Congenital Asplenia Interrupts Immune Homeostasis and Leads to Excessive Systemic Inflammation in Zebrafish
- 1Institute of Three Gorges Ecological Fisheries of Chongqing, College of Fisheries, Southwest University, Chongqing, China
- 2Key Laboratory of Freshwater Fish Reproduction and Development (Ministry of Education), The Key Laboratory of Aquatic Science of Chongqing, Southwest University, Chongqing, China
Splenectomy or congenital asplenia in humans increases susceptibility to infections. We have previously reported that congenital asplenia in zebrafish reduces resistance to Aeromonas hydrophila infection. However, the molecular mechanism of systemic immune response in congenitally asplenic individuals is largely unexplored. In this study, we found that pro-inflammatory cytokines were more highly induced in congenitally asplenic zebrafish than wild-type after pathogenic A. hydrophila infection and lipopolysaccharide exposure. In addition, a higher aggregation of apoptotic cells was observed in congenitally asplenic zebrafish than that in wild-type. Next, we examined the transcriptome profiles of whole kidneys from wild-type and congenitally asplenic zebrafish to investigate the effects of congenital asplenia on innate and adaptive immune responses induced by the inactivated A. hydrophila. Congenital asplenia inactivated the splenic anti-inflammatory reflex, disrupted immune homeostasis, and induced excessive inflammation as evidenced by the highly induced stress response–related biological processes, inflammatory and apoptosis-associated pathways, and pro-inflammatory cytokines/chemokines in congenitally asplenic zebrafish compared with wild-type after vaccination. In addition, complement component genes (c3a.1, c3a.6, c4, c6, and c9) and several important immune-related genes (tabp.1, tap1, hamp, prg4b, nfil3, defbl1, psmb9a, tfr1a, and sae1) were downregulated in congenitally asplenic zebrafish. Furthermore, congenital asplenia impaired adaptive immunity as demonstrated by downregulation of biological processes and signaling pathways involved in adaptive immune response after vaccination in congenitally asplenic zebrafish. The expression of MHCII/IgM was also significantly reduced in the congenitally asplenic zebrafish when compared with wild-type. Together, our study provides an in-depth understanding of spleen function in controlling immune homeostasis and may offer insight into the pathological response in splenectomized or congenitally asplenic patients after infections.
Introduction
The spleen is a secondary lymphatic and reticuloendothelial organ that performs several functions related to hemocatheresis, blood cell storage, and immune response against infections as well as extramedullary hematopoiesis (Mebius and Kraal, 2005; Bronte and Pittet, 2013). As an immune-related organ, the spleen is crucial in regulating immune homoeostasis through its ability to link innate and adaptive immunity and its function in protecting against infections. Over the past couple of years, the spleen has been considered an accessory organ because surgically splenectomized patients can live normal lives. However, the impact of splenectomy is equivocal. Studies show that the absence of the spleen, or hyposplenia and splenectomy in humans, often carries an increased risk of overwhelming infections caused by encapsulated bacteria (Thomsen et al., 2009; Ram et al., 2010; Kristinsson et al., 2014). Overwhelming postsplenectomy infection (OPSI) is a serious disease that may rapidly progress to fulminant infection and death in 50% of cases in a short time period for patients suspected to have splenectomy (Dahyot-Fizelier et al., 2013; Sinwar, 2014). Consequently, there has been renewed interest in preserving the spleen in circumstances in which splenectomy is thought to be necessary (Tracy and Rice, 2008).
Asplenia or hyposplenia may occur as a result of splenectomy following disease or as a congenital anomaly. Congenital asplenia is a rare disorder characterized by the absence of a spleen at birth and has been referred to as a primary immune deficiency. Patients with congenital asplenia have increased susceptibility to invasive infections along with a high mortality rate despite aggressive therapy. As with the splenectomized patients, congenitally asplenic individuals are susceptible to overwhelming pneumococcal infection throughout life (Mahlaoui et al., 2011). Studies on congenitally asplenic mice reveal that the generation and survival of B-1a cells, a B cell subset that cooperates with the innate immune system to control early bacterial and viral growth, was impaired, and asplenic mice exhibit slowed IgM production and clearance of injected bacteria, resulting in fatal sepsis (Karrer et al., 1997; Wardemann et al., 2002). Compared with the research on splenectomy, reports on the effects of congenital asplenia are relatively scarce. Thus, the physiological and pathological processes in congenitally asplenic patients under pathogen challenge has become the focus of attention.
Zebrafish (Danio rerio) are used for studying animal and human infectious disease (Novoa and Figueras, 2012) and are an accepted model system for filling an important gap between lower vertebrates and mammals in studies of host–pathogen interactions. The immune system of adult zebrafish is similar to that of mammals, including almost all lymphoid organs and immune cell types (Renshaw and Trede, 2012; van der Vaart et al., 2012). The similarities between zebrafish and mammalian immune systems enable numerous researchers to establish models of bacterial and viral infections in zebrafish. In our previous study, we generated a tlx1–/– zebrafish homozygous mutant, which exhibits congenital asplenia without any other obvious detected defects. Congenital asplenia reduces resistance to Aeromonas hydrophila infection in zebrafish as evidenced by the mortality of the congenitally asplenic zebrafish being significantly increased when compared with wild-type (WT) control after infection (Xie et al., 2019). In addition, with the outbreak of infectious diseases, the research around spleen function in fish immunity has been increasingly valued in recent years. Considering the importance of the spleen in mammals and in fish, the molecular mechanism of systemic immune response in congenitally asplenic individuals under pathogen infection must be fully explored.
In this study, the systemic inflammatory response induced by A. hydrophila challenge and lipopolysaccharide (LPS) treatment was investigated in both WT and congenitally asplenic zebrafish. In addition, to gain a full understanding of how congenital asplenia affects the immune response, we employed high-throughput sequencing technology to examine the global transcription profiles of whole kidneys (head-kidney and kidney) from both WT and congenitally asplenic zebrafish injected with an inactivated A. hydrophila vaccine at 6 h and 10 d, the time corresponding to the occurrence of innate and adaptive immune responses. Comparative transcriptome analyses were then conducted to identify differentially expressed genes between these groups. Our study provides a better understanding of the effects of congenital asplenia on systemic immunity, and in the meantime, understanding the molecular mechanism of systemic immune response in congenitally asplenic zebrafish after vaccination may contribute to pathological understanding and complication control in splenectomized or congenitally asplenic patients after infections.
Materials and Methods
Zebrafish
The WT AB and tlx1–/– mutant fish (Xie et al., 2019) strains were used in this study. Zebrafish were maintained in flow-through aquaria at 28°C ± 0.5°C with a photoperiod of 14 h light and 10 h dark. Fish were fed twice daily with newly hatched brine shrimp (Brine Shrimp Direct). All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Southwest University, China.
Pathogenic A. hydrophila Infection and LPS Exposure
A. hydrophila strains, which were used in our previous study, were grown in LB medium for 14 h at 30°C with shaking at 170 rpm. Bacterial cells were harvested via centrifugation at 5000 rpm for 5 min and then resuspended in phosphate buffered saline (PBS) (Beyotime, China), and the concentration was first determined via a turbidity meter (Qiwei, China). Then, bacteria were serially diluted and counted by plating on LB solid medium. About 108 CFU mL−1 bacteria were added to the tank water. Fifty zebrafish larvae, randomly selected at 4 days postfertilization (dpf) from each group (WT or congenitally asplenic zebrafish), were exposed to A. hydrophila as an immune challenge. Animals were sampled at 6 h after exposure to A. hydrophila for qRT-PCR. Another 100 larvae at 4 dpf from each WT or congenitally asplenic zebrafish were also randomly selected for exposure to LPS (50 μg/mL) for 2 d. To evaluate the differences in inflammatory response among WT and congenitally asplenic zebrafish, animals sampled at 6 h after exposure to LPS were used for qRT-PCR; animals sampled at 2 d after exposure to LPS were used for apoptosis assay. Zebrafish larvae treated with PBS were used as a control group.
Enzyme-Linked Immunosorbent Assay
Triplicate groups of at least 50 WT and congenitally asplenic larval zebrafish were challenged with A. hydrophila and exposed to LPS, respectively. Animals were sampled at 0 (blank control), 12, and 24 h after pathogenic A. hydrophila infection and LPS exposure for measurement of immune parameters. Cytokines (e.g., Il1β, Il6, and Tnfα) were detected with enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s protocol. The zebrafish tumor necrosis factor α (Tnf-α), Interleukin 6 (Il-6), and Interleukin 1β (Il-1β) ELISA kits were purchased from Sinobestbio (China). The cytokines were expressed as pg/mg protein.
Apoptosis Assay
For the apoptosis assay, the in situ cell death detection kit (C1089, Beyotime, Shanghai, China) was used in our work. Briefly, samples were collected and fixed with 4% PFA overnight. Next, larvae samples were washed with PBS at least three times and then treated with 1% protease K (without DNase) for 30 min. Subsequently, larvae were rinsed with PBS three times again and then labeled with terminal deoxynucleotidyl transferase reaction mixture for 60 min at 37°C under dark conditions. Finally, samples were washed again with PBS, and then, photographs were taken using a LEICA DM6000 microscope (Leica, Germany).
Vaccination and Sampling
Because A. hydrophila challenge results in high mortality, most of the challenged zebrafish do not survive before the activation of adaptive immunity (data not shown). A previous study shows that the vaccine is able to induce both innate and adaptive immune responses in zebrafish (Zhang et al., 2012). Thus, an inactivated A. hydrophila vaccine was used. Briefly, A. hydrophila cells were inactivated via addition of 0.3% formalin to the culture, followed by incubation at 4°C for 48 h with gentle agitation. After incubation with formalin, 100 μl of formalin-treated bacteria were plated in LB solid medium and cultured at 30°C for 24 h to ensure the effectiveness of bacterial inactivation. Completely inactivated bacterial cells were washed thrice with PBS. For the vaccination, triplicate groups of 30 WT and congenitally asplenic adult zebrafish were injected with 10 μl of inactivated bacterial cells (108 CFU mL−1). After euthanasia with 300 ng/ml MS-222, whole kidneys, liver, intestine, gill, and skin were sampled at 0 (blank control), 6 h, and 10 d following intraperitoneal injection with duplicates of 10 fish in each treatment group. The samples were stored at -80°C until RNA extraction.
cDNA Library Construction and Illumina Deep Sequencing
Total RNA was extracted using the RNAiso Plus kit (Takara, Japan) according to the manufacturer’s protocol and treated with RNase-free DNase I to remove genomic DNA contamination. For each sample, total RNA content was measured using a SmartSpecTM Plus spectrophotometer (Bio-Rad, Hercules, CA, USA), and the quality was assessed by agarose gel electrophoresis. Thereafter, the quality and quantity of RNA were assessed using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). Each sample must meet the conditions of 28S/18S RNA ratio ≥ 1.8 and RNA integrity number > 8.5 for further processing. The high-quality RNA samples were subsequently submitted to the Sangon Biotech Co. Ltd. (Shanghai, china) for library preparation and sequencing. A total amount of 2 μg RNA per sample was used as input material for the RNA sample preparations. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. First-strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H). Second-strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, the adaptor was ligated to prepare for the library. To select cDNA fragments of preferentially 150~200 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, USA). RNA-sequencing data were filtered to remove low-quality reads containing reads with adaptors, reads with >10% of the unknown nucleotides (N), and reads with >50% low-quality (Q-value ≤ 20) bases. Clean reads were mapped to the reference genome (GRCz11) by HISAT2 (version 2.0) with default parameters.
Expression Analysis
Gene expression values of the transcripts were computed by StringTie (version 1.3.3b). Principal component analysis (PCA) and principal coordinate analysis were performed to reflect the distance and difference between samples. The transcripts per million (TPM) eliminates the influence of gene lengths and sequencing discrepancies to enable direct comparison of gene expression between samples. DESeq2 (version 1.12.4) was used to determine differentially expressed genes (DEGs) between two samples. Genes were considered as significant differentially expressed if q-value < 0.001 and |Fold-Change| > 1.5. Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with false discovery rate (q-value) < 0.05 were considered as significantly altered.
Quantitative RT-PCR (qRT-PCR)
All qRT-PCRs were carried out in a CFX96 Real-Time PCR Detection System (Bio-Rad, USA) in 20-μl reactions containing 10 μl of SsoFast™ EvaGreen® SuperMix (Bio-Rad, USA), 1 μl of diluted cDNA or nuclease-free water as a negative control, 8 μl of nuclease-free water, and 0.5 μl of 10 μM stock solutions of each primer. The PCRs were initiated by denaturation at 95°C for 30 s, followed by 40 amplification cycles of 95°C for 5 s and 60°C for 5 s. The melting curve for the PCR products was generated by heating from 65°C to 95°C in 0.5°C increments with a 5-s dwell time, and a plate read was performed at each temperature. The relative expression levels of the target genes were evaluated using the formula R = 2−ΔΔCt with ef1α as a reference gene to normalize the expression values. Three biological replicates were performed for each sample. nfkb1and nfkb2 primers were used as described by Wang et al. (2018). MHCII, IgM, IgZ1, and IgZ2 primers were used as described by Liu et al. (2015). The primer sequences used for the PCRs are listed in Table S1.
Statistical Analysis
Data are expressed as the mean ± SD. Student’s t-test and one-way ANOVA followed by the Duncan post-hoc test were performed to determine statistical significance at P <.05.
Results
Effects of Congenital Asplenia on Inflammatory Response in Zebrafish Larva
In this part, zebrafish larvae at 4 dpf (mouthopening stage) were exposed to A. hydrophila. To evaluate the early immune response in WT and congenitally asplenic zebrafish after pathogen infection, expression levels of several innate immunity-related genes were analyzed at 6 h after A. hydrophila challenge. As shown in Figure 1A, pro-inflammatory cytokines (il1β, il6, tnfα, and tnfβ) and NF-κB subunits (nfkb2) were more highly induced in congenitally asplenic zebrafish when compared with WT. However, the expression of the anti-inflammatory cytokine gene (il10) failed to upregulate, indicating the uncontrolled inflammatory response in congenitally asplenic zebrafish. To test this hypothesis, inflammatory response was examined in zebrafish larvae after exposure to LPS (activator of the inflammatory response). As shown in Figure 1B, except for il1β, the expression of il6, tnfα, tnfβ, and nfkb2 were also found to be more highly induced after LPS treatment as the same expression patterns as in the A. hydrophila challenge. Then, ELISA was performed to examine the Il1β, Il6, and Tnfα proteins after A. hydrophila challenge and LPS exposure. As shown in Figure 1C, Il1β, Il6, and Tnfα protein levels were significantly upregulated, and they exhibited higher expression patterns in congenitally asplenic zebrafish when compared with WT after A. hydrophila challenge at 12 and 24 h. In addition, Il6 also exhibited significant upregulation and higher expression patterns in congenitally asplenic zebrafish after being exposed to LPS at 12 h. Then, Il1β and Tnfα were more highly induced in congenitally asplenic zebrafish at 24 h post-LPS exposure (Figure 1D). Because excessive inflammatory cytokine release possibly induces apoptosis, we further measured the apoptosis after LPS treatment by the TUNEL assay. As shown in Figure 1E, an increased apoptosis signal was founded in congenitally asplenic zebrafish when compared with WT before LPS treatment. After exposure to LPS for 2 d, a high aggregation of apoptotic cells was observed in the trunk of congenitally asplenic zebrafish. In contrast, only a few TUNEL-positive cells were found in WT zebrafish after LPS exposure.
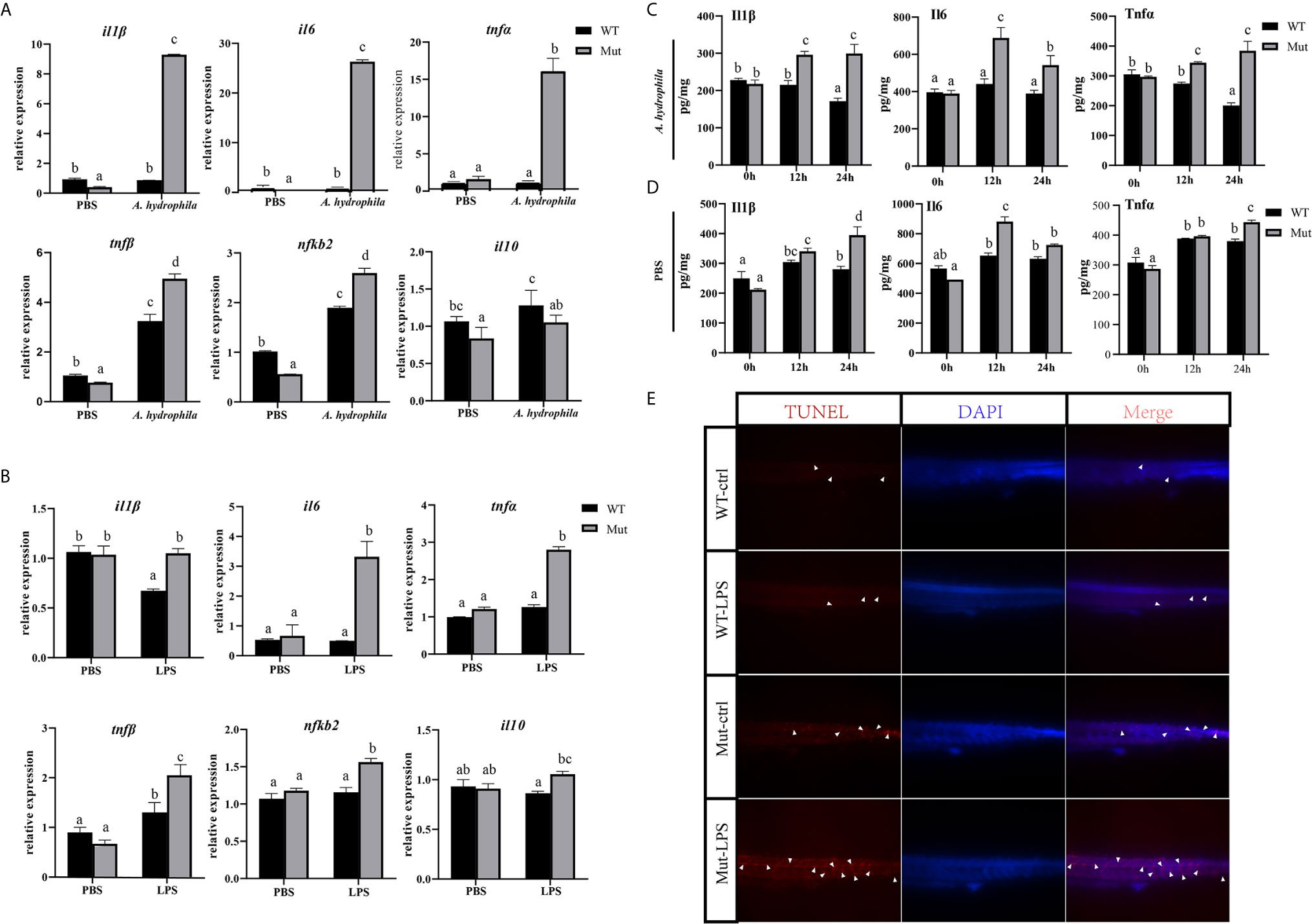
Figure 1 Effects of congenital asplenia on inflammatory response in zebrafish larvae. (A) The expression of inflammatory response–related genes after pathogenic A. hydrophila infection in zebrafish larvae. (B) The expression of inflammatory response–related genes after LPS exposure. (C, D) The expression of Il1β, Il6, and Tnfα proteins after A. hydrophila challenge and LPS by ELISA. (E) A high aggregation of apoptotic cells was observed in the trunk of congenitally asplenic zebrafish by TUNEL assay. The white arrowhead indicates TUNEL-positive cells. Different letters (a, b, c, etc.) indicate significant differences (p < .05).
RNA Sequencing, Read Mapping, and Differential Expression Analysis
The adaptive immunity of zebrafish occurs until 4 weeks after fertilization; thereby, adult zebrafish were used in this part. To gain full understanding of the effects of congenital asplenia on systemic immunity, RNA-Seq was applied to compare the innate and adaptive immune response between WT and congenitally asplenic zebrafish in the whole kidney (kidney and head-kidney) tissue. Samples (WT_0h and Mut_0h) were collected before vaccination, and samples (WT_6h and Mut_6h; WT_10d and Mut_10d) were obtained after vaccination for 6 h and 10 d, respectively. The library construction, quality control analyses, and reads mapped information of the 18 libraries are summarized in Table 1. In total, these results indicate that the RNA-seq data are of high quality and can be used for further analysis. The PCA score plot shows that the three RNA-seq samples as biological replicates were clustered (Figure S1). All the sequencing data were submitted to the Sequence Read Archive in the National Center for Biotechnology Information under the accession numbers PRJNA688352 and PRJNA688655.
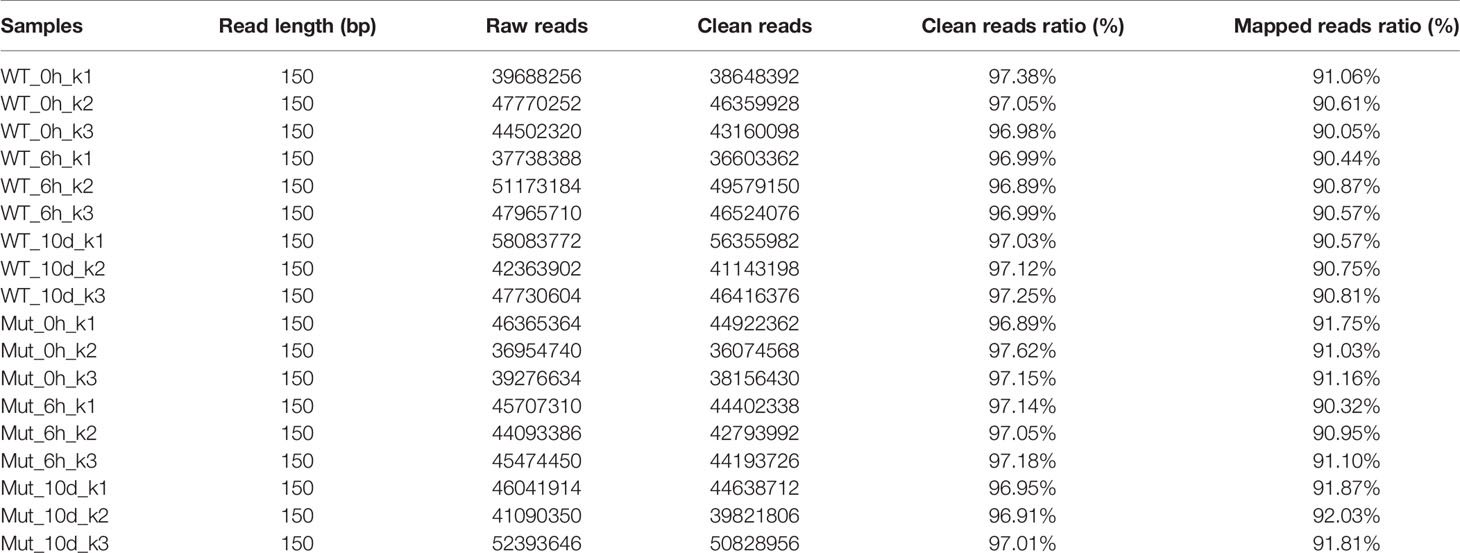
Table 1 Summary of the sequencing data. Each sample was triplicated as indicated by the number after the hyphen, e.g., WT_0h_k1, WT_0h_k2, WT_0h_k3.
Based on the following criteria: |Fold-Change| > 1.5 and adjusted q-value < 0.001, the number of DEGs was analyzed, and the data are shown in Figure S2. The number of DEGs identified in WT-6h_vs_WT-0h was 4388 with 2764 being upregulated DEGs and 1634 being downregulated DEGs. The number of DEGs identified in Mut-6h_vs_Mut-0h was 4494 with 2343 upregulated DEGs and 2153 downregulated DEGs. The number of DEGs identified in WT-10d_vs_WT-0h was 1681 with 1075 being upregulated DEGs and 606 being downregulated DEGs, and the number of DEGs in Mut-10d_vs_Mut-0h was 1231 with 197 upregulated DEGs and 1034 downregulated DEGs. When comparing the Mut group with the WT group, the number of DEGs in Mut-6h_vs_WT-6h was 1735 with 846 DEGs being upregulated and 889 DEGs being downregulated in the Mut group; the number of DEGs in Mut-10d_vs_WT-10d was 1963 with 640 DEGs being upregulated and 1323 DEGs being downregulated in the Mut group. Then, all of the DEGs with significant change were subject to further GO and KEGG enrichment analysis.
Zebrafish With Congenital Asplenia Are More Hyperresponsive After Vaccination
To investigate whether vaccination could successfully induce immune response in WT and asplenic fish, comparative transcriptome analysis was performed on WT-6h_vs_WT-0h and Mut-6h_vs_Mut-0h. In KEGG analysis, the top 30 enriched pathways of WT-6h_vs_WT-0h and Mut-6h_vs_Mut-0h are shown in Figure 2. The KEGG enrichment analysis showed that 14 and 12 pathways were significantly affected in asplenic and WT zebrafish, respectively. Both of the two contrasts clustered several conserved immune-related pathways, including the Jak-STAT and toll-like receptor signaling pathways and glutathione metabolism. The P53 signaling pathway was uniquely enriched as the topmost represented KEGG categories in Mut-6h_vs_Mut-0h. In addition, the TNF, RIG-I-like receptor, and FoxO signaling pathways were also uniquely and significantly enriched in Mut-6h_vs_Mut-0h (Figure 2). Detailed information of significantly enriched KEGG pathways in WT-6h_vs_WT-0h and Mut-6h_vs_Mut-0h is listed in Table S2.
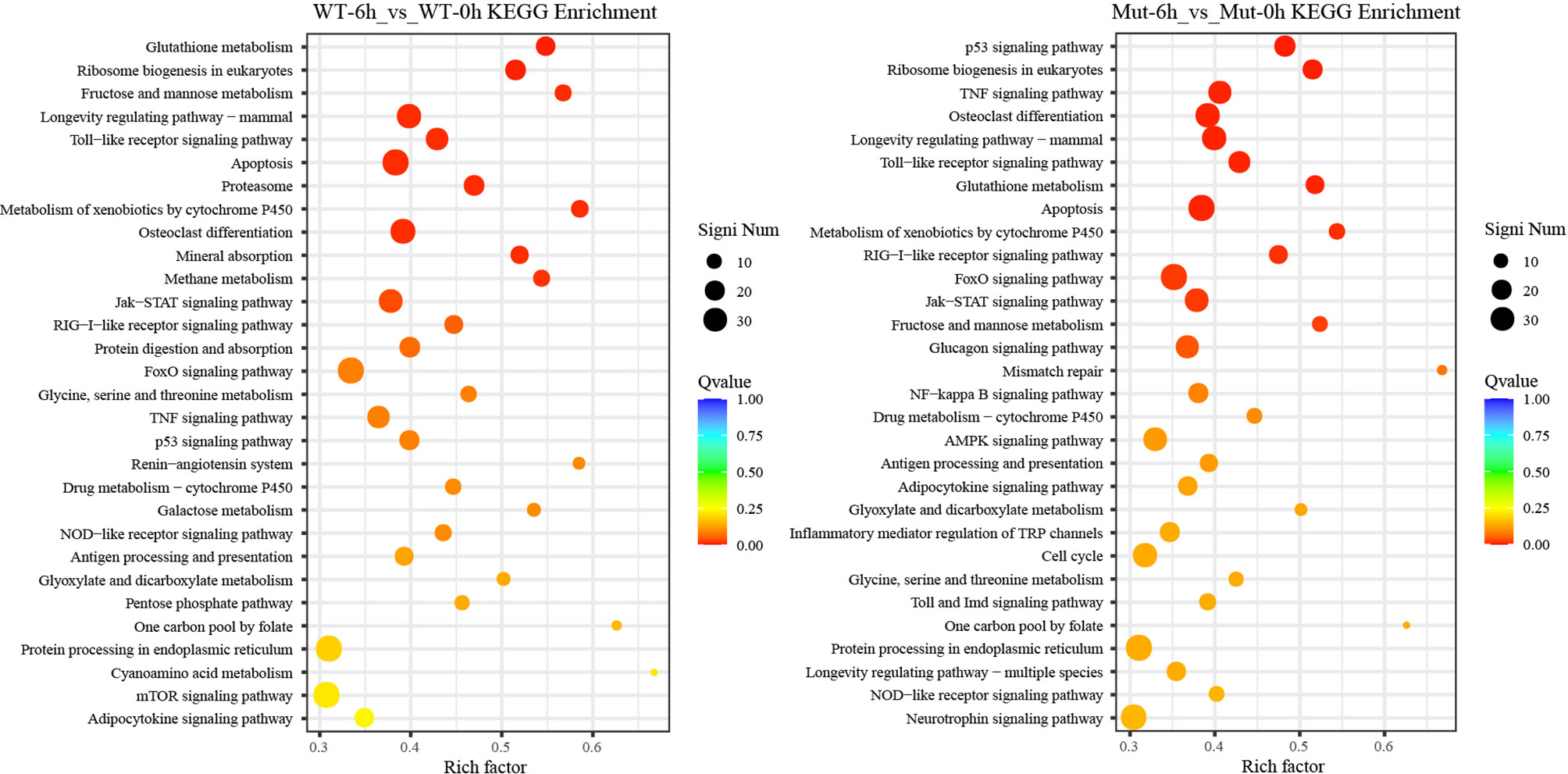
Figure 2 The top 30 enriched KEGG pathways in WT-6h_vs_WT-0h and Mut-6h_vs_Mut-0h. The color and size of the dots indicate Q-values and DEG numbers in pathways, respectively.
To gain a comprehensive understanding of the mechanisms involved and to directly understand the effects of congenital asplenia on innate immunity in zebrafish, comparative transcriptome analyses were further performed on the DEGs of Mut-6h_vs_WT-6h. For the GO enrichment analysis, most of the GO terms that were significantly enriched from the upregulated DEGs were involved in early stress response, including defense response, cell chemotaxis, response to bacterium, response to external biotic stimulus, response to other organism, response to biotic stimulus, and chemokine-mediated signaling pathway as well as response to chemical, leukocyte chemotaxis, leukocyte migration, and inflammatory response (Figure 3A). The complete information of enriched GO terms from the upregulated DEG in Mut-6h _vs_WT-6h is listed in Table S3. In KEGG analysis, KEGG categories significantly enriched from the upregulated genes in Mut-6h_vs_WT-6h describe metabolism of xenobiotics by cytochrome P450, glutathione metabolism, drug metabolism–cytochrome P450, steroid hormone biosynthesis, pentose and glucuronate interconversions, and valine, leucine and isoleucine degradation (Figure 3B and Table S4).
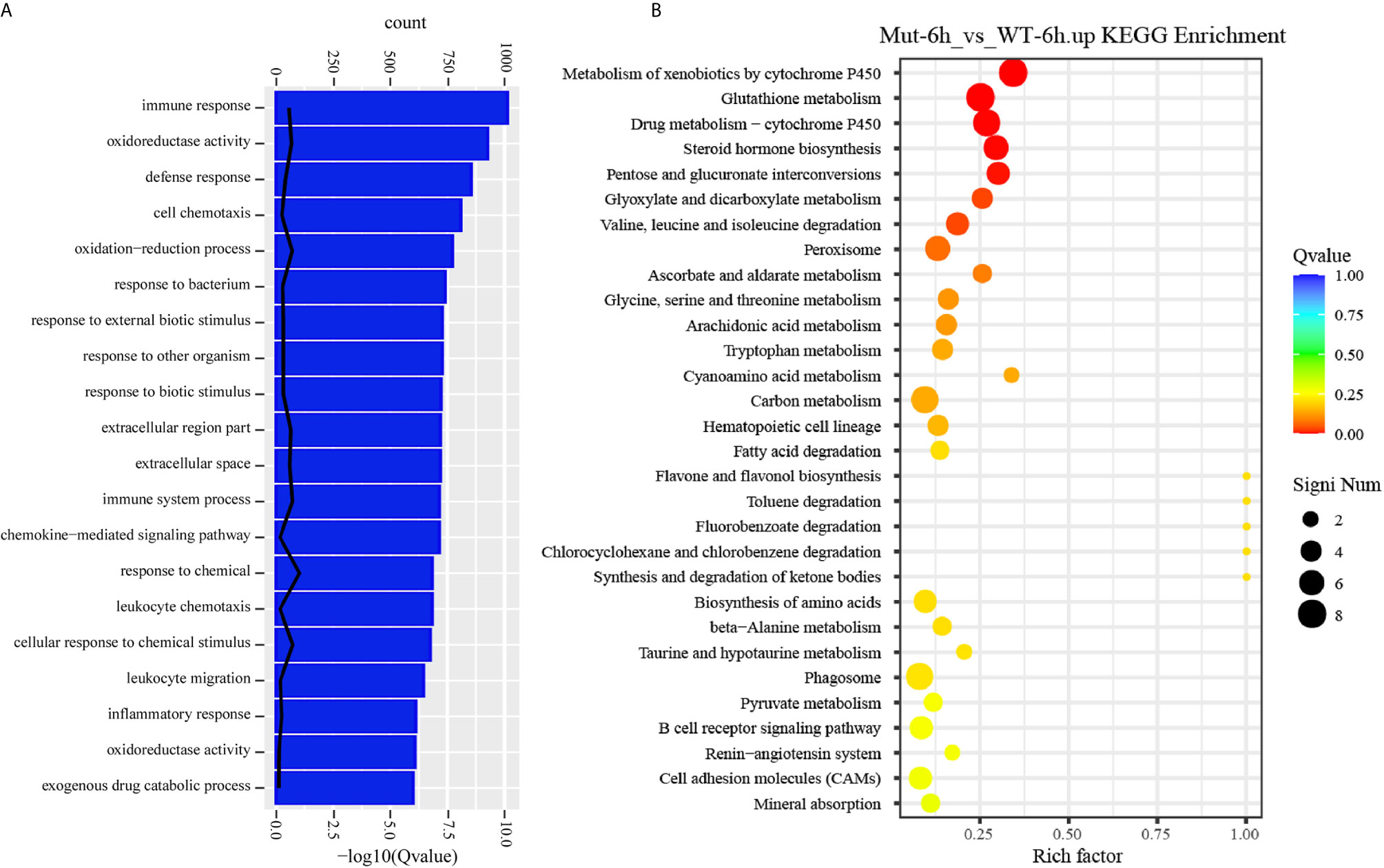
Figure 3 GO functional enrichment and KEGG analysis from upregulated DEGs in Mut-6h_vs_WT-6h. (A) The top 20 significantly upregulated GO categories in Mut-6h_vs_WT-6h. The X- and Y-axes represent the significantly enriched GO terms and the corresponding number of DEGs, respectively. (B) The top 30 upregulated KEGG pathways in Mut-6h_vs_WT-6h. The numbers of DEGs in each pathway are counted and rich factors and P-values are displayed.
Congenital Asplenia Induces Excessive Systemic Inflammation After Vaccination in Zebrafish
The TNF and RIG-I-like receptor signaling pathways were major signaling pathways involved in inflammatory response, and both of them were uniquely and significantly enriched in Mut-6h_vs_Mut-0h (Figures S3, S4).We hypothesized that vaccination might induce excessive inflammation in asplenia zebrafish. To understand the differences of inflammatory response in WT and congenitally asplenic zebrafish, inflammatory cytokines/chemokines were identified. Our RNA-seq analysis revealed that most regulated pro-inflammatory inflammatory cytokines/chemokines (il1β, il6, tnfα, tnfβ, il13, il13ra2, cxcr4a, elf3, cxcl8a, cxcl18b, csf1b, ccl20a.3, ccl39.3, etc.) are more highly induced in congenitally asplenic zebrafish than WT following vaccination (Figure 4A). The expression patterns of regulated cytokines/chemokines from all other groups are displayed in Figure S5. To confirm the excessive inflammatory response in congenitally asplenic zebrafish, the expression pattern of pro-inflammatory cytokines (il1β, il6, tnfα, tnfβ, and ifnγ), NF-κB subunits (nfkb1 and nfkb2), and an anti-inflammatory cytokine (il10) was also identified in liver and intestine tissue. In liver, il6, tnfα, tnfβ, ifnγ, and nfkb2 expression were more highly induced in congenitally asplenic zebrafish (Figure 4B). In intestine, the expression level of il1β, il6, tnfα, tnfβ, ifnγ, and nfkb2 were also significantly higher in congenitally asplenic zebrafish when compared with WT (Figure 4C).
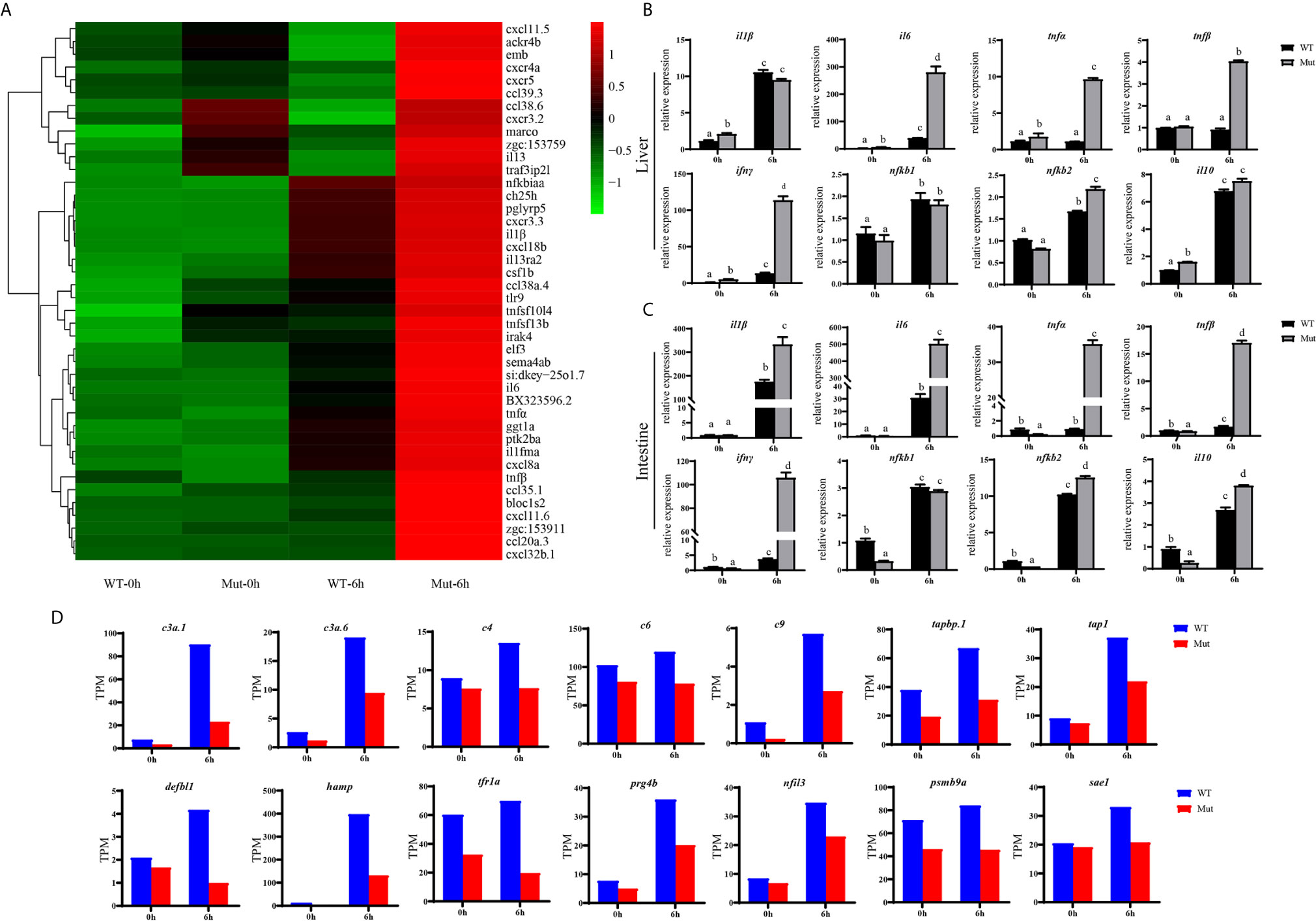
Figure 4 Congenital asplenia induces excessive systemic inflammation after vaccination in zebrafish. (A) The expression level of inflammatory cytokines/chemokines displayed with a heat map. (B) The expression of inflammation-related genes in liver after vaccination by qRT-PCR. (C) The expression of inflammation-related genes in intestine after vaccination by qRT-PCR. (D) The expression level of other important immune-related genes by RNA-seq. Different letters (a, b, c, etc.) indicate significant differences (p < .05).
Other important immune-related genes were also identified. As shown in Figure 4D, genes encoding complement components, including c3a.1, c3a.6, c4, c6, and c9 are downregulated in asplenic fish when compared with WT. In addition, other genes, including tabp.1, tap1, hamp, prg4b, nfil3, defbl1, psmb9a, tfr1a, and sae1, which are crucial for immune response, are also downregulated in asplenia zebrafish compared with WT.
Congenital Asplenia Results in an Impaired Adaptive Immune Response After Vaccination in Zebrafish
To explore the effects of congenital asplenia on adaptive immunity in zebrafish, comparative transcriptome analysis was performed on the DEGs of WT-10d_vs_WT-0h and Mut-10d_vs_Mut-0h. For the GO enrichment analysis, a total of 350 GO terms significantly enriched from the upregulated genes were identified, and the top 20 upregulated GO terms in WT-10d_vs_WT-0h are shown in Figure 5A. The GO terms of the immune system process, immune response, response to external biotic stimulus, response to other organisms, response to biotic stimulus, defense response, and response to cytokines were significantly enriched (Figure 5A). In addition, 31 adaptive immune response–related GO terms (T cell activation, B cell mediated immunity, B cell activation, lymphocyte-mediated immunity immunoglobulin production, etc.) were also significantly upregulated in WT-10d_vs_WT-0h (Table S5). The complete GO terms significantly enriched from the upregulated genes in WT-10d_vs_WT-0h are shown in Table S6. However, only 28 GO terms were significantly enriched from the upregulated genes in Mut-10d_vs_Mut-0h, and most of them were more often associated with hormone/steroid biosynthetic and metabolic process as well as several immune-related biological process (Figure 5B). The complete information of the significantly upregulated GO terms in Mut-10d_vs_Mut-0h are shown in Table S7. In KEGG analysis, pathways significantly enriched from the upregulated genes in WT-10d_vs_WT-0h include phagosome, lysosome, collecting duct acid secretion, osteoclast differentiation, T cell receptor signaling pathway, NF-kappa B signaling pathway, cytosolic DNA-sensing pathway, apoptosis, and proteasome (Figure 5C and Table S8). However, no immune-related pathways significantly enriched from upregulated genes were identified in Mut-10d_vs_Mut-0h (Figure 5D). The protein–protein interactions based on DEGs annotated in the immune system process of two vaccination groups also displayed a different immune response interaction network (Figure 6).
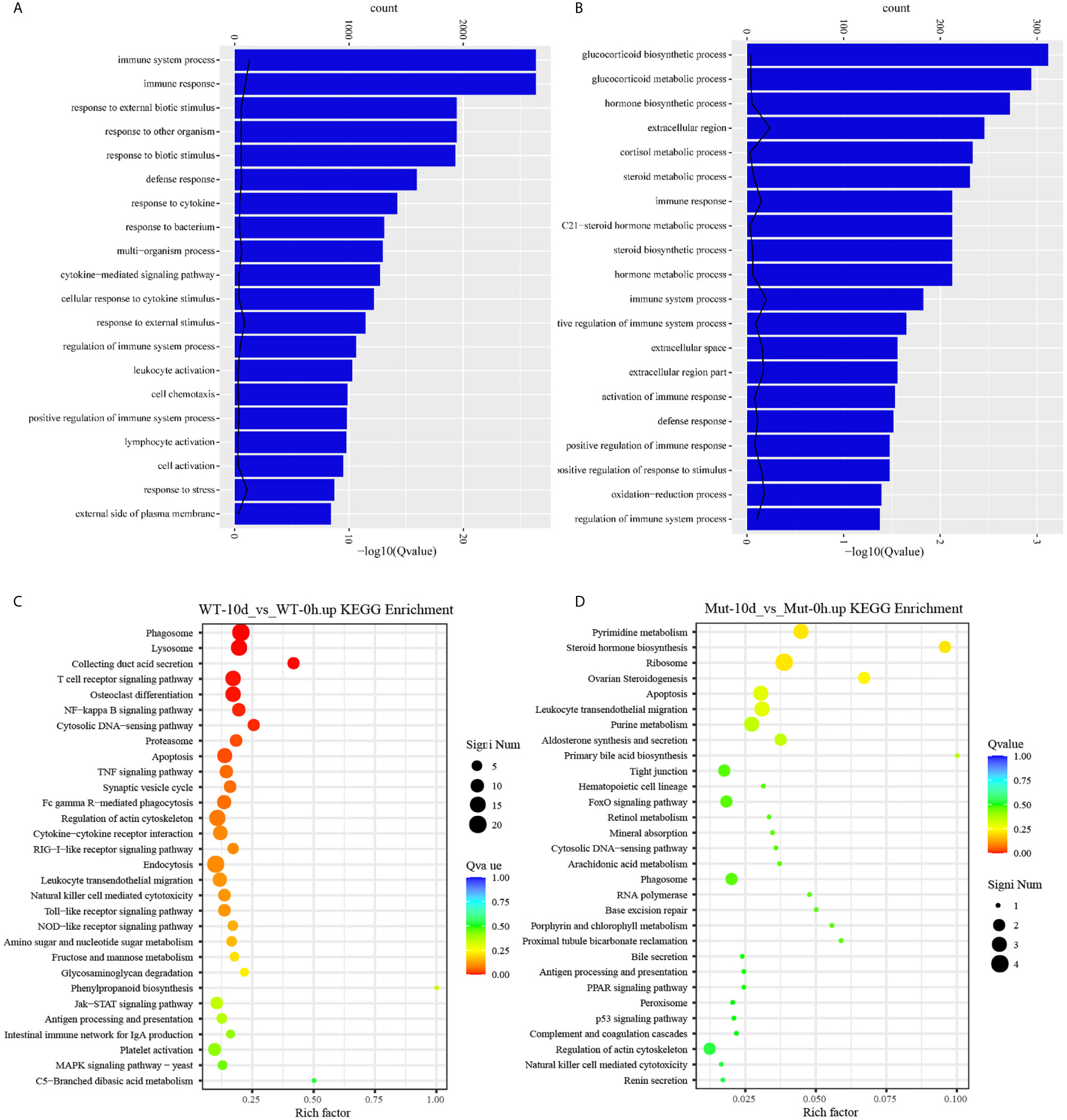
Figure 5 GO functional enrichment and KEGG analysis from upregulated DEGs in WT-10d_vs_WT-0h and Mut-10d_vs_Mut-0h. (A, B) The top 20 significantly upregulated GO categories in WT-10d_vs_WT-0h and Mut-10d_vs_Mut-0h, respectively. The X- and Y-axes represent the significantly enriched GO terms and the corresponding number of DEGs, respectively. (C, D) The top 30 upregulated KEGG pathways in WT-10d_vs_WT-0h and Mut-10d_vs_Mut-0h, respectively. The numbers of DEGs in each pathway are counted, and rich factors and P-values are displayed.
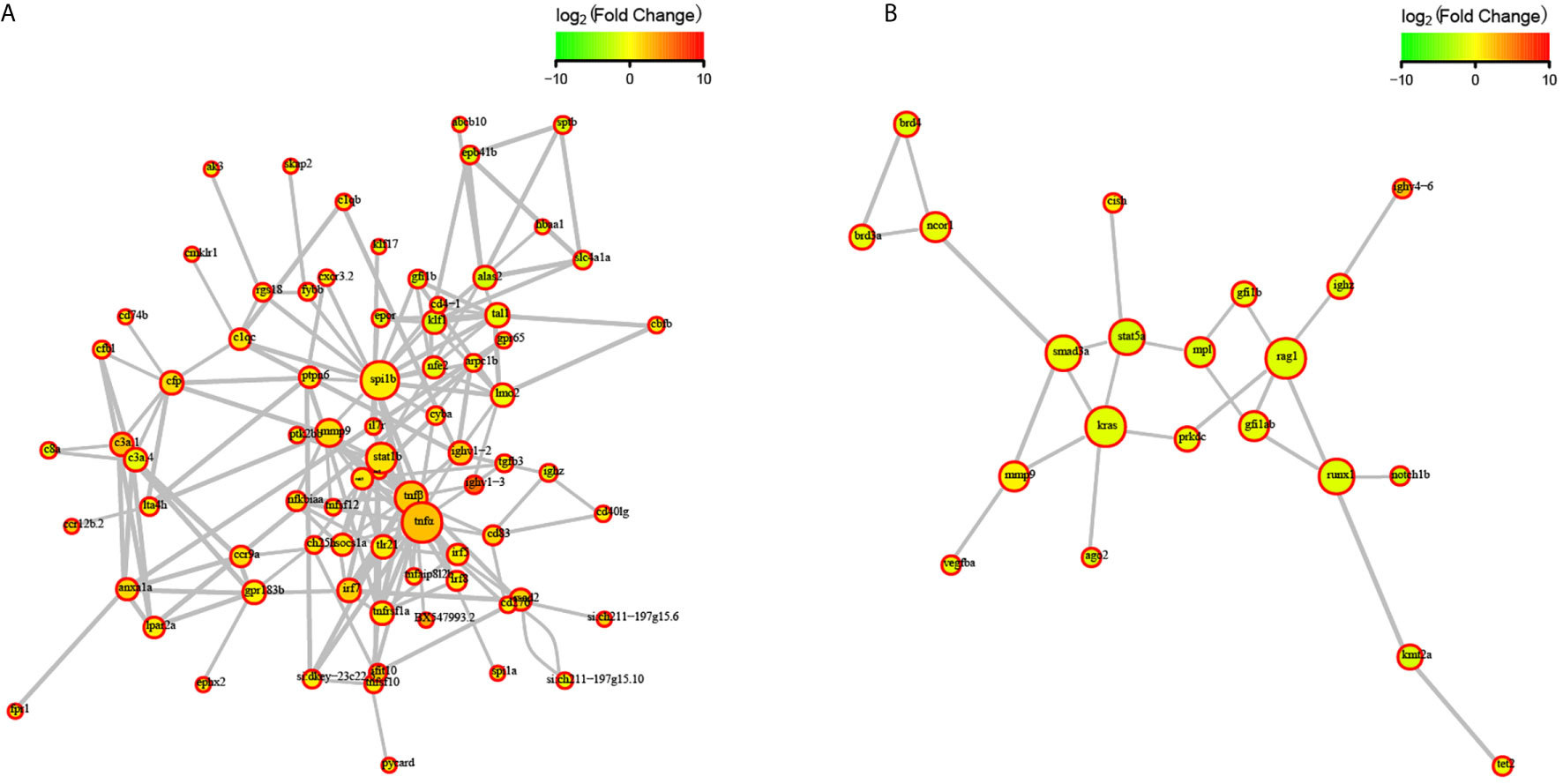
Figure 6 STRING network analysis on DEGs annotated in GO: immune system process. (A) WT-10d_vs_WT-0h. (B) Mut-10d_vs_Mut-0h. The nod represents proteins, bigger means more interaction with others. The red color means upregulated DEGs, and the green means downregulated DEGs.
Next, comparative transcriptome analysis of DEGs of Mut-10d_vs_WT-10d were done to further investigate the difference of adaptive immune responses in WT and asplenic zebrafish after vaccination. GO functional enrichment analysis indicated that GO terms, such as immune system process, immune response, response to cytokine, response to bacterium, and defense response, were significantly downregulated in asplenia zebrafish (Figure 7A). In addition, multiple adaptive immunity–associated GO terms (lymphocyte activation, T cell activation, regulation of T cell activation, negative regulation of T cell activation, positive regulation of T cell proliferation, positive regulation of lymphocyte proliferation, positive regulation of lymphocyte activation, regulation of T cell proliferation, regulation of lymphocyte proliferation, lymphocyte costimulation, positive regulation of T cell activation, T cell costimulation, T cell proliferation, lymphocyte proliferation, etc.) were significantly downregulated in congenital asplenic zebrafish (Table 2). The complete information of significantly downregulated GO terms in Mut-10d_vs_ WT-10d are shown in Table S9. In KEGG analysis, NF-kappa B signaling pathway, lysosome, osteoclast differentiation, cytokine-cytokine receptor interaction, TNF signaling pathway, phagosome, antigen processing and presentation, Jak-STAT signaling pathway, Toll-like receptor signaling pathway, and RIG-I-like receptor signaling pathway were significantly downregulated in congenital asplenic zebrafish (Figure 7B and Table S10).
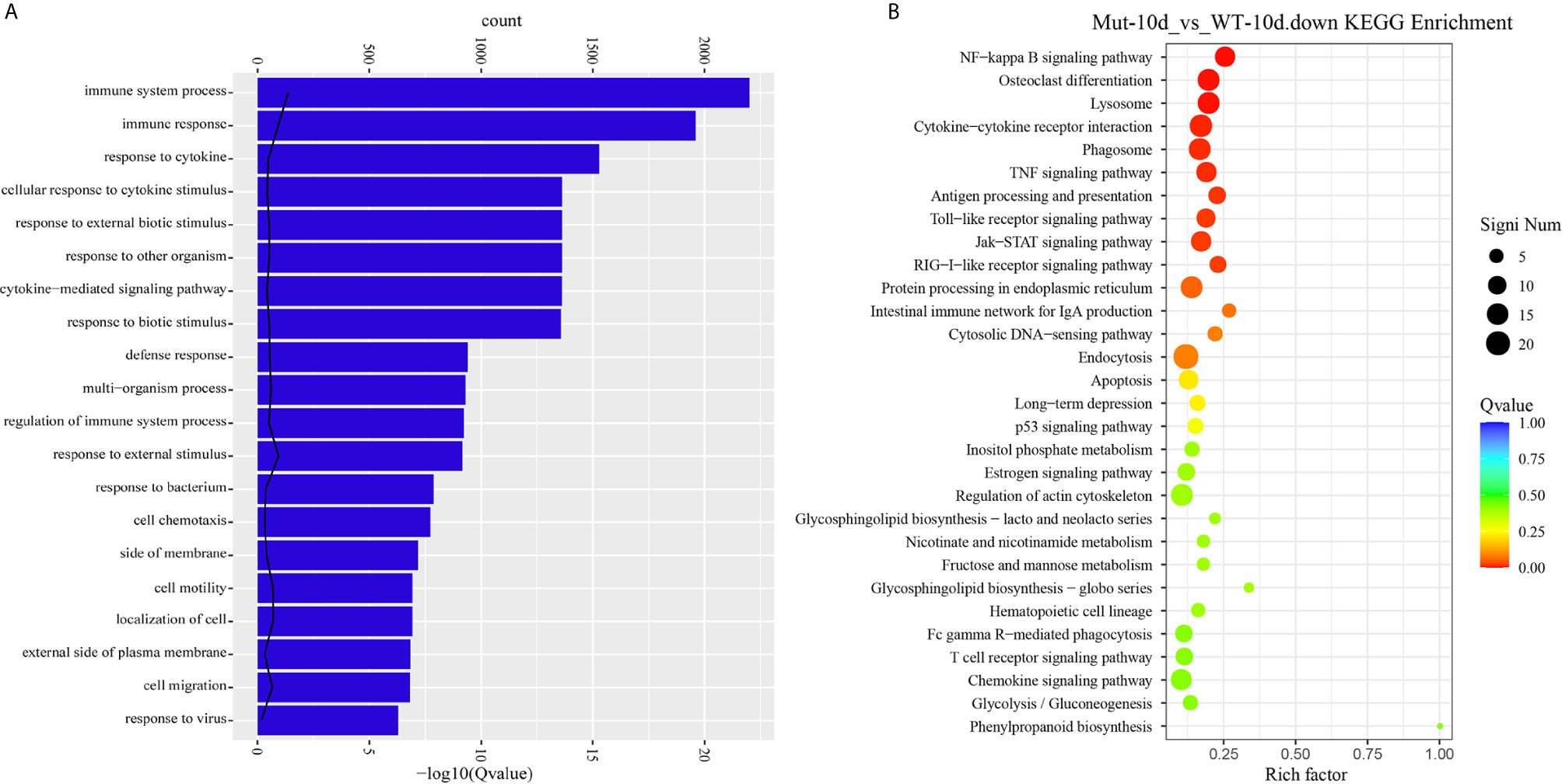
Figure 7 GO functional enrichment and KEGG analysis from downregulated DEGs in Mut-10d_vs_WT-10d. (A) The top 20 significantly downregulated GO categories in Mut-10d_vs_WT-10d. The X- and Y-axes represent the significantly enriched GO terms and the corresponding number of DEGs, respectively. (B) The top 30 downregulated KEGG pathways in Mut-10d_vs_WT-10d. The numbers of DEGs in each pathway are counted, and rich factors and P-values are displayed.
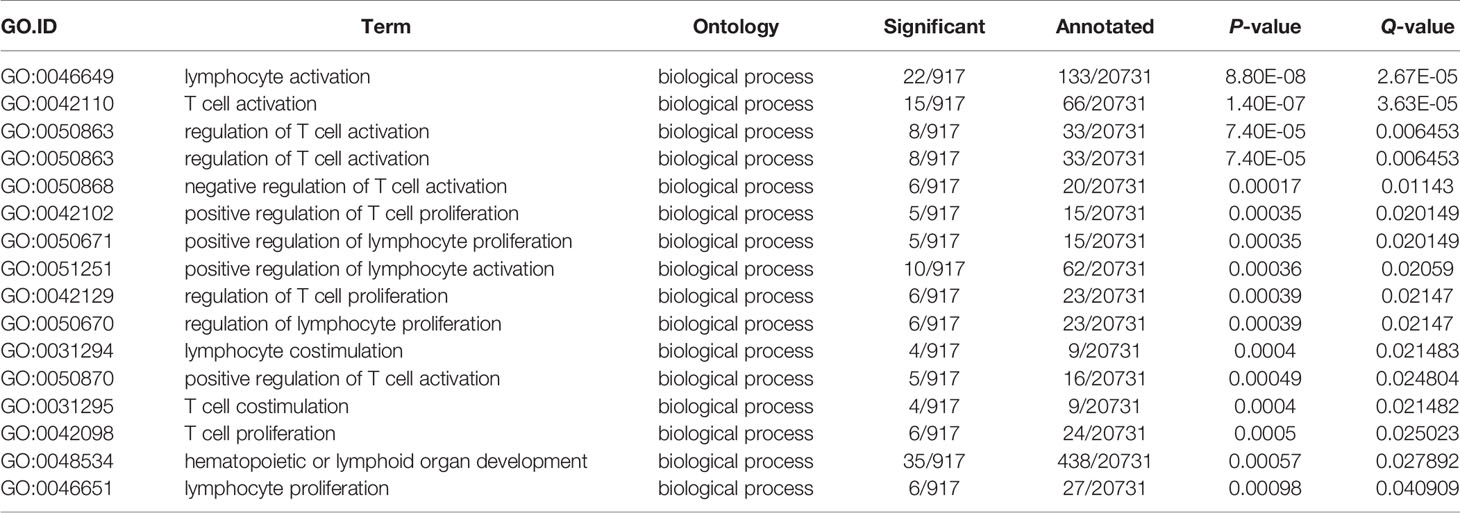
Table 2 The significantly downregulated GO terms involved in adaptive immunity in Mut-10d_vs_WT-10d.
Experimental Validation of DEGs
To validate the results of RNA-seq, 12 immune response–related genes, including il1β, il6, tnfα, tnfβ, ch25h, cxcr3.3, cxcl8a, cxcl18b, ccl20a.3, c3a.1, c3a.6, and c4 were chosen for qPCR detection. The gene expression patterns detected by qPCR were similar to those obtained from the RNA-seq results (Figure 8). Furthermore, expression of MHCII and IgM were also identified to compare the changes in adaptive immune responses in WT and asplenic zebrafish. The expression of MHCII exhibited a higher expression level in WT animals compared with its level in the asplenic zebrafish. IgM showed upregulation, and its expression reached the highest level in the 10 days postvaccination in both WT animals and asplenic zebrafish, and its expression was significantly reduced in the asplenic zebrafish compared with its expression in WT animals (Figure 9).
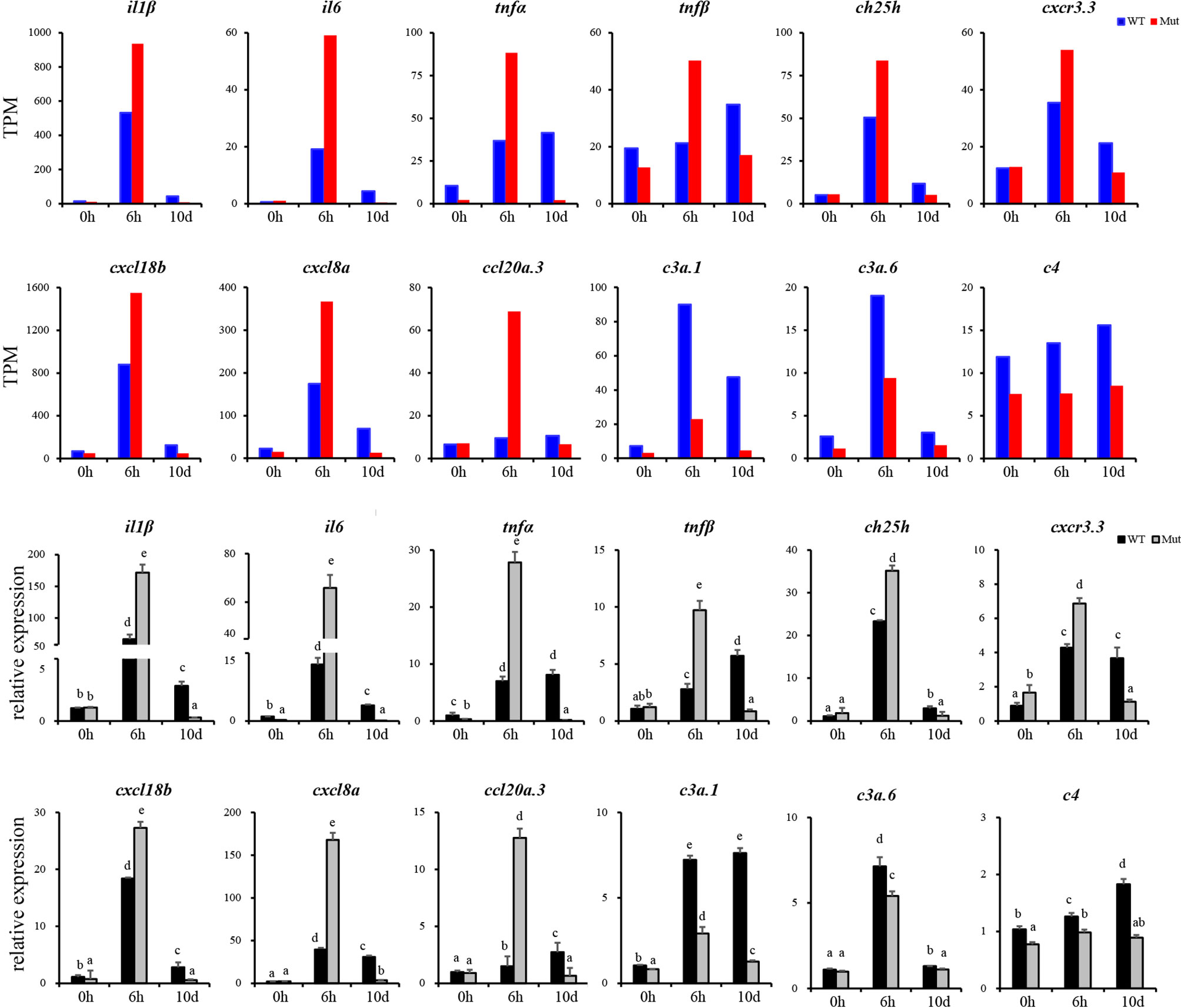
Figure 8 Validation of the 12 DEGs by qRT-PCR. WT animals and congenitally asplenic zebrafish sampled at 0 h were used as controls. The data were normalized to the expression level of WT-0h. The data are reported as the mean ± SEM. Different letters (a, b , c etc.) indicate significant differences (p <.05).
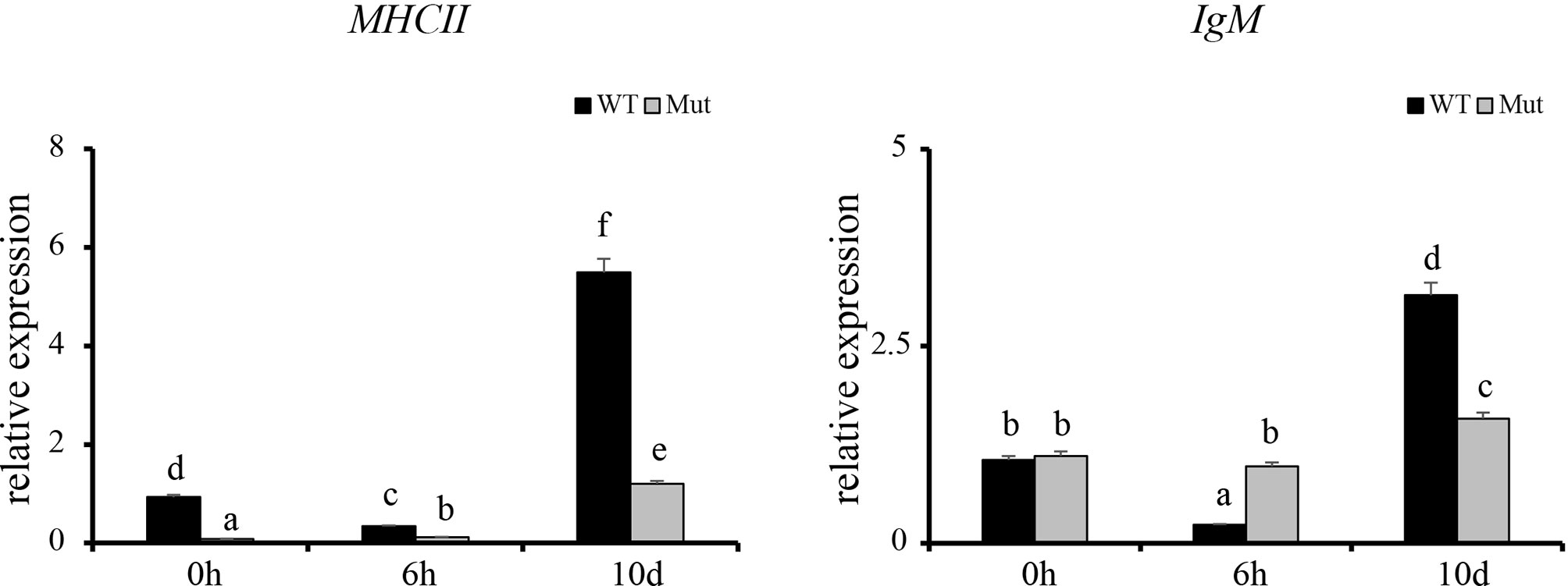
Figure 9 Expression profiles of MHCII and IgM after vaccination in whole kidneys. WT animals and congenitally asplenic zebrafish sampled at 0 h were used as controls. The data were normalized to the expression level of WT-0h. The data are reported as the mean ± SEM. Different letters (a, b, c, etc.) indicate significant differences (p < .05).
Discussion
Although the spleen is the largest immune organ in the human body, it has long been ignored until more and more surgically splenectomized patients and congenitally asplenic individuals were found to have immune-related deficiencies. In recent years, a growing number of studies have been conducted on the role of the spleen in inflammation. However, its role in controlling the systemic inflammatory response is still controversial. It is reported that the spleen is the main source of TNF production, and rats with splenectomy reduced the levels of inflammatory cytokines and leukocyte infiltration and developed acute pancreatitis more slowly than rats without splenectomy (Zhou et al., 2019). On the contrary, splenectomized mice with acute kidney injury resulted in increased serum inflammatory cytokines and worse lung injury as evidenced by the higher lung chemokines and the increased lung capillary leak (Andres-Hernando et al., 2011), implying the spleen may also play roles in downregulating the inflammatory response. Hence, it is necessary to establish a new model to explain the role of the spleen in inflammation. In our previous study, we generated a congenitally asplenic zebrafish model that was more susceptible to A. hydrophila infection (Xie et al., 2019). In this study, the inflammatory response of the congenitally asplenic zebrafish was identified after pathogenic A. hydrophila infection and LPS exposure. We found that pro-inflammatory cytokines and NF-κB subunits were more highly induced in congenitally asplenic zebrafish after exposure to A. hydrophila and LPS. Consistent with the mRNA expression, Il1β, Il6, and Tnfα proteins also exhibited higher expression patterns in congenitally asplenic zebrafish after exposure to A. hydrophila and LPS. It is reported that cytokines and chemokines, including il1β, il6, and tnfα are main pro-inflammatory factors involved in regulation of inflammation, and the excessive production of these molecules can be deleterious and associated with disease progression, inflammatory disorders, tissue damage, and apoptosis (Tracey, 2002; Takeshita and Ransohoff, 2012; Bleau et al., 2016; Hasegawa et al., 2017; Kany et al., 2019). Thus, our study suggested that congenital asplenia might induce uncontrolled inflammatory response. In addition, an apoptosis assay revealed the inflammatory injury in congenitally asplenic zebrafish. Taken together, our results suggested the anti-inflammatory effects of spleen in zebrafish as with the study in mice (Andres-Hernando et al., 2011).
In teleosts, the head-kidney and spleen are the major sites producing immune complexes and responsible for the trapping and eliminating pathogens from the whole circulation (Espenes et al., 1996; Hansen and Zapata, 1998). To gain full understanding of how congenital asplenia affects the systemic immune response, we performed comparative transcriptome analysis of whole kidneys (head-kidney and kidney) from both WT and congenitally asplenic zebrafish at the time points corresponding to the occurrence of innate and adaptive immunity after vaccination. According to KEGG enrichment analysis, several conserved immune-related pathways were upregulated in both WT and asplenic zebrafish by 6 h postvaccination, indicating the vaccination successfully activated the immune system. Interestingly, we found that zebrafish with congenital asplenia were more hyperresponsive as evidenced by the highly reduced stress response–associated biological processes (e.g., cell chemotaxis, leukocyte chemotaxis, leukocyte migration, inflammatory response) in asplenic zebrafish after vaccination when compared with WT. Thus, our data indicate that congenital asplenia unbalances the transcriptional regulation in early stress response.
Inflammation is an immune system response to external physical or chemical stimuli or bacterial infection, and it is usually considered a defensive reaction and protects the host from pathogens. However, excessive or uncontrolled systemic inflammation can disrupt immune function and aggravate disease status (Cohen, 2002; Calder et al., 2009). In RNA-seq analysis and qRT-PCR in liver and intestine, pro-inflammatory cytokines/chemokines were more highly induced in asplenic zebrafish. In accordance with the overproduction of these cytokines/chemokines, the TNF and RIG-I-like receptor signaling pathways were uniquely and significantly activated in asplenic zebrafish. It is well documented that TNF signaling pathway is one of the major signaling pathways involved in inflammatory response, and optimal regulation of TNF signaling is essential to maintain tissue homeostasis and prevent inflammatory pathology (Brenner et al., 2015; Van Quickelberghe et al., 2018). The RIG-I-like receptor signaling pathway is known to be essential for triggering inflammatory response upon infections (Zhao et al., 2020). Thus, our data indicate that congenital asplenia induces excessive inflammation after vaccination in zebrafish. In addition, we only observed the activation of the p53 signaling pathway in asplenic zebrafish (Figure S6), which is one of the major pathways to induce apoptosis (Wang et al., 2015). Combined with the characteristic of hyperresponsiveness to stress, overproduction of cytokines/chemokines and activation of inflammatory-related signaling pathways, our data suggest congenital asplenia might disrupt immune homeostasis and induce inflammatory damage in zebrafish, which is consistent with the experiment described above.
Splanchnic anti-inflammatory reflex has been described as a powerful mechanism involved in the suppression of inflammatory response in mammals. During systemic inflammation, the production of pro-inflammatory mediators is down-modulated by this reflex, which requires the activation of neural circuits (Tracey, 2002; Martelli et al., 2014b). It is well accepted that the splanchnic anti-inflammatory reflex relies on efferent neural connections from the brain to the viscera, especially the spleen (known as the splenic anti-inflammatory reflex), reducing the production of pro-inflammatory cytokines (Okusa et al., 2017). In rats, splenectomy disrupts the efferent neural connection to the brain and inactivates the splenic anti-inflammatory pathway, leading overproduction of circulating cytokines during systemic inflammation (Huston et al., 2006; Martelli et al., 2014a). Our data suggest that the overproduction of cytokines/chemokines and excessive inflammatory response in congenitally asplenic zebrafish might result from inactivation of splenic anti-inflammatory reflex caused by congenital asplenia. In addition, our study is the first to present findings that the splenic anti-inflammatory reflex plays an essential role in the regulation of inflammation in fish.
Other important immune-related genes were also identified to study the effects of congenital asplenia on innate immunity. It is reported that the complement system plays essential roles in microbial killing, phagocytosis, antibody production, and cell lysis (Boshra et al., 2006). Children with asplenia/hyposplenism have severe combined immune deficiency, including complement C2 deficiency (Scheuerman et al., 2014). In the present study, a number of complement components including c3a.1, c3a.6, c4, c6, and c9 showed a reduced expression level in the congenitally asplenic zebrafish, indicating that the complement system was partially impaired. Defbl1 (also known as β-def-1) is a crucial antimicrobial peptide that plays an essential role in protecting the intestine against infection as a result of antibacterial and immunomodulatory properties (Cederlund et al., 2011). Hamp is able to inhibit growth of certain pathogenic microorganisms, including E. coli, V. anguillarum, S. aureus, and B. subtilis (Lin et al., 2014). In our present study, defbl1 and hamp were reduced in congenitally asplenic zebrafish compared with WT. In addition, tap1 and tapbp.1, which are involved in antigen processing and presentation (Xu et al., 2017), were also reduced in congenitally asplenic zebrafish. Thus, our data suggest congenital asplenia might impair the antibacterial ability in zebrafish. Hematopoiesis is the synthesis and development of all types of immune cells. In this study, hematopoiesis-related genes, including tfr1a, gfi1b, nfil3, prg4b, and sae1 were downregulated in asplenia zebrafish compared with WT. All of these results indicate that congenital asplenia might impair the fish immune system.
Establishment of adaptive immunity needs 1–2 weeks and is important for host defense during the latter phases of an infection (Farber et al., 2016). The adaptive immune response is associated with the activation of lymphocytes and adaptive immune mechanisms, which allows specific recognition and elimination of the pathogen (Farber et al., 2016). Previous studies reveal that mice with congenital asplenia display impaired B-1a cell generation and delayed or lower IgM antibody secretion (Karrer et al., 1997; Wardemann et al., 2002). According to GO and KEGG enrichment analysis, adaptive immune response–associated biological processes and signaling pathways were highly activated in WT after vaccination although they showed reduced activation or failed to activate in congenitally asplenic zebrafish, indicating that the adaptive immunity was impaired by congenital asplenia. In addition, zebrafish with congenital asplenia displayed a low expression level of MHCII/IgM. In fish, antigen-presenting cells ingest, process, and present antigens for T cells via MHCII. IgM is the first antibody produced during the immune response, and it provides a crucial line of defense in the immune system. Therefore, our data suggest that antigen processing and presentation/antibody secretion might be retarded due to the congenital asplenia, which is consistent with previous research in mice (Karrer et al., 1997). Splenectomized or congenitally asplenic patients often have an increased risk of overwhelming infections (Dahyot-Fizelier et al., 2013; Sinwar, 2014). Thus, proper immunization might be effective to prevent the risk of OPSI in splenectomized and asplenic patients. The low or retarded expression of IgM after vaccination in our study might provide theoretical support for a vaccination strategy in splenectomized and asplenic patients. IgZ is the only teleost Ig isotype with a specialized mucosal immune response function in zebrafish, and it shows a rapid induction after bacterial infection in gill and skin (Hu et al., 2010; Ji et al., 2021). In this study, IgZ/IgZ2 were slightly increased at 10 d in gill and skin, and there was no significant difference in expression of IgZ/IgZ2 in WT and congenitally asplenic zebrafish after vaccination (Figure S7). One possible explanation is that intraperitoneal injection of an inactivated vaccine might not able to reduce high expression of the IgZ/IgZ2 gene. Further study will be conducted to study the effects of congenital asplenia on the mucosal immune response of skin and gill by pathogen challenge.
In summary, we present findings related to the molecular mechanism of systemic immune response in congenitally asplenic zebrafish and provide an in-depth understanding of spleen function in controlling immune homeostasis. To the best of our knowledge, we are the first to perform comparative transcriptome analysis to study the effects of congenital asplenia on innate and adaptive immune response by using a congenitally asplenic zebrafish model. Congenital asplenia might impair efferent neural connections from the brain to the spleen, inactivating the splenic anti-inflammatory reflex that, in turn, induces excessive cytokine/chemokine release and inflammatory damage and results in an impaired immune response.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA688352; https://www.ncbi.nlm.nih.gov/, PRJNA688655.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC) of Southwest University.
Author Contributions
Conceptualization, LX and YL. Methodology, LX and RW. Validation, LX and ZC. Resources, YL. Data curation, LX, ZC, and HG. Writing—original draft preparation, LX. Writing—review and editing, RW and YL. Visualization, LX. Supervision, YL. Funding acquisition, YL and RW. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by the Ecological Fishery Technological System of Chongqing Municipal Agricultural and Rural Committee under Grant (No. 4322000101) and Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0526).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Hao, Xu, Shi-Qi Fan, Zhe-Yu Chen, GuoWang, and Bi-Cheng Duan for their help on fish care and mutant maintenance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.668859/full#supplementary-material
Supplementary Figure 1 | PCA among samples.
Supplementary Figure 2 | The number of DEGs for different groups. WT-0h, WT zebrafish without vaccination; Mut-0h, congenitally asplenic zebrafish without vaccination; WT-6h, WT zebrafish with vaccination sampled at 6 h; Mut-6h, congenitally asplenic zebrafish with vaccination sampled at 6 h; WT-10d, WT zebrafish with vaccination sampled at 10d; Mut-10d, congenitally asplenic zebrafish with vaccination sampled at 10 d.
Supplementary Figure 3 | The DEGs involved in the TNF signaling pathway were annotated by KEGG in Mut-6h_vs_Mut-0h.
Supplementary Figure 4 | The DEGs involved in the RIG-I-like receptor signaling pathway were annotated by KEGG in Mut-6h_vs_Mut-0h.
Supplementary Figure 5 | The heat map with the expression pattern of inflammatory cytokines/chemokines at 0, 6 h and 10 d.
Supplementary Figure 6 | The differentially expressed genes involved in the P53 signaling pathway were annotated by KEGG in Mut-6h_vs_Mut-0h.
Supplementary Figure 7 | Expression profiles of IgZ and IgZ2 after vaccination in gill and skin. WT animals and congenitally asplenic zebrafish sampled at 0 h were used as controls. The data were normalized to the expression level of WT-0h. The data are reported as the mean ± SEM. Different letters (a, b, c, etc.) indicate significant differences (p < .05).
References
Andres-Hernando, A., Altmann, C., Ahuja, N., Lanaspa, M. A., Nemenoff, R., He, Z. B., et al. (2011). Splenectomy Exacerbates Lung Injury After Ischemic Acute Kidney Injury in Mice. Am. J. Physiol-Renal. Physiol. 301, F907–F916. doi: 10.1152/ajprenal.00107.2011
Bleau, C., Burnette, M., Filliol, A., Piquet-Pellorce, C., Samson, M., Lamontagne, L. (2016). Toll-Like Receptor-2 Exacerbates Murine Acute Viral Hepatitis. Immunology 149, 204–224. doi: 10.1111/imm.12627
Boshra, H., Li, J., Sunyer, J. O. (2006). Recent Advances on the Complement System of Teleost Fish. Fish Shellfish Immunol. 20, 239–262. doi: 10.1016/j.fsi.2005.04.004
Brenner, D., Blaser, H., Mak, T. W. (2015). Regulation of Tumour Necrosis Factor Signalling: Live or Let Die. Nat. Rev. Immunol. 15, 362–374. doi: 10.1038/nri3834
Bronte, V., Pittet, M. J. (2013). The Spleen in Local and Systemic Regulation of Immunity. Immunity 39, 806–818. doi: 10.1016/j.immuni.2013.10.010
Calder, P. C., Albers, R., Antoine, J. M., Blum, S., Bourdet-Sicard, R., Ferns, G. A., et al. (2009). Inflammatory Disease Processes and Interactions With Nutrition. Br. J. Nutr. 101 (Suppl 1), S1–45. doi: 10.1017/S0007114509377867
Cederlund, A., Gudmundsson, G. H., Agerberth, B. (2011). Antimicrobial Peptides Important in Innate Immunity. FEBS J. 278, 3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x
Dahyot-Fizelier, C., Debaene, B., Mimoz, O. (2013). [Management of Infection Risk in Asplenic Patients]. Ann. Fr Anesth. Reanim. 32, 251–256. doi: 10.1016/j.annfar.2013.01.025
Espenes, A., Press, C. M., Reitan, L. J., Landsverk, T. (1996). The Trapping of Intravenously Injected Extracellular Products From Aeromonas Salmonicida in Head Kidney and Spleen of Vaccinated and Nonvaccinated Atlantic Salmon. Fish Shellfish Immunol. 6, 413–426. doi: 10.1006/fsim.1996.0040
Farber, D. L., Netea, M. G., Radbruch, A., Rajewsky, K., Zinkernagel, R. M. (2016). Immunological Memory: Lessons From the Past and a Look to the Future. Nat. Rev. Immunol. 16, 124–128. doi: 10.1038/nri.2016.13
Hansen, J. D., Zapata, A. G. (1998). Lymphocyte Development in Fish and Amphibians. Immunol. Rev. 166, 199–220. doi: 10.1111/j.1600-065X.1998.tb01264.x
Hasegawa, T., Hall, C. J., Crosier, P. S., Abe, G., Kawakami, K., Kudo, A., et al. (2017). Transient Inflammatory Response Mediated by interleukin-1beta is Required for Proper Regeneration in Zebrafish Fin Fold. Elife 6, e22716. doi: 10.7554/eLife.22716
Huston, J. M., Ochani, M., Rosas-Ballina, M., Liao, H., Ochani, K., Pavlov, V. A., et al. (2006). Splenectomy Inactivates the Cholinergic Antiinflammatory Pathway During Lethal Endotoxemia and Polymicrobial Sepsis. J. Exp. Med. 203, 1623–1628. doi: 10.1084/jem.20052362
Hu, Y. L., Xiang, L. X., Shao, J. Z. (2010). Identification and Characterization of a Novel Immunoglobulin Z Isotype in Zebrafish: Implications for a Distinct B Cell Receptor in Lower Vertebrates. Mol. Immunol. 47, 738–746. doi: 10.1016/j.molimm.2009.10.010
Ji, J. F., Hu, C. B., Shao, T., Fan, D. D., Zhang, N., Lin, A. F., et al. (2021). Differential Immune Responses of Immunoglobulin Z Subclass Members in Antibacterial Immunity in a Zebrafish Model. Immunology 162, 105–120. doi: 10.1111/imm.13269
Kany, S., Vollrath, J. T., Relja, B. (2019). Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 20, 6008. doi: 10.3390/ijms20236008
Karrer, U., Althage, A., Odermatt, B., Roberts, C. W., Korsmeyer, S. J., Miyawaki, S., et al. (1997). On the Key Role of Secondary Lymphoid Organs in Antiviral Immune Responses Studied in Alymphoplastic (Aly/Aly) and Spleenless (Hox11(-)/-) Mutant Mice. J. Exp. Med. 185, 2157–2170. doi: 10.1084/jem.185.12.2157
Kristinsson, S. Y., Gridley, G., Hoover, R. N., Check, D., Landgren, O. (2014). Long-Term Risks After Splenectomy Among 8,149 Cancer-Free American Veterans: A Cohort Study With Up to 27 Years Follow-Up. Haematologica 99, 392–398. doi: 10.3324/haematol.2013.092460
Lin, W. J., Liu, S. S., Hu, L. L., Zhang, S. C. (2014). Characterization and Bioactivity of Hepcidin-2 in Zebrafish: Dependence of Antibacterial Activity Upon Disulfide Bridges. Peptides 57, 36–42. doi: 10.1016/j.peptides.2014.04.014
Liu, X., Wu, H., Liu, Q., Wang, Q., Xiao, J., Chang, X., et al. (2015). Profiling Immune Response in Zebrafish Intestine, Skin, Spleen and Kidney Bath-Vaccinated With a Live Attenuated Vibrio Anguillarum Vaccine. Fish Shellfish Immunol. 45, 342–345. doi: 10.1016/j.fsi.2015.04.028
Mahlaoui, N., Minard-Colin, V., Picard, C., Bolze, A., Ku, C. L., Tournilhac, O., et al. (2011). Isolated Congenital Asplenia: A French Nationwide Retrospective Survey of 20 Cases. J. Pediatr. 158, 142–8, 148.e1. doi: 10.1016/j.jpeds.2010.07.027
Martelli, D., Yao, S. T., Mancera, J., McKinley, M. J., McAllen, R. M. (2014a). Reflex Control of Inflammation by the Splanchnic Anti-Inflammatory Pathway is Sustained and Independent of Anesthesia. Am. J. Physiol-Regul. Integr. Comp. Physiol. 307, R1085–R1091. doi: 10.1152/ajpregu.00259.2014
Martelli, D., Yao, S. T., McKinley, M. J., McAllen, R. M. (2014b). Reflex Control of Inflammation by Sympathetic Nerves, Not the Vagus. J. Physiol-London 592, 1677–1686. doi: 10.1113/jphysiol.2013.268573
Mebius, R. E., Kraal, G. (2005). Structure and Function of the Spleen. Nat. Rev. Immunol. 5, 606–616. doi: 10.1038/nri1669
Novoa, B., Figueras, A. (2012). Zebrafish: Model for the Study of Inflammation and the Innate Immune Response to Infectious Diseases. Adv. Exp. Med. Biol. 946, 253–275. doi: 10.1007/978-1-4614-0106-3_15
Okusa, M. D., Rosin, D. L., Tracey, K. J. (2017). Targeting Neural Reflex Circuits in Immunity to Treat Kidney Disease. Nat. Rev. Nephrol. 13, 669–680. doi: 10.1038/nrneph.2017.132
Ram, S., Lewis, L. A., Rice, P. A. (2010). Infections of People With Complement Deficiencies and Patients Who Have Undergone Splenectomy. Clin. Microbiol. Rev. 23, 740. doi: 10.1128/Cmr.00048-09
Renshaw, S. A., Trede, N. S. (2012). A Model 450 Million Years in the Making: Zebrafish and Vertebrate Immunity. Dis. Model Mech. 5, 38–47. doi: 10.1242/dmm.007138
Scheuerman, O., Bar-Sever, Z., Hoffer, V., Gilad, O., Marcus, N., Garty, B. Z. (2014). Functional Hyposplenism is an Important and Underdiagnosed Immunodeficiency Condition in Children. Acta Paediatrica 103, E399–E403. doi: 10.1111/apa.12697
Sinwar, P. D. (2014). Overwhelming Post Splenectomy Infection Syndrome - Review Study. Int. J. Surg. 12, 1314–1316. doi: 10.1016/j.ijsu.2014.11.005
Takeshita, Y., Ransohoff, R. M. (2012). Inflammatory Cell Trafficking Across the Blood-Brain Barrier: Chemokine Regulation and In Vitro Models. Immunol. Rev. 248, 228–239. doi: 10.1111/j.1600-065X.2012.01127.x
Thomsen, R. W., Schoonen, W. M., Farkas, D. K., Riis, A., Jacobsen, J., Fryzek, J. P., et al. (2009). Risk for Hospital Contact With Infection in Patients With Splenectomy: A Population-Based Cohort Study. Ann. Intern. Med. 151, 546–555. doi: 10.7326/0003-4819-151-8-200910200-00008
Tracy, E. T., Rice, H. E. (2008). Partial Splenectomy for Hereditary Spherocytosis. Pediatr. Clin. North Am. 55, 503. doi: 10.1016/j.pcl.2008.02.001
van der Vaart, M., Spaink, H. P., Meijer, A. H. (2012). Pathogen Recognition and Activation of the Innate Immune Response in Zebrafish. Adv. Hematol. 2012:159807. doi: 10.1155/2012/159807
Van Quickelberghe, E., De Sutter, D., van Loo, G., Eyckerman, S., Gevaert, K. (2018). A Protein-Protein Interaction Map of the TNF-induced NF-Kappab Signal Transduction Pathway. Sci. Data 5, 180289. doi: 10.1038/sdata.2018.289
Wang, C., Chen, Y. L., Bian, W. P., Xie, S. L., Qi, G. L., Liu, L., et al. (2018). Deletion of Mstna and Mstnb Impairs the Immune System and Affects Growth Performance in Zebrafish. Fish Shellfish Immunol. 72, 572–580. doi: 10.1016/j.fsi.2017.11.040
Wang, X., Simpson, E. R., Brown, K. A. (2015). P53: Protection Against Tumor Growth Beyond Effects on Cell Cycle and Apoptosis. Cancer Res. 75, 5001–5007. doi: 10.1158/0008-5472.CAN-15-0563
Wardemann, H., Boehm, T., Dear, N., Carsetti, R. (2002). B-1a B Cells That Link the Innate and Adaptive Immune Responses are Lacking in the Absence of the Spleen. J. Exp. Med. 195, 771–780. doi: 10.1084/jem.20011140
Xie, L., Tao, Y. X., Wu, R. H., Ye, Q., Xu, H., Li, Y. (2019). Congenital Asplenia Due to a Tlx1 Mutation Reduces Resistance to Aeromonas Hydrophila Infection in Zebrafish. Fish Shellfish Immunol. 95, 538–545. doi: 10.1016/j.fsi.2019.10.065
Xu, H., Liu, E. X., Li, Y., Li, X. J., Ding, C. Y. (2017). Transcriptome Analysis Reveals Increases in Visceral Lipogenesis and Storage and Activation of the Antigen Processing and Presentation Pathway During the Mouth-Opening Stage in Zebrafish Larvae. Int. J. Mol. Sci. 18, 1634. doi: 10.3390/ijms18081634
Zhang, Z. H., Wu, H. Z., Xiao, J. F., Wang, Q. Y., Liu, Q., Zhang, Y. X. (2012). Immune Responses of Zebrafish (Danio Rerio) Induced by Bath-Vaccination With a Live Attenuated Vibrio Anguillarum Vaccine Candidate. Fish Shellfish Immunol. 33, 36–41. doi: 10.1016/j.fsi.2012.03.031
Zhao, X., Chu, H., Wong, B. H., Chiu, M. C., Wang, D., Li, C., et al. (2020). Activation of C-Type Lectin Receptor and (RIG)-I-Like Receptors Contributes to Proinflammatory Response in Middle East Respiratory Syndrome Coronavirus-Infected Macrophages. J. Infect. Dis. 221, 647–659. doi: 10.1093/infdis/jiz483
Keywords: congenital asplenia, transcriptome, excessive inflammation, splenic anti-inflammatory reflex, systemic immunity
Citation: Xie L, Chen Z, Guo H, Tao Y, Miao X, Wu R and Li Y (2021) Congenital Asplenia Interrupts Immune Homeostasis and Leads to Excessive Systemic Inflammation in Zebrafish. Front. Cell. Infect. Microbiol. 11:668859. doi: 10.3389/fcimb.2021.668859
Received: 17 February 2021; Accepted: 10 May 2021;
Published: 28 June 2021.
Edited by:
Natarajaseenivasan Kalimuthusamy, Bharathidasan University, IndiaReviewed by:
Hong-Yi Gong, National Taiwan Ocean University, TaiwanRaja Veerapandian, University of Texas at San Antonio, United States
Copyright © 2021 Xie, Chen, Guo, Tao, Miao, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, aquatics@swu.edu.cn
 Lang Xie
Lang Xie Zheyu Chen1
Zheyu Chen1  Yun Li
Yun Li