The Pivotal Role of Chemical Modifications in mRNA Therapeutics
- 1Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, United States
- 2Broad Institute of MIT and Harvard, Cambridge, MA, United States
After over a decade of development, mRNA has recently matured into a potent modality for therapeutics. The advantages of mRNA therapeutics, including their rapid development and scalability, have been highlighted due to the SARS-CoV-2 pandemic, in which the first two clinically approved mRNA vaccines have been spotlighted. These vaccines, as well as multiple other mRNA therapeutic candidates, are modified to modulate their immunogenicity, stability, and translational efficiency. Despite the importance of mRNA modifications for harnessing the full efficacy of mRNA drugs, the full breadth of potential modifications has yet to be explored clinically. In this review, we survey the field of mRNA modifications, highlighting their ability to tune the properties of mRNAs. These include cap and tail modifications, nucleoside substitutions, and chimeric mRNAs, each of which represents a component of mRNA that can be exploited for modification. Additionally, we cover clinical and preclinical trials of the modified mRNA platform not only to illustrate the promise of modified mRNAs but also to call attention to the room for diversifying future therapeutics.
Introduction
mRNA has emerged as an important platform for gene therapies and vaccines, presenting a new opportunity to target previously challenging diseases. Although the concept of mRNA drugs was envisioned over 30 years ago (Wolff et al., 1990), they were considered too unstable and immunotoxic for clinical use (Weng et al., 2020). Nonetheless, research into the chemical modifications of mRNA has shown that it can be used as an effective therapeutic agent. Moreover, mRNA offers distinct advantages over traditional drugs (Sahin et al., 2014). Compared to DNA technology, mRNA avoids the risk of genomic integration, circumvents the need to enter the nucleus, and has a transient activity profile, desirable in many gene therapy applications. mRNA vaccines can also be developed rapidly, can produce high quantities of antigen with relatively low dosages, and are safer and more readily produced at scale than traditional vaccines. Such benefits have been showcased in the first clinically approved mRNA vaccines against SARS-CoV-2 (Dolgin, 2021a; Kis et al., 2021).
The use of unmodified mRNA as a therapeutic agent is presented with several challenges and risks. Exogenously delivered mRNA is intrinsically immunogenic, triggering several innate immune sensing pathways, which leads to the production of inflammatory cytokines and suppression of cellular translation, undesirable for the production of the therapeutic protein (Tatematsu et al., 2018). Although the immunostimulatory nature of RNA could provide adjuvant activity for vaccinations, the translational inhibition and directed degradation caused by unmodified exogenous mRNAs mitigate their success (Morais et al., 2021). Other therapeutic strategies employing mRNAs, such as protein replacement therapy or regenerative therapy, are even less amenable to the strong stimulation of the immune system. The short half-life of mRNA, owing to its instability to degradation by ribonucleases, further obstructs the therapeutic application of mRNAs, limiting the protein production possible by delivered drugs. Improving both the lifespan and the translational efficiency of mRNA, in addition to removing its immune-activating nature, is thus necessary for successful therapeutics.
These technical challenges have been met by the development of mRNA modifications. Natural RNA contains many types of modifications, hundreds of which have been characterized (Boccaletto et al., 2018; Nachtergaele and He, 2018). Additionally, it has long been known that various viruses and bacteria decorate their genetic material with modifications to evade immune recognition by their host. With this motivation, several modified nucleotides have been incorporated during the in vitro transcription of RNA to make a synonymous modified transcript. Prominent among these substitutions is the replacement of uridine with pseudouridine (Ψ) and its methylated analog N1-methyl-pseudouridine (m1Ψ), which have been shown to dramatically reduce the stimulation caused by transcripts carrying these modified nucleotides (Dolgin, 2021b; Morais et al., 2021). Other work focusing on the translational capacity of mRNA have yielded longer-lasting, more highly translated transcripts through both nucleotide substitutions as well as targeted modifications of the 5′-cap and poly(A) tail, important protective structures against mRNA degradation. This enhancement has been attributed to a combination of increased resistance to exonucleases, decreased immune-triggered repression of translation, and greater rates of initiation, giving rise to much more effective protein production per transcript, enabling the burgeoning field of mRNA therapeutics. In this Review, we provide an overview of mRNA modifications relevant to mRNA therapeutics, as well as the current state of modified mRNA in clinical and preclinical studies.
Overview of mRNA Therapeutics
Conceptually, mRNA therapeutics relies on the delivery of a synthetic transcript and subsequent translation of the encoded pharmacologically active protein product (Sahin et al., 2014). They are typically designed to be similar to natural mRNA, being able to harness the intracellular translational machinery in a functionally analogous or identical way. Natural mRNA is generally single-stranded, containing a coding sequence (CDS) which is translated to the protein product, flanked on either side by untranslated regions (UTRs). The 5′-end of mRNA in eukaryotes is marked with a 5′-cap, a modified 7-methyl-guanosine (m7G) residue, which modulates mRNA stability and lifespan (Charenton and Graille, 2018). Ribosomal translation typically is also cap-dependent, beginning with the association of eukaryotic initiation factor eIF4E to the transcript, after which the remainder of the translational machinery assembles and translates the encoded protein (Jackson et al., 2010). At the 3′-end, a chain of adenosine residues termed the poly(A) tail buffers against 3′ degradation and further regulates mRNA stability.
The synthesis of mRNA drugs is predominantly achieved by in vitro transcription (IVT) from a DNA template, using T3, T7, or SP6 polymerase in the presence of cap precursor and free nucleoside triphosphates. The transcript can alternatively be capped and polyadenylated post-IVT to produce a functional mRNA (Weissman, 2015; Muttach et al., 2017). During these stages, mRNA modifications can be introduced enzymatically through the incorporation of modified nucleotides and cap analogs in the reaction mixture (as discussed below). After purification of the newly synthesized mRNA, it is delivered to target cells to produce the pharmacologically active protein product, which is post-translationally modified and processed naturally. Substantial research has gone into delivering mRNAs, given their large molecular weight and highly negatively charged nature (Kowalski et al., 2019; Hou et al., 2021). In some applications, including cancer immunotherapy and stem cell therapy, mRNA can be electroporated into cells ex vivo, after which the transfected cells can be returned to the patient. More commonly, mRNA is encapsulated in a shell of neutrally or positively charged lipids, termed a lipid nanoparticle (LNP), which is endocytosed and promotes the release of the mRNA drug into the cytosol. A wide variety of LNPs has been designed to shield mRNAs from degradation, enhance cell transfection, and facilitate endosomal escape, resulting in overall increased delivery efficiency in preclinical models and demonstrating clinical success in the SARS-CoV-2 vaccines (Hou et al., 2021).
Once the mRNA drug reaches the site of interest, it begins producing of the desired protein, which can be used in a variety of therapeutic ways (Sahin et al., 2014). mRNA vaccines encode an antigenic-protein to stimulate the immune system. The vaccine can either be directly administered, through injection to intradermal, intramuscular, subcutaneous, and other locations; alternatively, ex vivo transfection of professional antigen-presenting cells, especially dendritic cells (DCs), has shown promise in treatments against cancer as a form of cell therapy. In either case, the translated protein is used to prime T cells and B cells in order to elicit protective immunity. Self-amplifying mRNAs, containing positive-sense RNA viral sequences that allow the mRNA to replicate, have also been tested for use in mRNA vaccines in order to increase the effective dose size and enable greater protein production (Bloom et al., 2021). Alternatively, mRNA can be used in protein replacement therapy to supplement the deficiency of a necessary protein or in regenerative medicine and gene therapy, reprogramming and gene-editing cells in order to restore function to target tissues and organs. The use of mRNA for remodeling otherwise untreatable tissues is promising for treating heart failure, neurodegeneration, etc. A more thorough description of mRNA therapeutic strategies is beyond the scope of this review and has been covered elsewhere (Sahin et al., 2014; Chandler, 2019; Zhang H.-X. et al., 2019; Damase et al., 2021).
Immunogenicity of Exogenous mRNA
Exogenously delivered, unmodified IVT mRNA is an inherent immunostimulant, which poses a challenge to the efficacy of exogenously delivered mRNA drugs. Innate immune sensor detection of mRNA leads to inhibition of the cellular translational machinery and increased degradation of the mRNA, preventing effective protein production (Figure 1). Studies outlined below have revealed not only the underlying pathways relevant to mRNA-induced activation of the immune system but also that modifications can suppress the immune response. Pathogen-associated molecular patterns recognized by immune sensors have been studied; double-stranded RNA and double-stranded secondary structures have been highly investigated (Chen and Hur, 2022). Meanwhile, single-stranded mRNA recognition patterns are still not well understood. The following sections summarize key pathways in mRNA-associated immune regulation and how modifications help synthetic mRNA escape immune activation.
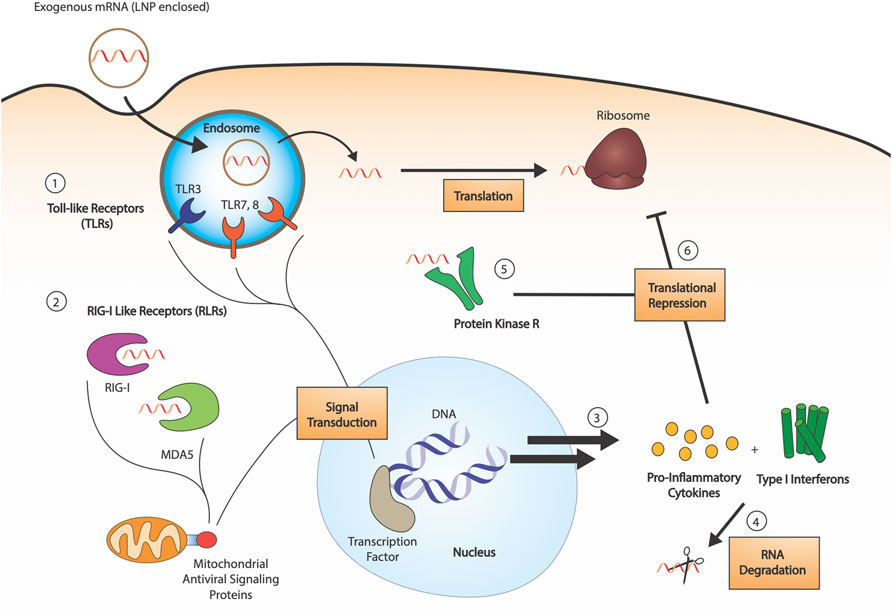
FIGURE 1. RNA sensing by the innate immune system. 1) RNA sensing Toll-like receptors (TLR3, TLR7, TLR8) are endosomal compartment receptors in sentinel cells, which activate upon late-endosomal acidification. Exogenous RNA is endocytosed by the cell, and pathogen associated molecular patterns are detected by the TLRs (dsRNAs, uridine-rich ribonucleosides, etc.). 2) RIG-I like receptors (RLRs) are cytosolic receptors present in all cell types. Both RIG-I and MDA5 are 5′-triphosphate dependent sensors, with some affinity for both dsRNA and ssRNA. Their activation leads to signal transduction through mitochondrial antiviral signaling proteins. 3) Innate immune detection of exogenous RNA leads to production of pro-inflammatory cytokines and type I interferons, which activate RNA degradation 4). 5) Protein kinase R (PKR) is a cytosolic sensor also involved in dsRNA sensing, the activation of which leads to phosphorylation of eukaryotic initiation factor eIF2α. 6) The combined action of produced cytokines and PKR leads to translational repression.
Toll-Like Receptors
Toll-Like Receptors (TLRs) are a class of membrane-bound receptors present in sentinel cells of the immune system, such as dendritic cells and macrophages. Ten functional TLR family members have been identified in humans, four of which are responsible for the detection of nucleic acids: TLR9 recognizes unmethylated CpG DNA, TLR3 recognizes double-stranded RNA (dsRNA), and TLR7 and TLR8 recognize single-stranded RNA (ssRNA) (Kawasaki and Kawai, 2014). More specifically, TLR7 has shown to be activated by uridine-containing ribonucleosides, in addition to dsRNA (Diebold et al., 2006), whereas TLR8 responds to various ssRNA oligonucleotides and RNA degradation products. Nonetheless, the particular sequence preference of these ssRNA sensors is still unknown (Schlee and Hartmann, 2016). The four nucleic acid specific receptors are localized to the endosomal compartment and rely on endosomal acidification for activation (Figure 1). Upon TLR engagement, interferons (IFNs) and other inflammatory cytokines are secreted, causing the upregulation of a variety of interferon-stimulated genes (ISGs), including RNA degrading enzymes such as 2′-5′-oligoadenylate synthase (OAS) and RNase L (Anderson et al., 2011).
Various nucleotide modifications have been shown to be impactful in evading TLR activation. The replacement of all uridine residues with modified nucleotides, including pseudouridine (Ψ) and 2-thiouridine (s2U), was shown by Karikó and coworkers to ablate the TLR immunogenicity of IVT mRNA (Karikó et al., 2005). Transcripts containing these modifications had decreased inflammatory signaling, corresponding to an enhanced translational capacity. Later work demonstrated that N1-methyl-pseudouridine (m1Ψ) substitution exhibited an even better performance, attributed to decreased activation of TLR3 compared to other modifications (Andries et al., 2015). Other modifications, such as 5-methylcytidine (m5C), 5-methyluridine (m5U), and N6-methyladenosine (m6A), have also been shown to have some immunosuppressive effects on TLR activity (Lou et al., 2021). Altogether, nucleotide replacement effectively suppresses TLR-associated immune signaling.
Retinoic Acid-Inducible Gene I Like Receptors
The retinoic acid-inducible gene I (RIG-I) like receptor family is a class of cytosolic pattern recognition receptors expressed in all cell types (Rehwinkel and Gack, 2020). This family consists of two primary receptors: the namesake RIG-I and melanoma differentiation-associated protein 5 (MDA5) (Figure 1). The two sensors are primarily associated with the detection of dsRNA: RIG-I senses short dsRNA segments containing 5′-triphosphates (Hornung et al., 2006), whereas MDA5 preferentially binds long dsRNAs. RIG-I can also detect 5′-triphosphate-containing ssRNAs, and the precise requirements for its activation are still being determined. MDA5 also has been suggested to detect the RNA of some ssRNA viruses, potentially due to the formation of secondary structures (Schlee, 2013). Despite the expanding understanding of their ligand range, the RIG-I-like receptors are a major part of the interferon response to RNA.
As a 5′-triphosphate is important for activation of RIG-I, the addition of a synthetic cap to IVT mRNA plays a critical role in evading RIG-I detection. The installation of an N7-methylguanosine (m7G) residue to the 5′ end of triphosphate mRNA decreases the RIG-I-dependent IFN secretion by synthetic transcripts (Hornung et al., 2006). Furthermore, modified nucleotide substitutions can also play an inhibitory role in RIG-I signaling, with Ψ, s2U, and 2′-O-methyluridine all reducing the total inflammatory cytokine-induced by 5′-triphosphate-containing mRNA (Karikó et al., 2008).
However, capping and nucleotide replacement are unable to fully abrogate the RIG-I dependent response to 5′-triphosphate mRNA (Schuberth-Wagner et al., 2015). Structural studies on RIG-I binding revealed that the receptor can accommodate the presence of an m7G moiety without drastic disruption of its triphosphate recognition (Devarkar et al., 2016). On the other hand, methylation of the 5′-most nucleotide of capped mRNA strongly interferes with RIG-I binding, and methylation of the second nucleotide is also implicated in decreasing RIG-I’s activation. Indeed, higher eukaryotic mRNA generally contain a 2′-O-methylated first nucleotide, termed a cap-1 structure in contrast with the unmethylated cap-0’s, and coronaviruses and poxviruses have been shown to employ cap one modifications to evade the innate immune system (Daffis et al., 2010). MDA5, although also 5′-triphosphate dependent, induces IFN production even in the presence of cap-0 structures but is inactive in the presence of cap-1 mRNA (Züst et al., 2011). Moreover, IFIT1, a major interferon induced gene, further recognizes 5′-triphosphates and cap-0 mRNA, inhibiting translation by competing with eIF4E, a cap-binding translation initiation factor (Habjan et al., 2013). Cap-1 demonstrated decreased IFIT1 binding activity, further assisting immune system evasion of modified mRNAs.
Protein Kinase R and eIF2α Phosphorylation
Protein kinase R (PKR) is an interferon-induced protein kinase (Figure 1), capable of being activated by either dsRNA (>33 bp) or ssRNA containing an exposed 5′-triphosphate (Nallagatla and Bevilacqua, 2008). Upon activation and autophosphorylation, PKR then phosphorylates the α-subunit of eIF2, the GTP-dependent translation initiation factor responsible for mediating binding of the first aminoacyl-tRNA (Met-tRNA) to the ribosome. Phosphorylation enhances the binding affinity of eIF2 for its GTP exchange factor, eIF2B, causing sequestration which results in impaired translation. Substitution of uridine using Ψ, s2U, 2′-dU, and other modifications are able to inhibit PKR signaling (Anderson et al., 2010). Notably, m1Ψ exhibited strong repression of PKR activation, outperforming Ψ and other modifications (Svitkin et al., 2017).
Future Directions
Much progress has been made in understanding the immune mechanisms and modifications relevant to mRNA therapeutics. Nonetheless, multiple confounding factors have complicated the research. Indeed, dsRNA contaminants cause residual stimulation of multiple innate immune sensors, and multiple purification methods have been developed to counteract this, including HPLC (Karikó et al., 2011) and RNase III digestion (Foster et al., 2019). Differences in the manufacturing process, such as the ratio of modified to unmodified nucleotides present in the IVT reaction mixture, also lead to differences in dsRNA byproduct formation (Nelson et al., 2020). Additionally, the immune response to mRNAs is highly dependent on the system under study, with variable results depending on target cell type, temperature, etc. (Uchida et al., 2015; Li et al., 2016) For example, whereas RNAs containing both m1Ψ substitution for uridine and m5C for cytidine had a higher translational yield in vitro, mRNA with only m1Ψ demonstrated higher performance in vivo in mice (Andries et al., 2015). As such, more investigation with standardized conditions and preparation is necessary to comprehensively understand the effects of mRNA modifications on immune responses.
Stability and Translational Efficiency of mRNA
The effectiveness of mRNA therapeutics depends highly on the amount of protein that can be produced from a given transcript. This translational yield is dependent both on the lifespan of mRNA as well as the rate of translational initiation. Years ago, significant doubt arose over the capability of mRNA as a drug, primarily due to its instability from both immune-induced degradation and its intrinsically shorter half-life from other therapeutic modalities. However, chemical modifications of mRNA targeted at decreasing its susceptibility to enzymatic degradation have been able to greatly increase the lifetime of IVT RNAs (Figure 2A). Additionally, the same modifications also affect the translational efficiency of delivered transcripts, leaving further potential for increasing the protein yield of mRNA drugs. Here, we review various modification strategies in order to improve the translational capacity of IVT mRNA.
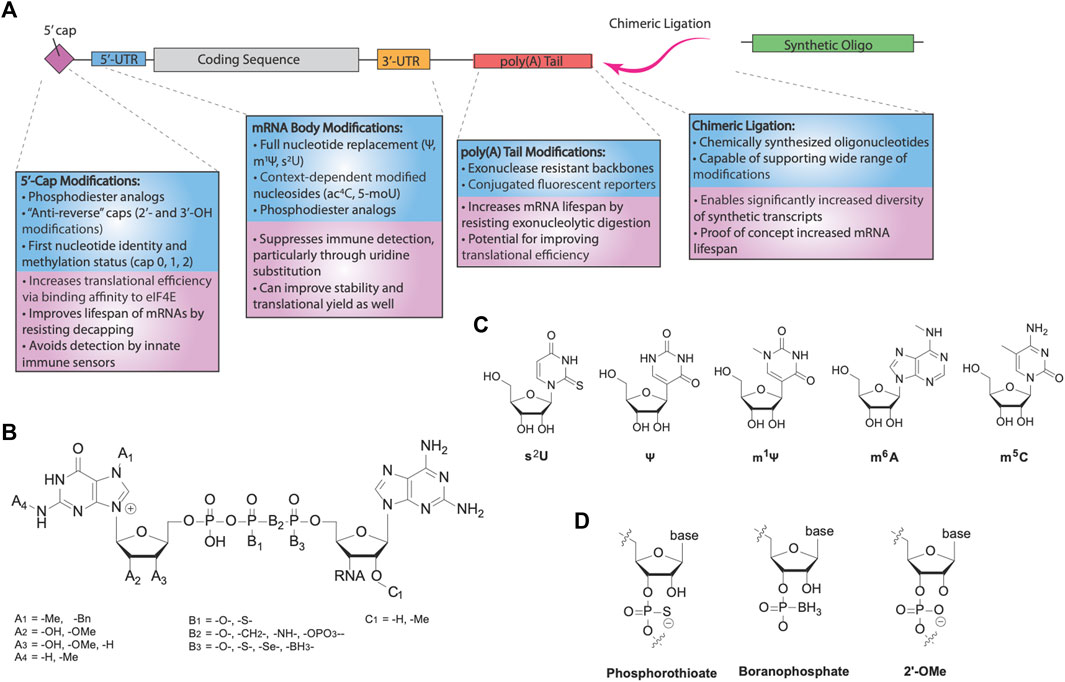
FIGURE 2. RNA modifications for mRNA therapeutics. (A) Categories of different modifications for mRNA. Modification of the cap and nucleotide substitution of the mRNA body are important for innate immune avoidance. Translational efficiency and mRNA stability are further modulated by various modifications, via increased eIF4E binding and reduced hydrolysis by nucleases. Additionally, chimeric ligation is a separate class of modification enabling incorporation of highly modified synthetic oligonucleotides, forming chimeric mocRNAs. (B) Chemical structure of 5′-caps. Eukaryotic caps are typically modified on the first base (A’s), triphosphate (B’s), or second base (C’s). (C) Common modified bases used for modification of mRNA. 2-thiouridine (s2U), pseudouridine (Ψ), and N1-methylpseudouridine (m1Ψ) are uridine substitutes, whereas N6-methyladenosine (m6A) is an adenosine substituent and 5-methylcytosine (m5C) is a cytosine substituent. (D) Common backbone modifications used for modification of mRNA. The phosphate backbone and 2′-OH are frequently modified.
The 5′-Cap
The degradation of mRNA is mediated primarily through two pathways: 5′ → 3′ and 3′ → 5′ degradation. In the 5′ → 3′ pathway, decapping of the 5′-end via cleavage of the α-β phosphodiester bond by the Dcp1/2 decapping complex precedes exonucleolytic degradation of the mRNA, primarily by the ribonuclease Xrn1 (Charenton and Graille, 2018). Thus, the stability of the 5′-cap is essential for controlling the lifespan of mRNA. The 5′-cap also exerts an effect on translational yield through modulating translational efficiency (Jackson et al., 2010). Translation is typically rate limited by the initiation step, a generally cap-dependent process reliant on binding of initiation factor eIF4E to the 5′-cap. Given its significance in both translational efficiency and mRNA stability, optimizing the 5′-cap of mRNA is crucial for designing more effective mRNA drugs (Figure 2A).
Two strategies are generally employed to cap synthetic mRNAs (Muttach et al., 2017). Recombinant viral capping enzymes, such as the vaccinia virus capping enzyme (VCE), can be used in conjunction with a methyltransferase in the presence of GTP and the 5′-triphosphate IVT mRNA to add a cap-1 structure. More commonly, however, co-transcriptional capping can be performed using a cap dinucleotide in the presence of the IVT polymerase mixture. The 3′-OH of the cap dinucleotide nucleophilically attacks the α phosphate of the next nucleotide, and elongation by the polymerase continues onwards. However, due to the similarity between the two 3′-OH’s present in the dinucleotide, capping with unmodified dinucleotides results in the wrong orientation at least half of the time, reducing the translational efficiency of the product mRNAs (Stepinski et al., 2001). To address this, Rhoads and others designed anti-reverse cap analogs (ARCAs), modified dinucleotides containing a 3′-O, 7′-dimethylguanosine or 3′-deoxy-7-methylguanosine, preventing incorrect incorporation into synthetic transcripts and more than doubling their translational efficiencies relative to unmodified cap dinucleotides. Alternative modifications were also shown to enforce the correct orientation, including 2′-O-methylation (Jemielity et al., 2003) and N7-benzyl-N2-methyl- dual modification (Grudzien et al., 2004). In all, the use of ARCAs allows for improved synthesis and function of mRNA drugs.
A series of ARCAs have since been synthesized and explored to improve the performance of synthetic transcripts while maintaining the anti-reverse function of these analogs (Figure 2B). For example, tetraphosphate analogs of the first-generation ARCA dinucleotides improved the translational yield of mRNAs, associated with the higher binding affinity for eIF4E (Muttach et al., 2017). Surprisingly, pentaphosphate counterparts did not recapitulate this trend, with a lower translational efficiency despite even higher binding affinities for eIF4E. This effect was attributed to slower release kinetics of eIF4E after initiation, indicating the strength of eIF4E binding does not directly imply higher translational efficiency. Meanwhile, modifications targeted towards improving IVT mRNA stability to decapping focused on altering the phosphodiester moiety. Grudzien et al. (2006) demonstrated that Dcp1/2 acts primarily on the α, β phosphodiester bond and replacement of the bridging oxygen with a methylene group (-CH2) blocked 5′ → 3′ degradation, albeit with some cost towards translational efficiency. Motivated by evidence that phosphorothioate modification of the mRNA backbone could also increase stability, later generations of cleavage-resistant caps used modifications of either the α or β phosphates with a phosphorothioate (Grudzien et al., 2007). Phosphorothioate modified caps yielded higher translational efficiencies than unmodified ARCAs, while simultaneously greatly improving the half-life of synthetic transcripts. Polysome profiling studies revealed that a greater rate of initiation is responsible for the increased translation rate, and phosphorothioate cap analogs have also been demonstrated to be effective in dendritic cells and in vivo in mice for vaccination and immune system priming (Kuhn et al., 2010). 1,2-dithiodiphosphates were also tested and demonstrated even higher stability profiles than phosphorothioate caps (Strenkowska et al., 2016).
A slew of other analogs have been explored as well, including phosphorothiolate (Wojtczak et al., 2018), phosphoroselenoate (Kowalska et al., 2009), boranophosphate (Kowalska et al., 2014), imidodiphosphate modified caps (Rydzik et al., 2012), etc. (Warminski et al., 2013; Shanmugasundaram et al., 2016; Dülmen et al., 2021; Wojcik et al., 2021) Locked nucleic acid (LNA) caps have also been investigated, in which the ribose is locked in an C3′-endo conformation by a bridging methylene group between the 2′ oxygen and 4′ carbon (Kore et al., 2009). Although LNAs have primarily been used in oligonucleotides, mRNAs capped by an LNA analog have recently been demonstrated to have increased translational efficiency and stability (Senthilvelan et al., 2021). Given the promise many of these modifications have demonstrated in in vitro experiments, the optimization of capped mRNAs using these analogs in vivo and in clinical applications holds promise for even more effective future drugs.
mRNA Body Modifications
Nucleoside and backbone modifications of the DNA encoded mRNA body are critical to enhance the protein production of mRNAs (Figures 2A,C,D). Ψ and m1Ψ are the most widely used body modifications for mRNA therapeutics. When incorporated as 100% replacement for U, they significantly increase the translational efficiency of mRNAs by turning off the innate immune-triggered eIF2α phosphorylation-dependent inhibition of translation (Karikó et al., 2008; Svitkin et al., 2017). Moreover, in comparison with Ψ, m1Ψ showed further enhancement of translational capacity, which has been linked to its capability of increasing ribosome density on the mRNA. Specifically, the additional methyl group on m1Ψ blocks hydrogen bonding at the N1 position, despite resulting in ribosome pausing, dramatically increasing the ribosome loading per mRNA (Svitkin et al., 2017), which may potentially increase translation initiation and prevent mRNA from entering degradation pathways. Thus, full-length body modifications using immunosuppressive and translation-enhancing modified nucleosides can generate mRNA drugs with greatly improved translational capacity.
Earlier attempts of backbone modification via IVT incorporation of phosphorothioates showed successful translation in reconstituted E. coli in vitro translation system (Ueda et al., 1991; Tohda et al., 1994). A recent study further uncovered that introduction of phosphorothioates to the 5′-UTR at either cytidine or both cytidine and uridine increases translational efficiency via faster initiation, even at the expense of elongation processivity (Kawaguchi et al., 2020). Other familiar modifications, including m6A and s2U, can increase RNA stability by decreased activation of the 2′-5′-oligoadenylate synthetase system (OAS), an interferon associated pathway that leads to RNase L activation (Anderson et al., 2011). In addition, some modifications have been revealed to exert a context-dependent effect on mRNA translational yield. The first nucleotide after the 5′-cap appears to play an important role in protein production (Sikorski et al., 2020). Adenosine and m6A residues at this site demonstrate higher translational yields, and 2′-O-methylation of the first nucleotide modulates protein production based on the identity of the first nucleotide. N4-acetylcytidine (ac4C) also increases transcript stability and translational yield in a position specific manner, increasing the speed of mRNA decoding when positioned at a wobble site (Arango et al., 2018). Another study revealed that 5-methoxyuridine (5-moU) is also capable of increasing mRNA stability, though further research is required to unravel the underlying mechanism of its enhancement (Li et al., 2016). In all, the diversity of potential chemical modifications gives substantial promise for even better-performing mRNA therapeutics.
The poly(A) Tail
The poly(A) tail is a chain of adenosine residues at the 3′-end of mRNA, which buffers it from degradation in a length-dependent fashion. Poly(A) shortening is catalyzed by the Pan2-Pan3 deadenylation complex, preceding both 3′ → 5′ and 5′ → 3′ degradation. Moreover, the tail and cap of actively translated mRNA interact, providing a mechanism by which the poly(A) tail can affect translational efficiency (Gallie, 1991; Goss and Kleiman, 2013). Although earlier works attempting to modify the poly(A) tail were met with disappointment (Rabinovich et al., 2006), more recent studies have indicated that there is still potential for improving stability and translational yield through poly(A) tail modifications (Figure 2A). Indeed, initial efforts to modify the poly(A) tail employed 3′-deoxyadenosine (cordycepin) or 8-aza-adenosine, which were shown to stabilize mRNA similarly to lengthening of the poly(A) tail, but were unable to outperform them in translational assays. Nonetheless, phosphorothioate modification of the poly(A) tail was able to exhibit increased stability and translational yield in some systems (Strzelecka et al., 2020). Boranophosphate substitution has also been tested, but underperformed compared to phosphorothioate functionalization. Interestingly, attachment of sulforhodamine B (SRB), a fluorescent small-molecule label, using click chemistry with incorporated 2′-azido-2′-dATP was able to substantially increase translational efficiency, though the mechanism of such enhancement has yet to be determined (Anhäuser et al., 2019). In all, despite the rather limited exploration of poly(A) tail modifications, future research into the poly(A) tail can likely further improve mRNA therapeutics.
Chimeric RNA
Recently, our group has demonstrated the generation of chimeric mRNAs, formed by the enzymatic ligation of an IVT synthesized mRNA transcript with a chemically synthesized oligonucleotide (Aditham et al., 2022) (Figure 2A). Termed mRNA-oligonucleotide conjugated RNA (mocRNA), this platform presents a novel method of circumventing translational restrictions on incorporating modified nucleotides and expands the possible space of synthetic transcripts for therapeutics. In our work, nuclease-resistant oligonucleotides were ligated to the poly(A) tail, resulting in 3–10 folds higher expression in human HeLa cells and rat primary neurons. The programmable and modular nature of mocRNAs enabled engineering mRNAs without interfering with the coding region. Future work into diversifying the ligated oligonucleotides will likely further illustrate the potential of chimeric RNAs.
Clinical and Preclinical Examples of Modified RNA
Various candidate mRNA therapeutic drugs have been examined both preclinically and clinically in the past years and have been reviewed extensively. Here, we highlight a number of these which employed modified mRNAs.
Vaccines
A number of vaccines based on modified mRNA have been developed (Zhang C. et al., 2019). Most prominent of the modified mRNA vaccines are those against SARS-CoV-2, advanced by Moderna (mRNA-1273) and BioNTech in partnership with Pfizer (BNT-162b2). Both vaccines encode the prefusion conformation of spike glycoprotein using N1-methyl-pseudouridine encoding mRNAs containing a 5' cap-1 (Corbett et al., 2020; World Health Organization 2020). mRNA modifications proved to be critical for the success of these vaccines, with similar products with unmodified mRNAs underperforming expectations (Morais et al., 2021; Nance and Meier, 2021). Moreover, the use of mRNA as a platform for a vaccine during the COVID-19 pandemic proved advantageous, owing to the rapid development and manufacturing speed of mRNA (Kis et al., 2021). Indeed, both vaccines were able to be produced within 10 months after the sequencing of the SARS-CoV-2 genome and proved to be over 90% effective (Polack et al., 2020; El Sahly et al., 2021). mRNA is also easily adaptable to new strains and mutations. The prefusion spike protein encoded in the aforementioned vaccines uses missense mutations at two loci in the original sequence to enforce the proper immunogenic conformation. As the SARS-CoV-2 virus continues to evolve, the adjustability of the mRNA vaccine platform will be critical.
Various influenza virus vaccines using modified mRNA have also been under development (Dolgin, 2021c). A vaccine candidate against H10N8 and H7N9 entered phase I trials in 2015 (Feldman et al., 2019), and two other candidates (mRNA-1010 and PF-07252220) entered phase I trials in late 2021. mRNA-1010 is a quadrivalent vaccine against the H1N1, H3N2, Yamagata, and Victoria strains, whereas the PF-07252220 is currently a monovalent vaccine, which is planned to be combined into a bivalent or quadrivalent product in the future. A slew of other mRNA influenza vaccine candidates have also undergone preclinical testing. The advent of mRNA vaccines against the flu is particularly exciting, as traditional flu vaccines are often ineffective and inconsistently manufactured (Wu et al., 2017). Additionally, due to constraints on the time necessary to develop traditional vaccines, the yearly influenza vaccines are often disappointingly ineffective. On the other hand, mRNA can easily be adjusted to encode antigens for the precise strain of influenza relevant, and its scalability bypasses the error-prone egg-based method for producing traditional vaccines. Altogether, the growing interest in modified mRNA vaccines holds promise for flu vaccinations in the future.
Clinical trials have also been initiated for a number of other diseases, which have posed a challenge for traditional vaccines. Phase III trials for a modified mRNA vaccine against cytomegalovirus (CMV) began late in 2021, after promising early results (John et al., 2018). Phase I trials of a modified mRNA vaccine against HIV have also recently begun in January 2022. Preclinical studies have also been performed for modified mRNA vaccine candidates against Ebola (Meyer et al., 2018), Zika (Pardi et al., 2017) human metapneumovirus (hMPV) (Shaw et al., 2019), etc.
Due to the highly polymorphic nature of cancer profiles, effective therapeutic vaccines against cancer often require individualization. Modified mRNA has been used in multiple preclinical and clinical applications against cancer, primarily in direct vaccine injections. LNP encapsulated modified mRNAs encoding bispecific antibodies (Stadler et al., 2017), cytokines (Hewitt et al., 2019; Kranz et al., 2019; Vormehr, 2019), or chimeric antigens (Foster et al., 2019) have been investigated. A full coverage of mRNA in cancer therapeutics can be found in other reviews (Beck et al., 2021; Miao et al., 2021).
Replacement and Gene Therapy
Protein production from mRNA has also been investigated as a tool for replacement and gene therapies. As opposed to DNA-based therapies, modified mRNAs demonstrate a pulse-like expression profile and do not risk genomic integration - problems that hindered previous efforts in such therapeutic approaches.
Potential use of modified mRNA as a vector for reprogramming and regenerative medicine was first demonstrated in 2010, when Warren and others used repeated transfections of reprogramming factor-encoding mRNAs to generate pluripotent stem cells (iPSCs) from fibroblasts with relatively high efficiency (Warren et al., 2010). These mRNAs were modified with m5C and Ψ substitutions for C and U, respectively, reducing the innate immune response against ectopic mRNA, and improving viability of targeted cells. Interestingly, evidence suggests that some residual inflammatory signaling may actually play a role in assisting reprogramming (Lee et al., 2012), but the presence of immunosuppressive modification nonetheless helped avoid translational silencing of the transcripts and overstimulation of the immune system. Indeed, the repeated transfection regime was only made possible by suppression of the innate immune system, indicating an essential role for mRNA modifications. The use of mRNA reprogramming for iPSC generation therapeutically has been covered elsewhere (Shi et al., 2017; Warren and Lin, 2019).
In addition to reprogramming, modified mRNAs have significant therapeutic potential in regenerative medicine, especially in organs and tissues with little regenerative capacity. In the heart, VEGF-A expression from modified mRNA resulted in healthy regeneration of cardiac vasculature after myocardial infarction in mice and swine (Zangi et al., 2013; Carlsson et al., 2018). In contrast, DNA-based expression was prolonged and resulted in edema and death. Additionally, Phase II a trials of a modified mRNA encoding VEGF-A have also been performed in patients with coronary artery disease, with generally positive results (Anttila et al., 2020). Expression of other proteins, including PKM2, FSTL1, and IGF-1 were used to promote cell survival and cardiomyocyte regeneration in vivo, improving general pathophysiology (Kaur and Zangi, 2020). VEGF-A mRNA has also been tested for the treatment of type II diabetes, yielding enhancements in skin blood flow in a phase I trial (Gan et al., 2019). Thus, mRNA holds potential as a platform for VEGF-A induced revascularization.
Modified mRNAs have been used in a variety of other regenerative medicinal applications. Attempts to prevent cell death in neuronal tissue after ischemic attack (Fukushima et al., 2021), to induce regeneration following liver damage (Rizvi et al., 2021), etc. have been successful preclinically. Delivery of gene editing enzymes through the expression of modified mRNAs have also presented an opportunity for gene therapies, circumventing many previous challenges of such strategies (Zhang H.-X. et al., 2019). Finally, modified mRNAs may also be used for direct replacement therapies for deficient proteins, including surfactant protein B (SP-B) (Kormann et al., 2011), arginase 1 (ARG1) (Asrani et al., 2018), cytochrome c oxidase (SCO2) (Miliotou et al., 2021), etc. In all, modified mRNA-based gene therapies provide an opportunity to treat many previously challenging diseases.
Conclusion and Perspectives
With the increasing popularity and maturation of mRNA therapeutics, significant progress has been made in understanding the role of mRNA modifications in attuning their immunogenicity, stability, and translational efficiency. Nucleotide substitutions and cap modifications play important parts in reducing innate immune sensing of IVT mRNA. Furthermore, modifications promoting translational initiation increase the translational yield of modified RNAs, and modifications resisting degradation by decapping or deadenylation increase the half-life of mRNA drugs for more sustained expression. Research into mRNA modifications has yielded multiple candidate mRNA therapeutics undergoing clinical or preclinical trials, as well as effective SARS-CoV vaccines.
However, mRNA modifications have yet to be fully employed in therapeutics. The diversity of known modifications has not been reflected in current mRNA drug candidates, which primarily focus on substitutions of uridine with N1-methyl-pseudouridine and cap methylation state. Given the evidence that phosphodiester modifications, labeling of the poly(A) tail, and other nucleoside substitutions are capable of increasing the stability and translational yield of mRNA, many optimizations can likely be made to future mRNA therapeutics. Indeed, in addition to altering the necessary dosage of mRNA drugs, modifications could also foreseeably increase their shelf-life, which is currently one of the major criticisms of their practicality. Nonetheless, the sensitivity and context-dependence of modified mRNAs’ performance requires further efforts to parse the precise effects of mRNA modifications on immunosuppression, translation, and stability.
Additionally, further insight into biological pathways relevant to mRNA therapeutics may motivate the targeted use of modifications. The importance of poly(A) tail modifications on translational initiation have yet to be fully understood and leaves room for potential improvements. Similarly, advances and new techniques in sequencing technology have enabled the discovery of new therapeutically relevant modifications. Surveying the effects of these new modifications and the mechanisms underlying them could lead the way to even more effective therapeutics. Finally, on a more cautionary note, further research into the long-term effects of highly modified mRNAs (including downstream byproducts of modified bases) are desired for the safe use in mRNA therapeutics. Nonetheless, given recent advances in modified mRNAs, future mRNA therapies will likely be shaped by progress in RNA modifications and have unlimited potentials in treating other diseases beyond mRNA vaccines.
Author Contributions
Conceptualization, AL and XW; writing, review, and editing, AL and XW. Both authors have read and agreed to the published version of the manuscript.
Funding
The research performed in the Wang lab is funded by the Searle Scholars Program, Thomas D. and Virginia W. Cabot Professorship, Edward Scolnick Professorship, Merkin Institute Fellowship, Ono Pharma Breakthrough Science Initiative Award, and NIH DP2 New Innovator Award.
Conflict of Interest
XW is an inventor of chimeric-RNA related patent application.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Huilin Huang and Chenqi Yi for handling our manuscript. We also thank the reviewers for their constructive feedback during the revision.
References
Aditham, A., Shi, H., Guo, J., Zeng, H., Zhou, Y., Wade, S. D., et al. (2022). Chemically Modified mocRNAs for Highly Efficient Protein Expression in Mammalian Cells. ACS Chem. Biol. Artic. ASAP. doi:10.1021/acschembio.1c00569
Anderson, B. R., Muramatsu, H., Jha, B. K., Silverman, R. H., Weissman, D., and Kariko, K. (2011). Nucleoside Modifications in RNA Limit Activation of 2'-5'-oligoadenylate Synthetase and Increase Resistance to Cleavage by RNase L. Nucleic Acids Res. 39 (21), 9329–9338. doi:10.1093/nar/gkr586
Anderson, B. R., Muramatsu, H., Nallagatla, S. R., Bevilacqua, P. C., Sansing, L. H., Weissman, D., et al. (2010). Incorporation of Pseudouridine into mRNA Enhances Translation by Diminishing PKR Activation. Nucleic Acids Res. 38 (17), 5884–5892. doi:10.1093/nar/gkq347
Andries, O., Mc Cafferty, S., De Smedt, S. C., Weiss, R., Sanders, N. N., and Kitada, T. (2015). N1-methylpseudouridine-incorporated mRNA Outperforms Pseudouridine-Incorporated mRNA by Providing Enhanced Protein Expression and Reduced Immunogenicity in Mammalian Cell Lines and Mice. J. Control. Release 217, 337–344. doi:10.1016/j.jconrel.2015.08.051
Anhäuser, L., Hüwel, S., Zobel, T., and Rentmeister, A. (2019). Multiple Covalent Fluorescence Labeling of Eukaryotic mRNA at the Poly(A) Tail Enhances Translation and Can Be Performed in Living Cells. Nucleic Acids Res. 47 (7), e42.
Anttila, V., Saraste, A., Knuuti, J., Jaakkola, P., Hedman, M., Svedlund, S., et al. (2020). Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol. Ther. - Methods & Clin. Dev. 18, 464–472. doi:10.1016/j.omtm.2020.05.030
Arango, D., Sturgill, D., Alhusaini, N., Dillman, A. A., Sweet, T. J., Hanson, G., et al. (2018). Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 175 (7), 1872–1886. doi:10.1016/j.cell.2018.10.030
Asrani, K. H., Cheng, L., Cheng, C. J., and Subramanian, R. R. (2018). Arginase I mRNA Therapy - a Novel Approach to Rescue Arginase 1 Enzyme Deficiency. RNA Biol. 15 (7), 914–922. doi:10.1080/15476286.2018.1475178
Beck, J. D., Reidenbach, D., Salomon, N., Sahin, U., Türeci, Ö., Vormehr, M., et al. (2021). mRNA Therapeutics in Cancer Immunotherapy. Mol. Cancer 20, 69. doi:10.1186/s12943-021-01348-0
Bloom, K., van den Berg, F., and Arbuthnot, P. (2021). Self-amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 28, 117–129. doi:10.1038/s41434-020-00204-y
Boccaletto, P., Machnicka, M. A., Purta, E., Piątkowski, P., Bagiński, B., Wirecki, T. K., et al. (2018). MODOMICS: a Database of RNA Modification Pathways. 2017 Update. Nucleic Acids Res. 46, D303–D307. doi:10.1093/nar/gkx1030
Carlsson, L., Clarke, J. C., Yen, C., Gregoire, F., Albery, T., Billger, M., et al. (2018). Biocompatible, Purified VEGF-A mRNA Improves Cardiac Function after Intracardiac Injection 1 Week Post-myocardial Infarction in Swine. Mol. Ther. - Methods & Clin. Dev. 9, 330–346. doi:10.1016/j.omtm.2018.04.003
Chandler, R. J. (2019). Messenger RNA Therapy as an Option for Treating Metabolic Disorders. Proc. Natl. Acad. Sci. U.S.A. 116 (42), 20804–20806. doi:10.1073/pnas.1914673116
Charenton, C., and Graille, M. (2018). mRNA Decapping: Finding the Right Structures. Philos. Trans. R. Soc. Lond B Biol. Sci. 373. doi:10.1098/rstb.2018.0164
Chen, Y. G., and Hur, S. (2022). Cellular Origins of dsRNA, Their Recognition and Consequences. Nat. Rev. Mol. Cell Biol. 23, 286–301. doi:10.1038/s41580-021-00430-1
Corbett, K. S., Edwards, D. K., Leist, S. R., Abiona, O. M., Boyoglu-Barnum, S., Gillespie, R. A., et al. (2020). SARS-CoV-2 mRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature 586, 567–571. doi:10.1038/s41586-020-2622-0
Daffis, S., Szretter, K. J., Schriewer, J., Li, J., Youn, S., Errett, J., et al. (2010). 2′-O Methylation of the Viral mRNA Cap Evades Host Restriction by IFIT Family Members. Nature 468, 452–456. doi:10.1038/nature09489
Damase, T. R., Sukhovershin, R., Boada, C., Taraballi, F., Pettigrew, R. I., and Cooke, J. P. (2021). The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 9, 628137. doi:10.3389/fbioe.2021.628137
Devarkar, S. C., Wang, C., Miller, M. T., Ramanathan, A., Jiang, F., Khan, A. G., et al. (2016). Structural Basis for m7G Recognition and 2′-O-Methyl Discrimination in Capped RNAs by the Innate Immune Receptor RIG-I. Proc. Natl. Acad. Sci. U.S.A. 113 (3), 596–601. doi:10.1073/pnas.1515152113
Diebold, S. S., Massacrier, C., Akira, S., Paturel, C., Morel, Y., and Reis e Sousa, C. (2006). Nucleic Acid Agonists for Toll-like Receptor 7 Are Defined by the Presence of Uridine Ribonucleotides. Eur. J. Immunol. 36 (12), 3256–3267. doi:10.1002/eji.200636617
Dolgin, E. (2021c). CureVac COVID Vaccine Let-Down Spotlights mRNA Design Challenges. Nature 594, 483. doi:10.1038/d41586-021-01661-0
Dolgin, E. (2021a). How COVID Unlocked the Power of RNA Vaccines. Nature 589, 189–191. doi:10.1038/d41586-021-00019-w
Dolgin, E. (2021b). mRNA Flu Shots Move into Trials. Nat. Rev. Drug Discov. 20, 801–803. doi:10.1038/d41573-021-00176-7
Dülmen, M., Muthmann, N., and Rentmeister, A. (2021). Chemo-enzymatic Modification of the 5’-cap Maintains Translation and Increases Immunogenic Properties of mRNA. Angew. Chem. Int. Ed. 60, 13280–13286.
El Sahly, H. M., Baden, L. R., Essink, B., Doblecki-Lewis, S., Martin, J. M., Anderson, E. J., et al. (2021). Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 385, 1774–1785. doi:10.1056/nejmoa2113017
Feldman, R. A., Fuhr, R., Smolenov, I., Ribeiro, A., Panther, L., Watson, M., et al. (2019). mRNA Vaccines against H10N8 and H7N9 Influenza Viruses of Pandemic Potential Are Immunogenic and Well Tolerated in Healthy Adults in Phase 1 Randomized Clinical Trials. Vaccine 37 (25), 3326–3334. doi:10.1016/j.vaccine.2019.04.074
Foster, J. B., Choudhari, N., Perazzelli, J., Storm, J., Hofmann, T. J., Jain, P., et al. (2019). Purification of mRNA Encoding Chimeric Antigen Receptor Is Critical for Generation of a Robust T-Cell Response. Hum. Gene Ther. 30 (2), 168–178. doi:10.1089/hum.2018.145
Fukushima, Y., Uchida, S., Imai, H., Nakatomi, H., Kataoka, K., Saito, N., et al. (2021). Treatment of Ischemic Neuronal Death by Introducing Brain-Derived Neurotrophic Factor mRNA Using Polyplex Nanomicelle. Biomaterials 270, 120681. doi:10.1016/j.biomaterials.2021.120681
Gallie, D. R. (1991). The Cap and Poly(A) Tail Function Synergistically to Regulate mRNA Translational Efficiency. Genes Dev. 5 (11), 2108–2116. doi:10.1101/gad.5.11.2108
Gan, L.-M., Lagerström-Fermér, M., Carlsson, L. G., Arfvidsson, C., Egnell, A.-C., Rudvik, A., et al. (2019). Intradermal Delivery of Modified mRNA Encoding VEGF-A in Patients with Type 2 Diabetes. Nat. Commun. 10, 871. doi:10.1038/s41467-019-08852-4
Goss, D. J., and Kleiman, F. E. (2013). Poly(A) Binding Proteins: Are They All Created Equal? WIREs RNA 4 (2), 167–179. doi:10.1002/wrna.1151
Grudzien, E., Kalek, M., Jemielity, J., Darzynkiewicz, E., and Rhoads, R. E. (2006). Differential Inhibition of mRNA Degradation Pathways by Novel Cap Analogs. J. Biol. Chem. 281 (4), 1857–1867. doi:10.1074/jbc.m509121200
Grudzien, E., Stepinski, J., Jankowska-anyszka, M., Stolarski, R., Darzynkiewicz, E., and Rhoads, R. E. (2004). Novel Cap Analogs for In Vitro Synthesis of mRNAs with High Translational Efficiency. RNA 10 (9), 1479–1487. doi:10.1261/rna.7380904
Grudzien-Nogalska, E., Jemielity, J., Kowalska, J., Darzynkiewicz, E., and Rhoads, R. E. (2007). Phosphorothioate Cap Analogs Stabilize mRNA and Increase Translational Efficiency in Mammalian Cells. RNA 13 (10), 1745–1755. doi:10.1261/rna.701307
Habjan, M., Hubel, P., Lacerda, L., Benda, C., Holze, C., Eberl, C. H., et al. (2013). Sequestration by IFIT1 Impairs Translation of 2'O-Unmethylated Capped RNA. PLoS Pathog. 9 (10), e1003663. doi:10.1371/journal.ppat.1003663
Hewitt, S. L., Bai, A., Bailey, D., Ichikawa, K., Zielinski, J., Karp, R., et al. (2019). Durable Anticancer Immunity from Intratumoral Administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 11 (477), eaat9143. doi:10.1126/scitranslmed.aat9143
Hornung, V., Ellegast, J., Kim, S., Brzozka, K., Jung, A., Kato, H., et al. (2006). 5'-Triphosphate RNA Is the Ligand for RIG-I. Science 314, 994–997. doi:10.1126/science.1132505
Hou, X., Zaks, T., Langer, R., and Dong, Y. (2021). Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater 6, 1078–1094. doi:10.1038/s41578-021-00358-0
Jackson, R. J., Hellen, C. U. T., and Pestova, T. V. (2010). The Mechanism of Eukaryotic Translation Initiation and Principles of its Regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127. doi:10.1038/nrm2838
Jemielity, J., Fowler, T., Zuberek, J., Stepinski, J., Lewdorowicz, M., Niedzwiecka, A., et al. (2003). Novel "Anti-reverse" Cap Analogs with Superior Translational Properties. RNA 9 (9), 1108–1122. doi:10.1261/rna.5430403
John, S., Yuzhakov, O., Woods, A., Deterling, J., Hassett, K., Shaw, C. A., et al. (2018). Multi-antigenic Human Cytomegalovirus mRNA Vaccines that Elicit Potent Humoral and Cell-Mediated Immunity. Vaccine 36 (12), 1689–1699. doi:10.1016/j.vaccine.2018.01.029
Karikó, K., Buckstein, M., Ni, H., and Weissman, D. (2005). Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 23, 165–175. doi:10.1016/j.immuni.2005.06.008
Karikó, K., Muramatsu, H., Ludwig, J., and Weissman, D. (2011). Generating the Optimal mRNA for Therapy: HPLC Purification Eliminates Immune Activation and Improves Translation of Nucleoside-Modified, Protein-Encoding mRNA. Nucleic Acids Res. 39 (21), e142. doi:10.1093/nar/gkr695
Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., et al. (2008). Incorporation of Pseudouridine into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 16 (11), 1833–1840. doi:10.1038/mt.2008.200
Kaur, K., and Zangi, L. (2020). Modified mRNA as a Therapeutic Tool for the Heart. Cardiovasc Drugs Ther. 34, 871–880. doi:10.1007/s10557-020-07051-4
Kawaguchi, D., Kodama, A., Abe, N., Takebuchi, K., Hashiya, F., Tomoike, F., et al. (2020). Phosphorothioate Modification of mRNA Accelerates the Rate of Translation Initiation to Provide More Efficient Protein Synthesis. Angew. Chem. Int. Ed. 59 (40), 17403–17407. doi:10.1002/anie.202007111
Kawasaki, T., and Kawai, T. (2014). Toll-like Receptor Signaling Pathways. Front. Immunol. 5, 461. doi:10.3389/fimmu.2014.00461
Kis, Z., Kontoravdi, C., Shattock, R., and Shah, N. (2021). Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand. Vaccines 9, 3. doi:10.3390/vaccines9030205
Kore, A. R., Shanmugasundaram, M., Charles, I., Vlassov, A. V., and Barta, T. J. (2009). Locked Nucleic Acid (LNA)-modified Dinucleotide mRNA Cap Analogue: Synthesis, Enzymatic Incorporation, and Utilization. J. Am. Chem. Soc. 131 (18), 6364–6365. doi:10.1021/ja901655p
Kormann, M. S. D., Hasenpusch, G., Aneja, M. K., Nica, G., Flemmer, A. W., Herber-Jonat, S., et al. (2011). Expression of Therapeutic Proteins after Delivery of Chemically Modified mRNA in Mice. Nat. Biotechnol. 29 (2), 154–157. doi:10.1038/nbt.1733
Kowalska, J., Lukaszewicz, M., Zuberek, J., Darzynkiewicz, E., and Jemielity, J. (2009). Phosphoroselenoate Dinucleotides for Modification of mRNA 5′ End. ChemBioChem 10, 2469–2473. doi:10.1002/cbic.200900522
Kowalska, J., Wypijewska del Nogal, A., Darzynkiewicz, Z. M., Buck, J., Nicola, C., Kuhn, A. N., et al. (2014). Synthesis, Properties, and Biological Activity of Boranophosphate Analogs of the mRNA Cap: Versatile Tools for Manipulation of Therapeutically Relevant Cap-dependent Processes. Nucleic Acids Res. 42 (16), 10245–10264. doi:10.1093/nar/gku757
Kowalski, P. S., Rudra, A., Miao, L., and Anderson, D. G. (2019). Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 27 (4), 710–728. doi:10.1016/j.ymthe.2019.02.012
Kranz, L. M., Vormehr, M., Diesckmann, J., Lindemann, C., Eisel, D., Muik, A., et al. (2019). Complementary Effects of RNA Encoded, Extended Half-Life IL2 and IL7 Synergize in Modulating T Cell Responses and Anti-tumoral Efficacy. SITC. Abstract P620.
Kuhn, A. N., Diken, M., Kreiter, S., Selmi, A., Kowalska, J., Jemielity, J., et al. (2010). Phosphorothioate Cap Analogs Increase Stability and Translational Efficiency of RNA Vaccines in Immature Dendritic Cells and Induce Superior Immune Responses In Vivo. Gene Ther. 17, 961–971. doi:10.1038/gt.2010.52
Lee, J., Sayed, N., Hunter, A., Au, K. F., Wong, W. H., Mocarski, E. S., et al. (2012). Activation of Innate Immunity Is Required for Efficient Nuclear Reprogramming. Cell 151, 547–558. doi:10.1016/j.cell.2012.09.034
Li, B., Luo, X., and Dong, Y. (2016). Effects of Chemically Modified Messenger RNA on Protein Expression. Bioconjugate Chem. 27 (3), 849–853. doi:10.1021/acs.bioconjchem.6b00090
Lou, X., Wang, J.-J., Wei, Y.-Q., and Sun, J.-J. (2021). Emerging Role of RNA Modification N6-Methyladenosine in Immune Evasion. Cell Death Dis. 12, 300. doi:10.1038/s41419-021-03585-z
Meyer, M., Huang, E., Yuzhakov, O., Ramanathan, P., Ciaramella, G., and Bukreyev, A. (2018). Modified mRNA-Based Vaccines Elicit Robust Immune Responses and Protect Guinea Pigs from Ebola Virus Disease. J. Infect. Dis. 217 (3), 451–455. doi:10.1093/infdis/jix592
Miao, L., Zhang, Y., and Huang, L. (2021). mRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 20, 41. doi:10.1186/s12943-021-01335-5
Miliotou, A. N., Pappas, I. S., Spyroulias, G., Vlachaki, E., Tsiftsoglou, A. S., Vizirianakis, I. S., et al. (2021). Development of a Novel PTD-Mediated IVT-mRNA Delivery Platform for Potential Protein Replacement Therapy of Metabolic/genetic Disorders. Mol. Ther. - Nucleic Acids 26, 694–710. doi:10.1016/j.omtn.2021.09.008
Morais, P., Adachi, H., and Yu, Y. T. (2021). The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 9, 789427. doi:10.3389/fcell.2021.789427
Muttach, F., Muthmann, N., and Rentmeister, A. (2017). Synthetic mRNA Capping. Beilstein J. Org. Chem. 13, 2819–2832. doi:10.3762/bjoc.13.274
Nachtergaele, S., and He, C. (2018). Chemical Modifications in the Life of an mRNA Transcript. Annu. Rev. Genet. 52, 349–372. doi:10.1146/annurev-genet-120417-031522
Nallagatla, S. R., and Bevilacqua, P. C. (2008). Nucleoside Modifications Modulate Activation of the Protein Kinase PKR in an RNA Structure-specific Manner. RNA 14 (6), 1201–1213. doi:10.1261/rna.1007408
Nance, K. D., and Meier, J. L. (2021). Modifications in an Emergency: the Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent. Sci. 7, 748–756. doi:10.1021/acscentsci.1c00197
Nelson, J., Sorensen, E. W., Mintri, S., Rabideau, A. E., Zheng, W., Besin, G., et al. (2020). Impact of mRNA Chemistry and Manufacturing Process on Innate Immune Activation. Sci. Adv. 6 (26), eaaz6893. doi:10.1126/sciadv.aaz6893
Pardi, N., Hogan, M. J., Pelc, R. S., Muramatsu, H., Andersen, H., DeMaso, C. R., et al. (2017). Zika Virus Protection by a Single Low-Dose Nucleoside-Modified mRNA Vaccination. Nature 543, 248–251. doi:10.1038/nature21428
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615. doi:10.1056/nejmoa2034577
Rabinovich, P. M., Komarovskaya, M. E., Ye, Z.-J., Imai, C., Campana, D., Bahceci, E., et al. (2006). Synthetic Messenger RNA as a Tool for Gene Therapy. Hum. Gene Ther. 17 (10), 1027–1035. doi:10.1089/hum.2006.17.1027
Rehwinkel, J., and Gack, M. U. (2020). RIG-I-like Receptors: Their Regulation and Roles in RNA Sensing. Nat. Rev. Immunol. 20, 537–551. doi:10.1038/s41577-020-0288-3
Rizvi, F., Everton, E., Smith, A. R., Liu, H., Osota, E., Beattie, M., et al. (2021). Murine Liver Repair via Transient Activation of Regenerative Pathways in Hepatocytes Using Lipid Nanoparticle-Complexed Nucleoside-Modified mRNA. Nat. Commun. 12, 613. doi:10.1038/s41467-021-20903-3
Rydzik, A. M., Kulis, M., Lukaszewicz, M., Kowalska, J., Zuberek, J., Darzynkiewicz, Z. M., et al. (2012). Synthesis and Properties of mRNA Cap Analogs Containing Imidodiphosphate Moiety-Fairly Mimicking Natural Cap Structure, yet Resistant to Enzymatic Hydrolysis. Bioorg. Med. Chem. 20 (5), 1699–1710. doi:10.1016/j.bmc.2012.01.013
Sahin, U., Karikó, K., and Türeci, Ö. (2014). mRNA-based Therapeutics - Developing a New Class of Drugs. Nat. Rev. Drug Discov. 13, 759–780. doi:10.1038/nrd4278
Schlee, M., and Hartmann, G. (2016). Discriminating Self from Non-self in Nucleic Acid Sensing. Nat. Rev. Immunol. 16, 566–580. doi:10.1038/nri.2016.78
Schlee, M. (2013). Master Sensors of Pathogenic RNA - RIG-I like Receptors. Immunobiology 218 (11), 1322–1335. doi:10.1016/j.imbio.2013.06.007
Schuberth-Wagner, C., Ludwig, J., Bruder, A. K., Herzner, A.-M., Zillinger, T., Goldeck, M., et al. (2015). A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1-2′o-Methylated Self RNA. Immunity 43, 41–51. doi:10.1016/j.immuni.2015.06.015
Senthilvelan, A., Vonderfecht, T., Shanmugasundaram, M., Pal, I., Potter, J., and Kore, A. R. (2021). Trinucleotide Cap Analogue Bearing a Locked Nucleic Acid Moiety: Synthesis, mRNA Modification, and Translation for Therapeutic Applications. Org. Lett. 23 (11), 4133–4136. doi:10.1021/acs.orglett.1c01037
Shanmugasundaram, M., Charles, I., and Kore, A. R. (2016). Design, Synthesis and Biological Evaluation of Dinucleotide mRNA Cap Analog Containing Propargyl Moiety. Bioorg. Med. Chem. 24 (6), 1204–1208. doi:10.1016/j.bmc.2016.01.048
Shaw, C., Lee, H., Knightly, C., Kalidindi, S., Zaks, T., Smolenov, I., et al. (2019). Phase 1 Trial of an mRNA-Based Combination Vaccine against hMPV and PIV3. Open Forum Infect. Dis. 6 (Suppl. 2), S970.
Shi, Y., Inoue, H., Wu, J. C., and Yamanaka, S. (2017). Induced Pluripotent Stem Cell Technology: a Decade of Progress. Nat. Rev. Drug Discov. 16, 115–130. doi:10.1038/nrd.2016.245
Sikorski, P. J., Warminski, M., Kubacka, D., Ratajczak, T., Nowis, D., Kowalska, J., et al. (2020). The Identity and Methylation Status of the First Transcribed Nucleotide in Eukaryotic mRNA 5′ Cap Modulates Protein Expression in Living Cells. Nucleic Acids Res. 48 (4), 1607–1626. doi:10.1093/nar/gkaa032
Stadler, C. R., Bähr-Mahmud, H., Celik, L., Hebich, B., Roth, A. S., Roth, R. P., et al. (2017). Elimination of Large Tumors in Mice by mRNA-Encoded Bispecific Antibodies. Nat. Med. 23, 815–817. doi:10.1038/nm.4356
Stepinski, J., Waddell, C., Stolarski, R., Darzynkiewicz, E., and Rhoads, R. E. (2001). Synthesis and Properties of mRNAs Containing the Novel “Anti-reverse” Cap Analogs 7-Methyl(3’-O-methyl)GpppG and 7-methyl (3’-deoxy)GpppG. RNA 7 (10), 1486–1495.
Strenkowska, M., Grzela, R., Majewski, M., Wnek, K., Kowalska, J., Lukaszewicz, M., et al. (2016). Cap Analogs Modified with 1,2-dithiodiphosphate Moiety Protect mRNA from Decapping and Enhance its Translational Potential. Nucleic Acids Res. 44 (20), 9578–9590. doi:10.1093/nar/gkw896
Strzelecka, D., Smietanski, M., Sikorski, P. J., Warminski, M., Kowalska, J., and Jemielity, J. (2020). Phosphodiester Modifications in mRNA Poly(A) Tail Prevent Deadenylation without Compromising Protein Expression. RNA 26 (12), 1815–1837. doi:10.1261/rna.077099.120
Svitkin, Y. V., Cheng, Y. M., Chakraborty, T., Presnyak, V., John, M., and Sonenberg, N. (2017). N1-methyl-pseudouridine in mRNA Enhances Translation through eIF2α-dependent and Independent Mechanisms by Increasing Ribosome Density. Nucleic Acids Res. 45 (10), 6023–6036. doi:10.1093/nar/gkx135
Tatematsu, M., Funami, K., Seya, T., and Matsumoto, M. (2018). Extracellular RNA Sensing by Pattern Recognition Receptors. J. Innate Immun. 10, 398–406. doi:10.1159/000494034
Tohda, H., Chikazumi, N., Ueda, T., Nishikawa, K., and Watanabe, K. (1994). Efficient Expression of E. coli Dihydrofolate Reductase Gene by an In Vitro Translation System Using Phosphorothioate mRNA. J. Biotechnol. 34 (1), 61–69. doi:10.1016/0168-1656(94)90166-x
Uchida, S., Kataoka, K., and Itaka, K. (2015). Screening of mRNA Chemical Modification to Maximize Protein Expression with Reduced Immunogenicity. Pharmaceutics 7 (3), 137–151. doi:10.3390/pharmaceutics7030137
Ueda, T., Tohda, H., Chikazumi, N., Eckstein, F., and Watanabe, K. (1991). Phosphorothioate-containing RNAs Show mRNA Activity in the Prokaryotic Translation Systemsin Vitro. Nucl. Acids Res. 19 (3), 547–552. doi:10.1093/nar/19.3.547
Vormehr, M. (2019). Substantial Improvement of Cancer Immunotherapy by an RNA Encoded Extend-Ed Half-Life Interleukin-2 Variant. SITC. Abstract P626.
Warminski, M., Kowalska, J., Buck, J., Zuberek, J., Lukaszewicz, M., Nicola, C., et al. (2013). The Synthesis of Isopropylidene mRNA Cap Analogs Modified with Phosphorothioate Moiety and Their Evaluation as Promoters of mRNA Translation. Bioorg. Med. Chem. Lett. 23 (13), 3753–3758. doi:10.1016/j.bmcl.2013.05.001
Warren, L., and Lin, C. (2019). mRNA-based Genetic Reprogramming. Mol. Ther. 27 (4), 729–734. doi:10.1016/j.ymthe.2018.12.009
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y.-H., Li, H., Lau, F., et al. (2010). Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell 7 (5), 618–630. doi:10.1016/j.stem.2010.08.012
Weissman, D. (2015). mRNA Transcript Therapy. Expert Rev. vaccines 14 (2), 265–281. doi:10.1586/14760584.2015.973859
Weng, Y., Li, C., Yang, T., Hu, B., Zhang, M., Guo, S., et al. (2020). The Challenge and Prospect of mRNA Therapeutics Landscape. Biotechnol. Adv. 40, 107534. doi:10.1016/j.biotechadv.2020.107534
Wojcik, R., Baranowski, M. R., Markiewicz, L., Kubacka, D., Bednarczyk, M., Baran, N., et al. (2021). Novel N7-Arylmethyl Substituted Dinucleotide mRNA 5' Cap Analogs: Synthesis and Evaluation as Modulators of Translation. Pharmaceutics 13 (11). doi:10.3390/pharmaceutics13111941
Wojtczak, B. A., Sikorski, P. J., Fac-Dabrowska, K., Nowicka, A., Warminski, M., Kubacka, D., et al. (2018). 5′-Phosphorothiolate Dinucleotide Cap Analogues: Reagents for Messenger RNA Modification and Potent Small-Molecular Inhibitors of Decapping Enzymes. J. Am. Chem. Soc. 140 (18), 5987–5999. doi:10.1021/jacs.8b02597
Wolff, J. A., Malone, R. W., Williams, P., Chong, W., Acsadi, G., Jani, A., et al. (1990). Direct Gene Transfer into Mouse Muscle In Vivo. Science 247, 1465–1468. doi:10.1126/science.1690918
World Health Organization (2020). International Nonproprietary Names Programme. Messenger RNA Encoding the Full-Length SARS-CoV-2 Spike Glycoprotein, 11889.
Wu, N. C., Zost, S. J., Thompson, A. J., Oyen, D., Nycholat, C. M., McBride, R., et al. (2017). A Structural Explanation for the Low Effectiveness of the Seasonal Influenza H3N2 Vaccine. PLoS Pathog. 13 (10), e1006682. doi:10.1371/journal.ppat.1006682
Zangi, L., Lui, K. O., von Gise, A., Ma, Q., Ebina, W., Ptaszek, L. M., et al. (2013). Modified mRNA Directs the Fate of Heart Progenitor Cells and Induces Vascular Regeneration after Myocardial Infarction. Nat. Biotechnol. 31, 898–907. doi:10.1038/nbt.2682
Zhang, C., Maruggi, G., Shan, H., and Li, J. (2019). Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 10, 594. doi:10.3389/fimmu.2019.00594
Zhang, H.-X., Zhang, Y., and Yin, H. (2019). Genome Editing with mRNA Encoding ZFN, TALEN, and Cas9. Mol. Ther. 27 (4), 735–746. doi:10.1016/j.ymthe.2019.01.014
Keywords: mRNA, chemical modifications, RNA modifications, mRNA therapeutics, mRNA vaccine, mocRNA, chimeric mRNA
Citation: Liu A and Wang X (2022) The Pivotal Role of Chemical Modifications in mRNA Therapeutics. Front. Cell Dev. Biol. 10:901510. doi: 10.3389/fcell.2022.901510
Received: 22 March 2022; Accepted: 26 May 2022;
Published: 13 July 2022.
Edited by:
Huilin Huang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Jia Sheng, University at Albany, United StatesYun Bai, ShanghaiTech University, China
Yimeng Gao, Yale University, United States
Copyright © 2022 Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Wang, xwangx@mit.edu
 Albert Liu
Albert Liu Xiao Wang
Xiao Wang