Progress in Synthesis of Conductive Polymer Poly(3,4-Ethylenedioxythiophene)
- 1China-Australia Institute for Advanced Materials and Manufacturing, Jiaxing University, Jiaxing, China
- 2National Engineering Lab of Textile Fiber Materials and Processing Technology (Zhejiang), Zhejiang Sci-Tech University, Hangzhou, China
PEDOT is the most popularly used conductive polymer due to its high conductivity, good physical and chemical stability, excellent optical transparency, and the capabilities of easy doping and solution processing. Based on the advantages above, PEDOT has been widely used in various devices for energy conversion and storage, and bio-sensing. The synthesis method of PEDOT is very important as it brings different properties which determine its applications. In this mini review, we begin with a brief overview of recent researches in PEDOT. Then, the synthesis methods of PEDOT are summarized in detail, including chemical polymerization, electrochemical polymerization, and transition metal-mediated coupling polymerization. Finally, research directions in acquiring high-quality PEDOT are discussed and proposed.
1 Introduction
Conductive polymer (CP) was discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid in 1977 (Shirakawa et al., 1977; Park et al., 1980; Park, 1988). They demonstrated that the conductivity of polyacetylene can be adjusted over a few orders of magnitude. The CPs not only share similar electrical properties with metal or semiconductor, but also have good mechanical properties like polymer (Heeger, 2001). Therefore, CPs have attracted wide attention in the printable electronics, energy conversion and storage devices, biological electronics and so on (Guimard et al., 2007; Rozlosnik, 2009; da Silva and de Torresi, 2019; Jiang et al., 2020). Up to now, four CPs comprising polyacetylene (PA), polyaniline (PANi), polypyrrole (PPy), and polythiophene (PTh) have been reported and studied intensively. Although CPs have decent conductivity and flexibility, the poor stability caused by the doping state and insufficient half-life of conductivity pose an important challenge to the commercialization of CPs (Kudoh et al., 1999). The problem was not solved until the invention of Poly (3, 4-ethylenedioxythiophene) (PEDOT) in the late 1980s (Bayer, 1988).
Among CPs, PEDOT has drawn most of the attention in both academic and industrial communities due to its relatively high conductivity and remarkable stability in ambient conditions, as well as its potential to be transparent in the visible range (Bayer, 1988). A lot of works have been done to improve the conductivity of PEDOT, the highest conductivity of 6,259 S cm−1 for thin films and 8,797 S cm−1 for single crystals have been reported (Cho et al., 2014; Gueye et al., 2016). Conductivity is an important parameter for CPs, as it directly determines their applications. Meanwhile, the improvement of conductivity is mainly due to the crystallinity and doping of PEDOT, which is directly caused by the different synthesis methods. The polymerization process of PEDOT is complicated, as it involves many oxidants and additives, thus a slight change might produce great influence on the properties of the final products. Over the last decades, researchers have made huge progress in the synthesis of PEDOT, but precisely control its crystallinity to achieve superior performance is still a major challenge in the field of PEDOT synthesis. In order to fully understand the properties of PEDOT, conjugated oligomer of EDOT are attractive materials as a model system. Oligo-EDOT derivatives were synthesized and investigated. It was found that the properties of the product could be tuned by the oligomer length, end group, and monomer composition with fine control (Spicer et al., 2017). In addition to the conductivity, the biocompatibility and non-toxicity of PEDOT are also deeply concerned by researchers (da Silva et al., 2018; Meng et al., 2019; Dominguez-Alfaro et al., 2021). In recent studies, a new type of conductive polymer-based biosensor has been obtained through monomer modification and polymerization (da Silva et al., 2018; Meng et al., 2020; Ritzau-Reid et al., 2020). In this review, we summarized the most used synthesis methods of PEDOT and their development trends. The synthesis method and corresponding conductivity are reviewed and discussed. Finally, the challenges and research directions are proposed.
2 Synthesis Methods for PEDOT
The properties of PEDOT (optical transparency, electrical conductivity, work function) are highly dependent on the counterion and packing of PEDOT polymer. Electronic structure and optical absorption spectra of PEDOT for different oxidation levels have been studied using density functional theory (DFT) and time-dependent DFT (Zozoulenko et al., 2018). Therefore, the design and preparation of PEDOT with excellent performance are critical to realizing its wide application. Initially, PEDOT was prepared by oxidative polymerization of 3, 4-ethylenedioxythiophene (EDOT). So far, the polymerization methods of PEDOT can be classified into three categories (Figure 1): 1) chemical polymerization; 2) electrochemical polymerization; 3) transition metal-mediated coupling polymerization.
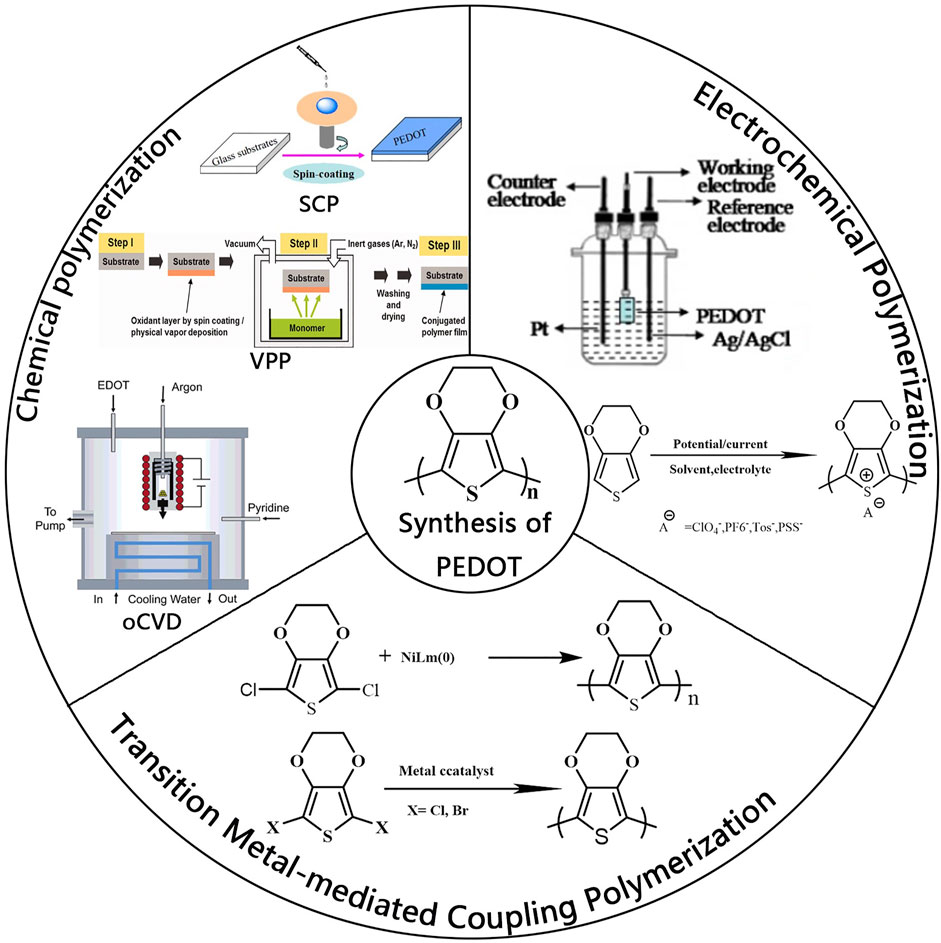
FIGURE1. Three kinds of polymerization methods for PEDOT, chemical polymerization, electrochemical polymerization, and transition metal-mediated coupling polymerization. Reproduced with permission (Lock et al., 2006; Wen and Xu, 2017; Jiang et al., 2020).
2.1 Chemical Polymerization
Chemical polymerization is the most basic and commonly used method for the synthesis of PEDOT. With continuous development, this method has become the main method for the preparation of PEDOT and its derivatives. The oxidative polymerization mechanism of PEDOT can be divided into two steps (Figure 2A) (Ha et al., 2004). First, the EDOT monomer is oxidized to form cationic radicals followed by free radicals dimerization. The achieved dimer consequently experiences a deprotonation process, resulting in an active neutral dimer, which facilitates the dimer to react in the following oxidation process for chain growth. The neutral PEDOT is doped by the oxidants, anions of the oxidants are acted as counterions to stabilize the charged PEDOT (Jiang et al., 2020). The oxidants are usually utilized in the chemical polymerization process. However, PEDOT can also be prepared without oxidant by acid-assisted-polycondensation or self-polymerization of EDOT (Yin et al., 2013; Tomšík et al., 2020). Oxidative chemical polymerization is divided into oxidative polymerization of PEDOT dispersion and in-situ chemical polymerization according to the different usage of products. As for the above two methods, CPs with high conductivity can be obtained since the oxidant can simultaneously dope the conjugated PEDOT during the reaction process.
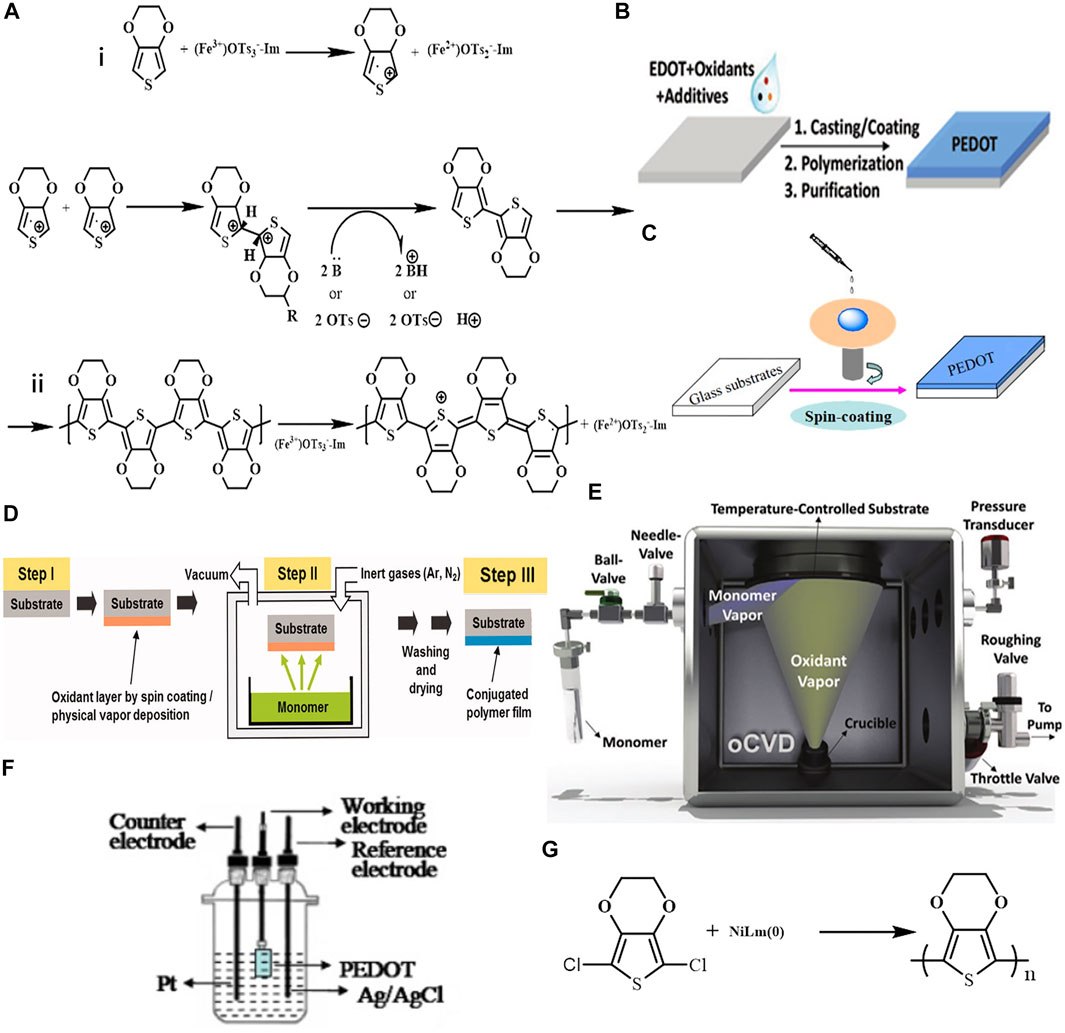
FIGURE2. (A) schematic diagram of oxidative polymerization mechanism of PEDOT; (B,C) PEDOT in-situ solution polymerization process; (D) flow chart of vapor-phase polymerization; (E) schematic diagram of oCVD reactor; (F) a three-electrode device for electrochemical synthesis; (G) PEDOT is obtained by coupling polymerization of transition metals. Reproduced with permission (Ha et al., 2004; Bhattacharyya et al., 2012; Wen and Xu, 2017; Gharahcheshmeh and Gleason, 2018; Jiang et al., 2020).
2.1.1 Oxidative Chemical Polymerization of PEDOT Dispersion
The synthesis of PEDOT by oxidative chemical polymerization method is similar to the preparation of PPy. In this method, the iron (III)-chloride was introduced as the oxidant, resulting in insoluble PEDOT powders with a high conductivity (Heywang and Jonas, 1992; Jiang et al., 2012). Furthermore, metal ions Cerium (IV) (Corradi and Armes, 1997), Manganese (IV) (Hupe et al., 1995), and Cooper (II) (Im et al., 2008) have also been used as the oxidant to synthesize PEDOT. A breakthrough in preparing highly conductive PEDOT films was achieved after using iron (III)-sulfonates as oxidants (Jonas et al., 1990). The sulfonates are soluble in common organic solvents like ethanol or n-butanol, thus the PEDOT dispersions could be obtained through mixing EDOT and Fe (III)-sulfonates in these solvents. PEDOT with different microstructure could be synthesized by adjusting the polymerization method. Manohar et al., developed a reverse emulsion polymerization method, where sodium bis(2-ethylhexyl) sulfosuccinate and FeCl3 were used as the template and oxidant, to obtain PEDOT nanotubes with tube diameters in the range of 50–100 nm (Zhang et al., 2006). Zhang and collaborators proposed a facile soft-template-assisted self-assembled method to prepare PEDOT nanofibers and nanocubes with controlled morphology and size by simply adding solvent and tuning the ratio of monomer and solvent (Sun et al., 2017). Further research demonstrated that the addition of organic bases as an inhibitor in the reaction mixture could not only reduce the activity of the oxidant and thus slow down the polymerization rate, but also adjust the PH of the reaction, thus improving the pot-life and conductivity at the same time (De Leeuw et al., 1994; Winther-Jensen et al., 2005). These observations were mainly attributed to the formation of toluene sulfonic acid and its anions function as the counterions for the positively charged PEDOT. In addition, the acidity of the reaction solution is also regulated by the added inhibitors. The conductivity of PEDOT is also highly related to the reaction temperature and time. In the process of gas phase polymerization, the introduction of monomers and additives is closely related to the ambient temperature of the reaction. Water vapor as a proton cleaner plays a very important role in conductivity, while the removal of protons is directly determined by temperature (Goktas et al., 2015). Peroxides such as hydrogen peroxide, alkyl hydroperoxides, and diacyl peroxides can also be used as the alternative oxidants to prepare highly conductive PEDOT (Jiang et al., 2012).
2.1.2 In-Situ Chemical Polymerization of PEDOT
In-situ polymerization refers to the oxidative chemical polymerization of EDOT directly on the substrates under film-forming conditions. PEDOT with regular molecular structure and high conductivity could be obtained by in-situ polymerization (Cho et al., 2014). The reaction mechanism of in-situ polymerization is roughly parallel to the oxidative polymerization (Fichou, 2008). Generally, the in-situ oxidative polymerization is preferably performed with iron (III), Manganese (IV), or other metal ions with a suitable higher oxidation state. The alcohol-soluble iron salts of sulfonic acids are ideal oxidants due to the limited solubility of EDOT in water. The p-toluenesulfonate has been taken as a very suitable anion and the corresponding iron (III) toluenesulfonate has become the most widely used oxidant in the preparation of PEDOT through in-situ method (Hong et al., 2005). Other metal salt oxidants and peroxides are also effective alternatives.
Solution-Cast Polymerization of PEDOT
There are three types of in-situ polymerizations: solution-cast polymerization (SCP), vapor phase polymerization (VPP), and oxidative chemical vapor deposition (oCVD). SCP is the simplest method for in-situ polymerizations of PEDOT, which was first reported by Bayer AG (Jonas et al., 1988). A typical procedure is performed as follows: the EDOT and oxidant were firstly dissolved in alcohol, then the mixture was cast onto the target substrate, followed by an annealing treatment to assist the polymerization. Finally, substrates were thoroughly rinsed to remove excess reagents (Figures 2B,C) (Gueye et al., 2020).
Kinetic studies show that the polymerization of PEDOT is determined by the slowest step of reaction rate, while the rate of EDOT monomer being oxidized by oxidant to generate free radicals is the slowest (Ha et al., 2004; Kim and Zozoulenko, 2019). Therefore, the oxidant plays a very important role in polymerization. In particular, the solubility, oxidation strength and stability of the oxidant have an important influence on the polymerization process. Anions in oxidants such as Cl−, tosylate (Tos−) and sulfonates also play important roles in the polymerization of PEDOT. These anions can not only neutralize the charge and further stabilize PEDOT (Brooke et al., 2017), but also affect the reaction rate, the molecular arrangement, as well as conductivity of PEDOT (Winther-Jensen et al., 2008). For iron-based oxidants, the standard electrode potential of cation reduction is constant, but the different anions can adjust the polymerization rate. Compared with Cl−, tosylate (Tos−) leads to lower effective oxidation strength of the oxidant. The polymerization rate of PEDOT can be reduced by iron p-toluenesulfonic acid (III) (Fe(Tos)3), which leads to a longer conjugated chain and smoother microstructure of PEDOT. Moreover, the conductivity of PEDOT is improved (Brooke et al., 2017). The polymerization rate is also related to the concentration of the oxidant. The PEDOT films obtained under low oxidant concentration exhibit higher conductivity and lower roughness. However, a challenge remains to prepare PEDOT films with conductivity over 1,000 S cm−1 by optimizing oxidants. There are two reasons that restrict the improvement of the conductivity of PEDOT. One is the influence of protons, which can accelerate the polymerization rate and decrease the conductivity. The generation of protons is inevitable, as shown in Figure 2A. The other is the side reaction caused by protons, which makes a large number of EDOT free radicals react into dimers or trimers, hindering the formation of long-chain PEDOT. The alkaline inhibitor as an additive was first proposed by De Leeuw et al. (1994). Imidazole was utilized as an alkaline inhibitor in the synthesis of PEDOT, which can increase the polymer chains by slowing down the reaction rate and restricting excessive doping. The PEDOT film achieved a high conductivity of 500 S cm−1 (Ha et al., 2004). In addition to alkaline additives, surfactants and co-solvents are also used as additives to assist the synthesis of high conductivity PEDOT. The conductivity of synthesized PEDOT can be up to 3,000 S cm−1 with polymeric surfactant poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG-PPG-PEG) and high boiling point cosolvent (N-methyl-2-pyrro-lidone) as additives (Gueye et al., 2016).
Vapor-Phase Polymerization of PEDOT
VPP is another common synthesis method for in-situ polymerizations, in which reactants participate in the reaction under gas state. PEDOT films obtained by VPP showed excellent photoelectric properties (Rahman et al., 2011; Howden et al., 2013; Li et al., 2015; Brooke et al., 2017). VPP involves three steps (Figure 2D): 1) depositing a solvent containing an oxidant and/or additive on a substrate by solution processing method; 2) exposing the coated substrate to EDOT monomer vapor in a closed chamber for polymerization; 3) washing the deposited film to remove residual oxidant and adsorbed monomer (Bhattacharyya et al., 2012; Brooke et al., 2017). Oxidation reaction occurs at the interface between oxidants and monomers in gas phase. The PEDOT film-forming mechanism could be “bottom-up” when the oxidant mixture diffuses upward or “top-down” when the monomer diffuses downward, but this speculation is still debated (Jiang et al., 2020).
In VPP method, the problems brought by oxidants are the same as those in SCP, so it is necessary to use an inhibitor to control the reaction rate. Winther-Jensen and Keld West initially added pyridine as an alkali inhibitor in 2004. The use of pyridine can not only slow down the polymerization rate but also eliminate acidic side reactions. The conductivity of the finally obtained PEDOT film is improved, and the highest conductivity exceeds 1000 S cm−1 (Winther-Jensen and West, 2004). In terms of processing conditions, such as the pressure, temperature, and humidity in the reaction chamber directly affect the photoelectric properties of PEDOT. A highly ordered crystal structure can be obtained at 60°C, which significantly improves the conductivity (Kim et al., 2003). In terms of humidity, the existence of water is helpful to repeat the polymerization cycle, because it can be used as a proton scavenger on EDOT dimer (Fabretto et al., 2009; Mueller et al., 2012; Goktas et al., 2015). The conductivity of PEDOT was significantly improved, and the top surface of the film was smoother. However, the existence of water also brings serious problems to polymerization. Excessive load leads to the formation of microcrystals in the oxidant layer, which makes the oxidant inactive. Common oxidant salts (FeCl3 (Cho et al., 2014), Fe(Tos)3 (Bubnova et al., 2014; Fabretto et al., 2012), Fe(OTf)3 (Massonnet et al., 2015; Brooke et al., 2018)) showed high water affinity, which led to easy formation of hydrate crystallites. These crystalline regions are bad for the synthesized PEDOT and seriously affect the conductivity. To solve this problem, Zuber et al. used an amphiphilic copolymer inhibitor of PEG–PPG–PEG, which can inhibit the hydrate crystal growth of oxidant (Zuber et al., 2008). The most significant benefit of the VPP method is that the conductivity of the product is greatly improved. The conductivity of the synthesized single crystal PEDOT nanowires reaches the highest value at present, up to 8,797 S cm−1 (Cho et al., 2014).
Oxidative Chemical Vapor Deposition of PEDOT
The oCVD method is a one-step steam synthesis process in which oxidant and monomer meet on the target substrate in a steam state and undergo oxidative polymerization. This method can avoid the deposition of oxidants on the substrate, and the oxidant with good volatility can be selected. oCVD was initiated in 2006 (Lock et al., 2006) and fiber-shaped PEDOT with high conductivity was obtained. As shown in Figure 2E, to prevent the accumulation of oxidant, the substrate in the reactor is inverted above the oxidant crucible. Since the commonly used solid oxidants have low volatility, and oCVD requires oxidants to have certain volatility, the choice of oxidants is limited. Although Fe(Tos)3 is widely used in VPP, it cannot be used in oCVD because of its poor volatility. Therefore, metal halogen salts with good volatility (e.g., FeCl3 (Gharahcheshmeh and Gleason, 2018), CuCl2 (Im et al., 2008)) have become the mainstream in oCVD. In order to better control the concentration of the oxidant, liquid oxidants such as antimony pentoxide (SbCl5) (Nejati et al., 2014) and vanadium trioxide (VOCl3) (Nejati and Lau, 2011) have been developed for oCVD method in recent years. Compared with the solid oxidant, the surface concentration and flow rate of liquid oxidant VOCl3 can be conveniently adjusted during the reaction (Gharahcheshmeh and Gleason, 2018). The orientation of PEDOT (face-on and edge-on) has a strong correlation with the conductivity. Grissom and his colleagues used liquid and solid oxidants, respectively. Liquid oxidants can easily adjust the saturation ratio of oxidants (OSR). PEDOT obtained using liquid oxidants has face-on orientation, while PEDOT obtained using solid oxidants has edge-on orientation (Gharahcheshmeh et al., 2019). The PEDOT film with face-on orientation exhibits the highest in-plane electrical conductivity of 2,800 S cm−1 and the largest optical bandgap of 2.9 eV. In addition to liquid oxidants, volatile oxidants such as halogen gases have also been reported. The conductivity of the prepared Br-PEDOT film without post-treatment is 380 S cm−1 at 80°C, which is significantly higher than that of the PEDOT film obtained with ferric chloride as the oxidant at the same temperature, and the Br-PEDOT film is more stable (Chelawat et al., 2010). However, halogen gases have been shown to damage the apparatus through corrosion during the reaction. In VPP, the conductivity of PEDOT film can be greatly improved by using some additives. However, the lack of suitable volatile additives limits the further increase in conductivity in oCVD. Therefore, it is necessary to optimize the deposition parameters such as substrate temperature, pressure, and vapor flow to improve the performance of PEDOT films in oCVD. Ugur et al. (2015) reported that the grain size of PEDOT can be adjusted by adjusting the substrate temperature.
2.2 Electrochemical Polymerization
Electrochemical polymerization (Figure 2F) to prepare PEDOT was first demonstrated in 1988 (Heywang et al., 1988), and its concept is similar to that of oxidative chemical polymerization. The biggest difference with oxidative chemical polymerization is that no oxidants are used. In electro-polymerization, EDOT is oxidized by an applied potential and polymerization takes place at the electrode. Electro-polymerization requires a three-electrode system (counter electrode, reference electrode, and working electrode) and electrolyte solution. The electrolyte solution usually contains small molecules as electrolytes, and the commonly used electrolytes are lithium perchlorate (LiClO4), 1-butyl-3-methylimidazolium hexaphonate (BMIMPF6), and lithium bis (trifluoromethosulfonyl) amide (LiTFSI) (Zotti et al., 2003; Culebras et al., 2014). In the process of electro-polymerization, the anions of electrolyte are doped into PEDOT as counterions to stabilize the charge in PEDOT. Furthermore, anions greatly affect the morphologies, photoelectric properties, and mechanical properties of PEDOT films (Zotti et al., 2003; Poverenov et al., 2010; Culebras et al., 2014). Therefore, the electrolyte can be changed to introduce different counterions, and then the conductivity of PEDOT film can be adjusted. The maximum conductivity of PEDOT obtained by electro-polymerization is about 2,000 S cm−1 by adjusting electrolytes (Culebras et al., 2014). The doping and dedoping of PEDOT films can be realized by changing the applied potential and direction of the electro-polymerization reaction. Accordingly, the optoelectronic properties (e.g., ionic/electric conductivity, transparency, and Seebeck coefficient) can be tuned (Bubnova et al., 2012; Nguyen and Lee, 2016; Teran and Reynolds, 2017; van de Burgt et al., 2017; Jiang et al., 2020).
2.3 Transition Metal-Mediated Coupling Polymerization
Inspired by the role of transition metals in the coupling polymerization of thiophene polymers, Yamamoto et al. used transition metal nickel complexes to prepare neutral PEDOT using (Figure 2G) (Yamamoto and Abla, 1999). The PEDOT obtained by this method is in black, insoluble in water, and non-conductive. Therefore, this method is not widely used.
3 Conclusion and Outlook
PEDOT, as a very unique CP, possesses high conductivity, high environmental stability, high visible spectrum transparency, and multi-purpose processing ability, which makes it has great application potential in many fields. In this review, we mainly summarized the polymerization methods of PEDOT, such as chemical polymerization (E.g., SCP, VPP, oCVD), electrochemical polymerization, and transition metal-mediated coupling polymerization. The factors affecting the performance of PEDOT were briefly analyzed. The development of PEDOT conductivity under different polymerization methods was summarized. The existing problems and solutions of PEDOT under different polymerization methods were discussed. Although great progress has been made in the synthesis of PEDOT, there are still great challenges in the synthesis of high quality PEDOT. First, the conductivity of synthesized PEDOT is not high enough, and how to improve the conductivity of synthesized PEDOT to 104 S cm−1 or even higher remains a challenge. Although many strategies have been used to improve the conductivity, the PEDOT cannot be mass-produced and the preparation conditions are harsh. In addition, the conductive mechanism of the synthesized PEDOT deserves further study to fundamentally optimize the conductivity of PEDOT. Finally, for different synthesis methods, we should develop new application directions and reach the full potential of PEDOT.
Author Contributions
ZL and YJ conceived the concept of the review. SN drafted the manuscript, searched for updated bibliography, and prepared the figures. ZL, YY, and YJ revised, corrected, and edited the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Jiaxing University (No. CDN70518005, No. CD70520065), the Jiaxing Public Welfare Research Program in 2019 (No. 2019AY11007), Innovation Jiaxing Elite leading plan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bayer, A. J. E. P. (1988). Polythiophenes, Process for Their Preparation and Their Use. US-4959430-A, 339340.
Bhattacharyya, D., Howden, R. M., Borrelli, D. C., and Gleason, K. K. (2012). Vapor Phase Oxidative Synthesis of Conjugated Polymers and Applications. J. Polym. Sci. B Polym. Phys. 50, 1329–1351. doi:10.1002/polb.23138
Brooke, R., Cottis, P., Talemi, P., Fabretto, M., Murphy, P., and Evans, D. (2017). Recent Advances in the Synthesis of Conducting Polymers from the Vapour Phase. Prog. Mater. Sci. 86, 127–146. doi:10.1016/j.pmatsci.2017.01.004
Brooke, R., Franco-Gonzalez, J. F., Wijeratne, K., Pavlopoulou, E., Galliani, D., Liu, X., et al. (2018). Vapor Phase Synthesized Poly(3,4-Ethylenedioxythiophene)-Trifluoromethanesulfonate as a Transparent Conductor Material. J. Mater. Chem. A. 6, 21304–21312. doi:10.1039/c8ta04744h
Bubnova, O., Berggren, M., and Crispin, X. (2012). Tuning the Thermoelectric Properties of Conducting Polymers in an Electrochemical Transistor. J. Am. Chem. Soc. 134, 16456–16459. doi:10.1021/ja305188r
Bubnova, O., Khan, Z. U., Wang, H., Braun, S., Evans, D. R., Fabretto, M., et al. (2014). Semi-metallic Polymers. Nat. Mater. 13, 190–194. doi:10.1038/nmat3824
Chelawat, H., Vaddiraju, S., and Gleason, K. (2010). Conformal, Conducting Poly(3,4-Ethylenedioxythiophene) Thin Films Deposited Using Bromine as the Oxidant in a Completely Dry Oxidative Chemical Vapor Deposition Process. Chem. Mater. 22, 2864–2868. doi:10.1021/cm100092c
Cho, B., Park, K. S., Baek, J., Oh, H. S., Lee, Y-E. K., and Sung, M. M. (2014). Single-crystal Poly(3,4-Ethylenedioxythiophene) Nanowires with Ultrahigh Conductivity. Nano Lett. 14, 3321–3327. doi:10.1021/nl500748y
Corradi, R., and Armes, S. (1997). Chemical Synthesis of Poly (3, 4-ethylenedioxythiophene). Synth. Met. 84, 453–454. doi:10.1016/S0379-6779(97)80828-4
Culebras, M., Gómez, C. M., and Cantarero, A. (2014). Enhanced Thermoelectric Performance of PEDOT with Different Counter-ions Optimized by Chemical Reduction. J. Mater. Chem. A. 2, 10109–10115. doi:10.1039/C4TA01012D
da Silva, A. C., and de Torresi, S. I. C. (2019). Advances in Conducting, Biodegradable and Biocompatible Copolymers for Biomedical Applications. Front. Mater. 6, 98. doi:10.3389/fmats.2019.00098
da Silva, A. C., Augusto, T., Andrade, L. H., and de Torresi, S. I. C. (2018). One Pot Biocatalytic Synthesis of a Biodegradable Electroactive Macromonomer Based on 3,4-ethylenedioxytiophene and Poly(l-Lactic Acid). Mater. Sci. Eng. C. Mater. Biol. Appl. 83, 35–43. doi:10.1016/j.msec.2017.09.007
De Leeuw, D. M., Kraakman, P., Bongaerts, P., Mutsaers, C., and Klaassen, D. (1994). Electroplating of Conductive Polymers for the Metallization of Insulators. Synth. Met. 66, 263–273. doi:10.1016/0379-6779(94)90076-0
Dominguez-Alfaro, A., Gabirondo, E., Alegret, N., DeLeón-Almazán, C. M., Hernandez, R., Vallejo-Illarramendi, A., et al. (2021). 3D Printable Conducting and Biocompatible PEDOT-Graft-PLA Copolymers by Direct Ink Writing. Macromol. Rapid Commun. 42, e2100100. doi:10.1002/marc.202100100
Fabretto, M. V., Evans, D. R., Mueller, M., Zuber, K., Hojati-Talemi, P., and Short, R. D. (2012). Polymeric Material with Metal-like Conductivity for Next Generation Organic Electronic Devices. Chem. Mater. 24, 3998–4003. doi:10.1021/cm302899v
Fabretto, M., Zuber, K., Hall, C., Murphy, P., and Griesser, H. J. (2009). The Role of Water in the Synthesis and Performance of Vapour Phase Polymerised PEDOT Electrochromic Devices. J. Mater. Chem. 19, 7871–7878. doi:10.1039/b912324e
Fichou, D. (2008). Handbook of Oligo-And Polythiophenes (Weinheim: John Wiley & Sons). doi:10.1002/9783527611713
Gharahcheshmeh, M. H., and Gleason, K. K. (2018). Device Fabrication Based on Oxidative Chemical Vapor Deposition (oCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Inter. 6, 1801564. doi:10.1002/admi.201801564
Gharahcheshmeh, M. H., Tavakoli, M. M., Gleason, E. F., Robinson, M. T., Kong, J., and Gleason, K. K. (2019). Tuning, Optimization, and Perovskite Solar Cell Device Integration of Ultrathin Poly(3,4-Ethylene Dioxythiophene) Films via a Single-step All-Dry Process. Sci. Adv. 5, 0414. doi:10.1126/sciadv.aay0414
Goktas, H., Wang, X., Ugur, A., and Gleason, K. K. (2015). Water-Assisted Vapor Deposition of PEDOT Thin Film. Macromol. Rapid Comm. 36, 1283–1289. doi:10.1002/marc.201500069
Gueye, M. N., Carella, A., Faure-Vincent, J., Demadrille, R., and Simonato, J.-P. (2020). Progress in Understanding Structure and Transport Properties of PEDOT-Based Materials: A Critical Review. Prog. Mater. Sci. 108, 100616. doi:10.1016/j.pmatsci.2019.100616
Gueye, M. N., Carella, A., Massonnet, N., Yvenou, E., Brenet, S., Faure-Vincent, J., et al. (2016). Structure and Dopant Engineering in PEDOT Thin Films: Practical Tools for a Dramatic Conductivity Enhancement. Chem. Mater. 28, 3462–3468. doi:10.1021/acs.chemmater.6b01035
Guimard, N. K., Gomez, N., and Schmidt, C. E. (2007). Conducting Polymers in Biomedical Engineering. Prog. Mater. Sci. 32, 876–921. doi:10.1016/j.progpolymsci.2007.05.012
Ha, Y. H., Nikolov, N., Pollack, S. K., Mastrangelo, J., Martin, B. D., and Shashidhar, R. (2004). Towards a Transparent, Highly Conductive Poly(3,4-Ethylenedioxythiophene). Adv. Funct. Mater. 14, 615–622. doi:10.1002/adfm.200305059
Heeger, A. J. (2001). Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chem. Int. Edit. 40, 2591–2611. doi:10.1002/1521-3773(20010716)40:14<2591:AID-ANIE2591>3.0.CO;2-0
Heywang, G., Jonas, F., Heinze, J., and Dietrich, M. J. P. D. (1988). 412 A1 (Bayer AG). DE 38, 4323.
Heywang, G., and Jonas, F. (1992). Poly (Alkylenedioxythiophene) S-New, Very Stable Conducting Polymers. Adv. Mater. 4, 116–118. doi:10.1002/adma.19920040213
Hong, K. H., Oh, K. W., and Kang, T. (2005). Preparation and Properties of Electrically Conducting Textiles by In Situ Polymerization of Poly (3, 4-ethylenedioxythiophene). J. Appl. Polym. Sci. 97, 1326–1332. doi:10.1002/app.21835
Howden, R. M., Flores, E. J., Bulović, V., and Gleason, K. K. (2013). The Application of Oxidative Chemical Vapor Deposited (oCVD) PEDOT to Textured and Non-planar Photovoltaic Device Geometries for Enhanced Light Trapping. Org. Electron. 14, 2257–2268. doi:10.1016/j.orgel.2013.05.004
Hupe, J., Wolf, G., and Jonas, F. (1995). DMS-E--a Recognised Principle with a Novel Basis. Through-Hole Contacting of Printed Circuit Boards Using Conductive Polymers. Galvanotechnik(Germany) 86, 3404–3411.
Im, S. G., Kusters, D., Choi, W., Baxamusa, S. H., Van de Sanden, M., and Gleason, K. K. (2008). Conformal Coverage of Poly (3, 4-ethylenedioxythiophene) Films with Tunable Nanoporosity via Oxidative Chemical Vapor Deposition. ACS Nano 2, 1959–1967. doi:10.1021/nn800380e
Jiang, C., Chen, G., and Wang, X. (2012). High-conversion Synthesis of Poly(3,4-Ethylenedioxythiophene) by Chemical Oxidative Polymerization. Synth. Met. 162, 1968–1971. doi:10.1016/j.synthmet.2012.09.008
Jiang, Y., Liu, T., and Zhou, Y. (2020). Recent Advances of Synthesis, Properties, Film Fabrication Methods, Modifications of Poly(3,4‐ethylenedioxythiophene), and Applications in Solution‐Processed Photovoltaics. Adv. Funct. Mater. 30, 2006213. doi:10.1002/adfm.202006213
Jonas, F., Heywang, G., Schmidtberg, W., Heinze, J., and Dietrich, M. J. P. A. (1988). (Bayer AG). EP 339 340, 22.
Jonas, F., Heywang, G., Schmidtberg, W., Heinze, J., and Dietrich, M. (1990). Polythiophenes, Process for Their Preparation and Their Use (Google Patents). US-4959430-A.
Kim, D., and Zozoulenko, I. (2019). Why Is Pristine PEDOT Oxidized to 33%? A Density Functional Theory Study of Oxidative Polymerization Mechanism. J. Phys. Chem. B. 123, 5160–5167. doi:10.1021/acs.jpcb.9b01745
Kim, J., Kim, E., Won, Y., Lee, H., and Suh, K. (2003). The Preparation and Characteristics of Conductive Poly(3,4-Ethylenedioxythiophene) Thin Film by Vapor-phase Polymerization. Synth. Met. 139, 485–489. doi:10.1016/s0379-6779(03)00202-9
Kudoh, Y., Akami, K., and Matsuya, Y. (1999). Solid Electrolytic Capacitor with Highly Stable Conducting Polymer as a Counter Electrode. Synth. Met. 102, 973–974. doi:10.1016/S0379-6779(98)01012-1
Li, Y., Mao, L., Tang, F., Chen, Q., Wang, Y., Ye, F., et al. (2015). Ambient Stable Large-Area Flexible Organic Solar Cells Using Silver Grid Hybrid with Vapor Phase Polymerized Poly(3,4-Ethylenedioxythiophene) Cathode. Sol. Energ. Mat. Sol. C. 143, 354–359. doi:10.1016/j.solmat.2015.07.022
Lock, J. P., Im, S. G., and Gleason, K. K. (2006). Oxidative Chemical Vapor Deposition of Electrically Conducting Poly(3,4-Ethylenedioxythiophene) Films. Macromolecules 16, 5326–5329. doi:10.1021/ma060113o
Massonnet, N., Carella, A., de Geyer, A., Faure-Vincent, J., and Simonato, J. P. (2015). Metallic Behaviour of Acid Doped Highly Conductive Polymers. Chem. Sci. 6, 412–417. doi:10.1039/c4sc02463j
Meng, L., Turner, A. P. F., and Mak, W. C. (2019). Modulating Electrode Kinetics for Discrimination of Dopamine by a PEDOT:COOH Interface Doped with Negatively Charged Tricarboxylate. ACS Appl. Mater. Inter. 11, 34497–34506. doi:10.1021/acsami.9b12946
Meng, L., Turner, A. P. F., and Mak, W. C. (2020). Tunable 3D Nanofibrous and Bio-Functionalised PEDOT Network Explored as a Conducting Polymer-Based Biosensor. Biosens. Bioelectron. 159, 112181. doi:10.1016/j.bios.2020.112181
Mueller, M., Fabretto, M., Evans, D., Hojati-Talemi, P., Gruber, C., and Murphy, P. (2012). Vacuum Vapour Phase Polymerization of High Conductivity PEDOT: Role of PEG-PPG-PEG, the Origin of Water, and Choice of Oxidant. Polymer 53, 2146–2151. doi:10.1016/j.polymer.2012.03.028
Nejati, S., and Lau, K. K. (2011). Chemical Vapor Deposition Synthesis of Tunable Unsubstituted Polythiophene. Langmuir 27, 15223–15229. doi:10.1021/la203318f
Nejati, S., Minford, T. E., Smolin, Y. Y., and Lau, K. K. S. (2014). Enhanced Charge Storage of Ultrathin Polythiophene Films within Porous Nanostructures. ACS Nano 8, 5413–5422. doi:10.1021/nn500007c
Nguyen, V. C., and Lee, P. S. (2016). Resistive Switching Memory Phenomena in PEDOT PSS: Coexistence of Switchable Diode Effect and Write Once Read Many Memory. Sci. Rep. 6, 19594. doi:10.1038/srep19594
Park, Y. W., Park, C., Lee, Y. S., Yoon, C. O., Shirakawa, H., Suezaki, Y., et al. (1988). Electrical Conductivity of Highly-Oriented-Polyacetylene. Solid State. Commun. 65 (2), 147–150. doi:10.1016/0038-1098(88)90675-8
Park, Y. W., Heeger, A. J., Druy, M. A., and MacDiarmid, A. G. (1980). Electrical Transport in Doped Polyacetylene. J. Chem. Phys. 73, 946–957. doi:10.1063/1.440214
Poverenov, E., Li, M., Bitler, A., and Bendikov, M. (2010). Major Effect of Electropolymerization Solvent on Morphology and Electrochromic Properties of PEDOT Films. Chem. Mater. 22, 4019–4025. doi:10.1021/cm100561d
Rahman, M. A., Rahim, A., Maniruzzaman, M., Yang, K., Lee, C., Nam, H., et al. (2011). ITO-free Low-Cost Organic Solar Cells with Highly Conductive Poly(3,4 Ethylenedioxythiophene): P-Toluene Sulfonate Anodes. Sol. Energ. Mat. Sol. C. 95, 3573–3578. doi:10.1016/j.solmat.2011.09.019
Ritzau-Reid, K. I., Spicer, C. D., Gelmi, A., Grigsby, C. L., Ponder, J. F., Bemmer, V., et al. (2020). An Electroactive Oligo-EDOT Platform for Neural Tissue Engineering. Adv. Funct. Mater. 30, 2003710. doi:10.1002/adfm.202003710
Rozlosnik, N. (2009). New Directions in Medical Biosensors Employing Poly(3,4-Ethylenedioxy Thiophene) Derivative-Based Electrodes. Anal. Bioanal. Chem. 395, 637–645. doi:10.1007/s00216-009-2981-8
Shirakawa, H., Louis, E. J., MacDiarmid, A. G., Chiang, C. K., and Heeger, A. J. (1977). Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of polyacetylene,(CH)X. J. Chem. Soc. Chem. Commun. 16, 578–580. doi:10.1039/C39770000578
Spicer, C. D., Booth, M. A., Mawad, D., Armgarth, A., Nielsen, C. B., and Stevens, M. M. (2017). Synthesis of Hetero-Bifunctional, End-Capped Oligo-EDOT Derivatives. Chem 2, 125–138. doi:10.1016/j.chempr.2016.12.003
Sun, T., Wang, J.-J., Khoso, N. A., Yu, L., and Zhang, Y. (2017). Facile Synthesis of Poly(3,4-Ethylenedioxythiophene) Nanostructure with Controlled Morphologies by Using an Aqueous Surfactant Soft-Template-Assisted Technique. Mater. Lett. 191, 61–64. doi:10.1016/j.matlet.2016.12.135
Teran, N. B., and Reynolds, J. R. (2017). Discrete Donor–Acceptor Conjugated Systems in Neutral and Oxidized States: Implications toward Molecular Design for High Contrast Electrochromics. Chem. Mate. 29, 1290–1301. doi:10.1021/acs.chemmater.6b04725
Tomšík, E., Ivanko, I., Svoboda, J., Šeděnková, I., Zhigunov, A., Hromádková, J., et al. (2020). Method of Preparation of Soluble PEDOT: Self‐Polymerization of EDOT without Oxidant at Room Temperature. Macromol. Chem. Phys. 221, 2000219. doi:10.1002/macp.202000219
Ugur, A., Katmis, F., Li, M., Wu, L., Zhu, Y., Varanasi, K. K., et al. (2015). Low-Dimensional Conduction Mechanisms in Highly Conductive and Transparent Conjugated Polymers. Adv. Mater. 27, 4604–4610. doi:10.1002/adma.201502340
Van de Burgt, Y., Lubberman, E., Fuller, E. J., Keene, S. T., Faria, G. C., Agarwal, S., et al. (2017). A Non-volatile Organic Electrochemical Device as a Low-Voltage Artificial Synapse for Neuromorphic Computing. Nat. Mater. 16, 414–418. doi:10.1038/nmat4856
Wen, Y., and Xu, J. (2017). Scientific Importance of Water-Processable PEDOT-PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Pol. Chem. 55, 1121–1150. doi:10.1002/pola.28482
Winther-Jensen, B., Breiby, D., and West, K. (2005). Base Inhibited Oxidative Polymerization of 3,4-ethylenedioxythiophene with iron(III)tosylate. Synth. Met. 152, 1–4. doi:10.1016/j.synthmet.2005.07.085
Winther-Jensen, B., and West, K. (2004). Vapor-Phase Polymerization of 3,4-Ethylenedioxythiophene: A Route to Highly Conducting Polymer Surface Layers. Macromolecules 37, 4538–4543. doi:10.1021/ma049864l
Winther-Jensen, B., Forsyth, M., West, K., Andreasen, J. W., Bayley, P., Pas, S., et al. (2008). Order–disorder Transitions in Poly(3,4-Ethylenedioxythiophene). Polymer 49, 481–487. doi:10.1016/j.polymer.2007.11.055
Yamamoto, T., and Abla, M. (1999). Synthesis of Non-doped Poly(3,4-Ethylenedioxythiophene) and its Spectroscopic Data. Synth. Met. 100, 237–239. doi:10.1016/S0379-6779(99)00005-3
Yin, Y., Li, Z., Jin, J., Tusy, C., and Xia, J. (2013). Facile Synthesis of Poly(3,4-Ethylenedioxythiophene) by Acid-Assisted Polycondensation of 5-Bromo-2,3-Dihydro-Thieno[3,4-B][1,4]dioxine. Synth. Met. 175, 97–102. doi:10.1016/j.synthmet.2013.05.001
Zhang, X., Lee, J.-S., Lee, G. S., Cha, D.-K., Kim, M. J., Yang, D. J., et al. (2006). Chemical Synthesis of PEDOT Nanotubes. Macromolecules 39, 470–472. doi:10.1021/ma051975c
Zotti, G., Zecchin, S., Schiavon, G., Louwet, F., Groenendaal, L., Crispin, X., et al. (2003). Electrochemical and XPS Studies toward the Role of Monomeric and Polymeric Sulfonate Counterions in the Synthesis, Composition, and Properties of Poly(3,4-Ethylenedioxythiophene). Macromolecules 36, 3337–3344. doi:10.1021/ma021715k
Zozoulenko, I., Singh, A., Singh, S. K., Gueskine, V., Crispin, X., and Berggren, M. (2018). Polarons, Bipolarons, and Absorption Spectroscopy of PEDOT. ACS Appl. Polym. Mater. 1, 83–94. doi:10.1021/acsapm.8b00061
Keywords: conducting polymer, PEDOT, doping, synthesis method, conductivity
Citation: Nie S, Li Z, Yao Y and Jin Y (2021) Progress in Synthesis of Conductive Polymer Poly(3,4-Ethylenedioxythiophene). Front. Chem. 9:803509. doi: 10.3389/fchem.2021.803509
Received: 29 October 2021; Accepted: 03 December 2021;
Published: 24 December 2021.
Edited by:
Alfonso Jiménez, University of Alicante, SpainReviewed by:
Aruã Clayton Da Silva, The University of Sheffield, United KingdomCopyright © 2021 Nie, Li, Yao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaifang Li, zaifang.li@zjxu.edu.cn; Yingzhi Jin, yingzhi.jin@zjxu.edu.cn
 Shisong Nie1,2
Shisong Nie1,2  Zaifang Li
Zaifang Li Yingzhi Jin
Yingzhi Jin