: 10.3389/fcvm.2018.00031
Received: 05 February 2018; Accepted: 16 March 2018;
Published: 28 March 2018
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Guoping Li, Massachusetts General Hospital and Harvard Medical School, United StatesMaurizio Acampa, Azienda Ospedaliera Universitaria Senese, Italy
Copyright © 2018 OURY, Nchimi, Lancellotti and Bergler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cécile Oury, cecile.oury@uliege.be
 Cécile Oury
Cécile Oury Alain Nchimi
Alain Nchimi Patrizio Lancellotti
Patrizio Lancellotti Jutta Bergler-Klein3
Jutta Bergler-Klein3