Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China
- 1State Key Laboratory of Crop Biology, Shandong Agricultural University, Taian, China
- 2College of Agronomy, Shandong Agricultural University, Taian, China
Lignocellulose content is an important factor affecting the conversion efficiency of biomass energy plants. In this study, 179 Miscanthus accessions in China were used to determine the content of lignocellulose components in stems via acid hydrolysis and high-performance liquid chromatography. Results showed that the average lignocellulose content of wild Miscanthus germplasm resources was 80.27 ± 6.51%, and the average content of cellulose, hemicellulose, lignin, extracts, and total ash was 38.38 ± 3.52, 24.23 ± 4.21, 17.66 ± 1.56, 14.50 ± 5.60, and 2.53 ± 0.59%, respectively. The average lignocellulose content of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 77.94 ± 6.06, 75.16 ± 4.98, 75.68 ± 3.02, 83.71 ± 4.78, 81.50 ± 5.23, and 74.72 ± 7.13%, respectively. In all the tested materials, the highest cellulose content was 48.52%, and the lowest was 29.79%. Hemicellulose had the maximum content of 34.23% and a minimum content of 15.71%. The highest lignin content was 23.75%, and the lowest was 13.01%. The lignocellulosic components of different ploidy materials were compared. The content of lignocellulosic components of diploid M. sacchariflorus was higher than that of tetraploid M. sacchariflorus, and the content of lignocellulosic components of diploid M. lutarioriparius was lower than that of tetraploid M. lutarioriparius. Analysis of the relationship between the changes in lignocellulosic components and geographical locations of Miscanthus showed that the holocellulose and hemicellulose content was significantly positive correlated with the latitude of the original growth location. Results indicated that the lignocellulosic components of Miscanthus resources in China are rich in genetic diversity.
Introduction
Miscanthus is a tall perennial herbaceous plant. It belongs to the subtribe Saccharinae, tribe Andropogoneae, subfamily Panicoideae, and family Poaceae. It originated in East Asia and Southeast Asia and is now widely distributed in China, Japan, and Pacific Islands (Hodkinson et al., 2002; Hastings et al., 2009; Jensen et al., 2011) China is an important origin and distribution center of Miscanthus (Clifton-Brown et al., 2001; Clifton et al., 2015; Li et al., 2019), with extensive wild germplasm resources and abundant genetic diversity (Hodkinson et al., 2002; Anzoua et al., 2011; Ge et al., 2019). Seven species of Miscanthus are found in China, namely, M. sinensis, M. floridulus, M. sacchariflorus, M. lutarioriparius, M. paniculatus, M. nepalensis, and M. nudipes. The widely distributed species are M. sinensis, M. floridulus, M. sacchariflorus, and M. lutarioriparius. Miscanthus has 19 chromosomes, with diploidy, triploidy, and tetraploidy occurring in nature. M. sacchariflorus and M. lutarioriparius have both diploid and tetraploid resources (Ge et al., 2017), and natural hybrids exist in nature (Lewandowski et al., 2003; Cichorz et al., 2015).
Miscanthus is a lignocellulosic crop with highly efficient C4 photosynthesis, high biomass production, strong stress resistance, and wide adaptability. Miscanthus has high water and fertilizer efficiency, excellent cellulose quality, extensive cultivation, eco-friendly environment, and low production costs (Beale and Long, 1995; Clifton-Brown et al., 2001; Lewandowski and Schmidt, 2005; Clifton et al., 2015). The biological yield of Miscanthus (3 × 104 kg/ha) is about three times higher than that of switchgrass (Heaton et al., 2008). Compared with other grasses, the lignocellulose of Miscanthus is closer to that of wooden materials (Villaverde et al., 2010; Lygin et al., 2011), where the contents of cellulose, hemicellulose, and lignin are ~30–50, 10–40, and 5–30%, respectively (McKendry, 2002; Yang et al., 2007; Kleinert and Barth, 2008). Miscanthus is a grassy lignocellulosic material used for converting heat, electricity, and liquid fuels (Cherubini, 2010), as well as for producing aromatic products (Pauly and Keegstra, 2008; Luo et al., 2016; Upton and Kasko, 2016). Miscanthus has a higher energy ratio than natural gas and coal, thus it has lower greenhouse gas emissions (Moukamnerd et al., 2010; McCalmont et al., 2017). Compared with high starch or high sugar crops such as sweet sorghum, Miscanthus has low moisture and sugar content during harvesting, making it more convenient to store and transport. In addition, unlike cereal crops, harvesting Miscanthus as a biofuel does not directly increase the price of cereals (Ziolkowska, 2014). These characteristics make it stand out among many energy crops and have made it one of the most promising non-grain energy plants. Hence, research on Miscanthus has sparked wide interest (Feltus and Vandenbrink, 2012; Cao et al., 2019).
With the increase in energy demand, the conversion of lignocellulosic biomass to fuels, such as ethanol, has been the focus of research in many countries. Plants can convert light energy into monosaccharides through photosynthesis and then use CO2 to fix monosaccharides into high-energy polymers and generate composite cell walls composed of cellulose, hemicellulose, and lignin (Rubin, 2008). The important factors that make saccharifying lignocellulose raw materials difficult are the degree of polymerization and lignification and cellulose crystallinity (Abramson et al., 2010). Therefore, the main obstacle hindering the accurate determination of lignocellulose content is how to effectively decompose cell walls into fermentable sugars. Five methods are commonly used for determining lignocellulose, including washing cellulose analysis methods, which can measure neutral detergent fiber, acid detergent fiber, and acid detergent lignin. The Klason method is a classic technique for determining lignocellulose, but it overestimates the true lignin value of raw materials. The application of this method is limited because it cannot determine soluble fiber and remove farinaceous substance (Hatfield et al., 1994; Wang et al., 2020). New and state-of-the art technologies, such as near-infrared spectroscopy and nuclear magnetic resonance, have been widely used in determining lignocellulose. Near-infrared spectroscopy can determine the concentration of various plant components, such as fat, grease, protein, and total fiber. However, the detection result of this method is not sufficiently accurate because the spectral measurement value has no direct relation to lignin concentration, and the influence of comparison parameter on the measurement value is high (Li et al., 2015; Ramirez et al., 2015; Hayes et al., 2017; Jin et al., 2017; Elle et al., 2019). Nuclear magnetic resonance spectroscopy is an analytical technique for detecting the composition and structural characteristics of lignin. Considering its inability to obtain good and clear spectra from complex plant samples, this method is currently not widely used (Capanema et al., 2004; Balakshin et al., 2011). The National Renewable Energy Laboratory (NREL) of the United States proposed the NREL method (Sluiter et al., 2008, 2012). Samples are hydrolyzed with sulfuric acid after extracting the extract of the sample to be tested. Glucose content is measured by high-performance liquid chromatography (HPLC). Cellulose content is quantified using the substitution ratio of glucose and cellulose. Lignin content is determined using the differential weight of the residue after hydrolysis of the sample. This method is not only experimentally operable but also provides accurate detection results.
Major breakthroughs in terms of unit biomass production and optimization of biomass conversion efficiency are needed to make the products of second-generation lignocellulosic energy crops economically competitive (Sims et al., 2010; Feltus and Vandenbrink, 2012). The biomass composition of energy plants affects the conversion efficiency. In addition, using Miscanthus as a feedstock for bioenergy requires that the biomass composition is adapted to various bioenergy conversion processes (Arnoult and Brancourt-Hulmel, 2015). The development of breeding programs also requires a clear understanding of the content of biomass composition. In order to investigated the biomass composition of Miscanthus, the components of lignocellulose in different ecological types of wild resources were determined by using the NREL method. Our research shows that the content of Miscanthus lignocellulose is affected by both genetic factors and environmental factors. The results are of great importance for the development and utilization of Miscanthus resources in China, genetic breeding of superior energy plants, and the conversion and utilization of biomass energy.
Materials and Methods
Materials
From 2011 to 2012, 156 wild Miscanthus germplasms in different ecological environments were collected from 23 provinces in China. These germplasms included 5 wild Miscanthus species, such as M. sinensis M. floridulus, M. sacchariflorus, M. lutarioriparius, and M. nudipes, and 23 hybrids. M. sacchariflorus and M. lutarioriparius have diploid and tetraploid plants in the wild, whereas the other species are only diploid. These materials were planted at the Miscanthus germplasm resource nursery (36°09′ N, 117°10′ E) of the Agricultural Experiment Station of Shandong Agricultural University. Each germplasm resource material was subjected to vegetative propagation by subterraneous stem with a planting density of 2 × 2 m. The stems of Miscanthus were harvested in March 2013 for the determination of lignocellulosic components.
Methods
The experimental method followed the NREL method for determining lignocellulose (Thygesen et al., 2005; Sluiter et al., 2012; Kuchelmeister and Bauer, 2015). This method was modified and improved.
Sample Pretreatment
Stems were dried to constant weight, crushed, and passed through a 40-mesh sieve. The ground sample (m1 = 0.6 ± 0.010 g) was weighed, reflowed in a Soxhlet extractor (a traditional glass apparatus, Shandong, Hualu) containing water for 8 h, and then dried in a drying oven at 40°C. Then, the sample was refluxed in a Soxhlet extractor containing absolute ethyl alcohol for 16 h and dried in a drying (DHG-9140A, Shanghai) oven at 40°C. After extraction, the remaining solid material (m2) was mainly lignocellulose; the part lost during the process was the extract, and its content was calculated using the equation:
Acidolysis of Samples
The extracted sample (m0 = 0.3000 g) was weighed and placed in a pressure-resistant tube (89063-334, VWR). Exactly 3.00 mL 72% H2SO4 was added, and the mixture was thoroughly stirred and mixed. Then, the sample was placed in a water bath (2321, Fisher Scientific) at 30°C for 60 min. Thereafter, 84.00 mL ddH2O was added, and the sample was sterilized in an autoclave (GI80TR, ZEALWAY) (121°C, 1 h).
After acidification of the sample, cellulose was degraded to glucose, whereas hemicellulose was degraded to xylan, arabinose, galactose, and mannose. Lignin was divided into acid-insoluble lignin (AIL) and acid-soluble lignin (ASL). The residue was used for the determination of AIL, whereas the filtrate was used for the determination of ASL and monosaccharides.
Determination of Lignin Content
Use a filter crucible (89038-050, VWR) with 15 μm Pore Diameter to filter the hydrolyzed sample to collect the filtrate and residue. AIL was determined via the ashing method. The residue was dried to a constant weight (m3) and then placed in a box-type electrical resistance furnace (SX2-G/T, Shanghai Yuejin). The sample was turned to ash at 575 ± 25°C for 10 h, cooled to room temperature, and weighed (m4). The percent AIL content was determined using the equation :
ASL was determined using an UV-Vis spectrophotometer (Nanodrop2000c, Thermo). The absorbance of the filtrate was determined at λ = 205 nm. The percent ASL content was calculated using the equation:
Where ε represents the absorption value, D represents dilution factor, V is the total liquid volume (87 mL), and K = 110 represents the absorption coefficient of acid-soluble lignin (Hayes, 2012).
Lignin content was calculated using the equation:
Determination of Cellulose and Hemicellulose
Monosaccharide content was determined via HPLC [Chromatographic conditions: chromatographic column (Biorad Aminex HPX-87P), Deashing packed column, Detector (evaporative light scattering detector), Injection volume (35 μL), mobile phase (Ultrapure water), flow velocity (0.6 mL/min), Nitrogen pressure (30 psi); drift tube (heating mode, 80 ± 25°C), Sprayer (60%); running time (20min)]. Exactly 4 mL of the filtrate was obtained, and the pH was adjusted to 5–6 with CaCO3. The supernatant was collected by centrifugation and filtered through a 0.22 μm filter membrane. Then, HPLC was used to determine the content of monosaccharides. Both monosaccharides and calcium carbonate are pure reagents (Sigma) for chromatographic analysis.
The cellulose and hemicellulose contents were calculated from the monosaccharide content as follows:
where Ac is the dehydration correction coefficient. The Ac values of pentose and hexose were 0.88 and 0.90, respectively. %Glu, %Xyl, %Ara, %Gal, and %Man represent the contents of the corresponding monosaccharides obtained by the regression curve method.
Determination of Total Ash
Weigh the mass of the empty crucible, record it as m5. Then weigh about 0.5 g of the sample, put it in a filter crucible and weigh it (m6), then put it into a box-type electric furnace, 575 ± 25°C, 24 h, cool it to room temperature in a desiccator and weigh m7.
Data Processing
The determination results of each Miscanthus sample were expressed as the average of three replicates. Data statistics were completed and coefficient of variation was calculated using Excel. Maps, mapdata, and ggplot2 software packages in R (3.6.0) were used to draw the distribution of material sources. SPSS software (statistics 24.0) was used to perform single-factor ANOVA test and obtain the boxplot of component content. Pearson correlation analysis was used to determine the material geographic location and lignocellulosic component content.
Result
Original Geographic Distribution of Materials
The original location of the Miscanthus accessions analyzed in this study included 23 provinces (spanning 21°31′ N, 46°07′ N from south to north and 102°32′ E, 128°91′ E from west to east, with an altitude of 1–1,650 m above sea level). The experimental materials contained 86 M. sacchariflorus materials, among which 72 were diploid and 14 were tetraploid. M. lutarioriparius had eight accessions of diploid and eight accessions of tetraploid. We found 31 M. sinensis, 19 M. floridulus, 23 hybrids, 4 M. nudipes. Among them, tetraploid M. sacchariflorus was mainly distributed in Shandong and Henan Provinces, whereas tetraploid M. lutarioriparius was mostly distributed in Hunan, Jiangsu, and Hubei Provinces. It can be seen from the distribution map that the distribution of five species of Miscanthus in China has a certain regionality. It is not difficult to see that the distribution range of M. sinensis and M. sacchariflorus is the widest, while the distribution range of M. nudipes is relatively concentrated. In addition, the sources and detailed distribution of all materials are shown in Supplemental Material. These materials cover the main distribution areas of Miscanthus species in China (Figure 1).
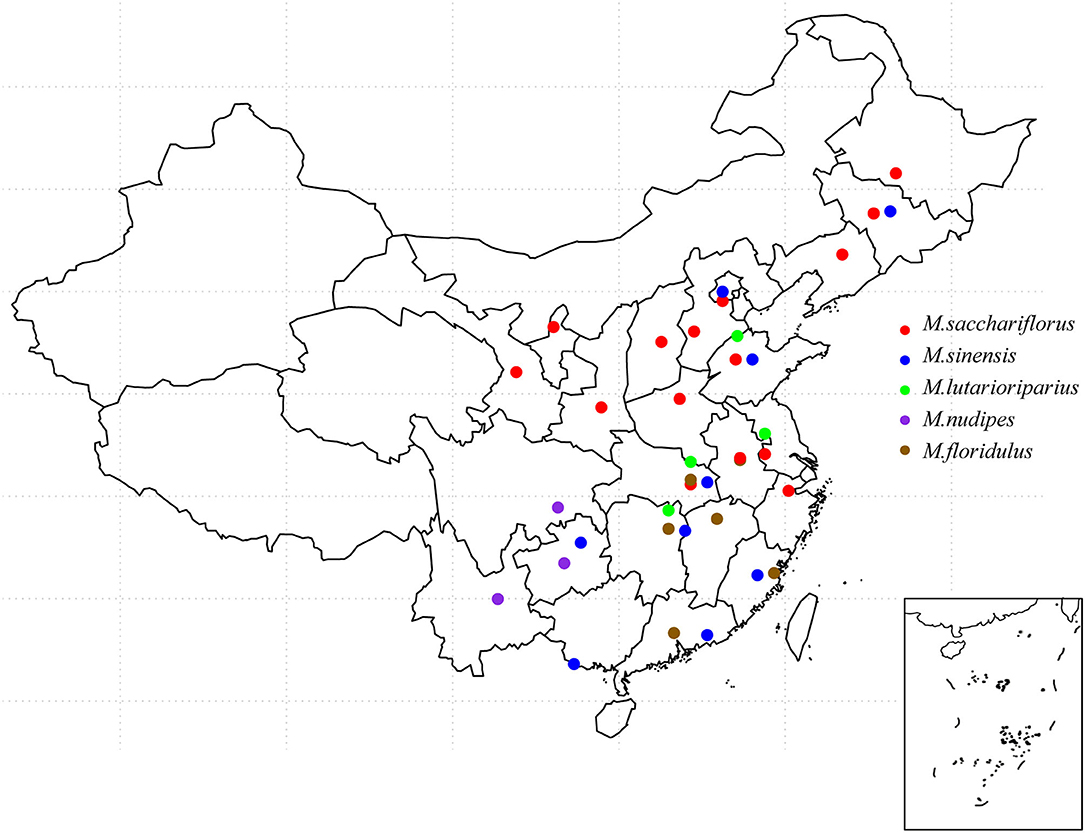
Figure 1. Distribution of Miscanthus species in China. The picture on the bottom right shows the South China Sea Islands.
Analysis of Lignocellulosic Components of Miscanthus
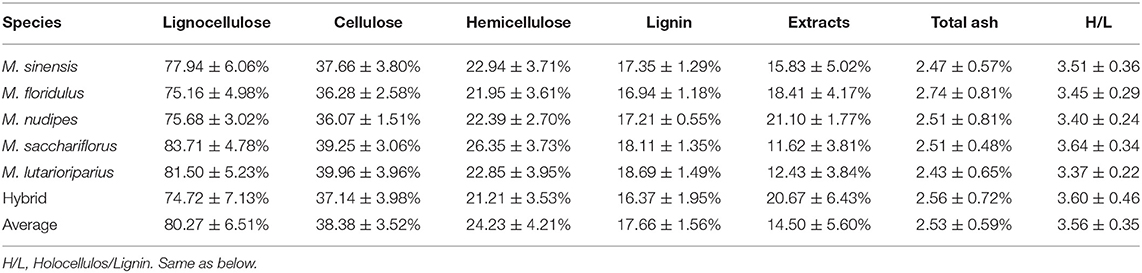
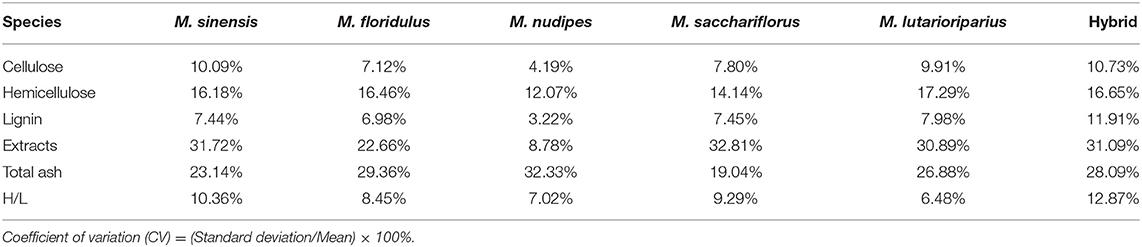
The results of the determination of lignocellulosic components of 179 Miscanthus materials showed that the average content of all lignocelluloses (the sum of cellulose, hemicellulose, and lignin) was 80.27 ± 6.51%, of which the content of cellulose, hemicellulose, lignin, extracts, and total ash was 38.38 ± 3.52, 24.23 ± 4.21, 17.66 ± 1.56, 14.50 ± 5.60, and 2.53 ± 0.59%, respectively. The average lignocellulose content (the sum of cellulose, hemicellulose, and lignin) of Miscanthus can be arranged in descending order as follows: M. sacchariflorus (83.71 ± 4.78%), M. lutarioriparius (81.50 ± 5.23%), M. sinensis (77.94 ± 6.06%), M. nudipes (75.68 ± 3.02%), M. floridulus (75.16 ± 4.98%), and hybrids (74.72 ± 7.13%) (Table 1, Supplementary Table 1). Univariate analysis of variance was used to analyze the difference in content among species (Figure 2), and the difference in lignocellulosic compositions of Miscanthus species was analyzed using the coefficient of variation to determine their potential genetic diversity (Table 2).
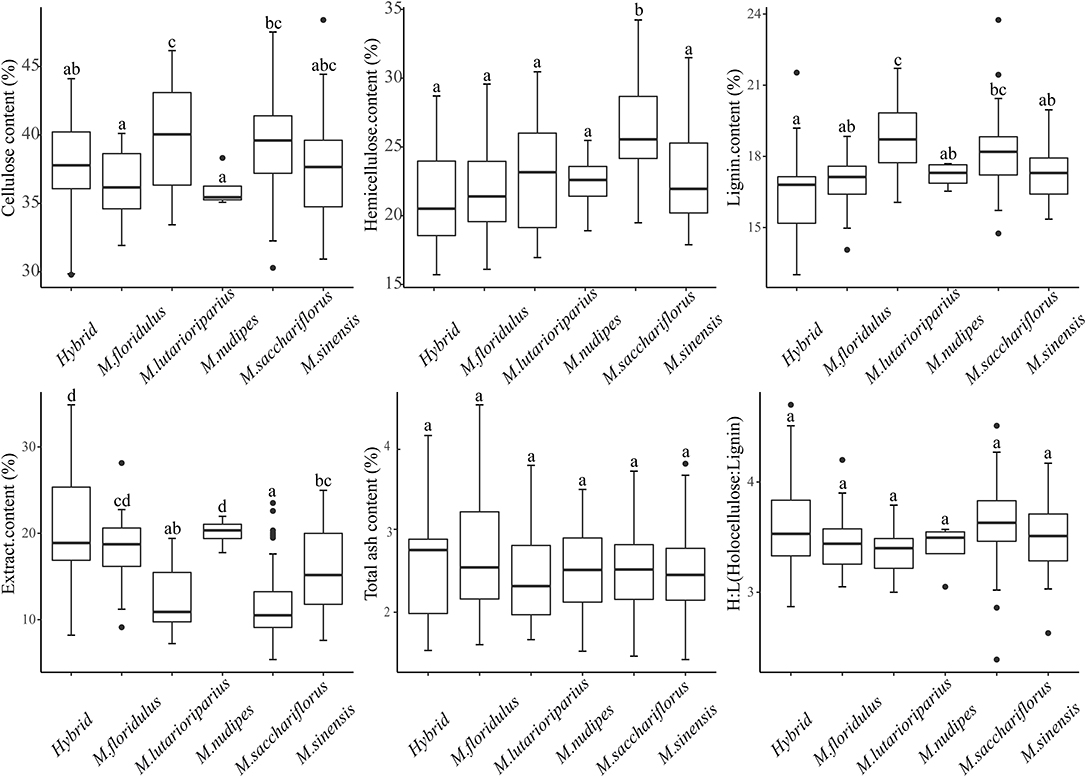
Figure 2. Statistical comparison of stem components in different Miscanthus species. Different small letters indicate significant differences at the P < 0.05 level. The upper limit of Whisker represents the largest non-outlier value, and the lower limit is the smallest non-outlier value. The upper border line of the box represents the upper quartile, the middle line represents the median, and the lower border line represents the lower quartile. The dots around the box represent outliers.
The average cellulose content of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 37.66 ± 3.80, 36.28 ± 2.58, 36.07 ± 1.51, 39.25 ± 3.06, 39.96 ± 3.96, and 37.14 ± 3.98%, respectively. Among all Miscanthus plant materials, M020 (M. sinensis, from Fujian Province) had the highest cellulose content of 48.52%, whereas M311 (hybrid, from Hunan Province) had the lowest cellulose content of 23.62% (Table 1, Supplementary Table 1). One-way ANOVA test found that the cellulose content of M. lutarioriparius was significantly different from that of M. floridulus, M. nudipes, and the hybrids but not significantly different from that of M. sinensis and M. sacchariflorus (Figure 2). The coefficients of variation were ranked from large to small as follows: hybrids (10.73%), M. sinensis (10.09%), M. lutarioriparius (9.91%), M. sacchariflorus (7.80%), M. floridulus (7.12%), and M. nudipes (4.19%).
The average hemicellulose content of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 22.94 ± 3.71, 21.95 ± 3.61, 22.39 ± 2.70, 26.35 ± 3.73, 22.85 ± 3.95, and 21.21 ± 3.53%, respectively. Among all the tested materials of Miscanthus, M137 (M. sacchariflorus, from Liaoning Province) had the highest hemicellulose content of 34.23%, whereas M010 (hybrid, from Hunan Province) had the lowest hemicellulose content of 15.71% (Table 1, Supplementary Table 1). The hemicellulose content of M. sacchariflorus was significantly different from that of M. sinensis, M. floridulus, M. lutarioriparius, M. nudipes, and the hybrids based on the results of ANOVA test (Figure 2). The coefficient of variation of hemicellulose can be arranged as follows: M. lutarioriparius (17.29%), hybrids (16.65%), M. floridulus (16.46%), M. sinensis (16.18%), M. sacchariflorus (14.14%), and M. nudipes (12.07%).
The average lignin content of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 17.35 ± 1.29, 16.94 ± 1.18, 17.21 ± 0.55, 18.11 ± 1.35, 18.69 ± 1.49, and 16.37 ± 1.95%, respectively. Of all the determined plant materials, the lignin content of M123 (M. sacchariflorus, from Heilongjiang Province) was the highest at 23.75%, whereas the lignin content of M322 (hybrid, from Hunan Province) was the lowest at 13.01% (Table 1, Supplementary Table 1). ANOVA test revealed significant differences between the lignin content of M. lutarioriparius and that of M. sinensis, M. floridulus, M. nudipes, and the hybrids. The difference between M. lutarioriparius and M. sacchariflorus was not significant (Figure 2). The coefficient of variation of lignin was 11.91% for the hybrids, 7.98% for M. lutarioriparius, 7.45% for M. sacchariflorus, 7.44% for M. sinensis, 6.98% for M. floridulus, and 3.22% for M. nudipes.
The average content of the extracts of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 15.83 ± 5.02, 18.41 ± 4.17, 21.10 ± 1.77, 11.62 ± 3.81, 12.43 ± 3.84, and 20.67 ± 6.43%, respectively. Among all the tested species, M322 (hybrid, from Hunan Province) had the highest extract content of 34.88%, whereas M177 (M. sacchariflorus, from Shandong Province) had the lowest extract content of 5.38% (Table 1, Supplementary Table 1). In the ANOVA test, the contents of extracts of M. nudipes and hybrids were significantly different from M. sinensis, M. floridulus, M. sacchariflorus, M. lutarioriparius, respectively (Figure 2). The coefficient of variation of the extracts had the largest difference, and this parameter cam be arranged in the following order: M. sacchariflorus (32.81%), M. sinensis (31.72%), hybrids (31.09%), M. lutarioriparius (30.89%), M. floridulus (22.66%), and M. nudipes (8.78%). The total ash content of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 2.47 ± 0.57, 2.74 ± 0.81, 2.51 ± 0.81, 2.51 ± 0.48, 2.43 ± 0.65, and 2.56 ± 0.72%, respectively. Among all the measured materials, M214 (M. sinensis, from Zhejiang Province) had the highest total ash content of 4.5%, whereas M165 (M. sinensis, from Guangxi) had the lowest total ash content of 1.43% (Table 1, Supplementary Table 1). ANOVA test showed no significant difference in total ash content between each species (Figure 2). The order of coefficient of variation of total ash content can be arranged as follows: M. nudipes (32.33%), M. floridulus (29.36%), hybrid (28.09%), M. lutarioriparius (26.88%), M. sinensis (23.14%), and M. sacchariflorus (19.04%).
The holocellulose-to-lignin (H/L) ratio of M. sinensis, M. floridulus, M. nudipes, M. sacchariflorus, M. lutarioriparius, and the hybrids was 3.51 ± 0.36, 3.45 ± 0.29, 3.40 ± 0.24, 3.64 ± 0.34, 3.37 ± 0.22, and 3.60 ± 0.46, respectively. M171 (hybrid, from Hunan Province) had the highest value at 4.70, whereas M123 (M. sacchariflorus, from Heilongjiang Province) had the lowest value at 2.39 (Table 1, Supplementary Table). ANOVA test showed that the H/L ratio did not differ significantly between species (Figure 2). The coefficient of variation of H/L ratios can be arranged as follows: hybrids (12.87%) > M. sinensis (10.36%) > M. sacchariflorus (9.29%) > M. floridulus (8.45%) > M. nudipes (7.02%) > M. lutarioriparius (6.48%).
Comparative Analysis Between Different Ploidies of M. sacchariflorus and M. lutarioriparius
A total of 86 parts of M. sacchariflorus (including 72 diploids and 14 tetraploids) and 18 parts of M. lutarioriparius (eight parts of diploids and eight parts of tetraploids) were collected for this test. We conducted a comparative analysis of the materials with different ploidies (Table 3). We found that diploid M. sacchariflorus had higher lignocellulose content than tetraploid M. sacchariflorus (4X), whereas diploid (2X) M. lutarioriparius had less lignocellulose content than tetraploid (4X) M. lutarioriparius. The content of cellulose, hemicellulose, and lignin of diploid M. sacchariflorus ranged from 32.60 to 47.52, 19.49 to 34.23, and 14.75 to 23.75%, respectively, whereas that of tetraploid M. sacchariflorus ranged from 30.29 to 42.63, 20.23 to 33.55, and 17.14 to 20.28%, respectively. The content of cellulose, hemicellulose, and lignin ranged from 35.46 to 46.16, 16.96 to 26.73, and 16.06 to 21.71%, respectively, in diploid M. lutarioriparius, whereas it ranged from 33.44 to 44.87, 18.74 to 30.46, and 17.78 to 20.31%, respectively, in triploid M. lutarioriparius.
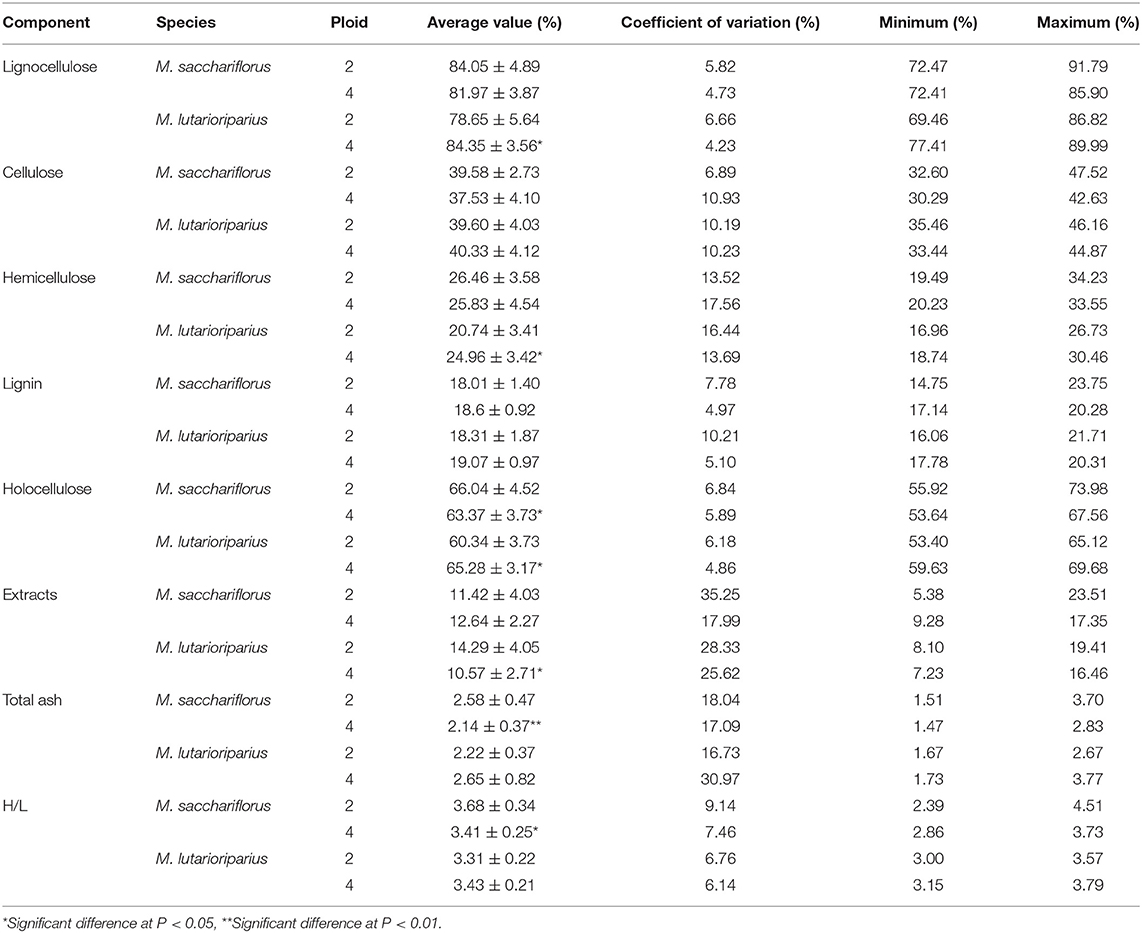
Table 3. Statistical results of lignocellulose fractions of different ploidies of M. sacchariflorus and M. lutarioriparius.
A comparison of the content of lignocellulosic components revealed significant differences in total cellulose content and H/L index and very significant differences in total ash content between 2X M. sacchariflorus and 4X M. sacchariflorus. The content of hemicellulose, holocellulose, lignocellulose, and extracts of 2X M. lutarioriparius was significantly different from that of 4X M. lutarioriparius. The coefficient of variation of cellulose and hemicellulose content of 2X M. sacchariflorus was less than that of 4X M. sacchariflorus, whereas that of lignin content of 2X M. sacchariflorus was greater than that of 4X M. sacchariflorus. The coefficient of variation of cellulose and lignin content of 2X M. lutarioriparius was less than that of 4X M. lutarioriparius, whereas the coefficient of variation of hemicellulose content of 2X M. lutarioriparius was greater than that of 4X M. lutarioriparius. Owing to ploidy changes, the cellulose and hemicellulose content of 4X M. sacchariflorus tended to diversify, whereas and its lignin content was more stable. The cellulose and lignin content of 4X M. lutarioriparius tended to diversify, whereas its hemicellulose content remained stable.
Comparative Analysis of Miscanthus Materials From Different Ecological Regions
The correlation between the differences in geographical factors (longitude, latitude, and altitude) and lignocellulosic components of M. sacchariflorus were analyzed. Results showed that the hemicellulose and holocellulose content increased with the increase in latitude, and a very significant positive correlation with latitude was observed. The correlation coefficients were 0.409 and 0.441 for hemicellulose and holocellulose, respectively. A significant positive correlation was found between total ash content and longitude and latitude, with correlation coefficients of 0.342 and 0.361, respectively. The extract content had a significant negative correlation with longitude and latitude, with coefficients of −0.346 and −0.523, respectively. In addition, no correlation was found between each lignocellulosic component of Miscanthus and altitude (Table 4). Further linear analysis was performed. Results showed that the difference in latitude would cause changes in hemicellulose and holocellulose content (the sum of hemicellulose and cellulose). The values of R2 were 0.7924 and 0.7654, respectively, indicating that the linear fitting equation was credible, and the high credibility reflects the correlation between total cellulose and hemicellulose content and latitude (Figure 3). This result was consistent with that of Pearson correlation analysis.
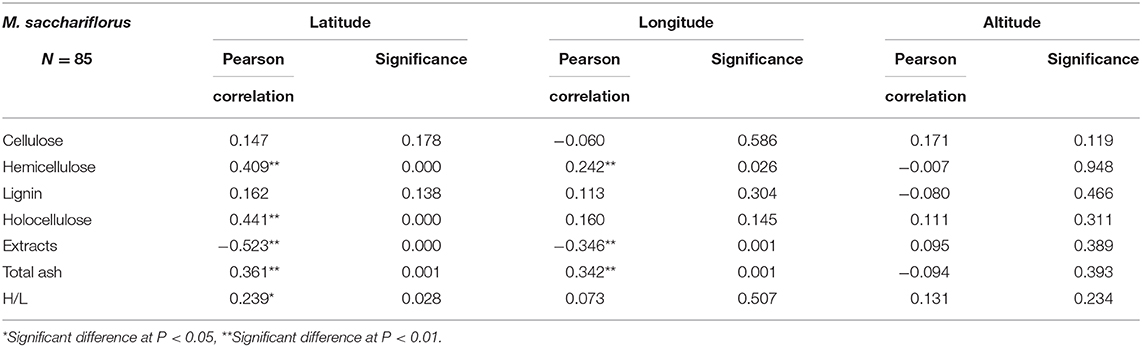
Table 4. Correlation analysis between lignocellulose content and geographical distribution of M. sacchariflorus.
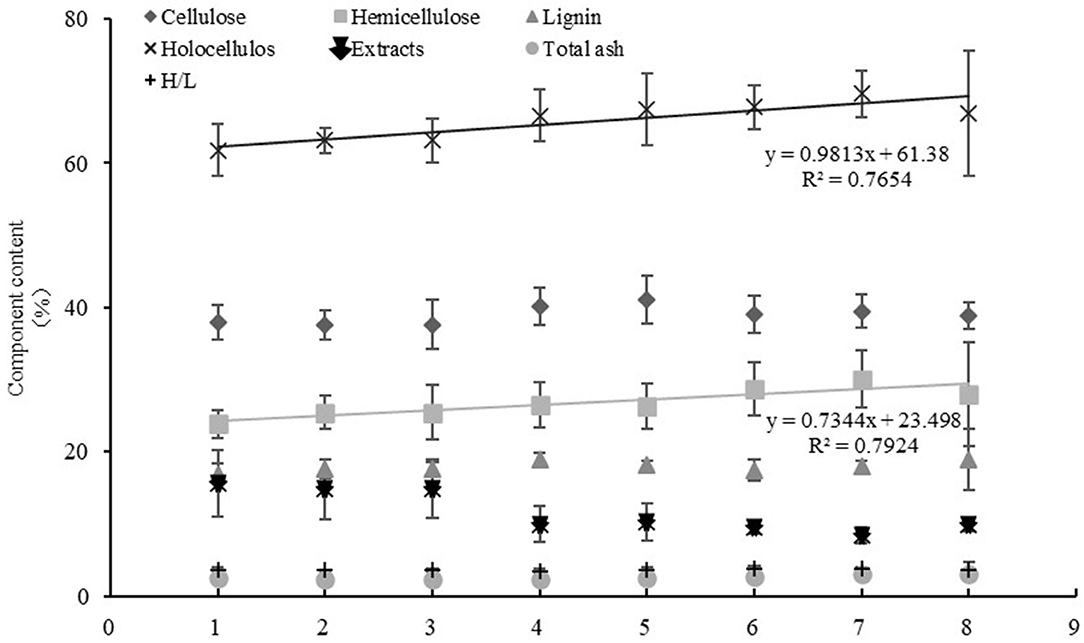
Figure 3. Linear analysis of latitude and lignocellulosic components. 1: 30–32 N°, 2: 32–34 N°, 3: 34–36 N°, 4: 36–38 N°, 5: 38–40 N°, 6: 40–42 N°, 7: 42–44 N°, 8: 44–46 N°.
Discussion
Effects of Geographical Factors on Lignocellulose Content
China is an important place of origin and distribution center of Miscanthus, with extensive wild germplasm resources and abundant genetic diversity. Four species of Miscanthus are found in China, namely, M. sinensis, M. sacchariflorus, M. lutarioriparius, and M. floridulus. M. sinensis and M. sacchariflorus are widely distributed in northern and southern China. M. lutarioriparius and M. floridulus are mainly located south of the Yangtze River, where the climate is relatively warm. M. lutarioriparius is a unique variant in China and the primary raw material for papermaking in the country. M. sacchariflorus and M. lutarioriparius have two types of diploids and tetraploids in China. The tetraploid M. sacchariflorus is mainly distributed in Shandong and Henan Provinces, whereas the tetraploid M. lutarioriparius is mainly distributed in Hunan, Jiangsu, and Hubei Provinces. Few other species of Miscanthus, such as M. nudipes, are found in the arid mountainous areas at high altitudes in southwest China. We found that related geographical factors, such as latitude, had an important selection effect on Miscanthus species, and the holocellulose and hemicellulose content increased with latitude. This result was consistent with that obtained by Zhao et al. (2014). Youngmi Kim also found similar patterns on the lignocellulose content of switchgrass (Kim et al., 2011). Low temperature increases the content of soluble sugar in plants to protect the stability of cell membranes (Jan et al., 2009; Pompeiano et al., 2015). Papini-Terzi found a correlation between some genes associated with cell wall metabolism and sugar content in plants. Plants under cold weather conditions increase their soluble sugar content, thereby indirectly inducing the synthesis of related lignocelluloses (Papini-Terzi et al., 2009; Vicentini et al., 2009; Waclawovsky et al., 2010). The holocellulose content of Miscanthus in high latitudes is relatively high. A statistical field phenotypic survey revealed significant differences in the flowering and maturity stages of Miscanthus plants transplanted to the Shandong experimental base due to the influence of photoperiod (Imaizumi and Kay, 2006). Light time increases with the increase in latitude, resulting in short flowering time. Analysis of M. sacchariflorus showed that in the Yangtze River Basin and south of the region, the flowering period is mainly concentrated in mid-to-late September and early October, but the flowering period in Shandong and Beijing mainly occurs in mid-to-late August and early September. Blooming in Heilongjiang, Jilin, and Liaoning mostly occurs in mid-to-late June. Hence, geographical location has an obvious selective effect on the genetic variation in Miscanthus, and this variation is closely related to phenological conditions corresponding to geographical location, such as photoperiod, accumulated temperature, and rainfall. Considering the vastness of China's geographical locations, the species of Miscanthus in China have rich diversity.
Comparative Analysis of the Determination Results of Lignocellulosic Components of Miscanthus
Jung compared the lignocellulose content of Miscanthus, switchgrass, sorghum, and reeds and found that the lignin content of Miscanthus was significantly lower than that of reeds. Therefore, Miscanthus is a more suitable energy plant than reeds. Moreover, M. sacchariflorus contains 14.12% lignin and 64.23% holocellulose (Heaton, 2008). Kim determined the lignocellulose content of 12 species of Miscanthus, including M. sinensis, M. sacchariflorus, and Miscanthus × giganteus, with a cellulose content of 36.1–44.9%, hemicellulose content of 17.1–30.5%, and lignin content of 13.8–31.1% (Kim et al., 2012). The cellulose, hemicellulose, and lignin content of all Miscanthus plants measured in this test ranged from 29.79 to 48.52, 15.71 to 34.23, and 13.01 to 23.75%, respectively. The measured lignin content was slightly lower than that obtained by Kim probably due to differences in measurement methods. The rest of the results are consistent with those of previous studies. The value range of the content of lignocellulosic components of Miscanthus plants is quite different, a result that also confirms the richness of Miscanthus germplasm resources in China.
The content of lignocellulosic components of Miscanthus plants has obvious differences within species. For example, the cellulose, hemicellulose, and lignin content of M. sinensis ranged from 30.93 to 48.40, 17.89 to 31.49, and 15.35 to 19.96%, respectively, whereas that of M. sacchariflorus ranged from 30.29 to 47.52, 19.49 to 34.23, and 14.75 to 23.75%, respectively. The maximum cellulose, hemicellulose, and lignin content was 1.5 times or higher than it, indicating that Miscanthus plants have abundant diversity within each species. Obvious differences were observed among various species of Miscanthus. For example, the content of hemicellulose and other components of M. sacchariflorus was considerably different from that of M. sinensis and M. floridulus according to single-factor ANOVA analysis. Significant differences were also observed in cellulose and lignin content among species. However, the contents of major lignocellulosic components, such as cellulose, hemicellulose, and lignin, between M. sinensis and M. floridulus and between M. sacchariflorus and M. lutarioriparius were slightly different. This result arose because the evolutionary relationship between M. sinensis and M. floridulus is close, as well as the genetic evolution between M. sacchariflorus and M. lutarioriparius. Ge described this evolutionary relationship in detail (Ge et al., 2017).
The coefficient of variation was calculated to determine the difference and potential genetic diversity of the various lignocellulosic components of Miscanthus. Generally, the coefficient of variation of each species of Miscanthus was high, indicating that its lignocellulosic components in China have rich diversity. The coefficient of variation of the content of each component of the hybrids was high in all species, proving that selecting varieties with high cellulose and hemicellulose contents and low lignin content from hybrids is easier than from wild types. The coefficient of variation of each component (except total ash) of M. nudipes was relatively low among all species, and this observation may explain the relatively concentrated geographical distribution of this species.
Significance and Application of the Determination of Lignocellulose Content of Miscanthus
In a 3-year field trial in Illinois, USA, Jung found that Miscanthus × giganteus has a biotransformation efficiency about 2.7 times higher than that of corn (Jung et al., 2015). Therefore, Miscanthus is widely studied as a second-generation biomass energy source. Lignocellulosic biomass is mainly a complex structure composed of cellulose, hemicellulose, lignin, and some extractable components. The amount, proportion, and type of each ingredient largely depend on the type of raw material (Pauly and Keegstra, 2008; Zhang et al., 2012; Yu et al., 2018). Cellulose and hemicellulose belong to polysaccharides. Cellulose and hemicellulose are used for the conversion of biomass energy, and their content determines the efficiency of fuel conversion (Bosch and Hazen, 2013). Lignin is an amorphous high-molecular organic polymer with a three-dimensional network structure composed of carbon–oxygen and carbon–carbon bonds. It cross-links cellulose and hemicellulose to provide good support for the stem. The complex chemical structure of lignin hinders the degradation of cellulose and hemicellulose and makes the conversion and use of biomass energy difficult (Boudet et al., 2003; Sticklen, 2006, 2008; Chang, 2007; Chen and Dixon, 2007; Li et al., 2008). Therefore, choosing varieties with high cellulose and hemicellulose content and low lignin content is beneficial to improve energy conversion efficiency. In view of this analysis, we proposed the H/L index, which can reflect the difficulty in converting cellulose energy plants into energy substances to a certain extent. Miscanthus with a high H/L value is suitable for conversion to alcohols by fermentation. The H/L value of Miscanthus did not significantly differ among species, but obvious differences were observed among varieties. The H/L index also has an important reference value in breed selection.
M. giganteus is the most studied species in terms of production applications. M. giganteus has high cellulose content and strong adaptability. Hence, it is widely cultivated in European countries. We obtained 23 hybrids (M. sinensis × M. sacchaflorus, M. floridulus × M. sacchariflorus, and M. floridulus × M. lutarioriparius) through artificial crosses. The yield of hybrids has obvious advantages compared with other Miscanthus species. Moreover, the lignin content of the hybrids was higher than that of M. sinensis and M. floridulus. Therefore, selecting varieties with high total cellulose content and low lignin content from hybrids is easier than from wild types. Hence, artificial hybrid breeding is an effective way of selecting excellent energy plants. According to the determination results of lignocellulose combined with the growth adaptability characteristics of Miscanthus, and by making full use of the abundant resources of Miscanthus in China, selective artificial breeding was performed to select energy plants with optimized content of stem components and broad growth adaptability.
Conclusion
From the overall results, Miscanthus is a good bioenergy plant with high lignocellulose content. At present, the biological production of various platform chemicals has been realized, such as ethanol, butanol, lactic acid, levulinic acid, sorbitol, glycerol, 1,3-propanediol, itaconic acid, succinic acid, and 2,5-FDCA. Therefore, suitable accessions of Miscanthus can be selected based on their biomass composition and different transformation processes. Further analyses found that Miscanthus there is obvious differences among intra- and interspecies of Miscanthus, and the content of lignocellulosic components has a wide range of values, in consistent with rich genetic diversity. More interestingly, our research shows that the content of Miscanthus lignocellulose is not only affected by genetic factors (such as ploidy), but also by environmental factors, such as Miscanthus in high latitudes with higher hemicellulose content. Overall, this research laid a solid foundation for the efficient development of Miscanthus biomass conversion, genetic breeding, and utilization in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
PX and SC analysis of experimental data and article writing. HL and DZ collection of experimental materials. YH and YW designed the experimental method. CC and GZ guidance of experimental ideas. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the Agricultural Variety Improvement Project of Shandong Province (Grant Nos. 2019LZGC010 and 2017LZN028) and National Natural Science Foundation of China (Grant No. 31871267) for the financial support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.595143/full#supplementary-material
References
Abramson, M., Shoseyov, O., and Shani, Z. (2010). Plant cell wall reconstruction toward improved lignocellulosic production and processability. Plant Sci. 178, 61–72. doi: 10.1016/j.plantsci.2009.11.003
Anzoua, K. G., Yamada, T., and Henry, R. J. (2011). Wild Crop Relatives: Genomic and Breeding Resources. Berlin Heidelberg: Springer, 157–164. doi: 10.1007/978-3-642-21102-7_9
Arnoult, S., and Brancourt-Hulmel, M. (2015). A review on miscanthus biomass production and composition for bioenergy use: genotypic and environmental variability and implications for breeding. Bioenergy Res. 8, 502–526. doi: 10.1007/s12155-014-9524-7
Balakshin, M., Capanema, E., Gracz, H., Chang, H. M., and Jameel, H. (2011). Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 233, 1097–1110. doi: 10.1007/s00425-011-1359-2
Beale, C. V., and Long, S. P. (1995). Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates. Plant Cell Environ. 18, 641–650. doi: 10.1111/j.1365-3040.1995.tb00565.x
Bosch, M., and Hazen, S. P. (2013). Lignocellulosic feedstocks: research progress and challenges in optimizing biomass quality and yield. Front. Plant Sci. 4:474. doi: 10.3389/fpls.2013.00474
Boudet, A. M., Kajita, S., Grima-Pettenati, J., and Goffner, D. (2003). Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends Plant Sci. 8, 576–581. doi: 10.1016/j.tplants.2003.10.001
Cao, W., Li, J., Martí-Rosselló, T., and Zhang, X. (2019). Experimental study on the ignition characteristics of cellulose, hemicellulose, lignin and their mixtures. J. Energy Inst. 92, 1303–1312. doi: 10.1016/j.joei.2018.10.004
Capanema, E. A., Balakshin, M. Y., and Kadla, J. F. (2004). A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J. Agric. Food Chem. 52, 1850–1860. doi: 10.1021/jf035282b
Chang, M. C. (2007). Harnessing energy from plant biomass. Curr. Opin. Chem. Biol. 11, 677–684. doi: 10.1016/j.cbpa.2007.08.039
Chen, F., and Dixon, R. A. (2007). Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 25, 759–761. doi: 10.1038/nbt1316
Cherubini, F. (2010). The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Conv. Manag. 51, 1412–1421. doi: 10.1016/j.enconman.2010.01.015
Cichorz, S., Gośka, M., and Rewers, M. (2015). Miscanthus: inter- and intraspecific genome size variation among M. × Giganteus, M. Sinensis, M. sacchariflorus accessions. acta biologica Cracoviensia s. Botanica 57, 104–113. doi: 10.1515/abcsb-2015-0013
Clifton, B., Schwarz, K.-U., and Hastings, A. (2015). History of the development of Miscanthus as a bioenergy crop: from small beginnings to potential realisation. Biol. Environ. 115, 1–13. doi: 10.3318/bioe.2015.05
Clifton-Brown, J. C., Lewandowski, I., Andersson, B., Basch, G., Christian, D. G., Kjeldsen, J. B., et al. (2001). Performance of 15 miscanthus genotypes at five sites in Europe. Agron. J. 93, 1013–1019. doi: 10.2134/agronj2001.9351013x
Elle, O., Richter, R., Vohland, M., and Weigelt, A. (2019). Fine root lignin content is well predictable with near-infrared spectroscopy. Sci. Rep. 9:6396. doi: 10.1038/s41598-019-42837-z
Feltus, F. A., and Vandenbrink, J. P. (2012). Bioenergy grass feedstock: current options and prospects for trait improvement using emerging genetic, genomic, and systems biology toolkits. Biotechnol. Biofuels 5:80. doi: 10.1186/1754-6834-5-80
Ge, C., Ai, X., Jia, S., Yang, Y., Che, L., Yi, Z., et al. (2019). Interspecific genetic maps in Miscanthus floridulus and M. sacchariflorus accelerate detection of QTLs associated with plant height and inflorescence. Mol. Genet. Genomics 294, 35–45. doi: 10.1007/s00438-018-1486-6
Ge, C., Liu, X., Liu, S., Xu, J., Li, H., Cui, T., et al. (2017). Miscanthus sp.: genetic diversity and phylogeny in China. Plant Mol. Biol. Rep. 35, 600–610. doi: 10.1007/s11105-017-1048-9
Hastings, A., Clifton-Brown, J., Wattenbach, M., Mitchell, C. P., Stampfl, P., and Smith, P. (2009). Future energy potential of miscanthusin Europe. GCB Bioenergy 1, 180–196. doi: 10.1111/j.1757-1707.2009.01012.x
Hatfield, R. D., Jung, H.-J. G., Ralph, J., Buxton, D. R., and Weimer, P. J. (1994). A comparison of the insoluble residues produced by the klason lignin and acid detergent lignin procedures. J. Sci. Food Agric. 65, 51–58. doi: 10.1002/jsfa.2740650109
Hayes, D. J. M. (2012). Development of near infrared spectroscopy models for the quantitative prediction of the lignocellulosic components of wet miscanthus samples. Bioresour. Technol. 119, 393–405. doi: 10.1016/j.biortech.2012.05.137
Hayes, D. J. M., Hayes, M. H. B., and Leahy, J. J. (2017). Use of near infrared spectroscopy for the rapid low-cost analysis of waste papers and cardboards. Faraday Discuss. 202, 465–482. doi: 10.1039/C7FD00081B
Heaton, E. A., Dohleman, F. G., and Long, S. P. (2008). Meeting US biofuel goals with less land: the potential of Miscanthus. Glob. Chang. Biol. 14, 2000–2014. doi: 10.1111/j.1365-2486.2008.01662.x
Heaton, J. (2008). Secondary analysis of qualitative data: an overview. Hist. Soc. Res. 33, 33–45. doi: 10.12759/hsr.33.2008.3.33-45
Hodkinson, T. R., Chase, M. W., Lledo, M. D., Salamin, N., and Renvoize, S. A. (2002). Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnLintron and trnL-F intergenic spacers. J. Plant Res. 115, 381–392. doi: 10.1007/s10265-002-0049-3
Imaizumi, T., and Kay, S. A. (2006). Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 11, 550–558. doi: 10.1016/j.tplants.2006.09.004
Jan, N., Ul-Hussain, M., and Andrabi, K. I. (2009). Cold resistance in plants: a mystery unresolved. Electr. J. Biotechnol. 12:3. doi: 10.2225/vol12-issue3-fulltext-3
Jensen, E., Farrar, K., Thomas-Jones, S., Hastings, A., Donnison, I., and Clifton-Brown, J. (2011). Characterization of flowering time diversity in miscanthus species. GCB Bioenergy 3, 387–400. doi: 10.1111/j.1757-1707.2011.01097.x
Jin, X., Chen, X., Shi, C., Li, M., Guan, Y., Yu, C. Y., et al. (2017). Determination of hemicellulose, cellulose and lignin content using visible and near infrared spectroscopy in Miscanthus sinensis. Bioresour. Technol. 241, 603–609. doi: 10.1016/j.biortech.2017.05.047
Jung, S.-J., Kim, S.-H., and Chung, I.-M. (2015). Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 83, 322–327. doi: 10.1016/j.biombioe.2015.10.007
Kim, S. J., Kim, M. Y., Jeong, S. J., Jang, M. S., and Chung, I. M. (2012). Analysis of the biomass content of various miscanthus genotypes for biofuel production in Korea. Ind. Crops Prod. 38, 46–49. doi: 10.1016/j.indcrop.2012.01.003
Kim, Y., Mosier, N. S., Ladisch, M. R., Pallapolu, V. R., Lee, Y. Y., Garlock, R., et al. (2011). Comparative study on enzymatic digestibility of switchgrass varieties and harvests processed by leading pretreatment technologies. Bioresour. Technol. 102, 11089–11096. doi: 10.1016/j.biortech.2011.06.054
Kleinert, M., and Barth, T. (2008). Phenols from Lignin. Chem. Eng. Technol. 31, 736–745. doi: 10.1002/ceat.200800073
Kuchelmeister, C., and Bauer, S. (2015). Rapid small-scale determination of extractives in biomass. Bioenergy Res. 8, 68–76. doi: 10.1007/s12155-014-9493-x
Lewandowski, I., and Schmidt, U. (2005). Nitrogen, energy and land use efficiencies of miscanthus, reed canary grass and triticale as determined by the boundary line approach. Agric. Ecosyst. Environ. 112, 335–346. doi: 10.1016/j.agee.2005.08.003
Lewandowski, I., Scurlock, J. M. O., Lindvall, E., and Christou, M. (2003). The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 25, 335–361. doi: 10.1016/S0961-9534(03)00030-8
Li, S. S., Zhou, H. F., Chen, W. L., Yan, J., Cai, Z., Wei, R. X., et al. (2019). Population genetics and evolutionary history of Miscanthus species in China. J. Syst. Evol. 57, 530–542. doi: 10.1111/jse.12497
Li, X., Sun, C., Zhou, B., and He, Y. (2015). Determination of hemicellulose, cellulose and lignin in moso bamboo by near infrared spectroscopy. Sci. Rep. 5:17210. doi: 10.1038/srep17210
Li, X., Weng, J. K., and Chapple, C. (2008). Improvement of biomass through lignin modification. Plant J. 54, 569–581. doi: 10.1111/j.1365-313X.2008.03457.x
Luo, H., Klein, I. M., Jiang, Y., Zhu, H., Liu, B., Kenttämaa, H. I., et al. (2016). Total utilization of miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain. Chem. Eng. 4, 2316–2322. doi: 10.1021/acssuschemeng.5b01776
Lygin, A. V., Upton, J., Dohleman, F. G., Juvik, J., Zabotina, O. A., Widholm, J. M., et al. (2011). Composition of cell wall phenolics and polysaccharides of the potential bioenergy crop -Miscanthus. GCB Bioenergy 3, 333–345. doi: 10.1111/j.1757-1707.2011.01091.x
McCalmont, J. P., Hastings, A., McNamara, N. P., Richter, G. M., Robson, P., Donnison, I. S., et al. (2017). Environmental costs and benefits of growing miscanthus for bioenergy in the UK. Glob Change Biol. Bioenergy 9, 489–507. doi: 10.1111/gcbb.12294
McKendry, P. (2002). Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 37–46. doi: 10.1016/S0960-8524(01)00118-3
Moukamnerd, C., Kino-oka, M., Sugiyama, M., Kaneko, Y., Boonchird, C., Harashima, S., et al. (2010). Ethanol production from biomass by repetitive solid-state fed-batch fermentation with continuous recovery of ethanol. Appl. Microbiol. Biotechnol. 88, 87–94. doi: 10.1007/s00253-010-2716-y
Papini-Terzi, F. S., Rocha, F. R., Vencio, R. Z., Felix, J. M., Branco, D. S., Waclawovsky, A. J., et al. (2009). Sugarcane genes associated with sucrose content. BMC Genomics 10:120. doi: 10.1186/1471-2164-10-120
Pauly, M., and Keegstra, K. (2008). Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 54, 559–568. doi: 10.1111/j.1365-313X.2008.03463.x
Pompeiano, A., Vita, F., Miele, S., and Guglielminetti, L. (2015). Freeze tolerance and physiological changes during cold acclimation of giant reed [Arundo donax(L.)]. Grass Forage Sci. 70, 168–175. doi: 10.1111/gfs.12097
Ramirez, J. A., Posada, J. M., Handa, I. T., Hoch, G., Vohland, M., Messier, C., et al. (2015). Near-infrared spectroscopy (NIRS) predicts non-structural carbohydrate concentrations in different tissue types of a broad range of tree species. Methods Ecol. Evol. 6, 1018–1025. doi: 10.1111/2041-210X.12391
Sims, R. E., Mabee, W., Saddler, J. N., and Taylor, M. (2010). An overview of second generation biofuel technologies. Bioresour. Technol. 101, 1570–1580. doi: 10.1016/j.biortech.2009.11.046
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., et al. (2012). Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory.NREL/TP-510-42618.
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008). Determination of Extractives in Biomass. Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory, 1-9.NREL/TP-510-42619. Available online at: http://www.nrel.gov/biomass/analytical_procedures.html
Sticklen, M. (2006). Plant genetic engineering to improve biomass characteristics for biofuels. Curr. Opin. Biotechnol. 17, 315–319. doi: 10.1016/j.copbio.2006.05.003
Sticklen, M. B. (2008). Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat. Rev. Genet. 9, 433–443. doi: 10.1038/nrg2336
Thygesen, A., Oddershede, J., Lilholt, H., Thomsen, A. B., and Ståhl, K. (2005). On the determination of crystallinity and cellulose content in plant fibres. Cellulose 12, 563–576. doi: 10.1007/s10570-005-9001-8
Upton, B. M., and Kasko, A. M. (2016). Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306. doi: 10.1021/acs.chemrev.5b00345
Vicentini, R., Felix Jde, M., Dornelas, M. C., and Menossi, M. (2009). Characterization of a sugarcane (Saccharum spp.) gene homolog to the brassinosteroid insensitive1-associated receptor kinase 1 that is associated to sugar content. Plant Cell Rep. 28, 481–491. doi: 10.1007/s00299-008-0656-0
Villaverde, J. J., Ligero, P., and de Vega, A. (2010). Miscanthus x giganteus as a source of biobased products through organosolv fractionation: a mini review. Open Agric. J. 4, 102–110. doi: 10.2174/1874331501004010102
Waclawovsky, A. J., Sato, P. M., Lembke, C. G., Moore, P. H., and Souza, G. M. (2010). Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 8, 263–276. doi: 10.1111/j.1467-7652.2009.00491.x
Wang, C., He, G., Meng, J., Wang, S., Kong, Y., Jiang, J., et al. (2020). Improved lignocellulose saccharification of a Miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy. doi: 10.1111/GCBB.12748. [Epub ahead of print].
Yang, H., Yan, R., Chen, H., Lee, D. H., and Zheng, C. (2007). Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86, 1781–1788. doi: 10.1016/j.fuel.2006.12.013
Yu, H., Wu, Z., and Chen, G. (2018). Catalytic gasification characteristics of cellulose, hemicellulose and lignin. Renew. Energy 121, 559–567. doi: 10.1016/j.renene.2018.01.047
Zhang, T., Wyman, C. E., Jakob, K., and Yang, B. (2012). Rapid selection and identification of Miscanthus genotypes with enhanced glucan and xylan yields from hydrothermal pretreatment followed by enzymatic hydrolysis. Biotechnol. Biofuels 5, 56–56. doi: 10.1186/1754-6834-5-56
Zhao, H., Li, Q., He, J., Yu, J., Yang, J., Liu, C., et al. (2014). Genotypic variation of cell wall composition and its conversion efficiency Inmiscanthus sinensis, a potential biomass feedstock crop in China. GCB Bioenergy 6, 768–776. doi: 10.1111/gcbb.12115
Keywords: Miscanthus, HPLC, cellulose, hemicellulose, lignin
Citation: Xu P, Cheng S, Han Y, Zhao D, Li H, Wang Y, Zhang G and Chen C (2020) Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China. Front. Chem. 8:595143. doi: 10.3389/fchem.2020.595143
Received: 15 August 2020; Accepted: 07 October 2020;
Published: 05 November 2020.
Edited by:
Uroš Novak, National Institute of Chemistry, SloveniaReviewed by:
Ashish Bohre, National Institute of Chemistry Slovenia, SloveniaKazimierz Warmiński, University of Warmia and Mazury in Olsztyn, Poland
Ming-Guo Ma, Beijing Forestry University, China
Copyright © 2020 Xu, Cheng, Han, Zhao, Li, Wang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuixia Chen, cxchen@sdau.edu.cn
†These authors have contributed equally to this work
 Pingping Xu1,2†
Pingping Xu1,2†  Cuixia Chen
Cuixia Chen