“In-Group” Communication in Marine Vibrio: A Review of N-Acyl Homoserine Lactones-Driven Quorum Sensing
- Central Laboratory, Navy General Hospital of Chinese People's Liberation Army, Beijing, China
N-Acyl Homoserine Lactones (N-AHLs) are an important group of small quorum-sensing molecules generated and released into the surroundings by Gram-negative bacteria. N-AHLs play a crucial role in various infection-related biological processes of marine Vibrio species, including survival, colonization, invasion, and pathogenesis. With the increasing problem of antibiotic abuse and subsequently the emergence of drug-resistant bacteria, studies on AHLs are therefore expected to bring potential new breakthroughs for the prevention and treatment of Vibrio infections. This article starts from AHLs generation in marine Vibrio, and then discusses the advantages, disadvantages, and trends in the future development of various detection methods for AHLs characterization. In addition to a detailed classification of the various marine Vibrio-derived AHL types that have been reported over the years, the regulatory mechanisms of AHLs and their roles in marine Vibrio biofilms, pathogenicity and interaction with host cells are also highlighted. Intervention measures for AHLs in different stages are systematically reviewed, and the prospects of their future development and application are examined.
Quorum Sensing (QS) is a phenomenon that allows bacterial communities to sense small auto-secreting molecules in the environment, allowing monitoring of population density and then regulating expressions of related genes (Bassler, 1999). These small molecules involved in bacterial QS, also known as AutoInducers (AIs) (Nealson, 1977), are classified into three types based on their synthesis pathways, namely AutoInducer-1 (AI-1), AutoInducer-2 (AI-2), and AutoInducing Peptides (AIPs) (Williams, 2007). Different bacterial species generate different AIs to carry out their QS-dependent regulatory functions.
Gram-negative bacteria can mainly produce AI-1 and AI-2 signaling molecules that mediate QS signal transduction via different pathways (Mok et al., 2003; Liaqat et al., 2014). For example, they produce N-Acyl Homoserine Lactones (N-AHLs, AI-1) to mediate QS, to regulate various functions such as biofilm formation, toxin expression, and to escape from host immune response. Increasing studies on the role and underlying mechanism of AHLs in recent years revealed that AHLs are closely associated with the survival and the pathogenicity of most bacteria (Horng et al., 2002; Lumjiaktase et al., 2006; García-Aljaro et al., 2012a).
Vibrio are Gram-negative bacteria commonly found in the marine environment, and 12 of them have been reported as marine pathogen (Balows et al., 1991). They are not only pathogenic to many animal species used in the aquaculture industry, but also are responsible for a number of human gastrointestinal, wound, and even severe acute infections (Tarr et al., 2015). Since QS is common among marine Vibrio, understanding the generation, characteristics, functional regulation, and intervention means of AHLs will help increase knowledge not only on the species but also on the prevention and treatment of infections caused by Vibrio. This article provides an overview of the current progress and knowledge gaps on the generation characteristics, detection, regulatory functions of AHLs in marine Vibrio, and several different AHL-related intervention measures as well.
Characteristics and Detection of Marine Vibrio AHLs
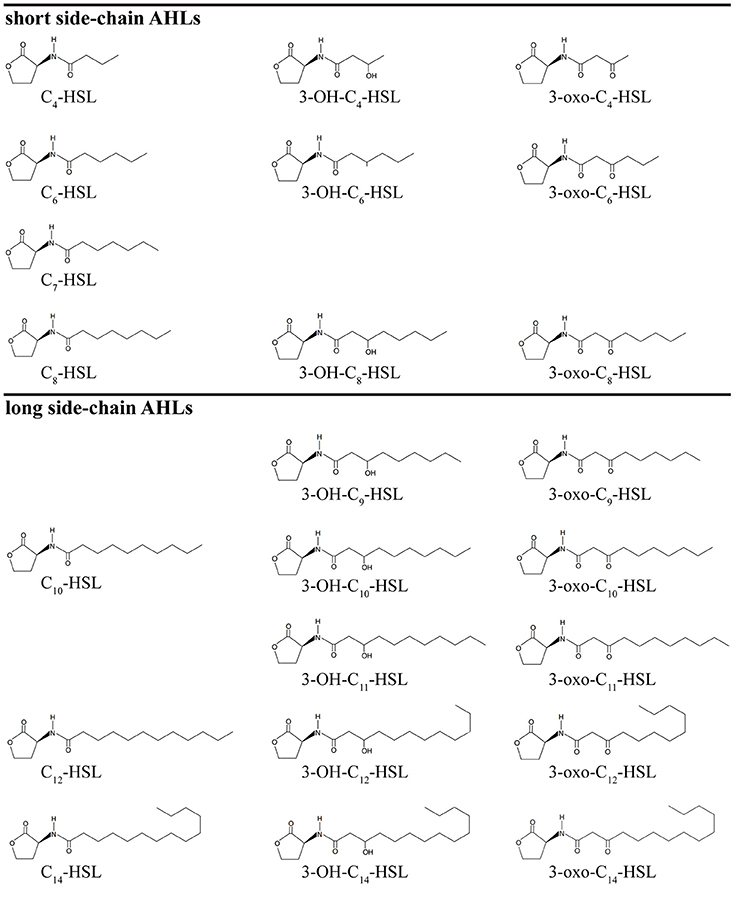
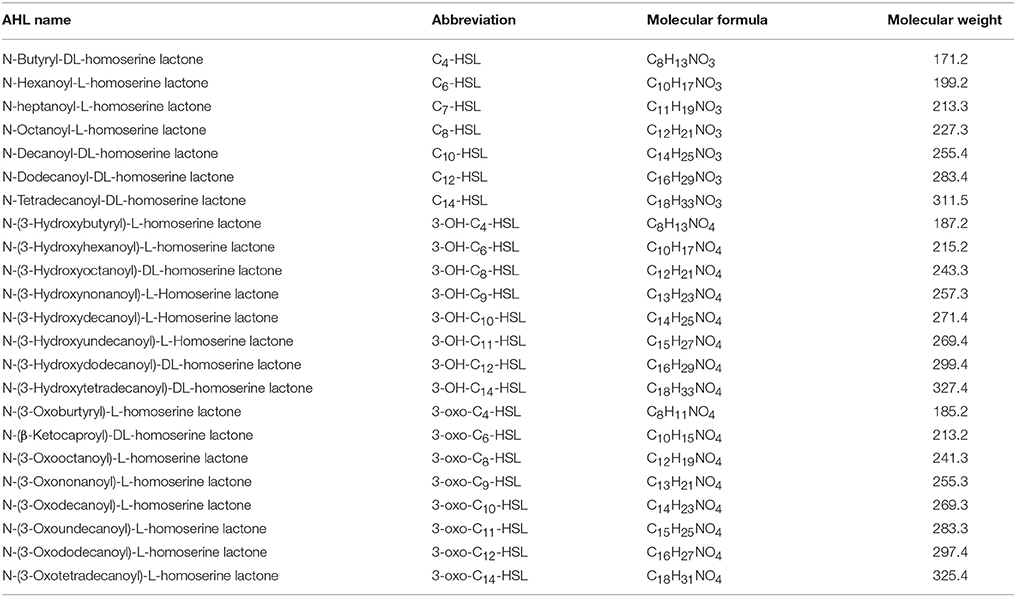
AHLs are a group of amphipathic small molecules (Figure 1), and their common structure is comprised of a hydrophilic homoserine lactone ring and a hydrophobic acyl side chain (O'Connor et al., 2015). Differences in molecular structures depend on the number of carbon (4–18), the substituent group on the third carbon (-H, -OH or -oxo), and the presence or absence of unsaturated double bonds in the acyl side chains (Kumari et al., 2006). These differences cause the diversity in the molecular structures of AHLs and in their secretion pathways. While short side-chain AHLs (<8 carbon atoms on acyl side chain, C4−8-HSL) can directly penetrate cell membrane and be released into the surrounding environment upon synthesis, long side-chain AHLs (>8 carbons on acyl side chain, C10−18-HSL) on the contrary can only be released through active efflux pathways, such as 3-oxo-C12-HSL being exported from membranes via an active mexAB-oprM-encoded MexAB-OprM pump (Pearson et al., 1999). Therefore, diversity of AHLs not only indicates differences in the application of detection methods, but also serves as the basis for various functional regulation.
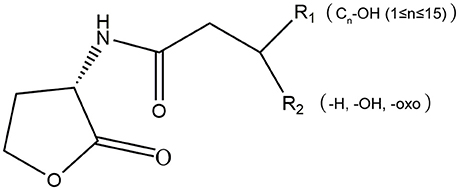
Figure 1. The molecular structure of AHLs. R1: one at least and 15 at most are included from the 4th carbon atom on the acyl side chain of AHL molecule; R2: the unsubstituted (-H) or substituent groups (-OH, -oxo) on the 3rd carbon of the acyl side chain.
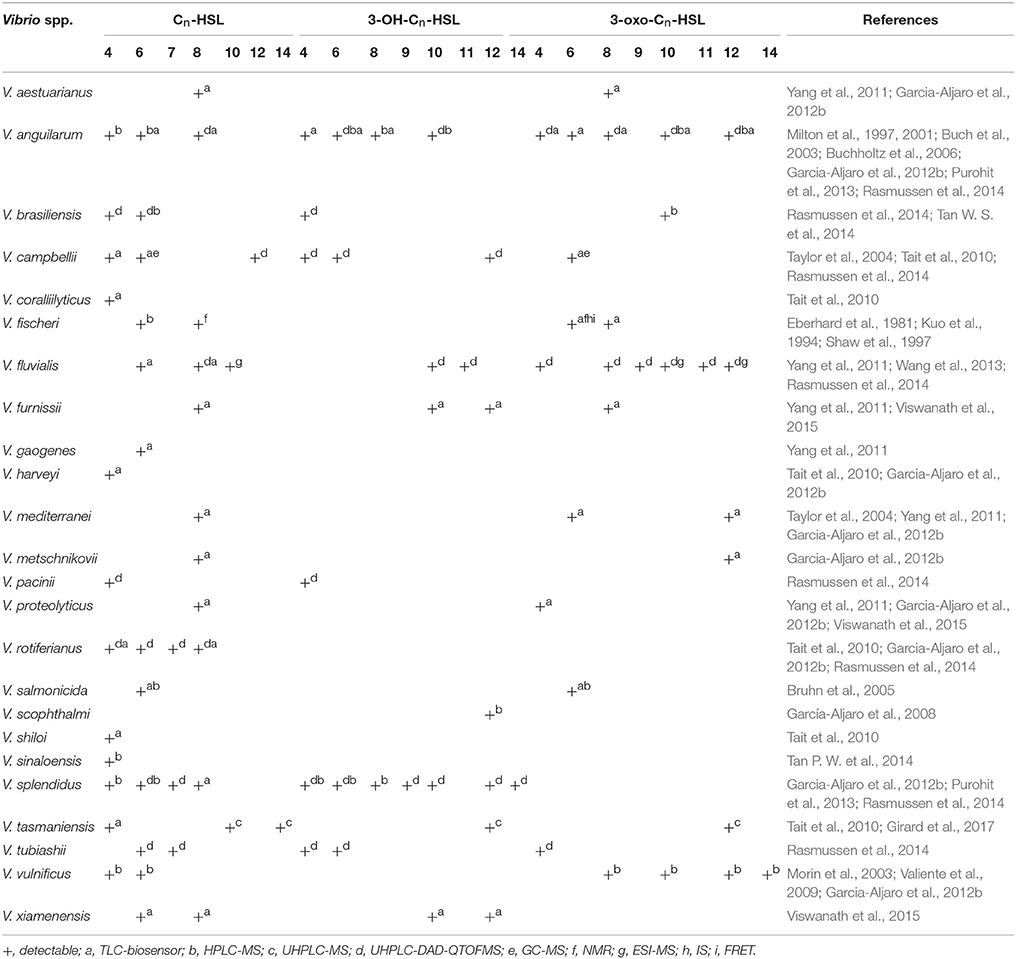
AHLs being generated by Vibrio species and AHL types being accurately detected are two important questions in QS-related studies in marine Vibrio. Common detection methods for AHLs include microbiosensor-based biological detection and chromatography/Mass Spectrometry (MS)-based physicochemical detection. Based on current literatures, a total of 32 AHLs-producing marine Vibrio species have already been identified using different detection methods. Out of the 32, 23 AHLs were definitely classified, including 10 short side-chain and 13 long side-chain AHLs (Figure 2;Tables 1,2).
Characteristics of AHLs Generation in Marine Vibrio
AHLs generation differs among different marine Vibrio species (Eberhard, 1972; Nealson, 1977). For example, V. anguillarum produces more than 12 types of AHLs (Milton et al., 1997, 2001; Buch et al., 2003; Buchholtz et al., 2006; Purohit et al., 2013; Rasmussen et al., 2014), whereas V. scophthalmi and V. harveyi only produce one type of detectable AHL (Tait et al., 2010; Garcia-Aljaro et al., 2012b), indicating that the number of AHLs generated largely varies among marine Vibrio. Furthermore, only long side-chain AHLs have so far been detected in V. scophthalmi (García-Aljaro et al., 2008). In contrast, only short side-chain AHLs are produced in 12 Vibrio spp., such as V. tubiashii and V. fischeri (Eberhard et al., 1981; Kuo et al., 1994; Shaw et al., 1997; Rasmussen et al., 2014), further indicating that the AHL types and proportions also largely differ among marine Vibrio.
AHLs generation is also significantly different between various strains of the same species (Greenberg et al., 1979). Tait et al. (2010) isolated several strains of V. campbelii from coral-associated Vibrio (Tait et al., 2010), and found out that the AHLs detected and identified in the different strains of V. campbelii varied significantly, indicating that AHL generation is diverse and complex even within the same environment. This pattern of AHL generation may be associated with the rapid adaptation of Vibrio to environmental changes (Persat et al., 2014).
The composition of AHLs generated by marine Vibrio is significantly different from those found in terrestrial bacteria. Apart from the AHLs that are commonly generated in terrestrial bacteria, marine Vibrio generate many types of ultra-long side-chain AHLs, such as C14-HSL (Girard et al., 2017), 3-OH-C14-HSL (Rasmussen et al., 2014), and 3-oxo-C14-HSL (Morin et al., 2003). On the other hand, AHLs such as C7-HSL, 3-OH-C9-HSL, 3-oxo-C9-HSL, 3-OH-C11-HSL, and 3-oxo-C11-HSL are rarely identified or reported in terrestrial bacteria, but are also detected in marine Vibrio (Rasmussen et al., 2014).
The environmental conditions that induce generation of AHLs in marine Vibrio are also different from those required by terrestrial bacteria. Firstly, the optimum temperature needed in marine Vibrio is lower than that of common terrestrial bacteria to produce AHLs. In fact, marine Vibrio produce more types and higher concentrations of AHLs at lower temperatures (< 16°C). Thus, the AHLs diversity and concentration decrease with increasing temperature (Tait et al., 2010). Secondly, marine Vibrio-derived AHL types are affected more by changes in ion levels than those generated by common terrestrial bacteria (Buchholtz et al., 2006), which may be associated with greater seasonal variation in temperature and ion levels in marine environment because of complex ocean hydrography. In addition, the dominant AHL alters as the colonization state of marine Vibrio changes, and no report has been described by evidence on this alteration in dominant AHL in terrestrial bacteria to this day. For example, when free V. anguillarum infects the host, its dominant AHL changes from 3-oxo-C10-HSL to 3-OH-C6-HSL (Buchholtz et al., 2006). This change in dominant AHL types could be associated with the various regulatory mechanisms in which AHLs are involved.
Biological Detection of AHLs
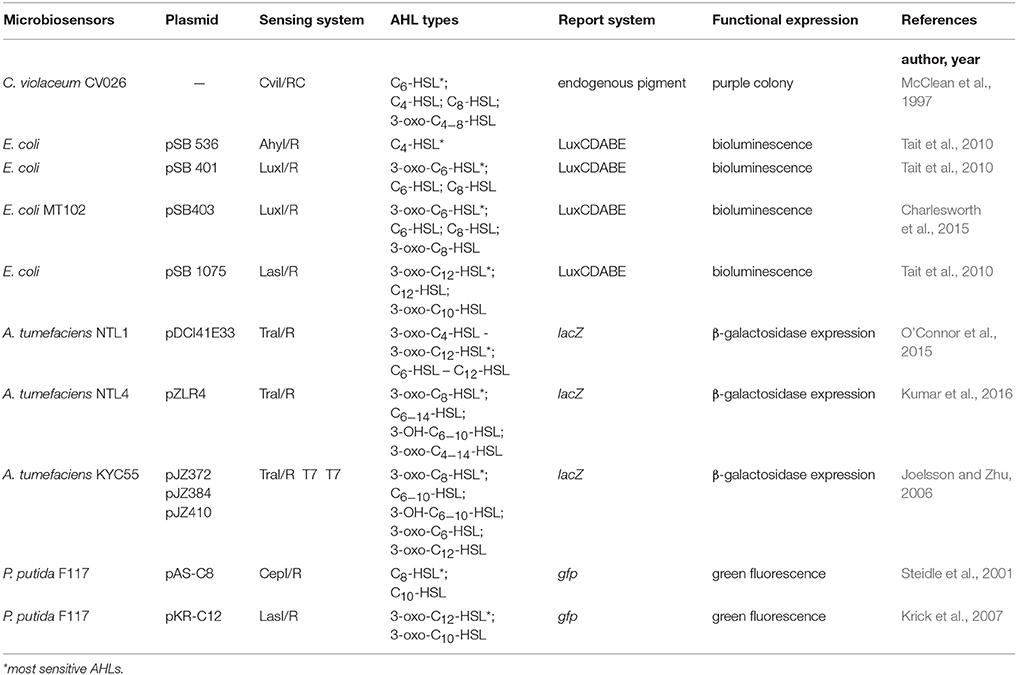
Previously, AHLs generation was measured indirectly by real-time monitoring of bacterial growth rate and AHL-related gene expression, which are time and energy consuming, and has low efficiency and poor accuracy (Bainton et al., 1992; Pearson et al., 1994). With the increasing understanding of the AHL-QS regulatory mechanisms, microbial-derived biosensors gradually replaced the above detection methods and became the conventional and standard technique for AHLs identification (O'Connor et al., 2015). Microbiosensors lack AHLs synthesis proteins but still contain the related AHL receptor proteins and functional genes. Under exogeneous AHLs stimulation, the expression of reporter genes can be initiated, which are then reflected by the changes in colony color, luminescence or enzyme activities. Microbiosensors are mainly obtained in two ways: (1) natural environmental mutation, and (2) genome editing.
For the mutation, although bacterial strains can no longer synthesize AHLs and have lost the characteristic functional expression due to gene mutation, they are still able to initiate QS regulation via exogenous AHLs recognition, leading to characteristic changes in pigments, bioluminescence and protease activities. An example of this type of biosensor is Chromobacterium violaceum CV026, which is a mini-Tn5 mutant of C. violaceum ATCC31532. C. violaceum CV026 has lost the ability to synthesize purple pigments itself but can proliferate purple colonies under exogenous AHLs stimulation. It is highly sensitive to short side-chain AHLs without substituents on the 3rd carbon of the acyl side chain, and with C6-HSL as the AHL having the strongest activating capability. Furthermore, its sensitivity to short side-chain AHLs is decreased by approximately 10-fold when carbonyl substituent is present on the 3rd carbon of the acyl side chains. In contrast, short side-chain AHLs with hydroxyl substituents on the 3rd carbon of the acyl side chain are not recognized by C. violaceum CV026 (McClean et al., 1997).
In the second type of microbiosensors construction, artificial plasmid insertion based on direct genome editing is carried out in bacterial cells to sense exogenous AHLs that induce reporter gene expression via the recombinant plasmid, leading to changes in biological characteristics of microbiosensors of this type. Agrobacterium tumefaciens KYC55 is a biosensor with broad-spectrum AHLs detection capacity acquired artificially, and is highly sensitive to long side-chain AHLs. A. tumefaciens KYC55 contains the pT7-traR plasmid, which has a ptral-lacZ promotor triggered by broad-spectrum AHLs to initiate the expression of the lacZ gene. The lacZ gene encodes β-galactosidase, which then hydrolyzes 5-bromo-4-chloro-3-indole-β-D-galactopyranoside (X-Gal) to produce a blue color in the bacterial colony (Zhu et al., 2003).
Given the diversity in microbiosensors, the types of detectable AHLs and their Limits of Detection (LOD) would also vary. Therefore, the detection of different AHLs generated by marine Vibrio would then be performed one by one across multiple microbiosensors for several times, with the concentrations of the generated AHLs being indirectly calculated. Although this method is not precise, its low cost and ease of use make it a popular technique for the crude screening of AHLs in marine Vibrio. Previously reported microbiosensors used for detecting AHLs generated by marine Vibrio are listed in Table 3.
Physicochemical Detection of AHLs
Thin Layer Chromatography (TLC) is a type of Liquid-Solid Absorption Chromatography (LSAC) commonly used in combination with microbiosensors. Generally, AHL standards and test samples are loaded onto the TLC plate and immersed in the developing solution, causing samples to migrate with different speed. After the plate is dried, the culture media containing microbiosensor is then added onto the plate for culture. Color changes or luminescence in the colonization sites of the microbiosensor are used as the reporter signals for determining the types of AHL via comparison with the AHL standards (Huang et al., 2012). The TLC-biosensor combination is a cheap, rapid and highly efficient detection method that qualitatively and semi-quantitatively identifies the types and concentrations of AHLs in mixtures (Sun et al., 2010). This makes it a favored common preliminary screening technique for AHLs detection in marine Vibrio. Shaw et al. (1997) was the first to utilize the TLC-biosensor method to show the generation of 3-oxo-C6-HSL and 3-oxo-C8-HSL from V. fischeri (Shaw et al., 1997). In 2015, Viswanath et al. (2015) also used the same method to identify two other AHLs (3-OH-C10-HSL, 3-OH-C12-HSL) synthesized by V. fischeri, and accurately classified the AHLs generated by V. xiamenensis and V. proteolyticus (Viswanath et al., 2015). To date, 18 marine Vibrio species were shown to generate AHLs using the TLC-biosensor method (Table 1). Despite the approval of many researchers on its qualitative detection capacity, some drawbacks remain. For example, due to the poor specificity of microbiosensor strains, migrations without matching any of colored or luminous colonization sites of the microbiosensors can easily occur when used in combination with TLC, leading to the inaccurate identification of AHL types (Buch et al., 2003).
Lately, High-Performance Liquid Chromatography tandem Mass Spectrometry (HPLC-MS) with higher sensitivity and specificity was introduced and widely applied for AHLs detection in marine Vibrio. HPLC-MS is a physicochemical detection method based on the different retention times of AHLs due to their molecular weights. The AHLs successively enter the mass spectrometer, and their molecular structures are determined based on ion charge-to-mass ratio. HPLC-MS has the LOD in pg level and can provide abundant information on the structures of AHLs. In 1981, Eberhard et al. used HPLC-MS to confirm that 3-oxo-C6-HSL was the dominant AHL type produced by V. fischeri regulating the bioluminescence of the bacterial community (Eberhard et al., 1981). Kuo et al. (1994) subsequently demonstrated that 3-oxo-C6-HSL was superior to C6-HSL and C8-HSL in inducing the bioluminescence in V. fischeri (Kuo et al., 1994). Since the 1990s, the TLC-biosensor method combined with HPLC-MS was commonly used across numerous AHL detection-related studies of marine Vibrio for the preliminary screening of AHLs generation and the accurate determination of AHLs types and concentrations (Table 1).
In recent years, Ultra-High-Performance Liquid Chromatography (UHPLC) has been used increasingly as the faster and more sensitive chromatography in detecting AHLs for its great application potentials. UHPLC-MS or UHPLC-Diode Array Detector-Quadrupole Time-Off-Flight Mass Spectrometer (DAD-QTOFMS) even provides accurate identification and quantification of the tested AHLs. Its ultra-high precision, stability and scan quality do not only detect common AHL types but also AHLs with ultra-long acyl side chains (>C14) or covalent double bonds (Rasmussen et al., 2014; Table 1). In 2017, Girard et al. first reported the generation of C14-HSL in V. tasmaniensis, and used UHPLC-MS/MS to confirm the presence of an unsaturated double bond in its acyl side chain (Girard et al., 2017). UHPLC not only overcomes the time-consuming disadvantage of HPLC, but it also greatly increases the types of detectable AHLs, and is therefore a milestone in the study of marine Vibrio AHLs.
Moreover, other physicochemical and photochemical methods, such as Gas Chromatograph tandem MS (GC-MS) and Electrospray Ionization tandem MS (ESI-MS), are also widely applied in the detection of marine Vibrio AHLs (Taylor et al., 2004; Wang et al., 2013; Table 1). GC-MS analyzes the molecular structures of AHLs based on the differences in adsorption intensity to inert gas and thereby their sequential entrance into the mass spectrometer. However, certain AHL types in the mixture sample may be lost during this process since they are sensitive to temperature change and may degrade during gasification. On the other hand, ESI-MS accurately determines the structure of AHLs via AHL gasification and analysis of the resulting ion fragments. Since AHLs are often a mixture of various types, it is difficult to accurately isolate each of them during gasification and could be missed. Furthermore, other physicochemical methods such as Nuclear Magnetic Resonance (NMR) (Kuo et al., 1994), Infrared Spectroscopy (IS), and Fluorescence Resonance Energy Transfer (FRET)were also used in some studies (Zhang and Ye, 2014). However, these methods are unable to meet the demands of rapid AHLs detection owing to their complicated operation procedures and high requirements on sample preparation. As a result, only few laboratories were able to use these methods for AHLs detection and analysis in marine Vibrio, making them unpopular in the research field.
Immunological Approaches
Apart from the aforementioned methods, some studies also attempted detection using immunological approaches. Although several antibodies against AHLs are now available, many limitations still exist. The RS2-IG9 antibody for example, developed against 3-oxo-C12-HSL (antigen) from Pseudomonas aeruginosa by Kaufmann et al. (2008), has a limited AHL detection range due to its inability to bind other AHLs (Kaufmann et al., 2008). Despite the subsequent emergence of several patented monoclonal Antibodies (mAbs) targeting the homoserine lactone ring or carboxylic acid derivatives on the acyl side chain of AHLs (Janda et al., 2010; Charlton and Porter, 2012; Bhardwaj et al., 2013), many of these mAbs are still under experimental investigation and are therefore not yet applicable for conventional detection.
Synthesis and Regulatory Mechanisms of Marine Vibrio AHLs
Synthesis of AHLs
AHLs can be catalytically synthesized by LuxI homologous proteins (Gilson et al., 1995; Henke and Bassler, 2004; Bruhn et al., 2005; Rasmussen et al., 2014; O'Connor et al., 2015). While some synthtic proteins of AHLs were found in terrestrial bacterial species, such as TraI in A. tumefaciens (White and Winans, 2007), RhlI and LasI in P. aeruginosa (Brint and Ohman, 1995; Seed et al., 1995), other synthtic proteins of AHLs were present in Vibrio species, such as VanI in V. anguilarum (Milton et al., 1997), LuxI and AinS in V. fischeri (Schaefer et al., 1996; Hanzelka et al., 1999). As Figure 3 shows, LuxI-type proteins first synthesize AHL precursors via the acylation of S-Adenosyl-Methionine (SAM), which removes methylthioadenosine through internal nucleophilic substitution to form the homoserine lactone ring of AHL. Then, Acyl Carrier Protein (ACP)-fatty acyl group derivatives are transferred onto the amino groups of SAM to form acyl side chains with various carbon numbers and chain lengths, which ultimately forms the entire AHL molecule. Difference in the geometric location of the binding site among different LuxI-type proteins determines the status of the third carbon on the acyl side chain, such as saturation (C3-H) or oxidation (C3-OH, C3-oxo), as well as the degree of methylation.
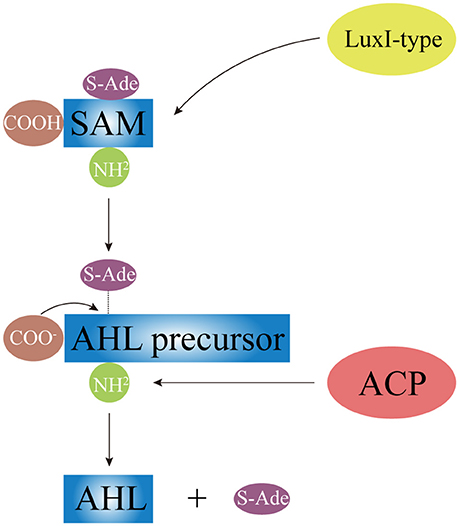
Figure 3. The schematic diagram of AHL synthesis. Under the catalysis of LuxI-type protein, AHL precursor (the HSL ring) is formed via acylating of SAM and removing the S-Ade; the acyl side chain is formed by the transferring of ACP-fatty acyl group derivative onto SAM. SAM, S-Adenosyl-Methionine; S-Ade, methylthioadenosine; ACP, Acyl Carrier Protein.
Regulatory Mechanisms of AHLs
As Chapter 1 has mentioned, AHLs are secreted to environment immediately via different secretion pathways after being produced. Short side-chain AHLs are directly released out of the cell upon synthesis while long side-chain AHLs are actively secreted to the environment. Both AHL types are involved in QS signal transduction. There are more than three QS transduction systems existed in Vibrio, which present the complexity of diversification and precise regulatory mechanisms in Vibrio species (see a review by Milton, 2006). Among all the QS systems, there are two AHL-mediated QS transduction systems in Vibrio including the direct “LuxI/R” system and the cascade regulatory system. Of the two the direct “LuxI/R” system first explored in V. fischeri is the most known one (Engebrecht and Silverman, 1984). The bioluminescence of V. fischeri as an example is the result of LuxR-mediated activation of the LuxCDABE protein, which was also the first QS regulation identified in bacteria. In the LuxI/R QS system, LuxI protein acted as the AHL synthase, and LuxR protein acted as the direct ligand protein of AHLs. The “LuxI/R” system allows bacterial cells to form AHL-receptor complex, which could then bind the functional DNA domain to the subsequent QS related genes (Choi and Greenberg, 1992).
Studies on V. fluvialis, V. harveyi, and V. cholerae showed that marine Vibrio species share similar AHLs regulatory cascades. In an intact AHLs regulatory cascade, the concentration of AHLs increasing to a sensing threshold level of LuxN protein is the key to form AHL-receptor complex in subsequently and to lead a successful QS signal transduction. There are three key molecule types involved in the regulatory cascade of AHLs. The first one is the “two-component” phosphorelay system (Ronson et al., 1987; Parkinson and Kofoid, 1992), where AHLs-sensing LuxN (a cytoplasmic membrane-bound protein) presents as the “input” element and its response regulator LuxO protein as the “output” element (Freeman et al., 2000). LuxN is responsible for sensing AHLs using its “input” domain and for modulating the transmitter activity by changing phosphorylation status of the histidine residue using its transmitter domain (Freeman and Bassler, 1999). LuxO is in response to receive and pass the transmitter signals to the “output” domain by changing phosphorylation status of the aspartate residue. The second one is the Quorum regulatory small RNAs (Qrr sRNAs), and they are in response to degrade the LuxR-type receptor proteins of AHLs via interacting with the chaperone molecule Hfq. The last one is the LuxR-type proteins, which are in charge of activate downstream signaling cascade.
As shown in Figure 4, when the concentration of AHLs is too low to be detected, LuxN presents as kinase, and the autophosphorylation of it occurs normally, activating its downstream transcription factor LuxO via the prior phosphorylation of histidine phosphotransfer protein LuxU (Freeman and Bassler, 1999). This then leads to the expression of Qrr sRNAs (Lilley and Bassler, 2000; Lenz et al., 2004), which sustains the degradation of LuxR-type AHL receptor proteins via interaction with Hfq (Tu and Bassler, 2007). In addition, under the role of two-component phosphorelay system, the phosphorylation of LuxO protein directly promotes the competitive binding of the downstream transcriptional regulators AphA (against OpaR) to the membrane fusion operon mfpABC via the activation of Qrr sRNAs expression, which finally inhibits bacterial biofilm formation (Zhou et al., 2013). There are also several feedback mechanisms on Qrr sRNA-related QS cascade (Figure 4; Ball et al., 2017). The increased expression of Qrr sRNAs could directly suppress the expression of LuxO and LuxN protein to maintain the whole dynamic accommodation system (Feng et al., 2015). Coincidentally, the feedback regulation between transcriptional regulator AphA and Qrr sRNAs is almost the same (Rutherford et al., 2011).
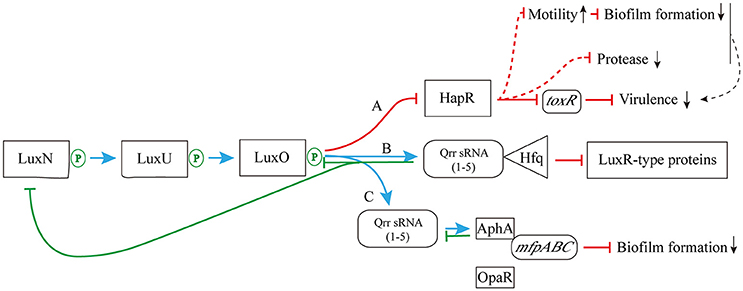
Figure 4. The pathway of phosphorylation for LuxN protein. (A) The LuxN phosphorylation inhibits the expression of HapR, and further influences the expression of virulence factor ToxR; (B) The LuxN phosphorylation promotes the combination of Qrr sRNA and Hfq, and it constantly degrades LuxR-type proteins; (C) The LuxN phosphorylation activates the expression of Qrr sRNAs, allowing competitive combination of transcriptional regulator AphA and membrane fusion operon mfpABC, and inhibiting biofilm formation. Blue arrow: positive regulation; red solid T-connector: direct negative regulation; red dashed T-connector: indirect negative regulation; green solid T-connector: direct negative regulation in the feedback pathway; black dashed arrow: indirect positive regulation; green P-circle: phosphorylation; down arrow: weakened expression.
On the other hand, when the concentration of environmental AHLs gradually increases to the sensing threshold of LuxN protein, its spontaneous autophosphorylation is inhibited. That in turn inhibits the phosphorylation of downstream proteins (Timmen et al., 2006) such as LuxU protein, which further interferes the phosphorylation of LuxO, leading to the inhibition of Qrr sRNAs expression. As a result, LuxR-type receptor protein is continuously synthesized, and it binds to the acyl side chain of free AHLs to form AHL-receptor transcription complex. The complex regulates the expression of multiple downstream target genes, such as the master regulating gene luxR of V. harveyi (Van Kessel et al., 2013), the elastase coding gene lasR of P. aeruginosa (Gambello and Iglewski, 1991), the curvature coding gene crvA of V. cholerae (Bartlett et al., 2017), and the QS regulon coding gene esaR of Pantoea stewartii (Ramachandran et al., 2014). Thus, those aforementioned regulations ultimately initiate or silence RNA transcription and protein translation to express related functions (Bassler et al., 1994; Anetzberger et al., 2009; Figure 5).
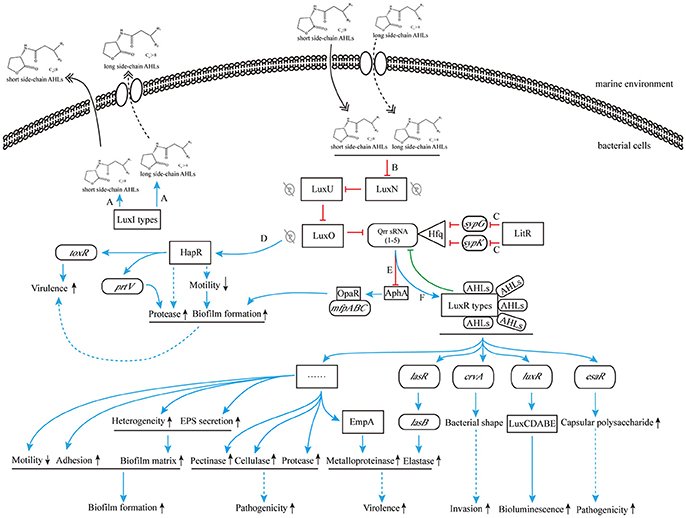
Figure 5. The cascade control mechanism of AHLs produced by marine Vibrio. (A) LuxI-type proteins synthesize and release AHLs to the environment; (B) high concentration of AHLs inhibit the phosphorylation for LuxN protein; (C) the transcription inhibition of sypG and sypK by LitR inhibit the combination of Qrr sRNAs and Hfq, and promote the production of LuxR-type protein; (D) the inhibition of phosphorylation for LuxN protein removes the surpression of HapR, resulting to direct increased ToxR expression and indirect down regulation of bacterial motility and subsequent increased regulation of biofilm formation and protease production, and promotes bacterial virulence; (E) the inhibition of Qrr sRNAs expression is in favor of combining OpaR to mfpABC, further increases biofilm formation; (F) the AHL-LuxR protein complex activates downstream functional pathways. Blue solid arrow: positive regulation; blue dashed arrow: indirect positive regulation; red T-connector: negative regulation; green T-connector: negative regulation in the feedback pathway; double-headed solid arrow: direct release; double-headed dashed arrow: active transmembrane transport; gray P-circle with a strikethrough: unhappened phosphorylation; up arrow: enhanced expression; down arrow: weakened expression.
Regulatory Functions of Marine Vibrio AHLs
AHL and Biofilm Formation
When the environmental surface is suitable for bacterial survival, biofilm formation starts from the adhesion of bacterial cells. Along with the enhanced secretion of extracellular enzymes, biofilm matrix builds and biofilm reaches to its mature stage eventually. At the end stage of biofilm formation circle, biofilm starts to collapse, leading to the increased motility of the bacterial cells within the matrix. The collapsing allows bacteria to attach to suitable environmental surfaces, followed by a new period of biofilm formation, including enhanced secretion of extracellular enzymes and formation of biofilm matrix (Figure 6). Biofilm formation is also connected to changes in colony morphology, proliferative metabolism, and drug resistance. Bacteria within the biofilm have significantly slower metabolism and present antibiotic resistance properties. The structure of the biofilm matrix protects bacteria from host cell-mediated or drug-induced phagocytic clearance, allowing the bacteria to evade the host's immune system (Bhardwaj et al., 2013).
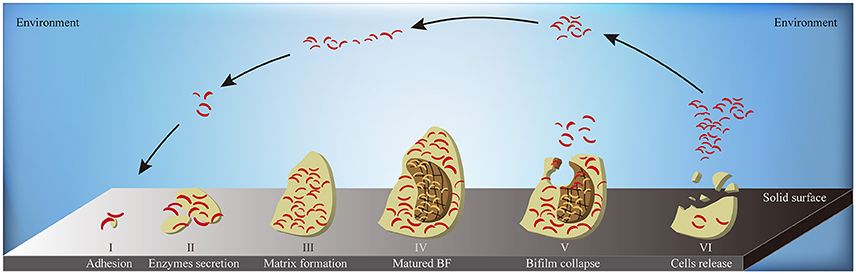
Figure 6. The schematic diagram of biofilm formation. A complete circle of biofilm formation contains: (I) the adhesion of bacterial cells to suitable solid surface; (II) the enhanced secretion of extracellular enzymes; (III) the formation of biofilm matrix; (IV) the formation of matured biofilms; (V) the collapse of biofilm in the middle-end stage; (VI) the release of bacterial cells inside the biofilm to environment in the end stage. BF, biofilm; yellow flat, the entire biofilm; borrow flat, the biofilm matrix; red bend pole, bacterial cells.
Indeed, previous studies have shown that AHLs increases the survival of marine Vibrio by regulating key processes of biofilm formation in many ways (McDougald et al., 2006). First, AHLs regulate the excretion of Extracellular Polymeric Substances (EPS) to constitute the cage construction of biofilm matrix. The matrix provides a suitable space for bacterial colonization and stable metabolism. Its porous nature and complex structure allow bacteria cells to hide deeply within the matrix, allowing avoidance of host immune cell-mediated cytotoxicity or phagocytosis and to effectively block the permeation of antibiotics. AHLs such as C4-HSL and C6-HSL also upregulate the expression of ESP-related genes via binding to AHL receptor proteins, which in turn increase EPS production by forming denser biofilm matrix and reinforced defense barrier (Jamuna and Ravishankar, 2016). Second, AHLs regulate the ability of adhesion or detachment of marine Vibrio, allowing the colonization changes for a better adaption to the environment, thus EPS excretion changes in order to quicken or reduce a new period of biofilm formation (Phippen and Oliver, 2015). When the bacteria are in a harsh environment, AHLs enhance bacterial adhesion to adjacent solid surfaces so as to promote clonal proliferation and to speed up the EPS excretion. Once the environmental condition is improved, Qrr sRNAs degrade LuxR-type AHL receptor proteins to reduce Vibrio adhesion and enhance their mobility (Phippen and Oliver, 2015), resulting to migration and proliferation of colony to compatible environments. Third, AHLs alter Vibrio colony morphology to facilitate biofilm formation. Changes in the colony morphology dynamically regulate the surface area of the biofilm. Compared with a smooth colony, a wrinkled one can effectively increase its surface area to enhance bacterial adhesion to the biofilm. Furthermore, active proliferation of the surface bacteria increases the heterogeneity of the biofilm, which in turns positively regulates its associated functions (Anetzberger et al., 2009). LitR, a transcription inhibitor of the syp gene family, inhibits syp-mediated transcription of Qrr sRNAs to promote the formation of the AHL-LuxR receptor transcription complex (Miyashiro et al., 2014), which regulates the transformation of smooth to wrinkled V. salmonicida colonies, and thus, enhancing biofilm formation (Hansen et al., 2014).
AHL and Bacterial Pathogenicity
Bacterial virulence is associated with the strength of bacterial pathogenicity in the host. The invasiveness level and the expression of virulence genes are two critical factors on bacterial virulence, which could exercise either combined effects or solo effects. In most Vibrio species, the above-mentioned factors often coordinate with each other. Taken wound infection by V. vulnificus as an example, the strong invasive capacity of V. vulnificus determines the accurate path and rapid efficiency when entering host bloodstream, and along with V. vulnificus proliferation, the subsequent accumulation and regulation of toxin expression via various virulence genes would lead to a high risk of V. vulnificus-related death (Lubin et al., 2015). However, as a non-bacteremia Vibrio species, V. cholerae doesn't cause septicemia but severe diarrhea, acute acidosis and vomiting, which is resulted in the solo-effects of its toxins (Rai and Chattopadhyay, 2014), such as the canonical Vibrio cholerae Cytolysin (VCC; He and Olson, 2010).
Substances related to bacterial invasiveness include extracellular enzymes, capsular polysaccharides and other proteins, which play crucial roles in breaking the defense barrier of the host. Bacterial virulence-related proteins encoded by virulence genes could induce the apoptosis of host cells during pathogen infection and lead to the development of various symptoms such as systemic infection and multiple-organ failure. For example, the transcriptional activator ExsA activates the expression of Type III Secretion System 1 (T3SS1 system) and causes disease progression of V. parahaemolyticus (Zhou et al., 2008); the pore forming toxin Vibrio vulnificus Hemolysin A (VvhA) is an important exotoxin of V. vulnificus and causes apoptosis in epithelial cells (Lohith et al., 2015); VCC of V. cholerae has potent cell-killing activity and is listed as its prominent membrane-damaging cytolysin (Khilwani and Chattopadhyay, 2015). Early in 1993, the study of Jones et al. (1993) had already revealed the virulence regulation of QS system, and in recent years, more studies have further shown that the expression of virulence-related pathogenic factors of Vibrio is strictly regulated by QS system and the environment (Bhardwaj et al., 2013; Lee et al., 2014; Hema et al., 2015; Jung et al., 2015).
Bacterial Extracellular Metalloproteases (BEMPs) are an important type of invasive exocytotic enzymes, and the dependence on the iron acquisition is a key factor for the expression and regulation of BEMPs (Nguyen and Jacq, 2014). BEMPs can be divided into 63 families based on differences in the homologous sequence (Zhang Y. Y. et al., 2017). Elastase is currently the most widely studied enzyme despite no studies or reports of it being produced by Vibrio, the production of which is closely related to QS system. As one of the important pathogenic determinants in P. aeruginosa, the elastase production is regulated by lasB gene, which is activated by the AHL-LasR receptor complex mediated through the binding of the QS signaling molecule 3-oxo-C12-HSL to its receptor LasR (Gambello and Iglewski, 1991). During this process, 3-oxo-C12-HSL reaching threshold concentration and activating relevant regulatory cascade provide an important intercellular transport pathway for the expression of elastase (Passador et al., 1993).
As reported in the newly published MEROPS database (January 2017)1, Vibrio species can produce multiple families of BEMPs including M4, M48 and S1. Taken EmpA—a metalloprotease in the BEMP family—as an example, it is a AHL-regulated virulence factor of Vibrio (Denkin and Nelson, 2004). Croxatto et al. (2002) have demonstrated that LuxR-type QS transcriptional regulator VanT is required for EmpA expression in V. anguillarum. The positive regulation of VanT could enhance EmpA expression, and thereby increase the total secretion of BEMPs from Vibrio (Croxatto et al., 2002). QS-mediated regulation of BEMPs has been well exploited in some terrestrial bacteria, such as lasB-mediated regulation of elastase via AHL-LasR receptor complex in P. aeruginosa (Gambello and Iglewski, 1991; Wei et al., 2015); Clostridium perfringens hemolysins CPA and PFO are regulated by the CpAL QS system (Vidal et al., 2015). In Vibrio species, HapR protein, a master regulator in V. cholerae, has been found to positively regulate V. cholerae protease production via upgrading the coding activity of a downstream BEMP related gene prtV under the high cell density condition (Figure 5; Nguyen and Jacq, 2014). However, since there are fewer studies on the AHLs-mediated regulation of Vibrio BEMPs than there are on common terrestrial bacteria, further supplementary data and investigation are required to elucidate the relevant phenotypes and mechanisms.
Besides the regulation of AHLs on BEMPs, many studies have shown that AHLs participate in the regulation of marine Vibrio pathogenicity via regulating other virulence-related proteins. For example, ToxR, a classic Vibrio virulence factor encoded by the virulence-related gene toxR, is directly regulated by AHLs. ToxR was first discovered in V. cholerae, and subsequent studies showed that homologous genes of toxR also exist in many pathogenic Vibrio species such as V. parahaemolyticus, V. vulnificus, and V. alginolyticus. When AHLs concentration is below threshold, the regulator LuxO affects the expression of toxR gene through inhibition of the production of its upstream protein—HapR, thereby keeping Vibrio virulence at a relatively attenuated level (Figure 4). In contrast, when AHLs concentration is higher than the threshold, LuxO is no longer capable of inhibiting the generation of HapR protein, leading to increased toxin synthesis by the toxR gene. Meanwhile, bacterial pathogenicity is also synergistically enhanced by the combined regulation of various biological activities, including the down regulation of bacterial motility and subsequent increased regulation of biofilm formation and protease production (Figure 5; Ball et al., 2017).
The curvature determinant protein CrvA, which is another virulence factor, is also regulated by QS signaling molecules. CrvA could alter the vibrioid shape of V. cholerae based on the changes occurring in the environment. V. cholerae invasiveness increases when CrvA changing the cell from a rod to a bent shape, allowing effective entrance into the host's gut for colonization and proliferation (Bartlett et al., 2017).
Apart from virulence factors ToxR and CrvA, the expressions of various Vibrio toxins, such as Vibrio vulnificus Hemolysin (VVH) and toxin A (Elgaml et al., 2014), are also directly regulated by AHLs. The synergistic expression of Vibrio toxins and virulence factors could enhance bacterial pathogenicity. However, relevant reports on this field remain scarce compared to those done on terrestrial bacteria, which merits further investigation and research.
Interaction Between AHL and Host
With an interaction called inter-kingdom signaling (Hughes and Sperandio, 2008), AHLs could modify various types of eukaryotic host cells and modulate host's defense system so as to exert multiple regulatory functions to higher organisms (Hartmann and Schikora, 2012). AHL-mediated regulation of the immune system is a common topic among many current studies.
AHLs directly regulate immune cell proliferation. Taken the signaling molecule 3-oxo-C12-HSL as an example, it could inhibit the proliferation of T lymphocytes and human dendritic cells in a dose-dependent manner (10–100 μm; Boontham et al., 2008). Besides, different AHLs have significantly different regulatory efficiency on host cells. AHLs-related comparative study by Gupta et al. (2011) showed that C4-HSL and 3-oxo-C12-HSL within a certain dose range (1–30 μm) could both inhibit splenic T cell proliferation in mice, while this inhibitory effect could only be detected at high concentration of C4-HSL addition alone. In contrast, low concentration of 3-oxo-C12-HSL alone was sufficient to inhibit T cell proliferation (Gupta et al., 2011). The study of Gupta et al. (2011) indicates that long side-chain AHLs have better regulatory efficiency than short side-chain AHLs, and the combination of both types has greater inhibitory effect than either AHLs alone.
As well as the regulation of cell proliferation, 3-oxo-C12-HSL also affects immune cell survival by inducing apoptosis of neutrophils (Tateda et al., 2003; Li et al., 2010), mast cells and phagocytes in a dose-dependent manner (10–100 μm). 3-oxo-C12-HSL could as well activate phagocytosis in human phagocytes via activation of the p38-MAPK pathway, leading to inflammation (Vikström et al., 2005). In the meantime, 3-oxo-C12-HSL acts as a chemokine to promote neutrophil migration to the inflammation site and induces host inflammatory response (Zimmermann et al., 2006).
AHLs could alter the host immune response pattern (Rumbaugh et al., 2004). Specifically, high concentration of synthesized AHLs could modulate the immune response of host cells by switching from the Th1 immune response, which protects host cells, to the Th2 immune response, which is more suitable for bacteria survival (Moser et al., 2002; Hooi et al., 2004). At the same time, these signaling molecules inhibit the activation of Th1-type immune response to enhance the AHLs-QS phenomenon (Gupta et al., 2011). During host immune response, other than inducing changes in immune cells, 3-oxo-C12-HSL also acts on several cells such as epithelial cells, fibroblasts and lung fibroblasts to synergistically mediate transformation to Th2 immune response (Smith et al., 2001, 2002).
In addition to the immune system, AHLs also regulate other cell types such as epithelial cells. 3-oxo-C12-HSL is the most commonly studied AHL molecule as it could (1) directly damage the barrier function of intestinal epithelial cells Caco-2 (Vikström et al., 2006), and (2) modify the integrity of epithelial cells via altering tyrosine, serine and threonine phosphorylation in Adherens Junction (AJ) transmembrane protein E-cadherin, cytoplasmic protein β-catenin, Tight Junction (TJ) transmembrane protein occluding, and TJ cytoplasmic protein Zonula Occludens-1 (ZO-1) in a time-dependent manner (Vikström et al., 2009).
AHLs-Related Intervention Measures
Vibrio genus included pathogenic species that are widely found in the marine environment (see a review by Milton, 2006), and their infections cause a series of diseases such as acute gastroenteritis (Shimohata and Takahashi, 2010), septicemia (Horseman and Surani, 2011), and Skin and Soft Tissue Infections (SSTIs; Diaz, 2014). These diseases have acute onset, rapid progression, and may lead to multiple organ failure or even death in severe cases (Janda et al., 2015). As a consequence of increased antibiotic abuse worldwide, the gradual development of Multi-Drug Resistance (MDR) in marine Vibrio renders the current measures for Vibrio infection less effective, making the search for new anti-Vibrio infection measures an urgent focus of research (Elmahdi et al., 2016). As previously discussed in this article, AHLs not only regulate many physiological functions in marine Vibrio, but also cause damages to the host cells and immune system, thereby playing a key role in the infection process. With the increased understanding of the regulatory mechanisms of AHLs, blocking key factors in their regulatory pathways and hence inhibiting the downstream effects of AHLs may serve as potential prevention measures and treatments for Vibrio infections.
Intervention measures for AHLs-mediated regulation reported in the current literature could be divided into three strategies. The first involves the inhibition of AHLs generation via blocking the synthesis pathway (including the synthesis proteins and the two-component phosphorelay system; Kalia and Purohit, 2011). Specifically, triclosan could inhibit the production of ACP protein by disrupting the chromosomal fabI gene, which in turn hinders the function of AHL synthase RhlI and blocks C4-HSL synthesis (Hoang and Schweizer, 1999); closantel inhibits the two-component phosphorelay system by altering the structure of histidine kinase sensor “in-put” element (Stephenson et al., 2000), leading to a suppression of AHL synthesis genes (Zhang, 2003). Furthermore, V. harveyi R-21446 and V. harveyi Fav 2-50-7 isolated from coral-associated microbial colonies could either interfere with the color change of bacterial colony or inhibit biofilm formation of other bacterial groups by blocking AHLs synthesis of the tested bacteria, while the AHL production of themselves was not influenced (Tait et al., 2010; Golberg et al., 2013), and are then considered natural anti-AHL Vibrio.
The second intervention type involves the degradation of synthesized AHLs. For example, the AHL-degrading enzyme AiiA produced by Bacillus spp. is a lactonase presenting broad spectrum anti-AHL characteristics and displays natural tolerance to acidic environments (Augustine et al., 2010). AiiA inhibits biofilm formation by degrading AHL and blocking its signaling pathway, and significantly reducing the pathogenicity of Vibrio in the host, making it a potential AHL inhibitor (Augustine et al., 2010). Further, some higher organisms, such as brine shrimp, have the ability to inactivate AHLs by at least two methods: (1) by providing a highly alkaline intestinal environment, the synthesized AHLs could be hydrolyzed immediately; (2) by producing AHL-inactivating enzymes to reduce the formation of AHL-receptor complex (Defoirdt et al., 2008).
The third-class functions by interfering with AHL-receptor complex formation. Based on the mechanistic pattern of AHL inhibitors, AHL-receptor complex formation could be intervened via four main approaches. (A) Via reducing the binding efficiency of AHL-receptor to its promoter sequence. For example, cinnamylaldehyde and its derivatives block the binding efficiency of transcriptional regulator LuxR-type protein to its promoter sequence, which then affects the expression of the latter, leading to the forming inhibition of AHL-receptor complex, and eventually hinder the downstream regulatory functions of AHLs (Brackman et al., 2008). (B) Via performing the competitive binding to AHL receptor between AHL inhibitor and AHL, and the downstream pathway of gene expression could be eventually blocked. For example, thiazolidinedione analog competitively binds to the binding sites on the amino group or carboxyl group of LuxR protein to block the formation of AHL-LuxR receptor complex (Rajamanikandan et al., 2017), ultimately decreasing the expression of downstream genes. (C). Via changing the structure of AHL receptors. QS inhibitors such as Furanone C-30, which reduces the stability of LuxR receptor and facilitate the structural change of the latter prevent the formation of the AHL-receptor complex (Ren et al., 2001; Lowery et al., 2008). (D) Via modulating AHL receptor-mediated regulation of downstream genes. For example, coumarin significantly reduces LuxR-mediated regulation of downstream genes, and alters the protease activity and hemolytic capacity of V. splendidus, resulting in reduced virulence expression (Zhang et al., 2017).
The three major intervention measures target different parts of the AHLs regulatory cascade to inhibit regulation of downstream functions, leading to interference of the infection process and reduced pathogenicity of marine Vibrio (Bhardwaj et al., 2013; Chu and McLean, 2016).
Conclusion and Prospects
AHLs are important QS signaling molecules produced by many bacteria genera, especially as the foremost type of QS molecules in a variety of Gram negative bacteria, such as P. aeruginosa and Acinetobacter baumannii (Smith et al., 2002; Chan et al., 2014). AHLs are not solely restricted to terrestrial bacteria, but are commonly found among marine Vibrio. They are involved in many key regulations and play crucial roles in the progress of Vibrio infections. With the increased emergence of antibiotic-resistant Vibrio species in recent years, studies on QS system have become the new breakthrough for the prevention and treatment of marine Vibrio infections.
From the detection methods of AHLs to their production diversity, there are several features about AHLs characterization in Vibrio summarized in this article, including types, concentrations, and dominance alteration. Among these features of Vibrio, an article by Buchholtz et al. (2006) discussed about the dominant AHL change in V. anguillarum along with differing environments, which so far has only been reported once. Yet, is this dominance changing of AHLs only happening in Vibrio? Or, is there any close relationship between this phenomenon and Vibrio adaption to different environments? There is still no further research continuing with these hypotheses. Currently, from AHLs regulating functions to AHL-related QS prevention strategies, most studies focus on common terrestrial pathogens such as P. aeruginosa and other bacteria especially on the interaction between AHLs and host cells, while the fewer studies in Vibrio are still on their exploration stage based on the similar researches in terrestrial bacteria. This means either a stagnate or a slow-moving forward in this field, which is expected to be seen for a breakthrough in the future.
Since the research direction is somewhat limited as above-mentioned, further studies will be required to determine other specific AHLs-related functions and regulatory mechanisms that may be present in Vibrio species. Therefore, expansion of research on the generation, regulation and relevant functions of AHLs in marine Vibrio has great application potentials and deserve further in-depth investigations.
Author Contributions
JL and LZ conceptualized this review. KF and LZ designed the frame structure of this review based on the idea. JL and KF organized the original writing of this review. CW and KQ confirmed the logic validity of this review. FL and LZ proofread the paper. JL and KF contributed equally to this work. All authors discussed the conclusion and commented on the manuscript.
Funding
This work is supported by the Key Foundation of Navy General Hospital (Grant No.: 14J004) and National Natural Science Foundation of China (Grant No.: 81273311 and 31400107).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YT and handling Editor declared their shared affiliation.
Acknowledgments
We thank our colleagues, MS. Yuxiao Wang, Dr. Xiaojie Yu, Dr. Weizheng Shuai, MS. Yan Liu, MS. Qian Pu, for their generous suggestions to this paper and the great help in the preparation of this review.
Abbreviations
N-AHL, N-Acyl Homoserine Lactone; QS, Quorum Sensing; AI, AutoInducer; AI-1, AutoInducer-1; AI-2, AutoInducer-2; AIP, AutoInducing Peptide; MS, Mass Spectrometry; X-Gal, 5-bromo-4-chloro-3-indole-β-D-galactopyranoside; LOD, Limits of Detection; TLC, Thin Layer Chromatography; LSAC, Liquid-Solid Absorption Chromatography; HPLC, High-Performance Liquid Chromatography; UHPLC, Ultra-High-Performance Liquid Chromatography; DAD, Diode Array Detector; QTOFMS, Quadrupole Time-Off-Flight Mass Specrometer; GC, Gas Chromatography; ESI, Electrospray Ionization; NMR, Nuclear Magnetic Resonance; IS, Infrared Spectroscopy; FRET, Fluorescence Resonance Energy Transfer; mAb, monoclonal Antibody; SAM, S-Adenosyl-Methionine; ACP, Acyl Carrier Protein; Qrr sRNA, Quorum regulatory small RNA; EPS, Extracellular Polysaccharides; T3SS1 system, Type III Secretion System 1; BEMPs, Bacterial Extracellular Metalloproteases; VvhA, Vibrio vulnificus Hemolysin A; VCC, Vibrio Cholerae Cytolysin; AJ, Adherens Junction; TJ, Tight Junction; SSTIs, Skin and Soft Tissue Infections; MDR, Multi-Drug Resistance.
Footnotes
References
Anetzberger, C., Pirch, T., and Jung, K. (2009). Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol. Microbiol. 73, 267–277. doi: 10.1111/j.1365-2958.2009.06768.x
Augustine, N., Kumar, P., and Thomas, S. (2010). Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch. Microbiol. 192, 1019–1022. doi: 10.1007/s00203-010-0633-1
Bainton, N. J., Bycroft, B. W., Chhabra, S. R., Stead, P., Gledhill, L., Hill, P. J., et al. (1992). A general role for the lux autoinducer in bacterial cell signaling: control of antibiotic biosynthesis in Erwinia. Gene 116, 87–91. doi: 10.1016/0378-1119(92)90633-Z
Ball, A. S., Chaparian, R. R., and van Kessel, J. C. (2017). Quorum sensing gene regulation by LuxR/HapR master regulators in vibrios. J. Bacteriol. 199, e00105–e00117. doi: 10.1128/JB.00105-17
Balows, A., Hausler, W. J. Jr., Herrmann, K. L., and Isenberg, H. D. (1991). Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology.
Bartlett, T. M., Bratton, B. P., Duvshani, A., Miguel, A., Sheng, Y., Martin, N. R., et al. (2017). A periplasmic polymer curves Vibrio cholerae and promotes pathogenesis. Cell 168, 172.e115–185.e115. doi: 10.1016/j.cell.2016.12.019
Bassler, B. L. (1999). How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2, 582–587. doi: 10.1016/S1369-5274(99)00025-9
Bassler, B. L., Wright, M., and Silverman, M. R. (1994). Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13, 273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x
Bhardwaj, A. K., Vinothkumar, K., and Rajpara, N. (2013). Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat. Antiinfect. Drug Discov. 8, 68–83. doi: 10.2174/1574891X11308010012
Boontham, P., Robins, A., Chandran, P., Pritchard, D., Cámara, M., Williams, P., et al. (2008). Significant immunomodulatory effects of Pseudomonas aeruginosa quorum-sensing signal molecules: possible link in human sepsis. Clin. Sci. 115, 343–351. doi: 10.1042/CS20080018
Brackman, G., Defoirdt, T., Miyamoto, C., Bossier, P., Van Calenbergh, S., Nelis, H., et al. (2008). Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 8:149. doi: 10.1186/1471-2180-8-149
Brint, J. M., and Ohman, D. E. (1995). Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177, 7155–7163.
Bruhn, J. B., Dalsgaard, I., Nielsen, K. F., Buchholtz, C., Larsen, J. L., and Gram, L. (2005). Quorum sensing signal molecules (acylated homoserine lactones) in Gram-negative fish pathogenic bacteria. Dis. Aquat. Org. 65, 43–52. doi: 10.3354/dao065043
Buch, C., Sigh, J., Nielsen, J., Larsen, J. L., and Gram, L. (2003). Production of acylated homoserine lactones by different serotypes of Vibrio anguillarum both in culture and during infection of rainbow trout. Syst. Appl. Microbiol. 26, 338–349. doi: 10.1078/072320203322497365
Buchholtz, C., Nielsen, K. F., Milton, D. L., Larsen, J. L., and Gram, L. (2006). Profiling of acylated homoserine lactones of Vibrio anguillarum in vitro and in vivo: influence of growth conditions and serotype. Syst. Appl. Microbiol. 29, 433–445. doi: 10.1016/j.syapm.2005.12.007
Chan, K. G., Cheng, H. J., Chen, J. W., Yin, W. F., and Ngeow, Y. F. (2014). Tandem mass spectrometry detection of quorum sensing activity in multidrug resistant clinical isolate Acinetobacter baumannii. Sci. World J. 2014:891041. doi: 10.1155/2014/891041
Charlesworth, J., Kimyon, O., Manefield, M., and Burns, B. P. (2015). Detection and characterization of N-acyl-l-homoserine lactones using GFP-based biosensors in conjunction with thin-layer chromatography. J. Microbiol. Methods 118, 164–167. doi: 10.1016/j.mimet.2015.09.012
Charlton, K. A., and Porter, A. J. R. (2012). Methods for the Treatment of an Infectious Bacterial Disease With an Anti-lactone or Lactone Derived Signal Molecules Antibody. U.S. Patent No 8,168,397. Washington, DC: U.S. Patent and Trademark Office.
Choi, S. H., and Greenberg, E. P. (1992). Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J. Bacteriol. 174, 4064–4069. doi: 10.1128/jb.174.12.4064-4069.1992
Chu, W., and McLean, R. J. (2016). Quorum signal inhibitors and their potential use against fish diseases. J. Aquat. Anim. Health 28, 91–96. doi: 10.1080/08997659.2016.1150907
Croxatto, A., Chalker, V. J., Lauritz, J., Jass, J., Hardman, A., Williams, P., et al. (2002). VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184, 1617–1629. doi: 10.1128/JB.184.6.1617-1629.2002
Defoirdt, T., Boon, N., Sorgeloos, P., Verstraete, W., and Bossier, P. (2008). Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J. 2, 19–26. doi: 10.1038/ismej.2007.92
Denkin, S. M., and Nelson, D. R. (2004). Regulation of Vibrio anguillarum empA metalloprotease expression and its role in virulence. Appl. Environ. Microbiol. 70, 4193–4204. doi: 10.1128/aem.70.7.4193-4204.2004
Diaz, J. H. (2014). Skin and soft tissue infections following marine injuries and exposures in travelers. J. Travel Med. 21, 207–213. doi: 10.1111/jtm.12115
Eberhard, A. (1972). Inhibition and activation of bacterial luciferase synthesis. J. Bacteriol. 109, 1101–1105.
Eberhard, A., Burlingame, A. L., Eberhard, C., Kenyon, G. L., Nealson, K. H., and Oppenheimer, N. J. (1981). Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20, 2444–2449. doi: 10.1021/bi00512a013
Elgaml, A., Higaki, K., and Miyoshi, S. (2014). Effects of temperature, growth phase and luxO-disruption on regulation systems of toxin production in Vibrio vulnificus strain L-180, a human clinical isolate. World J. Microbiol. Biotechnol. 30, 681–691. doi: 10.1007/s11274-013-1501-3
Elmahdi, S., DaSilva, L. V., and Parveen, S. (2016). Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 57, 128–134. doi: 10.1016/j.fm.2016.02.008
Engebrecht, J., and Silverman, M. (1984). Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. U.S.A. 81, 4154–4158. doi: 10.1073/pnas.81.13.4154
Feng, L., Rutherford, S. T., Papenfort, K., Bagert, J. D., van Kessel, J. C., Tirrell, D. A., et al. (2015). A qrr noncoding RNA deploys four different regulatory mechanisms to optimize quorum-sensing dynamics. Cell 160, 228–240. doi: 10.1016/j.cell.2014.11.051
Freeman, J. A., and Bassler, B. L. (1999). A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31, 665–677. doi: 10.1046/j.1365-2958.1999.01208.x
Freeman, J. A., Lilley, B. N., and Bassler, B. L. (2000). A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol. Microbiol. 35, 139–149. doi: 10.1046/j.1365-2958.2000.01684.x
Gambello, M. J., and Iglewski, B. H. (1991). Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173, 3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991
García-Aljaro, C., Eberl, L., Riedel, K., and Blanch, A. R. (2008). Detection of quorum-sensing-related molecules in Vibrio scophthalmi. BMC Microbiol. 8:138. doi: 10.1186/1471-2180-8-138
García-Aljaro, C., Melado-Rovira, S., Milton, D. L., and Blanch, A. R. (2012a). Quorum-sensing regulates biofilm formation in Vibrio scophthalmi. BMC Microbiol. 12:287. doi: 10.1186/1471-2180-12-287
Garcia-Aljaro, C., Vargas-Cespedes, G. J., and Blanch, A. R. (2012b). Detection of acylated homoserine lactones produced by Vibrio spp. and related species isolated from water and aquatic organisms. J. Appl. Microbiol. 112, 383–389. doi: 10.1111/j.1365-2672.2011.05199.x
Gilson, L., Kuo, A., and Dunlap, P. V. (1995). AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 177, 6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995
Girard, L., Blanchet, É., Intertaglia, L., Baudart, J., Stien, D., Suzuki, M., et al. (2017). Characterization of N-Acyl homoserine lactones in Vibrio tasmaniensis LGP32 by a biosensor-based UHPLC-HRMS/MS Method. Sensors 17:906. doi: 10.3390/s17040906
Golberg, K., Pavlov, V., Marks, R. S., and Kushmaro, A. (2013). Coral-associated bacteria, quorum sensing disrupters, and the regulation of biofouling. Biofouling 29, 669–682. doi: 10.1080/08927014.2013.796939
Greenberg, E. P., Hastings, J. W., and Ulitzur, S. (1979). Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 120, 87–91. doi: 10.1007/BF00409093
Gupta, R. K., Chhibber, S., and Harjai, K. (2011). Acyl homoserine lactones from culture supernatants of Pseudomonas aeruginosa accelerate host immunomodulation. PLoS ONE 6:e20860. doi: 10.1371/journal.pone.0020860
Hansen, H., Bjelland, A. M., Ronessen, M., Robertsen, E., and Willassen, N. P. (2014). LitR is a repressor of syp genes and has a temperature-sensitive regulatory effect on biofilm formation and colony morphology in Vibrio (Aliivibrio) salmonicida. Appl. Environ. Microbiol. 80, 5530–5541. doi: 10.1128/AEM.01239-14
Hanzelka, B. L., Parsek, M. R., Val, D. L., Dunlap, P. V., Cronan, J. E. Jr., and Greenberg, E. P. (1999). Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J. Bacteriol. 181, 5766–5770.
Hartmann, A., and Schikora, A. (2012). Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J. Chem. Ecol. 38, 704–713. doi: 10.1007/s10886-012-0141-7
He, Y., and Olson, R. (2010). Three-dimensional structure of the detergent-solubilized Vibrio cholerae cytolysin (VCC) heptamer by electron cryomicroscopy. J. Struct. Biol. 169, 6–13. doi: 10.1016/j.jsb.2009.07.015
Hema, M., Balasubramanian, S., and Princy, S. A. (2015). Meddling Vibrio cholerae murmurs: a neoteric advancement in cholera research. Indian J. Microbiol. 55, 121–130. doi: 10.1007/s12088-015-0520-1
Henke, J. M., and Bassler, B. L. (2004). Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 186, 6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004
Hoang, T. T., and Schweizer, H. P. (1999). Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J. Bacteriol. 181, 5489–5497.
Hooi, D. S., Bycroft, B. W., Chhabra, S. R., Williams, P., and Pritchard, D. I. (2004). Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect. Immun. 72, 6463–6470. doi: 10.1128/IAI.72.11.6463-6470.2004
Horng, Y. T., Deng, S. C., Daykin, M., Soo, P. C., Wei, J. R., Luh, K. T., et al. (2002). The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45, 1655–1671. doi: 10.1046/j.1365-2958.2002.03117.x
Horseman, M. A., and Surani, S. (2011). A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 15, e157–e166. doi: 10.1016/j.ijid.2010.11.003
Huang, Y., Zhang, J., Yu, Z., Zeng, Y., and Chen, Y. (2012). Isolation and characterization of acyl homoserine lactone-producing bacteria during an urban river biofilm formation. Arch. Microbiol. 194, 1043–1048. doi: 10.1007/s00203-012-0849-3
Hughes, D. T., and Sperandio, V. (2008). Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 6, 111–120. doi: 10.1038/nrmicro1836
Jamuna, B. A., and Ravishankar, R. V. (2016). Effect of small chain N acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J. Food Sci. Technol. 53, 3609–3614. doi: 10.1007/s13197-016-2346-1
Janda, J. M., Newton, A. E., and Bopp, C. A. (2015). Vibriosis. Clin. Lab. Med. 35, 273–288. doi: 10.1016/j.cll.2015.02.007
Janda, K. D., Kaufmann, G. F., and Park, J. (2010). Antibody-Mediated Disruption of Quorum Sensing in Bacteria. U.S. Patent No 9,394,371. Washington, DC: U.S. Patent and Trademark Office.
Joelsson, A. C., and Zhu, J. (2006). LacZ-based detection of acyl-homoserine lactone quorum-sensing signals. Curr. Protoc. Microbiol. Chapter 1:Unit 1C.2. doi: 10.1002/9780471729259.mc01c02s3
Jones, S., Yu, B., Bainton, N. J., Birdsall, M., Bycroft, B. W., Chhabra, S. R., et al. (1993). The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12, 2477–2482.
Jung, S. A., Chapman, C. A., and Ng, W. L. (2015). Quadruple quorum-sensing inputs control Vibrio cholerae virulence and maintain system robustness. PLoS Pathog. 11:e1004837. doi: 10.1371/journal.ppat.1004837
Kalia, V. C., and Purohit, H. J. (2011). Quenching the quorum sensing system: potential antibacterial drug targets. Crit. Rev. Microbiol. 37, 121–140. doi: 10.3109/1040841X.2010.532479
Kaufmann, G. F., Park, J., Mee, J. M., Ulevitch, R. J., and Janda, K. D. (2008). The quorum quenching antibody RS2-1G9 protects macrophages from the cytotoxic effects of the Pseudomonas aeruginosa quorum sensing signalling molecule N-3-oxo-dodecanoyl-homoserine lactone. Mol. Immunol. 45, 2710–2714. doi: 10.1016/j.molimm.2008.01.010
Khilwani, B., and Chattopadhyay, K. (2015). Signaling beyond punching holes: modulation of cellular responses by Vibrio cholerae cytolysin. Toxins 7, 3344–3358. doi: 10.3390/toxins7083344
Krick, A., Kehraus, S., Eberl, L., Riedel, K., Anke, H., Kaesler, I., et al. (2007). A marine Mesorhizobium sp. produces structurally novel long-chain N-acyl-L-homoserine lactones. Appl. Environ. Microbiol. 73, 3587–3594. doi: 10.1128/AEM.02344-06
Kumar, J. S., Umesha, S., Prasad, K. S., and Niranjana, P. (2016). Detection of quorum sensing molecules and biofilm formation in Ralstonia solanacearum. Curr. Microbiol. 72, 297–305. doi: 10.1007/s00284-015-0953-0
Kumari, A., Pasini, P., Deo, S. K., Flomenhoft, D., Shashidhar, H., and Daunert, S. (2006). Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 78, 7603–7609. doi: 10.1021/ac061421n
Kuo, A., Blough, N. V., and Dunlap, P. V. (1994). Multiple N-acyl-L-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J. Bacteriol. 176, 7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994
Lee, M. N., Kim, S. K., Li, X. H., and Lee, J. H. (2014). Bacterial virulence analysis using brine shrimp as an infection model in relation to the importance of quorum sensing and proteases. J. Gen. Appl. Microbiol. 60, 169–174. doi: 10.2323/jgam.60.169
Lenz, D. H., Mok, K. C., Lilley, B. N., Kulkarni, R. V., Wingreen, N. S., and Bassler, B. L. (2004). The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118, 69–82. doi: 10.1016/j.cell.2004.06.009
Li, G., Dong, S., Qu, J., Sun, Z., Huang, Z., Ye, L., et al. (2010). Synergism of hydroxyapatite nanoparticles and recombinant mutant human tumour necrosis factor-α in chemotherapy of multidrug-resistant hepatocellular carcinoma. Liver Int. 30, 585–592. doi: 10.1111/j.1478-3231.2009.02113.x
Liaqat, I., Bachmann, R. T., and Edyvean, R. G. (2014). Type 2 quorum sensing monitoring, inhibition and biofilm formation in marine microrganisms. Curr. Microbiol. 68, 342–351. doi: 10.1007/s00284-013-0484-5
Lilley, B. N., and Bassler, B. L. (2000). Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36, 940–954. doi: 10.1046/j.1365-2958.2000.01913.x
Lohith, G. K., Kingston, J. J., Singh, A. K., Murali, H. S., and Batra, H. V. (2015). Evaluation of recombinant leukocidin domain of VvhA exotoxin of Vibrio vulnificus as an effective toxoid in mouse model. Immunol. Lett. 167, 47–53. doi: 10.1016/j.imlet.2015.06.015
Lowery, C. A., Dickerson, T. J., and Janda, K. D. (2008). Interspecies and interkingdom communication mediated by bacterial quorum sensing. Chem. Soc. Rev. 37, 1337–1346. doi: 10.1039/b702781h
Lubin, J. B., Lewis, W. G., Gilbert, N. M., Weimer, C. M., Almagro-Moreno, S., Boyd, E. F., et al. (2015). Host-like carbohydrates promote bloodstream survival of Vibrio vulnificus in vivo. Infect. Immun. 83, 3126–3136. doi: 10.1128/IAI.00345-15
Lumjiaktase, P., Diggle, S. P., Loprasert, S., Tungpradabkul, S., Daykin, M., Cámara, M., et al. (2006). Quorum sensing regulates dpsA and the oxidative stress response in Burkholderia pseudomallei. Microbiology 152(Pt 12), 3651–3659. doi: 10.1099/mic.0.29226-0
McClean, K. H., Winson, M. K., Fish, L., Taylor, A., Chhabra, S. R., Camara, M., et al. (1997). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143 (Pt 12), 3703–3711. doi: 10.1099/00221287-143-12-3703
McDougald, D., Lin, W. H., Rice, S. A., and Kjelleberg, S. (2006). The role of quorum sensing and the effect of environmental conditions on biofilm formation by strains of Vibrio vulnificus. Biofouling 22, 161–172. doi: 10.1080/08927010600743431
Milton, D. L. (2006). Quorum sensing in vibrios: complexity for diversification. Int. J. Med. Microbiol. 296, 61–71. doi: 10.1016/j.ijmm.2006.01.044
Milton, D. L., Chalker, V. J., Kirke, D., Hardman, A., Cámara, M., and Williams, P. (2001). The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J. Bacteriol. 183, 3537–3547. doi: 10.1128/JB.183.12.3537-3547.2001
Milton, D. L., Hardman, A., Camara, M., Chhabra, S. R., Bycroft, B. W., Stewart, G. S., et al. (1997). Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-L-homoserine lactone. J. Bacteriol. 179, 3004–3012. doi: 10.1128/jb.179.9.3004-3012.1997
Miyashiro, T., Oehlert, D., Ray, V. A., Visick, K. L., and Ruby, E. G. (2014). The putative oligosaccharide translocase SypK connects biofilm formation with quorum signaling in Vibrio fischeri. Microbiologyopen 3, 836–848. doi: 10.1002/mbo3.199
Mok, K. C., Wingreen, N. S., and Bassler, B. L. (2003). Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 22, 870–881. doi: 10.1093/emboj/cdg085
Morin, D., Grasland, B., Vallée-Réhel, K., Dufau, C., and Haras, D. (2003). On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acylhomoserine lactones, quorum sensing signal molecules, in the presence of biological matrices. J. Chromatogr. A 1002, 79–92. doi: 10.1016/S0021-9673(03)00730-1
Moser, C., Jensen, P. O., Kobayashi, O., Hougen, H. P., Song, Z., Rygaard, J., et al. (2002). Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin. Exp. Immunol. 127, 206–213. doi: 10.1046/j.1365-2249.2002.01731.x
Nealson, K. H. (1977). Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch. Microbiol. 112, 73–79. doi: 10.1007/BF00446657
Nguyen, A. N., and Jacq, A. (2014). Small RNAs in the Vibrionaceae: an ocean still to be explored. Wiley Interdiscip. Rev. RNA 5, 381–392. doi: 10.1002/wrna.1218
O'Connor, G., Knecht, L. D., Salgado, N., Strobel, S., Pasini, P., and Daunert, S. (2015). Whole-cell biosensors as tools for the detection of quorum-sensing molecules: uses in diagnostics and the investigation of the quorum-sensing mechanism. Adv. Biochem. Eng. Biotechnol. 3, 181–200. doi: 10.1007/10_2015_337
Parkinson, J. S., and Kofoid, E. C. (1992). Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26, 71–112. doi: 10.1146/annurev.ge.26.120192.000443
Passador, L., Cook, J. M., Gambello, M. J., Rust, L., and Iglewski, B. H. (1993). Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260, 1127–1130.
Pearson, J. P., Gray, K. M., Passador, L., Tucker, K. D., Eberhard, A., Iglewski, B. H., et al. (1994). Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. U.S.A. 91, 197–201. doi: 10.1073/pnas.91.1.197
Pearson, J. P., Van Delden, C., and Iglewski, B. H. (1999). Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181, 1203–1210.
Persat, A., Stone, H. A., and Gitai, Z. (2014). The curved shape of Caulobacter crescentus enhances surface colonization in flow. Nat. Commun. 5:3824. doi: 10.1038/ncomms4824
Phippen, B. L., and Oliver, J. D. (2015). Clinical and environmental genotypes of Vibrio vulnificus display distinct, quorum-sensing-mediated, chitin detachment dynamics. Pathog. Dis. 73:ftv072. doi: 10.1093/femspd/ftv072
Purohit, A. A., Johansen, J. A., Hansen, H., Leiros, H. K., Kashulin, A., Karlsen, C., et al. (2013). Presence of acyl-homoserine lactones in 57 members of the Vibrionaceae family. J. Appl. Microbiol. 115, 835–847. doi: 10.1111/jam.12264
Rai, A. K., and Chattopadhyay, K. (2014). Trapping of Vibrio cholerae cytolysin in the membrane-bound monomeric state blocks membrane insertion and functional pore formation by the toxin. J. Biol. Chem. 289, 16978–16987. doi: 10.1074/jbc.M114.567099
Rajamanikandan, S., Jeyakanthan, J., and Srinivasan, P. (2017). Binding mode exploration of LuxR-thiazolidinedione analogues, e-pharmacophore-based virtual screening in the designing of LuxR inhibitors and its biological evaluation. J. Biomol. Struct. Dyn. 35, 897–916. doi: 10.1080/07391102.2016.1166455
Ramachandran, R., Burke, A. K., Cormier, G., Jensen, R. V., and Stevens, A. M. (2014). Transcriptome-based analysis of the Pantoea stewartii quorum-sensing regulon and identification of EsaR direct targets. Appl. Environ. Microbiol. 80, 5790–5800. doi: 10.1128/AEM.01489-14
Rasmussen, B. B., Nielsen, K. F., Machado, H., Melchiorsen, J., Gram, L., and Sonnenschein, E. C. (2014). Global and phylogenetic distribution of quorum sensing signals, acyl homoserine lactones, in the family of Vibrionaceae. Mar. Drugs 12, 5527–5546. doi: 10.3390/md12115527
Ren, D., Sims, J. J., and Wood, T. K. (2001). Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 3, 731–736. doi: 10.1046/j.1462-2920.2001.00249.x
Ronson, C. W., Nixon, B. T., and Ausubel, F. M. (1987). Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell 49, 579–581.
Rumbaugh, K. P., Hamood, A. N., and Griswold, J. A. (2004). Cytokine induction by the P. aeruginosa quorum sensing system during thermal injury. J. Surg. Res. 116, 137–144. doi: 10.1016/j.jss.2003.08.009
Rutherford, S. T., van Kessel, J. C., Shao, Y., and Bassler, B. L. (2011). AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 25, 397–408. doi: 10.1101/gad.2015011
Schaefer, A. L., Val, D. L., Hanzelka, B. L., Cronan, J. E. Jr., and Greenberg, E. P. (1996). Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. U.S.A. 93, 9505–9509. doi: 10.1073/pnas.93.18.9505
Seed, P. C., Passador, J., and Iglewski, B. H. (1995). Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177, 654–659. doi: 10.1128/jb.177.3.654-659.1995
Shaw, P. D., Ping, G., Daly, S. L., Cha, C., Cronan, J. E. Jr., Rinehart, K. L., et al. (1997). Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. U.S.A. 94, 6036–6041. doi: 10.1073/pnas.94.12.6036
Shimohata, T., and Takahashi, A. (2010). Diarrhea induced by infection of Vibrio parahaemolyticus. J. Med. Invest. 57, 179–182. doi: 10.2152/jmi.57.179
Smith, R. S., Fedyk, E. R., Springer, T. A., Mukaida, N., Iglewski, B. H., and Phipps, R. P. (2001). IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167, 366–374. doi: 10.4049/jimmunol.167.1.366
Smith, R. S., Harris, S. G., Phipps, R., and Iglewski, B. (2002). The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184, 1132–1139. doi: 10.1128/jb.184.4.1132-1139.2002
Steidle, A., Sigl, K., Schuhegger, R., Ihring, A., Schmid, M., Gantner, S., et al. (2001). Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 67, 5761–5770. doi: 10.1128/AEM.67.12.5761-5770.2001
Stephenson, K., Yamaguchi, Y., and Hoch, J. A. (2000). The mechanism of action of inhibitors of bacterial two–component signal transduction systems. J. Biol. Chem. 275, 38900–38904. doi: 10.1074/jbc.M006633200
Sun, L., Tang, C., and Leng, Y. P. (2010). The research progress of the novel thin layer chromatography expansion agent. Sci. Technol. West China 9, 47–48. doi: 10.3969/j.issn.1671-6396.2010.36.025
Tait, K., Hutchison, Z., Thompson, F. L., and Munn, C. B. (2010). Quorum sensing signal production and inhibition by coral-associated vibrios. Environ. Microbiol. Rep. 2, 145–150. doi: 10.1111/j.1758-2229.2009.00122.x
Tan, P. W., Tan, W. S., Yunos, N. Y., Mohamad, N. I., Adrian, T. G., Yin, W. F., et al. (2014). Short chain N-acyl homoserine lactone production in tropical marine Vibrio sinaloensis strain T47. Sensors 14, 12958–12967. doi: 10.3390/s140712958
Tan, W. S., Yunos, N. Y., Tan, P. W., Mohamad, N. I., Adrian, T. G., Yin, W. F., et al. (2014). Characterisation of a marine bacterium Vibrio brasiliensis T33 producing N-acyl homoserine lactone quorum sensing molecules. Sensors 14, 12104–12113. doi: 10.3390/s140712104
Tarr, C. L., Bopp, C. A., and Farmer, J. J. (2015). “Vibrio and related organisms,” in Manual of Clinical Microbiology, 11th Edn, eds J. H. Jorgensen, M. A. Pfaller, K. C. Carroll, G. Funke, M. L. Landry, S. S. Richter, and D. W. Warnock (Washington, DC: American Society of Microbiology), 762–772.
Tateda, K., Ishii, Y., Horikawa, M., Matsumoto, T., Miyairi, S., Pechere, J. C., et al. (2003). The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71, 5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003
Taylor, M. W., Schupp, P. J., Baillie, H. J., Charlton, T. S., de Nys, R., Kjelleberg, S., et al. (2004). Evidence for acyl homoserine lactone signal production in bacteria associated with marine sponges. Appl. Environ. Microbiol. 70, 4387–4389. doi: 10.1128/AEM.70.7.4387-4389.2004
Timmen, M., Bassler, B. L., and Jung, K. (2006). AI-1 influences the kinase activity but not the phosphatase activity of LuxN of Vibrio harveyi. J. Biol. Chem. 281, 24398–24404. doi: 10.1074/jbc.M604108200
Tu, K. C., and Bassler, B. L. (2007). Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev. 21, 221–233. doi: 10.1101/gad.1502407
Valiente, E., Bruhn, J. B., Nielsen, K. F., Larsen, J. L., Roig, F. J., Gram, L., et al. (2009). Vibrio vulnificus produces quorum sensing signals of the AHL-class. FEMS Microbiol. Ecol. 69, 16–26. doi: 10.1111/j.1574-6941.2009.00691.x
Van Kessel, J. C., Ulrich, L. E., Zhulin, I. B., and Bassler, B. L. (2013). Analysis of activator and repressor functions reveals the requirements for transcriptional control by LuxR, the master regulator of quorum sensing in Vibrio harveyi. mBio 4, e00378-13. doi: 10.1128/mBio.00378-13
Vidal, J. E., Shak, J. R., and Canizalez-Roman, A. (2015). The CpAL quorum sensing system regulates production of hemolysins CPA and PFO to build Clostridium perfringens biofilms. Infect. Immun. 83, 2430–2442. doi: 10.1128/IAI.00240-15
Vikström, E., Bui, L., Konradsson, P., and Magnusson, K. E. (2009). The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp. Cell Res. 315, 313–326. doi: 10.1016/j.yexcr.2008.10.044
Vikström, E., Magnusson, K. E., and Pivoriunas, A. (2005). The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone stimulates phagocytic activity in human macrophages through the p38 MAPK pathway. Microbes Infect. 7, 1512–1518. doi: 10.1016/j.micinf.2005.05.012
Vikström, E., Tafazoli, F., and Magnusson, K. E. (2006). Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett. 580, 6921–6928. doi: 10.1016/j.febslet.2006.11.057
Viswanath, G., Jegan, S., Baskaran, V., Kathiravan, R., and Prabavathy, V. R. (2015). Diversity and N-acyl-homoserine lactone production by Gammaproteobacteria associated with Avicennia marina rhizosphere of South Indian mangroves. Syst. Appl. Microbiol. 38, 340–345. doi: 10.1016/j.syapm.2015.03.008
Wang, Y., Wang, H., Liang, W., Hay, A. J., Zhong, Z., Kan, B., et al. (2013). Quorum sensing regulatory cascades control Vibrio fluvialis pathogenesis. J. Bacteriol. 195, 3583–3589. doi: 10.1128/JB.00508-13
Wei, G., Tian, N., Valery, A. C., Zhong, Y., Schuppan, D., and Helmerhorst, E. J. (2015). Identification of pseudolysin (lasB) as an aciduric gluten-degrading enzyme with high therapeutic potential for celiac disease. Am. J. Gastroenterol. 110, 899–908. doi: 10.1038/ajg.2015.97
White, C. E., and Winans, S. C. (2007). Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1135–1148. doi: 10.1098/rstb.2007.2040
Williams, P. (2007). Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153(Pt 12), 3923–3938. doi: 10.1099/mic.0.2007/012856-0
Yang, Q., Han, Y., and Zhang, X. H. (2011). Detection of quorum sensing signal molecules in the family Vibrionaceae. J. Appl. Microbiol. 110, 1438–1448. doi: 10.1111/j.1365-2672.2011.04998.x
Zhang, C., and Ye, B. C. (2014). Real-time measurement of quorum-sensing signal autoinducer 3OC6HSL by a FRET-based nanosensor. Bioprocess Biosyst. Eng. 37, 849–855. doi: 10.1007/s00449-013-1055-7
Zhang, L. H. (2003). Quorum quenching and proactive host defense. Trends Plant Sci. 8, 238–244. doi: 10.1016/S1360-1385(03)00063-3
Zhang, S., Liu, N., Liang, W., Han, Q., Zhang, W., and Li, C. (2017). Quorum sensing-disrupting coumarin suppressing virulence phenotypes in Vibrio splendidus. Appl. Microbiol. Biotechnol. 101, 3371–3378. doi: 10.1007/s00253-016-8009-3
Zhang, Y. Y., Yan, J., and Ge, Y. M. (2017). Advances in bacterial extracellular metalloproteases and their pathogenic roles. Chin. J. Microbiol. Immunol. 37, 161–164. doi: 10.3760/cma.j.issn.0254-5101.2017.02.013
Zhou, D., Yan, X., Qu, F., Wang, L., Zhang, Y., Hou, J., et al. (2013). Quorum sensing modulates transcription of cpsQ-mfpABC and mfpABC in Vibrio parahaemolyticus. Int. J. Food Microbiol. 166, 458–463. doi: 10.1016/j.ijfoodmicro.2013.07.008
Zhou, X., Shah, D. H., Konkel, M. E., and Call, D. R. (2008). Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol. Microbiol. 69, 747–764. doi: 10.1111/j.1365-2958.2008.06326.x
Zhu, J., Chai, Y., Zhong, Z., Li, S., and Winans, S. C. (2003). Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl. Environ. Microbiol. 69, 6949–6953. doi: 10.1128/AEM.69.11.6949-6953.2003
Keywords: N-acyl homoserine lactone, quorum sensing (QS), Vibrio, pathogenicity, intervention
Citation: Liu J, Fu K, Wu C, Qin K, Li F and Zhou L (2018) “In-Group” Communication in Marine Vibrio: A Review of N-Acyl Homoserine Lactones-Driven Quorum Sensing. Front. Cell. Infect. Microbiol. 8:139. doi: 10.3389/fcimb.2018.00139
Received: 17 January 2018; Accepted: 18 April 2018;
Published: 07 May 2018.
Edited by:
Rodolfo García-Contreras, Universidad Nacional Autónoma de México, MexicoReviewed by:
Efstathios D. Giaouris, University of the Aegean, GreeceYael González Tinoco, Universidad Nacional Autónoma de México, Mexico
Copyright © 2018 Liu, Fu, Wu, Qin, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Zhou, hzzhoulj@126.com
†These authors have contributed equally to this work.
 Jianfei Liu
Jianfei Liu Kaifei Fu
Kaifei Fu Chenglin Wu
Chenglin Wu Kewei Qin
Kewei Qin Fei Li
Fei Li Lijun Zhou
Lijun Zhou