Effect of CeH2.73-CeO2 Composites on the Desorption Properties of Mg2NiH4
- Institute of Advanced Wear and Corrosion Resistant and Functional Materials, Jinan University, Guangzhou, China
A series of CeH2.73/CeO2 composites with different ratios of hydride and oxide phases are prepared from the pure cerium hydride via oxidation treatments in the air at room temperature, and they are subsequently doped into Mg2NiH4 by ball milling. The desorption properties of the as-prepared Mg2NiH4+CeH2.73/CeO2 composites are studied by thermogravimetry and differential scanning calorimetery. Microstructures are studied by scanning electron microscopy and transmission electron microscopy, and the phase transitions during dehydrogenation are analyzed through in situ X-ray diffraction. Results show that the initial dehydrogenation temperature and activation energy of Mg2NiH4 are maximally reduced by doping the CeH2.73/CeO2 composite with the same molar ratio of cerium hydride and oxide. In this case, the CeH2.73/CeO2 composite has the largest density of interface among them, and the hydrogen release effect at the interface between cerium hydride and oxide plays an efficient catalytic role in enhancing the hydrogen desorption properties of Mg2NiH4.
Introduction
With the advantages of abundant natural resources and no pollution to the environment, hydrogen has been widely considered as an ideal carbon-free energy carrier. Hydrogen energy storage technology is a prerequisite for the large-scale utilization of hydrogen energy. Light-weight solid-state hydrogen storage materials have been considered to be ideal candidates for hydrogen storage because of the high hydrogen storage density and security consideration (Mohtadi and Orimo, 2016; Wang et al., 2016). Among the existing solid-state hydrogen storage materials, Mg-based hydrogen storage materials are widely studied and considered as promising solid-state hydrogen storage materials due to the high hydrogen storage capacity, abundance on the earth and low production cost (Ouyang et al., 2013, 2017; Rusman and Dahari, 2016; Shao et al., 2018).
MgH2 and Mg2NiH4 are two typical Mg-based hydrogen storage materials with hydrogen densities of 7.6 wt and 3.6 wt%, respectively (Reilly and Wiswall, 1968; Bogdanović, 1984; Liu et al., 2016, 2019; Zhan et al., 2016; Chen et al., 2018; Ding et al., 2019). Nevertheless, the stable thermodynamics and slow kinetics of hydrogen storage properties of Mg-based materials lead to harsh conditions for dehydrogenation (Bogdanović et al., 2010; Jain et al., 2010). The hydrogen desorption temperatures of MgH2 and Mg2NiH4 are usually as high as 250–350C. Many efforts have been made by researchers to improve the kinetics and reduce the desorption temperatures of MgH2 and Mg2NiH4. The commonly conducted methods include mechanical alloying, doping catalysts, nanostructuring, surface modification and so on (Terashita et al., 1999; Lin et al., 2012a, 2016; Shao et al., 2012; Eleskandarany et al., 2016; Zhang et al., 2017; Khan et al., 2018; Xu et al., 2019).
Doping catalysts by ball milling is a commonly-used and efficient method to enhance the hydrogenation/dehydrogenation kinetics of Mg-based materials (Ouyang et al., 2014a,b; Wang and Wang, 2017). To date, many additives have been explored, including oxides (Barkhordarian et al., 2003), transition metals (Hanada et al., 2005), hydrides (Ma et al., 2017), carbon-based materials (Lototskyy et al., 2013) and so on. It has been demonstrated that compounds with higher valences show higher catalytic effect on the hydrogen storage performances of Mg-based materials than that of the lower valence compounds (Bobet et al., 2002). Because of the unique 4f electron of the Ce element, Ce-based compounds are widely used in the catalysis field (Trovarelli, 1996). Long et al. (2013) reported Mg-Ce oxide powders produced by an arc plasma evaporation method. As a result, enthalpies of hydrogenation and dehydrogenation for Mg/MgH2 reduce to −71.0 and 75.4 kJ/mol H2, respectively. Moreover, the hydrogenation activation energy is reduced to only 47.75 kJ/mol. The composite can absorb 4.07 wt%-H at 323 K in 10 h. Their study indicates that minor addition of Ce oxide can remarkably improve the hydrogenation kinetics of Mg/MgH2. We previously reported that CeF4 was an efficient catalyst to enhance the hydrogen storage properties of MgH2 (Lin et al., 2015), which can lead to reduced dehydrogenation temperature and activation energy because of the formation of new Mg-Ce-F species on the surface of MgH2. Gulicovski (Gulicovski et al., 2012) et al. prepared MgH2-CeO2 composite by ball milling of MgH2 and nano-CeO2 particles. The dehydrogenation activation energy is reduced to 60 ± 10 kJ/mol, indicating that the activation energy is sufficiently decreased by the catalytic effect of vacant CeO2 particles. We also developed a new symbiotic CeH2.73/CeO2 nano-catalyst, which was in-situ produced by controlling hydrogenation and oxidation treatments upon the amorphous Mg-Ce-Ni alloys (Lin et al., 2014), leading to significantly improved dehydrogenation performance of MgH2-based composite. Moreover, in situ TEM and DFT study show that the remarkable catalysis effect is attributed to the spontaneous hydrogen release effect at the interface between cerium oxide and hydride. Because the composite contains only major MgH2 but also minor Mg2NiH4, the effect of CeH2.73/CeO2 composite on the dehydrogenation properties of Mg2NiH4 has not been well-understood.
In order to clarify the effect of cerium hydride/oxide composites on the dehydrogenation properties of Mg2NiH4, in the present study, a series of CeH2.73/CeO2 composites with different ratios of cerium oxide and hydride were synthesized from pure cerium hydride via controlled oxidation treatments in the air at room temperature, and then they were doped into Mg2NiH4 by ball milling. The dehydrogenation properties of cerium hydride and cerium oxide-doped Mg2NiH4 were studied, and the initial dehydrogenation temperature and activation energy were characterized by TG-DSC and a Kissinger's method. Moreover, the phase transitions during dehydrogenation were analyzed through in situ XRD experiments.
Experimental Details
Materials
The Mg2NiH4 used in this experiment was prepared by a method of hydrogenation combustion synthesis method (HCS), which was carried out in Prof. Yunfeng Zhu's group in Nanjing Tech University (Gu et al., 2009; Zhu et al., 2017). CeH2.73 (purity >99%) was purchased from Hunan Research Institute of Non-ferrous metals. About 0.1 g CeH2.73 powder was located in a plastic bottle of 3 ml, in the Ar glove-box. Then it was transferred into the small side box of glove-box. The door of side box was then open to let air in for different time: 0.5, 1.5, 3, 10, and 60 min. The oxidized CeH2.73 became CeH2.73/CeO2 composites, and 2 mol% of CeH2.73/CeO2 composites were then doped into Mg2NiH4 by ball milling at 400 rpm for 4 h.
Characterizations
The phase structures were analyzed by X-ray diffraction (XRD), and the phase transition during dehydrogenation was analyzed by in situ XRD analysis. The XRD analysis was carried out by an X-ray diffractometer apparatus (UItima IV, Rigaku, Japan) with Cu-K α (λ = 0.15405 nm). The tube voltage and tube current were 40 kV and 40 mA, respectively. Thermogravimetry (TG) and Differential Scanning Calorimetery (DSC) were used to study the hydrogen desorption behaviors at different heating rates on a METTLER TGA/DSC 3+ synchronous thermal analyzer. The microstructures of the Mg2NiH4-Ce2.73/CeO2 composites were observed by backscattered electron imaging using scanning electron microscope (SEM) and transition electronic microscopy (TEM).
Results and Discussion
Preparation of the CeH2.73/CeO2 Catalysts
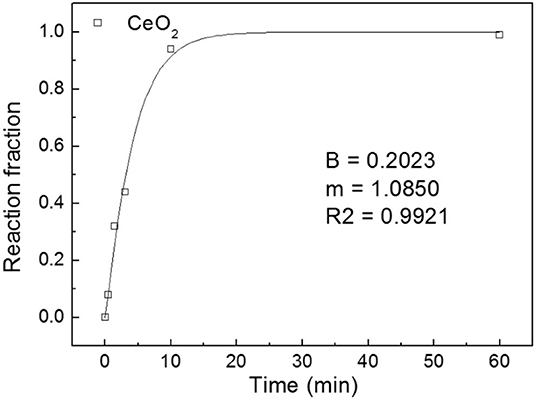
Six sets of CeH2.73/CeO2 composites prepared by oxidation treatments for different durations were analyzed by XRD. The CeH2.73/CeO2 composites are marked as S1, S2, S3, S4, S5, and S6, according to the oxidation duration of CeH2.73 of 0, 0.5, 1.5, 3, 10, and 60 min, respectively. The diffraction patterns are shown in Figure 1. With the increase of oxidation time, CeH2.73 gradually transforms into CeO2. After oxidation of 60 min, no CeH2.73 has left. The obtained XRD patterns were refined by a Jade 6.0 software and the phase contents were calculated by the K value (RIR) method. The relative contents of the CeO2 phase as increase of the oxidation time are shown in Figure 2. The oxidation data can be fitted by the Avrami–Erofeev equation deduced from the nucleation and growth process:
where α is the ratio of reacted material to total material, m and B are constants. The fitted m is 1.085, which is very close to 1.07, indicating the oxidation of CeH2.73 at room temperature is a three-dimensional interface reaction process (Lin et al., 2012b). For the S4 sample, the relative content of CeH2.73 is 54.2 wt%, and the relative content of CeO2 phase is 45.8 wt%, indicating almost the same molar ratio of cerium hydride and oxide when the oxidation time is around 3 min. After the oxidation treatments, the samples were doped into Mg2NiH4 by ball milling.
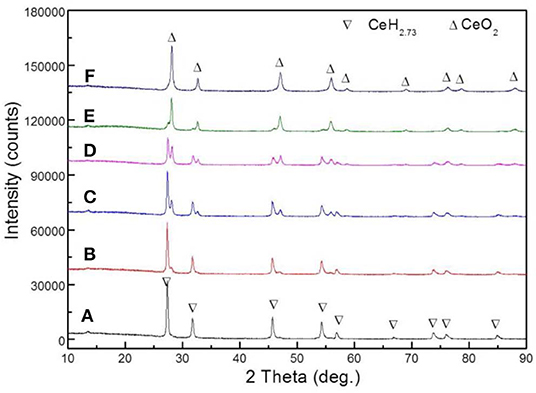
Figure 1. XRD images of six groups of CeH2.73/CeO2 composite catalysts obtained by oxidation of CeH2.73 for different durations: (A) 0 min, S1, (B) 0.5 min, S2, (C) 1.5 min, S3, (D) 3 min, S4, (E) 10 min, S5, (F) 60 min, S6.
In order to understand the morphologies of the CeH2.73/CeO2 composite doped Mg2NiH4, back scattering scanning electron microscopy (BSEM) was carried out. Because the S1–S4 sample will continue to oxidize in the air, the S5 doped Mg2NiH4 sample was selected as the experimental material. The morphologies of the as-prepared CeH2.73/CeO2 composite (S5 sample) and the ball-milled Mg2NiH4 + S5 sample are shown in Figure 3. It could be clearly seen that the particle size of the as-prepared CeH2.73/CeO2 composite is about 200–400 nm. After ball milling, the particle size of the CeH2.73/CeO2 composite is greatly reduced to below 30–100 nm. Figure 3B shows that the CeH2.73/CeO2 composites, which are brighter particles, are uniformly distributed in the Mg2NiH4 matrix. The homogeneous CeH2.73/CeO2 composites could be beneficial for catalyzing hydrogen storage properties for the Mg-based materials (Hong et al., 2009; Shao et al., 2011; Li et al., 2018).
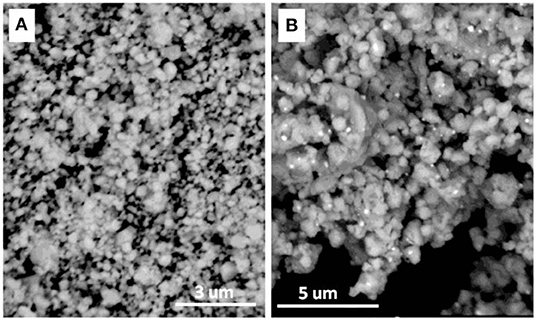
Figure 3. BSEM image of (A) the as-oxidized CeH2.73/CeO2 composite (S5) and (B) the ball-milled Mg2NiH4 + S5 sample.
Dehydrogenation of Mg2NiH4 + CeH2.73/CeO2 Composites
The decomposition behaviors of the ball-milled Mg2NiH4+CeH2.73/CeO2 composites are studied by TG-DSC synchronous thermal analyzer as shown in Figure 4. The DSC study of Mg2NiH4 + CeH2.73/CeO2 composites are at a heating rate of 20 K/min, showing the dehydrogenation initial temperature of Mg2NiH4 decreases after addition of CeH2.73/CeO2 composites. Among the six sets of catalysts, the S4 sample obtained by oxidation of CeH2.73 for 3 min, exhibits the highest catalytic effect on reducing the dehydrogenation temperature of Mg2NiH4. The dehydrogenation temperature is decreased to 267°C, which is about 17°C lower than the that of as-milled Mg2NiH4 (284°C).
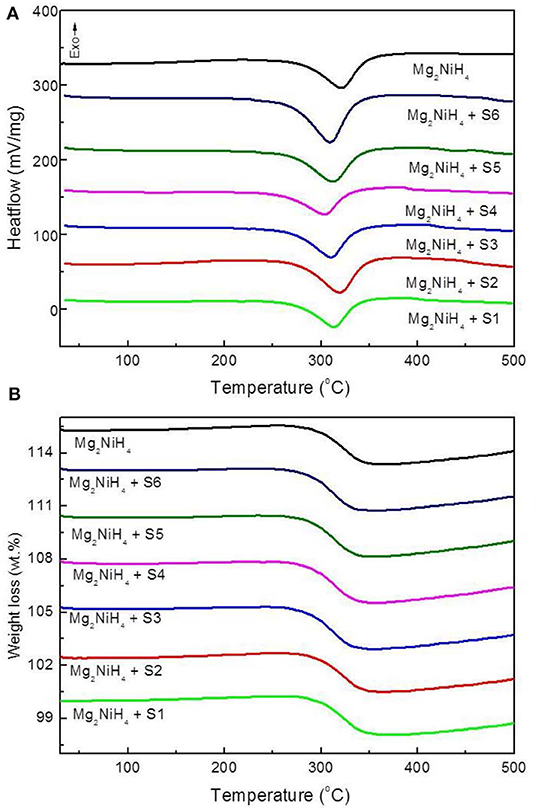
Figure 4. (A) DSC and (B) TG curves of the ball-milled Mg2NiH4-CeH2.73/CeO2 composites materials, at a heating rate of 20 K/min.
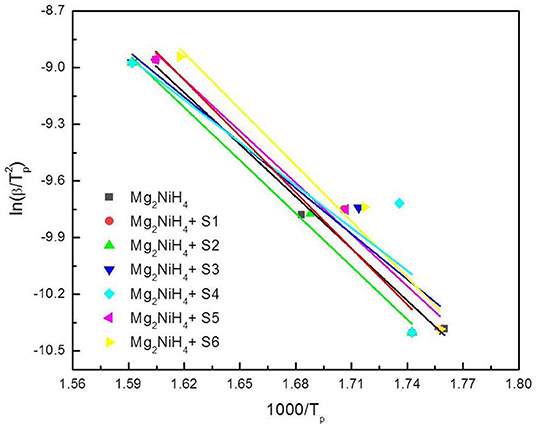
To further elucidate the dehydrogenation activation energy of the Mg2NiH4-CeH2.73/CeO2 composites, DSC experiments for the Mg2NiH4 + CeH2.73/CeO2 composites at heating rates of 10 K/min and 50 K/min were carried out (results not shown). The activation energy of the dehydrogenation process was calculated by using the Kissinger's method (Kissinger, 1957),
where Edes is the dehydrogenation activation energy, Tm, β, R, and C are the peak temperature, heating rate of DSC experiments, gas constant and another constant, respectively. The relation between Tm and β is linearly fitted as plotted in Figure 5, and the activation energy was summarized in Table 1. Results show that the S4 sample by oxidation of CeH2.73 for 3 min exhibits the best catalytic effect on reducing the dehydrogenation activation energy of Mg2NiH4 (from 76.3 kJ/mol to 62.6 kJ/mol). Zhang (Zhang et al., 2018) et al. suggested that alloying Mg2Ni with Ti, V, Fe, or Si could reduce the hydrogenation and dehydrogenation activation energies to 60–70 kJ/mol. Our study suggests that the CeH2.73/CeO2 composites are also efficient to reduce the dehydrogenation activation energy of Mg2NiH4.
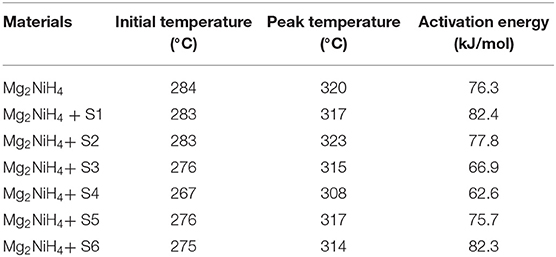
Table 1. Initial temperature, peak temperature and activation energy of dehydrogenation for the ball-milled Mg2NiH4 + CeH2.73/CeO2 composites (20 K/min).
Combining XRD and TG-DSC study, it indicates clearly that the initial dehydrogenation temperature and activation energy of Mg2NiH4 are maximally reduced as the cerium hydride and cerium oxide are in the same amount. These results well-accord with our previous finding on the effect of CeH2.73/CeO2 composites on the dehydrogenation properties of MgH2 (Lin et al., 2014), which means the CeH2.73/CeO2 composite with the same molar ratio of cerium hydride and oxide could be a good catalyst for the dehydrogenation properties of Mg-based hydrogen storage materials, including both MgH2 and Mg2NiH4.
Microstructures of the Mg2NiH4 + CeH2.73/CeO2 Composite
The morphology and microstructure of Mg2NiH4 and as-oxidized 10 min CeH2.73/CeO2 composite (S5) are shown in Figure 6a. From the HRTEM images in Figure 6b, it could be found that the composite matrix is Mg2NiH4, which lattice fringe corresponds to the lattice plane of (111) and (220). Particle sizes of the CeO2 nanoparticles are in a large range from 50–200 to 5–10 nm, which have been clearly shown in Figures 6a,d, respectively. Moreover, it could be seen that the in-situ generated CeO2 particles are embedded homogeneously in the Mg2NiH4 matrix after ball milling. The interfaces between Mg2NiH4 and CeO2 composite, as shown in Figures 6c,d, are contacted closely with each other. The homogeneously distributed catalysts can lead to high catalysis effect, and thus be beneficial for the hydrogenation/dehydrogenation properties of Mg2NiH4. Because of the too small amount of CeH2.73, we could not clarify their microstructures in the TEM study.
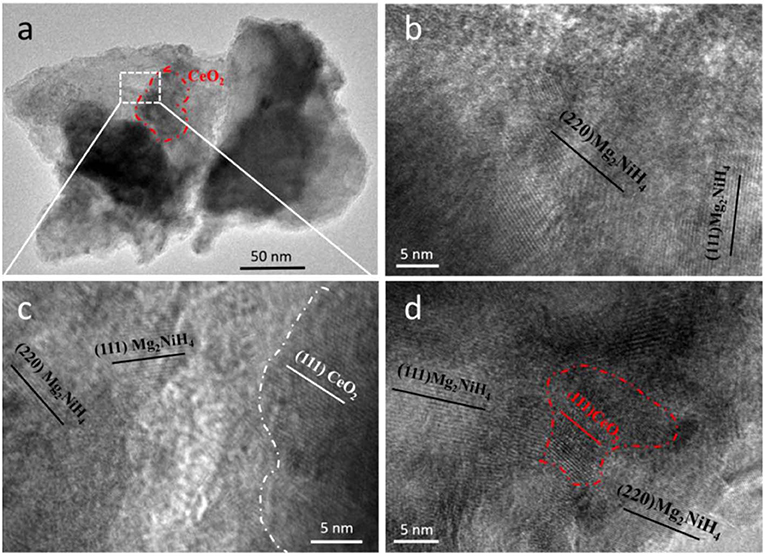
Figure 6. (a) TEM and (b–d) HRTEM images of the Mg2NiH4 + S5 composite. (b) shows the Mg2NiH4 matrix, (c) shows the magnified interface between Mg2NiH4 and CeO2, (d) shows a CeO2 particle with size of 5–10 nm.
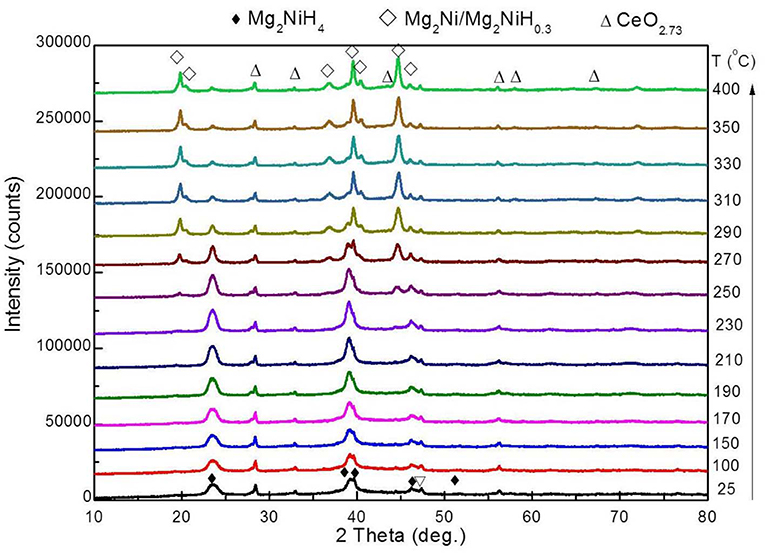
In situ XRD of Dehydrogenation
The phase transition of the Mg2NiH4 + S5 sample during dehydrogenation was further studied by in situ XRD. The obtained in situ XRD patterns are shown in Figure 7. The XRD pattern of Mg2NiH4 + S5 sample at room temperature contains the diffraction peaks of Mg2NiH4 and CeO2 because the XRD intensity of CeH2.73 is too weak. The diffraction peaks of CeO2 do not obviously change during the whole dehydrogenation process, indicating that the CeO2 phase might act as a catalyst of Mg2NiH4 dehydrogenation. When the temperature was raised to 250°C, the diffraction peak of Mg2NiH0.3/Mg2Ni phase appears, which means the dehydrogenation of Mg2NiH4 starts. As temperature increases, the intensity of Mg2NiH0.3/Mg2Ni phase gradually enhances, and that of the Mg2NiH4 peaks decreases. As temperature reaches about 330°C, the diffraction peaks of CeO2 remains unchanged and no MgO is found.
The in situ XRD patterns are then refined using Jade 6.0, and the relative contents of phases are calculated by the RIR method. At 270°C, the relative content of Mg2NiH0.3 phase is 47.1 wt%, and the relative content of Mg2NiH4 phase is 52.9 wt%. After heating to 290°C, the relative content of the two phases changes rapidly, and the relative content of Mg2NiH0.3 phase increases to 81.8 wt% and Mg2NiH4 The relative content of the phase was reduced to 18.2 wt%. Subsequently, the rate of phase change during the heating process was significantly slowed down. The relative content of Mg2NiH0.3 phase was 89.1 wt% at 400°C, and the relative content of Mg2NiH4 phase reduces to about 10.9 wt%.
Conclusion
In summary, CeH2.73/CeO2 composites with different proportions of cerium hydride and oxide are synthesized from pure cerium hydride via oxidation treatments in the air at room temperature. Oxidation time of 3 min leads to formation of CeH2.73/CeO2 composite with the same molar ratio of cerium hydride and oxide, which maximally reduces the initial dehydrogenation temperature and activation energy of Mg2NiH4. The CeH2.73/CeO2 composite with the same molar ratio is a good catalyst for reducing dehydrogenation temperatures of Mg-based materials.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
KW and DC: methodology and writing - original draft. KS and TX: methodology and formal analysis. PZ: resources and supervision. WL: supervision and project administration. H-JL: conceptualization, resources, writing - review and editing, supervision, and funding acquisition.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Yunfeng Zhu for providing the Mg2NiH4. Financial supports from National Natural Science Foundation of China (No. 51601090), Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011985) and the Fundamental Research Funds for the Central Universities (No. 21619415) are appreciated.
References
Barkhordarian, G., Klassen, T., and Bormann, R. (2003). Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 49, 213–217. doi: 10.1016/S1359-6462(03)00259-8
Bobet, J. L., Chevalier, B., Song, M. Y., Darriet, B., and Etourneau, J. (2002). Hydrogen sorption of Mg-based mixtures elaborated by reactive mechanical grinding. J. Alloys Comp. 336, 292–296. doi: 10.1016/S0925-8388(01)01883-7
Bogdanović, B. (1984). Magnesium hydride: a homogeneous-catalysed synthesis and its use in hydrogen storage. Int. J. Hydrog. Energ. 9, 937–941. doi: 10.1016/0360-3199(84)90159-9
Bogdanović, B., Bohmhammel, K., Christ, B., Reiser, A., Schlichte, K., Vehlen, R., et al. (2010). Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 282, 84–92. doi: 10.1016/S0925-8388(98)00829-9
Chen, G., Zhang, Y., Chen, J., Guo, X., Zhu, Y., and Li, L. (2018). Enhancing hydrogen storage performances of MgH2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 29:265705. doi: 10.1088/1361-6528/aabcf3
Ding, X., Ding, H., Song, Y., Xiang, C., Li, Y., and Zhang, Q. (2019). Activity-tuning of supported co–ni nanocatalysts via composition and morphology for hydrogen storage in MgH2. Front. Chem. 7:937. doi: 10.3389/fchem.2019.00937
Eleskandarany, M. S., Shabaan, E., Ali, N., Aldakheel, F., and Alkandary, A. (2016). In-situ catalyzation approach for enhancing the hydrogenation/dehydrogenation kinetics of MgH2 powders with Ni particles. Sci. Rep. 6:37335. doi: 10.1038/srep37335
Gu, H., Zhu, Y., and Li, L. (2009). Hydrogen storage properties of Mg–Ni–Cu prepared by hydriding combustion synthesis and mechanical milling (HCS+MM). Int. J. Hydrog. Energ. 34, 2654–2660. doi: 10.1016/j.ijhydene.2009.01.068
Gulicovski, J., Rašković-Lovre, Ž., Kurko, S., Vujasin, R., Jovanović, Z., Matović, L., et al. (2012). Influence of vacant CeO2 nanostructured ceramics on MgH2 hydrogen desorption properties. Ceram. Int. 38, 1181–1186. doi: 10.1016/j.ceramint.2011.08.047
Hanada, N., Ichikawa, T., and Fujii, H. (2005). Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 109, 7188–7194. doi: 10.1021/jp044576c
Hong, S-H., Kwon, S. N., Bae, J-S., and Song, M. Y. (2009). Hydrogen-storage properties of gravity cast and melt spun Mg-Ni-Nb2O5 alloys. Int. J. Hydrog. Energ. 34, 1944–1950. doi: 10.1016/j.ijhydene.2008.12.015
Jain, I. P., Lal, C., and Jain, A. (2010). Hydrogen storage in Mg: a most promising material. Int. J. Hydrog. Energ. 35, 5133–5144. doi: 10.1016/j.ijhydene.2009.08.088
Khan, D., Zou, J., Zeng, X., and Ding, W. (2018). Hydrogen storage properties of nanocrystalline Mg2Ni prepared from compressed 2MgH2Ni powder. Int. J. Hydrog. Energ. 43, 22391–22400. doi: 10.1016/j.ijhydene.2018.10.055
Kissinger, H. E. (1957). Reaction kinetics in differential thermal analysis. Anal. Chem. 29, 1702–1706.
Li, J., Li, B., Shao, H., Li, W., and Lin, H. (2018). Catalysis and downsizing in mg-based hydrogen storage materials. Catalysts 8:89. doi: 10.3390/catal8020089
Lin, H. -J., Matsuda, J., Li, H. -W., Zhu, M., and Akiba, E. (2015). Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides. J. Alloys Compd. 645, S392–S396. doi: 10.1016/j.jallcom.2014.12.102
Lin, H. J., Ouyang, L. Z., Wang, H., Zhao, D. Q., Wang, W. H., Sun, D. L., et al. (2012a). Hydrogen storage properties of Mg–Ce–Ni nanocomposite induced from amorphous precursor with the highest Mg content. Int. J. Hydrog. Energ. 37, 14329–14335. doi: 10.1016/j.ijhydene.2012.07.073
Lin, H. -J., Tang, J. -J., Yu, Q., Wang, H., Ouyang, L. -Z., Zhao, Y. -J., et al. (2014). Symbiotic CeH2.73/CeO2 catalyst: a novel hydrogen pump. Nano Energ. 9, 80–87. doi: 10.1016/j.nanoen.2014.06.026
Lin, H. J., Wang, W. H., and Zhu, M. (2012b). Room temperature gaseous hydrogen storage properties of Mg-based metallic glasses with ultrahigh Mg contents. J Non-Crystalline Solids 358, 1387–1390. doi: 10.1016/j.jnoncrysol.2012.03.015
Lin, H. -J., Zhang, C., Wang, H., Ouyang, L., Zhu, Y., Li, L., et al. (2016). Controlling nanocrystallization and hydrogen storage property of Mg-based amorphous alloy via a gas-solid reaction. J. Alloys Compd. 685, 272–277. doi: 10.1016/j.jallcom.2016.05.286
Liu, Y., Du, H., Zhang, X., Yang, Y., Gao, M., and Pan, H. (2016). Superior catalytic activity derived from a two-dimensional Ti3 C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 52, 705–708. doi: 10.1039/C5CC08801A
Liu, Y., Zhu, J., Liu, Z., Zhu, Y., Zhang, J., and Li, L. (2019). Magnesium nanoparticles with Pd decoration for hydrogen storage. Front. Chem. 7:949. doi: 10.3389/fchem.2019.00949
Long, S., Zou, J., Liu, Y., Zeng, X., and Ding, W. (2013). Hydrogen storage properties of a Mg–Ce oxide nano-composite prepared through arc plasma method. J. Alloys Compd. 580 (Suppl. 1), S167–S170. doi: 10.1016/j.jallcom.2013.02.063
Lototskyy, M., Sibanyoni, J. M., Denys, R. V., Williams, M., Pollet, B. G., and Yartys, V. A. (2013). Magnesium–carbon hydrogen storage hybrid materials produced by reactive ball milling in hydrogen. Carbon 57, 146–160. doi: 10.1016/j.carbon.2013.01.058
Ma, X., Xie, X., Liu, P., Xu, L., and Liu, T. (2017). Synergic catalytic effect of Ti hydride and Nb nanoparticles for improving hydrogenation and dehydrogenation kinetics of Mg-based nanocomposite. Prog. Nat. Sci. Mater. Int. 27, 99–104. doi: 10.1016/j.pnsc.2016.12.013
Mohtadi, R., and Orimo, S. -I. (2016). The renaissance of hydrides as energy materials. [Review Article]. Nat. Rev. Mater. 2:16091. doi: 10.1038/natrevmats.2016.91
Ouyang, L., Cao, Z., Wang, H., Hu, R., and Zhu, M. (2017). Application of dielectric barrier discharge plasma-assisted milling in energy storage materials – a review. J. Alloys Compd. 691, 422–435. doi: 10.1016/j.jallcom.2016.08.179
Ouyang, L., Cao, Z., Wang, H., Liu, J., Sun, D., Zhang, Q., et al. (2014b). Enhanced dehydriding thermodynamics and kinetics in Mg (In)–MgF2 composite directly synthesized by plasma milling. J. Alloys Compd. 586, 113–117. doi: 10.1016/j.jallcom.2013.10.029
Ouyang, L. Z., Cao, Z. J., Wang, H., Liu, J. W., Sun, D. L., Zhang, Q. A., et al. (2013). Dual-tuning effect of In on the thermodynamic and kinetic properties of Mg2Ni dehydrogenation. Int. J. Hydrogen Energ. 38, 8881–8887. doi: 10.1016/j.ijhydene.2013.05.027
Ouyang, L. Z., Yang, X. S., Zhu, M., Liu, J. W., Dong, H. W., Sun, D. L., et al. (2014a). enhanced hydrogen storage kinetics and stability by synergistic effects of in situ formed CeH2.73 and Ni in CeH2.73-MgH2-Ni nanocomposites. J. Phys. Chem. C 118, 7808–7820. doi: 10.1021/jp500439n
Reilly, J. J., and Wiswall, R. H. (1968). Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 7, 2254–2256. doi: 10.1021/ic50069a016
Rusman, N. A. A., and Dahari, M. (2016). A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrog. Energ. 41, 12108–12126. doi: 10.1016/j.ijhydene.2016.05.244
Shao, H., Felderhoff, M., Schüth, F., and Weidenthaler, C. (2011). Nanostructured Ti-catalyzed MgH2 for hydrogen storage. Nanotechnology 22:235401. doi: 10.1088/0957-4484/22/23/23540
Shao, H., He, L., Lin, H., and Li, H.-W. (2018). Progress and trends in magnesium-based materials for energy-storage research: a review. Energ. Technol. 6, 445–458. doi: 10.1002/ente.201700401
Shao, H., Xin, G., Zheng, J., Li, X., and Akiba, E. (2012). Nanotechnology in Mg-based materials for hydrogen storage. Nano Energ. 1, 590–601. doi: 10.1016/j.nanoen.2012.05.005
Terashita, N., Takahashi, M., Kobayashi, K., Sasai, T., and Akiba, E. (1999). Synthesis and hydriding/dehydriding properties of amorphous Mg2 Ni1.9 M0.1 alloys mechanically alloyed from Mg2Ni0.9M0.1 (M=none, Ni, Ca, La, Y, Al, Si, Cu and Mn) and Ni powder. 5, 541–545.
Trovarelli, A. (1996). Catalytic properties of ceria and CeO2-containing materials. Catalysis Rev. 38, 439–520. doi: 10.1080/01614949608006464
Wang, H., Lin, H. J., Cai, W. T., Ouyang, L. Z., and Zhu, M. (2016). Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems – a review of recent progress. J. Alloys Compds. 658, 280–300. doi: 10.1016/j.jallcom.2015.10.090
Wang, Y., and Wang, Y. (2017). Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 27, 41–49. doi: 10.1016/j.pnsc.2016.12.016
Xu, C., Lin, H.-J., Wang, Y., Zhang, P., Meng, Y., Zhang, Y., et al. (2019). Catalytic effect of in situ formed nano-Mg2Ni and Mg2Cu on the hydrogen storage properties of Mg-Y hydride composites. J. Alloys Compds. 782, 242–250. doi: 10.1016/j.jallcom.2018.12.223
Zhan, L., Zhang, Y., Zhu, Y., Zhuang, X., Dong, J., Guo, X., et al. (2016). The electrochemical hydrogen storage properties of Mg67– xPdxCo33 (x= 1, 3, 5, 7) electrodes with BCC phase. J. Alloys Compds. 662, 396–403. doi: 10.1016/j.jallcom.2015.12.068
Zhang, J., Zhu, Y., Lin, H., Liu, Y., Zhang, Y., Li, S., et al. (2017). Metal hydride nanoparticles with ultrahigh structural stability and hydrogen storage activity derived from microencapsulated nanoconfinement. Adv. Mater. 29:1700760. doi: 10.1002/adma.201700760
Zhang, Y., Zhang, H., Ding, X., Liu, D., Zhang, Q., and Si, T. (2018). Microstructure characterization and hydrogen storage properties study of Mg2Ni0.92M0.08 (M = Ti, V, Fe or Si) alloys. Prog. Nat. Sci. Mater. Int. 28, 464–469. doi: 10.1016/j.pnsc.2018.06.006
Keywords: Hydrogen storage materials, dehydrogenation, Mg2NiH4, CeH2.73/CeO2, catalysts
Citation: Wu K, Cai D, Shao K, Xue T, Zhang P, Li W and Lin H-J (2020) Effect of CeH2.73-CeO2 Composites on the Desorption Properties of Mg2NiH4. Front. Chem. 8:293. doi: 10.3389/fchem.2020.00293
Received: 22 February 2020; Accepted: 24 March 2020;
Published: 15 April 2020.
Edited by:
Yongfeng Liu, Zhejiang University, ChinaReviewed by:
Yao Zhang, Southeast University, ChinaRapee Utke, Suranaree University of Technology, Thailand
Copyright © 2020 Wu, Cai, Shao, Xue, Zhang, Li and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huai-Jun Lin, hjlin@jnu.edu.cn
 Kaiyao Wu
Kaiyao Wu  Huai-Jun Lin
Huai-Jun Lin